Abstract
Flow cytometry was used in the identification of human microsporidia belonging to the genus Encephalitozoon. Microsporidian spores of Encephalitozoon hellem, E. cuniculi, and E. intestinalis were propagated in axenic cultures of monkey kidney E6 cells, purified with Percoll, and exposed to homologous and heterologous rabbit antiserum and monoclonal antibody prepared against E. hellem spores. After reaction to goat anti-rabbit immunoglobulin G (IgG) or goat anti-mouse IgG conjugated to fluorescein isothiocyanate, fluorescence histograms from gated data on light-scatter profiles showed that rabbit anti-E. hellem serum was reactive to E. hellem spores but also had cross-reactivity to spores of E. cuniculi and E. intestinalis. On the other hand, fluorescence histograms showed that rabbit anti-E. cuniculi and rabbit anti-E. intestinalis sera were reactive with homologous spores only. Monoclonal antibody prepared against E. hellem reacted only with spores of E. hellem. Neither the polyclonal antibodies nor the monoclonal antibodies reacted with Cryptosporidium parvum oocysts. Fluorescence histograms of spores treated with 10% formalin also showed reactivity, but the number of events in the most intense peaks of fluorescence was fewer (7 to 42%, depending on species) than the number of events in the most intense peaks of fluorescence for nontreated spores. By flow cytometry, formalin-treated and nontreated spores of Encephalitozoon were identified to the species level by using gated data on light-scatter profiles and analyzing the fluorescence histograms from the indirect immunofluorescence of the spores. Once a procedure is established for the isolation of Encephalitozoon spores from clinical specimens, identification of spores by flow cytometry may be useful not only for diagnosis but also for epidemiologic studies.
The phylum Microsporidia (33) consists of a group of ancient, spore-forming, obligately parasitic, eukaryotic protozoans that lack mitochondria (8). A unique feature of microsporidia is the presence of a coiled polar tubule in the spore that, on extrusion, injects infective sporoplasm into a suitable host cell. Although microsporidia are known to infect principally insects and rodents, they are also known to parasitize members of every major phylum of the animal kingdom (8, 27). Ten species of microsporidia (Enterocytozoon bieneusi, Encephalitozoon cuniculi, Encephalitozoon hellem, Encephalitozoon intestinalis [synonym, Septata intestinalis], Nosema ocularum, Vittaforma corneae, Pleistophora sp., Trachipleistophora hominis, Trachipleistophora anthropohthera, and Brachiola vesicularum) have been identified as agents of human disease (6, 7, 19, 20, 27, 30, 31, 34).
Even though microsporidia have been recognized in human immunodeficiency virus-seronegative persons (5, 23–25, 39) as well as in recipients of liver (25) and heart-lung transplants (23) and have caused traveler’s diarrhea in immunocompetent persons (5, 24, 32, 39), microsporidia are now recognized as important emerging opportunistic agents in persons with AIDS (27). The species E. bieneusi is the most prevalent microsporidian that infects persons with AIDS, in whom it causes gastrointestinal disease (27). Encephalitozoon spp. have caused ocular as well as disseminated infections and have been identified with increasing frequency during the past decade, principally in patients with AIDS. E. cuniculi and E. hellem have caused ocular and disseminated infections without involving the gastrointestinal tract (13, 15, 27), while E. intestinalis has caused disseminated diseases, including diseases affecting the gastrointestinal tract (6, 14, 27, 36).
Identification of the genus and species of microsporidia is important for institution of the appropriate treatment regimens (13, 15, 27). However, identification to the species level is difficult and requires specialized and time-consuming techniques such as electron microscopy and PCR (9, 13, 14, 27). We have reported previously on the development of a species-specific monoclonal antibody (MAb) against E. hellem (12, 37) and highly specific polyclonal antibodies against E. cuniculi (11, 13) and E. intestinalis (4, 14, 36). These MAbs detect these agents in human and animal specimens, including stools (4, 26, 28, 29, 36). In this report we describe the use of flow cytometry, in conjunction with MAbs and polyclonal antibodies, as a tool that can be used to discriminate between the spores of the three species of Encephalitozoon on the basis of their light-scatter and indirect immunofluorescence properties.
MATERIALS AND METHODS
Parasites.
E. hellem CDC:V257, E. cuniculi CDC:V282, and E. intestinalis CDC:V297 were grown at 37°C on monolayers of monkey kidney cells (E6) as described previously (13, 36–38). The growth medium consisted of Eagle’s minimum essential medium containing 5% heat-inactivated fetal bovine serum, 50 μg of gentamicin per ml, and 1 μg of amphotericin B (Fungizone). All three parasites were isolated from the urine of three different male AIDS patients originating from different geographic locales (11–14, 36–38).
Parasite harvest and purification.
Spores that were periodically extruded into the culture medium were collected from several flasks and pooled. Most of the debris and unattached E6 cells were sedimented by low-speed centrifugation at 120 × g for 10 min at 4°C and discarded. The spores in the supernatant were sedimented by relatively high-speed centrifugation at 1,200 × g for 20 min at 4°C. After washing and suspension of the spores in cold phosphate-buffered saline (PBS), the spore suspension was layered over 45% Percoll containing 0.85% NaCl and centrifuged at 1,900 × g for 30 min at room temperature. Additional debris and dead E6 cells were trapped at the PBS-Percoll interface, while spores were sedimented through the Percoll. The spores were washed in cold PBS, quantitated with a hemacytometer, and stored at 4°C until use.
Cryptosporidium parvum oocysts (Iowa strain) were purified as described previously (2).
Spore measurement.
Approximately 50 spores of each of the three isolates (CDC:V257, CDC:V282, and CDC:V297) were measured with a stage micrometer. To obtain a uniform suspension of immovable spores, a drop (25 to 30 μl) of concentrated spore suspension was placed on a no. 1 coverslip and inverted over a drop of warm 1% agar solution on a microscope slide. The edges of the coverslip were sealed with paraffin. The spores were measured under an oil immersion lens of a microscope (Olympus [BH-2]; Olympus America, Inc., Melville, N.Y.) equipped with differential interference contrast optics.
Antibodies.
Polyclonal rabbit anti-E. hellem, anti-E. cuniculi, and anti-E. intestinalis sera and MAb ED4H10B11/B12 (B11/B12) against E. hellem were prepared as described previously (11, 36–38). Rabbit anti-E. hellem and rabbit anti-E. cuniculi sera were used at 1:750 dilutions against 2 × 106 spores in 100 μl of cold PBS containing 0.2% bovine serum albumin (BSA) and 0.02% sodium azide (PBS-BSA) for 30 min at 4°C. Rabbit anti-E. intestinalis serum and MAb B11/B12 (ascites fluid) were used similarly but were used at 1:200 dilutions. An irrelevant MAb and normal rabbit serum were used similarly. After washing with cold PBS-BSA, the spores were suspended in 100 μl of cold PBS-BSA containing a 1:750 dilution of either fluorescein isothiocyanate (FITC) conjugated to goat anti-rabbit immunoglobulin G (Cappel Laboratories, West Chester, Pa.) or FITC conjugated to goat anti-mouse immunoglobulin G (Cappel Laboratories) for 30 min at 4°C in the dark. The spores were washed in cold PBS, resuspended, and stored in about 700 μl of cold PBS in the dark until they were analyzed by flow cytometry. C. parvum oocysts were treated similarly.
Formalin treatment.
Spores were purified as described above and were suspended in 10% formalin for 25 days at room temperature. Afterward, the spores were washed and suspended in cold PBS and stored at 4°C until use. For formalin-treated spores, optimal fluorescence occurred at a 1:200 dilution for all rabbit sera and MAbs.
Flow cytometry.
Flow cytometric data were acquired and analyzed on a FACScan instrument (Becton Dickinson, San Jose, Calif.) equipped with a 488-nm argon laser and Lysis II software. The forward scatter (FSC) detector, side scatter (SSC) detector, and detector appropriate for detection of the emission of FITC were set to logarithmic scale. The voltages to the FITC detector were adjusted so that the autofluorescences of all species of spores exposed to normal rabbit serum or an irrelevant MAb were the same. Data on the FSC and SSC profiles from gated events (5,000 or 10,000) were collected and analyzed. The number of events in the peak fluorescence histograms was obtained with Lysis II analysis software.
RESULTS
Spore measurement.
The E. hellem isolate was slightly larger than the isolates of E. cuniculi and E. intestinalis. The average size of E. hellem CDC:V257 was 2.5 μm (range, 1.8 to 2.7 μm), while the average size of E. cuniculi CDC:V282 and E. intestinalis CDC:V297 was 2.2 μm (range, 1.4 to 2.5 μm for both organisms).
FSC and SSC profiles of Encephalitozoon spores and Cryptosporidium oocysts.
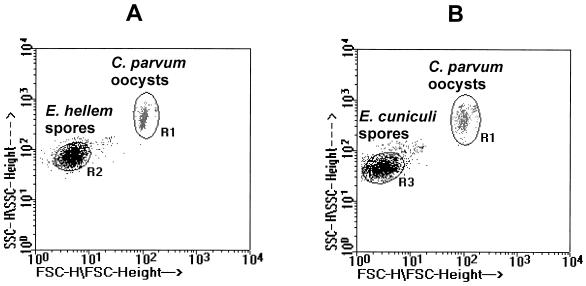
Figures 1A and B are double-axis dot plots showing the FSC and SSC profiles of E. hellem and E. cuniculi spores, respectively, along with those for C. parvum oocysts. The scatter profiles of E. intestinalis are not shown but were similar to those for E. cuniculi in Fig. 1B. The FSC profiles clearly separated the larger C. parvum oocysts (Fig. 1A or B, region 1 [R1]) from the smaller E. hellem spores (Fig. 1A, R2) and the E. cuniculi spores (Fig. 1B, R3). Although some overlap in the FSC profiles was observed between the E. hellem and E. cuniculi spores, R2 in Fig. 1A showed a slight increase in FSC intensity compared with that in R3 in Fig. 1B, indicating slightly larger E. hellem spores than E. cuniculi or E. intestinalis spores. All Encephilitozoon spores showed similar SSC profiles.
FIG. 1.
Double-axis dot plot showing FSC and SSC profiles. (A) E. hellem spores (R2) and C. parvum oocysts (R1); (B) E. cuniculi spores (R3) and C. parvum oocysts (R1).
Reactivity of rabbit anti-E. hellem serum.
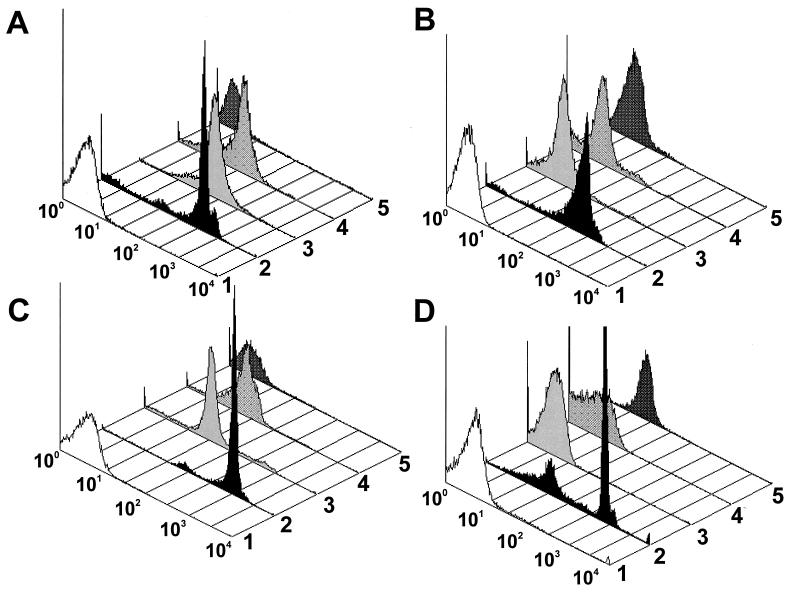
Figure 2A shows dot plot histograms of spores and oocysts exposed to rabbit anti-E. hellem serum. E. hellem spores (histogram 2) exposed to homologous serum showed a large peak and a small peak of fluorescence that were 2 and 1 log more intense, respectively, than those for the E. hellem spores exposed to normal rabbit serum (histogram 1). However, a few organisms exposed to homologous serum (histogram 2) showed the same fluorescence as those exposed to normal rabbit serum (histogram 1). Even though the fluorescence was slightly less than that for the spores of E. hellem, the spores of E. cuniculi (histogram 3) and E. intestinalis (histogram 4) showed considerable cross-reactivity with the rabbit anti-E. hellem serum. C. parvum oocysts (histogram 5) showed no cross-reactivity.
FIG. 2.
Dot plot histograms of Encephalitozoon spores and C. parvum oocysts exposed to homologous and heterologous rabbit antisera and MAbs. (A) Rabbit anti-E. hellem serum to E. hellem spores (histogram 2), E. cuniculi spores (histogram 3), E. intestinalis spores (histogram 4), and C. parvum oocysts (histogram 5). Histogram 1 is normal rabbit serum to E. hellem spores. (B) Rabbit anti-E. intestinalis serum to E. intestinalis spores (histogram 2), E. cuniculi spores (histogram 3), E. hellem spores (histogram 4), and C. parvum oocysts (histogram 5). Histogram 1 is normal rabbit serum to E. intestinalis spores. (C) Rabbit anti-E. cuniculi serum to E. cuniculi spores (histogram 2), E. hellem spores (histogram 3), E. intestinalis spores (histogram 4), and C. parvum oocysts (histogram 5). Histogram 1 is normal rabbit serum to E. cuniculi spores. (D) MAb B11/B12 to E. hellem spores (histogram 2), E. cuniculi spores (histogram 3), E. intestinalis spores (histogram 4), and C. parvum oocysts (histogram 5). Histogram 1 is an irrelevant MAb to E. hellem spores.
Reactivity of rabbit anti-E. intestinalis serum.
Dot plot histograms of spores and oocysts exposed to rabbit anti-E. intestinalis serum are shown in Fig. 2B. E. intestinalis spores exposed to homologous serum (histogram 2) showed a major peak of fluorescence that was about 1.5 logs more intense than that for E. intestinalis spores exposed to normal rabbit serum (histogram 1). A small population exposed to homologous serum (histogram 2) showed the same fluorescence as spores exposed to normal rabbit serum (histogram 1). No cross-reactivity of the rabbit anti-E. intestinalis serum was observed with spores of E. cuniculi (histogram 3), E. hellem (histogram 4), or C. parvum oocysts (histogram 5).
Reactivity of rabbit anti-E. cuniculi serum.
Histograms of spores and oocysts exposed to rabbit anti-E. cuniculi serum are shown in Fig. 2C. E. cuniculi spores exposed to homologous serum (histogram 2) showed a large peak and a small peak of fluorescence that were 2.5 and 1.5 logs more intense, respectively, than those for the E. cuniculi spores exposed to normal rabbit serum (histogram 1). A very small population exposed to the homologous serum (histogram 2) showed the same fluo rescence as spores exposed to normal rabbit serum (histogram 1). Although spores of E. hellem (histogram 3) and E. intestinalis (histogram 4) showed some cross-reactivity, their major peaks of fluorescence were at least 1.5 logs less than those for the homologous serum (histogram 2). C. parvum oocysts (histogram 5) showed no cross-reactivity.
Reactivity of MAb B11/B12.
Figure 2D shows histograms of spores and oocysts exposed to MAb B11/B12 prepared against E. hellem spores. Although this MAb (histogram 2) poorly recognized some spores with reactivity no greater than the autofluorescent range of the irrelevant MAb (histogram 1), most of the spores showed fluorescence that was 1.5 to 2 logs more than those for E. cuniculi (histogram 3), E. intestinalis (histogram 4), and C. parvum oocysts (histogram 5).
Antibody reactivity to formalin-treated spores.
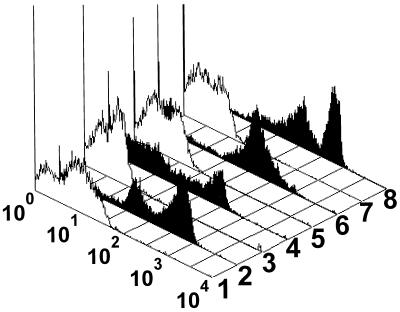
After treatment with 10% formalin at room temperature for 25 days, the spores were reacted with homologous rabbit antiserum or MAb B11/B12 at a 1:200 dilution and analyzed by flow cytometry (Fig. 3). For spores exposed to normal rabbit serum or an irrelevant MAb, the range for autofluorescence was broader (Fig. 3, white histograms) than the range for nontreated spores (Fig. 2A to D, white histograms). Although formalin-treated spores did react with homologous antiserum (Fig. 3, black histograms), there were fewer events in the most intense peak of fluorescence (7% for E. hellem [Fig. 3, histogram 2], 42% for E. intestinalis [Fig. 3, histogram 6], 37% for E. cuniculi [Fig. 3, histogram 8], and 34% for MAb B11/B12 [Fig. 3, histogram 4]) than in the most intense peak of fluorescence of nontreated spores (Fig. 2A to D, histograms for homologous sera). Formalin-treated spores of E. hellem and E. intestinalis showed slight increases in the most intense peaks of fluorescence compared with those for nontreated spores. On the other hand, formalin-treated spores of E. hellem exposed to MAb B11/B12 showed slight decreases in the most intense peak of fluorescence compared with those for nontreated spores.
FIG. 3.
Fluorescence histograms of 10% formalin-treated spores exposed to rabbit antiserum and MAb. Histograms 1 to 4 contain spores of E. hellem and normal rabbit serum (histogram 1), rabbit anti-E. hellem serum (histogram 2), an irrelevant MAb (histogram 3), and MAb B11/B12 (histogram 4). Histograms 5 and 6 contain spores of E. intestinalis and normal rabbit serum (histogram 5) and rabbit anti-E. intestinalis serum (histogram 6). Histograms 7 and 8 contain spores of E. cuniculi and normal rabbit serum (histogram 7) and rabbit anti-E. cuniculi serum (histogram 8).
DISCUSSION
Flow cytometry has been used in studies of various parasitic protozoa such as Plasmodium (16, 21), Eimeria (18), and Cryptosporidium (3, 35). In studies of free-living amoebae, flow cytometry has distinguished Naegleria fowleri from Acanthamoeba species (17). Furthermore, flow cytometry has been used to quantitate and determine the viability of E. cuniculi spores derived from culture (22), to isolate E. bieneusi spores from stool specimens (10), and to compare the nucleic acid contents of microsporidian spores isolated from fish (1).
In this study, we have used flow cytometry for the possible differentiation of the microsporidian species belonging to the genus Encephalitozoon. Although the light-scatter profiles of Encephalitozoon spores were similar, there was a slight increase in the intensity of the FSC profile of E. hellem, indicating that it has spores slightly larger than the spores of E. cuniculi and E. intestinalis. This was expected since this particular isolate of E. hellem (isolate CDC:V257) was about 2.5 μm, whereas the other two isolates, E. cuniculi CDC:V282 and E. intestinalis CDC:V297, measured about 2.2 μm. Also, as expected, the light-scatter profiles of all Encephalitozoon spores were clearly distinguishable from those of the C. parvum oocysts, which are larger (4 to 6 μm). Unfortunately, the light-scatter profiles of Encephalitozoon spores are similar to those of many bacteria, and thus, light-scatter profiles alone would not be sufficient for the identification or sorting of homogeneous populations of spores. Even though a procedure for the isolation of spores from clinical specimens could be established, interfering bacteria and debris will likely be present. Thus, light-scatter profiles alone would be of no value in clinical settings.
In flow cytometry, a parameter in addition to light-scatter profiles that can be used to aid in the identification or sorting of Encephalitozoon spores is immunofluorescence. By use of this parameter, all species could be identified. Overall, very little cross-reactivity was shown by polyclonal antibodies and MAbs, which showed no cross-reactivity to C. parvum oocysts. The exception was rabbit anti-E. hellem serum, which not only reacted well with homologous spores but also reacted slightly less but strongly with spores of E. cuniculi and E. intestinalis (Fig. 2A). This cross-reactivity could be useful in clinical settings, where the identification of Encephalitozoon spores at the genus level is important since infections with the Encephalitozoon spp. can be successfully treated with albendazole systemically (in cases of disseminated microsporidiosis) and with fumagillin topically (ocular microsporidiosis) (13, 15, 27). The anti-E. cuniculi and the anti-E. intestinalis sera reacted well with the E. cuniculi and E. intestinalis spores, respectively, and unlike the rabbit anti-E. hellem serum, these sera showed very little cross-reactivity with heterologous spores. For identification of E. hellem spores, epitopes reactive to MAb B11/B12 were present only on E. hellem spores.
In our previous studies, using the same antibodies in an indirect immunofluorescence (IIF) assay, we showed that the anti-E. hellem serum cross-reacted with E. cuniculi spores and that this cross-reactivity can easily be absorbed out, whereas MAb B11/B12 showed very little cross-reactivity (26, 37). Similarly, we showed that the anti-E. cuniculi sera as well as the anti-E. intestinalis sera reacted well with culture-derived spores of E. cuniculi and E. intestinalis, respectively, and showed very little cross-reactivity. By the IIF assay, these antibodies have successfully been used to identify microsporidial agents in clinical specimens, including stool specimens (4, 13–15, 26, 28, 29, 36–38).
The reason for multiple peaks in the fluorescence histograms is not known, but we believe that they represent mature and immature spores, with the latter showing lower fluorescence intensities than the former. By the IIF assay, we have observed spore-like structures, possibly sporogonic structures, with lower fluorescence intensities. Evidence from electron microscopy studies may answer this question.
Spores treated with 10% formalin remained reactive to polyclonal antibodies and MAbs. Although histograms with the most intense peaks of fluorescence showed fewer reactive spores than histograms with nontreated spores, a major portion of formalin-treated spores remained reactive to the antibodies, including the epitope that reacted to MAb B11/B12. These results are in parallel with those of the IIF assay for the identification of E. hellem, E. cuniculi, and E. intestinalis in formalin-fixed tissue sections and fecal specimens (4, 11, 13, 15, 26, 28, 29, 36–38). By the IIF assay, we have observed that spores treated with 1% formalin show greater fluorescence than spores treated with 10% formalin (data not shown). Furthermore, we have observed by the IIF assay that over time the fluorescence intensities of spores treated with 10% formalin diminish, suggesting that fluorescence intensity is dependent not only on the formalin concentration but also on the time of formalin treatment (data not shown). Thus, specimens treated with 1 or 10% formalin for at least 25 days could be used for the identification of Encephalitozoon by flow cytometry once a procedure for the isolation of the spores is established.
Microscopic analysis of clinical specimens containing microsporidia, besides being very subjective, is time-consuming, and thus, only a small number of specimens can be analyzed at any given time. Pending the establishment of a procedure for the isolation of Encephalitozoon spores from clinical specimens, flow cytometry in conjunction with immunofluorescence offers not only an objective means of analysis that can be performed quickly but also provides for a rapid assessment of clinical specimens for the presence of these opportunistic microsporidial organisms.
ACKNOWLEDGMENTS
We gratefully thank Patrick J. Lammie for his expertise in the revision of the manuscript. We also thank Michael J. Arrowood and Gordon J. Leitch for helpful suggestions and comments.
REFERENCES
- 1.Amigo J M, Gracia M, Comas J, Salvado H, Vivares C P. Comparative study of microsporidian spores by flow cytometric analysis. J Eukaryot Microbiol. 1994;41:210–214. doi: 10.1111/j.1550-7408.1994.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 2.Arrowood M J, Sterling C R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J Parasitol. 1987;73:314–319. [PubMed] [Google Scholar]
- 3.Arrowood M J, Hurd M R, Mead J R. A new method for evaluating experimental cryptosporidial parasite loads using immunofluorescent flow cytometry. J Parasitol. 1995;81:404–409. [PubMed] [Google Scholar]
- 4.Bornay-Llinnares F, da Silva A J, Moura H, Schwartz D A, Visvesvara G S, Pieniazek N J, Cruz-Lopez A, Hernandez-Jaúregui P, Guerrero J, Enriquez J. Immunologic, microscopic, and molecular evidence of Encephalitozoon (Septata) intestinalis infection in mammals other than humans. J Infect Dis. 1998;178:820–826. doi: 10.1086/515356. [DOI] [PubMed] [Google Scholar]
- 5.Bryan R T, Weber R, Schwartz D A. Microsporidiosis in persons without HIV. Clin Infect Dis. 1997;24:534–535. doi: 10.1093/clinids/24.3.534. [DOI] [PubMed] [Google Scholar]
- 6.Cali A, Kotler D P, Orenstein J M. Septata intestinalis n. g., n. sp., an intestinal microsporidian associated with chronic diarrhea and dissemination in AIDS patients. J Eukaryot Microbiol. 1993;40:101–112. doi: 10.1111/j.1550-7408.1993.tb04889.x. [DOI] [PubMed] [Google Scholar]
- 7.Cali A, Takvorian P M, Lewin S, Rendel M, Sian C S, Wittner M, Tanowitz H B, Keohane E, Weiss L M. Brachiola vesicularum, n. g., n. sp., a new microsporidium associated with AIDS and myositis. J Eukaryot Microbiol. 1998;45:240–251. doi: 10.1111/j.1550-7408.1998.tb04532.x. [DOI] [PubMed] [Google Scholar]
- 8.Canning E U, Lom J. The microsporidia of vertebrates. New York, N.Y: Academic Press, Inc.; 1986. [Google Scholar]
- 9.Canning E U, Hollister W S. In vitro and in vivo investigations of human microsporidia. J Protozool. 1991;38:631–635. [PubMed] [Google Scholar]
- 10.Challier S, Brown S, Ombrouck C, Desportes-Livage I, De Nay D, Gentilini M. Flow cytometry as a possible method of isolation of spores of the microsporidian Enterocytozoon bieneusi. J Eukaryot Microbiol. 1994;41:27S. [PubMed] [Google Scholar]
- 11.Croppo G P, Visvesvara G S, Leitch G J, Wallace S, De Groote M A. Western blot and immunofluorescence analysis of a human isolate of Encephalitozoon cuniculi established in culture from the urine of a patient with AIDS. J Parasitol. 1997;83:66–69. [PubMed] [Google Scholar]
- 12.Croppo G P, Visvesvara G S, Leitch G J, Wallace S, Schwartz D A. Identification of the microsporidian Encephalitozoon hellem using immunoglobulin G monoclonal antibodies. Arch Pathol Lab Med. 1998;122:182–186. [PubMed] [Google Scholar]
- 13.De Groote M A, Visvesvara G S, Wilson M, Pieniazek N J, Slemenda S, Da Silva A J, Leitch G J, Bryan R T, Reves R. Polymerase chain reaction and culture confirmation of disseminated Encephalitozoon cuniculi in a patient with AIDS: successful therapy with albendazole. J Infect Dis. 1995;171:1375–1378. doi: 10.1093/infdis/171.5.1375. [DOI] [PubMed] [Google Scholar]
- 14.Del Aguila C, Croppo G P, Moura H, da Silva A J, Leitch G J, Moss D M, Wallace S, Slemenda S B, Pieniazek N J, Visvesvara G S. Ultrastructure, immunofluorescence, Western blot, and PCR analysis of eight isolates of Encephalitozoon (Septata) intestinalis established in culture from sputum and urine samples and duodenal aspirates of five patients with AIDS. J Clin Microbiol. 1998;36:1201–1208. doi: 10.1128/jcm.36.5.1201-1208.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diesenhouse M C, Wilson L A, Corrent G C, Visvesvara G S, Grossniklaus H E, Bryan R T. Treatment of microsporidial keratoconjunctivitis with topical fumagillin. Am J Ophthalmol. 1993;115:293–298. doi: 10.1016/s0002-9394(14)73578-0. [DOI] [PubMed] [Google Scholar]
- 16.Field S P, Hempelmann E, Mendelow B V, Fleming A F. Glycophorin variants and Plasmodium falciparum: protective effect of the Dantu phenotype in vitro. Hum Genet. 1994;93:148–150. doi: 10.1007/BF00210600. [DOI] [PubMed] [Google Scholar]
- 17.Flores B M, Garcia C A, Stamm W E, Torian B E. Differentiation of Naegleria fowleri from Acanthamoeba species by using monoclonal antibodies and flow cytometry. J Clin Microbiol. 1990;28:1999–2005. doi: 10.1128/jcm.28.9.1999-2005.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller A L, Golden J, McDougald L R. Flow cytometric analysis of the response of Eimeria tenella (Coccidia) sporozoites to coccidiocidal effects of ionophores. J Parasitol. 1995;81:985–988. [PubMed] [Google Scholar]
- 19.Hartskeerl R A, van Gool T, Schuitema A R J, Didier E S, Tepstra W J. Genetic and immunological characterization of the microsporidian Septata intestinalis Cali, Kotler and Orenstein, 1993: reclassification to Encephalitozoon intestinalis. Parasitology. 1995;110:277–285. doi: 10.1017/s0031182000080860. [DOI] [PubMed] [Google Scholar]
- 20.Hollister W S, Canning E U, Weidner E, Field A S, Kench J, Marriott D J. Development and ultrastructure of Trachipleistophora hominis n.g., n. sp. after isolation from an AIDS patient and inoculation into athymic mice. Parasitology. 1996;112:143–154. doi: 10.1017/s0031182000065185. [DOI] [PubMed] [Google Scholar]
- 21.Pattanapanyasat K, Thaithong S, Kyle D E. Flow cytometric assessment of hydroxypyridinone iron chelators on in vitro growth of drug-resistant malaria. Cytometry. 1997;27:84–91. doi: 10.1002/(sici)1097-0320(19970101)27:1<84::aid-cyto11>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Peterson N, Jiuan L, Shadduck J. Encephalitozoon cuniculi: quantitation of parasites and evaluation of viability. J Protozool. 1988;35:430–434. doi: 10.1111/j.1550-7408.1988.tb04123.x. [DOI] [PubMed] [Google Scholar]
- 23.Rabodonirina M, Bertocchi M, Desportes-Livage I, Cotte L, Levrey H, Piens M A, Monneret G, Clelard M, Mornex J F, Mojon M. Enterocytozoon bieneusi as a cause of chronic diarrhea in a heart-lung transplant recipient who was seronegative for human immunodeficiency virus. Clin Infect Dis. 1996;23:114–117. doi: 10.1093/clinids/23.1.114. [DOI] [PubMed] [Google Scholar]
- 24.Sandfort J, Hannemann A, Gelderblom H, Stark D, Owen R L, Ruf B. Enterocytozoon bieneusi infection in an immunocompetent HIV-negative patient with acute diarrhea. Clin Infect Dis. 1994;19:514–516. doi: 10.1093/clinids/19.3.514. [DOI] [PubMed] [Google Scholar]
- 25.Sax P E, Rich J D, Pieciak W S, Trnka Y M. Intestinal microsporidiosis occurring in a liver transplant recipient. Transplantation. 1995;60:617–618. doi: 10.1097/00007890-199509270-00018. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz D A, Visvesvara G S, Diesenhouse M C, Weber R, Font R L, Wilson L A, Corrent G, Rosberger D F, Keenen P J, Grossniklaus H, Hewan-Lowe K, Bryan R T. Ocular pathology of microsporidiosis: role of immunofluorescent antibody for diagnosis of Encephalitozoon hellem in biopsies, smears, and intact globes from seven AIDS patients. Am J Ophthalmol. 1993;115:285–292. doi: 10.1016/s0002-9394(14)73577-9. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz D A, Bryan R T. Microsporidiosis. In: Horsburgh C R Jr, Nelson A, editors. Pathology of emerging infections. Washington, D.C: American Society for Microbiology; 1997. pp. 61–94. [Google Scholar]
- 28.Schwartz D A, Visvesvara G S, Leitch G J, Tashjian L, Pollack M, Holden J, Bryan R T. Pathology of symptomatic microsporidial (Encephalitozoon hellem) bronchiolitis in the acquired immunodeficiency syndrome: a new respiratory pathogen diagnosed from lung biopsy, bronchoalveolar lavage, sputum, and tissue culture. Hum Pathol. 1993;24:937–943. doi: 10.1016/0046-8177(93)90106-q. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz D A, Sobottka I, Leitch G J, Cali A, Visvesvara G S. Pathology of microsporidiosis. Emerging parasitic infections in patients with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1996;120:173–188. [PubMed] [Google Scholar]
- 30.Shadduck J A, Meccoli R A, Davis R, Font R L. First isolation of a microsporidian from a human patient. J Infect Dis. 1990;162:773–776. doi: 10.1093/infdis/162.3.773. [DOI] [PubMed] [Google Scholar]
- 31.Silveira H, Canning E U. Vittaforma corneae n. comb. for the human microsporidium Nosema corneum Shadduck, Meccoli, Davis & Font, 1990, based on its ultrastructure in the liver of experimentally infected athymic mice. J Eukaryot Microbiol. 1995;42:158–165. doi: 10.1111/j.1550-7408.1995.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 32.Sobottka I, Albrecht H, Schottelius J, Bentfeld M, Laufs R, Schwartz D A. Self-limited traveller’s diarrhea due to a dual infection with Enterocytozoon bieneusi and Cryptosporidium parvum in an immunocompetent HIV-negative child. Eur J Clin Microbiol Infect Dis. 1995;14:919–920. doi: 10.1007/BF01691502. [DOI] [PubMed] [Google Scholar]
- 33.Sprague V, Becnel J J. Note on the name-author-date combination for the taxon Microsporidies Balbiani, 1882, when ranked as a phylum. J Invertebr Pathol. 1998;71:91–94. doi: 10.1006/jipa.1997.4702. [DOI] [PubMed] [Google Scholar]
- 34.Vavra J, Yachnis A T, Shadduck J A, Orenstein J M. Microsporidia of the genus Trachipleistophora—causative agents or human microsporidiosis: description of Trachileistophora anthropophthera n. sp. (Protozoa: Microsporidia) J Eukaryot Microbiol. 1998;45:273–283. doi: 10.1111/j.1550-7408.1998.tb04536.x. [DOI] [PubMed] [Google Scholar]
- 35.Vesey G, Slade J S, Byrne M, Shepherd K, Dennis P J, Fricker C R. Routine monitoring of Cryptosporidium oocysts in water using flow cytometry. J Appl Bacteriol. 1993;75:87–90. doi: 10.1111/j.1365-2672.1993.tb03413.x. [DOI] [PubMed] [Google Scholar]
- 36.Visvesvara G S, da Silva A J, Croppo G P, Pieniazek N J, Leitch G J, Ferguson D, de Moura H, Wallace S, Slemenda S B, Tyrrell I, Moore D F, Meador J. In vitro culture and serologic and molecular characterization of Septata intestinalis isolated from the urine of a patient with AIDS. J Clin Microbiol. 1995;33:930–936. doi: 10.1128/jcm.33.4.930-936.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visvesvara G S, Leitch G J, Da Silva A J, Croppo G P, Moura H, Wallace S, Slemenda S B, Schwartz D A, Moss D, Bryan R T, Pieniazek N J. Polyclonal and monoclonal antibody and PCR-amplified small-subunit rRNA identification of a microsporidian, Encephalitozoon hellem, isolated from an AIDS patient with disseminated infection. J Clin Microbiol. 1994;32:2760–2768. doi: 10.1128/jcm.32.11.2760-2768.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Visvesvara G S, Leitch G J, Moura H, Wallace S, Weber R, Bryan R T. Culture, electron microscopy, and immunoblot studies on a microsporidian parasite isolated from the urine of a patient with AIDS. J Protozool. 1991;38:105S–111S. [PubMed] [Google Scholar]
- 39.Wanke C A, Degirolami P, Federman M. Enterocytozoon bieneusi infection and diarrheal disease in patients who were not infected with human immunodeficiency virus—case report and review. Clin Infect Dis. 1996;23:816–818. doi: 10.1093/clinids/23.4.816. [DOI] [PubMed] [Google Scholar]