Abstract
This study probed the largely unexplored regulation and role of fibronectin in Angiotensin II-stimulated cardiac fibroblasts. Using gene knockdown and overexpression approaches, Western blotting, and promoter pull-down assay, we show that collagen type I-activated Discoidin Domain Receptor 2 (DDR2) mediates Angiotensin II-dependent transcriptional upregulation of fibronectin by Yes-activated Protein in cardiac fibroblasts. Furthermore, siRNA-mediated fibronectin knockdown attenuated Angiotensin II-stimulated expression of collagen type I and anti-apoptotic cIAP2, and enhanced cardiac fibroblast susceptibility to apoptosis. Importantly, an obligate role for fibronectin was observed in Angiotensin II-stimulated expression of AT1R, the Angiotensin II receptor, which would link extracellular matrix (ECM) signaling and Angiotensin II signaling in cardiac fibroblasts. The role of fibronectin in Angiotensin II-stimulated cIAP2, collagen type I, and AT1R expression was mediated by Integrin-β1-integrin-linked kinase signaling. In vivo, we observed modestly reduced basal levels of AT1R in DDR2-null mouse myocardium, which were associated with the previously reported reduction in myocardial Integrin-β1 levels. The role of fibronectin, downstream of DDR2, could be a critical determinant of cardiac fibroblast-mediated wound healing following myocardial injury. In summary, our findings suggest a complex mechanism of regulation of cardiac fibroblast function involving two major ECM proteins, collagen type I and fibronectin, and their receptors, DDR2 and Integrin-β1.
Keywords: cardiac fibroblasts, DDR2, fibronectin, Integrin-β1, angiotensin II, AT1 receptor, cIAP2, collagen I
1. Introduction
Cardiac fibrosis, a hallmark of the senescent myocardium and the end stage of nearly all forms of heart disease, involves an excessive deposition of extracellular matrix by cardiac fibroblasts, leading to arrhythmias, pump dysfunction, and heart failure [1]. In a setting of myocardial damage, normally quiescent cardiac fibroblasts, under the influence of pro-fibrotic factors such as Angiotensin II (Ang II), are phenotypically transformed into α-smooth muscle actin (α-SMA)-positive myofibroblasts that infiltrate the site of injury, proliferate, and produce collagen and other extracellular matrix (ECM) components, leading to the formation of collagenous scar tissue that aids wound repair and preserves myocardial integrity in the short-term. However, unlike in non-cardiac tissues, active myofibroblasts in the heart resist apoptosis and persist in the infarct scar long after termination of the healing response, which results in excessive collagen deposition, adverse tissue fibrosis, and compromised ventricular compliance in the long-term [1]. Clearly, the delineation of molecular mechanisms underlying cardiac fibroblast function and cardiac fibrosis is a clinically relevant goal.
Although earlier studies on cardiac fibrosis had focused on collagen type I as a major contributor to the progression of tissue fibrosis [2], there is now increasing appreciation of the role of cellular fibronectin, an ECM glycoprotein, as an important regulator of ECM and tissue remodeling [3]. In fact, recent studies have considered fibronectin as a potential target to limit tissue fibrosis [4]. The inhibition of fibronectin polymerization is reported to reduce collagen deposition in the ECM and exert cardioprotective effects through attenuation of cardiac fibroblast proliferation and adverse fibrotic remodeling of the heart in an ischemia–reperfusion model [4]. These observations suggest a role for fibronectin in cardiac fibroblast function and cardiac fibrosis and provide compelling rationale for further studies on the regulation of fibronectin expression in cardiac fibroblasts and the mechanisms that underlie its regulatory role in these cells.
We had recently reported that Ang II, a potent pro-fibrotic factor whose intracardiac levels are increased following myocardial injury [5], enhances the expression of Discoidin Domain Receptor 2 (DDR2), a collagen receptor tyrosine kinase, which, upon activation by collagen, has an indispensable regulatory role in the expression of collagen type I in Ang II-stimulated cardiac fibroblasts [2,6]. Ang II also induces anti-apoptotic cIAP2 that protects cardiac fibroblasts against ambient stress [7]. Interestingly, the pro-survival role of Ang II in cardiac fibroblasts via cIAP2 was also found to be mediated by collagen type I-activated DDR2 (7).
As a sequel to our earlier findings, we now report that DDR2 has an obligate role in Yes-activated Protein (YAP)-mediated transcriptional upregulation of cellular fibronectin protein expression by Ang II in cardiac fibroblasts. Downstream of DDR2, fibronectin mediates Ang II-stimulated collagen type I protein expression and cIAP2-dependent resistance to apoptosis in cardiac fibroblasts exposed to oxidative stress. Importantly, fibronectin has an obligate role in Ang II-stimulated expression of the Ang II receptor, AT1R, which would link ECM signaling to Ang II signaling that profoundly impacts cardiac fibroblast function. Furthermore, a combination of gene knockdown and overexpression approaches revealed that the regulatory role of cellular fibronectin in cardiac fibroblasts is mediated by Integrin-β1 signaling. Notably, we observed a small but significant reduction in basal levels of AT1R in DDR2-null mouse myocardium, which is associated with a reduction in myocardial Integrin-β1 levels, reported by us earlier (6). We propose that the pro-survival role of cellular fibronectin and its regulatory role in collagen and AT1R expression, downstream of DDR2, could be critical determinants of cardiac fibroblast response to myocardial injury.
2. Results
2.1. DDR2 Mediates Ang II-Stimulated Fibronectin Expression in Cardiac Fibroblasts
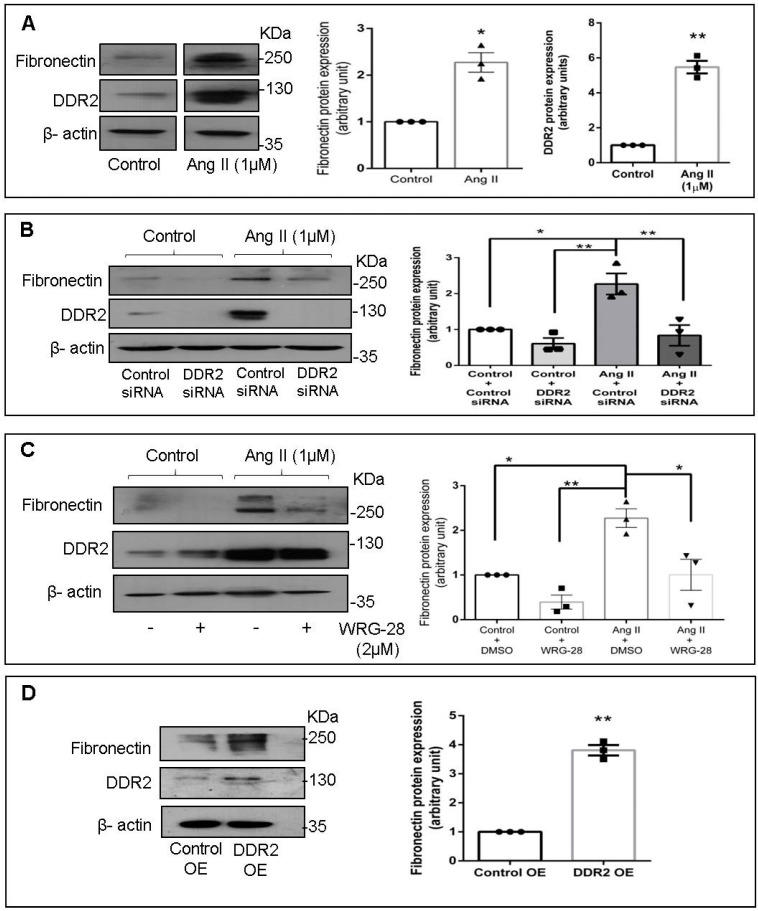
Treatment of cardiac fibroblasts with Ang II (1 µM) enhanced cellular fibronectin protein expression (≈2-fold), with concomitant increase (≈5-fold) in DDR2 protein expression (Figure 1A). Since enhanced DDR2 protein levels correlated with enhanced cellular fibronectin protein expression, the effect of siRNA-mediated DDR2 knockdown on fibronectin protein expression was checked to ascertain if DDR2 regulates fibronectin. DDR2 knockdown significantly diminished the Ang II-induced increase in fibronectin protein expression (Figure 1B). Furthermore, inhibition of collagen type I-dependent activation of DDR2 using WRG-28 [8] blocked Ang II-stimulated fibronectin protein expression (Figure 1C). Conversely, overexpression of DDR2 in unstimulated cardiac fibroblasts led to an upregulation of cellular fibronectin protein levels (Figure 1D), confirming the indispensable role of DDR2 in fibronectin protein expression.
Figure 1.
DDR2 mediates Ang II-stimulated fibronectin protein expression in cardiac fibroblasts. (A) Sub-confluent quiescent cultures of cardiac fibroblasts were stimulated with Angiotensin II (Ang II) (1 µM). Protein was isolated at 12 h of Ang II treatment and subjected to Western blot analysis for the detection of fibronectin and DDR2, with β-actin as loading control. * p < 0.05 and ** p < 0.01 vs. control. (B) Cardiac fibroblasts were transiently transfected with DDR2 siRNA (5 pmol) or control (scrambled) siRNA prior to treatment with Ang II for 12 h. Fibronectin protein expression was examined with β-actin as loading control. Validation of DDR2 silencing is also shown. * p < 0.05, ** p < 0.01 (comparisons as depicted in the figure). (C) Cardiac fibroblasts were treated with WRG-28 (2 µM) for 1 h prior to Ang II treatment. Cells were collected at 12 h post-Ang II treatment and fibronectin protein levels were examined, with β-actin as loading control. * p < 0.05 and ** p < 0.01 (comparisons as depicted in the Figure). (D) Cardiac fibroblasts were transfected with DDR2 cDNA overexpression plasmid (DDR2 OE) (with empty vector control, Control OE), as described under Materials and Methods, and fibronectin protein expression was examined, with β-actin as loading control. ** p < 0.01 vs. Control OE. Validation of DDR2 overexpression is also shown. Data are representative of three independent experiments, n = 3, Mean ± SEM.
2.2. Transcriptional Regulation of Fibronectin by DDR2-Activated YAP Transcription Co-Activator
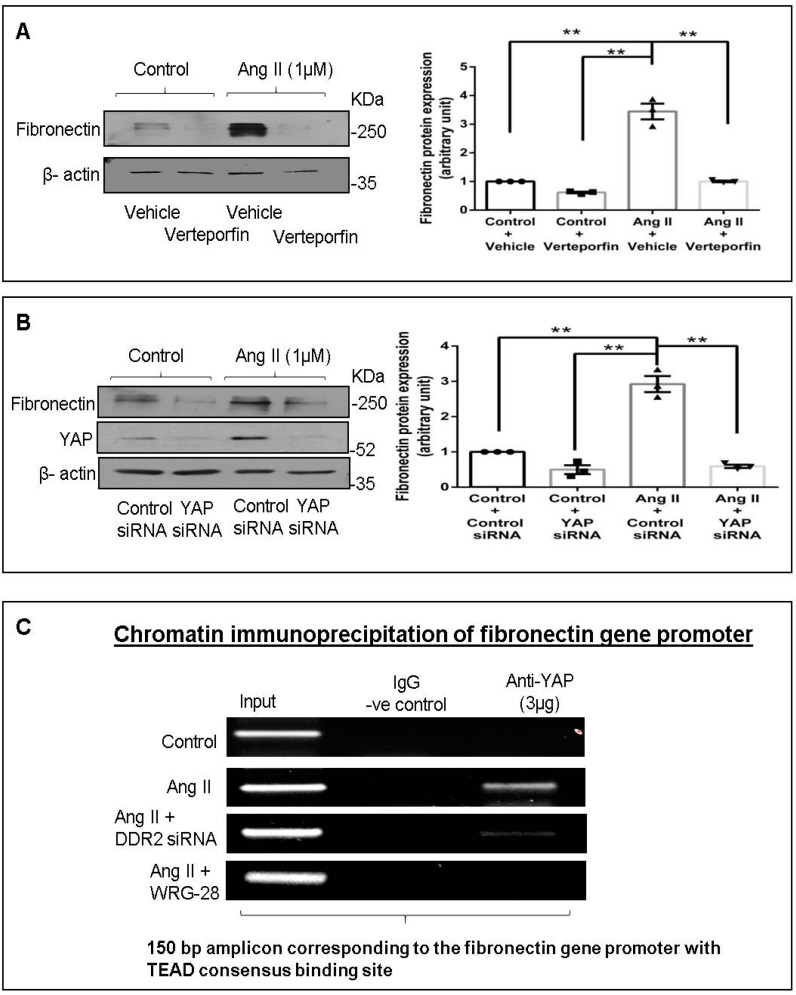
Since YAP was earlier reported to be activated downstream of DDR2 [6], and bioinformatics analysis of the fibronectin promoter region showed a consensus binding site for TEAD (a YAP-co-activated transcription factor), we checked whether YAP inhibition would have an effect on fibronectin protein expression. YAP inhibition using a chemical inhibitor (verteporfin) or siRNA significantly reduced Ang II-stimulated cellular fibronectin protein expression (Figure 2A,B). Furthermore, chromatin immunoprecipitation assay (ChIP) demonstrated YAP binding to the promoter region of fibronectin (containing the TEAD consensus sequence) in Ang II-treated cells, which was attenuated in DDR2-silenced (siRNA) or inhibited (WRG-28) cells (Figure 2C), confirming the transcriptional regulation of fibronectin by DDR2-activated YAP.
Figure 2.
Transcriptional regulation of fibronectin by DDR2-activated YAP transcription co-activator. (A) Cardiac fibroblasts were treated with verteporfin (10 µM) for 1 h prior to Ang II treatment. Cells were collected at 12 h post-Ang II treatment, and fibronectin protein levels were examined, with β-actin as loading control. ** p < 0.01 (comparisons as depicted in the Figure). (B) Cardiac fibroblasts were transiently transfected with YAP siRNA (10 pmol) or control (scrambled) siRNA prior to treatment with Ang II for 12 h. Fibronectin protein expression was examined, with β-actin as loading control. Validation of DDR2 silencing is also shown. ** p < 0.01 (comparisons as depicted in the Figure). Data are representative of three independent experiments, n = 3, Mean ± SEM. (C) Sub-confluent quiescent cultures of cardiac fibroblasts were transiently transfected with YAP siRNA (10 pmol) or control (scrambled) siRNA prior to treatment with Ang II (1 µM), and another set of cells was pre-treated with WRG-28 for 1 h. Cells were collected at 30 min post-Ang II treatment and chromatin was immunoprecipitated with anti-YAP antibody, followed by PCR amplification, and analyzed on a 2% agarose gel for presence of the 150 bp region of the fibronectin gene promoter containing the TEAD consensus binding site.
2.3. Functional Significance of Fibronectin Expression in Cardiac Fibroblasts
2.3.1. A Role for Fibronectin in Ang II-Dependent cIAP2 Expression and Apoptosis Resistance in Cardiac Fibroblasts Exposed to Oxidative Stress
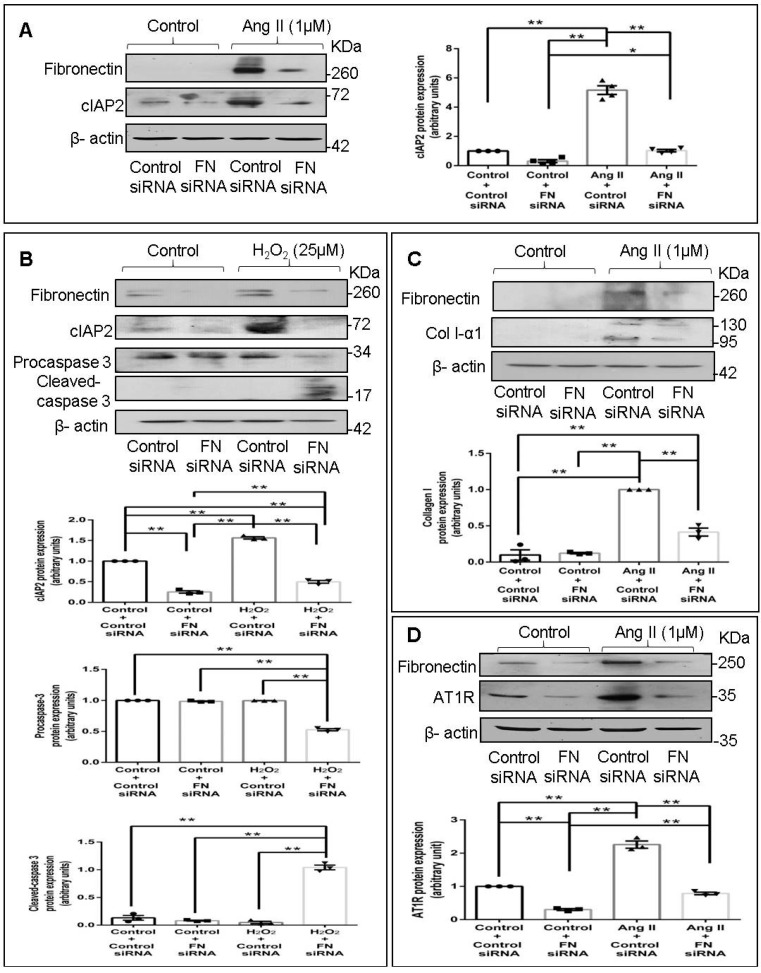
Fibronectin has been reported to enhance cell survival in various cell types [9,10,11,12]. Previous studies from our laboratory have demonstrated that Ang II enhances cIAP2 protein expression in cardiac fibroblasts that in turn protects these cells against ambient stress [7]. In the present study, the role of cellular fibronectin in the regulation of Ang II-dependent cIAP2 expression in cardiac fibroblasts was probed. Fibronectin knockdown using siRNA attenuated Ang II-stimulated cIAP2 protein expression (Figure 3A) and enhanced the apoptosis susceptibility of cardiac fibroblasts exposed to 25 µM H2O2, as indicated by decreased procaspase 3 and increased cleaved-caspase 3 levels (Figure 3B). These observations pointed to the role of cellular fibronectin in enhancing cardiac fibroblast survival under conditions of oxidative stress.
Figure 3.
Fibronectin mediates Ang II-stimulated protein expression of cIAP2, collagen type I, and AT1R in cardiac fibroblasts. (A) Cardiac fibroblasts were transiently transfected with fibronectin siRNA (20 pmol) or control (scrambled) siRNA prior to treatment with Ang II for 12 h. cIAP2 protein expression was examined, with β-actin as loading control. Validation of fibronectin silencing is also shown. * p < 0.05 and ** p < 0.01 (comparisons as depicted in the Figure). (B) Cardiac fibroblasts were transiently transfected with fibronectin siRNA (20 pmol) or control (scrambled) siRNA. Following treatment of cells with 25 µM H2O2 for 3 h, the cells were collected, and levels of procaspase 3 and cleaved-caspase 3 were determined, with β-actin as loading control. Validation of fibronectin silencing and associated changes in cIAP2 levels are also shown. ** p < 0.01 (comparisons as depicted in the figure). (C,D) Cardiac fibroblasts were transiently transfected with fibronectin siRNA (20 pmol) or control (scrambled) siRNA prior to treatment with Ang II for 12 h. (C) Collagen type 1 protein expression was examined with β-actin as loading control. Validation of fibronectin silencing is shown. ** p < 0.01 (comparisons as depicted in the Figure). (D) AT1R protein expression was examined, with β-actin as loading control. Validation of fibronectin silencing is shown. ** p < 0.01 (comparisons as depicted in the Figure). Data are representative of three independent experiments, n = 3. Mean ± SEM.
2.3.2. Fibronectin Mediates Ang II-Dependent Increases in Collagen Type I Expression
Fibronectin has been implicated in hepatic and pulmonary fibrosis [13,14,15,16], and global or fibroblast-specific ablation of fibronectin in adult mice post-injury resulted in significant cardio-protection with reduced hypertrophy and fibrosis [4,17]. Therefore, the possible role of cellular fibronectin in collagen type I expression in cardiac fibroblasts was probed next. Significant attenuation of Ang II-stimulated collagen type I protein expression (Figure 3C) upon RNA interference-mediated knockdown of fibronectin suggested a role for cellular fibronectin in the regulation of collagen expression in cardiac fibroblasts.
2.3.3. Fibronectin Mediates Ang II-Stimulated AT1 Receptor Expression in Cardiac Fibroblasts
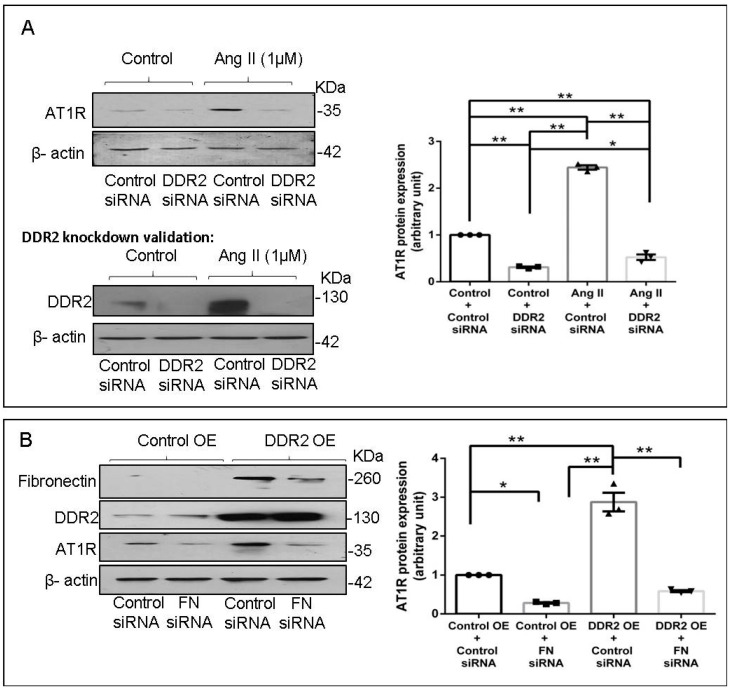
Previous studies from our laboratory have demonstrated that Ang II acts via the AT1 receptor (AT1R) to enhance cIAP2 and collagen type I protein expression in cardiac fibroblasts [7,18,19]. Therefore, we verified whether fibronectin plays a role in AT1R expression in Ang II-treated cardiac fibroblasts. Interestingly, fibronectin knockdown downregulated both basal and Ang II-stimulated AT1R protein expression (Figure 3D), clearly indicating the role of cellular fibronectin in AT1R expression in cardiac fibroblasts.
2.4. Fibronectin Regulates Ang II-Mediated AT1R Expression through Integrin-β1/Integrin-Linked Kinase Signaling Pathway
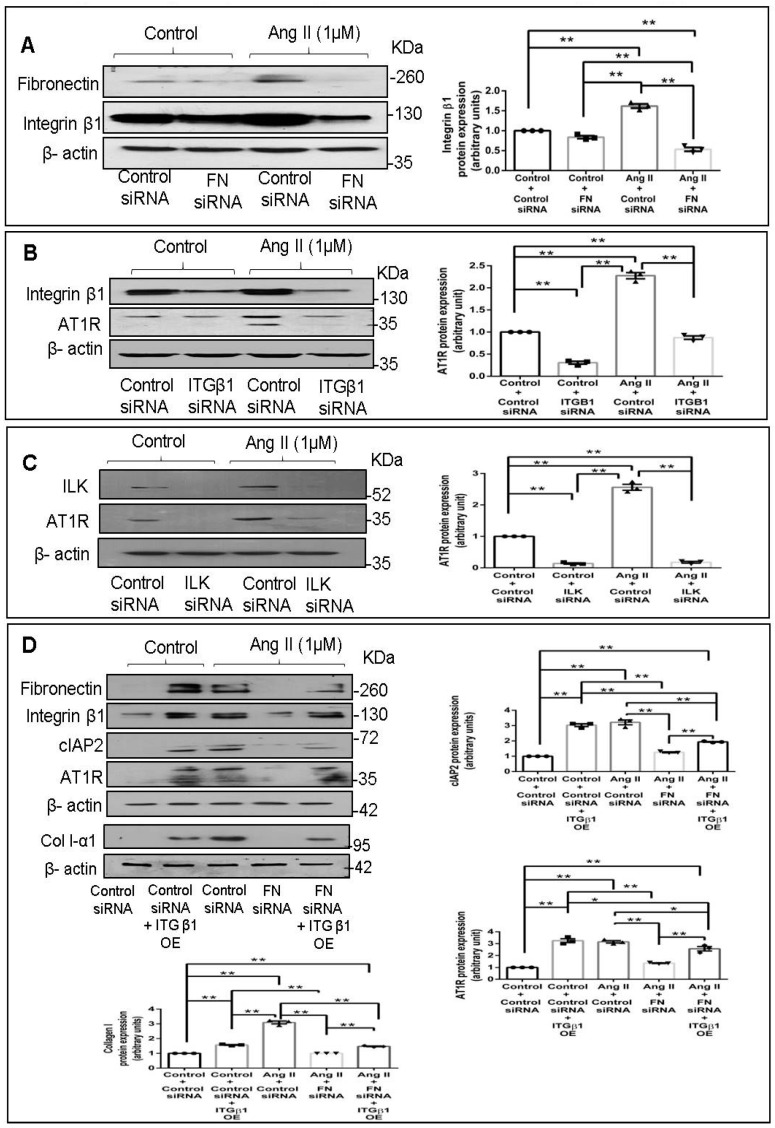
Fibronectin is reported to act via integrin α5β1 [20], and inhibition of the β1 subunit, the partially conserved catalytic subunit of integrins [21,22,23], rather than the α5 subunit, has a more pronounced inhibitory effect on the binding and assembly of exogenous fibronectin [20,24]. In the present study, we observed that fibronectin siRNA attenuates Integrin-β1 protein expression in Ang II-treated cardiac fibroblasts (Figure 4A), suggesting that cellular fibronectin has an obligate role in the regulation of its receptor. The effect of knockdown of Integrin-β1 and Integrin-linked kinase (ILK), a proximal effector of Integrin-β1 [25], on AT1R was probed next. Abrogation of Integrin-β1 signaling using Integrin-β1 or ILK siRNA led to attenuation of AT1R protein expression in Ang II-stimulated cardiac fibroblasts (Figure 4B,C). Notably, overexpression of Integrin-β1 in fibronectin-silenced cells partially restored the protein levels of cIAP2, collagen type I, and AT1R in Ang II-treated cells (Figure 4D), clearly demonstrating the involvement of fibronectin-dependent Integrin-β1 signaling in the regulation of cIAP2, collagen type I, and AT1R expression in cardiac fibroblasts.
Figure 4.
Fibronectin regulates Ang II-stimulated AT1R protein expression through Integrin β1/Integrin-linked kinase signaling pathway. (A) Cardiac fibroblasts were transiently transfected with fibronectin siRNA (20 pmol) or control (scrambled) siRNA prior to treatment with Ang II for 12 h. Integrin-β1 (ITGB1) protein expression was examined with β-actin as loading control. ** p < 0.01 (comparisons as depicted in the figure). (B) Cardiac fibroblasts were transiently transfected with ITGB1 siRNA (5 pmol) or control (scrambled) siRNA prior to treatment with Ang II for 12 h. AT1R protein expression was examined, with β-actin as loading control. Validation of Integrin-β1 silencing is also shown. ** p < 0.01 (comparisons as depicted in the Figure). (C) Cardiac fibroblasts were transiently transfected with ILK siRNA (5 pmol) or control (scrambled) siRNA prior to treatment with Ang II for 12 h. AT1R protein expression was examined, with β-actin as loading control. ** p < 0.01 (comparisons as depicted in the Figure). (D) Cardiac fibroblasts were co-transfected with fibronectin siRNA (10 pmol) and ITGB1 cDNA overexpression plasmid (2 µg). Following transfection, the cells were revived in M199 with 10% serum for 12 h. Post-revival, the cells were serum-deprived for 24 h prior to treatment with Ang II for 12 h. Cells were collected and protein levels of AT1R, cIAP2, and collagen type 1 were examined, with β-actin as loading control. * p < 0.05 and ** p < 0.01 (comparisons as depicted in the Figure). Validation of fibronectin knockdown and ITGB1 overexpression is also shown. Data are representative of three independent experiments, n = 3, Mean ± SEM.
2.5. DDR2 Knockdown Attenuates Ang II-Stimulated Expression of AT1R in Cardiac Fibroblasts
Since fibronectin expression is dependent on DDR2, we investigated the effect of DDR2 knockdown on Ang II-stimulated AT1R expression. siRNA-mediated DDR2 silencing reduced both basal and Ang II-stimulated AT1R protein expression (Figure 5A). However, DDR2 overexpression failed to restore AT1R in fibronectin-silenced cells, showing that DDR2 regulates AT1R via cellular fibronectin (Figure 5B).
Figure 5.
DDR2 regulates Ang II-stimulated protein expression of AT1R in cardiac fibroblasts. (A) Cardiac fibroblasts were transiently transfected with DDR2 siRNA (5 pmol) or control (scrambled) siRNA prior to treatment with Ang II for 12 h. AT1R protein expression was examined, with β-actin as loading control. Validation of DDR2 silencing is also shown. * p < 0.05, ** p < 0.01 (comparisons as depicted in the Figure). (B) Cardiac fibroblasts were co-transfected with fibronectin siRNA (10 pmol) and DDR2 cDNA overexpression plasmid (2 µg). Following transfection, the cells were revived in M199 with 10% serum for 12 h. Post-revival, the cells were serum-deprived for 24 h prior to treatment with Ang II for 12 h. Cells were collected, and AT1R protein levels were examined, with β-actin as loading control. * p < 0.05 and ** p < 0.01 (comparisons as depicted in the Figure). Validation of fibronectin knockdown and DDR2 overexpression is also shown. Data are representative of three independent experiments, n = 3, Mean ± SEM.
2.6. AT1R Levels in DDR2-Null Mouse Hearts
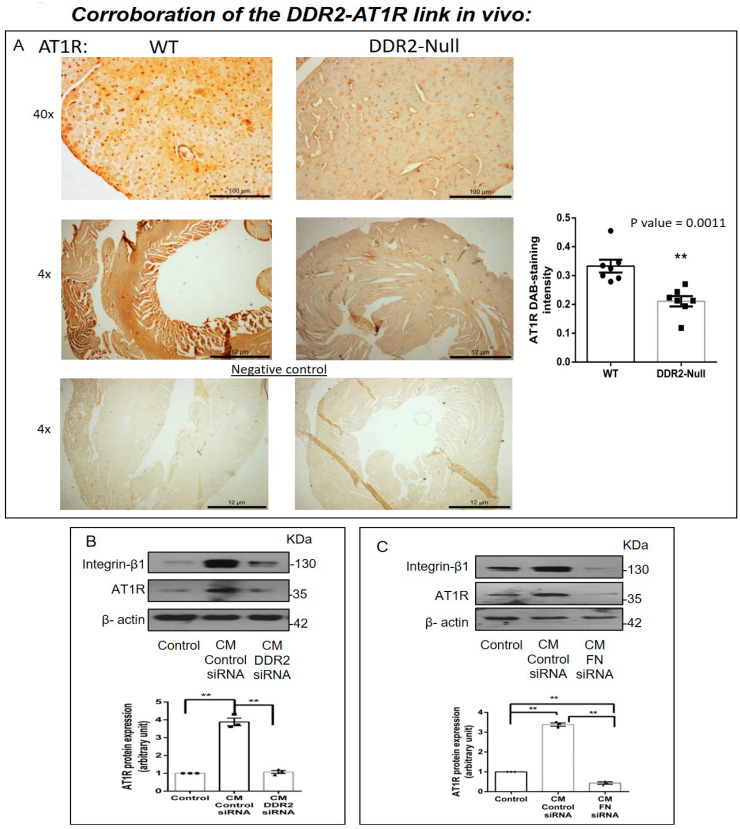
In view of the obligate regulatory role of DDR2 in Ang II-stimulated cardiac fibroblasts, we examined AT1R levels in DDR2-knockout mice carrying a germline deletion of DDR2. Knockin of a MerCreMer gene targeting exon 3 of the DDR2 allele was used for germline deletion of DDR2 in mice, and we had previously described the generation and validation of these mice, along with initial observations [26]. In the present study, immunohistochemical analysis of cardiac tissue sections using an anti-AT1R antibody demonstrated a small but significant reduction in myocardial AT1R expression in DDR2-null mice (Figure 6A). However, the global reduction in AT1R staining intensity suggested a likely decrease in AT1R in myocytes in addition to fibroblasts, which are reportedly fewer in mouse heart [27].
Figure 6.
Corroboration of the DDR2-AT1R link in vivo. (A) Representative image showing 3,3′-diaminobenzidine (DAB) staining of AT1R protein in myocardial tissue sections of 10-week-old WT and DDR2-null mice. ** p < 0.01 versus WT (n = 7). (B) Sub-confluent quiescent cultures of H9c2 cells were treated for 24 h with conditioned medium (CM) derived from control siRNA-treated cardiac fibroblasts or DDR2-silenced cardiac fibroblasts. Quiescent cultures of H9c2 in M199 without serum were used as the control for basal AT1R protein expression in these cells. AT1R protein expression in H9c2 cells was examined by Western blot analysis and normalized to β-actin. ** p < 0.01 (comparisons as depicted in the Figure), (n = 3, for the conditioned medium experiments, cardiac fibroblasts were from three isolations from three rats). (C) Sub-confluent quiescent cultures of H9c2 cells were treated for 24 h with conditioned medium (CM) derived from control siRNA-treated cardiac fibroblasts or fibronectin (FN)-silenced cardiac fibroblasts. Quiescent cultures of H9c2 in M199 without serum were used as the control for basal AT1R protein expression in H9c2 cells. AT1R protein expression was examined by Western blot analysis and normalized to β-actin. ** p < 0.01 (comparisons as depicted in the Figure), (n = 3, for the conditioned medium experiments, cardiac fibroblasts were from three isolations from three rats).
2.7. Cardiac Fibroblasts Influence AT1R Expression in H9c2 Cells via DDR2-Dependent Paracrine Mechanisms
Therefore, preliminary experiments were performed to explore the influence of fibroblast-specific DDR2 on myocyte AT1R expression. To this end, the effect of rat cardiac fibroblast-conditioned medium on AT1R expression in the rat ventricular H9c2 cell line (cardiomyoblasts) was analyzed. The exposure of H9c2 cells to fibroblast-conditioned medium for 24 h enhanced AT1R protein expression, but notably, conditioned medium from DDR2-silenced (Figure 6B) or fibronectin-silenced (Figure 6C) cardiac fibroblasts did not enhance AT1R protein expression in H9c2 cells, pointing to a DDR2/fibronectin-dependent paracrine effect of cardiac fibroblasts on AT1 expression in myocytes.
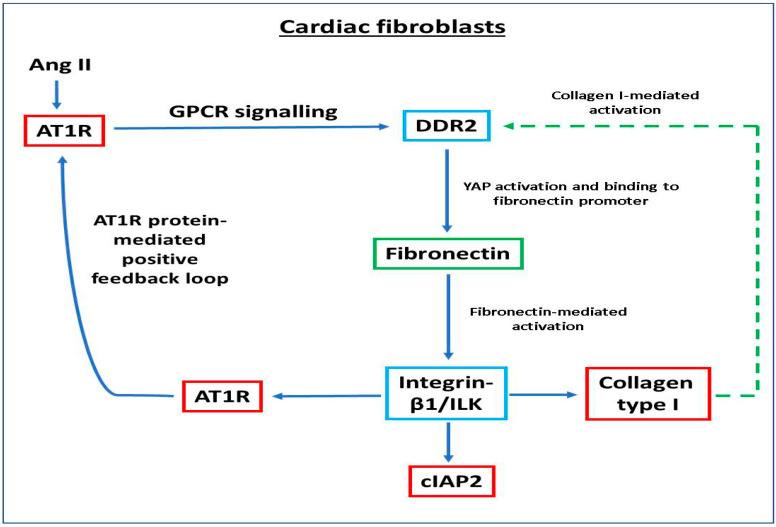
A schematic representation of the molecular events that link ECM and Ang II signaling pathways in cardiac fibroblasts is provided in Figure 7.
Figure 7.
Schematic representation of the molecular events that link ECM and Ang II signaling pathways in cardiac fibroblasts.
3. Discussion
Despite the centrality of interstitial fibrosis in heart disease and in the aging myocardium, and increasing appreciation that therapies directed at cardiac fibrosis may retard heart failure, there are limited therapeutic options to effectively target cardiac fibroblasts and attenuate cardiac fibrosis. Blocking the actions of Ang II either with AT1R blockers or ACE inhibitors is an accepted treatment strategy to reduce adverse cardiac remodeling following myocardial infarction, but it may entail unfavorable effects such as the paradoxical activation of a negative feedback loop within the Renin–Angiotensin cascade, leading to systemic elevation of Ang II [28,29,30]. Identification of alternate therapeutic targets would hinge around a sound understanding of the molecular basis of cardiac fibrogenesis.
In this regard, the critical role of ECM in myocardial pathophysiology, particularly in the pathogenesis of heart failure, has attracted increasing attention in recent years. ECM proteins significantly impact cellular responses, cell migration, differentiation, proliferation and survival through their ability to bind to multiple interacting partners, including other ECM proteins, and the transmission of growth factor signals [31,32]. Alterations in ECM are reported to play an important role in modulating fibroblast phenotype and function [33]. Major ECM proteins, collagen and fibronectin, and their receptors, DDR2 and integrins, participate in signaling events and determine cell fate [34]. Our previous studies had shown that collagen type I-activated DDR2 is a ‘master switch’ in cardiac fibroblasts with an obligate role in phenotypic transformation, proliferation, collagen expression, and apoptosis resistance in cardiac fibroblasts [2,6,7].
Fibronectin is a high molecular weight glycoprotein constituent of the ECM that interacts with several matrix and cell surface proteins to regulate cell adhesion, migration, metastasis, proliferation, and differentiation, as well as embryogenesis, wound healing, and blood coagulation [3]. It has also been reported that tissue fibrosis is associated with the upregulation of fibronectin [13,14,15,16]. In the present study, Ang II was found to enhance cellular fibronectin expression (Figure 1A). Although Ang II per se has not been shown to enhance fibronectin, our observation is consistent with scleraxis-mediated enhancement of fibronectin gene expression in cardiac fibroblasts by TGF-β1 [35], since it is known that Ang II exerts some of its effects via TGF-β signaling in these cells [36].
We have shown previously that Ang II acts via the AT1 receptor and the GPCR pathway to enhance DDR2 expression that in turn plays an indispensable role in collagen type I gene expression [2]. The findings reported here indicate that DDR2 mediates Ang II-stimulated expression of not only collagen type I but also of fibronectin (Figure 1B,C), which underscores its critical role in ECM homeostasis and ECM signaling. Furthermore, our data show the transcriptional upregulation of fibronectin by DDR2-dependent activation of YAP (Figure 2), which is a mechano-sensitive transcriptional co-activator [37]. The observation is consistent with previous studies that implicate the transcriptional activation of YAP in pro-fibrotic gene expression in fibroblasts [37]. Moreover, a previous study from our laboratory implicated Ang II-dependent activation of YAP in collagen type I expression [6], and the inhibition of YAP has been reported to attenuate cardiac fibrosis [38].
It was of obvious interest to explore the pathophysiological significance of augmented fibronectin expression in cardiac fibroblasts. Cardiac fibroblasts are relatively resistant to several death signals that prevail in the injured myocardium, which enables these cells to play a central role in tissue repair following myocyte loss but also facilitates their persistence in the infarct scar, resulting in disproportionate stromal growth and pump dysfunction [1,39]. While a role for constitutively expressed Bcl-2 in the resistance of cardiac fibroblasts to a variety of pro-apoptotic stimuli was reported by Mayorga et al. [40], our recent studies showed that anti-apoptotic cIAP2, induced by Ang II via a DDR2- and Serum Response Factor-mediated mechanism, protects cardiac fibroblasts from ambient stress [7]. The present study extends these earlier observations and points to a role for cellular fibronectin, downstream of DDR2, in Ang II-stimulated cIAP2 expression (Figure 3A) and protection of the cells from oxidative damage (Figure 3B). In a recent study by Valiente-Alandi et al., no significant change in fibroblast survival was observed upon inhibition of fibronectin polymerization under basal conditions [4]. However, it is possible that fibronectin is important for survival under stress conditions.
Collagen type I is the most abundant ECM protein in the heart and is a major constituent of the scar tissue formed following injury [41]. Excessive collagen deposition leads to diminished ventricular compliance that contributes to the initiation and progression of heart failure. The regulation of collagen expression has understandably been a subject of intense investigation for a long time. The findings presented here show that apart from a role in cIAP2 expression, cellular fibronectin has a role in collagen type I expression in Ang II-treated cells (Figure 3C), which is consistent with what was observed by Valiente-Alandi et al. in an ischemia–reperfusion injury model [4].
Probing the regulatory role of fibronectin further, we found that its knockdown attenuates the expression of not only cIAP2 and collagen type I but also of Integrin-β1 (Figure 4A), which is known to be importantly involved in transducing ECM signaling [42]. Thus, apart from acting as a ligand to trigger Integrin-β1 signaling, cellular fibronectin also regulates Integrin-β1 gene expression in Ang II-stimulated cardiac fibroblasts. Further, plasmid-mediated overexpression of Integrin-β1 in cells following fibronectin knockdown restores, at least in part, the expression of cIAP2 and collagen type I (Figure 4D). Together, the data indicate that cellular fibronectin is required for the expression of Integrin-β1 that in turn regulates cIAP2 and collagen type I in response to Ang II. The observed effect of Integrin-β1 overexpression in fibronectin-silenced cells suggests that fibronectin may not be indispensable as a ligand for Integrin-β1 signaling, and that other ligands such as collagen may also trigger Integrin-β1 signaling. Our earlier study had shown that a cross-talk between the two major collagen receptors, DDR2 and Integrin-β1, is a critical determinant of α-SMA-dependent collagen expression in Ang II-stimulated cardiac fibroblasts [6]. Moreover, cell survival through ECM–integrin interactions has long been recognized [43]. In light of the data presented here, it appears that fibronectin acts downstream of DDR2 to regulate Integrin-β1 signaling-dependent gene expression in cardiac fibroblasts. Our findings on the role of Integrin-β1 as an ‘effector molecule’ in a complex regulatory cascade are consistent with an earlier report that ECM-mediated mechanical stress activates TGF-β and triggers the expression of Integrins and the pro-fibrotic gene program in cardiac fibroblasts [44].
We also examined the mechanistic basis of fibronectin-mediated apoptosis resistance and collagen expression in Ang II-stimulated cardiac fibroblasts, downstream of DDR2. It is known that Ang II, an effector molecule of the Renin–Angiotensin system, acts via the AT1 receptor to exert its potent pro-fibrotic effects on cardiac fibroblasts, which makes Ang II–AT1 signaling a major regulator of cardiac fibroblast response to myocardial injury and hence a determinant of structural and functional alterations in the injured heart [45]. We had previously uncovered a complex mechanism of regulation of AT1R involving the redox-sensitive NF-κB and AP-1 transcription factors that are activated by the coordinated action of ERK1/2 MAPK, p38 MAPK, and JNK in cardiac fibroblasts exposed to oxidative stress [19]. Interestingly, Ang II produced endogenously in response to oxidative response was found to be responsible for increased AT1R expression [19]. Here, we present the novel finding that cellular fibronectin has an obligate role in the regulation of AT1R in Ang II-stimulated cardiac fibroblasts (Figure 3D). Notably, while knockdown of Integrin-β1 or ILK attenuated Ang II-stimulated AT1R expression, the overexpression of Integrin-β1 in fibronectin-silenced cells partially restored AT1R expression (Figure 4D), showing that Integrin-β1 signaling underlies, at least in part, the regulatory role of fibronectin in enhanced AT1R expression in Ang II-stimulated cardiac fibroblasts. Consistent with the fact that both fibronectin and Integrin-β1 are under the regulatory control of DDR2, Ang II-stimulated AT1R expression was found to be attenuated in DDR2-silenced cells (Figure 5A). However, DDR2 overexpression did not restore AT1R in fibronectin-silenced cells, showing that DDR2 regulates AT1R via fibronectin (Figure 5B).
The relationship between DDR2 and AT1R was further evident from the reduced AT1R levels in myocardial tissue from DDR2-null mice (Figure 6). Admittedly, the observed difference was modest, although significant, but it is pertinent to note that these were basal levels and hence noteworthy. Nonetheless, the regulatory role of DDR2 in AT1R expression in Ang II-stimulated cells could be far more pronounced, as borne out by the findings presented here. Our attempts to determine fibronectin levels in fixed myocardial tissue were not successful, which was possibly due to antigen masking, and it is also likely that only the injured myocardium may provide robust signals. However, we had previously reported a significant reduction in collagen [10] and Integrin-β1 [6] in cardiac tissue from DDR2-null mice. Additionally, data from the conditioned medium experiments presented here show that a loss of DDR2 or fibronectin in cardiac fibroblasts could repress paracrine factors regulating AT1R expression in myocytes. Considered in tandem, these data demonstrate the importance of the DDR2–fibronectin–Integrin-β1 axis in AT1R expression, pointing to a convergence of ECM signaling and Ang II signaling. These data unravel a novel mechanism by which ECM can impact cardiac fibroblast function via Ang II signaling.
Our findings indicate the existence of a complex mechanism of regulation of cardiac fibroblast function involving two major ECM proteins, collagen type I and cellular fibronectin, and their respective receptors, DDR2 and Integrin-β1. Future investigations should evaluate the possible role of other factors such as periostin within this complex mechanistic framework of molecular events under the regulatory control of DDR2 in cardiac fibroblasts. To uncover mechanisms relevant to cardiac fibrogenesis, it is also important to probe these DDR2-dependent signaling pathways in a setting of heart disease and in the senescent myocardium.
Lastly, as noted earlier, targeting ECM proteins could offer alternate therapeutic strategies to prevent or minimize adverse cardiac remodeling in pathological states. In this regard, it is significant that fibronectin inhibition is reported to exert cardioprotective effects through a significant reduction in cardiac fibrosis and adverse fibrotic remodeling in an ischemia/reperfusion injury model [4]. Viewed in tandem with these earlier observations in an in vivo model of myocardial injury, our findings support the postulation that increased susceptibility of cardiac fibroblasts to apoptosis and prevention of excessive collagen deposition may, in part, explain the cardioprotective effect of fibronectin inhibition observed in the animal model. Based on their findings, Valiente-Alandi et al. suggest that targeting fibronectin polymerization may be a new therapeutic strategy for treating cardiac fibrosis and heart failure. However, given the ubiquitous distribution and critical role of fibronectin and Integrin-β1 in the heart, targeting them may entail global consequences, compromising cardiac integrity and function. On the contrary, since fibronectin and Integrin-β1 are under the regulatory control of DDR2, it is logical to postulate that the predominant localization and regulatory role of DDR2 in cardiac fibroblasts mark it as a cardiac fibroblast-selective drug target to control cardiac fibrosis.
4. Materials and Methods
Angiotensin II and M199 were obtained from Sigma-Aldrich (St. Louis, MO, USA). Lipofectamine 2000 was from Invitrogen (Carlsbad, CA, USA). The Low Cell# ChIP kit protein A × 48 was from Diagenode (Denville, NJ, USA), and the Chemiluminescence Western blot detection reagent was from Thermo Fisher Scientific (Waltham, MA, USA). DDR2, Integrin-β1, ILK, and control siRNAs were from Ambion (Foster City, CA, USA). Fibronectin siRNA was custom-synthesized by Eurogentec (Liège, Belgium). The rat DDR2/CD167b Gene ORF cDNA clone expression plasmid was obtained from Sino Biologicals (Beijing, China). Pcax Itgb1-FLAG was a gift from Dennis Selkoe and Tracy Young-Pearse (Addgene plasmid 30153) [46]. Opti-MEM and fetal calf serum (FCS) were from GIBCO (Waltham, MA, USA). All cell culture ware was purchased from BD Falcon (Corning, NY, USA). Primary antibodies against DDR2 (Cat No. 12133S), Caspase 3 (Cat No. 9662), and cleaved-Caspase 3 (Cat No. 9661) were obtained from Cell Signaling Technology (Danvers, MA, USA). Primary antibodies against cIAP2 (Cat No. sc7944), Fibronectin (Cat No. sc9068), AT1R (Cat No. sc1173), and Collagen I (Cat No. sc293182) were from Santa Cruz Biotechnology (Dallas, TX, USA). Loading control β-Actin (Cat No. A2228) antibody was obtained from Sigma-Aldrich. ILK antibody was obtained from Elabscience (Houston, TX, USA). All antibodies were used after dilution (1:1000). XBT X-ray Film was from Carestream (Rochester, NY, USA). The study on rats was approved by the Institutional Animal Ethics Committees of Sree Chitra Tirunal Institute for Medical Sciences and Technology (B Form No: SCT/IAEC-233/AUGUST/2017/94 and SCT/IAEC-268/FEBRUARY/2018/95), and the study on mice was approved by the Institutional Animal Care and Use Committee of the University of California, San Diego (protocol S05478, current approval: 2/19/2020–2/19/2023).
4.1. Isolation of Cardiac Fibroblasts
Cardiac fibroblasts were isolated from young adult male Sprague–Dawley rats (2–3 months old) as described earlier [47]. Sub-confluent cultures of cardiac fibroblasts from passage 2 or 3 were used for the experiments. Cells were serum-deprived for 24 h prior to treatment with 1 μM Ang II. Cells were pre-incubated with 10 μM verteporfin for 1 h before the addition of 1 μM Ang II in the appropriate group. The cells were collected and processed further to analyze the expression of various genes and proteins.
4.2. Western Blot Analysis
Sub-confluent cultures of cardiac fibroblasts in serum-free M199 were treated with Ang II (1 μM), and relative protein abundance was determined by Western blot analysis following standard protocols, with β-actin as the loading control. Enhanced chemiluminescence reagent was used to detect the proteins with X-ray film.
4.3. RNA Interference and Overexpression
Cardiac fibroblasts at passage 3 were seeded on 60 mm dishes at equal density. After 24 h, the cells were incubated in Opti-MEM for 5–6 h with Ambion pre-designed Silencer-Select siRNA, custom-designed siRNA from Eurogentec, or scrambled siRNA (control siRNA) at the given concentrations (10 pmoles for both DDR2 and Integrin-β1, 20 pmoles for Fibronectin (mix of siRNA 1 and 2) and Lipofectamine 2000).
The constitutive expression of DDR2 and Integrin-β1 was achieved under the control of a CMV promoter. Both plasmids were verified by restriction mapping. For overexpression, the plasmid vector for DDR2 (2 μg/μL) was transfected using Lipofectamine 2000. Following a post-transfection recovery phase in M199 with 10% FCS for 12 h, the cells were serum-deprived for 24 h and then treated with Ang II (1 μM) for the indicated durations. Cell lysates were prepared in Laemmli sample buffer (with β-mercaptoethanol), denatured, and used for Western blot analysis.
4.4. Chromatin Immunoprecipitation (ChIP) Assay
The ChIP assay was performed with the Low Cell Number ChIP kit, according to the manufacturer’s protocol. Briefly, after treatment of cardiac fibroblasts with 1 μM Ang II for 30 min, the cells were cross-linked with 1% formaldehyde, lysed, and sonicated with Bioruptor® sonication device (Diagenode, Liege, Belgium) to generate ≈600 bp DNA fragments. The lysates were incubated with anti-YAP antibody overnight at 4 °C with rotation. Immune complexes were precipitated with protein A-coated magnetic beads. After digestion with proteinase K to remove the DNA-protein cross-links from the immune complexes, the DNA was isolated and subjected to PCR using primers for the specific promoter regions. In samples immunoprecipitated with the YAP antibody, the fibronectin promoter region was amplified using FP-5′-AAAACCGTTTTGTCAAGGGATG-3′ and RP-5′-TACCAGTTTCTTACAAGCGGTG-3′. DNA isolated from an aliquot of the total sheared chromatin was used as loading control for PCR (input control). ChIP with a non-specific antibody (normal rabbit IgG) served as negative control. The PCR products were subjected to electrophoresis on a 2% agarose gel.
4.5. In Vivo Study and Histology
The generation of DDR2-null mice and genotyping were described in our previous study [26]. Mouse tissue sections were prepared as described previously [6]. Briefly, hearts were collected from age-matched (10 weeks) WT and DDR2-null mice, fixed in 4% buffered paraformaldehyde for 2 days, embedded in paraffin, cross-sectioned, and mounted onto slides. AT1R levels in these sections were analyzed by 3,3′-diaminobenzidine staining and quantified using Fiji-Image J software [48].
4.6. Conditioned Medium Experiments
Rat ventricular H9c2 cells were used as an in vitro model for myocytes. The effect of cardiac fibroblast-conditioned medium on H9c2 cells was studied. Cardiac fibroblasts isolated from young adult (2–3 months) male Sprague–Dawley rats were transfected with scrambled siRNA, DDR2 siRNA, or fibronectin siRNA. Six hours post-transfection, the cells were revived in M199 with 10% serum for 12 h. Subsequently, the cells were transferred to M199 without serum for 24 h to generate fibroblast-conditioned medium. H9c2 cells were exposed to the fibroblast-derived conditioned medium from each of the transfected groups, collected after 24 h of incubation. H9c2 cells exposed to fresh M199 medium without serum served as controls for basal expression. Lysates of H9c2 cells were collected at 24 h following exposure to the conditioned medium, and AT1R protein expression was analyzed and quantified after normalization to β-actin expression.
4.7. Statistical Analysis
Data are expressed as Mean ± SEM (Standard Error of Mean). Statistical analysis was performed using Student’s t-test (unpaired, 2-tailed) for comparisons involving 2 groups. For comparisons involving more than 2 groups, the data were analyzed by one-way ANOVA (with one variable) or two-way ANOVA (with two variables). p ≤ 0.05 was considered significant. The in vitro data presented are representative of 3 or 4 independent experiments and the in vivo data are representative of 7 age-matched animals from each group.
Acknowledgments
This work was supported by a Research Grant (BT/PR23486/BRB/10/1589/2017) to S.K. from the Department of Biotechnology, Government of India, and Research Fellowships from SCTIMST, Trivandrum, to A.S.T. and the Department of Biotechnology, Government of India, to H.V. The source of funding for M.W. and E.G.L. was the Intramural Research Program of the National Institute on Aging, National Institutes of Health. S.K., A.S.T. and H.V. thank Ajay Kumar R of the Rajiv Gandhi Centre for Biotechnology, Trivandrum, for providing access to the Bioruptor Facility and acknowledge the facilities provided by SCTIMST, Trivandrum.
Author Contributions
A.S.T., S.K., E.G.L., H.V. and M.W. conceived the study; A.S.T. and S.K. designed the study; A.S.T., H.V. and M.G.U. performed the experiments; R.T.C. generated the DDR2-null mice and prepared the heart tissue; A.S.T., H.V. and S.K. analyzed the data; A.S.T., H.V. and S.K. interpreted the results of the experiments; A.S.T. prepared the figures; A.S.T. and S.K. drafted the manuscript; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Research Grant (BT/PR23486/BRB/10/1589/2017) to SK from the Department of Biotechnology, Government of India, and Research Fellowships from SCTIMST, Trivandrum, to AST and the Department of Biotechnology, Government of India, to HV. The source of funding for MW and EGL was the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Institutional Review Board Statement
The study on rats was approved by the Institutional Animal Ethics Committees of Sree Chitra Tirunal Institute for Medical Sciences and Technology (B form No: SCT/IAEC-233/AUGUST/2017/94 and SCT/IAEC-268/FEBRUARY/2018/95), and the study on mice was approved by the Institutional Animal Care and Use Committee of the University of California, San Diego (protocol S05478, current approval: 2/19/2020–2/19/2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Humeres C., Frangogiannis N.G. Fibroblasts in the Infarcted, Remodeling, and Failing Heart. JACC Basic Transl. Sci. 2019;4:449–467. doi: 10.1016/j.jacbts.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George M., Vijayakumar A., Dhanesh S.B., James J., Shivakumar K. Molecular Basis and Functional Significance of Angiotensin II-Induced Increase in Discoidin Domain Receptor 2 Gene Expression in Cardiac Fibroblasts. J. Mol. Cell. Cardiol. 2016;90:59–69. doi: 10.1016/j.yjmcc.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 3.To W.S., Midwood K.S. Plasma and Cellular Fibronectin: Distinct and Independent Functions during Tissue Repair. Fibrogenesis Tissue Repair. 2011;4:21. doi: 10.1186/1755-1536-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valiente-Alandi I., Potter S.J., Salvador A.M., Schafer A.E., Schips T., Carrillo-Salinas F., Gibson A.M., Nieman M.L., Perkins C., Sargent M.A., et al. Inhibiting Fibronectin Attenuates Fibrosis and Improves Cardiac Function in a Model of Heart Failure. Circulation. 2018;138:1236–1252. doi: 10.1161/CIRCULATIONAHA.118.034609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sopel M.J., Rosin N.L., Lee T.D.G., Légaré J.-F. Myocardial Fibrosis in Response to Angiotensin II Is Preceded by the Recruitment of Mesenchymal Progenitor Cells. Lab. Investig. 2011;91:565–578. doi: 10.1038/labinvest.2010.190. [DOI] [PubMed] [Google Scholar]
- 6.Harikrishnan V., Titus A.S., Cowling R.T., Kailasam S. Collagen Receptor Cross-Talk Determines α-Smooth Muscle Actin-Dependent Collagen Gene Expression in Angiotensin II-Stimulated Cardiac Fibroblasts. J. Biol. Chem. 2019;294:19723–19739. doi: 10.1074/jbc.RA119.009744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Titus A.S., Harikrishnan V., Kailasam S. Coordinated Regulation of Cell Survival and Cell Cycle Pathways by DDR2-Dependent SRF Transcription Factor in Cardiac Fibroblasts. Am. J. Physiol.-Heart Circ. Physiol. 2020;318:H1538–H1558. doi: 10.1152/ajpheart.00740.2019. [DOI] [PubMed] [Google Scholar]
- 8.Grither W.R., Longmore G.D. Inhibition of Tumor–Microenvironment Interaction and Tumor Invasion by Small-Molecule Allosteric Inhibitor of DDR2 Extracellular Domain. Proc. Natl. Acad. Sci. USA. 2018;115:E7786–E7794. doi: 10.1073/pnas.1805020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z., Vuori K., Reed J.C., Ruoslahti E. The Alpha 5 Beta 1 Integrin Supports Survival of Cells on Fibronectin and Up-Regulates Bcl-2 Expression. Proc. Natl. Acad. Sci. USA. 1995;92:6161. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Globus R.K., Doty S.B., Lull J.C., Holmuhamedov E., Humphries M.J., Damsky C.H. Fibronectin Is a Survival Factor for Differentiated Osteoblasts. J. Cell Sci. 1998;111:1385. doi: 10.1242/jcs.111.10.1385. [DOI] [PubMed] [Google Scholar]
- 11.Anwar A.R., Moqbel R., Walsh G.M., Kay A.B., Wardlaw A.J. Adhesion to Fibronectin Prolongs Eosinophil Survival. J. Exp. Med. 1993;177:839–843. doi: 10.1084/jem.177.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin F., Ren X.-D., Pan Z., Macri L., Zong W.-X., Tonnesen M.G., Rafailovich M., Bar-Sagi D., Clark R.A.F. Fibronectin Growth Factor-Binding Domains Are Required for Fibroblast Survival. J. Investig. Dermatol. 2011;131:84–98. doi: 10.1038/jid.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altrock E., Sens C., Wuerfel C., Vasel M., Kawelke N., Dooley S., Sottile J., Nakchbandi I.A. Inhibition of Fibronectin Deposition Improves Experimental Liver Fibrosis. J. Hepatol. 2015;62:625–633. doi: 10.1016/j.jhep.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Liu X., Liu R., Hou F., Cui L., Li C.-Y., Chi C., Yi E., Wen Y., Yin C. Fibronectin Expression Is Critical for Liver Fibrogenesis in Vivo and in Vitro. Mol. Med. Rep. 2016;14:3669–3675. doi: 10.3892/mmr.2016.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernnas J., Nettelbladt O., Bjermer L., Sarnstrand B., Malmstrom A., Hallgren R. Alveolar Accumulation of Fibronectin and Hyaluronan Precedes Bleomycin-Induced Pulmonary Fibrosis in the Rat. Eur. Respir. J. 1992;5:404. [PubMed] [Google Scholar]
- 16.Muro A.F., Moretti F.A., Moore B.B., Yan M., Atrasz R.G., Wilke C.A., Flaherty K.R., Martinez F.J., Tsui J.L., Sheppard D., et al. An Essential Role for Fibronectin Extra Type III Domain A in Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2008;177:638–645. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konstandin M.H., Völkers M., Collins B., Quijada P., Quintana M., Torre A., Ormachea L., Din S., Gude N., Toko H., et al. Fibronectin Contributes to Pathological Cardiac Hypertrophy but Not Physiological Growth. Basic Res. Cardiol. 2013;108:375. doi: 10.1007/s00395-013-0375-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philip L., Shivakumar K. CIAP-2 Protects Cardiac Fibroblasts from Oxidative Damage: An Obligate Regulatory Role for ERK1/2 MAPK and NF-ΚB. J. Mol. Cell. Cardiol. 2013;62:217–226. doi: 10.1016/j.yjmcc.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Anupama V., George M., Dhanesh S.B., Chandran A., James J., Shivakumar K. Molecular Mechanisms in H2O2-Induced Increase in AT1 Receptor Gene Expression in Cardiac Fibroblasts: A Role for Endogenously Generated Angiotensin II. J. Mol. Cell. Cardiol. 2016;97:295–305. doi: 10.1016/j.yjmcc.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Fogerty F.J., Akiyama S.K., Yamada K.M., Mosher D.F. Inhibition of Binding of Fibronectin to Matrix Assembly Sites by Anti-Integrin (Alpha 5 Beta 1) Antibodies. J. Cell Biol. 1990;111:699–708. doi: 10.1083/jcb.111.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balzac F., Belkin A.M., Koteliansky V.E., Balabanov Y.V., Altruda F., Silengo L., Tarone G. Expression and Functional Analysis of a Cytoplasmic Domain Variant of the Beta 1 Integrin Subunit. J. Cell Biol. 1993;121:171–178. doi: 10.1083/jcb.121.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reszka A.A., Hayashi Y., Horwitz A.F. Identification of Amino Acid Sequences in the Integrin Beta 1 Cytoplasmic Domain Implicated in Cytoskeletal Association. J. Cell Biol. 1992;117:1321–1330. doi: 10.1083/jcb.117.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sastry S.K., Horwitz A.F. Integrin Cytoplasmic Domains: Mediators of Cytoskeletal Linkages and Extra- and Intracellular Initiated Transmembrane Signaling. Curr. Opin. Cell Biol. 1993;5:819–831. doi: 10.1016/0955-0674(93)90031-K. [DOI] [PubMed] [Google Scholar]
- 24.Puzon-McLaughlin W., Yednock T.A., Takada Y. Regulation of Conformation and Ligand Binding Function of Integrin A5β1 by the Β1 Cytoplasmic Domain*. J. Biol. Chem. 1996;271:16580–16585. doi: 10.1074/jbc.271.28.16580. [DOI] [PubMed] [Google Scholar]
- 25.Dedhar S., Williams B., Hannigan G. Integrin-Linked Kinase (ILK): A Regulator of Integrin and Growth-Factor Signalling. Trends Cell Biol. 1999;9:319–323. doi: 10.1016/S0962-8924(99)01612-8. [DOI] [PubMed] [Google Scholar]
- 26.Cowling R.T., Yeo S.J., Kim I.J., Park J.I., Gu Y., Dalton N.D., Peterson K.L., Greenberg B.H. Discoidin Domain Receptor 2 Germline Gene Deletion Leads to Altered Heart Structure and Function in the Mouse. Am. J. Physiol. Heart Circ. Physiol. 2014;307:H773–H781. doi: 10.1152/ajpheart.00142.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto A.R., Ilinykh A., Ivey M.J., Kuwabara J.T., D’Antoni M.L., Debuque R., Chandran A., Wang L., Arora K., Rosenthal N.A., et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016;118:400–409. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nobakht N., Kamgar M., Rastogi A., Schrier R.W. Limitations of Angiotensin Inhibition. Nat. Rev. Nephrol. 2011;7:356–359. doi: 10.1038/nrneph.2011.29. [DOI] [PubMed] [Google Scholar]
- 29.Wolf G., Ritz E. Combination Therapy with ACE Inhibitors and Angiotensin II Receptor Blockers to Halt Progression of Chronic Renal Disease: Pathophysiology and Indications. Kidney Int. 2005;67:799–812. doi: 10.1111/j.1523-1755.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- 30.Baltatzi M., Savopoulos C., Hatzitolios A. Role of Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers in Hypertension of Chronic Kidney Disease and Renoprotection. Study Results. Hippokratia. 2011;15:27–32. [PMC free article] [PubMed] [Google Scholar]
- 31.Hastings J.F., Skhinas J.N., Fey D., Croucher D.R., Cox T.R. The Extracellular Matrix as a Key Regulator of Intracellular Signalling Networks. Br. J. Pharmacol. 2019;176:82–92. doi: 10.1111/bph.14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kusindarta D.L., Wihadmadyatami H. The Role of Extracellular Matrix in Tissue Regeneration. In: El-Sayed Kaoud H.A., editor. Tissue Regeneration. IntechOpen; Rijeka, Croatia: 2018. p. 65. Chapter 5. [Google Scholar]
- 33.Dobaczewski M., de Haan J.J., Frangogiannis N.G. The Extracellular Matrix Modulates Fibroblast Phenotype and Function in the Infarcted Myocardium. J. Cardiovasc. Transl. Res. 2012;5:837–847. doi: 10.1007/s12265-012-9406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sainio A., Järveläinen H. Extracellular Matrix-Cell Interactions: Focus on Therapeutic Applications. Cell. Signal. 2020;66:109487. doi: 10.1016/j.cellsig.2019.109487. [DOI] [PubMed] [Google Scholar]
- 35.Bagchi R.A., Lin J., Wang R., Czubryt M.P. Regulation of Fibronectin Gene Expression in Cardiac Fibroblasts by Scleraxis. Cell Tissue Res. 2016;366:381–391. doi: 10.1007/s00441-016-2439-1. [DOI] [PubMed] [Google Scholar]
- 36.Ehanire T., Ren L., Bond J., Medina M., Li G., Bashirov L., Chen L., Kokosis G., Ibrahim M., Selim A., et al. Angiotensin II Stimulates Canonical TGF-β Signaling Pathway through Angiotensin Type 1 Receptor to Induce Granulation Tissue Contraction. J. Mol. Med. Berl. Ger. 2015;93:289–302. doi: 10.1007/s00109-014-1211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu F., Lagares D., Choi K.M., Stopfer L., Marinković A., Vrbanac V., Probst C.K., Hiemer S.E., Sisson T.H., Horowitz J.C., et al. Mechanosignaling through YAP and TAZ Drives Fibroblast Activation and Fibrosis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014;308:L344–L357. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francisco J., Zhang Y., Jeong J.I., Mizushima W., Ikeda S., Ivessa A., Oka S., Zhai P., Tallquist M.D., Del Re D.P. Blockade of Fibroblast YAP Attenuates Cardiac Fibrosis and Dysfunction Through MRTF-A Inhibition. JACC Basic Transl. Sci. 2020;5:931–945. doi: 10.1016/j.jacbts.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furtado M.B., Nim H.T., Boyd S.E., Rosenthal N.A. View from the Heart: Cardiac Fibroblasts in Development, Scarring and Regeneration. Development. 2016;143:387–397. doi: 10.1242/dev.120576. [DOI] [PubMed] [Google Scholar]
- 40.Mayorga M., Bahi N., Ballester M., Comella J.X., Sanchis D. Bcl-2 Is a Key Factor for Cardiac Fibroblast Resistance to Programmed Cell Death. J. Biol. Chem. 2004;279:34882–34889. doi: 10.1074/jbc.M404616200. [DOI] [PubMed] [Google Scholar]
- 41.Weber K.T. Cardiac Interstitium in Health and Disease: The Fibrillar Collagen Network. J. Am. Coll. Cardiol. 1989;13:1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 42.Humphries J.D., Chastney M.R., Askari J.A., Humphries M.J. Signal Transduction via Integrin Adhesion Complexes. Curr. Opin. Cell Biol. 2019;56:14–21. doi: 10.1016/j.ceb.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Kadry Y.A., Calderwood D.A. Chapter 22: Structural and Signaling Functions of Integrins. Biochim. Biophys. Acta Biomembr. 2020;1862:183206. doi: 10.1016/j.bbamem.2020.183206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Margadant C., Sonnenberg A. Integrin–TGF-β Crosstalk in Fibrosis, Cancer and Wound Healing. EMBO Rep. 2010;11:97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunyady L., Catt K.J. Pleiotropic AT1 Receptor Signaling Pathways Mediating Physiological and Pathogenic Actions of Angiotensin II. Mol. Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 46.Young-Pearse T.L., Bai J., Chang R., Zheng J.B., LoTurco J.J., Selkoe D.J. A Critical Function for β-Amyloid Precursor Protein in Neuronal Migration Revealed by in Utero RNA Interference. J. Neurosci. 2007;27:14459. doi: 10.1523/JNEUROSCI.4701-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumaran C., Shivakumar K. Calcium- and Superoxide Anion-Mediated Mitogenic Action of Substance P on Cardiac Fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H1855–H1862. doi: 10.1152/ajpheart.00747.2001. [DOI] [PubMed] [Google Scholar]
- 48.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Method. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on request.