Abstract
This study evaluates whether patients enrolled in trials of aducanumab, EMERGE and ENGAGE, were representative of patients with dementia enrolled in Medicare by estimating the proportions of Medicare beneficiaries with Alzheimer disease (AD) or mild cognitive impairment (MCI) who would have been excluded from these trials.
In June 2021, the US Food and Drug Administration (FDA) granted accelerated approval for aducanumab to treat patients with mild cognitive impairment (MCI) or mild dementia due to Alzheimer disease (AD), despite limited evidence of clinical benefit. The 2 phase 3 clinical trials of aducanumab, EMERGE and ENGAGE, were stopped prematurely based on prespecified futility thresholds.1 Both trials also showed an increased risk of adverse events with aducanumab, including microhemorrhages and vasogenic brain edema, headache, and possibly falls.1 Though both trials excluded patients based on age, certain chronic diseases, and use of antiplatelet agents and anticoagulants, FDA approval was granted without contraindications or precautions for these unstudied patient populations. We evaluated whether patients enrolled in the trials of aducanumab were representative of patients with dementia enrolled in Medicare by estimating the proportions of Medicare beneficiaries with AD or MCI who would have been excluded from the trials.
Methods
We examined medical claims from the Centers for Medicare & Medicaid Services (CMS) for a 100% sample of adults enrolled in Medicare fee-for-service in 2018. We identified 3 cohorts of patients potentially eligible for aducanumab treatment: those with AD and related disorders (ADRDs), those with AD specifically, and those with MCI. ADRDs and AD were identified using CMS Chronic Condition Data Warehouse algorithms.2 We identified MCI based on the presence of International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes G3184 or R4181 in the preceding 3 years among patients without ADRDs. Sex, race, and ethnicity were examined given historic disparities in clinical trial enrollment and were identified using CMS Master Beneficiary Summary File variables. We used fixed categories for race and ethnicity from the Research Triangle Institute race code reported by CMS. Trial eligibility criteria were obtained from ClinicalTrials.gov.3,4 Methods for operationalizing claims data to identify exclusion criteria are included in the Table.
Table. Aducanumab Trial Exclusion Criteria and Operational Study Definitionsa.
| Clinical trial exclusion criteria | Operational study definition |
|---|---|
| Age >85 y | Age >85 y obtained from the Master Beneficiary Summary File |
| Age <50 y | Age <50 y obtained from the Master Beneficiary Summary File |
| Any medical or neurologic condition (other than Alzheimer disease) that might be a contributing cause of the patient’s cognitive impairment | Any inpatient, outpatient, or carrier claims with AHRQ CCS code for brain cancer (35), Parkinson disease (79), epilepsy (83), coma/brain damage (85), or intracranial injury (233) in 2017-2018 |
| Have had a stroke or TIA or unexplained loss of consciousness in the past 1 y | CMS CCW flag for stroke/TIA OR Any inpatient, outpatient, or carrier claims with AHRQ CCS code for syncope (245) in 2017-2018 |
| Clinically significant unstable psychiatric illness in past 6 mo | Any hospitalization in 2017-2018 with psychiatric primary discharge diagnosis (AHRQ CCS codes 650-659 OR 662) |
| History of unstable angina, myocardial infarction, advanced chronic heart failure, or clinically significant conduction abnormalities within 1 y prior to screening | CMS CCW flags for heart failure, ischemic heart disease, or atrial fibrillation OR Any hospitalization in 2017-2018 with cardiac primary discharge diagnosis (AHRQ CCS codes: 100 [acute myocardial infarction], 101 [coronary atherosclerosis and other heart disease], 103 [pulmonary heart disease], 104 [other and ill-defined heart disease], 105 [conduction disorders], 106 [cardiac dysrhythmias], 107 [cardiac arrest and ventricular fibrillation], 108 [congestive heart failure]) |
| Indication of impaired kidney function | CMS CCW flag for chronic kidney disease |
| Indication of impaired liver function | CMS CCW flag for liver disease, cirrhosis, and other liver conditions |
| Have HIV infection | CMS CCW flag for HIV and/or AIDS |
| Relevant brain hemorrhage, bleeding disorder, and cerebrovascular abnormalities | Any inpatient, outpatient, or carrier claims with AHRQ CCS code for coagulation and hemorrhagic disorders (62) in 2017-2018 |
| Alcohol or substance misuse in past 1 y | CMS CCW flag for drug use disorders OR Any hospitalization with substance use disorder primary discharge diagnosis (AHRQ CCS codes: 660 or 661) in 2017-2018 |
| Taking blood thinners (except for aspirin at a prophylactic dose or less) | Any inpatient, outpatient, or carrier claims with AHRQ CCS code for cardiac dysrhythmias (106) or phlebitis, thrombophlebitis, and thromboembolism (118) in 2017-2018 |
| Patients with history of ischemic heart disease, stroke, or TIA, who typically are treated with full-dose aspirin or other antiplatelets, are included in the prior exclusion criteria and not repeated | |
| Have a significant systemic illness or infection in past 30 d | Not examined |
| Any contraindications to brain magnetic resonance imaging or positron emission tomography scans | Not examined |
Abbreviations: AHRQ, Agency for Healthcare Research and Quality; CCS, Chronic Conditions Software; CCW, Chronic Conditions Warehouse; CMS, Centers for Medicare & Medicaid Services; TIA, transient ischemic attack.
Trial exclusion criteria identified from ClinicalTrials.gov as neither trial had been published in a peer-reviewed journal at the time of analysis.
We first calculated the number of adults in each cohort. We then identified the number and proportion of adults who met each exclusion criterion. Analyses were conducted using SAS version 9.2 (SAS Institute Inc). The study was approved by the Harvard Medical School Human Studies Committee and the CMS Privacy Board; informed consent was waived.
Results
The study included 27 785 076 Medicare beneficiaries, of whom 2 870 023 (10.3%) had been diagnosed with ADRDs (mean age, 80.7 [SD, 10.6] years; 62.7% female; 9.5% Black; 80.4% White), 1 026 387 (3.7%) with AD (mean age, 82.9 [SD, 8.6] years; 66.8% female; 9.0% Black; 80.5% White), and 396 251 (1.4%) with MCI (mean age, 74.9 [SD, 11.9] years; 56.6% female; 7.1% Black; 83.8% White).
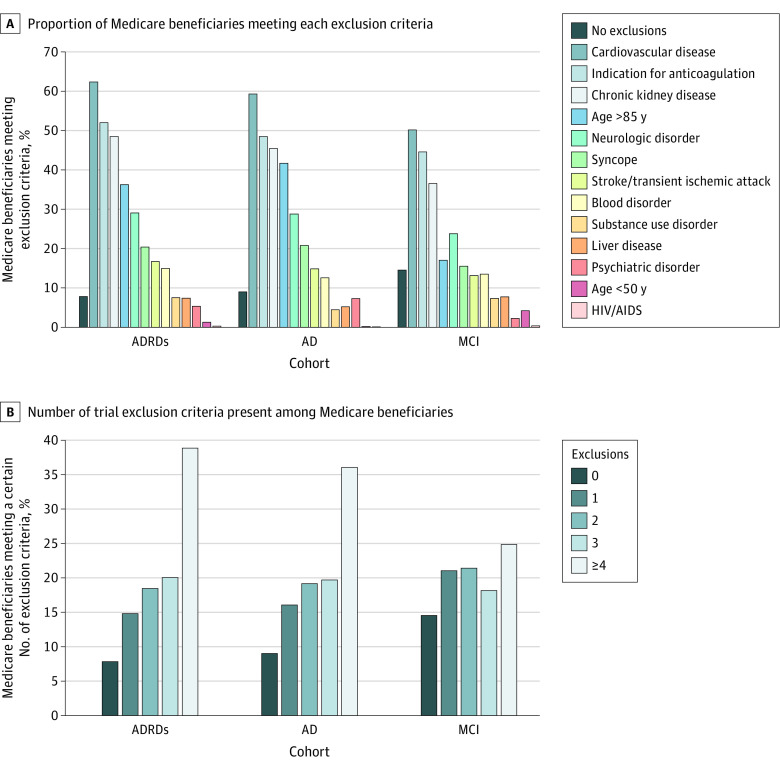
A total of 92.2% of patients with ADRDs, 91.0% with AD, and 85.5% with MCI met at least 1 trial exclusion criteria (Figure, A). The most common exclusion criteria were cardiovascular disease, conditions associated with anticoagulation, chronic kidney disease, and age older than 85 years. Most patients met multiple exclusion criteria, including 77.4% of patients with ADRDs and 64.4% with MCI (Figure, B).
Figure. Prevalence of Aducanumab Trial Exclusion Criteria Among Medicare Beneficiaries With Alzheimer Disease and Mild Cognitive Impairment.
ADRDs indicates Alzheimer disease and related disorders; AD, Alzheimer disease; and MCI, mild cognitive impairment.
Discussion
Clinical trials of aducanumab used exclusion criteria that would have excluded more than 92% of Medicare beneficiaries with ADRDs and 85% of those with MCI based on their age or comorbid conditions. These findings are concerning given the broad FDA labeling for aducanumab. Though labeling has recently been narrowed to focus on patients with MCI or mild-stage dementia, no contraindications to use have been added.5 The increased risk of vascular edema and hemorrhages observed in the clinical trials are likely to be higher in trial ineligible populations, particularly patients with prior stroke or chronic conditions treated with antiplatelets or anticoagulants.
This study had several limitations. First, it did not examine individuals enrolled in Medicare Advantage plans. Medicare claims algorithms for dementia have modest sensitivity and do not accurately capture disease severity6; thus, the study may overestimate comorbidity burden in patients with mild dementia who may face comorbidity burden more similar to patients with MCI. Second, claims do not allow for identification of positive amyloid positron emission tomography scans, which are necessary to determine trial inclusion criteria.
CMS should consider restricting coverage for aducanumab to populations meeting trial eligibility criteria and requiring additional evidence on clinical outcomes in groups excluded from the trials.
Section Editors: Jody W. Zylke, MD, Deputy Editor; Kristin Walter, MD, Associate Editor.
References
- 1.Alexander GC, Emerson S, Kesselheim AS. Evaluation of aducanumab for Alzheimer disease: scientific evidence and regulatory review involving efficacy, safety, and futility. JAMA. 2021;325(17):1717-1718. doi: 10.1001/jama.2021.3854 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Medicare & Medicaid Services . Chronic Conditions Data Warehouse. Accessed July 10, 2021. https://www2.ccwdata.org/web/guest/condition-categories
- 3.ClinicalTrials.gov . 221AD302 Phase 3 Study of Aducanumab (BIIB037) in Early Alzheimer’s Disease (EMERGE). Accessed July 10, 2021. https://clinicaltrials.gov/ct2/show/NCT02484547
- 4.ClinicalTrials.gov . 221AD301 Phase 3 Study of Aducanumab (BIIB037) in Early Alzheimer’s Disease (ENGAGE). Accessed July 10, 2021. https://clinicaltrials.gov/ct2/show/NCT02477800
- 5.US Food and Drug Administration. Aducanumab (marketed as Aduhelm) information. Accessed July 14, 2021. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/aducanumab-marketed-aduhelm-information
- 6.Lee E, Gatz M, Tseng C, et al. Evaluation of Medicare claims data as a tool to identify dementia. J Alzheimers Dis. 2019;67(2):769-778. doi: 10.3233/JAD-181005 [DOI] [PMC free article] [PubMed] [Google Scholar]