Abstract
Background
Refractory dyspnea or breathlessness is a common symptom in patients with advanced chronic obstructive pulmonary disease (COPD), with a high negative impact on quality of life (QoL). Low dosed opioids have been investigated for refractory dyspnea in COPD and other life-limiting conditions, and some positive effects were demonstrated. However, upon first assessment of the literature, the quality of evidence in COPD seemed low or inconclusive, and focused mainly on morphine which may have more side effects than other opioids such as fentanyl. For the current publication we performed a systematic literature search. We searched for placebo-controlled randomized clinical trials investigating opioids for refractory dyspnea caused by COPD. We included trials reporting on dyspnea, health status and/or QoL. Three of fifteen trials demonstrated a significant positive effect of opioids on dyspnea. Only one of four trials reporting on QoL or health status, demonstrated a significant positive effect. Two-thirds of included trials investigated morphine. We found no placebo-controlled RCT on transdermal fentanyl. Subsequently, we hypothesized that both fentanyl and morphine provide a greater reduction of dyspnea than placebo, and that fentanyl has less side effects than morphine.
Methods
We describe the design of a robust, multi-center, double blind, double-dummy, cross-over, randomized, placebo-controlled clinical trial with three study arms investigating transdermal fentanyl 12 mcg/h and morphine sustained-release 10 mg b.i.d. The primary endpoint is change in daily mean dyspnea sensation measured on a numeric rating scale. Secondary endpoints are change in daily worst dyspnea, QoL, anxiety, sleep quality, hypercapnia, side effects, patient preference, and continued opioid use. Sixty patients with severe stable COPD and refractory dyspnea (FEV1 < 50%, mMRC ≥ 3, on optimal standard therapy) will be included.
Discussion
Evidence for opioids for refractory dyspnea in COPD is not as robust as usually appreciated. We designed a study comparing both the more commonly used opioid morphine, and transdermal fentanyl to placebo. The cross-over design will help to get a better impression of patient preferences. We believe our study design to investigate both sustained-release morphine and transdermal fentanyl for refractory dyspnea will provide valuable information for better treatment of refractory dyspnea in COPD.
Trial registration NCT03834363 (ClinicalTrials.gov), registred at 7 Feb 2019, https://clinicaltrials.gov/ct2/show/NCT03834363.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-021-01647-8.
Keywords: COPD, Refractory dyspnea, Breathlessness, Opioids
Background
Refractory dyspnea or breathlessness is a common symptom in patients with advanced chronic obstructive pulmonary disease (COPD), with a prevalence of up to 94% in the last year of life [1, 2]. It is defined as persisting complaints of dyspnea despite optimal standard therapy including, but not limited to smoking cessation, education, inhaled bronchodilators and pulmonary physiotherapy [3]. Refractory dyspnea is known to severely impact quality of life and exercise tolerance, and to increase the risk of depression and anxiety [4]. As the prevalence of COPD is expected to rise during the upcoming decades [5], it is likely that the number of patients with COPD suffering from refractory dyspnea will also continue to grow.
Advanced treatments such as non-invasive ventilation, bronchoscopic lung volume reduction and lung transplantation can improve dyspnea and quality of life in patients with advanced COPD [6]. But these treatments are only available for a proportion of patients with advanced COPD, due to strict eligibility criteria, high health-care costs and sometimes scarcity. Therefore, there is still a need for more widely available treatments of refractory dyspnea. In this context low dosed opioids have previously been investigated, and some positive effect was demonstrated [7–9]. However, whether the quality of the evidence is sufficient is still a topic of discussion. Furthermore, despite a positive advice on opioids in palliative care guidelines for COPD, prescription appears to be low in clinical practice [10–12].
We performed a systematic literature search with respect to opioids for refractory dyspnea in COPD, which we updated for the current publication to include all recent trials. We searched for placebo-controlled randomized clinical trials investigating any type of opioid prescribed for dyspnea reduction in COPD (at least 50% of participants). We included trials reporting on dyspnea, health status and/or quality of life. Additional details on the search strategy can be found in the online supplement (Additional file 1: Online supplement MoreFoRCOPD), including a flow chart on the number of records identified, screened and included.
Table 1 shows an overview of the trials we identified as a result of our search strategy. In total, fifteen trials were included. A statistically significant positive effect on dyspnea of opioid versus placebo was demonstrated only in three studies [7, 8, 13]. Since the majority of these studies included a small number of patients, the lack of statistically significant results may in part be explained by a low statistical power to detect a treatment effect. This assumption is supported by a meta-analysis published by Ekström et al. in 2015, in which a positive effect on dyspnea was found for both systemically administered and nebulized opioids (analyses of combined data of 8 and 4 trials, respectively) [14]. Nevertheless, the three largest studies in our table, which all have been published more recently, demonstrated no significant change in dyspnea for sustained-release morphine and oxycodone [15–17]. While assessing this, it is important to note that in the studies of Currow et al. [15] and Ferriera et al. [16] (which were originally both part of a three-armed trial) all arms received immediate-release morphine as needed. For both studies, the immediate-release morphine was used significantly more frequently in the placebo group (8.7 vs. 5.8 and 7.0 vs. 4.2 daily doses, respectively) [15, 16] making an overall effect of the maintenance morphine more difficult to detect. Furthermore, in the study by Verberkt et al. there was a statistically significant effect on worst daily dyspnea measured on a numeric rating scale (NRS) in a subgroup of COPD patients with a modified Medical Research Council (mMRC) ≥ 3 (mean difference compared to placebo: − 1.33 (− 2.50 to − 0.16) points) [17].
Table 1.
Overview of randomized clinical trials investigating the effect of opioids on dyspnea, quality of life or health status in COPD
| References | Design | n (% COPD) | Setting | Comparison | Treatment duration | Breathlessness | Quality of life or health status | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurement (scale) | Opioid | Placebo | Measurement (scale) | Opioid | Placebo | ||||||
| Woodcock et al. [18] | Cross-over | 12 (100) | Outpatient | Dihydrocodeine | Single dose |
VAS (0–10 cm) 45 min after med |
5.54 ± 1.91 | 6.33 ± 2.0 | – | – | – |
| Light et al. [19] | Cross-over | 13 (100) | Outpatient | Oral morphine 0.8 mg/kg | Single dose |
Borg score (0–10) Rest |
0.29 ± 0.58 | 0.13 ± 0.23 | – | – | – |
| Jankelson et al. [20] | Cross-over | 16 (100) | Outpatient | Nebulized morphine 20/40 mg | Single dose |
Borg score (0–10) After 6MWT |
4.2 ± 2.1/4.3 ± 1.8 | 4.3 ± 2.2 | – | – | – |
| Noseda et al. [21] | Cross-over | 14 (79)# | Hospitalized | Nebulized morphine 10/20 mg ± oxygen | Single dose | VAS (− 100 to + 100%) | + 33 ± 28/+ 43 ± 27 | + 42 ± 27 | – | – | – |
| Jensen et al. [22] | Cross-over | 12 (100) | Outpatient | Nebulized fentanyl 50 µg | Single dose |
Borg score (0–10) Isotime CPET |
2.0 ± 0.5 | 2.6 ± 0.5 | – | – | – |
| Abdallah et al. [13] | Cross-over | 20 (100) | Outpatient | Morphine dose up to 10 mg | Single dose |
Borg score (0–10) Isotime CPET |
3.0 ± 1.6* | 4.2 ± 2.6 | – | – | – |
| Iupati et al. [23] |
Cross-over Multicenter |
21 (62) | Outpatient | Intranasal fentanyl 20 µg as needed | 1 day |
VAS (0–100 mm) 15 min after med |
26 ± 21 (Δ29 ± 25) | 21 ± 19 (Δ33 ± 24) | – | – | – |
| Abernethy et al. [7] |
Cross-over Multicenter |
48 (88)# | Outpatient | SR morphine 20 mg od | 4 days |
VAS (0–100 mm) Morning/evening |
40.1 ± 24*/40.3 ± 23* |
47.7 ± 26 49.9 ± 24 |
Data not presented | Data not presented | Data not presented |
| Janowiak et al. [24] | Cross-over | 10 (100) | Hospitalized | Nebulized morphine 3–5 mg qid | 4 days |
VAS (0–100 mm) Now (2dd) |
Δ25.4 ± 9.0$ | Δ6.3 ± 7.8 | – | – | – |
| Johnson et al. [8] | Cross-over | 18 (100) | Outpatient | Dihydrocodeine 15 mg as needed up to tds | 7 days |
VAS (0–10 cm) Mean daily |
4.6 ± 2.1* | 5.6 ± 2.3 | – | – | – |
| Currow et al. [15] |
Parallel Multicenter |
284 (58)# | Outpatient |
SR morphine 20 mg qd All arms: morphine 2.5 mg as needed |
7 days |
VAS (0–100 mm) Now (2dd) |
Δ-5.00 ± 2.13 | Δ-4.86 ± 2.07 | EORTC-QLQ-C15 PAL (0–100) | Δ1.8 ± 2.2 | Δ1.5 ± 2.2 |
| Ferriera et al. [16] |
Parallel Multicenter |
155 (60)# | Outpatient |
Oxycodone 5 mg tds All arms: morphine 2.5 mg as needed |
7 days |
VAS (0–100 mm) Now |
Δ-3.7 ± 2.9 | Δ-9.0 ± 2.7 | EORTC-QLQ-C15 PAL (0–100) | Δ-1.7 ± 3.1 | Δ2.82 ± 3.1 |
| Eiser et al. [25] | Cross-over | 14 (100) | Outpatient |
Diamorphine 2.5/5 mg qid |
14 days | VAS (0–10 cm) | 7.0 ± 0.7/7.0 ± 0.8 | 6.5 ± 0.7 | – | – | – |
| Verberkt et al. [17] |
Parallel Multicenter |
124 (100) | Outpatient | SR morphine 10 mg 1-tds | 28 days |
NRS (0–10 points) Mean |
Δ-0.60 (− 1.55 to 0.35) | CAT (0–40) | Δ-2.18 (− 4.14 to − 0.2)* | ||
| Poole et al. [9] | Cross-over | 16 (100) | Outpatient | SR morphine 10 mg od or bid | 42 days | DBS (0–5) | 2.22 | 2.26 | CRQ (20–140) | ∆2.08 ± 4.53 | ∆2.94 ± 3.46 |
Data presented as mean ± SD
od once a day, bid twice daily, tds three times a day, qid four times a day, SR sustained release, VAS visual analogue score, DBS daytime breathlessness score, NRS numeric rating scale, CRQ chronic respiratory questionnaire, EORTC-QLQ-C15 PAL Quality of life questionnaire developed by the European Organisation for Research and Treatment of Cancer, CAT COPD assessment test, CPET cardiopulmonary exercise testing, 6MWT 6-min walking test
*p < 0.05 opioid versus placebo, $p < 0.05 change after treatment. #Data not exclusively on COPD
Information on quality of life or health status was limited to four RCT’s. Of these, only the study by Verberkt et al. demonstrated a small positive, statistically significant effect on health status measured with the COPD assessment test (CAT) [17]. Our search identified no placebo-controlled RCT’s investigating transdermal fentanyl for refractory dyspnea in COPD.
Based on this assessment of available evidence, we designed a randomized, placebo-controlled clinical trial, on which we will further elaborate in the “Methods/design” section and “Discussion” section.
Methods/design
Overview
We designed a robust, multi-center, double blind, double-dummy, cross-over, randomized, placebo-controlled clinical trial with three study arms investigating transdermal fentanyl and sustained-release morphine. We hypothesize that both fentanyl and morphine provide a reduction of dyspnea which is greater than placebo, and that fentanyl has less side effects than morphine. A total of 60 patients with severe stable COPD and refractory dyspnea will be included in this study in ten Dutch hospitals. Patients will be recruited at the outpatient clinic of each participating hospital by chest physicians. The study is registered at clinicaltrials.gov (NCT03834363), where a full list of participating hospitals can be found, and the protocol is approved by the UMCG Ethics committee. Written informed consent will be obtained from all participants and the study will be performed in accordance with the Declaration of Helsinki.
Study duration and treatment
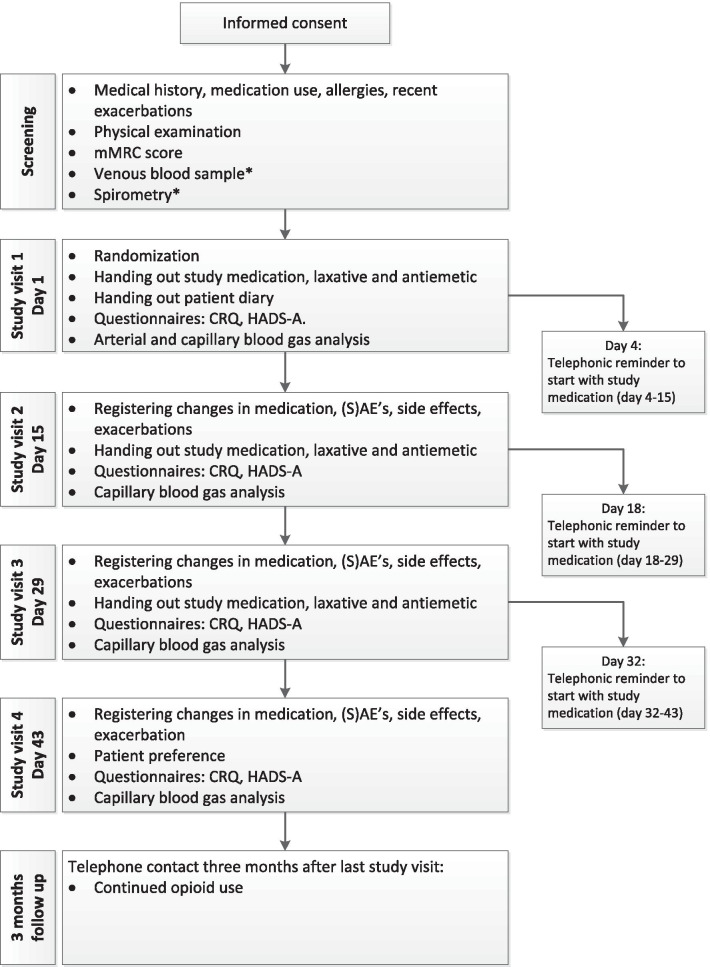
The study duration is 6 weeks for each participant, divided in three periods of 2 weeks. During each period the participant is treated for 11 days. During the first 3 days of every treatment period no study medication is used, to wash out medication of any previous treatment period. The fentanyl patches are dosed 12 µg/h and changed every 3 days. The morphine sustained-released capsules are dosed 10 mg b.i.d. Both an antiemetic (metoclopramide 10 mg as needed, up to thrice daily) and laxative (macrogol/electrolytes 13.7 g, started once daily, more or less sachets as needed) are prescribed. In total, there are four study visits. A complete study flowchart can be found in Fig. 1. After the end of the study treatment patients can discuss with their chest physician whether they would like to continue with low dosed morphine or transdermal fentanyl. At the time of this decision, the participants and physician are still blinded to the study treatment.
Fig. 1.
Study flowchart. mMRC modified Medical Research Council Score, CRQ chronic respiratory questionnaire, HADS-A hospital anxiety depression score—anxiety, (S)AE (serious) adverse event. *Unless already performed in the 6 months before screening
In- and exclusion criteria
All in- and exclusion criteria can be found in Table 2. In general, patients with COPD Gold class III or IV and a modified Medical Research Council score (mMRC) ≥ 3 who perceive dyspnea despite optimal standard therapy according to GOLD and the Dutch guideline for diagnosis and treatment of COPD can be included. If there is comorbidity substantially contributing to the breathlessness, for example severe heart failure, patients are excluded. Participants who have a moderate or severe exacerbation (requiring oral corticosteroids, antibiotics and/or hospital admission) during participation are discontinued from the trial. If they are stable for 8 weeks after recovery from the exacerbation, they are allowed to restart the study once more.
Table 2.
In- and exclusion criteria
| Inclusion criteria |
| Age ≥ 40 years |
| Read, understood and signed the Informed Consent form |
| COPD GOLD class III or IV, according to GOLD criteria |
| Post-bronchodilatation FEV1/FVC ≤ 70% and FEV1 < 50% pred.* |
| Complaints of refractory dyspnea as established by patient and doctor |
| mMRC score ≥ 3 |
| Life expectancy of ≥ 2 months |
| Optimized standard therapy according to Dutch LAN guideline for diagnosis and treatment of COPD |
| Exclusion criteria |
| Other severe disease with chronic pain or chronic dyspnea (a non-susbstantial component of left sided heart failure is acceptable) |
| Current use of opioids for whatever indication |
| Allergy/intolerance for opioids |
| Psychiatric disease, not related to severe COPD |
| Exacerbation of COPD 8 weeks prior to inclusion or between screening and randomization |
| Problematic (leading to medical help or social problems) substance abuse during the last 5 years |
| Active malignancy, with the exception of planocellular or basal cell carcinoma of the skin |
| eGFR < 15 ml/min* |
*Measured within 6 months of screening
COPD chronic obstructive pulmonary disease, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, GOLD global initiative for chronic obstructive lung disease, LAN Lung Alliance The Netherlands, mMRC modified Medical Research Council Dyspnea Scale, eGFR estimated Glomerular Filtration Rate
Outcome measurements
The primary outcome measurement is change in mean daily dyspnea sensation as measured on the numeric rating scale for Dyspnea [26]. Secondary outcome measurements are change in worst daily dyspnea sensation, health-related quality of life, anxiety, sleep quality, occurrence of respiratory depression and side effects, patient preference and continued opioid use. A more extensive description of the outcome measures can be found in Table 3. Patients who drop out will be followed as much as possible for vital status, hospitalization, and start of open label opioids during the intended 6 weeks period of the study.
Table 3.
Outcome measurements
| Measurement | Frequency of measurement | |
|---|---|---|
| Primary outcome measure | ||
| Change in mean dyspnea sensation | Numeric rating scale [26] | Once daily in patient diary |
| Secondary outcome measures | ||
| Change in worst dyspnea sensation | Numeric rating scale [26] | Once daily in patient diary |
| Change in Health-Related Quality of Life | CCQ [27] | Once daily in patient diary |
| CRQ [28] | During each study visit | |
| CRQ-mastery domain | During each study visit | |
|
HADS-A [29] Open en named side effects |
During each study visit Once daily in patient diary and asked during study visits |
|
|
Anxiety Side effects Change in hypercapnia, HCO3 and pH Change in sleep quality Patient preference Continued opioid use |
Capillary blood gas analysis Numeric rating scale [30] Asked during the final study visit Asked 3 months after the end of study |
During each study visit Once daily in patient diary Once Once |
CCQ clinical COPD questionnaire, CRQ chronic respiratory questionnaire, HADS-A hospital anxiety and depression scale—anxiety subscale
Randomization and unblinding
Randomization is tailor made for this study using a web based program (ALEA® DM version 17.1). Randomization can be performed online by the research team of each participating hospital. Participants will be randomized equally between the six possible treatment sequences, stratified for study location. Unblinding only occurs in the case of patient emergencies and at the conclusion of the study. Health authorities will be granted access to unblinded data if needed. The pharmacist on call of each participating hospital can unblind a participant using the web based program if requested by the researcher because of a patient emergency.
Statistical analysis
For the power calculation the difference in primary endpoint between fentanyl and placebo was used. The Minimal Clinical Important Difference (MCID) for the NRS score is 1 point, the standard deviation is 2.0 points [31]. With a two-sided alpha = 0.05 and a power of 0.90 in a cross-over design, 44 participants who complete the study are needed. Because this is a fragile patient group, we will aim to recruit 60 participants.
The primary endpoint analysis will be on an intention to treat basis and therefore all patients randomized. The primary endpoint is the NRS mean dyspnea score which we will treat as a continuous variable for day 7–14. This will not be calculated if less dan 2 days are available. Since it is a three way cross-over, the data for the available periods will also be used of not all periods were completed. No imputation will be used for the primary endpoint. There will be two comparisons: the difference in the mean dyspnea score of day 7–14 for fentanyl versus placebo and for morphine versus placebo. In this way, the risk of any remaining effect from the previous treatment periods influencing the outcome will be optimally reduced. The analysis will be by Student’s t-test. The analyses of secondary endpoints will be done by Student’s t-tests (or non-parametric tests where needed) or chi square, following the same scheme of main comparisons as for the primary endpoints. The analysis of side effects will be done by comparison of proportions of side effects by chi square tests between all three arms. Composite questionnaire data will be primarily analysed by total sum scores. Additionally, per protocol analyses will be performed. The study is not powered to determine equivalence of dyspnea relief of fentanyl compared to morphine: that comparison will consist of descriptive statistics only.
Safety
All (serious) adverse events will be monitored. The sponsor will report serious adverse events (SAEs) through the Dutch web portal ToetsingOnline to the accredited Ethics committee that approved the protocol, within 7 days of first knowledge for SAEs that result in death or are life threatening followed by a period of maximum of 8 days to complete the initial preliminary report. All other SAEs will be reported within a period of maximum 15 days after the sponsor has first knowledge of the serious adverse events. This is a short term study with 60 patients, entered parallel in a multi-centre study. Therefore, and since opioids in the form of morphine are in the guidelines, we will not perform interim analyses, even though the patient population of patients with severe COPD and in a palliative setting is at increased risk of death. For the same reasons, no Data Safety Monitoring Board (DSMB) will be instituted.
Study timeline
The study has started in November 2019. At this point the first participant was included at the University Medical Center Groningen. For the other participating hospitals the start of inclusion was delayed by one or more months because of a delay in the production of research medication and a delay in the issuing of a permit for scientific research with opioids for the participating hospital pharmacies. Unfortunately, starting March 2020 the inclusion was alternately put on hold or restricted in each participating hospital due to the COVID-19 pandemic. We aim to include all patients by the end of 2021, but whether this will be achieved is strongly depended on the course of the COVID-19 pandemic.
Discussion
Optimal reduction of dyspnea in patients with severe COPD is an important way to improve quality of life, yet can be very challenging. From our assessment of the literature, we found that even though opioids have found their way into COPD guidelines as a treatment option for refractory dyspnea, the evidence base can still be considered inconclusive. Furthermore, the majority of research has focused on morphine and we identified no placebo-controlled RCT investigating transdermal fentanyl. However, trials investigating fentanyl in the short-acting form, suggest that fentanyl could give a reduction of dyspnea [32, 33]. Additionally, studies on pain treatment indicate that patients may prefer transdermal fentanyl and experience less side effects in comparison to oral morphine [34]. Therefore, we believe that our current multi-center, double blind, cross-over, placebo-controlled study design to investigate sustained-release morphine and transdermal fentanyl for refractory dyspnea will provide valuable information on patient preference and the effectiveness of transdermal fentanyl and sustained-release morphine for refractory dyspnea in COPD.
By choosing a cross-over design for this study the participant is his or her own control, thus reducing the variability and the number of patients needed to participate. Additionally, this design helps to get a better impression of patient preferences. On the other hand, because of the cross-over design the treatment duration is 6 weeks instead of 11 days (which it would be if this study had a parallel design). This prolonged study duration will most likely increase the risk of participants that have to be discontinued from the trial because of the occurrence of COPD exacerbations, which occur frequently in advanced COPD. For this reason we aim to include 60 participants, which is sixteen more than the 44 participants calculated from the power analysis which need to fully complete the study. Furthermore, patients experiencing an exacerbation will discontinue the trial, but may be included once more if they are clinically stable for at least 8 weeks.
There are indications that prescription of opioids for refractory dyspnea in COPD can be a loaded topic for both patient and doctors, amongst others because of associations with terminal disease, possible adverse effects and addiction [10]. Although this has not been formally investigated in patients, we believe education is important to address any questions or worries patients may have regarding opioids. Therefore, both an animated short film for patients and their loved ones on facts and myths about opioids (developed by Indiveo B.V.) as well as an information leaflet with the same content are tested during our study. At the end of the trial, feedback from the participants will be used to adjust the animation and leaflet and these will be made widely available for patients with COPD. Additionally, both patients and physicians participating in the study are asked to share their experiences with opioids for refractory dyspnea in COPD during regional congresses and meetings.
Supplementary Information
Additional file 1. Online supplement MoreFoRCOPD.
Acknowledgements
The investigators would like to thank Daisy J. Janssen for advising on the research protocol. The investigators would like to thank the members of the investigational teams in all participating hospitals for all the time and effort it takes to carry out this study.
Protocol version
Protocol version 14, date 14th February 2020, has been approved by the ethics committee of the University Medical Center Groningen Medical Research.
Abbreviations
- AE
Adverse event
- CCMO
Central Committee on Research Involving Human Subjects
- CCQ
Clinical COPD Questionnaire
- CRQ
Chronic Respiratory Questionnaire
- COPD
Chronic obstructive pulmonary disease
- eGFR
Estimated Glomerular Filtration Rate
- FEV1
Forced expiratory volume in 1 s
- FVC
Forced vital capacity
- GOLD
Global initiative for chronic obstructive lung disease
- HR-QoL
Health Related Quality of Life
- HADS-A
Hospital Anxiety and Depression Scale—anxiety subscale
- IC
Informed consent
- LAN
Lung Alliance The Netherlands
- mMRC
Modified Medical Research Council Dyspnea Scale
- MREC
Medical Research Ethics Committee
- NRS
Numeric rating scale
- SAE
Serious adverse event
- SR
Sustained-release
Authors' contributions
HAK, SMH and KMM initiated the trial. MD and HAK wrote the research protocol and acquired funding for the trial. All authors were involved in the development of the study design and study protocol, and have approved the submitted version of the protocol. All authors include patients and collect and check data. MD is the coordinating researcher, and HAK is principal investigator. All authors read and approved the final manuscript.
Funding
Funding for this study is provided by the Innovatiefonds Zorgverzekeraars (Grant No. B18-110/Dossier 3.580) (Joint Dutch health insurers’ fund for innovation)’ and the Stichting Astma Bestrijding (Grant No. 2018/036) (Dutch Foundation for Asthma Prevention), and the Department of Pulmonology, University Medical Center Groningen. The Joint Dutch health insurers’ fund for innovation and Dutch Foundation for Asthma Prevention have no roll in the study design, analysis or interpretation of data, writing of the report or the decision to submit the report for publication.
Availability of data and materials
The data management plan is made available in de the online supplement (Additional file 1: Online supplement MoreFoRCOPD). Marlies (M.) van Dijk or Huib (H.A.M.) Kerstjens can be contacted to apply for permission to obtain access to the raw data that will be generated during the study.
Declarations
Ethics approval and consent to participate
The study is designed in accordance with the Declaration of Helsinki and approved by the ethics committee of the University Medical Center Groningen Medical Research Ethics Committee (Netherlands). All amendments will be communicated with relevant parties. Written informed consent will be obtained from all participants by a member of the research team of each participating hospital. Each participant will be covered by insurance in case of unexpected harm from participating in the trial.
Consent for publication
The ethical approval and patient information include consent to publish collected data.
Competing interests
MD, KMM, JWB, WB, RH, SMH, WYL, LEP, KP, HAK report no competing interests related to this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carette H, Zysman M, Morelot-Panzini C, Perrin J, Gomez E, Guillaumot A, et al. Prevalence and management of chronic breathlessness in COPD in a tertiary care center. BMC Pulm Med. 2019;19(1):95. doi: 10.1186/s12890-019-0851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edmonds P, Karlsen S, Khan S, Addington-Hall J. A comparison of the palliative care needs of patients dying from chronic respiratory diseases and lung cancer. Palliat Med. 2001;15(4):287–295. doi: 10.1191/026921601678320278. [DOI] [PubMed] [Google Scholar]
- 3.Mahler DA, Selecky PA, Harrod CG, Benditt JO, Carrieri-Kohlman V, Curtis JR, et al. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest. 2010;137(3):674–691. doi: 10.1378/chest.09-1543. [DOI] [PubMed] [Google Scholar]
- 4.Janssen DJ, Wouters EF, Spruit MA. Psychosocial consequences of living with breathlessness due to advanced disease. Curr Opin Support Palliat Care. 2015;9(3):232–237. doi: 10.1097/SPC.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 5.Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):190. doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 6.van Dijk M, Gan CT, Koster TD, Wijkstra PJ, Slebos DJ, Kerstjens HAM, et al. Treatment of severe stable COPD: the multidimensional approach of treatable traits. ERJ Open Res. 2020;6(3):00322-2019. doi: 10.1183/23120541.00322-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abernethy AP, Currow DC, Frith P, Fazekas BS, McHugh A, Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327(7414):523–528. doi: 10.1136/bmj.327.7414.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson MA, Woodcock AA, Geddes DM. Dihydrocodeine for breathlessness in "pink puffers". Br Med J (Clin Res Ed) 1983;286(6366):675–677. doi: 10.1136/bmj.286.6366.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poole PJ, Veale AG, Black PN. The effect of sustained-release morphine on breathlessness and quality of life in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1877–1880. doi: 10.1164/ajrccm.157.6.9711061. [DOI] [PubMed] [Google Scholar]
- 10.Janssen DJ, de Hosson SM, bij de Vaate E, Mooren KJ, Baas AA. Attitudes toward opioids for refractory dyspnea in COPD among Dutch chest physicians. Chron Respir Dis. 2015;12(2):85–92. doi: 10.1177/1479972315571926. [DOI] [PubMed] [Google Scholar]
- 11.Ahmadi Z, Bernelid E, Currow DC, Ekstrom M. Prescription of opioids for breathlessness in end-stage COPD: a national population-based study. Int J Chron Obstruct Pulmon Dis. 2016;11:2651–2657. doi: 10.2147/COPD.S112484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Currow DC, Johnson MJ, Pollack A, Ferreira DH, Kochovska S, Ekstrom M, et al. Breathlessness and opioid prescribing in COPD in general practice: a cross-sectional, observational study. ERJ Open Res. 2020;6(2):00299-2019. doi: 10.1183/23120541.00299-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdallah SJ, Wilkinson-Maitland C, Saad N, Li PZ, Smith BM, Bourbeau J, et al. Effect of morphine on breathlessness and exercise endurance in advanced COPD: a randomised crossover trial. Eur Respir J. 2017;50(4):1701235. doi: 10.1183/13993003.01235-2017. [DOI] [PubMed] [Google Scholar]
- 14.Ekstrom M, Nilsson F, Abernethy AA, Currow DC. Effects of opioids on breathlessness and exercise capacity in chronic obstructive pulmonary disease. A systematic review. Ann Am Thorac Soc. 2015;12(7):1079–1092. doi: 10.1513/AnnalsATS.201501-034OC. [DOI] [PubMed] [Google Scholar]
- 15.Currow D, Louw S, McCloud P, Fazekas B, Plummer J, McDonald CF, et al. Regular, sustained-release morphine for chronic breathlessness: a multicentre, double-blind, randomised, placebo-controlled trial. Thorax. 2020;75(1):50–56. doi: 10.1136/thoraxjnl-2019-213681. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira DH, Louw S, McCloud P, Fazekas B, McDonald CF, Agar MR, et al. Controlled-release oxycodone versus placebo in the treatment of chronic breathlessness—a multisite randomized placebo controlled trial. J Pain Symptom Manag. 2020;59(3):581–589. doi: 10.1016/j.jpainsymman.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Verberkt CA, van den Beuken-van Everdingen MHJ, Schols J, Hameleers N, Wouters EFM, Janssen DJA. Effect of sustained-release morphine for refractory breathlessness in chronic obstructive pulmonary disease on health status: a randomized clinical trial. JAMA Intern Med. 2020;180(10):1306–1314. doi: 10.1001/jamainternmed.2020.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodcock AA, Gross ER, Gellert A, Shah S, Johnson M, Geddes DM. Effects of dihydrocodeine, alcohol, and caffeine on breathlessness and exercise tolerance in patients with chronic obstructive lung disease and normal blood gases. N Engl J Med. 1981;305(27):1611–1616. doi: 10.1056/NEJM198112313052703. [DOI] [PubMed] [Google Scholar]
- 19.Light RW, Muro JR, Sato RI, Stansbury DW, Fischer CE, Brown SE. Effects of oral morphine on breathlessness and exercise tolerance in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1989;139(1):126–133. doi: 10.1164/ajrccm/139.1.126. [DOI] [PubMed] [Google Scholar]
- 20.Jankelson D, Hosseini K, Mather LE, Seale JP, Young IH. Lack of effect of high doses of inhaled morphine on exercise endurance in chronic obstructive pulmonary disease. Eur Respir J. 1997;10(10):2270–2274. doi: 10.1183/09031936.97.10102270. [DOI] [PubMed] [Google Scholar]
- 21.Noseda A, Carpiaux JP, Markstein C, Meyvaert A, de Maertelaer V. Disabling dyspnoea in patients with advanced disease: lack of effect of nebulized morphine. Eur Respir J. 1997;10(5):1079–1083. doi: 10.1183/09031936.97.10051079. [DOI] [PubMed] [Google Scholar]
- 22.Jensen D, Alsuhail A, Viola R, Dudgeon DJ, Webb KA, O'Donnell DE. Inhaled fentanyl citrate improves exercise endurance during high-intensity constant work rate cycle exercise in chronic obstructive pulmonary disease. J Pain Symptom Manag. 2012;43(4):706–719. doi: 10.1016/j.jpainsymman.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Iupati S, Bridge R, Allan S, Hewitt D. Intranasal fentanyl versus placebo for treatment of episodic breathlessness in hospice patients with advanced nonmalignant diseases. J Pain Symptom Manag. 2020;61:1035–1041. doi: 10.1016/j.jpainsymman.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Janowiak P, Krajnik M, Podolec Z, Bandurski T, Damps-Konstanska I, Sobanski P, et al. Dosimetrically administered nebulized morphine for breathlessness in very severe chronic obstructive pulmonary disease: a randomized, controlled trial. BMC Pulm Med. 2017;17(1):186. doi: 10.1186/s12890-017-0535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eiser N, Denman WT, West C, Luce P. Oral diamorphine: lack of effect on dyspnoea and exercise tolerance in the "pink puffer" syndrome. Eur Respir J. 1991;4(8):926–931. [PubMed] [Google Scholar]
- 26.Gift AG, Narsavage G. Validity of the numeric rating scale as a measure of dyspnea. Am J Crit Care. 1998;7(3):200–204. doi: 10.4037/ajcc1998.7.3.200. [DOI] [PubMed] [Google Scholar]
- 27.van der Molen T, Willemse BW, Schokker S, ten Hacken NH, Postma DS, Juniper EF. Development, validity and responsiveness of the Clinical COPD questionnaire. Health Qual Life Outcomes. 2003;1:13. doi: 10.1186/1477-7525-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wijkstra PJ, TenVergert EM, Van Altena R, Otten V, Postma DS, Kraan J, et al. Reliability and validity of the chronic respiratory questionnaire (CRQ) Thorax. 1994;49(5):465–467. doi: 10.1136/thx.49.5.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 30.Cappelleri JC, Bushmakin AG, McDermott AM, Sadosky AB, Petrie CD, Martin S. Psychometric properties of a single-item scale to assess sleep quality among individuals with fibromyalgia. Health Qual Life Outcomes. 2009;7:54. doi: 10.1186/1477-7525-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson MJ, Bland JM, Oxberry SG, Abernethy AP, Currow DC. Clinically important differences in the intensity of chronic refractory breathlessness. J Pain Symptom Manag. 2013;46(6):957–963. doi: 10.1016/j.jpainsymman.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Hui D, Hernandez F, Larsson L, Liu D, Kilgore K, Naberhuis J, et al. Prophylactic fentanyl sublingual spray for episodic exertional dyspnea in cancer patients: a pilot double-blind randomized controlled trial. J Pain Symptom Manag. 2019;58(4):605–613. doi: 10.1016/j.jpainsymman.2019.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon ST, Koskeroglu P, Gaertner J, Voltz R. Fentanyl for the relief of refractory breathlessness: a systematic review. J Pain Symptom Manag. 2013;46(6):874–886. doi: 10.1016/j.jpainsymman.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Payne R, Mathias SD, Pasta DJ, Wanke LA, Williams R, Mahmoud R. Quality of life and cancer pain: satisfaction and side effects with transdermal fentanyl versus oral morphine. J Clin Oncol. 1998;16(4):1588–1593. doi: 10.1200/JCO.1998.16.4.1588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Online supplement MoreFoRCOPD.
Data Availability Statement
The data management plan is made available in de the online supplement (Additional file 1: Online supplement MoreFoRCOPD). Marlies (M.) van Dijk or Huib (H.A.M.) Kerstjens can be contacted to apply for permission to obtain access to the raw data that will be generated during the study.