Abstract
Platelets play a critical role in hemostasis and thrombus formation. Platelets are small, anucleate, and short-lived blood cells that are produced by the large, polyploid, and hematopoietic stem cell (HSC)-derived megakaryocytes in bone marrow. Approximately 3000 platelets are released from one megakaryocyte, and thus, it is important to understand the physiologically relevant mechanism of development of mature megakaryocytes. Many genes, including several key transcription factors, have been shown to be crucial for platelet biogenesis. Mutations in these genes can perturb megakaryopoiesis or thrombopoiesis, resulting in thrombocytopenia. Metabolic changes owing to inflammation, ageing, or diseases such as cancer, in which platelets play crucial roles in disease development, can also affect platelet biogenesis. In this review, I describe the characteristics of platelets and megakaryocytes in terms of their differentiation processes. The role of several critical transcription factors have been discussed to better understand the changes in platelet biogenesis that occur during disease or ageing.
Keywords: megakaryocyte, platelet, transcription factor, differentiation, disease, ageing
1. Introduction
Platelets are small, anucleate blood cells produced from mature bone marrow megakaryocytes [1]. In humans, the reference range for normal platelet count is 150–400 × 109/L, and its life span is 8–12 days. Approximately 1011 platelets are generated daily, suggesting that a robust differentiation process is required [2]. Thrombocytopenia, a condition characterized by defective or low platelet generation, can be life-threatening owing to the risk of hemorrhage. Thus, many studies have been conducted to understand the mechanisms of thrombopoiesis, and novel factors involved in its mechanism are being discovered. Hematopoietic stem cells (HSCs) give rise to megakaryocyte–erythroid progenitors (MEPs) via common myeloid progenitors (CMPs), followed by differentiation into megakaryocytes (MKs) [3]. In addition, recent studies on mice suggest the existence of an MK-biased HSC population, which has been shown to play a critical role in thrombopoiesis during pathologic conditions [4,5,6]. Congenital platelet disorders occur due to quantitative or qualitative defects during megakaryocyte differentiation. Moreover, myeloproliferative neoplasm (MPN), a hematologic malignancy, is characterized by MK hyperplasia due to skewed MK differentiation in the bone marrow, resulting in an abnormal platelet count. Notably, many studies on inherited thrombocytopenia have revealed the accumulation of megakaryocyte precursors (MkPs) and/or early MKs in the bone marrow; this indicates the developmental arrest could result in MkP hyperplasia and affect platelet biogenesis. In this review, I focus on the molecular mechanisms of megakaryopoiesis in order to understand platelet disorders.
2. Platelet Biology and Platelet Biogenesis
2.1. Platelet Receptors
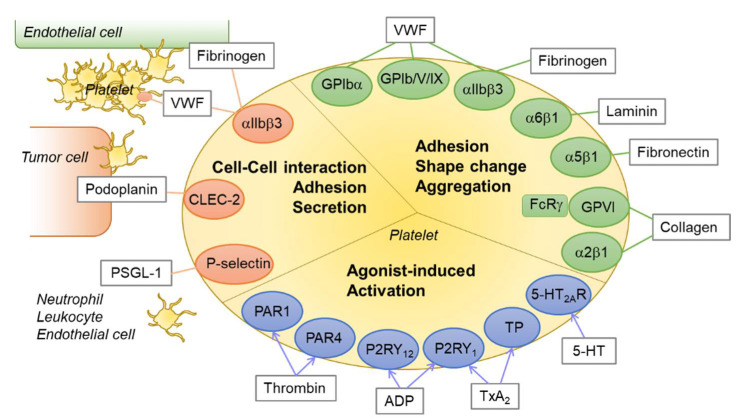
Although platelets are small and enucleated, they express various types of receptors, such as integrins, glycoproteins, selectins, G-protein coupled receptors (GPCRs), and receptors of the immunoglobulin type. These receptors interact with their endogenous ligands, which include agonists for the initiation of hemostasis, to enforce platelet activation. The receptor–ligand interactions are exemplified by integrin α5β1–fibrinogen [7], integrin α6β1–laminin [7], integrin α2β1–collagen [8], GP1bα–von Willebrand factor (VWF) [9], glycoprotein VI (GPVI)–collagen [10], GPIIb/IIIa (integrin α2bβ3, CD41/CD61)–fibrinogen [11], and p-selectin–p-selectin glycoprotein ligand1 (PSGL1) [12]. Platelets adhere to VWF on the subendothelial matrix via the GPIb/V/IX complex when vascular damage occurs [13]. This adhesion is stabilized by the exposed collagen, which recruits the binding of GPVI and integrin α2β1 to the platelets.
The heterodimeric integrin GPIIb/IIIa, which binds to fibrinogen, VWF, and fibronectin, comprises up to 15% by weight of total platelet plasma membrane proteins [14]. On initiation of GPVI signaling after vascular injury, crosslinking of platelets with platelets and platelets with endothelial cells occur via the interaction between GPIIb/IIIa and fibrinogen, leading to thrombus formation [15,16]. During hemostasis and thrombosis, agonists such as ADP, thromboxane A2 (TxA2), and thrombin, which are secreted by activated platelets or synthesized through the coagulation cascade in the plasma membrane, are released and stimulate the platelets further via P2Y purinoceptor 12 (P2RY12), TxA2 receptor, and protease activated receptor 1 (PAR1), respectively [17,18,19]. This supports platelet aggregation and thrombus formation [20]. Inhibitory signaling pathways, induced by nitric oxide (NO) and prostacyclin (PGI2), to avoid undesired platelet activation also exist [21].
Selectins are involved in the aggregation of platelets with other cell types, including endothelial cells, neutrophils, and circulating tumor cells [22,23]. Although it has been reported that tumor cell-induced platelet aggregation (TCIPA) or cancer-associated thrombocytosis is prevalent, its mechanism is not fully understood. Platelet p-selectin (CD62P), which is stored in platelet alpha granules, is exposed at the cell surface upon platelet activation via degranulation. A variety of p-selectin ligands, such as CEA, CA125, MUC1, and CD44 variants, are expressed in cancer cells [24,25]. In addition to the receptors for hemostasis, platelet CLEC-2 has also been identified as a receptor for podoplanin in tumor cells [26,27]. Podoplanin has been shown to mediate downstream signaling of CLEC-2 through an immunoreceptor tyrosine-based activation motif (ITAM), which modulates kinases for platelet activation [28]. Platelet GPIIb/IIIa also mediates platelet–tumor cell binding under shear stress [29,30]. Taken together, these results show that platelets could assist in tumor metastasis by crosslinking the circulating tumor cells, and thus, targeting the platelet receptors has been suggested as a potential anticancer therapy [31,32,33,34]. An overview of platelet receptor–ligand interactions and their roles is depicted in Figure 1.
Figure 1.
An overview of platelet receptor–ligand interactions. Platelets express various receptors for agonists or integrins and glycoproteins for ligands. The receptor–ligand interactions play critical roles in platelet activation, aggregation, shape change, adhesion, and thrombus formation. Green circle: integrins and glycoproteins for platelet adhesion and aggregation; blue circle: platelet receptors for agonists; orange circle: integrins and lectins for platelet–cell interaction; rectangle with gray line: endogenous ligands or agonists for each receptor. Abbreviations: PAR1, protease activated receptor 1; P2RY12, P2Y purinoceptor 12; TP, thromboxane receptor; 5-HT2AR, 5-hydroxytryptamine (serotonin) receptor 2A; CLEC-2, C-type lectin-like receptor 2; VWF, von Willebrand factor; PSGL-1, P-selectin glycoprotein ligand-1.
2.2. Platelet Metabolism
Platelet lifespan, which is approximately 8–12 days, likely depends on the mitochondria. The packaging of 5–8 mitochondria per platelet is critical for their survival and proper function. Mitochondria are not only involved in adenosine triphosphate (ATP) production [35], but also in reactive oxygen species (ROS) generation, calcium ion homeostasis, and cell processes and viability regulation [36,37]. Platelets are highly metabolically flexible, which enables the cells to function under various conditions [38]. Normally, glycolysis provides approximately 60% of the total ATP in platelets, whereas oxidative phosphorylation (OXPHOS), which occurs in mitochondria, compensates for the remaining 40% [39].
Disease or ageing could induce mitochondrial dysfunction, and are correlated with a decline in platelet survival and a higher risk of thrombosis [40]. For instance, enhanced production of mitochondrial ROS and activation of platelets, presumably due to hyperglycemia, have been observed in diabetes mellitus [41]. Mitochondrial damage and pathologic platelet activities have also been observed in other age-related diseases, such as Parkinson’s [42] and cardiovascular diseases [43], suggesting that platelet mitochondria may serve as therapeutic targets. In addition, higher methylation of platelet mitochondrial DNA (mtDNA) has been suggested as a possible biomarker for cardiovascular disease, as epigenetic regulation of platelet mtDNA could affect platelet activity [43]. Ageing is another factor that determines mitochondrial health [44]. Recently, TNFα has been implicated in increased mitochondrial mass and activity in megakaryocytes, resulting in thrombosis associated with ageing [45]. The alterations in platelet biogenesis and function with ageing will be discussed further.
2.3. Platelet Biogenesis
In 1906, MKs in the bone marrow were first identified as the precursors of platelets [46]. These cells are characterized by a large cell size (50–100 μm in diameter), multinuclei (64N DNA in a human cell), and consist of granules, mitochondria, and a demarcation membrane system (DMS). Thrombopoietin (TPO) plays a key role in megakaryopoiesis from hematopoietic stem cells by binding to its receptor myeloproliferative leukemia protein (c-MPL) in the MKs [46,47,48]. Receptor dimerization occurs by TPO binding, resulting in the activation of Janus kinase 2 (JAK2) and signal transducers and activators of transcription (STATs) that promote the maturation of MKs. The TPO-MPL signaling pathway also recruits phosphoinositide-3-kinase (PI3K) and mitogen-activated protein kinases (MAPKs) such as AKT [49]. Knockout of MPL resulted in the reduction of approximately 85% of the platelets, while the remaining 15% may be dependent on other mechanisms.
Mitochondria play an important role in megakaryocyte maturation as they are critical for platelet health. Many studies have demonstrated the involvement of mitochondrial fusion/fission dynamics in energy production, cell division, differentiation, and apoptosis [50,51]. Mitochondrial fission is mediated by dynamin-related protein 1 (DRP1), a cytosolic guanosine triphosphatase (GTPase), which localizes to the mitochondrial outer membrane [52]. This process can be induced by cellular stress, such as an increase in ROS, and is followed by mitochondrial fragmentation [53,54]. Aberrant mitochondrial shape may also lead to enhanced ROS formation, although the correlation between mitochondrial shape and redox homeostasis remains to be elucidated. Mitochondrial fusion is executed by mitofusin 1 and 2 (MFN1 and MFN2, respectively) and optic atrophy 1 (OPA1), and its importance in determining memory T-cell formation has been demonstrated [55,56,57]. Endomitosis is one of the most critical processes of megakaryopoiesis, followed by polyploidization. Thus, it is evident that mitochondrial dynamics and concomitant ROS play an important role in MK function, which is platelet production [58]. Recently, it was shown that mitochondrial ROS initiated proplatelet formation and terminal maturation of MKs [59,60]. In addition, MK deformation was found to be associated with mitochondrial ROS levels, and therefore, proplatelet formation [59], which is consistent with the findings of previous studies in which cytoskeletal reorganization was influenced by ROS [61].
Development of the DMS, which later forms the platelet membrane, is another feature of megakaryocyte maturation [62,63]. Since it is thought that one MK releases more than 3000 platelets, it is critical that the DMS divides the cytoplasm efficiently. The exact mechanism by which this unique membrane system is established is unclear. Hereditary diseases with loss-of-function mutations on GP1BA, GP1BB, or GP9, and MHC9-related disorders exhibit an abnormal DMS, resulting in thrombocytopenia [64,65]. Notably, DMS membranes were found to be enriched with phosphatidylinositol 4,5-bisphosphate (PI-4,5-P2), which is mediated by PI-5-P-4-kinase α (PIP4Kα) [66]. Knockdown of PIP4Kα resulted in impaired DMS formation with a reduction in the size of the MKs. The authors indicated that PI-4,5-P2 might be involved in the development of DMS, in association with the assembly of actin fibers.
3. Transcription Factors in Megakaryopoiesis
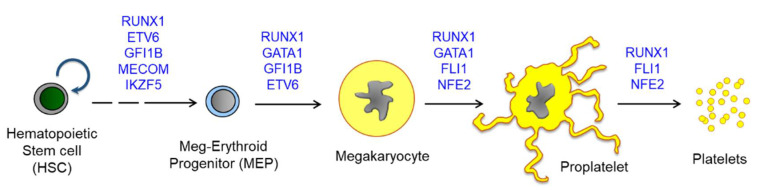
By investigating congenital thrombocytopenia, more than 30 genes have been implicated in megakaryopoiesis and platelet biogenesis [67]. Among them, seven genes, including RUNX1, GATA1, FLI1, GFI1B, MECOM, ETV6, and NFE2, are transcription factors [68,69,70]. During megakaryopoiesis, transcription factors are activated in a stepwise manner, and thus, different mutations induce different disease characteristics. For instance, GATA1 mutations could affect both megakaryopoiesis and erythropoiesis [71], whereas variants of RUNX1 and ETV6 are involved in leukemia predisposition [72,73]. Other genes such as HOXA11 and MECOM are associated with bone marrow failure [74,75]. Although defects in platelet production induced by these mutations have been investigated, it is also important to examine the alterations occurring in hematopoietic progenitor cells (HPCs) and subsequent megakaryopoiesis, as these are not fully understood. In this section, I describe the genomic landscape of megakaryopoiesis by examining the platelet disorders mediated by mutations in transcription factors. A scheme for megakaryopoiesis and key transcription factors that are associated with platelet biogenesis and platelet disorders are summarized in Figure 2 and Table 1.
Figure 2.
A scheme for megakaryopoiesis to show the involvement of transcription factors in a stepwise manner. Mutations on the transcription factors induce defective megakaryocyte maturation, resulting in congenital thrombocytopenia. Most except for MECOM are found to be associated with abnormal hyperplasia of MkPs or MKs upon mutation. HSCs produce all lineages of hematopoietic cells, and MEPs are differentiated into both megakaryocytes and erythroid cells. Megakaryocytes are further maturated to proplatelets and release platelets into blood flow. One megakaryocyte is known to produce more than 3000 platelets and approximately 1011 platelets are generated daily. Abbreviations: RUNX1, Runt-related transcription factor 1; FLI1, friend leukemia virus integration 1; NFE2, Nuclear factor erythroid 2; MECOM, MDS1 and EVI1 complex locus; ETV6, ETS variant 6.
Table 1.
Key transcription factors for platelet biogenesis and mutations found in inherited platelet disorders.
| Transcription Factor | Target Genes | Alterations of Megakaryopoiesis | Defects in Platelet Function | Disease | Other Features | Ref |
|---|---|---|---|---|---|---|
| RUNX1 | PF4, NR4A3, PRKCQ, MYL9 | Reduced polyploidization and proplatelet formation | Platelet granule deficiency | Familial platelet disorder with a predisposition to myeloid malignancy (FPDMM) | High risk (>40%) of acute myeloblastic leukemia or MDS at a young age, Absent to moderate bleeding tendency | [76,77,78] |
| GATA1 | NFE2, ITGA2B, Erythropoietic genes (ALAS2, BCL2L1, etc.) | Dysplasia of immature MK and MEP | Platelet alpha granule deficiency, Macrothrombocytopenia, Impaired platelet aggregation | GATA1-related disorders | Dyserythropoiesis with or without anemia, Risk of TMD, DS-AMKL | [79,80,81] |
| FLI1 | MPL, ITGA2B, PF4, GP9 | Dysmegakaryopoiesis, Reduced MK production from the patient-derived iPSCs | Macrothrombocytopenia with giant fused alpha granules, Defective platelet aggregation | Paris-Trousseau syndrome (PTS), FLI1-related thrombocytopenia, Jacobsen syndrome | Risk of bleeding | [82,83] |
| NFE2 | TUBB1, RAB27b, CASP12, HSD3B1 | Hyperplasia of immature MKs, Impaired DMS in MKs, Lack of binding activity to fibrinogen | Decreased levels of circulating platelets in Nfe2-null mice | Patients with MPNs show upregulation of NFE2 regardless of the presence of JAK2 mutation | Anemia with compensatory reticulocytosis and splenomegaly in Nfe2-null mice | [84,85,86] |
| MECOM | MPL | Hypomegakaryopoiesis | Severe bleeding tendency | MECOM-related thrombocytopenia | Bone marrow failure, Elevated TPO levels in plasma, Anemia, B-cell deficiency | [87,88,89] |
| ETV6 | GP1BA, GPIX | Hyperplasia of immature MKs, Dyserythropoiesis, Increased number of circulating HSPCs | Elongated alpha granules in platelets, Impaired adhesion, spreading and clot retraction activity | ETV6-related thrombocytopenia | Leukemia predisposition, Platelets with high levels of transcripts for erythrocytes, Absent to mild bleeding | [90,91,92] |
| GFI1B | RGS18 | Hyperplasia of MK and MkPs | Macrothrombocytopenia, Alpha granule deficiency, Defects in platelet aggregation, Reduced expression of GP1ba | GFI1B-related thrombocytopenia (GFI1B-RT) | Anisocytosis and poikilocytosis of RBCs, Mild to severe bleeding, Dyserythropoiesis, Severe phenotypes in mice model | [93,94,95] |
| IKZF5 | Platelet activation genes (LYN, P2RY12, etc.) | N/A | Platelets with reduced alpha and delta granules | IKZF5-related thrombocytopenia | Downregulation of gene expression for platelet biogenesis including GATA1, No bleeding tendency | [96] |
RUNX1, Runt-related transcription factor 1; PF4, Platelet Factor 4; NR4A3, Nuclear Receptor subfamily 4 group A member 3; PRKCQ, Protein Kinase C theta; MYL9, Myosin Light Chain 9; MDS, Myelodysplastic Syndrome; NFE2, Nuclear Factor Erythroid 2; ITGA2B, Integrin αIIb; ALAS2, 5′-Aminolevulinate Synthase 2; BCL2L1, BCL2 Like 1; MEP, Megakaryocyte-Erythroid Progenitor; TMD, Transient Myeloproliferative Disorder; DS-AMKL, Down Syndrome Acute Megakaryoblastic Leukemia; FLI1, Friend Leukemia virus Integration 1; MPL, thrombopoietin receptor; iPSC, induced Pluripotent Stem Cell; TUBB1, Tubulin Beta 1; CASP12, Caspase 12; HSD3B1, 3-beta-Hydroxysteroid Dehydrogenase; DMS, Demarcation System; MECOM, MDS1 and EVI1 Complex Locus; ETV6, ETS Variant 6; HSPC, Hematopoietic Stem and Progenitor Cell; RGS18, Regulator of G protein Signaling 18; RBC, Red Blood Cell; P2RY12, P2Y Purinoceptor 12; N/A, Not Applicable.
3.1. RUNX1
Runt-related transcription factor 1 (RUNX1; AML1) plays a critical role in the emergence of all definitive hematopoiesis and represents a common mutational target in leukemia. RUNX1 contributes to hematopoietic progenitor proliferation and is involved in the later stages of MK maturation. In addition, RUNX1, together with its cofactor core-binding factor beta (CBFβ), stimulates various platelet genes, such as platelet factor 4 (PF4) [97], nuclear receptor subfamily 4 group A member 3 (NR4A3) [98], protein kinase c theta (PRKCQ) [99], and myosin light chain 9 (MYL9) [76]. It can also generate a transcriptional repressor complex, which is inhibited by the arginine methyltransferase PRMT1, at megakaryocytic promoters [100]. Germline heterozygous mutations in RUNX1 lead to autosomal dominant human syndrome familial platelet disorder with a predisposition to myeloid malignancy (FPDMM), which presents with thrombocytopenia and bleeding [101]. The MKs of the patients with platelet disorders expressed nonmuscle myosin IIb (MYH10), which was supposed to be downregulated by RUNX1 during endomitosis, a hallmark of megakaryocyte differentiation [77,102,103]. Thus, RUNX1 is involved in polyploidization and proplatelet formation of megakaryocytes by regulating the expression of MYH9, MYL9, and MYH10.
Recently, single-cell RNA-sequencing (scRNA-SEQ) data revealed that haploinsufficiency of RUNX1 in induced pluripotent stem cell (iPSC)-derived MKs resulted in transcriptional deregulation, associated with various immune and cytokine response pathways, including TGF-β1 signaling [78]. The RUNX1 mutant iPSC-derived HPCs showed elevated c-Jun N-terminal kinase (JNK)-2 phosphorylation, a TGF-β1 signaling pathway. Small molecules that inhibited JNK or TGFβR-1 corrected MK yield, which was suppressed by the RUNX1 haploinsufficiency. Notably, growing evidence suggests that MK-biased hematopoietic stem and progenitor cells (HSPCs) display self-renewal and platelet production in response to stress and inflammation [5,104,105]. Therefore, RUNX1 may play an important role in these processes, together with other hematopoietic transcription factors [106].
3.2. GATA1
GATA1 is crucial for erythroid–megakaryocytic differentiation, and interacts with its cofactor ZFPM1 (FOG1; friend of GATA1) [107]. A lack of GATA1 function in the MK lineage leads to the accumulation of immature MKs with decreased polyploidization and cytoplasmic maturation, due to decreased cyclin D1 expression [108,109]. Mutations in GATA1 are known to cause several human disorders [110]. Missense mutations of GATA1 on FOG1-interacting sequences or nuclear binding sites also affect MK differentiation, resulting in aberrant numbers and maturation of MKs and platelets [111]. In addition, GATA1 interacts with RUNX1 in the zinc finger domain [112]. Down syndrome acute megakaryoblastic leukemia (DS-AMKL) is associated with somatic mutations in GATA1. Mutations in exon 2 result in an increase in the short form of GATA1 (GATA1s) protein via alternative splicing of the full-length GATA1 transcript. Many reports have revealed that GATA1s promotes the expansion of MK progenitors, but does not allow their terminal maturation, resulting in pre-leukemic transient myeloproliferative disorder (TMD) [79,80,113,114]. It is thought that the presence of GATA1s expression, trisomy 21, and additional hits, such as overexpression of the ETS family transcription factor ERG, could lead to the development of DS-AMKL [115].
Interestingly, reduced GATA1 levels is represented by the accumulation of MEP/MkPs in the bone marrow or proliferation of the precursors in ex vivo cell culture, in both mice and humans [81,116]. This has led to the development of the Gata1-deficient MEP-like cell line G1ME, from murine embryonic stem cells (ESCs), which have been widely used to understand the molecular mechanism of meg–erythroid differentiation, via perturbation of GATA1 expression during hematopoietic differentiation of stem cells [117]. However, G1ME cells failed to undergo terminal differentiation, presumably because GATA1 restoration via retroviral transduction is nonphysiological. This led to the development of G1ME2 cells using an inducible gene knockdown system, which greatly improved the previous system [118]. This indicates that the physiological levels of GATA1 are critical for meg–erythroid differentiation. Reduced Gata1 expression could lead to the emergence of leukemia and bone marrow fibrosis with the accumulation of immature MKs [119,120]. Although it has not been investigated whether GATA1 levels are correlated with MkP expansion and differentiation in human cells, many studies on GATA1s have demonstrated that the physiological regulation of GATA1 is crucial for these processes.
3.3. FLI1
Friend leukemia virus integration 1 (FLI1) increases the expression of megakaryocyte-specific genes, including MPL, ITGA2B (i.e., CD41), PF4, and GP9 (i.e., CD42a), together with that of GATA1, FOG1, and ETS1, in the late MkPs [121,122]. Several factors are involved in driving the MEPs toward either the erythroid or megakaryocytic lineage [2]. For instance, MYB is a proto-oncogene that induces the expression of the erythroid transcription factor, KLF1 [123]. It has been demonstrated that the knockdown of MYB enhances megakaryocyte differentiation and platelet generation, indicating its role in the inhibition of megakaryopoiesis [124]. The expression of miR-150-5p has been suggested as a mechanism of action to reduce MYB levels during megakaryopoiesis, following TPO-MPL signaling activation [125,126]. In addition, the downregulation of MYB induces FLI1 expression.
Paris–Trousseau syndrome (PTS), an inherited disorder associated with 11q chromosome deletion, is characterized by thrombocytopenia, with an increased risk of bleeding. It occurs owing to the hemizygous deletion of a region that encodes FLI1 and ETS1. It is also characterized by dysmegakaryopoiesis in the bone marrow and giant fused α granules in platelets [82,127]. Both PTS-specific (11q23.3 deletion) and genome-edited (FLI1 deletion, FLI1+/-) human iPSCs showed a decrease in the yield of megakaryocytes and platelets, indicating that the FLI1 is crucial for megakaryopoiesis [83]. It has also been discovered that the transcription factor ETS1, which is negatively regulated by FLI1, is critical in megakaryopoiesis [128]. Furthermore, during the later stages of megakaryopoiesis, the RUNX1/FLI1 complex suppresses ankyrin repeat domain 26 (ANKRD26), resulting in the development of blood platelets [129]. Point mutations in the 5′UTR of ANKRD26 have shown to result in familial thrombocytopenia 2 and leukemia predisposition, mainly mediated by the derepression of ANKRD26 due to insufficient RUNX1/FLI1 complex binding.
3.4. NFE2
Nuclear factor erythroid 2 (NFE2) is induced by GATA1 and promotes proplatelet formation by upregulating platelet genes, including tubulin β-1 chain (TUBB1) [84], RAB27b [85], caspase 12 (CASP12) [130], and 3-beta-hydroxysteroid dehydrogenase (HSD3B1) [131]. Moreover, decreased levels of circulating platelets and increased number of MKs in the bone marrow were observed in Nfe2 knockout mice [132,133]. However, the expanded MKs were impaired in terms of their aberrant granules, demarcation membrane, and lack of binding activity to fibrinogen [134]. In contrast, ectopic expression of NFE2 in bone marrow cells were found to enhance MK differentiation and platelet production, indicating that NFE2 is crucial for platelet biogenesis [135]. Interestingly, patients with myeloproliferative neoplasms showed upregulation of NFE2 regardless of the presence of the JAK2V617F mutation, presumably due to the increase in AML1 levels [86,136].
3.5. MECOM
Heterozygous mutations in MECOM (MDS1 and EVI1 complex locus) can cause congenital hypomegakaryocytic thrombocytopenia associated with inherited bone marrow failure syndromes [87,88]. Recently, a severe reduction in early CD34+CD38lo hematopoietic progenitors in the bone marrow and thrombocytopenia was reported in 12 patients with MECOM mutations [89]. In addition, the plasma levels of TPO were elevated although the TPO receptor MPL, which is known to be regulated by EVI1, was detected.
3.6. ETV6
The ETS variant 6 gene (ETV6), a member of the ETS family, has been demonstrated to play a role in megakaryopoiesis through transcriptional repression [137]. ETV6 is important for early hematopoiesis in the bone marrow; however, it has little effect on committed lymphoid progenitors. Interestingly, a drastic reduction in platelet counts was observed in MkP-specific knockout of Etv6, suggesting that ETV6 is involved in megakaryopoiesis [138]. Accordingly, germline mutations in ETV6 are related to familial thrombocytopenia (usually mild) and leukemia predisposition [73,90,91]. In addition, although ETV6 is a transcriptional repressor, it has been reported to interact with FLI1 [139].
Immature megakaryocyte hyperplasia and dyserythropoiesis were observed in the bone marrow of patients with the p.P214L mutation [90]. Erythroid transcripts were increased in the platelets of patients with ETV6 mutation, whereas the platelet transcripts were decreased. This indicates that ETV6 plays a lineage-specific role, by functioning as a transcriptional repressor in erythropoiesis and as a transcriptional activator in megakaryopoiesis [92].
3.7. GFI1B
GFI1B-related thrombocytopenia (GFI1B-RT) is a rare dominant congenital platelet disorder caused by mutations in the GFI1B gene. GFI1B-RT is characterized by the presence of truncated proteins lacking the zinc fingers of GFI1B, and mild to moderate bleeding disorder with macrothrombocytopenia. Platelets often show alpha granule deficiency, while anisocytosis and poikilocytosis are also observed [93,140]. The gene encodes two protein isoforms: GFI1B-p37 (isoform 1) and GFI1B-p32 (isoform 2). The long isoform 1 is required for megakaryocytic differentiation, while the shorter isoform induces proper erythropoiesis [94,141,142]. Mouse models with knockout or conditional knockout of Gfi1b revealed a severe phenotype [143], and thus, a novel Gfi1b dominant-negative mouse model was developed to obtain phenotypes similar to those found in humans [95]. Notably, patients with GFI1B-RT show hyperplasia of the MkPs and MKs in their bone marrow, which could also be observed in the mouse model. It was demonstrated that treatment with the TPO analog romiplostim, was able to rescue the disease phenotype in the mouse model.
3.8. IKZF5
The Ikaros family of transcription factor genes, IKZF1 through 5, is widely expressed in hematopoietic cells, and IKZF1, 2, and 3 play roles in lymphocyte development [144]. The immunomodulatory drugs lenalidomide and pomalidomide, were shown to induce the expansion of early megakaryocytic progenitors and thrombocytopenia in patients with multiple myeloma via suppression of IKZF1 [145]. This leads to the subsequent downregulation of GATA1, as it binds to IKZF1, which further decreases the expression of megakaryocyte genes, including ZFPM1 and NFE2 [146].
Recently, it has been found that the rare missense variants of IKZF5 are associated with thrombocytopenia [96]. The authors performed RNA sequencing of platelets, monocytes, neutrophils, and CD4+ T cells, and the results revealed that the platelets showed dramatic differences in their gene expression. The most significantly downregulated genes, such as FERMT3, PLA2G4A, P2RY12, TBXA2R, CDC42, GP1BA, and GP9, were related to platelet activation, aggregation, and platelet formation. About half of the differentially expressed genes (DEGs) that were upregulated are involved in immune system response and inflammation. GATA1 was found to be downregulated, which is consistent with its role in thrombopoiesis [147]. Among the variants of IKZF5, missense mutations of highly conserved residues in the N-terminal zinc fingers exhibited a strong reduction in chromatin binding and inhibited nuclear translocation, suggesting that the mechanism underlies the transcriptional activation of IKZF5 for platelet formation.
4. Clinical Implications of MK Hyperplasia
4.1. Thrombocytosis and/or Platelet Activation with Increased Thrombosis Risk
4.1.1. Clonal Hematopoiesis and Thrombocytosis
Clonal hematopoiesis (CH) can be observed in 10–20% of individuals older than 70 years [148,149]. The hematopoietic cells harbor mutations in the epigenetic regulators, such as DNMT3A and TET2, and CH is considered to increase risks of the developing hematological malignancies as well as cardiovascular diseases [150,151]. Although multiple factors involve in onset of the diseases, increased megakaryopoiesis and thrombopoiesis may be responsible for the CH-enhanced thrombosis risk and cardiovascular symptoms [152]. Among the CH mutations, genes encoding Additional sex combs like 1 (ASXL1) [153], Splicing factor 3B subunit 1 (SF3B1) [154], DNA methyltransferase 3a (DNMT3A) [155], Src homology 2 B3 (SH2B3, also named LNK) [156], and JAK2 [157] are considered to be associated with thrombocytosis and/or platelet hyper-reactivity.
Somatic mutations of ASXL1 are frequently found in hematological malignancies, such as acute myeloid leukemia (AML), chronic myelomonocytic leukemia (CML), and MPN [153]. ASXL1 acts as tumor suppressor in normal hematopoiesis. It has been demonstrated that transgenic expression of mutant Asxl1 could lead to thrombocytosis, age-related anemia, and myeloid dysplasia [153]. Among genes related to clonal hematopoiesis and predisposition to leukemia, loss-of-function mutation in gene encoding DNMT3A is one of the most prevalent. It plays a key role for DNA methylation and tumor suppression in hematopoiesis. Although the overall platelet count in AML patients is lower than healthy population, AML patients carrying DNMT3A mutations display higher thrombopoietic potential than patients with wild type DNMT3A, which could increase risk of thrombosis [155].
The gain-of-function mutations in JAK2, i.e., mutation V617F, are often detected in patients with MPN, which includes polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). JAK2 is a nonreceptor tyrosine kinase, and regulates cell survival and proliferation via cytokine receptor signaling. It has been reported that platelets from JAK2 V617F-positive patients displayed enhanced procoagulation activity [158]. The JAK2 V617F also promotes megakaryopoiesis, as JAK2 is critical in signal transduction of thrombopoietin [159]. Notably, the prevalence of mutations in ASXL1 or the JAK2 V617F variant is low in ageing healthy individuals; however, the risk of developing CVD has been estimated to twice or 10-fold increase, respectively [157].
4.1.2. Megakaryocyte Hyperplasia in ET and Thrombocytosis
The diagnostic criteria for ET have been announced to be platelet count ≥450 × 109/L, a proliferative bone marrow appearance with megakaryocyte predominance, not meeting the criteria for other myeloid neoplasms, and presence of a JAK2, CALR, or MPL mutation, by the World Health Organization (WHO) [152]. Approximately 55% of JAK2-V617F, 15–24% of CALR, and 4% of MPL mutations are found in ET. It has been suggested that JAK2 mutation after the first acquisition of DNMT3A mutation would lead to an ET phenotype [155]. Patients with ET may experience thrombosis, cardiovascular symptoms, or hemorrhage. Platelet-derived microvesicles (MVs) or microparticles (MPs) have been found to be elevated in peripheral blood of the patients [160]. Importantly, platelet-derived MV/MPs showed procoagulant and prothrombotic activities, which indicate that the risk of thrombosis in ET is correlated with the amount of platelet-derived MV/MPs [161]. Thus, it has been suggested that measurement of the MV/MPs for diagnosis of MPN, ET, or the thrombosis risk of ET might be feasible via liquid biopsy [162].
Chronic inflammation followed by oxidative stress in ET may be associated with the disease progression [163]. Constitutively active JAK-STAT signaling pathway promotes secretion of pro-inflammatory cytokines such as TNF-α and IL-1β, which could lead to increase of ROS production in ET [164]. Notably, ROS impacts on platelet biogenesis and platelet activation, as described in the Section 2. Moreover, leukemia or fibrosis could occur, presumably related to chronic myeloproliferative disorder and MK hyperplasia in the bone marrow [165]. Similar clinical implication has been reported in patients with hereditary thrombocytosis, who have mutations in TPO [166].
4.1.3. Cancer and Thrombocytosis
As described in the Section 2.1, various types of tumor cells are known to directly activate platelets and induce TCIPA to protect themselves. Thus, tumor-associated thrombosis is considered to worsen disease prognosis and survival [167]. It is estimated that approximately 20% of cancer patients suffer from vascular thromboembolism like pulmonary embolism, which indicates a greater risk of venous thrombosis in patients with malignancy [168]. Not only platelet hyper-reactivity, but also increased platelet counts can be associated with the severity of cancers, including breast [169], colorectal [170], and lung cancers [171]. Notably, it has been demonstrated that tumor-derived interleukin-6 promoted thrombopoietin production by the liver in ovarian cancer patients [172]. Efforts have been made to elucidate the underlying mechanisms and to develop novel antiplatelet therapeutics for cancer management. In addition to thrombocytosis risk in cancers, chemotherapy for treating cancers is responsible for severe thrombocytopenia. Recently, it has been demonstrated that damage on platelet mitochondria was induced by chemotherapy, and therefore, maintenance of bone marrow megakaryocytes may be crucial for the recovery [173].
4.2. MK Hyperplasia and Reduced Platelet Biogenesis
4.2.1. Ageing and Thrombocytopenia
Thrombocytopenia can occur not only in congenital diseases, but also in conditions that induce a low blood platelet count, such as leukemia, immune system disorder, and surgery. Ageing is another factor that affects platelet count and function. In humans, the platelet count is maintained consistently between the ages of 25 to 60, and may later decrease by approximately 8% [174,175,176]. In a study involving 1058 men and 1363 women aged between 26–82 years, it was shown that platelet aggregation, which is induced by epinephrine, ADP, and collagen, tends to decrease with age [177]. Although the platelets remain intact in the elderly population, there is a high prevalence of morbidity caused by other metabolic diseases or immunologic disorders, which may affect platelet biology. Considering the rapid growth of ageing population worldwide and the limited number of studies on patients over 75 years of age, there is an urgent need to elucidate platelet biogenesis and function in the elderly. Moreover, it has been shown that 129 mRNAs and 15 microRNAs are differentially expressed in platelets in the elderly [178].
Age-dependent changes are often associated with changes in bone marrow MKs. For instance, MK-biased hematopoiesis, which is either due to the transcriptional activity of MK precursors or an increase in metabolic stress in the bone marrow microenvironment, has been observed in old mice [6,179,180]. As previously mentioned, ROS can also affect platelet biogenesis. Moreover, ageing-related oxidative stress is higher in the platelets of elderly population [181]. For instance, the levels of thioredoxin-interacting protein (TXNIP), which hinders the endogenous antioxidant thioredoxin-1, were found to be increased in the platelets of older subjects (mean age 67 years) [182]. Platelets from 18-month-old mice have been shown to upregulate peroxide levels [183], suggesting that oxidative stress is related to the changes in platelet characteristics with ageing. Interestingly, the older MkPs harbor a greater capacity to engraft, expand, and generate platelets than young MkPs, presumably due to the enhanced proliferative potential of MkPs [184]. However, the old MkPs could not induce long-term engraftment of myeloid cells and lacked lymphocyte generation. Furthermore, age-related changes in gene expression levels, including that of Fli1, Gabpa, Mpl, Pf4, and Runx1, indicate that old MkPs may serve as a reservoir for mutations in clonal hematopoiesis during ageing [185].
4.2.2. Primary Myelofibrosis (PMF)
PMF is classified as a BCR-ABL1-negative MPN that harbor driver mutations in JAK2, CALR, or MPL, representing clonal hematopoiesis [186]. The median age of diagnosis of PMF ranges from 69 to 79 years, and most patients are over 60 years old [187]. Notably, chronic inflammation and ageing may be implicated in diseases such as MPN [188,189].
The levels of inflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β), are elevated in aged mice and elderly humans [190,191]. Recently, scRNA-SEQ analysis revealed that the genes related to inflammation, mitochondrial dysfunction, and oxidative phosphorylation were enriched in the MKs isolated from aged mice (>18 months), compared to that of young mice (2–3 months) [45]. In addition, a high incidence of thrombotic events was observed on stimulating the platelets with increased levels of circulating TNF-α [192], while ageing-associated platelet hyperactivity, thrombosis, and mitochondrial dysfunction was reversed by TNF-α blockade [45]. In addition to platelet activity, there were changes in the MKs, including increased MkP population and ploidy of MKs in the bone marrow, indicating that ageing and inflammation enhanced the potential of megakaryopoiesis.
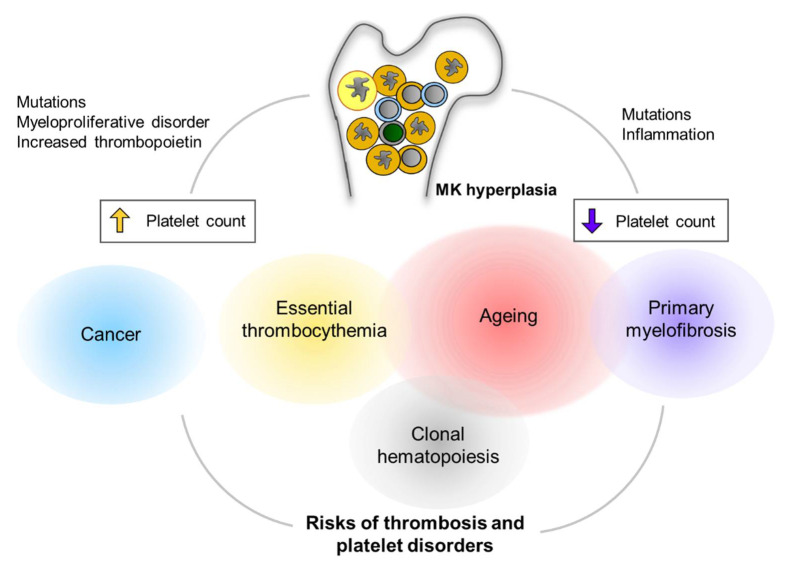
In both mice and humans, PMF is characterized by increased levels of TNF-α along with an increase in the incidence of thrombosis, caused by platelet hyperactivity [193]. Notably, patients with PMF show MK-biased hematopoiesis, resulting in the accumulation of immature MKs in the bone marrow, extramedullary hematopoiesis, and fibrosis. The number of mitochondria per platelet was also increased in patients with JAK2 V617F MPN [194], which might be because of decreased autophagy, similar to that seen in the platelets of aged mice [195]. An overview of clinical implications of MK hyperplasia is depicted in Figure 3.
Figure 3.
Clinical implications of MK hyperplasia. MK hyperplasia can be seen in many clinical status including clonal hematopoiesis (CH) and myeloproliferative neoplasm (MPN). Mutations in genes that cause CH or MPN are found more frequently in the elderly population. Cancers are another critical risk for thrombocytosis and platelet activation. MK hyperplasia results in either thrombocytosis (increased platelet count) or thrombocytopenia (decreased platelet count), presumably depending on physiological relevance of megakaryopoiesis as well as fibrotic status in the bone marrow. Alterations in platelet number can be implicated to increased risks of thrombus formation and/or platelet disorders.
5. Conclusions
In this review, I describe the characteristics of platelets and megakaryocytes to understand the mechanism of platelet biogenesis. Although several key regulators of the process, including RUNX1, GATA1, have been identified, the effects of metabolic changes, microRNAs, and epigenetic regulation remain to be elucidated. Moreover, since MKs are very rare and reside in the bone marrow, there is a lack of proper sources of human MKs for further investigations. Thus, recent advancements in scRNA-SEQ technology have provided valuable information, which I also introduce in the current review. Moreover, emerging evidence suggests that ageing can affect megakaryopoiesis as well as platelet function. Although it is quite clear that chronic inflammation can affect the MKs and platelet biology, the importance of transcription factors, such as their physiological levels and interactions with other factors, have not been elucidated. In addition, although certain diseases are characterized by the occurrence of thrombocytopenia, the bone marrow of the affected patients shows hyperplasia of MKs and MkPs. Taken together, it can be inferred that transcription factors induce megakaryopoiesis, and that they may be associated with many diseases, including thrombocytopenia, metabolic diseases, and MPN.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1C1C1011899) and a grant from KRIBB Research Initiative Program (KGM5362111; KGM5502113).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Machlus K.R., Italiano J.E., Jr. Platelets. 4th ed. Elsevier; Amsterdam, The Netherlands: 2019. Megakaryocyte development and platelet formation; pp. 25–46. [Google Scholar]
- 2.Bianchi E., Norfo R., Pennucci V., Zini R., Manfredini R. Genomic landscape of megakaryopoiesis and platelet function defects. Blood. 2016;127:1249–1259. doi: 10.1182/blood-2015-07-607952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woolthuis C.M., Park C.Y. Hematopoietic stem/progenitor cell commitment to the megakaryocyte lineage. Blood. 2016;127:1242–1248. doi: 10.1182/blood-2015-07-607945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pla A.S., Macaulay I., Jensen C.T., Woll P.S., Luis T.C., Mead A., Moore S., Carella C., Matsuoka S., Jones T.B., et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature. 2013;502:232–236. doi: 10.1183/nature12495. [DOI] [PubMed] [Google Scholar]
- 5.Carrelha J., Meng Y., Kettyle L.M., Luis T.C., Norfo R., Alcolea V., Boukarabila H., Grasso F., Gambardella A., Grover A., et al. Hierarchically related lineage-restricted fates of multipotent haematopoietic stem cells. Nature. 2018;554:106–111. doi: 10.1038/nature25455. [DOI] [PubMed] [Google Scholar]
- 6.Psaila B., Mead A.J. Single-cell approaches reveal novel cellular pathways for megakaryocyte and erythroid differentiation. Blood. 2019;133:1427–1435. doi: 10.1182/blood-2018-11-835371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grüner S., Prostredna M., Schulte V., Krieg T., Eckes B., Brakebusch C., Nieswandt B. Multiple integrin-ligand inter-actions synergize in shear-resistant platelet adhesion at sites of arterial injury in vivo. Blood. 2003;102:4021–4027. doi: 10.1182/blood-2003-05-1391. [DOI] [PubMed] [Google Scholar]
- 8.Inoue O., Suzuki-Inoue K., Dean W.L., Frampton J., Watson S. Integrin α2β1 mediates outside-in regulation of platelet spreading on collagen through activation of Src kinases and PLCγ2. J. Cell Biol. 2003;160:769–780. doi: 10.1083/jcb.200208043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savage B., Saldívar E., Ruggeri Z.M. Initiation of Platelet Adhesion by Arrest onto Fibrinogen or Translocation on von Willebrand Factor. Cell. 1996;84:289–297. doi: 10.1016/S0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 10.Massberg S., Gawaz M., Grüner S., Schulte V., Konrad I., Zohlnhöfer D., Heinzmann U., Nieswandt B. A Crucial Role of Glycoprotein VI for Platelet Recruitment to the Injured Arterial Wall In Vivo. J. Exp. Med. 2002;197:41–49. doi: 10.1084/jem.20020945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bombeli T., Schwartz B.R., Harlan J.M. Adhesion of activated platelets to endothelial cells: Evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), αvβ3 integrin, and GPIbα. J. Exp. Med. 1998;187:329–339. doi: 10.1084/jem.187.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evangelista V., Manarini S., Sideri R., Rotondo S., Martelli N., Piccoli A., Totani L., Piccardoni P., Vestweber D., De Gaetano G. Platelet/polymorphonuclear leukocyte interaction: P-selectin triggers protein-tyrosine phosphorylation–dependent CD11b/CD18 adhesion: Role of PSGL-1 as a signaling molecule. Blood. 1999;93:876–885. doi: 10.1182/blood.V93.3.876. [DOI] [PubMed] [Google Scholar]
- 13.Ruggeri Z.M. Von Willebrand factor, platelets and endothelial cell interactions. J. Thromb. Haemost. 2003;1:1335–1342. doi: 10.1046/j.1538-7836.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- 14.Mccarty O.J., Calaminus S., Berndt M.C., Machesky L., Watson S.P. von Willebrand factor mediates platelet spreading through glycoprotein Ib and αIIbβ3 in the presence of botrocetin and ristocetin, respectively. J. Thromb. Haemost. 2006;4:1367–1378. doi: 10.1111/j.1538-7836.2006.01966.x. [DOI] [PubMed] [Google Scholar]
- 15.Siljander P.R.-M., Munnix I.C.A., Smethurst P.A., Deckmyn H., Lindhout T., Ouwehand W.H., Farndale R.W., Heemskerk J.W.M. Platelet receptor interplay regulates collagen-induced thrombus formation in flowing human blood. Blood. 2004;103:1333–1341. doi: 10.1182/blood-2003-03-0889. [DOI] [PubMed] [Google Scholar]
- 16.Nesbitt W.S., Westein E., Tovar-Lopez F.J., Tolouei E., Mitchell A., Fu J., Carberry J., Fouras A., Jackson S.P. A shear gradient–dependent platelet aggregation mechanism drives thrombus formation. Nat. Med. 2009;15:665–673. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- 17.Siess W., Siegel F.L., Lapetina E.G. Arachidonic acid stimulates the formation of 1,2-diacylglycerol and phosphatidic acid in human platelets. Degree of phospholipase C activation correlates with protein phosphorylation, platelet shape change, serotonin release, and aggregation. J. Biol. Chem. 1983;258:11236–11242. doi: 10.1016/S0021-9258(17)44408-5. [DOI] [PubMed] [Google Scholar]
- 18.Gachet C., Hechlel B., Léon C., Vial C., Leray C., Ohlmann P., Cazenave J.-P. Activation of ADP Receptors and Platelet Function. Thromb. Haemost. 1997;78:271–275. doi: 10.1055/s-0038-1657538. [DOI] [PubMed] [Google Scholar]
- 19.Coughlin S.R. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 20.Shattil S.J., Newman P.J. Integrins: Dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–1615. doi: 10.1182/blood-2004-04-1257. [DOI] [PubMed] [Google Scholar]
- 21.Jones C., Barrett N., Moraes L.A., Gibbins J.M., Jackson D.E. Endogenous inhibitory mechanisms and the regulation of platelet function. Platelets Megakaryocytes. 2011;788:341–366. doi: 10.1007/978-1-61779-307-3_23. [DOI] [PubMed] [Google Scholar]
- 22.Hanley W., McCarty O., Jadhav S., Tseng Y., Wirtz D., Konstantopoulos K. Single Molecule Characterization of P-selectin/Ligand Binding. J. Biol. Chem. 2003;278:10556–10561. doi: 10.1074/jbc.M213233200. [DOI] [PubMed] [Google Scholar]
- 23.Tao D.L., Yunga S.T., Williams C.D., McCarty O.J.T. Aspirin and antiplatelet treatments in cancer. Blood. 2021;137:3201–3211. doi: 10.1182/blood.2019003977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alves C.S., Burdick M., Thomas S.N., Pawar P., Konstantopoulos K. The dual role of CD44 as a functional P-selectin ligand and fibrin receptor in colon carcinoma cell adhesion. Am. J. Physiol. Physiol. 2008;294:C907–C916. doi: 10.1152/ajpcell.00463.2007. [DOI] [PubMed] [Google Scholar]
- 25.Aigner S., Ramos C.L., Hafezi-Moghadam A., Lawrence M.B., Friederichs J., Altevogt P., Ley K. CD24 mediates rolling of breast carcinoma cells on P-selectin. FASEB J. 1998;12:1241–1251. doi: 10.1096/fasebj.12.12.1241. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki-Inoue K., Kato Y., Inoue O., Kaneko M.K., Mishima K., Yatomi Y., Yamazaki Y., Narimatsu H., Ozaki Y. Involvement of the Snake Toxin Receptor CLEC-2, in Podoplanin-mediated Platelet Activation, by Cancer Cells. J. Biol. Chem. 2007;282:25993–26001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki-Inoue K., Fuller G.L., García A., Eble J.A., Pöhlmann S., Inoue O., Gartner T.K., Hughan S.C., Pearce A.C., Laing G.D., et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki-Inoue K. Roles of the CLEC-2–podoplanin interaction in tumor progression. Platelets. 2018;29:786–792. doi: 10.1080/09537104.2018.1478401. [DOI] [PubMed] [Google Scholar]
- 29.Mitrugno A., Yunga S.T., Sylman J.L., Zilberman-Rudenko J., Shirai T., Hebert J.F., Kayton R., Zhang Y., Nan X., Shatzel J.J., et al. The role of coagulation and platelets in colon cancer-associated thrombosis. Am. J. Physiol. Cell Physiol. 2019;316:C264–C273. doi: 10.1152/ajpcell.00367.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King M.R., Phillips K.G., Mitrugno A., Lee T.-R., De Guillebon A.M.E., Chandrasekaran S., McGuire M.J., Carr R.T., Baker-Groberg S.M., Rigg R.A., et al. A physical sciences network characterization of circulating tumor cell aggregate transport. Am. J. Physiol. Cell Physiol. 2015;308:C792–C802. doi: 10.1152/ajpcell.00346.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y.J., Borsig L., Varki N.M., Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc. Natl. Acad. Sci. USA. 1998;95:9325–9330. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirai T., Inoue O., Tamura S., Tsukiji N., Sasaki T., Endo H., Satoh K., Osada M., Sato-Uchida H., Fujii H., et al. C-type lectin-like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor-bearing mice. J. Thromb. Haemost. 2016;15:513–525. doi: 10.1111/jth.13604. [DOI] [PubMed] [Google Scholar]
- 33.Mammadova-Bach E., Gil-Pulido J., Sarukhanyan E., Burkard P., Shityakov S., Schonhart C., Stegner D., Remer K., Nurden P., Nurden A.T. Platelet glycoprotein VI promotes metastasis through interaction with cancer cell–derived ga-lectin-3. Blood. 2020;135:1146–1160. doi: 10.1182/blood.2019002649. [DOI] [PubMed] [Google Scholar]
- 34.Yang E., Boire A., Agarwal A., Nguyen N., O’Callaghan K., Tu P., Kuliopulos A., Covic L. Blockade of PAR1 Signaling with Cell-Penetrating Pepducins Inhibits Akt Survival Pathways in Breast Cancer Cells and Suppresses Tumor Survival and Metastasis. Cancer Res. 2009;69:6223–6231. doi: 10.1158/0008-5472.CAN-09-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonora M., Patergnani S., Rimessi A., De Marchi E., Suski J.M., Bononi A., Giorgi C., Marchi S., Missiroli S., Poletti F., et al. ATP synthesis and storage. Purinergic Signal. 2012;8:343–357. doi: 10.1007/s11302-012-9305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kholmukhamedov A., Jobe S. Platelet respiration. Blood Adv. 2019;3:599–602. doi: 10.1182/bloodadvances.2018025155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H.-E., Grant A., Simic M., Kohnz R.A., Nomura D.K., Durieux J., Riera C.E., Sanchez M., Kapernick E., Wolff S., et al. Lipid Biosynthesis Coordinates a Mitochondrial-to-Cytosolic Stress Response. Cell. 2016;166:1539–1552.e16. doi: 10.1016/j.cell.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aibibula M., Naseem K.M., Sturmey R.G. Glucose metabolism and metabolic flexibility in blood platelets. J. Thromb. Haemost. 2018;16:2300–2314. doi: 10.1111/jth.14274. [DOI] [PubMed] [Google Scholar]
- 39.Wang L., Wu Q., Fan Z., Xie R., Wang Z., Lu Y. Platelet mitochondrial dysfunction and the correlation with human diseases. Biochem. Soc. Trans. 2017;45:1213–1223. doi: 10.1042/BST20170291. [DOI] [PubMed] [Google Scholar]
- 40.Shokolenko I.N., Wilson G.L., Alexeyev M.F. Aging: A mitochondrial DNA perspective, critical analysis and an update. World J. Exp. Med. 2014;4:46–57. doi: 10.5493/wjem.v4.i4.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamagishi S.-I., Edelstein D., Du X.-L., Brownlee M. Hyperglycemia potentiates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes. 2001;50:1491–1494. doi: 10.2337/diabetes.50.6.1491. [DOI] [PubMed] [Google Scholar]
- 42.Lee S.H., Lee S., Du J., Jain K., Ding M., Kadado A.J., Atteya G., Jaji Z., Tyagi T., Kim W., et al. Mitochondrial MsrB2 serves as a switch and transducer for mitophagy. EMBO Mol. Med. 2019;11:e10409. doi: 10.15252/emmm.201910409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baccarelli A.A., Byun H.-M. Platelet mitochondrial DNA methylation: A potential new marker of cardiovascular disease. Clin. Epigenetics. 2015;7:1–9. doi: 10.1186/s13148-015-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jain K., Tyagi T., Patell K., Xie Y., Kadado A.J., Lee S.H., Yarovinsky T., Du J., Hwang J., Martin K.A., et al. Age associated non-linear regulation of redox homeostasis in the anucleate platelet: Implications for CVD risk patients. EBioMedicine. 2019;44:28–40. doi: 10.1016/j.ebiom.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davizon-Castillo P., McMahon B., Aguila S., Bark D., Ashworth K., Allawzi A., Campbell R.A., Montenont E., Nemkov T., D’Alessandro A. TNF-α–driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging. Blood. 2019;134:727–740. doi: 10.1182/blood.2019000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel S.R., Hartwig J.H., Italiano J.E. The biogenesis of platelets from megakaryocyte proplatelets. J. Clin. Investig. 2005;115:3348–3354. doi: 10.1172/JCI26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hitchcock I.S., Chen M.M., King J.R., Kaushansky K. YRRL motifs in the cytoplasmic domain of the thrombopoietin receptor regulate receptor internalization and degradation. Blood. 2008;112:2222–2231. doi: 10.1182/blood-2008-01-134049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bunting S., Widmer R., Lipari T., Rangell L., Steinmetz H., Carver-Moore K., Moore M.W., Keller G.-A., De Sauvage F.J. Normal Platelets and Megakaryocytes Are Produced In Vivo in the Absence of Thrombopoietin. Blood. 1997;90:3423–3429. doi: 10.1182/blood.V90.9.3423. [DOI] [PubMed] [Google Scholar]
- 49.Behrens K., Alexander W.S. Cytokine control of megakaryopoiesis. Growth Factors. 2018;36:89–103. doi: 10.1080/08977194.2018.1498487. [DOI] [PubMed] [Google Scholar]
- 50.Willems P.H., Rossignol R., Dieteren C.E., Murphy M.P., Koopman W.J. Redox homeostasis and mitochondrial dynamics. Cell Metab. 2015;22:207–218. doi: 10.1016/j.cmet.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Jezek J., Cooper K.F., Strich R. Reactive Oxygen Species and Mitochondrial Dynamics: The Yin and Yang of Mitochondrial Dysfunction and Cancer Progression. Antioxidants. 2018;7:13. doi: 10.3390/antiox7010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan D.C. Fusion and Fission: Interlinked Processes Critical for Mitochondrial Health. Annu. Rev. Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 53.Wang K., Yan R., Cooper K.F., Strich R. Cyclin C mediates stress-induced mitochondrial fission and apoptosis. Mol. Biol. Cell. 2015;26:1030–1043. doi: 10.1091/mbc.E14-08-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horbay R., Bilyy R. Mitochondrial dynamics during cell cycling. Apoptosis. 2016;21:1327–1335. doi: 10.1007/s10495-016-1295-5. [DOI] [PubMed] [Google Scholar]
- 55.Cao Y.-L., Meng S., Chen Y., Feng J.-X., Gu D.-D., Yu B., Li Y.-J., Yang J.-Y., Liao S., Chan D.C., et al. MFN1 structures reveal nucleotide-triggered dimerization critical for mitochondrial fusion. Nature. 2017;542:372–376. doi: 10.1038/nature21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schrepfer E., Scorrano L. Mitofusins, from mitochondria to metabolism. Mol. Cell. 2016;61:683–694. doi: 10.1016/j.molcel.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 57.Buck M., O’Sullivan D., Geltink R.K., Curtis J.D., Chang C.-H., Sanin D.E., Qiu J., Kretz O., Braas D., Van Der Windt G.J., et al. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell. 2016;166:63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen S., Su Y., Wang J. ROS-mediated platelet generation: A microenvironment-dependent manner for megakaryocyte proliferation, differentiation, and maturation. Cell Death Dis. 2013;4:e722. doi: 10.1038/cddis.2013.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poirault-Chassac S., Nivet-Antoine V., Houvert A., Kauskot A., Lauret E., Lai-Kuen R., Dusanter-Fourt I., Baruch D. Mitochondrial dynamics and reactive oxygen species initiate thrombopoiesis from mature megakaryocytes. Blood Adv. 2021;5:1706–1718. doi: 10.1182/bloodadvances.2020002847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Q., Dong T., Li P., Wu M.X. Noninvasive low-level laser therapy for thrombocytopenia. Sci. Transl. Med. 2016;8:349ra101. doi: 10.1126/scitranslmed.aaf4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen H., Chan D.C. Mitochondrial Dynamics in Regulating the Unique Phenotypes of Cancer and Stem Cells. Cell Metab. 2017;26:39–48. doi: 10.1016/j.cmet.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Behnke O. An electron microscope study of the megacaryocyte of the rat bone marrow: I. The development of the demarcation membrane system and the platelet surface coat. J. Ultrastruct. Res. 1968;24:412–433. doi: 10.1016/S0022-5320(68)80046-2. [DOI] [PubMed] [Google Scholar]
- 63.Eckly A., Heijnen H., Pertuy F., Geerts W.J., Proamer F., Rinckel J.-Y., Leon C., Lanza F., Gachet C. Biogenesis of the demarcation membrane system (DMS) in megakaryocytes. Blood. 2014;123:921–930. doi: 10.1182/blood-2013-03-492330. [DOI] [PubMed] [Google Scholar]
- 64.Eckly A., Strassel C., Freund M., Cazenave J.-P., Lanza F., Gachet C., Leon C. Abnormal megakaryocyte morphology and proplatelet formation in mice with megakaryocyte-restricted MYH9 inactivation. Blood. 2009;113:3182–3189. doi: 10.1182/blood-2008-06-164061. [DOI] [PubMed] [Google Scholar]
- 65.Strassel C., Eckly A., Léon C., Petitjean C., Freund M., Cazenave J.-P., Gachet C., Lanza F. Intrinsic impaired pro-platelet formation and microtubule coil assembly of megakaryocytes in a mouse model of Bernard-Soulier syndrome. Haematologica. 2009;94:800–810. doi: 10.3324/haematol.2008.001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schulze H., Korpal M., Hurov J., Kim S.-W., Zhang J., Cantley L., Graf T., Shivdasani R.A. Characterization of the megakaryocyte demarcation membrane system and its role in thrombopoiesis. Blood. 2006;107:3868–3875. doi: 10.1182/blood-2005-07-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noris P., Pecci A. Hereditary thrombocytopenias: A growing list of disorders. Hematology Am. Soc. Hematol. Educ. Program. 2017;2017:385–399. doi: 10.1182/asheducation-2017.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Al Mazni I., Stapley R., Morgan N.V. Inherited Thrombocytopenia: Update on Genes and Genetic Variants Which may be Associated with Bleeding. Front. Cardiovasc. Med. 2019;6:80. doi: 10.3389/fcvm.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balduini C.L., Melazzini F., Pecci A. Inherited thrombocytopenias—Recent advances in clinical and molecular aspects. Platelets. 2016;28:3–13. doi: 10.3109/09537104.2016.1171835. [DOI] [PubMed] [Google Scholar]
- 70.Palma-Barqueros V., Revilla N., Sánchez A., Cánovas A.Z., Rodriguez-Alén A., Marín-Quílez A., González-Porras J., Vicente V., Lozano M., Bastida J., et al. Inherited Platelet Disorders: An Updated Overview. Int. J. Mol. Sci. 2021;22:4521. doi: 10.3390/ijms22094521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freson K., Wijgaerts A., Van Geet C. GATA1 gene variants associated with thrombocytopenia and anemia. Platelets. 2017;28:731–734. doi: 10.1080/09537104.2017.1361525. [DOI] [PubMed] [Google Scholar]
- 72.Song W.-J., Sullivan M.G., Legare R.D., Hutchings S., Tan X., Kufrin D., Ratajczak J., Resende I.C., Haworth C., Hock R. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat. Genet. 1999;23:166–175. doi: 10.1038/13793. [DOI] [PubMed] [Google Scholar]
- 73.Zhang M., Churpek J.E., Keel S.B., Walsh T., Lee M.K., Loeb K.R., Gulsuner S., Pritchard C.C., Sanchez-Bonilla M., Delrow J.J., et al. Germline ETV6 mutations in familial thrombocytopenia and hematologic malignancy. Nat. Genet. 2015;47:180–185. doi: 10.1038/ng.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thompson A.A., Nguyen L.T. Amegakaryocytic thrombocytopenia and radio-ulnar synostosis are associated with HOXA11 mutation. Nat. Genet. 2000;26:397–398. doi: 10.1038/82511. [DOI] [PubMed] [Google Scholar]
- 75.Niihori T., Ouchi-Uchiyama M., Sasahara Y., Kaneko T., Hashii Y., Irie M., Sato A., Saito-Nanjo Y., Funayama R., Nagashima T., et al. Mutations in MECOM, Encoding Oncoprotein EVI1, Cause Radioulnar Synostosis with Amegakaryocytic Thrombocytopenia. Am. J. Hum. Genet. 2015;97:848–854. doi: 10.1016/j.ajhg.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jalagadugula G., Mao G., Kaur G., Goldfinger L.E., Dhanasekaran D.N., Rao A.K. Regulation of platelet myosin light chain (MYL9) by RUNX1: Implications for thrombocytopenia and platelet dysfunction in RUNX1 haplodeficiency. Blood. 2010;116:6037–6045. doi: 10.1182/blood-2010-06-289850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bluteau D., Glembotsky A.C., Raimbault A., Balayn N., Gilles L., Rameau P., Nurden P., Alessi M.C., Debili N., Vainchenker W., et al. Dysmegakaryopoiesis of FPD/AML pedigrees with constitutional RUNX1 mutations is linked to myosin II deregulated expression. Blood. 2012;120:2708–2718. doi: 10.1182/blood-2012-04-422337. [DOI] [PubMed] [Google Scholar]
- 78.Estevez B., Borst S., Jarocha D.J., Sudunagunta V.S., Gonzalez M., Garifallou J., Hakonarson H., Gao P., Tan K., Liu P.P., et al. RUNX-1 haploinsufficiency causes a marked deficiency of megakaryocyte-biased hematopoietic progenitor cells. Blood. 2021;137:2662–2675. doi: 10.1182/blood.2020006389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wechsler J., Greene M.E., McDevitt M.A., Anastasi J., Karp J.E., Le Beau M.M., Crispino J. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat. Genet. 2002;32:148–152. doi: 10.1038/ng955. [DOI] [PubMed] [Google Scholar]
- 80.Gialesaki S., Mahnken A.K., Schmid L., Labuhn M., Bhayadia R., Heckl D., Klusmann J.-H. GATA1s exerts develop-mental stage-specific effects in human hematopoiesis. Haematologica. 2018;103:e336. doi: 10.3324/haematol.2018.191338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nichols K.E., Crispino J., Poncz M., White J.G., Orkin S.H., Maris J.M., Weiss M. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA1. Nat. Genet. 2000;24:266–270. doi: 10.1038/73480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raslova H., Komura E., Le Couédic J.P., Larbret F., Debili N., Feunteun J., Danos O., Albagli O., Vainchenker W., Favier R. FLI1 monoallelic expression combined with its hemizygous loss underlies Paris-Trousseau/Jacobsen thrombo-penia. J. Clin. Investig. 2004;114:77–84. doi: 10.1172/JCI21197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vo K.K., Jarocha D.J., Lyde R.B., Hayes V., Thom C.S., Sullivan S.K., French D.L., Poncz M. FLI1 level during megakaryopoiesis affects thrombopoiesis and platelet biology. Blood. 2017;129:3486–3494. doi: 10.1182/blood-2017-02-770958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lecine P., Italiano Jr J.E., Kim S.-W., Villeval J.-L., Shivdasani R.A. Hematopoietic-specific β1 tubulin participates in a pathway of platelet biogenesis dependent on the transcription factor NF-E2. Blood. 2000;96:1366–1373. doi: 10.1182/blood.V96.4.1366. [DOI] [PubMed] [Google Scholar]
- 85.Tiwari S., Italiano Jr J.E., Barral D.C., Mules E.H., Novak E.K., Swank R.T., Seabra M.C., Shivdasani R.A. A role for Rab27b in NF-E2-dependent pathways of platelet formation. Blood. 2003;102:3970–3979. doi: 10.1182/blood-2003-03-0977. [DOI] [PubMed] [Google Scholar]
- 86.Wang W., Schwemmers S., Hexner E.O., Pahl H.L. AML1 is overexpressed in patients with myeloproliferative neoplasms and mediates JAK2V617F-independent overexpression of NF-E2. Blood. 2010;116:254–266. doi: 10.1182/blood-2009-11-254664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mazzi S., Lordier L., Debili N., Raslova H., Vainchenker W. Megakaryocyte and polyploidization. Exp. Hematol. 2017;57:1–13. doi: 10.1016/j.exphem.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 88.Tijssen M.R., Ghevaert C. Transcription factors in late megakaryopoiesis and related platelet disorders. J. Thromb. Haemost. 2013;11:593–604. doi: 10.1111/jth.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Germeshausen M., Ancliff P., Estrada J., Metzler M., Ponstingl E., Rütschle H., Schwabe D., Scott R.H., Unal S., Wawer A., et al. MECOM-associated syndrome: A heterogeneous inherited bone marrow failure syndrome with amegakaryocytic thrombocytopenia. Blood Adv. 2018;2:586–596. doi: 10.1182/bloodadvances.2018016501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noetzli L., Lo R., Sherick A.L., Callaghan M., Noris P., Savoia A., Rajpurkar M., Jones K., Gowan K., Balduini C.L., et al. Germline mutations in ETV6 are associated with thrombocytopenia, red cell macrocytosis and predisposition to lymphoblastic leukemia. Nat. Genet. 2015;47:535–538. doi: 10.1038/ng.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Poggi M., Canault M., Favier M., Bassols E.T., Saultier P., Ghalloussi D., Baccini V., Vidal L., Mezzapesa A., Chelghoum N., et al. Germline variants in ETV6 underlie reduced platelet formation, platelet dysfunction and increased levels of circulating CD34+ progenitors. Haematologica. 2018 doi: 10.3324/haematol.2016.147694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Di Paola J., Porter C.C. ETV6-related thrombocytopenia and leukemia predisposition. Blood. 2019;134:663–667. doi: 10.1182/blood.2019852418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Monteferrario D., Bolar N.A., Marneth A.E., Hebeda K.M., Bergevoet S.M., Veenstra H., Laros-van Gorkum B.A., MacKenzie M.A., Khandanpour C., Botezatu L.A. A Dominant-Negative GFI1B Mutation in Gray Platelet Syndrome. N. Engl. J. Med. 2014;370:245–253. doi: 10.1056/NEJMoa1308130. [DOI] [PubMed] [Google Scholar]
- 94.Rabbolini D.J., Morel-Kopp M.-C., Chen Q., Gabrielli S., Dunlop L.C., Chew L.P., Blair N., Brighton T.A., Singh N., Ng A., et al. Thrombocytopenia and CD34 expression is decoupled from α-granule deficiency with mutation of the first growth factor-independent 1B zinc finger. J. Thromb. Haemost. 2017;15:2245–2258. doi: 10.1111/jth.13843. [DOI] [PubMed] [Google Scholar]
- 95.Beauchemin H., Shooshtharizadeh P., Pinder J., Dellaire G., Möröy T. Dominant negative Gfi1b mutations cause moderate thrombocytopenia and an impaired stress thrombopoiesis associated with mild erythropoietic abnormalities in mice. Haematologica. 2020;105:2457–2470. doi: 10.3324/haematol.2019.222596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lentaigne C., Greene D., Sivapalaratnam S., Favier R., Seyres D., Thys C., Grassi L., Mangles S., Sibson K., Stubbs M. Germline mutations in the transcription factor IKZF5 cause thrombocytopenia. Blood. 2019;134:2070–2081. doi: 10.1182/blood.2019000782. [DOI] [PubMed] [Google Scholar]
- 97.Aneja K., Jalagadugula G., Mao G., Singh A., Rao A.K. Mechanism of platelet factor 4 (PF4) deficiency with RUNX1 haplodeficiency: RUNX1 is a transcriptional regulator of PF4. J. Thromb. Haemost. 2010;9:383–391. doi: 10.1111/j.1538-7836.2010.04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bluteau D., Gilles L., Hilpert M., Antony-Debré I., James C., Debili N., Camara-Clayette V., Wagner-Ballon O., Cordette-Lagarde V., Robert T. Down-regulation of the RUNX1-target gene NR4A3 contributes to hematopoiesis dereg-ulation in familial platelet disorder/acute myelogenous leukemia. Blood. 2011;118:6310–6320. doi: 10.1182/blood-2010-12-325555. [DOI] [PubMed] [Google Scholar]
- 99.Jalagadugula G., Mao G., Kaur G., Dhanasekaran D.N., Rao A.K. Platelet protein kinase C-θ deficiency with human RUNX1 mutation: PRKCQ is a transcriptional target of RUNX1. Arterioscler. Thromb. Vasc. Biol. 2011;31:921–927. doi: 10.1161/ATVBAHA.110.221879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao X., Jankovic V., Gural A., Huang G., Pardanani A., Menendez S., Zhang J., Dunne R., Xiao A., Erdjument-Bromage H., et al. Methylation of RUNX1 by PRMT1 abrogates SIN3A binding and potentiates its transcriptional activity. Genes Dev. 2008;22:640–653. doi: 10.1101/gad.1632608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schlegelberger B., Heller P.G. RUNX1 deficiency (familial platelet disorder with predisposition to myeloid leukemia, FPDMM) Semin. Hematol. 2017;54:75–80. doi: 10.1053/j.seminhematol.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 102.Lordier L., Bluteau D., Jalil A., Legrand C., Pan J., Rameau P., Jouni D., Bluteau O., Mercher T., Leon C., et al. RUNX1-induced silencing of non-muscle myosin heavy chain IIB contributes to megakaryocyte polyploidization. Nat. Commun. 2012;3:717. doi: 10.1038/ncomms1704. [DOI] [PubMed] [Google Scholar]
- 103.Shin J.-W., Swift J., Spinler K.R., Discher D.E. Myosin-II inhibition and soft 2D matrix maximize multinucleation and cellular projections typical of platelet-producing megakaryocytes. Proc. Natl. Acad. Sci. USA. 2011;108:11458–11463. doi: 10.1073/pnas.1017474108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haas S., Hansson J., Klimmeck D., Loeffler D., Velten L., Uckelmann H., Wurzer S., Prendergast Á.M., Schnell A., Hexel K. Inflammation-induced emergency megakaryopoiesis driven by hematopoietic stem cell-like megakaryocyte pro-genitors. Cell Stem Cell. 2015;17:422–434. doi: 10.1016/j.stem.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 105.Miyawaki K., Iwasaki H., Jiromaru T., Kusumoto H., Yurino A., Sugio T., Uehara Y., Odawara J., Daitoku S., Kunisaki Y., et al. Identification of unipotent megakaryocyte progenitors in human hematopoiesis. Blood. 2017;129:3332–3343. doi: 10.1182/blood-2016-09-741611. [DOI] [PubMed] [Google Scholar]
- 106.Tijssen M.R., Cvejic A., Joshi A., Hannah R.L., Ferreira R., Forrai A., Bellissimo D.C., Oram S.H., Smethurst P.A., Wilson N.K. Genome-wide analysis of simultaneous GATA1/2, RUNX1, FLI1, and SCL binding in megakaryocytes identifies hematopoietic regulators. Dev. Cell. 2011;20:597–609. doi: 10.1016/j.devcel.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mancini E., Sanjuan-Pla A., Luciani L., Moore S., Grover A., Zay A., Rasmussen K.D., Luc S., Bilbao D., O’carroll D. FOG-1 and GATA-1 act sequentially to specify definitive megakaryocytic and erythroid progenitors. EMBO J. 2012;31:351–365. doi: 10.1038/emboj.2011.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Muntean A.G., Pang L., Poncz M., Dowdy S.F., Blobel G.A., Crispino J.D. Cyclin D–Cdk4 is regulated by GATA-1 and required for megakaryocyte growth and polyploidization. Blood. 2007;109:5199–5207. doi: 10.1182/blood-2006-11-059378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vyas P., McDevitt M., Cantor A., Katz S., Fujiwara Y., Orkin S. Different sequence requirements for expression in erythroid and megakaryocytic cells within a regulatory element upstream of the GATA-1 gene. Development. 1999;126:2799–2811. doi: 10.1242/dev.126.12.2799. [DOI] [PubMed] [Google Scholar]
- 110.Ciovacco W.A., Raskind W.H., Kacena M.A. Human phenotypes associated with GATA-1 mutations. Gene. 2008;427:1–6. doi: 10.1016/j.gene.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ferreira R., Ohneda K., Yamamoto M., Philipsen S. GATA1 function, a paradigm for transcription factors in hemato-poiesis. Mol. Cell. Biol. 2005;25:1215–1227. doi: 10.1128/MCB.25.4.1215-1227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Elagib K.E., Racke F.K., Mogass M., Khetawat R., Delehanty L.L., Goldfarb A.N. RUNX1 and GATA-1 coexpression and cooperation in megakaryocytic differentiation. Blood. 2003;101:4333–4341. doi: 10.1182/blood-2002-09-2708. [DOI] [PubMed] [Google Scholar]
- 113.Wagenblast E., Azkanaz M., Smith S.A., Shakib L., McLeod J.L., Krivdova G., Araújo J., Shultz L.D., Gan O.I., Dick J.E., et al. Functional profiling of single CRISPR/Cas9-edited human long-term hematopoietic stem cells. Nat. Commun. 2019;10:1–11. doi: 10.1038/s41467-019-12726-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Byrska-Bishop M., VanDorn D., Campbell A.E., Betensky M., Arca P.R., Yao Y., Gadue P., Costa F.F., Nemiroff R.L., Blobel G.A., et al. Pluripotent stem cells reveal erythroid-specific activities of the GATA1 N-terminus. J. Clin. Investig. 2015;125:993–1005. doi: 10.1172/JCI75714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stankiewicz M.J., Crispino J.D. ETS2 and ERG promote megakaryopoiesis and synergize with alterations in GATA-1 to immortalize hematopoietic progenitor cells. Blood. 2009;113:3337–3347. doi: 10.1182/blood-2008-08-174813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vyas P., Ault K., Jackson C.W., Orkin S.H., Shivdasani R.A. Consequences of GATA-1 deficiency in megakaryocytes and platelets. Blood. 1999;93:2867–2875. doi: 10.1182/blood.V93.9.2867.409k24_2867_2875. [DOI] [PubMed] [Google Scholar]
- 117.Stachura D.L., Chou S.T., Weiss M.J. Early block to erythromegakaryocytic development conferred by loss of transcription factor GATA-1. Blood. 2006;107:87–97. doi: 10.1182/blood-2005-07-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Noh J.-Y., Gandre-Babbe S., Wang Y., Hayes V., Yao Y., Gadue P., Sullivan S.K., Chou S.T., Machlus K., Italiano J.E., et al. Inducible Gata1 suppression expands megakaryocyte-erythroid progenitors from embryonic stem cells. J. Clin. Investig. 2015;125:2369–2374. doi: 10.1172/JCI77670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kanezaki R., Toki T., Terui K., Xu G., Wang R., Shimada A., Hama A., Kanegane H., Kawakami K., Endo M. Down syndrome and GATA1 mutations in transient abnormal myeloproliferative disorder: Mutation classes correlate with pro-gression to myeloid leukemia. Blood. 2010;116:4631–4638. doi: 10.1182/blood-2010-05-282426. [DOI] [PubMed] [Google Scholar]
- 120.Vannucchi A.M., Bianchi L., Cellai C., Paoletti F., Rana R.A., Lorenzini R., Migliaccio G., Migliaccio A.R.F. Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1low mice) Blood. 2002;100:1123–1132. doi: 10.1182/blood-2002-06-1913. [DOI] [PubMed] [Google Scholar]
- 121.Wang X., Crispino J., Letting D.L., Nakazawa M., Poncz M., Blobel G.A. Control of megakaryocyte-specific gene expression by GATA-1 and FOG-1: Role of Ets transcription factors. EMBO J. 2002;21:5225–5234. doi: 10.1093/emboj/cdf527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jackers P., Szalai G., Moussa O., Watson D.K. Ets-dependent regulation of target gene expression during megakaryo-poiesis. J. Biol. Chem. 2004;279:52183–52190. doi: 10.1074/jbc.M407489200. [DOI] [PubMed] [Google Scholar]
- 123.Bianchi E., Zini R., Salati S., Tenedini E., Norfo R., Tagliafico E., Manfredini R., Ferrari S. c-myb supports erythropoiesis through the transactivation of KLF1 and LMO2 expression. Blood. 2010;116:e99–e110. doi: 10.1182/blood-2009-08-238311. [DOI] [PubMed] [Google Scholar]
- 124.Carpinelli M.R., Hilton D.J., Metcalf D., Antonchuk J.L., Hyland C.D., Mifsud S.L., Di Rago L., Hilton A.A., Willson T.A., Roberts A.W. Suppressor screen in Mpl-/-mice: C-Myb mutation causes supraphysiological production of platelets in the absence of thrombopoietin signaling. Proc. Natl. Acad. Sci. USA. 2004;101:6553–6558. doi: 10.1073/pnas.0401496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barroga C.F., Pham H., Kaushansky K. Thrombopoietin regulates c-Myb expression by modulating micro RNA 150 ex-pression. Exp. Hematol. 2008;36:1585–1592. doi: 10.1016/j.exphem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lu J., Guo S., Ebert B.L., Zhang H., Peng X., Bosco J., Pretz J., Schlanger R., Wang J.Y., Mak R.H. MicroRNA-mediated control of cell fate in megakaryocyte-erythrocyte progenitors. Dev. Cell. 2008;14:843–853. doi: 10.1016/j.devcel.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Favier R., Jondeau K., Boutard P., Grossfeld P., Reinert P., Jones C., Bertoni F., Cramer E.M. Paris-Trousseau syndrome: Clinical, hematological, molecular data of ten new cases. Thromb. Haemost. 2003;90:893–897. doi: 10.1160/TH03-02-0120. [DOI] [PubMed] [Google Scholar]
- 128.Lulli V., Romania P., Morsilli O., Gabbianelli M., Pagliuca A., Mazzeo S., Testa U., Peschle C., Marziali G. Overex-pression of Ets-1 in human hematopoietic progenitor cells blocks erythroid and promotes megakaryocytic differentiation. Cell Death Differ. 2006;13:1064–1074. doi: 10.1038/sj.cdd.4401811. [DOI] [PubMed] [Google Scholar]
- 129.Bluteau D., Balduini A., Balayn N., Currao M., Nurden P., Deswarte C., Leverger G., Noris P., Perrotta S., Solary E., et al. Thrombocytopenia-associated mutations in the ANKRD26 regulatory region induce MAPK hyperactivation. J. Clin. Investig. 2014;124:580–591. doi: 10.1172/JCI71861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kerrigan S.W., Gaur M., Murphy R.P., Shattil S.J., Leavitt A.D. Caspase-12: A developmental link between G-protein–coupled receptors and integrin αIIbβ3 activation. Blood. 2004;104:1327–1334. doi: 10.1182/blood-2003-10-3633. [DOI] [PubMed] [Google Scholar]
- 131.Nagata Y., Yoshikawa J., Hashimoto A., Yamamoto M., Payne A.H., Todokoro K. Proplatelet formation of megakar-yocytes is triggered by autocrine-synthesized estradiol. Genes Dev. 2003;17:2864–2869. doi: 10.1101/gad.1128003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shivdasani R.A., Rosenblatt M.F., Zucker-Franklin D., Jackson C.W., Hunt P., Saris C.J., Orkin S.H. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoeitin/MGDF in megakaryocyte development. Cell. 1995;81:695–704. doi: 10.1016/0092-8674(95)90531-6. [DOI] [PubMed] [Google Scholar]
- 133.Lecine P., Villeval J.-L., Vyas P., Swencki B., Xu Y., Shivdasani R.A. Mice lacking transcription factor NF-E2 provide in vivo validation of the proplatelet model of thrombocytopoiesis and show a platelet production defect that is intrinsic to megakaryocytes. Blood. 1998;92:1608–1616. doi: 10.1182/blood.V92.5.1608. [DOI] [PubMed] [Google Scholar]
- 134.Shiraga M., Ritchie A., Aidoudi S., Baron V., Wilcox D., White G., Ybarrondo B., Murphy G., Leavitt A., Shattil S. Primary Megakaryocytes Reveal a Role for Transcription Factor Nf-E2 in Integrin αiibβ3 Signaling. J. Cell Biol. 1999;147:1419–1430. doi: 10.1083/jcb.147.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Leo V.C., Morgan N., Bem D., Jones M.L., Lowe G.C., Lordkipanidzé M., Drake S., Simpson M.A., Gissen P., Mumford A., et al. Use of next-generation sequencing and candidate gene analysis to identify underlying defects in patients with inherited platelet function disorders. J. Thromb. Haemost. 2015;13:643–650. doi: 10.1111/jth.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Goerttler P.S., Kreutz C., Donauer J., Faller D., Maiwald T., März E., Rumberger B., Sparna T., Schmitt-Gräff A., Wilpert J. Gene expression profiling in polycythaemia vera: Overexpression of transcription factor NF-E2. Br. J. Haematol. 2005;129:138–150. doi: 10.1111/j.1365-2141.2005.05416.x. [DOI] [PubMed] [Google Scholar]
- 137.Lopez R.G., Carron C., Oury C., Gardellin P., Bernard O., Ghysdael J. TEL is a sequence-specific transcriptional repressor. J. Biol. Chem. 1999;274:30132–30138. doi: 10.1074/jbc.274.42.30132. [DOI] [PubMed] [Google Scholar]
- 138.Hock H., Meade E., Medeiros S., Schindler J.W., Valk P.J., Fujiwara Y., Orkin S.H. Tel/Etv6 is an essential and selective regulator of adult hematopoietic stem cell survival. Genes Dev. 2004;18:2336–2341. doi: 10.1101/gad.1239604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Boily G., LaRose J., Langlois S., Sinnett D. Identification of transcripts modulated by ETV6 expression. Br. J. Haematol. 2007;136:48–62. doi: 10.1111/j.1365-2141.2006.06377.x. [DOI] [PubMed] [Google Scholar]
- 140.Uchiyama Y., Ogawa Y., Kunishima S., Shiina M., Nakashima M., Yanagisawa K., Yokohama A., Imagawa E., Miyatake S., Mizuguchi T. A novel GFI1B mutation at the first zinc finger domain causes congenital macrothrombocy-topenia. Br. J. Haematol. 2018;181:843–847. doi: 10.1111/bjh.14710. [DOI] [PubMed] [Google Scholar]
- 141.Polfus L.M., Khajuria R.K., Schick U.M., Pankratz N., Pazoki R., Brody J.A., Chen M.-H., Auer P.L., Floyd J.S., Huang J., et al. Whole-Exome Sequencing Identifies Loci Associated with Blood Cell Traits and Reveals a Role for Alternative GFI1B Splice Variants in Human Hematopoiesis. Am. J. Hum. Genet. 2016;99:481–488. doi: 10.1016/j.ajhg.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Laurent B., Randrianarison-Huetz V., Frisan E., Andrieu-Soler C., Soler E., Fontenay M., Dusanter-Fourt I., Duménil D. A short Gfi-1B isoform controls erythroid differentiation by recruiting the LSD1–CoREST complex through the dimethylation of its SNAG domain. J. Cell Sci. 2012;125:993–1002. doi: 10.1242/jcs.095877. [DOI] [PubMed] [Google Scholar]
- 143.Saleque S., Cameron S., Orkin S.H. The zinc-finger proto-oncogene Gfi-1b is essential for development of the erythroid and megakaryocytic lineages. Genes Dev. 2002;16:301–306. doi: 10.1101/gad.959102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Georgopoulos K., Winandy S., Avitahl N. The role of the ikaros gene in lymphocyte development and homeostasis. Annu. Rev. Immunol. 1997;15:155–176. doi: 10.1146/annurev.immunol.15.1.155. [DOI] [PubMed] [Google Scholar]
- 145.Li S., Fu J., Mapara M., Lentzsch S. IMiD® Compounds Affect the Hematopoiesis Via CRBN Dependent Degradation of IKZF1 Protein in CD34+ Cells. Blood. 2014;124:418. doi: 10.1182/blood.V124.21.418.418. [DOI] [Google Scholar]
- 146.Liu A., Li S., Donnenberg V., Fu J., Gollin S.M., Ma H., Lu C., Stolz D.B., Mapara M.Y., Monaghan S.A. Immuno-modulatory drugs downregulate IKZF1 leading to expansion of hematopoietic progenitors with concomitant block of megakaryocytic maturation. Haematologica. 2018;103:1688. doi: 10.3324/haematol.2018.188227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wijgaerts A., Wittevrongel C., Thys C., Devos T., Peerlinck K., Tijssen M.R., Van Geet C., Freson K. The transcription factor GATA1 regulates NBEAL2 expression through a long-distance enhancer. Haematologica. 2017;102:695–706. doi: 10.3324/haematol.2016.152777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jaiswal S., Fontanillas P., Flannick J., Manning A., Grauman P.V., Mar B., Lindsley C., Mermel C., Burtt N., Chavez A., et al. Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes. N. Engl. J. Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Young A.L., Challen G.A., Birmann B., Druley T.E. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat. Commun. 2016;7:12484. doi: 10.1038/ncomms12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jaiswal S., Natarajan P., Silver A.J., Gibson C.J., Bick A.G., Shvartz E., McConkey M., Gupta N., Gabriel S., Ardissino D. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Abplanalp W.T., Cremer S., John D., Hoffmann J., Schuhmacher B., Merten M., Rieger M.A., Vasa-Nicotera M., Zeiher A.M., Dimmeler S. Clonal Hematopoiesis–Driver DNMT3A Mutations Alter Immune Cells in Heart Failure. Circ. Res. 2021;128:216–228. doi: 10.1161/CIRCRESAHA.120.317104. [DOI] [PubMed] [Google Scholar]
- 152.Veninga A., De Simone I., Heemskerk J.W., Cate H.T., Van Der Meijden P.E. Clonal hematopoietic mutations linked to platelet traits and the risk of thrombosis or bleeding. Haematologica. 2020;105:2020–2031. doi: 10.3324/haematol.2019.235994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nagase R., Inoue D., Pastore A., Fujino T., Hou H.-A., Yamasaki N., Goyama S., Saika M., Kanai A., Sera Y. Ex-pression of mutant Asxl1 perturbs hematopoiesis and promotes susceptibility to leukemic transformation. J. Exp. Med. 2018;215:1729–1747. doi: 10.1084/jem.20171151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lin C.-C., Hou H.-A., Chou W.-C., Kuo Y.-Y., Wu S.-J., Liu C.-Y., Chen C.-Y., Tseng M.-H., Huang C.-F., Lee F.-Y., et al. SF3B1mutations in patients with myelodysplastic syndromes: The mutation is stable during disease evolution. Am. J. Hematol. 2014;89:E109–E115. doi: 10.1002/ajh.23734. [DOI] [PubMed] [Google Scholar]
- 155.Yang L., Rau R., Goodell M.A. DNMT3A in haematological malignancies. Nat. Rev. Cancer. 2015;15:152–165. doi: 10.1038/nrc3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang W., Tang Y., Wang Y., Tascau L., Balcerek J., Tong W., Levine R.L., Welch C.L., Tall A.R., Wang N. LNK/SH2B3 Loss of Function Promotes Atherosclerosis and Thrombosis. Circ. Res. 2016;119:e91–e103. doi: 10.1161/CIRCRESAHA.116.308955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Acuna-Hidalgo R., Sengul H., Steehouwer M., van de Vorst M., Vermeulen S.H., Kiemeney L., Veltman J., Gilissen C., Hoischen A. Ultra-sensitive Sequencing Identifies High Prevalence of Clonal Hematopoiesis-Associated Mutations throughout Adult Life. Am. J. Hum. Genet. 2017;101:50–64. doi: 10.1016/j.ajhg.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Barbui T., Finazzi G., Falanga A. Myeloproliferative neoplasms and thrombosis. Blood. 2013;122:2176–2184. doi: 10.1182/blood-2013-03-460154. [DOI] [PubMed] [Google Scholar]
- 159.Hobbs C.M., Manning H., Bennett C., Vasquez L., Severin S., Brain L., Mazharian A., Guerrero J.A., Li J., Soranzo N. JAK2V617F leads to intrinsic changes in platelet formation and reactivity in a knock-in mouse model of essential throm-bocythemia. Blood. 2013;122:3787–3797. doi: 10.1182/blood-2013-06-501452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Barone M., Ricci F., Sollazzo D., Ottaviani E., Romano M., Auteri G., Bartoletti D., Reggiani M.L.B., Vianelli N., Tazzari P.L., et al. Circulating megakaryocyte and platelet microvesicles correlate with response to ruxolitinib and distinct disease severity in patients with myelofibrosis. Br. J. Haematol. 2018;185:987–991. doi: 10.1111/bjh.15682. [DOI] [PubMed] [Google Scholar]
- 161.Trappenburg M.C., Van Schilfgaarde M., Marchetti M., Spronk H.M., Cate H.T., Leyte A., Terpstra W.E., Falanga A. Elevated procoagulant microparticles expressing endothelial and platelet markers in essential thrombocythemia. Haematologica. 2009;94:911–918. doi: 10.3324/haematol.13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Găman M.-A., Cozma M.-A., Dobrică E.-C., Crețoiu S., Găman A., Diaconu C. Liquid Biopsy and Potential Liquid Biopsy-Based Biomarkers in Philadelphia-Negative Classical Myeloproliferative Neoplasms: A Systematic Review. Life. 2021;11:677. doi: 10.3390/life11070677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moisă C., Găman M.-A., Pascu E.G., Assani A., Drăgusin O., Epîngeac M.-E., Găman A.-M. The role of oxidative stress in essential thrombocythemia. Arch. Balk. Med. Union. 2018;53:70–75. [Google Scholar]
- 164.Gaman A.M., Moisa C., Diaconu C.C., Gaman M.A. Crosstalk between Oxidative Stress, Chronic Inflammation and Disease Progression in Essential Thrombocythemia. Rev. Chim. 2019;70:3486–3489. doi: 10.37358/RC.19.10.7581. [DOI] [Google Scholar]
- 165.Tefferi A., Pardanani A. Myeloproliferative neoplasms: A contemporary review. JAMA Oncol. 2015;1:97–105. doi: 10.1001/jamaoncol.2015.89. [DOI] [PubMed] [Google Scholar]
- 166.Teofili L., Foà R., Giona F., Larocca L.M. Childhood polycythemia vera and essential thrombocythemia: Does their pathogenesis overlap with that of adult patients? Haematologica. 2008;93:169–172. doi: 10.3324/haematol.12002. [DOI] [PubMed] [Google Scholar]
- 167.Ferroni P., Martini F., Portarena I., Grenga I., Riondino S., La Farina F., Laudisi A., Roselli M., Guadagni F. An acti-vated protein C-dependent thrombin generation assay predicts chemotherapy-associated venous thromboembolism in cancer patients. Thromb. Haemost. 2011;105:931–932. doi: 10.1160/TH10-11-0757. [DOI] [PubMed] [Google Scholar]
- 168.Khorana A.A., Francis C.W., Culakova E., Kuderer N.M., Lyman G.H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 2007;5:632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 169.Taucher S., Salat A., Gnant M., Kwasny W., Mlineritsch B., Menzel R.-C., Schmid M., Smola M.G., Stierer M., Tausch C. Impact of pretreatment thrombocytosis on survival in primary breast cancer. Thromb. Haemost. 2003;89:1098–1106. [PubMed] [Google Scholar]
- 170.Sasaki K., Kawai K., Tsuno N.H., Sunami E., Kitayama J. Impact of Preoperative Thrombocytosis on the Survival of Patients with Primary Colorectal Cancer. World J. Surg. 2011;36:192–200. doi: 10.1007/s00268-011-1329-7. [DOI] [PubMed] [Google Scholar]
- 171.Pedersen L.M., Milman N. Prognostic significance of thrombocytosis in patients with primary lung cancer. Eur. Respir. J. 1996;9:1826–1830. doi: 10.1183/09031936.96.09091826. [DOI] [PubMed] [Google Scholar]
- 172.Stone R.L., Nick A.M., McNeish I., Balkwill F., Han H.D., Bottsford-Miller J., Rupaimoole R., Armaiz-Pena G.N., Pecot C.V., Coward J., et al. Paraneoplastic Thrombocytosis in Ovarian Cancer. N. Engl. J. Med. 2012;366:610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Baaten C.C., Moenen F.C., Henskens Y.M., Swieringa F., Wetzels R.J., van Oerle R., Heijnen H.F., Ten Cate H., Hol-loway G.P., Beckers E.A. Impaired mitochondrial activity explains platelet dysfunction in thrombocytopenic cancer pa-tients undergoing chemotherapy. Haematologica. 2018;103:1557–1567. doi: 10.3324/haematol.2017.185165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Daly M.E. Determinants of platelet count in humans. Haematologica. 2010;96:10–13. doi: 10.3324/haematol.2010.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Biino G., Santimone I., Minelli C., Sorice R., Frongia B., Traglia M., Ulivi S., Di Castelnuovo A., Gögele M., Nutile T. Age-and sex-related variations in platelet count in Italy: A proposal of reference ranges based on 40987 subjects’ data. PLoS ONE. 2013;8:e54289. doi: 10.1371/journal.pone.0054289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Segal J.B., Moliterno A.R. Platelet Counts Differ by Sex, Ethnicity, and Age in the United States. Ann. Epidemiol. 2006;16:123–130. doi: 10.1016/j.annepidem.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 177.O’Donnell C.J., Larson M.G., Feng D., Sutherland P.A., Lindpaintner K., Myers R.H., D’Agostino R.A., Levy D., Tofler G.H. Genetic and environmental contributions to platelet aggregation: The Framingham heart study. Circulation. 2001;103:3051–3056. doi: 10.1161/01.CIR.103.25.3051. [DOI] [PubMed] [Google Scholar]
- 178.Simon L.M., Edelstein L.C., Nagalla S., Woodley A.B., Chen E.S., Kong X., Ma L., Fortina P., Kunapuli S., Holinstat M. Human platelet microRNA-mRNA networks associated with age and gender revealed by integrated plateletomics. Blood. 2014;123:e37–e45. doi: 10.1182/blood-2013-12-544692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Grover A., Pla A.S., Thongjuea S., Carrelha J., Giustacchini A., Gambardella A., Macaulay I., Mancini E., Luis T.C., Mead A., et al. Single-cell RNA sequencing reveals molecular and functional platelet bias of aged haematopoietic stem cells. Nat. Commun. 2016;7:11075. doi: 10.1038/ncomms11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Frisch B.J., Hoffman C.M., Latchney S.E., LaMere M.W., Myers J., Ashton J., Li A.J., Saunders J. Aged marrow macrophages expand platelet-biased hematopoietic stem cells via interleukin-1B. JCI insight. 2019;4:e124213. doi: 10.1172/jci.insight.124213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nordberg J., Arnér E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free. Radic. Biol. Med. 2001;31:1287–1312. doi: 10.1016/S0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 182.Sverdlov A., Chan W.P., Procter N.E., Chirkov Y.Y., Ngo D.T., Horowitz J. Reciprocal regulation of NO signaling and TXNIP expression in humans: Impact of aging and ramipril therapy. Int. J. Cardiol. 2013;168:4624–4630. doi: 10.1016/j.ijcard.2013.07.159. [DOI] [PubMed] [Google Scholar]
- 183.Dayal S., Wilson K.M., Motto D.G., Miller F.J., Jr., Chauhan A.K., Lentz S.R. Hydrogen peroxide promotes aging-related platelet hyperactivation and thrombosis. Circulation. 2013;127:1308–1316. doi: 10.1161/CIRCULATIONAHA.112.000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Poscablo D.M., Worthington A.K., Smith-Berdan S., Forsberg E.C. Megakaryocyte progenitor cell function is enhanced upon aging despite the functional decline of aged hematopoietic stem cells. Stem Cell Rep. 2021;16:1598–1613. doi: 10.1016/j.stemcr.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pardali E., Dimmeler S., Zeiher A.M., Rieger M.A. Clonal hematopoiesis, aging, and cardiovascular diseases. Exp. Hematol. 2020;83:95–104. doi: 10.1016/j.exphem.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 186.Vannucchi A.M., Lasho T.L., Guglielmelli P., Biamonte F., Pardanani A., Pereira A., Finke C., Score J., Gangat N., Mannarelli C., et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27:1861–1869. doi: 10.1038/leu.2013.119. [DOI] [PubMed] [Google Scholar]
- 187.Moulard O., Mehta J., Fryzek J., Olivares R., Iqbal U., Mesa R.A. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur. J. Haematol. 2014;92:289–297. doi: 10.1111/ejh.12256. [DOI] [PubMed] [Google Scholar]
- 188.Lai H.Y., Brooks S.A., Craver B.M., Morse S.J., Nguyen T.K., Haghighi N., Garbati M.R., Fleischman A.G. Defective negative regulation of Toll-like receptor signaling leads to excessive TNF-α in myeloproliferative neoplasm. Blood Adv. 2019;3:122–131. doi: 10.1182/bloodadvances.2018026450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Macedo L.C., de Cesare Quintero F., Pagliari-E.-Silva S., Pagnano K.B.B., Rodrigues C., de Alencar J.B., Sell A.M., Visentainer J.E.L. Association of TNF polymorphisms with JAK2 (V617F) myeloproliferative neoplasms in Brazilian patients. Blood Cells Mol. Dis. 2016;57:54–57. doi: 10.1016/j.bcmd.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 190.Bruunsgaard H., Andersen-Ranberg K., Hjelmborg J., Pedersen B.K., Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am. J. Med. 2003;115:278–283. doi: 10.1016/S0002-9343(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 191.Henry C.J., Casás-Selves M., Kim J., Zaberezhnyy V., Aghili L., Daniel A.E., Jimenez L., Azam T., McNamee E.N., Clambey E.T., et al. Aging-associated inflammation promotes selection for adaptive oncogenic events in B cell progenitors. J. Clin. Investig. 2015;125:4666–4680. doi: 10.1172/JCI83024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Roselli M., Ferroni P., Rolfo C., Peeters M., Palmirotta R., Formica V., Ludovici G., Laudisi A., De Marchis M.L., La Farina F., et al. TNF-α gene promoter polymorphisms and risk of venous thromboembolism in gastrointestinal cancer patients undergoing chemotherapy. Ann. Oncol. 2013;24:2571–2575. doi: 10.1093/annonc/mdt251. [DOI] [PubMed] [Google Scholar]
- 193.Kleppe M., Koche R., Zou L., van Galen P., Hill C.E., Dong L., De Groote S., Papalexi E., Somasundara A.V.H., Cordner K. Dual targeting of oncogenic activation and inflammatory signaling increases therapeutic efficacy in myeloproliferative neoplasms. Cancer Cell. 2018;33:29–43.e27. doi: 10.1016/j.ccell.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ts’ao C., Rossi E., Lestina F. Abnormalities in platelet function and morphology in a case of thrombocythemia. Arch. Pathol. Lab. Med. 1977;101:526–533. [PubMed] [Google Scholar]
- 195.Jang J.Y., Blum A., Liu J., Finkel T. The role of mitochondria in aging. J. Clin. Investig. 2018;128:3662–3670. doi: 10.1172/JCI120842. [DOI] [PMC free article] [PubMed] [Google Scholar]