Abstract
Simple Summary
Cell fusion as a fundamental biological process is required for various physiological processes, including fertilization, placentation, myogenesis, osteoclastogenesis, and wound healing/tissue regeneration. However, cell fusion is also observed during pathophysiological processes like tumor development. Mesenchymal stroma/stem-like cells (MSC) which play an important role within the tumor microenvironment like other cell types such as macrophages can closely interact and hybridize with cancer cells. The formation of cancer hybrid cells can involve various different mechanisms whereby the genomic parts of the hybrid cells require rearrangement to form a new functional hybrid cell. The fusion of cancer cells with neighboring cell types may represent an important mechanism during tumor development since cancer hybrid cells are detectable in various tumor tissues. During this rare event with resulting genomic instability the cancer hybrid cells undergo a post-hybrid selection process (PHSP) to reorganize chromosomes of the two parental nuclei whereby the majority of the hybrid population undergoes cell death. The remaining cancer hybrid cells survive by displaying altered properties within the tumor tissue.
Abstract
The generation of cancer hybrid cells by intra-tumoral cell fusion opens new avenues for tumor plasticity to develop cancer stem cells with altered properties, to escape from immune surveillance, to change metastatic behavior, and to broaden drug responsiveness/resistance. Genomic instability and chromosomal rearrangements in bi- or multinucleated aneuploid cancer hybrid cells contribute to these new functions. However, the significance of cell fusion in tumorigenesis is controversial with respect to the low frequency of cancer cell fusion events and a clonal advantage of surviving cancer hybrid cells following a post-hybrid selection process. This review highlights alternative processes of cancer hybrid cell development such as entosis, emperipolesis, cannibalism, therapy-induced polyploidization/endoreduplication, horizontal or lateral gene transfer, and focusses on the predominant mechanisms of cell fusion. Based upon new properties of cancer hybrid cells the arising clinical consequences of the subsequent tumor heterogeneity after cancer cell fusion represent a major therapeutic challenge.
Keywords: hybrid cell formation, horizontal gene transfer/lateral gene transfer, mesenchymal stroma-/stem-like cells, heterokaryon-to-synkaryon transition, cell fusion
1. Introduction
Cell fusion is a fundamental biological mechanism that describes the plasma membrane merging of two or more cells, thereby giving rise to one new hybrid cell displaying altered functionalities. Several physiological processes, such as fertilization, placentation, myogenesis, osteoclastogenesis, and wound healing/repair activities require the fusion of distinct somatic cells (for review see: [1,2,3,4,5,6,7,8,9]). Thereby, the formation of fused hybrid cells increases tissue plasticity. Moreover, the generation of new hybrid cell populations contributes to tissue homeostasis and elevates regenerative potential. However, cell fusion is also observed in a pathophysiological environment, including virus-infected host cells or during tumor development and subsequently evolving metastases (for review see: [1,3,4,5,6,7,8]).
Even though the process of cell fusion appears simple, like two soap bubbles merging, it is a tightly regulated, energy dependent, and not yet fully understood and characterized process. For instance, only certain cell types, such as cytotrophoblasts [10,11,12,13], myoblasts [14,15,16,17], or macrophages [9,18,19] possess fusogenic properties, whereby these cells are not per se fusogenic. To enable fusion the participating cells need to undergo a pre-hybrid preparation process (PHPP) accompanied by the formation of an associated hybrid-permissive environment. Accordingly, hybrid cells after cell fusion undergo a post-hybrid selection process (PHSP) to remodel DNA stability and to adapt to the normal cell metabolism [20,21].
But what is the fate of fusion-derived hybrid cells? Some of them remain in a stable multinucleated or so-called heterokaryon state, such as syncytiotrophoblasts, multinucleated muscle fibers, and osteoclasts [10,11,12,13,14,15,16,17,19,22,23,24]. In contrast, some bi- or multinucleated cells can also give rise to mononucleated daughter cells via a process that has been independently from the other named the “heterokaryon-to-synkaryon transition” (HST) [25] or synonymously “ploidy reductions” (PR) [26,27,28,29] and which has been observed in several cell types, including hepatic cells [26,27,28], hematopoietic cells [29], epithelial cells [30], and fibroblasts [31,32]. In accordance with the not yet fully understood process of cell fusion, the phenomenon of HST/PR also remains ambiguous.
The complexity of HST/PR is displayed by the transition of a cellular state carrying two discrete diploid nuclei from different parental cells to merge to a new functional nuclear unit with initially a tetraploid chromosomal phenotype. During this transition, the mis-segregation of the chromosomes from the two (or more) paternal nuclei can occur whereby HST/PR can lead to the induction of aneuploidy [27,33,34,35,36,37,38]. Finally, different mechanisms exist, such as entosis, emperipolesis, cannibalism, therapy-induced polyploidization/endoreduplication, and horizontal gene transfer/lateral gene transfer (HGT/LGT), which could also give rise to cell fusion like-derived cells.
2. Cell Fusion in General
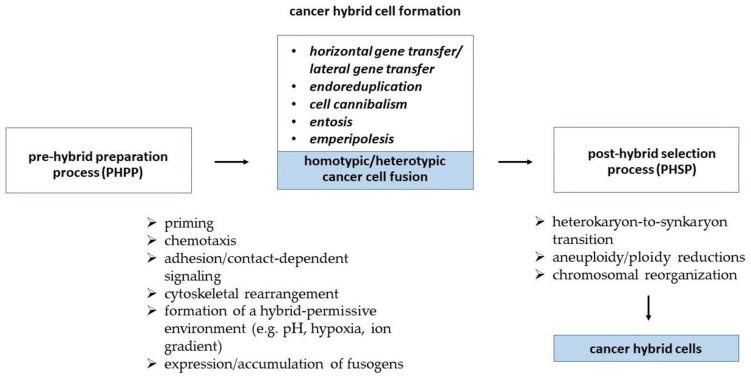
Cell fusion is a tightly regulated process, which can be distinguished by an initiation and a termination phase. Zhou and Platt postulated that cell fusion can be subdivided into five discrete steps, including (i) priming, (ii) chemotaxis, (iii) adhesion, (iv) fusion, and (v) post-fusion [39]. This suggestion has been supplemented by further cellular programs including a PHPP and a PHSP [20,21] which are summarized in a hypothetic scheme.
During the priming step cells acquire the ability to fuse, which is paralleled by an expression of adhesion molecules and alterations in the lipid composition of the plasma membranes. These changes are performed by a translocation of inner-leaflet lipids and the loss of an inhibitory state as a consequence of extracellular matrix degradation allowing facilitated cell migration and close cell–cell contact as a physical requirement of cell fusion [39]. The fusion step represents a complex orchestration of thermodynamic and biochemical processes which are yet not fully understood. Fusion-specific/-related proteins termed fusogens (for reviews see: [4,6,8,40]) play a pivotal role in overcoming certain energetic barriers. In particular, fusogens are mandatory for the contribution to steric formation of several cell fusion-related lipid intermediates named “the hallmarks of cell–cell fusion” [4]. These include (i) dehydration (thereby bringing phospholipid heads of the two cellular fusion partners in close vicinity of less than one nanometer), (ii) merging of the outer monolayers of hemifusion via a stalk and/or diaphragm intermediate, and (iii) opening and expansion of fusion pore(s) from nanometer diameters to multiple microns to enable fusion [4]. Finally, once the cells have merged they enter a post-hybrid state and become non-fusogenic again. In accordance with the still unresolved complexity of the fusion step it remains unclear which inter- and intracellular signaling pathways are involved in turning a cell from a fusogenic into a non-fusogenic state (Figure 1).
Figure 1.
The formation of cancer hybrid cells is composed of an orchestrated sequence of distinct cellular programs which can be distinguished as (i) a pre-hybrid preparation process (PHPP), (ii) the cancer cell hybridization process, and (iii) a subsequent post-hybrid selection process (PHSP). Several events can lead to cancer cell hybridization including the fusion of cancer cells with neighboring cancer cells (homotypic fusion) or with other cell types (e.g., macrophages, cancer-associated fibroblasts (CAFs), MSC) within the tumor microenvironment (heterotypic fusion).
Well-known fusogens in physiological human processes are syncytin-1 and syncytin-2, which are involved in the fusion of villous cytotrophoblasts to multinucleated syncytiotrophoblasts [13,41,42,43,44], myomaker and myomerger, that play a role in myogenesis [45,46,47,48] and Izumo1 (sperm) and Juno (oocyte), which are crucial for fertilization [49,50,51,52]. In contrast, despite the fact that several proteins that are involved in macrophage fusion, such as CD9, CD47, CD81, CD200, DC-STAMP, OC-STAMP, and MMP9 (for review see: [1,7,9]), no macrophage specific fusogens have been identified so far. Interestingly, syncytin-1 expression was also found in differentiating osteoclasts suggesting an involvement of this fusogen in osteoclastogenesis [22,53].
In addition to fusogens it is well known that cell fusion also depends on the reorganization of the actin cytoskeleton and crosstalk with plasma membrane phospholipids, in particular phosphatidylserine (PS), PS-binding annexins, and further membrane proteins (Figure 1) (for review see: [1,4,8,39,54,55,56,57,58,59]). Notably, PS signaling has been suggested as a uniquely conserved signaling module in cell–cell fusion, as PS exposure in the outer leaflet of plasma membranes has been found in myoblasts (myogenesis), macrophages (osteoclastogenesis), trophoblasts (placentation), sperm (fertilization), and even cancer cells (for review see: [57]). The translocation of PS from the inner leaflet to the outer leaflet of the plasma membrane is facilitated by the large family of professional phospholipid scramblases via at least three independent pathways, such as Ca2+-activated phospholipid scrambling (Ca2+-PLS), caspase-activated phospholipid scrambling (Cas-PLS), and constitutive phospholipid scrambling (for review see: [57]). Indeed, studies have revealed the involvement of transmembrane member 16 (TMEM16) Ca2+-PLSases family members in myogenesis [60,61], osteoclastogenesis [62], and placentation [63]. Anoctamin 5/TMEM16E knockout mice (Ano5−/−) muscle progenitor cells (MPCs) exhibited defective cell fusion in culture and produced muscle fibers with significantly fewer nuclei compared with controls, which could be corrected by the viral introduction of the ANO5 gene in Ano5−/−MPCs [60]. Likewise, fusion among the human choriocarcinoma trophoblastic BeWoc cell line was abolished by the genetic ablation of the TMEM16F Ca2+-PLSase and the placentas of TMEM16F-deficient mice exhibited deficiencies in trophoblast fusion and fetal blood vessel development [63]. How PS facilitates cell–cell fusion is not yet clear. As excellently reviewed by Whitlock and Chernomordik, PS could be either recognized by cells expressing PS receptors and/or PS binding proteins, and/or functions at the remodeling stage of cell fusion [57]. For instance, data from Hochreiter-Hufford and colleagues and Park et al. revealed that the fusion of myoblasts was mediated by PS-exposing apoptotic cells via the PS receptor BAI1 and associated protein complexes with ELMO and Dock180 [64,65]. Interestingly, apoptotic cells did not directly fuse with the healthy myoblasts, but rather induced a contact-dependent signaling with neighbors to promote fusion among the healthy myoblasts [64]. Likewise, the PS receptor CD36 facilitates the fusion of myoblasts [66], macrophages [67], and cancer cells [68]. Data from Verma and colleagues revealed that the inhibition of TMEM16 PLSases or the perturbation of the expression or activity of annexins A1 and A5 markedly impaired the fusion between osteoclast precursors, suggesting that PS exposure played a role in the membrane remodeling stages of cell fusion [62].
Regeneration studies of bone marrow-derived cells (BMDC) have revealed an elevated frequency of cell fusion events in inflamed tissues as compared to a non-inflammatory environment [69,70,71], indicating that inflammation can trigger cell fusion. This assumption is supported by the findings of the pro-inflammatory cytokine tumor necrosis factor-α (TNF-α) to increase fusion and osteoclastogenesis [72]. Further evidences have been provided by several studies also in a pathophysiological environment demonstrating enhanced rates of fusion events between cancer cells and normal cells, including epithelial cells, endothelial cells, or mesenchymal stroma/stem-like cells (MSC) in the presence of TNF-α [72,73,74,75,76,77,78]. Alternative to these processes, several studies have revealed that cell fusion can also occur in the absence of inflammation [29,69,70] adding to the complexity of the entire phenomenon.
In summary, the process of cell fusion and how it is initiated and terminated is still only scarcely understood. Fusogenic proteins in combination with cytoskeletal reorganization which can be induced by inflammation/inflammatory cytokines and apoptosis can support cell fusion, e.g., by converting cells from a non-fusogenic into a fusogenic state or by increasing cell–cell contacts (Figure 1). In this context, a pro-inflammatory environment generated by viral or bacterial infection can also contribute to cell fusion and neoplastic development.
3. DNA Reorganization during Cancer Cell Fusion
3.1. DNA Transfer by Horizontal or Lateral Gene Transfer
Horizontal gene transfer (HGT), also known as lateral gene transfer (LGT), is the transfer of genetic material between unicellular and multicellular organisms of all living matter and has been suggested as an important factor in the evolution of many organisms [79,80,81]. Thereby, DNA as well as RNAs (mRNA and microRNA) may be transferred by different mechanisms, such as (1) transduction (including viruses and bacteriophages), (2) transformation (of cell-free DNA), (3) conjugation (via F-pili), (4) gene transfer agents, (5) nanotubes, and (6) extracellular vesicles (EVs, such as apoptotic bodies, microvesicles, and exosomes) [79,80,81]. In this connection, it is well known that HGT/LGT is one of the driving mechanisms for the rapid spread of multidrug resistance bacteria worldwide [82].
Even though HGT/LGT is best studied in bacteria, fungi, insects, and plants, this process also occurs in mammals, whereby genetic material is predominantly transferred by extracellular vesicles and even cell-free DNA (for review see: [80,83]). However, with regard to the size of extracellular vesicles and the underlying mechanisms that will result in the release of cell-free DNA, it can be concluded that not intact chromosomes, but rather DNA fragments and even circular DNA, will be transferred. Thereby, the size of cell-free DNA could vary between 166 bp to 498 bp of apoptotic-derived nucleosomes and polynucleosomes and up to 20,000 bp of extrachromosomal circular DNA [83]. Indeed, some studies have revealed normal murine NIH 3T3 fibroblasts to be transformed by HGT/LGT due to the uptake of tumor cell-derived cell-free DNA harboring oncogenes [84,85,86].
In addition to cell-free DNA, it is well recognized that DNA, mRNAs, miRNAs, and proteins are transferred via extracellular vesicles, including apoptotic bodies, microvesicles, and exosomes (for review see: [80,83]). In accordance with cell-free DNA, whole genes can be transferred via apoptotic bodies, which are functionally integrated into the recipient cell genome. For instance, Bergsmedh and colleagues showed that DNA encapsulated in apoptotic bodies derived from H-rasV12- and human c-myc-transfected rat fibroblasts was found in the nuclei of recipient murine cells, which was further associated with a loss of contact inhibition in vitro and a tumorigenic phenotype in vivo [87]. Likewise, the co-cultivation of cell lines containing integrated copies of Epstein-Barr virus (EBV) resulted in a rapid uptake and transfer of EBV-DNA as well as genomic DNA to the nucleus of the phagocyting cells [88]. Moreover, data from Ehnfors and colleagues revealed that both fibroblasts and endothelial cells are capable of acquiring and replicating DNA derived from apoptotic SV40 large T antigen positive tumor cells [89]. In vivo data demonstrated that xenotransplanted tumors in severe combined immunodeficient mice exhibited a sub-population of endothelial cells containing tumor DNA, which maintained the ability of vessel formation and concurrently expressed tumor-encoded endothelial-specific genes [89]. With regard to cell fusion, a study of de la Taille et al. demonstrated that the co-cultivation of neomycin-resistant LNCaP prostate cancer cells with hygromycin-resistant LNCaP (LNCaP-HygR) cells resulted in the origin of dual antibiotic resistant cells after the induction of apoptosis in LNCaP-HygR cells [84]. The dual antibiotic selection assay is commonly used for the generation and selection of cancer hybrid cells. Hence, cancer hybrids have to be further validated by additional methods, such as short tandem repeat analysis, to exclude the possibility of HGT/LGT-mediated dual antibiotic resistance.
3.2. Role of Extracellular Vesicles (EVs) in Cancer Cell Fusion
EVs are well-known carriers for DNA, various types of RNA, lipids, and proteins, playing an essential role in intercellular communication and the regulation of diverse physiological and pathophysiological processes (for review see: [80,90,91,92]). In contrast to apoptotic bodies, microvesicles (50 to 500 nm) and exosomes (50 to 150 nm) are much smaller and are actively secreted by cells (for review see: [90,91,92]). Thereby, microvesicles are formed by budding of the plasma membrane, whereas exosomes are formed as intraluminal vesicles from multivesicular endosomes that subsequently fuse with the plasma membrane to release their content (for review see: [90,91,92]).
Microvesicles and exosomes, in addition to apoptotic bodies, can transfer genomic donor DNA to recipient cells, whereby double stranded DNA fragments of up to 17,000 bp have been detected [93,94,95,96]. For instance, exosomes isolated from the peripheral blood of chemotherapy-treated melanoma patients contained double stranded “self-DNA” derived from intestinal tissue, which were internalized by macrophages and dendritic cells [96]. The release of “self-DNA” into the cytoplasm activated the melanoma 2 (AIM2) inflammasome concomitant with the secretion of the pro-inflammatory cytokines IL-1β and IL-18 [96].
High copy numbers of Arabidopsis thaliana-DNA (A.t.-DNA) were detected in EVs derived from lentivirally A.t.-DNA-transduced human bone marrow MSCs [95]. A thorough analysis of recipient cells treated with these extracellular vesicles finally revealed a stable integration of A.t.-DNA in the cells’ genome, indicating that HGT/LGT could be mediated by EVs [95].
These findings are also in line with data from Cai and colleagues demonstrating that AT1-DNA and the BCR/ABL hybrid gene were found in EVs and could be transferred to HEK293 cells and neutrophils [93,94]. Additional findings revealed the in vivo relevance of EV-mediated DNA transfer, since BCR/ABL DNAs-containing EVs display pathophysiological characteristics of CML, such as feeble, febrile, splenomegaly, and neutrophilia two months after injection [94].
In addition to transfer of biological materials, EVs have been associated with the process of cell fusion. Whether EVs can directly mediate cell–cell fusion by acting as a linker to bridge two individual cells remains ambiguous. In any case, exosomes released by trophoblasts carry molecules involved in placental physiology, including syncytins as fusogens [97]. These fusogenic factors include sequestered or truncated genes originating from viral functions such as the HERV-W retroviral envelope genes that have been domesticated in the mammalian genome. Accordingly, EVs can carried syncytin-1 [98] and may contribute to cancer cell fusion [99].
4. Cell Cannibalism, Entosis, and Emperipolesis
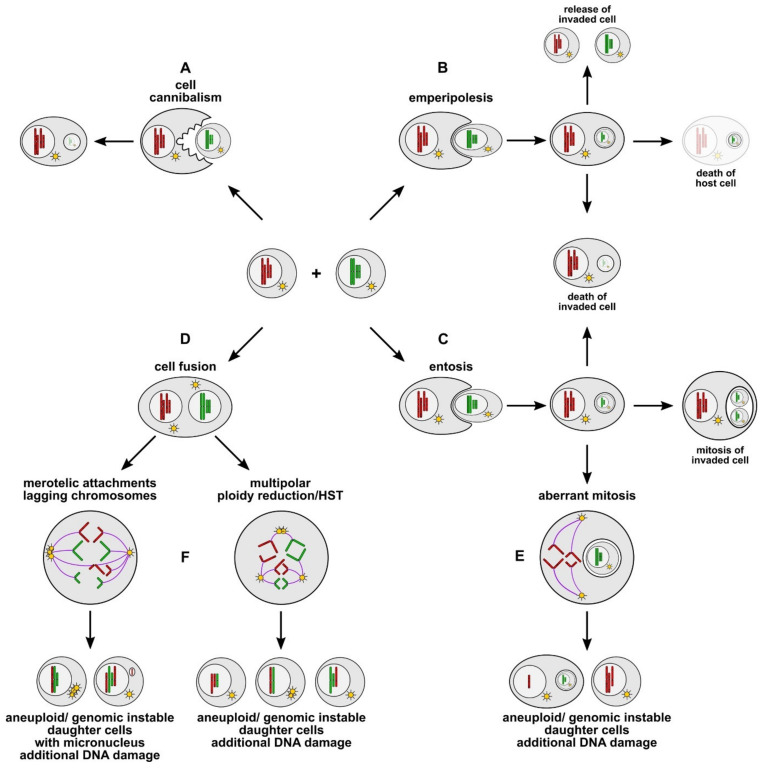
Alternative processes of cell merging predominantly observed in cancer cells include cell cannibalism, entosis, and emperipolesis (Figure 1).
During cell fusion various cell types can hybridize mediated by different fusogens and the resulting combined plasma membranes give rise to a bi- or multinucleated heterokaryon with a mixed cytoplasm. In contrast, cell cannibalism, entosis, and emperipolesis of cancer cells are among those so-called cell-in-cell phenomena (for review see: [100,101,102,103,104,105,106]).
4.1. Cell Cannibalism
Cell cannibalism displays similarities to phagocytosis (Figure 1). However, in addition to the engulfment of cell debris and apoptotic cells/bodies, cannibalistic cancer cells also have the capability to actively “phagocytose” living cells, such as other cancer cells (homotypic cannibalism) or leukocytes/lymphocytes and MSCs (heterotypic cannibalism). This process is supported by factors including the vacuolar-type ATPase proton pump-activating transmembrane 9 superfamily 4 protein TM9SF4, the plasma membrane and actin cytoskeleton linker protein Ezrin (cytovillin, villin-2), and the integrin subunits to tyrosine kinases linker and scaffolding protein caveolin-1, usually resulting in a complete lysosomal digestion of the cannibalized cells [100,105,107,108,109,110,111,112,113,114]. It is commonly assumed that the reason for cannibalism among cancer cells is the lack of nutrition in proliferating neoplastic tissues whereby cannibalizing other living cells appears to be the only alternative for cancer cells to survive in this environment [113,115,116]. In addition, cannibalism might also play a role in the immune escape of cancer cells [117,118].
4.2. Entosis and Emperipolesis
In contrast to cell engulfment and subsequent digestion in cannibalism, cells actively invade host cells in entosis and emperipolesis, thereby building a cell-in-cell structure (Figure 1 and Figure 2; for review see: [100,101,102,104]). Although both phenomena display morphological similarities, some functional differences have been characterized. Emperipolesis is observed mostly in cells of hematopoietic origin, such as lymphocytes or NK cells, by invading into host cells like megakaryocytes, thymic nurse cells, liver cells, or cancer cells [100,102,119,120]. Proteins like Ezrin, the leukocyte function-associated antigen-1 integrin LFA-1, and its ligand the intercellular adhesion molecule-1 ICAM-1, contribute to emperipolesis. Different possible outcomes accompany emperipolesis: invaded cells can either escape from the host cell, or may be destroyed by the host cell, or vice versa may destroy the host cell [100,102] (Figure 2).
Figure 2.
Different fates of merged cells. Cell cannibalism (A), emperipolesis (B), and entosis (C) belong to the so-called cell-in-cell phenomena, which are characterized by the engulfment of intact cells. In contrast, cell fusion (D) gives rise to bi- or multinucleated heterokaryons due to merging of the cells’ plasma membranes. Cell cannibalism (A) resembles “phagocytosis” and usually results in the lysosomal digestion of the engulfed cell. In contrast, in emperipolesis (B) and entosis (C) one cell actively invades another cell, whereby the fate of the engulfed cell differs markedly between processes. Emperipolesis is characterized by cells either escaping from the host cell, being destroyed by the host cell, or vice versa destroying the host cell (B). On the contrary, most entotic cells are usually destroyed by lysosomal degradation, whereas some internalized cells may also survive and can even divide within the host cell (C). Moreover, entosis may be associated with aberrant mitosis (E) and the origin of aneuploid and genomic instable daughter cells. A characteristic of hybrid cells is the merging of parental chromosomes and their random segregation to daughter cells during bi- and multipolar divisions (F), which is also associated with aneuploidy, genomic instability, and micronucleus formation.
In entosis, internalized cells such as cancer or epithelial cells (named “loser cells”) actively invade other epithelial or cancer cells (named “winner cells”) [100,102]. Entosis depends on various factors such as glucose, E- or P-Cadherin, the G-Protein Rho A and the associated kinase ROCK, and the AMP-activated protein kinase (AMPK). Compared to emperipolesis, the fates of entosis-internalized cells are different [100,102,121]. While most entotic cells are usually destroyed [122] by lysosomal degradation, some internalized cells can survive and even divide within host cells [101,102] (Figure 2).
Similar to cell fusion, the entosis of cancer cells is associated with the induction of aneuploidy and chromosomal instability, although via different effects. The entotic cell acts as a barrier, which leads to a failed cytokinesis in its host cell [100,101,102,106,121]. Whether entosis can develop a hybrid cell as occurs after fusion remains to be elucidated. This would require a recombination of the internalized cell genome with the nucleus of the host cell. Data from Sottile et al. revealed that the co-cultivation of embryonic stem cells (ESCs) and MSCs results in both cell fusion-derived hybrids and entotic hybrids, which were able to proliferate and to form ESC- and MSC-like colonies [123]. In contrast to cell fusion-derived hybrids undergoing HST/PR, however, entotic cells were either released or degraded [123]. Similar effects were observed in co-cultivation experiments using human MSCs and human U87 glioblastoma cells whereby entotic cells were degraded [124]. While entosis was also observed in co-cultivation experiments using human breast cancer cell lines, little is known about the fate of entotic cells [125].
5. Cell Fusion in Tumors
The hypothesis that cell fusion might play a role in tumor development had already been postulated by the German physician Otto Aichel more than 100 years ago in 1911 [126]. Aichel assumed that the aneuploidy of cancer cells and leukocyte-like properties of metastatic tumors were attributed to fusion events between tumor-invading leukocytes and cancer cells [126]. Since then, a plethora of in vitro and in vivo studies on human tumors have been published supporting Aichel’s hypothesis that cancer cells are fusogenic. Moreover, hybridization with other cancer cells or with adjacent non-tumor cell types, including macrophages, fibroblasts, and stem cells have generated new cancer hybrid cells with increased metastatic potential and more chemotherapy and radiotherapy resistant properties than parental cancer cells [32,73,123,125,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199]. Consequently, tumor progression could be fostered due to the generation of more metastatic tumor hybrids, which can evade from the primary tumor and form secondary lesions both in the lymph nodes and in distant organs. Likewise, chemotherapy and/or radiotherapy resistant tumor hybrids may survive cancer therapy and become the seeds for recurrences. Appropriate examples are given in the following subchapters (see below). Additionally, cell–cell fusion has been suggested as a driver in tumor heterogeneity, which is related to genomic instability and a hallmark of solid tumors [200]. Assuming a fusion probability of 6.6 × 10−3 in vitro and 6.6 × 10−5 in vivo together with values of genetic mutation rates of 10−6 to 10−3 as well as the number of potential driver genes of about 300 mathematical calculations revealed a substantially enhanced increase in clonal richness and overall mutation rate in a 3D environment as compared to only “mutated” cells [125]. Hence, despite a relatively low frequency of spontaneous somatic cell–cell fusion events and the impact of spatial constraint, fusion-mediated recombination could have a profound impact on somatic evolution through the accelerated diversification of tumor cell populations and the generation of rare mutational variants capable of exploring larger swathes of adaptive landscapes [125]. Such cell–cell fusion derived populations could then be the seeds for metastatic lesions and/or could be resistant to chemo- and/or radiotherapy.
How cancer cells fuse is still not clear, but it is most likely to be similar to the fusion of normal cells and facilitated by fusogens, such as syncytin-1 [77,98,168,201,202,203], PS and PS-binding proteins [98,136], and inflammation/inflammatory cytokines [76,77]. Likewise, tumor cells are not per se fusogenic, but have to be transferred into a fusogenic state first. In this connection, the co-cultivation of human prostate cancer cells with muscle cells resulted in an IL-4 and IL-13 dependent upregulation of syncytin-1 and annexin 5 expression and enhanced frequency of homotypic cancer cell–cell fusion [98] suggesting that both cytokines might be involved in the conversion towards a pro-fusogenic state of (cancer) cells. This would be in view with findings showing that the exposure of macrophages to IL-4 and IL-13 induced homokaryon formation [9]. However, the detection of cancer hybrids in vitro and in vivo is challenging and depends on valid and reliable fusion markers since other multinucleoli- or aneuploidy-generated mechanisms such as entosis, emperipolesis, cannibalism, therapy-induced polyploidization/endoreduplication, and HGT/LGT could also give rise to cancer hybrid-like cells.
5.1. Evidence for Cancer Cell Fusion by In Vitro Studies
Several studies using genetically modified cancer cells and transgenic mouse models have revealed cancer cell fusion among themselves (homotypic fusion) or with other cell types in the tumor microenvironment, such as macrophages, CAFs, or MSC (heterotypic fusion) [31,32,73,123,125,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,198,204,205,206].
Markers to identify cancer cell fusion and to isolate cancer hybrid cells include resistance to different antibiotics, or the co-expression of two different fluorescent reporter proteins [31,32,73,123,128,129,133,134,137,138,140,141,142,143,144,145,147,148,149,150,151,152,154,155,158,161,162,164,167,169,171,172,173,174,178], or the use of hypoxanthine, aminopterin, or thymidine (HAT) medium [125,160,173,175], respectively.
In more detail, a variety of different cell systems provide evidence for the acquisition of fusion-derived new properties in cancer hybrid cells, e.g., the co-cultivation of bone- and lung-tropic sublines of the human MDA-MB-231 breast cancer cell line with resistance to either puromycin or hygromycin in media supplemented with both antibiotics resulted in the appearance of double resistant hybrid cells exhibiting a dual metastatic organotropism [158]. Likewise, a dual antibiotic selection strategy was used for the generation of hybrid cells derived from spontaneous fusion events between human breast epithelial cells and human breast cancer cells [128,129,178]. Functional changes were also observed in M13HS-2 and M13HS-8 hybrid cells that were derived after the fusion of human M13SV1-EGFP-Neo breast epithelial cells with human HS578T-Hyg breast cancer cells. These cancer hybrid cells responded to the chemokine CCL21 with an increased migratory activity, whereas the parental cells did not [127]. Since CCL21 has been associated with lymph node metastases in breast cancer [207], these findings likely indicate that the fusion of CCL21-insensitive cancer cells could give rise to CCL21-sensitive cancer hybrid cells. In addition, M13HS hybrids display certain cancer stem/initiating cell properties, such as an increased mammosphere and colony formation capacity as well as elevated ALDH1 expression [208]. This is in line with previous suggestions that cancer stem/initiating cell properties may be acquired during cell fusion events [178].
A dual antibiotic selection/fluorescence reporter strategy has also been used to isolate hybrid cells derived from lung IMR90 E6 E7 H-RASG12V CFP-Blast cells and IMR90 E6 E7 SmallT hTERT DsRed-Puro cells [32]. The aim of this study was to prove whether the fusion-mediated oncogenic combination of hTERT, SV40 SmallT antigen, and H-RASG12V would result in a malignant conversion of non-transformed immortalized fibroblasts. Indeed, hybrids harboring all five oncogenes not only exhibited an increased and aneuploid mean chromosomal number but were also highly tumorigenic [32]. In a similar study, cancer hybrid cells derived from neoplastic IMR90 E6E7 H-RASG12V SmallT hTERT DsRed PuroR and non-transformed IMR90 E6E7 CFP BlastR cells were selected based on their co-resistance to blasticidin and puromycin [172]. These hybrids were highly aneuploid and revealed non-recurrent, large-scale genomic rearrangements including interchromosomal translocations [172].
In a sarcoma study, cancer hybrids exhibited novel phenotypic traits such as metastatic spreading capabilities and were the only cells that recapitulated in vivo all features of pleomorphic sarcomas [172]. Similar findings were reported for hybrid cells that were derived from immortalized myoblasts and transformed fibroblasts by exhibiting clonogenic ability and dissemination properties [31]. Moreover, hybrid tumors were found to mimic the histological characteristics of undifferentiated pleomorphic sarcomas with incomplete muscular differentiation, suggesting that cell fusion might favor specific sarcoma development according to the differentiation lineage of parent cells [31].
The co-cultivation of RFP-tagged G418 resistant LNCaP prostate cancer cells with GFP-labeled puromycin-resistant prostate stromal cells resulted in the origin of RFP/GFP double positive and dual antibiotic-resistant hybrids displaying divergent behaviors and exhibiting permanent genomic hybridization [142]. Lindstrom and colleagues co-cultivated human M2-macrophages and GFP-labeled MCF-7 human breast cancer cells and spontaneously formed GFP+, CD163+, and CD45+ tumor hybrids were sorted by flow cytometry [132]. After treatment with γ-radiation, tumor hybrids showed an increased survival fraction and colony formation ability, which was accompanied by an overall lesser DNA damage as compared to parental MCF-7 breast cancer cells [132] indicating that cell–cell fusion could give rise to radioresistant cells.
5.2. Cancer Cell Fusion as a Rapid Process and In Vivo Studies
Previous work has demonstrated that RFP-labeled omental adipose-derived stromal (O-ASC) cells spontaneously fused with GFP-marked endometrial cancer cells (ECC), whereby the resulting cancer hybrid cells displayed a mesenchymal phenotype [169]. These hybrids proliferated through bipolar and multipolar divisions and showed an increased migratory capacity, suggesting epithelial-to-mesenchymal-associated changes including the down-modulation of E-cadherin and the up-regulation of Vimentin [169]. Time-lapse video microscopy could verify the direct fusion of RFP-O-ASCs with GFP-ECCs [169]. Similar morphological documentations were provided by Melzer and colleagues demonstrating that the fusion of GFP-tagged MSCs with mCherry-tagged MCF10A non-tumorigenic breast epithelial cells or mCherry-tagged human mammary epithelial cells (HMECs) occurred within less than five minutes [73].
In vivo studies have revealed a significantly elevated tumor growth of human breast cancer hybrid populations (MDA-MSC-hyb1 and –hyb2) together with the development of multiple distant organ metastases in a much shorter period of time than the parental breast cancer cells [153]. Similar findings have been reported by Gast and colleagues who demonstrated the spontaneous fusion of GFP-tagged murine macrophages with H2B-RFP-labeled MC38 mouse colon carcinoma cells, thereby giving rise to highly motile and metastatic hybrids [164]. Time-lapse movie data substantiated this fusion of H2B-RFP MC38 cells and a GFP-tagged macrophage with the subsequent division of the evolving hybrid cell into two daughter cells [164]. An altered tumor behavior was observed in MDA-MSC-hyb5 breast cancer hybrid populations displaying an initial tumor dormancy. Following tumor initiation after dormancy, however, tumor growth and the formation of various different organ metastases occurred much faster as compared to the parental breast cancer cells [209]. A Cre-LoxP recombination strategy has been used in additional studies either to visualize cell fusion [166,198] or to quantify cell fusion events between human breast epithelial cells and human breast cancer cell lines [177]. Beside these cell fusion strategies, the further hybridization of cancer cells and normal cells was also visualized by bimolecular fluorescence complementation (BiFC) [136], dual split reporter assays [210], co-expression of lineage specific surface markers [138,164,165,191,192,193,194], or karyotypization [131,156,165,187].
Although these data support the occurrence of homotypic and heterotypic cancer cell fusion events in vitro and in vivo, further characterization and distinction of cancer hybrid cells require karyotypization, short tandem repeat (STR) analysis, or FISH analysis. This, however, is limited in cancer hybrid cells of homotypic fusions originating from parental cells with an identical genomic background [211]. Thus, it cannot be ruled out that the acquisition of parental genes was mediated by HGT/LGT due to the up-take of apoptotic bodies or extracellular vesicles such as exosomes rather than by cell fusion [87,89,212].
5.3. Indirect Evidence for Cell Fusion by Clinical Observations of Hybrid Cells in Human Tumors
Several in vitro and in vivo animal studies have demonstrated that cancer cells exhibit fusogenic properties and may either homo- or hetero-hybridize with the generation of new cancer hybrid cells displaying altered properties [138,164,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197].
A common approach to search for putative heterotypic fusion is to seek the expression of non-cancer specific epitopes in cancer hybrid cells, such as macrophage, hematopoietic, or epithelial antigens [138,182,183,184,191,194]. However, the expression of hematopoietic and epithelial lineage markers of cancer cells can also be traced back to genomic instability, which limits this strategy.
In any case, Shabo and colleagues detected the expression of macrophage antigens DAP12, MAC387, and CD163 in human breast cancer and colorectal cancer tissues [182,183,184,197]. Increased CD163 expression levels were related to early recurrence and reduced overall survival of afflicted patients [183,184], suggesting CD163 as a suitable prognostic factor.
Putative cell fusion-derived cancer hybrid cells co-expressing EpCAM/CD45 and CD125/CD45, respectively, were found in the ascites of ovarian carcinoma patients [138]. About 50% of these cancer hybrid cells also expressed the promigratory chemokine receptor CXCR4 [138], suggesting an acquired metastatic capacity. The expression of CD117 and CD44, which has been associated with a cancer stem cell phenotype in ovarian cancer, was found in up to 90% of EpCAM+/CD45+ ovarian cancer hybrid cells [138] indicating an association of cell fusion with the origin of cancer stem/initiating cells. Indeed, cell fusion has been proposed as a potential mechanism that could give rise to cancer stem/initiating cells [25,128,146,213].
Alternatively to the detection of non-cancer specific epitopes in cancer hybrid cells the identification of cancer-specific oncogenes/markers in non-cancerous cells represents a further strategy to identify putative cancer hybrid cells in vivo, e.g., a melanoma-derived mutated BRAFV600E oncoprotein was detectable not only among cells surrounding the primary tumor but was also present in the stroma of melanoma metastases as well as in a histologically tumor-free re-excision sample from a patient who subsequently developed a local recurrence [190].
In addition to the identification within solid tumor tissues, cancer hybrid cells were also detectable in the circulation of cancer patients. Thus, CK+/CD45+ cancer hybrid cells were identified by gradient centrifugation in the peripheral blood of patients with melanoma, pancreatic, and colorectal cancer [191]. Thereby, cultured circulating melanoma-derived cancer hybrid cells concomitantly expressed macrophage markers (CD14, CD68, CD163, CD204, and CD206), melanocyte-specific markers (ALCAM, MLANA, and melanoma-specific BRAFV600E mutations), epithelial cell markers (CK, EpCAM), and some stem cell-related markers (CXCR4, CD44) [192]. Similar findings were obtained with cultured circulating pancreatic cancer hybrid cells derived from pancreatic ductal adenocarcinoma patients [193]. Such cells were characterized by a co-expression of pancreatic cancer (stem cell) markers (CD44, ALDH1A1, CXCR4, ZG16B, S100PBP) and macrophage markers (CD204, CD206) [193]. Moreover, metastatic spreading along multiple tissues, including liver, spleen, and lung, was observed for cultured circulating pancreatic cancer hybrid cells following orthotropic transplantation into nude mice [193]. Rather, micro-metastases consisting of single cells or small groups of cells were formed within the organs, potentially serving as “niches” for subsequent colonization by metastasis-initiating cells [193]. Supportive findings were observed for non-small cell lung cancer (NSCLC). Thus, Manjunath et al. isolated circulating EpCAM+/CK+/CD14+/CD45+ cancer hybrid cells from the peripheral blood of NSCLC patients by immunomagnetic separation [194]. Circulating cancer hybrid cells were identified in 76.5% of NSCLC patients and the number of cancer hybrid cells in the circulation was correlated with AJCC tumor stages [194]. In addition to the overall number of circulating cancer hybrid cells, the authors also identified some giant circulating cancer hybrid cells with a diameter of more than 50 µm. These findings were associated with a significantly shorter overall and cancer-specific disease-free survival after curative surgery for stages I–IIIA and, hence, might be used as an independent survival predictor [194]. The appearance of multi-nucleated giant cells was also observed during the fusion of HIV-infected T cells with macrophages evolving virus-productive multinucleated immune hybrid cells [214]. Moreover, the fusion of cancer cells with leukocytes such as macrophages also contributes to increased tumor heterogeneity [164].
In sum, these data indicate that cancer cells expressing non-cancer specific epitopes (e.g., from macrophages, MSCs, or epithelial cells) were identified in human cancers indicating an origination of former cancer cell fusion events [132,133,137,156,160,164,165,215].
5.4. Direct Evidence for Cell Fusion by Clinical Observations of Hybrid Cells in Human Tumors
Besides the limitations of lineage markers as indicators for cell fusion, some studies have focused on cancer patients with a former bone marrow transplantation (BMT) history or have specifically searched for tumor-specific DNA in commonly normal cells [164,185,186,188,189,196,216]. A combination of immunohistochemistry and FISH analysis in patient samples identified B-cell lymphoma-specific chromosomal translocations in 15 to 85% of microvascular endothelial cells, likely indicating that such cells originated from cell fusion [216]. In myeloma patients FISH analysis of bone-resorbing osteoclasts revealed that more than 30% of these multinucleated cells contained nuclei with translocated chromosomes of myeloma B-cell clone origin [187]. Moreover, these nuclei were fully integrated amongst the nuclei and were transcriptionally active [187]. Additional findings suggested that the multiple myeloma-induced disruption of bone remodeling compartments resulted in osteolytic lesions and provided a fusion permissive environment enabling the generation of bone-destructive osteoclast-myeloma hybrid cells [188].
The to-date gold standard for identifying cancer cell fusion in human tumors represents a mixed genomic phenotype of donor and recipient DNA in cancer hybrid cells from patients with a former BMT. Unfortunately, the number of such cancer patients is pretty rare. An analysis of a renal cell carcinoma metastasis for BMT DNA (recipient blood group A+; donor blood group O+) revealed that the tumor genotype was A/O indicating that such cells have been originated by cell fusion [189]. Likewise, donor Y chromosome was found in the renal carcinoma cells of a female BMT patient also suggesting that these cells were generated from hybridization events between “recipient” cancer cells and “donor” bone marrow-derived cells [196]. Similar findings were reported by Gast et al. demonstrating that Y chromosome-positive cancer cells were identified in a variety of different tumors, such as pancreatic ductal adenocarcinoma (PDAC), lung cancer, renal cell carcinoma, and head and neck squamous carcinoma (HNSCC) in women with previous sex-mismatch BMT [164]. In addition, EpCAM, CD45, and Y chromosome-positive circulating cancer hybrid cells were detected in PDAC patients whereby high cell numbers were correlated with a poor survival [164]. These results are in agreement with the overall assumption that cancer hybrid cells can acquire novel properties such as an altered metastatic behavior and a modified chemoresistance [217]. Strong evidences for cell fusion-derived cancer hybrid cells in human tumors were also provided by Lazova et al. and LaBerge et al. [185,186,218]. In both studies laser microdissected tumor hybrid cells from cancer biopsies were investigated by short tandem repeat (STR) analysis and revealed an overlap of different donor and recipient alleles [185,186]. Moreover, in both studies cancer hybrid cells were found in metastases, suggesting that the hybridization between donor and patient cells contributed to the promotion of secondary lesions [185,186]. A brief summary of the identification of tumor hybrid cells in human cancers is given in Table 1.
Table 1.
Identification of tumor hybrid cells in human cancers.
| Tumor Type | Normal Cells | Marker | Reference |
|---|---|---|---|
| Breast cancer | Macrophages | CD163, MAC387, DAP12 | [182,184,197] |
| Colon/colorectal cancer | Macrophages | CD163, MAC387, DAP12, CD45, CD14, Cytokeratin | [183,192,197] |
| Epithelial ovarian carcinoma | BMDCs | CD45, CXCR4 | [138] |
| Melanoma | Macrophages | CD14, CD45, Cytokeratin | [192] |
| Stromal cells | BRAF (V600E) mutation | [190] | |
| BMDCs | STR analysis * | [185,186] | |
| Multiple myeloma | Osteoclasts | Myeloma specific translocations | [187,188,219] |
| Non-small cell lung cancer | Macrophages | CD14, CD45, Cytokeratin, EpCAM | [194] |
| Pancreatic ductal adenocarcinoma | Macrophages | CD45, Cytokeratin | [191] |
| BMDCs | CD45, EpCAM, | [164] | |
| Y chromosome * | |||
| Renal cell carcinoma | BMDCs | blood group alleles * | [189] |
| BMDCs | Y chromosome * | [196] |
* Cancer patients with a BMT history.
6. Conclusions
Besides several distinct mechanisms of cancer hybrid cell formation the development of cell fusion can significantly contribute to tumor heterogeneity and the generation of distal metastases. After preparation of cell fusion by PHPP and following fusion the surviving cancer hybrid cells during clonal convergence of a subsequent PHSP display altered functionalities. Consequently, the initially rare cancer hybrid cells may eventually develop a selection advantage to overgrow other cancer cells and contribute to increased tumor plasticity. Thus, cancer hybrid cells can eventually represent a prominent population within the tumor tissue to influence further tumor growth and to support enhanced metastatic diversity. Accordingly, molecular mechanisms of cancer cell fusion or specific molecular patterns of cancer hybrid cells may provide preferred targets for tumor-therapeutic interventions of identified fusion-based tumors.
Abbreviations
| BMT | bone marrow transplantation |
| CAFs | cancer-associated fibroblasts |
| HGT/LGT | horizontal gene transfer/lateral gene transfer |
| HST | heterokaryon-to-synkaryon transition |
| MSC | mesenchymal stroma-/stem-like cells |
| NSCLC | non-small cell lung cancer |
| PHPP | pre-hybrid preparation process |
| PHSP | post-hybrid selection process |
| PR | ploidy reductions |
| PS | phosphatidylserine |
| TNF-α | tumor necrosis factor-α |
Author Contributions
Conceptualization, R.H. and T.D.; writing—original draft preparation, R.H. and T.D.; writing—review and editing, R.H. and T.D.; visualization, J.v.d.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aguilar P.S., Baylies M.K., Fleissner A., Helming L., Inoue N., Podbilewicz B., Wang H., Wong M. Genetic basis of cell-cell fusion mechanisms. Trends Genet. 2013;29:427–437. doi: 10.1016/j.tig.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dittmar T., Zänker K.S. Cell Fusion in Health and Disease: Volume I. Volume 1. Springer; Dordrecht, The Netherlands: 2011. [DOI] [PubMed] [Google Scholar]
- 3.Dittmar T., Zanker K.S. Cell Fusion in Health and Disease: Volume II. Volume 2. Springer; Dordrecht, The Netherlands: 2011. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez J.M., Podbilewicz B. The hallmarks of cell-cell fusion. Development. 2017;144:4481–4495. doi: 10.1242/dev.155523. [DOI] [PubMed] [Google Scholar]
- 5.Larsson L.I., Bjerregaard B., Talts J.F. Cell fusions in mammals. Histochem. Cell Biol. 2008;129:551–561. doi: 10.1007/s00418-008-0411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Vargas J., Krey T., Valansi C., Avinoam O., Haouz A., Jamin M., Raveh-Barak H., Podbilewicz B., Rey F.A. Structural basis of eukaryotic cell-cell fusion. Cell. 2014;157:407–419. doi: 10.1016/j.cell.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Willkomm L., Bloch W. State of the art in cell-cell fusion. Methods Mol. Biol. 2015;1313:1–19. doi: 10.1007/978-1-4939-2703-6_1. [DOI] [PubMed] [Google Scholar]
- 8.Brukman N.G., Uygur B., Podbilewicz B., Chernomordik L.V. How cells fuse. J. Cell Biol. 2019;218:1436–1451. doi: 10.1083/jcb.201901017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helming L., Gordon S. Molecular mediators of macrophage fusion. Trends Cell Biol. 2009;19:514–522. doi: 10.1016/j.tcb.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Frendo J.L., Cronier L., Bertin G., Guibourdenche J., Vidaud M., Evain-Brion D., Malassine A. Involvement of connexin 43 in human trophoblast cell fusion and differentiation. J. Cell Sci. 2003;116:3413–3421. doi: 10.1242/jcs.00648. [DOI] [PubMed] [Google Scholar]
- 11.Getsios S., MacCalman C.D. Cadherin-11 modulates the terminal differentiation and fusion of human trophoblastic cells in vitro. Dev. Biol. 2003;257:41–54. doi: 10.1016/S0012-1606(03)00041-1. [DOI] [PubMed] [Google Scholar]
- 12.Huppertz B., Gauster M. Trophoblast fusion. Adv. Exp. Med. Biol. 2011;713:81–95. doi: 10.1007/978-94-007-0763-4_6. [DOI] [PubMed] [Google Scholar]
- 13.Vargas A., Moreau J., Landry S., LeBellego F., Toufaily C., Rassart E., Lafond J., Barbeau B. Syncytin-2 plays an important role in the fusion of human trophoblast cells. J. Mol. Biol. 2009;392:301–318. doi: 10.1016/j.jmb.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Abmayr S.M., Pavlath G.K. Myoblast fusion: Lessons from flies and mice. Development. 2012;139:641–656. doi: 10.1242/dev.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simionescu A., Pavlath G.K. Molecular mechanisms of myoblast fusion across species. Adv. Exp. Med. Biol. 2011;713:113–135. doi: 10.1007/978-94-007-0763-4_8. [DOI] [PubMed] [Google Scholar]
- 16.Hindi S.M., Shin J., Gallot Y.S., Straughn A.R., Simionescu-Bankston A., Hindi L., Xiong G., Friedland R.P., Kumar A. MyD88 promotes myoblast fusion in a cell-autonomous manner. Nat. Commun. 2017;8:1624. doi: 10.1038/s41467-017-01866-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pizza F.X., Martin R.A., Springer E.M., Leffler M.S., Woelmer B.R., Recker I.J., Leaman D.W. Intercellular adhesion molecule-1 augments myoblast adhesion and fusion through homophilic trans-interactions. Sci. Rep. 2017;7:5094. doi: 10.1038/s41598-017-05283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vignery A. Osteoclasts and giant cells: Macrophage-macrophage fusion mechanism. Int. J. Exp. Pathol. 2000;81:291–304. doi: 10.1046/j.1365-2613.2000.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vignery A. Macrophage fusion: The making of osteoclasts and giant cells. J. Exp. Med. 2005;202:337–340. doi: 10.1084/jem.20051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melzer C., Ohe J.V., Hass R. Altered Tumor Plasticity after Different Cancer Cell Fusions with MSC. Int. J. Mol. Sci. 2020;21:8347. doi: 10.3390/ijms21218347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hass R., von der Ohe J., Ungefroren H. Impact of the Tumor Microenvironment on Tumor Heterogeneity and Consequences for Cancer Cell Plasticity and Stemness. Cancers. 2020;12:3716. doi: 10.3390/cancers12123716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moller A.M., Delaisse J.M., Soe K. Osteoclast Fusion: Time-Lapse Reveals Involvement of CD47 and Syncytin-1 at Different Stages of Nuclearity. J. Cell. Physiol. 2017;232:1396–1403. doi: 10.1002/jcp.25633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soe K. Osteoclast Fusion: Physiological Regulation of Multinucleation through Heterogeneity-Potential Implications for Drug Sensitivity. Int. J. Mol. Sci. 2020;21:7717. doi: 10.3390/ijms21207717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teitelbaum S.L. Osteoclasts: What do they do and how do they do it? Am. J. Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjerkvig R., Tysnes B.B., Aboody K.S., Najbauer J., Terzis A.J. Opinion: The origin of the cancer stem cell: Current controversies and new insights. Nat. Rev. 2005;5:899–904. doi: 10.1038/nrc1740. [DOI] [PubMed] [Google Scholar]
- 26.Duncan A.W., Hanlon Newell A.E., Bi W., Finegold M.J., Olson S.B., Beaudet A.L., Grompe M. Aneuploidy as a mechanism for stress-induced liver adaptation. J. Clin. Investig. 2012;122:3307–3315. doi: 10.1172/JCI64026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duncan A.W., Hickey R.D., Paulk N.K., Culberson A.J., Olson S.B., Finegold M.J., Grompe M. Ploidy reductions in murine fusion-derived hepatocytes. PLoS Genet. 2009;5:e1000385. doi: 10.1371/journal.pgen.1000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto T., Wakefield L., Tarlow B.D., Grompe M. In Vivo Lineage Tracing of Polyploid Hepatocytes Reveals Extensive Proliferation during Liver Regeneration. Cell Stem Cell. 2020;26:34–47.e3. doi: 10.1016/j.stem.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skinner A.M., Grompe M., Kurre P. Intra-hematopoietic cell fusion as a source of somatic variation in the hematopoietic system. J. Cell Sci. 2012;125:2837–2843. doi: 10.1242/jcs.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou X., Merchak K., Lee W., Grande J.P., Cascalho M., Platt J.L. Cell Fusion Connects Oncogenesis with Tumor Evolution. Am. J. Pathol. 2015;185:2049–2060. doi: 10.1016/j.ajpath.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delespaul L., Gelabert C., Lesluyes T., Le Guellec S., Perot G., Leroy L., Baud J., Merle C., Lartigue L., Chibon F. Cell-cell fusion of mesenchymal cells with distinct differentiations triggers genomic and transcriptomic remodelling toward tumour aggressiveness. Sci. Rep. 2020;10:21634. doi: 10.1038/s41598-020-78502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delespaul L., Merle C., Lesluyes T., Lagarde P., Le Guellec S., Perot G., Baud J., Carlotti M., Danet C., Fevre M., et al. Fusion-mediated chromosomal instability promotes aneuploidy patterns that resemble human tumors. Oncogene. 2019;38:6083–6094. doi: 10.1038/s41388-019-0859-6. [DOI] [PubMed] [Google Scholar]
- 33.Duncan A.W., Taylor M.H., Hickey R.D., Hanlon Newell A.E., Lenzi M.L., Olson S.B., Finegold M.J., Grompe M. The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature. 2010;467:707–710. doi: 10.1038/nature09414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganem N.J., Godinho S.A., Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Godinho S.A., Kwon M., Pellman D. Centrosomes and cancer: How cancer cells divide with too many centrosomes. Cancer Metastasis Rev. 2009;28:85–98. doi: 10.1007/s10555-008-9163-6. [DOI] [PubMed] [Google Scholar]
- 36.Holland A.J., Cleveland D.W. Boveri revisited: Chromosomal instability, aneuploidy and tumorigenesis. Nat. Rev. Mol. Cell Biol. 2009;10:478–487. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chunduri N.K., Storchova Z. The diverse consequences of aneuploidy. Nat. Cell Biol. 2019;21:54–62. doi: 10.1038/s41556-018-0243-8. [DOI] [PubMed] [Google Scholar]
- 38.Passerini V., Ozeri-Galai E., de Pagter M.S., Donnelly N., Schmalbrock S., Kloosterman W.P., Kerem B., Storchova Z. The presence of extra chromosomes leads to genomic instability. Nat. Commun. 2016;7:10754. doi: 10.1038/ncomms10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou X., Platt J.L. Molecular and cellular mechanisms of Mammalian cell fusion. Adv. Exp. Med. Biol. 2011;713:33–64. doi: 10.1007/978-94-007-0763-4_4. [DOI] [PubMed] [Google Scholar]
- 40.Podbilewicz B. Virus and cell fusion mechanisms. Annu. Rev. Cell Dev. Biol. 2014;30:111–139. doi: 10.1146/annurev-cellbio-101512-122422. [DOI] [PubMed] [Google Scholar]
- 41.Huppertz B., Bartz C., Kokozidou M. Trophoblast fusion: Fusogenic proteins, syncytins and ADAMs, and other prerequisites for syncytial fusion. Micron. 2006;37:509–517. doi: 10.1016/j.micron.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Malassine A., Handschuh K., Tsatsaris V., Gerbaud P., Cheynet V., Oriol G., Mallet F., Evain-Brion D. Expression of HERV-W Env glycoprotein (syncytin) in the extravillous trophoblast of first trimester human placenta. Placenta. 2005;26:556–562. doi: 10.1016/j.placenta.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Mi S., Lee X., Li X., Veldman G.M., Finnerty H., Racie L., LaVallie E., Tang X.Y., Edouard P., Howes S., et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 44.Muir A., Lever A.M., Moffett A. Human endogenous retrovirus-W envelope (syncytin) is expressed in both villous and extravillous trophoblast populations. J. Gen. Virol. 2006;87:2067–2071. doi: 10.1099/vir.0.81412-0. [DOI] [PubMed] [Google Scholar]
- 45.Millay D.P., O’Rourke J.R., Sutherland L.B., Bezprozvannaya S., Shelton J.M., Bassel-Duby R., Olson E.N. Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature. 2013;499:301–305. doi: 10.1038/nature12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leikina E., Gamage D.G., Prasad V., Goykhberg J., Crowe M., Diao J., Kozlov M.M., Chernomordik L.V., Millay D.P. Myomaker and Myomerger Work Independently to Control Distinct Steps of Membrane Remodeling during Myoblast Fusion. Dev. Cell. 2018;46:767–780.e7. doi: 10.1016/j.devcel.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bi P., Ramirez-Martinez A., Li H., Cannavino J., McAnally J.R., Shelton J.M., Sanchez-Ortiz E., Bassel-Duby R., Olson E.N. Control of muscle formation by the fusogenic micropeptide myomixer. Science. 2017;356:323–327. doi: 10.1126/science.aam9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quinn M.E., Goh Q., Kurosaka M., Gamage D.G., Petrany M.J., Prasad V., Millay D.P. Myomerger induces fusion of non-fusogenic cells and is required for skeletal muscle development. Nat. Commun. 2017;8:15665. doi: 10.1038/ncomms15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inoue N., Ikawa M., Isotani A., Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–238. doi: 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- 50.Chalbi M., Barraud-Lange V., Ravaux B., Howan K., Rodriguez N., Soule P., Ndzoudi A., Boucheix C., Rubinstein E., Wolf J.P., et al. Binding of sperm protein Izumo1 and its egg receptor Juno drives Cd9 accumulation in the intercellular contact area prior to fusion during mammalian fertilization. Development. 2014;141:3732–3739. doi: 10.1242/dev.111534. [DOI] [PubMed] [Google Scholar]
- 51.Bianchi E., Wright G.J. Cross-species fertilization: The hamster egg receptor, Juno, binds the human sperm ligand, Izumo1. Philos. Trans. R. Soc. B Biol. Sci. 2015;370:20140101. doi: 10.1098/rstb.2014.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kato K., Satouh Y., Nishimasu H., Kurabayashi A., Morita J., Fujihara Y., Oji A., Ishitani R., Ikawa M., Nureki O. Structural and functional insights into IZUMO1 recognition by JUNO in mammalian fertilization. Nat. Commun. 2016;7:12198. doi: 10.1038/ncomms12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soe K., Andersen T.L., Hobolt-Pedersen A.S., Bjerregaard B., Larsson L.I., Delaisse J.M. Involvement of human endogenous retroviral syncytin-1 in human osteoclast fusion. Bone. 2011;48:837–846. doi: 10.1016/j.bone.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 54.Chernomordik L.V., Zimmerberg J., Kozlov M.M. Membranes of the world unite! J. Cell Biol. 2006;175:201–207. doi: 10.1083/jcb.200607083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jahn R., Lang T., Sudhof T.C. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/S0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 56.Ogle B.M., Cascalho M., Platt J.L. Biological implications of cell fusion. Nat. Rev. Mol. Cell Biol. 2005;6:567–575. doi: 10.1038/nrm1678. [DOI] [PubMed] [Google Scholar]
- 57.Whitlock J.M., Chernomordik L.V. Flagging fusion: Phosphatidylserine signaling in cell-cell fusion. J. Biol. Chem. 2021;296:100411. doi: 10.1016/j.jbc.2021.100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melzer C., von der Ohe J., Hass R. Involvement of Actin Cytoskeletal Components in Breast Cancer Cell Fusion with Human Mesenchymal Stroma/Stem-Like Cells. Int. J. Mol. Sci. 2019;20:876. doi: 10.3390/ijms20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bevers E.M., Williamson P.L. Getting to the Outer Leaflet: Physiology of Phosphatidylserine Exposure at the Plasma Membrane. Physiol. Rev. 2016;96:605–645. doi: 10.1152/physrev.00020.2015. [DOI] [PubMed] [Google Scholar]
- 60.Whitlock J.M., Yu K., Cui Y.Y., Hartzell H.C. Anoctamin 5/TMEM16E facilitates muscle precursor cell fusion. J. Gen. Physiol. 2018;150:1498–1509. doi: 10.1085/jgp.201812097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Griffin D.A., Johnson R.W., Whitlock J.M., Pozsgai E.R., Heller K.N., Grose W.E., Arnold W.D., Sahenk Z., Hartzell H.C., Rodino-Klapac L.R. Defective membrane fusion and repair in Anoctamin5-deficient muscular dystrophy. Hum. Mol. Genet. 2016;25:1900–1911. doi: 10.1093/hmg/ddw063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verma S.K., Leikina E., Melikov K., Gebert C., Kram V., Young M.F., Uygur B., Chernomordik L.V. Cell-surface phosphatidylserine regulates osteoclast precursor fusion. J. Biol. Chem. 2018;293:254–270. doi: 10.1074/jbc.M117.809681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y., Le T., Grabau R., Mohseni Z., Kim H., Natale D.R., Feng L., Pan H., Yang H. TMEM16F phospholipid scramblase mediates trophoblast fusion and placental development. Sci. Adv. 2020;6:eaba0310. doi: 10.1126/sciadv.aba0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hochreiter-Hufford A.E., Lee C.S., Kinchen J.M., Sokolowski J.D., Arandjelovic S., Call J.A., Klibanov A.L., Yan Z., Mandell J.W., Ravichandran K.S. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature. 2013;497:263–267. doi: 10.1038/nature12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park D., Tosello-Trampont A.C., Elliott M.R., Lu M., Haney L.B., Ma Z., Klibanov A.L., Mandell J.W., Ravichandran K.S. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 66.Park S.Y., Yun Y., Kim I.S. CD36 is required for myoblast fusion during myogenic differentiation. Biochem. Biophys. Res. Commun. 2012;427:705–710. doi: 10.1016/j.bbrc.2012.09.119. [DOI] [PubMed] [Google Scholar]
- 67.Helming L., Winter J., Gordon S. The scavenger receptor CD36 plays a role in cytokine-induced macrophage fusion. J. Cell Sci. 2009;122:453–459. doi: 10.1242/jcs.037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aguirre L.A., Montalban-Hernandez K., Avendano-Ortiz J., Marin E., Lozano R., Toledano V., Sanchez-Maroto L., Terron V., Valentin J., Pulido E., et al. Tumor stem cells fuse with monocytes to form highly invasive tumor-hybrid cells. Oncoimmunology. 2020;9:1773204. doi: 10.1080/2162402X.2020.1773204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davies P.S., Powell A.E., Swain J.R., Wong M.H. Inflammation and proliferation act together to mediate intestinal cell fusion. PLoS ONE. 2009;4:e6530. doi: 10.1371/journal.pone.0006530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johansson C.B., Youssef S., Koleckar K., Holbrook C., Doyonnas R., Corbel S.Y., Steinman L., Rossi F.M., Blau H.M. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat. Cell Biol. 2008;10:575–583. doi: 10.1038/ncb1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nygren J.M., Liuba K., Breitbach M., Stott S., Thoren L., Roell W., Geisen C., Sasse P., Kirik D., Bjorklund A., et al. Myeloid and lymphoid contribution to non-haematopoietic lineages through irradiation-induced heterotypic cell fusion. Nat. Cell Biol. 2008;10:584–592. doi: 10.1038/ncb1721. [DOI] [PubMed] [Google Scholar]
- 72.Hotokezaka H., Sakai E., Ohara N., Hotokezaka Y., Gonzales C., Matsuo K., Fujimura Y., Yoshida N., Nakayama K. Molecular analysis of RANKL-independent cell fusion of osteoclast-like cells induced by TNF-alpha, lipopolysaccharide, or peptidoglycan. J. Cell. Biochem. 2007;101:122–134. doi: 10.1002/jcb.21167. [DOI] [PubMed] [Google Scholar]
- 73.Melzer C., von der Ohe J., Hass R. In vitro fusion of normal and neoplastic breast epithelial cells with human mesenchymal stroma/stem cells (MSC) partially involves TNF receptor signaling. Stem Cells. 2018;36:977–989. doi: 10.1002/stem.2819. [DOI] [PubMed] [Google Scholar]
- 74.Skokos E.A., Charokopos A., Khan K., Wanjala J., Kyriakides T.R. Lack of TNF-alpha-induced MMP-9 production and abnormal E-cadherin redistribution associated with compromised fusion in MCP-1-null macrophages. Am. J. Pathol. 2011;178:2311–2321. doi: 10.1016/j.ajpath.2011.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weiler J., Dittmar T. Minocycline impairs TNF-alpha-induced cell fusion of M13SV1-Cre cells with MDA-MB-435-pFDR1 cells by suppressing NF-kappaB transcriptional activity and its induction of target-gene expression of fusion-relevant factors. Cell Commun. Signal. 2019;17:71. doi: 10.1186/s12964-019-0384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weiler J., Mohr M., Zanker K.S., Dittmar T. Matrix metalloproteinase-9 (MMP9) is involved in the TNF-alpha-induced fusion of human M13SV1-Cre breast epithelial cells and human MDA-MB-435-pFDR1 cancer cells. Cell Commun. Signal. 2018;16:14. doi: 10.1186/s12964-018-0226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yan T.L., Wang M., Xu Z., Huang C.M., Zhou X.C., Jiang E.H., Zhao X.P., Song Y., Song K., Shao Z., et al. Up-regulation of syncytin-1 contributes to TNF-alpha-enhanced fusion between OSCC and HUVECs partly via Wnt/beta-catenin-dependent pathway. Sci. Rep. 2017;7:40983. doi: 10.1038/srep40983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song K., Zhu F., Zhang H.Z., Shang Z.J. Tumor necrosis factor-alpha enhanced fusions between oral squamous cell carcinoma cells and endothelial cells via VCAM-1/VLA-4 pathway. Exp. Cell Res. 2012;318:1707–1715. doi: 10.1016/j.yexcr.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 79.Sinkovics J.G. Horizontal Gene Transfers with or without Cell Fusions in All Categories of the Living Matter. Cell Fusion Health Dis. 2011;714:5–89. doi: 10.1007/978-94-007-0782-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Emamalipour M., Seidi K., Zununi Vahed S., Jahanban-Esfahlan A., Jaymand M., Majdi H., Amoozgar Z., Chitkushev L.T., Javaheri T., Jahanban-Esfahlan R., et al. Horizontal Gene Transfer: From Evolutionary Flexibility to Disease Progression. Front. Cell Dev. Biol. 2020;8:229. doi: 10.3389/fcell.2020.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Husnik F., McCutcheon J.P. Functional horizontal gene transfer from bacteria to eukaryotes. Nat. Rev. Microbiol. 2018;16:67–79. doi: 10.1038/nrmicro.2017.137. [DOI] [PubMed] [Google Scholar]
- 82.Cairns J., Ruokolainen L., Hultman J., Tamminen M., Virta M., Hiltunen T. Ecology determines how low antibiotic concentration impacts community composition and horizontal transfer of resistance genes. Commun. Biol. 2018;1:35. doi: 10.1038/s42003-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bronkhorst A.J., Ungerer V., Holdenrieder S. The emerging role of cell-free DNA as a molecular marker for cancer management. Biomol. Detect. Quantif. 2019;17:100087. doi: 10.1016/j.bdq.2019.100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De la Taille A., Chen M.W., Burchardt M., Chopin D.K., Buttyan R. Apoptotic conversion: Evidence for exchange of genetic information between prostate cancer cells mediated by apoptosis. Cancer Res. 1999;59:5461–5463. [PubMed] [Google Scholar]
- 85.Serrano-Heras G., Dominguez-Berzosa C., Collantes E., Guadalajara H., Garcia-Olmo D., Garcia-Olmo D.C. NIH-3T3 fibroblasts cultured with plasma from colorectal cancer patients generate poorly differentiated carcinomas in mice. Cancer Lett. 2012;316:85–90. doi: 10.1016/j.canlet.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 86.Garcia-Olmo D.C., Dominguez C., Garcia-Arranz M., Anker P., Stroun M., Garcia-Verdugo J.M., Garcia-Olmo D. Cell-free nucleic acids circulating in the plasma of colorectal cancer patients induce the oncogenic transformation of susceptible cultured cells. Cancer Res. 2010;70:560–567. doi: 10.1158/0008-5472.CAN-09-3513. [DOI] [PubMed] [Google Scholar]
- 87.Bergsmedh A., Szeles A., Henriksson M., Bratt A., Folkman M.J., Spetz A.L., Holmgren L. Horizontal transfer of oncogenes by uptake of apoptotic bodies. Proc. Natl. Acad. Sci. USA. 2001;98:6407–6411. doi: 10.1073/pnas.101129998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holmgren L., Bergsmedh A., Spetz A.L. Horizontal transfer of DNA by the uptake of apoptotic bodies. Vox Sang. 2002;83(Suppl. 1):305–306. doi: 10.1111/j.1423-0410.2002.tb05323.x. [DOI] [PubMed] [Google Scholar]
- 89.Ehnfors J., Kost-Alimova M., Persson N.L., Bergsmedh A., Castro J., Levchenko-Tegnebratt T., Yang L., Panaretakis T., Holmgren L. Horizontal transfer of tumor DNA to endothelial cells in vivo. Cell Death Differ. 2009;16:749–757. doi: 10.1038/cdd.2009.7. [DOI] [PubMed] [Google Scholar]
- 90.Van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 91.Yanez-Mo M., Siljander P.R., Andreu Z., Zavec A.B., Borras F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J., et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Colombo M., Raposo G., Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 93.Cai J., Han Y., Ren H., Chen C., He D., Zhou L., Eisner G.M., Asico L.D., Jose P.A., Zeng C. Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J. Mol. Cell Biol. 2013;5:227–238. doi: 10.1093/jmcb/mjt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cai J., Wu G., Tan X., Han Y., Chen C., Li C., Wang N., Zou X., Chen X., Zhou F., et al. Transferred BCR/ABL DNA from K562 extracellular vesicles causes chronic myeloid leukemia in immunodeficient mice. PLoS ONE. 2014;9:e105200. doi: 10.1371/journal.pone.0105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fischer S., Cornils K., Speiseder T., Badbaran A., Reimer R., Indenbirken D., Grundhoff A., Brunswig-Spickenheier B., Alawi M., Lange C. Indication of Horizontal DNA Gene Transfer by Extracellular Vesicles. PLoS ONE. 2016;11:e0163665. doi: 10.1371/journal.pone.0163665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lian Q., Xu J., Yan S., Huang M., Ding H., Sun X., Bi A., Ding J., Sun B., Geng M. Chemotherapy-induced intestinal inflammatory responses are mediated by exosome secretion of double-strand DNA via AIM2 inflammasome activation. Cell Res. 2017;27:784–800. doi: 10.1038/cr.2017.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Record M. Intercellular communication by exosomes in placenta: A possible role in cell fusion? Placenta. 2014;35:297–302. doi: 10.1016/j.placenta.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 98.Uygur B., Melikov K., Arakelyan A., Margolis L.B., Chernomordik L.V. Syncytin 1 dependent horizontal transfer of marker genes from retrovirally transduced cells. Sci. Rep. 2019;9:17637. doi: 10.1038/s41598-019-54178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Howcroft T.K., Zhang H.G., Dhodapkar M., Mohla S. Vesicle transfer and cell fusion: Emerging concepts of cell-cell communication in the tumor microenvironment. Cancer Biol. Ther. 2011;12:159–164. doi: 10.4161/cbt.12.3.17032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fais S., Overholtzer M. Cell-in-cell phenomena in cancer. Nat. Rev. 2018;18:758–766. doi: 10.1038/s41568-018-0073-9. [DOI] [PubMed] [Google Scholar]
- 101.Overholtzer M., Brugge J.S. The cell biology of cell-in-cell structures. Nat. Rev. Mol. Cell Biol. 2008;9:796–809. doi: 10.1038/nrm2504. [DOI] [PubMed] [Google Scholar]
- 102.Wang X., Li Y., Li J., Li L., Zhu H., Chen H., Kong R., Wang G., Wang Y., Hu J., et al. Cell-in-Cell Phenomenon and Its Relationship With Tumor Microenvironment and Tumor Progression: A Review. Front. Cell Dev. Biol. 2019;7:311. doi: 10.3389/fcell.2019.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Janssen A., Medema R.H. Entosis: Aneuploidy by invasion. Nat. Cell Biol. 2011;13:199–201. doi: 10.1038/ncb0311-199. [DOI] [PubMed] [Google Scholar]
- 104.Xia P., Wang S., Guo Z., Yao X. Emperipolesis, entosis and beyond: Dance with fate. Cell Res. 2008;18:705–707. doi: 10.1038/cr.2008.64. [DOI] [PubMed] [Google Scholar]
- 105.Krajcovic M., Overholtzer M. Mechanisms of ploidy increase in human cancers: A new role for cell cannibalism. Cancer Res. 2012;72:1596–1601. doi: 10.1158/0008-5472.CAN-11-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.He M.F., Wang S., Wang Y., Wang X.N. Modeling cell-in-cell structure into its biological significance. Cell Death Dis. 2013;4:e630. doi: 10.1038/cddis.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sarode S.C., Sarode G.S. Neutrophil-tumor cell cannibalism in oral squamous cell carcinoma. J. Oral Pathol. Med. 2014;43:454–458. doi: 10.1111/jop.12157. [DOI] [PubMed] [Google Scholar]
- 108.Sarode G.S., Sarode S.C., Karmarkar S. Complex cannibalism: An unusual finding in oral squamous cell carcinoma. Oral Oncol. 2012;48:e4–e6. doi: 10.1016/j.oraloncology.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 109.Caruso R.A., Muda A.O., Bersiga A., Rigoli L., Inferrera C. Morphological evidence of neutrophil-tumor cell phagocytosis (cannibalism) in human gastric adenocarcinomas. Ultrastruct. Pathol. 2002;26:315–321. doi: 10.1080/01913120290104593. [DOI] [PubMed] [Google Scholar]
- 110.Cano C.E., Sandi M.J., Hamidi T., Calvo E.L., Turrini O., Bartholin L., Loncle C., Secq V., Garcia S., Lomberk G., et al. Homotypic cell cannibalism, a cell-death process regulated by the nuclear protein 1, opposes to metastasis in pancreatic cancer. EMBO Mol. Med. 2012;4:964–979. doi: 10.1002/emmm.201201255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arya P., Khalbuss W.E., Monaco S.E., Pantanowitz L. Salivary duct carcinoma with striking neutrophil-tumor cell cannibalism. Cytojournal. 2011;8:15. doi: 10.4103/1742-6413.84222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kinoshita M., Matsuda Y., Arai T., Soejima Y., Sawabe M., Honma N. Cytological diagnostic clues in poorly differentiated squamous cell carcinomas of the breast: Streaming arrangement, necrotic background, nucleolar enlargement and cannibalism of cancer cells. Cytopathology. 2018;29:22–27. doi: 10.1111/cyt.12461. [DOI] [PubMed] [Google Scholar]
- 113.Lugini L., Matarrese P., Tinari A., Lozupone F., Federici C., Iessi E., Gentile M., Luciani F., Parmiani G., Rivoltini L., et al. Cannibalism of live lymphocytes by human metastatic but not primary melanoma cells. Cancer Res. 2006;66:3629–3638. doi: 10.1158/0008-5472.CAN-05-3204. [DOI] [PubMed] [Google Scholar]
- 114.Bartosh T.J., Ullah M., Zeitouni S., Beaver J., Prockop D.J. Cancer cells enter dormancy after cannibalizing mesenchymal stem/stromal cells (MSCs) Proc. Natl. Acad. Sci. USA. 2016;113:E6447–E6456. doi: 10.1073/pnas.1612290113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lozupone F., Fais S. Cancer Cell Cannibalism: A Primeval Option to Survive. Curr. Mol. Med. 2015;15:836–841. doi: 10.2174/1566524015666151026100916. [DOI] [PubMed] [Google Scholar]
- 116.Sharma N., Dey P. Cell cannibalism and cancer. Diagn. Cytopathol. 2011;39:229–233. doi: 10.1002/dc.21402. [DOI] [PubMed] [Google Scholar]
- 117.Huber V., Fais S., Iero M., Lugini L., Canese P., Squarcina P., Zaccheddu A., Colone M., Arancia G., Gentile M., et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: Role in immune escape. Gastroenterology. 2005;128:1796–1804. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 118.Caruso R.A., Fedele F., Finocchiaro G., Arena G., Venuti A. Neutrophil-tumor cell phagocytosis (cannibalism) in human tumors: An update and literature review. Exp. Oncol. 2012;34:306–311. [PubMed] [Google Scholar]
- 119.Sierro F., Tay S.S., Warren A., Le Couteur D.G., McCaughan G.W., Bowen D.G., Bertolino P. Suicidal emperipolesis: A process leading to cell-in-cell structures, T cell clearance and immune homeostasis. Curr. Mol. Med. 2015;15:819–827. doi: 10.2174/1566524015666151026102143. [DOI] [PubMed] [Google Scholar]
- 120.Chen Y.H., Wang S., He M.F., Wang Y., Zhao H., Zhu H.Y., Yu X.M., Ma J., Che X.J., Wang J.F., et al. Prevalence of heterotypic tumor/immune cell-in-cell structure in vitro and in vivo leading to formation of aneuploidy. PLoS ONE. 2013;8:e59418. doi: 10.1371/journal.pone.0059418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Janssen A., Medema R.H. Genetic instability: Tipping the balance. Oncogene. 2013;32:4459–4470. doi: 10.1038/onc.2012.576. [DOI] [PubMed] [Google Scholar]
- 122.Overholtzer M., Mailleux A.A., Mouneimne G., Normand G., Schnitt S.J., King R.W., Cibas E.S., Brugge J.S. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–979. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 123.Sottile F., Aulicino F., Theka I., Cosma M.P. Mesenchymal stem cells generate distinct functional hybrids in vitro via cell fusion or entosis. Sci. Rep. 2016;6:36863. doi: 10.1038/srep36863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oliveira M.N., Pillat M.M., Motaln H., Ulrich H., Lah T.T. Kinin-B1 Receptor Stimulation Promotes Invasion and is Involved in Cell-Cell Interaction of Co-Cultured Glioblastoma and Mesenchymal Stem Cells. Sci. Rep. 2018;8:1299. doi: 10.1038/s41598-018-19359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miroshnychenko D., Baratchart E., Ferrall-Fairbanks M.C., Velde R.V., Laurie M.A., Bui M.M., Tan A.C., Altrock P.M., Basanta D., Marusyk A. Spontaneous cell fusions as a mechanism of parasexual recombination in tumour cell populations. Nat. Ecol. Evol. 2021;5:379–391. doi: 10.1038/s41559-020-01367-y. [DOI] [PubMed] [Google Scholar]
- 126.Aichel O. Über Zellverschmelzung mit quantitativ abnormer Chromosomenverteilung als Ursache der Geschwulstbildung. In: Roux W., editor. Vorträge und Aufsätze über Entwicklungsmechanik der Organismen. Wilhelm Engelmann; Leipzig, Germany: 1911. pp. 1–115. [Google Scholar]
- 127.Berndt B., Haverkampf S., Reith G., Keil S., Niggemann B., Zanker K.S., Dittmar T. Fusion of CCL21 non-migratory active breast epithelial and breast cancer cells give rise to CCL21 migratory active tumor hybrid cell lines. PLoS ONE. 2013;8:e63711. doi: 10.1371/journal.pone.0063711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dittmar T., Nagler C., Schwitalla S., Reith G., Niggemann B., Zanker K.S. Recurrence cancer stem cells--made by cell fusion? Med. Hypotheses. 2009;73:542–547. doi: 10.1016/j.mehy.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 129.Dittmar T., Schwitalla S., Seidel J., Haverkampf S., Reith G., Meyer-Staeckling S., Brandt B.H., Niggemann B., Zanker K.S. Characterization of hybrid cells derived from spontaneous fusion events between breast epithelial cells exhibiting stem-like characteristics and breast cancer cells. Clin. Exp. Metastasis. 2011;28:75–90. doi: 10.1007/s10585-010-9359-3. [DOI] [PubMed] [Google Scholar]
- 130.Duelli D.M., Padilla-Nash H.M., Berman D., Murphy K.M., Ried T., Lazebnik Y. A virus causes cancer by inducing massive chromosomal instability through cell fusion. Curr. Biol. 2007;17:431–437. doi: 10.1016/j.cub.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 131.Jacobsen B.M., Harrell J.C., Jedlicka P., Borges V.F., Varella-Garcia M., Horwitz K.B. Spontaneous fusion with, and transformation of mouse stroma by, malignant human breast cancer epithelium. Cancer Res. 2006;66:8274–8279. doi: 10.1158/0008-5472.CAN-06-1456. [DOI] [PubMed] [Google Scholar]
- 132.Lindstrom A., Midtbo K., Arnesson L.G., Garvin S., Shabo I. Fusion between M2-macrophages and cancer cells results in a subpopulation of radioresistant cells with enhanced DNA-repair capacity. Oncotarget. 2017;8:51370–51386. doi: 10.18632/oncotarget.17986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lizier M., Anselmo A., Mantero S., Ficara F., Paulis M., Vezzoni P., Lucchini F., Pacchiana G. Fusion between cancer cells and macrophages occurs in a murine model of spontaneous neu+ breast cancer without increasing its metastatic potential. Oncotarget. 2016;7:60793–60806. doi: 10.18632/oncotarget.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Luo F., Liu T., Wang J., Li J., Ma P., Ding H., Feng G., Lin D., Xu Y., Yang K. Bone marrow mesenchymal stem cells participate in prostate carcinogenesis and promote growth of prostate cancer by cell fusion in vivo. Oncotarget. 2016;7:30924–30934. doi: 10.18632/oncotarget.9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Melzer C., von der Ohe J., Hass R. In Vivo Cell Fusion between Mesenchymal Stroma/Stem-Like Cells and Breast Cancer Cells. Cancers. 2019;11:185. doi: 10.3390/cancers11020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Noubissi F.K., Harkness T., Alexander C.M., Ogle B.M. Apoptosis-induced cancer cell fusion: A mechanism of breast cancer metastasis. FASEB J. 2015;29:4036–4045. doi: 10.1096/fj.15-271098. [DOI] [PubMed] [Google Scholar]
- 137.Powell A.E., Anderson E.C., Davies P.S., Silk A.D., Pelz C., Impey S., Wong M.H. Fusion between Intestinal epithelial cells and macrophages in a cancer context results in nuclear reprogramming. Cancer Res. 2011;71:1497–1505. doi: 10.1158/0008-5472.CAN-10-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ramakrishnan M., Mathur S.R., Mukhopadhyay A. Fusion derived epithelial cancer cells express hematopoietic markers and contribute to stem cell and migratory phenotype in ovarian carcinoma. Cancer Res. 2013;73:5360–5370. doi: 10.1158/0008-5472.CAN-13-0896. [DOI] [PubMed] [Google Scholar]
- 139.Shabo I., Svanvik J., Lindstrom A., Lechertier T., Trabulo S., Hulit J., Sparey T., Pawelek J. Roles of cell fusion, hybridization and polyploid cell formation in cancer metastasis. World J. Clin. Oncol. 2020;11:121–135. doi: 10.5306/wjco.v11.i3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Su Y., Subedee A., Bloushtain-Qimron N., Savova V., Krzystanek M., Li L., Marusyk A., Tabassum D.P., Zak A., Flacker M.J., et al. Somatic Cell Fusions Reveal Extensive Heterogeneity in Basal-like Breast Cancer. Cell Rep. 2015;11:1549–1563. doi: 10.1016/j.celrep.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 141.Suetsugu A., Matsumoto T., Hasegawa K., Nakamura M., Kunisada T., Shimizu M., Saji S., Moriwaki H., Bouvet M., Hoffman R.M. Color-coded Live Imaging of Heterokaryon Formation and Nuclear Fusion of Hybridizing Cancer Cells. Anticancer Res. 2016;36:3827–3831. [PubMed] [Google Scholar]
- 142.Wang R., Lewis M.S., Lyu J., Zhau H.E., Pandol S.J., Chung L.W.K. Cancer-stromal cell fusion as revealed by fluorescence protein tracking. Prostate. 2019;80:274–283. doi: 10.1002/pros.23941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang R., Sun X., Wang C.Y., Hu P., Chu C.Y., Liu S., Zhau H.E., Chung L.W. Spontaneous cancer-stromal cell fusion as a mechanism of prostate cancer androgen-independent progression. PLoS ONE. 2012;7:e42653. doi: 10.1371/journal.pone.0042653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yao J., Zhang L., Hu L., Guo B., Hu X., Borjigin U., Wei Z., Chen Y., Lv M., Lau J.T., et al. Tumorigenic potential is restored during differentiation in fusion-reprogrammed cancer cells. Cell Death Dis. 2016;7:e2314. doi: 10.1038/cddis.2016.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yin L., Hu P., Shi X., Qian W., Zhau H.E., Pandol S.J., Lewis M.S., Chung L.W.K., Wang R. Cancer cell’s neuroendocrine feature can be acquired through cell-cell fusion during cancer-neural stem cell interaction. Sci. Rep. 2020;10:1216. doi: 10.1038/s41598-020-58118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zeng C., Zhang Y., Park S.C., Eun J.R., Nguyen N.T., Tschudy-Seney B., Jung Y.J., Theise N.D., Zern M.A., Duan Y. CD34 Liver Cancer Stem Cells Were Formed by Fusion of Hepatobiliary Stem/Progenitor Cells with Hematopoietic Precursor-Derived Myeloid Intermediates. Stem Cells Dev. 2015;24:2467–2478. doi: 10.1089/scd.2015.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang L.N., Kong C.F., Zhao D., Cong X.L., Wang S.S., Ma L., Huang Y.H. Fusion with mesenchymal stem cells differentially affects tumorigenic and metastatic abilities of lung cancer cells. J. Cell. Physiol. 2018:3570–3582. doi: 10.1002/jcp.27011. [DOI] [PubMed] [Google Scholar]
- 148.Ding J., Jin W., Chen C., Shao Z., Wu J. Tumor associated macrophage x cancer cell hybrids may acquire cancer stem cell properties in breast cancer. PLoS ONE. 2012;7:e41942. doi: 10.1371/journal.pone.0041942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mukhopadhyay K.D., Bandyopadhyay A., Chang T.T., Elkahloun A.G., Cornell J.E., Yang J., Goins B.A., Yeh I.T., Sun L.Z. Isolation and characterization of a metastatic hybrid cell line generated by ER negative and ER positive breast cancer cells in mouse bone marrow. PLoS ONE. 2011;6:e20473. doi: 10.1371/journal.pone.0020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nagler C., Zanker K.S., Dittmar T. Murine breast-cancer-cell/mesenchymal-stem-cell hybrids exhibit enhanced drug resistance to different cytostatic drugs. J. Stem Cells Regen. Med. 2010;6:133. [PubMed] [Google Scholar]
- 151.Rappa G., Mercapide J., Lorico A. Spontaneous Formation of Tumorigenic Hybrids between Breast Cancer and Multipotent Stromal Cells Is a Source of Tumor Heterogeneity. Am. J. Pathol. 2012;180:2504–2515. doi: 10.1016/j.ajpath.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xu M.H., Gao X., Luo D., Zhou X.D., Xiong W., Liu G.X. EMT and acquisition of stem cell-like properties are involved in spontaneous formation of tumorigenic hybrids between lung cancer and bone marrow-derived mesenchymal stem cells. PLoS ONE. 2014;9:e87893. doi: 10.1371/journal.pone.0087893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Melzer C., von der Ohe J., Hass R. Enhanced metastatic capacity of breast cancer cells after interaction and hybrid formation with mesenchymal stroma/stem cells (MSC) Cell Commun. Signal. 2018;16:2. doi: 10.1186/s12964-018-0215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Melzer C., von der Ohe J., Hass R. MSC stimulate ovarian tumor growth during intercellular communication but reduce tumorigenicity after fusion with ovarian cancer cells. Cell Commun. Signal. 2018;16:67. doi: 10.1186/s12964-018-0279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Melzer C., Yang Y., Hass R. Interaction of MSC with tumor cells. Cell Commun. Signal. 2016;14:20. doi: 10.1186/s12964-016-0143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chakraborty A.K., Sodi S., Rachkovsky M., Kolesnikova N., Platt J.T., Bolognia J.L., Pawelek J.M. A spontaneous murine melanoma lung metastasis comprised of host x tumor hybrids. Cancer Res. 2000;60:2512–2519. [PubMed] [Google Scholar]
- 157.Goldenberg D.M., Pavia R.A., Tsao M.C. In vivo hybridisation of human tumour and normal hamster cells. Nature. 1974;250:649–651. doi: 10.1038/250649a0. [DOI] [PubMed] [Google Scholar]
- 158.Lu X., Kang Y. Efficient acquisition of dual metastasis organotropism to bone and lung through stable spontaneous fusion between MDA-MB-231 variants. Proc. Natl. Acad. Sci. USA. 2009;106:9385–9390. doi: 10.1073/pnas.0900108106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mekler L.B., Drize O.B., Osechinskii I.V., Shliankevich M.A. Transformation of a normal differentiated cell of an adult organism, induced by the fusion of this cell with another normal cell of the same organism but with different organ or tissue specificity. Vestn. Akad. Meditsinskikh Nauk. SSSR. 1971;26:75–80. [PubMed] [Google Scholar]
- 160.Rachkovsky M., Sodi S., Chakraborty A., Avissar Y., Bolognia J., McNiff J.M., Platt J., Bermudes D., Pawelek J. Melanoma x macrophage hybrids with enhanced metastatic potential. Clin. Exp. Metastasis. 1998;16:299–312. doi: 10.1023/A:1006557228604. [DOI] [PubMed] [Google Scholar]
- 161.Van Golen K.L., Risin S., Staroselsky A., Berger D., Tainsky M.A., Pathak S., Price J.E. Predominance of the metastatic phenotype in hybrids formed by fusion of mouse and human melanoma clones. Clin. Exp. Metastasis. 1996;14:95–106. doi: 10.1007/BF00121206. [DOI] [PubMed] [Google Scholar]
- 162.Yan B., Wang J., Liu L. Chemotherapy promotes tumour cell hybridization in vivo. Tumour Biol. 2015;37:5025–5030. doi: 10.1007/s13277-015-4337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li G.C., Ye Q.H., Dong Q.Z., Ren N., Jia H.L., Qin L.X. Mesenchymal stem cells seldomly fuse with hepatocellular carcinoma cells and are mainly distributed in the tumor stroma in mouse models. Oncol. Rep. 2013;29:713–719. doi: 10.3892/or.2012.2174. [DOI] [PubMed] [Google Scholar]
- 164.Gast C.E., Silk A.D., Zarour L., Riegler L., Burkhart J.G., Gustafson K.T., Parappilly M.S., Roh-Johnson M., Goodman J.R., Olson B., et al. Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Sci. Adv. 2018;4:eaat7828. doi: 10.1126/sciadv.aat7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Martin-Padura I., Marighetti P., Gregato G., Agliano A., Malazzi O., Mancuso P., Pruneri G., Viale A., Bertolini F. Spontaneous cell fusion of acute leukemia cells and macrophages observed in cells with leukemic potential. Neoplasia. 2012;14:1057–1066. doi: 10.1593/neo.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tal A., Tal R., Shaikh S., Gidicsin S., Mamillapalli R., Taylor H.S. Characterization of Cell Fusion in an Experimental Mouse Model of Endometriosis. Biol. Reprod. 2018;100:390–397. doi: 10.1093/biolre/ioy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Carloni V., Mazzocca A., Mello T., Galli A., Capaccioli S. Cell fusion promotes chemoresistance in metastatic colon carcinoma. Oncogene. 2013;32:2649–2660. doi: 10.1038/onc.2012.268. [DOI] [PubMed] [Google Scholar]
- 168.Strick R., Ackermann S., Langbein M., Swiatek J., Schubert S.W., Hashemolhosseini S., Koscheck T., Fasching P.A., Schild R.L., Beckmann M.W., et al. Proliferation and cell-cell fusion of endometrial carcinoma are induced by the human endogenous retroviral Syncytin-1 and regulated by TGF-beta. J. Mol. Med. 2007;85:23–38. doi: 10.1007/s00109-006-0104-y. [DOI] [PubMed] [Google Scholar]
- 169.Li M., Li X., Zhao L., Zhou J., Cheng Y., Xu B., Wang J., Wei L. Spontaneous formation of tumorigenic hybrids between human omental adipose-derived stromal cells and endometrial cancer cells increased motility and heterogeneity of cancer cells. Cell Cycle. 2019;18:320–332. doi: 10.1080/15384101.2019.1568743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kerbel R.S., Lagarde A.E., Dennis J.W., Donaghue T.P. Spontaneous fusion in vivo between normal host and tumor cells: Possible contribution to tumor progression and metastasis studied with a lectin-resistant mutant tumor. Mol. Cell. Biol. 1983;3:523–538. doi: 10.1128/MCB.3.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Duelli D.M., Hearn S., Myers M.P., Lazebnik Y. A primate virus generates transformed human cells by fusion. J. Cell Biol. 2005;171:493–503. doi: 10.1083/jcb.200507069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lartigue L., Merle C., Lagarde P., Delespaul L., Lesluyes T., Le Guellec S., Perot G., Leroy L., Coindre J.M., Chibon F. Genome remodeling upon mesenchymal tumor cell fusion contributes to tumor progression and metastatic spread. Oncogene. 2020;39:4198–4211. doi: 10.1038/s41388-020-1276-6. [DOI] [PubMed] [Google Scholar]
- 173.Wakeling W.F., Greetham J., Devlin L.M., Bennett D.C. Suppression of properties associated with malignancy in murine melanoma-melanocyte hybrid cells. Br. J. Cancer. 1992;65:529–537. doi: 10.1038/bjc.1992.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Spees J.L., Olson S.D., Ylostalo J., Lynch P.J., Smith J., Perry A., Peister A., Wang M.Y., Prockop D.J. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc. Natl. Acad. Sci. USA. 2003;100:2397–2402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wakeling W.F., Greetham J., Bennett D.C. Efficient spontaneous fusion between some co-cultured cells, especially murine melanoma cells. Cell Biol. Int. 1994;18:207–210. doi: 10.1006/cbir.1994.1063. [DOI] [PubMed] [Google Scholar]
- 176.Wang R., Chen S., Li C., Ng K.T., Kong C.W., Cheng J., Cheng S.H., Li R.A., Lo C.M., Man K., et al. Fusion with stem cell makes the hepatocellular carcinoma cells similar to liver tumor-initiating cells. BMC Cancer. 2016;16:56. doi: 10.1186/s12885-016-2094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mohr M., Tosun S., Arnold W.H., Edenhofer F., Zanker K.S., Dittmar T. Quantification of cell fusion events human breast cancer cells and breast epithelial cells using a Cre-LoxP-based double fluorescence reporter system. Cell. Mol. Life Sci. 2015;72:3769–3782. doi: 10.1007/s00018-015-1910-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fahlbusch S.S., Keil S., Epplen J.T., Zanker K.S., Dittmar T. Comparison of hybrid clones derived from human breast epithelial cells and three different cancer cell lines regarding in vitro cancer stem/initiating cell properties. BMC Cancer. 2020;20:446. doi: 10.1186/s12885-020-06952-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Uygur B., Leikina E., Melikov K., Villasmil R., Verma S.K., Vary C.P.H., Chernomordik L.V. Interactions with Muscle Cells Boost Fusion, Stemness, and Drug Resistance of Prostate Cancer Cells. Mol. Cancer Res. 2019;17:806–820. doi: 10.1158/1541-7786.MCR-18-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Larsson L.I., Holck S., Christensen I.J. Prognostic role of syncytin expression in breast cancer. Hum. Pathol. 2007;38:726–731. doi: 10.1016/j.humpath.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 181.Shabo I., Midtbo K., Andersson H., Akerlund E., Olsson H., Wegman P., Gunnarsson C., Lindstrom A. Macrophage traits in cancer cells are induced by macrophage-cancer cell fusion and cannot be explained by cellular interaction. BMC Cancer. 2015;15:922. doi: 10.1186/s12885-015-1935-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shabo I., Olsson H., Stal O., Svanvik J. Breast cancer expression of DAP12 is associated with skeletal and liver metastases and poor survival. Clin. Breast Cancer. 2013;13:371–377. doi: 10.1016/j.clbc.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 183.Shabo I., Olsson H., Sun X.F., Svanvik J. Expression of the macrophage antigen CD163 in rectal cancer cells is associated with early local recurrence and reduced survival time. Int. J. Cancer. 2009;125:1826–1831. doi: 10.1002/ijc.24506. [DOI] [PubMed] [Google Scholar]
- 184.Shabo I., Stal O., Olsson H., Dore S., Svanvik J. Breast cancer expression of CD163, a macrophage scavenger receptor, is related to early distant recurrence and reduced patient survival. Int. J. Cancer. 2008;123:780–786. doi: 10.1002/ijc.23527. [DOI] [PubMed] [Google Scholar]
- 185.LaBerge G.S., Duvall E., Grasmick Z., Haedicke K., Pawelek J. A Melanoma Lymph Node Metastasis with a Donor-Patient Hybrid Genome following Bone Marrow Transplantation: A Second Case of Leucocyte-Tumor Cell Hybridization in Cancer Metastasis. PLoS ONE. 2017;12:e0168581. doi: 10.1371/journal.pone.0168581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lazova R., Laberge G.S., Duvall E., Spoelstra N., Klump V., Sznol M., Cooper D., Spritz R.A., Chang J.T., Pawelek J.M. A Melanoma Brain Metastasis with a Donor-Patient Hybrid Genome following Bone Marrow Transplantation: First Evidence for Fusion in Human Cancer. PLoS ONE. 2013;8:e66731. doi: 10.1371/journal.pone.0066731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Andersen T.L., Boissy P., Sondergaard T.E., Kupisiewicz K., Plesner T., Rasmussen T., Haaber J., Kolvraa S., Delaisse J.M. Osteoclast nuclei of myeloma patients show chromosome translocations specific for the myeloma cell clone: A new type of cancer-host partnership? J. Pathol. 2007;211:10–17. doi: 10.1002/path.2078. [DOI] [PubMed] [Google Scholar]
- 188.Andersen T.L., Soe K., Sondergaard T.E., Plesner T., Delaisse J.M. Myeloma cell-induced disruption of bone remodelling compartments leads to osteolytic lesions and generation of osteoclast-myeloma hybrid cells. Br. J. Haematol. 2010;148:551–561. doi: 10.1111/j.1365-2141.2009.07980.x. [DOI] [PubMed] [Google Scholar]
- 189.Chakraborty A., Lazova R., Davies S., Backvall H., Ponten F., Brash D., Pawelek J. Donor DNA in a renal cell carcinoma metastasis from a bone marrow transplant recipient. Bone Marrow Transplant. 2004;34:183–186. doi: 10.1038/sj.bmt.1704547. [DOI] [PubMed] [Google Scholar]
- 190.Kurgyis Z., Kemeny L.V., Buknicz T., Groma G., Olah J., Jakab A., Polyanka H., Zanker K., Dittmar T., Kemeny L., et al. Melanoma-Derived BRAF(V600E) Mutation in Peritumoral Stromal Cells: Implications for in Vivo Cell Fusion. Int. J. Mol. Sci. 2016;17:980. doi: 10.3390/ijms17060980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Clawson G.A., Kimchi E., Patrick S.D., Xin P., Harouaka R., Zheng S., Berg A., Schell T., Staveley-O’Carroll K.F., Neves R.I., et al. Circulating tumor cells in melanoma patients. PLoS ONE. 2012;7:e41052. doi: 10.1371/journal.pone.0041052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Clawson G.A., Matters G.L., Xin P., Imamura-Kawasawa Y., Du Z., Thiboutot D.M., Helm K.F., Neves R.I., Abraham T. Macrophage-Tumor Cell Fusions from Peripheral Blood of Melanoma Patients. PLoS ONE. 2015;10:e0134320. doi: 10.1371/journal.pone.0134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Clawson G.A., Matters G.L., Xin P., McGovern C., Wafula E., dePamphilis C., Meckley M., Wong J., Stewart L., D’Jamoos C., et al. Stealth dissemination of macrophage-tumor cell fusions cultured from blood of patients with pancreatic ductal adenocarcinoma. PLoS ONE. 2017;12:e0184451. doi: 10.1371/journal.pone.0184451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Manjunath Y., Mitchem J.B., Suvilesh K.N., Avella D.M., Kimchi E.T., Staveley-O’Carroll K.F., Deroche C.B., Pantel K., Li G., Kaifi J.T. Circulating giant tumor-macrophage fusion cells are independent prognosticators in non-small cell lung cancer patients. J. Thorac. Oncol. 2020;15:1460–1471. doi: 10.1016/j.jtho.2020.04.034. [DOI] [PubMed] [Google Scholar]
- 195.Manjunath Y., Porciani D., Mitchem J.B., Suvilesh K.N., Avella D.M., Kimchi E.T., Staveley-O’Carroll K.F., Burke D.H., Li G., Kaifi J.T. Tumor-Cell-Macrophage Fusion Cells as Liquid Biomarkers and Tumor Enhancers in Cancer. Int. J. Mol. Sci. 2020;21:1872. doi: 10.3390/ijms21051872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yilmaz Y., Lazova R., Qumsiyeh M., Cooper D., Pawelek J. Donor Y chromosome in renal carcinoma cells of a female BMT recipient: Visualization of putative BMT-tumor hybrids by FISH. Bone Marrow Transplant. 2005;35:1021–1024. doi: 10.1038/sj.bmt.1704939. [DOI] [PubMed] [Google Scholar]
- 197.Shabo I., Svanvik J. Expression of macrophage antigens by tumor cells. Adv. Exp. Med. Biol. 2011;714:141–150. doi: 10.1007/978-94-007-0782-5_7. [DOI] [PubMed] [Google Scholar]
- 198.Sprangers A.J., Freeman B.T., Kouris N.A., Ogle B.M. A Cre-Lox P recombination approach for the detection of cell fusion in vivo. J. Vis. Exp. JoVE. 2012;59:e3581. doi: 10.3791/3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Koulakov A.A., Lazebnik Y. The Problem of Colliding Networks and its Relation to Cell Fusion and Cancer. Biophys. J. 2012;103:2011–2020. doi: 10.1016/j.bpj.2012.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 201.Bjerregaard B., Holck S., Christensen I.J., Larsson L.I. Syncytin is involved in breast cancer-endothelial cell fusions. Cell. Mol. Life Sci. 2006;63:1906–1911. doi: 10.1007/s00018-006-6201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Larsson L.I., Bjerregaard B., Wulf-Andersen L., Talts J.F. Syncytin and cancer cell fusions. Sci. World J. 2007;7:1193–1197. doi: 10.1100/tsw.2007.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yu H., Liu T., Zhao Z., Chen Y., Zeng J., Liu S., Zhu F. Mutations in 3′-long terminal repeat of HERV-W family in chromosome 7 upregulate syncytin-1 expression in urothelial cell carcinoma of the bladder through interacting with c-Myb. Oncogene. 2014;33:3947–3958. doi: 10.1038/onc.2013.366. [DOI] [PubMed] [Google Scholar]
- 204.Hass R., Otte A. Mesenchymal stem cells as all-round supporters in a normal and neoplastic microenvironment. Cell Commun. Signal. 2012;10:26. doi: 10.1186/1478-811X-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Melzer C., von der Ohe J., Hass R. Concise Review: Crosstalk of Mesenchymal Stroma/Stem-Like Cells with Cancer Cells Provides Therapeutic Potential. Stem Cells. 2018;36:951–968. doi: 10.1002/stem.2829. [DOI] [PubMed] [Google Scholar]
- 206.Hass R. Role of MSC in the Tumor Microenvironment. Cancers. 2020;12:2107. doi: 10.3390/cancers12082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Muller A., Homey B., Soto H., Ge N., Catron D., Buchanan M.E., McClanahan T., Murphy E., Yuan W., Wagner S.N., et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 208.Gauck D., Keil S., Niggemann B., Zanker K.S., Dittmar T. Hybrid clone cells derived from human breast epithelial cells and human breast cancer cells exhibit properties of cancer stem/initiating cells. BMC Cancer. 2017;17:515. doi: 10.1186/s12885-017-3509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Melzer C., Ohe J.V., Luo T., Hass R. Spontaneous Fusion of MSC with Breast Cancer Cells Can Generate Tumor Dormancy. Int. J. Mol. Sci. 2021;22:5930. doi: 10.3390/ijms22115930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ishikawa H., Meng F., Kondo N., Iwamoto A., Matsuda Z. Generation of a dual-functional split-reporter protein for monitoring membrane fusion using self-associating split GFP. Protein Eng. Des. Sel. 2012;25:813–820. doi: 10.1093/protein/gzs051. [DOI] [PubMed] [Google Scholar]
- 211.Weiler J., Dittmar T. Cell Fusion in Human Cancer: The Dark Matter Hypothesis. Cells. 2019;8:132. doi: 10.3390/cells8020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Trejo-Becerril C., Perez-Cardenas E., Taja-Chayeb L., Anker P., Herrera-Goepfert R., Medina-Velazquez L.A., Hidalgo-Miranda A., Perez-Montiel D., Chavez-Blanco A., Cruz-Velazquez J., et al. Cancer progression mediated by horizontal gene transfer in an in vivo model. PLoS ONE. 2012;7:e52754. doi: 10.1371/journal.pone.0052754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hass R., von der Ohe J., Ungefroren H. Potential Role of MSC/Cancer Cell Fusion and EMT for Breast Cancer Stem Cell Formation. Cancers. 2019;11:1432. doi: 10.3390/cancers11101432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bracq L., Xie M., Lambele M., Vu L.T., Matz J., Schmitt A., Delon J., Zhou P., Randriamampita C., Bouchet J., et al. T Cell-Macrophage Fusion Triggers Multinucleated Giant Cell Formation for HIV-1 Spreading. J. Virol. 2017;91:e01237-17. doi: 10.1128/JVI.01237-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kemeny L.V., Kurgyis Z., Buknicz T., Groma G., Jakab A., Zanker K., Dittmar T., Kemeny L., Nemeth I.B. Melanoma Cells Can Adopt the Phenotype of Stromal Fibroblasts and Macrophages by Spontaneous Cell Fusion in Vitro. Int. J. Mol. Sci. 2016;17:826. doi: 10.3390/ijms17060826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Streubel B., Chott A., Huber D., Exner M., Jager U., Wagner O., Schwarzinger I. Lymphoma-specific genetic aberrations in microvascular endothelial cells in B-cell lymphomas. N. Engl. J. Med. 2004;351:250–259. doi: 10.1056/NEJMoa033153. [DOI] [PubMed] [Google Scholar]
- 217.Duelli D., Lazebnik Y. Cell fusion: A hidden enemy? Cancer Cell. 2003;3:445–448. doi: 10.1016/S1535-6108(03)00114-4. [DOI] [PubMed] [Google Scholar]
- 218.LaBerge G., Duvall E., Grasmick Z., Haedicke K., Galan A., Pawelek J. A melanoma patient with macrophage-cancer cell hybrids in the primary tumor, a lymph node metastasis and a brain metastasis. Cancer Genet. 2021;256–257:162–164. doi: 10.1016/j.cancergen.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 219.Silvestris F., Ciavarella S., Strippoli S., Dammacco F. Cell fusion and hyperactive osteoclastogenesis in multiple myeloma. Adv. Exp. Med. Biol. 2011;714:113–128. doi: 10.1007/978-94-007-0782-5_5. [DOI] [PubMed] [Google Scholar]