Abstract
Background: the neoplastic B cells of the Helicobacter pylori-related low-grade gastric mucosa-associated lymphoid tissue (MALT) lymphoma proliferate in response to H. pylori, however, the nature of the H. pylori antigen responsible for proliferation is still unknown. The purpose of the study was to dissect whether CagY might be the H. pylori antigen able to drive B cell proliferation. Methods: the B cells and the clonal progeny of T cells from the gastric mucosa of five patients with MALT lymphoma were compared with those of T cell clones obtained from five H. pylori–infected patients with chronic gastritis. The T cell clones were assessed for their specificity to H. pylori CagY, cytokine profile and helper function for B cell proliferation. Results: 22 of 158 CD4+ (13.9%) gastric clones from MALT lymphoma and three of 179 CD4+ (1.7%) clones from chronic gastritis recognized CagY. CagY predominantly drives Interferon-gamma (IFN-γ) and Interleukin-17 (IL-17) secretion by gastric CD4+ T cells from H. pylori-infected patients with low-grade gastric MALT lymphoma. All MALT lymphoma-derived clones dose dependently increased their B cell help, whereas clones from chronic gastritis lost helper activity at T-to-B-cell ratios greater than 1. Conclusion: the results obtained indicate that CagY drives both B cell proliferation and T cell activation in gastric MALT lymphomas.
Keywords: Helicobacter pylori, CagY, B cells, T cells, cytokines, MALT, gastric lymphoma
1. Introduction
Helicobacter pylori is a spiral-shaped Gram-negative bacterium that chronically infects the stomach of more than 50% of the human population, and is the leading cause of gastric cancer, gastric lymphoma, gastric autoimmunity and peptic ulcer diseases [1,2,3,4,5]. A strong association between Helicobacter pylori infection and the development of gastric mucosa-associated lymphoid tissue (MALT) lymphoma has been demonstrated [6,7,8]. A prerequisite for lymphomagenesis is the development of secondary inflammatory MALT, which is induced by chronic H. pylori infection [7,8]. In the early stages, this tumor is sensitive to the withdrawal of H. pylori-induced T cell help, providing an explanation for both the tendency of the tumor to remain localized at the primary site and its regression after eradication of H. pylori with antibiotics. The tumor cells of low-grade gastric MALT lymphoma are memory B lymphocytes that still respond to differentiation signals, such as CD40 costimulation and cytokines produced by antigen-stimulated T helper (Th) cells [9,10] and their growth depends on antigen-stimulation by H. pylori-specific T cells [11,12]. An important unanswered question remains the chemical nature of the H. pylori factors responsible for the induction of gastric Th cells which can promote the proliferation of B cells. Bacterial products are known to possess immunomodulatory properties and induce B cell responses as well as different types of innate and adaptive responses [13].
Among the bacterial components, some factors associated with malignancy have been identified, although the high degree of genomic variability of H. pylori strains has prevented the complete identification of the factors involved. The major virulence factor of H. pylori is the cag pathogenicity island (cagPAI), an approximately 40 kb genetic locus, containing 31 genes [14,15] and encoding for the so-called type IV secretion system (T4SS). This forms a syringe-like structure that injects bacterial components (mainly peptidoglycan and the oncoprotein cagA) into the host target cell [16]. H. pylori strains harboring the cagPAI pathogenicity locus show a significantly increased ability to induce severe pathological outcomes in infected individuals, such as gastric cancer and gastric lymphoma, compared to cagPAI-negative strains [17,18,19,20]. Recently, it was reported that among H. pylori-infected patients, those with gastric low-grade MALT lymphoma are preferentially seropositive for H. pylori CagY protein [21]. CagY, a VirB10-homologous protein, also known as Cag7 or HP0527, is able to activate innate cells in a flagellin-independent manner. CagY is a TLR5 agonist and five interaction sites have been identified in the CagY repeat domains [16,22,23]. HP0527 encodes a large protein of 1927 amino acids that is expressed on the surface and has been described as one of the main components of H. pylori cag T4SS-associated pilum; it may act as a molecular switch that modifies the proinflammatory host responses by modulating the T4SS function and tuning CagA injection [24,25].
The aims of this study were (1) to investigate the presence of H. pylori CagY-specific Th cells in the context of low-grade gastric MALT lymphomas, (2) to define the cytokine patterns of these cells, and (3) to assess whether gastric CagY-specific T cells from MALT lymphomas are able to provide help for B cell proliferation.
2. Results
2.1. H. pylori CagY-Specific CD4+ T Cells Predominate in Gastric Low-Grade MALT Lymphoma
To characterize at the clonal level the in vivo activated T cells present in the gastric inflammatory infiltrates of H. pylori-infected patients, two cohorts were collected: five untreated patients with gastric low-grade MALT (MALT) and five patients with H. pylori-induced uncomplicated chronic gastritis (CG). All patients were infected with CagA1, VacA1 H. pylori type I strains and were ELISA positive for anti-CagA serum IgG antibodies. The T cells from all enrolled patients were obtained by multiple biopsies and expanded by culturing in Interleukin-2 (IL-2)-conditioned medium for 10 days. Then, T cell blasts were recovered and cloned by limiting dilution.
Comparable numbers of clones were obtained from both cohorts: a total of 158 CD4+ and 17 CD8+ clones were obtained from the gastric biopsy specimens of MALT lymphoma. 179 CD4+ and 22 CD8+ T cell clones from chronic gastritis. All clones were tested for their ability to respond to either H. pylori lysate or purified CagY protein. The data obtained are summarized in Table 1 and show that none of the CD8+ clones from MALT and CG responded to H. pylori CagY antigen or to H. pylori lysate. Analyzing the proliferative response of CD4+ clones to H. pylori lysate, 23 and 22% of the clones showed positivity for MALT and CG, respectively. A marked difference was observed when CagY was used. While 22 CD4+ (corresponding to 13.9%) of clones from MALT lymphoma showed antigen-induced proliferation, only three CD4+ clones (1.7% of the clones) from chronic gastritis were CagY-specific (Table 1).
Table 1.
Number (%) of CagY or H. pylori lysate-specific T cell clones isolated from biopsy specimens of MALT lymphoma (MALT) and chronic gastritis (CG) patients.
| Patient ID | CagY-Specific T CD4+ Clones |
H. pylori Lysate-Specific T CD4+ Clones |
CagY-Specific T CD8+ Clones |
H. pylori Lysate-Specific T CD8+ Clones |
|---|---|---|---|---|
| MALT 1 | 4/30 (13.3) | 5/30 (16.7) | 0/3 (0) | 0/3 (0) |
| MALT 2 | 5/28 (17.9) | 7/28 (25) | 0/4 (0) | 0/4 (0) |
| MALT 3 | 5/35 (14.3) | 8/35 (22.9) | 0/2 (0) | 0/2 (0) |
| MALT 4 | 4/22 (18.2) | 7/22 (31.8) | 0/4 (0) | 0/4 (0) |
| MALT 5 | 4/43 (9.3) | 10/43 (23.2) | 0/4 (0) | 0/4 (0) |
| Total | 22/158 (13.9) | 37/158 (23.4) | 0/17 (0) | 0/17 (0) |
| CG 1 | 1/37 (2.7) | 6/37 (16.2) | 0/3 (0) | 0/3 (0) |
| CG 2 | 1/28 (3.6) | 8/28 (28.6) | 0/4 (0) | 0/4 (0) |
| CG 3 | 0/35 (0) | 9/35 (25.7) | 0/5 (0) | 0/5 (0) |
| CG 4 | 1/44 (2.3) | 7/44 (15.9) | 0/5 (0) | 0/5 (0) |
| CG 5 | 0/35 (0) | 9/35 (25.7) | 0/5 (0) | 0/5 (0) |
| Total | 3/179 (1.7) | 39/179 (21.8) | 0/22 (0) | 0/22 (0) |
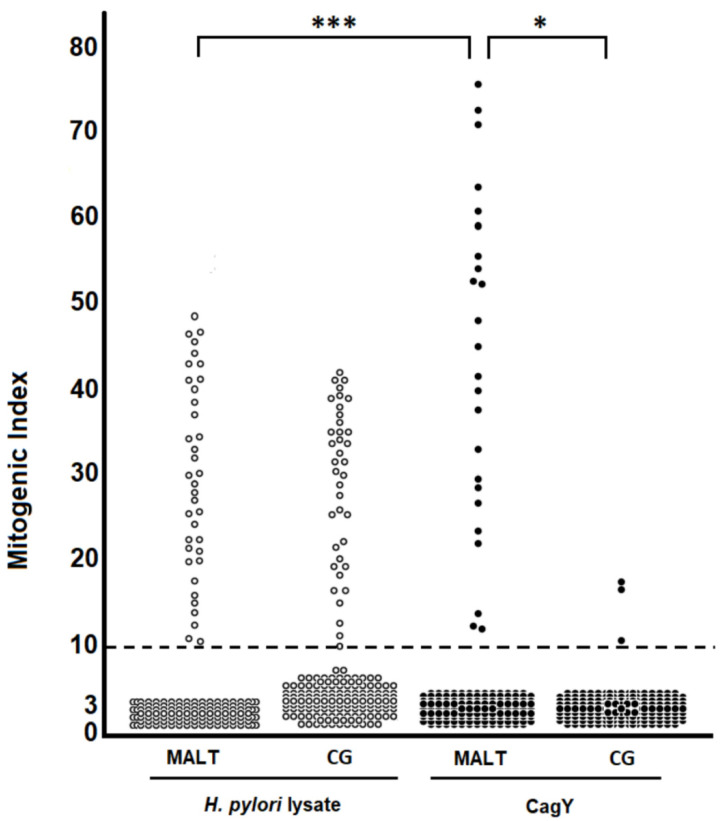
The percentage of MALT lymphoma-derived T clones reactive for CagY was higher than CG. A significant difference (p = 0.012) was also found between the mitogenic index of CagY-specific MALT lymphoma-derived T clones (mean mitogenic index 44.48 ± 18.46) and CG-derived ones (mean mitogenic index 14.63 ± 3.89). A significantly higher proliferation (p < 0.001) to CagY than H. pylori lysate was found in MALT lymphoma-derived T clones (mean CagY mitogenic index 44.48 ± 18.46; mean H. pylori lysate mitogenic index 29.14 ± 11.67) (Figure 1).
Figure 1.
Antigen specificity of H. pylori-reactive T cell clones derived from the gastric mucosa of H. pylori-infected patients with low-grade MALT B cell lymphoma (MALT) or uncomplicated chronic gastritis (CG). In vivo activated T cells were recovered from biopsy specimens of gastric mucosa and cloned by limiting dilution. T cell blasts from each clone were seeded in triplicate cultures with irradiated autologous peripheral blood mononuclear cells in the presence of medium alone or optimal doses of H. pylori lysate (10 μg/mL), or CagY (1 μg/mL). After 60 h, [3H] thymidine uptake was measured and expressed as mitogenic index. A significant difference (*) was found between the mitogenic index of CagY-specific MALT lymphoma-derived T clones and CG-derived ones. A highly significant (***) proliferation to CagY than to H.pylori lysate was found in MALT lymphoma-derived T clones.
2.2. H. pylori CagY Predominantly Drives IFN-γ and IL-17 Secretion by Gastric CD4+ T Cells from H. pylori-Infected Patients with Gastric Low-Grade MALT Lymphoma
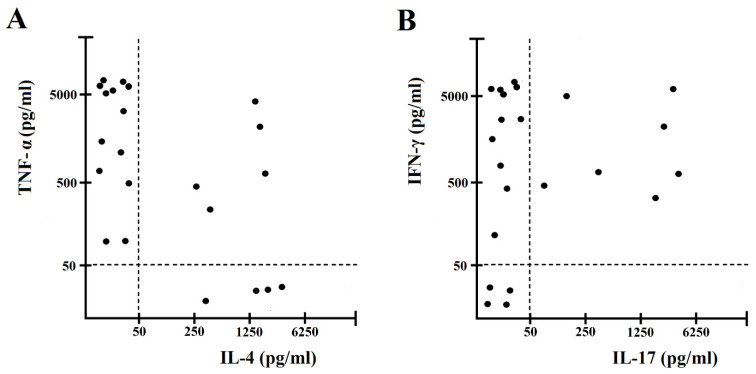
To evaluate cytokine production by gastric-derived H. pylori CagY–specific Th clones, each clone was co-cultured in duplicate with autologous antigen presenting cells (APCs) and H. pylori CagY for 48 h. After antigen stimulation, 32% of clones from MALT lymphoma gastritis produced both Interferon-gamma (IFN-γ) and Interleukin-17 (IL-17), but not Interleukin-4 (IL-4) (Th1/Th17 profile), 27% of clones produced IFN-γ, but not IL-17, nor IL-4 (Th1 profile), 23% secreted both Tumor necrosis factor- alpha (TNF-α) and IL-4, but not IL-17 (Th0 profile), and 18% produced IL-4, but not TNF-α, nor IL-17 (Th2 profile) (Figure 2). Among the three gastric T cell clones obtained from chronic gastritis, two were Th1, and one Th1/Th17.
Figure 2.
Cytokine profile of gastric mucosa CagY-specific CD4+ T cell clones obtained from H. pylori-infected patients with gastric low-grade MALT lymphoma. Th clones were tested for cytokine production (A,B). CagY-specific Th clones were stimulated with CagY and TNF-α and IL-4, IFN-γ and IL-17 production was measured in culture supernatants. In unstimulated cultures, levels of TNF-α, IL-4, IFN-γ and IL-17 were consistently < 20 pg/mL. CD4+ T cell clones producing IFN-γ, but not IL-17 nor IL-4, were coded as Th1. CD4+ T cell clones producing IL-17, but not IFN-γ nor IL-4, were coded as Th17. CD4+ T cell clones producing IFN-γ, and IL-17, but not IL-4, were coded as Th17/Th1. CD4+ T cell clones producing TNF-α and IL-4, but not IL-17, were coded as Th0.
2.3. Antigen-Dependent B Cell Help by H. pylori CagY-Specific Th Clones
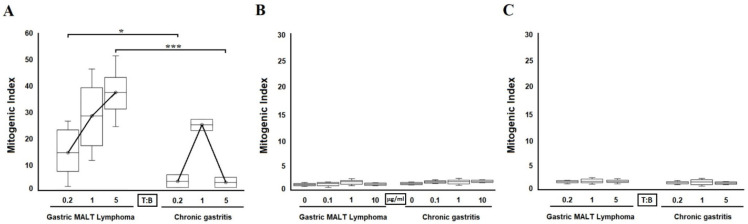
To assess the ability of H. pylori CagY-specific T cell clones to provide antigen-triggered B cell help, irradiated T cell blasts of each clone were co-cultured with autologous peripheral blood B cells at a ratio of 0.2, 1, and 5 to 1. At a T-to-B cell ratio of 0.2 to 1, all CagY-specific clones from MALT lymphoma patients but none from patients with chronic gastritis, provided significant help (p < 0.05) to B cell proliferation under H. pylori CagY stimulation (mean mitogenic index, 14 and 3.5; range, 1–26 and 1–6, respectively; Figure 3A). At a T-to-B cell ratio of 1 to 1, all CagY-specific clones from both MALT lymphoma and chronic gastritis patients provided significant help for B cell proliferation under H. pylori CagY stimulation (mean mitogenic index, 28 and 25; range, 11–46 and 22.8–27.2, respectively; Figure 3A). Finally, at a T-to-B cell ratio of 5 to 1, all 22 Th clones from MALT lymphoma further increased B cell proliferation with a mean mitogenic index of 37 (range 24–51; Figure 3A). A significant decrease (p < 0.001) in B cell proliferation was observed in the presence of all three Th clones from chronic gastritis with a mean mitogenic index of 3 (range 1–5; Figure 3A). B cells alone cultured with or without CagY did not proliferate unless autologous MALT lymphoma-derived T cells were added (Figure 3B). B cells cultured with autologous MALT lymphoma-derived T cells, without CagY did not proliferate at any T-to-B cell ratio (Figure 3C).
Figure 3.
B cell proliferation to CagY. CagY-stimulated T cell clones derived from the gastric mucosa of patients with MALT lymphoma provide huge help for proliferation to autologous B cells (A). Irradiated T cell blasts of each CagY-reactive clone derived from patients with gastric MALT lymphoma or chronic gastritis were co-cultured for four days with peripheral blood autologous B cells (3 × 104) at 0.2, 1, and 5 to 1 T-to-B-cell ratios in the presence of medium alone or CagY. Sixteen hours before harvesting, 0.5 μCi of [3H] thymidine was added, and its uptake was measured as mitogenic index. B cells cultured with or without CagY did not show any proliferation both in gastric low-grade MALT lymphoma and chronic gastritis patients at any CagY concentration used (0.1, 1, 10 μg/mL) (B). B cells cultured without CagY, with autologous irradiated gastric T cells did not show any proliferation both in gastric low-grade MALT lymphoma and in chronic gastritis patients at any T-to-B-cell ratios (0.2, 1, and 5 to 1) (C). * p < 0.05; *** p < 0.001.
Gastric T cell clones specific for H. pylori lysate but not specific for CagY (15/37 from MALT lymphoma and 36/39 from patients with chronic gastritis) showed no helper activity on B cell proliferation when co-cultured with CagY antigen and autologous B cells (mean mitogenic index was 1, range 0.9–1.3 both for MALT and for CG) (data not shown).
3. Discussion
H. pylori induces a strong inflammatory response that is directed at clearing the infection, but if not controlled, the response can be harmful to the host, and eventually lead to the development of gastric MALT lymphoma [26,27,28]. It has been shown that CagA activates the mTOR Complex 1 (mTORC1) which, in turn, promotes the expression and release of proinflammatory cytokines, chemokines, and of an antimicrobial peptide from gastric epithelial cells [29]. H. pylori stimulates macrophages, both in vitro and in vivo, to produce the proliferation-inducing ligand (APRIL), a crucial cytokine able to promote lymphomagenesis and B cell proliferation and abundantly expressed in gastric MALT lymphoma [30]. Lymphoma-infiltrating macrophages are a major gastric source of APRIL. By using a model of lymphomagenesis, based on the Helicobacter sp. infection of transgenic C57BL6 mice expressing the human form of the APRIL cytokine (Tg-hAPRIL), Blosse et al. [31] characterized the gastric mucosal inflammatory response associated with gastric MALT lymphoma and highlighted that all T cell subtypes infiltrate gastric MALT lymphoma, including regulatory T cells, both in the animal model and in human gastric MALT lymphoma patients. Regulatory T cell response might contribute to the persistence of the pathogen in the gastric mucosa by delaying the inflammatory response to allow the chronic antigen stimulation necessary for lymphoid proliferation. According to a previous report [30], authors found APRIL significantly dysregulated in human gastric MALT lymphoma and revealed that the cytokine is mainly expressed by eosinophils, suggesting the pro-tumorigenic potential of these cells. By using an antibody which recognizes the secreted and internalized form of APRIL, they also confirmed that the target cells of the cytokine are B cells [32]. Besides APRIL, also the cytokine BAFF contributes to the B cell lymphomagenesis during chronic H. pylori infection [31]. Chonwerawong et al. revealed that H pylori upregulates NLRC5 expression in the macrophages and gastric tissues of mice and humans and that this expression correlates with gastritis severity. However, by taking advantage of NLRC5-deficient macrophages, and of knockout mice with nonfunctional Nlrc5 within the myeloid cell lineage, the authors found that NLRC5 negatively modulates the production of proinflammatory cytokines, including BAFF and protects against the formation of mucosal B cell lymphoid tissue formation in response to chronic Helicobacter infection in mice [33].
In vivo activated T cells in the lesional gastric mucosa of five patients with H. pylori–associated low-grade MALT lymphoma were expanded in our study and efficiently cloned to assess their specificity for H. pylori CagY protein and functional profile. This procedure has proved useful and accurate for in vitro studies of tissue-infiltrating T cells in many diseases [13,34,35]. In the progeny of gastric T cells from MALT lymphoma, but not chronic gastritis, a high proportion of T cell clones were reactive to the H. pylori CagY protein. Whether infection with more aggressive CagA-positive H. pylori strains is associated with MALT lymphoma is controversial [36,37], present data suggest that CagY is one of the immunodominant targets of gastric T cells in gastric low-grade MALT lymphoma, but not in uncomplicated chronic gastritis. On the other hand, we cannot exclude that other still undefined antigens of H. pylori may be involved in driving gastric T cell and B cell responses in patients with low-grade gastric MALT lymphoma.
The main limitation of this study is the small sample size enrolled, composed of five gastric MALT lymphoma patients and five chronic gastritis subjects. This is a consequence of both the fact that MALT lymphoma is not frequently found in the population and the restricted sampling caused by the difficult COVID times we are facing nowadays. This study has, however, the power to detect a relevant characterization of the cellular immune response to the H. pylori protein CagY given the high number of T cell clones obtained from T lymphocytes infiltrating the gastric mucosa of our tested subjects. H. pylori CagY-activated T cell clones from MALT lymphoma showed higher helper activity for B cell proliferation than clones generated from chronic gastritis. This supports the concept that H. pylori-CagY-specific T cells are responsible for the abnormal B cell growth, which probably precedes and favors the development of low-grade B cell lymphoma at gastric level in some H. pylori-infected patients. A possible mechanism for enhanced B cell proliferation might be the abnormal production of Th-derived cytokines active on B cell growth. MALT lymphoma-like lesions of the gastric mucosa were found after long-term Helicobacter felis infection in aged BALB/c mice, a strain genetically prone to high production of Th2 cytokines and B cell response [38]. Th2-skewed cytokine production in the local T cell response might account for enhanced B cell proliferation in MALT lymphoma. The majority of CagY-specific T cells in gastric MALT lymphoma produced IFN-γ and TNF-α. A significant proportion of T cells from MALT lymphoma produced IL-17 together with IFN-γ. Some CagY-specific T cells were able to produce IL-4. We can speculate that almost all CagY-specific T cells were able to produce several cytokines, such as TNF-α, and IL-4, able to promote B cell proliferation. Moreover it has been recently showed that IL-17 is also able to promote the growth of human germinal center-derived non-Hodgkin B cell lymphoma [39].
Based on the results obtained so far we can conclude that the H. pylori CagY protein, in patients with H. pylori infection and gastric low-grade MALT lymphoma, was able to promote gastric Th1 and Th17 inflammation through the production of various cytokines that can promote B cell proliferation. H. pylori CagY-specific Th cells derived from the gastric mucosa of H. pylori-infected patients with gastric low-grade MALT lymphoma were able to provide significantly higher B cell help compared to T cells obtained from patients with uncomplicated chronic gastritis.
4. Materials and Methods
4.1. Patients
Five untreated patients (three men and two women; mean age, 69 years; range, 63–75 years) with low-grade B cell lymphoma of gastric MALT (MALToma) and five patients (three men and two women; mean age, 59 years; range, 55–68 years) with uncomplicated chronic gastritis provided informed consent for this study, which was performed after approval by the local ethical committee (protocol number 14936_bio, approved on 8 October 2019). Multiple biopsy specimens were obtained from the gastric antrum of patients with chronic gastritis. In patients with low-grade MALT lymphoma, biopsy specimens were obtained from perilesional regions. Biopsy specimens were used for diagnosis (positive urease test, typing of H. pylori strain, and histology) and culture of tumor-infiltrating T lymphocytes. All patients with chronic gastritis or MALT lymphoma were infected with CagA1 VacA1 H. pylori type I strains and were positive for anti-CagA serum immunoglobulin (Ig) G antibodies, as assessed by specific ELISA (MyBioSource, San Diego, CA, USA).
4.2. Reagents
Helicobacter pylori CagY was produced as described [21]. We ruled out the presence of contaminants by a limulus test. The H. pylori CagY used resulted limulus test negative throughout the whole study.
4.3. Generation of H. pylori-Specific T Cell Clones
Biopsy specimens were cultured for 10 days in RPMI 1640 medium (Biochrom AG, Berlin, Germany) supplemented with human IL-2 (PeproTech, London, UK) (50 U/mL) to expand in vivo activated T cells [10]. Mucosal specimens were disrupted and single T-cell blasts were cloned under limiting dilution (0.3 cells/well) as reported previously [40]. Each clone was screened (in triplicate cultures for each condition) for responsiveness to H. pylori by measuring [3H] thymidine (Perkin Elmer, Waltham, MA, US) uptake after 60 h’ stimulation with medium, H. pylori lysate (aqueous extract of NCTC11637 strain, 10 μg/mL being optimal), recombinant CagY protein (1 μg/mL) in the presence of irradiated autologous mononuclear cells as APCs [40]. A mitogenic index greater than 10 was considered a positive result.
4.4. Cytokine Profile of H. pylori CagY–Specific Gastric T Cell Clones
To assess the cytokine production of CagY-specific Th clones, 106 T cell blasts of each clone were co-cultured in duplicate cultures for 48 h in 1 mL of medium with 5 × 105 irradiated autologous peripheral blood mononuclear cells as APCs and CagY (1 μg/mL). To induce cytokine production by gastric T cell clones, T cell blasts were stimulated for 36 h with phorbol-12-myristate 13-acetate (PMA, 10 ng/mL) (BioLegend, San Diego, CA, USA) plus anti-CD3 monoclonal antibody (200 ng/mL) (BioLegend, San Diego, CA, USA) [10]. Duplicate samples of each supernatant were assayed by ELISA for IFN-γ, IL-4, TNF-α, and IL-17 (R&D Systems, Minneapolis, MN, USA).
4.5. Helper Activity of T Cell Lones for B Cell Proliferation
B cells were prepared from each patient as described [13]. Briefly, PBMCs isolated by Ficoll Hypaque gradient method (Lymphoprep, Alere Technologies, Oslo, Norway) from each enrolled patient were processed to obtain a purified population of B lymphocytes by positive selection magnetic labelling with anti-CD19 microbeads (MACS, Miltenyi biotec, Bergisch Gladbach, Germany). The target population was incubated with specific microbeads and the cell suspension was loaded onto MACS columns placed in a magnetic field; B cells were then retained within the column and the other PBMCs were eluted away. Purified B lymphocytes were then harvested, washed, and used for the subsequent experiments.
The ability of gastric Th clones to induce B cell proliferation under CagY stimulation was assessed by measuring [3H] thymidine uptake by peripheral blood B cells (3 × 104) alone or co-cultured for four days with different concentrations of irradiated (2000 rad) autologous clonal T cell blasts (0.2, 1, and 5 T-to-B cell ratio) with or without H. pylori CagY antigen (1 μg/mL), as described previously [10].
4.6. Statistical Analysis
Descriptive statistics were used for the calculation of absolute frequencies and percentages of qualitative data, as well as for mean and standard deviation of quantitative data. After evaluating the homogeneity of variance with Hartley’s test, the t-test (IC 95%) was performed and a p < 0.05 was considered statistically significant. Statistical analysis was computed using the IBM SPSS Statistics software, version 27.
5. Conclusions
The results obtained so far suggest that H. pylori CagY is an important factor involved in the genesis of Th1 and Th17 response in H. pylori-infected patients with gastric MALT lymphoma. We show that H. pylori CagY-specific Th cells derived from the gastric mucosa of H. pylori-infected patients with gastric low-grade MALT lymphoma can provide B cell help in a dose-dependent manner. Taken together, these results suggest that H. pylori CagY is an important factor in generating Th1 and Th17 responses in H. pylori-infected patients with gastric MALT lymphoma and that the T cell-dependent B cell proliferation induced by H. pylori CagY may represent an important link between bacterial infection and gastric lymphoma. CagY, Th1 and Th17 pathways might be useful for the design of novel diagnostics, novel vaccines and therapeutics for gastric MALT lymphomas related to H. pylori infection.
Acknowledgments
We thank Mary Wilkins for the English editing of the manuscript.
Author Contributions
Conceptualization, C.D.B. and M.M.D.; methodology, C.D.B., M.B., A.G., M.F.S. and D.S.; software, S.P. and J.B.; investigation, C.D.B., F.C., M.F.S., C.P., M.B., J.B. and A.G. formal analysis and data curation, C.D.B., M.F.S. and S.P.; writing—original draft preparation, C.D.B. and M.M.D.; writing—review and editing, C.D.B., M.F.S., S.P., M.B., A.G., J.B., F.C., D.S., C.P. and M.M.D.; supervision, M.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Careggi Hospital in Florence (protocol number 14936_bio, approved on 8 October 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting reported results are available from the coordinator of the study, Mario Milco D’Elios, corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marshall B., Warren J. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;323:1311–1315. doi: 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Parsonnet J., Friedman G.D., Vandersteen D.P., Chang Y., Vogelman J.H., Orentreich N., Sibley R.K. Helicobacter pyloriInfection and the Risk of Gastric Carcinoma. N. Engl. J. Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 3.Wotherspoon A., Diss T., Pan L., Isaacson P., Doglioni C., Moschini A., De Boni M. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. doi: 10.1016/0140-6736(93)91409-F. [DOI] [PubMed] [Google Scholar]
- 4.D’Elios M.M., Appelmelk B.J., Amedei A., Bergman M.P., Del Prete G. Gastric autoimmunity: The role of Helicobacter pylori and molecular mimicry. Trends Mol. Med. 2004;10:316–323. doi: 10.1016/j.molmed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Pellicano R., Ribaldone D.G., Fagoonee S., Astegiano M., Saracco G.M., Mégraud F. A 2016 panorama of Helicobacter pylori infection: Key messages for clinicians. Panminerva Med. 2016;58:304–317. [PubMed] [Google Scholar]
- 6.Plummer M., de Martel C., Vignat J., Ferlay J., Bray F., Franceschi S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health. 2016;4:e609–e616. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 7.Filip P.V., Cuciureanu D., Diaconu L.S., Vladareanu A.M., Pop C.S. MALT lymphoma: Epidemiology, clinical diagnosis and treatment. J. Med. Life. 2018;11:187–193. doi: 10.25122/jml-2018-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herlevic V., Morris J.D. Gastric Lymphoma. StatPearls; Treasure Island, FL, USA: 2021. [Google Scholar]
- 9.Greiner A., Knörr C., Qin Y., Sebald W., Schimpl A., Banchereau J., Müller-Hermelink H.K. Low-grade B cell lymphomas of mucosa-associated lymphoid tissue (MALT-type) require CD40-mediated signaling and Th2-type cytokines for in vitro growth and differentiation. Am. J. Pathol. 1997;150:1583–1593. [PMC free article] [PubMed] [Google Scholar]
- 10.D’Elios M.M., Amedei A., Manghetti M., Costa F., Baldari C., Quazi A.S., Telford J.L., Romagnani S., del Prete G. Impaired T-cell regulation of B-cell growth in Helicobacter pylori–related gastric low-grade MALT lymphoma. Gastroenterology. 1999;117:1105–1112. doi: 10.1016/S0016-5085(99)70395-1. [DOI] [PubMed] [Google Scholar]
- 11.Hussell T., Isaacson P., Spencer J., Crabtree J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet. 1993;342:571–574. doi: 10.1016/0140-6736(93)91408-E. [DOI] [PubMed] [Google Scholar]
- 12.Hussell T., Isaacson P.G., Crabtree J.E., Spencer J. Helicobacter pylori–specific tumor-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J. Pathol. 1996;178:122–127. doi: 10.1002/(SICI)1096-9896(199602)178:2<122::AID-PATH486>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 13.Capitani N., Codolo G., Vallese F., Minervini G., Grassi A., Cianchi F., Troilo A., Fischer W., Zanotti G., Baldari C.T., et al. The lipoprotein HP1454 of Helicobacter pylori regulates T-cell response by shaping T-cell receptor signalling. Cell Microbiol. 2019;21:e13006. doi: 10.1111/cmi.13006. [DOI] [PubMed] [Google Scholar]
- 14.Tomb J.-F., White O., Kerlavage A.R., Clayton R.A., Sutton G.G., Fleischmann R.D., Ketchum K.A., Klenk H.P., Gill S., Dougherty B.A., et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 15.Alm R.A., Ling L.-S.L., Moir D.T., King B., Brown E.D., Doig P.C., Smith D.R., Noonan B., Guild B.C., Dejonge B.L., et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 16.Rohde M., Püls J., Buhrdorf R., Fischer W., Haas R. A novel sheathed surface organelle of the Helicobacter pylori cag type IV secretion system. Mol. Microbiol. 2003;49:219–234. doi: 10.1046/j.1365-2958.2003.03549.x. [DOI] [PubMed] [Google Scholar]
- 17.Blaser M.J., Perez G.P., Kleanthous H., Cover T., Peek R.M., Chyou P.H., Stemmermann G.N., Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 18.Kuipers E.J., Pérez-Pérez G.I., Meuwissen S.G.M., Blaser M.J. Helicobacter pylori and Atrophic Gastritis: Importance of the cagA Status. J. Natl. Cancer Inst. 1995;87:1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- 19.Noto J.M., Peek R.M. The Helicobacter pylori cag Pathogenicity Island. Methods Mol. Biol. 2012;921:41–50. doi: 10.1007/978-1-62703-005-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi-Kanemitsu A., Knight C.T., Hatakeyama M. Molecular anatomy and pathogenic actions of Helicobacter pylori CagA that underpin gastric carcinogenesis. Cell. Mol. Immunol. 2019;17:50–63. doi: 10.1038/s41423-019-0339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soluri M.F., Puccio S., Caredda G., Edomi P., D’Elios M.M., Cianchi F., Troilo A., Santoro C., Sblattero D., Peano C. Defining the Helicobacter pylori Disease-Specific Antigenic Repertoire. Front. Microbiol. 2020;11:1551. doi: 10.3389/fmicb.2020.01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tegtmeyer N., Neddermann M., Lind J., Pachathundikandi S.K., Sharafutdinov I., Gutiérrez-Escobar A.J., Brönstrup M., Tegge W., Hong M., Rohde M., et al. Toll-like Receptor 5 Activation by the CagY Repeat Domains of Helicobacter pylori. Cell Rep. 2020;32:108–159. doi: 10.1016/j.celrep.2020.108159. [DOI] [PubMed] [Google Scholar]
- 23.Pachathundikandi S.K., Tegtmeyer N., Backert S. Signal transduction of Helicobacter pylori during interaction with host cell protein receptors of epithelial and immune cells. Gut Microbes. 2013;4:454–474. doi: 10.4161/gmic.27001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrozo R.M., Cooke C.L., Hansen L.M., Lam A.M., Gaddy J.A., Johnson E.M., Cariaga T.A., Suarez G., Peek R.M., Jr., Cover T., et al. Functional Plasticity in the Type IV Secretion System of Helicobacter pylori. PLoS Pathog. 2013;9:e1003189. doi: 10.1371/journal.ppat.1003189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrozo R.M., Hansen L.M., Lam A.M., Skoog E., Martin M.E., Cai L.P., Lin Y., Latoscha A., Suerbaum S., Canfield D.R., et al. CagY Is an Immune-Sensitive Regulator of the Helicobacter pylori Type IV Secretion System. Gastroenterology. 2016;151:1164–1175.e3. doi: 10.1053/j.gastro.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isaacson P.G., Wright D.H. Malignant lymphoma of mucosaassociated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52:1410–1416. doi: 10.1002/1097-0142(19831015)52:8<1410::AID-CNCR2820520813>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Parsonnet J., Hansen S., Rodriguez L., Gelb A.B., Warnke R.A., Jellum E., Orentreich N., Vogelman J.H., Friedman G.D. Helicobacter pylori Infection and Gastric Lymphoma. N. Engl. J. Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 28.Isaacson P.G. Gastrointestinal lymphoma. Hum. Pathol. 1994;25:1020–1029. doi: 10.1016/0046-8177(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 29.Feng G., Chen Y., Li K. Helicobacter pylori promote inflammation and host defense through the cagA-dependent activation of mTORC1. J. Cell. Physiol. 2020;235:10094–10108. doi: 10.1002/jcp.29826. [DOI] [PubMed] [Google Scholar]
- 30.Munari F., Lonardi S., Cassatella M.A., Doglioni C., Cangi M.G., Amedei A., Facchetti F., Eishi Y., Rugge M., Fassan M., et al. Tumor-associated macrophages as major source of APRIL in gastric MALT lymphoma. Blood. 2011;117:6612–6616. doi: 10.1182/blood-2010-06-293266. [DOI] [PubMed] [Google Scholar]
- 31.Munari F., Fassan M., Capitani N., Codolo G., Caballer M.A.V., Pizzi M., Rugge M., Della Bella C., Troilo A., D’Elios S., et al. Cytokine BAFF Released by Helicobacter pylori–Infected Macrophages Triggers the Th17 Response in Human Chronic Gastritis. J. Immunol. 2014;193:5584–5594. doi: 10.4049/jimmunol.1302865. [DOI] [PubMed] [Google Scholar]
- 32.Blosse A., Peru S., Levy M., Marteyn B., Floch P., Sifré E., Giese A., Prochazkova-Carlotti M., Martin L.A., Dubus P., et al. APRIL-producing eosinophils are involved in gastric MALT lymphomagenesis induced by Helicobacter sp infection. Sci. Rep. 2020;10:14858. doi: 10.1038/s41598-020-71792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chonwerawong M., Ferrand J., Chaudhry H.M., Higgins C., Tran L.S., Lim S.S., Walker M.M., Bhathal P.S., Dev A., Moore G.T., et al. Innate Immune Molecule NLRC5 Protects Mice from Helicobacter-induced Formation of Gastric Lymphoid Tissue. Gastroenterology. 2020;159:169–182. doi: 10.1053/j.gastro.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Codolo G., Bossi F., Durigutto P., Della Bella C., Fischetti F., Amedei A., Tedesco F., D’Elios S., Cimmino M., Micheletti A., et al. Orchestration of Inflammation and Adaptive Immunity inBorrelia burgdorferi-Induced Arthritis by Neutrophil-Activating Protein A. Arthritis Rheum. 2013;65:1232–1242. doi: 10.1002/art.37875. [DOI] [PubMed] [Google Scholar]
- 35.De Jong D., Van Der Hulst R.W., Pals G., Van Dijk W.C., Van Der Ende A., Tytgat G.N., Taal B.G., Boot H. Gastric Non-Hodgkin Lymphomas of Mucosa-Associated Lymphoid Tissue Are not Associated with More Aggressive Helicobacter pyloriStrains as Identified by CagA. Am. J. Clin. Pathol. 1996;106:670–675. doi: 10.1093/ajcp/106.5.670. [DOI] [PubMed] [Google Scholar]
- 36.Eck M., Schmausser B., Haas R., Greiner A., Czub S., Müller-Hermelink H.K. MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology. 1997;112:1482–1486. doi: 10.1016/S0016-5085(97)70028-3. [DOI] [PubMed] [Google Scholar]
- 37.Enno A., O’Rourke J.L., Howlett C.R., Jack A., Dixon M.F., Lee A. MALToma-like lesions in the murine gastric mucosa after longterminfection with Helicobacter felis. A mouse model of Helicobacter pylori–induced gastric lymphoma. Am. J. Pathol. 1995;47:217–222. [PMC free article] [PubMed] [Google Scholar]
- 38.Ferretti E., DI Carlo E., Ognio E., Guarnotta C., Bertoni F., Corcione A., Prigione I., Orcioni G.F., Ribatti D., Ravetti J.L., et al. Interleukin-17A promotes the growth of human germinal center derived non-Hodgkin B cell lymphoma. OncoImmunology. 2015;4:e1030560. doi: 10.1080/2162402X.2015.1030560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Elios M.M., Manghetti M., De Carli M., Costa F., Baldari C., Burroni D., Telford J.L., Romagnani S., Del Prete G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J. Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- 40.D’Elios M.M., Bergman M.P., Azzurri A., Amedei A., Benagiano M., De Pont J.J., Cianchi F., Vandenbroucke-Grauls C.M., Romagnani S., Appelmelk B.J., et al. H+, K+-ATPase (proton pump) is the target autoantigen of Th1-type cytotoxic T cells in autoimmune gastritis. Gastroenterology. 2001;120:377–386. doi: 10.1053/gast.2001.21187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting reported results are available from the coordinator of the study, Mario Milco D’Elios, corresponding author.