Abstract
The performance of a commercial microplate latex agglutination assay, the Verotox-F assay, was compared with that of the Vero cell assay for the detection and characterization of Escherichia coli verocytotoxins (VTs). Culture filtrates of 68 VT-positive E. coli strains (65 human isolates [33 of serotype O157:H7/H−, 32 of non-O157 serotypes] and 3 reference strains) and 104 VT-negative strains (100 human isolates and 4 reference strains) were investigated. The toxin phenotypes and genotypes of the 68 VT-positive isolates were VT1 only (18 strains), VT2 and/or VT2c (33 strains), and VT1 plus VT2 (17 strains). The Verotox-F assay involved incubation of serial dilutions of culture filtrates with equal volumes of latex particles sensitized with anti-VT1 antibody or anti-VT2 antibody in 96-well microtiter plates with appropriate controls and examination for latex agglutination after 20 to 24 h. Compared to the results of the Vero cell assay, the Verotox-F assay was 100% sensitive and 100% specific for the detection of VTs in culture filtrates and correctly identified the toxin types of all 68 VT producers. By checkerboard titration with purified toxins, the sensitivity of the Verotox-F assay was found to be 14 pg (0.7 ng/ml) for VT1, 12 pg (0.6 ng/ml) for VT2, and 350 pg (17.5 ng/ml) for VT2c; this sensitivity is comparable to that of the bioassay. The anti-VT2 latex reagent detected both VT2 and VT2c and did not cross-react with VT1. The anti-VT1 reagent showed a low-level cross-reaction with VT2c only at levels (≥4.5 μg/ml) that were about 1,000-fold higher than those found in culture filtrates. We conclude that the Verotox-F assay is highly sensitive and specific for the detection and characterization of VTs in culture filtrates of human E. coli isolates. The test is rapid, reliable, and easy to perform; its results are easy to interpret; and it should allow testing for VT to become more widely performed.
Verocytotoxin (VT)-producing Escherichia coli (VTEC) (17), also referred to as Shiga-like toxin (SLT)-producing E. coli (20) or Shiga toxin (Stx)-producing E. coli (5), is associated with a spectrum of disease that includes diarrhea, hemorrhagic colitis, and the classical hemolytic-uremic syndrome (HUS) (8, 13, 14). HUS results from systemic VT-mediated damage of capillary endothelial cells in the kidneys, gastrointestinal tract, central nervous system, and other organs (21, 25, 26). Human VTEC isolates produce one or more VTs (14, 28, 29) which are called VT1 (SLT-I; Stx1), VT2 (SLT-II; Stx2), and VT2c (SLT-IIc; Stx2c) (5, 20). VT1 is serologically distinct from VT2 (and VT2c), and the toxins are not cross-neutralized by heterologous antisera in tissue culture assays (9, 11, 28). Conversely, VT2 is completely neutralized by antiserum to VT2c, whereas VT2c is only partially neutralized by antiserum to VT2 (9, 11).
Although the VTEC isolates associated with human disease belong to a broad spectrum of serotypes including O157:H7 and more than 100 others (8, 14), VT production is a common virulence trait of these strains (14). Consequently, the use of assays that detect VT production represents a more reliable approach to the diagnosis of VTEC infection than the use of assays that rely on the detection of a specific serotype such as O157:H7 (8, 14). However, the standard Vero cell cytotoxicity assay for the detection of VT (13, 17) is slow, labor-intensive, and difficult to standardize and requires cell culture facilities and additional VT neutralization experiments for identification of the VT type (11, 13). These drawbacks limit the applicability of the assay in clinical microbiological laboratories. The objective of this study was to compare the performance of a commercial reverse passive latex agglutination (RPLA) assay (the Verotox-F assay) with that of the Vero cell cytotoxicity assay for the detection of VT in E. coli culture filtrates and for the characterization of toxin phenotypes.
(Material from the manuscript was presented in part at the 62nd Conjoint Meeting on Infectious Diseases, Montreal, Canada, 20 to 24 November 1994 [abstr. 13a], and at the 95th General Meeting of the American Society for Microbiology, Washington D.C., 21 to 25 May 1995 [12a].)
MATERIALS AND METHODS
Bacterial strains.
The 172 E. coli strains investigated comprised 68 well-characterized VTEC strains and 104 VT-negative strains. The VTEC strains included three VT-positive reference strains from our collection, namely, H.30 (VT1), C600(933W) (VT2), and E32511 (VT2c) (17, 20), and 65 clinical isolates. The latter were isolated from Canadian and Czech pediatric patients with HUS or diarrhea in previous studies (2, 3, 13, 15) and belonged to the serotypes O157:H7/H− (nonmotile) (n = 33 strains), O1:H− (n = 1), O26:H11/H− (n = 12), O55:H? (H antigen not determined) (n = 2), O91:H21 (n = 1), O111:H8/H− (n = 11), O113:H21 (n = 2), O117:H4 (n = 1), O118:H30 (n = 1), and O121:H19 (n = 1).) The VT-negative strains consisted of four reference strains and 100 clinical isolates. The reference strains were ATCC 25922 (nonpathogenic), TD213C2 (producer of heat-stable enterotoxin [ST]), TD427C2 (producer of heat-labile enterotoxin [LT]), and CL114 (enteroinvasive). The VT-negative clinical isolates were E. coli strains from the stools of children who were hospitalized for respiratory tract infections or from the urine of children suspected of having urinary tract infection.
Culture filtrates.
To obtain culture filtrates, the strains were grown overnight in Penassay broth (antibiotic medium 3; Difco Laboratories, Detroit, Mich.), and the supernatants were filtered through 0.22-μm-pore-size membrane filters (Millipore Corp., Bedford, Mass.).
Vero cell cytotoxicity assay and VT phenotyping.
The Vero cell assay was performed as described previously (13). The VT titer was expressed as the reciprocal of the highest sample dilution that caused a cytotoxic effect in 50% of the cells in the Vero cell monolayer after 3 days of incubation. The breakpoint for a positive result was a titer of ≥4. The VT phenotype was determined by neutralization assays (11, 13, 19) with antisera to purified VT1, VT2, and VT2c (4, 10).
VT genotyping.
The presence of the VT1, VT2, and VT2c genes in the Canadian VTEC strains was investigated by using the PCR methods of Pollard et al. (24) and Tyler et al. (31), and their presence in the Czech isolates was investigated by the PCR method of Rüssmann et al. (27).
Verotox-F assay.
The Verotox-F assay (Denka Seiken Co., Ltd., Tokyo, Japan) was performed according to the manufacturer’s instructions. By using 96-well V-bottom microtiter plates (Gamedium, Říčany, Czech Republic) or U-bottom Immulon 2 plates (Dynatech, Inc., McLean, Va.), serial twofold dilutions of culture filtrates were mixed with equal volumes (25 μl) of latex particles sensitized with rabbit polyclonal anti-VT1 or anti-VT2 immunoglobulin G antibody. The plates were covered, incubated at room temperature, and examined for latex agglutination after 20 to 24 h. The titer of VT1 and VT2 was expressed as the reciprocal of the highest filtrate dilution that caused agglutination of the respective latex reagent; a titer of ≥2 was considered a positive result. The positive and negative controls included in the kit (purified VT1 and VT2 and latex particles sensitized with normal rabbit immunoglobulin G, respectively) were run with each assay.
Toxin purification.
VT1 was purified as described previously (22) from JB28, an E. coli TB1 strain (kindly provided by J. Brunton) that had been transformed with recombinant plasmid pUC19B containing VT1 genes cloned from bacteriophage H19B (6). VT2 was purified from E. coli DH5α(pJES120), which was kindly provided by J. E. Samuel. VT2c was purified from strain E32511 (11), as described by Head et al. (10).
Checkerboard titration.
Serial twofold dilutions of purified VT preparations and of culture filtrates of reference strains H.30, C600(933W), and E32511 were examined for their Verotox-F VT titers and Vero cell cytotoxicity titers. The starting concentrations of purified VT1, VT2, and VT2c for checkerboard titrations were 3.6 ng (0.18 μg/ml), 6 ng (0.3 μg/ml), and 180 ng (9.0 μg/ml), respectively.
RESULTS
Comparison of Verotox-F assay with Vero cell assay for detection of purified VTs and VTs in culture filtrates of reference strains.
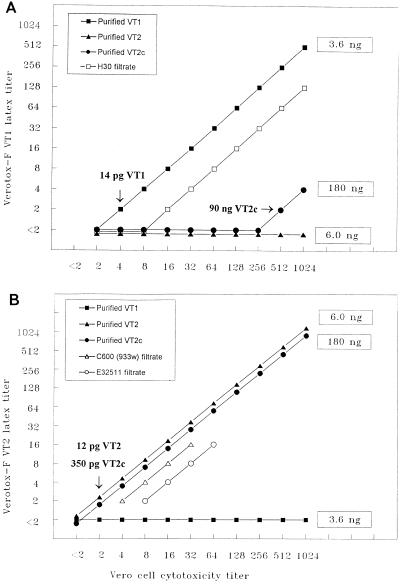
The sensitivities of the Verotox-F anti-VT1 and anti-VT2 latex reagents for the detection of purified VTs were assessed and compared with that of the Vero cell assay by using checkerboard titrations (Fig. 1). The Verotox-F anti-VT1 latex reagent and the bioassay detected purified VT1 in a dose-dependent manner, with the limits of VT1 detection being 14 pg per well (0.7 ng/ml) (Fig. 1A). The anti-VT1 latex reagent showed no cross-reaction with purified VT2 but cross-reacted with large amounts (≥90 ng per well [≥4.5 μg/ml]) of purified VT2c (Fig. 1A). The Verotox-F anti-VT2 latex reagent (Fig. 1B) showed dose-dependent reactivity with both purified VT2 and purified VT2c, but it did not cross-react with purified VT1. The sensitivity of the anti-VT2 latex reagent for the detection of purified VT2 (12 pg per well; 0.6 ng/ml) was 30-fold higher than that for the detection of purified VT2c (350 pg per well; 17.5 ng/ml) (Fig. 1B). For assays with both toxins, this sensitivity was two-fold higher than that of the Vero cell assay (Fig. 1B).
FIG. 1.
Comparison of the assay with the Verotox-F anti-VT1 latex reagent (A) and anti-VT2 latex reagent (B) with the Vero cell cytotoxicity assay for the detection of purified VTs and VTs in culture filtrates of reference strains H.30 (VT1), C600(933W) (VT2), and E32511 (VT2c). Serial twofold dilutions of purified VT preparations starting from the indicated toxin concentrations (boxed numbers) and of culture filtrates were titrated with the Verotox-F anti-VT1 latex reagent (A) and/or with the Verotox-F anti-VT2 latex reagent (B) and by the Vero cell assay. The Verotox-F assay VT1 and VT2 titer and the Vero cell cytotoxicity titer indicate the reciprocals of the highest dilutions of the samples that caused agglutination of the Verotox-F assay latex reagents overnight and a 50% cytotoxic effect in the Vero cell monolayer after 3 days of incubation, respectively. The arrows indicate the smallest amounts of purified VTs detectable with the Verotox-F assay anti-VT1 and anti-VT2 latex reagent.
The sensitivity of the Verotox-F assay for the detection of VT1 and VT2 purified in our laboratory was in agreement with that for the detection of purified VT1 and VT2 included in the kit as positive assay controls. The control VT preparations (concentration, 50 ng/ml) had titers of 64 (VT1) and 128 (VT2) in assays with the corresponding VT latex reagents, which indicated that the anti-VT1 and anti-VT2 reagents detected 20 pg of the homologous control VT per well (0.8 ng/ml) and 10 pg of the homologous control VT per well (0.4 ng/ml), respectively; no cross-reactivity was seen between the latex reagents and the heterologous control VTs (data not shown).
In checkerboard titration experiments with culture filtrates of VT reference strains H.30 (Fig. 1A), C600(933W) (Fig. 1B), and E32511 (Fig. 1B), the Verotox-F anti-VT1 and anti-VT2 latex reagents detected corresponding crude VTs in a dose-dependent manner and with a sensitivity that was the same as that of the bioassay [C600(933W) filtrate] or with a sensitivity that was twofold (E32511 filtrate) or fourfold (H.30 filtrate) lower. None of the culture filtrates reacted with the heterologous VT latex reagent (data not shown). No reaction of the Verotox-F anti-VT1 or anti-VT2 latex reagent was observed with culture filtrates of four VT-negative E. coli reference strains, including nonpathogenic strain ATCC 25922, enterotoxigenic strains TD427C2 (LT positive) and TD213C2 (ST positive), and enteroinvasive strain CL114 (data not shown).
Comparison of Verotox-F assay with Vero cell assay for detection and characterization of VT in culture filtrates of clinical E. coli isolates.
As demonstrated in Table 1, the Verotox-F assay was 100% sensitive and 100% specific compared with the results of the bioassay for the detection of VT in culture filtrates of clinical E. coli isolates.
TABLE 1.
Comparison of Verotox-F assay with Vero cell cytotoxicity assay for detection of VT in culture filtrates of clinical E. coli isolates
| Verotox-F result | No. of isolates with the following Vero cell cytotoxicity result:
|
Total | |
|---|---|---|---|
| Positivea | Negative | ||
| Positiveb | 65 | 0 | 65 |
| Negative | 0 | 100 | 100 |
| Total | 65 | 100 | 165 |
Titer, ≥4.
Titer, ≥2.
The performance of the Verotox-F assay for the identification of the VT types of 65 clinical VTEC isolates was then compared with that of the Vero cell phenotyping assay; in addition, VT genotyping of the isolates was performed to confirm the VT phenotypes. On initial testing, 61 (93.8%) of 65 VTEC isolates of phenotype and genotype VT1 (16 strains), VT2 (17 strains), VT2c (6 strains), VT2 plus VT2c (7 strains), and VT1 plus VT2 (19 strains) were of the same toxin type also by the Verotox-F assay, with all 30 producers of VT2 and/or VT2c being detected by the anti-VT2 latex reagent. Retesting of the four strains with discrepant results (Table 2) confirmed the initial VT type detected by the Verotox-F assay for all of them; moreover, it was found that the VT types determined by the Verotox-F assay showed 100% correlation with those found on repeat phenotyping by the Vero cell assay (Table 2) and on repeat genotyping (data not shown). The Verotox-F assay thus correctly identified the VT types of all 65 VTEC strains, showing a specificity of 100% for the characterization of the two major VT phenotypes, VT1 and VT2, in culture filtrates of clinical VTEC isolates. The VT titers found in the culture filtrates by the Verotox-F assay are presented in Table 3. Most of the 65 clinical VTEC isolates had VT1 and VT2 titers of ≥16 by the Verotox-F assay. A titer of 4 was observed for eight VT2 producers and none of VT1 producers. A titer of 8 was found for four VT1 producers and six VT2 producers.
TABLE 2.
Analysis of discrepant VT phenotyping results between Verotox-F assay and Vero cell assay
TABLE 3.
VT1 and VT2 titers in culture filtrates of 65 clinical VTEC isolates by Verotox-F assay
| VT type by Verotox-F assay | No. of strains | VT titera
|
||
|---|---|---|---|---|
| Range | Median | Geometric mean | ||
| VT1 | 17 | 8–512 | 64 | 47 |
| VT2 | 31 | 4–512 | 32 | 26 |
| VT1 + VT2 | 17 | 8–128 (VT1) | 32 | 32 |
| 4–512 (VT2) | 32 | 35 | ||
The VT titer by the Verotox-F assay was defined as the reciprocal of the highest filtrate dilution that caused agglutination of the respective latex reagent overnight.
DISCUSSION
Since the detection of VT represents a rational, serotype-independent strategy for the diagnosis of VTEC infections, simple and reliable procedures that allow the detection of VT production by clinical isolates are required. For this purpose, we evaluated the performance of a microplate latex agglutination assay, the Verotox-F assay, for the detection and characterization of VTs in E. coli culture filtrates by comparing the results of the assay with those of the standard Vero cell cytotoxicity assay (13, 17). The Verotox-F assay was found to reliably detect the three VTs produced by human VTEC strains, namely, VT1, VT2, and VT2c, showing no cross-reactivity with LT and ST of E. coli. The sensitivity of the Verotox-F assay for the detection of purified VTs was nearly identical to that of the Vero cell assay (Fig. 1A and B). By detecting 0.7 and 0.6 ng of VT1 and VT2 purified in our laboratory per ml, respectively, and 0.8 and 0.4 ng of control VT1 and VT2 provided by the manufacturer per ml, respectively, in our hands the sensitivity of the Verotox-F assay for the detection of the toxins agreed well with that declared by the manufacturer (1 to 2 ng/ml). The absence of cross-reactivity of the anti-VT1 and anti-VT2 latex reagents with the heterologous purified toxin, namely, VT2 and VT1 (Fig. 1A and B), respectively, is consistent with a lack of cross-neutralization between VT1 and VT2 in the Vero cell assay (9, 11, 28). Similarly, the ability of the anti-VT2 latex reagent to detect purified VT2c, even though the sensitivity was significantly lower than that for the detection of VT2 (Fig. 1B), corresponds to only partial cross-neutralization of VT2c by the anti-VT2 antibody in Vero cells (9, 11). Given the lack of cross-neutralization between VT1 and VT2c in the Vero cell culture (9, 11), we observed low-level cross-reactivity between the anti-VT1 latex reagent and large quantities of purified VT2c (Fig. 1A), but this is not of diagnostic significance.
When used for investigation of clinical E. coli isolates, the Verotox-F assay was found to be 100% sensitive and 100% specific compared with the results of the Vero cell assay for the detection of VT in culture filtrates (Table 1) and 100% specific compared with the results of phenotyping by the Vero cell assay and VT genotyping for the characterization of two major VT types, VT1 and VT2. The discrepancies in the results of the initial Vero cell VT phenotyping assay and the Verotox-F VT typing assay for four O157:H7 strains (Table 2) probably resulted from the loss of the VT1 or VT2 gene before testing by the Verotox-F assay for three of the strains; the spontaneous loss of VT genes during laboratory storage and subcultures has previously been shown to be quite frequent for non-O157 VTEC strains (12). Important for the practical diagnostic use of the Verotox-F assay is the finding that the anti-VT2 latex reagent, even though it is 30-fold less sensitive to purified VT2c than to VT2 (Fig. 1B), gave a positive result with culture filtrates of all six clinical isolates that produced VT2c only. On the other hand, although the anti-VT1 latex reagent showed cross-reactivity with large amounts of purified VT2c (Fig. 1A), it did not cross-react with any of VT2c-containing culture filtrates, suggesting that the amount of VT2c produced in culture filtrates was low enough to avoid the cross-reactivity.
The results of our study corroborate and extend the earlier findings of Beutin et al. (1), who used the VTEC-RPLA assay kit (Denka Seiken Co., Ltd.), which is identical to the Verotox-F assay (18). We tested a larger collection of strains with better-defined controls and negative samples to determine the sensitivity and specificity of the assay; moreover, we defined the limits of detection of verotoxins in culture filtrates compared to those of highly purified toxins. Beutin et al. (1) performed the VTEC-RPLA assay with polymyxin B extracts of bacterial colonies, whereas we performed the test with bacterial culture filtrates. VTEC isolates that are associated with pig edema disease produce the toxin VT2e (8, 14), which is serologically related to VT2 (11). Beutin et al. (1) tested toxin extracts from three pig edema disease strains and found that none was detected by the VTEC-RPLA assay. This is of practical diagnostic interest given that VT2e-producing strains have been isolated, albeit rarely, from humans with enteritis (23) and HUS (30).
In addition to the latex agglutination assay described in our study, other immunospecific assays for the detection of VT have been developed (7, 16). In particular, an enzyme-linked immunosorbent assay system, the Premier EHEC Assay (Meridian Diagnostics, Inc.), was shown to be useful for the detection of VT both in bacterial isolates and in fecal filtrates (16); however, the assay does not allow identification of the toxin type. In contrast, the Verotox-F test has yet to be validated for the detection of the toxin in fecal filtrates. On the other hand, the Verotox-F assay kit is particularly useful for the testing of individual bacterial isolates for VT production, and its ability to type toxins may be of epidemiological significance. Laboratorians may thus select either assay to address specific needs.
ACKNOWLEDGMENTS
The study was supported by a grant from McDonalds Restaurants of Canada, Limited, and by grant IGA 2063-3 from the Ministry of Health of the Czech Republic. The Verotox-F kits were kindly provided by Denka Seiken Co., Ltd.
We thank Helge Karch, University of Würzburg, Würzburg, Germany, for genotyping of the Czech VTEC isolates included in the study. The excellent technical assistance of M. Winkler and K. McDowell is appreciated.
REFERENCES
- 1.Beutin L, Zimmermann S, Gleier K. Rapid detection and isolation of Shiga-like toxin (Verocytotoxin)-producing Escherichia coli by direct testing of individual enterohemolytic colonies from washed sheep blood agar plates in the VTEC-RPLA assay. J Clin Microbiol. 1996;34:2812–2814. doi: 10.1128/jcm.34.11.2812-2814.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bielaszewska M, Janda J, Bláhová K, Šrámková L, Havlík J, Potužník V. Vero cytotoxin-producing Escherichia coli in children with hemolytic uremic syndrome and diarrhea in the Czech Republic. In: Karmali M A, Goglio A G, editors. Recent advances in verocytotoxin-producing Escherichia coli infections. Amsterdam, The Netherlands: Elsevier Science; 1994. pp. 37–40. [Google Scholar]
- 3.Bielaszewska M, Janda J, Bláhová K, Feber J, Potužník V, Součková A. Verocytotoxin-producing Escherichia coli in children with hemolytic uremic syndrome in the Czech Republic. Clin Nephrol. 1996;46:42–44. [PubMed] [Google Scholar]
- 4.Bielaszewska M, Clarke I, Karmali M A, Petric M. Localization of intravenously administered verocytotoxins (Shiga-like toxins) 1 and 2 in rabbits immunized with homologous and heterologous toxoids and toxin subunits. Infect Immun. 1997;65:2509–2516. doi: 10.1128/iai.65.7.2509-2516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calderwood S B, Acheson D W K, Keusch G T, Barrett T J, Griffin P M, Strockbine N A, Swaminathan B, Kaper J B, Levine M M, Kaplan B S, Karch H, O’Brien A D, Obrig T G, Takeda Y, Tarr P I, Wachsmuth I K. Proposed new nomenclature for Shiga-like toxin (verotoxin) family. ASM News. 1996;62:118–119. [Google Scholar]
- 6.DeGrandis S A, Ginsberg J, Toone M, Climie S, Friesen J, Brunton J. Nucleotide sequence and promoter mapping of the Escherichia coli Shiga-like toxin operon of bacteriophage H-19B. J Bacteriol. 1987;169:4313–4319. doi: 10.1128/jb.169.9.4313-4319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng P. Impact of molecular biology on the detection of foodborne pathogens. Mol Biotechnol. 1997;7:267–268. doi: 10.1007/BF02740817. [DOI] [PubMed] [Google Scholar]
- 8.Griffin P M, Tauxe R V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 9.Head S C, Karmali M A, Roscoe M E, Petric M, Strockbine N A, Wachsmuth I K. Serological differences between verocytotoxin 2 and Shiga-like toxin II. Lancet. 1988;ii:751. doi: 10.1016/s0140-6736(88)90228-0. [DOI] [PubMed] [Google Scholar]
- 10.Head S C, Petric M, Richardson S, Roscoe M, Karmali M A. Purification and characterization of verocytotoxin 2. FEMS Microbiol Lett. 1988;51:211–216. [Google Scholar]
- 11.Hii J H, Gyles C, Morooka T, Karmali M A, Clarke R, DeGrandis S, Brunton J L. Development of verotoxin 2- and verotoxin 2 variant (VT2v)-specific oligonucleotide probes on the basis of the nucleotide sequence of the B cistron of VT2v from Escherichia coli E32511 and B2F1. J Clin Microbiol. 1991;29:2704–2709. doi: 10.1128/jcm.29.12.2704-2709.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karch H, Meyer T, Rüssmann H, Heesemann J. Frequent loss of Shiga-like toxin genes in clinical isolates of Escherichia coli upon subcultivation. Infect Immun. 1992;60:3464–3467. doi: 10.1128/iai.60.8.3464-3467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Karmali M, Winkler M, Petric M, McDowell C, Penn L. Abstracts of the 95th General Meeting of the American Society for Microbiology 1995. Washington, D.C: American Society for Microbiology; 1995. Evaluation of a microplate latex method (VEROTOX-F) in Escherichia coli, abstr. C-178; p. 32. [Google Scholar]
- 13.Karmali M A, Petric M, Lim C, Fleming P C, Arbus G S, Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 13a.Karmali M A, Winkler M, Petric M. Abstracts of the 62nd Conjoint meeting on Infectious Diseases. 1994. Evaluation of a microplate latex method (Verotox-F) for detecting and characterizing verotoxins (VT) in Escherichia coli, abstr. O-Z. [Google Scholar]
- 14.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karmali M A, Petric M, Winkler M, Bielaszewska M, Brunton J, van de Kar N, Morooka T, Nair G B, Richardson S E, Arbus G S. Enzyme-linked immunosorbent assay for detection of immunoglobulin G antibodies to Escherichia coli Vero cytotoxin 1. J Clin Microbiol. 1994;32:1457–1463. doi: 10.1128/jcm.32.6.1457-1463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kehl K S, Havens P, Behnke C E, Acheson D W K. Evaluation of the Premier EHEC assay for detection of Shiga toxin-producing Escherichia coli. J Clin Microbiol. 1997;35:2051–2054. doi: 10.1128/jcm.35.8.2051-2054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konowalchuk J, Speirs J I, Stavric S. Vero response to a cytotoxin of Escherichia coli. Infect Immun. 1977;18:775–779. doi: 10.1128/iai.18.3.775-779.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mangan, K. (Denka Seiken Company, Ltd.). Personal communication.
- 19.Morooka T, Petric M, Arbus G S, Winkler M, Karmali M A. Distribution of Verocytotoxins (Shiga-like toxins) among clinical isolates of Verocytotoxin-producing Escherichia coli. Med Bull Fukuoka Univ. 1994;21:263–268. [Google Scholar]
- 20.O’Brien A D, Karmali M A, Scotland S M. A proposal for rationalizing the nomenclature of the Escherichia coli cytotoxins. In: Karmali M A, Goglio A G, editors. Recent advances in verocytotoxin-producing Escherichia coli infections. Amsterdam, The Netherlands: Elsevier Science; 1994. pp. 147–149. [Google Scholar]
- 21.Obrig T G, Vecchio P J D, Brown J E, Moran T P, Rowland B M, Judge T K, Rothman S W. Direct cytotoxic action of Shiga toxin on human vascular endothelial cells. Infect Immun. 1988;56:2373–2378. doi: 10.1128/iai.56.9.2373-2378.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petric M, Karmali M A, Richardson S, Cheung R. Purification and biological properties of Escherichia coli verocytotoxin. FEMS Microbiol Lett. 1987;41:63–68. [Google Scholar]
- 23.Pierard D, Huyghens L, Lauwers S. Diarrhoea associated with Escherichia coli producing porcine oedema disease verotoxin. Lancet. 1991;338:762. doi: 10.1016/0140-6736(91)91487-f. [DOI] [PubMed] [Google Scholar]
- 24.Pollard D R, Johnson W M, Lior H, Tyler S D, Rozee K R. Differentiation of Shiga toxin and Vero cytotoxin type 1 genes by polymerase chain reaction. J Infect Dis. 1990;162:1195–1198. doi: 10.1093/infdis/162.5.1195. [DOI] [PubMed] [Google Scholar]
- 25.Richardson S E, Karmali M A, Becker L E, Smith C R. The histopathology of the hemolytic uremic syndrome associated with verocytotoxin-producing Escherichia coli infections. Hum Pathol. 1988;19:1102–1108. doi: 10.1016/s0046-8177(88)80093-5. [DOI] [PubMed] [Google Scholar]
- 26.Richardson S E, Rotman T A, Jay V, Smith C R, Becker L E, Petric M, Olivieri N F, Karmali M A. Experimental verocytotoxemia in rabbits. Infect Immun. 1992;60:4154–4167. doi: 10.1128/iai.60.10.4154-4167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rüssmann H, Schmidt H, Heesemann J, Caprioli A, Karch H. Variants of Shiga-like toxin II constitute a major toxin component in Escherichia coli O157 strains from patients with haemolytic uraemic syndrome. J Med Microbiol. 1994;40:338–343. doi: 10.1099/00222615-40-5-338. [DOI] [PubMed] [Google Scholar]
- 28.Scotland S M, Smith H R, Rowe B. Two distinct toxins active on Vero cells from Escherichia coli O157:H7. Lancet. 1985;ii:885–886. doi: 10.1016/s0140-6736(85)90146-1. [DOI] [PubMed] [Google Scholar]
- 29.Thomas A, Smith H R, Rowe B. Use of digoxigenin-labelled oligonucleotide DNA probes for VT2 and VT2 variant genes to differentiate Vero cytotoxin-producing Escherichia coli strains of serogroup O157. J Clin Microbiol. 1993;31:1700–1703. doi: 10.1128/jcm.31.7.1700-1703.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas A, Cheasty T, Chart H, Rowe B. Isolation of Vero cytotoxin-producing Escherichia coli serotype O9ab:H− carrying VT2 variant gene sequences from a patient with haemolytic uraemic syndrome. Eur J Microbiol Infect Dis. 1994;13:1074–1076. doi: 10.1007/BF02111832. [DOI] [PubMed] [Google Scholar]
- 31.Tyler S D, Johnson W M, Lior H, Wang G, Rozee K R. Identification of verotoxin type 2 variant B subunit genes in Escherichia coli by the polymerase chain reaction and restriction fragment length polymorphism analysis. J Clin Microbiol. 1991;29:1339–1343. doi: 10.1128/jcm.29.7.1339-1343.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]