Abstract
We evaluated new features from biosignals comprising diverse physiological response information to predict the outcome of weaning from mechanical ventilation (MV). We enrolled 89 patients who were candidates for weaning from MV in the intensive care unit and collected continuous biosignal data: electrocardiogram (ECG), respiratory impedance, photoplethysmogram (PPG), arterial blood pressure, and ventilator parameters during a spontaneous breathing trial (SBT). We compared the collected biosignal data’s variability between patients who successfully discontinued MV (n = 67) and patients who did not (n = 22). To evaluate the usefulness of the identified factors for predicting weaning success, we developed a machine learning model and evaluated its performance by bootstrapping. The following markers were different between the weaning success and failure groups: the ratio of standard deviations between the short-term and long-term heart rate variability in a Poincaré plot, sample entropy of ECG and PPG, α values of ECG, and respiratory impedance in the detrended fluctuation analysis. The area under the receiver operating characteristic curve of the model was 0.81 (95% confidence interval: 0.70–0.92). This combination of the biosignal data-based markers obtained during SBTs provides a promising tool to assist clinicians in determining the optimal extubation time.
Keywords: weaning, prediction, mechanical ventilator, biosignal, machine learning, digital biomarker
1. Introduction
Attempting to wean critically ill patients from mechanical ventilation (MV) is crucial. Reducing the duration of MV decreases ventilator-related pneumonia, muscle weakness, length of stay in the intensive care unit (ICU), and health care costs [1,2,3,4,5]. However, premature weaning may result in harmful outcomes such as complications during reintubation, deconditioning of the patient, an increased need for tracheostomy, and a potential increase in mortality [6,7,8,9,10,11,12]. Therefore, identifying the precise time for weaning from MV is a critical decision.
Although many predictive indices and clinical tools are already in use [1,13,14,15,16,17,18,19,20,21,22], >20% of the patients who have fulfilled the classic weaning criteria require reintubation [14,23,24,25]. The predictive performance decreases in patients with multi-organ dysfunction, older age, prolonged MV, and severe illness [26,27,28,29,30,31]. The lack of reliable weaning parameters is related to the heterogeneity of critically ill patients and their ever-changing clinical courses [32,33,34,35]. The causes of weaning failure are not exclusively attributable to oxygenation or ventilation insufficiency; cardiac function, volume status, muscle deconditioning, and the presence of delirium also affect weaning outcomes [9,23,36,37,38,39,40]. Most of the indices are based on the clinical situation recorded at a single time point, although each patient’s oxygenation, ventilation, hemodynamic, musculoskeletal, and mental statuses are often unstable and vary over time.
In this study, we hypothesized that features extracted from biosignals collected during the weaning process would provide better predictive information than the commonly used rapid shallow breathing index (RSBI); specifically, biosignal-based features would include more diverse physiological information regarding the patient’s status (i.e., information not limited to the pulmonary system). This hypothesis has been supported by recent studies, in which useful digital biomarkers were present in biosignal data [26,41,42,43,44]. These markers may predict or detect cardiovascular and other clinical events [45,46,47]. Electrocardiogram (ECG) data reflect heart status, and specific morphology of the arterial blood pressure waveform could reflect the status of the cardiovascular system [48,49,50,51,52]. Photoplethysmogram (PPG) data are used to measure oxygen saturation and provide information regarding oxygen transfer [53,54,55,56]. A healthy biosystem is characterized by complexity and variability, and alterations in variability and reduced complexity are related to pathological conditions [57,58,59,60,61,62]. For ventilator weaning, breathing pattern variability analysis has been performed for the estimation of weaning readiness in many studies; reductions in variability indices during a spontaneous breathing trial (SBT) were reportedly associated with extubation failure [63,64,65,66,67].
Here, we proposed a biosignal-based weaning prediction approach, which would continuously reflect the patient’s clinical and physiological progression over time. This study aimed to compare the distribution of values of biosignal data between the weaning success and failure groups during an SBT. It also aimed to evaluate the additional value of the biosignal data for the prediction of extubation outcomes, compared with the commonly used RSBI.
2. Materials and Methods
This retrospective study was conducted using anonymized data. The Institutional Review Board of Ajou University Hospital approved the study (IRB No. AJIRB-MED-MDB-20-090) and waived the requirement for informed consent.
2.1. Data Sources
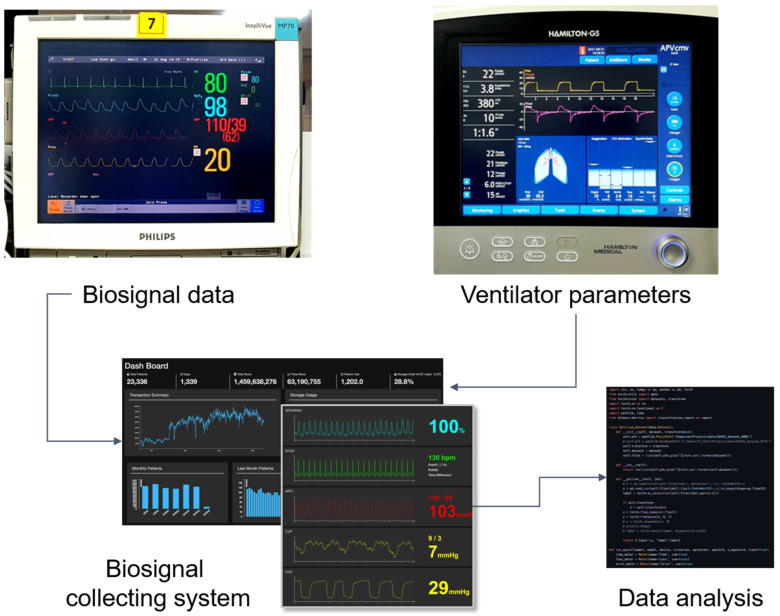
We collected clinical and biosignal data from patients who were admitted to the ICU and underwent MV at Ajou University Hospital, a tertiary teaching hospital in South Korea, from January 2019 to November 2020. Clinical data obtained from electronic medical records and biosignal data were collected using our custom biosignal collecting platform, which we developed for research purposes [68]. We also collected ventilator parameters directly from the ventilators following every SBT to accurately identify the breathing patterns and their variability (Figure 1).
Figure 1.
Biosignal data collecting and data analysis process. Biosignal data from patient monitor devices and parameters from mechanical ventilators were collected via our biosignal collecting system. Collected data were analyzed retrospectively to find out features for predicting weaning success.
2.2. Study Population
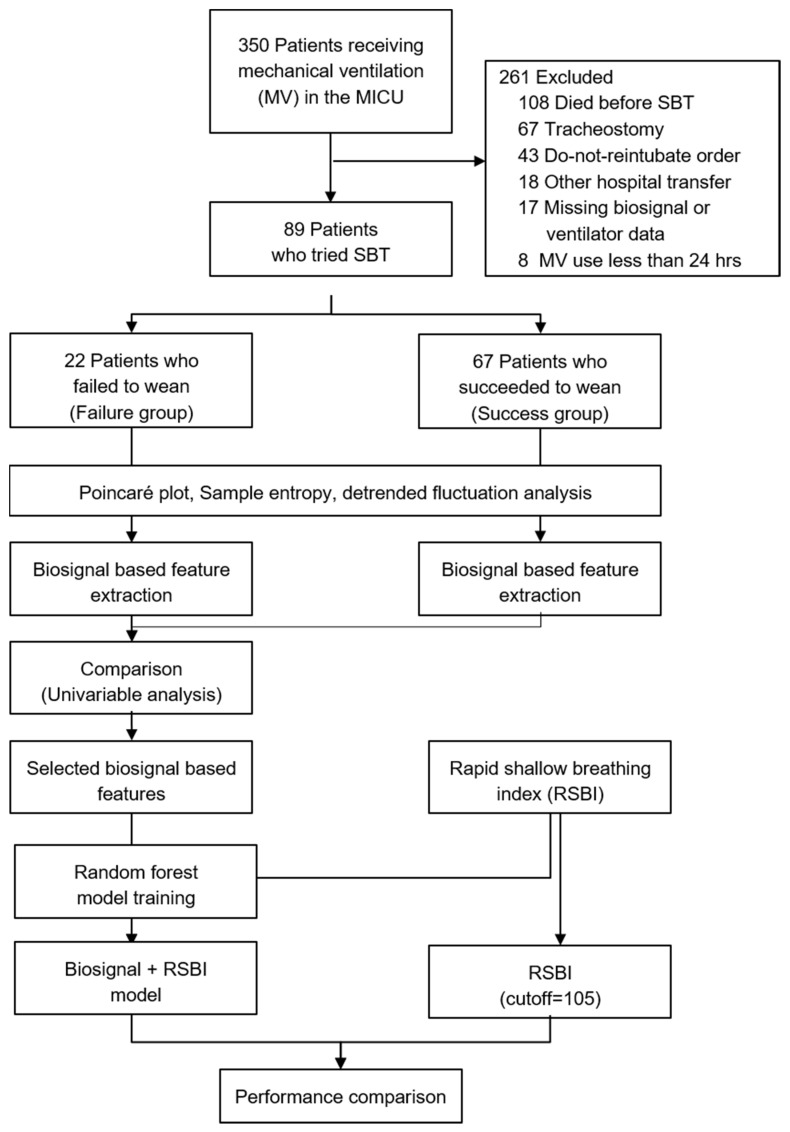
Patients aged ≥ 18 years who had undergone MV for at least 24 h and who fulfilled the weaning criteria were included (Figure 2). Weaning criteria were applied according to our institution’s ventilator weaning protocol, which was based on the guidelines developed by the American College of Chest Physicians, the American Association for Respiratory Care, and the American College of Critical Care Medicine, with reference to additional research methods [1,10,69,70]. The weaning criteria were as follows: resolution or improvement of the condition leading to intubation; hemodynamic stability, which was defined as systolic blood pressure between 90 and 160 mmHg, and heart rate <140 beats/min with low or no doses of vasopressors; stable neurological status (no deterioration in Glasgow Coma Scale during the prior 24 h); respiratory stability (oxygen saturation >90% with fraction of inspired oxygen [FiO2] ≤ 0.4), respiratory rate < 35/min, spontaneous tidal volume >5 mL/kg; and intact cough and gag reflexes. Patients with a tracheostomy or a do-not-reintubate order were excluded.
Figure 2.
Study overview. We selected biosignal-based features that showed differences between the weaning failure and success groups. Their usefulness was evaluated by applying these features to predict weaning success, followed by comparison with the pre-existing RSBI. ICU, intensive care unit; RSBI, rapid shallow breading index; SBT, spontaneous breathing trial.
All the patients underwent a 30-min SBT with ≤6 cm H2O pressure support ventilation and positive end-expiratory pressure; the FiO2 remained unchanged from the MV period prior to the SBT. When the patients successfully passed the 30-min SBT, they were extubated and provided with a high-flow nasal cannula or air entrainment mask for oxygen therapy. Patients who did not tolerate the SBT were reconnected to a ventilator. The criteria for failure to tolerate the SBT were agitation, anxiety, deterioration of consciousness, respiratory rate > 35/min and/or use of accessory muscles, oxygen saturation by pulse oximetry <90% with FiO2 > 0.5, heart rate > 140/min or >20% increase from baseline, systolic blood pressure <90 mmHg, or development of an arrhythmia.
2.3. Study Design
This study focused on the variability of the physiological responses to the following abrupt changes in the external environment: support with MV, reduced ventilator support, and increased respiratory demand because of the SBT. To compare the biosignal features between the weaning success and failure groups, we defined the two groups (as described below), and then calculated the biosignal features representing those variabilities (Figure 2).
We defined the weaning failure group as patients who failed to wean before extubation and patients who were reintubated within 48 h following extubation. Failed extubation was defined as reintubation within 48 h of extubation. Respiratory failure within 48 h of extubation was defined as the occurrence of at least one of the following: respiratory acidosis with pH <7.3 and partial pressure of carbon dioxide (PaCO2) >45 mmHg, oxygen saturation <90% with FiO2 >0.5, respiratory rate >35/min, deterioration of consciousness, severe agitation, or clinical signs of respiratory fatigue. We defined the extubation success group as patients free from MV for >48 h following extubation. Two pulmonologists (W.Y.C. and J.E.P.) reviewed the clinical data of all the enrolled patients and confirmed whether the patients were included in the case (success) or control (failure) groups.
2.4. Feature Extraction
To extract biosignal-based features, we used waveform data including ECG, PPG, respiratory impedance, and invasive arterial blood pressure measurements, as well as numerical measurements including heart rate, respiratory rate, and mean arterial pressure. All the waveform data were down-sampled as 62.5 Hz to ensure that they have the same data format and to reduce computational complexity in the analysis. We also collected and used ventilator parameters for every breath from mechanical ventilators including tidal volume, inspiration time, and the ratio of inspiratory and expiratory time during the 30-min SBT.
We calculated the features that represented the variability of time-series data using Poincaré plots, sample entropy (SampEn), and detrended fluctuation analysis in the middle 10 min of the SBT.
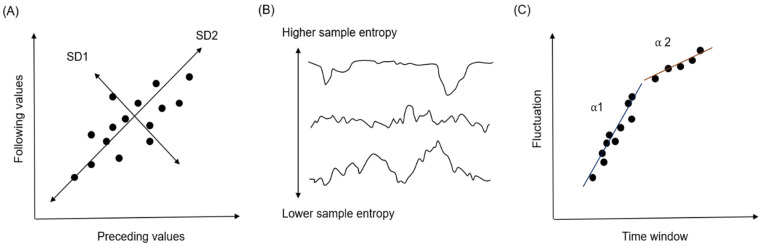
A Poincaré plot is a scatter plot of the current value (e.g., the R-R interval in an ECG) against the immediately preceding value (Figure 3A). Standard deviation 1 (SD1) in the plot is defined as the level of deviation against the line of identity (y = x). SD1 represents how consecutive values differ from previous values (short-term variability). SD2 is calculated as the level of deviation together with the line of identity (i.e., how all values are distributed; long-term variability). The ratio of SD1 and SD2 (SD1/SD2) represents the level of short-term variability, compared with long-term variability.
Figure 3.
Methods used for the feature extraction process. (A) Poincaré plot, (B) Sample entropy, and (C) Detrended fluctuation analysis. All three approaches evaluate the level of variability in time-series data. For numerical values, the Poincaré plot method was used; for waveform data, sample entropy and detrended fluctuation analysis methods were used.
SampEn is an index that represents the level of complexity of a particular dataset (Figure 3B). It calculates the probability that the same findings are observed in different time windows; the calculated value is then used as input in a negative logarithm. A low SampEn value indicates a high level of regularity; a high SampEn value indicates an irregular state.
Detrended fluctuation analysis is used to quantify the level of fractal-like correlation of the time-series data. When the patterns observed in some time windows are also observed in the larger or smaller time windows, this is regarded as fractal-like correlation. An α = 0.5 indicates random data with no pattern, while α >0.5 indicates data with fractal correlation. Usually, two indicators are calculated, α1 and α2, indicating short- and long-term fractal-like correlation (fluctuation) (Figure 3C).
2.5. Statistical Analyses
Categorical variables are presented as numbers and percentages. Continuous variables are summarized as means and standard deviations. To compare categorical variables, the χ2 test or Fisher’s exact test was used. Mann–Whitney U test was used for continuous variables. In the comparison of the baseline characteristics between the success and failure group, p < 0.05 was considered significant. In the comparison of the biosignal-based features, p < 0.1 was used to include more diverse variables as input values for the machine learning model for weaning prediction. However, to control the false discovery rate owing to multiple comparisons, we used the Benjamini and Hochberg correction. Thresholds for statistical significance αadj were adjusted as α*i/m, where α = 0.1, m is the number of comparisons, and i is the position in an ordered p-value list from smallest to largest (1, …, m). Statistical significance was defined as p < αadj.
2.6. Development of the Machine Learning Model
To evaluate whether the composite of biosignal-based features is useful to predict the probability of weaning success, we developed a machine learning model using biosignal features. We included not only biosignal features that showed significant differences but also features that showed near significant differences between the case and control groups, to include all variables that could have additional information in weaning prediction. The RSBI value, which is currently used for weaning prediction, was also included in the input values.
A Random Forest classifier was used to predict the weaning failure. The Random Forest classifier is consisted of series of independent decision trees. Each tree has a hierarchical decision rules, and it separates input data into N(t) leaves ∈ [Nt,1, …, Nt,N(t)], where t ∈ (1, …, T) means each tree and Nt,i contains a probability of weaning success πt,i ∈ [0,1]. The Random Forest model collects all prediction from each of decision models, and it returns the majority of votes as final output. The character of the classifier can be determined by hyperparameters. In this study, the following hyperparameters were set: the function to measure the quality of a split was “Gini Impurity,” the number of estimators was 50, the minimum number of samples required to constitute a leaf node was one, and the minimum number of samples required to split an internal node was two. The Gini Impurity was calculated using the following formula:
where C is the number of total classes and p(i) is the probability of selecting data with class i (weaning success or failure). Each internal node was trained to have best splits the space of training data to lead to the greatest reduction in Gini Impurity defined above.
We determined the relative importance of the features after training. The feature importance of a Random Forest indicates the degree to which the overall classification impurity is reduced if the feature is used in the model. To calculate importance of each feature, the importance of each node was calculated using the following formula:
where nij, wj, Gj, left(j), right(j) means the importance of node j, weighted number of samples reaching node j (Nj/N), the impurity value of node j, child node from left split on node j, child node from right split on node j, respectively. Further, the importance of each feature was then calculated using the following formula:
where fii and ni means the importance of feature i and node j. Then fii was normalized by dividing by the sum of all feature importance value to make all fii values be ranged between 0 and 1. Finally, the averaged fii over all trees was used to evaluate fii at the random forest level.
Performance of the model was measured by sensitivity, specificity, positive predictive value, negative predictive value, F1 score, and area under the receiver operating characteristic (AUROC). To compare the usefulness of our model with the existing RSBI alone, the weaning prediction performance of the RSBI was evaluated using the same performance measures. The dataset was randomly separated into training and test datasets in the ratio of 7:3. The average performance and 95% confidence intervals of performance indices were calculated using bootstrap resampling procedures with 1000 iterations. For performance comparison, we also conducted multiple logistic regression with the same process.
2.7. Software Used in the Study
Acquisition software (Hamilton Medical Ventilator data logger version 5.0, Bonaduz, Switzerland) was used to obtain the numerical data, such as tidal volume, inspiratory time, and the ratio of inspiratory and expiratory time from the mechanical ventilators, during the trial. Microsoft SQL Server and Python were used for data management and statistical analyses.
3. Results
During the study period, 350 patients underwent MV in the ICU. Of these 350 patients, 106 fulfilled the inclusion criteria, 17 were excluded due to missing biosignal or ventilator data, and 89 were finally included in the study (Figure 2). Among the 89 included patients, 67 successfully discontinued MV and were able to breath by themselves without the aid of a ventilator for at least 48 h following extubation (case group); and 22 patients failed and resumed MV within 48 h (control group). The baseline characteristics of the two groups are provided in Table 1. They were similar in terms of age, sex, main cause of ICU admission, APACHE II score, and length of MV before the SBT. In particular, in the case of pneumonia, which accounts for a large proportion of the reasons for ICU admission, there was no difference between the two groups in the comparison according to the type of pneumonia and the causative pathogen.
Table 1.
Baseline characteristics of the study population according to the outcome of weaning.
| Characteristics | Total (N = 89) |
Success Group (N = 67) |
Failure Group (N = 22) |
p Value |
|---|---|---|---|---|
| Age, mean ± SD, year | 69.3 ± 14.3 | 69.8 ± 13.5 | 67.59 ± 16.5 | 0.533 |
| Sex (males/females), n | 54/35 | 40/27 | 14/8 | 0.743 |
| Body weight, mean ± SD, kg | 59.2 ± 11.7 | 59.6 ± 12.2 | 57.9 ± 10.3 | 0.568 |
| Height, mean ± SD, cm | 164.5 ± 9.6 | 163.8 ± 10.0 | 166.8 ± 8.0 | 0.193 |
| BMI, mean ± SD, kg/m2 | 21.9 ± 4.2 | 22.3 ± 4.3 | 20.8 ± 3.7 | 0.152 |
| Main cause of ICU admission, n(%) | 0.897 | |||
| Pneumonia | 59 (66.3) | 45 (67.2) | 14 (63.6) | |
| COPD/Asthma AE | 8 (9.0) | 6 (9.0) | 2 (9.1) | |
| Pulmonary hemorrhage | 4 (4.5) | 3 (4.5) | 1 (4.5) | |
| Sepsis | 3 (3.4) | 3 (4.5) | 0 (0) | |
| Gastrointestinal bleeding | 1 (1.1) | 1 (1.5) | 0 (0) | |
| Neurologic disease | 2 (2.2) | 1 (1.5) | 1 (4.5) | |
| Pulmonary edema | 7 (7.9) | 5 (7.5) | 2 (9.1) | |
| Others | 5 (5.6) | 3 (4.5) | 2 (9.1) | |
| Comorbidity, n(%) | ||||
| Cardiovascular disease | 52 (58.4) | 40 (59.7) | 12 (54.5) | 0.670 |
| Diabetes mellitus | 25 (28.1) | 20 (29.9) | 5 (22.7) | 0.519 |
| Chronic obstructive pulmonary disease | 16 (18.0) | 11 (16.4) | 5 (22.7) | 0.530 |
| Neurological disease | 24 (27.0) | 20 (29.9) | 4 (18.2) | 0.285 |
| Malignancy | 18 (20.2) | 14 (20.9) | 4 (18.2) | >0.99 |
| Renal disease | 10 (11.2) | 9 (13.4) | 1 (4.5) | 0.440 |
| Liver disease | 4 (4.5) | 4 (6.0) | 0 (0) | 0.568 |
| APACHE II score, mean ± SD | 21.8 ± 8.1 | 22.3 ± 8.3 | 20.2 ± 7.3 | 0.288 |
| Length of mechanical ventilation before SBT, mean ± SD, d | 7.3 ± 5.3 | 7.0 ± 5.5 | 8.1 ± 4.7 | 0.393 |
| Duration of MV ≥ 72 h, n(%) | 68 (76.4) | 50 (74.6) | 18 (81.8) | 0.491 |
| Use of neuromuscular blocker, n(%) | 18 (20.2) | 13 (19.4) | 5 (22.7) | 0.764 |
| Excess secretion, n(%) | 9 (10.1) | 6 (9.0) | 3 (13.6) | 0.684 |
| Arterial blood gas ananlysis, mean ± SD | ||||
| PaO2, mmHg | 107.8 ± 34.8 | 108.0 ± 31.4 | 106.9 ± 44.4 | 0.891 |
| PaCO2, mmHg | 38.7 ± 11.0 | 37.6 ± 10.4 | 41.9 ± 12.4 | 0.116 |
| PaO2/FiO2 ratio | 317.3 ± 102.3 | 320.5 ± 91.4 | 307.3 ± 132.1 | 0.666 |
| Upper airway disorder after extubation, n(%) | 2 (2.2) | 2 (3) | 0 (0) | >0.99 |
| Prior failed weaning attempt, n(%) | 15 (16.9) | 9 (13.4) | 6 (27.3) | 0.187 |
Data are presented as mean ± standard deviation or number (%). BMI, body mass index; ICU, intensive care unit; COPD, chronic obstructive pulmonary disease; AE, acute exacerbation; APACHE, acute physiology and chronic health evaluation; SBT, spontaneous breathing trial; MV, mechanical ventilation; PaO2, partial pressure of oxygen in arterial blood; PaCO2, partial pressure of carbon dioxide; FiO2 ratio, fraction of inspired oxygen.
Among the biosignal-based features, we could detect significant differences between the two groups in the following features (Table 2): SampEns in ECG and PPG. We also included the additional following variables that showed near significant level of difference for further analysis (machine learning model development): ratio of SD2 and SD1 in heart rate; ɑ1 values in ECG and respiratory impedance, ɑ2 values and ɑ1/ɑ2 in ECG.
Table 2.
Univariate analysis results of biosignal features between the weaning success and failure groups.
| Items | Variability Index | Success Group | Failure Group | p Value | αadj † |
|---|---|---|---|---|---|
| Heart rate | SD1 (mean ± SD) | 2.52 (1.45) | 2.17 (1.15) | 0.316 | 0.035 |
| SD2 (mean ± SD) | 6.74 (4.56) | 8.63 (6.98) | 0.741 | 0.076 | |
| SD1/SD2 (mean ± SD) | 0.43 (0.18) | 0.32 (0.13) | 0.015 * | 0.009 | |
| Respiratory rate | SD1 (mean ± SD) | 2.76 (1.22) | 1.77 (0.49) | 0.617 | 0.068 |
| SD2 (mean ± SD) | 2.94 (1.25) | 3.08 (1.25) | 0.561 | 0.059 | |
| SD1/SD2 (mean ± SD) | 0.62 (0.16) | 0.65 (0.24) | 0.592 | 0.065 | |
| Tidal volume | SD1 (mean ± SD) | 52.58 (32.96) | 27.64 (10.9) | 0.237 | 0.029 |
| SD2 (mean ± SD) | 72.90 (40.18) | 45.40 (9.86) | 0.747 | 0.079 | |
| SD1/SD2 (mean ± SD) | 0.72 (0.2) | 0.62 (0.19) | 0.496 | 0.053 | |
| IE ratio | SD1 (mean ± SD) | 61.23 (47.58) | 164.57 (297.75) | 0.882 | 0.094 |
| SD2 (mean ± SD) | 61.23 (47.58) | 189.32 (268.39) | 0.408 | 0.044 | |
| SD1/SD2 (mean ± SD) | 0.63 (0.22) | 0.55 (0.23) | 0.318 | 0.038 | |
| Inspiratory time | SD1 (mean ± SD) | 96.20 (68.04) | 79.98 (53.70) | 0.750 | 0.082 |
| SD2 (mean ± SD) | 146.79 (79.75) | 156.47 (117.67) | 0.567 | 0.062 | |
| SD1/SD2 (mean ± SD) | 0.66 (0.24) | 0.58 (0.28) | 0.511 | 0.056 | |
| Mean ABP | SD1 (mean ± SD) | 5.37 (4.58) | 5.99 (9.42) | 0.340 | 0.041 |
| SD2 (mean ± SD) | 10.80 (6.09) | 15.98 (23.12) | 0.832 | 0.088 | |
| SD1/SD2 (mean ± SD) | 0.5 (0.21) | 0.4 (0.17) | 0.087 | 0.026 | |
| ECG | SampEn (mean ± SD) | 2.04 (0.61) | 2.50 (0.46) | 0.005 ** | 0.006 |
| ɑ1 (mean ± SD) | 1.29 (0.15) | 1.22 (0.08) | 0.033 * | 0.021 | |
| ɑ2 (mean ± SD) | 0.57 (0.23) | 0.43 (0.20) | 0.016 * | 0.012 | |
| ɑ1/ɑ2 (mean ± SD) | 2.83 (2.46) | 3.47 (1.57) | 0.026 * | 0.018 | |
| Respiratory impedance | SampEn (mean ± SD) | 0.21 (0.05) | 0.22 (0.05) | 0.413 | 0.047 |
| ɑ1 (mean ± SD) | 2.03 (0.04) | 2.01 (0.03) | 0.018 * | 0.015 | |
| ɑ2 (mean ± SD) | 1.11 (0.27) | 1.07 (0.31) | 0.719 | 0.074 | |
| ɑ1/ɑ2 (mean ± SD) | 2.04 (1.11) | 2.70 (3.64) | 0.973 | 0.1 | |
| PPG | SampEn (mean ± SD) | 0.14 (0.05) | 0.18 (0.11) | 0.002 ** | 0.003 |
| ɑ1 (mean ± SD) | 1.96 (0.12) | 1.91 (0.19) | 0.062 | 0.024 | |
| ɑ2 (mean ± SD) | 1.96 (0.12) | 0.78 (0.49) | 0.429 | 0.05 | |
| ɑ1/ɑ2 (mean ± SD) | 5.61 (10.72) | −2.15 (29.37) | 0.947 | 0.097 | |
| ABP | SampEn (mean ± SD) | 0.36 (0.39) | 0.43 (0.46) | 0.247 | 0.032 |
| ɑ1 (mean ± SD) | 2.09 (0.01) | 2.08 (0.02) | 0.627 | 0.071 | |
| ɑ2 (mean ± SD) | 1.83 (0.10) | 1.82 (0.12) | 0.775 | 0.085 | |
| ɑ1/ɑ2 (mean ± SD) | 1.14 (0.07) | 1.15 (0.08) | 0.853 | 0.091 |
Data are presented as mean ± standard deviation. IE ratio, inspiratory to expiratory time ratio; ABP, arterial blood pressure; ECG, electrocardiogram; PPG, photoplethysmogram; SD1, standard deviations between short-term heart rate variability; SD2, standard deviations between long-term heart rate variability; SampEn, sample entropy. † αadj is the adjusted statistically significant threshold α = 0.1 by multiple testing by Benjamini and Hochberg (BH) correction. ** Statistically significant variables after BH correction. * Statistically near significant variables after BH correction.
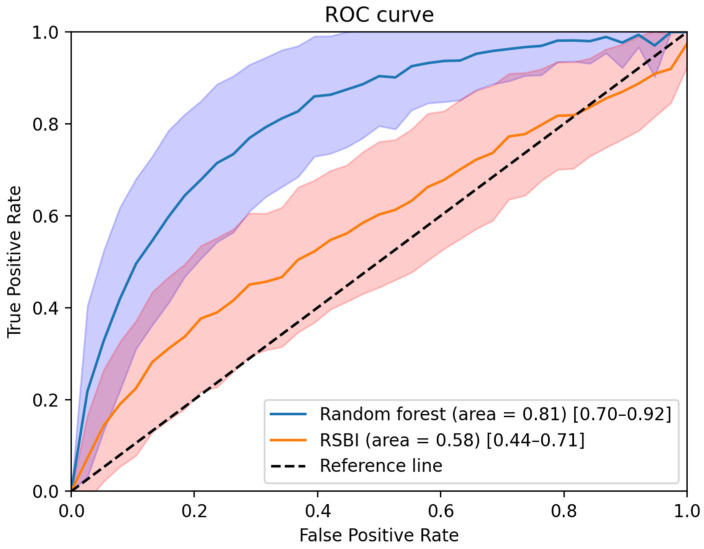
When the RSBI value alone was used for weaning prediction, its AUROC was 0.58 (95% confidence interval: 0.44–0.71). When the biosignal-based features were combined with RSBI for weaning prediction, the AUROC value increased to 0.81 (95% confidence interval: 0.70–0.92). The performance comparison of the models (based on biosignals with the RSBI score and based on RSBI alone) is shown in Table 3. The combined model demonstrated improved specificity (+26%), accuracy (+4%), negative predictive value (+18%), and F1 score (+23%), with a similar level of sensitivity. The details of model performance are provided in Table 3 and Figure 4.
Table 3.
Performance indices (accuracy, sensitivity, specificity, positive predictive value, negative predictive value, and F-1 score).
| Sensitivity | Specificity | Accuracy | PPV | NPV | F-1 Score | |
|---|---|---|---|---|---|---|
| RSBI (≥105) | 0.91 (0.87–0.96) | 0.26 (0.13–0.38) | 0.80 (0.75–0.84) | 0.85 (0.83–0.88) | 0.40 (0.20–0.61) | 0.30 (0.17–0.44) |
| RSBI + biosignal (Random Forest) |
0.91 (0.85–0.97) | 0.52 (0.36–0.69) | 0.84 (0.79–0.89) | 0.90 (0.87–0.93) | 0.58 (0.40–0.76) | 0.53 (0.40–0.66) |
| RSBI + biosignal (Multiple regression) |
0.91 (0.86–0.97) | 0.41 (0.25–0.57) | 0.82 (0.78–0.87) | 0.88 (0.85–0.91) | 0.53 (0.33–0.73) | 0.44 (0.30–0.58) |
Mean values of areas under the curve with 95% confidence intervals. PPV, positive predictive value; NPV, negative predictive value; RSBI, rapid shallow breathing index.
Figure 4.
Performance comparison between the model using RSBI alone and the model using RSBI and biosignal-based features. After 1000 iterations of bootstrapping, the mean AUROC and 1 standard deviation of each group are shown as a solid line and shaded area.
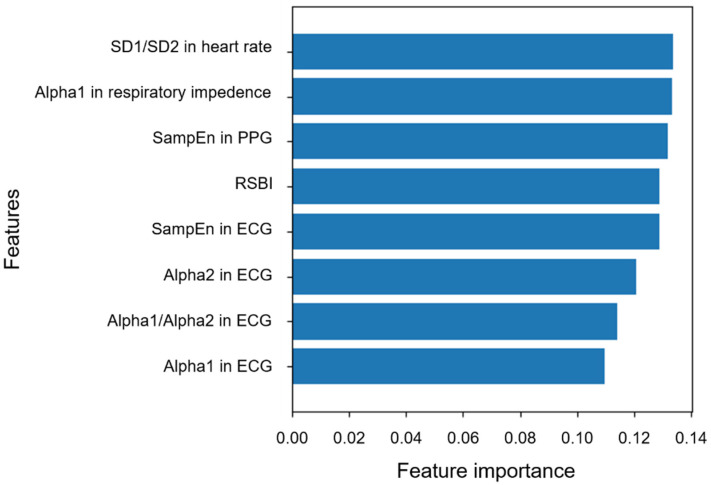
As shown in Figure 5, biosignal-based features selected in this study exhibited value similar to the existing RSBI for predicting weaning success in the Random Forest model. In particular, SD1/SD2 in heart rate, α1 in respiration impedance, and SampEn in PPG were more valuable than RSBI.
Figure 5.
Feature importance in the Random Forest model to predict weaning success. All biosignal features were demonstrated to have value similar to RSBI.
4. Discussion
Our study successfully incorporated novel biosignal-based features into classic weaning prediction tools to provide a more accurate marker for MV discontinuation. SD1/SD2 in heart rate, SampEn in ECG, α1 in respiratory impedance, and SampEn in PPG were significant discriminants of MV weaning success. The addition of this panel of new parameters to the RSBI yielded better predictive performance, compared with RSBI alone.
During the weaning process, we observed differences between the success and failure groups based on the biosignal features extracted from heart rate, ECG, respiratory impedance, and PPG. In the analysis of numerical data, heart rate distribution using the Poincaré plot method for patients with weaning failure showed reduced variability of measured parameters. A decrease in variability reportedly indicates reduced adaptive capacity in a stressful environment (e.g., reduced ventilator support) and has been described in several pathological conditions [71,72]. Heart rate variability (HRV) is the time interval between consecutive heartbeats, and is a commonly used variable for predicting weaning outcomes using biosignal data analysis. HRV is associated with the balance between parasympathetic and sympathetic regulation, thermoregulation, baroreflexes, and respiration; altered HRV is reportedly associated with failed weaning trials [46,73]. Huang et al. reported that when analyzing HRV in the pre-SBT, SBT, and post-extubation periods, decreased HRV was significantly associated with SBT failure; the inability to increase HRV following extubation was correlated with subsequent reintubation [74]. Seely et al. also reported that alterations in the HRV during SBTs significantly correlated with weaning failure [64]. The weaning process is associated with increased breathing effort; Seely et al. suggested that the inability to tolerate the increased breathing effort in patients who were not ready for extubation could be used to improve the prediction of failed extubation. In our study, we used numerical values of heart rate per minute measured every second, which would be difficult to compare directly using HRV. However, the increased variability of the cardiovascular system to adapt to environmental changes is presumably through a similar mechanism.
In analysis of the waveform data, patients who were successfully extubated had lower complexities in the ECG, respiratory impedance, and PPG during the SBTs. We presume that patients with weaning success exhibited better preservation of regularity and reproducibility in biosignal features, compared with patients with weaning failure; patients with weaning success presented low complexity and predictable features. In contrast, the biological rhythms became more irregular and unpredictable in patients who did not tolerate the weaning process in our study. Engoren et al. reported that the weaning failure group showed increased irregularity in biosignal analysis of approximate entropy of tidal volume, which reflects enhanced external inputs to the respiratory control center; increased regularity in the weaning success group indicated a better adaptive mechanism of an autonomous system [75]. El Khatib et al. reported that Kolmogorov entropy and dimensions of the spontaneous breathing pattern were increased in patients who failed weaning trials; they also suggested that complexity during the SBTs was enhanced in patients with weaning failure [41]. In another study, Papaioannou et al. assessed the respiratory pattern complexity in critically ill surgical patients during weaning trials; they reported that patients with weaning failure exhibited significantly decreased respiratory pattern complexity, reduced SampEn, and increased detrended fluctuation analysis exponents, compared with patients with weaning success [76]. Discrepancies in the results, compared with previous studies, are presumably associated with different protocols for weaning and different patient characteristics. Papaioannou et al. compared the before and after SBTs in both successful and unsuccessful groups. Our study directly compared the biosignal features of the weaning success and failure groups. We suspect that the success group showed predictable variability owing to control by the internal regulatory system, while the failure group exhibited a more chaotic behavior since these patients were unable to tolerate environmental changes.
In this study, we also analyzed the variability and complexity of respiratory rate, tidal volume, arterial blood pressure, and inspiratory to expiratory time ratio (I:E ratio) over time using the machine learning model; however, they failed to show any significant results in our study.
Respiratory rate and tidal volume are the main variables of RSBI, which is the most coveted weaning predictor in the ICU so far, and their stability were expected to demonstrate a significant role in the prediction of weaning success. Their limited role in our study could be because once they are maintained under a certain value, the change over time may have little significance. A ratio of respiratory rate over tidal volume (i.e., RSBI) under 105 is considered adequate for considering extubation in many critical care guidelines, and if the value is maintained under 105, the stability should not be a major factor in weaning success [77,78]. Moreover, since the values are included in the RSBI itself, the comparison of these biosignals with RSBI would not incur any difference. Furthermore, in order to collect the ventilator associated biosignals, we provided minimal ventilatory support until extubation, instead of disconnecting MV during SBTs. This should have maintained stable tidal volume to maintain the volume over the minimally required value to reduce the predictive performance of the breathing pattern variability. According to Otaguro et al. respiratory rate and tidal volume were the least important weaning predictors in their study comparing different machine learning models for successful extubation prediction [79].
Similar to heart rate, blood pressure reflects the patient’s cardiovascular reserve. Its instability over time is more a matter of poor cardiovascular function or inadequate volume status than the patient’s adaptation mechanism to decreasing ventilatory support. Before selecting weaning candidates, we carefully achieved optimal volume control and checked their sufficient cardiovascular function in order to protect patients from negative cardiovascular events during the weaning procedure. Inspiratory time to expiratory time ratio in self-breathing patients is a function of respiratory system resistance [80]. Therefore, before undergoing the weaning procedure, it is fundamental to control pathologic conditions, which can increase the bronchial resistance, such as bronchial spasm or exacerbation of airway disease. This conservative approach is endowed with a low discriminating value to inspiratory time complexity as well as I:E ratio variability.
The prediction of weaning outcome improved with the combination of biosignal markers and RSBI, compared with RSBI alone. RSBI is derived from the respiratory frequency divided by tidal volume, and thus directly represents breathing characteristics. Furthermore, RSBI is the most used index for the estimation of weaning readiness during an SBT [77,78]. If the patient has adequate tidal volume with deep and regular breathing, the RSBI will be low, which suggests weaning success. However, the purposes of breathing are to successfully inhale air into the lungs and transfer oxygen to each tissue in the body. Breathing quality may be affected by many diverse variables, including the cardiovascular system, autonomic nervous system, and musculoskeletal capacity [81,82]. In this study, the prediction model that integrated RSBI and biosignal data demonstrated higher values of performance indices, compared with RSBI alone.
Previous studies have suggested that clinically important information remained undiscovered in the biosignal data [83,84]. In the ECG waveform data, atrial fibrillation could be detected regardless of whether the ECG waveforms maintained normal sinus rhythm [48]. Normal sinus rhythm (presumed to indicate completely normal status) is expected to include information for mortality prediction [49]. Morphological changes of P waves suggest an increased risk of hemorrhage in patients with ischemic stroke [50]. ECG waveform information is also useful when screening for cardiac contractile dysfunction [51,85]. Our results in this study suggest that biosignal data (e.g., ECG, PPG, and arterial blood pressure) provide useful information, specifically regarding whether patients attempting an SBT have sufficient ability to breath without MV assistance.
Incorporation of biosignal-based features when monitoring patients during the weaning process enables physicians to make rapid clinical decisions based on real-time, continuous medical information [86,87,88]. Continuous monitoring of biosignal information, such as ECG, respiratory rate, PPG, and arterial blood pressure is routine practice in ICU care. Thus, biosignal-based measurements are easily accessible and would be helpful when assessing patients attempting SBTs. Our model did not use any clinical information other than the biosignal data obtained from the patient monitoring devices. Therefore, our model can easily be used in the ICU setting.
A limitation of this study was its single-center design (i.e., single ICU at a single institute). We did not include patients admitted for surgical or trauma-related ICU care, owing to their ventilator use characteristics. Post-surgical use of MV in the ICU rarely causes weaning failure associated with a primary problem in the pulmonary system. We included only patients who received respiratory support with MV because of respiratory problems, while excluding other ICU patients. The medical records of all enrolled patients were reviewed by two board-certified pulmonologists. Owing to this process, we found no significant differences in the baseline characteristics between the weaning success and failure groups.
The small number of patients was another limitation in this study, although we identified meaningful biosignal-based digital biomarkers. Previous studies discovered novel biosignal-based digital biomarkers using deep learning [41,42,64,75]. Current deep learning techniques can identify hidden patterns with large amounts of data; however, we could not collect sufficient data to support a deep learning model. We presume that further valuable information for determining the possibility of weaning success can be discovered when more data are obtained, supporting the development of a deep learning model.
5. Conclusions
MV weaning failure is usually multifactorial; thus, the weaning parameters that assess a single physiological function may have limited predictive accuracy. In the weaning process, changes in biosignal markers can serve as predictive indicators of each patient’s extubation outcome. These changes offer a noninvasive and valuable tool to characterize cardiorespiratory function and autonomic system interactions. We identified a new biosignal-based combination of markers to determine the possibility of weaning success. By using these digital biomarkers, clinicians can select the appropriate earliest weaning time, which could decrease the risks of both unnecessarily prolonged ventilator support and premature weaning. Therefore, new marker-based biosignal data obtained during SBTs provide a promising tool to assist clinicians in determining the optimal extubation time for the treatment of critically ill patients undergoing ventilator care. However, confirmation of the model generalizability warrants additional studies in other institutions to obtain larger numbers of patients.
Author Contributions
Conceptualization, W.Y.C. and D.Y.; methodology, D.Y.; software, T.Y.K.; validation, T.Y.K., C.H. and C.M.P.; formal analysis, T.Y.K.; investigation, J.E.P., Y.J.J. and W.Y.C.; resources, J.E.P., J.H.P. and K.J.P.; data curation, J.E.P., Y.J.J. and W.Y.C.; writing—original draft preparation, D.Y. and J.E.P.; writing—review and editing, J.E.P.; visualization, J.E.P. and T.Y.K.; supervision, D.Y and W.Y.C.; project administration, W.Y.C.; funding acquisition, D.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT; Ministry of Trade, Industry and Energy; Ministry of Health and Welfare; and Ministry of Food and Drug Safety) (Project Number: 1711138152, KMDF_PR_20200901_0095).
Institutional Review Board Statement
This retrospective study was conducted using anonymized data. The Institutional Review Board of Ajou University Hospital approved the study (IRB No. AJIRB-MED-MDB-20-090) and waived the requirement for informed consent.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study and the analysis used anonymous clinical data.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
T.Y.K. and D.Y. are employees of BUD.on Inc. BUD.on Inc. did not have any role in the study design, analysis, decision to publish, or the preparation of the manuscript. There are no patents, products in development, or marketed products to declare. The other authors declare that they have no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.MacIntyre N.R., Cook D.J., Ely E.W., Jr., Epstein S.K., Fink J.B., Heffner J.E., Hess D., Hubmayer R.D., Scheinhorn D.J., American College of Chest P., et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: A collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120:375S–395S. doi: 10.1378/chest.120.6_suppl.375S. [DOI] [PubMed] [Google Scholar]
- 2.De Jonghe B., Bastuji-Garin S., Durand M.C., Malissin I., Rodrigues P., Cerf C., Outin H., Sharshar T. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit. Care Med. 2007;35:2007–2015. doi: 10.1097/01.ccm.0000281450.01881.d8. [DOI] [PubMed] [Google Scholar]
- 3.Rello J., Ollendorf D.A., Oster G., Vera-Llonch M., Bellm L., Redman R., Kollef M.H. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 4.Rajakaruna C., Rogers C.A., Angelini G.D., Ascione R. Risk factors for and economic implications of prolonged ventilation after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2005;130:1270–1277. doi: 10.1016/j.jtcvs.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 5.Russotto V., Myatra S.N., Laffey J.G., Tassistro E., Antolini L., Bauer P., Lascarrou J.B., Szuldrzynski K., Camporota L., Pelosi P., et al. Intubation practices and adverse peri-intubation events in critically ill patients from 29 countries. JAMA. 2021;325:1164–1172. doi: 10.1001/jama.2021.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epstein S.K., Ciubotaru R.L., Wong J.B. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997;112:186–192. doi: 10.1378/chest.112.1.186. [DOI] [PubMed] [Google Scholar]
- 7.Esteban A., Alía I., Gordo F., Fernández R., Solsona J.F., Vallverdú I., Macías S., Allegue J.M., Blanco J., Carriedo D., et al. Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. The spanish lung failure collaborative group. Am. J. Respir. Crit. Care Med. 1997;156:459–465. doi: 10.1164/ajrccm.156.2.9610109. [DOI] [PubMed] [Google Scholar]
- 8.Capdevila X., Perrigault P.F., Ramonatxo M., Roustan J.P., Peray P., d’Athis F., Prefaut C. Changes in breathing pattern and respiratory muscle performance parameters during difficult weaning. Crit. Care Med. 1998;26:79–87. doi: 10.1097/00003246-199801000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Jubran A., Tobin M.J. Pathophysiologic basis of acute respiratory distress in patients who fail a trial of weaning from mechanical ventilation. Am. J. Respir. Crit. Care Med. 1997;155:906–915. doi: 10.1164/ajrccm.155.3.9117025. [DOI] [PubMed] [Google Scholar]
- 10.Esteban A., Frutos F., Tobin M.J., Alía I., Solsona J.F., Valverdú I., Fernández R., de la Cal M.A., Benito S., Tomás R., et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N. Engl. J. Med. 1995;332:345–350. doi: 10.1056/NEJM199502093320601. [DOI] [PubMed] [Google Scholar]
- 11.Epstein S.K. Predicting extubation failure: Is it in (on) the cards? Chest. 2001;120:1061–1063. doi: 10.1378/chest.120.4.1061. [DOI] [PubMed] [Google Scholar]
- 12.Gershengorn H.B. international variation in intubation and extubation practices and adverse events among critically ill patients receiving mechanical ventilation. JAMA. 2021;325:1157–1159. doi: 10.1001/jama.2021.1178. [DOI] [PubMed] [Google Scholar]
- 13.Vassilakopoulos T., Roussos C., Zakynthinos S. Weaning from mechanical ventilation. J. Crit. Care. 1999;14:39–62. doi: 10.1016/S0883-9441(99)90007-2. [DOI] [PubMed] [Google Scholar]
- 14.Boles J.M., Bion J., Connors A., Herridge M., Marsh B., Melot C., Pearl R., Silverman H., Stanchina M., Vieillard-Baron A., et al. Weaning from mechanical ventilation. Eur. Respir. J. 2007;29:1033–1056. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- 15.Burns K.E.A., Rizvi L., Cook D.J., Dodek P., Slutsky A.S., Jones A., Villar J., Kapadia F.N., Gattas D.J., Epstein S.K., et al. Variation in the practice of discontinuing mechanical ventilation in critically ill adults: Study protocol for an international prospective observational study. BMJ Open. 2019;9:e031775. doi: 10.1136/bmjopen-2019-031775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns K.E.A., Rizvi L., Cook D.J., Lebovic G., Dodek P., Villar J., Slutsky A.S., Jones A., Kapadia F.N., Gattas D.J., et al. Ventilator weaning and discontinuation practices for critically ill patients. JAMA. 2021;325:1173–1184. doi: 10.1001/jama.2021.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perkins G.D., Mistry D., Lall R., Gao-Smith F., Snelson C., Hart N., Camporota L., Varley J., Carle C., Paramasivam E., et al. Protocolised non-invasive compared with invasive weaning from mechanical ventilation for adults in intensive care: The Breathe RCT. Health Technol. Assess. 2019;23:1–114. doi: 10.3310/hta23480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns K.E.A., Rizvi L., Cook D.J., Seely A.J.E., Rochwerg B., Lamontagne F., Devlin J.W., Dodek P., Mayette M., Tanios M., et al. Frequency of screening and SBT technique trial—North American weaning collaboration (FAST-NAWC): A protocol for a multicenter, factorial randomized trial. Trials. 2019;20:587. doi: 10.1186/s13063-019-3641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis K.A., Chaudhuri D., Guyatt G., Burns K.E.A., Bosma K., Ge L., Karachi T., Piraino T., Fernando S.M., Ranganath N., et al. Comparison of ventilatory modes to facilitate liberation from mechanical ventilation: Protocol for a systematic review and network meta-analysis. BMJ Open. 2019;9:e030407. doi: 10.1136/bmjopen-2019-030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vu P.H., Tran V.D., Duong M.C., Cong Q.T., Nguyen T. Predictive value of the negative inspiratory force index as a predictor of weaning success: A crosssectional study. Acute Crit. Care. 2020;35:279–285. doi: 10.4266/acc.2020.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appavu S.K. Prediction of extubation failure following mechanical ventilation: Where are we and where are we going? Crit. Care Med. 2020;48:1536–1538. doi: 10.1097/CCM.0000000000004536. [DOI] [PubMed] [Google Scholar]
- 22.da Silva Neto A.E., de Souza L.C., Costa H.L., Guimarães B.L., de Azeredo L.M., Godoy M.D.P., Lugon J.R. The timed inspiratory effort index as a weaning predictor: Analysis of intra- and interobserver reproducibility. Respir. Care. 2020;65:636–642. doi: 10.4187/respcare.07225. [DOI] [PubMed] [Google Scholar]
- 23.Frutos-Vivar F., Ferguson N.D., Esteban A., Epstein S.K., Arabi Y., Apezteguía C., González M., Hill N.S., Nava S., D’Empaire G., et al. Risk factors for extubation failure in patients following a successful spontaneous breathing trial. Chest. 2006;130:1664–1671. doi: 10.1378/chest.130.6.1664. [DOI] [PubMed] [Google Scholar]
- 24.Epstein S.K. Weaning from ventilatory support. Curr. Opin. Crit. Care. 2009;15:36–43. doi: 10.1097/MCC.0b013e3283220e07. [DOI] [PubMed] [Google Scholar]
- 25.Yang K.L., Tobin M.J. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N. Engl. J. Med. 1991;324:1445–1450. doi: 10.1056/NEJM199105233242101. [DOI] [PubMed] [Google Scholar]
- 26.Casaseca-de-la-Higuera P., Martín-Fernández M., Alberola-López C. Weaning from mechanical ventilation: A retrospective analysis leading to a multimodal perspective. IEEE Trans. Biomed. Eng. 2006;53:1330–1345. doi: 10.1109/TBME.2006.873695. [DOI] [PubMed] [Google Scholar]
- 27.El-Khatib M.F., Zeineldine S.M., Jamaleddine G.W. Effect of pressure support ventilation and positive end expiratory pressure on the rapid shallow breathing index in intensive care unit patients. Intensive Care Med. 2008;34:505–510. doi: 10.1007/s00134-007-0939-x. [DOI] [PubMed] [Google Scholar]
- 28.Krieger B.P., Isber J., Breitenbucher A., Throop G., Ershowsky P. Serial measurements of the rapid-shallow-breathing index as a predictor of weaning outcome in elderly medical patients. Chest. 1997;112:1029–1034. doi: 10.1378/chest.112.4.1029. [DOI] [PubMed] [Google Scholar]
- 29.Shang P., Zhu M., Baker M., Feng J., Zhou C., Zhang H.L. Mechanical ventilation in Guillain-Barré syndrome. Expert Rev. Clin. Immunol. 2020;16:1053–1064. doi: 10.1080/1744666X.2021.1840355. [DOI] [PubMed] [Google Scholar]
- 30.Demiralp B., Koenig L., Xu J., Soltoff S., Votto J. Time spent in prior hospital stay and outcomes for ventilator patients in long-term acute care hospitals. BMC Pulm. Med. 2021;21:104. doi: 10.1186/s12890-021-01454-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villalba D., Gil Rossetti G., Scrigna M., Collins J., Rocco A., Matesa A., Areas L., Golfarini N., Pini P., Hannun M., et al. Prevalence of and risk factors for mechanical ventilation reinstitution in patients weaned from prolonged mechanical ventilation. Respir. Care. 2020;65:210–216. doi: 10.4187/respcare.06807. [DOI] [PubMed] [Google Scholar]
- 32.Meade M., Guyatt G., Cook D., Griffith L., Sinuff T., Kergl C., Mancebo J., Esteban A., Epstein S. Predicting success in weaning from mechanical ventilation. Chest. 2001;120:400S–424S. doi: 10.1378/chest.120.6_suppl.400S. [DOI] [PubMed] [Google Scholar]
- 33.Videtta W., Vallejos J., Roda G., Collazos H., Naccarelli N., Tamayo A., Calderón N., Bairaclioti A., Yoshida M., Vandaele G., et al. Predictors of successful extubation in neurocritical care patients. Acta Neurochir. Suppl. 2021;131:91–93. doi: 10.1007/978-3-030-59436-7_20. [DOI] [PubMed] [Google Scholar]
- 34.Baptistella A.R., Mantelli L.M., Matte L., Carvalho M., Fortunatti J.A., Costa I.Z., Haro F.G., Turkot V.L.O., Baptistella S.F., de Carvalho D., et al. Prediction of extubation outcome in mechanically ventilated patients: Development and validation of the extubation predictive score (ExPreS) PLoS ONE. 2021;16:e0248868. doi: 10.1371/journal.pone.0248868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leonov Y., Kisil I., Perlov A., Stoichev V., Ginzburg Y., Nazarenko A., Gimelfarb Y. Predictors of successful weaning in patients requiring extremely prolonged mechanical ventilation. Adv. Respir. Med. 2020;88:477–484. doi: 10.5603/ARM.a2020.0151. [DOI] [PubMed] [Google Scholar]
- 36.Wu T.J., Shiao J.S., Yu H.L., Lai R.S. An integrative index for predicting extubation outcomes after successful completion of a spontaneous breathing trial in an adult medical intensive care unit. J. Intensive Care Med. 2019;34:640–645. doi: 10.1177/0885066617706688. [DOI] [PubMed] [Google Scholar]
- 37.Liu J., Wang C.J., Ran J.H., Lin S.H., Deng D., Ma Y., Xu F. The predictive value of brain natriuretic peptide or N-terminal pro-brain natriuretic peptide for weaning outcome in mechanical ventilation patients: Evidence from SROC. J. Renin Angiotensin Aldosterone Syst. 2021;22:1–12. doi: 10.1177/1470320321999497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deschamps J., Webber J., Featherstone R., Sebastianski M., Vandermeer B., Senaratne J., Bagshaw S.M. Brain natriuretic peptide to predict successful liberation from mechanical ventilation in critically ill patients: Protocol for a systematic review and meta-analysis. BMJ Open. 2019;9:e022600. doi: 10.1136/bmjopen-2018-022600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fontela P.C., Glaeser S.S., Martins L.F., Condessa R.L., Prediger D.T., Forgiarini S.G., Forgiarini L.A., Jr., Lisboa T.C., Friedman G. Medical research council scale predicts spontaneous breathing trial failure and difficult or prolonged weaning of critically ill individuals. Respir. Care. 2021;66:733–741. doi: 10.4187/respcare.07739. [DOI] [PubMed] [Google Scholar]
- 40.Mallat J., Baghdadi F.A., Mohammad U., Lemyze M., Temime J., Tronchon L., Thevenin D., Fischer M.O. Central venous-to-arterial PCO2 difference and central venous oxygen saturation in the detection of extubation failure in critically ill patients. Crit. Care Med. 2020;48:1454–1461. doi: 10.1097/CCM.0000000000004446. [DOI] [PubMed] [Google Scholar]
- 41.El-Khatib M., Jamaleddine G., Soubra R., Muallem M. Pattern of spontaneous breathing: Potential marker for weaning outcome. Spontaneous breathing pattern and weaning from mechanical ventilation. Intensive Care Med. 2001;27:52–58. doi: 10.1007/s001340000758. [DOI] [PubMed] [Google Scholar]
- 42.Bien M.Y., Hseu S.S., Yien H.W., Kuo B.I., Lin Y.T., Wang J.H., Kou Y.R. Breathing pattern variability: A weaning predictor in postoperative patients recovering from systemic inflammatory response syndrome. Intensive Care Med. 2004;30:241–247. doi: 10.1007/s00134-003-2073-8. [DOI] [PubMed] [Google Scholar]
- 43.Fabregat A., Magret M., Ferré J.A., Vernet A., Guasch N., Rodríguez A., Gómez J., Bodí M. A Machine Learning decision-making tool for extubation in intensive care unit patients. Comput. Methods Programs Biomed. 2021;200:105869. doi: 10.1016/j.cmpb.2020.105869. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida T., Fujino Y. Monitoring the patient for a safe-assisted ventilation. Curr. Opin. Crit. Care. 2021;27:1–5. doi: 10.1097/MCC.0000000000000788. [DOI] [PubMed] [Google Scholar]
- 45.Kennedy H.L. Heart rate variability—A potential, noninvasive prognostic index in the critically ill patient. Crit. Care Med. 1998;26:213–214. doi: 10.1097/00003246-199802000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Camm A.J., Malik M., Bigger J.T., Breithardt G., Cerutti S., Cohen R.J., Coumel P., Fallen E.L., Kennedy H.L., Kleiger R.E., et al. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 47.Kwon J.M., Cho Y., Jeon K.H., Cho S., Kim K.H., Baek S.D., Jeung S., Park J., Oh B.H. A deep learning algorithm to detect anaemia with ECGs: A retrospective, multicentre study. Lancet Digit. Health. 2020;2:e358–e367. doi: 10.1016/S2589-7500(20)30108-4. [DOI] [PubMed] [Google Scholar]
- 48.Attia Z.I., Noseworthy P.A., Lopez-Jimenez F., Asirvatham S.J., Deshmukh A.J., Gersh B.J., Carter R.E., Yao X., Rabinstein A.A., Erickson B.J., et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet. 2019;394:861–867. doi: 10.1016/S0140-6736(19)31721-0. [DOI] [PubMed] [Google Scholar]
- 49.Raghunath S., Ulloa Cerna A.E., Jing L., van Maanen D.P., Stough J., Hartzel D.N., Leader J.B., Kirchner H.L., Stumpe M.C., Hafez A., et al. Prediction of mortality from 12-lead electrocardiogram voltage data using a deep neural network. Nat. Med. 2020;26:886–891. doi: 10.1038/s41591-020-0870-z. [DOI] [PubMed] [Google Scholar]
- 50.He J., Tse G., Korantzopoulos P., Letsas K.P., Ali-Hasan-Al-Saegh S., Kamel H., Li G., Lip G.Y.H., Liu T. P-wave indices and risk of ischemic stroke: A systematic review and meta-analysis. Stroke. 2017;48:2066–2072. doi: 10.1161/STROKEAHA.117.017293. [DOI] [PubMed] [Google Scholar]
- 51.Attia Z.I., Kapa S., Lopez-Jimenez F., McKie P.M., Ladewig D.J., Satam G., Pellikka P.A., Enriquez-Sarano M., Noseworthy P.A., Munger T.M., et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat. Med. 2019;25:70–74. doi: 10.1038/s41591-018-0240-2. [DOI] [PubMed] [Google Scholar]
- 52.Zhu H., Cheng C., Yin H., Li X., Zuo P., Ding J., Lin F., Wang J., Zhou B., Li Y., et al. Automatic multilabel electrocardiogram diagnosis of heart rhythm or conduction abnormalities with deep learning: A cohort study. Lancet Digit. Health. 2020;2:e348–e357. doi: 10.1016/S2589-7500(20)30107-2. [DOI] [PubMed] [Google Scholar]
- 53.Kei Fong M.W., Ng E.Y.K., Er Zi Jian K., Hong T.J. SVR ensemble-based continuous blood pressure prediction using multi-channel photoplethysmogram. Comput. Biol. Med. 2019;113:103392. doi: 10.1016/j.compbiomed.2019.103392. [DOI] [PubMed] [Google Scholar]
- 54.Lin C.T., Wang C.Y., Huang K.C., Horng S.J., Liao L.D. Wearable, Multimodal, Biosignal Acquisition System for Potential Critical and Emergency Applications. Emerg Med. Int. 2021;2021:1–10. doi: 10.1155/2021/9954669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elgendi M., Fletcher R., Liang Y., Howard N., Lovell N.H., Abbott D., Lim K., Ward R. The use of photoplethysmography for assessing hypertension. NPJ Digit. Med. 2019;2:1–11. doi: 10.1038/s41746-019-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomita K., Nakada T.A., Oshima T., Oami T., Aizimu T., Oda S. Non-invasive monitoring using photoplethysmography technology. J. Clin. Monit. Comput. 2019;33:637–645. doi: 10.1007/s10877-018-0205-5. [DOI] [PubMed] [Google Scholar]
- 57.Buchman T.G. The community of the self. Nature. 2002;420:246–251. doi: 10.1038/nature01260. [DOI] [PubMed] [Google Scholar]
- 58.Annane D., Trabold F., Sharshar T., Jarrin I., Blanc A.S., Raphael J.C., Gajdos P. Inappropriate sympathetic activation at onset of septic shock: A spectral analysis approach. Am. J. Respir. Crit. Care Med. 1999;160:458–465. doi: 10.1164/ajrccm.160.2.9810073. [DOI] [PubMed] [Google Scholar]
- 59.Tobin M.J., Mador M.J., Guenther S.M., Lodato R.F., Sackner M.A. Variability of resting respiratory drive and timing in healthy subjects. J. Appl. Physiol. (1985) 1988;65:309–317. doi: 10.1152/jappl.1988.65.1.309. [DOI] [PubMed] [Google Scholar]
- 60.Tobin M.J., Yang K.L., Jubran A., Lodato R.F. Interrelationship of breath components in neighboring breaths of normal eupneic subjects. Am. J. Respir. Crit. Care Med. 1995;152:1967–1976. doi: 10.1164/ajrccm.152.6.8520764. [DOI] [PubMed] [Google Scholar]
- 61.Jonkman A.H., Rauseo M., Carteaux G., Telias I., Sklar M.C., Heunks L., Brochard L.J. Proportional modes of ventilation: Technology to assist physiology. Intensive Care Med. 2020;46:2301–2313. doi: 10.1007/s00134-020-06206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gertler R. Respiratory Mechanics. Anesthesiol. Clin. 2021;39:415–440. doi: 10.1016/j.anclin.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brochard L. Breathing: Does regular mean normal? Crit. Care Med. 1998;26:1773–1774. doi: 10.1097/00003246-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 64.Seely A.J., Bravi A., Herry C., Green G., Longtin A., Ramsay T., Fergusson D., McIntyre L., Kubelik D., Maziak D.E., et al. Do heart and respiratory rate variability improve prediction of extubation outcomes in critically ill patients? Crit. Care. 2014;18:R65. doi: 10.1186/cc13822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chakraborty M., Watkins W.J., Tansey K., King W.E., Banerjee S. Predicting extubation outcomes using the heart rate characteristics index in preterm infants: A cohort study. Eur. Respir. J. 2020;56:1–9. doi: 10.1183/13993003.01755-2019. [DOI] [PubMed] [Google Scholar]
- 66.Lilitsis E., Stamatopoulou V., Andrianakis E., Petraki A., Antonogiannaki E.M., Georgopoulos D., Vaporidi K., Kondili E. Inspiratory effort and breathing pattern change in response to varying the assist level: A physiological study. Respir. Physiol. Neurobiol. 2020;280:103474. doi: 10.1016/j.resp.2020.103474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grasselli G., Brioni M., Zanella A. Monitoring respiratory mechanics during assisted ventilation. Curr. Opin. Crit. Care. 2020;26:11–17. doi: 10.1097/MCC.0000000000000681. [DOI] [PubMed] [Google Scholar]
- 68.Yoon D., Lee S., Kim T.Y., Ko J., Chung W.Y., Park R.W. System for collecting biosignal data from multiple patient monitoring systems. Healthc. Inform. Res. 2017;23:333–337. doi: 10.4258/hir.2017.23.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roh J.H., Synn A., Lim C.M., Suh H.J., Hong S.B., Huh J.W., Koh Y. A weaning protocol administered by critical care nurses for the weaning of patients from mechanical ventilation. J. Crit. Care. 2012;27:549–555. doi: 10.1016/j.jcrc.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt G.A., Girard T.D., Kress J.P., Morris P.E., Ouellette D.R., Alhazzani W., Burns S.M., Epstein S.K., Esteban A., Fan E., et al. Liberation from mechanical ventilation in critically ill adults: Executive summary of an official american college of chest physicians/American thoracic society clinical practice guideline. Chest. 2017;151:160–165. doi: 10.1016/j.chest.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 71.Shen H.N., Lin L.Y., Chen K.Y., Kuo P.H., Yu C.J., Wu H.D., Yang P.C. Changes of heart rate variability during ventilator weaning. Chest. 2003;123:1222–1228. doi: 10.1378/chest.123.4.1222. [DOI] [PubMed] [Google Scholar]
- 72.La Rovere M.T., Pinna G.D., Maestri R., Mortara A., Capomolla S., Febo O., Ferrari R., Franchini M., Gnemmi M., Opasich C., et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107:565–570. doi: 10.1161/01.CIR.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- 73.Malpas S.C. Neural influences on cardiovascular variability: Possibilities and pitfalls. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H6–H20. doi: 10.1152/ajpheart.2002.282.1.H6. [DOI] [PubMed] [Google Scholar]
- 74.Huang C.T., Tsai Y.J., Lin J.W., Ruan S.Y., Wu H.D., Yu C.J. Application of heart-rate variability in patients undergoing weaning from mechanical ventilation. Crit. Care. 2014;18:R21. doi: 10.1186/cc13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Engoren M. Approximate entropy of respiratory rate and tidal volume during weaning from mechanical ventilation. Crit. Care Med. 1998;26:1817–1823. doi: 10.1097/00003246-199811000-00021. [DOI] [PubMed] [Google Scholar]
- 76.Papaioannou V.E., Chouvarda I.G., Maglaveras N.K., Pneumatikos I.A. Study of multiparameter respiratory pattern complexity in surgical critically ill patients during weaning trials. BMC Physiol. 2011;11:2. doi: 10.1186/1472-6793-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tanios M.A., Nevins M.L., Hendra K.P., Cardinal P., Allan J.E., Naumova E.N., Epstein S.K. A randomized, controlled trial of the role of weaning predictors in clinical decision making. Crit. Care Med. 2006;34:2530–2535. doi: 10.1097/01.CCM.0000236546.98861.25. [DOI] [PubMed] [Google Scholar]
- 78.Huang C.T., Yu C.J. Conventional weaning parameters do not predict extubation outcome in intubated subjects requiring prolonged mechanical ventilation. Respir. Care. 2013;58:1307–1314. doi: 10.4187/respcare.01773. [DOI] [PubMed] [Google Scholar]
- 79.Otaguro T., Tanaka H., Igarashi Y., Tagami T., Masuno T., Yokobori S., Matsumoto H., Ohwada H., Yokota H. Machine learning for the prediction of successful extubation among patients with mechanical ventilation in the intensive care unit: A retrospective observational study. J. Nippon Med. Sch. 2021 doi: 10.1272/jnms.JNMS.2021_88-508. [DOI] [PubMed] [Google Scholar]
- 80.Goldman S.L., McCann E.M., Lloyd B.W., Yup G. Inspiratory time and pulmonary function in mechanically ventilated babies with chronic lung disease. Pediatr. Pulmonol. 1991;11:198–201. doi: 10.1002/ppul.1950110303. [DOI] [PubMed] [Google Scholar]
- 81.Vassilakopoulos T., Zakynthinos S., Roussos C. Respiratory muscles and weaning failure. Eur. Respir. J. 1996;9:2383–2400. doi: 10.1183/09031936.96.09112383. [DOI] [PubMed] [Google Scholar]
- 82.Vassilakopoulos T., Zakynthinos S., Roussos C. The tension-time index and the frequency/tidal volume ratio are the major pathophysiologic determinants of weaning failure and success. Am. J. Respir. Crit. Care Med. 1998;158:378–385. doi: 10.1164/ajrccm.158.2.9710084. [DOI] [PubMed] [Google Scholar]
- 83.Yoon D., Jang J.H., Choi B.J., Kim T.Y., Han C.H. Discovering hidden information in biosignals from patients using artificial intelligence. Korean J. Anesthesiol. 2020;73:275–284. doi: 10.4097/kja.19475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ouyang D., Zou J. Deep learning models to detect hidden clinical correlates. Lancet Digit. Health. 2020;2:e334–e335. doi: 10.1016/S2589-7500(20)30138-2. [DOI] [PubMed] [Google Scholar]
- 85.Chen X., Guo W., Zhao L., Huang W., Wang L., Sun A., Li L., Mo F. Acute myocardial infarction detection using deep learning-enabled electrocardiograms. Front. Cardiovasc. Med. 2021;8:654515. doi: 10.3389/fcvm.2021.654515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jia Y., Kaul C., Lawton T., Murray-Smith R., Habli I. Prediction of weaning from mechanical ventilation using convolutional neural networks. Artif. Intell. Med. 2021;117:102087. doi: 10.1016/j.artmed.2021.102087. [DOI] [PubMed] [Google Scholar]
- 87.Chang Y.J., Hung K.C., Wang L.K., Yu C.H., Chen C.K., Tay H.T., Wang J.J., Liu C.F. A real-time artificial intelligence-assisted system to predict weaning from ventilator immediately after lung resection surgery. Int. J. Environ. Res. Public Health. 2021;18:2713. doi: 10.3390/ijerph18052713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hill B.L., Rakocz N., Rudas Á., Chiang J.N., Wang S., Hofer I., Cannesson M., Halperin E. Imputation of the continuous arterial line blood pressure waveform from non-invasive measurements using deep learning. Sci. Rep. 2021;11:15755. doi: 10.1038/s41598-021-94913-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.