Abstract
Simple Summary
In this study, we assess the prognostic potential of immune and inflammatory elements determined preoperatively in the peripheral blood of patients with oral squamous cell carcinoma (OSCC). Preoperative plasma fibrinogen (Fib) and platelet-to-lymphocyte ratio (PLR) show strong correlations with patients’ outcomes. Analyzed together, in a new parameter named Fibrinogen-PLR Algorithm (FiPLA), predictive power increases significantly. Clinicians can use this new, easy, cost-effective, and globally available tool for risk stratification of patients with OSCC, as early as from the moment of diagnosis.
Abstract
(1) Background: Oral squamous cell carcinoma (OSCC) is a common malignancy, and the impact of immune and inflammatory mechanisms in its development and progression are of major interest. The aim of our study is to assess the prognostic potential of circulating immune and inflammatory elements determined preoperatively in patients with OSCC, as well as the development of a new compound parameter with predictive value. (2) Methods: We assessed preoperative fibrinogen (Fib) and the platelet-to-lymphocyte ratio (PLR) in 111 OSCC patients. Using a mathematic algorithm, we determined a composite parameter with cumulative information from Fib and PLR, named Fibrinogen-PLR Algorithm (FiPLA). Survival analysis, followed by bivariate and multivariate analyses, was subsequently conducted. (3) Results: Increased preoperative Fib and PLR levels were associated with poor outcome in OSCC (p = 0.0001 and p = 0.0015, respectively). Preoperative FiPLA values were also associated with poor patient survival (p < 0.0001). Multivariate analysis confirmed the independent prognostic role for FiPLA only (CI95% 1.232–67.770, p = 0.03), showing the superior predictive value of FiPLA compared to its individual components. (4) Conclusions: Preoperative assessments of circulating immune and inflammatory elements can provide high-quality prognostic information, and they represent valuable tools in clinical practice, facilitating the early risk stratification of patients with OSCC.
Keywords: oral squamous cell carcinoma, head and neck cancer, preoperative, fibrinogen, platelet-to-lymphocyte ratio, prognostic
1. Introduction
Oral squamous cell carcinoma (OSCC) is a common malignancy worldwide, with a global incidence of 377.713 new cases in 2020 [1]. In 2018, Globocan reported that oral cancers were responsible for 2% of all malignancies and 1.9% of cancer-associated deaths [2]. Despite all forms of progress made in diagnosis and therapy for oral cancer, the age-adjusted mortality rates caused by this malignancy revealed an increasing trend during the last decade, with an average of 0.5% per year [3]. Currently, the only reliable prognostic element used worldwide in treatment-naïve patients is clinical TNM staging. Even though pathological TNM staging provides more accurate information, it is only available after therapy has been initiated and a patient has undergone surgical treatment [4]. Additional tumor characteristics that have confirmed a predictive value, such as the degree of differentiation, perineural invasion, lymph node micro-metastasis, or tumor immune infiltration, are available only after curative surgery has been performed and the entire specimen has undergone pathology assessment [5,6,7]. Thus, in the scientific world, there is a continuous quest for different elements with predictive potential that can increase the accuracy of disease characterization in terms of aggressiveness, response to therapy, and survival. Ideally, these biomarkers should be available as early as possible, even from the moment of diagnosis, and should be cost efficient for worldwide implementation, providing the advantage of optimal risk stratification and therapy selection before the initiation of any treatment. A wide variety of molecules that meet the criteria for biomarkers was investigated in scientific research conducted on OSCC; however, they were not introduced in standard clinical care due to the challenges raised by costs, the necessity of specific equipment, and specially trained personal [8,9,10,11,12].
Patients with OSCC undergo a general evaluation in their preparation for therapy, which usually includes blood tests. These peripheral blood tests allow for the assessment of the inflammatory and immune status of patients, two factors that are known to play major roles in most malignancies, including OSCC [13,14,15,16]. Cellular and molecular elements from the blood stream have revealed predictive attributes in patients with OSCC. Some studies have suggested the prognostic role of circulating cells assessed individually or in different ratios, such as the platelet-to-lymphocyte ratio (PLR) [17,18,19]. Acute phase inflammatory proteins have also been assessed for their predictive value in OSCC, with promising results [20,21]. However, in clinical practice it is challenging to interpret all this accessible but dispersed information. Thus, a unitary, easily applicable parameter available from the moment of diagnosis, which would incorporate different prognostic data within one numeric value and provide information regarding patient risk stratification, could be of great utility in clinical practice.
In our study, we aim to assess the predictive value of preoperative immune and inflammatory parameters in patients with OSCC with the development of a novel algorithm that can facilitate data interpretation for all clinicians in order to provide additional prognostic information to clinical TNM staging before the initiation of any curative therapy.
2. Materials and Methods
2.1. Patient Selection
We included patients with a confirmed diagnosis of OSCC who were subsequently treated in the department of Oral and Maxillofacial Surgery, “Carol Davila” Military University Emergency Hospital Bucharest. This study was carried out with the approval of the Local Ethics Committee from our hospital (No. 25/27 November 2017). All patients were newly diagnosed with OSCC and had not received any previous treatment. During the period of 2016–2019, in our department, we registered a total of 223 patients with OSCC who were considered for further eligibility in our study. Inclusion criteria were defined as follows: patients with operable disease, who received no previous treatment, with no history of other malignancies, and with complete follow-up data. Subjects with inoperable disease or other decompensated comorbidities, as well as patients who did not complete the treatment and patients with previous or concomitant neoplasms or incomplete medical records, were excluded from the study.
2.2. Preoperative Assessment
Within the preoperative work-up protocol, patients underwent clinical, imagistic, and general status assessments, including preoperative blood tests. Blood samples were collected at an interval of 1 to 5 days before surgery. Preoperative fibrinogen levels (Fib), as marker of systemic inflammatory status, and platelet-to-lymphocyte ratio (PLR), as a marker of systemic immune status, were selected for our study analysis. After preoperative workup, patients underwent radical surgical treatment with or without adjuvant chemo-/radiotherapy, in accordance with national oncological guidelines. All patients entered a follow-up program, with periodic visits and complete clinical and imagistic assessment.
2.3. Statistical Analysis
Statistical analysis was carried out with Prism 9 software (GraphPad) and SPSS version 23 (IBM). We used Shapiro–Wilk test to assess the normality of data distribution in our group. Data with parametric distribution were analyzed using Student’s t-test, while data with no Gaussian distribution were analyzed using Mann–Whitney test. Correlation analysis was carried out with chi-square or Fisher’s exact test. We selected two elements for further analysis: Fib and PLR, and we conducted a receiver operating characteristic (ROC) curve analysis. Cut-off values were determined using the Youden index method. Prognostic data, defined as disease specific survival (DSS), were calculated as the time interval from diagnosis to death caused by disease progression. Overall survival (OS) was defined as the time interval from diagnosis until the last follow-up, for all living patients, or until death by any other cause except the disease. Recurrence was defined as local, regional, or distant metastatic disease progression. Kaplan–Meier method was used for survival analysis, comparing results from log-rank test for each group. Univariate and multivariate analyses were subsequently performed. Statistical significance was considered to be p < 0.05.
3. Results
3.1. Patient Characteristics
From 223 subjects diagnosed with OSCC in our department between 2016 and 2019, 111 patients met the eligibility criteria and were included in this study. Primary tumors involved oral mucosa sites: tongue, floor of the mouth, gingiva, buccal, and palate mucosa. The mean age of the patients was 60.68 years old. Most of the patients were male (79%), with a male:female ratio of almost 4:1. Advanced disease at the time of diagnosis (stages TNM III and IVA) was found in 72% of patients. Exposure to classic risk factors: tobacco and alcohol, was confirmed in 72% and 57% of cases, respectively. Neck dissection, unilateral or bilateral, in tumors involving midline, was performed in 87 patients (78%), and lymph node metastasis was confirmed in 55 patients (63%). Perineural invasion was found in 22% of patients, while 9% had vascular invasion. Positive margins after tumor resection were present in 17 patients (15%). The mean follow-up time was 36 months (4 to 60 months). During the follow-up interval, disease recurrence was recorded in 40% of patients, and 34 patients (31%) died due to disease progression. Results are shown in Table 1.
Table 1.
Descriptive statistics in OSCC.
| Age, Years (Mean, SD) | 60.68 (10.19) | ||
|---|---|---|---|
| No | % | ||
| Sex | |||
| Male | 88 | 79% | |
| Female | 23 | 21% | |
| TNM stage * | |||
| I | 6 | 5% | |
| II | 25 | 23% | |
| III | 22 | 20% | |
| IVA | 58 | 52% | |
| Smoking | |||
| Smokers | 80 | 72% | |
| Nonsmokers | 25 | 23% | |
| Missing | 6 | 5% | |
| Alcohol consumption | |||
| Drinkers | 57 | 51% | |
| Nondrinkers | 48 | 43% | |
| Missing | 6 | 5% | |
| T stage * | |||
| T1 | 13 | 12% | |
| T2 | 43 | 39% | |
| T3 | 31 | 28% | |
| T4 | 24 | 22% | |
| Nodal status a | |||
| pN0 | 32 | 37% | |
| pN+ | 55 | 63% | |
| Histological differentiation | |||
| High | 23 | 21% | |
| Intermediate | 65 | 59% | |
| Low | 23 | 21% | |
| Perineural invasion | |||
| Confirmed | 24 | 22% | |
| Not confirmed | 87 | 78% | |
| Vascular invasion | |||
| Confirmed | 10 | 9% | |
| Not confirmed | 101 | 91% | |
| Resection margins | |||
| Positive | 17 | 15% | |
| Negative | 94 | 85% | |
| Loco-regional recurrence | |||
| Present | 44 | 40% | |
| Absent | 67 | 60% | |
| Follow-up | |||
| Alive | 77 | 69% | |
| Dead due to disease | 34 | 31% | |
a neck dissection was performed in 87 patients; * AJCC, Eighth Edition.
3.2. Assessment of Preoperative Inflammatory and Immune Parameters in Peripheral Blood
Analysis of the preoperative peripheral blood values for fibrinogen (Fib), white blood cells count (WBC), lymphocyte count (Ly#), lymphocyte ratio (Ly%), platelet count (PLT), and platelet-to-lymphocyte ratio (PLR) revealed significant differences for several of these parameters in those patients who died due to disease progression compared to surviving patients. The results are presented in Table 2. Increased preoperative Fib levels were found in patients who died secondary to disease progression (p < 0.001), with a median (IQR) of 566.5 mg/dL in the deceased group compared to 480 mg/dL in the surviving group of patients. WBC, Ly#, Ly%, and PLT did not exhibit statistically significant differences between the two groups. However, the ratio between peripheral lymphocytes and platelets (PLR) was significantly lower in the surviving group of patients, with a median of 119.85 compared to the deceased group, which revealed a median of 150.73 for this parameter (p = 0.005).
Table 2.
Preoperative inflammatory and immune parameters in peripheral blood.
| Parameter | Survivors | Deceased | |
|---|---|---|---|
| Mean/Median (SD/IQR) | Mean/Median (SD/IQR) | p Value | |
| Fibrinogen, mg/dL | 480 (145.5) | 566.5 (143) | <0.001 a |
| White blood cells, k/µL | 8.42 (2.565) | 8.285 (3.61) | 0.919 a |
| Lymphocytes, k/µL | 2.043 (0.61) | 1.83 (0.646) | 0.164 b |
| Ly, % | 25.3 (9.5) | 20.95 (12.325) | 0.378 a |
| Platelets, k/µL | 255 (94) | 278 (93.5) | 0.108 a |
| Platelet-to-lymphocyte ratio | 119.85 (49.44) | 150.73 (84.738) | 0.005 a |
SD: standard deviation, IQR interquartile range, a Mann–Whitney test; statistical significance, b Student’s-t test, p < 0.05.
3.3. Determination of Cut-Off Points for Fibrinogen (Fib) and Platelet/Lymphocyte Ratio (PLR)
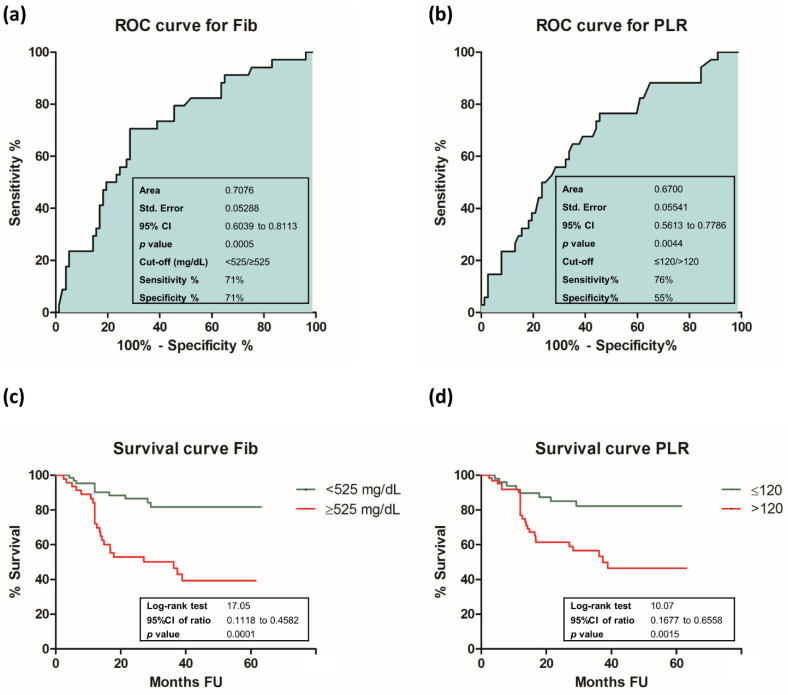
For further analysis, we selected Fib and PLR as preoperative markers of the systemic inflammatory and immune status in OSCC. ROC curve analysis revealed that the area under the curve (AUC) for preoperative Fib levels was 0.7076 (95% CI 0.6039 to 0.8113, p < 0.001), while for PLR, we calculated an AUC of 0.6700 (95% CI 0.5613 to 0.7786, p = 0.004). Using the Youden index method, we identified the optimal cut-off values for each parameter. The cut-off value for preoperative Fib was 525 mg/dL and it was 120 for PLR. Results are shown in Figure 1.
Figure 1.
ROC and Survival curves for Fib and PLR: (a) ROC curve for Fib; (b) ROC curve for PLR; (c) survival curve for Fib; (d) survival curve for PLR. Statistical significance was considered at p < 0.05.
3.4. Correlation Analysis between Clinico-Pathological Features and Immuno-Inflammatory Peripheral Blood Parameters in OSCC
Based on the cut-off values, we divided our patients in two groups for each parameter and conducted a correlation analysis with clinico-pathological features in OSCC. Results are shown in Table 3. The two groups according to Fib were: Group 1, which included a total of 65 patients with Fib below cut-off and Group 2, which included 46 patients with Fib above cut-off. According to PLR cut-off value, there were the following: Group A, which included 49 patients with PLR ≤ 120 and Group B, which included 62 patients with PLR > 120. Our correlation analysis with clinico-pathological characteristics in OSCC revealed no significant correlations except for tumor recurrence, which was more frequently seen in patients with Fib and PLR levels above cut-off value (p < 0.001 and p = 0.002, respectively). In addition, smaller size tumors were associated with preoperative Fib levels below cut-off (p = 0.002).
Table 3.
Correlation analysis with clinico-pathological features in OSCC.
| Variable | Fib | p Value | PLR | p Value | |||
|---|---|---|---|---|---|---|---|
| <525 | ≥525 | ≤120 | >120 | ||||
| Sex | 0.056 | 0.643 | |||||
| Male | 56 | 32 | 40 | 48 | |||
| Female | 9 | 14 | 9 | 14 | |||
| T stage | 0.002 | 0.197 | |||||
| T1 | 10 | 3 | 7 | 6 | |||
| T2 | 30 | 13 | 21 | 22 | |||
| T3 | 19 | 12 | 15 | 16 | |||
| T4A | 6 | 18 | 6 | 18 | |||
| Nodal status a | 0.824 | 0.999 | |||||
| pN0 | 19 | 13 | 15 | 17 | |||
| pN+ | 34 | 21 | 25 | 30 | |||
| TNM stage | 0.635 | 0.677 | |||||
| I | 4 | 2 | 3 | 3 | |||
| II | 17 | 8 | 11 | 14 | |||
| III | 13 | 9 | 12 | 10 | |||
| IVA | 31 | 27 | 23 | 35 | |||
| Smoking | 0.221 | 0.869 | |||||
| Smokers | 46 | 34 | 36 | 44 | |||
| Nonsmokers | 15 | 10 | 10 | 15 | |||
| Missing | 4 | 2 | 3 | 3 | |||
| Acohol consumption | 0.863 | 0.693 | |||||
| Drinkers | 34 | 23 | 23 | 34 | |||
| Nondrinkers | 27 | 21 | 23 | 25 | |||
| Missing | 4 | 2 | 3 | 3 | |||
| Histological differentiation | 0.253 | 0.358 | |||||
| High | 14 | 9 | 12 | 11 | |||
| Intermediate | 41 | 24 | 25 | 40 | |||
| Low | 10 | 13 | 12 | 11 | |||
| Perineural invasion | 0.646 | 0.495 | |||||
| Confirmed | 13 | 11 | 9 | 15 | |||
| Not confirmed | 52 | 35 | 40 | 47 | |||
| Loco-regional recurrence | <0.001 | 0.002 | |||||
| Present | 15 | 29 | 11 | 33 | |||
| Absent | 50 | 17 | 38 | 29 | |||
a from a total of 87 neck dissections; statistical significance p < 0.05.
3.5. Survival Analysis for Fibrinogen and Platelet/Lymphocyte Ratio in OSCC
Survival analysis showed that both Fib and PLR exhibited statistically significant correlations with patients’ outcomes. According to preoperative Fib levels, at the end of the follow-up interval, 85% of patients from Group 1 were alive compared to only 48% of patients from Group 2 (95% CI 0.1118 to 0.4582, p < 0.001). According to preoperative PLR values, survival analysis revealed that 84% of patients from Group A were alive compared to 58% of patients from Group B (95% CI 0.1677 to 0.6558, p = 0.002) Result are shown in Figure 1.
3.6. Assessment of Cumulative Prognostic Potential for Preoperative Fib and PLR
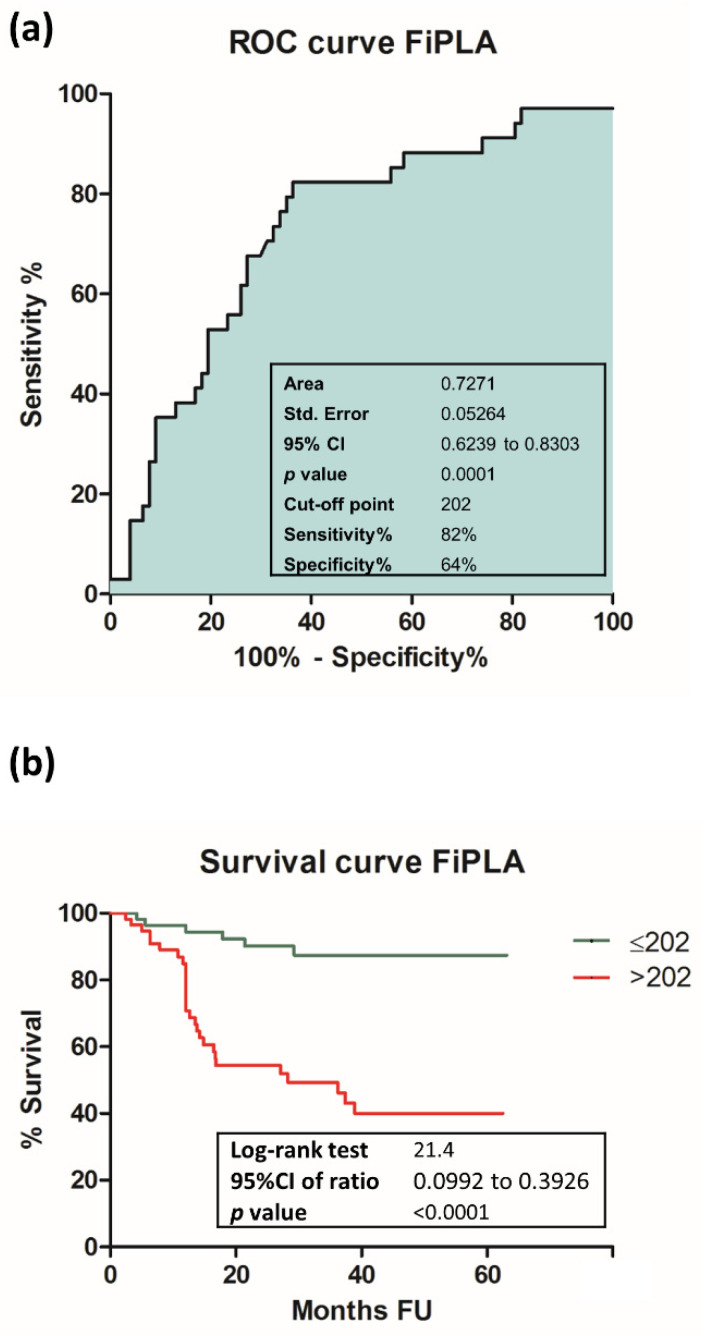
The cumulative power of Fib and PLR was calculated using, for each patient, percentage values from the previously determined cut-off levels for Fib and PLR, which were subsequently summed within a new parameter named Fibrinogen-PLR Algorithm (FiPLA). This calculation method allows for the mathematical addition of two otherwise distinct values—Fib, measured in mg/dL, and PLR, a cellular report—within a single numerical unit. The mean value of FiPLA was 222 (ranging between 123–523). ROC curve analysis for FiPLA resulted in an AUC of 0.7271 (95% CI 0.6239 to 0.8303, p < 0.001), with an increased value compared to its individual elements. The optimal cut-off point for FiPLA was 202 (sensitivity 82% and specificity 64%). Survival analysis based on preoperative FiPLA levels revealed that 89% of patients with FiPLA bellow cut-off and 51% of patients with FiPLA values above cut-off were alive at the end of the follow-up interval (95% CI 0.0992 to 0.3926, p < 0.001). Results are shown in Figure 2.
Figure 2.
ROC and survival curves for FiPLA: (a) ROC curve for FiPLA; (b) survival curve for FiPLA. Statistical significance was considered at p < 0.05.
3.7. Multivariate Analysis for Fib, PLR and FiPLA in OSCC
A binomial logistic regression was performed to ascertain the effects of Fib, PLR, and FiPLA on the likelihood that subjects suffered death related to disease. The logistic regression model was statistically significant, χ2(3) = 26.004, p < 0.0005. The model explained 29.5% (Nagelkerke R2) of the variance in death related to disease and correctly classified 77.5% of cases (sensitivity 67.6%, specificity 81.8%, positive predictive value 62.16% and negative predictive value 85.13%). Of the three predictors, only two were statistically significant: Fib and FiPLA. Subjects with Fib above cut-off had 3.129-times higher odds of dying due to disease progression than those under cut-off (95% CI 1.149–8.520, p = 0.026). FiPLA revealed a superior predictive power, as subjects with FiPLA values over cut-off were 6.307-times more likely to die secondarily to disease than those under cut-off (95% CI 1.024–38.845, p = 0.047).
As certain clinico-pathological variables might affect the prediction pattern of these parameters, we ran a regression model controlling for age, TNM stage, smoking status, degree of differentiation, and surgical margin clearance. We excluded gender and recurrence from the analysis due to the high predominance of male sex and the complete separation of data for recurrence. The logistic regression model was statistically significant, χ2(11) = 39.021, p < 0.001. The model explained 41.8% (Nagelkerke R2) of the variance in death related to disease and correctly classified 77.5% of cases (sensitivity 55.9%, specificity 87%, positive predictive value 65.51% and negative predictive value 81.7%). Of the three predictors, only FiPLA maintained its statistical significance, independent of the controlling variables. Subjects with FiPLA over the cut-off value had 8.785-times higher odds of dying from disease progression (95% CI 1.133–68.115, p = 0.038).
4. Discussion
Controlling disease progression and improving patient survival have been the main goals in cancer research. These objectives led to an intense quest for biomarkers that have revolutionized cancer history in several types of malignancies [22,23]. However, despite all efforts to identify powerful biomarkers in OSCC, the survival of patients suffering from this disabling disease has not improved significantly in recent decades. Many simple and sophisticated, technologically challenging, and costly molecules have been investigated in OSCC, some of them displaying valid biomarker features [24,25]. In our study, we analyzed the predictive role of inflammatory and immune elements determined in peripheral blood in patients with operable OSCC. Our results revealed an enhanced systemic inflammatory status, expressed through elevated levels of fibrinogen found in most of the patients at the time of diagnosis. These values were significantly higher in patients with poor outcomes. Our results are in accordance with other studies that have confirmed the prognostic potential of fibrinogen in many types of malignancy [26,27,28]. Similar findings were reported in laryngeal, nasopharyngeal, oral, and oropharyngeal squamous cell carcinomas, where elevated preoperative levels of fibrinogen were associated with worse outcomes and resistance to therapy [29,30,31]. Increased fibrinogen values were correlated with an aggressive behavior in solid tumors, where deposits of fibrinogen organized in networks in the extracellular matrix facilitate the binding of growth factors, thus promoting tumor growth and invasion through angiogenesis and fibroblast proliferation [13]. Plasma fibrinogen, through an extravasation process, is the main source for these peritumor deposits. However, studies showed that some tumor cells have the ability to synthesize endogenous fibrinogen and release it in the peritumor environment [32]. This circulating glycoprotein provides protective assets for tumor cells released in the blood stream and facilitates their aggregation with platelets, preventing tumor cell clearance through immune effectors [33]. Analysis of the circulating blood cells conducted in our study group revealed no significant differences in relation to patient outcomes in OSCC. However, PLR was significantly higher in patients who died due to disease progression. The prognostic value of PLR is supported by other studies in different types of cancers, which have reported that increased PLR values act as a negative predictive factor in most malignancies [34]. Two meta-analyses conducted on OSCC and head and neck squamous cell carcinoma (HNSCC) patients reported similar results [18,35]. PLR is a composite parameter that encloses the information from two distinct cellular elements, platelets and lymphocytes, and the reported results suggest that the relationship between these two parameters have a major impact on patients’ survival. Platelets’ role in cancer progression is exerted through tumor cell–platelet aggregates that facilitate tumor cell survival and escape from immune clearance mechanisms [36]. Moreover, it has been shown that platelets support tumor metastasis and the systemic spread of cancers [37]. In the general population, elevated platelet counts, even within normal ranges, are associated with an increased incidence of cancers [38]. This suggests that platelets are involved not only in tumor progression, but also in the process of carcinogenesis. Lymphocytes—the second component of PLR—have been known for their major role in all phases of cancer development and progression. Lymphocytes, the main effectors of the antitumor immune response, exert their functions both at the tumor site and in the peripheral compartment. Extensive studies have been conducted during the last decades that have provided valuable information on the mechanisms that underlie and interfere with the systemic antitumor immune response, leading to one of the most important breakthroughs in cancer history—the discovery of immunotherapy [14,39,40]. Decreased lymphocyte densities in tumor tissue are associated with a poor outcome in OSCC [41,42]. However, for circulating lymphocytes, the results are not so consistent and divergent findings compared to our results have been reported [43]. It is interesting to observe that, even though the scientific literature lacks consistency in the reported results for peripheral blood platelet and lymphocyte counts regarding their prognostic potential in OSCC, when analyzed together as PLR, the situation changes completely, and most studies underscore their predictive role in OSCC [17,35]. The superior prognostic power of PLR compared to its individual components is probably due to the opposing roles of platelets and lymphocytes in cancer progression, and discrete changes in individual blood elements, which are not detectable in a routine assessment, become obvious when analyzed together in a unique parameter—PLR—that accumulates the information from each individual component. Based on this principle, we have further developed our analysis and evaluated the cumulative predictive potential of fibrinogen and PLR within the new parameter of FiPLA. To our knowledge, this is the first scientific report of an algorithm based on this principle of determination that is evaluated for its predictive power in cancer patients. Our analysis revealed that FiPLA proved its superior potential in detecting high-risk patients compared to its individual components. FiPLA can be considered a complex parameter that encompasses within one numeric value prognostic information provided by the inflammatory, immune, and coagulative systems in a cost-effective, easily applicable method that is available worldwide for the assessment of patients with OSCC. This new parameter can be used, in addition to clinical TNM staging, to stratify patients with OSCC based on prognostic risks, as early as from the moment of diagnosis. The limitations of our retrospective study are related to a single center location and a relatively reduced sample of patients; these are limitations that can be overcome by a large group in the future, as well as multicentric studies, both of which will evaluate the adequateness of cut-off values and validate these preliminary results.
5. Conclusions
Fibrinogen (Fib) and platelet-to-lymphocyte ratio (PLR) both act as prognostic factors for OSCC, and they demonstrate an increased predictive power when analyzed together within a composite parameter, the Fibrinogen-PLR Algorithm (FiPLA). In conclusion, we propose a new predictive tool that has several major advantages: a simple, cost-efficient, early available, reproducible method that is feasible for worldwide implementation and that can provide valuable prognostic information in patients with OSCC.
Acknowledgments
Authors wish to thank Esmeralda Horjea—chief of Medical Statistics Department, “Carol Davila” Central Military Emergency Hospital, for her continuous support during the course of this work.
Author Contributions
Conceptualization, A.C. and C.C.; methodology, A.C. and C.C.; investigation and data curation A.C., L.M. and L.T.; data analysis, A.C. and M.L.; resources, A.C., C.C. and C.T.; writing—original draft preparation, A.C., L.M., M.L., L.T., C.C. and C.T.; writing—review and editing, supervision, A.C. and C.C.; project administration, A.C., C.C. and C.T.; funding acquisition, C.C. and C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research and APC were partially funded by the Romanian Ministry of Research and Innovation Core Program PN 19.29.01.04.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the “Carol Davila” Central Military Emergency Hospital, Bucharest (No. 25/27 November 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global Cancer Observatory. [(accessed on 1 May 2021)]; Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&i.
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Stat Facts: Oral Cavity and Pharynx Cancer. [(accessed on 1 May 2021)]; Available online: https://seer.cancer.gov/statfacts/html/oralcav.html.
- 4.Kreppel M., Nazarli P., Grandoch A., Safi A.-F., Zirk M., Nickenig H.-J., Scheer M., Rothamel D., Hellmich M., Zöller J.E. Clinical and Histopathological Staging in Oral Squamous Cell Carcinoma—Comparison of the Prognostic Significance. Oral Oncol. 2016;60:68–73. doi: 10.1016/j.oraloncology.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Majumdar B., Patil S., Sarode S.C., Sarode G.S., Rao R.S. Clinico-Pathological Prognosticators in Oral Squamous Cell Carcinoma: An Update. Transl. Res. Oral Oncol. 2017;2:1–14. doi: 10.1177/2057178X17738912. [DOI] [Google Scholar]
- 6.Alkhadar H., Macluskey M., White S., Ellis I. Perineural Invasion in Oral Squamous Cell Carcinoma: Incidence, Prognostic Impact and Molecular Insight. J. Oral Pathol. Med. 2020;49:994–1003. doi: 10.1111/jop.13069. [DOI] [PubMed] [Google Scholar]
- 7.Caruntu A., Moraru L., Lupu M., Ciubotaru D.A., Dumitrescu M., Eftimie L., Hertzog R., Zurac S., Caruntu C., Voinea O.C. Assessment of Histological Features in Squamous Cell Carcinoma Involving Head and Neck Skin and Mucosa. J. Clin. Med. 2021;11:2343. doi: 10.3390/jcm10112343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiegnitz E., Kämmerer P.W., Schön H., Blatt S., Berres M., Sagheb K., Al-Nawas B. Proinflammatory Cytokines as Serum Biomarker in Oral Carcinoma—A Prospective Multi-Biomarker Approach. J. Oral Pathol. Med. 2018;38:42–49. doi: 10.1111/jop.12670. [DOI] [PubMed] [Google Scholar]
- 9.Zhao M., Ding L., Yang Y., Chen S., Zhu N., Fu Y., Ni Y., Wang Z. Aberrant Expression of PDCD4/EIF4A1 Signal Predicts Postoperative Recurrence for Early-Stage Oral Squamous Cell Carcinoma. Cancer Manag. Res. 2019;11:9553–9562. doi: 10.2147/CMAR.S223273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X., Gu W., Wang Q., Fu X., Wang Y., Xu X., Wen Y. C-MYC and BCL-2 Mediate YAP-Regulated Tumorigenesis in OSCC. Oncotarget. 2018;9:668–679. doi: 10.18632/oncotarget.23089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribeiro I.P., De Melo J.B., Carreira I.M. Head and Neck Cancer: Searching for Genomic and Epigenetic Biomarkers in Body Fluids—The State of Art. Mol. Cytogenet. 2019;12:4–9. doi: 10.1186/s13039-019-0447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caruntu A., Moraru L., Lupu M., Vasilescu F., Dumitrescu M., Cioplea M., Popp C., Dragusin A., Caruntu C., Zurac S. Prognostic Potential of Tumor-Infiltrating Immune Cells in Resectable Oral Squamous Cell Carcinoma. Cancers. 2021;13:2268. doi: 10.3390/cancers13092268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caruntu A., Scheau C., Tampa M., Georgescu S.R., Caruntu C., Tanase C. Complex Interaction Among Immune, Inflammatory, and Carcinogenic Mechanisms in the Head and Neck Squamous Cell Carcinoma. Adv. Exp. Med. Biol.-Clin. Exp. Biomed. 2021 doi: 10.1007/5584_2021_626. [DOI] [PubMed] [Google Scholar]
- 14.Thorsson V., Gibbs D.L., Brown S.D., Wolf D., Bortone D.S., Ou Yang T.H., Porta-Pardo E., Gao G.F., Plaisier C.L., Eddy J.A., et al. The Immune Landscape of Cancer. Immunity. 2018;48:812–830. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greten F.R., Grivennikov S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tampa M., Mitran M.I., Mitran C.I., Sarbu M.I., Matei C., Nicolae I., Caruntu A., Tocut S.M., Popa M.I., Caruntu C., et al. Mediators of Inflammation—A Potential Source of Biomarkers in Oral Squamous Cell Carcinoma. J. Immunol. Res. 2018;2018 doi: 10.1155/2018/1061780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malik A., Mishra A., Mair M., Chakrabarti S., Garg A., Singhvi H., Chopda P., Qayyumi B., Sawarkar N., Mathur Y., et al. Role of Neutrophil-To-Lymphocyte Ratio and Platelet-To-Lymphocyte Ratio as Prognostic Markers in Oral Cavity Cancers. Indian J. Med. Paediatr. Oncol. 2019;40:94–100. doi: 10.4103/ijmpo.ijmpo_5_18. [DOI] [Google Scholar]
- 18.Takenaka Y., Oya R., Kitamiura T., Ashida N., Shimizu K., Takemura K., Yamamoto Y., Uno A. Platelet Count and Platelet-Lymphocyte Ratio as Prognostic Markers for Head and Neck Squamous Cell Carcinoma: Meta-Analysis. Head Neck. 2018;40:2714–2723. doi: 10.1002/hed.25366. [DOI] [PubMed] [Google Scholar]
- 19.Mattavelli D., Lombardi D., Missale F., Calza S., Battocchio S., Paderno A., Bozzola A., Bossi P., Vermi W., Piazza C., et al. Prognostic Nomograms in Oral Squamous Cell Carcinoma: The Negative Impact of Low Neutrophil to Lymphocyte Ratio. Front. Oncol. 2019;9:339. doi: 10.3389/fonc.2019.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holzinger D., Danilovic I., Seemann R., Kornek G., Engelmann J., Pillerstorff R., Holawe S., Psyrri A., Erovic B.M., Farwell G., et al. Prognostic Impact of Pretreatment Plasma Fibrinogen in Patients with Locally Advanced Oral and Oropharyngeal Cancer. PLoS ONE. 2016;11:e0158697. doi: 10.1371/journal.pone.0158697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khandavilli S.D., Ceallaigh P.Ó., Lloyd C.J., Whitaker R. Serum C-Reactive Protein as a Prognostic Indicator in Patients with Oral Squamous Cell Carcinoma. Oral Oncol. 2009;45:912–914. doi: 10.1016/j.oraloncology.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Alsibai K.D., Meseure D. Histopathology—An Update. IntechOpen; London, UK: 2018. Significance of Tumor Microenvironment Scoring and Immune Biomarkers in Patient Stratification and Cancer Outcomes. [DOI] [Google Scholar]
- 23.Goossens N., Nakagawa S., Sun X., Hoshida Y. Cancer Biomarker Discovery and Validation. Transl. Cancer Res. 2015;4:256–269. doi: 10.3978/j.issn.2218-676X.2015.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyford-Pike S., Peng S., Young G.D., Taube J.M., Westra W.H., Akpeng B., Bruno T.C., Richmon J.D., Wang H., Bishop J.A., et al. Evidence for a Role of the PD-1:PD-L1 Pathway in Immune Resistance of HPV-Associated Head and Neck Squamous Cell Carcinoma. Cancer Res. 2013;73:1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao Y., Li H., Mao L., Yang Q.C., Fu L.Q., Wu C.C., Liu B., Sun Z.J. CD103(+) T and Dendritic Cells Indicate a Favorable Prognosis in Oral Cancer. J. Dent. Res. 2019;98:1480–1487. doi: 10.1177/0022034519882618. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., Cao J., Deng Y., Huang Y., Li R., Lin G., Dong M., Huang Z. Pretreatment Plasma Fibrinogen Level as a Prognostic Biomarker for Patients with Lung Cancer. Clinics. 2020;75:e993. doi: 10.6061/clinics/2020/e993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu X., Hu F., Yao Q., Li C., Zhang H., Xue Y. Serum Fibrinogen Levels Are Positively Correlated with Advanced Tumor Stage and Poor Survival in Patients with Gastric Cancer Undergoing Gastrectomy: A Large Cohort Retrospective Study. BMC Cancer. 2016;16:480. doi: 10.1186/s12885-016-2510-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu W.Y., Zhang H.H., Yang X.B., Bai Y., Lin J.Z., Long J.Y., Xiong J.P., Zhang J.W., Sang X.T., Zhao H.T. Prognostic Signifcance of Combined Preoperative Fbrinogen and CA199 in Gallbladder Cancer Patients. World J. Gastroenterol. 2018;24:1451–1463. doi: 10.3748/wjg.v24.i13.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He S.S., Wang Y., Yang L., Chen H.Y., Liang S.B., Lu L.X., Chen Y. Plasma Fibrinogen Correlates with Metastasis and Is Associated with Prognosis in Human Nasopharyngeal Carcinoma. J. Cancer. 2017;8:403–409. doi: 10.7150/jca.17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai H., Zhang Z., Zhou Y., Liu J. The Prognostic Value of Preoperative Plasma Fibrinogen and Neutrophil-to- Lymphocyte Ratio in Patients with Laryngeal Squamous Cell Carcinoma. Ear Nose Throat J. 2020 doi: 10.1177/0145561320920746. [DOI] [PubMed] [Google Scholar]
- 31.Selzer E., Grah A., Heiduschka G., Kornek G., Thurnher D. Pre-Therapeutic Fibrinogen Levels Are of Prognostic Significance in Locally Advanced Head and Neck Cancer. Wien. Klin. Wochenschr. 2016;128:320–328. doi: 10.1007/s00508-016-0963-3. [DOI] [PubMed] [Google Scholar]
- 32.Simpson-Haidaris P.J., Rybarczyk B. Tumors and Fibrinogen. The Role of Fibrinogen as an Extracellular Matrix Protein. Ann. N. Y. Acad. Sci. 2001;936:406–425. doi: 10.1111/j.1749-6632.2001.tb03525.x. [DOI] [PubMed] [Google Scholar]
- 33.Zheng S., Shen J., Jiao Y., Liu Y., Zhang C., Wei M., Hao S., Zeng X. Platelets and Fibrinogen Facilitate Each Other in Protecting Tumor Cells from Natural Killer Cytotoxicity. Cancer Sci. 2009;100:859–865. doi: 10.1111/j.1349-7006.2009.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou X., Du Y., Huang Z., Xu J., Qiu T., Wang J., Wang T., Zhu W., Liu P. Prognostic Value of PLR in Various Cancers: A Meta-Analysis. PLoS ONE. 2014;9:e101119. doi: 10.1371/journal.pone.0101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen S., Guo J., Feng C., Ke Z., Chen L., Pan Y. The Preoperative Platelet-Lymphocyte Ratio versus Neutrophil-Lymphocyte Ratio: Which Is Better as a Prognostic Factor in Oral Squamous Cell Carcinoma? Ther. Adv. Med. Oncol. 2016;8:160–167. doi: 10.1177/1758834016638019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Menter D.G., Tucker S.C., Kopetz S., Sood A.K., Crissman J.D., Honn K.V. Platelets and Cancer: A Casual or Causal Relationship: Revisited. Cancer Metastasis Rev. 2014;33:231–269. doi: 10.1007/s10555-014-9498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gay L.J., Felding-Habermann B. Contribution of Platelets to Tumour Metastasis. Nat. Rev. Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ankus E., Price S.J., Ukoumunne O.C., Hamilton W., Bailey S.E.R. Cancer Incidence in Patients with a High Normal Platelet Count: A Cohort Study Using Primary Care Data. Fam. Pract. 2018;35:671–675. doi: 10.1093/fampra/cmy018. [DOI] [PubMed] [Google Scholar]
- 39.Dudley M.E., Wunderlich J.R., Robbins P.F., Yang J.C., Hwu P., Schwartzentruber D.J., Topalian S.L., Sherry R., Restifo N.P., Hubicki A.M., et al. Cancer Regression and Autoimmunity in Patients after Clonal Repopulation with Antitumor Lymphocytes. Science (80-) 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dobosz P., Dzieciątkowski T. The Intriguing History of Cancer Immunotherapy. Front. Immunol. 2019;10:2965. doi: 10.3389/fimmu.2019.02965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Troiano G., Rubini C., Togni L., Caponio V.C.A., Zhurakivska K., Santarelli A., Cirillo N., Lo Muzio L., Mascitti M. The Immune Phenotype of Tongue Squamous Cell Carcinoma Predicts Early Relapse and Poor Prognosis. Cancer Med. 2020;9:8333–8344. doi: 10.1002/cam4.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spector M.E., Bellile E., Amlani L., Zarins K., Smith J., Brenner J.C., Rozek L., Nguyen A., Thomas D., McHugh J.B., et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma. JAMA Otolaryngol.-Head Neck Surg. 2019;145:1012–1019. doi: 10.1001/jamaoto.2019.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diao P., Wu Y., Ge H., Li J., Zhang W., Huang R., Wang Y., Cheng J. Preoperative Circulating Platelet, Neutrophil, and Lymphocyte Counts Predict Survival in Oral Cancer. Oral Dis. 2019;25:1057–1066. doi: 10.1111/odi.13049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author.