Abstract
The genetic diversity of 88 Streptococcus suis serotype 2 isolates which were recovered from various countries was examined by randomly amplified polymorphic DNA (RAPD) analysis with three primers. This bacterial collection included 80 isolates of porcine origin and 8 of human origin. This investigation allowed the identification of 23 RAPD types containing 1 to 30 isolates originating from one to six countries. Common RAPD patterns were found between human and pig isolates. The isolates were also tested for the production of virulent factors such as hemolysin, muramidase-released protein (MRP), and extracellular factor (EF). All isolates exhibiting the virulent phenotype hemolysin+ MRP+ EF+ clearly clustered on the basis of fingerprinting by RAPD analysis. In a similar way, most of isolates with the hemolysin− MRP− EF− phenotype were assigned to one RAPD cluster. Therefore, RAPD clusters are more related to the phenotype defined with hemolysin, MRP, and EF than to the geographic origin of the isolates. These data indicate that RAPD analysis used in conjunction with phenotypic methods provides a reliable method for the assessment of the clonal relationship between S. suis isolates responsible for infections in pigs or humans, especially for those exhibiting the classic “virulent” phenotype hemolysin+ MRP+ EF+.
Streptococcus suis serotype 2 is the causative agent of a wide range of infections in pigs such as septicemia, meningitis, arthritis, and pneumonia (20). This particular serotype has also been associated with severe infections in humans (1, 22).
Little is known about the pathogenesis of S. suis infections. Moreover, the virulence of S. suis isolates belonging to serotype 2 has been shown to be variable (3, 24). Two virulence markers have been described for European S. suis isolates: the first is a 136-kDa cell wall-associated protein called muramidase-released protein (MRP) and the second is a 110-kDa extracellular factor (EF) (24). Related proteins with higher (EF*, MRP*) or lower (MRPs) molecular masses have been described (26). According to these studies, most isolates from diseased pigs belonged to the MRP+ EF+ phenotype, whereas the majority of isolates from healthy pigs belonged to the MRP− EF− phenotype. MRP+ EF* isolates were associated with slight pathological changes (26). Most isolates from human patients exhibited the MRP+ EF− phenotype (25). Interestingly, most virulent North American isolates do not carry these two virulence proteins (7, 10). More recently, a hemolysin, designed suilysin, was shown to be produced by some S. suis serotype 2 strains (8, 13, 14), but its involvement in the pathogenesis of S. suis infections is still unknown. A correlation between hemolysin activity and virulence has been reported (21). As for proteins MRP and EF, the suilysin is not produced by all virulent strains (10).
Genetic differences between S. suis serotype 2 strains have previously been demonstrated. The use of multilocus enzyme electrophoresis to define the diversity among Australian strains indicated that S. suis serotype 2 strains from healthy and diseased Australian pigs originated from diverse genetic backgrounds (11, 16). The use of restriction endonuclease analysis and ribotyping to examine a collection of Canadian S. suis serotype 2 isolates has shown that those recovered from diseased pigs exhibited less genetic heterogeneity than those isolated from healthy pigs (12). Recently, it was demonstrated that strains synthesizing both MRP (or MRPs) and EF molecules have a unique ribotype profile and that strains with a MRP+ EF+ phenotype are genetically more homogeneous (18).
The purpose of this study was to clarify the genetic relatedness of a collection of S. suis serotype 2 isolates from various geographic origins in correlation with their phenotypes. Isolates had been recovered from diseased or healthy carrier pigs and from humans in Europe and North America. They were studied by random amplified polymorphic DNA (RAPD) analysis. The objectives were (i) to assess if isolates from different countries share common RAPD patterns; (ii) to evaluate the correlation between RAPD patterns and the production of MRP, EF, and hemolysin; (iii) to define if strains from diseased pigs exhibit distinct RAPD patterns from those identified in isolates from healthy carrier pigs; and (iv) to assess if human isolates and pig isolates exhibit similar RAPD patterns.
MATERIALS AND METHODS
S. suis isolates.
A total of 88 unrelated S. suis serotype 2 isolates were used in this study, and 80 were of porcine origin and 8 were of human origin. Samples were collected between 1989 and 1997. Seventy-two of the porcine isolates were from diseased animals, and the other 8 isolates originated from the upper respiratory tracts of clinically healthy pigs. All human isolates were from patients who were diagnosed with S. suis infection and who were all in close contact with pigs.
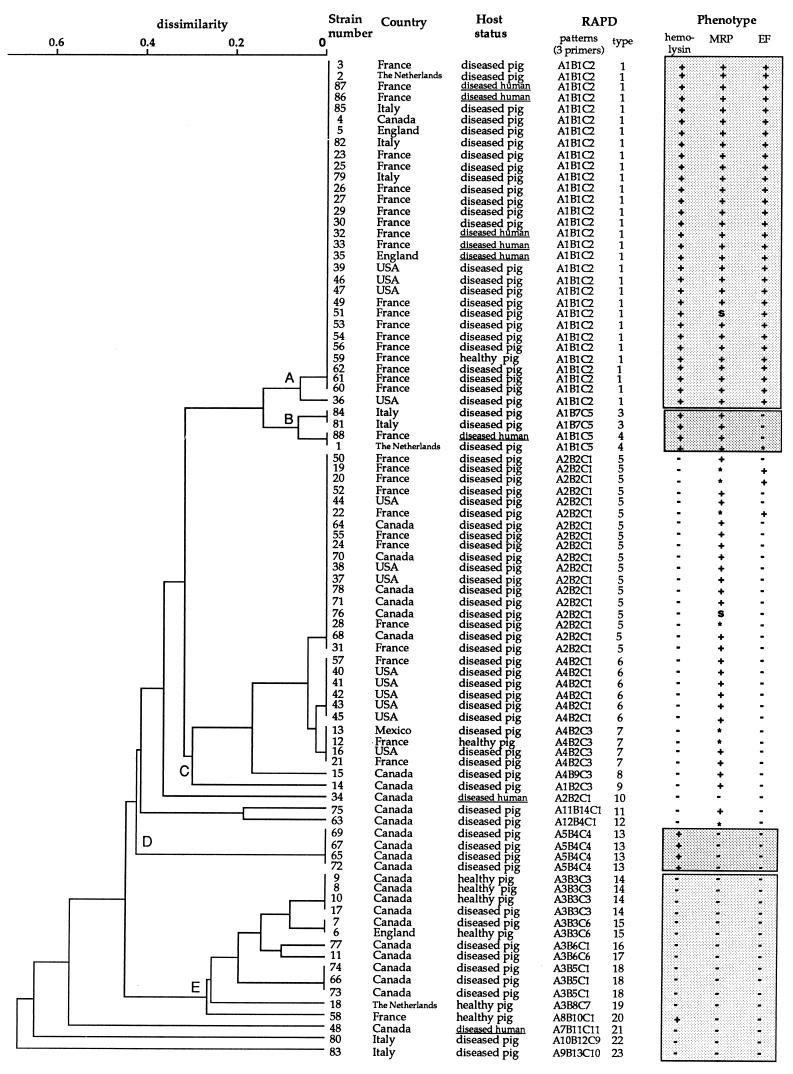
The isolates originated from different geographic areas: 34 isolates were from France, 3 were from England, 3 were from The Netherlands, 7 were from Italy, 27 were from Canada, 13 were from the United States, and 1 was from Mexico. The origin of each isolate is indicated in Fig. 2. The S. suis isolates were serotyed by a coagglutination test, based on polysaccharide capsular antigens, as described previously (9).
FIG. 2.
Genetic relationship between 88 S. suis serotype 2 isolates as estimated by clustering analysis of RAPD patterns obtained with three primers. The tree was generated by the unweighted pair group method with arithmetic means. The geographic origin, the host health status, and the phenotype regarding hemolysin, MRP, and EF are indicated for each strain. ∗, related proteins with a higher-molecular-mass form; s, MRP-like protein with a lower molecular mass.
Phenotyping.
The strains were tested for the production of MRP and EF by immunoblotting with monoclonal antibodies as described previously (24). They were also tested for the production of a hemolysin as described previously (8).
Fingerprinting by RAPD analysis.
PCR was conducted under a layer of mineral oil in a 25-μl reaction mixture containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2.5 mM MgCl2, 100 μM (each) dATP, dCTP, dGTP, and dTTP (Pharmacia, Uppsala, Sweden), 0.2 μM primer, 0.5 U of Taq DNA polymerase (Pharmacia), and 25 ng of DNA extracted and purified as described previously (17). The primers used in this study were purchased from Operon Technologies (Alameda, Calif.). The cycling program was 1 cycle of 94°C for 4 min, 36°C for 1 min, and 72°C for 2 min; 33 cycles of 94°C for 1 min, 36°C for 1 min, and 72°C for 2 min; and 1 cycle of 94°C for 2 min, 36°C for 1 min, and 72°C for 10 min in a DNA Thermal Cycler 480 (Perkin-Elmer Cetus, Norwalk, Conn.). Amplified products (20 μl) were analyzed by 1.4% agarose gel electrophoresis in Tris-borate-EDTA buffer and were visualized by UV transillumination following ethidium bromide staining. A negative control consisting of the same reaction mixture but with water instead of template DNA was included in each run.
Pattern analysis.
Photographs of each gel were digitized with a video camera connected to a microcomputer (AlphaEase; Alpha Innotech Corp., San Leandro, Calif.). Analysis of the RAPD patterns was performed with the Taxotron package (Taxolab, Institut Pasteur, Paris, France). This package is composed of the RestrictoScan, RestrictoTyper, Adanson, and Dendrograf programs. The digitized images were transferred to a Macintosh microcomputer, and the band migration distances for each lane were determined with the RestrictoScan program. The molecular size of each fragment was generated from migration distances by using cubic spline algorithms with the RestrictoTyper program. A distance matrix was calculated with the RestrictoTyper program, with the fragment length error tolerance set at 5%. A schematic representation of the electrophoretic patterns was also produced with the RestrictoTyper program. The relationships between RAPD types were calculated by the unweighted pair group method with arithmetic mean (19) with the Adanson clustering program (dissimilarity). A dendrogram of the tree description file was drawn with the Dendrograf program.
RESULTS
Identification of informative primers.
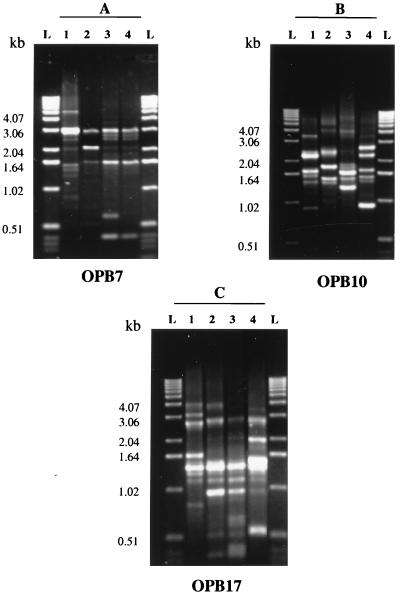
To identify primers that generate informative and discriminatory arrays of PCR products, 20 different 10-mer primers were tested with genomic DNA from 18 unrelated S. suis capsular type 2 isolates. Six of them were from healthy pigs and the others were identified among diseased pigs. The geographic origins of these isolates varied: 9 originated from Canada, 3 originated from The Netherlands, 2 originated from France, 2 originated from England, 1 originated from the United States, and 1 originated from Mexico. Three primers named OPB7 (5′-GGTGACGCAG-3′), OPB10 (5′-CTGCTGGGAC-3′), and OPB17 (5′-AGGGAACGAG-3′) were selected because they gave reproducible patterns containing fragments with a large size range and a small number of low-intensity bands (Fig. 1). Among the primers tested, they were also the most discriminative for the 18 unrelated isolates. The reproducibilities of the RAPD patterns were tested by using DNA preparations made from separate cultures of 18 strains on different days. Identical strain-specific patterns were obtained from the paired DNA preparations. In addition, the same DNA preparations from isolates tested at least three times exhibited identical and reproducible strain-specific patterns.
FIG. 1.
RAPD patterns most frequently generated with primers OPB7, OPB10, and OPB17. (A) Primer OPB7. Lanes 1 to 4, patterns A1, A3, A4, and A2, respectively. (B) Primer OPB10. Lanes 1 to 4, patterns B1, B2, B3, and B4, respectively. (C) Primer OPB17. Lanes 1 to 4, patterns C2, C1, C3, and C4, respectively. These primers were chosen because they generate patterns which contain a large range of fragment sizes and a small number of minor fragments. Lanes L, 1-kb DNA ladder (DNA molecular size markers).
Genetic diversity of isolates as defined by fingerprinting by RAPD analysis.
For the whole panel of 88 isolates, 12 RAPD patterns each composed of 3 to 7 bands with sizes of between 0.4 and 5 kbp were achieved with primer OPB7, 14 patterns each characterized by 4 to 9 bands in a 0.5- to 5-kbp size range resulted with OPB10, and 11 patterns each with 6 to 11 bands in a 0.4- to 6-kbp size range were observed with OPB17. Each pattern contained 1 to 36 isolates. By combining the data obtained with the three primers, 23 RAPD types were found among the 88 isolates. The genetic relationships between the isolates on the basis of their RAPD types are represented in the dendrogram shown in Fig. 2. This clustering analysis allowed the differentiation of five groups (groups A, B, C, D, and E), each of which contained 4 to 31 isolates, and grouped 82 of the 88 isolates (Fig. 2).
Genetic variations of isolates in relation to their geographic origins.
The RAPD types contained 1 to 30 isolates originating from one to six countries (Fig. 2). Strains isolated in the same country were not all characterized by the same RAPD type. Clustering analysis revealed that 42 of the 47 European isolates were assigned to six RAPD types belonging to the major groups A, B, and C (Fig. 2). The 13 isolates from the United States were assigned to five RAPD types of the groups A, B, and C. The 27 Canadian isolates, distributed in 14 RAPD types, defined the most heterogeneous population (Fig. 2).
Relation between genotypic and phenotypic characteristics.
The phenotypic characteristics of each isolate are presented in Fig. 2. Only 8% of the North American isolates had the phenotype MRP+ EF+, whereas 55% isolates of European origin had this phenotype. Similar differences were found for the hemolysin, for which 22.5 and 66% of North American and European isolates were positive, respectively. The two North American isolates of human origin were hemolysin− MRP− EF−, whereas five of the six European ones were hemolysin+ MRP+ EF+. The nine different phenotypes identified among the population studied were hemolysin+ MRP+ EF+, hemolysin+ MRP+ EF*, hemolysin+ MRP+ EF−, hemolysin− MRP* EF+, hemolysin− MRP+ EF−, hemolysin− MRP* EF−, hemolysin− MRPS EF−, hemolysin+ MRP− EF−, and hemolysin− MRP− EF−. A correlation between RAPD types and the production of MRP, EF, and hemolysin was observed (Table 1 and Fig. 2). All isolates exhibiting the hemolysin+ MRP+ EF+ phenotype clearly clustered on the basis of fingerprinting by RAPD analysis (group A, Fig. 2). In a similar way, most of isolates with the hemolysin− MRP− EF− phenotype were assigned to one RAPD cluster (group E, Fig. 2). Isolates with the hemolysin− MRP+ EF− phenotype were closely related (group C, Fig. 2). The four isolates with the hemolysin+ MRP+ EF− (or EF*) phenotype clustered together (group B, Fig. 2). Finally, the four isolates demonstrating the hemolysin+ MRP− EF− phenotype were identical on the basis of their RAPD types (group D, Fig. 2).
TABLE 1.
Distribution of S. suis isolates in the different RAPD types according to their phenotypes
| RAPD type
|
No. of strains | No. of strains with the following phenotypea:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hemolysin
|
MRP
|
EF
|
||||||||
| Pos | Neg | Pos | s | * | Neg | Pos | * | Neg | ||
| 1 | 29 | 29 | 28 | 1 | 29 | |||||
| 2 | 2 | 2 | 2 | 2 | ||||||
| 3 | 2 | 2 | 2 | 2 | ||||||
| 4 | 2 | 2 | 2 | 1 | 1 | |||||
| 5 | 18 | 1 | 17 | 13 | 1 | 4 | 4 | 14 | ||
| 6 | 6 | 6 | 6 | 6 | ||||||
| 7 | 4 | 4 | 2 | 2 | 4 | |||||
| 8 | 1 | 1 | 1 | 1 | ||||||
| 9 | 1 | 1 | 1 | 1 | ||||||
| 10 | 1 | 1 | 1 | 1 | ||||||
| 11 | 1 | 1 | 1 | 1 | ||||||
| 12 | 1 | 1 | 1 | 1 | ||||||
| 13 | 4 | 4 | 4 | 4 | ||||||
| 14 | 4 | 4 | 4 | 4 | ||||||
| 15 | 2 | 2 | 2 | 2 | ||||||
| 16 | 1 | 1 | 1 | 1 | ||||||
| 17 | 1 | 1 | 1 | 1 | ||||||
| 18 | 3 | 1 | 2 | 3 | 3 | |||||
| 19 | 1 | 1 | 1 | 1 | ||||||
| 20 | 1 | 1 | 1 | 1 | ||||||
| 21 | 1 | 1 | 1 | 1 | ||||||
| 22 | 1 | 1 | 1 | 1 | ||||||
| 23 | 1 | 1 | 1 | 1 | ||||||
Pos, positive; Neg, negative; *, related proteins with higher molecular masses (EF*, MRP*); s, related proteins with lower molecular masses (MRPs).
Genetic variations of isolates in relation to host status.
The eight isolates from pigs without clinical signs of infection were assigned to six RAPD types. Four of these types also corresponded to those of isolates recovered from diseased pigs. Three unrelated isolates from clinically healthy animals, all isolated in Canada, were characterized by the same RAPD types. Cluster analysis based on the fingerprints obtained by RAPD analysis could not distinguish isolates from pigs with meningitis and those from pigs with septicemia (data not shown).
Genetic variations of isolates in relation to host origin.
Clustering was not observed for the eight S. suis isolates from human patients (Fig. 2). In addition, common RAPD patterns between isolates from humans and pigs were identified. All isolates of human origin and with the hemolysin+ MRP+ EF+ phenotype clustered with pig isolates possessing the same phenotype.
DISCUSSION
Fingerprinting by RAPD analysis has successfully been used to analyze bacterial genomic DNAs (5, 6, 23). The results of the present study have shown a relative genetic diversity among the different isolates of S. suis serotype 2. This genetic heterogeneity among S. suis serotype 2 isolates had also been previously observed by other typing methods such as multilocus enzyme electrophoresis (11, 16), restriction endonuclease analysis (4), and ribotyping (12, 15, 18, 21). These findings suggest that different S. suis type 2 clones are associated with infections in pigs. Several RAPD clusters regrouped isolates from different countries, even from two different continents. These data suggest that the North American and European isolates that share the same RAPD cluster may have originated from a common ancestor. S. suis serotype 2 is usually transmitted by the respiratory route and colonizes the nasal cavities and tonsils of both diseased and healthy carrier pigs (2, 16). Therefore, the intensive swine production in developed countries is likely to be at the origin of the spread of particular S. suis “clones” worldwide.
Since European studies had demonstrated that the MRP and EF proteins were strongly associated with the virulence of S. suis serotype 2 isolates (26), the strain relatedness with phenotypic and genotypic characteristics was also investigated in this study. The present study confirmed previous reports which indicate that the MRP and EF proteins, as well as the hemolysin, are not frequently associated with North American strains (10, 21). It appears that RAPD clusters are more related to the phenotype defined with the hemolysin, MRP, and EF than to the geographic or host origin of the strains. Interestingly, 30 of the 31 isolates that synthesized the hemolysin, MRP (or MRPs), and EF belonged to a unique RAPD type; the other isolate was assigned to a RAPD type closely related to the previous one. Twenty-six of these 31 isolates originated from Europe, only 1 isolate was from Canada, and only 4 isolates were from the United States. Data indicate that these isolates are very closely related and may originate from a common ancestor. A recent study indicated that MRP+ (or MRPs+) EF+ isolates also exhibited a unique ribotype profile (18). This suggests that both ribotyping and fingerprinting by RAPD analysis can be used to detect specifically these pathogenic MRP+ EF+ hemolysin+ isolates. Compared with ribotyping, RAPD analysis is faster, technically less demanding, and more economical. However, since the MRP and EF proteins are not present in all virulent strains (e.g., Canadian strains) (7, 10), these alternative typing methods will not be sufficient for the tracking of all virulent strains. Another correlation between phenotypic and genotypic characteristics has been observed for strains which do not express hemolysin, MRP, or EF. All but one of these strains were closely related on the basis of fingerprinting by RAPD analysis. This RAPD cluster included nine isolates from diseased pigs and six isolates from healthy pigs. The fact that isolates from healthy pigs and diseased pigs were closely related at the genomic level emphasizes the importance of the host factors in the occurrence of the infection.
Interestingly, S. suis serotype 2 isolates recovered from human patients in different cities or countries appeared to have identical RAPD types (Fig. 2). This suggests the existence of a clonal relationship and that some S. suis clones could commonly be the cause of human infections. Moreover, six human isolates were identical to some of the pig isolates, confirming a possible transmission of S. suis from pigs to humans. This conclusion agrees with the results of Arends and Zanen (1), who indicated that people who are in close contact with pigs are at greater risk for S. suis infection.
In summary, the results of this study indicate that fingerprinting by RAPD analysis is a useful technique for the characterization of virulent strains exhibiting the classic “virulent” phenotype hemolysin+ MRP+ EF+. When used in conjunction with phenotypic methods, RAPD analysis provides a reliable method for assessment of the clonal relationship of S. suis strains responsible for infections in pigs or humans.
ACKNOWLEDGMENTS
We are in debt to Sonia Lacouture for expert technical assistance in the MRP, EF, and hemolysin studies. We also thank L. Brasme (Centre Hospitalier Universitaire de Reims, France), M. M. Chengappa (Kansas University, Manhattan), M. Kobisch (Centre National d’Études Vétérinaires et Alimentaires, Ploufragan, France), P. Norton (Institute for Animal Health, Compton, United Kingdom), and U. Vecht (DLO-Institute for Animal Science and Health, Lelystad, The Netherlands) for providing the strains. U. Vecht also provided the monoclonal antibodies for the detection of MRP and EF proteins. We also thank A. Nassar (Faculté de Médecine Vétérinaire, Saint-Hyacinthe, Québec, Canada) for critical reading of the manuscript.
This research was supported by a grant (grant STRO 181154) from the National Sciences and Engineering Research Council of Canada and a postdoctoral fellowship offered by AUPELF.UREF to S.C.
REFERENCES
- 1.Arends J P, Zanen H C. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis. 1988;10:131–137. doi: 10.1093/clinids/10.1.131. [DOI] [PubMed] [Google Scholar]
- 2.Arends J P, Harwig N, Rudolphy M, Zanen H C. Carrier state of Streptococcus suis capsular type 2 in palatine tonsils of slaughtered pigs. J Clin Microbiol. 1984;20:945–947. doi: 10.1128/jcm.20.5.945-947.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaudoin M, Higgins R, Harel J, Gottschalk M. Studies on a murine model for evaluation of virulence of Streptococcus suis capsular type 2. FEMS Microbiol Lett. 1992;78:111–116. doi: 10.1016/0378-1097(92)90011-c. [DOI] [PubMed] [Google Scholar]
- 4.Beaudoin M, Harel J, Higgins R, Gottschalk M, Frenette M, MacInnes J. Molecular analysis of isolates of Streptococcus suis capsular type 2 by restriction-endonuclease-digested DNA separated on SDS-PAGE and by hybridization with an rDNA probe. J Gen Microbiol. 1992;138:2639–2645. doi: 10.1099/00221287-138-12-2639. [DOI] [PubMed] [Google Scholar]
- 5.Brousseau R, Saint-Onge A, Prefontaine G, Masson L, Cabana J. Arbitrary primer polymerase chain reaction, a powerful method to identify Bacillus thuringiensis serovars and strains. Appl Environ Microbiol. 1993;59:114–119. doi: 10.1128/aem.59.1.114-119.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatellier S, Ramanantsoa C, Harriau P, Rolland K, Rosenau A, Quentin R. Genetic characterization of Streptococcus agalactiae strains by RAPD fingerprinting: identification of particularities of virulent clone families that cause meningitis. J Clin Microbiol. 1997;35:2573–2579. doi: 10.1128/jcm.35.10.2573-2579.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galina L, Vecht U, Wisselink H J, Pijoan C. Prevalence of various phenotypes of Streptococcus suis isolated from swine in the U.S.A. based on the presence of muraminidase-released protein and extracellular factor. Can J Vet Res. 1996;60:72–74. [PMC free article] [PubMed] [Google Scholar]
- 8.Gottschalk G, Lacouture S, Dubreuil D. Characterization of Streptococcus suis capsular type 2 haemolysin. Microbiology. 1995;141:189–195. doi: 10.1099/00221287-141-1-189. [DOI] [PubMed] [Google Scholar]
- 9.Gottschalk M, Higgins R, Boudreau M. Use of polyvalent coagglutination reagents for serotyping of Streptococcus suis. J Clin Microbiol. 1993;31:2192–2194. doi: 10.1128/jcm.31.8.2192-2194.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottschalk M, Lebrun A, Wisselink H, Dubreuil J D, Smith H, Vetch U. Production of virulence-related proteins by Canadian strains of Streptococcus suis capsular type 2. Can J Vet Res. 1998;62:75–79. [PMC free article] [PubMed] [Google Scholar]
- 11.Hampson D J, Trott D J, Clarke L T, Mwaniki C G, Robertson I D. Population structure of Australian isolates of Streptococcus suis. J Clin Microbiol. 1993;31:2895–2900. doi: 10.1128/jcm.31.11.2895-2900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harel J, Higgins R, Gottschalk M, Bigras-Poulin M. Genomic relatedness among reference strains of different Streptococcus suis serotypes. Can J Vet Res. 1994;58:259–262. [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs A A C, van den Berg A J G, Storm P. Protection of experimentally infected pigs by suilysin, the thiol-activated haemolysin of Streptococcus suis. Vet Rec. 1996;139:225–228. doi: 10.1136/vr.139.10.225. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs A A C, van den Berg A J G, Baars J C, Nielsen B, Johannsen L W. Production of suilysin, the thiol-activated haemolysin of Streptococcus suis, by field isolates from diseased pigs. Vet Rec. 1995;137:295–296. doi: 10.1136/vr.137.12.295. [DOI] [PubMed] [Google Scholar]
- 15.Mogollon J D, Pijoan C, Murthaug M P, Kaplan E L, Collins E J, Cleary P P. Characterization of prototype and clinically defined strains of Streptococcus suis by genomic fingerprinting. J Clin Microbiol. 1990;28:2462–2466. doi: 10.1128/jcm.28.11.2462-2466.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mwaniki C G, Robertson I D, Trott D J, Atyeo R F, Lee B J, Hampson D J. Clonal analysis and virulence of Australian isolates of Streptococcus suis type 2. Epidemiol Infect. 1994;113:321–334. doi: 10.1017/s095026880005175x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitcher D G, Saunders N A, Owen R J. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol. 1989;8:151–156. [Google Scholar]
- 18.Smith H E, Rijnsburger M, Stockhofe-Zurwieden N, Wisselink H J, Vetch U, Smits M A. Virulent strains of Streptococcus suis serotype 2 and highly virulent strains of Streptococcus suis serotype 1 can be recognized by a unique ribotype profile. J Clin Microbiol. 1997;35:1049–1053. doi: 10.1128/jcm.35.5.1049-1053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sneath P H A, Sokal R R. Numerical taxonomy. W. H. San Francisco, Calif: Freeman & Co.; 1973. [Google Scholar]
- 20.Staats J J, Feder I, Okwumabua O, Chengappa M M. Streptococcus suis: past and present. Vet Res Commun. 1997;21:381–407. doi: 10.1023/a:1005870317757. [DOI] [PubMed] [Google Scholar]
- 21.Staats J J, Plattner B L, Nietfeld J, Dritz S, Chengappa M M. Use of ribotyping and hemolysin activity to identify highly virulent Streptococcus suis type 2 isolates. J Clin Microbiol. 1998;36:15–19. doi: 10.1128/jcm.36.1.15-19.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trottier S, Higgins R, Brochu G, Gottschalk M. A case of human endocarditis due to Streptococcus suis in North America. Rev Infect Dis. 1991;13:1251–1252. doi: 10.1093/clinids/13.6.1251. [DOI] [PubMed] [Google Scholar]
- 23.Van Belkum A, Kluytmans J, Van Leeuwen W, Bax R, Quint W, Peters E, Fluit A, Vandenbroucke-Grauls C, Van Den Brule A, Koeleman H, Melchers W, Meis J, Elaichouni A, Vaneechoutte M, Moonens F, Maes N, Struelens M, Tenover F, Verbrugh H. Multicenter evaluation of arbitrarily primed PCR for typing of Staphylococcus aureus strains. J Clin Microbiol. 1995;33:1537–1547. doi: 10.1128/jcm.33.6.1537-1547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vecht U, Wisselink H J, Jellema M L, Smith H E. Identification of two proteins associated with virulence of Streptococcus suis type 2. Infect Immun. 1991;59:3156–3162. doi: 10.1128/iai.59.9.3156-3162.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vecht U, Wisselink H J, van Dijk J E, Smith H E. Virulence of Streptococcus suis type 2 strains in newborn germfree pigs depends on phenotype. Infect Immun. 1992;60:550–556. doi: 10.1128/iai.60.2.550-556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vecht U, Wisselink H J, Anakotta J, Smith H E. Discrimination between virulent and non-virulent Streptococcus suis type 2 strains by enzyme-linked immunosorbent assay. Vet Microbiol. 1993;34:71–82. doi: 10.1016/0378-1135(93)90008-u. [DOI] [PubMed] [Google Scholar]