Abstract
Objective: To investigate the efficacy of recombinant human basic fibroblast growth factor (rh-bFGF) combined with local application of insulin (INS) in diabetic patients with deep II degree burns, and to analyze the changes in pain and healing. Methods: Seventy-eight diabetic patients with deep II degree burns treated in our hospital were divided into the observation group (n=39) for combination therapy of rh-bFGF and local INS, and the control group (n=39) for rh-bFGF alone. The two groups were compared regarding treatment effect, wound healing time, pain degree (Visual Analogue Scale, VAS), wound exudate rate, vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1α (HIF-1α), as well as adverse reactions. Results: Compared with the control group, the total clinical effective rate was evidently higher in the observation group (P<0.05). The wound healing time was shorter and the VAS score was lower in the observation group as compared to the control group (both P<0.05). The observation group showed significantly lower wound exudate rate and higher HIF-1α and VEGF protein levels in wound tissue than the control group (all P<0.05). No adverse reactions such as abnormalities in blood routine and hepatorenal function were observed in the two groups during treatment. Conclusions: rh-bFGF combined with local application of INS is markedly effective in diabetic patients with deep II degree burns. It can relieve pain and shorten healing time, with low wound exudate rate and high safety profile.
Keywords: Recombinant human basic fibroblast growth factor, insulin, diabetes, deep II degree burn, therapeutic effect
Introduction
Burn is an accidental injury, which mainly refers to the tissue damage arisen from heat, including hydrothermal solution (water, soup, oil, etc.), high-temperature gas, hot metal liquid, flame, steam or solid (molten steel, steel ingot, etc.). Generally, the skin and/or mucosa can be damaged when the burn is mild; but in severe cases, subcutaneous or/and submucosal tissues, such as muscles, joints and even internal organs, may be injured [1]. In the United States, approximately 0.8% of the total population suffers from burns every year, and about 55,000 patients (2 million with burns) are hospitalized, of which 5,000 died [2]. Burns are divided into three degrees and four categories, namely first, shallow second, deep second, and third degree burns, depending on how deep and severe they penetrate the skin’s surface. Among them, patients with deep second-degree burns present thermal damage to the deep layer of the dermis; the local manifestation is blisters, which are relatively flat, with a fine reticular embolized vascular network, and few residual epithelial cells on the wound surface, so it was easy to develop into a third degree wound [3]. This is particularly true in patients complicated with diabetes mellitus, as their blood sugar rises, endocrine disorders, with microvascular and peripheral neuropathy. As a result, their wound repair ability is worse following burn, which can easily cause infection, increasing the suffering of patients and seriously threaten human health [4]. Hence, the selection of effective topical drugs to fight infection and promote wound healing is the focus of clinical attention. Widely used in the treatment of burn patients, recombinant human basic fibroblast growth factor (rh-bFGF) is shown to be able to promote regeneration, repair and improved microcirculation, with favorable therapeutic effects [5]. It is also reported that local application of insulin (INS) can improve wound outcomes of patients [6]. Although there are reports about the application of rh-bFGF combined with INS in diabetic patients with deep II degree burns, no attention has been paid to the effect on hypoxia-inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF) proteins in wound tissue, as they can induce neovascularization and promote wound repair [7]. Therefore, this study mainly focuses on the clinical efficacy of the combination therapy of rh-bFGF and local application of INS in diabetic patients with deep II degree burns, and focuses on the analysis of the role of the combined use in controlling wound healing time, pain, wound exudate rate, HIF-1α and VEGF protein expression as well as the incidence of adverse reactions, in order to provide theoretical direction for clinical treatment.
Materials and methods
General data
In this prospective study, 78 diabetic patients with deep II degree burns treated in our hospital from December 6, 2018 to February 25, 2020 were randomized into observation group and control group, each with 39 cases. Patient general information is presented in Table 1. The Medical Ethics Committee of our hospital approved the study protocol.
Table 1.
Comparison of general baseline information between the two groups of patients (n, x̅ ± sd)
| Indicators | Observation group (n=39) | Control group (n=39) | χ2/t | P |
|---|---|---|---|---|
| Age (year) | 47.4±4.0 | 46.6±5.6 | 0.726 | 0.470 |
| Gender (n) | 0.053 | 0.817 | ||
| Male | 24 | 23 | ||
| Female | 15 | 16 | ||
| BMI (kg/m2) | 23.75±2.18 | 23.50±3.08 | 0.414 | 0.680 |
| Trauma area (cm2) | 173.55±7.12 | 174.53±8.80 | 0.541 | 0.590 |
| Duration of diabetes mellitus (years) | 5.15±2.18 | 5.30±3.08 | 0.248 | 0.805 |
| Co-morbidities (n) | ||||
| Hypertension (n) | 15 | 13 | 0.222 | 0.637 |
| Coronary heart disease (n) | 11 | 9 | 0.269 | 0.604 |
| Cause of burn injury (n) | 0.286 | 0.908 | ||
| Chemical burns (n) | 20 | 22 | ||
| Flame burns (n) | 19 | 17 | ||
| Diabetes treatment drugs (n) | ||||
| Biguanides (n) | 13 | 14 | 0.056 | 0.811 |
| Sulfonylureas (n) | 9 | 9 | 0.000 | 1.000 |
| Thiazolidinediones (n) | 8 | 9 | 0.075 | 0.783 |
| Benzoic acid derivatives (n) | 10 | 8 | 0.288 | 0.590 |
| Insulin (n) | 19 | 17 | 0.206 | 0.649 |
Inclusion criteria: (1) Patients who meet the diagnostic criteria of type 2 diabetes and deep II degree burns [8,9]; (2) Patients with hydrothermal or flame burns; (3) Patients older than 18 years old; (4) Patients with stable blood glucose levels; (5) Patients whose burn area is not more than 30% and not less than 1%; (6) Patients with burns located in the extremities; (7) Patients without other burns or internal burns; (8) Patients without co-infection; (9) Patients who understand the purpose of this research with the signed informed consent provided.
Exclusion criteria: (1) Patients engaging in other studies; (2) Patients with severe heart, liver and kidney dysfunction; (3) Patients with cognitive impairment; (4) Patients with severe mental illness; (5) Patients with other diseases that may affect healing, such as rheumatism, vascular diseases, mobility inconvenience, bedsores, pressure sores or stroke.
Methods
All wounds were debrided and routinely disinfected before treatment with rh-bFGF and local INS.
In the control group, rh-bFGF was sprayed on the wound surface immediately upon admission, twice a day, 50 IU each time, with no drug loss as the principle. The outer layer was wrapped with sterile gauze (Manufacturer: Beijing SL Pharmaceutical Co., Ltd., Approval No. 20180910, Specification: 3500 IU ×1 bottle/box).
The observation group was given rh-bFGF and INS (Manufacturer: Xuzhou Wanbang Jinqiao Pharmaceutical Co., Ltd., State Food and Drug Administration Approval No. 20180907, Specification: 4 units of INS/piece). After rh-bFGF spraying, the wound was covered with INS soaked sterile gauze, twice a day, half a tube each time, and the outer layer was wrapped with sterile gauze. INS was discontinuing if there were INS-related adverse reactions and/or wound infection.
Patients with severe burn pain were injected with 50 mg pethidine intramuscularly, while those with craniocerebral trauma or dyspnea were given painkillers instead. If the wound was seriously contaminated and the burn area was more than 5%, 3000 U of tetanus antiviral serum was routinely injected. Patients who may develop hypovolemic shock were treated with fluid replacement.
The duration of treatment was 21 days. The medication was discontinued when the patient recovered early.
Outcome measures
Primary outcome measures: (1) The therapeutic effects, classified as markedly effective (the wound inflammation is well controlled within 1 week after medication, and the bacterial culture is negative), effective (the wound inflammation is well controlled after 1-2 weeks of medication, and the bacterial culture is negative), and ineffective (the wound inflammation is not controlled after 2 weeks of medication, or the bacterial culture was positive) according to the evaluation standard of “Practical Burn Plastic Surgery”, were compared between the two cohorts of patients. Total effective rate = (markedly effective + effective) cases/total cases ×100% [10]. (2) Pain assessment, performed with the visual analogue scale (VAS) with 0 being painless and 10 being severe pain, was compared between the two cohorts of patients before and 3 days after treatment [11]. (3) After 21 days of observation, the wound healing time of the two groups was compared, with wound epithelium >95% as the healing standard [12]. (4) The wound exudation rate (total incidence = wound exudation cases/total cases ×100%) was compared between the two groups.
Secondary outcome measures: (1) HIF-1α and VEGF protein levels in wound tissue of patients in two groups were compared. After 3 days of treatment, a few granulation tissues were collected from the unhealed edge of the wound for the determination of HIF-1α (HIF-1α antibody was purchased from Wuhan Yipu Biotechnology, Batch No. 20180211) and VEGF (VEGF antibody was purchased from Amyjet Scientific Co., Ltd., Batch No. 20180320) by immunohistochemistry. Identification: HIF-1α showed brown granules in nucleus and cytoplasm as positive cells, while VEGF showed brown granules in cytoplasm as positive cells. According to the staining intensity score, 0 was colorless, 1 was light yellow, 2 was pale brown, and 3 was brown. Based on the proportion of positive cells, it was divided into: 0=0, 1=less than 10%, 2=10%-50%, 3=51%-75%, and 4= >75%. (-), (+), (++), and (+++) corresponded to a total score of 0-1, 2, 3 and 4 point(s), respectively. (2) The incidence of adverse reactions such as blood routine and hepatorenal function abnormalities, infection and local wound edge reaction was compared between the two groups. Each complication is calculated independently, even if more than one complication occurs in one single patient. Total incidence = cases of complications/total cases ×100%.
Statistical processing
SPSS 20.0 was employed for data statistics. Counting data were presented in n (%) and analyzed by χ2. Measurement data following normal distribution were expressed as mean ± standard deviation (x̅ ± sd), and compared by the independent t test between groups or by the paired t test before and after treatment; otherwise, the data were recorded as median and interquartile range, and compared by the rank sum test between groups. Differences with P<0.05 were considered significant.
Results
Comparison of general baseline data
There was no evident difference in age, gender, body mass index, wound area, co-morbidities and anti-diabetic medications between the two groups (all P>0.05, Table 1).
Comparison of clinical efficacy
The observation group had a notably higher total effective rate than the control group (P<0.05, Table 2).
Table 2.
Comparison of clinical efficacy between two groups of patients [n (%)]
| Group | Apparently effective | Effective | Ineffective | Total effective rate |
|---|---|---|---|---|
| Observation group (n=39) | 30 (76.92) | 8 (20.51) | 1 (2.56) | 38 (97.43) |
| Control group (n=39) | 21 (53.85) | 10 (25.64) | 8 (20.51) | 31 (79.49) |
| χ2 | 7.255 | 6.152 | ||
| P | 0.027 | 0.013 |
Comparison of wound healing time
The follow-up period was 21 days, with a median healing time of 14 days in the observation group and 20 days in the control group. The results revealed a significant difference in wound healing time between the two groups (χ2=4.380, P=0.036, Figure 1).
Figure 1.
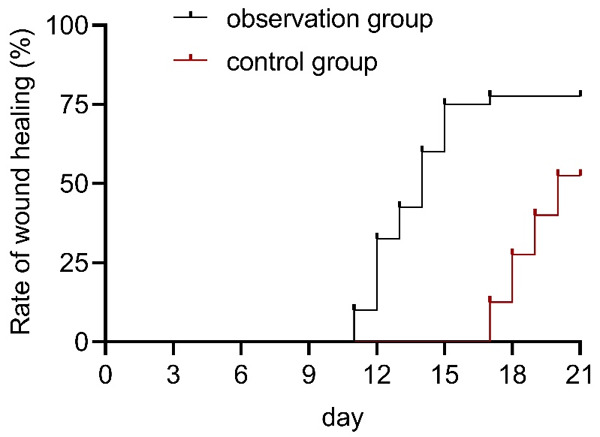
Wound healing time in both groups.
Comparison of VAS scores between the two groups before and after treatment
The two groups showed no distinct difference in VAS scores before treatment. However, VAS scores were significantly lower in the observation group than in the control group after treatment (P<0.05, Figure 2).
Figure 2.
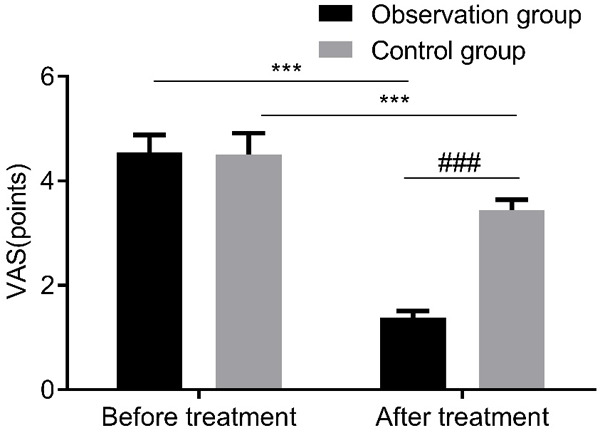
VAS scores of patients in both groups. VAS: Visual Analogue Scale. Compared with pre-treatment, ***P<0.001; compared with control group, ###P<0.001.
Comparison of wound exudate rate
In comparison with the control group, the wound exudate rate was statistically lower in the observation group (P<0.05, Figure 3).
Figure 3.
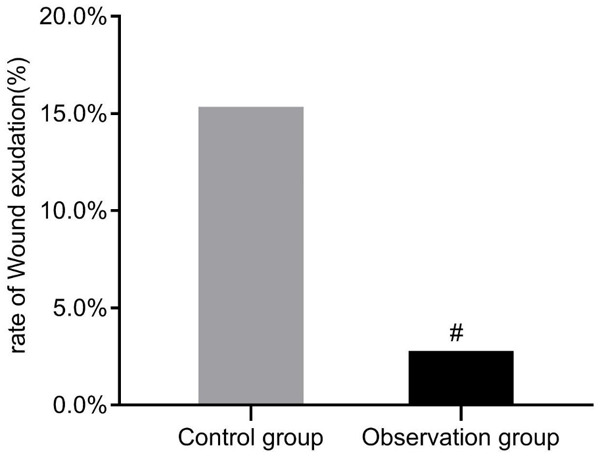
Comparison of wound exudate rate between two groups. Compared with the control group, #P<0.05.
Comparison of HIF-1α and VEGF protein expression
HIF-1α and VEGF protein levels were higher in the observation group as compared to the control group (both P<0.05, Table 3 and Figure 4).
Table 3.
Comparison of HIF-1α and VEGF proteins between two groups of patients (n)
| Group | Observation group (n=39) | Control group (n=39) | U | P |
|---|---|---|---|---|
| HIF-1α | 2.111 | 0.035 | ||
| - | 17 | 25 | ||
| + | 12 | 11 | ||
| ++ | 8 | 2 | ||
| +++ | 2 | 1 | ||
| VEGF | 2.035 | 0.048 | ||
| - | 2 | 4 | ||
| + | 13 | 16 | ||
| ++ | 14 | 18 | ||
| +++ | 10 | 1 |
Note: VEGF: vascular endothelial growth factor; HIF-1α: hypoxia-inducible factor-1α.
Figure 4.
Expression of HIF-1α and VEGF protein in patients in the observation group (100×). A: HIF-1α protein expression of patients in the observation group. B: VEGF protein expression of patients in the observation group. HIF-1α: hypoxia-inducible factor-1α; VEGF: vascular endothelial growth factor.
Occurrence of adverse reactions in the two groups
No abnormalities in blood routine and hepatorenal function or severe infection were observed in the two groups during treatment. Whereas, wound-edge reaction occurred in 4 and 2 cases in the observation group and the control group respectively, which subsided after appropriate use of antibiotics.
Discussion
Basic fibroblasts were first isolated and purified from bovine brain and pituitary gland [13]. Subsequent studies have confirmed that basic fibroblasts can stimulate the differentiation and proliferation of vascular endothelial cells and other cells originating from mesoderm and neuroectoderm, and play a vital role in tissue healing [14]. It has been shown that during wound repair, exogenous basic fibroblasts can promote the secretion of endogenous basic fibroblasts, which plays a significant role in promoting the repair of burn wounds [15,16]. As mentioned earlier, local application of INS can reduce the blood sugar around the wound, improve the wound microenvironment and promote wound healing [5]. Therefore, rh-bFGF combined with local application of INS was used in this study to treat diabetic patients with deep II degree burns. The results revealed a higher total effective rate in the observation group, which validate the advantages of the combined regime in the treatment of diabetic patients with deep II degree burns.
Li et al. conducted a study on 30 patients with diabetic foot, and intervened the control group by negative pressure suction, while the observation group by negative pressure suction combined with local INS [17]. They found that the average wound healing time was statistically lower in observation group than in control group. Ge et al. studied 80 patients with diabetic foot and found that local application of INS could reduce local inflammatory response and promote wound healing [18]. In the research of Zhang et al., 80 patients with deep II degree burns complicated with diabetes were enrolled and assigned to the control group for rh-bFGF therapy and the observation group for combination therapy of rh-bFGF and local INS [7]; and a lower wound exudate rate was determined in the observation group. In the present study, shorter healing time and lower wound exudate rate were determined in the observation group as compared to the control group, suggesting that rh-bFGF combined with local application of INS can shorten the wound healing time and lower the wound exudate rate compared with single application, which is consistent with the preceding research results.
Tenderness is a common symptom in patients with deep II degree burns, and its intensity is closely related to the individual psychological state. If the pain can’t be controlled in time, it may bring physiological and psychological troubles to the patients and affect their rehabilitation [19]. We have found that the VAS score in the observation group improved more significantly, which indicated that the combination of rh-bFGF and local INS could reduce the pain of patients compared with single drug. It may be that INS promotes wound healing, which shortens the healing time and the time for patients to feel tenderness, so the tenderness is reduced [20].
Burn, especially in diabetic patients, causes microcirculation disturbance in local wounds and distant tissues, resulting in tissue and organ ischemia and hypoxia, which in turn induces burn wound deterioration and various complications. Therefore, improving tissue cell ischemia and hypoxia and restoring organ blood supply are the key to prevent and treat complications after burns and to improve prognosis. HIF-1α is a major regulator of cell homeostasis in hypoxic environment, which exists in most mammalian cells and participates in pathophysiological processes such as vascular permeability change, intestinal mucosal barrier destruction, cell apoptosis, revascularization and cell migration after burns. It can mediate the formation of corneal neovascularization after alkali burn and promote the repair of burn wounds [21]. HIF-1α protein can act on the transcriptional regulatory region by binding with the DNA site of the target gene, such as regulating the expression of VEGF, thus promoting the formation of new blood vessels and initiating wound repair. While VEGF, regulated by HIF-1α protein, can induce angiogenesis and promote the growth and healing of wounds [22]. In our research, HIF-1α and VEGF protein levels were found to be higher in the observation group, which validated the higher healing effect of the combined local INS administration, suggesting that local INS application can promote angiogenesis and wound healing by regulating HIF-1α and VEGF protein levels. This may be due to the fact that HIF-1α promotes the mobilization and migration of angioblasts to the burn site by up-regulating the expression of VEGF, thus promoting angiogenesis and finally accelerating the recovery of blood supply of the burned skin and wound healing.
Finally, we investigated the adverse reactions such as abnormalities in blood routine and hepatorenal function as well as local wound margin in the two groups, and found no evident difference, indicating that the combined therapy had a high safety profile, which is consistent with the study of Zhang [23]. However, further investigation is warranted to elucidate the mechanism through which local INS improves HIF-1α and VEGF protein levels. Besides, due to the small sample size of this study, a large sample study is needed in the later stage for further exploration.
To sum up, given the significant effect of rh-bFGF combined with local application of INS in diabetic patients with deep II degree burns, mainly manifested in relieving pain, shortening healing time, reducing wound exudate rate, and a high safety profile.
Disclosure of conflict of interest
None.
References
- 1.Cuono C, Langdon R, McGuire J. Use of cultured epidermal autografts and dermal allografts as skin replacement after burn injury. Lancet. 1986;1:1123–4. doi: 10.1016/s0140-6736(86)91838-6. [DOI] [PubMed] [Google Scholar]
- 2.Papini R. Management of burn injuries of various depths. BMJ. 2004;329:158–60. doi: 10.1136/bmj.329.7458.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guillory AN, Porter C, Suman OE, Zapata-Sirvent RL, Finnerty CC, Herndon DN. 29-modulation of the hypermetabolic response after burn injury. In: Guillory AN, Porter C, Suman OE, Zapata-Sirvent RL, Finnerty CC, Herndon DN, editors. Total burn care. 5th, edition. Amsterdam: Elsevier; 2018. pp. 301–306. e3. [Google Scholar]
- 4.Kim S, Kwak I, Park GH. Effects of diabetes mellitus on the mortality, length of hospital stay and number of operations in burn patients. Ann Dermatol. 2019;31:51–58. doi: 10.5021/ad.2019.31.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao MC, Zhao MY, Yuan WJ, Li W, Wu H, Li ZH. Research progress of local application of insulin to promote diabetic wound healing. Med Recapitulate. 2018;24:4078–4085. 4091. [Google Scholar]
- 6.Han BX. Observation on the effect of external application of recombinant human basic fibroblast growth factor in the treatment of deep second degree burn. J Clin Ration Drug Use. 2018;11:60–61. [Google Scholar]
- 7.Zhang YB, Li H, Wang JL, Zhang L, Wang H. Effect of recombinant human basic fibroblast growth factor combined with local application of insulin in the treatment of diabetic deep second degree burn. Chin J Physician. 2019;21:1879–1881. [Google Scholar]
- 8.Tang LZ, Tong NW. Interpretation of ADA standers of medival care in diabetes-2018. West Chin Med J. 2018;33:513–519. [Google Scholar]
- 9.Wang DC, Zhao R. Pay attention to the application of dermabrasion treatment in early deep second degree burn. Chin J Burns. 2020;36:506–509. doi: 10.3760/cma.j.cn501120-20190115-00007. [DOI] [PubMed] [Google Scholar]
- 10.Li CC, Lin W, Wang CD, Wang C. Practical burns and plastic surgery. In: Li CC, Lin W, Wang CD, Wang C, editors. Beijing: World Publishing Corporation; 2013. [Google Scholar]
- 11.Maxwell C. Sensitivity and accuracy of the visual analogue scale: a psycho- physical classroom experiment. Br J Clin Pharmacol. 1978;6:15–24. doi: 10.1111/j.1365-2125.1978.tb01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu CX, Zhang C, Chen L. Effect of wound eschar and skin grafting on scar appearance and healing time in patients with deep burn wounds. J Clinl Surg. 2018;26:550–552. [Google Scholar]
- 13.Soeda S, Kozako T, Iwata K, Shimeno H. Oversulfated fucoidan inhibits the basic fibroblast growth factor-induced tube formation by human umbilical vein endothelial cells: its possible mechanism of action. Biochim Biophys Acta. 2000;1497:127–34. doi: 10.1016/s0167-4889(00)00052-5. [DOI] [PubMed] [Google Scholar]
- 14.Qu J, Wang L, Niu LX, Lin JM, Huang Q, Jiang XF, Li MZ. Porous silk fibroin microspheres sustainably releasing bioactive basic fibroblast growth factor. Materials (Basel) 2018;11:1280. doi: 10.3390/ma11081280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakrabarti S, Islam J, Hazarika H, Mazumder B, Raju PS, Chattopadhyay P. Safety profile of silver sulfadiazine-bFGF-loaded hydrogel for partial thickness burn wounds. Cutan Ocul Toxicol. 2018;37:258–266. doi: 10.1080/15569527.2018.1442843. [DOI] [PubMed] [Google Scholar]
- 16.Xu LXZ, Li M. Observation on the clinical effect of moist exposed burn ointment (MEBO) in the treatment of diabetes complicated with II degree scar on foot. Chin J Burns Wounds Surf Ulcers. 2019;31:113–116. [Google Scholar]
- 17.Li L, Mao YG, Liu DW. Effects of negative-pressure wound therapy combined with local application of insulin on the expression of β-catenin and cyclinD1 during the healing process of diabetic foot skin. Guangdong Med J. 2019;40:567–571. [Google Scholar]
- 18.Ge X, Zhou YT. Observation on the efficacy of insulin local application in the treatment of diabetic foot negative pressure. J Pre Med Chin People’s Liberation Army. 2019;37:22–24. [Google Scholar]
- 19.Huang ML, Huang YX. Analysis of influencing factors of pain related to continuous burn in burn patients. Nurs J Chin People’s Liberation Army. 2018;35:25–28. [Google Scholar]
- 20.Guang L, Chen BG, Song HF, Kang N, Wu Y. Effect of Xuebijing on pain and wound healing in burn patients. J Hainan Med Univ. 2020;26:1691–1694. [Google Scholar]
- 21.He JQ, Liao Y, Li L, Wang JY. Effects of rhGM CSF combined with insulin on the expression of CD31, HIF-1α and VEGF in deep second degree scald wound tissue of type 1 diabetic rats. Chin J Burns Wounds Surf Ulcers. 2018;30:101–112. [Google Scholar]
- 22.Ze K, Chen X, Yang YY, Chai YY, Li S, Kuai L, Ru Y, Lu Y, Zhao HB, Li X, Li FL, Li B. Effect of Quyu Shengji Recipe on wound healing and tissue PGT, PGE2 and VEGF contents in diabetic skin ulcer in rats. Liaoning J Tradit Chin Med. 2019;46:860–862. 900. [Google Scholar]
- 23.Zhang YB, Wang JL, Li H, Zhang L. Clinical observation of RH bFGF combined with topical insulin in the treatment of diabetic deep second degree burn wound. Chin J Aesthetic Med. 2019;28:32–35. [Google Scholar]


