Abstract
The human UV-damaged-DNA binding protein DDB has been linked to the repair deficiency disease xeroderma pigmentosum group E (XP-E), because a subset of XP-E patients lack the damaged-DNA binding function of DDB. Moreover, the microinjection of purified DDB complements the repair deficiency in XP-E cells lacking DDB. Two naturally occurring XP-E mutations of DDB, 82TO and 2RO, have been characterized. They have single amino acid substitutions (K244E and R273H) within the WD motif of the p48 subunit of DDB, and the mutated proteins lack the damaged-DNA binding activity. In this report, we describe a new function of the p48 subunit of DDB, which reveals additional defects in the function of the XP-E mutants. We show that when the subunits of DDB were expressed individually, p48 localized in the nucleus and p125 localized in the cytoplasm. The coexpression of p125 with p48 resulted in an increased accumulation of p125 in the nucleus, indicating that p48 plays a critical role in the nuclear localization of p125. The mutant forms of p48, 2RO and 82TO, are deficient in stimulating the nuclear accumulation of the p125 subunit of DDB. In addition, the mutant 2RO fails to form a stable complex with the p125 subunit of DDB. Our previous studies indicated that DDB can associate with the transcription factor E2F1 and can function as a transcriptional partner of E2F1. Here we show that the two mutants, while they associate with E2F1 as efficiently as wild-type p48, are severely impaired in stimulating E2F1-activated transcription. This is consistent with our observation that both subunits of DDB are required to stimulate E2F1-activated transcription. The results provide insights into the functions of the subunits of DDB and suggest a possible link between the role of DDB in E2F1-activated transcription and the repair deficiency disease XP-E.
The human UV-damaged-DNA binding protein has been linked to the repair deficiency disease xeroderma pigmentosum group E (XP-E). Cells from about 30% of XP-E patients (6 of 19) were shown to lack the damaged-DNA binding activity of DDB (3, 27). DDB was originally identified as an activity that binds to UV-damaged DNA (3). It has high affinities for the 6-4 photoproduct (14, 29, 36). In addition, DDB also binds to cisplatin-modified DNA (14). It has been proposed that the damaged-DNA binding activity of DDB is related to a potential DNA repair function (7, 15, 18, 20, 36). The microinjection of purified DDB overcomes the repair deficiencies in cells from XP-E patients lacking the damaged-DNA binding activity of DDB (20, 27). However, purified DDB has no significant effect in nucleotide excision repair assays in vitro (18). A recent study proposed that DDB functions as a repair protein in the context of chromatin structure (27). A repair function of DDB would be consistent with the fact that the p48 subunit of DDB possesses extensive sequence homology with the Cockayne syndrome protein CS-A, which is involved in transcription-coupled DNA repair (2, 9, 12, 19).
DDB also possesses a transcriptional function. The p125 subunit of DDB possesses homology with CPSF (4) and was implicated in the transcription of the apolipoprotein B gene. A highly purified preparation of p125 interacted with the apolipoprotein B promoter, and an antibody against p125 inhibited the transcriptional activity of this promoter in vitro (22). Hayes et al. (11) observed that DDB associated with the transcription factor E2F1 and stimulated E2F1-activated transcription. This study showed that DDB interacted with the activation domain of E2F1 in experiments that assayed interactions in vivo and in vitro. Moreover, E2F1 and DDB in HeLa nuclear extracts cofractionated through several steps of E2F1 purification, including an E2F-specific DNA affinity chromatography. More importantly, the coexpression of DDB with E2F1 resulted in a cooperative stimulation of transcription from an E2F1-regulated promoter. Using Gal4 fusion constructs, we also showed that a fusion construct containing the activation domain of E2F1 (residues 363 to 437) but not that of an unrelated transcription factor (hepatocyte nuclear factor 3), responded to the transcriptional stimulatory activity of DDB. The transcriptional stimulatory activity required both subunits (125 and 48 kDa) of DDB (11). These results clearly suggested a potential role for DDB in E2F1-activated transcription.
DDB is a target of several viral proteins. The large subunit of DDB, p125, was identified as a cellular target of the hepatitis B virus X protein (HBx) (23). The HBx protein, which is believed to be the oncoprotein encoded by hepatitis B virus, was shown to function as a transcriptional activator protein (35 and references therein). It was shown that the transcriptionally proficient HBx proteins were able to bind DDB (35). Moreover, mutants of HBx that are impaired in binding to p125 were also defective in their transcription activation function (35). Becker et al. (1) showed that cells expressing HBx exhibit a reduction in the DNA repair activity after UV irradiation. Mutational analysis of HBx also demonstrated a partial correlation between the reduction of repair activity in cells expressing HBx and the ability of HBx to bind p125 (1). The p125 subunit of DDB also interacts with the V proteins of paramyxovirus SV5, mumps virus, human parainfluenza virus, and measles virus (24). It has been postulated that the interaction between the V proteins and DDB plays a role in the pathogenic function of these viruses (24).
Nichols et al. (26) have characterized two naturally occurring mutants of DDB from XP-E patients. These are point mutations leading to single amino acid substitutions in the WD motif of the small subunit (p48) of DDB. These mutations in the small subunit of DDB correlated with a loss of the damaged-DNA binding activity of DDB. Hwang et al. (16) demonstrated that these naturally occurring mutants of DDB failed to activate damaged-DNA binding activity when expressed in mammalian cells. These authors also suggested that the two mutant p48 proteins were defective in activating the damaged-DNA binding function of p125.
We have now investigated the naturally occurring mutants of DDB for their abilities to participate in E2F1-activated transcription. Here, we show that the p48 subunit stimulated the nuclear localization of the p125 subunit of DDB. The naturally occurring mutants of p48 were severely impaired in stimulating the nuclear localization of p125. These mutant p48 proteins were able to interact with E2F1 but were unable to stimulate E2F1-activated transcription. The results are consistent with our previous observation that both subunits of DDB are required for stimulating E2F1-activated transcription, and they suggest a possible link between DDB’s role in E2F1-activated transcription and the repair deficiency disease XP-E.
MATERIALS AND METHODS
Cell culture.
U2OS and C33A cells were grown on 10-cm-diameter dishes in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum in 5% CO2.
Mammalian cell expression plasmids.
A mammalian cell expression vector, pCDNA3, was used to express the DDB subunits and E2F1 (11). The constructs expressing T7-tagged p48 and p125 were generated by PCR, in which the upstream primers contained sequences encoding the T7 epitope in frame with p48 or p125 amino acid sequences, as described previously (11). The PCR primers (11) used for wild-type T7-p48 were used to obtain mutants 2RO and 82TO in frame with T7. A similar PCR strategy was employed to generate the plasmids that express hemagglutinin (HA)-tagged p48. The pGEX-E2F1 construct has been described before (11). The construct expressing glutathione S-transferase (GST)-E2F4 was a gift from D. M. Livingston (Dana Farber Cancer Institute, Boston, Mass.). The construct expressing HBx was obtained by subcloning the X open reading frame (subtype adw2) from plasmid pSV-X (1) as a NotI-HindII fragment into the same restriction sites of the plasmid pRc/CMV (Invitrogen).
Cytosolic and nuclear extracts.
The cytosolic and nuclear extracts of U2OS cells were prepared following the procedure of Dignam et al. (6) with the following modifications. Cells in a hypotonic buffer were lysed by 30 strokes of a Kontes 2-ml tissue grinder. The nuclei were washed with hypotonic buffer and then extracted with high-salt buffer (0.5 M KCl). The extracts were not dialyzed. A second approach was also employed to obtain nuclear and cytosolic extracts, partly according to the method of Schreiber et al. (30). In this method cells were lysed in a hypotonic buffer containing 0.5% Nonidet P-40 (NP-40), the lysates were centrifuged for 30 s to pellet the nuclei, and the supernatant was directly assayed as cytosol. The nuclei were extracted by a 30-min incubation in ice with buffer A, which contained 20 mM HEPES (pH 7.9), 400 mM NaCl, 1 mM EDTA, 0.1% NP-40, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, and 20% glycerol. Extracted proteins were separated from the nuclei by centrifugation at 13,000 × g for 10 min.
p125 antisera and affinity purification.
Based on the observations made by Lee et al. (23), a chemically synthesized peptide, with the sequence QYDDGSGMKREATA, corresponding to p125 (peptide 2 in reference 23) was conjugated to maleimide-activated keyhole limpet hemocyanin protein (Pierce). The conjugate was used for rabbit immunizations and antiserum production. For affinity purification, the same peptide was coupled to cyanogen bromide-activated Sepharose 4B (Pharmacia), and the peptide-coupled Sepharose beads were used to purify the antibody according to a previously described procedure (10).
Immunoprecipitation and Western blotting.
Cells were harvested after DNA transfection. The harvested cells were washed twice with phosphate-buffered saline (PBS) and suspended in a lysis buffer (60 μl for cells from one 100-mm-diameter dish) that contained 20 mM HEPES (pH 7.9), 400 mM NaCl, 1 mM EDTA, 0.1% NP-40, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, and 10% glycerol. After an incubation at 4°C for 30 min, the lysates were centrifuged at 13,000 × g for 10 min. The supernatants were used for the immunoprecipitation experiments.
Agarose-linked T7 antibody (Novagen) and protein A-Sepharose-bound HA antibody (Santa Cruz) were employed. Cell lysates (0.5 mg) were incubated with beads containing antibodies for 2 h at 4°C. The beads were then collected by centrifugation. Precipitates were washed three times with 1 ml of buffer W (20 mM Tris-HCl [pH 7.8], 100 mM NaCl, 0.1% NP-40, and 1 mM EDTA). The bound proteins were subjected to Western blot analysis. Western blots were performed by using anti-rabbit and anti-mouse Fab fragments conjugated to horseradish peroxidase (Amersham) and Pierce Supersignal detection reagents according to the manufacturer’s instructions.
Immunostaining.
U2OS cells were plated on 10-cm-diameter dishes containing glass coverslips. After DNA transfection, cells were fixed with methanol at −20°C and permeabilized with 0.5% Triton X-100 in PBS for 10 min. Coverslips were blocked in 3% bovine serum albumin followed by incubation with anti-T7 tag (1:1,000 dilution) or affinity-purified anti-p125 antibodies (1:100) in PBS containing 3% bovine serum albumin and 0.1% Triton X-100. Cells were washed four times with PBS and 0.1% Triton X-100 for 5 min each and incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse (1:100 dilution; Gibco BRL) or donkey anti-rabbit immunoglobulin G (1:200 dilution; Jackson Laboratories, Inc.). After being washed, the coverslips were mounted on glass slides by using 20 μl of a mounting medium (15% [wt/vol] Vinol 205 polyvinyl alcohol, 33% [vol/vol] glycerol, 0.1% azide in PBS [pH 8.5]). Cells were visualized, and images were taken with a CLSM 510 microscope (Zeiss) and a 63× Achroplan water immersion objective.
DNA transfection and CAT assays.
Transient transfections were carried out by the calcium phosphate method as previously described (33, 34). Twenty micrograms of DNA was used per 100-mm-diameter plate. Each experiment was controlled for transfection efficiency by including 1 μg of pCMV–β-galactosidase in each transfection and then normalizing for β-galactosidase activity. Chloramphenicol acetyltransferase (CAT) assays were performed by the xylene extraction method of Seed and Sheen (31).
RESULTS
P48 stimulates nuclear accumulation of the p125 subunit of DDB.
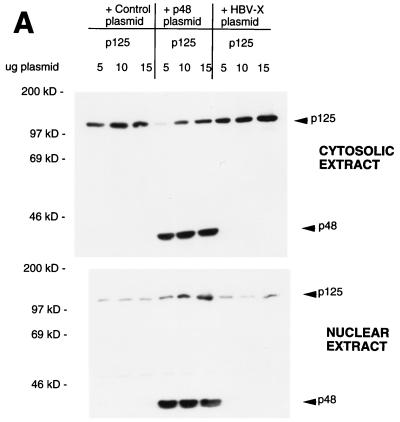
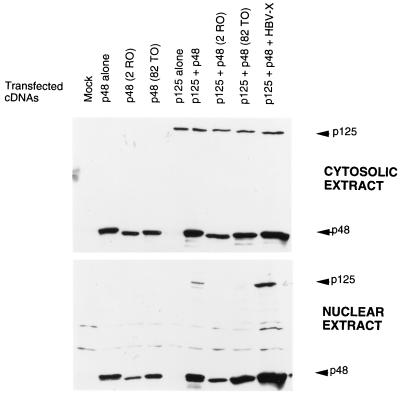
We observed that p125, when expressed alone, localizes in the cytosol, whereas p48 localizes in the nucleus. Because DDB is believed to possess nuclear functions, we suspected that p48 might play a role in the nuclear localization of p125. We investigated this possibility by looking at the distribution of p125 in the nuclear and cytosolic extracts in the presence and absence of p48 expression. U2OS cells were transfected with plasmids expressing T7-tagged p125 and p48. Transfected cells were lysed by using a hypotonic buffer; cytosolic S100 and high-salt nuclear extracts were prepared following the procedure of Dignam et al. (6). Because the distribution between nucleus and cytosol can vary with the level of expression, three levels of the p125 plasmid were used with the expectation that we would be able to obtain extracts with comparable levels of p125 in the presence and absence of p48. The HBx protein was shown to bind p125 (23). A plasmid expressing HBx was used as a control to see whether any p125-binding protein would have an effect on the distribution of p125. Cytosolic and nuclear extracts of the transfected cells were subjected to Western blot assays. The blots were probed with a T7 antibody that would detect both p125 and p48. As can be seen in Fig. 1A, the coexpression of p48 resulted in an increased accumulation of p125 in the nuclear extracts. In the absence of p48 coexpression there was very little increase in the nuclear accumulation of p125 with increasing levels of p125 plasmid. This result is consistent with the notion that p48 plays an important role in the nuclear localization of p125.
FIG. 1.
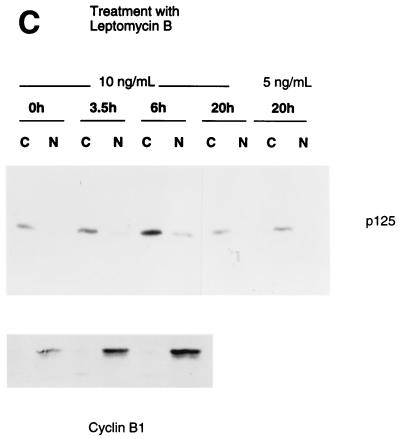
P48 stimulates nuclear entry of the p125 subunit of DDB. (A) Indicated amounts of the plasmid expressing T7-tagged p125 were transfected into U2OS cells alone or in combination with a plasmid expressing T7-tagged p48 or HBx. Cytosolic and nuclear extracts were prepared from the transfected cells according to the method of Dignam et al. (6). Fifty micrograms of the cytosolic and the nuclear extracts were subjected to Western blot assays with the T7 antibody. The blots were developed with enhanced chemiluminescence reagents. The bands corresponding to p125 and p48 are indicated. (B) Plasmids (1.5 μg) expressing T7-tagged p125 and p48 proteins were transfected into U2OS cells. Transfected cells were lysed in a hypotonic buffer containing 0.5% NP-40, and cytosolic and nuclear extracts were prepared as described in Materials and Methods. Fifty micrograms of the cytosolic and nuclear extracts were assayed for p125 and p48 with the antibody against the T7 tag. (C) Plasmid (1.5 μg) expressing T7-tagged p125 was transfected into U2OS cells. The transfected cells were treated with leptomycin B for the indicated time periods. The 0-h time point represents no leptomycin B treatment. Cytosolic (C) and nuclear (N) extracts were prepared according to the procedure of Dignam et al. (6). Twenty micrograms of the extracts was analyzed by Western blot assays. The blots were probed with a monoclonal antibody against the T7 epitope (upper panel). The extracts (20 μg) were also assayed for endogenous cyclin B1 with a polyclonal antibody (Santa Cruz). The blots were developed with enhanced chemiluminescence reagents.
To rule out the possibility that the distribution was an artifact of the extraction procedure, we employed a different method to prepare cytosolic and nuclear extracts. Transfected cells were lysed in the presence of 0.5% NP-40, and nuclei and cytosol were separated according to the procedure of Schreiber et al. (30). Nuclei were extracted with buffer A (see Materials and Methods). The nuclear and cytosolic extracts were assayed for p125. Clearly, the coexpression of p48 resulted in a quantitative increase in the nuclear accumulation of p125 (Fig. 1B). In these assays, we always detected a portion of p48 in the cytosolic extracts. We think that this is a result of leaking of p48 from nuclei during the extraction procedure, because in immunostaining experiments the majority of p48 was detected in the nucleus (Fig. 2). The additional bands in Fig. 1B detected by the T7 antibody are unrelated to DDB because they are also present in lanes corresponding to the mock-transfected cells.
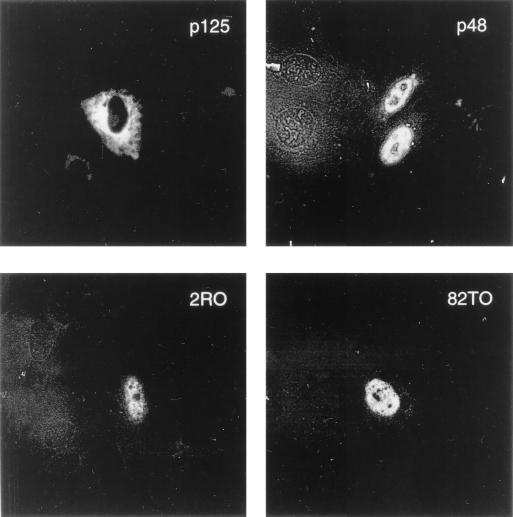
FIG. 2.
The naturally occurring mutants of p48, like the wild-type protein, localize in the nucleus. U2OS cells were grown in plates containing coverslips and transfected with plasmids (5 μg) expressing T7-tagged p125 or p48 protein. The coverslips containing transfected cells were fixed and probed with the T7 antibody and the FITC-labeled secondary antibody as described in Materials and Methods. The immunofluorescence was detected by using a CLSM 510 microscope.
It is possible that p125 is capable of nuclear entry, but in the absence of p48 it is exported out of the nucleus. P125 contains leucine-rich sequences, which often serve as signals for nuclear export (8a, 9a, 22a). Leucine-rich export signals are recognized by the nuclear export receptor CRM1 (8a, 9a, 22a). Therefore, we investigated the effect of leptomycin B, an inhibitor of CRM1, on the localization of p125. If in the absence of p48 expression p125 is exported out of the nucleus by the export receptor CRM1, leptomycin B is expected to increase the accumulation of p125 in the nucleus. U2OS cells were transfected with a plasmid expressing T7 epitope-tagged p125. The transfected cells were treated with leptomycin B for various lengths of time. Cytosolic and nuclear extracts were prepared by following the method of Dignam et al. (6). Extracts were assayed for p125 by Western blotting with a monoclonal antibody against the T7 epitope. The Western blots indicated very little or no significant effect of leptomycin B on the localization of p125 (Fig. 1C). The majority of p125 was detected in the cytosolic extracts after leptomycin B treatment. Under these experimental conditions, endogenous cyclin B1 was detected mainly in the nuclear extracts (Fig. 1C, lower panel). An increase in the band intensity of p125 was detected in the lane corresponding to 6 h of leptomycin B treatment (Fig. 1C); however, the nucleus/cytoplasm ratio of p125 levels remained unaltered. These results are consistent with the notion that p48 is required for the nuclear entry of p125.
The naturally occurring mutants of p48 (2RO and 82TO) are defective in stimulating nuclear import of p125.
Nichols et al. (26) characterized two naturally occurring mutants of p48 from cells of XP-E patients. These mutants, 2RO (R273H) and 82TO (K244E), harbor single amino acid substitutions within the WD motif of the p48 protein. We compared the mutant forms of p48 with the wild-type protein for their ability to localize in the nucleus by using an immunostaining procedure (see Materials and Methods). Cells were grown in plates containing coverslips and then transfected with plasmids expressing T7-tagged p48 or the mutants. T7-tagged p125, which localizes in the cytoplasm, was used as a negative control. Transfected cells on the coverslips were subjected to immunostaining with a monoclonal antibody against the T7 tag and an FITC-labeled secondary antibody. p48-expressing cells were visualized by indirect immunofluorescence. As can be seen in Fig. 2, the mutant p48 proteins localized in the nucleus as efficiently as the wild-type p48 protein.
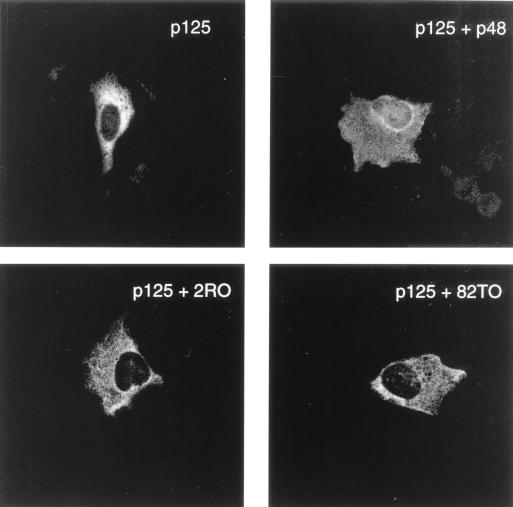
We then investigated the p48 mutants for their ability to import p125 into the nucleus. Under our assay conditions of immunostaining, p125 is mainly found in the cytoplasm (Fig. 2). Cells were transfected with plasmids expressing p125 and p48 or p48 mutants without any epitope tag. The coverslips containing transfected cells were subjected to immunostaining with an affinity-purified peptide antibody specific for p125 and an FITC-labeled secondary antibody. In this experimental setup, the immunostaining would detect only p125. As can be seen in Fig. 3, consistent with our results shown in Fig. 1, a significant amount of fluorescence is detectable in the nuclei of cells transfected with p125 and the wild-type p48 expression plasmid. Interestingly, the two mutant proteins were unable to increase the fluorescence of p125 in the nucleus (Fig. 3). This would be consistent with the notion that the two naturally occurring mutants are incapable of promoting the nuclear entry of p125.
FIG. 3.
The naturally occurring mutants of p48 are impaired in their ability to stimulate nuclear import of p125 as detected by immunostaining. U2OS cells on coverslips were transfected with a plasmid (5 μg) expressing p125 alone or in combination with plasmids expressing the wild-type or mutant p48 proteins. The cells on coverslips were fixed and probed with an affinity-purified antibody specific for p125 and then with an FITC-labeled secondary antibody. The immunofluorescence was detected by using a CLSM 510 microscope.
The immunostaining results were confirmed by biochemical studies. Cells were transfected with plasmids expressing T7-tagged p125 and T7-tagged p48 or the mutants. The transfected cells were lysed and fractionated to obtain cytoplasmic and nuclear extracts by following the procedure of Dignam et al. (6). To determine the distribution of p125 and p48 between the nucleus and cytoplasm, the extracts were subjected to Western blot assays with the T7 antibody. Clearly, p125 was detectable in the nuclear extracts of cells cotransfected with the wild-type p48 expression plasmid but not in the nuclear extracts of cells transfected with the mutant p48 expression plasmids (Fig. 4). Taken together, these results indicate that the naturally occurring mutants of p48 (2RO and 82TO) are defective in stimulating the nuclear import of p125.
FIG. 4.
The naturally occurring mutants of p48 are unable to increase the level of p125 in nuclear extracts. U2OS cells were transfected with a plasmid (5 μg) expressing T7-tagged p125 and plasmids (5 μg) expressing T7-tagged p48 proteins. Cytosolic and nuclear extracts of the transfected cells were prepared according to the procedure of Dignam et al. (6). Fifty micrograms of the nuclear and the cytosolic extracts was assayed for p125 and p48 proteins with the T7 antibody.
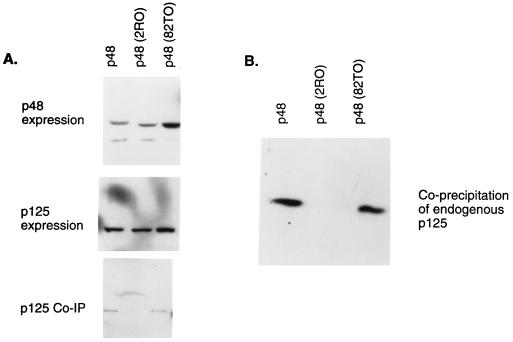
The p48 mutant 2RO is deficient in its ability to associate with the p125 subunit of DDB.
We sought to analyze the interaction between the mutant p48 proteins and p125, because they were unable to stimulate a nuclear accumulation of p125. Plasmids expressing HA-tagged p48 were transfected into U2OS cells along with a plasmid expressing T7-tagged p125 protein. Extracts of the transfected cells were subjected to immunoprecipitation with HA antibody and then Western blotting with the T7 antibody. As can be seen in Fig. 5A, the mutant 2RO failed to coprecipitate T7-tagged p125, whereas the other mutant (82TO) and wild-type p48 coprecipitated p125. The mutants and wild-type p48 were expressed at comparable levels, and no significant difference in the expression of p125 was detected (Fig. 5A). This observation was confirmed by another experiment in which the coprecipitation of endogenous p125 with transfected T7-tagged p48 was assayed. The endogenous p125 in U2OS cells also failed to coprecipitate with 2RO (Fig. 5B). Taken together, these results clearly indicate that the main defect in 2RO is its inability to associate with p125. The mutant 82TO bound p125 but did not stimulate a nuclear accumulation of p125, suggesting that the interaction with p48 is necessary but not sufficient for the nuclear accumulation of p125.
FIG. 5.
p48 mutant 2RO is impaired in its ability to associate with the p125 subunit of DDB. (A) Plasmids (5 μg) expressing the HA epitope-tagged p48 proteins (wild type and mutants) were transfected into U2OS cells along with the plasmid (5 μg) expressing T7 epitope-tagged p125. Extracts (50 μg) of the transfected cells were assayed for the expression of the p48 proteins (upper panel) and p125 (middle panel). The expression of p48 was assayed by using a monoclonal antibody against the HA epitope, and the expression of p125 was assayed by using a monoclonal antibody against the T7 epitope in Western blots. To assay for an interaction between the mutant p48 proteins and p125, 200 μg of the extracts was also subjected to immunoprecipitation (IP) with a monoclonal antibody against the HA epitope. The immunoprecipitates were assayed for the presence of p125 by Western blotting with the antibody against the T7 epitope (lower panel). The blots were developed with enhanced chemiluminescence reagents by following a procedure provided by the manufacturer. (B) The interactions between the mutant p48 proteins and endogenous p125 were assayed by transfecting U2OS cells with plasmids expressing T7-tagged p48 or the mutants 2RO and 82TO. Extracts (200 μg) of the transfected cells were subjected to immunoprecipitation with the T7 antibody. The immunoprecipitates were analyzed by Western blot assays for the presence of p125. A peptide antibody specific for p125 was used to detect p125 in the blot. The Western blot was developed with enhanced chemiluminescence reagents.
The mutant p48 proteins are defective in stimulating E2F1-activated transcription.
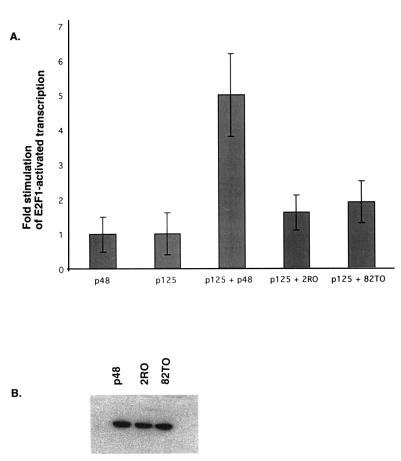
We have previously shown that the coexpression of DDB along with E2F1 resulted in a cooperative stimulation of transcription from an E2F1-regulated promoter (11). Both subunits of DDB were needed to observe the transcriptional stimulatory activity of DDB (11). Because the two mutants 82TO and 2RO failed to stimulate a nuclear accumulation of p125, we predicted that these mutants would be impaired in their ability to stimulate E2F1-activated transcription. The E2F1 gene promoter is a natural target of E2F1-activated transcription (13, 17) and we showed that a CAT gene construct containing the E2F1 promoter was activated by the expression of DDB (11). Moreover, the coexpression of DDB and E2F1 produced a much greater stimulation of E2F1-induced transcription from this promoter (15). We employed this transcription system to compare the activities of the two p48 mutant proteins, 2R0 (R273H) and 82TO (K244E), with that of wild-type p48. C33A cells were employed for this analysis because these cells contain a low endogenous level of the DDB proteins (not shown), and they exhibited a significant response to DDB expression in transcription assays (11).
Cells were transfected by using the calcium phosphate precipitation method. Plasmids expressing E2F1 and p125 were transfected along with the mutant or wild-type p48 expression plasmid and the reporter CAT gene. A plasmid expressing β-galactosidase was used to control for the transfection efficiencies. CAT gene activities in the extracts were normalized by using the β-galactosidase values. An average of fold stimulation by DDB over E2F1-activated transcription from three independent transfection experiments is shown in Fig. 6A. The results of these experiments clearly indicated that the two naturally occurring mutants of p48 are impaired in stimulating E2F1-activated transcription. Both mutants were expressed quite efficiently in these transfection experiments (Fig. 6B), indicating that the inactivity of the mutants is not due to an expression defect.
FIG. 6.
The naturally occurring mutants of p48 are impaired in stimulating E2F1-activated transcription. (A) Cells were transfected with the E2F1-CAT reporter gene plasmid (5 μg) along with 0.5 μg of the E2F1 expression plasmid. Plasmids (5 μg) expressing p125 and T7 epitope-tagged p48 or mutant p48 proteins (2RO and 82TO) were also included in some of the transfection mixtures. DNA transfection was carried out as described in Materials and Methods. A plasmid expressing β-galactosidase was included to control for the transfection efficiencies. Averages of fold stimulation by DDB from three independent experiments are shown. Error bars indicate standard deviations. (B) Extracts from one of the above transfection experiments were assayed for p48 expression. Fifty micrograms of the extract proteins was separated by SDS–12% PAGE and blotted onto nitrocellulose membranes. The blots were probed with the T7 antibody and developed with enhanced chemiluminescence reagents as described before (11).
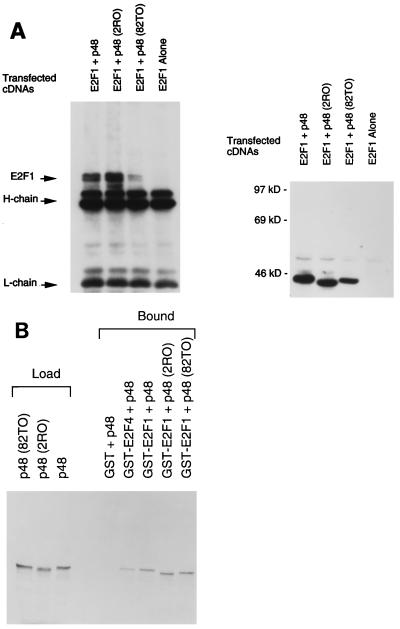
We have previously shown that wild-type p48 could associate with E2F1, which correlated with the DDB-mediated stimulation of E2F1-activated transcription (11). Therefore, we examined whether the mutant p48 proteins were impaired in their abilities to associate with E2F1. Plasmids expressing E2F1, p125, and T7-tagged p48 (wild-type or mutant form) were transfected into U2OS cells. Under these conditions of DNA transfection, the proteins are overexpressed, and the majority of E2F1 remains in its free form, as judged by gel retardation assays and coimmunoprecipitation experiments with Rb antibody (data not shown). Extracts of the transfected cells were subjected to immunoprecipitation with a monoclonal antibody against the T7 epitope. The immunoprecipitates were subjected to Western blot assays, which were probed with a monoclonal antibody against E2F1. The results of these experiments clearly indicate that the mutants, like wild-type p48, were able to associate with E2F1, as evidenced by coimmunoprecipitation (Fig. 7A, left panel). In Fig. 7A, 82TO coprecipitated E2F1 at a lower level, the result of lower-level expression of 82TO in this experiment (Fig. 7A, right panel). In other experiments, we did not see any difference in the coprecipitation of E2F1 by the p48 mutant forms (data not shown). The interactions between the mutant p48 proteins and E2F1 were further confirmed by additional binding experiments with a GST pull-down assay. The p48 proteins were synthesized in reticulocyte lysates as [35S]methionine-labeled proteins. The labeled proteins were incubated with GST-E2F1 and subjected to a pull-down assay with GSH-Sepharose beads (11). The bound proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography. The mutant p48 proteins bound GST-E2F1 as efficiently as wild-type p48 (Fig. 7B). We also observed an interaction between p48 and GST-E2F4; however, an interaction between these proteins is yet to be seen in vivo. These results are consistent with the notion that the mutant p48 proteins are impaired in their transcriptional function because they are deficient in stimulating a nuclear accumulation of p125.
FIG. 7.
The naturally occurring mutants of p48 are able to associate with E2F1. (A) Plasmids expressing E2F1 (5 μg) and T7 epitope-tagged wild-type or mutant p48 protein were transfected into U2OS cells by the Ca phosphate precipitation method (see Materials and Methods). Thirty-six hours after transfection, cells were harvested and extracts were prepared with buffer A (see Materials and Methods for details). Equal amounts (200 μg) of the extract proteins were subjected to immunoprecipitation with T7 antibody. The immunoprecipitates were subjected to Western blot assays. The blot was probed with a monoclonal antibody against E2F1 (left panel). The migrations of E2F1 and the immunoglobulin G bands are indicated. To assay for the expression of the p48 proteins in this particular experiment, the blot was stripped with 2% SDS and 100 mM 2-mercaptoethanol. Following an extensive wash to remove SDS, the blot was further probed with horseradish peroxidase-linked T7 antibody (right panel). (B) The p48 and mutant proteins (2RO and 82TO) were transcribed and translated in vitro by using T7 RNA polymerase and reticulocyte lysate. The translation was performed in the presence of [35S]methionine. The translated products (2 μl) were incubated with 1 μg of the indicated GST fusion proteins. Glutathione-Sepharose beads (10 μl) were added to each of the incubation mixtures, followed by incubation on a Nutator for 30 min at 4°C. The beads were collected by centrifugation and extensively washed with NETN buffer (150 mM NaCl, 20 mM Tris-HCl [pH 7.6], 1 mM EDTA, 0.5% NP-40). The bound proteins were eluted with SDS gel loading buffer and were subjected to SDS–10% PAGE followed by autoradiography. Two microliters of the translated products was analyzed in the same gel (Load).
DISCUSSION
The studies described here are significant with regard to our understanding of the function of DDB, which has been linked to a rare inheritable repair deficiency disease, XP-E. We showed that the p48 subunit of DDB, which is found to be mutated in XP-E, plays an important role in the nuclear accumulation of the p125 subunit of DDB. This appears to be a specific function of p48, because HBx, which binds to p125, was unable to stimulate the nuclear accumulation of p125. The nuclear accumulation of p125 in the presence of p48 most likely involves an increase in nuclear transport, because the nuclear level of p125 increased at the expense of its cytosolic level (Fig. 1). However, we have not ruled out the possibility that p48, in addition to stimulating nuclear import, also stabilizes p125 in the nucleus. We analyzed two naturally occurring mutants of p48, 2RO and 82TO. These mutants harbor single amino acid substitutions in the WD motif of the p48 protein and were isolated from XP-E patients lacking the damaged-DNA binding activity of DDB. Hwang et al. showed that unlike wild-type p48, these two naturally occurring mutants of p48 are unable to stimulate damaged-DNA binding activity when expressed in mammalian cells (16). This is consistent with the notion that p48 is required for the damaged-DNA binding activity of DDB. It has been postulated that p48 functions by activating the damaged-DNA binding activity of the p125 subunit by a “hit and run” mechanism in which p125 binds damaged DNA and p48 acts to activate the DNA-binding function of p125 (16). We observed that these naturally occurring mutants, which failed to bind damaged DNA, were also defective in stimulating the nuclear entry of the p125 subunit.
The mutant 82TO was isolated from cells of a 41-year-old Japanese patient. This patient exhibited an acute sun sensitivity but did not develop skin malignancies (21). The mutant 2RO corresponded to a Dutch patient who developed skin tumors at the age of 14 (5). The difference in severity of the disease phenotype might be a reflection of how severely the two mutations disrupted the function of p48. This is consistent with our results in that unlike 82TO, 2RO is severely impaired in its ability to associate with the p125 subunit of DDB. If p125 is an essential functional partner of p48, we would predict that the mutation in 2RO would severely compromise the function of p48. The mutation in 2RO (R273H) did not disrupt the structure of p48 because the mutant protein was able to associate with E2F1. The result also suggests that the arginine residue at position 273 within the WD motif of p48 is critical for binding to p125. Our assays of the nuclear localization of p125 and E2F1-activated transcription failed to distinguish between 82TO and 2RO. This could be a limitation of the sensitivity of our in vitro assays; however, other possibilities cannot be ruled out.
The mutant p48 82TO localizes in the nucleus, and it is able to associate with the p125 subunit. Therefore, it is surprising that this mutant did not enhance the nuclear import of p125. This would suggest that binding to p48 is not sufficient for the nuclear accumulation of p125. It is possible that this mutant is deficient in other interactions that are critical for the nuclear import or retention of p125 in the nucleus. p48 has three putative nuclear localization signals: two overlapping between residues 3 and 7 (PKKRP) and one between residues 241 and 244 (HKKK). It is important to note that the nuclear localization signal (HKKK) near the p125-binding site is mutated in 82TO (K244E). It is possible that this signal plays a role in carrying p125 into the nucleus. Other possibilities cannot be ruled out. A modification that is required for the stable nuclear localization of p125 may be missing in the context of 82TO. Clearly, further work is required to understand the mechanisms that control the nuclear localization of the DDB subunits.
We previously observed that DDB could functionally interact with the transcription factor E2F1, which is involved in the expression of a variety of cell cycle genes. In this study, we show that the two mutants of DDB, 82TO and 2RO, are capable of interacting with E2F1 but failed to functionally cooperate with E2F1 in transcription assays. This would be consistent with the observation that both subunits of DDB are necessary to stimulate E2F1-activated transcription because the mutant p48 proteins (2RO and 82TO) are unable to stimulate the nuclear accumulation of p125. The results presented here also suggest a link between the XP-E phenotype and the transcription factor E2F1. However, it is unclear how a lack of functional interaction between DDB and E2F1 would contribute to the disease phenotype found in XP-E patients.
It was shown that E2F1-deficient mice (E2F1−/−) develop tumors at a high frequency, and it has been suggested that E2F1 possesses tumor suppressor-like activity (8, 38). The tumor suppressor-like activity of E2F1 might be related to its role in p53-mediated apoptosis (25, 28, 32, 37). It is possible that DDB plays a role in activating the E2F1 target genes involved in apoptosis. However, a role for DDB in the apoptotic function of E2F1 is yet to be established. In addition, it will be interesting to determine whether the cells from XP-E patients harboring mutations in the DDB gene are deficient in E2F1-mediated apoptosis.
ACKNOWLEDGMENTS
We are grateful to Minoru Yoshida (University of Tokyo, Tokyo, Japan) for the kind gift of leptomycin B. We also thank S. Bagchi, R. Costa, and G. Adami for critically reviewing the manuscript.
The work was supported by grants from the American Cancer Society (RPG-94-041-04-TBE) and the National Cancer Institute (RO1 CA77637) to P.R. S.L. is supported by grants (RO1 GM30415 and P30 ES08196) from the National Institutes of Health.
REFERENCES
- 1.Becker S A, Lee T-H, Butel J S, Slagle B L. Hepatitis B virus X protein interferes with cellular DNA repair. J Virol. 1998;72:266–272. doi: 10.1128/jvi.72.1.266-272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohr V A, Okumoto D S, Hanawalt P C. Survival of UV-irradiated mammalian cells correlates with efficient DNA repair in an essential gene. Proc Natl Acad Sci USA. 1986;83:3830–3833. doi: 10.1073/pnas.83.11.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu G, Chang E. Xeroderma pigmentosum group E cells lack a nuclear factor that binds to damaged DNA. Science. 1988;242:564–567. doi: 10.1126/science.3175673. [DOI] [PubMed] [Google Scholar]
- 4.Dantonel J-C, Murthy K G K, Manley J L, Tora L. Transcription factor TFIID recruits factor CPSF for formation of 3′ end of mRNA. Nature. 1997;389:399–402. doi: 10.1038/38763. [DOI] [PubMed] [Google Scholar]
- 5.de Weerd-Kastelein E A, Keijzer W, Bootsma D. A third complementation group in xeroderma pigmentosum. Mutat Res. 1974;22:87–91. doi: 10.1016/0027-5107(74)90013-x. [DOI] [PubMed] [Google Scholar]
- 6.Dignam J D, Lebovitz R M, Roeder R. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dualan R, Brody T, Keeney S, Nichols A F, Admon A, Linn S. Chromosomal localization and cDNA cloning of the genes (DDB1 and DDB2) for the p127 and p48 subunits of a human damage-specific DNA binding protein. Genomics. 1995;29:62–69. doi: 10.1006/geno.1995.1215. [DOI] [PubMed] [Google Scholar]
- 8.Field S J, Tsai F Y, Kuo F, Zubiaga A M, Kaelin W G, Jr, Livingston D M, Orkin S H, Greenberg M E. E2F1 functions in mice to promote apoptosis and suppress proliferation. Cell. 1996;85:549–561. doi: 10.1016/s0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- 8a.Fornerod M, Ohno M, Yoshida M, Mattaj I W. Crm1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 9.Friedberg E C. Relationships between DNA repair and transcription. Annu Rev Biochem. 1996;65:15–42. doi: 10.1146/annurev.bi.65.070196.000311. [DOI] [PubMed] [Google Scholar]
- 9a.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 10.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 11.Hayes S, Shiyanov P, Chen X, Raychaudhuri P. DDB, a putative DNA repair protein, can function as a transcriptional partner of E2F1. Mol Cell Biol. 1998;18:240–249. doi: 10.1128/mcb.18.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henning K A, Li L, Iyer N, McDaniel L D, Reagan M S, Legerski R, Schultz R A, Stefanini M, Lehman A R, Mayne L V, Friedberg E C. The Cockayne syndrome group A gene product encodes a WD repeat protein that interacts with CSB protein and a subunit of RNA polymerase II TFIIH. Cell. 1995;82:555–564. doi: 10.1016/0092-8674(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 13.Hsiao K M, McMahon S L, Farnham P J. Multiple DNA elements are required for the growth regulation of the mouse E2F1 promoter. Genes Dev. 1994;8:1526–1537. doi: 10.1101/gad.8.13.1526. [DOI] [PubMed] [Google Scholar]
- 14.Hwang B J, Chu G. Purification and characterization of a human protein that binds to damaged DNA. Biochemistry. 1993;32:1657–1666. doi: 10.1021/bi00057a033. [DOI] [PubMed] [Google Scholar]
- 15.Hwang B J, Liao J C, Chu G. Isolation of a cDNA encoding a UV-damaged DNA binding factor defective in xeroderma pigmentosum group E cells. Mutat Res. 1996;362:105–117. doi: 10.1016/0921-8777(95)00040-2. [DOI] [PubMed] [Google Scholar]
- 16.Hwang B J, Toering S, Francke U, Chu G. p48 activates a UV-damaged-DNA binding factor and is defective in xeroderma pigmentosum group E cells that lack binding activity. Mol Cell Biol. 1998;18:4391–4399. doi: 10.1128/mcb.18.7.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson D G, Ohtani K, Nevins J R. Autoregulatory control of E2F1 expression in response to positive and negative regulators of cell cycle progression. Genes Dev. 1994;8:1514–1525. doi: 10.1101/gad.8.13.1514. [DOI] [PubMed] [Google Scholar]
- 18.Kazantsev A, Mu D, Nichols A F, Zhao X, Linn S, Sancar A. Functional complementation of xeroderma pigmentosum complementation group E by replication protein A in an in vitro system. Proc Natl Acad Sci USA. 1996;93:5014–5018. doi: 10.1073/pnas.93.10.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keeney S, Chang G J, Linn S. Characterization of a human DNA damage binding protein implicated in xeroderma pigmentosum E. J Biol Chem. 1993;268:21293–21300. [PubMed] [Google Scholar]
- 20.Keeney S, Eker A P, Brody T, Vermeulen W, Bootsma D, Hoeijmakers J H, Linn S. Correction of the DNA repair defect in xeroderma pigmentosum group E by injection of a DNA damage-binding protein. Proc Natl Acad Sci USA. 1994;91:4053–4056. doi: 10.1073/pnas.91.9.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo S J, Fukuro S, Mamada A, Kawada A, Satoh Y. Assignment of three patients with xeroderma pigmentosum to complementation group E and their characteristics. J Investig Dermatol. 1988;90:152–157. doi: 10.1111/1523-1747.ep12462130. [DOI] [PubMed] [Google Scholar]
- 22.Krishnamoorthy R R, Lee T-H, Butel J S, Das H K. Apolipoprotein B gene regulatory factor-2 (BRF-2) is structurally and immunologically highly related to hepatitis B virus S associated protein-1 (XAP-1) Biochemistry. 1997;36:960–969. doi: 10.1021/bi961407c. [DOI] [PubMed] [Google Scholar]
- 22a.Kudo N, Khochbin S, Nishi K, Kitano K, Yanagida M, Yoshida M, Horinouchi S. Molecular cloning and cell cycle dependent expression of mammalian CRM1, a protein involved in nuclear export. J Biol Chem. 1997;272:29742–29751. doi: 10.1074/jbc.272.47.29742. [DOI] [PubMed] [Google Scholar]
- 23.Lee T H, Elledge S J, Butel J S. Hepatitis B virus X protein interacts with a probable cellular DNA repair protein. J Virol. 1995;69:1107–1114. doi: 10.1128/jvi.69.2.1107-1114.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin G Y, Paterson R G, Richardson C D, Lamb R A. The V protein of the paramyxovirus SV5 interacts with damage-specific DNA binding protein. Virology. 1998;249:189–200. doi: 10.1006/viro.1998.9317. [DOI] [PubMed] [Google Scholar]
- 25.Nevins J R. Towards an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 1998;9:585–593. [PubMed] [Google Scholar]
- 26.Nichols A F, Ong P, Linn S. Mutations specific to the xeroderma pigmentosum group E Ddb-phenotype. J Biol Chem. 1996;271:24317–24320. doi: 10.1074/jbc.271.40.24317. [DOI] [PubMed] [Google Scholar]
- 27.Otrin V R, Kuraoka I, Nardo T, McLenigan M, Eker A P M, Stefanini M, Levine A S, Wood R D. Relationship of the xeroderma pigmentosum group E DNA repair defect to the chromatin and DNA binding proteins UV-DDB and replication protein A. Mol Cell Biol. 1998;18:3182–3190. doi: 10.1128/mcb.18.6.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin X Q, Livingston D M, Kaelin W, Jr, Adams P D. Deregulated transcription factor E2F1 expression leads to S phase entry and apoptosis. Proc Natl Acad Sci USA. 1994;91:10918–10922. doi: 10.1073/pnas.91.23.10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reardon J T, Nichols A F, Keeney S, Smith C A, Taylor J S, Linn S, Sancar A. Comparative analysis of binding of human damaged DNA-binding protein (XPE) and Escherichia coli damage recognition protein (UvrA) to the major ultraviolet photoproducts: T[c,s]T, T[t,s]T, T[6-4]T, and T[Dewar]T. J Biol Chem. 1993;268:21301–21308. [PubMed] [Google Scholar]
- 30.Schreiber E, Matthias P, Muller M M, Schaffner W. Rapid detection of octamer binding proteins with mini-extracts, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419–6420. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seed B, Sheen J Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988;67:271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- 32.Shan B, Lee W-H. Deregulated expression of E2F1 induces S phase entry and apoptosis. Mol Cell Biol. 1994;14:8166–8173. doi: 10.1128/mcb.14.12.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiyanov P, Bagchi S, Adami G, Kokontis J, Hay N, Arroyo M, Morozov A, Raychaudhuri P. p21 disrupts the interaction between cdk2 and the E2F-p130 complex. Mol Cell Biol. 1996;16:737–744. doi: 10.1128/mcb.16.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiyanov P, Hayes S, Chen N, Pestov D G, Lau L F, Raychaudhuri P. p27Kip1 induces an accumulation of the repressor complexes of E2F and inhibits expression of the E2F-regulated genes. Mol Biol Cell. 1997;8:1815–1827. doi: 10.1091/mbc.8.9.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sitterlin D, Lee T-H, Progent S, Tiollais P, Butel J S, Transy C. Interaction of the UV-damaged DNA-binding protein with hepatitis B virus X protein is conserved among mammalian hepadnaviruses and restricted to transactivation-proficient X-insertion mutants. J Virol. 1997;71:6194–6199. doi: 10.1128/jvi.71.8.6194-6199.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Assendelft G B, Rigney E M, Hickson I D. Purification of a HeLa cell nuclear protein that binds selectively to DNA irradiated with ultra-violet light. Nucleic Acids Res. 1993;21:3399–3404. doi: 10.1093/nar/21.15.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu X, Levine A J. p53 and E2F1 cooperate to mediate apoptosis. Proc Natl Acad Sci USA. 1994;91:3802–3808. doi: 10.1073/pnas.91.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamasaki L, Jacks T, Bronson R T, Harlow E, Dyson N. Tumor induction and tissue atrophy in mice lacking E2F1. Cell. 1996;85:537–548. doi: 10.1016/s0092-8674(00)81254-4. [DOI] [PubMed] [Google Scholar]