Abstract
This review aimed to study molecular mechanisms for high incidence of life-threatening mucormycosis infection in COVID19 cases during second wave of SARS CoV2 pandemic in India. Hyperglycaemia, impaired immunity, acidosis, raised ferritin, glucocorticoid therapy, and COVID19 specific other factors have been implicated in pathogenesis of COVID19 associated mucormycosis (CAMM). Endoplasmic reticulum chaperone ‘Glucose Related Protein 78' (GRP78), also involved in SARS CoV2 entry, is the host receptor for invasion by Mucorales. GRP78 is over-expressed by SARS CoV2, hyperglycaemia and ferritin. Delta variant of SARS CoV2 and indiscriminate use of steroids were distinguishing features of second wave and appear to upregulate GRP78 through intricate interplay between internal and external milieu. Common invasive fungal infections like candidiasis and aspergillosis, not utilizing GRP78 as receptor, were inconspicuous. Further molecular research to unravel mechanisms involved in the pathogenesis of CAMM shall effectively complement existing strategies for its prevention and treatment.
Keywords: CoTH, COVID19, Delta variant, GRP78, Mucormycosis
Introduction
The Severe Acute Respiratory Syndrome Corona Virus 2 (SARS CoV2) causing Corona Virus Disease (COVID19) has affected more than 30 million people and caused 433,589 deaths in India [1]. A second surge in COVID19 cases was witnessed in India almost 12 months after the declaration of SARS CoV2 pandemic. Delta variant, which was designated as strain of concern by Centre for Disease Control and Prevention (CDC) for its increased transmissibility and the severity of disease it causes [2], was the dominant strain [3]. An increased incidence of mucormycosis was observed in COVID19 patients during this second wave [4,5]. Mucormycosis being a rapidly spreading fungal infection with life-threatening consequences created concern among medical fraternity and public. Secondary infections with bacteria and fungi were not a common finding in COVID19 cases earlier. Studies reported very low incidences of bacterial co- infections and extremely low rates of fungal co-infections in COVID19 cases as compared to co-infections in influenza disease [[6], [7], [8]]. Similarly co-infections in MERS and SARS CoV1 were also uncommon [[6], [7], [8], [9]]. The fungal co-infections in COVID19 cases reported from other countries were mainly of Aspergillus and Candida infections [[10], [11], [12], [13]]. A review article on cases reports and case series published between December 2020 and April 2021 found 43 cases of mucormycosis in COVID19 cases of which 71% were from India [14]. The aim of the present review was to understand the mechanisms underlying mucormycosis co-infection in COVID19 cases and to explore further avenues for their prevention and treatment.
Method
Literature search for this narrative review was conducted on PubMed for articles published between the years 1980 and August 2021 and on google search-engine for current COVID19 information. The key words used included ‘COVID19’, ‘mucormycosis’, ‘GRP78’, ‘heat shock proteins’, ‘invasive fungal diseases’ and ‘delta variant’. Titles and abstracts including ‘pathogenesis of mucormycosis’, ‘immune mechanism against mucormycosis’, ‘invasive fungal infection’, ‘fungal infections in COVID19’, ‘haematological findings in COVID19’, ‘COVID19 associated mucormycosis’, ‘trends in second wave’ and ‘GRP78 in COVID19’ were identified and full papers were studied.
Mucormycosis
Mucormycosis or zygomycosis is a rare, aggressive, rapidly spreading, angio-invasive fungal infection [15]. This life-threatening infection is most frequently caused by Rhizopus oryzae (syn. Rhizopus arrhizus), a filamentous fungus belonging to the family Mucoraceae of the order Mucorales (Fig. 1 ). Its spores are ubiquitously present in soil, air, and in decaying fruits and vegetables. Unlike other filamentous fungi that are largely opportunistic infections in immunosuppressed hosts like cancer patients and organ recipients, mucormycosis can also affect those with no apparent immune impairment [16]. Mucormycosis has however been associated mainly with diabetes mellitus especially in those with diabetic ketoacidosis (DKA), haematological malignancies, organ transplant, immunocompromise, trauma and neutropenia [17,18]. The fungal hyphae erode the tissues and blood vessels causing endothelial damage, thrombosis and tissue necrosis [19]. Case fatality is high and disfigurement occurs in survivors due to debridement surgeries needed for treatment. Necrosis gives blackish appearance to the tissues. Hence, the term ‘black fungal disease’ is used for mucormycosis, although the fungus itself is not black.
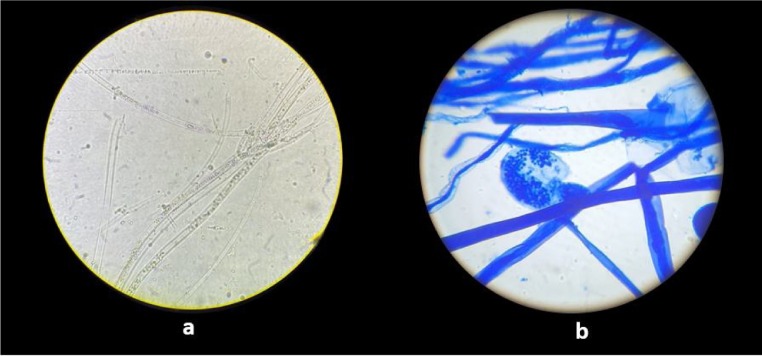
Fig. 1.
Microscopic view of Mucorales: (a) KOH examination showing broad, hyaline, aseptate, right angle branched fungal hyphae; (b) LPCB mount showing broad aseptate hyphae with long sporangiophore and terminal round sporangium.
Epidemiology of mucormycosis
The computational model-based method estimated the prevalence of mucormycosis of 0.14 cases per 1000 population in India [20], whereas the global prevalence is 0.02–9.5 cases (with median of 0.2 cases) per 100,000 population. Thus, the estimated prevalence of mucormycosis in India is 70 times higher than global data [21]. In India, the most common presentation is rhino-orbito-cerebral mucormycosis followed by pulmonary and cutaneous types [22,23] and diabetes mellitus is the most common risk factor.
There has been definitive increase in the incidence of COVID19 associated mucormycosis (CAMM) in India during the second wave. Although recently published scientific data was not available, this unequivocal rise was observed both locally and in other parts of country and was supported by the facts viz. setting up of special medical wards for mucormycosis patients, increase in the number of debridement surgeries, rising demands for antifungal drug amphotericin and declaration of mucormycosis as a notifiable disease in India on 20th May 2021. By the end of June 2021, 40,854 cases of mucormycosis were notified. [24] Such high incidence in a short span of time created an alarm and concern amongst health authorities.
Potential factors causing COVID19 associated mucormycosis
It is well documented that hyperglycaemia, acidosis and compromised immunity are favourable factors for mucormycosis infection, and iron enhances growth and survival of Mucorales in human host [16,18,19]. In the studies published on CAMM so far, most cases reported were diabetics and most received steroid [4,[25], [26], [27]]. High blood sugar in these cases may have occurred as a result of loss of sugar control in diabetics as a consequence of inadequate availability of routine medical services due to various restriction during the pandemic and inadvertent glucocorticoid treatment. Although steroids had generally been reserved for moderate and severe cases of COVID19 to suppress unwanted host immune responses and cytokine storm, injudicious use among mild and home isolated cases was also in practice. Use of steroids in mild COVID19 cases must have been a consequence of indiscriminate prescription and self-treatment by patients due to mis-information.
It is however worth scrutiny that although glucocorticoids have been used in much higher doses and for longer durations in various medical conditions, mucormycosis has not been a menace in these cases. Also, diabetes mellitus is common in India and uncontrolled diabetes is not uncommon [28]. Hence, although diabetes mellitus and steroids associated hyperglycaemia hold the centre stage as per current knowledge, a deeper understanding beyond this is mandatory in the light of above facts.
There are many factors peculiar to COVID19 that may be considered contributory to CAMM. It has been in scientific discussion that unclean water in humidifiers, bad hygiene of oxygen mask and its tubing and use of industrial oxygen could be the sources of Mucorales. However, local outbreaks of mucormycosis in admitted patients receiving oxygen therapy have not been reported from any medical facility. Use of protective face masks is another unique feature of this pandemic. Repetitive use of the same mask for prolonged duration has been suspected as another source of the fungus as many pathogens can grow due to the moisture trapped in the mask. Yet another probable source could be unclean beddings, unclean vicinity and poor hygiene due to compromised bed side patient care and hospitality accentuated by the fear of contracting COVID19 amongst caregivers. Swab collection for PCR testing with non-sterile and unhygienic nasopharyngeal swabs could be another possibility.
A breach in the integrity of the nasal mucosa due to (a) overenthusiastic use of home remedies like steam inhalation, (b) nasal instillation of substances like oils and lemon juice, and (c) injury from swab collection needs to be investigated as a possible route of entry. Rampant use of broad-spectrum antibiotics, which suppresses normal commensals and reduces bacterial competition on the surface, is another factor.
Zinc, commonly prescribed for its antiviral properties in Covid19, is also an essential micronutrient for fungal growth. The new SARS CoV2 mutant B.1.617.2 or the delta variant, which was the dominant strain during the second wave [29], is another probable factor. Higher viral load has been reported in these cases on RTPCR testing [30]. New variant and high viral loads need to be considered as an important distinguishing factor between this and previous wave. Various in-vitro studies, with dissimilar methodology, reported conflicting effects of glucocorticoid on viral replication. A study on effect of glucocorticoid treatment on respiratory tract cells found increased viral replication, altered inflammatory cell profiles and paradoxical increase of pro-inflammatory cytokines [31]. Elevated acute phase reactant ferritin in COVID19 cases needs to be investigated for being an important source of iron, which is essential nutrient for fungal growth and survival.
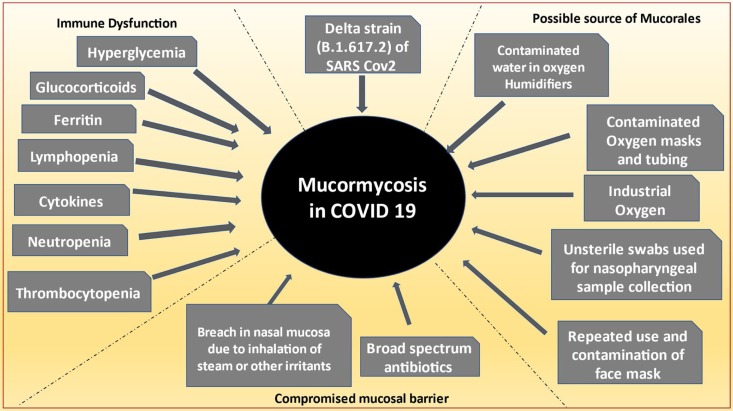
However, CAMM was also reported in patients who were not hospitalized, not given steroids, non-diabetic and treated at home with home remedies like steam inhalation, gargling and herbal substances [32], with or without the usual approved treatment regimens. Hence, there may be multifactorial causation and complex interplay therein, enabling the fungus to prosper when the host environment became conducive (Fig. 2 ).
Fig. 2.
Causal relevance between COVID19 and mucormycosis.
Evasion of host defences by Mucorales
Two steps are required for Mucorales to infect the host, first is to germinate and second is to invade the tissues. Ability of spores to germinate and form hyphae is the most critical step [17]. Animal studies have shown that the inhalation of Mucorale sporangiospores in immunocompetent animals does not produce mucormycosis [33]. The macrophages suppress spore germination and neutrophils kill the hyphae by oxidative burst in immunocompetent host [34]. The neutrophils start expressing Toll-Like-Receptors 2 (TLR2) on exposure to hyphae and phagocytose the fungus.
The type of immunosuppression determines the susceptibility to and virulence of the fungal infection [35]. 70–100% of the patients with haematological malignancies developing mucormycosis, have been reported to have neutropenia [36]. In diabetic ketoacidosis, there is dysfunctional phagocytosis, impaired chemotaxis and defective intracellular killing of fungus [37]. In a study of 658 COVID19 cases, ketoacidosis was noted in 5 cases and ketosis was present in 42 cases, of which only 15 were diabetics [38]. Thus the presence of ketosis and ketoacidosis in non-diabetics with COVID19 is a possibility. Experimental studies have shown that exposure to corticosteroids renders the alveolar macrophages incapable of preventing germination of spores and ketoacidotic environment prevents the cytotoxic action of macrophages [39]. High ferritin levels, as an acute phase reactant have been found in COVID19 [40,41] and ferritin associated iron induces neutrophil dysfunction [42].
Platelets have also been observed to inhibit fungal germination and hyphal growth, and induce hyphal damage in in-vitro studies [34,43]. Lymphopenia has been associated with disease severity in COVID19 and has also been a frequent finding in cases of mucormycosis with COVID19 [44,45]. T-Lymphocytes play an important role in regulating functions of other immune cells. Lymphopenia, thrombocytopenia and morphologically abnormal neutrophils have been observed in COVID19 cases [46] and any of them, alone or in combination may predispose to mucormycosis. It is however worth noticing that AIDS patients, who predominantly have lymphopenia, suffer from many opportunistic infections but mucormycosis has not been a concern among them. Dysfunctional phagocytosis by neutrophil and macrophage is next critical step for the Mucorales to escape destruction and germinate to form hyphae for host invasion.
In immunocompromised hosts, the main invasive fungal infections are Candidiasis, Aspergillosis, Mucormycosis and Cryptococcosis [35]. The unanswered pertinent observation herein is the extremely rare occurrence of other opportunistic infections usually expected in immunocompromised cases and the selective presence of mucormycosis in some COVID19 cases. Once the fungus germinates and escapes phagocytosis, damage of, and penetration through the endothelial cells or the extracellular matrix proteins lining the blood vessels is the final step for the fungal invasion [17].
GRP78 as receptor for invasion of Mucorales
The aggressive nature of mucormycosis is due to invasive property of fungal hyphae. Mucorales utilize host Glucose Regulated Protein 78 (GRP78) as receptor for invasion into tissues and endothelial cells [18]. GRP78, also known as Binding Immunoglobulin Protein (BiP) or Heat Shock Protein A5 (HSPA5), is a chaperone protein found mainly in endoplasmic reticulum (ER) and is concerned with folding, assembly and secretion of proteins. GRP78 is upregulated during ER stress and serves as an ER stress sensor. In addition to ER, it is also found on cell surface and extracellular environment [47]. The Spore Coat Protein Homologs CoTH3 and CoTH2 of the fungal hyphae act as ligands for cell surface GRP78 (csGRP78) (Fig. 3 ). CoTH3 and CoTH2 are widely present in Mucorales and absent in non-invasive fungi [[48], [49], [50]]. Diabetic Keto Acidosis has been found to upregulate CoTH3 and nasal GRP78, which explains the rhino-orbito-cerebral presentation of mucormycosis in these cases [51]. The fact that more common invasive fungi viz. Candida albicans and Aspergillus fumigatus do not utilize GRP78 for invasion needs special emphasis [18] (Table 1 ). Scope for both targeted anti-GRP78 and anti-CoTH drugs to treat mucormycosis is obvious [48,49].
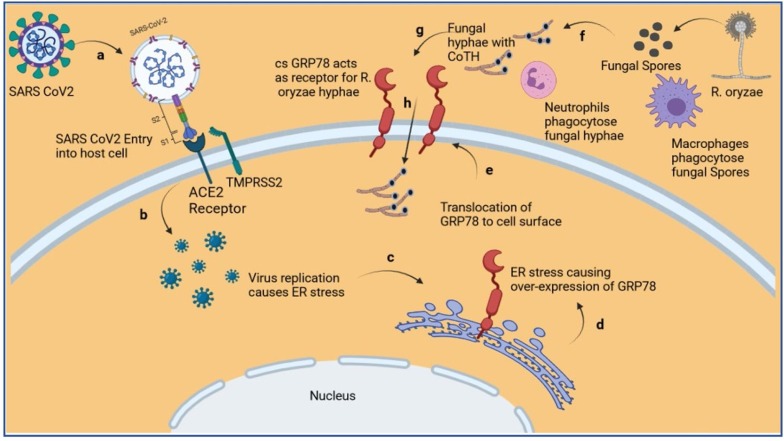
Fig. 3.
Diagram depicting over-expression and translocation of GRP78 to cell surface due to SARS CoV2 replication induced ER stress, and invasion of hyphae of R. oryzae using cs-GRP78 as its receptor. (a) Entry of SARS CoV2 into host utilizing ACE2 and TMPRSS2 as receptors. (b) Replication of SARS CoV2 inside host cell. (c) ER stress induced due to virus replication. (d) Over expression of GRP78. (e) Translocation of GRP78 to the cell surface. (f) Germination of R. oryzae spores into hyphae after escaping phagocytosis by macrophages. (g) R. oryzae hyphae escape phagocytosis by neutrophils and utilize cs-GRP78 as receptors, (h) to invade host cell. [18,34,39,42,[48], [49], [50],55,58] (ACE2-Converting Enzyme type 2; CoTH-Spore coat protein; cs-GRP78-cell surface GRP78; ER-endoplasmic reticulum; GRP78- Glucose Regulated Protein 78; R. oryzae-Rhizopus oryzae; SARS CoV2-Severe Acute Respiratory Syndrome Coronavirus 2; TMPRSS2-transmembrane protease, serine 2) [this figure was created with BioRender.com].
Table 1.
The fungal proteins and host receptors in common invasive fungal infections.
| Invasive fungi | Fungal protein mediating invasion | Host receptors mediating invasion |
|---|---|---|
| Mucorales * | CoTH3 & CoTH2 | GRP78 |
| Aspergillus# | Thaumatin-like protein CalA | Integrins |
| Candida ^ | Agglutinin-like sequence (Als) | Cadherins |
It has been observed that iron and glucose both enhance the susceptibility of endothelial cells to R. oryzae invasion and damage by inducing overexpression of GRP78, wherein the effect of iron was more drastic [18]. Acidosis causes release of free iron from iron binding proteins [18]. As ketosis, ketoacidosis [38] and high levels of ferritin were found in COVID19 cases [40,41] and since iron is essential for the fungal growth, survival and virulence [54], the role of ferritin in CAMM needs to be further investigated.
SARS COV2 and GRP78
The SARS CoV2 spike protein has two subunits S1 and S2. The S1 subunit binds with the host ACE2 (Angiotensin converting enzyme Type 2) receptors and S2 subunit is implicated in fusion of the virus with the host cells. The process of internalization of virus utilizes cathepsin, transmembrane protease, serine 2 (TMPRSS2) and human airway trypsin like proteases (HAT) as well as ACE2 [55], Several studies have shown the evidence of alternative receptors and cofactors that help in virus entry and fusion [[56], [57], [58]], Docking studies have shown interaction between receptor binding domain (RBD) of SARS CoV2 spike protein and GRP78 [56].
The csGRP78 is known to play a role in the infection of host cell by several viruses viz. MERS CoV, Ebola, Japanese encephalitis and Dengue [58]. In fact, GRP78 acts as an important chaperone required for life cycle of all mammalian viruses [59]. Viral glycoproteins of many viruses including SARS CoV2 are the main triggers for endoplasmic reticulum stress. They induce ER stress by accumulation of unfolded protein in the ER lumen and activate Unfolded Protein Response (UPR). The UPR signalling pathways cause upregulation of GRP78 synthesis to handle the unfolded and misfolded proteins. In this process GRP78 is exported out of ER and expressed on the cell surface. GRP78 was four times higher in SARS CoV2 positive pneumonia than SARS CoV2 negative pneumonia [58]. Significant elevation of GRP78 was observed in both SARS CoV2 positive and negative pneumonia as compared to controls but it is still higher in SARS CoV2 positive pneumonia [55].
The increased GRP78 expression on cell surface may further enhance viral entry by positive feedback cycle. Co-localization has been observed between endogenous GRP78 and ACE2 in perinuclear region typical of ER and also on the cell surface. GRP78 may be important for ACE2 trafficking, localization and stability on cell surface, as GRP78 knockdown by siRNA (Small interfering Ribonucleic acid) reduces the level of cell surface ACE2 in parallel with decrease in csGRP78 [57]. Co-localization of GRP78 and SARS CoV2 has also been established in live viral infection. AR-12 (2-amino-N-[4-[5-(2 phenanthrenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl] phenyl]-acetamide) a derivative of celecoxib and an inhibitor of chaperone GRP78 (along with many other chaperones) reduces the expression of cell surface ACE2 and GRP78 as well as total GRP78. In addition, AR-12 suppresses the ability of SARS CoV2 to produce virus spike protein and to generate infectious virion. AR-12 may hence be an antiviral against SARS CoV2 infection. [59]. Another study found imatinib as the top docking score drug in virtual screening on GRP78 nucleotide binding domain (NBD) [58].
GRP78 mediated damage of Beta cell of Pancreas
It had been reported that cytokine-exposed beta pancreatic cells cause secretion and cell surface translocation of GRP78 [60]. The csGRP78 can mediate cell signalling in pro-proliferative, pro-survival and pro-apoptotic pathways [47]. The secreted GRP78 itself acts as a ligand for csGRP78 on pancreatic β cell and activates the pre-apoptotic pathways leading to beta cell damage and hyperglycaemia [60]. COVID19 is associated with release of various cytokines depending on the stage and severity of the disease [40,41] and can cause hyperglycaemia via this pathway.
Summary
Increased incidence of CAMM during second wave of SARS CoV2 pandemic in India shows to have multifactorial causation. Hyperglycaemia, acidosis, impaired immunity and raised iron are important factors associated with pathogenesis of mucormycosis. Steroid therapy, raised ferritin and various factors unique to COVID19 viz. oxygen therapy, protective face masks, broad spectrum antibiotics and breached integrity of nasal mucosa have been implicated in creating a suitable environment for mucormycosis infection. Most CAMM cases had hyperglycaemia and history of glucocorticoid therapy.
The delta variant of SARS CoV2 and indiscriminate use of steroids were two unique features typical to second wave and appear to be drivers of CAMM. The endoplasmic reticulum chaperone GRP78 is over-expressed in viral infections and high viral load on RTPCR was observed in COVID19 during second wave. The higher viral load observed could be consequential to inadvertent glucocorticoids use, subsequently leading to higher ER stress and exaggerated GRP78 expression. Hyperglycaemia and raised ferritin also cause over-expression of GRP78.
Mucorales bind to host GRP78 via its CoTH proteins to invade tissues. On the contrary, these are not utilized by more common invasive fungi. Overexpressed csGRP78 is also a potential mediator of pancreatic beta cell damage leading to hyperglycaemia. Thus, hyperglycaemia is both cause and effect of over-expressed GRP78.
In COVID19 cases, phagocytic dysfunction can be caused by hyperglycaemia, glucocorticoids, raised ferritin and by virus itself. Owing to ubiquitous nature of Mucorales and the other implicated sources, peculiar to COVID19, escape from phagocytic destruction, upregulation of CoTH3 and availability of iron makes germination and tissue invasion possible in presence of abundant GRP78 receptors causing CAMM.
The limitation of this review in depicting comprehensive sequence of cellular events underlying CAMM necessitates further studies as published researches related to molecular mechanisms involved in its pathogenesis are scarce.
Conclusion
Phagocytic dysfunction, GRP78 over-expression, hyperglycaemia and ferritin derived iron are herein inferred as major determinants of mucormycosis in COVID19. Hyperglycaemia, both a cause and effect of GRP78 over-expression, has potentials of causing phagocytic dysfunction and CoTH3 upregulation in presence of acidosis. The delta variant and the rampant use of glucocorticoids qualify as most important factors for increased incidence of CAMM during the second wave owing to their potential in generating the prerequisites.
In view of recurring waves and unpredictable nature of the SARS CoV2, there is compelling need and urgency for exploring anti-GRP78 agents for treatment of COVID19, mucormycosis and CAMM. The synergistic effects of anti-GRP78, anti-CoTH3 and anti-CoTH2 agents also need investigations for treatment of mucormycosis, an extremely dreadful fungal infection. Intensive research on molecular mechanisms involved in the pathogenesis of CAMM shall bridge the gaps in our current understanding for exploring targeted prevention and treatment options.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgement
The authors thankfully acknowledge Dr Neeta Gade, Associate Professor, Department of Microbiology, All India Institute of Medical Sciences, Nagpur, Maharashtra, India, for contributing quality images of microscopic view of Mucorales as were seen in COVID19 cases with mucormycosis in India.
References
- 1.Website of Ministry of Health and Family welfare, Government of India website www.mohfw.gov.in [Accessed 20 August 2021].
- 2.The Tribune. US CDC classifies delta variant as a variant of concern. https://www.tribuneindia.com/news/world/us-cdc-classifies-delta-variant-as-variant-of-concern-269825 [Accessed 17 June 2021].
- 3.Yang Wan, Shaman Jeffrey. 2021. COVID-19 pandemic dynamics in India and impact of the SARS-CoV-2 Delta (B.1.617.2): COVID-19 SARS-CoV-2 preprints from medRxiv. [DOI] [Google Scholar]
- 4.Sharma S., Grover M., Bhargava S., Samdani S., Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;(April):1–6. doi: 10.1017/S0022215121000992. Epub ahead of print. PMID: 33827722; PMCID: PMC8060545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmadikia K., Hashemi S.J., Khodavaisy S., Getso M.I., Alijani N., Badali H., et al. The double-edged sword of systemic corticosteroid therapy in viral pneumonia: a case report and comparative review of influenza-associated mucormycosis versus COVID-19 associated mucormycosis. Mycoses. 2021;16(February) doi: 10.1111/myc.13256. Epub ahead of print. PMID: 33590551; PMCID: PMC8013756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes S., Troise O., Donaldson H., Mughal N., Moore L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26(October (10)):1395–1399. doi: 10.1016/j.cmi.2020.06.025. Epub 2020 Jun 27. PMID: 32603803; PMCID: PMC7320692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71(December (9)):2459–2468. doi: 10.1093/cid/ciaa530. PMID: 32358954; PMCID: PMC7197596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(August (2)):266–275. doi: 10.1016/j.jinf.2020.05.046. Epub 2020 May 27. PMID: 32473235; PMCID: PMC7255350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai C.C., Yu W.L. COVID-19 associated with pulmonary aspergillosis: a literature review. J Microbiol Immunol Infect. 2021;54(February (1)):46–53. doi: 10.1016/j.jmii.2020.09.004. Epub 2020 Sep 24. PMID: 33012653; PMCID: PMC7513876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoenigl M. Invasive fungal disease complicating COVID-19: when it rains it pours. Clin Infect Dis. 2020;(September) doi: 10.1093/cid/ciaa1342. Epub ahead of print. PMID: 32887998; PMCID: PMC7499555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gangneux J.P., Bougnoux M.E., Dannaoui E., Cornet M., Zahar J.R. Invasive fungal diseases during COVID-19: we should be prepared. J Mycol Med. 2020;30(June (2)) doi: 10.1016/j.mycmed.2020.100971. Epub 2020 Apr 6. PMID: 32307254; PMCID: PMC7136887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White P.L., Dhillon R., Cordey A., Hughes H., Faggian F., Soni S., et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis. 2020;(August) doi: 10.1093/cid/ciaa1298. Epub ahead of print. PMID: 32860682; PMCID: PMC7499527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellanger A.P., Navellou J.C., Lepiller Q., Brion A., Brunel A.S., Millon L., et al. Mixed mold infection with Aspergillus fumigatus and Rhizopus microsporus in a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patient. Infect Dis Now. 2021;(January) doi: 10.1016/j.idnow.2021.01.010. S2666-9919(21)00030-0. Epub ahead of print. PMID: 33527098; PMCID: PMC7839422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.John T.M., Jacob C.N., Kontoyiannis D.P. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J Fungi (Basel) 2021;7(April (4)):298. doi: 10.3390/jof7040298. PMID: 33920755; PMCID: PMC8071133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montaño D.E., Voigt K. Host immune defense upon fungal infections with mucorales: pathogen-immune cell interactions as drivers of inflammatory responses. J Fungi (Basel) 2020;6(September (3)):173. doi: 10.3390/jof6030173. PMID: 32957440; PMCID: PMC7557740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roden M.M., Zaoutis T.E., Buchanan W.L., Knudsen T.A., Sarkisova T.A., Schaufele R.L., et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(September (5)):634–653. doi: 10.1086/432579. Epub 2005 Jul 29. PMID: 16080086. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim A.S., Spellberg B., Walsh T.J., Kontoyiannis D.P. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(February (Suppl 1)):S16–S22. doi: 10.1093/cid/cir865. PMID: 22247441; PMCID: PMC3286196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu M., Spellberg B., Phan Q.T., Fu Y., Fu Y., Lee A.S., et al. Theendothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J Clin Invest. 2010;120(June (6)):1914–1924. doi: 10.1172/JCI42164. Epub 2010 May 17. PMID: 20484814; PMCID: PMC2877958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown J. Zygomycosis: an emerging fungal infection. Am J Health Syst Pharm. 2005;62(December (24)):2593–2596. doi: 10.2146/ajhp050188. PMID: 16333056. [DOI] [PubMed] [Google Scholar]
- 20.Chakrabarti A., Singh R. Mucormycosis in India: unique features. Mycoses. 2014;57(December (Suppl 3)):85–90. doi: 10.1111/myc.12243. Epub 2014 Sep 3. PMID: 25187095. [DOI] [PubMed] [Google Scholar]
- 21.Prakash H., Ghosh A.K., Rudramurthy S.M., Singh P., Xess I., Savio J., et al. A prospective multicenter study on mucormycosis in India: epidemiology, diagnosis, and treatment. Med Mycol. 2019;57(June (4)):395–402. doi: 10.1093/mmy/myy060. PMID: 30085158. [DOI] [PubMed] [Google Scholar]
- 22.Prakash H., Chakrabarti A. Epidemiology of mucormycosis in India. Microorganisms. 2021;9(March (3)):523. doi: 10.3390/microorganisms9030523. PMID: 33806386; PMCID: PMC8000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel A., Kaur H., Xess I., Michael J.S., Savio J., Rudramurthy S., et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin Microbiol Infect. 2020;26(July (7)):944.e9–944.e15. doi: 10.1016/j.cmi.2019.11.021. Epub 2019 Dec 4. PMID: 31811914. [DOI] [PubMed] [Google Scholar]
- 24.Sharma N.C. 2021. India reports 40,854 cases of black fungus so far, MINT. Updated: 28 Jun 2021. https://www.livemint.com/news/india-records-over-40k-cases-of-mucormycosis-11624875874985.html (Accessed 19 August 2021) [Google Scholar]
- 25.Sen M., Lahane S., Lahane T.P., Parekh R., Honavar S.G. Mucor in a viral land: a tale of two pathogens. Indian J Ophthalmol. 2021;69(February (2)):244–252. doi: 10.4103/ijo.IJO_3774_20. PMID: 33463566; PMCID: PMC7933891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta S., Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12(September (9)):e10726. doi: 10.7759/cureus.10726. PMID: 33145132; PMCID: PMC7599039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moorthy A., Gaikwad R., Krishna S., Hegde R., Tripathi K.K., Kale P.G., et al. SARS-CoV-2, uncontrolled diabetes and corticosteroids-an unholy trinity in invasive fungal infections of the maxillofacial region? A retr p;hbospective, multi-centric analysis. J Maxillofac Oral Surg. 2021;(March):1–8. doi: 10.1007/s12663-021-01532-1. Epub ahead of print. PMID: 33716414; PMCID: PMC7936599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tripathy J.P., Thakur J.S., Jeet G., Chawla S., Jain S., Pal A., et al. Prevalence and risk factors of diabetes in a large community-based study in North India: results from a STEPS survey in Punjab, India. Diabetol Metab Syndr. 2017;9(January):8. doi: 10.1186/s13098-017-0207-3. PMID: 28127405; PMCID: PMC5259959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gayathri Vaidyanathan. News in Nature 593, 321–322 (2021). 10.1038/d41586-021-01274-7 [Accessesd 16 June 2021]. [DOI]
- 30.Singh J., Rahman S.A., Ehtesham N.Z., Hira S., Hasnain S.E. SARS-CoV-2 variants of concern are emerging in India. Nat Med. 2021;27:1131–1133. doi: 10.1038/s41591-021-01397-4. [DOI] [PubMed] [Google Scholar]
- 31.Thomas B.J., Porritt R.A., Hertzog P.J., Bardin P.G., Tate M.D. Glucocorticosteroids enhance replication of respiratory viruses: effect of adjuvant interferon. Sci Rep. 2014;4(November):7176. doi: 10.1038/srep07176. PMID: 25417801; PMCID: PMC5384105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chugh H., Awasthi A., Agarwal Y., Gaur R.K., Dhawan G., Chandra R. A comprehensive review on potential therapeutics interventions for COVID-19. Eur J Pharmacol. 2021;890(January) doi: 10.1016/j.ejphar.2020.173741. Epub 2020 Nov 20. PMID: 33227287; PMCID: PMC7677683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waldorf A.R., Ruderman N., Diamond R.D. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. J Clin Invest. 1984;74(July (1)):150–160. doi: 10.1172/JCI111395. PMID: 6736246; PMCID: PMC425195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghuman H., Voelz K. Innate and adaptive immunity to mucorales. J Fungi. 2017;3(3):48. doi: 10.3390/jof3030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ben-Ami R., Lewis R.E., Kontoyiannis D.P. Enemy of the (immunosuppressed) state: an update on the pathogenesis of Aspergillus fumigatus infection. Br J Haematol. 2010;150(August (4)):406–417. doi: 10.1111/j.1365-2141.2010.08283.x. Epub 2010 Jul 7. PMID: 20618330. [DOI] [PubMed] [Google Scholar]
- 36.Pagano L., Valentini C.G., Fianchi L., Caira M. The role of neutrophils in the development and outcome of zygomycosis in haematological patients. Clin Microbiol Infect. 2009;(October (Suppl 5)):33–36. doi: 10.1111/j.1469-0691.2009.02977.x. PMID: 19754754. [DOI] [PubMed] [Google Scholar]
- 37.Chinn R.Y., Diamond R.D. Generation of chemotactic factors by Rhizopus Oryza in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycaemia and ketoacidosis. Infect Immun. 1982;38(December (3)):1123–1129. doi: 10.1128/iai.38.3.1123-1129.1982. PMID: 6818145; PMCID: PMC347866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22(October (10)):1935–1941. doi: 10.1111/dom.14057. Epub 2020 May 18. PMID: 32314455; PMCID: PMC7264681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrikkos G., Tsioutis C. Recent advances in the pathogenesis of mucormycoses. Clin Ther. 2018;40(June (6)):894–902. doi: 10.1016/j.clinthera.2018.03.009. Epub 2018 Apr 6. PMID: 29631910. [DOI] [PubMed] [Google Scholar]
- 40.Wang J., Jiang M., Chen X., Montaner L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020;108(July (1)):17–41. doi: 10.1002/JLB.3COVR0520-272R. Epub 2020 Jun 13. PMID: 32534467; PMCID: PMC7323250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaur S., Bansal R., Kollimuttathuillam S., Gowda A.M., Singh B., Mehta D., et al. The looming storm: blood and cytokines in COVID-19. Blood Rev. 2021;46(March) doi: 10.1016/j.blre.2020.100743. Epub 2020 Aug 18. PMID: 32829962; PMCID: PMC7431319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cantinieaux B., Janssens A., Boelaert J.R., Lejeune M., Vermylen C., Kerrels V., et al. Ferritin-associated iron induces neutrophil dysfunction in hemosiderosis. J Lab Clin Med. 1999;133(April (4)):353–361. doi: 10.1016/s0022-2143(99)90066-5. PMID: 10218766. [DOI] [PubMed] [Google Scholar]
- 43.Perkhofer S., Kainzner B., Kehrel B.E., Dierich M.P., Nussbaumer W., Lass-Flörl C. Potential antifungal effects of human platelets against zygomycetes in vitro. J Infect Dis. 2009;200(October (7)):1176–1179. doi: 10.1086/605607. PMID: 19698079; PMCID: PMC3017871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garg D., Muthu V., Sehgal I.S., Ramachandran R., Kaur H., Bhalla A., et al. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186(May (2)):289–298. doi: 10.1007/s11046-021-00528-2. Epub 2021 Feb 5. PMID: 33544266; PMCID: PMC7862973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhatt K., Agolli A., Patel M.H., Garimella R., Devi M., Garcia E., et al. High mortality co-infections of COVID-19 patients: mucormycosis and other fungal infections. Discoveries (Craiova) 2021;9(March (1)):e126. doi: 10.15190/d.2021.5. PMID: 34036149; PMCID: PMC8137279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karimi Shahri M., Niazkar H.R., Rad F. COVID-19 and hematology findings based on the current evidences: a puzzle with many missing pieces. Int J Lab Hematol. 2021;43(April (2)):160–168. doi: 10.1111/ijlh.13412. Epub 2020 Dec 2. PMID: 33264492; PMCID: PMC7753300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ge R., Kao C. Cell surface GRP78 as a death receptor and an anticancer drug target. Cancers (Basel) 2019;11(November (11)):1787. doi: 10.3390/cancers11111787. PMID: 31766302; PMCID: PMC6896222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baldin C., Ibrahim A.S. Molecular mechanisms of mucormycosis—the bitter and the sweet. PLoS Pathog. 2017;13(August (8)) doi: 10.1371/journal.ppat.1006408. PMID: 28771587; PMCID: PMC5542377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gebremariam T., Liu M., Luo G., Bruno V., Phan Q.T., Waring A.J., et al. CotH3 mediates fungal invasion of host cells during mucormycosis. J Clin Invest. 2014;124(January (1)):237–250. doi: 10.1172/JCI71349. Epub 2013 Dec 20. PMID: 24355926; PMCID: PMC3871245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allam L., Ghrifi F., Mohammed H., El Hafidi N., El Jaoudi R., El Harti J., et al. Targeting the GRP78-dependant SARS-CoV-2 cell entry by peptides and small molecules. Bioinform Biol Insights. 2020;14(October) doi: 10.1177/1177932220965505. 1177932220965505. PMID: 33149560; PMCID: PMC7585878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alqarihi A., Gebremariam T., Gu Y., Swidergall M., Alkhazraji S., Soliman S.S.M., et al. GRP78 and integrins play different roles in host cell invasion during mucormycosis. mBio. 2020;11(June (3)) doi: 10.1128/mBio.01087-20. e01087–20. PMID: 32487760; PMCID: PMC7267888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu H., Lee M., Solis N., Phan Q.T., Swidergall M., Ralph B., et al. Aspergillus fumigatus CalA binds to integrin α5β1 and mediates host cell invasion. Nat Microbiol. 2017;2:16211. doi: 10.1038/nmicrobiol.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wächtler B., Citiulo F., Jablonowski N., Förster S., Dalle F., Schaller M., et al. Candida albicans-epithelial interactions: dissecting the roles of active penetration, induced endocytosis and host factors on the infection process. PLoS One. 2012;7(5):e36952. doi: 10.1371/journal.pone.0036952. Epub 2012 May 14. PMID: 22606314; PMCID: PMC3351431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stanford F.A., Voigt K. Iron assimilation during emerging infections caused by opportunistic fungi with emphasis on mucorales and the development of antifungal resistance. Genes (Basel) 2020;11(October (11)):1296. doi: 10.3390/genes11111296. PMID: 33143139; PMCID: PMC7693903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu J., Wei C., He J., Zhang L., Zhou J., Balaji K.S., et al. Evaluation and characterization of HSPA5 (GRP78) expression profiles in normal individuals and cancer patients with COVID-19. Int J Biol Sci. 2021;17(February (3)):897–910. doi: 10.7150/ijbs.54055. PMID: 33767597; PMCID: PMC7975696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabirli R., Koseler A., Goren T., Turkcuer I., Kurt O. High GRP78 levels in Covid-19 infection: a case-control study. Life Sci. 2021;265(January) doi: 10.1016/j.lfs.2020.118781. Epub 2020 Nov 19. PMID: 33220289; PMCID: PMC7674149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carlos A.J., Ha D.P., Yeh D.W., Van Krieken R., Tseng C.C., Zhang P., et al. The chaperone GRP78 is a host auxiliary factor for SARS-CoV-2 and GRP78 depleting antibody blocks viral entry and infection. J Biol Chem. 2021;296(May) doi: 10.1016/j.jbc.2021.100759. Epub ahead of print. PMID: 33965375; PMCID: PMC8102082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmeira A., Sousa E., Köseler A., Sabirli R., Gören T., Türkçüer İ, et al. Preliminary virtual screening studies to identify GRP78 inhibitors which may interfere with SARS-CoV-2 infection. Pharmaceuticals (Basel) 2020;13(June (6)):132. doi: 10.3390/ph13060132. PMID: 32630514; PMCID: PMC7345920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rayner J.O., Roberts R.A., Kim J., Poklepovic A., Roberts J.L., Booth L., et al. AR12 (OSU-03012) suppresses GRP78 expression and inhibits SARS-CoV-2 replication. Biochem Pharmacol. 2020;182(December) doi: 10.1016/j.bcp.2020.114227. Epub 2020 Sep 20. PMID: 32966814; PMCID: PMC7502229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vig S., Buitinga M., Rondas D., Crèvecoeur I., van Zandvoort M., Waelkens E., et al. Cytokine-induced translocation of GRP78 to the plasma membrane triggers a pro-apoptotic feedback loop in pancreatic beta cells. Cell Death Dis. 2019;10(April (4)):309. doi: 10.1038/s41419-019-1518-0. PMID: 30952835; PMCID: PMC6450900. [DOI] [PMC free article] [PubMed] [Google Scholar]