Abstract
Repetitive sequence-based (Rep)-PCR genotyping as described here is based on the presence of homologues of Mycoplasma pneumoniae repeat-like elements in Staphylococcus. In this study we comparatively evaluated the usefulness of rep-PCR typing with two sets of well-defined collections of Staphylococcus aureus strains. Rep-PCR analysis of the first collection of S. aureus strains (n = 59) and one Staphylococcus intermedius strain showed 14 different rep-PCR patterns, with each pattern harboring 6 to 15 DNA fragments. The discriminatory power of rep-PCR typing compared well to those of arbitrarily primed PCR (average of 20 types) and pulsed-field gel electrophoresis (11 types). S. aureus strain collection I comprised four outbreak-related groups of isolates. The isolates in only one group were found to have identical rep-PCR profiles. However, in an analysis of isolates from three additional independent local outbreaks (n for outbreaks 1 and 2 = 5, n for outbreak 3 = 12), identical rep-PCR types were found among strains isolated during each outbreak. Therefore, we conclude that rep-PCR genotyping may be an easy and fast method for monitoring of the epidemiology of nosocomial Staphylococcus infections. Rep-PCR analysis of strain collection II, which consisted of epidemic and nonepidemic methicillin-resistant S. aureus (MRSA) strains, revealed that a cluster of similar rep-PCR profiles was found among MRSA isolates which were more frequently isolated and which were most often associated with outbreaks.
Staphylococcus aureus is one of the most significant pathogens causing nosocomial infections. The emergence of methicillin-resistant S. aureus (MRSA) in particular has become a major clinical problem. In Europe, the incidence of MRSA varies from <1% in The Netherlands, Sweden, and Denmark to >30% in the southern European countries such as Spain, France, and Italy (16). Outbreaks in hospitals in countries with a low incidence of MRSA are often initiated by the migration of patients from hospitals in countries with a high prevalence of MRSA. Also, carriage of S. aureus among hospital personnel may be hazardous to the neutropenic patient (8). On the other hand, S. aureus is often carried asymptomatically and does not always cause disease. Strains may vary considerably in their epidemiological potentials, and those strains that have been known to spread widely and rapidly among patients have been designated epidemic S. aureus strains (4, 9).
At present, several molecular typing systems are in use for the monitoring of outbreaks of infections caused by S. aureus. In particular, pulsed-field gel electrophoresis (PFGE) and arbitrarily primed (AP)-PCR have been shown to be well-suited methods with regard to their discriminatory abilities (12, 13). However, each method has experimental drawbacks: PFGE is a reliable and reproducible method but is very tedious and time-consuming; AP-PCR is easy and can be performed very fast but lacks intercenter reproducibility. The genotyping results obtained by both these methods do not overlap completely, and if the results are combined, they will give additional resolution (13).
The ideal system for the typing of S. aureus strains should be easy, rapid, reliable, highly discriminatory, and reproducible. Furthermore, it should be suitable for widespread use, so that the genotyping results obtained in different laboratories or countries can be compared. In Europe, different laboratories use different typing systems. To allow identification of possible epidemic strains which are spread by migration of patients, it is of great importance that the same suitable typing system be used. A PCR-based typing system would be most appropriate because of its ease and speed of performance. Because AP-PCR has its limitations for widespread use, another more reproducible PCR method should be considered. A repetitive element sequence-based PCR (rep-PCR) has been described for the molecular genotyping of S. aureus (2). This high-stringency PCR fingerprinting method is based on a repetitive sequence found in Mycoplasma pneumoniae (17), but it also generates strain-specific DNA fragments when S. aureus DNA is used as an amplification template.
We optimized the rep-PCR and investigated its performance and discriminatory abilities by using a well-defined collection of Staphylococcus strains which were previously analyzed by many different methods. In addition, we studied a collection of MRSA isolates which consisted of epidemic and nonepidemic strains that were previously analyzed by assessment of protein A gene polymorphism.
MATERIALS AND METHODS
Bacterial strains.
Two sets of Staphylococcus isolates were included in this study. The first set consisted of 60 isolates which were divided into three groups (isolates SA-01 to SA-20, SB-01 to SB-20, and SC-01 to SC-20) and which were described in great detail by Tenover et al. (12), Deplano et al. (3), and Van Belkum et al. (13). A single isolate of Staphylococcus intermedius was included in this set (isolate SA-16 in Table 1). In addition, 46 MRSA strains previously described by Frénay et al. (4) were investigated. Thirty-two of these 46 strains were isolated during an ongoing MRSA surveillance study in Dutch hospitals. These strains were not epidemiologically related and were imported by different patients after a stay in a hospital abroad. Nineteen of the 32 strains were classified by Frénay et al. (4) as epidemic MRSA strains on the basis of their association with outbreaks. In addition to the 32 strains from the Dutch survey, 14 well-documented epidemic MRSA strains from England and Wales were included in this study (4, 9). Further details on these strains are provided by Frénay et al. (4) and Marples and Reith (9).
TABLE 1.
Molecular genotyping results for Staphylococcus strains
| Straina | Outbreak | Oxacillin susceptibilityb | Inter-IS256 typec | AP-PCR Ia type | PFGE type | Rep-PCR type |
|---|---|---|---|---|---|---|
| SA-16 | NO | S | HEH | I | S.i | |
| SA-04 | NO | S | BBB | E | E | |
| SA-12 | NO | R | 43 | AAA | J | A |
| SA-18 | NO | R | 43 | AAA | J | B |
| SA-20 | NO | R | 43 | AAA | J | A |
| SA-06 | NO | I | CAC | C | A | |
| SA-07 | NO | S | DAD | B | A | |
| SA-08 | NO | R | 42 | ECE | G | A |
| SA-11 | NO | R | 42 | ECF | F | F |
| SA-01 | NH1 | R | 44 | AAA | K1 | A |
| SA-09 | NH1 | R | 44 | AAA | K2 | A |
| SA-03 | NH1 | R | 43 | AAA | A | A |
| SA-13 | NH1 | R | 44 | AAG | A | A |
| SA-14 | NH1 | S | GDG | H | C | |
| SA-19 | NH1 | R | 44 | AAA | K3 | A |
| SA-17 | NH2 | R | 44 | AAA | A | A |
| SA-02 | NH2 | R | 43 | AAA | A | B |
| SA-15 | NH2 | R | 43 | AAA | A | A |
| SA-05 | NH2 | R | 44 | AAA | A | A |
| SA-10 | NH2 | R | 44 | FAA | D | A |
| SB-07 | NO | S | BBB | D | J | |
| SB-03 | I | R | 44 | AAA | A | A |
| SB-05 | I | R | 44 | AAA | A | A |
| SB-10 | I | R | 44 | AAA | A | H |
| SB-12 | I | R | 44 | AAA | A1 | H |
| SB-15 | I | R | 44 | AAA | A | A |
| SB-19 | I | R | 44 | AAA | A | A |
| SB-20 | I | R | 44 | AAA | A | A |
| SB-01 | NO | R | 44 | AAA | A1 | A |
| SB-16 | NO | R | 44 | AAA | A1 | A |
| SB-18 | NO | R | 44 | AAA | A | A |
| SB-17 | NO | I | LDL | E | C | |
| SB-14 | NO | R | 44 | AAG | A2 | A |
| SB-08 | NO | S | JGJ | F | K | |
| SB-02 | II | S | IFI | B | G | |
| SB-04 | II | S | IFI | B | G | |
| SB-06 | II | S | IFI | B | B | |
| SB-11 | II | S | FFK | C | H | |
| SB-09 | NO | S | BFI | B | I | |
| SB-13 | NO | S | KFI | B1 | L | |
| SC-03 | NO | S | BBB | C | D | |
| SC-01 | III | R | 44 | MFB | A | M |
| SC-04 | III | R | 44 | MFB | A | M |
| SC-05 | III | R | 44 | MFB | A | M |
| SC-09 | III | R | 44 | MFB | A | M |
| SC-11 | III | R | 44 | MFB | A | M |
| SC-12 | III | R | 44 | MFB | A | M |
| SC-14 | III | R | 44 | MFB | A | M |
| SC-15 | III | R | 44 | MFB | A | M |
| SC-17 | III | R | 44 | MFB | A | M |
| SC-20 | III | R | 44 | MFB | A | M |
| SC-08 | NO | S | JHD | B1 | K | |
| SC-02 | IV | S | NGJ | B | K | |
| SC-06 | IV | S | JHD | B | M | |
| SC-07 | IV | S | JHD | B | K | |
| SC-10 | IV | S | JHD | B | K | |
| SC-13 | IV | S | JHD | B | K | |
| SC-16 | IV | S | JHD | B | K | |
| SC-18 | IV | S | JHD | B | K | |
| SC-19 | IV | S | JHD | B | K | |
| No. of types | 20 | 11 | 14 |
Rep-PCR typing procedure.
The rep-PCR was carried out in 25-μl reaction volumes. Each reaction mixture contained 75 pmol of primer RW3A (2), 1 U of SuperTaq DNA polymerase (HT Biotechnology Ltd., Cambridge, United Kingdom), 2.5% dimethyl sulfoxide, and each deoxynucleoside triphosphate at a concentration of 200 μM in SuperTaq PCR buffer (50 mM Tris-HCl [pH 9.0], 50 mM KCl, 1.5 mM MgCl2, 0.01% gelatin, 0.1% Triton X-100; HT Biotechnology Ltd.). Subsequently, 2.5 μl (200 to 500 ng) of chromosomal S. aureus DNA was added to the PCR mixture. Chromosomal DNA for rep-PCR was isolated from S. aureus strains essentially as described elsewhere (2). Cycling was performed in a Perkin-Elmer thermocycler 9600 by using the following program: 3 min at 94°C, followed by 30 cycles of 1 min at 94°C, 1 min at 54°C, and 2 min at 72°C. The program finished with an additional 5-min extension step at 72°C.
Analysis of rep-PCR-generated banding patterns.
The PCR products were separated by electrophoresis of 12 μl of the reaction mixtures in ethidium bromide-stained 1.5% agarose gels in 0.5× TBE (Tris-borate-EDTA) buffer for 3.15 h at 5 V/cm. A 1-kb DNA ladder (Life Technologies, GIBCO BRL, Breda, The Netherlands) was used as a DNA size standard. The DNA was visualized on a UV transilluminator and photographed. The gels were analyzed both by visual inspection and by computer-aided methods. The bands used to determine the rep-PCR type were arbitrarily chosen to range from 200 to 4,000 bp. Strains that had PCR-generated DNA banding patterns that had more than one band difference in terms of size or intensity were considered distinct types. Banding patterns were digitized with a Hewlett-Packard Scanjet IIcx/T scanner and were analyzed with GelCompar software (version 4.0; Applied Maths, Kortrijk, Belgium). Degrees of homology were determined by Dice comparisons, and clustering correlation coefficients were calculated by the unweighted pair group method with arithmetic averages.
RESULTS
Genotyping of Staphylococcus strains.
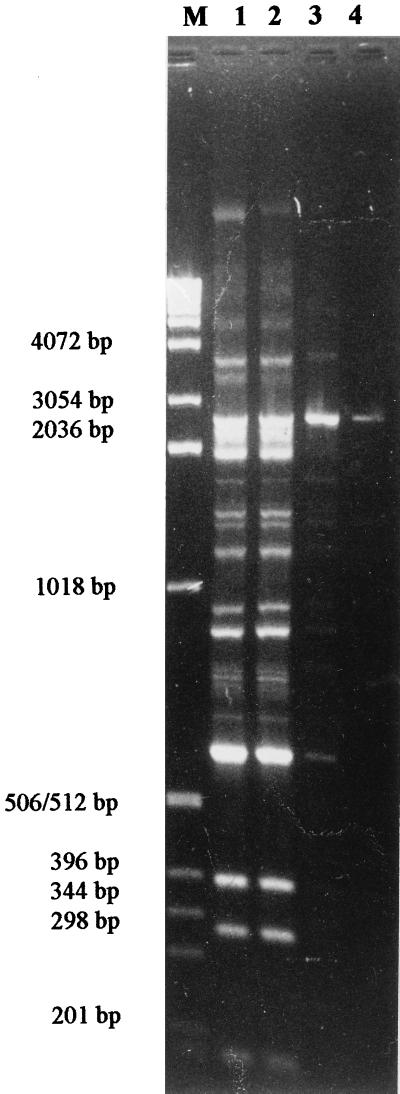
We optimized and modified the PCR conditions as described by DelVecchio et al. (2). The addition of 2.5% dimethyl sulfoxide was found to result in highly consistent PCR banding patterns with template DNA concentrations ranging from 1 μg down to 100 pg (Fig. 1). To ensure visualization of all low-intensity bands on the gel, template concentrations were chosen as described in the Materials and Methods section.
FIG. 1.
Rep-PCR profiles generated with a range of concentrations of template DNA. Lane M, marker DNA; lanes 1 to 4 template DNA at 20 ng, 2 ng, 200 pg, and 20 pg, respectively.
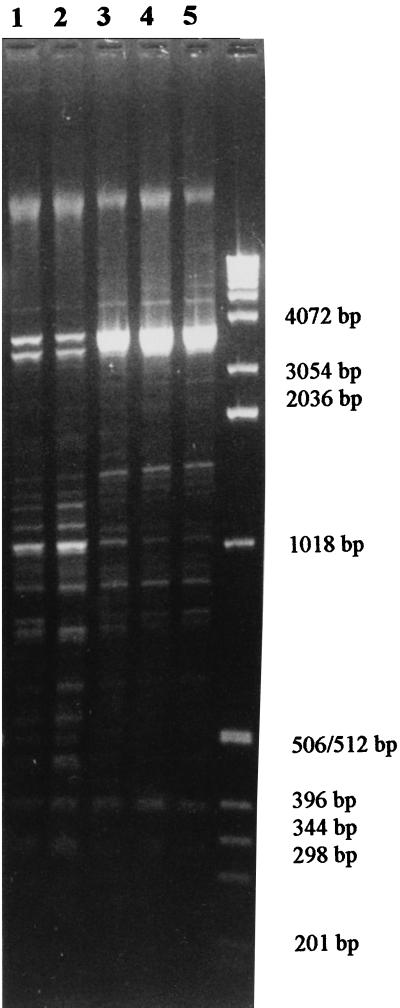
By analyzing the S. aureus strain collections described before, various rep-PCR banding patterns with from 5 to 15 bands with various intensities were found. Reproducible patterns were obtained with different DNAs isolated from one strain on different occasions, as well as with DNAs from different strains belonging to the same rep-PCR type (Fig. 2). Furthermore, multiple colonies of separate isolates were repeatedly tested over a time span of 1 year and resulted in consistent rep-PCR profiles. Even faint bands were amplified reproducibly (Fig. 2). Each pattern was assigned a specific type that was designated with a letter (A through Z; see Fig. 3 and 4).
FIG. 2.
Rep-PCR profiles of five different strains judged to be of two distinctive rep-PCR types. Lanes 1 and 2, strains SB-02 and SB-04 (type G), respectively; lanes 3 to 5, strains SB-10, SB-11, and SB-12 (type H), respectively. The sizes of the marker DNA are given on the right.
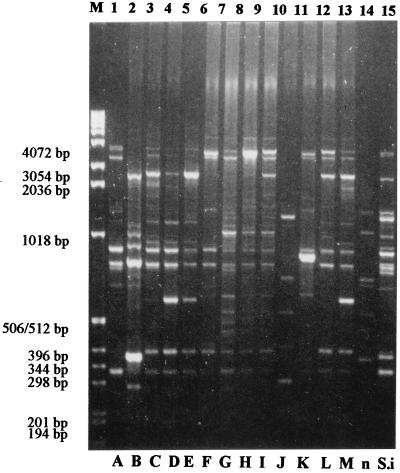
FIG. 3.
Rep-PCR fingerprints of Staphylococcus strains. Lanes 1 to 13: isolates SB-03 (type A), SA-18 (type B), SB-17 (type C), SC-03 (type D), SA-04 (type E), SA-11 (type F), SB-04 (type G), SB-11 (type H), SB-09 (type I), SB-07 (type J), SB-08 (type K), SB-13 (type L), and SC-04 (type M), respectively; lane 14, type n (not included); lane 15, S. intermedius SA-16 (type S.i). The sizes of the marker DNA (lane M) are given on the left.
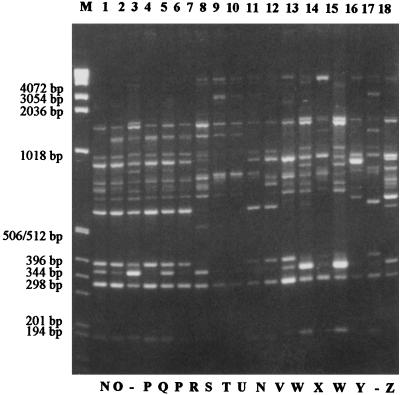
FIG. 4.
Rep-PCR fingerprints of epidemic and nonepidemic MRSA strains. Lanes 1 to 18, isolates 89-00640 (type N), 89-00423 (type O), 94-00533 (not included), 90-05882 (type P), 89-00227 (type Q), 89-06639 (type P), 91-10150 (type R), 91-10151 (type S), 91-10153 (type T), 89-07616 (type U), 90-01145 (type N), 90-06609 (type V), 90-02473 (type W), 90-02341 (type X), 90-02473 (type W), 89-03706 (type Y), 94-00533 (not included), and 90-02308 (type Z), respectively. The sizes of the marker DNA (lane M) are given on the left.
Comparison of rep-PCR with AP-PCR and PFGE performed with a well-defined S. aureus strain collection.
To investigate the usefulness of rep-PCR in the molecular genotyping of S. aureus strains, a well-characterized set of strains was analyzed. The Staphylococcus strains mentioned in Table 1 have previously been described in detail and were typed by a broad range of typing methods (13). In the present study, the rep-PCR typing results (Table 1) were mainly compared to the results obtained by inter-IS256 PCR (3), AP-PCR, and PFGE (13).
Among the 60 Staphylococcus strains, 14 different rep-PCR banding patterns were found (Fig. 3), and each different pattern was assigned a letter (A through M; n and S.i were used for additional rep-PCR types) (Table 1). The only S. intermedius strain (strain SA-16) showed a unique rep-PCR type (type S.i). Among the S. aureus strains associated with outbreaks (outbreaks I to IV; Table 1), only the isolates from outbreak III were identical by rep-PCR typing (type M). This result was consistent with the results of inter-IS256 PCR, AP-PCR, and PFGE. Among the isolates from outbreak I (n = 7), two isolates (SB-10 and SB-12) had a rep-PCR profile (type H) that differed from those of the other isolates (type A). Strains from outbreak II (n = 4) were found to have three different rep-PCR types (types B, G, and H), whereas by AP-PCR and PFGE the type for only one strain (SB-11) was different from that for the other three strains, which were isolated from the same patient. All of the isolates from outbreak IV except strain SC-06 (type M) had rep-PCR fingerprints of type K.
To assess further the ability of rep-PCR typing to cluster isolates from given outbreaks, we analyzed related strains from three local outbreaks in Dutch hospitals. Isolates within each outbreak (n = 5, n = 5, and n = 12) had identical rep-PCR fingerprints (data not shown). According to Tenover et al. (12), strain ATCC 12600 was included in the strain collection three times (SA-04, SB-07, and SC-03). By rep-PCR, these strains were found to be of types E, J, and D, respectively. This result is concordant with the result of PFGE in which three different types were also found (types E, D, and C, respectively). Of duplicate strains (SA-01 and SA-09, SA-02 and SA-15, and SC-17 and SC-20) only SA-02 and SA-15 were of different rep-PCR types, but they also showed some variation by other typing methods (13) that are not discussed here.
By rep-PCR typing, no strict discrimination could be made between oxacillin (methicillin)-resistant or -sensitive S. aureus strains. However, rep-PCR types A, B, F, H, and M were more often observed among methicillin-resistant strains (95%) than among methicillin-sensitive S. aureus strains (21%). Other rep-PCR types were found only among methicillin-sensitive S. aureus strains. In general, a strong correlation was observed between the results of rep-PCR typing, AP-PCR, and PFGE genotyping. The discriminatory power of rep-PCR genotyping, which discriminates 14 different types, was less than that of AP-PCR (average of 21 types) but greater than that of PFGE (11 types) (13).
Rep-PCR typing of epidemic and nonepidemic S. aureus strains.
To investigate whether certain rep-PCR fingerprints would have prognostic value for epidemic or nonepidemic MRSA strains, we genotyped the 46 S. aureus strains described by Frénay et al. (4) (Table 2). Of these strains, Frenay et al. classified 13 as nonepidemic based on the fact that these strains were isolated only once. If strains were known to have caused hospital outbreaks involving at least two patients or staff members, they were classified as epidemic (4). On the basis of these criteria, 32 strains were defined as epidemic. The numbers and the DNA sequences of spa gene repeats were listed, but only the numbers and not the sequences of these repeats were previously suggested to be correlated with the epidemic potentials of certain strains (4).
TABLE 2.
Genotyping results for epidemic and nonepidemic MRSA strains
| MRSA groupa | Strain | No. of isolates | No. of spa gene repeats | spa typeb | Rep-PCR type |
|---|---|---|---|---|---|
| Epidemic MRSA | 89-09138 | 6 | 6 | ----bcmf--kh--- | N |
| 92-01866 | 4 | 7 | ----bcmf--khi-- | N | |
| 91-10155 | 5 | 7 | ----bcmf--khi-- | N | |
| 91-10147 | 243 | 7 | ----bcmf--khi-- | U | |
| 91-10160 | 7 | 7 | -a---heiejk---- | Q | |
| 91-10159 | 3 | 7 | -a---heiejk---- | Q | |
| 91-10148 | 30 | 7 | -a---heiejk---- | O | |
| 91-10158 | 47 | 7 | -a---heiejk---- | O | |
| 91-10152 | 9 | 7 | -a---heiejk---- | O | |
| 91-04772 | 18 | 9 | -a-cbheiejk---- | O | |
| 90-02348 | 69 | 9 | -a-cbheiejk---- | X | |
| 89-06639 | 2 | 9 | aa--bheiejk---- | P | |
| 91-07490 | 2 | 9 | ----ehehrhchm-- | O | |
| 91-01611 | 14 | 9 | ----ehehrhchm-- | V | |
| 90-03415 | 8 | 10 | ---txhehrhchm-- | N | |
| 91-10149 | 19 | 10 | ---tshehrhchm-- | X | |
| 90-02473 | 2 | 10 | ---eohehrhchm-- | W | |
| 90-03140 | 10 | 10 | -abcbheiejk---- | O | |
| 92-00354 | 8 | 10 | -abcbheiejk---- | O | |
| 91-10157 | 3 | 11 | aaccbheieok---- | O | |
| 90-05857 | 6 | 11 | aabcbheiejk---- | O | |
| 91-04739 | 18 | 11 | aabcbheiejk---- | O | |
| 92-00889 | 18 | 11 | aabcbheiejk---- | O | |
| 92-06062 | Unknown | 11 | aabcbheiejk---- | O | |
| 89-00640 | 39 | 11 | aabcbheiejk---- | N | |
| 90-05882 | 18 | 11 | aabcbheiejk---- | P | |
| 89-00227 | 43 | 11 | aabcbheiejk---- | Q | |
| 91-10154 | 3 | 11 | aaccceeiejk---- | O | |
| 90-02308 | 3 | 11 | ----bcmfmfkhiii | Z | |
| 91-10156 | 3 | 13 | -a-cbheiejiejlk | O | |
| 91-10150 | 17 | 15 | eodopqhpqhkhkmn | R | |
| 91-10151 | 12 | 15 | eodopqhpqhkhkmn | S | |
| 91-10153 | 11 | 15 | eodopqhpqhkhkmn | T | |
| Nonepidemic MRSA | 90-06609 | 1 | 3 | dod------------ | V |
| 89-07616 | 1 | 6 | ----bcmf--kh--- | U | |
| 89-05856 | 1 | 7 | ----bcmf--khi-- | O | |
| 89-06597 | 1 | 7 | ----bcmf--khi-- | O | |
| 89-06928 | 1 | 7 | ----bcmf--khi-- | O | |
| 90-05366 | 1 | 7 | ----bcmf--khi-- | O | |
| 90-04649 | 1 | 7 | ----bcmf--khi-- | N | |
| 90-01145 | 1 | 7 | ----bcmf--khi-- | N | |
| 89-00423 | 1 | 7 | -a---heiejk---- | O | |
| 89-03706 | 1 | 9 | ---eohe-rhchm-- | Y | |
| 90-02341 | 1 | 10 | ---eohehrhchm-- | X | |
| 89-02036 | 1 | 11 | aabcbheiejk---- | O | |
| 90-05145 | 1 | 11 | babcbheiejk---- | O |
Discrimination of epidemic strains was made on the basis of an association with hospital outbreaks involving at least two patients or staff members. MRSA strains that were isolated only once were defined as nonepidemic (4).
The DNA repeats of the X region of the spa gene are coded by letters, as described previously (5). Hyphens indicate gaps in the alignment.
Among 46 strains, 13 different rep-PCR fingerprints, designated N through Z, were found (Fig. 4; Table 2). The distribution of the different rep-PCR types among epidemic and nonepidemic MRSA strains is presented in Table 3. Among epidemic MRSA strains, rep-PCR types N, O, P, Q, and X were found for more than one isolate. In addition, strains represented by rep-PCR types N, O, P, Q, U, and X were isolated 64, 182, 20, 53, 89, and 244 times, respectively. Strains with rep-types R through Z were isolated less frequently (Table 3).
TABLE 3.
Relationship between rep-PCR type and epidemic potentials of MRSA strains
| Rep-PCR type | N | O | P | Q | R | S | T | U | V | W | X | Y | Z |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of epidemic MRSA isolatesa | 5 | 14 | 2 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 1 |
| No. of associated isolatesb | 62 | 175 | 20 | 53 | 17 | 12 | 11 | 243 | 14 | 2 | 88 | 0 | 3 |
| No. of MRSA isolates | 2 | 7 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 |
Isolates investigated in this study.
Representative strains associated with outbreaks involving the indicated number of isolates (4).
GelCompar analysis of rep-PCR fingerprints.
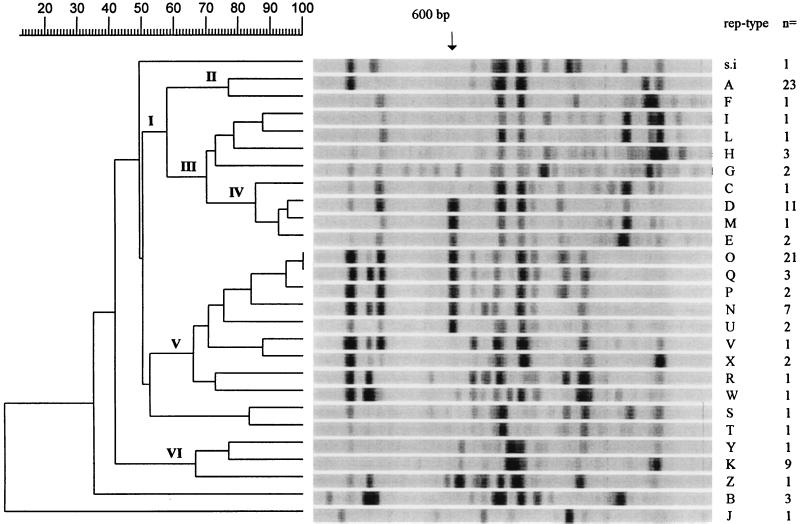
The rep-PCR banding patterns were scanned and subjected to GelCompar analysis. All strains with the same rep-PCR type were recognized to be identical by GelCompar analysis. In these cases, one representative strain of a given rep-PCR type was selected (Fig. 5). The similarity of the majority of the rep-PCR-generated fingerprints indicates a high degree of relatedness between strains. Several groups of strains with a similar rep-PCR banding patterns were clustered (Fig. 5). Cluster I comprises three subclusters of the rep-PCR types of strains which are predominantly methicillin sensitive; this does not include strains in subcluster II (rep-PCR types A and F) and those of rep-PCR types H and M in subclusters III and IV, respectively. Subcluster IV consisted of strains of rep-PCR types which generated a 600-bp DNA fragment in connection with a 980-bp DNA fragment; these fragments are not seen for the other isolates within cluster I. These bands are also present in strains of five rep-PCR types belonging to cluster V. GelCompar analysis correctly classified rep-PCR types O and Q as being identical, whereas after repeated checking, we deliberately differentiated them into two types because of a reproducible difference in the intensity of one band. Cluster V consisted of strains of rep-PCR types associated with outbreaks with large numbers of isolates except strains of rep-PCR types S and T, which show a relatively large genetic distance to the remainder of isolates in cluster V. Cluster VI comprises strains of three rep-PCR types; one type (type K) is methicillin sensitive and outbreak related. Strains with rep-PCR types B and J are most divergent, even more so than S. intermedius, whose rep-PCR type is genetically closer to the rep-PCR types of other S. aureus strains.
FIG. 5.
GelCompar software analysis of all rep-PCR types within Staphylococcus strain collections I (mostly outbreak-related isolates) and II (epidemic and nonepidemic MRSA strains). The photographs in Fig. 3 and 4 were digitized, and the degree of homology was calculated by Dice comparisons. Correlation coefficients were calculated by the unweighted pair group method with arithmetic averages. Clusters of related strains are indicated as clusters I to VI. The molecular sizes of the bands are indicated at the top. On the right, the rep-PCR types are indicated and the number of strains from this study that had that type are indicated.
DISCUSSION
This study was performed to assess the usefulness of rep-PCR genotyping of Staphylococcus strains. Rep-PCR typing is based on the presence in Staphylococcus of DNA sequences that are homologous to M. pneumoniae repeat MP3 (17). This high-stringency PCR typing method has been described previously by DelVecchio et al. (2) and simultaneously detects the mecA gene which encodes the oxacillin- or methicillin-resistant phenotype. They detected the mecA PCR products with fluorescent dyes, which requires more sophisticated equipment than is usually found in a clinical microbiology laboratory. We separated mecA detection from rep-PCR genotyping to be able to analyze the typing results by agarose gel electrophoresis. Consistent rep-PCR results were achieved with a broad range of template DNA concentrations. Because PCRs performed by different technicians with DNAs separated from one strain on different occasions yielded identical profiles, the intralaboratory reproducibility of the method was judged to be excellent. Thus, the results of different rep-PCR runs can be compared. The discriminatory ability of rep-PCR genotyping was shown to be excellent, with 31 distinctive patterns among 108 strains.
Analysis of the strains in Staphylococcus strain collection I, described earlier by Tenover et al. (12) and Van Belkum et al. (13), showed that the rep-PCR typing method is as discriminatory as AP-PCR and PFGE. In comparison with AP-PCR, rep-PCR requires an additional chromosomal DNA purification step, and thus, performance of AP-PCR is a little faster than performance of rep-PCR. However, the reproducibility of rep-PCR, which is highly important when used for epidemiological purposes, was found to be excellent, in contrast to the reproducibility of AP-PCR. If rep-PCR is compared to PFGE, the most important advantage of rep-PCR is its easy and fast performance. The reproducibility of rep-PCR is comparable to that of PFGE. Another PCR-based typing method, inter-IS256 PCR, which compares best to rep-PCR typing with regard to ease of performance and reproducibility, has been described by Deplano et al. (3). Among a selection of 36 Staphylococcus strains analyzed by inter-IS256 PCR, three different types were identified (Table 1), while rep-PCR typing of the same selection of strains identified five different types. Rep-PCR typing also compares favorably to other repetitive element-based PCR methods such as the amplification of the inter-16S-23S rRNA gene spacer region (6), and the Tn916-16S rRNA gene spacer region (1), which both were found to be moderately discriminatory (10). Among the strains in Staphylococcus strain collection I, in general a high correlation was found between rep-PCR typing and the typing results obtained by AP-PCR and PFGE. Among epidemiologically unrelated S. aureus strains, rep-PCR typing results were discordant in a few cases with the combined data of AP-PCR and PFGE, for example, for strains SA-18 and SA-02 (Table 1). On the other hand, there is also no complete conformity between the results of AP-PCR and PFGE. For example, PFGE types A and B are differentiated into three and five AP-PCR types, respectively, and AP-PCR types AAA and BBB are each differentiated into three different PFGE types. Compared to the results of AP-PCR and PFGE, rep-PCR also had a few discrepant results with regard to outbreak-related isolates as well, i.e., strains SB-10 and SB-12 (outbreak I), SB-06 (outbreak II), and SC-06 (outbreak IV). These isolates had rep-PCR types different from those of other isolates from the same outbreak. Although the stability of the rep-PCR genotyping method was demonstrated by continuous subculturing of strains (2), intrastrain variability caused by repeated conservation and “revival” cannot be excluded completely. This phenomenon has been brought forward before to explain the extensive variability of the same American Type Culture Collection strain which was included in the collection of strains studied three times and which was typed differently by each method used. In addition, sampling errors may have occurred. Because the strains of collection I were subcultured many times in different laboratories, there is a realistic chance that contaminants instead of subcultures of certain strains were typed by rep-PCR. This would explain the finding that strain SC-06 (the only strain with rep-PCR type M within an outbreak of methicillin-sensitive strains of type K) appeared to possess the mecA gene (data not shown). Nevertheless, analysis of strains from three local outbreaks revealed that rep-PCR genotyping proved to be adequate in the identification of strains belonging to the same outbreak. Therefore, we may conclude that rep-PCR genotyping is a suitable method for the monitoring of outbreaks of S. aureus infections.
Analysis of MRSA strain collection II, described by Frénay et al. (4), did not result in a correlation between rep-PCR typing results with those of spa gene typing. We found no significant correlation between the number of repeats in the spa gene and the rep-PCR types or between the spa types and the rep-PCR types. Due to its location in the outer membrane, protein A is liable to selection pressure and may therefore present a hypervariable target not suitable for use in genotyping and determination of interstrain relationships (14). In contrast to PCR analysis of single gene (such as spa gene) polymorphisms, rep-PCR typing, like AP-PCR and PFGE, is expected to generate a random genomic fingerprint, which is better suited for epidemiological studies.
The analysis of epidemic and nonepidemic MRSA strains described by Frénay et al. (4) revealed that the identification of highly epidemic strains may be possible by rep-PCR genotyping. The definition of the epidemic phenotype versus the nonepidemic phenotype on the basis of the criteria described by Frénay et al. (4) is difficult to establish and may depend on local hygienic measures or the susceptibilities of individual patients. Many different circumstances determine whether a strain causes disease or leads only to enigmatic carriage and whether a strain is isolated from only one patient and is therefore classified as nonepidemic or spreads to two or more patients and is therefore classified as epidemic. Hence, the discrimination between epidemic and nonepidemic strains among those in the strain collection studied may be a major point of dispute. An alternative measure of the epidemic phenotype could be presented by the frequency of isolation of strains with identical genotypes among both epidemiologically related and epidemiologically unrelated isolates. Among the strains in the collections described here, those of rep-PCR types P and Q and rep-PCR types N and O were either strictly or most often associated with outbreaks, respectively. The fact that rep-PCR types N and O were also frequently observed among strains that were isolated only once may also be taken as an indication of their potential to spread.
Analysis of banding patterns by using GelCompar software clustered the fingerprints of outbreak-related strains (types N, O, P, Q, and U) within the same cluster of related strains (cluster V). Strikingly, almost all of the epidemic MRSA strains analyzed are recognized by their rep-PCR patterns, which include 600- and 980-bp DNA fragments. These most conspicuous DNA fragments are also seen in isolates in cluster IV and include the MRSA strains from outbreak III (type M).
It is likely that the MRSA strains but not the methicillin-sensitive S. aureus strains within these clusters of strains with strongly related fingerprints have higher epidemic potentials. We propose the following hypothesis to explain these findings: When an S. aureus strain is mecA gene positive and its rep-PCR pattern includes 600- and 980-bp DNA fragments, this strain is probably an epidemic strain. Whether these fragments are generated by differential locations of the homologous MP3 sequences on the chromosome of S. aureus or represent strain-specific sequences is under investigation. Further analysis of outbreak-related strains in addition to epidemiologically unrelated isolates may help to identify possibly epidemic S. aureus strains, regardless of whether they are methicillin resistant or sensitive.
Rep-PCR typing appears to be superior to AP-PCR, since the rep-PCR results obtained in this study are highly reproducible due to the high annealing conditions of the primer. Thus, it is expected that consistent results can be obtained when the method is performed in another laboratory. The interlaboratory reproducibility of the method is under investigation. On the basis of the results of this study, rep-PCR typing also appears to be more useful than PFGE because of its higher resolution power and ease of performance. We conclude that rep-PCR typing may be suitable for widespread use in the clinical microbiology laboratory for epidemiological typing of S. aureus strains. Furthermore, rep-PCR patterns are suitable for analysis with GelCompar software and storage, provided that (i) PCR is performed with isolated chromosomal DNA and not with lysed cells and (ii) the gel electrophoresis conditions are standardized. Stored rep-PCR patterns and interlaboratory exchange of digitized fingerprints by electronic mail can play an important role in the analysis of future nosocomial MRSA outbreaks and in the monitoring of the international spread of strains with high epidemic potentials.
ACKNOWLEDGMENTS
Han de Neeling, Corrie Schot, Willem van Leeuwen, Marco Janssens, Jan Kluytmans, and Rob Wintermans are gratefully acknowledged for providing strains.
REFERENCES
- 1.Cuny C, Witte W. Typing of Staphylococcus aureus by PCR for DNA sequences flanked by transposon Tn916 target region and ribosomal binding site. J Clin Microbiol. 1996;34:1502–1505. doi: 10.1128/jcm.34.6.1502-1505.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DelVecchio V G, Petroziello J M, Gress M J, McCleskey F K, Melcher G P, Crouch H K, Lupski J R. Molecular genotyping of methicillin-resistant Staphylococcus aureus via fluorophore-enhanced repetitive-sequence PCR. J Clin Microbiol. 1995;33:2141–2144. doi: 10.1128/jcm.33.8.2141-2144.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deplano A, Vaneechoutte M, Verschraegen G, Struelens M J. Typing of Staphylococcus aureus and Staphylococcus epidermidis strains by PCR analysis of inter-IS256 spacer length polymorphisms. J Clin Microbiol. 1997;35:2580–2586. doi: 10.1128/jcm.35.10.2580-2587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frénay H M E, Theelen J P G, Schouls L M, Vandenbroucke-Grauls C M J E, Verhoef J, van Leeuwen W J, Mooi F R. Discrimination of epidemic and nonepidemic methicillin-resistant Staphylococcus aureus strains on the basis of protein A gene polymorphism. J Clin Microbiol. 1994;32:846–847. doi: 10.1128/jcm.32.3.846-847.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frénay H M E, Bunschoten A E, Schouls L M, van Leeuwen W J, Vandenbroucke-Grauls C M J E, Verhoef J, Mooi F R. Molecular typing of methicillin-resistant Staphylococcus aureus on the basis of protein A gene polymorphism. Eur J Clin Microbiol Infect Dis. 1996;15:60–64. doi: 10.1007/BF01586186. [DOI] [PubMed] [Google Scholar]
- 6.Gürtler V, Barrie H D. Typing of Staphylococcus aureus strains by PCR-amplification of variable-length 16S-23S rDNA spacer regions: characterization of spacer sequences. Microbiology. 1995;141:1255–1265. doi: 10.1099/13500872-141-5-1255. [DOI] [PubMed] [Google Scholar]
- 7.Ichiyama S, Ohta M, Shimokata K, Kato N, Takeuchi J. Genomic DNA fingerprinting by pulsed-field gel electrophoresis as an epidemiological marker for study of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1991;29:2690–2695. doi: 10.1128/jcm.29.12.2690-2695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kluytmans J, van Leeuwen W, Goessens W, Hollis R, Messer S, Herwaldt L, Bruining H, Heck M, Rost J, van Leeuwen N, van Belkum A, Verbrugh H. Food-initiated outbreak of methicillin-resistant Staphylococcus aureus, analyzed by geno- and phenotyping. J Clin Microbiol. 1995;33:1121–1128. doi: 10.1128/jcm.33.5.1121-1128.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marples R R, Reith S. Methicillin-resistant Staphylococcus in England and Wales. Commun Dis Rep. 1992;2:25–29. [PubMed] [Google Scholar]
- 10.Prasanna Kumari D N, Keer V, Hawkey P M, Parnell P, Joseph N, Richardson J F, Cookson B. Comparison and application of ribosome spacer DNA amplicon and pulsed-field gel electrophoresis for differentiation of methicillin-resistant Staphylococcus aureus strains. J Clin Microbiol. 1997;35:881–885. doi: 10.1128/jcm.35.4.881-885.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saulnier P, Bourneix C, Prevost G, Andremont A. Random amplified polymorphic DNA assay is less discriminant than pulsed-field gel electrophoresis for typing strains of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1993;31:982–985. doi: 10.1128/jcm.31.4.982-985.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenover F C, Arbeit R, Archer G, Biddle J, Byrne S, Goering R, Hancock G, Hebert A, Hill B, Hollis R, Jarvis W, Kreiswirth B, Eisner W, Maslow J, McDougal L, Miller M, Mulligan M, Pfaller M. Comparison of traditional and molecular methods of typing isolates of Staphylococcus aureus. J Clin Microbiol. 1994;32:407–415. doi: 10.1128/jcm.32.2.407-415.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Belkum A, Kluytmans J, van Leeuwen W, Bax R, Quint W, Peters E, Fluit A, Vandenbroucke-Grauls C, van den Brule A, Koeleman H, Melchers W, Meis J, Elaichouni A, Vaneechoute M, Moonens F, Maes N, Struelens M, Tenover F, Verbrugh H. Multicenter evaluation of arbitrarily primed PCR for typing of Staphylococcus aureus strains. J Clin Microbiol. 1995;33:1537–1547. doi: 10.1128/jcm.33.6.1537-1547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Belkum A, Riewerts Eriksen N, Sijmons M, van Leeuwen W, VandenBergh M, Kluytmans J, Espersen F, Verbrugh H. Are variable repeats in the spa gene suitable targets for epidemiological studies of methicillin-resistant Staphylococcus aureus strains? Eur J Clin Microbiol Infect Dis. 1996;15:768. doi: 10.1007/BF01691972. [DOI] [PubMed] [Google Scholar]
- 15.Vandenbroucke-Grauls C M, Frenay H M, van Klingeren B, Savelkoul T H, Verhoef J. Control of epidemic methicillin resistant Staphylococcus aureus in a Dutch university hospital. Eur J Clin Microbiol Infect Dis. 1991;10:6–11. doi: 10.1007/BF01967090. [DOI] [PubMed] [Google Scholar]
- 16.Voss A, Milatovic D, Wallrauch-Schwarz C, Rosdahl V T, Braveny I. Methicillin resistant Staphylococcus aureus in Europe. Eur J Clin Microbiol Infect Dis. 1994;13:50–55. doi: 10.1007/BF02026127. [DOI] [PubMed] [Google Scholar]
- 17.Wenzel R, Hermann R. Repetitive DNA sequences in Mycoplasma pneumoniae. Nucleic Acids Res. 1988;16:8337–8350. doi: 10.1093/nar/16.17.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]