Abstract
Covalent organic frameworks (COFs) are promising materials for gas storage and separation; however, the potential of COFs for separation of CH4 from industrially relevant gases such as H2, N2, and C2H6 is yet to be investigated. In this work, we followed a multiscale computational approach to unlock both the adsorption- and membrane-based CH4/H2, CH4/N2, and C2H6/CH4 separation potentials of 572 COFs by combining grand canonical Monte Carlo (GCMC) and molecular dynamics (MD) simulations and density functional theory (DFT) calculations. Adsorbent performance evaluation metrics of COFs, adsorption selectivity, working capacity, regenerability, and adsorbent performance score were calculated for separation of equimolar CH4/H2, CH4/N2, and C2H6/CH4 mixtures at vacuum swing adsorption (VSA) and pressure swing adsorption (PSA) conditions to identify the best-performing COFs for each mixture. Results showed that COFs could achieve selectivities of 2–85, 1–7, and 2–23 for PSA-based CH4/H2, CH4/N2, and C2H6/CH4 separations, respectively, outperforming conventional adsorbents such as zeolites and activated carbons for each mixture. Structure–performance relations revealed that COFs with pore sizes <10 Å are promising adsorbents for all mixtures. We identified the gas adsorption sites in the three top-performing COFs commonly identified for each mixture by DFT calculations and computed the binding strength of gases, which were found to be on the order of C2H6 > CH4 > N2 > H2, supporting the GCMC results. Nucleus-independent chemical shift (NICS) indexes of aromaticity for adsorption sites were calculated, and the results revealed that the degree of linker aromaticity could be a measure for the selection or design of highly alkane-selective COF adsorbents over N2 and H2. Finally, COF membranes were shown to achieve high H2 permeabilities, 4.57 × 103–1.25 × 106 Barrer, and decent membrane selectivities, as high as 4.3, outperforming polymeric and MOF-based membranes for separation of H2 from CH4.
1. Introduction
Natural gas, as one of the main energy resources, is gaining more and more attention due its clean energy supply compared to other types of fossil fuels. Natural gas is mostly composed of methane; however, it also contains some impurities such as hydrogen, nitrogen, carbon dioxide, and long-chain hydrocarbons depending on its source. These impurities either decrease the energy density of the natural gas or increase the CO2 emission of natural gas; thus, removal of the contaminants from methane prior to its utilization in energy applications is crucial.1,2 Methods like pressure swing adsorption (PSA) and vacuum swing adsorption (VSA) have been widely used for the purification of natural gas.3−5 Traditional porous materials like zeolites and activated carbons have been used as adsorbents for selective separation of methane from other gases; however, they mostly suffer from low selectivities and low adsorption capacities.6,7 Thus, there is an ongoing search for new materials with promising adsorption properties.
Metal–organic frameworks (MOFs) are crystalline porous materials in which metallic nodes are connected to each other with organic ligands.8 Due to the vast number of available metallic nodes and organic ligands, MOFs have diverse physical and chemical properties such as variable surface areas and porosities, which can be tuned by in situ or post-synthesis modifications to achieve the best-performing materials for a specific application.9−11 MOFs have been considered as promising materials for both adsorption- and membrane-based CH4 separation from various gases.12−16 Since the number of experimentally synthesized MOFs and MOF-like materials has reached ∼100 000, identifying the best possible material for a specific gas separation using purely experimental manners is not practical.17 Thus, high-throughput computational screening (HTCS) methods have been utilized to assess the adsorption-based CH4/H2, CH4/N2, CO2/CH4, C2H6/CH4, and C3H8/CH4 separation performances of MOFs.18−22 A total of 4350 MOFs were studied for CH4/H2 mixture separation at VSA and PSA conditions, and CH4/H2 selectivities of MOFs were predicted based on the porosity and the largest cavity diameter (LCD) of MOFs, and the difference between the heat of adsorption values of gases (ΔQst) in MOFs.23 Yan et al.24 used molecular simulations to examine CH4/N2 mixture separation potentials of 5109 MOFs and revealed that MOFs with pore-limiting diameters (PLDs) in the range of 3.8–4.7 Å achieve high selectivities. Borah and Ponraj screened about 12 000 MOFs for C2H6/CH4 separation and highlighted that MOFs with the Zr4+ cation are promising candidates for purification of CH4.25 MOFs have also been investigated for membrane-based CH4/C2H6 and CH4/H2 separations,26,27 and all of these works have highlighted the high potential of MOFs for CH4 purification.
Covalent organic frameworks (COFs) have recently been considered as an emerging class of porous materials and have become alternatives to MOFs with their immense chemical and thermal stabilities due to the strong covalent bonds holding the structure, low densities, high porosities, and high gas separation performances.28−30 Studies for the design of new COFs have gained pace, and the number of experimentally synthesized COFs is rapidly growing. COFs are composed of light elements, such as hydrogen, nitrogen, carbon, oxygen, and boron.30−32 COFs, especially three-dimensional (3D) ones, offer higher surface areas and a higher number of adsorption sites than MOFs.33 Similar to MOFs, structural properties of COFs can be tuned using in situ or post-synthetic modifications, granting the materials a diverse range of structural features.28 Cao and Yang investigated the effect of Li doping on CH4/H2 separation performance of four different COFs using molecular simulations and showed that Li doping significantly increases the adsorption selectivity of COFs.34 Tong et al.35 screened 46 COFs to assess their CH4/H2, CO2/H2, and CO2/CH4 separation performances at PSA conditions and revealed that COFs can achieve higher selectivities (>100) and working capacities (>3 mol/kg) than zeolites and common MOFs.
The establishment of a computation-ready experimental COF (CoRE COF) database,36 which currently consists of 449 solvent-free COF structures, promoted the utilization of HTCS for COFs.37 Yan et al.37 studied CO2/CH4 separation performance of 290 functionalized CoRE COF membranes at 10 bar, 298 K, and showed that 137 of them could outperform traditional polymers for CO2/CH4 separation. Ongari et al.38 created the CURATED (Clean, Uniform, and Refined with Automatic Tracking from Experimental Database) COF database consisting of 324 structures with high-quality partial charges. They screened the CURATED COFs38 along with the hypothetical COFs39 for CO2/N2 separation and reported that many of the COFs have lower parasitic energies than the amine scrubbing process, which is conventionally used for CO2 capture. Our group recently screened 295 COFs for CO2/N2 separation at VSA, PSA, and TSA conditions and reported that COFs with pore sizes < 10 Å, 0.6 < porosity < 0.8, and surface area < 4500 m2/g are the most promising candidates for flue gas separation.40 A total of 288 COFs were studied using HTCS methods for CO2/H2 separation at five different operating conditions, and COFs with narrow pores and low porosities were identified as the best-performing candidates for CO2 separation from H2.41
As evidenced from this literature review, COFs have been extensively studied for CO2-related separations including CO2/N2, CO2/H2, and CO2/CH4, but we still have very limited information about the CH4 separation potentials of COFs from other gases such as H2, N2, and C2H6. Motivated by this, we used an HTCS approach combining grand canonical Monte Carlo (GCMC) and molecular dynamics (MD) simulations to unlock the potential of COFs for adsorption- and membrane-based CH4 separation from H2, N2, and C2H6. First, we performed GCMC simulations for the experimentally reported 572 COFs to compute equimolar CH4/H2, CH4/N2, and C2H6/CH4 mixture adsorption properties of COFs at VSA and PSA conditions. Using this adsorption data, various adsorbent evaluation metrics were computed, and COFs were ranked according to these metrics to identify the top 10 materials for each separation process. The common top adsorbents for the three gas separation processes were studied in detail by performing density functional theory (DFT) calculations to understand the underlying gas adsorption dynamics. Adsorption-based CH4 separation performances of COFs were compared with those of MOFs and zeolites to reveal their potential. Relations between structural properties and gas separation performances of COFs were examined to describe the best combination of structural characteristics leading to the most promising COF adsorbents for separation of each gas mixture. We finally computed membrane-based CH4/H2, CH4/N2, and C2H6/CH4 separation performances of COFs by performing MD simulations. Gas permeability and selectivity of COF membranes were compared with those of MOF membranes and conventional polymer membranes. Our results will be useful in (i) providing a comparison between CH4 purification performances of COFs with those of MOFs, zeolites, and polymers; (ii) highlighting the most promising COFs for CH4 separation from various gases to guide the future experimental studies; and (iii) contributing to the design of new and novel COFs with greater potentials for CH4 purification than existing COFs.
2. Computational Details
We used the CURATED COF38 database, which consists of 598 distinct COF structures. The physical properties of COFs such as PLD, LCD, porosity (ϕ), accessible surface area (Sacc), and density were computed using Zeo++ software.42 A probe radius of 1.86 Å (kinetic radius of the N2 molecule) was employed for the calculations of surface area, and the COFs with zero Sacc were eliminated so that the gas molecules studied in this work can adsorb into the remaining COFs. CH4/H2, CH4/N2, and C2H6/CH4 mixtures were assumed to be equimolar as most of the experimental studies in the literature that we compared our results with consider equimolar mixtures.43,44 Moreover, Guo et al.45,46 recently showed that composition does not significantly change the simulated C2H6/CH4 and CH4/H2 selectivities of ZIFs. To compute the mixture adsorption data in COFs, such as gas uptakes (N) and isosteric heat of adsorption (Qst), we performed GCMC simulations using the RASPA simulation code.47 The fluctuation method48 was employed to calculate Qst values of gases, and the difference between the isosteric heats of adsorption of gases (ΔQst) was computed by subtracting the Qst value of the weakly adsorbed gas molecule from the Qst value of the strongly adsorbed gas molecule in a mixture.
Two different operating conditions were considered. In VSA (PSA) conditions, the adsorption pressure was set to 1 bar (10 bar), while the desorption pressure was set to 0.1 bar (1 bar). The temperature was fixed at 298 K for both operating conditions. Pressure-to-fugacity conversion was utilized using the Peng–Robinson equation of state.49 The non-bonded interactions were defined with the Lennard-Jones (LJ) 12–6 potential, and a cutoff radius of 14 Å was employed for the truncation of those interactions.50 The number of unit cells for COFs was adjusted so that the dimension of the simulation box is at least twice that of the cutoff distance. Electrostatic interactions, which were defined by the Coulomb potential,51 were considered for the separation of the CH4/N2 mixture since the N2 molecule has a quadrupole moment. COFs in the CURATED database were reported with high-quality density-derived electrostatic and chemical (DDEC) charges, and these charges were used in the calculations of electrostatic interactions between N2 and COF atoms. The Ewald summation was used for the long-range interactions.52 A total of 10 000 and 20 000 cycles were set for initialization and taking ensemble averages of GCMC simulations, respectively. The H2 molecule was modeled as a single-site LJ 12–6 potential, and its potential parameters were taken from the Buch model.53 CH4 and C2H6 molecules were modeled using the united atom model of the TraPPE force field,54 where CH4 was modeled as a single-site spherical LJ 12–6 potential and C2H6 was modeled as a two-site LJ 12–6 potential.55 A three-site model was employed for N2 where two N atoms are residing on the sides and partial charges are at the center of mass.56 Potential parameters of COF atoms were taken from the DRIEDING force field57 since a good agreement between simulated and experimentally reported CO2 isotherms of COFs was found in our previous works.40,41 A good agreement between simulations and experimentally measured CH4 adsorption isotherms of COFs58 is also seen in Figure S1 of the Supporting Information (SI). Using the results obtained from GCMC simulations, we calculated various adsorbent performance evaluation metrics including adsorption selectivity (Sads), working capacity (ΔN), adsorbent performance score (APS), and percent regenerability (R%), as listed in Table 1.
Table 1. Calculation of Metrics Used to Assess Adsorption- and Membrane-Based Gas Separation Performances of COFsa.
| metrics | formula | |
|---|---|---|
| adsorption selectivity | ||
| working capacity (mol/kg) | ||
| adsorbent performance score (mol/kg) | ||
| percent regenerability | ||
| diffusion selectivity | ||
| membrane selectivity | ||
| permeability (Barrer) |
i: Selected component, CH4 for CH4/H2 and CH4/N2 mixtures, C2H6 for the C2H6/CH4 mixture. Nads (mol/kg): Gas uptake at adsorption conditions. Ndes (mol/kg): Gas uptake at desorption conditions. y: Composition of the gas species in the bulk phase. f (Pa): Partial pressure of gas species in the mixture. c (mol/m3): Gas loading obtained from GCMC simulations. D (m2/s): Self-diffusivity of gas obtained from MD simulations. 1 Barrer = 3.348 × 10–16 mol·m/(m2·s·Pa).
The quantitative analyses of CH4, C2H6, N2, and H2 interactions with the three common top COFs for C2H6/CH4, CH4/N2, and CH4/H2 separations were performed using DFT calculations. Cluster models were generated to represent the linkers of these three common top COFs. To mimic the constrained environment of the COFs, selected key atoms in cluster models were kept frozen in their crystalline positions, and the rest of the atoms were allowed to be fully optimized. All possible conformations of the COF–gas pairs were located with the Becke–three-parameter-Lee–Yang–Parr (B3LYP)59,60 functional including Grimme’s D2 correction61 employing the all-electron 6-31G* basis set using the Gaussian0962 program package. The vibrational frequency calculations were performed to ensure that no imaginary frequency remained. The binding energies between the gases and the COFs were calculated using ΔEbind= ECOF+Gas – (ECOF+ EGas) + δBSSE, where ECOF+Gas is the energy of the COF–gas complex, ECOF and EGas denote the COF and gas energies, respectively, and δBSSE is the basis set superposition error correction employed by the Counter Poise approach. The magnetic properties of the COFs were calculated by the gauge-independent atomic orbital (GIAO) method with the 6-31+G** basis set.
MD simulations were performed for 3 × 106 cycles in the NVT ensemble to compute the self-diffusivities of CH4, H2, N2, and C2H6 gases in COFs. A total of 5 × 105 cycles were set for both initialization and equilibration of NVT-MD simulations, where the Nosé–Hoover thermostat was employed.63 The slope of mean-square displacements of gases was used to calculate the self-diffusivities by the Einstein equation.64 All COFs were assumed to be rigid to save computational time. Since the pore apertures of the COF structures in this study are larger than the kinetic diameters of the gas molecules, the results of our molecular simulations are not expected to be affected by the flexibility of the structures.27 The gas loadings computed with GCMC simulations at 1 bar and 298 K were taken as the number of gas molecules in the simulation box for NVT-MD simulations to calculate the self-diffusivities of gases through the pores of COFs. Gas permeabilities (P), diffusion selectivities (Sdiff), and membrane selectivities (Smem) were computed as shown in Table 1. We performed MD simulations for all of the COFs for membrane-based CH4/H2 mixture separation and identified the top 10 membranes based on their permeabilities and selectivities. Considering the expense of MD simulations, only these top 10 COF membranes were studied for membrane-based separation of CH4/N2 and C2H6/CH4 mixtures.
3. Results and Discussion
First, we analyzed adsorption-based CH4 separation performances of 572 COFs. Figure 1 shows the adsorption selectivity (Sads) of COFs as a function of their working capacities (ΔN) for CH4/H2, CH4/N2, and C2H6/CH4 mixture separations at VSA and PSA conditions. In CH4/H2 and CH4/N2 mixtures, CH4 interacts more strongly with the framework than H2 and N2, making all COFs CH4-selective adsorbents. Thus, we reported the selectivities and working capacities based on CH4 for CH4/H2 and CH4/N2 separations. On the other hand, for the C2H6/CH4 mixture, selectivities and working capacities were reported based on C2H6 since the two site-represented C2H6 molecules were more strongly adsorbed into COFs than the single sited-CH4. Sads,CH4/H2 values of COFs were calculated to be in the ranges of 2–102 and 2–85 at VSA and PSA conditions, respectively. Higher selectivities were observed for COFs at VSA conditions compared to PSA conditions for CH4/H2 separation, as shown in Figure 1a,b, which can be attributed to the packing effects favoring adsorption of the smaller molecule at higher pressures.65 ΔNCH4 values of COFs at VSA conditions were between 0.03 and 1.15 mol/kg, whereas at PSA conditions, higher values, 0.22–4.97 mol/kg, were observed. These higher working capacities were the result of higher gas uptakes achieved at PSA conditions.
Figure 1.
Selectivity and working capacity of 572 COFs for separation of (a, b) CH4/H2: 50/50 mixture, (c, d) CH4/N2: 50/50 mixture, and (e, f) C2H6/CH4: 50/50 mixture at VSA (1–0.1 bar at 298 K) and PSA (10–1 bar at 298 K) conditions. Colors represent APSs of COFs. MOFs from our previous works21,23,88 (black data points, 4350 MOFs for CH4/H2, 4738 MOFs for CH4/N2, and 281 MOFs for C2H6/CH4 separations) were included in (b), (d), and (f) for comparison.
A promising adsorbent for gas separation applications should achieve the combination of high selectivity and high working capacity.66 Therefore, we calculated the APS of COFs, the multiplication of Sads and ΔN, to assess the gas separation performances of COFs and presented with the color scale in Figure 1a,b. Higher ΔNCH4 values were obtained for COFs at PSA conditions rather than at VSA conditions, whereas there was no significant difference between the selectivities achieved at VSA and PSA conditions. Therefore, higher APSs were obtained at PSA conditions. These results suggest that using COFs in PSA-based CH4/H2 separation would be more efficient than VSA-based separation. The COFs with the highest APS at PSA conditions mostly have lower ΔNCH4 compared to other COFs. Therefore, for CH4/H2 separation, Sads has a more pronounced impact than ΔN to achieve a high APS in the PSA process. Similar results were obtained for CH4/N2 separation at PSA and VSA conditions in Figure 1c,d. Due to the higher ΔNCH4 values achieved at PSA conditions (0.03–1.10 mol/kg at VSA, 0.21–4.70 mol/kg at PSA), APSs of COFs were higher than those at VSA conditions; thus, PSA can be considered as a desirable condition for CH4/N2 separation.
The largest difference between the performances of COFs for CH4/H2 and CH4/N2 separations was observed in the selectivities. Sads values of COFs were between 2 and 85 for PSA-based CH4/H2 separation, whereas Sads values of COFs were in the range of 1–7 for CH4/N2 separation at the same conditions. To investigate this difference, in Figure S2, we analyzed the ΔQst values of mixtures, which can be related to selectivities of materials.67,68 We observed that ΔQst,CH4/H2 values of COFs (2.72–17.14 kJ/mol) are always higher than ΔQst,CH4/N2 values (1.14–6.42 kJ/mol), leading to lower selectivities for the CH4/N2 mixture. This can be explained by two reasons: (i) LJ energy parameter of N2 is larger than that of H2, which leads to larger LJ interactions between COF atoms and N2 molecules; and (ii) N2 molecules have electrostatic interactions with the COF atoms, which are absent for H2 molecules. Thus, the competition between N2 and CH4 is stronger compared to H2 and CH4, leading to lower selectivities for the CH4/N2 mixture. To better understand which of these interactions contribute more to the adsorption of the N2 molecule, we switched off the Coulombic interactions between COF atoms and N2 molecules and reperformed GCMC simulations. Sads values of COFs computed with and without considering the Coulombic interactions between COFs and N2 are presented in Figure S3. Sads,CH4/N2 values do not significantly change when the electrostatic interactions were neglected, which can be attributed to the absence of strong metal atoms in COFs.38,40 Therefore, we concluded that LJ interactions are the main contributors to the adsorption of N2 molecules in COFs.
In Figure 1e,f, selectivities and working capacities of COFs for separation of CH4 from C2H6 are shown at VSA and PSA conditions, respectively. Sads,C2H6/CH4 (ΔNC2H6) values of COFs at VSA and PSA conditions were computed as 2–33 and 2–23 (0.1–4.3 and 0.3–16.5 mol/kg), respectively. COFs have slightly lower selectivities at PSA conditions than VSA conditions; however, the high ΔNC2H6 achieved by COFs at PSA conditions outweighs their selectivities, resulting in higher APSs for COFs at PSA conditions. Contrary to the CH4/H2 mixture, the COFs with the highest APSs were identified to have high ΔNC2H6. Thus, it can be concluded that ΔN has a greater importance than Sads for COFs to achieve high APSs for C2H6/CH4 separation.
We also included Sads and ΔN values of MOFs that we computed in our previous works21,23 in Figure 1b,d,f to compare the separation performance of COFs with that of MOFs. CH4/H2 selectivities of MOFs at PSA conditions were between 1 and 2028, which are significantly higher than the most selective COF investigated in this study (CTF-FUM, Sads: 85.3). The lower CH4/H2 selectivity of COFs compared to MOFs can be attributed to the larger pore apertures of COFs, which allow both gases to be adsorbed inside pores. Of the COFs used in this work, 80% have PLD > 10 Å, while this ratio was only 5% for MOFs. However, ΔNCH4 values of MOFs (0.01–7.27 mol/kg) are comparable to those of COFs (0.22–4.97 mol/kg). Still, MOFs outperform COFs for adsorption-based CH4/H2 separation due to their high selectivities. Selectivities and ΔNCH4 of COFs (1–7; 0.2–4.7 mol/kg) are comparable to those of MOFs (1–14; 0.1–6.6 mol/kg) for PSA-based CH4/N2 separation. This is due to the similarity in the physical features of both gases limiting the CH4/N2 mixture selectivity.24 Nevertheless, APSs of MOFs (0.2–30.7 mol/kg) are slightly higher than those of COFs (0.7–16.2 mol/kg). Still, COFs can be used as alternative adsorbents because they show better stabilities than MOFs under harsh chemical conditions, which may make COFs preferable for practical applications over MOFs.28 Similar results were obtained for PSA-based C2H6/CH4 separation, as shown in Figure 1f, where APSs of MOFs (0.4–150.5 mol/kg) are slightly higher than APSs of COFs (1.4–109.4 mol/kg); however, COFs can be good alternatives to MOFs.
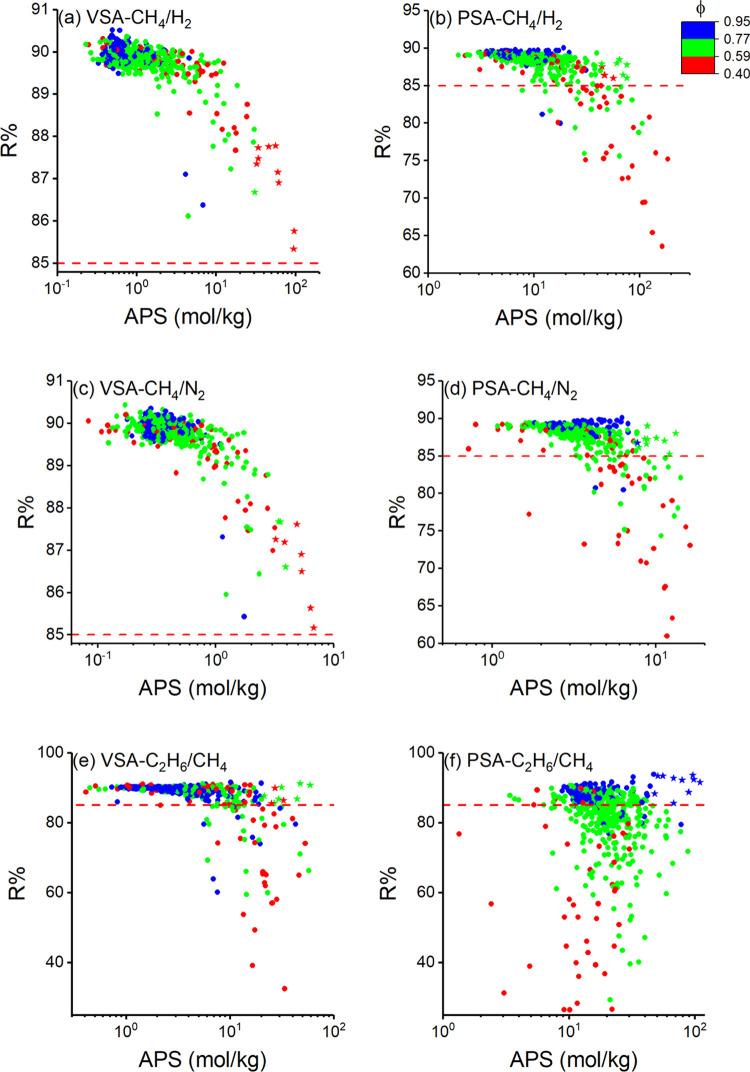
Regenerability (R%) should be considered in the selection of the top materials for cyclic VSA and PSA processes since materials offering high APSs usually have low R% values.69,70 Calculated R% values of COFs as a function of their APSs are given in Figure 2a,b for CH4/H2, in Figure 2c,d for CH4/N2, and in Figure 2e,f for C2H6/CH4 separation at VSA and PSA conditions, respectively. We set a desired value, R% > 85% (red dotted line), to easily identify the promising COFs. For CH4/H2 and CH4/N2 mixtures, all of the COFs are over the R% > 85% line at VSA conditions, as shown in Figure 2a,c. This is because of the low amount of adsorbed gas in the pores at low pressure, which can easily desorb at 0.1 bar. However, as the pressure increases, gases prefer to remain inside the pores, especially for COFs with lower porosities, due to the strong confinement inside the narrow pores.71 Therefore, R% values of COFs at PSA conditions are lower than those at VSA conditions (Figure 2b,d). Although most of the COFs could still surpass the R% > 85% target at PSA conditions, COFs offering the best APSs are under the R% > 85 line and are unfeasible for use in practical applications. This trend was previously observed for COFs in adsorption-based CO2/N2 separation.40 For example, a COF (CTF-FUM) could be a top material for both CH4/H2 and CH4/N2 separations due to its high APS (85.3 mol/kg for CH4/H2, 11.7 mol/kg for CH4/N2) at PSA conditions; however, its R% values were calculated as 64 and 61%, respectively, making the material inefficient for a cyclic adsorption process. R% values of COFs are generally lower for C2H6/CH4 separation compared to CH4/H2 and CH4/N2 separations, as shown in Figure 2e,f. This is due to the stronger adsorption of C2H6, which decreases the R%. Similar to other mixtures, low R% values were observed for COFs with the highest APSs for C2H6/CH4 at VSA conditions. Interestingly, this trend changed in PSA conditions, where R% of COFs increases directly proportional to their APSs. This can be attributed to the dominance of ΔNC2H6 in the assessment of APS of COFs for PSA-based C2H6/CH4 separation as discussed before. The COFs with the highest APSs for PSA-based C2H6/CH4 separation have the highest porosities leading to high ΔNC2H6 and R% values. Thus, COFs can achieve a combination of high APSs with high R% values for PSA-based C2H6/CH4 separation.
Figure 2.
R% and APSs of 572 COFs for separation of (a, b) CH4/H2 mixture, (c, d) CH4/N2 mixture, and (e, f) C2H6/CH4 mixture at VSA and PSA conditions. Colors represent the porosity of COFs, and stars represent the top 10 COFs identified for each condition. Red dashed line represents the R% = 85% line.
We identified the top 10 promising COF adsorbents for each gas separation and condition by ranking the materials based on their APSs and focusing on the ones having R% > 85%. The 3D structural representations of the top COF adsorbents, shown with stars in Figure 2, are given in Figure S4, and their performance metrics are given in Tables S1–S3, along with the selectivities and working capacities of some representative conventional adsorbents for the comparison of COFs with industrially relevant materials. For PSA-based CH4/H2 and CH4/N2 separations and VSA-based C2H6/CH4 separation, COF-303 was identified to be the most promising adsorbent. COF-303 has a small pore size (LCD of 8.31 Å) relative to other COFs and low Sacc (1821 m2/g) leading to high selectivities. For PSA-based C2H6 separation, the most promising COF candidate was identified as COF-102, which has a high porosity (0.78) and high Sacc (5049 m2/g) resulting in a high working capacity. Both COF-303 and COF-102 were reported to have excellent stabilities; thus, they can be ideal materials for utilization in practical applications.33,72 Most of the top COF candidates identified for CH4/H2 separation have higher Sads and ΔN values than commercial adsorbents. For example, Sads,CH4/H2 of commercial zeolite 13X is 22 at 1 bar and 308 K, while its ΔNCH4 is 0.32 mol/kg.73 Selectivity and working capacity of the top COFs for VSA-based CH4/H2 separation were in the ranges of 40–102 and 0.59–1.15 mol/kg, respectively. Similar results were obtained for COFs when CH4/N2 and C2H6/CH4 separations were considered. For instance, selectivity and working capacity of the top COFs (4.1–8.2 and 0.6–1.1 mol/kg, respectively) identified for VSA-based CH4/N2 separation were higher than those of Linde 4A (Sads,CH4/N2: 3.4 and ΔNCH4: 0.4 mol/kg for an equimolar mixture) measured at 1 bar and 302 K.74 Also, the top COFs identified for VSA-based C2H6/CH4 separation achieved higher selectivities and working capacities than zeolite CaX (ideal selectivity: 5; ΔNC2H6: 0.9 mol/kg) at the same conditions.75 Thus, these results suggest that COFs can outperform conventional adsorbents for the purification of CH4 from H2, N2, and C2H6.
We then examined the relation between the structural properties of 572 COF adsorbents and their gas separation performances to highlight the best combination of structural features. Figure 3 shows the APSs of COFs as a function of their PLDs and porosities for CH4/H2, CH4/N2, and C2H6/CH4 mixture separations at PSA conditions. Results for CH4/H2 and CH4/N2 mixtures at VSA conditions are given in Figure S5. APSs increase with a decrease in PLDs of COFs for all mixtures at all conditions. As the pore aperture becomes smaller, selectivity increases in favor of the strongly adsorbed gas.71 As a result, the top COFs identified for each separation have PLDs < 10 Å, except for C2H6/CH4 separation at PSA conditions in which ΔN is the dominating factor in APSs of COFs as discussed earlier. Changes in porosity have two different effects on the adsorption properties of COFs: a decrease in porosity may (i) lead to an increase in selectivities of COFs due to the stronger confinement of gases, and (ii) result in an increase in working capacities of COFs due to higher gas uptakes. For PSA-based CH4/H2 separation and VSA-based C2H6/CH4 separation, APSs are negatively correlated with COFs’ porosities since Sads is the dominant factor in the assessment of APSs for these separations. Therefore, most of the top COFs have porosity < 0.6. On the other hand, ΔN is the dominating factor for the assessment of APSs in PSA-based C2H6/CH4 separation, and porosity is positively correlated with APSs. Thus, COFs with high porosities, >0.8, are identified as the most promising materials.
Figure 3.
PLDs and porosities of 572 COFs as a function of their APSs calculated for (a) CH4/H2 and (b) CH4/N2 separation at PSA conditions, and (c, d) C2H6/CH4 separation at VSA and PSA conditions. Big circles with thick edges represent the top 10 COFs identified for each mixture at each condition.
Among the top COFs identified for each mixture, COF-303, COF-300, and PZ-COF2 are common for PSA-based CH4/H2 and CH4/N2 separations and VSA-based C2H6/CH4 separation. Thus, these three common top materials were studied in detail by the DFT calculations. We prepared cluster models consisting of the organic linker fragments and optimized them in the presence of gas molecules. As an important tool for the interpretation of electrostatic interactions of a material with adsorbate molecules, electrostatic potential maps (ESPs) were generated and are presented in Figure 4 together with the 3D representations of the cluster models. All three COFs are composed of the same atoms, which differ in their arrangements in space. They have low PLDs (5.6–8.9 Å), leading to selective separation of gases and similar porosities (0.61–0.76). In COF-303, the repeating unit is the benzene-1,4-diamine double-bonded to phenyl groups by two amine ends. According to the ESP map, the most negative regions are located around conjugated N bonds, which act as good electron-donor sites. Aromatic rings, which are located at the linkers’ joint, are also expected to exhibit good adsorption capability due to the electrostatic potentials generated. 1,4-phenylenedimethanimine scaffolds are bonded to phenyl groups by amine ends to form the linker fragments in COF-300. Electron-donating sites are located on the N atoms and the benzene rings. PZ-COF2 consists of phenazine scaffolds in which the most electronegative regions are concentrated on the N atoms, whereas the phenyl rings show less electronegativity.
Figure 4.
Three-dimensional representations and electrostatic potential maps of the linker fragments of the three common top COFs. White, hydrogen; gray, carbon; blue, nitrogen. Negative (positive) regions are depicted in red (blue) color.
From ESP maps, possible adsorption sites were determined and then the interactions between the gas molecules and the linker scaffolds were investigated to identify the most probable adsorption sites for each COF–gas pair. The binding energies for the COF–gas pair conformations were calculated, and the results are given in Table 2. To elucidate the relationship between the magnetic index of aromaticity of the rings and the stabilization of the gas molecules upon interaction, nucleus-independent chemical shift (NICS)76,77 indexes were calculated for the linker fragments. Aromaticity of an organic or inorganic compound can be quantified by measuring the molecular magnetic response properties, such as chemical shifts, nuclear magnetic shieldings, and magnetic susceptibility, in the presence of an external magnetic field. The NICS method has been extensively employed for the evaluation of aromaticity, antiaromaticity, and nonaromaticity of ring systems, and it uses the induced current densities to quantify the aromaticity indices.78−80 It is computed by introducing a dummy atom at the ring center (the average coordinates of the nonweighted heavy atoms) or at a certain distance below or above the ring, and then, the magnitude of the shielding constant is obtained. The negative value of the calculated absolute shielding at some point of the system is defined as NICS, and the more aromatic character of the ring corresponds to the more negative NICS value. It has been previously reported that NICS(1), which is obtained at 1 Å above the molecular plane, reflects the π-electron density better than NICS(0).81 As presented in Table 2, the central phenyl fragment (P1) in COF-303 was calculated to be slightly less aromatic (−8.82 ppm) than the phenyl fragment in the edge (P2) (−9.19 ppm) due to the presence of two nitrogen atoms bonded to P1, which help in the conjugation of the π-electrons in the ring system through the imine bonds. This was confirmed by Cring–Nimide (1.400 Å) and Cring–Cring (1.408 Å) distances of the P1 ring, which are almost equal, and Cring–Cimide (1.460 Å) and Cring–Cring (1.403 Å) distances of the P2 fragment, which are not equal. All of the gases are adsorbed stronger by the more aromatic P2 site, as illustrated in Figure S6. Figure S6a displays that two hydrogens of CH4 form C–H···π interactions with the center of masses of P2 and P3 rings, which are on the C6 axis of benzene, and one hydrogen of CH4 forms a H-bond with the electron-rich N atom of COF-303. The shorter C–H···π bond lengths (2.60 Å with P2 and 2.62 Å with P3) compared to the C–H···N bond (3.68 Å) indicate that the contribution of the C–H···π interaction to the stabilization of the CH4 molecule is larger. Similar interactions are observed between COF-303 and C2H6, as shown in Figure S6b. C2H6 interacts slightly stronger with the P3 fragment (2.57 Å) than with the P2 fragment (2.78 Å), and the H-bond length of C–H···N is 2.81 Å, shorter than the C–H···N bond in COF-303-CH4. This can be attributed to the larger polarizability of C2H6 (α = 4.226 Å3),82 which allows C2H6 to form stronger dispersion interactions with P3 compared to CH4 (α = 2.448 Å3),82 and thus stronger adsorption of C2H6 (ΔEbind = 15.99 kJ/mol) was observed with respect to CH4 (ΔEbind = 10.38 kJ/mol). Figure S6c displays the interactions between N2 and COF-303. The internuclear axis of N2 is oriented parallel to the C6 axis of benzene rings at a distance of 3.23 Å above the P2 plane and 3.17 Å above the P3 plane, making an attractive quadrupole–quadrupole interaction. The lower polarizability of N2 (α = 1.710 Å3)82 compared to C2H6 and CH4 results in the formation of weaker dispersion forces with COF-303, and eventually weaker adsorption was observed (ΔEbind = 9.39 kJ/mol). The geometrical representations of H2 and COF-303, which are aligned to form the most favorable electrostatic interaction, are illustrated in Figure S6d. The interatomic distance between one H atom of H2 and the center of mass of P2 is 2.78 Å and the other H atom of H2 and the center of mass of P3 is 2.67 Å. H2 is the least polarizable (α = 0.787 Å3)82 and the least adsorbed molecule by COF-303 among the gases considered in this study (ΔEbind = 0.62 kJ/mol).
Table 2. Calculated NICS(1) Values (ppm) for the Respective Linker Fragments and Calculated Binding Energies (kJ/mol) of the COF–Gas Pairs with Respect to the Positions of the Gases along with the Qst (kJ/mol) of COF–Gas Pairs Obtained from GCMC Simulationsa.
| CH4 |
C2H6 |
N2 |
H2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| fragment | NICS(1) | Ebind | Qst | Ebind | Qst | Ebind | Qst | Ebind | Qst | |
| COF-303 | P1 | –8.82 | –5.30 | –12.14 | –11.10 | –19.54 | –5.63 | –9.12 | –0.06 | –3.39 |
| P2 | –9.19 | –10.38 | –15.99 | –9.39 | –0.62 | |||||
| COF-300 | P1 | –8.83 | –5.50 | –11.56 | –12.03 | –18.46 | –4.44 | –8.59 | 0.55 | –3.27 |
| P2 | –8.94 | –12.07 | –21.05 | –9.85 | –0.72 | |||||
| PZ-COF2 | P1 | –11.02 | –7.12 | –14.22 | –9.82 | –23.52 | –5.23 | –11.03 | 0.45 | –3.52 |
| P2 | 0.40 | –7.66 | –14.99 | –5.83 | –0.20 | |||||
| P3 | –9.11 | –6.23 | –14.99 | –5.68 | 0.37 | |||||
Negative signs of binding energies imply attractive interaction.
In COF-300, NICS assigns almost equal aromaticities to the P2 scaffold in the edge (−8.94 ppm) and the central P1 fragment (−8.83 ppm). The nitrogen atom bonded to P2 enriches the delocalized π-electron density in the ring system (Cring–Nimide = 1.400 Å and Cring–Cring = 1.409 Å), but the tetrahedral carbon at the joint of the linkers does not take place in delocalization of the ring electrons (Cring–Cjoint = 1.524 Å). P1 is bonded to N atoms through doubly bonded carbon atoms (Cring–Cimide = 1.461 Å and Cring–Cring = 1.408 Å). The interactions of the gases with COF-300 are very similar to their interactions with COF-303, as displayed in Figure S7a–d. All of the gas molecules are adsorbed stronger by P2 fragments (Table 2) due to the stronger electrostatic potential generated by the more electron-rich P2 and P3 scaffolds, as represented in Figure 4b. While the adsorption energies of N2 and H2 with COF-300 (9.85 and 0.72 kJ/mol, respectively) are very similar to their binding energies with COF-303, C2H6 and CH4 are adsorbed strongly in COF-300 (21.05 and 12.07 kJ/mol, respectively) due to the presence of more electron-rich P2 and P3 regions in COF-300, as can be deduced from the ESP maps in Figure 4b. Since N2 and H2 have very low polarizabilities, they are not affected by the dispersion forces induced by P2 and P3 as much as C2H6 and CH4.
While P1 and P3 fragments were calculated to be aromatic (−11.02 and −9.11 ppm) in PZ-COF2, the P2 fragment was determined as nonaromatic/antiaromatic (0.40 ppm). According to the binding energies given in Table 2, CH4, N2, and H2 are adsorbed almost equally in P1, P2, and P3 regions, whereas C2H6 is adsorbed significantly stronger by P2–P3 fragments. CH4 is stabilized on the P2 ring since it can form two C–H···π bonds with adjacent aromatic P3 rings at distances of 3.34 and 3.12 Å from the C6 axis of P3 rings, as shown in Figure S8a. C2H6 is adsorbed stronger by PZ-COF2 (ΔEbind = 14.99 kJ/mol) than CH4 (ΔEbind = 7.66 kJ/mol) since the former can form three C–H···π bonds with the adjacent P3 fragments as a result of its larger size and larger polarizability as discussed above and shown in Figure S8b. Similar to the interactions formed between N2 and COF-300 and COF-303, the N2 molecule’s internuclear axis is oriented parallel to the C6 axis of the P2 ring at a distance of 3.01 Å (Figure S8c). The almost equal binding energies calculated between N2 and P1, P2, P3 regions, which possess different degrees of aromaticities, point out that adsorption of N2 is not related to the aromatic/nonaromatic character of a ring. The same conclusion was valid for H2 since the affinities of H2 toward P1, P2, and P3 sites are very similar, as can be tracked from Table 2. Figure S8d illustrates the intermolecular interaction between the P2 ring of PZ-COF2 and H2, which is driven by the weak van der Waals forces.
The snapshots taken from GCMC simulations show that all of the gas molecules primarily occupy the P2 and P3 regions, as shown in Figure S9, in harmony with the DFT calculations. As shown in Table 2, although Ebind values between C2H6, CH4, H2, N2 and COF-303, PZ-COF2, COF-300 obtained from the B3LYP-D2/6-31G* level are slightly different from the Qst values calculated by GCMC simulations, adsorption strengths of the gases are on the order of C2H6 > CH4 > N2 > H2 for all COFs, showing a good agreement with our GCMC simulations. Analysis of gas–COF pairs reveals that adsorption is mainly driven by the dispersion interactions between the COFs and gases, which are directly related to the polarizabilities of the molecules. On the other hand, as stated by Levitt and Perutz,83 aromatic rings act as H-bond acceptors, and thus, C–H···π bonds formed between the linkers and C2H6 and CH4 increase the strength of their adsorption. This was proven by comparing the binding energies calculated for each conformation of the gases with respect to their locations on P1, P2, and P3 regions of the COFs. Therefore, besides increasing the number of aromatic sites in a linker of COF, increasing the extent of aromaticity of the linker rings may result in stronger interactions and stronger adsorption of C2H6 and CH4 in a COF.
Finally, we analyzed membrane-based CH4 separation performances of COFs. We performed MD simulations for all COFs and computed gas permeabilities and selectivities for CH4/H2 separation, as shown in Figure 5. Since H2 is weakly adsorbed and has a smaller kinetic radius than CH4, H2 diffusion is favored over CH4 in all COFs. Therefore, we reported the diffusion selectivities of COFs based on H2. Sdiff,H2/CH4 values of COFs mostly dominate their Sads,CH4/H2, resulting in H2-selective membranes. H2/CH4 adsorption, diffusion, and membrane selectivities of 572 COFs are given in Figure 5a. A trade-off between Sads,H2/CH4 and Sdiff,H2/CH4 is observed since materials with high adsorption selectivities offer low diffusion selectivities and vice versa. For example, in nine of the COFs, diffusion of H2 was exceptionally faster than that of CH4, resulting in Sdiff,H2/CH4 > 20, as shown by the blue points. Due to very low H2/CH4 adsorption selectivities, mediocre Smem,H2/CH4 were obtained for those COFs (0.01–4.30). The highest membrane selectivities were achieved for COFs with a combination of high Sads,H2/CH4 (>0.4) and mediocre Sdiff,H2/CH4 (>7). For 223 COFs, high CH4/H2 adsorption selectivities dominated the H2/CH4 diffusion selectivities, leading to CH4-selective membranes (Smem,CH4/H2 > 1). Interestingly, 12 of those COFs were offering high CH4/H2 adsorption selectivities (31.1 < Sads,CH4/H2 < 102.7) and diffusion selectivities close to 1 (0.25 < Sdiff,CH4/H2 < 0.70), leading to high CH4/H2 membrane selectivities (10.4 < Smem,CH4/H2 < 70.3). Therefore, these 12 COFs can be used as CH4-selective membranes for separating CH4/H2 mixtures.
Figure 5.

(a) Calculated membrane, diffusion, and adsorption selectivities of 572 COFs at 1 bar. Hollow circles represent the top COF membranes identified for H2/CH4 separation. Sads,CH4/H2 of COFs are provided in purple under the x-axis. (b) H2 permeabilities and H2/CH4 membrane selectivities of COFs calculated at 1 bar along with Robeson’s upper bound.85 Colors represent the porosities of COFs. Membrane-based H2/CH4 separation performances of 4240 MOFs obtained from our previous work are given in gray diamonds for comparison.84
Gas permeabilities of 572 COFs are given as a function of their membrane selectivities in Figure 5b. H2/CH4 separation metrics for MOF membranes computed at infinite dilution from our previous work84 and Robeson’s upper bound,85 which represents the performance limit of polymeric membranes, were also included in Figure 5b for comparison of COFs with MOFs and polymers. We extended the upper bound for permeabilities that are out of the reach of polymeric membranes. H2/CH4 membrane selectivities and H2 permeabilities of COFs were in the range of 0.01–4.30 and 4.6 × 103–1.2 × 106 Barrer, respectively. We observed that PH2 values of COFs increase as porosities of COFs increase. This is due to the high self-diffusivity of H2 in large pore spaces. Interestingly, Smem,H2/CH4 values of COFs also increase as a function of their porosities, which can be attributed to the low Sads,CH4/H2 achieved for COFs with high porosities. Thus, we conclude that COFs with high porosities are promising membrane candidates for separation of H2 from CH4. The top 10 COF membranes having PH2 > 7.5 × 105 Barrer together with the highest membrane selectivities (2.93–4.14) for selective separation of H2 from CH4 are displayed in Figure 5. As expected, all of the top COF membranes have high porosities (>0.92) and large pore sizes (LCD > 17.5 Å). Many COFs in Figure 5b were observed to surpass Robeson’s upper bound, indicating that COFs can outperform polymeric membranes. When compared with MOF membranes, COF membranes are shown to offer lower Smem,H2/CH4; however, large pore sizes and high porosities of COFs allow them to achieve high H2 permeabilities. It is also important to note that the membrane properties of MOFs were computed for single-component gases at the infinite dilution condition without considering any gas–gas interactions, and therefore, we can conclude that COFs have comparable and even better membrane-based H2/CH4 separation potentials than MOFs.
C2H6/CH4 and N2/CH4 separation potentials of the top 10 COFs identified for H2/CH4 separation are given in Figure S10. N2 permeabilities of COFs were observed to be high (1.9 × 105–2.7 × 105 Barrer); however, all 10 COFs were identified to be unselective materials since their Smem,N2/CH4 values are between 0.66 and 0.87. This is attributed to the similar kinetic diameters of both gases (σN2 = 3.6 Å, σCH4 = 3.8 Å)86 and the very large pores of the COFs, which result in similar self-diffusivities for N2 and CH4 in these COFs. Therefore, with respect to the data obtained from the 10 COFs investigated, we concluded that COF membranes are not proper materials for selective N2/CH4 separation. COF membranes could achieve very high C2H6 permeabilities (1.9 × 105–3.2 × 105 Barrer) for C2H6/CH4 separation, which is higher than the unary C2H6 permeability of zeolite MFI membranes experimentally measured at 298 K and 1 bar (2340 GPU corresponding to 1.9 × 103 Barrer).87 Although COFs could also offer higher C2H6/CH4 membrane selectivities (0.63–1.19) than zeolite MFI (ideal selectivity: 0.57), these selectivities are still very low for selective separation of C2H6 from CH4. Overall, COF membranes were found to offer strong potential only for CH4/H2 separation among all separations considered in this study.
4. Conclusions
In this work, GCMC and MD simulations were performed to reveal the potentials of 572 COFs for both adsorption- and membrane-based CH4 separation from H2, N2, and C2H6. Adsorption properties of COFs for equimolar CH4/H2, CH4/N2, and C2H6/CH4 mixture separations were calculated for VSA and PSA processes. PSA-based separation was found to be preferable for all of the mixtures due to the high working capacities of COFs. The top 10 COF adsorbents having R% > 85% were identified according to their APSs, which outperform traditional adsorbents, zeolites, activated carbon, and mesoporous carbon, for all of the mixture separations considered in this work, indicating that COFs have the potential to replace traditional materials for CH4 purification. Structure–performance relations revealed that COFs with PLDs < 10 Å are promising adsorbents for CH4 separation. Moreover, COFs with low porosities (<0.6) are observed to have the best separation potential for VSA- and PSA-based CH4/H2 separation, whereas COFs with high porosities (>0.8) are found to be the best materials for PSA-based C2H6/CH4 separation. Among the top COFs, COF-303, COF-300, and PZ-COF2 were found to be common materials for PSA-based CH4/H2 and CH4/N2 separations and VSA-based C2H6/CH4 separation. DFT calculations were carried out for these three common top materials to investigate the adsorption dynamics of CH4, H2, N2, and C2H6. The binding energies of gases obtained from DFT calculations were found to be in the order of C2H6 > CH4 > N2 > H2, in accordance with the results of GCMC simulations. For the first time, we applied NICS as an aromaticity criterion to COFs to determine the dependency of adsorption strength of the gases on the strength of aromaticity of the rings on the linkers. We showed that introducing multiple aromatic fragments to the linkers and increasing the extent of aromaticity of the rings in the linkers result in stronger adsorption of C2H6 and CH4, whereas N2 and H2 are not directly affected by the aromaticity degree of the rings. Therefore, calculated NICS indexes may guide experimentalists for the design of new COFs with improved CH4 and C2H6 selectivities over N2 and H2. MD simulations were performed to assess membrane-based H2/CH4 separation performances of COFs. Results showed that there is a trade-off between Sads,H2/CH4 and Sdiff,H2/CH4 values of COFs and COF membranes can achieve high H2 permeabilities (4.69 × 103–1.24 × 106 Barrer) and mediocre selectivities (0.01–4.3), outperforming traditional polymers and high-performing MOFs for membrane-based separation of H2 from CH4. Moreover, 12 COFs were shown to have high Smem,CH4/H2 (10.4–70.3), demonstrating the potential of COFs to act as CH4-selective membranes. The top 10 COF membranes identified for H2/CH4 separation were found to be unselective for N2/CH4 and C2H6/CH4 separations, but they can achieve higher N2 and C2H6 permeabilities than polymer and zeolite membranes. In conclusion, COFs were shown to be promising in PSA-based CH4/N2 and C2H6/CH4 separations, outperforming commercial adsorbents, and in membrane-based CH4/H2 separation, exceeding the performance limit of polymers. The results obtained from this multiscale computational study will open new horizons for the selection and design of high-performance COFs as an emerging class of porous materials for CH4 purification applications.
Acknowledgments
S.K. acknowledges the ERC-2017-Starting Grant. This study has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (ERC-2017-Starting Grant, grant agreement No. 756489-COSMOS).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.iecr.1c01742.
Comparison of C2H6 and CH4 adsorption isotherms obtained from GCMC simulations with experimental results; selectivities and ΔQst of COFs for CH4/H2 and CH4/N2 mixtures at 1 bar; comparison of CH4/N2 selectivities of COFs with and without considering electrostatic interactions between COF atoms and N2; lists of the top 10 COF adsorbents for CH4/H2, CH4/N2, and C2H6/CH4 separations; structural representation of the top COF adsorbents for CH4/H2, CH4/N2, and C2H6/CH4 separation applications; APSs of COFs as a function of their porosities and PLDs for CH4/H2 and CH4/N2 separations at VSA conditions; 3D representations of the interactions between the most favorable adsorption sites on the top three COFs and CH4, C2H6, N2, and H2; adsorption snapshots of C2H6, CH4, N2, and H2 in COF-300; PC2H6/CH4, PN2/CH4, Smem,C2H6/CH4, and Smem,N2/CH4 of the COF membranes; Cartesian coordinates of cluster models and gas molecules calculated at the B3LYP-D2/6-31G* level of theory (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Litvinenko V. The Role of Hydrocarbons in the Global Energy Agenda: The Focus on Liquefied Natural Gas. Resources 2020, 9, 59 10.3390/resources9050059. [DOI] [Google Scholar]
- Ponraj Y. K.; Borah B. Separation of Methane from Ethane and Propane by Selective Adsorption and Diffusion in MOF Cu-BTC: A Molecular Simulation Study. J. Mol. Graphics Modell. 2020, 97, 107574 10.1016/j.jmgm.2020.107574. [DOI] [PubMed] [Google Scholar]
- Bhadra S.; Farooq S. Separation of Methane–Nitrogen Mixture by Pressure Swing Adsorption for Natural Gas Upgrading. Ind. Eng. Chem. Res. 2011, 50, 14030–14045. 10.1021/ie201237x. [DOI] [Google Scholar]
- Knaebel S. P.; Ko D.; Biegler L. T. Simulation and Optimization of a Pressure Swing Adsorption System: Recovering Hydrogen from Methane. Adsorption 2005, 11, 615–620. 10.1007/s10450-005-5994-4. [DOI] [Google Scholar]
- Olajossy A.; Gawdzik A.; Budner Z.; Dula J. Methane Separation from Coal Mine Methane Gas by Vacuum Pressure Swing Adsorption. Chem. Eng. Res. Des. 2003, 81, 474–482. 10.1205/026387603765173736. [DOI] [Google Scholar]
- Lin Y.; Kong C.; Zhang Q.; Chen L. Metal-Organic Frameworks for Carbon Dioxide Capture and Methane Storage. Adv. Energy Mater. 2017, 7, 1601296 10.1002/aenm.201601296. [DOI] [Google Scholar]
- Alonso A.; Moral-Vico J.; Markeb A. A.; Busquets-Fité M.; Komilis D.; Puntes V.; Sánchez A.; Font X. Critical Review of Existing Nanomaterial Adsorbents to Capture Carbon Dioxide and Methane. Sci. Total Environ. 2017, 595, 51–62. 10.1016/j.scitotenv.2017.03.229. [DOI] [PubMed] [Google Scholar]
- Jiang J. Molecular Simulations in Metal–Organic Frameworks for Diverse Potential Applications. Mol. Simul. 2014, 40, 516–536. 10.1080/08927022.2013.832247. [DOI] [Google Scholar]
- Deng H.; Grunder S.; Cordova K. E.; Valente C.; Furukawa H.; Hmadeh M.; Gándara F.; Whalley A. C.; Liu Z.; Asahina S.; et al. Large-Pore Apertures in a Series of Metal-Organic Frameworks. Science 2012, 336, 1018–1023. 10.1126/science.1220131. [DOI] [PubMed] [Google Scholar]
- Daglar H.; Gulbalkan H. C.; Avci G.; Aksu G. O.; Altundal O. F.; Altintas C.; Erucar I.; Keskin S. Effect of MOF Database Selection on the Assessment of Gas Storage and Separation Potentials of MOFs. Angew. Chem., Int. Ed. 2021, 60, 7828–7837. 10.1002/anie.202015250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daglar H.; Keskin S. Recent Advances, Opportunities, and Challenges in High-Throughput Computational Screening of MOFs for Gas Separations. Coord. Chem. Rev. 2020, 422, 213470 10.1016/j.ccr.2020.213470. [DOI] [Google Scholar]
- Belmabkhout Y.; Bhatt P. M.; Adil K.; Pillai R. S.; Cadiau A.; Shkurenko A.; Maurin G.; Liu G.; Koros W. J.; Eddaoudi M. Natural Gas Upgrading Using a Fluorinated MOF with Tuned H2S and CO2 Adsorption Selectivity. Nat. Energy 2018, 3, 1059–1066. 10.1038/s41560-018-0267-0. [DOI] [Google Scholar]
- Qiao Z.; Xu Q.; Jiang J. High-Throughput Computational Screening of Metal-Organic Framework Membranes for Upgrading of Natural Gas. J. Membr. Sci. 2018, 551, 47–54. 10.1016/j.memsci.2018.01.020. [DOI] [Google Scholar]
- Li J.-R.; Kuppler R. J.; Zhou H.-C. Selective Gas Adsorption and Separation in Metal–Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. 10.1039/b802426j. [DOI] [PubMed] [Google Scholar]
- Lin R.-B.; Xiang S.; Xing H.; Zhou W.; Chen B. Exploration of Porous Metal–Organic Frameworks for Gas Separation and Purification. Coord. Chem. Rev. 2019, 378, 87–103. 10.1016/j.ccr.2017.09.027. [DOI] [Google Scholar]
- Basdogan Y.; Sezginel K. B.; Keskin S. Identifying Highly Selective Metal Organic Frameworks for CH4/H2 Separations Using Computational Tools. Ind. Eng. Chem. Res. 2015, 54, 8479–8491. 10.1021/acs.iecr.5b01901. [DOI] [Google Scholar]
- Moghadam P. Z.; Li A.; Wiggin S. B.; Tao A.; Maloney A. G.; Wood P. A.; Ward S. C.; Fairen-Jimenez D. Development of a Cambridge Structural Database Subset: A Collection of Metal–Organic Frameworks for Past, Present, and Future. Chem. Mater. 2017, 29, 2618–2625. 10.1021/acs.chemmater.7b00441. [DOI] [Google Scholar]
- Han S.; Huang Y.; Watanabe T.; Dai Y.; Walton K. S.; Nair S.; Sholl D. S.; Meredith J. C. High-Throughput Screening of Metal–Organic Frameworks for CO2 Separation. ACS Comb. Sci. 2012, 14, 263–267. 10.1021/co3000192. [DOI] [PubMed] [Google Scholar]
- Bobbitt N. S.; Chen J.; Snurr R. Q. High-Throughput Screening of Metal–Organic Frameworks for Hydrogen Storage at Cryogenic Temperature. J. Phys. Chem. C 2016, 120, 27328–27341. 10.1021/acs.jpcc.6b08729. [DOI] [Google Scholar]
- Altintas C.; Avci G.; Daglar H.; Azar A. N. V.; Erucar I.; Velioglu S.; Keskin S. An Extensive Comparative Analysis of Two MOF Databases: High-Throughput Screening of Computation-Ready MOFs for CH4 and H2 Adsorption. J. Mater. Chem. A 2019, 7, 9593–9608. 10.1039/C9TA01378D. [DOI] [Google Scholar]
- Altintas C.; Keskin S. Computational Screening of MOFs for C2H6/C2H4 and C2H6/CH4 Separations. Chem. Eng. Sci. 2016, 139, 49–60. 10.1016/j.ces.2015.09.019. [DOI] [Google Scholar]
- Wu D.; Wang C.; Liu B.; Liu D.; Yang Q.; Zhong C. Large-Scale Computational Screening of Metal-Organic Frameworks for CH4/H2 Separation. AIChE J. 2012, 58, 2078–2084. 10.1002/aic.12744. [DOI] [Google Scholar]
- Altintas C.; Erucar I.; Keskin S. High-Throughput Computational Screening of the Metal Organic Framework Database for CH4/H2 Separations. ACS Appl. Mater. Interfaces 2018, 10, 3668–3679. 10.1021/acsami.7b18037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T.; Lan Y.; Liu D.; Yang Q.; Zhong C. Large-Scale Screening and Design of Metal–Organic Frameworks for CH4/N2 Separation. Chem. - Asian J. 2019, 14, 3688–3693. 10.1002/asia.201900732. [DOI] [PubMed] [Google Scholar]
- Ponraj Y. K.; Borah B. High-Throughput Computational Screening of Metal–Organic Frameworks for the Separation of Methane from Ethane and Propane. J. Phys. Chem. C 2021, 125, 1839–1854. 10.1021/acs.jpcc.0c09117. [DOI] [Google Scholar]
- Altintas C.; Keskin S. Molecular Simulations of MOF Membranes for Separation of Ethane/Ethene and Ethane/Methane Mixtures. RSC Adv. 2017, 7, 52283–52295. 10.1039/C7RA11562H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erucar I.; Keskin S. Computational Assessment of MOF Membranes for CH4/H2 Separations. J. Membr. Sci. 2016, 514, 313–321. 10.1016/j.memsci.2016.04.070. [DOI] [Google Scholar]
- Song Y.; Sun Q.; Aguila B.; Ma S. Opportunities of Covalent Organic Frameworks for Advanced Applications. Adv. Sci. 2019, 6, 1801410 10.1002/advs.201801410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz U.; Corma A. Ordered Covalent Organic Frameworks, COFs and PAFs. From Preparation to Application. Coord. Chem. Rev. 2016, 311, 85–124. 10.1016/j.ccr.2015.12.010. [DOI] [Google Scholar]
- Feng X.; Ding X.; Jiang D. Covalent Organic Frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022. 10.1039/c2cs35157a. [DOI] [PubMed] [Google Scholar]
- Waller P. J.; Gándara F.; Yaghi O. M. Chemistry of Covalent Organic Frameworks. Acc. Chem. Res. 2015, 48, 3053–3063. 10.1021/acs.accounts.5b00369. [DOI] [PubMed] [Google Scholar]
- Ding S.-Y.; Wang W. Covalent Organic Frameworks (COFs): From Design to Applications. Chem. Soc. Rev. 2013, 42, 548–568. 10.1039/C2CS35072F. [DOI] [PubMed] [Google Scholar]
- El-Kaderi H. M.; Hunt J. R.; Mendoza-Cortés J. L.; Côté A. P.; Taylor R. E.; O’Keeffe M.; Yaghi O. M. Designed Synthesis of 3D Covalent Organic Frameworks. Science 2007, 316, 268–272. 10.1126/science.1139915. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Cao D. Effect of Li Doping on Diffusion and Separation of Hydrogen and Methane in Covalent Organic Frameworks. J. Phys. Chem. C 2012, 116, 12591–12598. 10.1021/jp302175d. [DOI] [Google Scholar]
- Tong M.; Yang Q.; Zhong C. Computational Screening of Covalent Organic Frameworks for CH4/H2, CO2/H2 and CO2/CH4 Separations. Microporous Mesoporous Mater. 2015, 210, 142–148. 10.1016/j.micromeso.2015.02.034. [DOI] [Google Scholar]
- Tong M. M.; Lan Y. S.; Yang Q. Y.; Zhong C. L. Exploring the Structure-Property Relationships of Covalent Organic Frameworks for Noble Gas Separations. Chem. Eng. Sci. 2017, 168, 456–464. 10.1016/j.ces.2017.05.004. [DOI] [Google Scholar]
- Yan T.; Lan Y.; Tong M.; Zhong C. Screening and Design of Covalent Organic Framework Membranes for CO2/CH4 Separation. ACS Sustainable Chem. Eng. 2019, 7, 1220–1227. 10.1021/acssuschemeng.8b04858. [DOI] [Google Scholar]
- Ongari D.; Yakutovich A. V.; Talirz L.; Smit B. Building a Consistent and Reproducible Database for Adsorption Evaluation in Covalent–Organic Frameworks. ACS Cent. Sci. 2019, 5, 1663–1675. 10.1021/acscentsci.9b00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg K. S.; Damasceno Borges D.; Ongari D.; Rampal N.; Talirz L.; Yakutovich A. V.; Huck J. M.; Smit B. In Silico Discovery of Covalent Organic Frameworks for Carbon Capture. ACS Appl. Mater. Interfaces 2020, 12, 21559–21568. 10.1021/acsami.0c01659. [DOI] [PubMed] [Google Scholar]
- Altundal O. F.; Altintas C.; Keskin S. Can COFs Replace MOFs in Flue Gas Separation? High-Throughput Computational Screening of COFs for CO2/N2 Separation. J. Mater. Chem. A 2020, 8, 14609–14623. 10.1039/D0TA04574H. [DOI] [Google Scholar]
- Aksu G. O.; Daglar H.; Altintas C.; Keskin S. Computational Selection of High-Performing Covalent Organic Frameworks for Adsorption and Membrane-Based CO2/H2 Separation. J. Phys. Chem. C 2020, 124, 22577–22590. 10.1021/acs.jpcc.0c07062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems T. F.; Rycroft C. H.; Kazi M.; Meza J. C.; Haranczyk M. Algorithms and Tools for High-Throughput Geometry-Based Analysis of Crystalline Porous Materials. Microporous Mesoporous Mater. 2012, 149, 134–141. 10.1016/j.micromeso.2011.08.020. [DOI] [Google Scholar]
- Junior M. J. C.; Wang Y.; Wu X.; Cai W. Computational Screening of Metal-Organic Frameworks with Open Copper Sites for Hydrogen Purification. Int. J. Hydrogen Energy 2020, 45, 27320–27330. 10.1016/j.ijhydene.2020.07.041. [DOI] [Google Scholar]
- Javani R.; Maghsoudi H.; Darvishi Gilan S.; Majidpour M. Study on Adsorption Performance of Different Adsorbents in Nitrogen/Methane Separation. Sep. Sci. Technol. 2020, 1–16. 10.1080/01496395.2020.1842889. [DOI] [Google Scholar]
- Guo H.-c.; Shi F.; Ma Z.-f.; Liu X.-q. Molecular Simulation for Adsorption and Separation of CH4/H2 in Zeolitic Imidazolate Frameworks. J. Phys. Chem. C 2010, 114, 12158–12165. 10.1021/jp908978q. [DOI] [Google Scholar]
- Guo H.; Shi F.; Ma Z.; Liu X. Simulation of Separation of C2H6 from CH4 Using Zeolitic Imidazolate Frameworks. Mol. Simul. 2014, 40, 349–360. 10.1080/08927022.2013.811722. [DOI] [Google Scholar]
- Dubbeldam D.; Calero S.; Ellis D. E.; Snurr R. Q. Raspa: Molecular Simulation Software for Adsorption and Diffusion in Flexible Nanoporous Materials. Mol. Simul. 2016, 42, 81–101. 10.1080/08927022.2015.1010082. [DOI] [Google Scholar]
- Karavias F.; Myers A. L. Isosteric Heats of Multicomponent Adsorption: Thermodynamics and Computer Simulations. Langmuir 1991, 7, 3118–3126. 10.1021/la00060a035. [DOI] [Google Scholar]
- Peng D.-Y.; Robinson D. B. A New Two-Constant Equation of State. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. 10.1021/i160057a011. [DOI] [Google Scholar]
- Tee L. S.; Gotoh S.; Stewart W. E. Molecular Parameters for Normal Fluids. Lennard-Jones 12-6 Potential. Ind. Eng. Chem. Fundam. 1966, 5, 356–363. 10.1021/i160019a011. [DOI] [Google Scholar]
- Goldstein H.; Poole C.; Safko J.. Classical Mechanics; American Association of Physics Teachers, 2002. [Google Scholar]
- Ewald P. P. Die Berechnung Optischer Und Elektrostatischer Gitterpotentiale. Ann. Phys. 1921, 369, 253–287. 10.1002/andp.19213690304. [DOI] [Google Scholar]
- Buch V. Path Integral Simulations of Mixed Para-D2 and Ortho-D2 Clusters: The Orientational Effects. J. Chem. Phys. 1994, 100, 7610–7629. 10.1063/1.466854. [DOI] [Google Scholar]
- Eggimann B. L.; Sunnarborg A. J.; Stern H. D.; Bliss A. P.; Siepmann J. I. An Online Parameter and Property Database for the Trappe Force Field. Mol. Simul. 2014, 40, 101–105. 10.1080/08927022.2013.842994. [DOI] [Google Scholar]
- Martin M. G.; Siepmann J. I. Transferable Potentials for Phase Equilibria. 1. United-Atom Description of N-Alkanes. J. Phys. Chem. B 1998, 102, 2569–2577. 10.1021/jp972543+. [DOI] [Google Scholar]
- Murthy C.; Singer K.; Klein M.; McDonald I. Pairwise Additive Effective Potentials for Nitrogen. Mol. Phys. 1980, 41, 1387–1399. 10.1080/00268978000103611. [DOI] [Google Scholar]
- Mayo S. L.; Olafson B. D.; Goddard W. A. Dreiding: A Generic Force Field for Molecular Simulations. J. Phys. Chem. A 1990, 94, 8897–8909. 10.1021/j100389a010. [DOI] [Google Scholar]
- Furukawa H.; Yaghi O. M. Storage of Hydrogen, Methane, and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. 10.1021/ja9015765. [DOI] [PubMed] [Google Scholar]
- Becke A. D. Density-Functional Thermochemistry. Iii. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]
- Lee C.; Yang W.; Parr R. G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- Grimme S.; Antony J.; Ehrlich S.; Krieg H. A Consistent and Accurate ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H.; Li X.; Caricato M.; Marenich A. V.; Bloino J.; Janesko B. G.; Gomperts R.; Mennucci B.; Hratchian H. P.; Ortiz J. V.; Izmaylov A. F.; Sonnenberg J. L.; Williams; Ding F.; Lipparini F.; Egidi F.; Goings J.; Peng B.; Petrone A.; Henderson T.; Ranasinghe D.; Zakrzewski V. G.; Gao J.; Rega N.; Zheng G.; Liang W.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Throssell K.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M. J.; Heyd J. J.; Brothers E. N.; Kudin K. N.; Staroverov V. N.; Keith T. A.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A. P.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Millam J. M.; Klene M.; Adamo C.; Cammi R.; Ochterski J. W.; Martin R. L.; Morokuma K.; Farkas O.; Foresman J. B.; Fox D. J.. Gaussian 09, rev. E.01; Gaussian Inc.: Wallingford, CT.
- Frenkel D.; Smit B.. Understanding Molecular Simulation: From Algorithms to Applications; Elsevier, 2001; Vol. 1. [Google Scholar]
- Einstein A.Investigations on the Theory of the Brownian Movement; Courier Corporation, 1956. [Google Scholar]
- Liu B.; Smit B. Molecular Simulation Studies of Separation of CO2/N2, CO2/CH4, and CH4/N2 by ZIFs. J. Phys. Chem. C 2010, 114, 8515–8522. 10.1021/jp101531m. [DOI] [Google Scholar]
- Gulbalkan H. C.; Haslak Z. P.; Altintas C.; Uzun A.; Keskin S. Assessing CH4/N2 separation potential of MOFs, COFs, IL/MOF, MOF/Polymer, and COF/Polymer composites. Chem. Eng. J. 2022, 428, 131239. 10.1016/j.cej.2021.131239. [DOI] [Google Scholar]
- Chung Y. G.; Gómez-Gualdrón D. A.; Li P.; Leperi K. T.; Deria P.; Zhang H.; Vermeulen N. A.; Stoddart J. F.; You F.; Hupp J. T.; et al. In Silico Discovery of Metal-Organic Frameworks for Precombustion CO2 Capture Using a Genetic Algorithm. Sci. Adv. 2016, 2, e1600909 10.1126/sciadv.1600909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D.; Yang Q.; Zhong C.; Liu D.; Huang H.; Zhang W.; Maurin G. Revealing the Structure–Property Relationships of Metal–Organic Frameworks for CO2 Capture from Flue Gas. Langmuir 2012, 28, 12094–12099. 10.1021/la302223m. [DOI] [PubMed] [Google Scholar]
- Wilmer C. E.; Farha O. K.; Bae Y.-S.; Hupp J. T.; Snurr R. Q. Structure–Property Relationships of Porous Materials for Carbon Dioxide Separation and Capture. Energy Environ. Sci. 2012, 5, 9849–9856. 10.1039/c2ee23201d. [DOI] [Google Scholar]
- Basdogan Y.; Sezginel K. B.; Keskin S. Identifying Highly Selective Metal Organic Frameworks for CH4/H2 Separations Using Computational Tools. Ind. Eng. Chem. Res. 2015, 54, 8479–8491. 10.1021/acs.iecr.5b01901. [DOI] [Google Scholar]
- Avci G.; Erucar I.; Keskin S. Do New MOFs Perform Better for CO2 Capture and H2 Purification? Computational Screening of the Updated MOF Database. ACS Appl. Mater. Interfaces 2020, 12, 41567–41579. 10.1021/acsami.0c12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitakbunkate T.; Blasingame T.; Moridis G.; Balbuena P. Phase Behavior of Methane–Ethane Mixtures in Nanopores. Ind. Eng. Chem. Res. 2017, 56, 11634–11643. 10.1021/acs.iecr.7b01913. [DOI] [Google Scholar]
- Ma T.; Kapustin E. A.; Yin S. X.; Liang L.; Zhou Z.; Niu J.; Li L.-H.; Wang Y.; Su J.; Li J.; et al. Single-Crystal X-Ray Diffraction Structures of Covalent Organic Frameworks. Science 2018, 361, 48–52. 10.1126/science.aat7679. [DOI] [PubMed] [Google Scholar]
- Delgado J. A.; Águeda V.; Uguina M.; Sotelo J.; Brea P.; Grande C. A. Adsorption and Diffusion of H2, CO, CH4, and CO2 in BPL Activated Carbon and 13X Zeolite: Evaluation of Performance in Pressure Swing Adsorption Hydrogen Purification by Simulation. Ind. Eng. Chem. Res. 2014, 53, 15414–15426. 10.1021/ie403744u. [DOI] [Google Scholar]
- Jensen N. K.; Rufford T. E.; Watson G.; Zhang D. K.; Chan K. I.; May E. F. Screening Zeolites for Gas Separation Applications Involving Methane, Nitrogen, and Carbon Dioxide. J. Chem. Eng. Data 2012, 57, 106–113. 10.1021/je200817w. [DOI] [Google Scholar]
- Hosseinpour S.; Fatemi S.; Mortazavi Y.; Gholamhoseini M.; Ravanchi M. T. Performance of CaX Zeolite for Separation of C2H6, C2H4, and CH4 by Adsorption Process; Capacity, Selectivity, and Dynamic Adsorption Measurements. Sep. Sci. Technol. 2010, 46, 349–355. 10.1080/01496395.2010.508478. [DOI] [Google Scholar]
- Schleyer P. V. R.; Maerker C.; Dransfeld A.; Jiao H.; Van Eikema Hommes N. J. R. Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. 10.1021/ja960582d. [DOI] [PubMed] [Google Scholar]
- Stanger A. Nucleus-Independent Chemical Shifts (Nics): Distance Dependence and Revised Criteria for Aromaticity and Antiaromaticity. J. Org. Chem. 2006, 71, 883–893. 10.1021/jo051746o. [DOI] [PubMed] [Google Scholar]
- Gomes J. A. N. F.; Mallion R. B. Aromaticity and Ring Currents. Chem. Rev. 2001, 101, 1349–1384. 10.1021/cr990323h. [DOI] [PubMed] [Google Scholar]
- Lazzeretti P. Ring Currents. Prog. Nucl. Magn. Reson. Spectrosc. 2000, 36, 1–88. 10.1016/S0079-6565(99)00021-7. [DOI] [Google Scholar]
- Lazzeretti P. Assessment of Aromaticity via Molecular Response Properties. Phys. Chem. Chem. Phys. 2004, 6, 217–223. 10.1039/B311178D. [DOI] [Google Scholar]
- Schleyer P. v. R.; Jiao H.; Hommes N. J. R. vE.; Malkin V. G.; Malkina O. L. An Evaluation of the Aromaticity of Inorganic Rings: Refined Evidence from Magnetic Properties. J. Am. Chem. Soc. 1997, 119, 12669–12670. 10.1021/ja9719135. [DOI] [Google Scholar]
- Olney T. N.; Cann N. M.; Cooper G.; Brion C. E. Absolute Scale Determination for Photoabsorption Spectra and the Calculation of Molecular Properties Using Dipole Sum-Rules. Chem. Phys. 1997, 223, 59–98. 10.1016/S0301-0104(97)00145-6. [DOI] [Google Scholar]
- Levitt M.; Perutz M. F. Aromatic Rings Act as Hydrogen Bond Acceptors. J. Mol. Biol. 1988, 201, 751–754. 10.1016/0022-2836(88)90471-8. [DOI] [PubMed] [Google Scholar]
- Altintas C.; Avci G.; Daglar H.; Gulcay E.; Erucar I.; Keskin S. Computer Simulations of 4240 MOF Membranes for H2/CH4 Separations: Insights into Structure–Performance Relations. J. Mater. Chem. A 2018, 6, 5836–5847. 10.1039/C8TA01547C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robeson L. M. The Upper Bound Revisited. J. Membr. Sci. 2008, 320, 390–400. 10.1016/j.memsci.2008.04.030. [DOI] [Google Scholar]
- Tagliabue M.; Farrusseng D.; Valencia S.; Aguado S.; Ravon U.; Rizzo C.; Corma A.; Mirodatos C. Natural Gas Treating by Selective Adsorption: Material Science and Chemical Engineering Interplay. Chem. Eng. J. 2009, 155, 553–566. 10.1016/j.cej.2009.09.010. [DOI] [Google Scholar]
- Min B.; Korde A.; Yang S.; Kim Y.; Jones C. W.; Nair S. Separation of C2–C4 Hydrocarbons from Methane by Zeolite MFI Hollow Fiber Membranes Fabricated from 2D Nanosheets. AIChE J. 2021, 67, e17048 10.1002/aic.17048. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.