Abstract
An interlaboratory study was conducted through the Vitamin D Standardization Program (VDSP) to assess commutability of Standard Reference Materials® (SRMs) and proficiency testing/external quality assessment (PT/EQA) samples for determination of serum total 25-hydroxyvitamin D [25(OH)D] using ligand binding assays and liquid chromatography – tandem mass spectrometry (LC-MS/MS). A set of 50 single-donor serum samples were assigned target values for 25-hydroxyvitamin D2 [25(OH)D2] and 25-hydroxyvitamin D3 [25(OH)D3] using Reference Measurement Procedures (RMPs). SRM and PT/EQA samples evaluated included SRM 972a (four levels), SRM 2973, six College of American Pathologists (CAP) Accuracy-Based Vitamin D (ABVD) samples, and nine Vitamin D External Quality Assessment Scheme (DEQAS) samples. Results were received from 28 different laboratories using 20 ligand binding assays and 14 LC-MS/MS methods. Using the test assay results for total serum 25(OH)D [i.e., the sum of 25(OH)D2 and 25(OH)D3] determined for the single-donor samples and the RMP target values, the linear regression and 95% prediction intervals (PIs) were calculated. Using a subset of 42 samples that had concentrations of 25(OH)D2 below 30 nmol/L, one or more of the SRM and PT/EQA samples with high concentrations of 25(OH)D2 were deemed non-commutable using 5 of 11 unique ligand binding assays. SRM 972a (Level 4), which has high exogenous concentration of 3-epi-25(OH)D3 was deemed non-commutable for 50% of the LC-MS/MS assays.
Keywords: 25-hydroxyvitamin D3, 25-hydroxyvitamin D2, total 25-hydroxyvitamin D, liquid chromatography – tandem mass spectrometry (LC-MS/MS), ligand binding assay, Vitamin D Standardization Program (VDSP)
Graphical Abstract
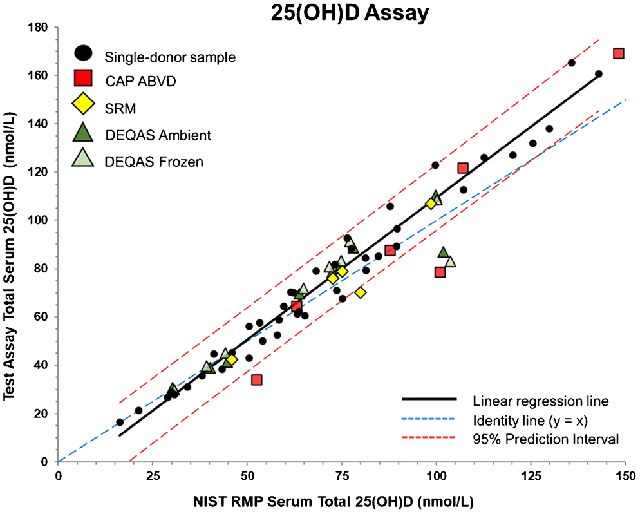
Introduction
Concerns about comparability of measurements among laboratories for the determination of total serum 25-hydroxyvitamin D, [25(OH)D], which is defined as the sum of 25-hydroxyvitamin D2 [25(OH)D2] and 25-hydroxyvitamin D3 [25(OH)D3] excluding the 3-epimer of 25(OH)D3 and is the primary marker of vitamin D status, led to the creation of the Vitamin D Standardization Program (VDSP) in 2010 by the U.S. National Institutes of Health, Office of Dietary Supplements (NIH-ODS) [1]. The VDSP is a collaboration among NIH-ODS, the U.S. National Institute of Standards and Technology (NIST) [2], the U.S. Centers for Disease Control and Prevention (CDC), national survey laboratories in several countries, and vitamin D researchers worldwide [1]. With a goal of standardizing measurements of 25(OH)D among laboratories worldwide to improve clinical practice and public health [1], the VDSP established a reference measurement system that includes reference measurement procedures (RMPs) based on isotope dilution liquid chromatography – tandem mass spectrometry (ID LC-MS/MS) at NIST [3,4], Ghent University [5], and CDC [6]; NIST Standard Reference Materials® (SRMs) [7–9]; the CDC Vitamin D Standardization – Certification Program [10]; and collaborations with two accuracy-based performance testing/external quality assessment (PT/EQA) programs, i.e., the U.S. College of American Pathologists (CAP) accuracy-based vitamin D (ABVD) program [11] and the Vitamin D External Quality Assessment Scheme (DEQAS) [12,13]. The VDSP also implemented performance criteria and guidelines to establish traceability [14] and developed approaches for the prospective and retrospective standardization of serum total 25(OH)D, serum 24R,25-dihydroxyvitamin D3 [24,25(OH)2D3], and vitamin D binding protein [15–19].
In 2011, the VDSP coordinated an interlaboratory study to address two major areas related to measurements of 25(OH)D using ligand binding assays and LC-MS/MS. The first aspect of the study was a comparison of different assays to assess performance regarding variability and bias [20]. A second focus of the study was to assess commutability of SRMs and PT/EQA study materials from CAP ABVD and DEQAS. Establishing the commutability of SRMs and test materials used in accuracy-based PT/EQA programs is an essential task in establishing a reference measurement system for 25(OH)D. Commutability is defined as the equivalence of mathematical relationships between results obtained using different measurement procedures for a reference material and for representative patient samples [21–23]. SRMs and PT/EQA materials typically use pooled serum samples and commutability of these materials cannot be assumed but must be demonstrated through an appropriately designed study. The results of this first commutability study, denoted as VDSP Commutability Study 1, were reported by Phinney et al. [24] and demonstrated that, with few exceptions, SRM 972a Vitamin D Metabolites in Frozen Human Serum (four levels), CAP ABVD, and DEQAS materials were commutable with multiple assays. Unfortunately, the conclusions of Commutability Study 1 were limited in that all participants did not agree to identification of their laboratories or assays used.
A second set of VDSP intercomparison/commutability studies, designated as Intercomparsion Study 2 and Commutability Study 2, was undertaken in late 2016 with all participants agreeing to identification of laboratories and assays used. For Intercomparison Study 2 and Commutability Study 2, 50 single-donor serum samples were obtained and characterized for 25(OH)D2, 25(OH)D3, and 24R,25-dihydroxyvitamin D3 [24,25(OH)2D3] using RMPs [3,4], and for 3-epi-25(OH)D3 using an isotope dilution (ID) LC-MS/MS method similar to the RMPs [3]. To identify specific challenges for the determination of serum total 25(OH)D and to expand the boundaries for commutability assessment, 8 of the 50 single-donor samples had high concentrations of 25(OH)D2 (>30 nmol/L).
For Commutability Study 2, SRM 972a (four levels) [8] was evaluated again as well as a more recent SRM with a higher level of 25(OH)D3, SRM 2973 Vitamin D Metabolites in Frozen Human Serum (High Level) [9], and six CAP ABVD samples [11] and nine DEQAS samples [12]. For the DEQAS samples, two sets of the same nine samples were shipped frozen and at ambient temperature to investigate the effect of shipping temperature on the commutability assessment. For Commutability Study 2, results were received from 28 different institutions/laboratories using 20 ligand binding assays methods and 14 LC-MS/MS methods. In this paper, we report the results of VDSP Commutability Study 2. The results of Intercomparison Study 2 are reported by Wise et al. for the LC-MS/MS assays [25] and for the ligand binding assays [26].
Methods
Measurands
The measurand for Commutability Study 2 was serum total 25(OH)D in concentration units of nanomoles per liter (nmol/L). Serum total 25(OH)D is defined as the sum of the concentrations of 25(OH)D2 and 25(OH)D3, without the inclusion of the concentration of 3-epi-25(OH)D3. The concentrations of two additional metabolites, 3-epi-25(OH)D3 and 24R,25(OH)2D3, were also determined in the 50 single-donor and SRM/PT/EQA samples.
Commutability Study 2 - Coordination and Responsibilities
Commutability Study 2 was co-designed and coordinated by NIST and NIH-ODS through the VDSP, including acquisition and distribution of 50 single-donor serum samples, recruitment of participating laboratories, and compilation of the results [27]. Samples were distributed to the participating laboratories in November 2016 and results were received in January/February 2017. NIST was responsible for analysis of the 50 single-donor serum samples and the SRM, ABVD, and DEQAS samples to assign target values for 25(OH)D2, 25(OH)D3, 3-epi-25(OH)D3, and 24R,25(OH)2D3. NIH-ODS and VDSP LLC were responsible for conducting the data analyses.
Single-Donor Serum Samples
The 50 single-donor serum samples used in Commutability Study 2 were procured from Solomon Park Research Laboratories (Seattle, WA). Single-donor serum samples from 50 healthy human donors (i.e., no known disease states, pregnant, renal failure patients) were prepared according to the Clinical Laboratory Standards Institute (CLSI) C37-A guidelines [28,29] and contained only endogenous vitamin D metabolites with a distribution of total 25(OH)D concentrations across the clinically-relevant range of 15 nmol/L to 150 nmol/L. All single-donor samples were anonymous to the study. A number of serum samples were requested with high levels of 25(OH)D2. The details of the preparation of and distribution of 25(OH)D concentrations for these 50 human single-donor samples are described elsewhere [25].
SRMs and PT/EQA Samples
The samples evaluated in Commutability Study 2 included: SRM 972a (Four Levels), SRM 2973, six CAP ABVD samples, and nine DEQAS samples. With the exception of SRM 972a L4 (fortified with 3-epi-25(OH)D3), all SRM/PT/EQA samples were unprocessed serum pools containing only endogenous 25(OH)D2, 25(OH)D3, and 3-epi-25(OH)D3. The DEQAS samples were from serum pools previously distributed as part of DEQAS quarterly exercises. DEQAS samples 1 through 9 were the following samples from DEQAS exercises conducted in April 2015 and July 2015: (1) 472, (2) 473, (3) 474, (4), 475 (5) 479, (6), 476, (7) 478, (8) 477, (9) 480. In DEQAS, the quarterly exercise samples are shipped to the participants at ambient temperature [12]. To assess the commutability of samples shipped differently, two sets of the same nine samples were shipped to the study participants; one set was shipped at ambient temperature (i.e., no temperature control) and the second set was shipped frozen on dry ice. Therefore, participating laboratories were not blinded as to the identity of the DEQAS samples that were shipped at ambient temperature since they arrived separately from the other samples shipped frozen. The 29 SRM and PT/EQA samples were assigned target values for serum total 25(OH)D using the NIST RMPs [3,4] as described below.
Commutability Study 2 Design
Participating laboratories agreed prior to the study that their results, including the identification of laboratory and assay, would be included in publications reporting the results. VDSP Commutability Study 2 was designed and conducted according to CLSI EP14-A3 and EP30-A guidelines prior to the publication of new recommendations for commutability assessment by the IFCC Working Group on Commutability [30–32]. Each participant received the 50 single-donor serum sample set (one cryovial containing 0.50 mL of serum per single-donor sample) and one vial each of 6 CAP ABVD samples (each containing approximately 1.0 mL serum), 5 NIST SRM samples (each containing approximately 1.1 mL of serum), and 18 DEQAS samples (each containing 0.6 mL of serum). Prior to distribution, all CAP ABVD, DEQAS, and SRM samples were stored at < −40 °C. All samples (except 9 DEQAS samples) were shipped to the participants frozen on dry ice and participants were instructed to store all samples frozen at −60 °C or lower (including the DEQAS samples shipped at ambient temperature) until the time of analysis.
Participants were to analyze the 50 single-donor samples (DS) and the 29 SRM and PT/EQA samples in duplicate on the same day. Duplicate measurements (i.e., sample preparations) were to be made from one vial. For laboratories that needed a greater volume of serum than provided in the single-donor sample vial (i.e., 0.5 mL), an additional vial was provided to facilitate the duplicate measurements. The second vial was to be pooled with the first vial prior to analysis. The protocol specified a run order of analyzing the samples first in ascending (DS01-DS50) and then descending order (DS50-DS01) with the SRMs and PT/EQA materials interspersed among the donor samples. Participants were requested to use their routine laboratory operation procedures with normal internal QC criteria.
Participants were requested to provide results using a reporting template provided by NIST. Results for 25(OH)D2, 25(OH)D3, 3-epi-25(OH)D3, and total 25(OH)D were requested in units of nmol/L with three significant figures. The following information was also requested: (1) instrument description and measurement technology, (2) assay performance characteristics such as limit of detection and measurement range, and (3) lot numbers of reagent(s), calibrators, and controls used in the analysis.
NIST Value Assignment of 50 Single-Donor Serum Samples and SRMs and PT/EQA Samples
Mass fractions (ng/g) of 25(OH)D2, 25(OH)D3, and 24R,25(OH)2D3 were determined in each of the 50 single-donor serum samples using the ID LC-MS/MS-based RMPs as described by Tai et al. [3,4]. Although not designated as an RMP, a similar ID LC-MS/MS method was used for determination of the 3-epi-25(OH)D3. The 50 single-donor serum samples and the DEQAS, CAP ABVD, and SRM samples were distributed among 15 sample sets (a total of 79 samples in duplicate). SRM samples also served as control materials for the analyses of the single-donor samples. Each of the 15 sample sets was analyzed separately as described in detail elsewhere [25]. The results for 25(OH)D2, 25(OH)D3, 3-epi-25(OH)D3 and 24R,25(OH)2D3 in the 50 single-donor samples are reported elsewhere [25] and the results for the SRM and PT/EQA samples are summarized in Table 1. The results for 25(OH)D2, 25(OH)D3, 3-epi-25(OH)D3, and 24R,25(OH)2D3 were determined as mass fraction (ng/g), converted to mass concentration (ng/mL) using a universal serum sample density value of 1.02 g/mL, and converted to molar concentration (nmol/L) using the appropriate molecular mass ratios of 25(OH)D2 (2.42), 25(OH)D3 (2.50), 3-epi-25(OH)D3 (2.50), and 24R,25(OH)2D3 (2.40). The results for the CAP ABVD and DEQAS samples as determined in ng/g and converted to nmol/L are reported in Tables S1 through S4 (see Supplementary Information, ESM) for 25(OH)D2, 25(OH)D3, 3-epi-25(OH)D3 and 24R,25(OH)2D3, respectively. The analyses of SRM 972a (L1-L4) and SRM 2973 served as controls as well as providing target values for 25(OH)D2, 25(OH)D3, 3-epi-25(OH)D3, and 24R,25(OH)2D3, and the results are summarized in Tables S5 through S8, respectively (see ESM). All results for 25(OH)D2, 25(OH)D3, 3-epi-25(OH)D3, and 24R,25(OH)2D3 determined in the SRMs as controls were within the uncertainties of the certified and reference values as demonstrated in ESM, Tables S5 through S8.
Table 1.
Summary of NIST Determination of 25(OH)D2, 25(OH)D3, Total 25(OH)D, 3-epi-25(OH)D3, and 24R,25(OH)2D3 in SRM, CAP ABVD, and DEQAS Samples
| SRM and PT/EQA Samples | 25(OH)D2 (nmol/L | 25(OH)D3 (nmol/L) | Total 25(OH)D (nmol/L) | 3-epi-25(OH)D3 (nmol/L) | 24R,25(OH)2D3 (nmol/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SDa | %CV | Mean | SDa | %CV | Mean | SDa | %CV | Mean | SDa | %CV | Mean | SDa | %CV | |
| SRM 972a L1 | 1.15 | 0.09 | 7.8 | 71.5 | 2.3 | 3.2 | 72.7 | 2.4 | 3.3 | 4.53 | 0.05 | 1.1 | 6.24 | 0.06 | 1.0 |
| SRM 972a L2 | 1.87 | 0.04 | 2.1 | 44.1 | 0.4 | 0.9 | 46.0 | 0.4 | 0.9 | 3.21 | 0.09 | 2.8 | 3.28 | 0.06 | 1.8 |
| SRM 972a L3 | 31.69 | 0.83 | 2.6 | 48.2 | 0.7 | 1.5 | 79.9 | 0.7 | 0.9 | 2.99 | 0.04 | 1.3 | 3.81 | 0.09 | 2.4 |
| SRM 972a L4 | 1.15 | 0.11 | 9.6 | 74.0 | 1.0 | 1.4 | 75.2 | 1.0 | 1.3 | 64.3 | 1.8 | 2.8 | 6.17 | 0.05 | 0.8 |
| SRM 2973 | 1.59 | 0.03 | 1.9 | 96.9 | 1.4 | 1.4 | 98.5 | 1.4 | 1.4 | 5.23 | 0.06 | 1.1 | 7.44 | 0.10 | 1.3 |
| CAP ABVD-1 | 56.3 | 0.8 | 1.4 | 44.53 | 0.26 | 0.6 | 100.9 | 0.7 | 0.7 | 4.09 | 0.16 | 3.9 | 3.83 | 0.08 | 2.1 |
| CAP ABVD-2 | 1.99 | 0.04 | 2.0 | 85.76 | 0.64 | 0.7 | 87.8 | 0.7 | 0.8 | 4.57 | 0.04 | 0.9 | 7.00 | 0.06 | 0.9 |
| CAP ABVD-3 | 1.60 | 0.40 | 25 | 61.35 | 0.80 | 1.3 | 63.0 | 0.4 | 0.6 | 3.27 | 0.03 | 0.9 | 3.81 | 0.08 | 2.1 |
| CAP ABVD-4 | 23.8 | 0.3 | 1.3 | 28.68 | 0.21 | 0.7 | 52.5 | 0.2 | 0.4 | 1.96 | 0.05 | 2.6 | 1.81 | 0.03 | 1.7 |
| CAP ABVD-6 | 3.94 | 0.05 | 1.3 | 103.1 | 0.95 | 0.9 | 107.0 | 0.9 | 0.8 | 7.54 | 0.29 | 4.0 | 8.33 | 0.13 | 1.6 |
| CAP ABVD-7 | 0.85 | 0.02 | 2.4 | 147.3 | 0.90 | 0.6 | 148.1 | 0.9 | 0.6 | 12.8 | 0.2 | 1.6 | 13.46 | 0.16 | 1.2 |
| DEQAS-1Fb | 1.92 | 0.20 | 10.4 | 38.18 | 0.28 | 0.7 | 40.1 | 0.3 | 0.7 | 1.78 | 0.09 | 5.1 | 1.82 | 0.01 | 0.5 |
| DEQAS-1Ab | 1.88 | 0.10 | 5.3 | 37.35 | 0.90 | 2.4 | 39.2 | 1.0 | 2.6 | 1.85 | 0.06 | 3.2 | 1.79 | 0.03 | 1.7 |
| DEQAS-2F | 2.89 | 0.02 | 0.7 | 60.91 | 0.27 | 0.4 | 63.8 | 0.3 | 0.5 | 4.00 | 0.08 | 2.0 | 6.11 | 0.02 | 0.3 |
| DEQAS-2A | 2.97 | 0.05 | 1.7 | 61.90 | 0.22 | 0.4 | 64.9 | 0.2 | 0.3 | 3.94 | 0.02 | 0.5 | 6.12 | 0.05 | 0.9 |
| DEQAS-3F | 1.46 | 0.06 | 4.1 | 76.59 | 0.66 | 0.9 | 78.0 | 0.7 | 0.9 | 5.72 | 0.03 | 0.5 | 6.72 | 0.08 | 1.2 |
| DEQAS-3A | 1.39 | 0.05 | 3.6 | 75.77 | 1.08 | 1.4 | 77.2 | 1.1 | 1.4 | 5.70 | 0.08 | 1.4 | 6.73 | 0.06 | 0.9 |
| DEQAS-4F | 1.29 | 0.04 | 3.1 | 73.00 | 0.63 | 0.9 | 74.3 | 0.7 | 0.9 | 5.30 | 0.10 | 1.9 | 5.94 | 0.07 | 1.2 |
| DEQAS-4A | 1.21 | 0.04 | 3.3 | 73.62 | 0.84 | 1.1 | 74.8 | 0.8 | 1.1 | 5.34 | 0.04 | 0.7 | 6.02 | 0.05 | 0.8 |
| DEQAS-5F | 1.33 | 0.03 | 2.3 | 28.83 | 0.86 | 3.0 | 30.2 | 0.8 | 2.6 | 1.41 | 0.04 | 2.8 | 1.09 | 0.02 | 1.8 |
| DEQAS-5A | 1.25 | 0.11 | 8.8 | 29.50 | 0.28 | 0.9 | 30.8 | 0.4 | 1.3 | 1.68 | 0.08 | 4.8 | 1.07 | 0.01 | 0.9 |
| DEQAS-6F | 0.77 | 0.03 | 3.9 | 99.04 | 0.47 | 0.5 | 99.8 | 0.5 | 0.5 | 7.30 | 0.10 | 1.4 | 9.47 | 0.06 | 0.6 |
| DEQAS-6A | 0.76 | 0.06 | 7.9 | 99.34 | 1.24 | 1.2 | 100.1 | 1.2 | 1.2 | 7.49 | 0.12 | 1.6 | 9.44 | 0.06 | 0.6 |
| DEQAS-7F | 1.20 | 0.01 | 0.8 | 71.28 | 1.10 | 1.5 | 72.5 | 1.1 | 1.5 | 3.03 | 0.03 | 1.0 | 5.94 | 0.04 | 0.7 |
| DEQAS-7A | 1.13 | 0.06 | 5.3 | 70.61 | 0.32 | 0.5 | 71.7 | 0.3 | 0.4 | 3.13 | 0.04 | 1.3 | 5.97 | 0.05c | 0.8 |
| DEQAS-8F | 1.94 | 0.05 | 2.6 | 42.67 | 0.39 | 0.9 | 44.6 | 0.4 | 0.9 | 2.32 | 0.04 | 1.7 | 2.37 | 0.03 | 1.3 |
| DEQAS-8A | 1.84 | 0.05 | 2.7 | 42.41 | 0.45 | 1.1 | 44.2 | 0.4 | 0.9 | 2.27 | 0.06 | 2.6 | 2.33 | 0.02 | 0.9 |
| DEQAS-9F | 57.0 | 0.6 | 1.1 | 44.76 | 0.75 | 1.7 | 101.8 | 1.2 | 1.2 | 4.66 | 0.05 | 1.1 | 4.46 | 0.07 | 1.6 |
| DEQAS-9A | 57.1 | 0.8 | 1.4 | 46.57 | 1.38 | 3.0 | 103.6 | 1.0 | 1.0 | 4.65 | 0.05 | 1.1 | 4.53 | 0.09 | 2.0 |
For SRM 972a and SRM 2973, n = 12 (3 samples × 2 sample preparations × 2 LC-MS/MS injections); for CAP ABVD and DEQAS samples, n = 4 (1 sample × 2 sample preparations × 2 LC-MS/MS injections).
DEQAS Samples denoted as “F” were shipped frozen and “A” were shipped at ambient conditions.
For DEQAS-7A, n = 6, (1 sample × 3 sample preparations × 2 LC-MS/MS injections).
Participants and Assays Used in This Study
Forty sets of the 50 single-donor samples were distributed to 34 laboratories. Results were received from 28 laboratories for 34 assays including 20 ligand binding assays (12 unique assays) and 14 LC-MS/MS assays. The results of the analysis of the 50 single-donor samples and the SRMs and PT/EQA samples reported by all laboratories are provided as Excel file in ESM identified as Data VDSP Commutability Study 2. The laboratories, assay manufacturers, and assay name/model used in this study are listed in Table 2. Several laboratories used the same assay resulting in two or more data sets including (number in parentheses is number of labs using this assay): Abbott (4), bioMérieux (2), DiaSorin (2), Roche (2), Siemens (2), and SNIBE (2). The ligand binding assays evaluated in this study included the most frequently represented assays in recent DEQAS exercises [12], i.e., DiaSorin, Roche, Siemens, IDS, and Abbott. To avoid repetition of the assay name/model, the assay will be referred to by the manufacturer’s name.
Table 2.
Participants and Assays Used in VDSP Commutability Study 2 (Grouped by Assay Alphabetically)
| Lab No. | Participant | Assay Manufacturer | Assay Model | Assay Type |
|---|---|---|---|---|
| 1 | Abbott Diagnostics, DE | Abbott | Architect 25-OH Vitamin D; Architect i2000SR | CLIA/CMIA |
| 18 | Golwilkar Metropolis Health Services Pvt. Ltd., IN | Abbott | Architect 25-OH Vitamin D; Architect i2000 SR | CLIA/CMIA |
| 23 | Imperial College Healthcare, UK | Abbott | Architect 25-OH Vitamin D; Architect i2000 SR | CLIA/CMIA |
| 27 | National University of Medical Sciences, PK | Abbott | Architect 25-OH Vitamin D; Architect i2000 SR | CLIA/CMIA |
| 26 | National Institute of Public Health, NL | Beckman Coul. | Access 25(OH) Vitamin D Total; Access-2 | CLIA |
| 3 | bioMérieux, FR | bioMérieux | VIDAS 25 OH Vitamin D Total; Vidas Legacy | ELFA |
| 34 | University of Chester, UK | bioMérieux | VIDAS 25 OH Vitamin D Total; Mini-Vidas | ELFA |
| 4 | Bio-Rad Laboratories, Inc., US | Bio-Rad | BioPlex 2200 25(OH) Vitamin D; BioPlex 2200 | FCIA |
| 9 | CHU de Liège, University of Liège, BE | DiaSorin | Liaison 25 OH Vitamin D Total; Liaison XL | ECLIA |
| 24 | Imperial College Healthcare, UK | DiaSorin | Liaison 25 OH Vitamin D Total; Liaison XL | ECLIA |
| 2 | Awareness Technology, US | DIAsource | 25OH Vitamin D Total ELISA; ChemWell 2910 | ELISA |
| 21 | Immunodiagnostic Systems (IDS), UK | IDS | 25-Hydroxy Vitamin DS EIA; IDS | EIA |
| 20 | Immunodiagnostic Systems (IDS), UK | IDS | IDS-iSYS 25VitDS (IDS-iSYS-2)a | CLIA |
| 39 | Yale University, USA | IDS | IDS-iSYS 25-Hydroxy Vitamin DS (IDS-iSYS-1)a | CLIA |
| 19 | Hospital Israelita Albert Einstein, BR | Roche | Vitamin D Total II; Modular Analytics E-170 | ECLIA |
| 29 | St. Vincent’s University Hospital, IE | Roche | Vitamin D Total II; Cobas e602 | ECLIA |
| 30 | Siemens-Healthineers, US | Siemens | Vitamin D Total (VitD); ADVIA Centaur XP | CLIA |
| 40 | CHU de Liège, University of Liège, BE | Siemens | Vitamin D Total (VitD); ADVIA Centaur XPT | CLIA |
| 31 | SNIBE, CN | SNIBEb,c | Prototype MAGLUMI 25-OH Vitamin D; MAGLUMI 2000 | CLIA |
| 5 | Care S.r.l., IT | SNIBEb,c | Prototype MAGLUMI 25-OH Vitamin D; MAGLUMI 2000 | CLIA |
| Lab No. | Participant | Assay | Mass Spectrometer (LC column) | Assay Type |
| 6 | Manchester Royal Infirmary (MRI), UK | Chromsystems | AB Sciex 5500 (Chromsystems column) | LC-MS/MS |
| 7 | Chromsystems Instruments & Chemicals, DE | Chromsystems | AB Sciex API4000 (Chromsystems column) | LC-MS/MS |
| 11 | Canisius Wilhelmina Hospital, NL | LC-MS/MS | Waters Quattro Premier XE (HSS PFP 2.1 × 100 mm, 1.8 μm) | LC-MS/MS |
| 12 | Prince of Wales Hospital, HK | LC-MS/MS | Waters Xevo TQ-S micro (CSH Fluoro-Phenyl, 2.1 × 100 mm, 1.7 μm) | LC-MS/MS |
| 16 | Endoceutics Inc., CA | LC-MS/MS | AB Sciex (C18-PFP 100 × 3 mm, 2 μm) | LC-MS/MS |
| 17 | Endocrine Sciences (LabCorp), US | LC-MS/MS | AB Sciex API5000 (Proprietary) | LC-MS/MS |
| 22 | Imperial College Healthcare, UK | LC-MS/MS | Waters Acquity TQD (BEH Phenyl 2.1 × 50 mm, 1.8 μm) | LC-MS/MS |
| 25–1 | Medical Research Council (MRC) Elsie Widdowson Laboratoryd, Cambridge, UK | LC-MS/MS | AB Sciex 4000, Waters Acquity UPLC (Hypersil PFP 2.1 × 100 mm, 1.9 μm) | LC-MS/MS |
| 25–2 | Medical Research Council (MRC) Elsie Widdowson Laboratoryd, Cambridge, UK | LC-MS/MS | AB Sciex 5500, Waters Acquity UPLC (Hypersil PFP 2.1 × 100 mm, 1.9 μm) | LC-MS/MS |
| 28 | Penn State University, College of Medicine, US | LC-MS/MS | Agilent 1260 HPLC and 6460 QQQ (Poroshell 120 EC-18, (2.1 × 50 mm, 2.7 μm) | LC-MS/MS |
| 33 | University of California at San Diego, US | LC-MS/MS | Waters XevoTSQ (HSS T3 2.1 × 75 mm, 2.5 μm) | LC-MS/MS |
| 36 | University of Washington, US | LC-MS/MS | Waters Quattro Micro, Acquity UPLC, (PFP, 3.2 × 100 mm, 2.5 μm) | LC-MS/MS |
| 37 | University of Western Australia, AU | LC-MS/MS | Agilent 6460 QQQ (PFP × 2) | LC/MS/MS |
| 38 | Waters Technologies Ireland Ltd., IE | LC-MS/MS | Waters Xevo TQD Acquity UPLC (BEH Phenyl, 2.1 × 50 mm, 1.7 μm) | LC-MS/MS |
CLIA = Chemiluminescence Immunoassay
CMIA = Chemiluminescence Microparticle Immunoassay
ELFA = Enzyme-Linked Fluorescence Assay
ECLIA = Electrochemiluminescence immunoassay
ELISA = Enzyme-Linked Immunosorbent Assay
EIA = Electrochemical Immunoassay
FCIA = Flow Competitive Immunoassay
ITA = Immunoturbidimetric assay
Two different IDS-iSYS kits were used in the study, 25-Hydroxy Vitamin DS and 25VitDS, which are designated as IDS-iSYS-1 and IDS-iSYS-2, respectively. The 25VitDS (IDS-iSYS-2) is the currently available kit.
SNIBE = Shenzhen New Industries Biomedical Engineering Co., Ltd.
The MAGLUMI 25-OH Vitamin D kit used in this study was a prototype kit, which is not equivalent to current MAGLUMI 25-OH Vitamin D kit per personal communication from SNIBE.
Medical Research Council (MRC) Elsie Widdowson Laboratory closed December 2018; Researchers are now associated with NIHR BRC Nutritional Biomarker Laboratory, MRC Epidemiology Unit, University of Cambridge, Cambridge, UK.
Data Analysis
Statistical assessment of the results was based on CLSI EP09c-ED3, EP14-A3, and EP30-A guidelines [33–35]. Using only the first of the two replicates for the determination of 25(OH)D in each of the single-donor samples and the NIST assigned target values for total serum 25(OH)D, Ordinary Deming regression analysis and 95% prediction intervals (PIs) were calculated for each assay as recommended by CLSI EP14-A3 [34]. All calculations for the Deming regression with 95% PIs were performed using Analyse-it (Analyse-it Software, Leeds, UK), a statistical analysis add-in for Microsoft Excel.
Commutability was assessed using the Methods Comparison tab within Analyse-it using the Ordinary Deming Regression. The Analyse-it Methods Comparison function is based on the CSLI EP14-A3 and EP30-A guidelines and provides a regression line with 95% PIs based on the patient samples and places the SRMs and PT/EQA samples on the plot and determines whether they are located within the 95% PI. The Deming regression requires input of the ratio of the variances of the NIST RMP versus the test assay (X/Y = λ). For consistency in the assessment of all assays, we used λ = 0.1, which was the mean of all the individual λ values (N = 34) for each of the ligand binding assays (λ = 0.07, N =20) and LC-MS/MS assays (λ = 0.15, N = 14). However, changes in the values for λ between 0.01 and 1 had little impact on the regression analysis plots and ultimately the results of the commutability assessment. The standard deviation of the measurements (two replicates) from each method was calculated and the mean SD for the methods ranged from 1.2 nmol/L to 8.2 nmol/L (mean 4.4 nmol/L) for the ligand binding assays and from 1.6 nmol/L to 4.2 nmol/L (mean 2.8 nmol/L) for the LC-MS/MS assays. The mean variance (SD2) was calculated for the ligand binding assays (17.6 nmol/L) and for the LC-MS/MS (8.5 nmol/L). The variance of the NIST RMP was determined to be 1.2. Use of the Analyse-it Methods Comparison function for commutability assessment eliminated potential bias associated with visual examination of the plots to determine commutability.
Results and Discussion
NIST Assignment of Target Values for Single-Donor, SRM, and PT/EQA Samples
The 50 single-donor serum samples and the SRMs and PT/EQA samples were assigned target values using RMPs for 25(OH)D2 [3], 25(OH)D3 [3], and 24R,25(OH)2D3 [4], and an ID LC-MS/MS method for the determination of 3-epi-25(OH)D3. The results for the 50 single-donor serum samples are reported in Intercomparison Study 2 Part 1 [25]. The results for the determination of the same vitamin D metabolites in the SRM, CAP ABVD, and DEQAS samples are summarized in Table 1. The distribution of 25(OH)D2, 25(OH)D3, 3-epi-25(OH)D3, and 24R,25(OH)2D3 concentrations in the SRM, CAP ABVD, and DEQAS samples is shown graphically, arranged as increasing concentration of 25(OH)D, in Figure S1 (see ESM). The relationship between the concentrations of 3-epi-25(OH)D3 and 24R,25(OH)2D3 with the concentration of 25(OH)D3 in the SRM/PT/EQA samples is illustrated in Figure S2 (see ESM).
The 50 single-donor samples had serum total 25(OH)D concentrations across a clinically-relevant range of 16 nmol/L to 148 nmol/L, with 25(OH)D3 ranging from 10 nmol/L to 141 nmol/L. For the 50 single-donor samples, concentrations for 25(OH)D2 ranged from 0.3 nmol/L to 7.6 nmol/L (only 3 samples between 3 nmol/Land 7.6 nmol/L) with the exception of 8 samples with 25(OH)D2 concentrations of > 30 nmol/L ranging from 32 nmol/L to 137 nmol/L (with 4 samples ≥ 99 nmol/L), which were obtained from donors who were taking ergocalciferol supplements. Although levels of 25(OH)D2 >30 nmol/L are rare in the healthy U.S. population [17,36], these extremely high concentration 25(OH)D2 single-donor samples provided an opportunity to better assess assay response to both 25(OH)D2 and 25(OH)D3. Because these higher concentration 25(OH)D2 samples were problematic for 9 of the 12 different ligand binding assays evaluated, as described below, the assays were evaluated using both the 50-sample set and the 42-sample subset (excluding samples with concentrations of 25(OH)D2 >30 nmol/L).
Regression Analysis
The regression models (i.e., slope, intercept, and R2) are summarized in Table 3 for all assays for both the 50- and 42-sample sets. Of particular interest for comparison of the 50- and 42-samples sets are the y-intercepts for the 95% PIs (Min and Max), which were used to determine the width of the PI. These regression models and PIs based on only the first replicate measurement were used as the basis for the commutability assessment; however, similar regression models using the mean of the two replicates were used to assess assay performance in a broader scope as described elsewhere [25,26].
Table 3.
Ordinary Deming Regression Analysis for Ligand Binding Assays and LC-MS/MS Assays using Replicate 1 for Commutability Assessment
| Lab No. | Assay | 50 Sample set | 42 Sample Set | Difference 50 – 42 Sample Setsc | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regression Linea | 95% PIb (nmol/L) | Regression Linea | 95% PIb (nmol/L) | |||||||||||||
| Slope | Int. | R2 | Min | Max | Width | Slope | Int. | R2 | Min | Max | Width | Slope | Width | R2 | ||
| 1 | Abbott | 0.880 | 7.26 | 0.707 | −33.5 | 48.0 | 81.5 | 1.192 | −8.24 | 0.970 | −21.5 | 5.02 | 26.5 | −0.312 | 55.0 | −0.263 |
| 18 | Abbott | 0.881 | 6.88 | 0.710 | −33.6 | 47.4 | 81.0 | 1.184 | −8.11 | 0.966 | −22.1 | 5.8 | 27.9 | −0.303 | 53.1 | −0.256 |
| 23 | Abbott | 0.874 | 6.85 | 0.703 | −32.1 | 45.8 | 77.9 | 1.177 | −8.33 | 0.970 | −20.3 | 3.6 | 23.9 | −0.303 | 54.0 | −0.267 |
| 27 | Abbott | 0.858 | 8.65 | 0.702 | −31.7 | 49.0 | 80.7 | 1.160 | −6.24 | 0.972 | −18.6 | 6.1 | 24.7 | −0.302 | 56.0 | −0.27 |
| 26 | Beckman | 1.196 | −10.3 | 0.911 | −37.0 | 16.4 | 53.4 | 1.170 | −8.70 | 0.871 | −39.0 | 21.6 | 60.6 | 0.026 | −7.2 | 0.04 |
| 3 | BioMérieux | 1.017 | 0.67 | 0.653 | −50.6 | 51.0 | 101.6 | 1.336 | −15.7 | 0.867 | −48.1 | 16.7 | 64.8 | −0.319 | 36.8 | −0.214 |
| 34 | BioMérieux | 1.059 | −1.21 | 0.605 | −58.4 | 56.0 | 114.4 | 1.431 | −21.0 | 0.851 | −56.8 | 14.8 | 71.6 | −0.372 | 42.8 | −0.246 |
| 4 | Bio-Rad | 0.952 | 0.59 | 0.846 | −29.0 | 30.2 | 59.2 | 1.054 | −4.56 | 0.852 | −34.7 | 25.6 | 60.3 | −0.102 | −1.1 | −0.006 |
| 9 | DiaSorin | 0.846 | 3.70 | 0.811 | −25.7 | 33.1 | 58.8 | 1.021 | −5.13 | 0.908 | −26.3 | 16.0 | 42.3 | −0.175 | 16.5 | −0.097 |
| 24 | DiaSorin | 0.945 | 7.62 | 0.770 | −29.7 | 44.9 | 74.6 | 1.179 | −4.04 | 0.917 | −26.9 | 18.8 | 45.7 | −0.234 | 28.9 | −0.147 |
| 2 | DIAsource | 1.482 | −4.07 | 0.443 | −100.1 | 92.0 | 192.1 | 1.971 | −4.78 | 0.531 | −1.2 | 63.9 | 65.1 | −0.489 | 127.0 | −0.088 |
| 20 | IDS-iSYS-2 | 1.236 | −11.6 | 0.901 | −40.5 | 17.3 | 57.8 | 1.042 | −1.69 | 0.950 | −17.1 | 13.8 | 30.9 | 0.194 | 26.9 | −0.049 |
| 39 | IDS-iSYS-1 | 0.837 | 13.0 | 0.837 | −15.8 | 41.8 | 57.6 | 1.039 | 6.52 | 0.867 | −19.7 | 32.8 | 52.5 | −0.202 | 5.1 | −0.03 |
| 21 | IDS-EIA | 0.952 | 14.0 | 0.740 | −26.1 | 54.1 | 80.2 | 1.175 | 2.49 | 0.861 | −26.7 | 31.7 | 58.4 | −0.223 | 21.8 | −0.121 |
| 19 | Roche | 1.181 | −11.6 | 0.707 | −64.9 | 41.7 | 106.6 | 1.475 | −26.5 | 0.824 | −70.7 | 17.7 | 88.4 | −0.294 | 6 | −0.117 |
| 29 | Roche | 1.102 | −5.53 | 0.720 | −54.2 | 43.2 | 97.4 | 1.378 | −19.5 | 0.853 | −57.0 | 17.9 | 74.9 | −0.276 | 22.5 | −0.133 |
| 30 | Siemens | 1.207 | −9.23 | 0.872 | −42.0 | 23.6 | 65.6 | 1.024 | 1.28 | 0.848 | −27.7 | 30.2 | 57.9 | 0.183 | 7.7 | 0.024 |
| 40 | Siemens | 1.161 | −10.6 | 0.871 | −42.5 | 21.4 | 63.9 | 1.009 | −1.76 | 0.846 | −31.1 | 27.6 | 58.7 | 0.152 | 5.2 | 0.025 |
| 5 | SNIBE | 0.848 | 25.5 | 0.618 | −22.7 | 73.8 | 96.5 | 1.129 | 11.4 | 0.808 | −24.6 | 47.4 | 72.0 | −0.281 | 24.5 | −0.19 |
| 31 | SNIBE | 1.129 | −0.76 | 0.608 | −63.3 | 61.8 | 125.1 | 1.509 | −19.8 | 0.834 | −61.9 | 22.3 | 84.2 | −0.38 | 40.9 | −0.226 |
| 6 | LC-MS/MS | 1.109 | 7.83 | 0.918 | −15.1 | 30.8 | 45.9 | 1.082 | 9.42 | 0.906 | −14.2 | 33.1 | 47.3 | 0.027 | −1.4 | 0.012 |
| 7 | LC-MS/MS | 1.138 | −0.69 | 0.984 | −10.7 | 9.6 | 20.3 | 1.119 | 0.20 | 0.978 | −10.1 | 10.5 | 20.6 | 0.019 | −0.3 | 0.006 |
| 11 | LC-MS/MS | 1.030 | −0.52 | 0.981 | −10.7 | 10.5 | 21.2 | 1.036 | −0.52 | 0.979 | −10.5 | 9.5 | 20.0 | −0.006 | 1.2 | 0.002 |
| 12 | LC-MS/MS | 1.061 | 0.59 | 0.978 | −10.3 | 11.5 | 21.8 | 1.025 | 2.15 | 0.980 | −7.1 | 11.4 | 18.5 | 0.036 | 3.3 | −0.002 |
| 16 | LC-MS/MS | 1.091 | −4.09 | 0.980 | −15.2 | 7.0 | 22.2 | 1.029 | −1.34 | 0.990 | −7.7 | 5.0 | 12.7 | 0.062 | 9.5 | −0.01 |
| 17 | LC-MS/MS | 0.997 | −2.36 | 0.981 | −12.3 | 7.6 | 19.9 | 0.965 | −0.73 | 0.975 | −11.0 | 9.5 | 20.5 | 0.032 | −0.06 | 0.006 |
| 22 | LC-MS/MS | 1.192 | −2.08 | 0.983 | −13.4 | 9.2 | 21.6 | 1.163 | −0.91 | 0.986 | −9.8 | 8.0 | 17.0 | 0.029 | 4.6 | −0.003 |
| 25–1 | LC-MS/MS | 1.033 | 0.42 | 0.980 | −10.2 | 11.1 | 21.3 | 1.000 | 2.33 | 0.978 | −7.2 | 11.9 | 19.1 | 0.033 | 2.2 | 0.002 |
| 25–2 | LC-MS/MS | 0.968 | 3.59 | 0.967 | −9.2 | 16.4 | 25.6 | 0.979 | 2.19 | 0.990 | −4.3 | 8.7 | 13.0 | −0.011 | 12.6 | −0.023 |
| 28 | LC-MS/MS | 1.219 | −6.89 | 0.974 | −20.7 | 6.9 | 27.6 | 1.145 | −3.30 | 0.976 | −14.5 | 7.9 | 22.4 | 0.074 | 5.2 | −0.002 |
| 33 | LC-MS/MS | 1.059 | −1.77 | 0.973 | −14.5 | 10.9 | 25.4 | 1.056 | −2.13 | 0.978 | −12.7 | 8.4 | 21.1 | 0.003 | 4.3 | −0.005 |
| 36 | LC-MS/MS | 1.041 | 1.46 | 0.990 | −6.0 | 8.9 | 14.9 | 1.044 | 1.0 | 0.988 | −5.3 | 7.3 | 12.6 | −0.003 | 2.3 | 0.002 |
| 37 | LC-MS/MS | 1.047 | −0.71 | 0.984 | −10.3 | 8.8 | 19.1 | 1.024 | −0.11 | 0.992 | −6.4 | 6.2 | 13.3 | 0.023 | 5.8 | −0.008 |
| 38 | LC-MS/MS | 1.055 | −0.25 | 0.974 | −12.4 | 11.9 | 24.1 | 1.038 | 0.58 | 0.968 | −11.2 | 12.4 | 23.6 | 0.017 | 0.5 | 0.006 |
For the regression line, Int. = y-intercept in nmol/L
Min = minimum y-intercept and Max = maximum y-intercept in nmol/L; width = Min + Max values in nmol/L
Difference in the values for the 50-sample set minus the 42-sample set for slope, R2, and width of PI
The regression plots with PIs are shown in Figure 1 for three assays to illustrate the differences in slope and PI widths with removal of the high concentration 25(OH)D2 samples. For the Bio-Rad assay (Lab 4) shown in Figure 1A and 1B, removal of the higher concentration 25(OH)D2 samples does not significantly change the slope or PIs for the regression line. The regression analysis plot for an LC-MS/MS assay (Lab 7), shown in Figure 1E and 1F, also indicates only minor changes for the 50- versus 42-sample sets. However, as shown in Figure 1C and 1D, removal of the higher concentration 25(OH)D2 samples significantly alters the slope and PIs for the Abbott assay and would therefore influence the assessment of commutability. Because the 42-sample subset is more representative of the distribution of 25(OH)D2 concentrations in clinical assays, the assessment and discussion of commutability will focus on using the 42-sample subset. In addition, the significantly wider PIs for the 50-sample set for several ligand binding assays due to the influence of the high 25(OH)D2 concentration samples from donors supplemented with ergocalciferol would provide an overly liberal assessment of commutability of the SRM and PT/EQA samples for several of the assays. Similar regression plots comparing the 50- versus 42-samples sets are provided for all remaining assays as Figures S3 through S13 (see ESM).
Figure 1.
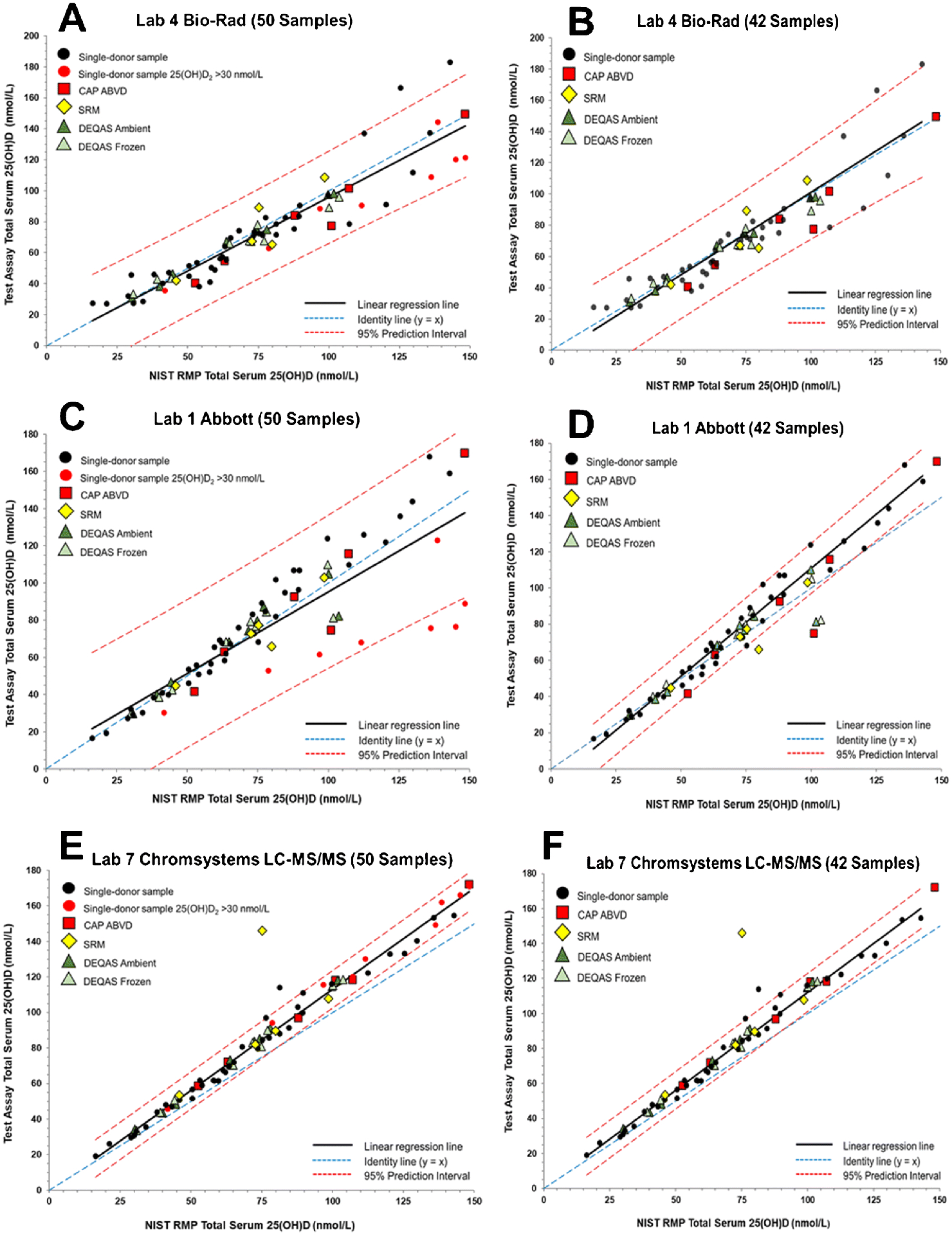
Assay results for determination of serum total 25(OH)D in single-donor samples versus the NIST assigned target value for Laboratory 4 (Bio-Rad) using the BioPlex 2200 25(OH) Vitamin D assay for 50 samples (A) and 42 samples (B), Laboratory 1 (Abbott Diagnostics) using the Abbott Architect 25-OH Vitamin D assay for 50 samples (C) and 42 samples (D), and Laboratory 7 (Chromsystems Instruments & Chemicals) using the Chromsystems LC-MS/MS assay for 50 samples (E) and 42 samples (F).
Commutability Assessment
Regression analysis plots for the 42-sample subset used to assess commutability are shown in Figures 2 through 5 for each of the 12 unique ligand binding assays and for the 14 different LC-MS/MS assays. A summary of the commutability assessment by laboratory/assay and SRMs and PT/EQA materials for the 42-sample subset is provided in Table 4. A similar commutability assessment summary using the 50-sample set is provided in Table S9 (see ESM). Commutability was assessed for both the frozen and ambient DEQAS samples; however, the determination of the equivalence of the frozen versus ambient samples with respect to the various 25(OH)D assay responses is described elsewhere [37].
Figure 2.
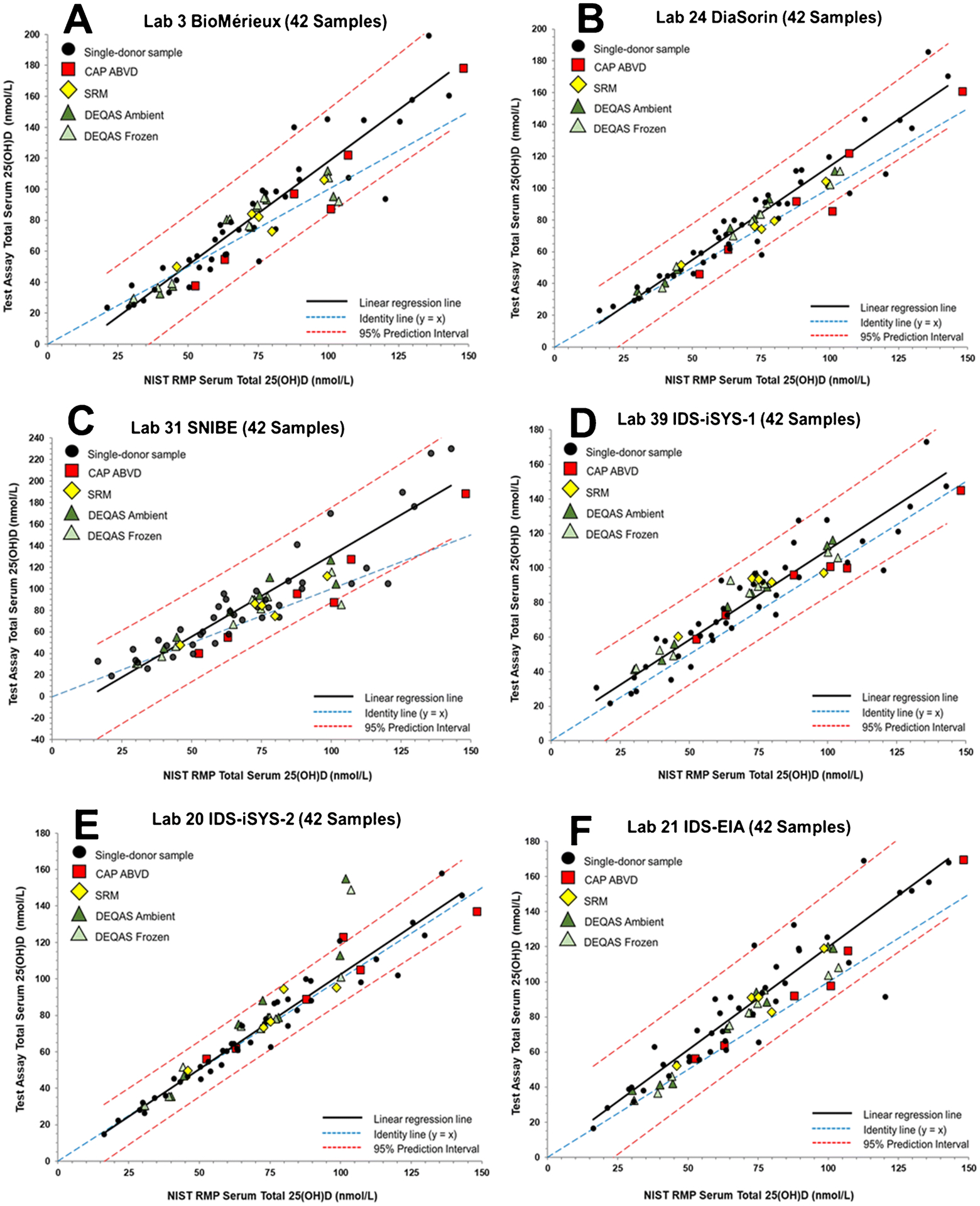
Assay results for determination of serum total 25(OH)D in the 42 single-donor sample subset versus the NIST assigned target value for (A) Laboratory 3 (bioMérieux) using the VIDAS 25OH Vitamin D Total assay, (B) Laboratory 24 (Imperial College Healthcare) using an DiaSorin Liaison 25 OH Vitamin D Total assay, (C) Laboratory 31 (SNIBE) using a prototype MAGLUMI 25-OH Vitamin D assay, (D) Laboratory 39 (Yale University) using the IDS-iSYS 25 Vitamin DS (IDS-iSYS-1), (E) Laboratory 20 (Immunodiagnostic Systems) using the IDS-iSYS 25 VitDS (IDS-iSYS-2), and (F) Laboratory 21 (Immunodiagnostic Systems) using the IDS-EIA 25-Hydroxy Vitamin DS EIA assay.
Figure 5.
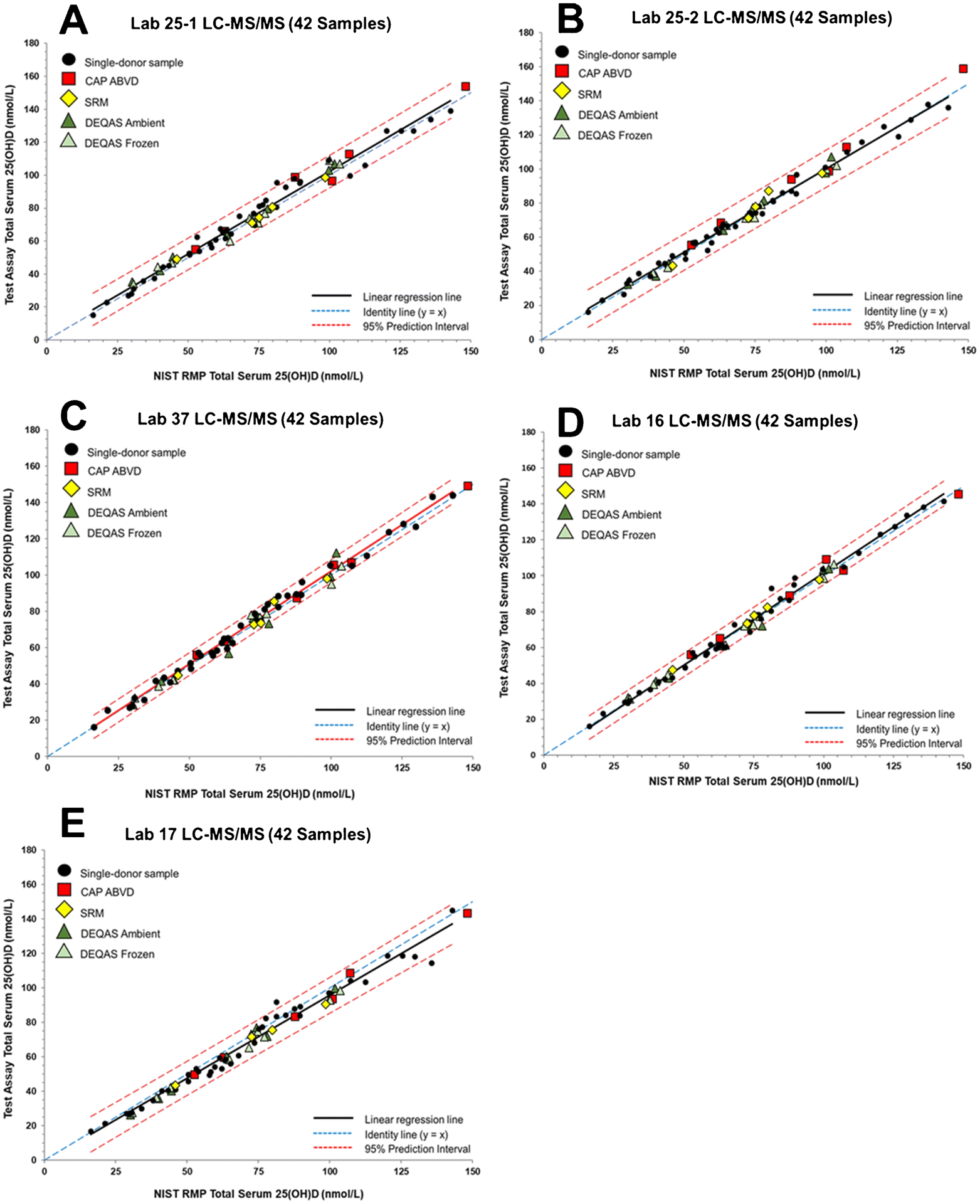
Assay results for determination of serum total 25(OH)D in the 42 single-donor sample subset versus the NIST assigned target value for (A) Laboratory 25–1 (University of Cambridge) using an LC-MS/MS assay, (B) Laboratory 25–2 (University of Cambridge) using an LC-MS/MS assay, (C) Laboratory 37 (University of Western Australia) using an LC-MS/MS assay, (D) Laboratory 16 (Endoceutics Inc.) using an LC-MS/MS assay, and (E) Laboratory 17 (Endocrine Sciences - LabCorp) using an LC-MS/MS assay.
Table 4.
Summary of Commutability Study Results by Laboratory, Assay Type, and Test Material for 42 Samples (excluding 8 samples with 25(OH)D2 > 30 nmol/L)a
| Samples | SRMsb,c,d | CAP ABVDd,e | DEQASd,e,f | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 6 | 7 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||||||||||
| Lab | Assay | A | F | A | F | A | F | A | F | A | F | A | F | A | F | A | F | A | F | |||||||||||
| LIGAND BINDING ASSAYS | ||||||||||||||||||||||||||||||
| 1 | Abbott | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N |
| 18 | Abbott | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N |
| 23 | Abbott | Y | Y | N | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N |
| 27 | Abbott | Y | Y | N | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N |
| 26 | Beckman | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 3 | BioMérieux | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 34 | BioMérieux | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 4 | Bio-Rad | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 9 | DiaSorin | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 24 | DiaSorin | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 2 | DIAsource | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 20 | IDS-iSYS-2 | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N |
| 39 | IDS-iSYS-1 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 21 | IDS-EIA | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 19 | Roche | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 29 | Roche | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 30 | Siemens | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 40 | Siemens | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 31 | SNIBE | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y |
| 5 | SNIBE | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y |
| LC-MS/MS | ||||||||||||||||||||||||||||||
| 6 | Chromsystems | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 7 | Chromsystems | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 11 | LC-MS/MS | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 12 | LC-MS/MS | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 16 | LC-MS/MS | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 17 | LC-MS/MS | Y | Y | Y | -g | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 22 | LC-MS/MS | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 25–1 | LC-MS/MS | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 25–2 | LC-MS/MS | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 28 | LC-MS/MS | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 33 | LC-MS/MS | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N |
| 36 | LC-MS/MS | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 37 | LC-MS/MS | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | N |
| 38 | LC-MS/MS | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
Y, Yes, sample is commutable for the assay; N, No, sample is non-commutable for the assay
SRMs: L1 = SRM 972a Level 1; L2 = SRM 972a Level 2; L3 = SRM 972a Level 3; L4 = SRM 972a Level 4; L5 = SRM 2973
Sample contains high exogenous concentration of 3-epi-25(OH)D3 (64 nmol/L) = Green
Sample contains endogenous concentration of 25(OH)D2 > 20 nmol/L = Yellow
Sample contains endogenous concentration of 3-epi-25(OH)D3 > 7 nmol/L = Blue
DEQAS = commutability determined for samples shipped at both ambient (A) and frozen (F) temperatures
Lab 17 intentionally did not analyze SRM 972a L4 due to presence of high concentration of 3-epi-25(OH)D3
For the Bio-Rad assay (Figure 1B), all of the CAP ABVD, DEQAS, and SRM samples are assessed as commutable. The results for the Bio-Rad assay are similar to results for several other ligand binding assays that are not significantly influenced by the higher concentration 25(OH)D2 samples including IDS-EIA (Figure 2F), Beckman Coulter (Figure 3A), Roche (Figure 3C), and Siemens (Figure 3D). All SRM and PT/EQA samples were deemed to be commutable for these assays.
Figure 3.
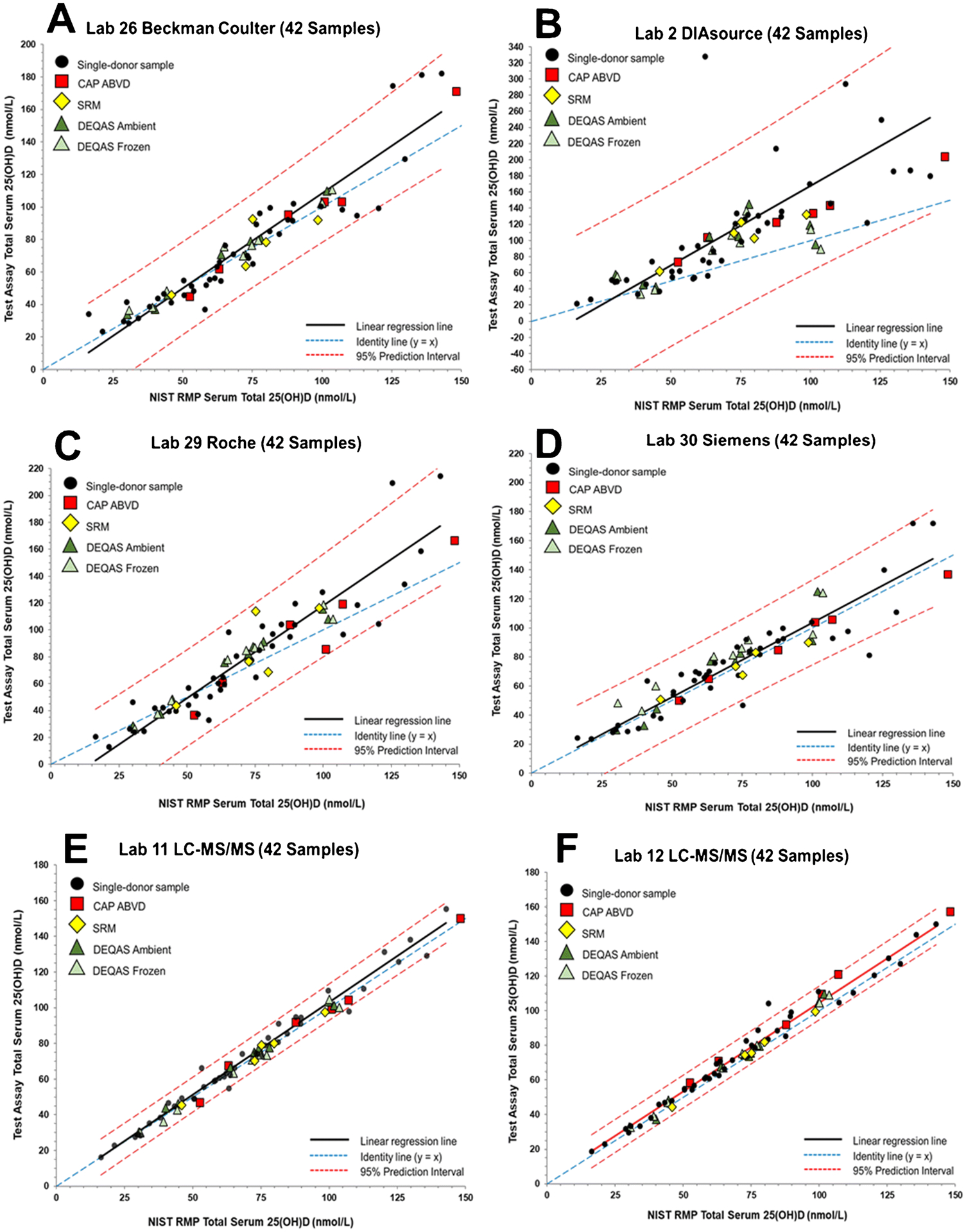
Assay results for determination of serum total 25(OH)D in the 42 single-donor sample subset versus the NIST assigned target value for (A) Laboratory 26 (National Institute of Public Health) using the Beckman Coulter Access 25(OH) Vitamin D Total assay, (B) Laboratory 2 (Awareness Technology) using an DIAsource 25 OH Vitamin D Total ELISA assay, (C) Laboratory 29 (St. Vincent’s University Hospital) using a Roche Vitamin D Total II assay, (D) Laboratory 30 (Siemens) using the Siemens 25 Vitamin D (VitD), (E) Laboratory 11 (Canisius Wilhelmina Hospital) using an LC-MS/MS assay, and (F) Laboratory 12 (Prince of Wales Hospital) using an LC-MS/MS assay.
As shown in Figure 1D, CAP ABVD-1, DEQAS-9 (frozen and ambient), and SRM 972a L3 are non-commutable using the Abbott assay. These four samples all have concentrations of 25(OH)D2 >30 nmol/L (see Table 1). CAP ABVD-04 also has a relatively high 25(OH)D2 concentration (23.8 nmol/L) and was assessed as non-commutable by two of the laboratories using the Abbott assay (see Table 4 and ESM Figure S3D and S3F). The IDS-iSYS-2 assay (Lab 20) also had difficulties with samples with high concentrations of 25(OH)D2, as shown in Figure 2E indicating that the two materials with the highest 25(OH)D2 concentrations, CAP ABVD-1 and DEQAS-9, are non-commutable. Interestingly, for the IDS-iSYS-1 (Lab 39) assay, which was an earlier version of the IDS-iSYS-2 assay (see Table 2), all SRM and PT/EQA samples were found to be commutable (see Figure 2D). DEQAS-9 was also assessed as non-commutable with the SNIBE assay (Figure 2C) but only for the ambient shipped sample. The SNIBE assay was one of four assays that were found to have statistically different results for the ambient versus frozen DEQAS samples as described elsewhere [37].
As summarized in Table 4, CAP ABVD-1 was assessed as non-commutable for Abbott, bioMérieux (Lab 34), DiaSorin (Lab 24), IDS-iSYS-2, and SNIBE (Lab 31). For the two laboratories reporting results for the bioMérieux, DiaSorin, and SNIBE assays, one laboratory provided results indicating that all SRMs and PT/EQA samples were commutable, whereas the results from the second laboratory indicated that CAP ABVD-1 was non-commutable. In all three cases, the CAP ABVD-1 sample is near the 95% PI line. When all 50 single-donor samples are used to assess commutability (see Table S10 in ESM), only three samples were found to be non-commutable, and all three samples were measured using the IDS-iSYS-2 (Lab 20) assay.
For the LC-MS/MS assays, the main issue related to commutability of the SRMs and PT/EQA samples is whether 3-epi-25(OH)D3 and 25(OH)D3 are chromatographically separated. The results shown in Figure 1F illustrate this non-commutability issue for one LC-MS/MS assay (Lab 7). As shown in Figure 1E and 1F, the width of the PI does not change significantly between the 50-sample (E) and 42-sample (F) sets for Lab 7 LC-MS/MS assay when compared to the ligand binding assays (see Table 3). However, because of the high exogeneous concentration of 3-epi-25(OH)D3, SRM 972a L4 is readily assessed as non-commutable. In Figure 4, the results for six additional LC-MS/MS assays are shown for which SRM 972a L4 is assessed as non-commutable. For Lab 36 (Figure 4E), the result for SRM 972a L4 is only slightly outside the 95% PIs whereas for the other LC-MS/MS assays for which this sample is non-commutable, the results are significantly outside the 95% PIs. Of the 14 different LC-MS/MS assays evaluated, 6 assays do not appear to separate the 3-epi-25(OH)D3 and 25(OH)D3, and therefore, SRM 972a L4 becomes non-commutable for these assays (Table 4). Lab 17 (Figure 5E) intentionally did not analyze SRM 972a L4 because these VDSP samples were not analyzed with the alternative test method offered by LapCorp (Lab 17) that separates and quantifies the epimers of both 25(OH)D2 and 25(OH)D3.
Figure 4.
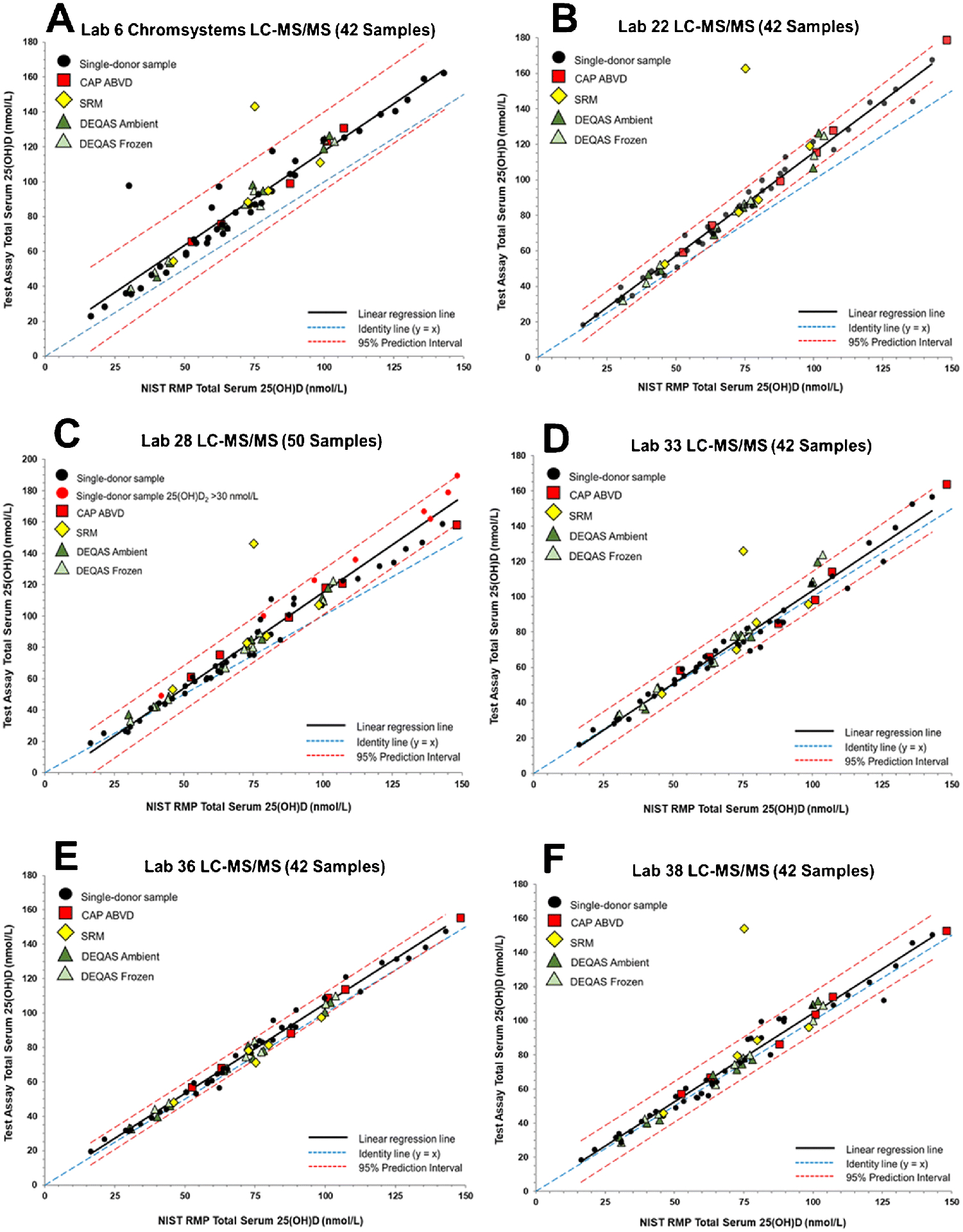
Assay results for determination of serum total 25(OH)D in the 42 single-donor sample subset versus the NIST assigned target value for (A) Laboratory 6 (Manchester Royal Infirmary) using an LC-MS/MS assay, (B) Laboratory 22 (Imperial College Healthcare) using an LC-MS/MS assay, (C) Laboratory 28 (Penn State University) using an LC-MS/MS assay, (D) Laboratory 33 (University of California at San Diego) using an LC-MS/MS assay, (E) Laboratory 36 (University of Washington) using an LC-MS/MS assay, and (F) Laboratory 38 (Waters Technologies Ireland Ltd.) using an LC-MS/MS assay.
Several additional samples are assessed as non-commutable with three LC-MS/MS assays. DEQAS-9 (both frozen and ambient) with high 25(OH)D2 concentration is also non-commutable for Lab 33’s LC-MS/MS assay as shown in Figure 4D. For Lab 16’s LC-MS/MS assay (Figure 5D), CAP ABVD-01 [high 25(OH)D2] and DEQAS-4A were assessed as non-commutable. Four samples, including DEQAS-9F [high 25(OH)D2], were found to be non-commutable using the LC-MS/MS assay at Lab 37 (see Figure 5C). However, these commutability assessments for samples other than SRM 972a L4 [high exogeneous 3-epi-25(OH)D3] and DEQAS-09 [high 25(OH)D2] may be attributed to the narrow width of the PI for the 42-sample set rather than an actual commutability problem. When all 50 single-donor samples are used for the assessment of commutability for LC-MS/MS assays (see ESM Table S10), the results are similar to using the 42-sample subset, i.e., SRM 972a L4 is deemed non-commutable using seven LC-MS/MS assays, and DEQAS-9 is assessed as non-commutable with one assay (Lab 33).
Comparison to Commutability Study 1
Beyond the identification of the laboratories and assays in Commutability Study 2, there were several other significant improvements over Commutability Study 1 [24]. Results from only 9 laboratories (6 ligand binding assays and 3 LC-MS/MS assays) were reported in Commutability Study 1 compared with 34 sets of results (20 ligand binding assays and 14 LC-MS/MS) in this second study. In Commutability Study 2, target concentrations of 25(OH)D2 were reported for all 50 single-donor samples, ranging from 0.3 nmol/L to 137 nmol/L with eight samples > 30 nmol/L, compared with Commutability Study 1, where only 17 of 50 samples had concentrations of 25(OH)D2 above the LOQ, and there were no samples with concentrations > 20 nmol/L.
The 20 SRM, CAP ABVD, and DEQAS samples in Commutability Study 2 (compared to 17 samples in the first study) provided a greater range of serum total 25(OH)D concentrations ranging from 30 nmol/L to 148 nmol/L (with 5 samples > 98 nmol/L) compared with a range of 28 nmol/L to 127 nmol/L (with only one sample > 98 nmol/L) in the first study. Five samples in Commutability Study 2 had endogenous concentrations of 25(OH)D2 >20 nmol/L compared with only three samples in the first study. The 3-epi-25(OH)D3 concentrations in the SRM/PT/EQA samples were similar in both studies with endogenous levels between 1 nmol/L and 7 nmol/L (study 2 had one sample at 12.8 nmol/L); however, the first study had three samples with high concentrations of 3-epi-25(OH)D3 (all exogenous), whereas the second study had only one sample with exogenous 3-epi-25(OH)D3 (i.e., SRM 972a L4).
For Commutability Study 1, all SRMs and PT/EQA samples were deemed commutable using the ligand binding assays with the exception of one CAP ABVD sample (inconclusive commutability for two assays). For the second commutability study, the SRM and PT/EQA samples with concentrations of 25(OH)D2 > 20 nmol/L were deemed as non-commutable by five assays (i.e., Abbott, bioMérieux, DiaSorin, IDS-iSYS-2, and SNIBE). For the three LC-MS/MS assays in the first study, two samples with high exogenous 3-epi-25(OH)D3 concentrations were identified as inconclusively commutable for one assay, and SRM 972a L3 and DEQAS-8 with high 25(OH)D2 concentrations (33.1 nmol/L and 47.3 nmol/L, respectively) were determined to be inconclusively commutable and non-commutable, respectively, for the two remaining assays. In the current study, 50% of the LC-MS/MS assays did not adequately separate 25(OH)D3 and 3-epi-25(OH)D3 resulting in non-commutability for SRM 972a L4. While SRM 972a L4 provides an excellent performance challenge for LC-MS/MS assays, endogenous concentrations of 3-epi-25(OH)D3 at this high level would rarely, if ever, be encountered in actual patient samples. Significant positive biases observed for 50% of the LC-MS/MS assays in the interlaboratory comparison study [25] also point to the lack of separation of 25(OH)D3 and 3-epi-25(OH)D3 as the major LC-MS/MS performance issue.
Conclusions
VDSP Commutability Study 2 is a comprehensive commutability assessment of SRMs and PT/EQA samples for 25(OH)D measurements based on results from 12 unique ligand binding assays and 14 different LC/MS/MS assays. For several ligand binding assays (i.e., Abbott, bioMérieux, DiaSorin, IDS-iSYS-2, and SNIBE), SRM and PT/EQA samples with high concentrations of 25(OH)D2 were assessed as non-commutable, which may be attributed to unequal responses of these assays to 25(OH)D2 and 25(OH)D3 resulting in significant bias for the measurement of total 25(OH)D when concentrations of 25(OH)D2 > 20 nmol/L [26]. SRM and PT/EQA samples with high 3-epi-25(OH)D3 concentrations were found to be non-commutable for 7 of the 14 LC-MS/MS assays evaluated indicating that these assays probably do not adequately separate the 3-epi-25(OH)D3 and 25(OH)D3. While this lack of specificity for 25(OH)D3 and 3-epi-25(OH)D3 may or may not be an issue for clinical testing and patient care, it is a limitation that can be easily addressed with modifications to the LC-MS/MS assay.
Supplementary Material
Acknowledgements
The Office of Dietary Supplements at the National Institutes of Health (NIH-ODS) provided partial funding for this study to the National Institute of Standards and Technology (NIST). The laboratory study participants agreed to the publication of their measurements data, laboratory identification, and measurement assay platform identification. The authors acknowledge Gary L. Myers (Myers Consulting, Smyrna GA) and Gregory W. Miller (Virginia Commonwealth University, Richmond, VA) for input in the planning of this study. MWC is affiliated to Metabolomics Australia, University of Western Australia, Perth, Western Australia, Australia. This work was supported by infrastructure funding from the Western Australian State Government in partnership with the Australian Federal Government, through Bioplatforms Australia and the National Collaborative Research Infrastructure Strategy (NCRIS). Bruno Emanuelli and Angelo Maggio (Care S.r.l) and Manisha Patwardhan (Golwilkar Metropolis Health Services Pvt. Ltd.) are acknowledged for contributing results to this study.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
S.A. Wise is an Editor of the journal Analytical and Bioanalytical Chemistry and was not involved in peer reviewing this manuscript. Several of the coauthors on the manuscript are employees of companies who produced the assays evaluated in this study. There are no financial or nonfinancial conflicts of interest for any of the coauthors.
Human Subjects Ethics
The National Institute of Standards and Technology Research Protections Office reviewed the protocol for this project and determined it is “not human subjects research” as defined in 15 CFR 27, the Common Rule for the Protection of Human Subjects.
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: Certain commercial equipment or materials are identified in this paper to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology or the National Institutes of Health, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
References
- 1.Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM, VDSP (2012) Vitamin D status as an international issue: National surveys and the problem of standardization. Scand J Clin Lab Invest 72:32–40. doi: 10.3109/00365513.2012.681935 [DOI] [PubMed] [Google Scholar]
- 2.Wise SA, Tai SSC, Burdette CQ, Camara JE, Bedner M, Lippa KA, Nelson MA, Nalin F, Phinney KW, Sander LC, Betz JM, Sempos CT, Coates PM (2017) Role of the National Institute of Standards and Technology (NIST) in support of the vitamin D initiative of the National Institutes of Health, Office of Dietary Supplements. J AOAC Int 100 (5):1260–1276. doi: 10.5740/jaoacint.17-0305 [DOI] [PubMed] [Google Scholar]
- 3.Tai SSC, Bedner M, Phinney KW (2010) Development of a candidate reference measurement procedure for the determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem 82 (5):1942–1948. doi: 10.1021/ac9026862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tai SSC, Nelson MA (2015) Candidate reference measurement procedure for the determination of (24R),25-dihydroxyvitamin D3 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem 87 (15):7964–7970. doi: 10.1021/acs.analchem.5b01861 [DOI] [PubMed] [Google Scholar]
- 5.Stepman HCM, Vanderroost A, Van Uytfanghe K, Thienpont LM (2011) Candidate reference measurement procedures for serum 25-hydroxyvitamin D-3 and 25-hydroxyvitamin D-2 by using isotope-dilution liquid chromatography-tandem mass spectrometry. Clin Chem 57 (3):441–448. doi: 10.1373/clinchem.2010.152553 [DOI] [PubMed] [Google Scholar]
- 6.Mineva EM, Schleicher RL, Chaudhary-Webb M, Maw KL, Botelho JC, Vesper HW, Pfeiffer CM (2015) A candidate reference measurement procedure for quantifying serum concentrations of 25-hydroxyvitamin D-3 and 25-hydroxyvitamin D-2 using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 407 (19):5615–5624.doi: 10.1007/s00216-015-8733-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phinney KW, Bedner M, Tai SSC, Vamathevan VV, Sander LC, Sharpless KE, Wise SA, Yen JH, Schleicher RL, Chaudhary-Webb M, Pfeiffer CM, Betz JM, Coates PM, Picciano MF (2012) Development and certification of a Standard Reference Material for vitamin D metabolites in human serum. Anal Chem 84 (2):956–962. doi: 10.1021/ac202047n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phinney KW, Tai SSC, Bedner M, Camara JE, Chia RRC, Sander LC, Sharpless KE, Wise SA, Yen JH, Schleicher RL, Chaudhary-Webb M, Maw KL, Rahmani Y, Betz JM, Merkel J, Sempos CT, Coates PM, Durazo-Arvizu RA, Sarafin K, Brooks SPJ (2017) Development of an improved Standard Reference Material for vitamin D metabolites in human serum. Anal Chem 89 (9):4907–4913. doi: 10.1021/acs.analchem.6b05168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tai SSC, Nelson MA, Bedner M, Lang BE, Phinney KW, Sander LC, Yen JH, Betz JM, Sempos CT, Wise SA (2017) Development of Standard Reference Material (SRM) 2973 vitamin D metabolites in frozen human serum (high level). J AOAC Int 100 (5):1294–1303. doi: 10.5740/jaoacint.17-0182 [DOI] [PubMed] [Google Scholar]
- 10.CDC Vitamin D Standardization — Certification Program. https://www.cdc.gov/labstandards/vdscp.html.
- 11.Erdman P, Palmer-Toy DE, Horowitz G, Hoofnagle A (2019) Accuracy-based vitamin D survey six years of quality improvement guided by proficiency testing. Arch Pathol Lab Med 143 (12):1531–1538. doi: 10.5858/arpa.2018-0625-CP [DOI] [PubMed] [Google Scholar]
- 12.Carter GD, Berry J, Durazo-Arvizu R, Gunter E, Jones G, Jones J, Makin HLJ, Pattni P, Sempos CT, Twomey P, Williams EL, Wise SA (2018) Hydroxyvitamin D assays: An historical perspective from DEQAS. J Steroid Biochem Mol Biol 177:30–35. doi: 10.1016/j.jsbmb.2017.07.018 [DOI] [PubMed] [Google Scholar]
- 13.Burdette CQ, Camara JE, Nalin F, Pritchett J, Sander LC, Carter GD, Jones J, Betz JM, Sempos CT, Wise SA (2017) Establishing an accuracy basis for the Vitamin D External Quality Assessment Scheme (DEQAS). J AOAC Int 100 (5):1277–1287. doi: 10.5740/jaoacint.17-0306 [DOI] [PubMed] [Google Scholar]
- 14.Sempos CT, Betz JM, Camara JE, Carter GD, Cavalier E, Clarke MW, Dowling KG, Durazo-Arvizu RA, Hoofnagle AN, Liu A, Phinney KW, Sarafin K, Wise SA, Coates PM (2017) General steps to standardize the laboratory measurement of serum total 25-hydroxyvitamin D. J AOAC Int 100 (5):1230–1233. doi: 10.5740/jaoacint.17-0259 [DOI] [PubMed] [Google Scholar]
- 15.Cashman KD, Dowling KG, Skrabakova Z, Kiely M, Lamberg-Allardt C, Durazo-Arvizu RA, Sempos CT, Koskinen S, Lundqvist A, Sundvall J, Linneberg A, Thuesen B, Husemoen LLN, Meyer HE, Holvik K, Gronborg IM, Tetens I, Andersen R (2015) Standardizing serum 25-hydroxyvitamin D data from four Nordic population samples using the Vitamin D Standardization Program protocols: Shedding new light on vitamin D status in Nordic individuals. Scand J Clin Lab Invest 75 (7):549–561. doi: 10.3109/00365513.2015.1057898 [DOI] [PubMed] [Google Scholar]
- 16.Cashman KD, Kiely M, Kinsella M, Durazo-Arvizu RA, Tian L, Zhang Y, Lucey A, Flynn A, Gibney MJ, Vesper HW, Phinney KW, Coates PM, Picciano MF, Sempos CT (2013) Evaluation of Vitamin D Standardization Program protocols for standardizing serum 25-hydroxyvitamin D data: a case study of the program’s potential for national nutrition and health surveys. Am J Clin Nutr 97 (6):1235–1242. doi: 10.3945/ajcn.112.057182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schleicher RL, Sternberg MR, Lacher DA, Sempos CT, Looker AC, Durazo-Arvizu RA, Yetley EA, Chaudhary-Webb M, Maw KL, Pfeiffer CM, Johnson CL (2016) The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am J Clin Nutr 104 (2):454–461. doi: 10.3945/ajcn.115.127985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Binkley N, Dawson-Hughes B, Durazo-Arvizu R, Thamm M, Tian L, Merkel JM, Jones JC, Carter GD, Sempos CT (2017) Vitamin D measurement standardization: The way out of the chaos. J Steroid Biochem Mol Biol 173:117–121. doi: 10.1016/j.jsbmb.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 19.Rabenberg M, Scheidt-Nave C, Busch MA, Thamm M, Rieckmann N, Durazo-Arvizu RA, Dowling KG, Skrabakova Z, Cashman KD, Sempos CT, Mensink GBM (2018) Implications of standardization of serum 25-hydroxyvitamin D data for the evaluation of vitamin D status in Germany, including a temporal analysis. BMC Public Health 18:14. doi: 10.1186/s12889-018-5769-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wise SA, Phinney KW, Tai SSC, Camara JE, Myers GL, Durazo-Arvizu R, Tian L, Hoofnagle AN, Bachmann LM, Young IS, Pettit J, Caldwell G, Liu A, Brooks SPJ, Sarafin K, Thamm M, Mensink GBM, Busch M, Rabenberg M, Cashman KD, Kiely M, Kinsella M, Galvin K, Zhang JY, Oh K, Lee SW, Jung CL, Cox L, Goldberg G, Guberg K, Prentice A, Carter GD, Jones J, Brannon PM, Lucas RM, Crump PM, Cavalier E, Merkel J, Betz JM, Sempos CT (2017) Baseline assessment of 25-hydroxyvitamin D assay performance: A Vitamin D Standardization Program (VDSP) interlaboratory comparison study. J AOAC Int 100 (5):1244–1252. doi: 10.5740/jaoacint.17-0258 [DOI] [PubMed] [Google Scholar]
- 21.ISO (2003) In vitro diagnostic medical devices — Measurement of quantities in biological samples — Metrological traceability of values assigned to calibrators and control materials. International Standards Organization, Geneva, Switzerland [Google Scholar]
- 22.ISO (2009) In vitro diagnostic medical devices — Measurement of quantities in samples of biological origin — Requirements for certified reference materials and the content of supporting documentation.. vol ISO 15194. International Standards Organization, Geneva Switzerland [Google Scholar]
- 23.Vesper HW, Miller WG, Myers GL (2007) Reference materials and commutability. Clinical Biochemist Reviews 28:139–147 [PMC free article] [PubMed] [Google Scholar]
- 24.Phinney KW, Sempos CT, Tai SSC, Camara JE, Wise SA, Eckfeldt JH, Hoofnagle AN, Carter GD, Jones J, Myers GL, Durazo-Arvizu R, Miller WG, Bachmann LM, Young IS, Pettit J, Caldwell G, Liu A, Brooks SPJ, Sarafin K, Thamm M, Mensink GSM, Busch M, Rabenberg M, Cashman KD, Kiely M, Galvin K, Zhang JY, Kinsella M, Oh K, Lee SW, Jung CL, Cox L, Goldberg G, Guberg K, Meadows S, Prentice A (2017) Baseline assessment of 25-hydroxyvitamin D reference material and proficiency testing/external quality assurance material commutability: A Vitamin D Standardization Program Study. J AOAC Int 100 (5):1288–1293. doi: 10.5740/jaoacint.17-0291 [DOI] [PubMed] [Google Scholar]
- 25.Wise SA, Camara JE, Sempos CT, Burdette CQ, Hahm G, Nalin F, Kuszak AJ, Merkel J, Durazo-Arvizu R, Hoofnagle AN, Williams EL, Ivison F, Fischer R, Van den Ouweland JMW, Ho CS, Law EWK, Simard J-N, Gonthier R, Holmquist B, Meadows S, Cox L, Robyak K, Creer MH, Fitzgerald R, Clarke MW, Breen N, Lukas P, Cavalier E (2022) Interlaboratory comparison of 25-hydroxyvitamin D assays: Vitamin D Standardization Program (VDSP) intercomparison study 2 – Part 1 Liquid chromatography – tandem mass spectrometry (LC-MS/MS) assays – Impact of 3-epi-25-hydroxyvitamin D3 on assay performance. Submitted to Anal Bioanal Chem [DOI] [PubMed] [Google Scholar]
- 26.Wise SA, Camara JE, Sempos CT, Burdette CQ, Hahm G, Nalin F, Kuszak AJ, Merkel J, Durazo-Arvizu R, Williams EL, Popp C, Beckert C, Schultess C, Van Slooten G, Tourneur C, Pease C, Kaul R, Villarreal A, Batista MC, Pham H, Bennett A, Jansen E, Khan DA, Kilbane M, Freeman J, Parker N, Yuan J, Mushtaq S, Simpson C, Lukas P, Cavalier E (2022) Interlaboratory comparison of 25-hydroxyvitamin D assays: Vitamin D Standardization Program (VDSP) intercomparison study 2 - Part 2 Ligand binding assays – Impact of 25-hydroxyvitamin D2 and 24R,25-dihydroxyvitamin D3 on assay performance. Submitted to Anal Bioanal Chem [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camara J, Hoofnagle A, Carter G, Sempos C (2015) Take Two: Gearing up for the next vitamin D commutability study. Clinical Laboratory News (February 1, 2015)
- 28.CLSI (1999) Preparation and Validation of Commutable Frozen Human Serum Pools as Secondary Reference Materials for Cholesterol Measurement Procedures. CLSI Document C37-A Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 29.Danilenko U, Vesper HW, Myers GL, Clapshaw PA, Camara JE, Miller WG (2020) An updated protocol based on CLSI document C37 for preparation of off-the-clot serum from individual units for use alone or to prepare commutable pooled serum reference materials. Clin Chem Lab Med 58 (3):368–374. doi: 10.1515/cclm-2019-0732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Budd JR, Weykamp C, Rej R, MacKenzie F, Ceriotti F, Greenberg N, Camara JE, Schimmel H, Vesper HW, Keller T, Delatour V, Panteghini M, Burns C, Miller WG, Commutability IWGo (2018) IFCC working group recommendations for assessing commutability part 3: Using the calibration effectiveness of a reference material. Clin Chem 64 (3):465–474. doi: 10.1373/clinchem.2017.277558 [DOI] [PubMed] [Google Scholar]
- 31.Miller WG, Schimmel H, Rej R, Greenberg N, Ceriotti F, Burns C, Budd JR, Weykamp C, Delatour V, Nilsson G, MacKenzie F, Panteghini M, Keller T, Camara JE, Zegers I, Vesper HW, Commutability IWG (2018) IFCC working group recommendations for assessing commutability part 1: General experimental design. Clin Chem 64 (3):447–454. doi: 10.1373/clinchem.2017.277525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nilsson G, Budd JR, Greenberg N, Delatour V, Rej R, Panteghini M, Ceriotti F, Schimmel H, Weykamp C, Keller T, Camara JE, Burns C, Vesper HW, MacKenzie F, Miller WG, Commutability IWG (2018) IFCC working group recommendations for assessing commutability part 2: Using the difference in bias between a reference material and clinical samples. Clin Chem 64 (3):455–464. doi: 10.1373/clinchem.2017.277541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CLSI (2010) Characterization and Qualification of Commutable Reference Materials for Laboratory Medicine. CLSI document EP30A Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 34.CLSI (2014) Evaluation of Commutability of Processed Samples; Approved Guideline. CLSI document EP14-A3, 3rd edn. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 35.CLSI (2018) Measurement Procedure Comparison and Bias Estimation using Patient Samples,. CSLI Document EP09c, 3rd edn. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 36.Schleicher RL, Sternberg MR, Looker AC, Yetley EA, Lacher DA, Sempos CT, Taylor CL, Durazo-Arvizu RA, Maw KL, Chaudhary-Webb M, Johnson CL, Pfeiffer CM (2016) National estimates of serum total 25-hydroxyvitamin D and metabolite concentrations measured by liquid chromatography-tandem mass spectrometry in the US population during 2007–2010. J Nutr 146 (5):1051–1061. doi: 10.3945/jn.115.227728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sempos CT, Williams EL, Carter GD, Jones J, Camara JE, Burdette CQ, Hahm G, Nalin F, Kuszak AJ, Hoofnagle AN, Lukas P, Cavalier E, Durazo-Arvizu R, Crump PM, Popp C, Beckert C, Schultess J, Van Slooten G, Briand H, Pease C, Kual R, Villarreal A, Emanuelli B, Maggio A, Ivison F, Fischer R, van den Ouweland JMW, Ho CS, Law EWK, Simard J-N, Gonthier R, Holmquist B, Patwardhan MS, Batista MC, Meadows S, Cox L, Jansen E, D.A L,Robyak K, Creer MH, Kilbane M, Twomey P, Freeman J, Parker N, Chen V, Fitzgerald R, Mushtaq S, Clarke MW, Breen N, Simpson C, Wise SA (2021) Assessment of serum total 25-hydroxyvitamin D assays for vitamin D external quality assessment scheme (DEQAS) materials distributed at ambient and frozen conditions. To be submitted to J Steroid Biochem Mol Biol [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


