Abstract
Stress granules (SGs) are small membrane-free cytosolic liquid-phase ordered entities in which mRNAs are protected and translationally silenced during cellular adaptation to harmful conditions (e.g., hypoxia, oxidative stress). This function is achieved by structural and functional SG components such as scaffold proteins and RNA-binding proteins controlling the fate of mRNAs. Increasing evidence indicates that the capacity of cells to assemble/disassemble functional SGs may significantly impact the onset and the development of metabolic and inflammatory diseases, as well as cancers. In the liver, the abnormal expression of SG components and formation of SG occur with chronic liver diseases, hepatocellular carcinoma (HCC), and selective hepatic resistance to anti-cancer drugs. Although, the role of SG in these diseases is still debated, the modulation of SG assembly/disassembly or targeting the expression/activity of specific SG components may represent appealing strategies to treat hepatic disorders and potentially cancer. In this review, we discuss our current knowledge about pathophysiological functions of SGs in HCC as well as available molecular tools and drugs capable of modulating SG formation and functions for therapeutic purposes.
Keywords: stress granules, liver diseases, hepatitis, Adenylate-Uridylate-rich element-binding proteins, oncogenes, tumor suppressors, post-transcriptional regulation
1. Introduction
Hepatocellular carcinoma (HCC) is the seventh most common cancer and the second biggest cause of cancer mortality worldwide [1,2]. HCC can arise in the context of various chronic liver diseases, including chronic hepatitis B and C viral infections (HBV and HCV, respectively), alcoholic liver disease (ALD), and non-alcoholic fatty liver disease (NAFLD), a metabolic liver disorder tightly associated with obesity, diabetes, and sedentary lifestyle [1,2]. Most of these hepatic diseases start with the aberrant accumulation of fat in hepatocytes, a condition called steatosis, and are thus referred as fatty liver disease (FLD). With time, lipotoxicity, endoplasmic reticulum (ER) stress, and mitochondrial dysfunctions lead to hepatocyte death and inflammation (steatohepatitis) with the associated accumulation of fibrotic tissues in the liver [2,3]. If unresolved, inflammation and fibrosis can progress with time and regenerative nodules of poorly differentiated hepatocytes develop in the parenchyma leading to a loss of hepatic functions and portal hypertension [4]. This end stage of FLD is a life-threatening conditions per se, but also an important risk factor for HCC development [5]. Of note, HCC can also arise in non-cirrhotic conditions, directly from early stages of FLD (steatohepatitis/fibrosis) in the absence of cirrhosis [6]. Importantly, given the rapid worldwide increase of the prevalence of NAFLD with obesity and diabetes [7] and the high prevalence of ALD in developed countries, HCC incidence is expected to dramatically increase in the future [8], thus representing a major public health concern and an economic burden. HCC is one of the less curable cancers, due to the limited number of available therapeutic options and the high resistance of this cancer to conventional chemotherapy and radiotherapy. Surgical resection or liver transplantation remain the most efficient strategies but not all patients are eligible for surgery and these interventions are associated with life-threatening issues [9,10]. Understanding the molecular mechanisms of FLD and HCC is therefore required to develop new and efficient preventive and therapeutic approaches.
A myriad of molecular alterations is associated to the development of FLD and HCC, among which abnormal post-transcriptional regulation of gene expression is a key pathological mechanism driving hepatic metabolic diseases and carcinogenesis. Alterations of microRNAs (miRNAs) and RNA-binding proteins (RBPs) expression and/or activity promote mRNA decay or impair translation of transcripts controlling hepatic metabolism, inflammation, and carcinogenesis [11]. Similarly, to genetic mutations, alterations of these post-transcriptional regulators of gene expression may lead to an overexpression of oncogenes or the silencing of tumor suppressors, thereby favoring cancer development. Some of these regulatory mechanisms take place within small cytoplasmic ribonucleoprotein foci, such as processing bodies (P-bodies) or stress granules (SGs), where the fate of mRNAs is determined (i.e., translation, degradation, etc.) [12]. SGs, like P-bodies, are small cytosolic compartments devoid of membranes and containing translationally stalled mRNAs [13,14,15]. SGs form in stress conditions (e.g., nutrient deprivation, hypoxia), likely to protect mRNAs from degradation and spare energy to re-synthesize them when required again [14]. Consistent with this function, SGs contain several components of the translation initiation complex (e.g., 40S subunit). P-bodies share similar biophysical properties (liquid–liquid phase separation) and common protein components with SGs (e.g., tristetraprolin, BRF1) [16]. However, P-bodies differ from SG by the presence of several factors triggering mRNA decay, such as decapping enzymes (e.g., DCP1A, EDC4), deadenylases (i.e., Ccr4-Not, Lsm1-7), and exonucleases (e.g., Xrn1) [16]. P-bodies may be present in physiological conditions but increase in number and size during cellular stress [16]. Several stress stimuli promote the formation of both P-bodies and SGs (e.g., heat shock or oxidative stress), while others (e.g., viral infection) are specific for SGs [16].
The molecular mechanisms governing SG assembly are still incompletely understood, but the interaction of several RBPs with mRNAs appear to be determinant [13,14]. SG formation is usually reversible, and in case of prolonged stress, mRNAs located within SGs can be translocated and degraded within P-bodies [12]. The biogenesis and stability of SGs have been associated to the development of a wide range of diseases and cancers [14]. Consistent with a functional relevance of SGs in cancer, the expression/activity of several SG components are often deregulated in cancer cells, thus likely modulating their ability to adapt to stress conditions usually affecting transformed cells within tumors or metastatic cells. However, whether SG formation and functions display an oncogenic or tumor-suppressive role in cancer cells remains debated. In this regard, the ability of SGs to counteract cellular senescence and apoptosis by sequestering PAI-1 [17] and pro-apoptotic factors, such as TRAF2, or RACK1, may contribute to cancer cell survival [18]. Intense efforts aiming at characterizing the mRNA/protein content of these granules further uncovered numerous cancer-related factors suggesting that SGs may have the ability to sequester and/or stabilize the mRNA of oncogenes and tumor suppressors, thus affecting their impact on carcinogenesis [12]. Finally, whether SG formation can render cancer cells more resistant to various anti-cancerous approaches (e.g., chemotherapy, radiotherapy) remains to be clearly demonstrated but is supported by the ability of various anti-cancer molecules, e.g., sorafenib, one of the few drugs available for the treatment of advanced HCC, to trigger SG assembly [19]. Based on these observations, the concept emerges that modulating the formation of SGs with specific molecules may thus represent a potential therapeutic approach alone, or in combination with other treatments (i.e., chemotherapy, radiotherapy, etc.). In this review, we discuss our current knowledge on the function of SGs and their main components in HCC development, as well as currently available molecular tools targeting SGs for therapeutic purposes.
2. Molecular Bases and Complexity of SG Biogenesis
SGs are membrane-less cytoplasmic compartments made of ribonucleoproteins complexes and exhibiting a liquid-like property, which allowing the rapid exchange of mRNAs and proteins with the cytosol. The mechanisms regulating the dynamic assembly/disassembly of SGs are complex but appear to be widely conserved across species [20]. SG formation is triggered by a variety of cellular stresses including nutrients deprivation, hypoxia, heat shock, UV irradiation, oxidative and ER stresses, impaired protein degradation, and many others [21]. Specific circulating mediators (e.g., prostaglandins PGJ2/PGA1, oxidized-low density lipoproteins LDLs) or dietary factors such as obesogenic diets were also shown to promote SG formation in mouse macrophages and liver tissues [22], thus suggesting an important impact of dietary habits and chronic inflammatory/metabolic diseases on SG formation. As well, several anti-cancerous treatments, e.g., sorafenib, oxaliplatin, carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone, or radiotherapy, trigger SG assembly in various cancer types (see Mahboubi et al. [23] for the list of anti-cancer molecules triggering SG assembly), which appears to protect them from death, leading to the concept that SG formation may represent an important survival mechanism for cancer cells [12].
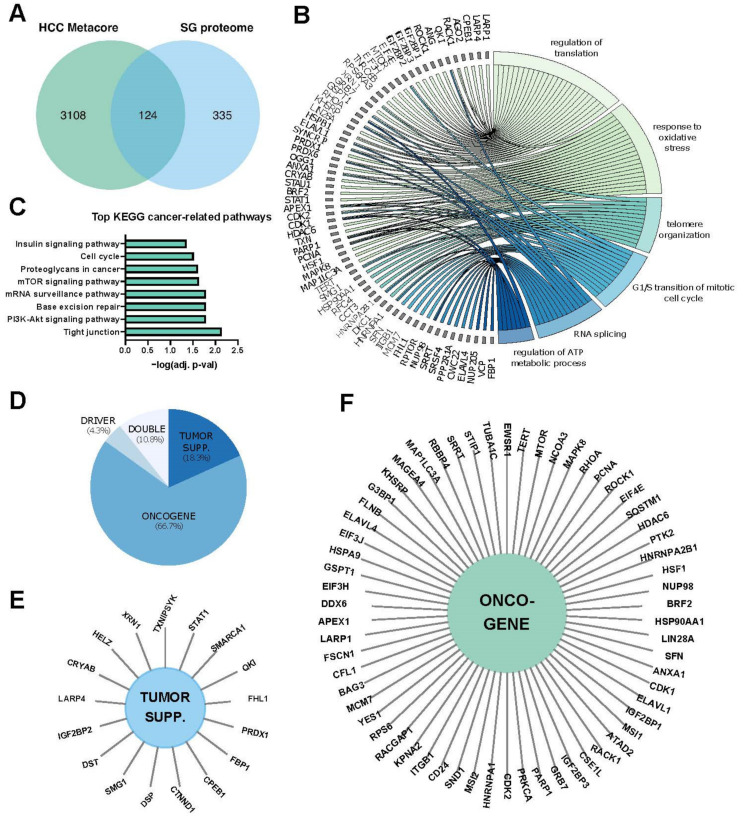
More than 400 proteins have been identified in isolated SGs by proteomic-based approaches (http://rnagranuledb.lunenfeld.ca/, accessed on 1 April 2021). About 50% of them are RNA-binding proteins (RBPs), while the others are involved in a wide range of cellular processes (e.g., metabolism, stress responses and cancer-related processes) (Figure 1). The composition of the SG proteome may, however, probably depend on several factors including the genetic context (i.e., mutations) or environmental factors (e.g., dietary factors, physical activity, inflammation). Structurally, SG are not uniform and are composed of internal dense structures referred to as “cores” that can be biochemically purified and contain a high amount of RNA and proteins. These cores are surrounded by a less concentrated shell, termed the “dynamic shell”, allowing a dynamic exchange of mRNPs with the cytosol or other cytoplasmic compartments (e.g., P-bodies) [20].
Figure 1.
The SG proteome. (A) The stress granule (SG) proteome (https://msgp.pt/, accessed on 1 April 2021) was crossed with genes associated with hepatocellular carcinoma (HCC) obtained with the Metacore database (https://portal.genego.com/, accessed on 1 April 2021). (B) Gene ontology (GO) enrichment for biological processes (BP), with a cutoff of adjusted p-value at 0.05 was performed on the 124 genes found in (A). A chord-plot highlighting the most relevant processes and genes was constructed using the GOplot R package [24]. (C) KEGG pathway enrichment of 124 genes at a cut-off of 0.05 of the adj. p-value. (D) Annotation of cancer functions of the 124 genes using CancerMine [25]. (E,F), Sun diagrams showing annotated tumor suppressors (E) and oncogenes (F).
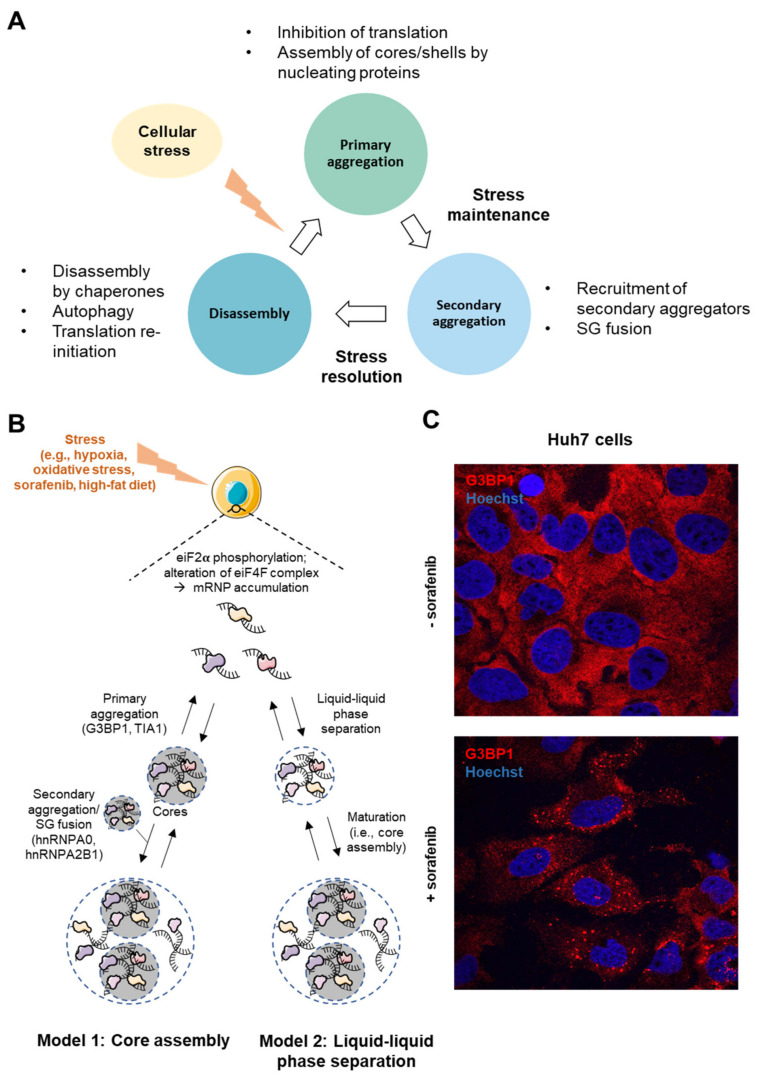
The precise mechanisms and temporal/structural sequences of SGs biogenesis are still not fully understood but two models have been proposed to date. In both models, the first step appears to start with eIF2α phosphorylation by various kinases (e.g., PERK, eIF2α kinase 3, Protein Kinase R), which inhibits its activity, thus preventing the formation of eIF2/GTP/tRNAi ternary complex and triggering the dissociation of mRNAs from polysomes [12,21,26]. eIF2α-independent mechanisms initiating SG formation have also been proposed such as the alteration of the eIF4E or eIF4F complex, which promotes cap-dependent translation [26,27]. Then, two variations of the subsequent steps leading to SG assembly have been proposed (Figure 2):
In the “core first” model, the increased pool of untranslated mRNAs is bound and oligomerized by RBPs (e.g., G3BP1, TIA1, FMRP) bearing either a prion-like domain (PLD) or an intrinsically disordered domain (IDD), necessary for the recruitment of other proteins. This step, forming stable core structures is called “primary aggregation” [20]. The PLD and IDD domains of RBPs are enriched in glycine and uncharged polar residues (i.e., asparagine, glutamine, serine), which promote electrostatic interactions and liquid–liquid phase separation (LLPS)” [28]. Due to these special biophysical properties, SGs behave as hydrogel-like structures and are often considered as “viscous liquid droplets”. Then, the recruitment of additional ribonucleoproteins with weaker interactions (e.g., hnRNPA0, hnRNPA2B1, EWSR1) contributes to the formation of a dynamic shell (secondary aggregation) [20]. During the primary aggregation step, the transport of RBPs within SGs requires functional microtubules and motor proteins (i.e., dyneins and kinesins) [29,30]. Consistent with the role of microtubules in this process, HDAC6, which is a microtubule-associated deacetylase, reduces tubulin-α acetylation (Lys40) and promotes SG formation [31]. Finally, when the stress persists, other SGs components are recruited, allowing the growth and fusion of SGs in a process called “coalescence”, wherein several cores are embedded in a dynamic shell.
In a second model called “LLPS First”, oligomerization of mRNAs with proteins containing IDD is believed to promote LLPS. Then, in a further step, the high density of core components stabilizes the core structures, which are assembled inside LLPS.
Figure 2.
Stress Granule assembly. (A) The cycle of stress granule formation and disassembly. (B) SGs in the liver can form during stressful events such as hypoxia, oxidative stress, sorafenib treatment or high-fat diet. Two models of SG assembly have been described. In the “core first” model, nucleating proteins (e.g., G3BP1 and TIA1) form a stable core, and later, other SG-associated proteins are recruited to form the dynamic shell. Alternatively, in the ‘LLPS first’ model, proteins bound to transcripts assemble through interactions of their IDD domains. Further on, highly dense fractions form SG cores. (C) Confocal microscopy images of SG formation (G3BP1 staining in red; Hoechst-33342 staining in blue) in hepatic Huh7 cancer cells after 24 h treatment with 5 μM sorafenib (63× magnification).
Finally, reversibility of SG assembly is ensured by several clearance mechanisms proposed to be mediated by (i) autophagy, (ii) translation re-initiation, (iii) chaperone proteins, (iv) mRNA decay in processing bodies (P-Bodies), and/or (v) proteasome-dependent degradation of SG proteins.
The dynamic of SG assembly is tightly regulated by specific signaling pathways, such as the PI3K/AKT or the p38/MAPK pathways [32], which induce the assembly of SG components by activating the S6 Kinases-1 (S6K1), a downstream effector of mTORC1 signaling, which in turn phosphorylates and inhibits eiF2α [33]. mTORC1 was also shown to phosphorylate and activate 4E-BP1, which binds and inhibits the eIF4E initiation complex [34]. Of note, mutations and/or non-genomic alterations in cancer, often lead to the constitutive activation of these signaling pathways, which are strong promoters of SG formation. Various post-translational modifications (e.g., acetylation, phosphorylation, methylation) of key SG components were further reported to also govern the dynamic formation of functional SGs in pathological conditions [14,35,36,37].
Based on our current knowledge, it is likely that there is still much to be discovered about the pleiotropic mechanisms, functions, and relevance of SGs assembly in the cellular physiology and diseases. However, abnormal formation and functions of SGs occur in hepatic diseases and likely HCC, as supported by the altered expression of specific SG components, deregulated signaling pathways involved in SG assembly, as well as a deficient mechanism of SGs clearance, which are observed in these diseases.
3. SGs in Hepatic Carcinogenesis
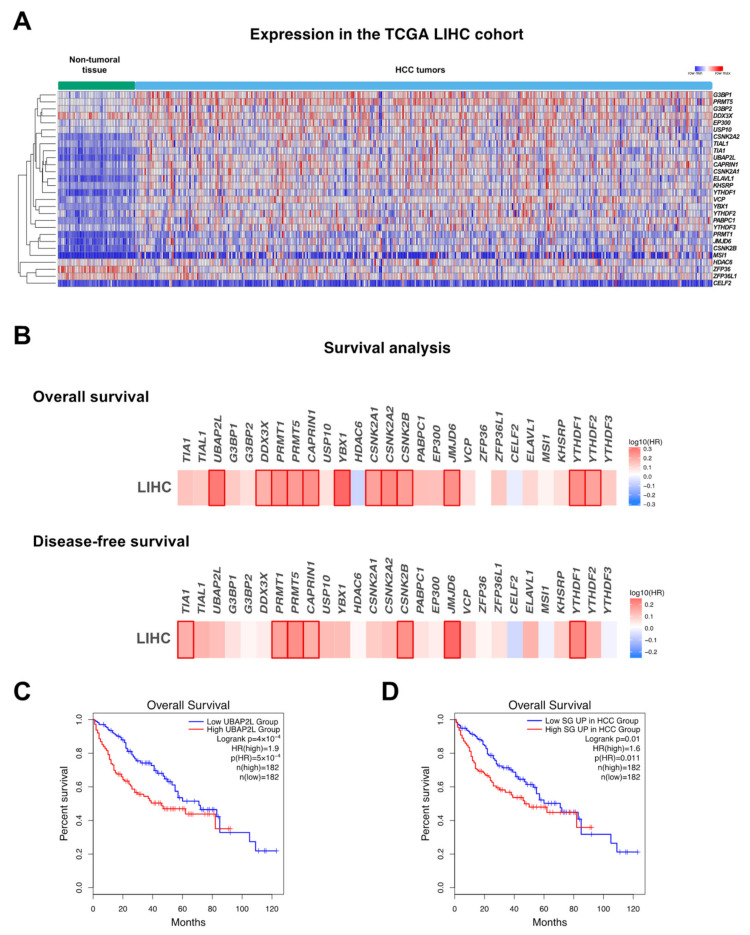
As previously discussed, SG formation is increased in many types of cancer cells, where they are believed to potentially exert an oncogenic function. However, an oncogenic role of SGs in cancer was not firmly demonstrated but is rather deduced from a set of observations, mostly derived from in vitro experimental approaches, such as bioimaging analyses, gain and loss of function analyses of specific SG components, or biochemical isolations and characterization of SG-containing subcellular fractions. As illustrated in Figure 1, approximately one third of transcripts/proteins present in SGs is functionally involved in classical cancer-related processes, thus supporting a significant role for SGs in carcinogenesis. In HCC particularly, only a few studies have provided correlative links between SGs biogenesis and hepatic carcinogenesis. This includes evidence showing that in vitro hepatic cancer cell resistance to sorafenib, a multi-kinases inhibitor used for the treatment of advanced HCC, is associated with the formation of SGs [19] and the observation that SG formation in HCV-infected hepatocytes is required for an efficient viral replication [38]. Furthermore, key factors intimately involved in the SG biogenesis or RBPs located in SGs and controlling the expression of hepatic metabolism, inflammation, and cancer-related genes [39] are significantly deregulated in hepatic diseases and cancer (Figure 1 and Figure 3A). As well, oncogenic signaling pathways typically overactivated in HCC (e.g., PI3K, p38/MAPK) were shown to drive SG formation in cells. Importantly, whether SG formation and presence is increased in vivo in HCC animal models or in patients with HCC was never firmly established in contrast to other cancer types. In the following sections, we discuss key factors and processes regulating SG biogenesis and function that are significantly altered in hepatic diseases and cancer and which may potentially affect the onset and/or the progression of these pathologies.
Figure 3.
SG assembly in hepatocellular carcinoma. (A) mRNA expression levels of genes associated with stress granules in the LIHC TCGA cohort. (B) Heat map showing hazard ratio (HR) for overall and disease-free survival based on the expression of genes associated with stress granules in the LIHC TCGA cohort (http://gepia.cancer-pku.cn/, accessed on 1 April 2021). Positive hazard ratio indicates lower possibility of survival. Frames indicate significance. (C) Survival curve showing overall survival of patients expressing high vs. low levels of UBAP2L. (D) Survival curve showing overall survival of patients expressing high vs. low levels of SG-associated genes that are significantly upregulated in the LIHC TCGA cohort.
3.1. Nucleic Acids and Proteins Involved in SG Formation
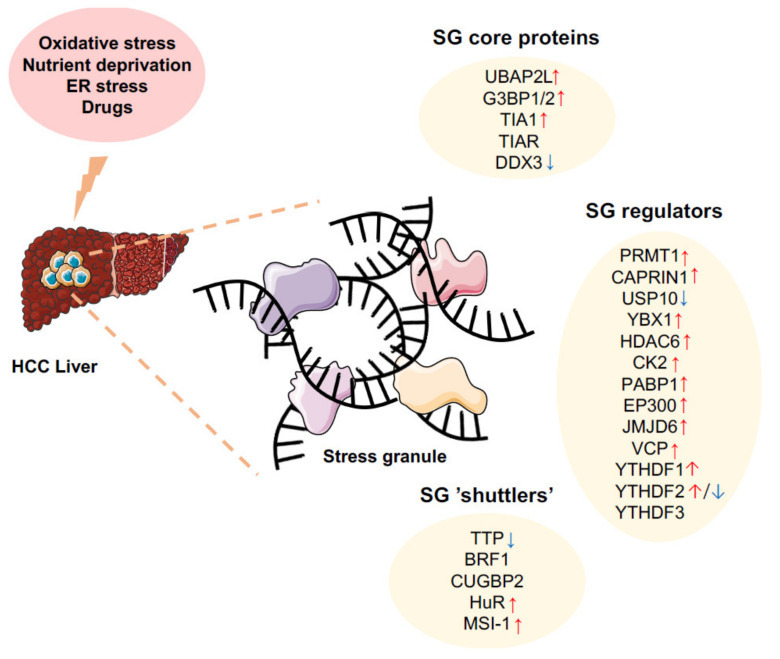
Overexpression of specific factors involved in the aggregation phase of SG formation is sufficient per se to trigger SG assembly, even in the absence of any cellular stress, while reducing their expression or activity considerably prevents SG formation in stress conditions [12,13]. Deregulated expressions/activities of these SG nucleators by various mechanisms are frequently observed in HCC (Figure 3A and Figure 4). Below are discussed key factors implicated in SGs biogenesis and for which experimental evidence suggests that they might be associated with the onset and development of HCC.
Figure 4.
Alterations of SG components in hepatocellular carcinoma. Hepatocellular carcinoma is associated to the alteration of several SG components (core proteins, SG regulators, and SG shuttlers). Together, these alterations may importantly contribute to SG formation upon cellular stresses (e.g., oxidative stress, nutrients deprivation, ER stress, drugs). SG may in turn alter cell survival and death, angiogenesis, and other cancer-related processes. Arrows indicate higher (red) or lower (blue) expression in HCC based on literature (see respective paragraphs).
3.1.1. UBAP2L (Ubiquitin-Associated Protein 2-Like)
UBAP2L is involved in the ubiquitin-proteasome pathway, but functions also as an essential component of SG assembly. It contains several specific domains including a ubiquitin-associated domain (UBA) required for the binding to ubiquitin chains [40,41] and an Arg-Gly-Gly (RGG) motif allowing the recruitment of other SG components. Another domain of UBAP2L, called the domain of unknown function (DUF), has poorly known functions but appears to be necessary to form complexes with other SG nucleators, such as G3BP1/2 (discussed in the next section) [40]. UBAP2L can, however, form SGs independently of G3BP1/2 [42] and its expression is sufficient per se to trigger SG formation, even in absence of any cellular stress [43,44]. UBAP2L has oncogenic properties in various cancers [43,44], but in the liver, its role is poorly defined and UBAP2L was never associated to SG formation. Nevertheless, emerging evidence indicates that the overexpression of UBAP2L in HCC correlates with a poor clinical outcome [45,46]. In vitro studies further indicate a tumor promoting function of UBAP2L through its ability to promote SMMC-7721 hepatocarcinoma cell proliferation, survival, and migration/invasion [45,47]. The RGG-containing domain can undergo various post-translational modifications, which regulate SG assembly. For example, PRMT1, a protein arginine methyltransferase overexpressed in HCC, promotes carcinogenesis, but in the meantime, methylates UBAP2L on the RGG motif, thereby inhibiting SG formation [40,48,49,50] and thus questioning the necessity to assemble SGs for the oncogenic functions of UBAP2L.
3.1.2. G3BPs (Ras GTPase-Activating Protein-Binding Proteins)
The G3BP family has three members (i.e., G3BP1, G3BP2a, and G3BP2b), which interact with the SH3 domain of the Ras GTPase activating protein (RasGAP) and promote Ras signaling [51]. As for UBAP2L, G3BPs lack a PLD, but appear to act as key regulators of SG assembly [52]. The RNA recognition motif (RRM) of G3BPs allows their interaction with the 40S ribosomal subunit, while an RGG domain mediates mRNA binding [51]. G3BPs promote SG assembly through poorly characterized mechanisms triggering interactions with other SG components such as USP10 or CAPRIN1, which inhibits and promotes SG formation, respectively [53]. Of note, CAPRIN1 is overexpressed in HCC and correlates with a poor prognosis [54,55,56], while USP10 is downregulated in HCC and possesses various tumor suppressive functions [57]. Most of available studies are focused on G3BP1, which is frequently upregulated in a variety of cancers, where it seems to exert an oncogenic function [58]. Recent findings showed that RBPs, such as the Y-box binding protein (YBX1), which is overexpressed in many cancers including HCC [59,60], can upregulate G3BP1 expression by promoting its translation [61]. In HCC, G3BP1 induction was shown to contribute to cancer cells migration by increasing SLUG expression [62], but whether this was associated with increased SG formation was not investigated.
G3BPs are tightly regulated by post-translational modifications. Among them, phosphorylation of G3BP1 on Ser149 by Casein Kinase-2 (CK2) inhibits SG formation, as evidenced in osteosarcoma cells (U20S) [63]. CK2 is frequently overexpressed in HCC, correlates with a poor clinical outcome [64] and triggers various carcinogenic processes, including cell proliferation [65], resistance to death stimuli [66], and cancer cell migration/invasion [67]. Moreover, CK2 is also involved in hepatitis delta virus (HDV) replication [68] and NAFLD by promoting SIRT1 phosphorylation [69]. Acetylation of G3BP1 represents another regulatory mechanism of SG assembly. This regulation is mostly mediated by the CBP/P300 acetylase and the histone deacetylase 6 (HDAC6), which, respectively, inhibits and promotes SG assembly through acetylation/deacetylation of G3BP1 on lysine 376 (K376). Acetylation of K376 impairs G3BP1 interaction with its partners (e.g., USP10, CAPRIN1 or PABP1) and thus SG assembly [35]. Surprisingly, CPB/P300, HDAC6 and PABP1 are all upregulated in human HCC and correlate with a poor prognosis (Figure 2A,B) [70,71,72]. Finally, other post-translational modifications of G3BP1 can also modulate SG assembly, such as methylation by the oncogenes PRMT1 and 5, which are highly expressed in HCC and impair SG assembly [48,49,50] or demethylation by JMJD6 (Jumonji domain-containing 6), a tumor-promoting histone arginine demethylase favoring SG assembly and also overexpressed in HCC [37]. How these highly complex and antagonistic post-translational modifications of G3BP1, which are strongly deregulated in HCC, impact SG formation and functions in cancer cells was never experimentally investigated and outcomes in terms of formation of functional SGs in HCC cells remain purely speculative. Finally, the role of other G3BPs (2a and 2b), which may compensate or synergize with G3BP1, was likely underestimated in previous studies, and deserves further consideration [73].
3.1.3. T-Cell-Restricted Intracellular Antigen-1 (TIA1)
TIA1 is an important SG component, which binds to AU-rich sequences in the 3′UTRs of its target transcripts through three RRMs (RNA recognition motifs) [74]. TIA1 contains a PLD in its C-term required for its self-aggregation but also likely for interactions with other SG components. During cellular stress, TIA1, together with other co-factors (e.g., TIA1-related protein, TIAR), sequesters target mRNAs into SGs, where they are kept translationally silent [74]. TIA1 localization, and therefore presence in SGs, can be regulated through the control of its nuclear-cytoplasmic shuttling, where nuclear accumulation is Ran-GTP-dependent, while its export is Chromosomal Maintenance 1 (CRM1)-dependent [75]. However, whether this mechanism is important for SG formation in liver cells is currently unknown.
TIA1 is mostly considered as a tumor suppressor, due to its ability to reduce the translation of transcripts promoting carcinogenesis (e.g., cyclooxygenase-2, COX-2) in many cancers. Accordingly, TIA1 expression is frequently downregulated in human cancers and its loss correlates with a poor prognosis [76,77]. However, in the liver, TIA1 could exert a dual function since it appears also to behave as an oncogene. TIA1 mRNA expression is indeed upregulated in HCC and hepatic cancer cells [78] and can act as an oncogene due to its ability to silence the tumor suppressor IGFBP3 [79,80]. Such oncogenic activity remains to be confirmed in vivo as well as whether TIA1 is required for SG formation in hepatic cancer cells. Nevertheless, the activity of TIAR, which is an important co-factor of TIA1 involved in SG assembly, is inhibited in HCC by PHAROH lncRNA, thereby promoting MYC translation [81]. Whether this oncogenic effect is related to an impaired SG assembly remains, however, to be investigated.
3.1.4. DDX3 (DEAD-Box RNA Helicase 3 or CAP-Rf)
DDX3 is a ubiquitously expressed protein, which possesses ATPase and helicase activities and is involved in mRNA splicing and transcription [82]. Its helicase core contains two Recombinase A (RecA)-like domains, both of which display specific motifs responsible for RNA binding (reviewed in [83]). In Hela cells, DDX3 was shown to inhibit translation through its binding to eIF4E and PABP1 (Polyadenylate-Binding Protein 1) and thus to favor the first step of SGs formation [82]. This function was independent of its ATPase/helicase activity. Consistent with the role of SGs in this process, silencing of DDX3 importantly reduces SG formation and renders HeLa cells more sensitive to death stimuli [82]. In the liver, DDX3 appears to exert a tumor suppressive function in the liver [84] by promoting p21 upregulation in hepatic cancer cells or by repressing stemness [85]. Upon HCV infection, DDX3 interacts with the 3′UTR of HCV RNA and IKK-α and redistributes them into SGs [38], which then colocalize with the HCV core around lipid droplets. DDX3 functions importantly contribute to the HCV life cycle since silencing of DDX3 was shown to impair HCV replication. Moreover, DDX3 plays an important role in the HCV life cycle by interacting with the viral non-structural proteins NS5A and YBX1, as evidenced in Huh7.5.1 cells [86]. However, whether this function requires the formation of SGs is currently unknown. Surprisingly, whereas DDX3 is strongly downregulated in HBV-induced HCC, this is not the case in HCV-positive patients [87].
3.1.5. G4DNA (G-Quadruplex DNA Structures)
G4DNA are quartets of guanine organized as a planar ring and linked by hydrogen bonds [88]. Recent findings indicate that G4DNA structures promote SG formation in the context of oxidative stress and DNA damage, as evidenced in melanoma cells treated by hydrogen peroxide. Once formed, these structures are exported to the cytosol and interact with RBPs (e.g., TIA1, TIAR, YBX1, HuR). Similarly, to protein nucleators, overexpression of G4DNA is sufficient to trigger the formation of SGs [88]. It thus appears that G4DNA are important cellular factors involved in SG formation and cancer-related cellular processes, but their role in HCC has currently been not investigated.
3.1.6. tRNA-Derived Stress-Induced RNAs (tiRNAs)
tiRNAs are a class of non-coding RNAs, which displace the eIF4F complex from the m7GTP cap, thereby impairing cap-dependent translation and SG assembly [89]. This function has been observed with 5′-tiRNA but not 3′-tiRNA and relies on a 5′ terminal oligoguanine motif (5′-TOG), which fold in G-quadruplex structures [90]. This can interact with the translational silencer YBX1 via its cold shock domain [90]. tiRNAs are generated by angiogenin in harmful conditions by the cleavage of mature tRNAs within the anticodon loop [89]. Increasing evidence indicates that angiogenin is overexpressed in HCC and promotes cancer cells proliferation, migration, tumor vascularity, and EMT [91,92]. Whether these effects are linked to SG remains to be demonstrated, but it is likely that angiogenin overexpression may contribute to G4DNA synthesis and thus to SG formation.
3.1.7. m6A RNA-Related Proteins
N6-methyladenosine (m6A) is the most common RNA modification [93]. It regulates mRNA stability, splicing, and translation [94] and can promote the recruitment of modified RNAs to stress granules in response to oxidative stress [94]. RNA methylation is coordinated by a “writer” complex, composed of the methyltransferase-like 3 (METTL3), methyltransferase-like 14 (METTL14), and the Wilms’ tumor 1-associating protein (WTAP), while demethylation is enabled by an “eraser” complex composed of fat mass and obesity-associated protein (FTO) and AlkB homologue 5 (ALKBH5) [94]. A third class of proteins called “readers” recognize methylated RNA and can impact their fate. YTHDF1–3 proteins, for instance, bind to m6A RNA and interact with SG components (i.e., G3BPs) [95]. Recent studies show that YTHDF proteins contribute to stress granule formation and that SGs are enriched in m6A RNAs [96], but whether “readers” or other m6A RNA-related proteins regulate the formation of functional SGs was not investigated in HCC. However, alterations of their expression are frequently observed and correlate with a poor clinical outcome [97]. In particular, several m6A RNA-related proteins with relevant function in cancer were found upregulated in HCC, thus potentially contributing to SG assembly. These include (i) METTL3, which increases the glycolytic capacity of HCC cells [98]; (ii) WTAP, which promotes hepatic cancer cells proliferation and tumor growth in vivo [99]; and (iii) the demethylase FTO, which fosters HCC development [100]. Other members of these families were found on the contrary to be downregulated in HCC, such as METTL14 [101,102] and ALKBH5 [97,103], and to exert a tumor suppressive function. Alterations of “readers” were also uncovered in HCC, such as the upregulation of YTHDF1, which increases cell proliferation and migration [104,105,106,107]. YTHDF2′s role in liver cancer is still controversial, with some data showing its downregulation and consequential decreased cell proliferation during hypoxia in HCC cells lines [108], while others reported an upregulation of YTHDF2 in HCC correlating with a poor survival [109]. Finally, YTHDF3 has not yet been investigated in the context of liver cancer; however, in silico analyses do not demonstrate any unequivocal deregulation in HCC tumors (Figure 3). However, whether YTHDF members affects SG formation and function in liver cancer still remains obscure.
3.2. Mechanisms Regulating SG Clearance in HCC
Defective clearance of SGs may importantly contribute to their accumulation in cancer cells. Considering these potential mechanisms of SG clearance, the accumulation of SGs in cancer cells is again paradoxical. Indeed, autophagic fluxes are usually increased in HCC and contribute to cancer cell survival and resistance to therapeutic molecules (e.g., sorafenib) [19]. Moreover, the components of the HspB8-HSP70-Bag3 complex, which contribute to SG disassembly by promoting autophagic-dependent degradation of misfolded proteins, are frequently overexpressed in HCC [110]. The Valosin-containing protein (VCP/p97), an ATPase belonging to the AAA family (ATPase-associated with diverse cellular activities), also triggers SG disassembly by interacting with ubiquitinated proteins and promoting their degradation [111] but also by fostering autophagosome maturation [112]. Accordingly, alterations of VCP expression in several diseases (e.g., Paget disease, inclusion body myopathy) lead to an impairment of autophagy and SG accumulation. However, in HCC, VCP is upregulated, and its silencing reduces hepatic tumor progression in vivo [113]. Finally, kinases activating VCP and thus reducing SGs, such as ULK1 and ULK2 (Unc-51-like Kinase1/2) [114], are also upregulated in HCC tumors [115].
Based on these observations, it is currently unclear how SGs are stabilized in an environment favoring their clearance, indicating that additional but still unknown mechanisms regulating SG biogenesis and degradation remain to be uncovered.
3.3. RNA-Binding Proteins Controlling mRNA Stability/Translation
RBPs are major components of SGs and important regulators of gene expression. Among them, AU-rich element binding proteins (AUBPs) govern the mRNA stability and translation of 5 to 8% of the transcriptomes by binding to AU-rich elements present in the 3′UTR of target mRNAs [116,117,118,119]. AUBPs usually regulate the fate of target mRNAs by recruiting them within either P-bodies or SGs. Importantly, the expression/activity of several AUBPs is altered in pre-cancerous hepatic stages and HCC, thereby modulating the expression of cancer-related transcripts including those of key factors governing metabolic and inflammatory processes (Figure 2A and Figure 3). Below, the most important AUBPs and other RBPs present in SGs are discussed and are reported to play a significant role in HCC development.
3.3.1. TTP
TTP is encoded by ZFP36. It contains a zinc finger domain with a double zinc finger motif (Cys-Cys-Cys-His) responsible for RNA binding, three quadruple proline motifs responsible for the binding of the 4EHP (a.k.a. eIF4E2) -GYF2 (GRB10-interacting GYF protein 2) cap-binding complex, and a Not-1 binding domain [120,121,122]. It is usually described as a tumor suppressor, downregulated in many human cancers, and its level correlates with a poor clinical outcome [123,124,125]. TTP binds to mRNA transcripts and targets them for degradation in P-bodies. In physiological conditions, TTP is associated to P-bodies, whereas, upon stress, it can colocalize within SGs, as evidenced in Hela and COS7 cells [126,127]. It is therefore described as a protein shuttling between these two entities. TTP was proposed to be recruited to SGs upon specific cellular stress such as FCCP (carbonyl cyanide p-trifluoro-methoxyphenyl hydrazone)-induced energy deprivation (mitochondrial oxidative phosphorylation uncoupling) [126]. Translocation to SGs was prevented by MK2 kinase, which promotes TTP phosphorylation and sequestration by 14-3-3 protein, as evidenced in COS7 cells [126]. TTP was also reported to promote P-body and SG fusion [127]. In the liver, we recently showed that TTP promotes the development of non-alcoholic steatohepatitis (NASH), a condition from which HCC can develop [5]. In human HCC, TTP is downregulated at the protein level, and we could show using transgenic mouse models that TTP displays a dual oncogenic and tumor-suppressive role depending on the stage of the disease [125,128]. TTP overexpression was further shown by others to prevent activation of LX2 stellate cells suggesting that TTP may also restrain hepatic fibrosis development depending on the cell context and environment [129]. Different mechanisms regulating the expression/activity of TTP might in part be responsible for the different and sometimes antagonistic roles of TTP in liver pathologies. Indeed, suppression of TTP phosphorylation by MK2 inhibitor was shown to impair TTP activity in HCC cell lines [130], whereas TTP ability to regulate c-Myc was abrogated by methylation of a single CpG site in its promoter [131]. We also recently showed that the TTP expression is regulated by the HNF4α/EGR1 axis in Huh7 cells [125]. Although the precise role and functions of TTP in liver diseases and HCC have started to be delineated, whether TTP regulates SGs biogenesis or requires these entities for its functions, as suggested in non-hepatic cell lines [127], remains to be investigated in the liver.
3.3.2. BRF1 (Butyrate Response Factor 1, ZFP36L1)
BRF1 is encoded by ZFP36L1 and is a member of the ZFP36 family. Similarly to TTP, BRF1 binds to mRNAs through a tandem zinc finger domain with a double zinc finger motif [132,133], it promotes the mRNA decay of various cancer-related transcripts (e.g., VEGFA), and its expression is reduced in several cancers [132]. BRF1 was reported to decrease cell proliferation in a cyclin D1-dependent manner in colorectal cancer cells [134], and to regulate the expression of various mRNAs involved in hypoxia and cell cycle regulation (e.g., HIF1A, E2F1, etc.) in bladder cancer cells [135]. BRF-1 may also importantly participate to SG formation, as its overexpression can also trigger their assembly [127] but only fragmentary information is available about its role and functions in the liver. In hepatic cancer cells and primary hepatocytes, overexpression of BRF-1 was reported upon ethanol exposure, thus suggesting that it may contribute to ALD development and potentially HCC [136]. BRF1 was also suggested to regulate the bile acid metabolism, since it promotes the decay of CYP7A1 mRNA [137]. Similarly, to TTP, evidence indicates that BRF1 activity is regulated by phosphorylation. Protein kinase B (PKB) was shown to phosphorylate BRF1 on Ser203 and Ser92 phosphorylation in HIRc-B and mouse embryonic fibroblasts, thus inducing its binding to 14-3-3 and disruption of BRF1-dependent mRNA decay [138]. As for TTP, whether BRF1 and SG biogenesis/functions are linked in hepatic cells is currently unknown.
3.3.3. HuR
HuR is encoded by ELAVL1 and is an RNA-binding protein belonging to the embryonic-lethal abnormal vision in the drosophila (ELAV) family [139]. HuR is ubiquitously expressed and usually localized in the cell nucleus from where it translocates to the cytosol to control translation/stability of target mRNAs. The protein contains two tandem RRMs and a hinge region followed by a third RRM [140]. The hinge region is subjected to various post-translational modifications, including phosphorylation by various kinases within the HuR nucleocytoplasmic shuttling domain, which regulates the localization of the protein [140]. The stabilizing property of HuR on various mRNA transcripts relies on its ability to compete with or displace destabilizing factors, including miRNAs but also other AUBPs (i.e., TTP) sharing the same ARE binding site. HuR is overexpressed in several cancers including HCC and correlates with a poor clinical outcome [139,141,142]. The overexpression of HuR in cancer cells importantly promotes carcinogenesis by fostering the overexpression of oncogenes and the loss of tumor suppressors [141,142]. In stress conditions, HuR accumulates in SGs and promotes the stabilization of various oncogenic transcripts, thereby favoring cancer cell survival [143]. In the liver, HuR is upregulated in HCC patients and increases the expression of transcripts involved in cell cycle regulation (i.e., cyclin A and D1) [144], inhibits apoptosis through direct interaction with the FAS mRNA and by inhibiting caspase-3 activity [144,145], and facilitates hepatocyte de-differentiation by stabilizing the MAT2A transcript [146]. HuR is also an important promoter of sorafenib-induced ferroptosis, a type of cell death that can lead to fibrosis development in the liver [147]. However, despite the importance of SG formation in sorafenib resistance [19], no specific association between HuR and SG formation was reported in the liver. The regulation of HuR expression/activity has already been extensively reviewed elsewhere [140] and points to a strict control of its cellular localization and transcript binding, two processes that are also intimately linked to stress granule formation.
3.3.4. CUGBP2
CUGBP2 is encoded by CELF2. This RNA-binding protein contains three RNA recognition motif domains and modulates alternative splicing and mRNA translation. For instance, it regulates COX-2 mRNA translocation to stress granules in myoblastic H9c2 rat cells [148]. CUGBP2 expression is reduced in many cancers; however, its role in human HCC remains to be examined. Nevertheless, CUGBP2 was shown to regulate, together with APOBEC, C to U editing of apolipoprotein B (APOB), which is a protein important for sterol metabolism, i.e., VLDL, LDL, and HDL formation in the liver [149]. Of note, accumulation of cholesterol in hepatocytes fosters NAFLD progression toward NASH and HCC [150]. Inactivation of APOB is observed in human HCC and correlates with a poor prognosis [151]. However, whether the editing activity of CUGBP2/APOBEC significantly impact cholesterol metabolism in HCC cells remains to be demonstrated. The mechanisms of CUGBP2 regulation are poorly know, but its alternative splicing (i.e., exon 14 inclusion, which encodes for the first half of the third RRM) promotes the alternative splicing of insulin receptor transcript in HeLa cells [152]. The existence of such mechanisms is, however, currently unknown in the liver.
3.3.5. Musashi-1 (Msi-1)
Msi-1 is another important RBP promoting mRNA translation inhibition and decay. It contains two RRMs and a PABP binding domain [153]. Msi-1 expression is upregulated in a variety of cancers and promotes tumor development, due to its ability to control the expression of specific cancer-related factors (e.g., oncotachykinin) [154]. In the liver, little information is available, but recent studies indicate that Msi-1 is upregulated in HCC tissues as compared to non-tumoral adjacent tissues [155]. Furthermore, the overexpression of Msi-1 in hepatic cancer cells enhances cell proliferation [155]. This effect was associated with the ability of Msi-1 to downregulate the tumor suppressor APC, thereby triggering activation of the β-catenin pathway [155]. In stress conditions, Msi-1 is localized in SGs and contributes to chemoresistance, as evidenced in colorectal cancer [156], but such a functional link remains to be demonstrated in HCC.
4. Are SG Potential Therapeutic Targets in HCC?
Although it is far from clear whether SGs contribute to cancer survival and chemoresistance in HCC, studies in other cancers argue that this might indeed be the case [12,157]. Targeting the assembly of functional SGs may therefore represent a novel therapeutic approach to at least restore chemosensitivity of HCC towards drugs currently approved such as sorafenib.
4.1. Targeting SG Nucleators
Targeting SG nucleators may represent an efficient strategy to prevent SG assembly and thus potentially re-sensitize hepatic cancer cells to physiological death stimuli and anti-tumoral therapies (e.g., sorafenib) [19]. Moreover, given the reported roles of SGs in hepatic virus replication [158] targeting their assembly represents an alternative strategy to treat HCV and HBV infections [86,158]. The therapeutic virtues of various compounds affecting the expression/activity of SGs nucleators have been examined for various diseases (Table 1). In this regard, pharmacological inhibitors of SG nucleators are a promising strategy, although the effects of only a few of these compounds have been investigated in hepatic diseases. Alternatively, the delivery of siRNAs (i.e., aptamers) to reduce the expression of these SGs nucleating proteins, i.e., G3BP1, may represent an additional approach as demonstrated in colorectal cancer (CRC) [159]. Resveratrol [160], or epigallocatechin-gallate (EGCG) [161] were, for example, reported to reduce G3BP1 expression in lung (H1299 and CL13) cancer cells [162,163], while attenuating NAFLD [164] and restraining HCC development [165]. However, given the pleiotropic effects of these compounds in the cells, how G3BP1 inhibition contributes to their anti-tumoral activity remains to be evaluated. Small peptides inhibiting G3BP1 activity, such as GAP161, can also block SG assembly, as evidenced in colon cancer [58], but still not in HCC. Similarly, other compounds such as EMICORON or RHPS4, two G-quadruplex ligands or angiogenin inhibitors (e.g., chANG) can efficiently prevent SG formation [166,167], but their effects in HCC remain to be demonstrated. More recently, restoring DDX3 expression with rottlerin, a natural compound derived from Mallotus Philippinensis [168], or diosgenin [169] have been indicated for HCC treatment. However, if SGs are confirmed to mediate chemoresistance and tumor recurrence, the use of these compounds in clinic should be considered with care since DDX3 clearly promotes SG formation.
Table 1.
Drugs/therapeutics targeting SG components and key regulators of SG assembly.
| Molecule | Target | Cell Models | Tested in HCC Cells * |
|---|---|---|---|
| Resveratrol | G3BP1 [163] |
SK-MEL-5 human melanoma, HCT116 human colorectal carcinoma |
yes [170] |
| EGCG (epigallocatechin-gallate) | G3BP1 [162] |
H1299 and CL13 lung cancer cells | yes [165] |
| GAP161 | G3BP1/2 [58] |
HCT116 human colorectal carcinoma | no |
| EMICORON | G4DNA [167] |
BJ EHLT immortalized human fibroblasts, A90-LUC colorectal murine cells | no |
| chANG | Angiogenin [166] |
HT1080 (human fibrosarcoma), HM7 (human colorectal carcinoma), NIH/3T3 (Mouse fibroblast) | no |
| Rottlerin | DDX3 [168] |
QGY7703, SMMC7721 liver cancer cells | yes [168] |
| Diosgenin | DDX3 [169] |
HepG2, SMMC-7721 human liver cancer cells | yes [169] |
| Compound-C | AMPKα [21] |
COS7 cells monkey kidney fibroblasts | no |
| A452 | HDAC6 [171] |
Multiple myeloma cells: MM.1S, H929, BM-MSCs, PCS-500-012 cell lines | no |
| C1A | HDAC6 [172] |
Panel of cancer cell lines (colon, breast, endometrial, epidermal, lung, myeloma, neuroblastoma, ovarian, and prostate cancer cells) | no |
| ACY-1215 | HDAC6 [173] |
Lymphoma cells: OCI-LY10 | no |
| MPT0G612 | HDAC6 [174] |
Colon cancer cells: HCT-116, HT-29 and DLD-1 | no |
| OSS_128167 | SIRT6 [175] |
Large B-Cell Lymphoma: DLBCL cells | no |
| Vinblastine | Microtubules [30] |
CV-1 green monkey kidney fibroblasts | yes [176] |
| Nocodazole | Microtubules [30] |
CV-1 green monkey kidney fibroblasts | no |
| Paclitaxel | Microtubules [30] |
CV-1 green monkey kidney fibroblasts | yes [177] |
| Temsirolimus | mTOR inhibitor [176] |
Hep3B, HepG2, Huh7 | ys [176] |
| DHTS | HuR [178] |
Colon cancer cells, HCT116 | yes [179] |
| MS-444 | HuR [180] |
Colon cancer cells, HCT116 | no |
* This column indicates if the listed molecules have already been tested on HCC cells and does not necessarily indicate if the molecules are able to affect the targets in HCC.
4.2. Targeting Regulatory Pathways Involved in SG Assembly
Several pathways have been involved in the regulation of SG assembly. Among them, the AMPKα/mTORC1 pathway may represent an appealing target, as SG formation is usually associated to a decrease of mTORC1 and an activation of AMPKα [32]. Therefore, molecules activating or inhibiting mTORC1 or AMPKα may abrogate or promote SG assembly. In agreement, Compound-C, an inhibitor of AMPKα, prevents SGs induced by cold exposure in yeast [21] and displays anti-cancerous properties [181] (Table 1).
However, the role of mTORC1/AMPKα signaling on SG assembly is still unclear and controversial, as other studies have documented that mTORC1 activation is necessary for SG assembly in breast cancer [182]. In addition, in the liver, inhibition of the mTOR pathway with rapamycin-derived compounds to fight HCC is poorly effective [183]. Finally, the effect of AMPKα activators (e.g., metformin, or physical activity, which possess important anti-tumor properties in HCC [184,185]) on SG assembly, remains to be investigated.
4.3. Targeting Post-Translational Modification of SG Components
As discussed before, both HDAC6 and SIRT6 promote SG assembly [186]. HDAC6 inhibitors may therefore represent a potential strategy to impair SG formation in HCC. Among the HDAC6 inhibitors (e.g., A452 [171], C1A [187], ACY-1215 [173], MPT0G612 [174], several possess strong anti-tumor properties [188], but whether this effect is linked to an impairment of SG assembly is currently unknown (Table 1). Similarly, for SIRT6, very few inhibitors have been developed (e.g., OSS_128167) [175,189] and although they display anticancer properties (e.g., large B-cell lymphoma) [175], their impact on hepatic cancer cells is currently unknown.
4.4. Increasing SG Clearance
Favoring clearance of SGs may represent another relevant approach to limit SG accumulation in cancer cells. As already mentioned, the clearance of SGs is partially mediated by autophagy [190]; increasing autophagic flux in cancer cells may therefore lower the amount of SGs and re-sensitize cancer cells to chemotherapy. In the liver, autophagy regulates multiple functions, including lipid metabolism, insulin sensitivity, hepatocytes cell death, or sorafenib resistance in HCC [191,192]. In HCC, autophagy is currently considered as a survival mechanism of cancer cells [193]; therefore, several autophagic inhibitors have been developed and proposed for HCC treatment in preclinical models [194]. However, the impact of autophagy inhibition on SG dynamics is currently unknown and should be carefully considered given the potential role of SGs in HCC cells survival and tumor recurrence.
4.5. Targeting Microtubules
Functional microtubules and motor proteins are required for the transport of RNPs during SG assembly [29]. Destabilizing microtubules by pharmacologic approaches (e.g., vinblastine, nocodazole) efficiently prevents SG assembly [29,30]. On the contrary, stabilizing molecules, such as paclitaxel, trigger SG formation. In HCC, the combination of vinblastine with temsirolimus (an mTOR inhibitor) displays anticancer properties [176]. However, the impact of such a combination on SG dynamics remains to be investigated, as well as the real importance of SG modulation by these compounds as compared to other strongly affected cellular processes [195].
4.6. Targeting AUBPs Associated with SGs
Several AUBPs control the stability and translation of transcripts involved in inflammatory, metabolic, or cancer-related processes. Targeting these proteins may therefore represents a potential therapeutic option. Although these proteins were originally considered “undruggable”, specific inhibitors of HuR (e.g., DHTS, MS444) have been developed and inhibit HuR activity by interacting with its RNA-binding domains in the case of DHTS and by inhibiting its relocation to the cytoplasm, thereby enabling transcript association with P-bodies, in the case of MS-444 [178,180]. Interestingly, DHTS suppresses the growth of HCC cell lines through the JAK2/STAT3 pathway [179]. MS-444 has not yet been tested in liver diseases nor HCC. Given the anticancer properties of these molecules [196], investigating the precise mechanisms involved in their anti-tumoral activity and how the latter is related to SGs biogenesis deserves in-depth investigations prior to consideration for clinical applications.
Except for a few compounds targeting key components of SGs specifically (e.g., G3BP1, HuR, see Table 1), a number of drugs and clinically approved therapeutics have been found to affect, although mostly indirectly, the expression/activity of SG components or key regulators of these latter, which have been described in previous sections of this review. Most of the drugs/therapeutics described in Table 1 have well recognized anti-tumoral properties and have been documented to affect a wide variety of cellular cancer-related processes. However, to which extent inhibition of SG formation, or functional impairment of specific SG components, contribute to the anti-tumoral effects of these drugs/therapeutics remains unknown to date. Further investigations of the role and functions of SGs in HCC are therefore required prior to envisage any therapeutic interventions targeting these structures specifically. Finally, once the presence or absence of SGs in cancer cells, including HCC, has been firmly validated, their relevance as prognostic/diagnostic biomarkers for cancer risk, staging, and chemoresistance should also be evaluated and thus may represent potential biomarkers of chemoresistance, as suggested for sorafenib in HCC [19].
5. Conclusions
Although the formation of SGs has been associated with cell survival and resistance to anti-tumoral treatments in several cancers, the role of these cytoplasmic entities in HCC remains enigmatic. Several key components of the SGs, implicated in their nucleation and functions, are strongly deregulated in hepatic cancer cells, suggesting that both the formation and functions of these entities are modulated with cell transformation and the tumor microenvironment. However, based on our still fragmentary knowledge about the roles and functions of SG components and their involvement in mechanisms governing the formation and integrity of SGs, whether they represent a structural hallmark of cancer cells, e.g., in the liver, remains an open question. Several factors considered as key elements of SGs still have a poorly characterized function in HCC and most of them likely also exert part of their functions independently of their presence in mature SGs. In addition, it is probable that several factors exert redundant functions in nucleating and maturing SGs, which renders the analyses of the role of these structures in pathophysiological situations more complex. In HCC, the expression of SG components is clearly altered, and their functions appears to be regulated by multiple and complex antagonistic mechanisms, as well as the formation of mature SGs. In addition, most of the studies performed to date were using in vitro hepatic cell systems, which could be irrelevant as compared to the in vivo pathophysiological situation both in animal models of HCC or in patients. Future analyses should therefore first clearly investigate whether SG formation occurs in precancerous stages of the liver and/or preferentially in transformed hepatocytes as compared to normal cells. Then, additional work will be required to precisely understand how SG components regulate their assembly in a single entity and whether the liquid-phase organization of these SG components in the cytoplasm is required for their functions. Finally, the current dogmatic view of SGs as oncogenic cytoplasmic entities promoting chemoresistance and cell survival must be taken with caution, at least in the case of liver cancer. Verification of this hypothesis should set the basis for future efforts aiming at targeting SGs for therapeutic purposes or using these structures as biomarkers to predict cancer development stages and therapeutic outcomes.
Acknowledgments
Figures were created using Servier Medical Art (https://smart.servier.com/, accessed on 1 April 2021).
Abbreviations
ALD: alcoholic liver disease; AMPK: AMP-activated protein kinase; ARE: AU-rich Element; ATX-2: ataxin 2; AUBP: Adenylate-Uridylate-rich elements binding protein; Bag3: BCL2-associated athanogene 3; BRF1: Butyrate response factor 1; CK2: casein kinase 2; COX: cyclooxygenase; CUGBP2: CUG triplet repeat-binding protein 2; DDX3: DEAD-Box RNA helicase 3; DUF: domain of unknown function; DYRK3: Dual Specificity Tyrosine Phosphorylation Regulated Kinase 3; ELAV: embryonic-lethal abnormal vision in drosophila; ER: endoplasmic reticulum; G3BP: Ras GTPase-activating protein-binding protein; GO: gene ontology; HBV: hepatitis B virus; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; HDAC: histone deacetylase; HDV: hepatitis delta virus; HSP70: heat shock proteins 70; HspB8: Heat Shock Protein Family B (Small) Member 8; IDD: intrinsically disordered domain; JMJD6: Jumonji domain-containing 6; KEGG: Kyoto encyclopedia of genes and genomes; KSRP: K-homology splicing regulator protein; LDL: low density lipoprotein; LLPS: liquid–liquid phase separation; miRs: microRNAs; Msi-1: Musashi; mTORC1: mammalian target of rapamycin complex 1; NAFLD: non-alcoholic fatty liver disease; ONC: oncogenes; PABP1: Polyadenylate-Binding Protein 1; P-Bodies: Processing-Bodies; PI3K: phosphoinositide 3-kinase; PLD: prion-like domain; PRMT: protein arginine methyltransferase; PTEN: Phosphatase and TENsin homolog; RACK1: Receptor for Activated C Kinase; RBP: RNA-binding protein; RGG: Arginine-Glycine-Glycine; RNP: ribonucleoprotein; RRM: RNA-recognition motif; SG: stress granule; SIRT6: sirtuin 6; TDP-43: TAR DNA-binding protein 43; TIA1: T-cell-restricted intracellular antigen-1; TIAR: TIA-1-related; TTP: tristetraprolin; UBA: ubiquitin-associated domain; UBAP2L: Ubiquitin-associated protein 2-like; ULK: Unc-51-like kinase; USP: Ubiquitin Specific Peptidase; UTR: Untranslated Region; VCP: Valosin-containing protein; VEGF: Vascular endothelial growth factor; YBX1: Y-box binding protein
Author Contributions
C.S., D.D. and M.F.: conceptualization, writing—review and editing; C.S. and M.F.: supervision, C.S. and M.F.: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Geneva Cancer League (“Ligue Genevoise Contre le Cancer”) (Grant no. 1514 and 1711), the Swiss Cancer Research Foundation (Grant no. KFS-4094-02-2017) and by the Swiss National Science Foundation (Grant no. 320030-200530).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The Stress granule proteome (Figure 1) was obtained from https://msgp.pt/, accessed on 1 April 2021. Genes associated to hepatocellular carcinoma (HCC, Figure 1) were obtained with the MetaCore database (https://portal.genego.com/, accessed on 1 April 2021). Expression of SG components and survival information in HCC patients (Figure 3) were obtained from the CancerLivER database (https://webs.iiitd.edu.in/raghava/cancerliver/index.html, accessed on 1 April 2021) and the Gepia database (http://gepia.cancer-pku.cn/, accessed on 1 April 2021), respectively.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McGlynn K.A., Petrick J.L., El-Serag H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73(Suppl. S1):4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulik L., El-Serag H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477–491.e1. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown G.T., Kleiner D.E. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism. 2016;65:1080–1086. doi: 10.1016/j.metabol.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsochatzis E.A., Bosch J., Burroughs A.K. Liver cirrhosis. Lancet. 2014;383:1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 5.Fattovich G., Stroffolini T., Zagni I., Donato F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology. 2004;127(Suppl. S1):S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Cui J., Placzek W.J. Post-Transcriptional Regulation of Anti-Apoptotic BCL2 Family Members. Int. J. Mol. Sci. 2018;19:308. doi: 10.3390/ijms19010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., George J., Bugianesi E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 8.Seitz H.K., Bataller R., Cortez-Pinto H., Gao B., Gual A., Lackner C., Mathurin P., Mueller S., Szabo G., Tsukamoto H. Alcoholic liver disease. Nat. Rev. Dis. Primers. 2018;4:16. doi: 10.1038/s41572-018-0014-7. [DOI] [PubMed] [Google Scholar]
- 9.Wong T.C., Lo C.M. Resection strategies for hepatocellular carcinoma. Semin. Liver Dis. 2013;33:273–281. doi: 10.1055/s-0033-1351782. [DOI] [PubMed] [Google Scholar]
- 10.Santopaolo F., Lenci I., Milana M., Manzia T.M., Baiocchi L. Liver transplantation for hepatocellular carcinoma: Where do we stand? World J. Gastroenterol. 2019;25:2591–2602. doi: 10.3748/wjg.v25.i21.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sobolewski C., Calo N., Portius D., Foti M. MicroRNAs in fatty liver disease. Semin. Liver Dis. 2015;35:12–25. doi: 10.1055/s-0034-1397345. [DOI] [PubMed] [Google Scholar]
- 12.Anderson P., Kedersha N., Ivanov P. Stress granules, P-bodies and cancer. Biochim. Biophys. Acta. 2015;1849:861–870. doi: 10.1016/j.bbagrm.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson P., Kedersha N. Stress granules: The Tao of RNA triage. Trends Biochem. Sci. 2008;33:141–150. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Gao X., Jiang L., Gong Y., Chen X., Ying M., Zhu H., He Q., Yang B., Cao J. Stress granule: A promising target for cancer treatment. Br. J. Pharm. 2019;176:4421–4433. doi: 10.1111/bph.14790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubstenberger A., Courel M., Benard M., Souquere S., Ernoult-Lange M., Chouaib R., Yi Z., Morlot J.B., Munier A., Fradet M., et al. P-Body Purification Reveals the Condensation of Repressed mRNA Regulons. Mol. Cell. 2017;68:144–157.e5. doi: 10.1016/j.molcel.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Riggs C.L., Kedersha N., Ivanov P., Anderson P. Mammalian stress granules and P bodies at a glance. J. Cell Sci. 2020;133:jcs242487. doi: 10.1242/jcs.242487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omer A., Patel D., Lian X.J., Sadek J., Di Marco S., Pause A., Gorospe M., Gallouzi I.E. Stress granules counteract senescence by sequestration of PAI-1. EMBO Rep. 2018;19:e44722. doi: 10.15252/embr.201744722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arimoto K., Fukuda H., Imajoh-Ohmi S., Saito H., Takekawa M. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat. Cell Biol. 2008;10:1324–1332. doi: 10.1038/ncb1791. [DOI] [PubMed] [Google Scholar]
- 19.Adjibade P., St-Sauveur V.G., Quevillon Huberdeau M., Fournier M.J., Savard A., Coudert L., Khandjian E.W., Mazroui R. Sorafenib, a multikinase inhibitor, induces formation of stress granules in hepatocarcinoma cells. Oncotarget. 2015;6:43927–43943. doi: 10.18632/oncotarget.5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcelo A., Koppenol R., de Almeida L.P., Matos C.A., Nobrega C. Stress granules, RNA-binding proteins and polyglutamine diseases: Too much aggregation? Cell Death Dis. 2021;12:592. doi: 10.1038/s41419-021-03873-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann S., Cherkasova V., Bankhead P., Bukau B., Stoecklin G. Translation suppression promotes stress granule formation and cell survival in response to cold shock. Mol. Biol. Cell. 2012;23:3786–3800. doi: 10.1091/mbc.e12-04-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai Y., Dong Z., Shang Q., Zhao H., Wang L., Guo C., Gao F., Zhang L., Wang Q. Pdcd4 Is Involved in the Formation of Stress Granule in Response to Oxidized Low-Density Lipoprotein or High-Fat Diet. PLoS ONE. 2016;11:e0159568. doi: 10.1371/journal.pone.0159568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahboubi H., Stochaj U. Cytoplasmic stress granules: Dynamic modulators of cell signaling and disease. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:884–895. doi: 10.1016/j.bbadis.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 24.Walter W., Sanchez-Cabo F., Ricote M. GOplot: An R package for visually combining expression data with functional analysis. Bioinformatics. 2015;31:2912–2914. doi: 10.1093/bioinformatics/btv300. [DOI] [PubMed] [Google Scholar]
- 25.Lever J., Zhao E.Y., Grewal J., Jones M.R., Jones S.J.M. CancerMine: A literature-mined resource for drivers, oncogenes and tumor suppressors in cancer. Nat. Methods. 2019;16:505–507. doi: 10.1038/s41592-019-0422-y. [DOI] [PubMed] [Google Scholar]
- 26.Panas M.D., Ivanov P., Anderson P. Mechanistic insights into mammalian stress granule dynamics. J. Cell Biol. 2016;215:313–323. doi: 10.1083/jcb.201609081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kedersha N., Ivanov P., Anderson P. Stress granules and cell signaling: More than just a passing phase? Trends Biochem. Sci. 2013;38:494–506. doi: 10.1016/j.tibs.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molliex A., Temirov J., Lee J., Coughlin M., Kanagaraj A.P., Kim H.J., Mittag T., Taylor J.P. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell. 2015;163:123–133. doi: 10.1016/j.cell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chernov K.G., Barbet A., Hamon L., Ovchinnikov L.P., Curmi P.A., Pastre D. Role of microtubules in stress granule assembly: Microtubule dynamical instability favors the formation of micrometric stress granules in cells. J. Biol. Chem. 2009;284:36569–36580. doi: 10.1074/jbc.M109.042879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanov P.A., Chudinova E.M., Nadezhdina E.S. Disruption of microtubules inhibits cytoplasmic ribonucleoprotein stress granule formation. Exp. Cell Res. 2003;290:227–233. doi: 10.1016/S0014-4827(03)00290-8. [DOI] [PubMed] [Google Scholar]
- 31.Hubbert C., Guardiola A., Shao R., Kawaguchi Y., Ito A., Nixon A., Yoshida M., Wang X.F., Yao T.P. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 32.Heberle A.M., Razquin Navas P., Langelaar-Makkinje M., Kasack K., Sadik A., Faessler E., Hahn U., Marx-Stoelting P., Opitz C.A., Sers C., et al. The PI3K and MAPK/p38 pathways control stress granule assembly in a hierarchical manner. Life Sci. Alliance. 2019;2 doi: 10.26508/lsa.201800257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sfakianos A.P., Mellor L.E., Pang Y.F., Kritsiligkou P., Needs H., Abou-Hamdan H., Desaubry L., Poulin G.B., Ashe M.P., Whitmarsh A.J. The mTOR-S6 kinase pathway promotes stress granule assembly. Cell Death Differ. 2018;25:1766–1780. doi: 10.1038/s41418-018-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fournier M.J., Coudert L., Mellaoui S., Adjibade P., Gareau C., Cote M.F., Sonenberg N., Gaudreault R.C., Mazroui R. Inactivation of the mTORC1-eukaryotic translation initiation factor 4E pathway alters stress granule formation. Mol. Cell. Biol. 2013;33:2285–2301. doi: 10.1128/MCB.01517-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gal J., Chen J., Na D.Y., Tichacek L., Barnett K.R., Zhu H. The Acetylation of Lysine-376 of G3BP1 Regulates RNA Binding and Stress Granule Dynamics. Mol. Cell. Biol. 2019;39:e00052-19. doi: 10.1128/MCB.00052-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai W.C., Gayatri S., Reineke L.C., Sbardella G., Bedford M.T., Lloyd R.E. Arginine Demethylation of G3BP1 Promotes Stress Granule Assembly. J. Biol. Chem. 2016;291:22671–22685. doi: 10.1074/jbc.M116.739573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai W.C., Reineke L.C., Jain A., Jung S.Y., Lloyd R.E. Histone arginine demethylase JMJD6 is linked to stress granule assembly through demethylation of the stress granule-nucleating protein G3BP1. J. Biol. Chem. 2017;292:18886–18896. doi: 10.1074/jbc.M117.800706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pene V., Li Q., Sodroski C., Hsu C.S., Liang T.J. Dynamic Interaction of Stress Granules, DDX3X, and IKK-alpha Mediates Multiple Functions in Hepatitis C Virus Infection. J. Virol. 2015;89:5462–5477. doi: 10.1128/JVI.03197-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herman A.B., Silva Afonso M., Kelemen S.E., Ray M., Vrakas C.N., Burke A.C., Scalia R.G., Moore K., Autieri M.V. Regulation of Stress Granule Formation by Inflammation, Vascular Injury, and Atherosclerosis. Arter. Thromb. Vasc. Biol. 2019;39:2014–2027. doi: 10.1161/ATVBAHA.119.313034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang C., Chen Y., Dai H., Zhang H., Xie M., Zhang H., Chen F., Kang X., Bai X., Chen Z. UBAP2L arginine methylation by PRMT1 modulates stress granule assembly. Cell Death Differ. 2020;27:227–241. doi: 10.1038/s41418-019-0350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Youn J.Y., Dunham W.H., Hong S.J., Knight J.D.R., Bashkurov M., Chen G.I., Bagci H., Rathod B., MacLeod G., Eng S.W.M., et al. High-Density Proximity Mapping Reveals the Subcellular Organization of mRNA-Associated Granules and Bodies. Mol. Cell. 2018;69:517–532.e11. doi: 10.1016/j.molcel.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 42.Cirillo L., Cieren A., Barbieri S., Khong A., Schwager F., Parker R., Gotta M. UBAP2L Forms Distinct Cores that Act in Nucleating Stress Granules Upstream of G3BP1. Curr. Biol. 2020;30:698–707.e6. doi: 10.1016/j.cub.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 43.He J., Chen Y., Cai L., Li Z., Guo X. UBAP2L silencing inhibits cell proliferation and G2/M phase transition in breast cancer. Breast Cancer. 2018;25:224–232. doi: 10.1007/s12282-017-0820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li D., Huang Y. Knockdown of ubiquitin associated protein 2-like inhibits the growth and migration of prostate cancer cells. Oncol. Rep. 2014;32:1578–1584. doi: 10.3892/or.2014.3360. [DOI] [PubMed] [Google Scholar]
- 45.Li Q., Wang W., Hu Y.C., Yin T.T., He J. Knockdown of Ubiquitin Associated Protein 2-Like (UBAP2L) Inhibits Growth and Metastasis of Hepatocellular Carcinoma. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018;24:7109–7118. doi: 10.12659/MSM.912861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang W., Zhang M., Peng Y., He J. Ubiquitin Associated Protein 2-Like (UBAP2L) Overexpression in Patients with Hepatocellular Carcinoma and its Clinical Significance. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017;23:4779–4788. doi: 10.12659/MSM.907071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye T., Xu J., Du L., Mo W., Liang Y., Xia J. Downregulation of UBAP2L Inhibits the Epithelial-Mesenchymal Transition via SNAIL1 Regulation in Hepatocellular Carcinoma Cells. Cell Physiol. Biochem. 2017;41:1584–1595. doi: 10.1159/000470824. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X.P., Jiang Y.B., Zhong C.Q., Ma N., Zhang E.B., Zhang F., Li J.J., Deng Y.Z., Wang K., Xie D., et al. PRMT1 Promoted HCC Growth and Metastasis In Vitro and In Vivo via Activating the STAT3 Signalling Pathway. Cell Physiol. Biochem. 2018;47:1643–1654. doi: 10.1159/000490983. [DOI] [PubMed] [Google Scholar]
- 49.Wei H., Liu Y., Min J., Zhang Y., Wang J., Zhou M., Xiong E., Yu G., Zhou H., He J., et al. Protein arginine methyltransferase 1 promotes epithelial-mesenchymal transition via TGF-beta1/Smad pathway in hepatic carcinoma cells. Neoplasma. 2019;66:918–929. doi: 10.4149/neo_2018_181226N999. [DOI] [PubMed] [Google Scholar]
- 50.Ryu J.W., Kim S.K., Son M.Y., Jeon S.J., Oh J.H., Lim J.H., Cho S., Jung C.R., Hamamoto R., Kim D.S., et al. Novel prognostic marker PRMT1 regulates cell growth via downregulation of CDKN1A in HCC. Oncotarget. 2017;8:115444–115455. doi: 10.18632/oncotarget.23296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.French J., Stirling R., Walsh M., Kennedy H.D. The expression of Ras-GTPase activating protein SH3 domain-binding proteins, G3BPs, in human breast cancers. Histochem. J. 2002;34:223–231. doi: 10.1023/A:1021737413055. [DOI] [PubMed] [Google Scholar]
- 52.Tourriere H., Chebli K., Zekri L., Courselaud B., Blanchard J.M., Bertrand E., Tazi J. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J. Cell Biol. 2003;160:823–831. doi: 10.1083/jcb.200212128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Kedersha N., Panas M.D., Achorn C.A., Lyons S., Tisdale S., Hickman T., Thomas M., Lieberman J., McInerney G.M., Ivanov P., et al. G3BP-Caprin1-USP10 complexes mediate stress granule condensation and associate with 40S subunits. J. Cell Biol. 2016;212:845–860. doi: 10.1083/jcb.201508028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan N., Dai L., Liu X., Pan G., Chen H., Huang J., Xu Q. Upregulation of caprin1 expression is associated with poor prognosis in hepatocellular carcinoma. Pathol. Res. Pract. 2017;213:1563–1567. doi: 10.1016/j.prp.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 55.Li X.Q., Song J.Y., Lv W., Zhang D., Wu J.Z. Circular circ_0000885 promotes hepatocellular carcinoma proliferation by epigenetically upregulating Caprin1. Eur. Rev. Med. Pharm. Sci. 2019;23:7848–7854. doi: 10.26355/eurrev_201909_18994. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y., You W., Zhou H., Chen Z., Han G., Zuo X., Zhang L., Wu J., Wang X. Downregulated miR-621 promotes cell proliferation via targeting CAPRIN1 in hepatocellular carcinoma. Am. J. Cancer Res. 2018;8:2116–2129. [PMC free article] [PubMed] [Google Scholar]
- 57.Lu C., Ning Z., Wang A., Chen D., Liu X., Xia T., Tekcham D.S., Wang W., Li T., Liu X., et al. USP10 suppresses tumor progression by inhibiting mTOR activation in hepatocellular carcinoma. Cancer Lett. 2018;436:139–148. doi: 10.1016/j.canlet.2018.07.032. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H., Zhang S., He H., Zhao W., Chen J., Shao R.G. GAP161 targets and downregulates G3BP to suppress cell growth and potentiate cisplaitin-mediated cytotoxicity to colon carcinoma HCT116 cells. Cancer Sci. 2012;103:1848–1856. doi: 10.1111/j.1349-7006.2012.02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chao H.M., Huang H.X., Chang P.H., Tseng K.C., Miyajima A., Chern E. Y-box binding protein-1 promotes hepatocellular carcinoma-initiating cell progression and tumorigenesis via Wnt/beta-catenin pathway. Oncotarget. 2017;8:2604–2616. doi: 10.18632/oncotarget.13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liao L.Z., Chen C.T., Li N.C., Lin L.C., Huang B.S., Chang Y.H., Chow L.P. Y-Box Binding Protein-1 Promotes Epithelial-Mesenchymal Transition in Sorafenib-Resistant Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2020;22:224. doi: 10.3390/ijms22010224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Somasekharan S.P., El-Naggar A., Leprivier G., Cheng H., Hajee S., Grunewald T.G., Zhang F., Ng T., Delattre O., Evdokimova V., et al. YB-1 regulates stress granule formation and tumor progression by translationally activating G3BP1. J. Cell Biol. 2015;208:913–929. doi: 10.1083/jcb.201411047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dou N., Chen J., Yu S., Gao Y., Li Y. G3BP1 contributes to tumor metastasis via upregulation of Slug expression in hepatocellular carcinoma. Am. J. Cancer Res. 2016;6:2641–2650. [PMC free article] [PubMed] [Google Scholar]
- 63.Reineke L.C., Tsai W.C., Jain A., Kaelber J.T., Jung S.Y., Lloyd R.E. Casein Kinase 2 Is Linked to Stress Granule Dynamics through Phosphorylation of the Stress Granule Nucleating Protein G3BP1. Mol. Cell. Biol. 2017;37:e00596-16. doi: 10.1128/MCB.00596-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang H.X., Jiang S.S., Zhang X.F., Zhou Z.Q., Pan Q.Z., Chen C.L., Zhao J.J., Tang Y., Xia J.C., Weng D.S. Protein kinase CK2alpha catalytic subunit is overexpressed and serves as an unfavorable prognostic marker in primary hepatocellular carcinoma. Oncotarget. 2015;6:34800–34817. doi: 10.18632/oncotarget.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu W., Ding X., Chen F., Liu M., Shen S., Gu X., Yu L. The phosphorylation of SEPT2 on Ser218 by casein kinase 2 is important to hepatoma carcinoma cell proliferation. Mol. Cell. Biochem. 2009;325:61–67. doi: 10.1007/s11010-008-0020-2. [DOI] [PubMed] [Google Scholar]
- 66.Kim H.R., Kim K., Lee K.H., Kim S.J., Kim J. Inhibition of casein kinase 2 enhances the death ligand- and natural kiler cell-induced hepatocellular carcinoma cell death. Clin. Exp. Immunol. 2008;152:336–344. doi: 10.1111/j.1365-2249.2008.03622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu D., Sui C., Meng F., Tian X., Fu L., Li Y., Qi X., Cui H., Liu Y., Jiang Y. Stable knockdown of protein kinase CK2-alpha (CK2alpha) inhibits migration and invasion and induces inactivation of hedgehog signaling pathway in hepatocellular carcinoma Hep G2 cells. Acta Histochem. 2014;116:1501–1508. doi: 10.1016/j.acthis.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 68.Yeh T.S., Lo S.J., Chen P.J., Lee Y.H. Casein kinase II and protein kinase C modulate hepatitis delta virus RNA replication but not empty viral particle assembly. J. Virol. 1996;70:6190–6198. doi: 10.1128/jvi.70.9.6190-6198.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi S.E., Kwon S., Seok S., Xiao Z., Lee K.W., Kang Y., Li X., Shinoda K., Kajimura S., Kemper B., et al. Obesity-Linked Phosphorylation of SIRT1 by Casein Kinase 2 Inhibits Its Nuclear Localization and Promotes Fatty Liver. Mol. Cell. Biol. 2017;37:e00006-17. doi: 10.1128/MCB.00006-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanno K., Kanno S., Nitta H., Uesugi N., Sugai T., Masuda T., Wakabayashi G., Maesawa C. Overexpression of histone deacetylase 6 contributes to accelerated migration and invasion activity of hepatocellular carcinoma cells. Oncol. Rep. 2012;28:867–873. doi: 10.3892/or.2012.1898. [DOI] [PubMed] [Google Scholar]
- 71.Wang Y., Toh H.C., Chow P., Chung A.Y., Meyers D.J., Cole P.A., Ooi L.L., Lee C.G. MicroRNA-224 is up-regulated in hepatocellular carcinoma through epigenetic mechanisms. FASEB J. 2012;26:3032–3041. doi: 10.1096/fj.11-201855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu Y., Ming Q. Expression and prognostic roles of PABPC1 in hepatocellular carcinoma. Int. J. Surg. 2020;84:3–12. doi: 10.1016/j.ijsu.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 73.Matsuki H., Takahashi M., Higuchi M., Makokha G.N., Oie M., Fujii M. Both G3BP1 and G3BP2 contribute to stress granule formation. Genes Cells. 2013;18:135–146. doi: 10.1111/gtc.12023. [DOI] [PubMed] [Google Scholar]
- 74.Gilks N., Kedersha N., Ayodele M., Shen L., Stoecklin G., Dember L.M., Anderson P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol. Biol. Cell. 2004;15:5383–5398. doi: 10.1091/mbc.e04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang T., Delestienne N., Huez G., Kruys V., Gueydan C. Identification of the sequence determinants mediating the nucleo-cytoplasmic shuttling of TIAR and TIA-1 RNA-binding proteins. J. Cell Sci. 2005;118 Pt 23:5453–5463. doi: 10.1242/jcs.02669. [DOI] [PubMed] [Google Scholar]
- 76.Liu Y., Liu R., Yang F., Cheng R., Chen X., Cui S., Gu Y., Sun W., You C., Liu Z., et al. miR-19a promotes colorectal cancer proliferation and migration by targeting TIA1. Mol. Cancer. 2017;16:53. doi: 10.1186/s12943-017-0625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanchez-Jimenez C., Ludena M.D., Izquierdo J.M. T-cell intracellular antigens function as tumor suppressor genes. Cell Death Dis. 2015;6:e1669. doi: 10.1038/cddis.2015.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tak H., Kang H., Ji E., Hong Y., Kim W., Lee E.K. Potential use of TIA-1, MFF, microRNA-200a-3p, and microRNA-27 as a novel marker for hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2018;497:1117–1122. doi: 10.1016/j.bbrc.2018.02.189. [DOI] [PubMed] [Google Scholar]
- 79.Frankel J., Turf R.M., Nichols D. Complications of calcaneal osteotomies. Clin. Podiatr. Med. Surg. 1991;8:409–423. [PubMed] [Google Scholar]
- 80.Subramaniam K., Ooi L.L., Hui K.M. Transcriptional down-regulation of IGFBP-3 in human hepatocellular carcinoma cells is mediated by the binding of TIA-1 to its AT-rich element in the 3′-untranslated region. Cancer Lett. 2010;297:259–268. doi: 10.1016/j.canlet.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 81.Yu A.T., Berasain C., Bhatia S., Rivera K., Liu B., Rigo F., Pappin D.J., Spector D.L. PHAROH lncRNA regulates Myc translation in hepatocellular carcinoma via sequestering TIAR. Elife. 2021;10:e68263. doi: 10.7554/eLife.68263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shih J.W., Wang W.T., Tsai T.Y., Kuo C.Y., Li H.K., Wu Lee Y.H. Critical roles of RNA helicase DDX3 and its interactions with eIF4E/PABP1 in stress granule assembly and stress response. Biochem. J. 2012;441:119–129. doi: 10.1042/BJ20110739. [DOI] [PubMed] [Google Scholar]
- 83.Mo J., Liang H., Su C., Li P., Chen J., Zhang B. DDX3X: Structure, physiologic functions and cancer. Mol. Cancer. 2021;20:38. doi: 10.1186/s12943-021-01325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chao C.H., Chen C.M., Cheng P.L., Shih J.W., Tsou A.P., Lee Y.H. DDX3, a DEAD box RNA helicase with tumor growth-suppressive property and transcriptional regulation activity of the p21waf1/cip1 promoter, is a candidate tumor suppressor. Cancer Res. 2006;66:6579–6588. doi: 10.1158/0008-5472.CAN-05-2415. [DOI] [PubMed] [Google Scholar]
- 85.Li H.K., Mai R.T., Huang H.D., Chou C.H., Chang Y.A., Chang Y.W., You L.R., Chen C.M., Lee Y.H. DDX3 Represses Stemness by Epigenetically Modulating Tumor-suppressive miRNAs in Hepatocellular Carcinoma. Sci. Rep. 2016;6:28637. doi: 10.1038/srep28637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang W.T., Tsai T.Y., Chao C.H., Lai B.Y., Wu Lee Y.H. Y-Box Binding Protein 1 Stabilizes Hepatitis C Virus NS5A via Phosphorylation-Mediated Interaction with NS5A To Regulate Viral Propagation. J. Virol. 2015;89:11584–11602. doi: 10.1128/JVI.01513-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang P.C., Chi C.W., Chau G.Y., Li F.Y., Tsai Y.H., Wu J.C., Wu Lee Y.H. DDX3, a DEAD box RNA helicase, is deregulated in hepatitis virus-associated hepatocellular carcinoma and is involved in cell growth control. Oncogene. 2006;25:1991–2003. doi: 10.1038/sj.onc.1209239. [DOI] [PubMed] [Google Scholar]
- 88.Byrd A.K., Zybailov B.L., Maddukuri L., Gao J., Marecki J.C., Jaiswal M., Bell M.R., Griffin W.C., Reed M.R., Chib S., et al. Evidence That G-quadruplex DNA Accumulates in the Cytoplasm and Participates in Stress Granule Assembly in Response to Oxidative Stress. J. Biol. Chem. 2016;291:18041–18057. doi: 10.1074/jbc.M116.718478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tao E.W., Cheng W.Y., Li W.L., Yu J., Gao Q.Y. tiRNAs: A novel class of small noncoding RNAs that helps cells respond to stressors and plays roles in cancer progression. J. Cell Physiol. 2020;235:683–690. doi: 10.1002/jcp.29057. [DOI] [PubMed] [Google Scholar]
- 90.Lyons S.M., Achorn C., Kedersha N.L., Anderson P.J., Ivanov P. YB-1 regulates tiRNA-induced Stress Granule formation but not translational repression. Nucleic Acids Res. 2016;44:6949–6960. doi: 10.1093/nar/gkw418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hisai H., Kato J., Kobune M., Murakami T., Miyanishi K., Takahashi M., Yoshizaki N., Takimoto R., Terui T., Niitsu Y. Increased expression of angiogenin in hepatocellular carcinoma in correlation with tumor vascularity. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003;9:4852–4859. [PubMed] [Google Scholar]
- 92.Hengjuan L.V., Liu G., Kun L.I., Mingqiu L.I., Zhang D. Angiogenin regulates epithelial-mesenchymal transition of hepatocellular carcinoma through upregulation of HMGA2. Pharmazie. 2019;74:301–304. doi: 10.1691/ph.2019.8943. [DOI] [PubMed] [Google Scholar]
- 93.Fu Y., Dominissini D., Rechavi G., He C. Gene expression regulation mediated through reversible m(6)A RNA methylation. Nat. Rev. Genet. 2014;15:293–306. doi: 10.1038/nrg3724. [DOI] [PubMed] [Google Scholar]
- 94.Anders M., Chelysheva I., Goebel I., Trenkner T., Zhou J., Mao Y., Verzini S., Qian S.B., Ignatova Z. Dynamic m(6)A methylation facilitates mRNA triaging to stress granules. Life Sci. Alliance. 2018;1:e201800113. doi: 10.26508/lsa.201800113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Markmiller S., Soltanieh S., Server K.L., Mak R., Jin W., Fang M.Y., Luo E.C., Krach F., Yang D., Sen A., et al. Context-Dependent and Disease-Specific Diversity in Protein Interactions within Stress Granules. Cell. 2018;172:590–604.e13. doi: 10.1016/j.cell.2017.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fu Y., Zhuang X. m(6)A-binding YTHDF proteins promote stress granule formation. Nat. Chem. Biol. 2020;16:955–963. doi: 10.1038/s41589-020-0524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang P., Wang X., Zheng L., Zhuang C. Gene Signatures and Prognostic Values of m6A Regulators in Hepatocellular Carcinoma. Front. Genet. 2020;11:540186. doi: 10.3389/fgene.2020.540186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang H., Bai Y., Lu X., Xu Y., Zhao H., Sang X. N6-methyladenosine associated prognostic model in hepatocellular carcinoma. Ann. Transl. Med. 2020;8:633. doi: 10.21037/atm-20-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen Y., Peng C., Chen J., Chen D., Yang B., He B., Hu W., Zhang Y., Liu H., Dai L., et al. WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1. Mol. Cancer. 2019;18:127. doi: 10.1186/s12943-019-1053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li J., Zhu L., Shi Y., Liu J., Lin L., Chen X. m6A demethylase FTO promotes hepatocellular carcinoma tumorigenesis via mediating PKM2 demethylation. Am. J. Transl. Res. 2019;11:6084–6092. [PMC free article] [PubMed] [Google Scholar]
- 101.Ma J.Z., Yang F., Zhou C.C., Liu F., Yuan J.H., Wang F., Wang T.T., Xu Q.G., Zhou W.P., Sun S.H. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6)-methyladenosine-dependent primary MicroRNA processing. Hepatology. 2017;65:529–543. doi: 10.1002/hep.28885. [DOI] [PubMed] [Google Scholar]
- 102.Shi Y., Zhuang Y., Zhang J., Chen M., Wu S. METTL14 Inhibits Hepatocellular Carcinoma Metastasis Through Regulating EGFR/PI3K/AKT Signaling Pathway in an m6A-Dependent Manner. Cancer Manag. Res. 2020;12:13173–13184. doi: 10.2147/CMAR.S286275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen Y., Zhao Y., Chen J., Peng C., Zhang Y., Tong R., Cheng Q., Yang B., Feng X., Lu Y., et al. ALKBH5 suppresses malignancy of hepatocellular carcinoma via m(6)A-guided epigenetic inhibition of LYPD1. Mol. Cancer. 2020;19:123. doi: 10.1186/s12943-020-01239-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu X., Qin J., Gao T., Li C., He B., Pan B., Xu X., Chen X., Zeng K., Xu M., et al. YTHDF1 Facilitates the Progression of Hepatocellular Carcinoma by Promoting FZD5 mRNA Translation in an m6A-Dependent Manner. Mol. Ther. Nucleic Acids. 2020;22:750–765. doi: 10.1016/j.omtn.2020.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 105.Luo X., Cao M., Gao F., He X. YTHDF1 promotes hepatocellular carcinoma progression via activating PI3K/AKT/mTOR signaling pathway and inducing epithelial-mesenchymal transition. Exp. Hematol. Oncol. 2021;10:35. doi: 10.1186/s40164-021-00227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao X., Chen Y., Mao Q., Jiang X., Jiang W., Chen J., Xu W., Zhong L., Sun X. Overexpression of YTHDF1 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Biomark. 2018;21:859–868. doi: 10.3233/CBM-170791. [DOI] [PubMed] [Google Scholar]
- 107.Bian S., Ni W., Zhu M., Song Q., Zhang J., Ni R., Zheng W. Identification and Validation of the N6-Methyladenosine RNA Methylation Regulator YTHDF1 as a Novel Prognostic Marker and Potential Target for Hepatocellular Carcinoma. Front. Mol. Biosci. 2020;7:604766. doi: 10.3389/fmolb.2020.604766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhong L., Liao D., Zhang M., Zeng C., Li X., Zhang R., Ma H., Kang T. YTHDF2 suppresses cell proliferation and growth via destabilizing the EGFR mRNA in hepatocellular carcinoma. Cancer Lett. 2019;442:252–261. doi: 10.1016/j.canlet.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 109.Shao X.Y., Dong J., Zhang H., Wu Y.S., Zheng L. Systematic Analyses of the Role of the Reader Protein of N (6)-Methyladenosine RNA Methylation, YTH Domain Family 2, in Liver Hepatocellular Carcinoma. Front. Mol. Biosci. 2020;7:577460. doi: 10.3389/fmolb.2020.577460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang M., Wei K., Qian B., Feiler S., Lemekhova A., Buchler M.W., Hoffmann K. HSP70-eIF4G Interaction Promotes Protein Synthesis and Cell Proliferation in Hepatocellular Carcinoma. Cancers. 2020;12:2262. doi: 10.3390/cancers12082262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meyer H., Weihl C.C. The VCP/p97 system at a glance: Connecting cellular function to disease pathogenesis. J. Cell Sci. 2014;127 Pt 18:3877–3883. doi: 10.1242/jcs.093831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tresse E., Salomons F.A., Vesa J., Bott L.C., Kimonis V., Yao T.P., Dantuma N.P., Taylor J.P. VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes and this function is impaired by mutations that cause IBMPFD. Autophagy. 2010;6:217–227. doi: 10.4161/auto.6.2.11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu Y., Hei Y., Shu Q., Dong J., Gao Y., Fu H., Zheng X., Yang G. VCP/p97, down-regulated by microRNA-129-5p, could regulate the progression of hepatocellular carcinoma. PLoS ONE. 2012;7:e35800. doi: 10.1371/journal.pone.0035800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang B., Maxwell B.A., Joo J.H., Gwon Y., Messing J., Mishra A., Shaw T.I., Ward A.L., Quan H., Sakurada S.M., et al. ULK1 and ULK2 Regulate Stress Granule Disassembly Through Phosphorylation and Activation of VCP/p97. Mol. Cell. 2019;74:742–757.e8. doi: 10.1016/j.molcel.2019.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu H., Yu H., Zhang X., Shen X., Zhang K., Sheng H., Dai S., Gao H. UNC51-like kinase 1 as a potential prognostic biomarker for hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 2013;6:711–717. [PMC free article] [PubMed] [Google Scholar]
- 116.Barreau C., Paillard L., Osborne H.B. AU-rich elements and associated factors: Are there unifying principles? Nucleic Acids Res. 2005;33:7138–7150. doi: 10.1093/nar/gki1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bakheet T., Frevel M., Williams B.R., Greer W., Khabar K.S. ARED: Human AU-rich element-containing mRNA database reveals an unexpectedly diverse functional repertoire of encoded proteins. Nucleic Acids Res. 2001;29:246–254. doi: 10.1093/nar/29.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bakheet T., Williams B.R., Khabar K.S. ARED 2.0: An update of AU-rich element mRNA database. Nucleic Acids Res. 2003;31:421–423. doi: 10.1093/nar/gkg023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Legrand N., Dixon D.A., Sobolewski C. AU-rich element-binding proteins in colorectal cancer. World J. Gastrointest. Oncol. 2019;11:71–90. doi: 10.4251/wjgo.v11.i2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.DuBois R.N., McLane M.W., Ryder K., Lau L.F., Nathans D. A growth factor-inducible nuclear protein with a novel cysteine/histidine repetitive sequence. J. Biol. Chem. 1990;265:19185–19191. doi: 10.1016/S0021-9258(17)30642-7. [DOI] [PubMed] [Google Scholar]
- 121.Fu R., Olsen M.T., Webb K., Bennett E.J., Lykke-Andersen J. Recruitment of the 4EHP-GYF2 cap-binding complex to tetraproline motifs of tristetraprolin promotes repression and degradation of mRNAs with AU-rich elements. RNA. 2016;22:373–382. doi: 10.1261/rna.054833.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hsieh H.H., Chen Y.A., Chang Y.J., Wang H.H., Yu Y.H., Lin S.W., Huang Y.J., Lin S., Chang C.J. The functional characterization of phosphorylation of tristetraprolin at C-terminal NOT1-binding domain. J. Inflamm. 2021;18:22. doi: 10.1186/s12950-021-00288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brennan S.E., Kuwano Y., Alkharouf N., Blackshear P.J., Gorospe M., Wilson G.M. The mRNA-destabilizing protein tristetraprolin is suppressed in many cancers, altering tumorigenic phenotypes and patient prognosis. Cancer Res. 2009;69:5168–5176. doi: 10.1158/0008-5472.CAN-08-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Young L.E., Sanduja S., Bemis-Standoli K., Pena E.A., Price R.L., Dixon D.A. The mRNA binding proteins HuR and tristetraprolin regulate cyclooxygenase 2 expression during colon carcinogenesis. Gastroenterology. 2009;136:1669–1679. doi: 10.1053/j.gastro.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dolicka D., Sobolewski C., Gjorgjieva M., Correia de Sousa M., Berthou F., De Vito C., Colin D.J., Bejuy O., Fournier M., Maeder C., et al. Tristetraprolin Promotes Hepatic Inflammation and Tumor Initiation but Restrains Cancer Progression to Malignancy. Cell. Mol. Gastroenterol. Hepatol. 2021;11:597–621. doi: 10.1016/j.jcmgh.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stoecklin G., Stubbs T., Kedersha N., Wax S., Rigby W.F., Blackwell T.K., Anderson P. MK2-induced tristetraprolin: 14-3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J. 2004;23:1313–1324. doi: 10.1038/sj.emboj.7600163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kedersha N., Stoecklin G., Ayodele M., Yacono P., Lykke-Andersen J., Fritzler M.J., Scheuner D., Kaufman R.J., Golan D.E., Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Krohler T., Kessler S.M., Hosseini K., List M., Barghash A., Patial S., Laggai S., Gemperlein K., Haybaeck J., Muller R., et al. The mRNA-binding Protein TTP/ZFP36 in Hepatocarcinogenesis and Hepatocellular Carcinoma. Cancers. 2019;11:1754. doi: 10.3390/cancers11111754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu J.C., Luo S.Z., Liu T., Lu L.G., Xu M.Y. linc-SCRG1 accelerates liver fibrosis by decreasing RNA-binding protein tristetraprolin. FASEB J. 2019;33:2105–2115. doi: 10.1096/fj.201800098RR. [DOI] [PubMed] [Google Scholar]
- 130.Tran D.D.H., Koch A., Allister A., Saran S., Ewald F., Koch M., Nashan B., Tamura T. Treatment with MAPKAP2 (MK2) inhibitor and DNA methylation inhibitor, 5-aza dC, synergistically triggers apoptosis in hepatocellular carcinoma (HCC) via tristetraprolin (TTP) Cell. Signal. 2016;28:1872–1880. doi: 10.1016/j.cellsig.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 131.Sohn B.H., Park I.Y., Lee J.J., Yang S.J., Jang Y.J., Park K.C., Kim D.J., Lee D.C., Sohn H.A., Kim T.W., et al. Functional switching of TGF-beta1 signaling in liver cancer via epigenetic modulation of a single CpG site in TTP promoter. Gastroenterology. 2010;138:1898–1908. doi: 10.1053/j.gastro.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 132.Sanduja S., Blanco F.F., Dixon D.A. The roles of TTP and BRF proteins in regulated mRNA decay. Wiley Interdiscip. Rev. RNA. 2011;2:42–57. doi: 10.1002/wrna.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Blackshear P.J. Tristetraprolin and other CCCH tandem zinc-finger proteins in the regulation of mRNA turnover. Biochem. Soc. Trans. 2002;30 Pt 6:945–952. doi: 10.1042/bst0300945. [DOI] [PubMed] [Google Scholar]
- 134.Suk F.M., Chang C.C., Lin R.J., Lin S.Y., Liu S.C., Jau C.F., Liang Y.C. ZFP36L1 and ZFP36L2 inhibit cell proliferation in a cyclin D-dependent and p53-independent manner. Sci. Rep. 2018;8:2742. doi: 10.1038/s41598-018-21160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Loh X.Y., Sun Q.Y., Ding L.W., Mayakonda A., Venkatachalam N., Yeo M.S., Silva T.C., Xiao J.F., Doan N.B., Said J.W., et al. RNA-Binding Protein ZFP36L1 Suppresses Hypoxia and Cell-Cycle Signaling. Cancer Res. 2020;80:219–233. doi: 10.1158/0008-5472.CAN-18-2796. [DOI] [PubMed] [Google Scholar]
- 136.Zhong S., Machida K., Tsukamoto H., Johnson D.L. Alcohol induces RNA polymerase III-dependent transcription through c-Jun by co-regulating TATA-binding protein (TBP) and Brf1 expression. J. Biol. Chem. 2011;286:2393–2401. doi: 10.1074/jbc.M110.192955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tarling E.J., Clifford B.L., Cheng J., Morand P., Cheng A., Lester E., Sallam T., Turner M., de Aguiar Vallim T.Q. RNA-binding protein ZFP36L1 maintains posttranscriptional regulation of bile acid metabolism. J. Clin. Investig. 2017;127:3741–3754. doi: 10.1172/JCI94029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Benjamin D., Schmidlin M., Min L., Gross B., Moroni C. BRF1 protein turnover and mRNA decay activity are regulated by protein kinase B at the same phosphorylation sites. Mol. Cell. Biol. 2006;26:9497–9507. doi: 10.1128/MCB.01099-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brennan C.M., Steitz J.A. HuR and mRNA stability. Cell. Mol. Life Sci. 2001;58:266–277. doi: 10.1007/PL00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Grammatikakis I., Abdelmohsen K., Gorospe M. Posttranslational control of HuR function. Wiley Interdiscip. Rev. RNA. 2017;8:e1372. doi: 10.1002/wrna.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dixon D.A., Tolley N.D., King P.H., Nabors L.B., McIntyre T.M., Zimmerman G.A., Prescott S.M. Altered expression of the mRNA stability factor HuR promotes cyclooxygenase-2 expression in colon cancer cells. J. Clin. Investig. 2001;108:1657–1665. doi: 10.1172/JCI12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Srikantan S., Gorospe M. HuR function in disease. Front. Biosci. Landmark Ed. 2012;17:189–205. doi: 10.2741/3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Von Roretz C., Di Marco S., Mazroui R., Gallouzi I.E. Turnover of AU-rich-containing mRNAs during stress: A matter of survival. Wiley Interdiscip. Rev. RNA. 2011;2:336–347. doi: 10.1002/wrna.55. [DOI] [PubMed] [Google Scholar]
- 144.Embade N., Fernandez-Ramos D., Varela-Rey M., Beraza N., Sini M., Gutierrez de Juan V., Woodhoo A., Martinez-Lopez N., Rodriguez-Iruretagoyena B., Bustamante F.J., et al. Murine double minute 2 regulates Hu antigen R stability in human liver and colon cancer through NEDDylation. Hepatology. 2012;55:1237–1248. doi: 10.1002/hep.24795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhu H., Berkova Z., Mathur R., Sehgal L., Khashab T., Tao R.H., Ao X., Feng L., Sabichi A.L., Blechacz B., et al. HuR Suppresses Fas Expression and Correlates with Patient Outcome in Liver Cancer. Mol. Cancer Res. 2015;13:809–818. doi: 10.1158/1541-7786.MCR-14-0241. [DOI] [PubMed] [Google Scholar]
- 146.Vazquez-Chantada M., Fernandez-Ramos D., Embade N., Martinez-Lopez N., Varela-Rey M., Woodhoo A., Luka Z., Wagner C., Anglim P.P., Finnell R.H., et al. HuR/methyl-HuR and AUF1 regulate the MAT expressed during liver proliferation, differentiation, and carcinogenesis. Gastroenterology. 2010;138:1943–1953. doi: 10.1053/j.gastro.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang Z., Yao Z., Wang L., Ding H., Shao J., Chen A., Zhang F., Zheng S. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy. 2018;14:2083–2103. doi: 10.1080/15548627.2018.1503146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moraes K.C., Monteiro C.J., Pacheco-Soares C. A novel function for CUGBP2 in controlling the pro-inflammatory stimulus in H9c2 cells: Subcellular trafficking of messenger molecules. Cell Biol. Int. 2013;37:1129–1138. doi: 10.1002/cbin.10127. [DOI] [PubMed] [Google Scholar]
- 149.Anant S., Henderson J.O., Mukhopadhyay D., Navaratnam N., Kennedy S., Min J., Davidson N.O. Novel role for RNA-binding protein CUGBP2 in mammalian RNA editing. CUGBP2 modulates C to U editing of apolipoprotein B mRNA by interacting with apobec-1 and ACF, the apobec-1 complementation factor. J. Biol. Chem. 2001;276:47338–47351. doi: 10.1074/jbc.M104911200. [DOI] [PubMed] [Google Scholar]
- 150.Morales A., Mari M., Garcia-Ruiz C., Colell A., Fernandez-Checa J.C. Hepatocarcinogenesis and ceramide/cholesterol metabolism. Anticancer Agents Med. Chem. 2012;12:364–375. doi: 10.2174/187152012800228689. [DOI] [PubMed] [Google Scholar]
- 151.Lee G., Jeong Y.S., Kim D.W., Kwak M.J., Koh J., Joo E.W., Lee J.S., Kah S., Sim Y.E., Yim S.Y. Clinical significance of APOB inactivation in hepatocellular carcinoma. Exp. Mol. Med. 2018;50:1–12. doi: 10.1038/s12276-018-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Suzuki H., Takeuchi M., Sugiyama A., Alam A.K., Vu L.T., Sekiyama Y., Dam H.C., Ohki S.Y., Tsukahara T. Alternative splicing produces structural and functional changes in CUGBP2. BMC Biochem. 2012;13:6. doi: 10.1186/1471-2091-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ohyama T., Nagata T., Tsuda K., Kobayashi N., Imai T., Okano H., Yamazaki T., Katahira M. Structure of Musashi1 in a complex with target RNA: The role of aromatic stacking interactions. Nucleic Acids Res. 2012;40:3218–3231. doi: 10.1093/nar/gkr1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nahas G.R., Murthy R.G., Patel S.A., Ganta T., Greco S.J., Rameshwar P. The RNA-binding protein Musashi 1 stabilizes the oncotachykinin 1 mRNA in breast cancer cells to promote cell growth. FASEB J. 2016;30:149–159. doi: 10.1096/fj.15-278770. [DOI] [PubMed] [Google Scholar]
- 155.Li J., Yan K., Yang Y., Li H., Wang Z., Xu X. Musashi-1 positively regulates growth and proliferation of hepatoma cells in vitro. Nan Fang Yi Ke Da Xue Xue Bao. 2019;39:1436–1442. doi: 10.12122/j.issn.1673-4254.2019.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chiou G.Y., Yang T.W., Huang C.C., Tang C.Y., Yen J.Y., Tsai M.C., Chen H.Y., Fadhilah N., Lin C.C., Jong Y.J. Musashi-1 promotes a cancer stem cell lineage and chemoresistance in colorectal cancer cells. Sci. Rep. 2017;7:2172. doi: 10.1038/s41598-017-02057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhan Y., Wang H., Ning Y., Zheng H., Liu S., Yang Y., Zhou M., Fan S. Understanding the roles of stress granule during chemotherapy for patients with malignant tumors. Am. J. Cancer Res. 2020;10:2226–2241. [PMC free article] [PubMed] [Google Scholar]
- 158.Garaigorta U., Heim M.H., Boyd B., Wieland S., Chisari F.V. Hepatitis C virus (HCV) induces formation of stress granules whose proteins regulate HCV RNA replication and virus assembly and egress. J. Virol. 2012;86:11043–11056. doi: 10.1128/JVI.07101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu Y., Pang L., Wang H., Xu C., Shah H., Guo P., Shu D., Qian S.Y. Specific delivery of delta-5-desaturase siRNA via RNA nanoparticles supplemented with dihomo-gamma-linolenic acid for colon cancer suppression. Redox Biol. 2019;21:101085. doi: 10.1016/j.redox.2018.101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dai H., Li M., Yang W., Sun X., Wang P., Wang X., Su J., Wang X., Hu X., Zhao M. Resveratrol inhibits the malignant progression of hepatocellular carcinoma via MARCH1-induced regulation of PTEN/AKT signaling. Aging. 2020;12:11717–11731. doi: 10.18632/aging.103338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tang Y., Cao J., Cai Z., An H., Li Y., Peng Y., Chen N., Luo A., Tao H., Li K. Epigallocatechin gallate induces chemopreventive effects on rats with diethylnitrosamineinduced liver cancer via inhibition of cell division cycle 25A. Mol. Med. Rep. 2020;22:3873–3885. doi: 10.3892/mmr.2020.11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shim J.H., Su Z.Y., Chae J.I., Kim D.J., Zhu F., Ma W.Y., Bode A.M., Yang C.S., Dong Z. Epigallocatechin gallate suppresses lung cancer cell growth through Ras-GTPase-activating protein SH3 domain-binding protein 1. Cancer Prev. Res. 2010;3:670–679. doi: 10.1158/1940-6207.CAPR-09-0185. [DOI] [PubMed] [Google Scholar]
- 163.Oi N., Yuan J., Malakhova M., Luo K., Li Y., Ryu J., Zhang L., Bode A.M., Xu Z., Li Y., et al. Resveratrol induces apoptosis by directly targeting Ras-GTPase-activating protein SH3 domain-binding protein 1. Oncogene. 2015;34:2660–2671. doi: 10.1038/onc.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Naito Y., Ushiroda C., Mizushima K., Inoue R., Yasukawa Z., Abe A., Takagi T. Epigallocatechin-3-gallate (EGCG) attenuates non-alcoholic fatty liver disease via modulating the interaction between gut microbiota and bile acids. J. Clin. Biochem. Nutr. 2020;67:2–9. doi: 10.3164/jcbn.20-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sojoodi M., Wei L., Erstad D.J., Yamada S., Fujii T., Hirschfield H., Kim R.S., Lauwers G.Y., Lanuti M., Hoshida Y., et al. Epigallocatechin Gallate Induces Hepatic Stellate Cell Senescence and Attenuates Development of Hepatocellular Carcinoma. Cancer Prev. Res. 2020;13:497–508. doi: 10.1158/1940-6207.CAPR-19-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gho Y.S., Yoon W.H., Chae C.B. Antiplasmin activity of a peptide that binds to the receptor-binding site of angiogenin. J. Biol. Chem. 2002;277:9690–9694. doi: 10.1074/jbc.M105526200. [DOI] [PubMed] [Google Scholar]
- 167.Porru M., Artuso S., Salvati E., Bianco A., Franceschin M., Diodoro M.G., Passeri D., Orlandi A., Savorani F., D’Incalci M., et al. Targeting G-Quadruplex DNA Structures by EMICORON Has a Strong Antitumor Efficacy against Advanced Models of Human Colon Cancer. Mol. Cancer Ther. 2015;14:2541–2551. doi: 10.1158/1535-7163.MCT-15-0253. [DOI] [PubMed] [Google Scholar]
- 168.Wang Z., Shen G.H., Xie J.M., Li B., Gao Q.G. Rottlerin upregulates DDX3 expression in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2018;495:1503–1509. doi: 10.1016/j.bbrc.2017.11.198. [DOI] [PubMed] [Google Scholar]
- 169.Yu H., Liu Y., Niu C., Cheng Y. Diosgenin increased DDX3 expression in hepatocellular carcinoma. Am. J. Transl. Res. 2018;10:3590–3599. [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang B., Yin X., Sui S. Resveratrol inhibited the progression of human hepatocellular carcinoma by inducing autophagy via regulating p53 and the phosphoinositide 3kinase/protein kinase B pathway. Oncol. Rep. 2018;40:2758–2765. doi: 10.3892/or.2018.6648. [DOI] [PubMed] [Google Scholar]
- 171.Won H.R., Lee D.H., Yeon S.K., Ryu H.W., Kim G.W., Kwon S.H. HDAC6selective inhibitor synergistically enhances the anticancer activity of immunomodulatory drugs in multiple myeloma. Int. J. Oncol. 2019;55:499–512. doi: 10.3892/ijo.2019.4828. [DOI] [PubMed] [Google Scholar]
- 172.Kaliszczak M., van Hechanova E., Li Y., Alsadah H., Parzych K., Auner H.W., Aboagye E.O. The HDAC6 inhibitor C1A modulates autophagy substrates in diverse cancer cells and induces cell death. Br. J. Cancer. 2018;119:1278–1287. doi: 10.1038/s41416-018-0232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang F., Zhong B.W., Zhao Z.R. ACY 1215, a histone deacetylase 6 inhibitor, inhibits cancer cell growth in melanoma. J. Biol. Regul. Homeost. Agents. 2018;32:851–858. [PubMed] [Google Scholar]
- 174.Chen M.C., Lin Y.C., Liao Y.H., Liou J.P., Chen C.H. MPT0G612, a Novel HDAC6 Inhibitor, Induces Apoptosis and Suppresses IFN-gamma-Induced Programmed Death-Ligand 1 in Human Colorectal Carcinoma Cells. Cancers. 2019;11:1617. doi: 10.3390/cancers11101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang J., Li Y., Zhang Y., Fang X., Chen N., Zhou X., Wang X. Sirt6 promotes tumorigenesis and drug resistance of diffuse large B-cell lymphoma by mediating PI3K/Akt signaling. J. Exp. Clin. Cancer Res. 2020;39:142. doi: 10.1186/s13046-020-01623-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhou Q., Lui V.W., Lau C.P., Cheng S.H., Ng M.H., Cai Y., Chan S.L., Yeo W. Sustained antitumor activity by co-targeting mTOR and the microtubule with temsirolimus/vinblastine combination in hepatocellular carcinoma. Biochem. Pharm. 2012;83:1146–1158. doi: 10.1016/j.bcp.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 177.Okano J., Nagahara T., Matsumoto K., Murawaki Y. The growth inhibition of liver cancer cells by paclitaxel and the involvement of extracellular signal-regulated kinase and apoptosis. Oncol. Rep. 2007;17:1195–1200. doi: 10.3892/or.17.5.1195. [DOI] [PubMed] [Google Scholar]
- 178.Lal P., Cerofolini L., D’Agostino V.G., Zucal C., Fuccio C., Bonomo I., Dassi E., Giuntini S., Di Maio D., Vishwakarma V., et al. Regulation of HuR structure and function by dihydrotanshinone-I. Nucleic Acids Res. 2017;45:9514–9527. doi: 10.1093/nar/gkx623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hu X., Jiao F., Zhang L., Jiang Y. Dihydrotanshinone Inhibits Hepatocellular Carcinoma by Suppressing the JAK2/STAT3 Pathway. Front. Pharm. 2021;12:654986. doi: 10.3389/fphar.2021.654986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Blanco F.F., Preet R., Aguado A., Vishwakarma V., Stevens L.E., Vyas A., Padhye S., Xu L., Weir S.J., Anant S., et al. Impact of HuR inhibition by the small molecule MS-444 on colorectal cancer cell tumorigenesis. Oncotarget. 2016;7:74043–74058. doi: 10.18632/oncotarget.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yang W.L., Perillo W., Liou D., Marambaud P., Wang P. AMPK inhibitor compound C suppresses cell proliferation by induction of apoptosis and autophagy in human colorectal cancer cells. J. Surg. Oncol. 2012;106:680–688. doi: 10.1002/jso.23184. [DOI] [PubMed] [Google Scholar]
- 182.Mahboubi H., Koromilas A.E., Stochaj U. AMP Kinase Activation Alters Oxidant-Induced Stress Granule Assembly by Modulating Cell Signaling and Microtubule Organization. Mol. Pharm. 2016;90:460–468. doi: 10.1124/mol.116.105494. [DOI] [PubMed] [Google Scholar]
- 183.Ferrin G., Guerrero M., Amado V., Rodriguez-Peralvarez M., De la Mata M. Activation of mTOR Signaling Pathway in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2020;21:1266. doi: 10.3390/ijms21041266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Berzigotti A., Saran U., Dufour J.F. Physical activity and liver diseases. Hepatology. 2016;63:1026–1040. doi: 10.1002/hep.28132. [DOI] [PubMed] [Google Scholar]
- 185.Zhou J., Massey S., Story D., Li L. Metformin: An Old Drug with New Applications. Int. J. Mol. Sci. 2018;19:2863. doi: 10.3390/ijms19102863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kwon S., Zhang Y., Matthias P. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes Dev. 2007;21:3381–3394. doi: 10.1101/gad.461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kaliszczak M., Trousil S., Aberg O., Perumal M., Nguyen Q.D., Aboagye E.O. A novel small molecule hydroxamate preferentially inhibits HDAC6 activity and tumour growth. Br. J. Cancer. 2013;108:342–350. doi: 10.1038/bjc.2012.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li T., Zhang C., Hassan S., Liu X., Song F., Chen K., Zhang W., Yang J. Histone deacetylase 6 in cancer. J. Hematol. Oncol. 2018;11:111. doi: 10.1186/s13045-018-0654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Damonte P., Sociali G., Parenti M.D., Soncini D., Bauer I., Boero S., Grozio A., Holtey M.V., Piacente F., Becherini P., et al. SIRT6 inhibitors with salicylate-like structure show immunosuppressive and chemosensitizing effects. Bioorgan. Med. Chem. 2017;25:5849–5858. doi: 10.1016/j.bmc.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 190.Buchan J.R., Kolaitis R.M., Taylor J.P., Parker R. Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell. 2013;153:1461–1474. doi: 10.1016/j.cell.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lu S., Yao Y., Xu G., Zhou C., Zhang Y., Sun J., Jiang R., Shao Q., Chen Y. CD24 regulates sorafenib resistance via activating autophagy in hepatocellular carcinoma. Cell Death Dis. 2018;9:646. doi: 10.1038/s41419-018-0681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wu W.K.K., Zhang L., Chan M.T.V. Autophagy, NAFLD and NAFLD-Related HCC. Adv. Exp. Med. Biol. 2018;1061:127–138. doi: 10.1007/978-981-10-8684-7_10. [DOI] [PubMed] [Google Scholar]
- 193.Huang F., Wang B.R., Wang Y.G. Role of autophagy in tumorigenesis, metastasis, targeted therapy and drug resistance of hepatocellular carcinoma. World J. Gastroenterol. 2018;24:4643–4651. doi: 10.3748/wjg.v24.i41.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shimizu S., Takehara T., Hikita H., Kodama T., Tsunematsu H., Miyagi T., Hosui A., Ishida H., Tatsumi T., Kanto T., et al. Inhibition of autophagy potentiates the antitumor effect of the multikinase inhibitor sorafenib in hepatocellular carcinoma. Int. J. Cancer. 2012;131:548–557. doi: 10.1002/ijc.26374. [DOI] [PubMed] [Google Scholar]
- 195.Cermak V., Dostal V., Jelinek M., Libusova L., Kovar J., Rosel D., Brabek J. Microtubule-targeting agents and their impact on cancer treatment. Eur. J. Cell Biol. 2020;99:151075. doi: 10.1016/j.ejcb.2020.151075. [DOI] [PubMed] [Google Scholar]
- 196.Schultz C.W., Preet R., Dhir T., Dixon D.A., Brody J.R. Understanding and targeting the disease-related RNA binding protein human antigen R (HuR) Wiley Interdiscip. Rev. RNA. 2020;11:e1581. doi: 10.1002/wrna.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Stress granule proteome (Figure 1) was obtained from https://msgp.pt/, accessed on 1 April 2021. Genes associated to hepatocellular carcinoma (HCC, Figure 1) were obtained with the MetaCore database (https://portal.genego.com/, accessed on 1 April 2021). Expression of SG components and survival information in HCC patients (Figure 3) were obtained from the CancerLivER database (https://webs.iiitd.edu.in/raghava/cancerliver/index.html, accessed on 1 April 2021) and the Gepia database (http://gepia.cancer-pku.cn/, accessed on 1 April 2021), respectively.