Abstract
Simple Summary
Familial cancer can be defined through the occurrence of the same cancer in two or more family members. Hereditary cancer is a narrower definition of high-risk familial aggregation through identified predisposing genes. The absence of correlation between spouses for risk of most cancers, particularly those not related to tobacco smoking or solar exposure, suggests that familial cancers are mainly due to genetic causes. The aim of the present study was to define the frequency and increased risk for familial cancer. Data on 31 of the most common cancers were obtained from the Swedish Family-Cancer Database and familial relative risks (SIRs) were estimated between persons with or without family history of the same cancer in first-degree relatives. Practically all cancers showed a familial risk, with an SIR most commonly around two, or a doubling of the risk because of family history.
Abstract
Background: Familial cancer can be defined through the occurrence of the same cancer in two or more family members. We describe a nationwide landscape of familial cancer, including its frequency and the risk that it conveys, by using the largest family database in the world with complete family structures and medically confirmed cancers. Patients/methods: We employed standardized incidence ratios (SIRs) to estimate familial risks for concordant cancer among first-degree relatives using the Swedish Cancer Registry from years 1958 through 2016. Results: Cancer risks in a 20–84 year old population conferred by affected parents or siblings were about two-fold compared to the risk for individuals with unaffected relatives. For small intestinal, testicular, thyroid and bone cancers and Hodgkin disease, risks were higher, five-to-eight-fold. Novel familial associations included adult bone, lip, pharyngeal, and connective tissue cancers. Familial cancers were found in 13.2% of families with cancer; for prostate cancer, the proportion was 26.4%. High-risk families accounted for 6.6% of all cancer families. Discussion/Conclusion: High-risk family history should be exceedingly considered for management, including targeted genetic testing. For the major proportion of familial clustering, where genetic testing may not be feasible, medical and behavioral intervention should be indicated for the patient and their family members, including screening recommendations and avoidance of carcinogenic exposure.
Keywords: familial risk, familial proportion, high-risk families, nationwide study, family-cancer database
1. Introduction
Familial cancer can be defined through the occurrence of the same cancer in two or more family members. Hereditary cancer is a narrower definition of high-risk familial aggregation through identified predisposing genes or Mendelian type of inheritance. The absence of correlation between spouses for risk of most cancers, particularly those not related to tobacco smoking or solar exposure, suggests that familial cancers are mainly due to genetic causes [1,2]. Hereditary cancer has become an important issue in oncology clinics because of the success in implementing genetic testing and/or screening methods for cancer syndromes [3,4]. For the management of hereditary cancers, it is essential to identify individuals and families at risk and provide targeted surveillance and management for affected individuals and their family members [4]. High-risk individuals may be identified based on family history and patient-specific personal factors, such as age at diagnosis and tumor phenotype [3,4,5,6]. While ascertainment of family history is still important in most management recommendations, panel sequencing has been brought forward as an early diagnostic tool [7]. Multigene panels allow for the inclusion of a battery of susceptibility genes, which is important because emerging evidence suggests a wider distribution of pathogenic mutations than previously thought. Under-recognition of clinical criteria used to identify individuals with hereditary cancer may lead to incomplete risk assessments and insufficient surveillance recommendations [3].
Risk for familial cancer is a continuum from rare high-risk to common low-risk clustering [2,8,9]. While the need for action regarding high-risk families is generally accepted and management procedures are in practice, there are no consensus guidelines for the much larger group of families with lower risk lacking a dissected genetic background. This is a dilemma at the time when genetic testing is daily news and public awareness of familial cancer and demand for counseling are increasing. For the oncology community, the dilemma culminates in lacking operational guidelines, on how to obtain and judge the family history of various cancers, and if or how to counsel family members. Even though the guidance from professional organizations on diagnostics and management of low-risk familial cancer is lacking, the guidelines for population screening recommendations do consider family histories of breast, colorectal, and prostate cancers and earlier starting ages have been recommended [10,11,12,13]. We have used age-specific risks for familial cancer to empirically define the antedated starting age for population screening of breast and prostate cancers [14,15].
In the present article, we aim to describe a nationwide landscape of concordant familial cancer by defining the relative risk depending on the affected proband (parent or sibling), number of affected probands and, for common cancers, by exact diagnostic age of familial cases. In addition, we will be able to define familial proportions for each cancer thus indicating the share of familial cancers among all cancers. The results are discussed in terms of germline genetic background and clinical implication. The analyses are based on the latest version of the Swedish Family-Cancer Database (FCD), which is a unique source for family studies comprising 16.8 million people with clinical cancer data and other detailed personal information from 1958 through 2016.
2. Results
2.1. Case Numbers, Median Ages, Incidence, and Familial Proportions
Table 1 lists background data for 9.3 million index individuals and for their 31 cancer sites (colorectal cancer not counted as colon and rectal cancers are separately included) analyzed through the years 1958 to 2016. The ICD-7 codes specify the definitions of each cancer. Overall, 525,019 cancers were diagnosed in the second generation at ages between 20 and 84 years. Breast cancer accounted for 18.2% (crude incidence 30.85/100,000) and prostate cancer 17.5% (57.82/100,000) of all patients. The median age at diagnosis for these second generation patients was 60 years, which is about 10 years lower than that for all cancers in the Swedish Cancer Registry. The median diagnostic age was highest for squamous cell skin (67 years) and prostate cancers (66 years) and lowest for Hodgkin disease (32 years) and testicular cancer (33 years). A total of 69,104 patients had concordant cancers, accounting for 13.2% of all cancers in the offspring generation. More than 1/3 of these were prostate cancers, for which the familial proportion of 26.4% was highest. This was followed by breast (17.5%), colorectal (15.7%), and lung (13.0%) cancers. For rare cancers, such as salivary gland and bone cancers the proportions were 0.2% and 0.8%, respectively. The number of all families was 449,545 and of these 53,132 (11.8%) had concordant cancers.
Table 1.
Number of cancers, median ages, incidence rates, and number of familial cancer in the 20 to 84 year old index population (N = 9,338,882) during 1958–2016.
| Cancer Site | ICD-7 Codes | Total Numbers * | Median Age | Incidence Rate per 100,000 Person Years | Family History of Concordant Cancer |
||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | IR | 95% CI | No. | Familial Proportion % | ||||
| Lip, oral cavity | 140, 141, 143, 144 | 5772 | 1.1 | 60 | 1.86 | 1.81 | 1.91 | 119 | 2.1 |
| Salivary glands | 142 | 1299 | 0.2 | 53 | 0.42 | 0.40 | 0.44 | 3 | 0.2 |
| Pharynx | 145, 146, 147, 148 | 3718 | 0.7 | 58 | 1.20 | 1.16 | 1.24 | 45 | 1.2 |
| Esophagus | 150 | 3854 | 0.7 | 63 | 1.24 | 1.20 | 1.28 | 73 | 1.9 |
| Stomach | 151 | 6980 | 1.3 | 61 | 2.25 | 2.20 | 2.30 | 411 | 5.9 |
| Small intestine | 152 | 2442 | 0.5 | 60 | 0.79 | 0.76 | 0.82 | 54 | 2.2 |
| Colorectum | 153, 154, excluding anus | 48,803 | 9.3 | 63 | 15.72 | 15.58 | 15.86 | 7650 | 15.7 |
| Colon | 153 | 30,819 | 5.9 | 64 | 9.93 | 9.82 | 10.04 | 3462 | 11.2 |
| Rectum | 154, excluding anus | 17,984 | 3.4 | 62 | 5.79 | 5.71 | 5.88 | 1054 | 5.9 |
| Liver, primary | 155.0 | 4113 | 0.8 | 63 | 1.32 | 1.28 | 1.37 | 86 | 2.1 |
| Pancreas | 157 | 9557 | 1.8 | 64 | 3.08 | 3.02 | 3.14 | 465 | 4.9 |
| Lung | 162, 163 | 33,121 | 6.3 | 64 | 10.67 | 10.55 | 10.78 | 4291 | 13.0 |
| Breast, female | 170 | 95,756 | 18.2 | 55 | 30.85 | 30.65 | 31.04 | 16,718 | 17.5 |
| Cervix | 171 | 10,542 | 2.0 | 40 | 6.94 | 6.81 | 7.08 | 223 | 2.1 |
| Endometrium | 172, 174 | 16,012 | 3.0 | 61 | 10.55 | 10.38 | 10.71 | 821 | 5.1 |
| Ovary | 175 | 11,554 | 2.2 | 55 | 7.61 | 7.47 | 7.75 | 495 | 4.3 |
| Prostate | 177 | 91,696 | 17.5 | 66 | 57.82 | 57.44 | 58.19 | 24,238 | 26.4 |
| Testis | 178 | 8272 | 1.6 | 33 | 5.22 | 5.10 | 5.33 | 158 | 1.9 |
| Kidney parenchyma | 180.0 | 8574 | 1.6 | 60 | 2.76 | 2.70 | 2.82 | 325 | 3.8 |
| Bladder | 181.0 | 18,738 | 3.6 | 64 | 6.04 | 5.95 | 6.13 | 1319 | 7.0 |
| Melanoma | 190 | 37,842 | 7.2 | 52 | 12.19 | 12.07 | 12.31 | 2715 | 7.2 |
| Skin, squamous cell | 191 | 19,014 | 3.6 | 67 | 6.12 | 6.04 | 6.21 | 1476 | 7.8 |
| Eye | 192 | 1607 | 0.3 | 57 | 0.52 | 0.49 | 0.54 | 10 | 0.6 |
| Nervous system | 193 | 21,560 | 4.1 | 51 | 6.95 | 6.85 | 7.04 | 790 | 3.7 |
| Thyroid gland, adenocarcinoma | 194 + PAD 096 | 6044 | 1.2 | 42 | 1.95 | 1.90 | 2.00 | 136 | 2.3 |
| Endocrine glands | 195 | 11,713 | 2.2 | 53 | 3.77 | 3.70 | 3.84 | 341 | 2.9 |
| Bone | 196 | 1186 | 0.2 | 39 | 0.38 | 0.36 | 0.40 | 9 | 0.8 |
| Connective tissue | 197 | 3754 | 0.7 | 52 | 1.21 | 1.17 | 1.25 | 34 | 0.9 |
| Hodgkin disease | 201 | 4064 | 0.8 | 32 | 1.31 | 1.27 | 1.35 | 57 | 1.4 |
| Non-Hodgkin lymphoma | 200, 202 | 17,197 | 3.3 | 59 | 5.54 | 5.46 | 5.62 | 743 | 4.3 |
| Myeloma | 203 | 5618 | 1.1 | 63 | 1.81 | 1.76 | 1.86 | 140 | 2.5 |
| Leukemia | 204–209 | 14,617 | 2.8 | 60 | 4.71 | 4.63 | 4.78 | 643 | 4.4 |
| All above | 525,019 | 100.0 | 60 | 169.12 | 168.66 | 169.58 | 69,104 | 13.2 | |
*: Total number of cancers was 552,953 including 27,934 diverse rare cancers, which were not included in this study. IR: Incidence rate per 100,000 person years; PAD: Pathological anatomical diagnosis.
2.2. Risk Estimates for Offspring of Affected Patients and for Siblings
Familial standardized incidence ratio (SIR, also referred to as familial relative risk) for offspring of affected parents was increased for 29/31 cancers; the exceptions being salivary gland and pharyngeal cancers (Table 2). We show data only for combined sexes as none of the sex-specific SIRs were significant. The highest SIR for offspring of affected parents was observed for bone cancer (6.11), followed by small intestinal (5.37) and testicular cancer (4.56). For common familial cancers, the SIRs were 2.05 for prostate, 1.74 for breast and 1.66 for colorectal cancers. For the latter, risk for colon (1.79) was somewhat higher (95%CIs overlapped) than that for rectal cancer (1.61). The highest relative risks between siblings were observed for bone cancer (8.23), Hodgkin disease (6.77), and small intestinal cancer (6.11).
Table 2.
Familial risk for index persons whose parents or siblings were diagnosed with concordant cancer.
| Cancer Site | Only Parent Diagnosed with Concordant Cancer | Only Sibling Diagnosed with Concordant Cancer | ||||||
|---|---|---|---|---|---|---|---|---|
| O | SIR | 95% CI | O | SIR | 95% CI | |||
| Lip | 73 | 1.78 | 1.39 | 2.24 | 46 | 2.44 | 1.79 | 3.26 |
| Salivary gland | 3 | 1.80 | 0.34 | 5.34 | ||||
| Pharynx | 15 | 1.66 | 0.93 | 2.75 | 30 | 3.88 | 2.62 | 5.55 |
| Esophagus | 43 | 2.37 | 1.71 | 3.19 | 30 | 2.91 | 1.96 | 4.16 |
| Stomach * | 307 | 1.66 | 1.48 | 1.85 | 94 | 2.74 | 2.22 | 3.36 |
| Small intestine | 31 | 5.37 | 3.65 | 7.63 | 21 | 6.11 | 3.78 | 9.36 |
| Colorectum | 4981 | 1.66 | 1.62 | 1.71 | 2252 | 1.76 | 1.69 | 1.84 |
| Colon | 2249 | 1.79 | 1.71 | 1.86 | 1060 | 1.96 | 1.84 | 2.08 |
| Rectum | 696 | 1.61 | 1.49 | 1.73 | 341 | 1.69 | 1.52 | 1.88 |
| Liver, primary | 56 | 2.30 | 1.73 | 2.98 | 28 | 2.65 | 1.76 | 3.84 |
| Pancreas | 320 | 1.99 | 1.78 | 2.22 | 135 | 2.17 | 1.82 | 2.57 |
| Lung * | 2247 | 1.86 | 1.79 | 1.94 | 1875 | 2.50 | 2.39 | 2.62 |
| Breast | 9154 | 1.74 | 1.70 | 1.77 | 6573 | 1.73 | 1.69 | 1.77 |
| Cervix | 155 | 1.51 | 1.28 | 1.76 | 68 | 1.58 | 1.23 | 2.01 |
| Endometrium | 511 | 1.94 | 1.78 | 2.12 | 288 | 1.82 | 1.61 | 2.04 |
| Ovary | 319 | 2.32 | 2.08 | 2.59 | 162 | 2.31 | 1.97 | 2.70 |
| Prostate * | 11,791 | 2.05 | 2.01 | 2.09 | 9897 | 2.38 | 2.33 | 2.43 |
| Testis | 51 | 4.56 | 3.39 | 5.99 | 107 | 5.56 | 4.55 | 6.72 |
| Kidney | 211 | 1.77 | 1.54 | 2.03 | 108 | 2.38 | 1.95 | 2.87 |
| Bladder | 835 | 1.76 | 1.64 | 1.88 | 459 | 1.94 | 1.77 | 2.12 |
| Melanoma | 1360 | 2.37 | 2.24 | 2.49 | 1260 | 2.45 | 2.32 | 2.59 |
| Skin | 991 | 1.98 | 1.86 | 2.11 | 426 | 1.89 | 1.72 | 2.08 |
| Eye | 8 | 3.30 | 1.41 | 6.53 | 2 | 1.42 | 0.13 | 5.24 |
| Nervous system | 443 | 1.49 | 1.36 | 1.64 | 320 | 1.64 | 1.47 | 1.83 |
| Thyroid gland, adenocarcinoma | 71 | 3.78 | 2.95 | 4.77 | 65 | 4.98 | 3.84 | 6.35 |
| Endocrine glands | 201 | 2.03 | 1.76 | 2.34 | 124 | 2.00 | 1.67 | 2.39 |
| Bone | 5 | 6.11 | 1.93 | 14.36 | 4 | 8.23 | 2.14 | 21.29 |
| Connective tissue | 22 | 1.76 | 1.10 | 2.67 | 10 | 1.61 | 0.77 | 2.97 |
| Hodgkin disease * | 23 | 2.87 | 1.81 | 4.31 | 34 | 6.77 | 4.69 | 9.47 |
| Non-Hodgkin lymphoma | 443 | 1.66 | 1.51 | 1.82 | 294 | 1.80 | 1.60 | 2.02 |
| Myeloma | 96 | 1.92 | 1.56 | 2.35 | 44 | 2.00 | 1.45 | 2.69 |
| Leukemia | 409 | 1.83 | 1.66 | 2.02 | 217 | 1.97 | 1.72 | 2.26 |
| All above * | 38,120 | 1.86 | 1.84 | 1.88 | 26,374 | 2.07 | 2.04 | 2.09 |
O = Observed; SIR = Standardized incidence ratio; CI = Confidence intervals. *: SIRs between cases with parental and sibling probands were significant (non-overlapping 95%CIs).
Familial risks between offspring and parents and between siblings may have different causes, as for the latter recessive inheritance and shared childhood environment may play a role. Overall, the relative risk for siblings was higher (SIR 2.07) than it was for offspring and parents (1.86). For individual cancers, a significantly higher risk for siblings than for offspring of affected parents was noted for a total of four cancers. Hodgkin disease was characterized by a large difference (6.77 vs. 2.87), as was stomach cancer (2.74 vs. 1.66). The other sites with a significant difference were the lung and the prostate.
For bone and connective tissue cancer with previously unreported familial risks, we checked SNOMED histology, available in the Swedish Cancer Registry since 1993. For the nine bone cancers in Table 2, four were chondrosarcomas and two were osteosarcomas. Probands of two chondrosarcoma patients were also diagnosed with chondrosarcoma; for other probands, no SNOMED data were available (diagnosis before 1993). Among the 36 connective tissue cancer patients, eight were leiomyosarcomas, five were liposarcomas, and five were sarcomas not otherwise specified; the remaining cases were diverse sarcomas. Only two proband diagnoses matched case diagnoses but most SNOMED data for probands were missing.
2.3. Increased Risk When Several Family Members Were Affected
In Table 3, we compare concordant familial risks for those who have one first-degree relative (FDR) diagnosed with cancer to those who have two or more affected relatives. SIRs for persons with one affected FDR are the composite of SIRs from Table 2, with an overall SIR of 1.94. For persons with two or more affected relatives, the overall SIR was 3.33. However, such high-risk, ‘multiplex’ families were found for only 20 cancers (including colorectal cancer). Almost all SIRs for multiplex families were significantly higher than those for in single FDR families. Some SIRs were remarkably high, such as 112.36 for small intestinal and 55.25 for connective tissue cancers. Even for common cancers, such as colon (3.64), lung (3.42), and prostate (3.74) cancers, the SIRs were well above those with a single FDR. However, the total number of cases accounting for the multiplex families was only 4532 of all 69,104 familial cases (6.6%). The proportion for prostate cancer in such high-risk families was 10.5% (2550/24,238), for breast and colorectal cancers, these were 5.5% for both. Prostate cancer accounted for 56.4% of cases in multiplex families, followed by breast (20.1%) and colorectal (9.2%) cancers.
Table 3.
Concordant familial risks when one or at least two probands were diagnosed with cancer.
| Cancer Site | One Family Member Diagnosed with Concordant Cancer | Two or More Family Members Diagnosed with Concordant Cancer | ||||||
|---|---|---|---|---|---|---|---|---|
| O | SIR | 95% CI | O | SIR | 95% CI | |||
| Lip | 119 | 2.00 | 1.66 | 2.40 | ||||
| Salivary gland | 3 | 1.19 | 0.22 | 3.51 | ||||
| Pharynx | 45 | 2.70 | 1.97 | 3.62 | ||||
| Esophagus | 73 | 2.59 | 2.03 | 3.25 | ||||
| Stomach | 401 | 1.83 | 1.65 | 2.01 | 10 | 5.55 | 2.64 | 10.25 |
| Small intestine | 52 | 5.64 | 4.21 | 7.40 | 2 | 112.36 | 10.59 | 413.21 |
| Colorectum | 7233 | 1.70 | 1.66 | 1.74 | 417 | 2.76 | 2.51 | 3.04 |
| Colon | 3309 | 1.84 | 1.78 | 1.90 | 153 | 3.64 | 3.08 | 4.26 |
| Rectum | 1037 | 1.64 | 1.54 | 1.74 | 17 | 2.20 | 1.28 | 3.52 |
| Liver, primary | 84 | 2.40 | 1.92 | 2.98 | 2 | 12.57 | 1.19 | 46.23 |
| Pancreas | 455 | 2.04 | 1.86 | 2.24 | 10 | 4.96 | 2.36 | 9.15 |
| Lung | 4122 | 2.11 | 2.05 | 2.17 | 169 | 3.42 | 2.93 | 3.98 |
| Breast | 15,805 | 1.74 | 1.71 | 1.76 | 913 | 2.50 | 2.34 | 2.67 |
| Cervix | 223 | 1.54 | 1.35 | 1.76 | ||||
| Endometrium | 799 | 1.90 | 1.77 | 2.03 | 22 | 5.58 | 3.49 | 8.46 |
| Ovary | 481 | 2.32 | 2.12 | 2.54 | 14 | 8.99 | 4.90 | 15.13 |
| Prostate | 21,688 | 2.20 | 2.17 | 2.23 | 2550 | 3.74 | 3.60 | 3.89 |
| Testis | 158 | 5.24 | 4.45 | 6.12 | ||||
| Kidney parenchyma | 319 | 1.94 | 1.73 | 2.16 | 6 | 5.17 | 1.86 | 11.33 |
| Bladder | 1294 | 1.82 | 1.72 | 1.92 | 25 | 2.48 | 1.60 | 3.67 |
| Melanoma | 2620 | 2.41 | 2.32 | 2.50 | 95 | 5.68 | 4.59 | 6.94 |
| Skin | 1417 | 1.96 | 1.86 | 2.06 | 59 | 4.60 | 3.50 | 5.94 |
| Eye | 10 | 2.62 | 1.25 | 4.83 | ||||
| Nervous system | 763 | 1.55 | 1.44 | 1.67 | 27 | 6.24 | 4.11 | 9.09 |
| Thyroid gland, adenomcarcinoma | 136 | 4.33 | 3.63 | 5.12 | ||||
| Endocrine glands | 325 | 2.02 | 1.81 | 2.26 | 16 | 14.82 | 8.45 | 24.11 |
| Bone | 9 | 6.93 | 3.14 | 13.20 | ||||
| Connective tissue | 32 | 1.71 | 1.17 | 2.42 | 2 | 55.25 | 5.21 | 203.18 |
| Hodgkin disease | 57 | 4.40 | 3.33 | 5.71 | ||||
| Non-Hodgkin lymphoma | 737 | 1.71 | 1.59 | 1.84 | 6 | 1.51 | 0.54 | 3.31 |
| Myeloma | 140 | 1.97 | 1.66 | 2.32 | ||||
| Leukemia | 626 | 1.88 | 1.73 | 2.03 | 17 | 5.03 | 2.92 | 8.06 |
| All above | 64,572 | 1.94 | 1.93 | 1.96 | 4532 | 3.33 | 3.24 | 3.43 |
O = Observed; SIR = Standardized incidence ratio; CI = Confidence intervals.
2.4. Age-Incidence for Familial and Non-Familial Cancer
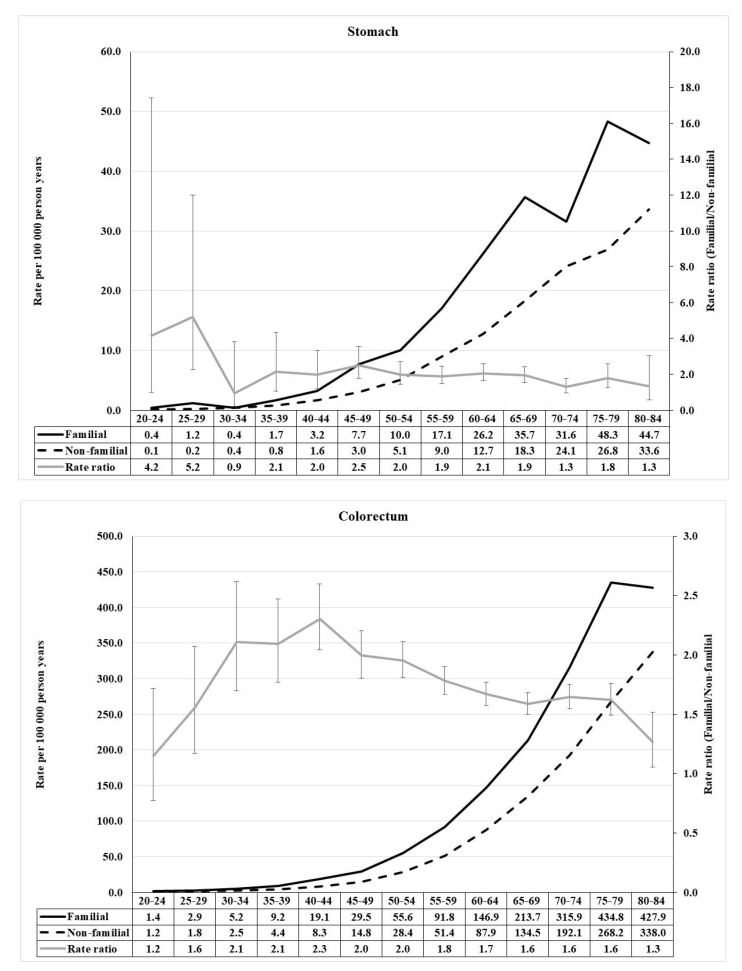
We selected 10 common cancers for a detailed study of age-incidence relationships. Familial risk was defined through FDRs. The details of the calculations for these 10 cancers (rate ratios, and 95%CIs) are shown in Supplementary Tables S1–S5. The first two cancer incidences of the stomach and colorectum are shown in Figure 1. Incidence rates and rate ratios (RRs, familial/non-familial) are shown under each panel. For stomach cancer, an early onset peak reachedRR of 5.2, while in the early middle age it was two and then declined at higher ages. Even for this rare familial cancer, the RRs were significant in almost all five-year age brackets. For colorectal cancer, the highest RR at age 40–44 reached 2.3, followed by a slow decline to 1.3.
Figure 1.
Age-specific incidence rate and rate ratio of stomach (upper) and colorectal (lower) cancer in population with and without family history of concordant cancer.
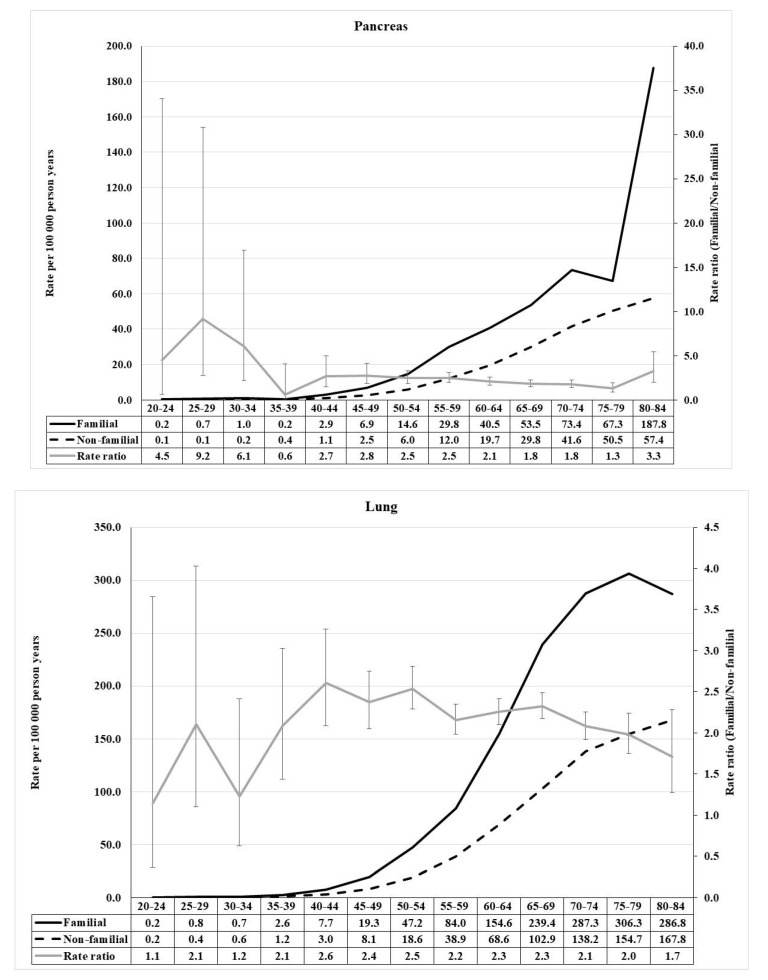
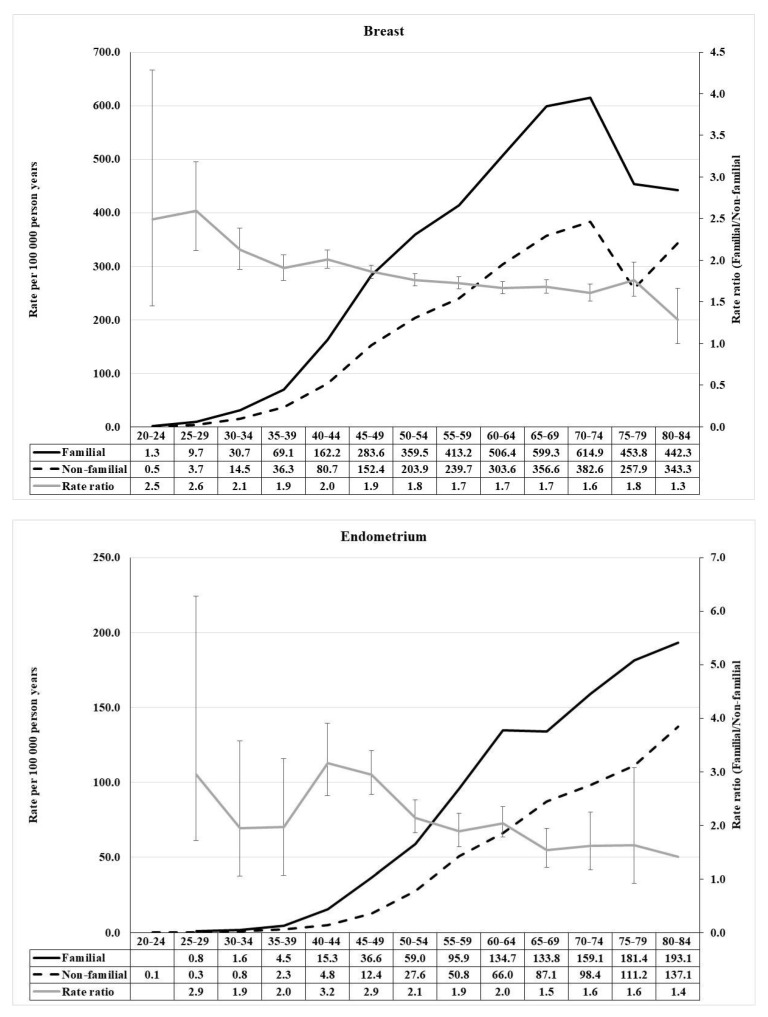
For pancreatic cancer, a prominent early age component reached RR of 9.2, while the middle age RR was below three and declined with age (Figure 2). For lung, the RRs peaked at 2.6 at age 40–44, and declined at higher age. Breast cancer showed a modest early onset maximum (2.6) at age 25–29, followed by a steady decline (Figure 3). Endometrial cancer was characterized by an early onset (2.9) and a higher (3.2) early middle age component.
Figure 2.
Age-specific incidence rate and rate ratio of pancreas (upper) and lung (lower) cancer in population with and without family history of concordant cancer.
Figure 3.
Age-specific incidence rate and rate ratio of female breast (upper) and endometrium (lower) cancer in population with and without family history of concordant cancer.
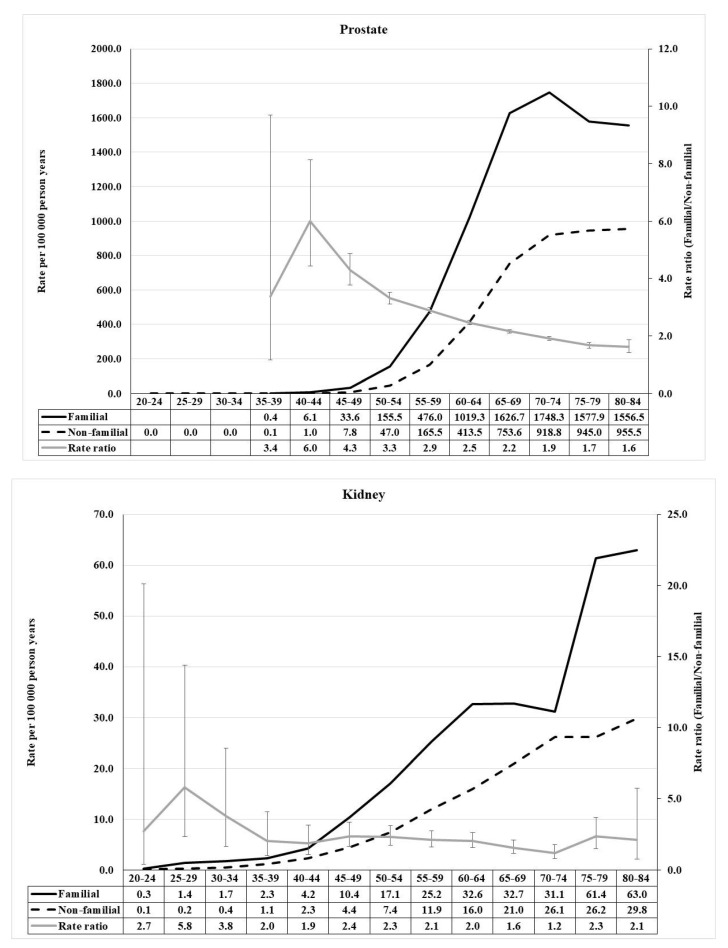
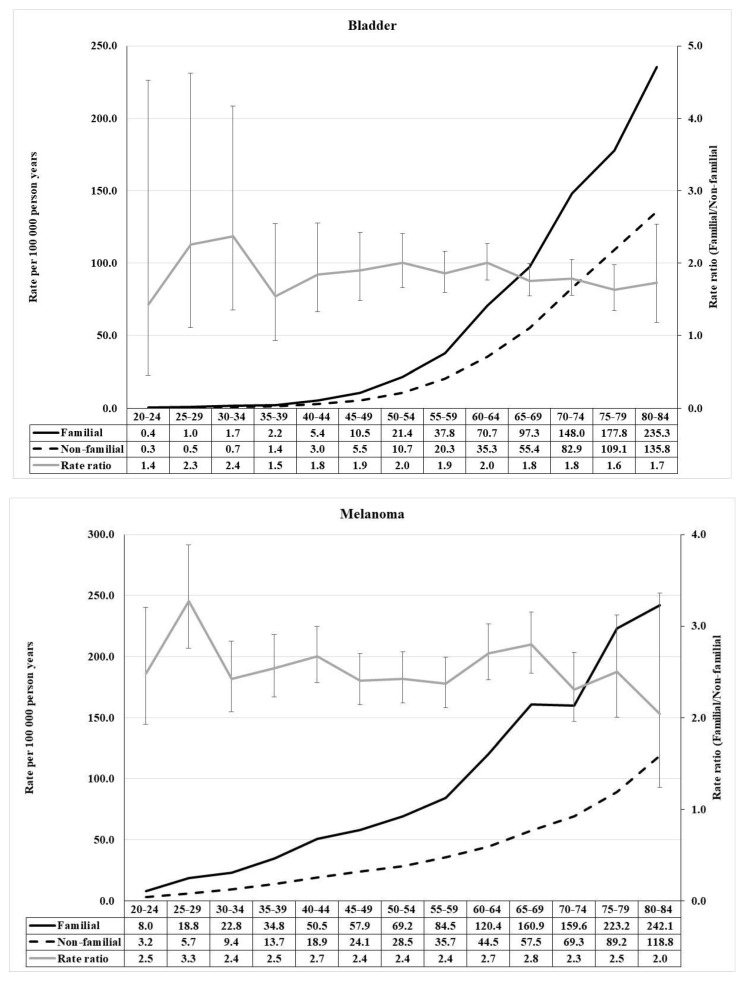
The distributions of RR for prostate and kidney cancers were similar, with the exception that the kidney cancer peak of RR 5.8 was at age 25–29 while the one of RR 6.0 for prostate cancer was at age 40–44 (Figure 4). Furthermore, the shapes of RR distributions for bladder cancer and melanoma showed similarities, RRs reaching 2.4 for bladder cancer and 3.3 for melanoma (Figure 5).
Figure 4.
Age-specific incidence rate and rate ratio of prostate (upper) and kidney (renal parenchyma) (lower) cancer in population with and without family history of concordant cancer.
Figure 5.
Age-specific incidence rate and rate ratio of bladder (urinary bladder) cancer (upper) and melanoma (lower) in population with and without family history of concordant cancer.
3. Discussion
The Swedish FCD is the largest family cancer database in the world, with premium features of nationwide family structures and practically complete data on cancers from the high-quality cancer registry [16,17,18]. The results are not biased by selection of families or inaccurate reporting of cancer in family members. These features guarantee the novelty of the present results: unbiased familial risk estimates and familial proportions even for rare cancers, by the type and number of probands. We discuss below the implications of these findings in terms of germline genetic landscape of familial cancer and the possible clinical impact.
3.1. Familial Risk Is a Feature of (All) Cancers
Cancer risks in a 20–84 year old population conferred by affected parents or siblings were about two-fold compared to the risk for individuals with unaffected relatives. The only site with no familial risk was salivary glands presenting only with three familial pairs. In a few sites, the risks were high, five-to-eight-fold, including cancers for which high risks were previously known (small intestinal, testicular, and thyroid cancers and Hodgkin disease) and a novel site (adult bone cancer) [8,9,19]. Furthermore, familial risks for lip and oral cavity, pharyngeal, and connective tissue cancers are novel from the Swedish FCD. Lip, oral cavity, and pharynx have been previously covered as part of upper aerodigestive tract but specific analysis of pharynx did not show significant results [20]. However, positive results have been published from case-control studies and from a cohort study [21,22,23,24,25]. Primary bone and connective tissue cancers present with diverse histologies, but we could not properly test if family members shared histological types of tumors because of missing data from years before 1993. However, two pairs of family members shared chondrosarcoma histology for bone cancer (which also includes tumors of cartilage).
Familial risks were higher for persons whose siblings were affected compared to those whose parents were affected. The difference was significant for a total of four cancers. Such results may indicate recessive genetic effects or deleterious influence of shared environmental risk factors during childhood or adolescence [26]. Recessive effects require mutations in both alleles but monoallelic mutations may also be a risk factor, as reported for the MUTYH gene [27]. Panel sequencing has revealed common monoallelic MUTYH mutations in colorectal, prostate, and lung cancers [28].
A non-genetic explanation for increased cancer risk is the family member’s concern for his/her own cancer risk when a relative is diagnosed with cancer. We have previously shown that cancer diagnosis in one family member leads to a higher chance of cancer diagnosis in another family member but the increase was limited to the same year. This transient increase was observed for many cancers, including colorectal, lung and prostate cancers; such detection bias was higher for siblings than for offspring of affected parents [29]. In the present study, the increased sibling risk for colon cancer could be related to detection bias. For stomach cancer, a higher concordance within a generation than between generations may be explained by a sharply declining incidence rate [30]. For the high sibling risk of lung cancer, early smoking start among siblings may contribute and facilitate persistent addiction [31]. For prostate cancer, recessive genetic effect cannot be excluded but the above detection bias and uptake of prostate specific antigen testing may preferentially influence brothers [29,32]. For Hodgkin lymphoma, shared childhood social environment has been proposed as a risk factor [33].
We showed in Table 3 that the risks increased by the number of affected relatives. For some common cancers, such as breast, colorectal, and prostate cancers, it has been possible to identify families with many affected relatives, and hence high risks have been shown in earlier studies [2,34]. In the extreme, for prostate cancer risk for a sibling with four affected brothers the cumulative risk was 50%, which would imply dominant inheritance with 100% penetrance [34].
3.2. Familial Proportions
Our data provide an answer to the question, ‘how common is familial cancer’. A casual reader may feel that such data are already known. We have been curious about how well this is known and reviewed the 18 chapters on site-specific cancers in the ‘classical’ multi-author treatise,’ The Genetic Basis of Human Cancer’ [35]. Half of the chapters made a reference to family history, but only two specified what was meant [36]. Unqualified statements about family history are commonplace even in expert reviews on specific cancers; unreferenced statements are made ‘X% of the patients have a family history of this cancer’, and such figures circulate in subsequent publications as proven facts [36]. Even if proper citations are given the accuracy of data is not controlled. Many familial risks reported in the literature are based on anecdotal reports on cancers in family members without case confirmation. The accuracy of reporting a family history is notoriously inaccurate for many internal cancers [37,38,39].
Familial proportions are a moving target because they depend on the magnitude of familial risk and population prevalence of each cancer [36]. Thus, it is not surprising that the highest proportions were found for common cancers. Yet, it may be surprising that for prostate cancer familial proportion was as high as 26.4% (every fourth prostate cancer is diagnosed in families of prostate cancer patients), and that for all concordant cancer it was as high as 13.4%.
3.3. Genetic Implications
The contribution of germline genetics to the prevalence of cancer is largely cancer specific, ranging from those with prominent high-risk genes on a polygenic background (colorectal and breast cancers and melanoma) to those with a predominant low-risk polygenic background (prostate cancer), and to those with limited contribution by either types of genes (lung and bladder cancer) [40,41,42]. Early onset is a characteristic of heritable cancer, and is also a prominent feature of most familial cancers [43]. Nevertheless, familial clustering is not limited by age and most familial cases may be found at ages when the background incidence is highest [44]. Family studies do not tell the reason for familial clustering but level of risks and detailed age-incidence data may suggest involvement of known germline genetics for the particular cancers.
Among the 10 cancers with detailed age-incidence data, clear early onset risks for stomach, pancreatic, and kidney cancers suggest contribution by known genes. Hereditary stomach cancer is associated with mutations in the CDH1 gene and it is also manifested in Lynch (hereditary nonpolyposis colorectal cancer) syndrome related to mutations in mismatch repair genes [45,46,47,48]. Next-generation sequencing studies have identified rare germline variants in several other genes, including BRCA2 and other DNA repair genes [49,50,51]. In kidney cancer, the contribution of von Hippel–Lindau syndrome is a likely explanation for the early onset risk component [52]. In pancreatic cancer, the early onset risk could be due to high risk genes such as CDKN2A, BRCA1, and BRCA2, or some known rare genes, while the second peak at age 40–49 matches the age of onset of Lynch syndrome [6]. Lynch syndrome is most likely prominently contributing to the main colorectal cancer peak at ages 30–44 years, to the main endometrial cancer peak and to bladder cancer susceptibility [53,54,55]. A recent New York study on bladder (urothelial) cancer found pathogenic variants in 14% of patients, and MSH2 and BRCA2 mutations were significantly associated with risk with odds ratios of about four [56]. CDKN2A is the main predisposing gene also for melanoma and it contributes to the genetic architecture of melanoma as mutations are found in about 30% of patients in rare families of three or more affected individuals [57]. According to a Swedish study, CDKN2A positive families accounted for 11.5% of all melanoma families, and the positivity correlated with the number of affected individuals; in the positive families a median of six melanomas were diagnosed compared to two in mutation negative families [58]. However, the broad age range that we observed suggests that other genes are involved including those associated with skin pigmentation, and hence with possible interactions with ultraviolet radiation [59]. The same kind of genetic background may also apply to breast cancers, for which BRCA1 and BRCA2 are prominent predisposing genes for the early onset component but at higher ages other genes are likely to contribute [60].
Lung cancer has the strongest environmental component among these cancers, as shown by spouse correlation [1,2]. In a modeling study, we have estimated, based on the assumptions of heritability of the smoking habit, that shared smoking would contribute to a familial risk of 1.19, which is well below the SIR of 2.14 observed here [61]. However, the problems with such estimates of genetic and environmental effects is that interactions cannot be reliably assessed and nicotine dependence plays an important role in tobacco carcinogenesis [62]. An interesting aspect of the age-incidence relationships for lung cancer was the relatively early peaking and modestly declining risk towards old age, implicating polygenic inheritance and probable interactions with smoking. Genetics of prostate cancer have shown population specific features, particularly related mutations in HOXB13, NBN, and CHEK2; however mutations in BRCA2, mismatch repair genes, BRCA1, and ATM appear to contribute to risk in many populations [63]. The detected high-risk peak at age 40–44 years could be due to HOXB13 and other of these genes [63].
3.4. Clinical Interventions and Familial Risk
In considering familial cancer, one needs to keep in mind that for certain cancers clinical interventions have probably influenced familial risks in Sweden. For melanoma prevention, national multi-level campaigns were started already in the late 1980s. These targeted physicians, nurses, and the general population [64]. Different population screening approaches were tested and, while being overall beneficial, they also showed that these programs did not reach people in all walks of life [65,66]. Genetic counseling and genetic testing for Lynch syndrome in Sweden was reviewed over a period of 20 years from 1994 to 2014, and 369 families were identified, which the authors assumed to account for one quarter of national Lynch families [67]. Another study reported over 500 Lynch families, and sequenced a cohort of 572 consecutive colorectal cancer patients for mismatch repair gene mutations; pathogenic variants were found in 1.9% of patients, suggesting that universal testing could be equally effective in identifying mutation carriers as the Bethesda criteria [68]. A recent Swedish study on more than 5000 unselected breast cancer patients reported that the prevalence of pathogenic BRCA1/2 mutations was 1.8%; six in ten BRCA1/2 mutation carriers were not detected by selective clinical screening of individuals [69]. Only 8% of all patients had been part of a previous clinical screening but their share of the detected mutations was 38%. For prostate cancer, Sweden resisted against prostate specific antigen testing, but once it started in the late 1990s, a huge incidence peak for prostate cancer was observed [32].
How did the above clinical and screening interventions change familial risks? An answer is offered by a comparison of our current study with our earliest comprehensive analysis of familial risk for which cancers were followed-up until 1999; we can assume that, with the exception of melanoma, none of the above interventions played a role for those results [70]. Melanoma risks for offspring with affected parents showed exactly the same SIR of 2.41 then and now. For colorectal and breast cancer the old SIRs were about one decimal unit higher than the present ones, which would be consistent with the younger age of the index population in the old study. For endometrial cancer, there was a larger decrease in the current SIR (old 2.86 to current 1.98), which was also true for prostate cancer (2.71 to 2.08). As for these cancers, familial risk decreased markedly with increasing age (cf. Figure 3 and Figure 4), and it is likely that the age truncation was the main cause for time-dependent decrease in risk. The differences were larger for sibling risks but the siblings were very young (below 62 years) in the old study.
The somewhat surprising conclusion is that the interventions have not influenced familial risks. The main reason may be that the high-risk families account for a small proportion of all familial cancers (6.6% of families had more than two concordant cancers), which was one of the main conclusions of the present study. Another reason is that only a proportion of affected families have been identified as referred to above for Lynch syndrome. Finally, prevention of familial risk is effective only when intervention is successful before cancer diagnosis in yet unaffected carriers. It is not known if preventive counseling of healthy family members in the identified families has been of high priority in Sweden.
3.5. Clinical Implications of Familial Risk
The population attributable fraction defines the proportion of a disease that would be prevented if the risk factor or the cause could be avoided, and it is often used in ranking causes of diseases. Familial risk is an important cause of cancer, and it has been ranked as the third most common population attributable fraction after tobacco smoking and unhealthy diet [8]. For known environmental causes of familial clustering, including smoking, alcohol, solar irradiation, infections and other causes, primary prevention or avoidance of risk factors is the most effective method of risk reduction. For genetic causes, however, primary prevention is more difficult and early detection may be the method of choice in catching precursor lesions. Family history has been and still is an important guide in identification of Lynch, breast-ovarian, or other cancer syndromes and in directing clinical management and counseling [3,4,71,72]. However, the power of family history is weakened when penetrance of the genetic effect is low and when families are small. This raises the question about utility of family histories as a guide to genetic background at the time when genetic testing through gene panels and next generation sequencing has entered the oncology routine. Below we discuss some clinical scenarios about management of familial/heritable cancer.
Family history may be redundant if management of cancer patients is based on universal genetic testing with panel (or other) sequencing methods of sufficient sensitivity and specificity [28]. While such approaches are able to find more pathogenic variants than guideline-directed targeted testing, they create a number of problems germane to panel sequencing, which include variants of uncertain significance (VUS), increasing need for counseling and costs [4,7]. VUS increase with increasing panel sizes, and more than one VUS may be detected in a single patient. Counseling is necessary before and after testing, and dealing with VUS is an unsolved problem [4,7]. A practical hindrance to large scale applications of panel sequencing is that the personnel and financial demands would be beyond most health care systems. Offering panel tests also to unaffected family members (cascade testing) would add another dimension to the costs. Even targeted sequencing demands health care resources, and it has been estimated that fewer than 10% of prevalent BRCA1/2 or mismatch repair mutation carriers have been identified in the UK [42,73]. Universal sequencing of cancer patients or of the total population may become more tractable when alternative technologies, such as array-based sequencing methods are being developed [42].
Another scenario, which we proposed based on the present results, is to intensify the application of family and personal history (age of onset and multiple primary cancers, considering also relatives) with separate strategies targeting high-risk families (two or more diagnosed family members and/or known predisposing genes) and low-risk families (one affected family member). The present high-risk (multiplex) families accounted for only 6.6% of all familial cancer cases. Colorectal and breast cancer accounted for 25.5% of these multiplex families, and these and ovarian cancer are the only cancers for which genetic testing has been organized. Predisposing genes for many other high-risk cancers are known, but genetic testing is done only on a case-by-case basis. Thus, there is scope to expand targeted high-risk testing to a larger number of high-risk families considering the most likely predisposing genes, and the likely benefits of gene identification in terms of therapeutic options and preventive counseling of family members. Oncology clinics should continue in managing high-risk families for which genetic testing is justified and counseling of patients and their relatives is required. Moreover, when a mutation test is negative, an appropriate surveillance plan should be developed considering the level of risk in each family. Starting age of screening for unaffected family members should be accordingly recommended [34,74]. In high-risk families multiple primaries are common and these should be considered in counseling. Managing all types of high-risk familial patients and their family members should be built into the clinical care routine with dedicated staff.
For the >90% of familial cancers with a relative risk of about two, genetic testing may not be indicated. However, it is important to be sensitive to rare cancers and small families because a single family member with a rare cancer may signal high risk. All cancer patients are at a risk of second primary cancers and family history is a risk factor for that particular cancer to be diagnosed as second primary cancer, observed for diverse primary cancers, such as breast cancer, melanoma and non-Hodgkin lymphoma [75,76,77]. Thus, patients need counseling about risk factors, surveillance and possible screening tailored to his/her primary cancer. If that cancer may appear as multiple primaries, such as breast or colorectal cancers or melanoma, the risk of second primaries at these sites should be considered in the management plan. Ideally, counseling should be extended to family members but reaching them may be problematic and could perhaps best be conducted through prevention campaigns at primary health care centers [78].
The entrance of large-scale DNA sequencing into oncology clinics may herald a paradigm change towards technology driven patient care as we described above. Powerful technologies often result in hype of promising health benefits which, however, remain to be demonstrated. Our proposed strategy is an extension of the existing practice, aiming at balancing the population burden of familial cancer between rare high-risk individuals with lower population risk. We have to admit that, in spite of recommendations by professional organizations, the evidence for targeted screening of hereditary/familial cancer is not overwhelming. The first and only controlled trial in Lynch syndrome, which was conducted in Finland, showed a significant reduction in subsequent colorectal cancers compared to the control arm of no screening [79]. For ethical reasons, no more such trials have been conducted but results from prospective surveillance have supported the reduction in cancers and deaths [80]. A systematic review on evidence relating to management of BRCA1/2-related cancers in women concluded that no single study evaluated the effectiveness of risk assessment, genetic counseling, or genetic testing in reducing incidence and mortality in of BRCA1/2-related cancers; a total of 103 studies were evaluated [81]. For melanoma, an Australian expert group found some justification of screening of high-risk groups but little supporting evidence [82]. Whichever strategies are followed in fighting familial cancer, more resources are required. The beneficial population impact should be a fair target for health policy decision makers.
3.6. Limitations and Need for Future Studies
The main limitations in this population-based approach are the descriptive nature of the results confined to the population under study. Most causes for familial clustering are unknown, and even though circumstantial evidence suggests involvement of known and unknown genes (or gene-environment interactions), their role remains speculative. We lack information on potential confounders and causes of familial clustering, including lifestyle factors. However, adjustment for socio-economic factors helps mitigate their influence [83]. In Discussion, we speculate about the predisposing genes that may contribute to the observed familial risks; we included only some of the most common predisposing genes as for rarer genes it would have been necessary to carry out detailed analysis, including discordant cancers and histologies which were beyond the present scope. Another limitation is that the population under study is not ‘fully aged’, i.e., the 20–84 year old index population is a segment of the total population, and the median age of cancers in the index population was some 10 years below that in the Swedish Cancer Registry [84]. This limited the use of children of the index persons as FDRs. Thus, true multigenerational studies are not possible in the current FCD, lacking information on past generations, which are available in population databases in Iceland and Utah [85,86,87]. In future studies, it will be possible to test more complex and extended family structures but this does not change the truth that genetic sharing is halved in every added generation. Thus, analysis by FDR is most powerful in showing genetic effects. Our experience from using a much smaller FCD up to 20 years ago, as discussed above, showed that the magnitude of familial risk was only marginally changed, dependent on age of the population. Yet, the benefit of the current expanded FCD was the statistical power of finding familial associations in rare cancers [70,88].
4. Conclusions
Next-generation sequencing methods have made germline analysis of cancer more tangible but also revealed complexities and new dilemmas. Pathogenic or likely pathogenic mutations in BRCA1/2, mismatch repair and other cancer predisposing genes are found in many cancers for which genetic association studies are not available, leaving the causal role of mutations open. The familial landscape of cancer, which we defined through this largest family study yet published, demarcated a familial proportion of 13.2% of all cancers, considering concordant cancer in FDR; 6.6% of cancer families (0.9% of all cancers) included at three discordant cancer patients. Among this 6.6%, systematic genetic testing is available only for a quarter of patients, and this share should be expanded based on family and personal histories. For the major proportion of familial clustering, genes remain unknown or include low-risk genes with possible environmental interactions; some familial clustering may be due to pure non-genetic factors including diet, tobacco and alcohol use and environmental carcinogens such as pollution or radon. Nevertheless, medical and behavioral intervention may be indicated, including screening recommendations and avoidance of carcinogenic exposure. The readily available information of family history deserves more attention from first diagnosis through referral to the oncology clinic, enabling establishment of clinical counseling strategies, individually tailored to screening and prevention needs of patients and their family members.
5. Methods
5.1. Data Source and Patients
The FCD was used to define the index population (second generation or offspring generation) born after 1931 and the parental generation who were parents of the index population. These two generations were used to estimate familial cancer risks for the 31 most common cancer sites and, in addition, colorectal cancer. The diagnostic age was defined to over 19 years in order to exclude childhood cancers with divergent etiologies compared to adult cancers [89]. FCD comprises information collected at the Center for Primary Health Care Research, Lund University, Malmö, from nationwide databases of the Multigeneration Register, censuses and death notifications maintained at Statistics Sweden, and information from the Swedish Cancer Registry. In its latest update from 2016, FCD includes 16.8 million individuals, where all people born in Sweden from 1932 onwards (the offspring generation) were linked to their biological parents (the parental generation). Register linkages were performed at the individual level via the national 10-digit civic registration number. In the linked dataset, civic registration numbers were replaced with serial numbers to ensure anonymity.
A total of 2,013,623 medically verified cancers were recorded in the Cancer Registry from 1958 to 2016. The 7th revision of the International Classification of Diseases (ICD-7) and later revisions were used to identify the cancer type. SNOMED histology was used in some analyses. In order to investigate cancer risks for offspring of affected parents as well as risk shared by siblings, all individuals with identified parents were selected for the analysis, totaling in 9,338,882 index individuals. Among them, 552,953 were diagnosed with one of the cancer sites under study.
5.2. Relative Risk Estimation
Familial risk was assessed for the offspring generation by estimating standardized incidence ratios (SIRs) as the ratio of observed (O) to expected number of cases. The observed numbers were cancers in the 20–84 year old index populations, whose FDRs were diagnosed with cancer; in the case of siblings, the observed number included all affected siblings. The expected numbers were calculated for all individuals without cancer in family members (i.e., the Swedish population minus familial cases), and the rates were standardized by 5-year-age, gender, period (5 years group), latest educational attainment (as proxy socioeconomic status), and residential area. As the SIR calculation was based on person-years at risk, it was independent of family size. The 95% confidence interval (95%CI) of the SIR was calculated assuming a Poisson distribution.
5.3. Familial Proportion, Different Levels of Family History, and Age-Incidence Ratios
Familial proportion was defined in the index population as the percentage of concordant familial cancers (including offspring of affected parents and siblings) of all cancer. Cancers in parents contributed only to the definition of family history but not to case numbers.
Independent groups for familial relationships were considered in order to examine the differences in the familial risk shared by parents and siblings, and the risk shared by multiple affected relatives. The dependence of familial risk on diagnostic age (age-incidence) was investigated considering all first-degree relatives (FDRs) as probands for 10 common cancers. Plots of familial and non-familial incidence rates in 5-year intervals were generated. RRs between familial and non-familial rates were given and 95%CIs were calculated for each age band and for any age.
5.4. Calling Significant Difference
The difference between two SIRs was called significant when their 95%CIs did not overlap. The word ‘significance’ was not always repeated but a reference to a difference implied that it was significant, unless the opposite was stated.
Acknowledgments
The authors thank Patrick Reilly for excellent language editing.
Abbreviations
| CI | Confidence interval |
| FCD | Family-Cancer Database |
| FDR | First-degree relatives |
| ICD | International Classification of Diseases. |
| RR | Relative risk |
| SIR | Standardized incidence ratios |
| SNOMED | Systematized Nomenclature of Medicine |
Supplementary Materials
The following are available online at www.mdpi.com/article/10.3390/cancers13174385/s1, Table S1: Age-specific incidence rate and rate ratio of stomach and colorectal cancer in population with and without family history of concordant cancer; Table S2: Age-specific incidence rate and rate ratio of pancreas and lung cancer in population with and without family history of concordant cancer; Table S3: Age-specific incidence rate and rate ratio of female breast and endometrium cancer in population with and without family history of concordant cancer; Table S4: Age-specific incidence rate and rate ratio of prostate and kidney (renal parenchyma) cancer in population with and without family history of concordant cancer; Table S5: Age-specific incidence rate and rate ratio of bladder (urinary bladder) cancer and melanoma in population with and without family history of concordant cancer.
Author Contributions
K.H. conceived the study; K.H., X.L., K.S., and J.S. were responsible for design of the study; K.S. and J.S. were responsible for procuring data; X.L. carried out statistical analysis; K.H., A.F. and A.H. were involved in the interpretation of the results; K.H. wrote the main manuscript text. All authors contributed to manuscript writing. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by the European Union’s Horizon 2020 research and innovation program, grant No 856620 (Chaperon), the German Jose Carreras Leukemia Foundation, The Swedish Research Council, Jane and Aatos Erkko Foundation, Sigrid Juselius Foundation, Finnish Cancer Organizations, University of Helsinki and Helsinki University Central Hospital.
Institutional Review Board
The study was approved by the Regional Ethical Review Board in Lund on 6 February 2013 (2012/795) without requirement for informed consent. Instead, the Ethical Review Board obliged us to advertise in the newspapers in order to inform the public that their data would be used for secondary purposes. After that, around 40 individuals were excluded (≈40 excluded individuals/≈10 million individuals in the Swedish population).
Informed Consent Statement
The study was approved by the Regional Ethical Review Board in Lund without requirement for informed consent because anonymous data were used.
Data Availability Statement
Data used were issued by the National Board of Health and Welfare, Stockholm, to Kristina and Jan Sundquist for exclusive institutional use. Any data requests should be directed to the National Board of Health and Welfare, Stockholm.
Conflicts of Interest
Akseli Hemminki is shareholder in Targovax ASA. Akseli Hemminki is employee and shareholder in TILT Biotherapeutics Ltd. Other authors declared no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weires M., Bermejo J.L., Sundquist J., Hemminki K. Clustering of concordant and discordant cancer types in Swedish couples is rare. Eur. J. Cancer. 2011;47:98–106. doi: 10.1016/j.ejca.2010.06.125. [DOI] [PubMed] [Google Scholar]
- 2.Frank C., Fallah M., Sundquist J., Hemminki K. Population Landscape of Familial Cancer. Sci. Rep. 2015;5:12891. doi: 10.1038/srep12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta S., Provenzale D., Llor X., Halverson A.L., Grady W., Chung D.C., Haraldsdottir S., Markowitz A.J., Slavin T.P., Jr., Hampel H., et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Colorectal, Version 2. 2019. J. Natl. Compr. Cancer Netw. 2019;17:1032–1041. doi: 10.6004/jnccn.2019.0044. [DOI] [PubMed] [Google Scholar]
- 4.Daly M.B., Pal T., Berry M.P., Buys S.S., Dickson P., Domchek S.M., Elkhanany A., Friedman S., Goggins M., Hutton M.L., et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021;19:77–102. doi: 10.6004/jnccn.2021.0001. [DOI] [PubMed] [Google Scholar]
- 5.Giri V.N., Knudsen K.E., Kelly W.K., Cheng H.H., Cooney K.A., Cookson M.S., Dahut W., Weissman S., Soule H.R., Petrylak D.P., et al. Implementation of Germline Testing for Prostate Cancer: Philadelphia Prostate Cancer Consensus Conference 2019. J. Clin. Oncol. 2020;38:2798–2811. doi: 10.1200/JCO.20.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aslanian H.R., Lee J.H., Canto M.I. AGA Clinical Practice Update on Pancreas Cancer Screening in High-Risk Individuals: Expert Review. Gastroenterology. 2020;159:358–362. doi: 10.1053/j.gastro.2020.03.088. [DOI] [PubMed] [Google Scholar]
- 7.Pilarski R. How Have Multigene Panels Changed the Clinical Practice of Genetic Counseling and Testing. J. Natl. Compr. Cancer Netw. 2021;19:103–108. doi: 10.6004/jnccn.2020.7674. [DOI] [PubMed] [Google Scholar]
- 8.Frank C., Fallah M., Ji J., Sundquist J., Hemminki K. The population impact of familial cancer, a major cause of cancer. Int. J. Cancer. 2013;134:1899–1906. doi: 10.1002/ijc.28510. [DOI] [PubMed] [Google Scholar]
- 9.Frank C., Sundquist J., Yu H., Hemminki A., Hemminki K. Concordant and discordant familial cancer: Familial risks, proportions and population impact. Int. J. Cancer. 2016;140:1510–1516. doi: 10.1002/ijc.30583. [DOI] [PubMed] [Google Scholar]
- 10.Grill S., Fallah M., Leach R., Thompson I.M., Freedland S., Hemminki K., Ankerst D.P. Incorporation of Detailed Family History from the Swedish Family Cancer Database into the PCPT Risk Calculator. J. Urol. 2015;193:460–465. doi: 10.1016/j.juro.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith R.A., Andrews K., Brooks D., DeSantis C.E., Fedewa S.A., Lortet-Tieulent J., Manassaram-Baptiste D., Brawley O.W., Wender R.C. Cancer screening in the United States, 2016: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2016;66:95–114. doi: 10.3322/caac.21336. [DOI] [PubMed] [Google Scholar]
- 12.Cheng H.H., Pritchard C.C., Montgomery B., Lin D.W., Nelson P.S. Prostate Cancer Screening in a New Era of Genetics. Clin. Genitourin. Cancer. 2017;15:625–628. doi: 10.1016/j.clgc.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Kolb J.M., Ahnen D.J., Samadder N.J. Evidenced-Based Screening Strategies for a Positive Family History. Gastrointest. Endosc. Clin. N. Am. 2020;30:597–609. doi: 10.1016/j.giec.2020.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandt A., Bermejo J.L., Sundquist J., Hemminki K. Age of onset in familial breast cancer as background data for medical sur-veillance. Br. J. Cancer. 2010;102:42–47. doi: 10.1038/sj.bjc.6605421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roudgari H., Hemminki K., Brandt A., Sundquist J., Fallah M. Prostate cancer risk assessment model: A scoring model based on the Swedish Family-Cancer Database. J. Med. Genet. 2012;49:345–352. doi: 10.1136/jmedgenet-2011-100290. [DOI] [PubMed] [Google Scholar]
- 16.Hemminki K., Ji J., Brandt A., Mousavi S.M., Sundquist J. The Swedish Family-Cancer Database 2009: Prospects for histolo-gy-specific and immigrant studies. Int. J. Cancer. 2010;126:2259–2267. doi: 10.1002/ijc.24795. [DOI] [PubMed] [Google Scholar]
- 17.Ji J., Sundquist K., Sundquist J., Hemminki K. Comparability of cancer identification among Death Registry, Cancer Registry and Hospital Discharge Registry. Int. J. Cancer. 2012;131:2085–2093. doi: 10.1002/ijc.27462. [DOI] [PubMed] [Google Scholar]
- 18.Pukkala E., Engholm G., Schmidt L.K.H., Storm H., Khan S., Lambe M., Pettersson D., Ólafsdóttir E., Tryggvadóttir L., Hakanen T., et al. Nordic Cancer Registries—An overview of their procedures and data comparability. Acta Oncol. 2017;57:440–455. doi: 10.1080/0284186X.2017.1407039. [DOI] [PubMed] [Google Scholar]
- 19.Ji J., Hemminki K. Familial risk for histology-specific bone cancers: An updated study in Sweden. Eur. J. Cancer. 2006;42:2343–2349. doi: 10.1016/j.ejca.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 20.Li X., Hemminki K. Familial upper aerodigestive tract cancers: Incidence trends, familial clustering and subsequent cancers. Oral Oncol. 2003;39:232–239. doi: 10.1016/S1368-8375(02)00091-X. [DOI] [PubMed] [Google Scholar]
- 21.Di Credico G., Edefonti V., Polesel J., Pauli F., Torelli N., Serraino D., Negri E., Luce D., Stucker I., Matsuo K., et al. Joint effects of intensity and duration of cigarette smoking on the risk of head and neck cancer: A bivariate spline model approach. Oral Oncol. 2019;94:47–57. doi: 10.1016/j.oraloncology.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Credico G., Polesel J., Dal Maso L., Pauli F., Torelli N., Luce D., Radoi L., Matsuo K., Serraino D., Brennan P., et al. Alcohol drinking and head and neck cancer risk: The joint effect of intensity and duration. Br. J. Cancer. 2020;123:1456–1463. doi: 10.1038/s41416-020-01031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negri E., Boffetta P., Berthiller J., Castellsagué X., Curado M.P., Maso L.D., Daudt A.W., Fabianova E., Fernandez L., Wünsch-Filho V., et al. Family history of cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Int. J. Cancer. 2008;124:394–401. doi: 10.1002/ijc.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radoï L., Paget-Bailly S., Guida F., Cyr D., Menvielle G., Schmaus A., Carton M., Cenée S., Sanchez M., Guizard A.-V., et al. Family history of cancer, personal history of medical conditions and risk of oral cavity cancer in France: The ICARE study. BMC Cancer. 2013;13:560. doi: 10.1186/1471-2407-13-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renkonen S., Lee M., Mäkitie A., Lindström L.S., Czene K. Site-specific familial risk and survival of familial and sporadic head and neck cancer. Int. J. Cancer. 2017;141:497–502. doi: 10.1002/ijc.30751. [DOI] [PubMed] [Google Scholar]
- 26.Carpenter D.O., Bushkin-Bedient S. Exposure to Chemicals and Radiation During Childhood and Risk for Cancer Later in Life. J. Adolesc. Health. 2013;52:S21–S29. doi: 10.1016/j.jadohealth.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 27.Win A.K., Reece J., Dowty J.G., Buchanan D., Clendenning M., Rosty C., Southey M.C., Young J.P., Cleary S., Kim H., et al. Risk of extracolonic cancers for people with biallelic and monoallelic mutations inMUTYH. Int. J. Cancer. 2016;139:1557–1563. doi: 10.1002/ijc.30197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samadder N.J., Riegert-Johnson D., Boardman L., Rhodes D., Wick M., Okuno S., Kunze K.L., Golafshar M., Uson P.L.S., Mountjoy L., et al. Comparison of Universal Genetic Testing vs Guideline-Directed Targeted Testing for Patients with Hereditary Cancer Syndrome. JAMA Oncol. 2021;7:230. doi: 10.1001/jamaoncol.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenzo Bermejo J., Hemminki K. Familial risk of cancer shortly after diagnosis of the first familial tumor. J. Natl. Cancer Inst. 2005;97:1575–1579. doi: 10.1093/jnci/dji338. [DOI] [PubMed] [Google Scholar]
- 30.Brenner H., Rothenbacher D., Arndt V. Epidemiology of Stomach Cancer. Methods Mol. Biol. 2009;472:467–477. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- 31.Paul S.L., Blizzard L., Patton G.C., Dwyer T., Venn A. Parental smoking and smoking experimentation in childhood increase the risk of being a smoker 20 years later: The Childhood Determinants of Adult Health Study. Addiction. 2008;103:846–853. doi: 10.1111/j.1360-0443.2008.02196.x. [DOI] [PubMed] [Google Scholar]
- 32.Hemminki K., Rawal R., Bermejo J.L. Prostate cancer screening, changing age-specific incidence trends and implications on familial risk. Int. J. Cancer. 2004;113:312–315. doi: 10.1002/ijc.20568. [DOI] [PubMed] [Google Scholar]
- 33.Chang E.T., Zheng T., Weir E.G., Borowitz M., Mann R.B., Spiegelman D., Mueller N.E. Childhood social environment and Hodgkin’s lymphoma: New findings from a population-based case-control study. Cancer Epidemiol. Biomark. Prev. 2004;13:1361–1370. [PubMed] [Google Scholar]
- 34.Brandt A., Bermejo J.L., Sundquist J., Hemminki K. Age-Specific Risk of Incident Prostate Cancer and Risk of Death from Prostate Cancer Defined by the Number of Affected Family Members. Eur. Urol. 2010;58:275–280. doi: 10.1016/j.eururo.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Vogelstein B., Kinzler K. The Genetic Basis of Human Cancer. 2nd ed. McGraw-Hill; New York, NY, USA: 2002. [Google Scholar]
- 36.Hemminki K., Sundquist J., Bermejo J.L. How common is familial cancer? Ann. Oncol. 2007;19:163–167. doi: 10.1093/annonc/mdm414. [DOI] [PubMed] [Google Scholar]
- 37.Chang E.T., Smedby K.E., Hjalgrim H., Glimelius B., Adami H.-O. Reliability of Self-Reported Family History of Cancer in a Large Case–Control Study of Lymphoma. J. Natl. Cancer Inst. 2006;98:61–68. doi: 10.1093/jnci/djj005. [DOI] [PubMed] [Google Scholar]
- 38.Murff H.J., Spigel D.R., Syngal S. Does this patient have a family history of cancer? An evidence-based analysis of the accuracy of family cancer history. JAMA. 2004;292:1480–1489. doi: 10.1001/jama.292.12.1480. [DOI] [PubMed] [Google Scholar]
- 39.Fiederling J., Shams A.Z., Haug U. Validity of self-reported family history of cancer: A systematic literature review on selected cancers. Int. J. Cancer. 2016;139:1449–1460. doi: 10.1002/ijc.30203. [DOI] [PubMed] [Google Scholar]
- 40.Rahman N. Realizing the promise of cancer predisposition genes. Nature. 2014;505:302–308. doi: 10.1038/nature12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sud A., Kinnersley B., Houlston R. Genome-wide association studies of cancer: Current insights and future perspectives. Nat. Rev. Cancer. 2017;17:692–704. doi: 10.1038/nrc.2017.82. [DOI] [PubMed] [Google Scholar]
- 42.Turnbull C., Sud A., Houlston R.S. Cancer genetics, precision prevention and a call to action. Nat. Genet. 2018;50:1212–1218. doi: 10.1038/s41588-018-0202-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brandt A., Bermejo J.L., Sundquist J., Hemminki K. Age of onset in familial cancer. Ann. Oncol. 2008;19:2084–2088. doi: 10.1093/annonc/mdn527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kharazmi E., Fallah M., Sundquist K., Hemminki K. Familial risk of early and late onset cancer: Nationwide prospective cohort study. BMJ. 2012;345:e8076. doi: 10.1136/bmj.e8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shenoy S. CDH1 (E-Cadherin) Mutation and Gastric Cancer: Genetics, Molecular Mechanisms and Guidelines for Management. Cancer Manag. Res. 2019;11:10477–10486. doi: 10.2147/CMAR.S208818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kharazmi E., Babaei M., Fallah M., Chen T., Sundquist K., Hemminki K. Importance of tumor location and histology in familial risk of upper gastrointestinal cancers: A nationwide cohort study. Clin. Epidemiol. 2018;10:1169–1179. doi: 10.2147/CLEP.S168152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Møller P., Seppälä T.T., Bernstein I., Holinski-Feder E., Sala P., Gareth Evans D., Lindblo A., Macrae F., Blanco I., Sijmons R.H., et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: A report from the Prospective Lynch Syndrome Database. Gut. 2018;67:1306–1316. doi: 10.1136/gutjnl-2017-314057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marwitz T., Hüneburg R., Spier I., Lau J.-F., Kristiansen G., Lingohr P., Kalff J.C., Aretz S., Nattermann J., Strassburg C.P. Hereditary Diffuse Gastric Cancer: A Comparative Cohort Study According to Pathogenic Variant Status. Cancers. 2020;12:3726. doi: 10.3390/cancers12123726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Z., Fu H., Wang S., Yu X., You Q., Shi M., Dai C., Wang G., Cha W., Wang W. Whole-exome sequencing identifies prognostic mutational signatures in gastric cancer. Ann. Transl. Med. 2020;8:1484. doi: 10.21037/atm-20-6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iyer P., Moslim M., Farma J.M., Denlinger C.S. Diffuse gastric cancer: Histologic, molecular, and genetic basis of disease. Transl. Gastroenterol. Hepatol. 2020;5:52. doi: 10.21037/tgh.2020.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slavin T.P., Weitzel J.N., Neuhausen S.L., Schrader K.A., Oliveira C., Karam R. Genetics of gastric cancer: What do we know about the genetic risks? Transl. Gastroenterol. Hepatol. 2019;4:55. doi: 10.21037/tgh.2019.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Leeuwaarde R.S., Ahmad S., Links T.P., Giles R.H. Von Hippel-Lindau Syndrome. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., editors. GeneReviews(®) University of Washington; Seattle, DC, USA: 1993. [PubMed] [Google Scholar]
- 53.Pearlman R., Frankel W.L., Swanson B., Zhao W., Yilmaz A., Miller K., Bacher J., Bigley C., Nelsen L., Goodfellow P.J., et al. Prevalence and Spectrum of Germline Cancer Sus-ceptibility Gene Mutations Among Patients with Early-Onset Colorectal Cancer. JAMA Oncol. 2017;3:464–471. doi: 10.1001/jamaoncol.2016.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dominguez-Valentin M., Sampson J.R., Seppälä T.T., Ten Broeke S.W., Plazzer J.P., Nakken S., Engel C., Aretz S., Jenkins M.A., Suende L., et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: Findings from the Prospective Lynch Syndrome Database. Genet. Med. 2020;22:15–25. doi: 10.1038/s41436-019-0596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valle L., de Voer R.M., Goldberg Y., Sjursen W., Försti A., Ruiz-Ponte C., Caldés T., Garré P., Olsen M.F., Nordling M., et al. Update on genetic predisposition to colorectal cancer and polyposis. Mol. Asp. Med. 2019;69:10–26. doi: 10.1016/j.mam.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Carlo M.I., Ravichandran V., Srinavasan P., Bandlamudi C., Kemel Y., Ceyhan-Birsoy O., Mukherjee S., Mandelker D., Chaim J., Knezevic A., et al. Cancer Susceptibility Mutations in Patients with Urothelial Malignancies. J. Clin. Oncol. 2020;38:406–414. doi: 10.1200/JCO.19.01395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldstein A.M., Chan M., Harland M., Hayward N.K., Demenais F., Bishop D.T., Azizi E., Bergman W., Scarrà G.B., Bruno W., et al. Features associated with germline CDKN2A mutations: A GenoMEL study of melanoma-prone families from three continents. J. Med Genet. 2006;44:99–106. doi: 10.1136/jmg.2006.043802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Helgadottir H., Hoiom V., Tuominen R., Nielsen K., Jonsson G., Olsson H., Hansson J. Germline CDKN2A Mutation Status and Survival in Familial Melanoma Cases. J. Natl. Cancer Inst. 2016;108:djw135. doi: 10.1093/jnci/djw135. [DOI] [PubMed] [Google Scholar]
- 59.Read J., Wadt K.A., Hayward N.K. Melanoma genetics. J. Med. Genet. 2016;53:1–14. doi: 10.1136/jmedgenet-2015-103150. [DOI] [PubMed] [Google Scholar]
- 60.Samadder N.J., Giridhar K.V., Baffy N., Riegert-Johnson D., Couch F.J. Hereditary Cancer Syndromes-A Primer on Diagnosis and Management: Part 1: Breast-Ovarian Cancer Syndromes. Mayo. Clin. Proc. 2019;94:1084–1098. doi: 10.1016/j.mayocp.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 61.Lorenzo Bermejo J., Hemminki K. Familial lung cancer and aggregation of smoking habits:a simulation of the effect of shared environmental factors on the familial risk of cancer. Cancer Epidemiol. Biomark. Prev. 2005;14:1738–1740. doi: 10.1158/1055-9965.EPI-05-0201. [DOI] [PubMed] [Google Scholar]
- 62.Quach B.C., Bray M.J., Gaddis N.C., Liu M., Palviainen T., Minica C.C., Zellers S., Sherva R., Aliev F., Nothnagel M., et al. Expanding the genetic architecture of nicotine de-pendence and its shared genetics with multiple traits. Nat. Commun. 2020;11:5562. doi: 10.1038/s41467-020-19265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ni Raghallaigh H., Eeles R. Genetic predisposition to prostate cancer: An update. Fam. Cancer. 2021 doi: 10.1007/s10689-021-00227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ringborg U., Lagerlof B., Broberg M., Mansson-Brahme E., Platz A., Thorn M. Early detection and prevention of cutaneous ma-lignant melanoma: Emphasis on Swedish activities. Med Oncol. Tumor Pharmacother. 1991;8:183–187. doi: 10.1007/BF02987178. [DOI] [PubMed] [Google Scholar]
- 65.Tornberg S., Mansson-Brahme E., Linden D., Ringborg U., Krakau I., Karnell R., Landegren J., Brandberg Y., Hakulien T. Screening for cutaneous malignant mela-noma: A feasibility study. J. Med. Screen. 1996;3:211–215. doi: 10.1177/096914139600300411. [DOI] [PubMed] [Google Scholar]
- 66.Eriksson H., Lyth J., Månsson-Brahme E., Frohm-Nilsson M., Ingvar C., Lindholm C., Naredi P., Stierner U., Wagenius G., Carstensen J., et al. Low level of education is associated with later stage at diagnosis and reduced survival in cutaneous malignant melanoma: A nationwide population-based study in Sweden. Eur. J. Cancer. 2013;49:2705–2716. doi: 10.1016/j.ejca.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 67.Lagerstedt-Robinson K., Rohlin A., Aravidis C., Melin B., Nordling M., Stenmark-Askmalm M., Lindblom A., Nilbert M. Mismatch repair gene mu-tation spectrum in the Swedish Lynch syndrome population. Oncol. Rep. 2016;36:2823–2835. doi: 10.3892/or.2016.5060. [DOI] [PubMed] [Google Scholar]
- 68.Keränen A., Ghazi S., Carlson J., Papadogiannakis N., Lagerstedt-Robinson K., Lindblom A. Testing strategies to reduce morbidity and mortality from Lynch syndrome. Scand. J. Gastroenterol. 2018;53:1535–1540. doi: 10.1080/00365521.2018.1542453. [DOI] [PubMed] [Google Scholar]
- 69.Li J., Wen W.X., Eklund M., Kvist A., Eriksson M., Christensen H.N., Torstensson A., Bajalica-Lagercrantz S., Dunning A.M., Decker B., et al. Prevalence of BRCA1 and BRCA2 pathogenic variants in a large, unselected breast cancer cohort. Int. J. Cancer. 2018;144:1195–1204. doi: 10.1002/ijc.31841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong C., Hemminki K. Modification of cancer risks in offspring by sibling and parental cancers from 2,112,616 nuclear families. Int. J. Cancer. 2001;92:144–150. doi: 10.1002/1097-0215(200102)9999:9999<::AID-IJC1147>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 71.Guttmacher A.E., Collins F.S., Carmona R.H. The family history--more important than ever. N. Engl. J. Med. 2004;351:2333–2336. doi: 10.1056/NEJMsb042979. [DOI] [PubMed] [Google Scholar]
- 72.Hemminki K., Eng C. Clinical genetic counselling for familial cancers requires reliable data on familial cancer risks and general action plans. J. Med. Genet. 2004;41:801–807. doi: 10.1136/jmg.2004.022731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manchanda R., Blyuss O., Gaba F., Gordeev V.S., Jacobs C., Burnell M., Gan C., Taylor R., Turnbull C., Legood R., et al. Current detection rates and time-to-detection of all identifiable BRCA carriers in the Greater London population. J. Med Genet. 2018;55:538–545. doi: 10.1136/jmedgenet-2017-105195. [DOI] [PubMed] [Google Scholar]
- 74.Brandt A., Lorenzo Bermejo J., Sundquist J., Hemminki K. Breast cancer risk in women who fulfill high-risk criteria: At what age should surveillance start? Breast Cancer Res. Treat. 2010;121:133–141. doi: 10.1007/s10549-009-0486-y. [DOI] [PubMed] [Google Scholar]
- 75.Chattopadhyay S., Hemminki A., Försti A., Sundquist K., Sundquist J., Hemminki K. Familial Risks and Mortality in Second Primary Cancers in Melanoma. JNCI Cancer Spectr. 2018;2:pky068. doi: 10.1093/jncics/pky068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chattopadhyay S., Zheng G., Sud A., Sundquist K., Sundquist J., Försti A., Houlston R., Hemminki A., Hemminki K. Second primary cancers in non-Hodgkin lym-phoma: Family history and survival. Int. J. Cancer. 2020;146:970–976. doi: 10.1002/ijc.32391. [DOI] [PubMed] [Google Scholar]
- 77.Zheng G., Hemminki A., Försti A., Sundquist J., Sundquist K., Hemminki K. Second primary cancer after female breast cancer: Familial risks and cause of death. Cancer Med. 2018;8:400–407. doi: 10.1002/cam4.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee S.I., Patel M., Dutton B., Weng S., Luveta J., Qureshi N. Effectiveness of interventions to identify and manage patients with familial cancer risk in primary care: A systematic review. J. Community Genet. 2019;11:73–83. doi: 10.1007/s12687-019-00419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Järvinen H.J., Aarnio M., Mustonen H.K., Aktan–Collan‡ K., Aaltonen L., Peltomäki P., De La Chapelle§ A., Mecklin J.-P. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. doi: 10.1016/S0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 80.Vasen H.F.A. Progress Report: New insights into the prevention of CRC by colonoscopic surveillance in Lynch syndrome. Fam. Cancer. 2021:1–8. doi: 10.1007/s10689-020-00225-x. [DOI] [PubMed] [Google Scholar]
- 81.Nelson H.D., Pappas M., Cantor A., Haney E., Holmes R. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer in Women: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2019;322:666–685. doi: 10.1001/jama.2019.8430. [DOI] [PubMed] [Google Scholar]
- 82.Janda M., Cust A.E., Neale R.E., Aitken J.F., Baade P., Green A.C., Khosrotehrani K., Mar V., Soyer H.P., Whiteman D.C. Early detection of melanoma: A consensus report from the Australian Skin and Skin Cancer Research Centre Melanoma Screening Summit. Aust. N. Z. J. Public Health. 2020;44:111–115. doi: 10.1111/1753-6405.12972. [DOI] [PubMed] [Google Scholar]
- 83.Hemminki K., Zhang H., Czene K. Socioeconomic factors in cancer in Sweden. Int. J. Cancer. 2003;105:692–700. doi: 10.1002/ijc.11150. [DOI] [PubMed] [Google Scholar]
- 84.CentreforEpidemiology . Cancer Incidence in Sweden Stockholm. The National Board of Health and Welfare; Stockholm, Sweden: 2013. [Google Scholar]
- 85.Amundadottir L.T., Thorvaldsson S., Gudbjartsson D.F., Sulem P., Kristjansson K., Arnason S., Gulcher J.R., Bjornsson J., Kong A., Steffanson K., et al. Cancer as a Complex Phe-notype: Pattern of Cancer Distribution within and beyond the Nuclear Family. PLoS Med. 2004;1:e65. doi: 10.1371/journal.pmed.0010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goldgar D.E., Easton D.F., Albright L., Skolnick M.H. Systematic Population-Based Assessment of Cancer Risk in First-Degree Relatives of Cancer Probands. J. Natl. Cancer Inst. 1994;86:1600–1608. doi: 10.1093/jnci/86.21.1600. [DOI] [PubMed] [Google Scholar]
- 87.Yu H., Frank C., Sundquist J., Hemminki A., Hemminki K. Common cancers share familial susceptibility: Implications for cancer genetics and counselling. J. Med. Genet. 2016;54:248–253. doi: 10.1136/jmedgenet-2016-103932. [DOI] [PubMed] [Google Scholar]
- 88.Hemminki K., Li X. Familial risk of cancer by site and histopathology. Int. J. Cancer. 2002;103:105–109. doi: 10.1002/ijc.10764. [DOI] [PubMed] [Google Scholar]
- 89.Pui C.-H., Nichols K.E., Yang J.J. Somatic and germline genomics in paediatric acute lymphoblastic leukaemia. Nat. Rev. Clin. Oncol. 2018;16:227–240. doi: 10.1038/s41571-018-0136-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used were issued by the National Board of Health and Welfare, Stockholm, to Kristina and Jan Sundquist for exclusive institutional use. Any data requests should be directed to the National Board of Health and Welfare, Stockholm.