Abstract
Two approaches have been utilized to investigate the role of individual SH2 domains in growth factor activation of phospholipase C-γ1 (PLC-γ1). Surface plasmon resonance analysis indicates that the individual N-SH2 and C-SH2 domains are able to specifically recognize a phosphotyrosine-containing peptide corresponding to Tyr 1021 of the platelet-derived growth factor (PDGF) β receptor. To assess SH2 function in the context of the full-length PLC-γ1 molecule as well as within the intact cell, PLC-γ1 SH2 domain mutants, disabled by site-directed mutagenesis of the N-SH2 and/or C-SH2 domain(s), were expressed in Plcg1−/− fibroblasts. Under equilibrium incubation conditions (4°C, 40 min), the N-SH2 domain, but not the C-SH2 domain, was sufficient to mediate significant PLC-γ1 association with the activated PDGF receptor and PLC-γ1 tyrosine phosphorylation. When both SH2 domains in PLC-γ1 were disabled, the double mutant did not associate with activated PDGF receptors and was not tyrosine phosphorylated. However, no single SH2 mutant was able to mediate growth factor activation of Ca2+ mobilization or inositol 1,4,5-trisphosphate (IP3) formation. Subsequent kinetic experiments demonstrated that each single SH2 domain mutant was significantly impaired in its capacity to mediate rapid association with activated PDGF receptors and become tyrosine phosphorylated. Hence, when assayed under physiological conditions necessary to achieve a rapid biological response (Ca2+ mobilization and IP3 formation), both SH2 domains of PLC-γ1 are essential to growth factor responsiveness.
Phospholipase Cγ1 (PLC-γ1) is a tyrosine kinase substrate for many receptor and nonreceptor tyrosine kinases (25, 40). Activation of the enzyme produces two second messenger molecules, inositol 1,4,5-trisphosphate (IP3) and diacylglycerol, which provoke the mobilization of intracellular Ca2+ and activation of protein kinase C. Although the mammalian genome encodes 10 known phosphoinositide-specific PLCs, only the γ1 and γ2 isoforms are regulated by tyrosine kinase activity. PLC-β isoform activity is controlled by heterotrimeric G protein-coupled receptors, while the mechanism of regulation of PLC-δ activity is unknown.
The extent to which PLC-γ1 is an essential signal transducing element depends on the biological system tested. In cell culture systems, a variety of experimental protocols have come to conflicting conclusions. In some instances PLC-γ1 activation has been deemed dispensable for mitogenesis or cell differentiation (18, 23, 28, 36, 44, 49, 53), while in other reports this signaling molecule has been concluded to be essential for cell proliferation or differentiation (3, 16, 19, 38, 48, 56, 58). In the mouse, however, PLC-γ1 is essential for embryonic development, as disrupted Plcg1 alleles produces embryonic lethality in the mouse at approximately embryonic day 9.0 (22). Hence, PLC-γ1 must be essential for the development or proliferation of at least one essential cell population in the embryo. Disruption of the Plcg1 gene in Drosophila is not lethal but leads to abnormal eye development, with the suggestion that this enzyme is involved in negative regulation (55).
The PLC-γ1 isozymes are distinct from PLC-β and -δ molecules in that the conserved catalytic subdomains (X + Y) are separated by a region that contains three src homology (SH) domains: two SH2 and one SH3 domain (25, 40). The two SH2 domains are nonidentical, with a 35% identity at the protein level. The SH2 domains facilitate association of PLC-γ1 with phosphotyrosine-containing proteins, especially activated growth factor receptor tyrosine kinases (4). The physiological function of the SH3 domain is unclear. Activation of PLC-γ1 is considered to require tyrosine phosphorylation at multiple sites (27, 39). Also, receptor association together with tyrosine phosphorylation may play a role in the activation process (29, 57).
In several growth factor receptors a single autophosphorylation site has been identified as crucial for PLC-γ1 association and in the absence of receptor association, PLC-γ1 is not tyrosine phosphorylated nor activated. The platelet-derived growth factor (PDGF) receptor contains multiple autophosphorylation sites which each mediate association with one or a few SH2 domain containing molecules. In the case of PLC-γ1, Tyr 1021 of the PDGF β receptor is the major association site, though a minor role for Tyr 1009 has been detected in some but not all reports (26, 31, 49, 56).
While several tyrosine kinase substrates or adapters (e.g., PLC-γ1, SHP, and p85) have two SH2 domains, this is not an essential feature, as others (e.g., GRB-2, nck, and STAT) have a single SH2 domain. Hence, the presence of two distinct SH2 domains in PLC-γ1 may be related to the protein’s capacity to associate with a wide spectrum of phosphotyrosine-containing proteins and hence allow PLC-γ1 to interact with an enlarged repertoire of receptors in various cell types. Alternatively, the second SH2 domain may function in the activation process in a manner not involving receptor association. Recently it has been reported that phosphatidylinositol 3,4,5-trisphosphate (PI 3,4,5-P3) binds to several SH2 domains (45) and the C-SH2 domain of PLC-γ1 (46). Also, it has been reported that phosphatidylinositol 3-kinase (PI-3 kinase) activity can, through direct and indirect mechanisms, modulate PLC-γ1 activation in various cell types (1, 6, 7, 9, 46, 50).
Analysis of the two SH2 domains in PLC-γ1 with phosphotyrosine-containing peptide libraries shows they have distinct receptor specificities (52). Initial studies with the isolated N-SH2 and C-SH2 domains, as fusion proteins, indicated that the N-SH2 bound to the activated PDGF receptor more effectively than did the C-SH2 (4). A role for the C-SH2 in receptor association was suggested by the fact that a fusion protein containing the N+C SH2 domains bound to activated PDGF receptors synergistically compared to binding of the single N-SH2 or C-SH2 domains. Also, a nuclear magnetic resonance structure of the C-SH2 domain complexed to a phosphopeptide representing Tyr 1021 of the PDGF receptor has been published (42).
To explore the functional role of the two SH2 domains present in PLC-γ1, we have undertaken a series of in vitro and intact-cell experiments. The former studies employ glutathione S-transferase–SH2 (GST-SH2) domain fusion constructs for BIAcore analysis, while the latter experiments utilize a novel intact-cell system. In this system, SH2 disabling site-directed mutations have been introduced into the intact PLC-γ1 molecule, which were then transfected into Plcg1−/− mouse embryo fibroblasts (MEF). This approach allows the assay of not only receptor association and PLC-γ1 tyrosine phosphorylation but also IP3 formation and Ca2+ mobilization in response to PDGF.
MATERIALS AND METHODS
Materials.
Dulbecco’s modified Eagle’s medium (DMEM) containing l-glutamine and high glucose and inositol-free DMEM were purchased from Life Technologies, Inc. Recombinant human PDGF-AA and recombinant rat PDGF-BB were from R & D Systems, Inc. Myo-[2-3H]inositol and 125I-protein A were purchased from New England Life Science Products. Antibodies to PDGF α and β receptors were from Upstate Biotechnology, while antibody to phosphotyrosine was from Zymed Laboratories Inc. Rabbit anti-PLC-γ1 serum was prepared as previously described (5). Fluo-4AM was purchased from Molecular Probes. Wortmannin, hygromycin B, aprotinin, leupeptin, pepstatin, phenylmethylsulfonyl fluoride, hydrogen peroxide, reagents for enhanced chemiluminescence (ECL), and protein A-Sepharose were from Sigma. Streptavidin-coated chips were obtained from BIAcore Inc. The pGEX-2TK vector was obtained from Pharmacia, and the ExSite mutagenesis kit was purchased from Stratagene. Glutathione-Sepharose beads were purchased from Pharmacia, and anti-GST monoclonal antibodies were from Transduction Laboratories. Prestained molecular weight markers were from Amersham Life Science Inc., while Immobilon-P membranes were from Micron Separations Inc. Peptides corresponding to the sequence surrounding Tyr 1021 in the PDGF β receptor (DNDYIIPLPDPK) were obtained from Quality Controlled Biochemicals, Inc. One peptide contained phosphotyrosine (pY 1021), while the second contained nonphosphorylated tyrosine (Y 1021). Both peptides were synthesized with biotin at the N terminus for coupling to streptavidin-coated BIAcore chips.
Cell culture.
Plcg1−/− MEF (23) were cultured in DMEM containing 10% fetal bovine serum, while ψ-2 cells were maintained in DMEM plus 5% calf serum. Cells were incubated at 37°C in a humidified atmosphere with 5% CO2.
Construction and expression of PLC-γ1 mutants in Plcg1−/− MEF.
The rat PLC-γ1 full-length cDNA was a generous gift of Sue Goo Rhee (National Institutes of Health). A double hemagglutinin (HA) epitope was added to generate HA–PLC-γ1. Using ExSite, a site-directed PCR mutagenesis kit, mutations of Arg 586 to Lys (R586K) within the N-SH2 domain and/or Arg 649 to Lys (R694K) within the C-SH2 domains were generated according to the manufacturer’s instruction and confirmed by DNA sequencing. The four HA–PLC-γ1 constructs produced are depicted in Fig. 1B and designated as follows: PLC-γ1wt (N+C+), PLC-γ1R586K (N−C+), PLC-γ1R694K (N+C−), and PLC-γ1R586,649K (N−C−). Each construct was then cloned into the retrovirus expression vector, pBabe-Hygro (a generous gift of Ronald Wisdom, Vanderbilt University), allowing for selection in hygromycin B. Proviral DNAs containing each PLC-γ1 construct were then transfected into ψ-2 cells, and stable cells were isolated according to standard protocols. Supernatants from these ψ-2 cells were then used to infect Plcg1−/− MEF. After selection in the presence of hygromycin B (400 μg/ml) for 2 weeks, resistant colonies were picked and placed into a 96-well tissue culture plate. After the cells were expanded, Western blotting was employed to screen the cells for expression of PLC-γ1 immunoreactive protein.
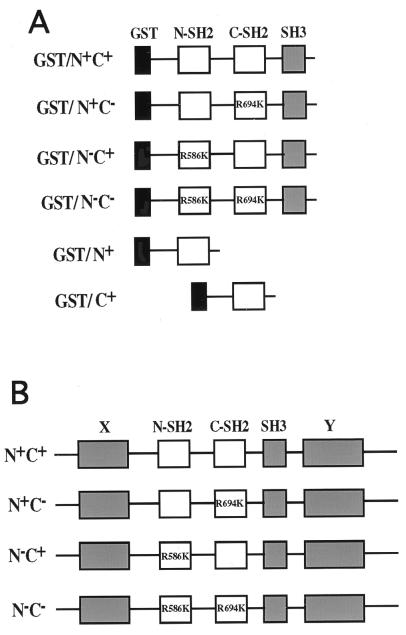
FIG. 1.
Schematic representations of SH2 domain fusion proteins and PLC-γ1 mutants. (A) GST fusion proteins with the SH region (SH2-SH2-SH3) of PLC-γ1 and having, as indicated, mutation of the Arg βB5 residue essential for phosphotyrosine recognition. Also shown are fusion proteins with the single N-SH2 or C-SH2 domain. (B) Full-length PLC-γ1 with the same SH2-disabling mutations in the N-SH2 and/or C-SH2 domain. Wild-type SH2 domains are indicated as N+ or C+, and mutants are indicated as N− or C−.
Construction and bacterial expression of PLC-γ1 SH fusion proteins.
GST fusion proteins were prepared to contain the central SH2-SH2-SH3 region of PLC-γ1 (encompassing residues 548 to 854) with the SH2 domain site-directed mutations described above. Also, fusion proteins containing the single N-SH2 domain (residues 548 to 661) or the single C-SH2 domain (residues 667 to 759) of PLC-γ1 were prepared. These fusion proteins are depicted in Fig. 1A.
To produce the GST constructs, the desired sequences within the rat PLC-γ1 cDNA were amplified by PCR using the PLC-γ1 wild type and the SH2 domain mutants described above as templates. These oligonucleotides contained BamHI and EcoRI restriction sites, which were employed to insert each PLC-γ1-derived sequence into the pGEX-2TK bacterial expression vector. The fidelity of all the PCR-amplified fragments was verified by DNA sequencing.
The recombinant GST constructs were introduced into an E. coli strain (XL1-blue), and the bacterial transformants were analyzed for the presence of the correct insert. The GST fusion proteins were then expressed by induction with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). Expressed fusion proteins were isolated by procedures described elsewhere (12). Following purification, fusion proteins were stored at −80°C in a buffer containing 10 mM Tris (pH 8.0), 150 mM NaCl, 1 mM EDTA, leupeptin (10 μg/ml), aprotinin (10 μg/ml), 1 mM phenylmethylsulfonyl fluoride, and 10% glycerol. Protein concentrations were determined by the modified method of Bradford (Bio-Rad Laboratories).
Surface plasmon resonance spectroscopy.
The binding kinetics of GST fusion proteins to immobilized peptides were measured with a BIAcore 2000 instrument. The BIAcore system and its use have been described elsewhere (41). Binding of GST fusion protein mass to immobilized peptide was observed in terms of resonance units (RU) (1,000 RU = 1 ng of protein bound/mm2 of flow cell surface). The running buffer used in this study was phosphate-buffered saline containing 0.05% Tween 20, and the same buffer was also used for diluting samples prior to injection. The streptavidin-coated CM-dextran chips were used to couple biotinylated peptides. Each chip contains four flow cells; one was coupled to the pY 1021 peptide, and a second was coupled to the Y 1021 peptide. The other flow cells were kept blank for background measurements. Hence, each fusion protein was assayed on one chip for binding to the pY 1021 peptide, the Y 1021 peptide, and a blank (no peptide) cell. To avoid erroneous results generated by the avidity influence of GST dimers, a low concentration of peptide (55 to 60 RU) was coupled to each BIAcore chip, as recommended by Ladbury et al. (30).
To measure binding, the GST fusion proteins, at different concentrations (100, 200, 300, 400, and 500 nM), were passed over the immobilized peptide surface at a flow rate of 10 μl/min for 10 min at 25°C. After each binding assay, flow cells were regenerated by running 0.1% sodium dodecyl sulfate (SDS) (flow rate, 10 μl/min [for 1 min]). To assess whether any degradation of the chip occurred between the experiments, the level of the response was checked with a GST-SH2-SH2-SH3 (N+C+) solution of fixed concentration immediately before and after every programmed run. In all cases there was no significant change in the response. The sensogram generated by each GST fusion protein’s binding to the pY 1021 peptide was adjusted by subtracting the binding to the Y 1021 peptide. Binding constants were then determined from the titration curves by using BIAevaluation software (version 3.0). Detailed methodology for the estimation of rate constants is presented in the software handbook (7a).
Growth factor treatment and preparation of cell lysates.
Subconfluent MEF in 150-mm-diameter dishes were incubated overnight in DMEM containing 0.5% fetal bovine serum. After removal from the medium and washing with ice-cold phosphate-buffered saline, cells were treated without or with PDGF-AA or PDGF-BB (25 ng/ml) at 4°C or at room temperature for the indicated period. Then, the cells were lysed in cold TGH buffer (1% Triton X-100, 10% glycerol, 50 mM HEPES [pH 7.2]) supplemented with 100 mM NaCl and proteinase and phosphatase inhibitors (aprotinin [10 μg/ml], leupeptin [10 μg/ml], 100 μM phenylmethylsulfonyl fluoride, 1 mM Na3VO4). The Triton-soluble fractions were collected by centrifugation (16,000 × g, 10 min) at 4°C.
Immunoprecipitation and Western blotting.
To detect the association of PLC-γ1 with PDGF receptors, 2 to 3 mg of each sample was incubated with PDGF receptor antibody. To detect PLC-γ1 tyrosine phosphorylation, 2 mg of each sample was incubated with PLC-γ1 antibody. These incubations with antibody were performed at 4°C with rocking. Then, protein A-agarose beads were added and the incubation continued at 4°C for 1 h. The protein A beads were then collected by centrifugation and washed three times with cold TGH buffer. The samples were boiled, and the immunoprecipitated proteins were separated on an SDS–7.5% polyacrylamide gel. Following transfer to nitrocellulose membranes, the proteins were probed with antibodies to either PLC-γ1 or phosphotyrosine. Where indicated, 125I-protein A was used to detect bound antibody. Otherwise, the bound antibody was detected by ECL. Where indicated, blots were stripped in a solution containing 62.5 mM Tris (pH 6.8), 2% SDS, and 0.7% β-mercaptoethanol and incubated at 55°C for 30 min. The stripped blots were reprobed with anti-PDGF receptor or anti-PLC-γ1 as indicated.
Where indicated, cell lysates (100 μg of protein) in TGH buffer were separated by SDS-polyacrylamide gel electrophoresis, and the proteins were transferred to nitrocellulose membranes. After blocking with 5% dry milk in TBST buffer (50 nM Tris Cl [pH 7.4], 150 mM NaCl, 0.05% Tween 20), antibody to PLC-γ1 or PDGF receptor was incubated with the membrane for 1 h at room temperature. Following washing, bound antibody was detected by ECL.
Intracellular Ca2+ mobilization and inositol 1,4,5-P3 formation.
Subconfluent MEF were plated on coverslips and incubated in DMEM containing 0.5% fetal bovine serum overnight. Then, the cells were rinsed with wash buffer (10 mM HEPES [pH 7.4], 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 0.55 mM glucose) and loaded with 1 μM Fluo-4AM for 20 min at room temperature. After washing with the wash buffer, the coverslips were placed into the microscope chamber followed by the addition of 1 ml of wash buffer plus 1 mM CaCl2. The chamber was placed on a Zeiss Axiovert 135 confocal microscope, and the cells were treated with PDGF (25 ng/ml) or 1% fetal bovine serum at room temperature. The number of cells emitting fluorescence at a wavelength of 488 nm was recorded, and the total number of cells present were counted. Data are expressed as the percent of cells mobilizing Ca2+. For wortmannin treatment, 100 nM wortmannin was added to the cells 15 min prior to PDGF stimulation.
Growth factor-stimulated IP3 was measured as described previously (17). The results are expressed as the fold increase in IP3 following PDGF stimulation. All experiments were performed in duplicate.
RESULTS
Surface plasmon resonance analysis.
To quantitatively assess the capacity of individual PLC-γ1 SH2 domains to interact with the known PDGF β receptor association site (Tyr 1021) for this tyrosine kinase substrate, the technique of surface plasmon resonance was employed. The biotinylated phosphotyrosine-containing peptide DNDpYIIPLPDPK, as well as the nonphosphorylated control peptide, was attached to streptavidin-coated chips under conditions that minimize the avidity influence of GST dimers (30). The real-time association and dissociation kinetics of GST fusion proteins, corresponding to the SH region of PLC-γ1 (Fig. 1A), with each peptide was then determined with increasing concentrations (100 to 500 nM) of each fusion protein. To analyze the binding capacity of SH2 domains within the context of the entire SH region of PLC-γ1, each fusion protein encoded both SH2 domains plus the SH3 domain present in PLC-γ1. Hence, these fusion proteins encompass residues 548 to 854 of PLC-γ1 and represent approximately 25% of the entire molecule. Site-directed mutagenesis of the βB5 Arg residue, which is essential in SH2 domains for phosphotyrosine recognition (8), was employed to disable one or both SH2 domains present in fusion proteins. Mutagenesis of the conserved Arg residue to Lys has been demonstrated in different proteins to be sufficient to oblate interaction of SH2 domains with phosphotyrosine (34, 35). To compare the binding contribution of a single SH2 domain within the context of the entire SH region to that of the isolated SH2 domain, fusion proteins representing only the N-SH2 or C-SH2 domains were also prepared (Fig. 1A) and analyzed. Isolated SH2 domain fusion proteins have usually been employed by others for similar BIAcore analyses of other proteins.
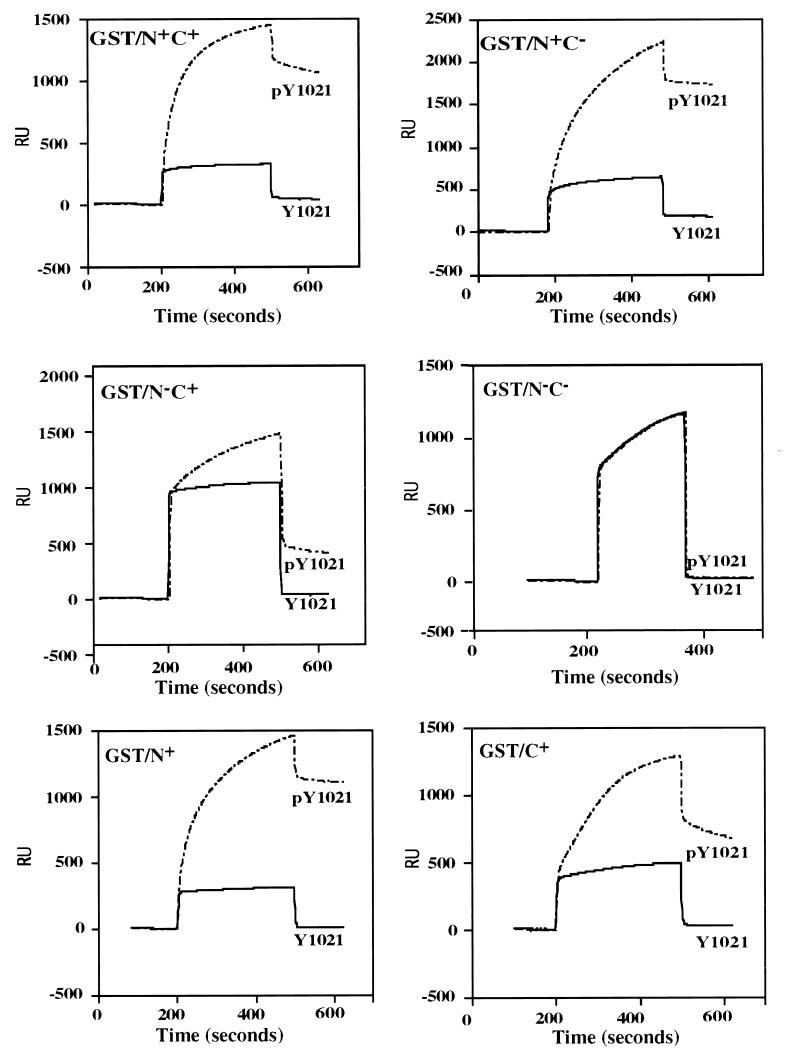
Representative sensograms for this binding reaction, at a fusion protein concentration of 500 nM, are shown in Fig. 2, and kinetic constants, derived from the binding data obtained at all fusion protein concentrations, are presented in Table 1. Comparison of the equilibrium constant KA for each fusion protein indicates that approximately 60% of the wild-type (GST/N+C+) binding capacity was lost when either the N-SH2 or C-SH2 domain was disabled (GST/N−C+ or GST/N+C−, respectively) by site-directed mutagenesis. When both SH2 domains were disabled (GST/N−C−), no binding to the target peptide was detectable. Analysis of individual rate constants indicates that within this group of constructs, the dissociation rate constants (kd) are comparable for each sample and the decrease in binding for the single SH2 mutants is attributable to decreases in rates of association (ka) relative to the wild-type protein.
FIG. 2.
Overlay sensograms for surface plasmon resonance analysis of GST SH2 domain fusion protein binding to immobilized Tyr 1021 PDGF receptor peptide. Biotinylated peptides corresponding to the pY 1021 or Y 1021 PDGF β receptor were immobilized on the sensor chip as described in Materials and Methods. The indicated GST fusion proteins were then passed over the chip, and the real-time binding responses were plotted as RU signals relative to time. For each GST fusion protein, increasing concentrations (100 to 500 nM) were tested. The sensograms shown are for 500 nM fusion protein. In each plot, the solid and broken lines, respectively, indicate interactions of each fusion protein with the Y 1021 and pY 1021 peptides.
TABLE 1.
Surface plasmon resonance binding constants of SH2 domain constructsa
| SH2 construct | ka (104) (M−1 s−1) | kd (10−3) (s−1) | KAb (107) | Relative bindingc |
|---|---|---|---|---|
| GST/N+C+ | 4.92 | 1.4 | 3.50 | 100 |
| GST/N+C− | 1.82 | 1.32 | 1.31 | 39 |
| GST/N−C+ | 1.47 | 1.26 | 1.17 | 33 |
| GST/N−C− | NDd | ND | ||
| GST/N+ | 3.66 | 2.20 | 1.66 | 47 |
| GST/C+ | 1.40 | 0.48 | 2.90 | 83 |
Binding constants were calculated from surface plasmon resonance data over all tested concentrations of fusion proteins (100, 200, 300, 400, and 500 nM) as described in Methods and Materials.
KA = ka/kd
The KA of GST/N+C+ was set at 100.
ND, not detectable.
These studies suggest that maximal PLC-γ1 association with pY 1021 of the PDGF β receptor requires both SH2 domains, but significant association levels might be expected in the presence of the single N-SH2 or C-SH2 domain.
Influence of SH2 domains on PLC-γ1 association with PDGF receptors in intact cells.
To test the in vitro results and predictions derived from the surface plasmon resonance studies, an intact cell system was employed. This cell system utilized fibroblasts derived from mouse embryos having targeted disruption of both Plcg1 alleles (23). As previously described, Plcg1−/− MEF express no intact PLC-γ1 protein and do not mobilize intracellular Ca2+ in response to epidermal growth factor or PDGF (17, 22).
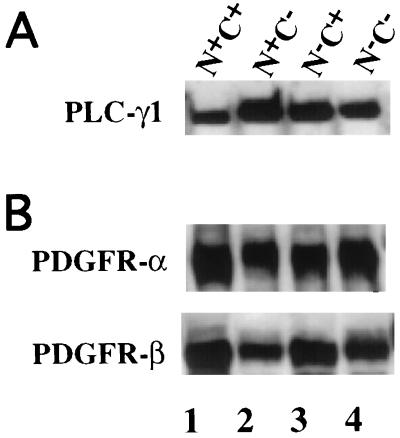
Plcg1−/− MEF were infected with retrovirus containing wild-type PLC-γ1 or PLC-γ1 constructs in which site-directed mutagenesis of the codon for the βB5 Arg residue was employed to disable the N-SH2 or C-SH2 domain or both SH2 domains (Fig. 1B). Stable cell lines expressing each PLC-γ1 construct were then selected, and the amount of PLC-γ1 protein was determined by Western blotting, as shown in Fig. 3A. Individual PLC-γ1 mutants are expressed at a slightly higher level, approximately twofold, relative to the expression of the wild-type proteins. The expression level of wild-type PLC-γ1 (N+C+) is comparable to the endogenous expression level of PLC-γ1 in Plcg1+/+ MEF (data not shown). The cell lines expressing PLC-γ1 isoforms were also assayed by Western blotting for PDGF α and β receptors, as shown in Fig. 3B.
FIG. 3.
Expression of PLC-γ1 mutants in Plcg1−/− MEF. As described in Materials and Methods, retroviral infection was employed to introduce wild-type and SH2 domain mutants of PLC-γ1 into MEF genetically deficient in PLC-γ1. After selection in hygromycin B, stable colonies were examined for their expression of PLC-γ1 (A) as well as PDGF α and β receptors (B). In both panels, bound antibody was detected by ECL.
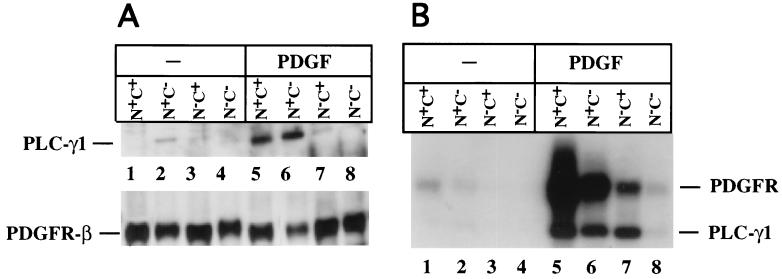
Cell lines reconstituted with PLC-γ1 or its mutants were then tested for the capacity of each PLC-γ1 construct to associate with PDGF receptors following the addition of PDGF. To maximize and achieve an equilibrium level of receptor association with PLC-γ1, the incubation with growth factor was performed at 4°C for 40 min. The results, shown in Fig. 4A, demonstrate that compared to the wild-type (N+C+) PLC-γ1, the N+C− mutant effectively associates with the PDGF β receptor. In this experiment there are low but detectable levels of the N−C+ mutant coprecipitated with the PDGF receptor in the presence of PDGF. Under these conditions, therefore, the N-SH2 domain of PLC-γ1 provides effective association with the PDGF receptor and the C-SH2 domain seems dispensable.
FIG. 4.
Equilibrium association and tyrosine phosphorylation of PLC-γ1 wild-type and mutants in response to PDGF. MEF expressing PLC-γ1 or each mutant were incubated at 4°C for 40 min in the absence or presence of PDGF-BB (25 ng/ml). (A) As described in Materials and Methods, cell lysates were then prepared and precipitated with anti-PDGF β receptor. The precipitates were subsequently probed by Western blotting for PLC-γ1 or PDGF β receptors. Bound antibody was detected by ECL. (B) Separate lysate aliquots were precipitated with anti-PLC-γ1 and blotted with antiphosphotyrosine. Bound antibody was detected with 125I-protein A.
Under the same conditions, the PDGF-dependent tyrosine phosphorylation of PLC-γ1 and the SH2 mutants was also assessed. Following PDGF treatment (4°C, 40 min), PLC-γ1 was immunoprecipitated and the precipitates were blotted with antiphosphotyrosine. The resulting data (Fig. 4B) demonstrate strong tyrosine phosphorylation of PLC-γ1 and also detect the coprecipitating tyrosine-phosphorylated PDGF receptor. In this experiment, PLC-γ1 tyrosine phosphorylation is apparent for wild-type, N+C−, and N−C+ PLC-γ1 species. The level of tyrosine phosphorylation of the N+C− and N−C+ PLC-γ1 mutant was approximately 50% of that of the wild-type molecule. These data also show that while tyrosine phosphorylation of the N+C− mutant is equivalent to that of the N−C+ mutants, there is significantly less receptor coprecipitated with the N−C+ mutant compared to the N+C− mutant. This is consistent with the data in Fig. 4A. No phosphorylation or receptor association was detectable for the N−C− double SH2 domain mutant.
The data in Fig. 4A indicate that equivalent levels of wild-type N+C+ and N+C− mutants of PLC-γ1 are coprecipitated with PDGF receptors. In Fig. 4B, precipitation of the N+C+ PLC-γ1 coprecipitates more tyrosine-phosphorylated PDGF receptors compared to the N+C− PLC-γ1 mutant. These data could be interpreted to indicate that a more highly phosphorylated receptor species is associated with wild-type PLC-γ1 than with the N+C− mutant. This conclusion, however, seems unlikely for several reasons. Quantitation of the tyrosine-phosphorylated receptor that coprecipitates with PLC-γ1 (Fig. 4B) shows only a 50% greater signal for the wild-type PLC-γ1 compared to the N+C− mutant. It is also possible that the high-molecular-mass band in Fig. 4B includes other molecules besides PDGF receptors. Furthermore, the experiments shown in Fig. 4 use different precipitating and blotting antibodies, which makes a quantitative comparison of the detected bands difficult.
These data do indicate that under these conditions the N-SH2 domain of PLC-γ1 is sufficient for effective receptor association and tyrosine phosphorylation. Also, the apparently lower level of receptor association mediated by the C-SH2 domain is able to produce an equivalent level of tyrosine phosphorylation, suggesting that the C-SH2 domain productively associates with the receptor, but the interaction is not as strong as that of the N-SH2 domain. Similar data were obtained when the cells were exposed to PDGF-AA (data not shown), indicating that both α and β PDGF receptors interact with PLC-γ1 SH2 mutants in a similar fashion.
SH2 mutants and PLC-γ1-mediated responses.
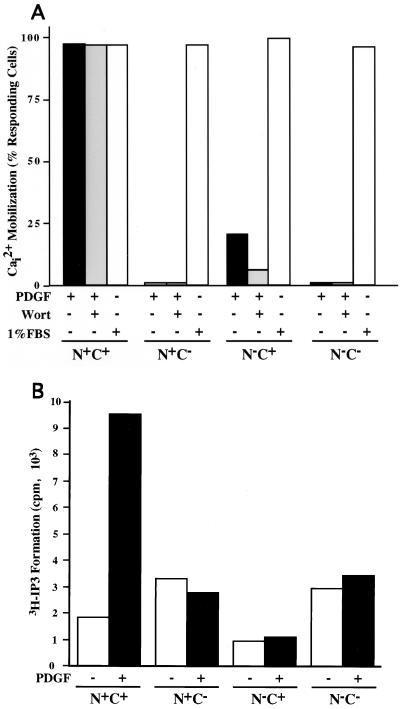
MEF Plcg1−/− cell lines expressing exogenous PLC-γ1 or its mutants were tested for their capacity to mobilize intracellular Ca2+ in response to PDGF. The results of this experiment are depicted in Fig. 5A and demonstrate that both SH2 domains are essential for significant Ca2+ mobilization. Although the presence of a functional N-SH2 domain was sufficient to facilitate effective PLC-γ1 association with activated PDGF receptors and tyrosine phosphorylation of PLC-γ1, the PLC-γ1 N+C− mutant was unable to elicit Ca2+ mobilization. Ca2+ mobilization in all cell lines was provoked by 1% fetal calf serum. Serum components, such as lysophatidic acid, activate PLC-β isoforms to provoke this mobilization and demonstrate that none of the cell lines are generally deficient in this response. In the experiment shown in Fig. 5A, Ca2+ mobilization data was collected for 2 min following the addition of PDGF. The lack of Ca2+ mobilization produced by the SH2 domain mutants does not reflect delayed mobilization, as analysis for 10 min following PDGF addition yielded the same results (data not shown).
FIG. 5.
Capacity of PLC-γ1 mutants to provoke intracellular Ca2+ mobilization and IP3 formation. (A) MEF expressing the indicated forms of PLC-γ1 were grown on coverslips and incubated overnight in medium supplemented with 0.5% serum. After loading with Fluo-4AM, Ca2+ mobilization was recorded in the absence (−) or presence (+) of PDGF-BB (25 ng/ml). One group of cells was incubated with wortmannin (100 nM) (Wort) for 15 min prior to the addition of PDGF. As a positive control, each cell line was exposed to 1% fetal bovine serum (FBS). Ca2+ mobilization was recorded over a 2-min period following the addition of PDGF. Approximately 400 total cells were analyzed for each condition. (B) Each MEF cell line was prelabeled with [3H]inositol for 24 h. The medium was changed to serum-free medium, the cells were incubated for 1 h, and LiCl (10 mM) was added. Approximately 20 min later, the cells were exposed to PDGF-BB (25 ng/ml) for 15 min at room temperature. Intracellular IP3 was then measured as described in Materials and Methods.
Recently published results have suggested that activation of PI-3 kinase positively regulates the activity of PLC-γ1 (6, 7, 9, 46, 50). Therefore, we employed wortmannin, a PI-3 kinase inhibitor, to determine whether Ca2+ mobilization in this system is sensitive to the known PDGF-dependent activation of PI-3 kinase. However, wortmannin (100 nM) had no measurable influence on PDGF-dependent Ca2+ mobilization by cells expressing wild-type PLC-γ1. Interestingly, the N−C+ PLC-γ1 mutant did mobilize a small amount of Ca2+ in several independent assays. The mobilization of Ca2+ by this mutant, which depends on the function of the C-SH2 domain, is partially sensitive to wortmannin. When the cells were activated with PDGF-AA, similar data for all the above results were obtained (data not presented).
A more direct assay of PLC-γ1 activity is the formation of IP3 from the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PI 4,5-P2). To determine whether the PLC-γ1 mutants that fail to provoke Ca2+ mobilization were able to hydrolyze PI 4,5-P2, the experiment shown in Fig. 5B was performed. Cells were preincubated with LiCl to block metabolic degradation of IP3 and then were treated with PDGF for 15 min. In the presence of PDGF, wild-type PLC-γ1 (N+C+) provoked an increase in IP3 levels of approximately fivefold. However, none of the mutants was able to stimulate IP3 formation in response to the growth factor. In this experiment, the cellular levels of inositol mono- and bisphosphate (IP1 and IP2) were also determined (data not shown). PDGF increased IP1 levels 70-fold and IP2 levels 4-fold in cells expressing wild-type PLC-γ1. However, there was no growth factor-mediated increase or accumulation of IP1 or IP2 for any of the SH2 domain mutants.
These data indicate that both SH2 domains of PLC-γ1 are essential for the activation of rapid intracellular responses to the PLC-dependent hydrolysis of PI 4,5-P2.
Kinetic analysis of PLC-γ1 function.
The preceding results suggest a paradox. The N-SH2 domain of PLC-γ1 is sufficient in the absence of the C-SH2 domain to mediate near-maximal association with and tyrosine phosphorylation by the PDGF receptor. Yet this mutant is not functionally activated by the PDGF receptor when IP3 or Ca2+ mobilization is measured. Since the receptor association and tyrosine phosphorylation data were obtained under incubation conditions that maximize these measurements, it was considered whether the SH2 function was more significant in the rapidity of PLC-γ1 interaction with the PDGF receptor. This would be biologically important, as Ca2+ mobilization and IP3 formation are provoked within minutes of PDGF addition.
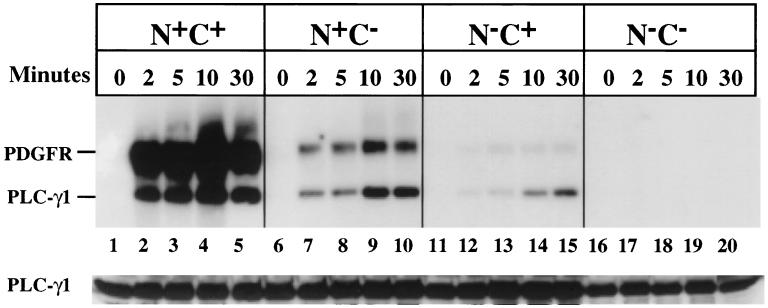
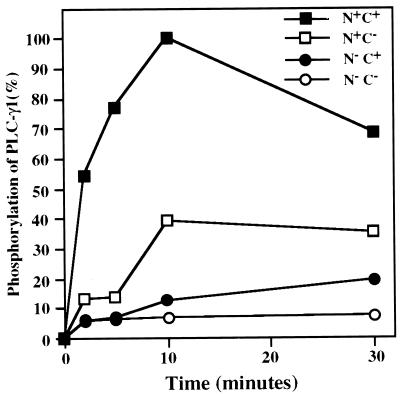
The data presented in Fig. 6 measure the capacity of PLC-γ1 and its mutants to be rapidly tyrosine phosphorylated following PDGF addition. The results are quite clear. None of the PLC-γ1 mutants are significantly phosphorylated compared to the wild-type enzyme, particularly within the first 5 min of PDGF addition, which is the time frame relevant for intracellular Ca2+ mobilization in response to the growth factor. The results of this experiment are quantitatively displayed in Fig. 7. In cells expressing wild-type PLC-γ1, the protein was half-maximally tyrosine phosphorylated within 2 min of PDGF addition. At this time point, the phosphorylation of the PLC-γ1 SH2 domain mutants (N+C− or N−C+) was approximately 5- to 10-fold less. At late times (i.e., 30 min) following PDGF addition, the levels of PLC-γ1 tyrosine phosphorylation resembled those obtained previously under equilibrium conditions in Fig. 4B. However, PLC-γ1 modifications at this point are no longer relevant to Ca2+ mobilization.
FIG. 6.
Time course of PDGF-dependent tyrosine phosphorylation of PLC-γ1 isoforms. MEF expressing PLC-γ1 isoforms were incubated overnight in medium containing 0.5% serum, and then PDGF-BB (25 ng/ml) was added for the indicated times. Lysates were prepared, and PLC-γ1 was immunoprecipitated. The precipitates were subsequently analyzed by Western blotting with antiphosphotyrosine, and bound antibody was detected with 125I-protein A. The membrane was then stripped and reprobed with anti-PLC-γ1, and bound antibody was detected by ECL.
FIG. 7.
Quantitation of the level of PDGF-induced tyrosine phosphorylation of PLC-γ1 isoforms. The original data in Fig. 6 were analyzed quantitatively by imaging densitometry. The maximal level of signal obtained was set at 100%, and other data points were expressed relative to this value.
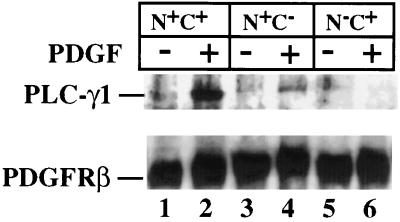
In view of the essential requirement for both SH2 domains for rapid PLC-γ1 tyrosine phosphorylation, an experiment was conducted to determine if both SH2 domains were also necessary for rapid association of the enzyme with the activated PDGF receptor. Following PDGF addition for 10 min, cells expressing each PLC-γ1 isoform were lysed and PDGF β receptors were immunoprecipitated. Subsequently, the level of PLC-γ1 present in the receptor precipitates was analyzed by Western blotting. As shown in Fig. 8, a small level of N+C− PLC-γ1 was receptor associated under these conditions, but this was only 10 to 20% of the level of wild-type PLC-γ1 associated with the receptor under the same conditions. In this experiment, no receptor association of the N−C+ PLC-γ1 mutant was detected.
FIG. 8.
Analysis of PLC-γ1 isoform capacity to associate with PDGF β receptors following ligand stimulation for a brief period. MEF expressing the indicated PLC-γ1 isoforms were incubated overnight in medium containing 0.5% serum. The cells were then exposed to PDGF-BB (25 ng/ml) for 10 min at room temperature. Thereafter, cell lysates were prepared and PDGF β receptors were immunoprecipitated. The precipitates were probed by Western blotting with anti-PLC-γ1. The blot was then stripped and reprobed with PDGF β receptor antibody. Both bound antibodies were detected by ECL.
DISCUSSION
The primary finding described in this manuscript is that both SH2 domains of PLC-γ1 participate in and are required for the activation of this enzyme. This requirement for both SH2 domains is especially apparent when PLC-γ1 function is measured under physiological circumstances related to its signal-transducing role. While the presence of the single N-SH2 domain does mediate a significant level of receptor association and tyrosine phosphorylation of PLC-γ1, this does not occur with sufficient rapidity for measurable IP3 formation and intracellular Ca2+ mobilization. It seems, therefore, that the two SH2 domains have overlapping functions. This conclusion is based on a novel approach for examining SH2 domain function in vivo. The contributions of individual SH2 domains of PLC-γ1 are evaluated by using single-residue mutagenesis within the intact molecule to disable each or both SH2 domains, and functional assays which allow a positive in vivo readout of PLC-γ1 functions are then conducted by expressing the mutants in Plcg1−/− cells.
When surface plasmon resonance is employed to measure SH2 interaction with a phosphotyrosine-containing peptide representing the PDGF β receptor association site for PLC-γ1, both the N-SH2 and C-SH2 domains are individually able to facilitate approximately equivalent levels of binding. The binding constants for these constructs (GST/N+C− and GST/N−C+) are nearly identical, particularly when measured within the context of the SH2-SH2-SH3 fragment of PLC-γ1. The amounts of each GST construct employed included both subsaturating and saturating concentrations (∼300 nM) for binding to the pY 1021 peptide. At all concentrations tested, both SH2 domains bound the peptide without dramatic differences becoming apparent. In all cases, however, the fusion protein containing both SH2 domains (GST/N+C+) was significantly more effective than either individual SH2 domain.
In studies of SH2 domain specificity with degenerate phosphotyrosine-containing peptide libraries (50), the N-SH2 and C-SH2 domains of PLC-γ1 are reported to prefer the sequences pYLEL and pYV/IIP, respectively. This assignment suggests that the C-SH2 domain would primarily mediate PLC-γ1 association at the position 1021 PDGF β receptor site (pYIIP). Both our data and the fact that a nuclear magnetic resonance structure of the C-SH2 domain in complex with a pY 1021 peptide was determined (42) suggest that this association occurs in vivo.
However, two different studies suggest that it is the N-SH2 domain that primarily mediates PLC-γ1 association with the PDGF receptor. In vitro studies with TrpE-SH2 fusion proteins and cell lysates containing activated PDGF β receptors showed effective receptor association with the PLC-γ1 N-SH2 domain but no detectable association with the PLC-γ1 C-SH2 domain (4). Our in vivo studies using PLC-γ1 site-directed mutants of individual SH2 domains reports the same preference for the N-SH2 domain within the context of the entire PLC-γ1 protein. We conclude, therefore, that it is the N-SH2 domain that primarily mediates association of PLC-γ1 with activated PDGF β receptors. Both of these studies also have shown that the presence of both N-SH2 and C-SH2 domains mediates binding more effectively than the N-SH2 domain alone. This occurs in the in vitro BIAcore measurements and in in vivo assays using both equilibrium and nonequilibrium receptor association conditions reported in this manuscript, as well as the in vitro system previously described (4). Hence it seems that the C-SH2 does have a function in receptor association. The studies described herein show that while the C-SH2 domain does not by itself mediate a relatively high level of receptor association, this domain does, in the absence of N-SH2, allow a measurable level of tyrosine phosphorylation of PLC-γ1 that is biochemically significant compared to the low level of phosphorylation in the double (N−C−) mutant. Hence, in vivo the C-SH2 domain may associate, but weakly, with the activated PDGF receptor.
That both SH2 domains of PLC-γ1 are required for PDGF-stimulated IP3 formation and Ca2+ mobilization may not be entirely explained solely by their participation in maximal and rapid receptor association. It seems likely that the C-SH2 domain has a second distinct function in PLC-γ1 activation. PDGF is known to provoke the formation of PIP3, and in vitro PIP3 is reported to directly associate with the C-SH2 domain of PLC-γ1 and increase the basal activity of the enzyme (6, 46). Also, inhibition of PI-3 kinase activity in vivo reduced PDGF activation of PLC-γ1, as measured by IP3 formation of Ca2+ mobilization (1, 6, 46). Our data do not show a similar inhibition of wild-type PLC-γ1-stimulated Ca2+ mobilization by the PI-3 kinase inhibitor wortmannin. Hence, if inhibition of PI-3 kinase does decrease IP3 formation, it does not seem to be sufficient to abrogate Ca2+ mobilization in MEF. We conclude that if PLC-γ1 is strongly tyrosine phosphorylated, then PIP3 is unlikely to be a significant physiological activator, at least for Ca2+ mobilization. In the absence of a functional N-SH2 domain in the PLC-γ1 mutant N−C+, however, PDGF does provoke a low level of PLC-γ1 tyrosine phosphorylation and a low level of Ca2+ mobilization, which is sensitive to wortmannin. This may suggest that if PLC-γ1 is weakly associated with PDGF receptors and not maximally phosphorylated, then PIP3 may, in fact, be a physiological activator. Based on published data, the R694K mutation in the C-SH2 domain should not abrogate its capacity to interact with PIP3 (45). The reason why the N−C+ mutant does promote some Ca2+ mobilization, while the N+C− mutant mediates no Ca2+ mobilization, is unclear.
The C-SH2 domain of PLC-γ1 has also been reported to associate with synaptojanin, which inhibits PLC enzyme activity in vitro (2), and with the actin cytoskeleton (43). Hence, there is evidence that the C-SH2 domain may be subjected to various effectors. Also it is possible that PIP3 acts in other ways to modulate PLC-γ1 activity. This could occur through the PLC-γ1 PH domain (9) or indirectly through profilin, which is reported to modulate PLC-γ1 activity (15) and bind PIP3 (32). Lastly, in B cells PIP3 participates in PLC-γ activation by activation of a tyrosine kinase that phosphorylates PLC-γ (11, 50).
There are other factors which also may contribute to PLC-γ1 through SH2 domains. Phosphatidic acid is a strong in vitro activator of PLC-γ1 (24). The extent to which phosphatidic acid produced by mitogens in vivo might bind SH2 domains is unknown. Several groups have identified tyrosine-phosphorylated proteins that associate with PLC-γ1. These include the p36/38 adapter in T cells (14, 37, 51), pp70 and pp68 molecules in B cells (13), and pp62 adapter in fibroblasts (33, 47). The pp36/38 adapter LAT, which is essential for PLC-γ1 activation in T cells (10), associates with the N-SH2 domain of PLC-γ1 (54) and functionally localizes PLC-γ1 to glycolipid-enriched membrane microdomains (59). Lastly, the microtubule protein Tau and arachidonic acid have been shown to associate with PLC-γ1 and stimulate its basal enzymatic activity (20, 21). It seems clear that understanding the physiological activation of PLC-γ1 will include numerous inputs. The use of genetically defined cell systems, such as Plcg1−/− cells, may be useful in deciphering the regulation of this signal-transducing molecule.
ACKNOWLEDGMENTS
We thank Sue Carpenter and Nicholas Garcia for assistance with manuscript preparation and technical assistance, respectively.
Additionally, the support of NIH grants CA24071 and CA75195 is acknowledged, as is assistance from the Vanderbilt Cancer (CA68485) and Diabetes (DK20593) Centers.
REFERENCES
- 1.Åhlén K, Berg A, Stiger F, Tengholm A, Siegbahn A, Gylfe E, Reed R K, Rubin K. Cell interactions with collagen matrices in vivo and in vitro depend on phosphatidylinositol 3-kinase and free cytoplasmic calcium. Cell Adhesion Commun. 1998;5:461–473. doi: 10.3109/15419069809005604. [DOI] [PubMed] [Google Scholar]
- 2.Ahn S J, Han S J, Mor H J, Chung J-K, Hong S H, Park T K, Kim C G. Interaction of phospholipase Cγ1 via its COOH-terminal SRC homology 2 domain with synaptojanin. Biochem Biophys Res Commun. 1998;244:62–67. doi: 10.1006/bbrc.1998.8220. [DOI] [PubMed] [Google Scholar]
- 3.Alimandi M, Heidaran M A, Gutkind J S, Zhang J, Ellmore N, Valius M, Kazlauskas A, Pierce J H, Li W. PLC-γ activation is required for PDGF-βR-mediated mitogenesis and monocytic differentiation of myeloid progenitor cells. Oncogene. 1997;15:585–593. doi: 10.1038/sj.onc.1201221. [DOI] [PubMed] [Google Scholar]
- 4.Anderson D, Koch C A, Grey L, Ellis C, Moran M F, Pawson T. Binding of SH2 domains of phospholipase Cγ1, GAP, and src to activated growth factor receptors. Science. 1990;250:979–982. doi: 10.1126/science.2173144. [DOI] [PubMed] [Google Scholar]
- 5.Arteaga C L, Johnson M D, Todderud G, Coffey R J, Carpenter G, Page D L. Elevated content of the tyrosine kinase substrate phospholipase C-γ1 in primary human breast carcinomas. Proc Natl Acad Sci USA. 1991;88:10435–10439. doi: 10.1073/pnas.88.23.10435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae Y S, Cantley L G, Chen C-S, Kim S-R, Kwon K-S, Rhee S G. Activation of phospholipase C-γ by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;172:4465–4469. doi: 10.1074/jbc.273.8.4465. [DOI] [PubMed] [Google Scholar]
- 7.Barker S A, Caldwell K K, Pfeiffer J R, Wilson B S. Wortmannin-sensitive phosphorylation, translocation, and activation of PLCγ1, but not PLCγ2, in antigen-stimulated RBL-2H3 mast cells. Mol Biol Cell. 1998;9:483–496. doi: 10.1091/mbc.9.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.BIAcore Inc. BIAevaluation version 3.0 software handbook. Uppsala, Sweden: BIAcore Inc.; 1997. [Google Scholar]
- 8.Cohen G B, Ren R, Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995;80:237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- 9.Falasca M, Logan S K, Lehot V P, Baccante G, Lemmon M A, Schlessinger J. Activation of phospholipase Cγ by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finco T S, Kadlececk T, Zhang W, Samelson L E, Weiss A. LAT is required for TCR-mediated activation of PLC-γ1 and the Ras pathway. Immunity. 1998;9:617–628. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 11.Fluckiger A-C, Li Z, Kato R M, Wahl M I, Ochs H D, Longnecker R, Kinet J-P, Witte W N, Scharenberg A M, Rawlings D J. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J. 1998;17:1973–1985. doi: 10.1093/emboj/17.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frangioni J V, Neel B G. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal Biochem. 1993;210:179–187. doi: 10.1006/abio.1993.1170. [DOI] [PubMed] [Google Scholar]
- 13.Fu C, Chan A C. Identification of two tyrosine phosphoproteins, pp70 and pp68, which interact with phospholipase Cγ, Grb2, and Vav after B cell antigen receptor activation. J Biol Chem. 1997;272:27362–27368. doi: 10.1074/jbc.272.43.27362. [DOI] [PubMed] [Google Scholar]
- 14.Fukazawa T, Reedquist K A, Panchamoorthy G, Soltoff S, Trub T, Druker B, Cantley L, Shoelson S E, Band H. T cell activation-dependent association between the p85 subunit of the phosphatidylinositol 3-kinase and Grb2/phospholipase C-γ1-binding phosphotyrosyl protein pp36/38. J Biol Chem. 1995;270:20177–20182. doi: 10.1074/jbc.270.34.20177. [DOI] [PubMed] [Google Scholar]
- 15.Goldschmidt-Clermont H J, Kim J W, Machesky L M, Rhee S G, Pollard T D. Regulation of phospholipase C-γ1 by profilin and tyrosine phosphorylation. Science. 1991;251:1231–1233. doi: 10.1126/science.1848725. [DOI] [PubMed] [Google Scholar]
- 16.Hall H, Williams E J, Moore S E, Walsh F S, Prochiantz A, Doherty P. Inhibition of FGF-stimulated phosphatidylinositol hydrolysis and neurite outgrowth by a cell-membrane permeable phosphopeptide. Curr Biol. 1996;6:580–587. doi: 10.1016/s0960-9822(02)00544-4. [DOI] [PubMed] [Google Scholar]
- 17.Hess J A, Ji Q-S, Carpenter G, Exton J H. Analysis of platelet-derived growth factor-induced phospholipase D activation in mouse embryo fibroblasts lacking phospholipase C-γ1. Biol Chem. 1998;273:20517–20524. doi: 10.1074/jbc.273.32.20517. [DOI] [PubMed] [Google Scholar]
- 18.Hill T D, Dean N M, Mordan L J, Lau A F, Kanemitsu M Y, Boynton A L. PDGF-induced activation of phospholipase C is not required for induction of DNA synthesis. Science. 1990;248:1660–1663. doi: 10.1126/science.2163545. [DOI] [PubMed] [Google Scholar]
- 19.Homma M K, Yamasaki M, Ohmi S, Homma Y. Inhibition of phosphoinositide hydrolysis and cell growth of Swiss 3T3 cells by myristoylated phospholipase C inhibitor peptides. Biochem. 1997;122:738–742. doi: 10.1093/oxfordjournals.jbchem.a021817. [DOI] [PubMed] [Google Scholar]
- 20.Hwang S C, Jhon D-Y, Bae Y S, Kim J H, Rhee S G. Activation of phospholipase C-γ1 by the concerted action of tau proteins and arachidonic acid. J Biol Chem. 1996;271:18342–18349. doi: 10.1074/jbc.271.31.18342. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins S M, Johnson G V W. Tau complexes with phospholipase C-γ in situ. Neuroreport. 1998;9:67–71. doi: 10.1097/00001756-199801050-00014. [DOI] [PubMed] [Google Scholar]
- 22.Ji Q-S, Winnier G E, Niswender K D, Horstman D, Wisdom R, Magnuson M A, Carpenter G. Essential role of the tyrosine kinase substrate phospholipase C-γ1 in mammalian growth and development. Proc Natl Acad Sci USA. 1997;94:2999–3003. doi: 10.1073/pnas.94.7.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji Q-S, Ermini S, Baulida J, Sun F-L, Carpenter G. Epidermal growth factor signaling and mitogenesis in Plcg1 null mouse embryonic fibroblasts. Mol Biol Cell. 1998;9:749–757. doi: 10.1091/mbc.9.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones G A, Carpenter G. The regulation of phospholipase C-γ1 by phosphatidic acid. Assessment of kinetic parameters. J Biol Chem. 1993;268:20845–20850. [PubMed] [Google Scholar]
- 25.Kamat A, Carpenter G. Phospholipase C-γ1: regulation of enzyme function and role in growth factor-dependent signal transduction. Cytokine Growth Factor Rev. 1997;8:109–117. doi: 10.1016/s1359-6101(97)00003-8. [DOI] [PubMed] [Google Scholar]
- 26.Kashishian A, Cooper J A. Phosphorylation sites at the C-terminus of the platelet-derived growth factor receptor bind phospholipase Cγ1. Mol Biol Cell. 1993;4:49–57. doi: 10.1091/mbc.4.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H K, Kim J W, Zilberstein A, Margolis B, Kim J G, Schlessinger J, Rhee S G. PDGF stimulation of inositol phospholipid hydrolysis requires PLC-γ1 phosphorylation on tyrosine residues 783 and 1254. Cell. 1991;65:435–441. doi: 10.1016/0092-8674(91)90461-7. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi S, Nishimura J, Kanaide H. Cytosolic Ca2+ transients are not required for platelet-derived growth factor to induce cell cycle progression of vascular smooth muscle cells in primary culture. Actions of tyrosine kinase. J Biol Chem. 1994;269:9011–9018. [PubMed] [Google Scholar]
- 29.Kumjian D A, Barnstein A, Rhee S G, Daniel T O. Phospholipase Cγ complexes with ligand-activated platelet-derived growth factor receptors. An intermediate implicated in phospholipase activation. J Biol Chem. 1991;266:3973–3980. [PubMed] [Google Scholar]
- 30.Ladbury J E, Lemmon M A, Zhou M, Green J, Botfield M C, Schlessinger J. Measurement of the binding of tyrosyl phosphopeptides to SH2 domains: a reappraisal. Proc Natl Acad Sci USA. 1995;92:3199–3203. doi: 10.1073/pnas.92.8.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larose L, Gish G, Shoelson S, Pawson T. Identification of residues in the β platelet-derived growth factor receptor that confer specificity for binding to phospholipase C-γ1. Oncogene. 1993;8:2493–2499. [PubMed] [Google Scholar]
- 32.Lu P-J, Shieh W R, Rhee S G, Yin H L, Chen C-S. Lipid products of phosphoinositide 3-kinase bind human profilin with high affinity. Biochemistry. 1996;35:14027–14034. doi: 10.1021/bi961878z. [DOI] [PubMed] [Google Scholar]
- 33.Maa M-C, Leu T-H, Trandel B J, Chang J-H, Parsons S J. A protein that is highly related to GTPase-activating protein-associated p62 complexes with phospholipase Cγ. Mol Cell Biol. 1994;14:5466–5473. doi: 10.1128/mcb.14.8.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marengere I E M, Pawson T. Identification of residues in GTPase activating protein Src homology 2 domains that control binding to tyrosine phosphorylated growth factor receptors and p62. J Biol Chem. 1992;267:22779–22786. [PubMed] [Google Scholar]
- 35.Mayer B J, Jackson P K, Van Etten R A, Baltimore D. Point mutations in the abl SH2 domain coordinately impair phosphotyrosine binding in vitro and transforming activity in vivo. Mol Cell Biol. 1992;12:609–618. doi: 10.1128/mcb.12.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohammadi M, Dionne C A, Li W, Li N, Spivak T, Honegger A M, Jaye M, Schlessinger J. Point mutation in FGF receptor eliminates phosphatidylinositol hydrolysis without affecting mitogenesis. Nature. 1992;358:681–684. doi: 10.1038/358681a0. [DOI] [PubMed] [Google Scholar]
- 37.Motto D G, Musci M A, Ross S E, Koretzky G A. Tyrosine phosphorylation of Grb2-associated proteins correlates with phospholipase Cγ1 activation in T cells. Mol Cell Biol. 1996;16:2823–2829. doi: 10.1128/mcb.16.6.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nebigil C G. Suppression of phospholipase C β, γ, and δ families alters cell growth and phosphatidylinositol 4,5-bisphosphate levels. Biochemistry. 1997;36:15949–15958. doi: 10.1021/bi971721m. [DOI] [PubMed] [Google Scholar]
- 39.Nishibe S, Wahl M I, Hernández-Sotomayor S M T, Tonks N K, Rhee S G, Carpenter G. Increase of the catalytic activity of phospholipase C-γ1 by tyrosine phosphorylation. Science. 1990;250:1253–1256. doi: 10.1126/science.1700866. [DOI] [PubMed] [Google Scholar]
- 40.Noh D-Y, Shin S H, Rhee S G. Phosphoinositide-specific phospholipase C and mitogenic signaling. Biochim Biophys Acta. 1995;1242:99–114. doi: 10.1016/0304-419x(95)00006-0. [DOI] [PubMed] [Google Scholar]
- 41.Panayotou A. Surface plasmon resonance. Measuring protein interactions in real time. Methods Mol Biol. 1998;88:1–10. doi: 10.1385/0-89603-487-9:1. [DOI] [PubMed] [Google Scholar]
- 42.Pascal S M, Singer A U, Gish G, Yamazaki Y, Shoelson S E, Pawson T, Kay L E, Forman-Kay J D. Nuclear magnetic resonance structure of an SH2 domain of phospholipase C-γ1 complexed with a high affinity binding peptide. Cell. 1994;77:461–472. doi: 10.1016/0092-8674(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 43.Pei Z, Yang L, Williamson J R. Phospholipase C-γ1 binds to actin-cytoskeleton via its C-terminal SH2 domain in vitro. Biochem Biophys Res Commun. 1996;228:802–806. doi: 10.1006/bbrc.1996.1735. [DOI] [PubMed] [Google Scholar]
- 44.Peters K G, Marie J, Wilson E, Ives H E, Escobedo J, Del Rosario M, Mirda D, Williams L T. Point mutation of an FGF receptor abolishes phosphatidylinositol turnover and Ca2+ flux but not mitogenesis. Nature. 1992;358:678–681. doi: 10.1038/358678a0. [DOI] [PubMed] [Google Scholar]
- 45.Rameh L E, Chen C-S, Cantley L C. Phosphatidylinositol (3,4,5)P3 interacts with SH2 domains and modulates PI 3-kinase association with tyrosine-phosphorylated proteins. Cell. 1995;83:821–830. doi: 10.1016/0092-8674(95)90195-7. [DOI] [PubMed] [Google Scholar]
- 46.Rameh L E, Rhee S G, Spokes K, Kazlauskas A, Cantley L C, Cantley L G. Phosphoinositide 3-kinase regulates phospholipase Cγ-mediated calcium signaling. J Biol Chem. 1998;273:23750–23757. doi: 10.1074/jbc.273.37.23750. [DOI] [PubMed] [Google Scholar]
- 47.Richard S, Yu D, Blumer K J, Hausladen D, Olszowy M W, Connelly P A, Shaw A S. Association of p62, a multifunctional SH2- and SH3-domain-binding protein, with src family tyrosine kinases, Grb2, and phospholipase Cγ-1. Mol Cell Biol. 1995;15:186–197. doi: 10.1128/mcb.15.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roche S, McGlade J, Jones M, Gish G D, Pawson T, Courtneidge S A. Requirement of phospholipase Cγ, the tyrosine phosphatase Syp and the adaptor proteins Shc and Nck for PDGF-induced DNA synthesis: evidence for Ras-independent pathways. EMBO J. 1996;15:4940–4948. [PMC free article] [PubMed] [Google Scholar]
- 49.Rönnstrand L, Mori S, Arridsson A-K, Eriksson A, Wernstedt C, Hellman U, Claesson-Welsh L, Heldin C-H. Identification of two C-terminal autophosphorylation sites in the PDGF β-receptor: involvement in the interaction with phospholipase C-γ. EMBO J. 1992;11:3911–3919. doi: 10.1002/j.1460-2075.1992.tb05484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scharenberg A M, El-Hillal O, Fruman D A, Beitz L O, Li Z, Lin S, Gout I, Cantley L C, Rawlings D J, Kinet J-P. Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase-dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. EMBO J. 1998;17:1961–1972. doi: 10.1093/emboj/17.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sieh M, Batzer A, Schlessinger J, Weiss A. GRB2 and phospholipase C-γ1 associate with a 36- to 38-kilodalton phosphotyrosine protein after T-cell receptor stimulation. Mol Cell Biol. 1994;14:4435–4442. doi: 10.1128/mcb.14.7.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Songyang Z, Shoelson S E, Chaudhuri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider R J, Neel B G, Birge R B, Fajardo J E, Chou M M, Hanafusa H, Schaffhausen B, Cantley L C. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 53.Spivak-Kroizman T, Mohammadi M, Hu P, Jaye M, Schlessinger J, Lax I. Point mutation in the fibroblast growth factor receptor eliminates phosphatidylinositol hydrolysis without affecting neuronal differentiation of PC12 cells. J Biol Chem. 1994;269:14419–14423. [PubMed] [Google Scholar]
- 54.Stoica B, DeBell K E, Graham L, Rellahan B L, Alava M A, Laborda J, Bonvini E. The amino-terminal Src homology 2 domain of phospholipase Cγ1 is essential for TCR-induced tyrosine phosphorylation of phospholipase Cγ1. J Immunol. 1998;160:1059–1066. [PubMed] [Google Scholar]
- 55.Thackeray J R, Gaines P C W, Ebert P, Carlson J R. small wing encodes a phospholipase C-γ that acts as a negative regulator of R7 development in Drosophila. Development. 1998;125:5033–5042. doi: 10.1242/dev.125.24.5033. [DOI] [PubMed] [Google Scholar]
- 56.Valius M, Bazenet C, Kazlauskas A. Tyrosines 1021 and 1009 are phosphorylation sites in the carboxy terminus of the platelet-derived growth factor receptor β subunit and are required for binding of phospholipase Cγ and a 64-kilodalton protein, respectively. Mol Cell Biol. 1993;13:133–143. doi: 10.1128/mcb.13.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vega Q C, Cochet C, Filhol O, Chang C-P, Rhee S G, Gill G N. A site of tyrosine phosphorylation in the C terminus of the epidermal growth factor receptor is required to activate phospholipase C. Mol Cell Biol. 1992;12:128–135. doi: 10.1128/mcb.12.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Z, Glück S, Zhang L, Moran M F. Requirement for phospholipase C-γ1 enzymatic activity in growth factor-induced mitogenesis. Mol Cell Biol. 1998;18:590–597. doi: 10.1128/mcb.18.1.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang W, Trible R P, Samelson L E. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]