Abstract
Parkinson’s disease (PD) is linked to the aberrant self-assembly of the amyloid protein, α-synuclein (αS), where αS monomers aggregate to form oligomers and fibrils. Out of the three conformers, αS oligomers are the major toxic agents in PD, while αS fibrils may work as a reservoir for toxic oligomeric conformers. Thus, compounds that inhibit aggregation of αS monomers and disaggregate αS oligomers and fibrils may serve as therapeutic agents against PD. In this regard, resveratrol and its synthetic derivatives (e.g., AM17, which contains a copper ion-selective ionophoric motif) have previously been examined for their inhibitory effects on aggregation of amyloid proteins, such as β-amyloid implicated in Alzheimer’s disease. In the current study, we employed an array of experimental tools, such as Thioflavin T fluorescence, transmission electron microscopy, immuno-dot blot assays, SDS- and native-PAGE, and circular dichroism, to determine the impact of AM17 and resveratrol on αS aggregation. To the best of our knowledge, we show for the first time that AM17 not only inhibits aggregation of αS monomers but also disaggregates αS oligomers and fibrils, independent of the copper ions. Similar αS aggregation inhibitory effects were observed with resveratrol only in the presence of the copper ion. The present study supports the high promise of applicability of AM17 as an effective amyloid aggregation inhibitor for various conformers and protein sequences.
Keywords: Aggregation, Alpha-synuclein, Amyloid, Fibril, Oligomer, Resveratrol
1. INTRODUCTION
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s disease (AD), and the most common movement disorder [1]. PD is characterized by the loss of substantia nigra dopamine neurons and the presence of Lewy bodies with intracellular protein inclusions that contain the 140-residue protein, α-synuclein (αS, Fig. 1A) [2, 3]. The pathology of PD is tightly connected to αS aggregation [1], where monomeric αS self-associates into soluble αS oligomers, which eventually aggregate into insoluble αS fibrils. The hydrophobic region, residues αS 61-95, known as the non-amyloid component (NAC) [4], is critical in αS self-assembly [5, 6]. While αS monomers are structurally disordered [7, 8], αS oligomers and fibrils are β sheet-structured [9-11] with the more extensive β-sheet structures found in fibrillar aggregates [12]. Among the three conformers, soluble oligomeric αS is the major toxic agent in PD [11, 13-15] and αS fibrils may serve as a reservoir for the toxic oligomeric forms [16]. In contrast, αS monomers are non-toxic. Therefore, inhibition of αS aggregation may be of significant therapeutic relevance. αS-related toxicity is mediated by various mechanisms, such as the generation of oxidative stress [17, 18]. Aggregation behaviors and oxidative stress-dependent toxic effects of αS may be modulated by transition metals, including Cu [19, 20]. Thus, the interactions between Cu and αS play an important role in PD etiology [21, 22].
Figure 1.
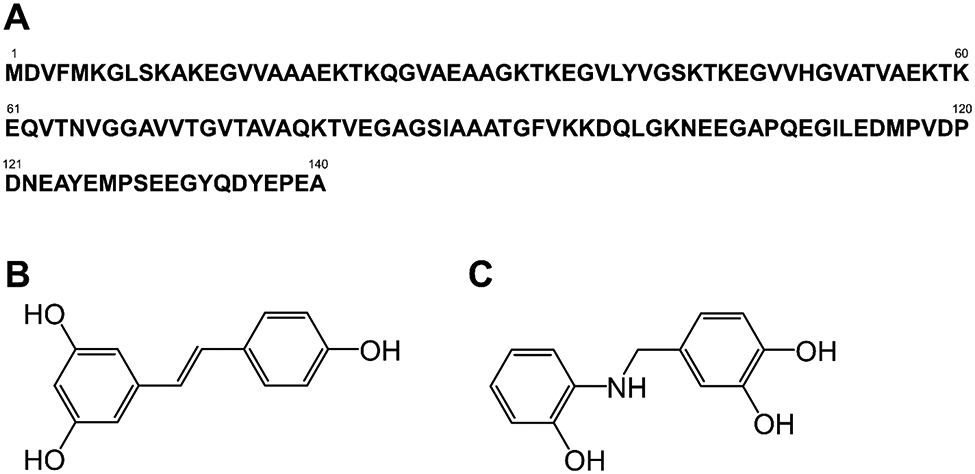
(A) Amino acid sequence of αS and chemical structures of (B) resveratrol and (C) AM17. In (A), several amino acid numbers are shown.
Natural polyphenols have been explored for their potential therapeutic applications in amyloid diseases (e.g., PD and AD) [23]. In this group of molecules, resveratrol (Fig. 1B) has garnered attention due to its modulatory effect on amyloid aggregation [24], antioxidant property [25] and ability to penetrate the blood-brain barrier [26]. Recently, a synthetic derivative of resveratrol, referred to as AM17 (Fig 1C), was created by incorporating a copper ion-selective ionophoric motif into the chemical backbone of resveratrol [27]. AM17 displays strong antioxidant properties, preventing the generation of reactive oxygen species mediated by Cu2+ [27]. AM 17 inhibits aggregation of β-amyloid (Aβ) implicated in AD in both the presence and absence of Cu2+ and disaggregates pre-formed Aβ fibrils [27]. In addition, AM17 can cross the blood-brain barrier, as judged by the parallel artificial membrane permeability assay [28]. Despite such promising observations, the effectiveness of AM17 on inhibiting αS aggregation has yet to be examined. Moreover, the impact of resveratrol on αS aggregation is ill-defined despite its therapeutic potential for PD [29, 30].
In the present study, we characterized effects of AM17 and resveratrol on αS aggregation in the presence and absence of Cu2+ using a comprehensive array of experimental tools. For thorough evaluation, the three major αS conformers found during αS aggregation, namely, monomers, oligomers and fibrils, were prepared and incubated with AM17 or resveratrol. The samples were then subjected to experimental characterizations for evaluation of αS aggregation states. Our study reveals that AM17 strongly inhibits fibrillization of αS monomers while dissociating preformed αS oligomers and αS fibrils, all independent of Cu2+. In contrast, resveratrol displays similar anti-aggregation effects only in the presence of Cu2+. Overall, this study demonstrates the strong anti-amyloidogenic potential of AM17 against αS aggregation and warrants further investigation into this molecule’s therapeutic efficacy.
2. MATERIALS AND METHODS
2.1. αS sample preparation
αS monomers, oligomers and fibrils were prepared, as described previously [6, 31]. To prepare αS monomer samples, lyophilized αS was solubilized in Tris-HCl (20 mM Tris-HCl, 150 mM NaCl, pH 7.4) at room temperature and then filtered through an Amicon Ultra centrifugal filter (100 kDa MWCO, Burlington, MA, USA). The filtrates were collected in a glass vial and kept on ice until use as αS monomer samples. Membrane filters with 100 kDa cutoff were used to isolate αS monomers (molecular weight: 14.5 kDa) from freshly prepared αS solution. This is due to the natively unfolded nature of αS monomers, making their hydrodynamic size similar to that of ~60 kDa globular proteins [7].
For preparation of αS oligomer samples, fresh αS solution was prepared at 350 μM at room temperature and then filtered through 0.45 μm filters into glass vials. The filtered αS solution was subsequently incubated at 37 °C under constant orbital shaking at 250 rpm for 6 hr, followed by filtration through 100 kDa cutoff filters. The retentate was collected at room temperature to obtain αS oligomers.
For αS monomer and oligomer samples, αS concentrations were determined by absorbance at 280 nm with correction for scattering effects [32] using a Varian Cary 50 UV-Vis Spectrophotometer (Agilent Technologies, Santa Clara, CA, USA).
αS fibril samples were prepared by incubating fresh αS solution, which was filtered through a 0.45 μm syringe filter, in glass vials for ~2-3 weeks with constant stirring at 37 °C. The samples were centrifuged to collect insoluble αS fibrils, which were subsequently washed and rinsed with buffer multiple times, followed by resuspension in buffer at room temperature. The αS fibril concentration was determined from concentrations of αS measured prior to the incubation and lost during the washing.
AM17 and resveratrol were dissolved in dimethyl sulfoxide, and CuCl2 was dissolved in Tris-HCl buffer. αS samples (i.e., monomers, oligomers, and fibrils) were then incubated at 37 °C under constant stirring at 100 rpm with and without AM17 or resveratrol in the presence and absence of CuCl2, unless otherwise mentioned. The final concentration in the samples was 100 μM for αS, 0 or 50 μM CuCl2, 0 or 100 μM for AM17 or resveratrol, unless otherwise mentioned. AM17 and Cu2+ were found to form a 2:1 complex [27] and thus the concentration ratio of AM17 to CuCl2 in samples was set accordingly. During the incubation, samples were aliquoted at specific time points for characterization of αS aggregation states. αS concentrations used in this study are monomer-equivalent concentrations.
2.2. Thioflavin T (ThT) fluorescence
Ten μL of ThT solution at 1.0 mM was mixed with 10 μL of αS samples and 180 μL of buffer. The ThT fluorescence of the samples was measured using a Photon Technology QuantaMaster QM-4 spectrofluorometer (Horiba, Kyoto, Japan). The excitation wavelength was set to 440 nm and the emission monitored at 485 nm.
2.3. Transmission electron microscopy (TEM)
Five μL of samples was placed on copper grids and negatively stained with 1% uranyl acetate. A Philips CM12 transmission electron microscope (FEI Corp. Hillsboro, OR, USA) at 120 kV was used for imaging the samples with a 4k x 2.67k GATAN digital camera located at the Image Core Facility of the NYU School of Medicine.
2.4. A11 dot blot assay
One μg of αS in samples was dotted onto nitrocellulose membrane and then air dried at room temperature for 5 min. The membrane was subsequently blocked with 10 mL of 10% milk in 1X TBST buffer (20 mM Tris, 50 mM NaCl2, 1 mM CaCl2 0.25% Tween-20) on a shaker at room temperature for 1 hr. After washing three times with 1X TBST buffer, the membrane was incubated on a shaker overnight in 10 mL of 5% milk with 10 μL of A11 antibody (Thermo Fisher Scientific, Waltham, MA, USA). After washing with 1X TBST buffer again, the membrane was incubated with 1.5 μL of F(ab’)2 goat anti-rabbit IgG (H+L) conjugated with alkaline phosphatase (Thermo Fisher Scientific) in 10 mL of 5% milk for 1 hr. After washing three additional times with 1X TBST buffer and five time with water, chemiluminescence was developed by the addition of CDP-Star substrate, diluted 1/20X in Nitro-Block-II enhancer (Thermo Fisher Scientific), on top of the membrane. Dot blot assays with anti-αS antibodies as primary antibodies were performed similarly according to the manufacturer’s protocols.
2.5. Statistical Analysis
All data are presented as mean ± 1 standard deviation of triplicates, unless otherwise mentioned. Significant differences were determined using two-tailed, unpaired Student’s t-test.
Other experimental details can be found in Supplementary Material.
3. RESULTS
3.1. Effects of AM17 and resveratrol on αS monomers
To determine effects of AM17 and resveratrol on αS aggregation, these compounds were added at an equimolar ratio to freshly prepared αS monomers at 100 μM. The control samples containing αS alone without AM17 or resveratrol were prepared similarly. These αS samples were incubated at 37 °C with constant stirring to initiate aggregation and then aliquoted during the incubation. αS aggregation of the aliquots was examined using an array of experimental tools, including (1) an assay with Thioflavin T (ThT), a dye that exhibits fluorescence ≥ ~100 times more strongly with αS fibrils compared to αS monomers and oligomers [9, 31, 33] (also see Fig. S1), (2) SDS-PAGE to determine the fractions of soluble αS (i.e., monomers and oligomers) vs. insoluble αS (i.e., fibrils), (3) Native-PAGE to monitor the distribution between αS monomers and αS oligomers, (4) circular dichroism (CD) for αS secondary structures, and (5) transmission electron microscopy (TEM) for αS morphology.
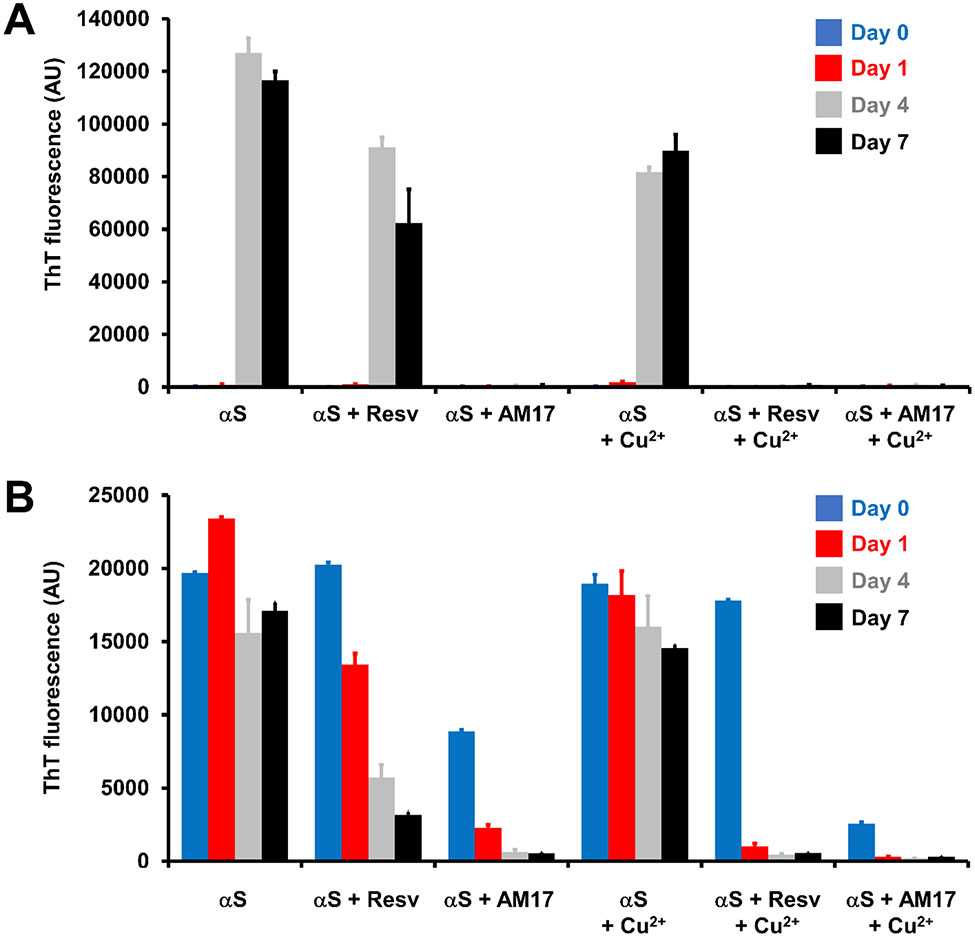
As reported elsewhere [11, 31, 33], the αS control samples showed low to no ThT fluorescence until Day 1, followed by a drastic increase in fluorescence intensity on Day 4 with its value levelling off thereafter (Fig. 2A). ThT fluorescence trends of αS samples measured from three independent experiments were qualitatively similar to each other (compare data on αS alone in Figs. 2A, S2 and S3). Together with the ThT fluorescence data, our results from SDS- and native-PAGE, CD spectroscopy and TEM collectively indicate that soluble, structurally disordered, monomeric αS was converted to insoluble, β-sheet structured, fibrillar aggregates during the 7-day incubation (Figs 3A, S4 and S5A).
Figure 2.
ThT fluorescence of (A) αS monomer and (B) αS fibril samples with and without resveratrol, AM17 and Cu2+ during 7-day incubation at 37 °C with (A) constant stirring and (B) no stirring. During the incubation, αS concentrations were (A) 100 μM or (B) 25 μM, and the concentration ratio was 1:(0 or 1):(0 or 1):(0 or 0.5) for αS:resveratrol:AM17:Cu2+. Error bars: 1 standard deviation of triplicates.
Figure 3.
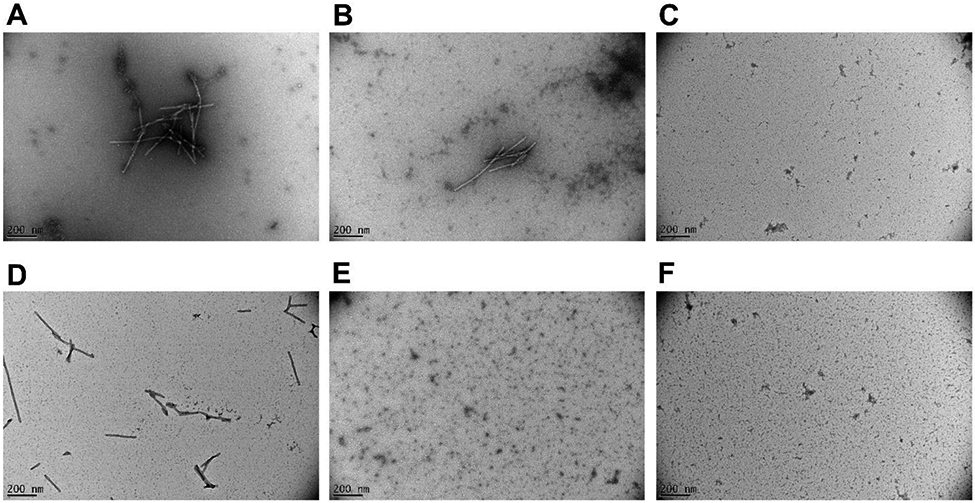
Representative TEM images of samples containing (A) αS monomer alone, (B) αS monomer + resveratrol, (C) αS monomer + AM17, (D) αS monomer + Cu2+, (E) αS monomer + resveratrol + Cu2+, and (F) αS monomer + AM17 + Cu2+ after 7-day incubation at 37 °C with constant stirring.
Compared to the αS control samples, a qualitatively similar ThT fluorescence trend was observed when resveratrol was added to the αS solution (Fig 2A), with the concomitant conversion of soluble, structurally disordered αS monomers to insoluble, β-sheet structured αS fibrils after the 7-day incubation (Figs 3B, S4 and S5B). In contrast, ThT fluorescence of αS samples was negligible in the presence of equimolar AM17 during the incubation (Fig 2A), indicating strong αS fibrillization-inhibitory effects of AM17. The absence of fibrillar aggregates, no loss of soluble αS monomers, and the lack of structural changes after the 7-day incubation were verified in αS samples containing equimolar AM17 (Figs 3C, S4 and S5C). At the substoichiometric ratio, the inhibitory effect of AM17 on αS aggregation appeared weaker (Fig S3). When measured with assumption of single site binding, Ka (the apparent association constant) for binding to αS monomers was calculated to be 1.4 ± 0.1 × 105 M−1 and 0.67 ± 0.01 × 105 M−1 for AM17 and resveratrol, respectively.
Motivated by the previous findings on binding of AM17 to Cu2+ [27] and the implication of this metal ion in PD etiology [21, 22], similar experiments were carried out with Cu2+ at 50 μM. The ThT fluorescence trend of the αS control samples was qualitatively similar whether Cu2+ was added or not (Figs 2A and S2). The formation of β-sheet structured, insoluble αS fibrils and the loss of structurally disordered, soluble αS monomers were observed after the 7-day incubation in the αS control samples containing Cu2+, similar to those without Cu2+ (Figs 3D, S4 and S5D). Interestingly, inhibition of αS aggregation by equimolar resveratrol was observed in the presence of Cu2+ (Figs 2A, 3E, S4 and S5E), but not in the absence of Cu2+ (see the text above). The Cu2+ - dependent αS aggregation inhibitory effect of resveratrol became weaker at the substoichiometric ratio (Fig S3). Similar to the finding without Cu2+, AM17 appeared as strong inhibitors of αS fibrillization when Cu2+ was co-present (Figs 2A, 3F, S4 and S5F) with the inhibitory effect being weaker at the substoichiometric ratio compared to the equimolar ratio (Fig S3). The Ka values for binding of Cu2+ to αS monomers were similar with and without the addition of equimolar AM17 or resveratrol (Table S1), suggesting no strong overlap between binding sites on αS for Cu2+ and these small molecules under our experimental conditions. ThT fluorescence reduction due to inner filter effects of resveratrol and AM17 was relatively minor (see “Inner filter effects” in Supplementary Material for details), indicating that the aforementioned ThT fluorescence changes in αS samples upon addition of these small molecules were driven by their aggregation modulatory effects.
3.2. Effects of AM17 and resveratrol on αS oligomers and αS fibrils
Motivated by inhibition of αS monomer aggregation by AM17 and resveratrol, independent and dependent on Cu2+ respectively, we set out to determine the ability of these compounds to destabilize preformed aggregates. To examine aggregation-inhibitory effects of AM17 and resveratrol on αS oligomeric conformers, preformed αS oligomers were incubated at 25 μM under a quiescent condition with these molecules in the presence and absence of Cu2+. For selective monitoring of oligomeric states in samples taken during the incubation, a dot blot assay was employed with A11, an antibody that selectively detects amyloid oligomers over monomeric and fibrillar species [13].
As expected, αS oligomers were detectable by A11, whereas no such detection was observed with αS monomers and fibrils (Fig S6). ThT fluorescence was not used here, as preformed αS oligomers at 25 μM showed negligible ThT fluorescence (data not shown). Overall, resveratrol and AM17 displayed inhibitory effects on αS oligomers in a similar manner to αS monomers (Fig 4). While αS oligomers remained oligomeric in αS control samples during the incubation, AM17 destabilized the preformed αS oligomers, regardless of the presence or absence of Cu2+ (Fig 4). Moreover, ThT fluorescence of the αS samples containing AM17 remained negligible (data not shown), indicating that AM17 dissociated αS oligomers into αS monomers rather than converting αS oligomers into αS fibrils. AM17 also disrupted oligomeric conformations formed during co-incubation of αS and Aβ monomers (Fig S7), a condition previously found to promote the formation of and enhance stability of amyloid oligomers via co-assembly [33]. For resveratrol, the αS oligomer-inhibitory effects were noticeable only in the presence of Cu2+ (Fig 4), similar to the case with αS monomers.
Figure 4.
A11 dot blot assays of αS oligomer samples with and without resveratrol, AM17 and Cu2+ during 7-day incubation at 37 °C under a quiescent condition. During the incubation, αS concentration was 25 μM with the concentration ratio of 1:(0 or 1):(0 or 1):(0 or 0.5) for αS:resveratrol:AM17:Cu2+.
We extended our study further to examine the impacts of resveratrol and AM17 on preformed αS fibrils. When preformed αS fibrils were diluted with buffer to 25 μM, they remained fibrillar during the 7-day incubation in the presence and absence of Cu2+, as judged by ThT fluorescence (Fig 2B). The addition of resveratrol lowered ThT fluorescence of αS fibril samples over time, indicating that resveratrol dissociated αS fibrillar aggregates, with the effects becoming greater in the presence of Cu2+ (Fig 2B). AM17 was effective for dissociating αS fibrils independent of Cu2+ with > 2-fold ThT fluorescence decreases observed immediately after addition of AM17 (Fig 2B).
4. DISCUSSION
The present study demonstrates that AM17 prevents aggregation of αS monomers while dissociating preformed αS oligomers and αS fibrils. The observed impacts of AM17 on the three αS conformers (representing the major αS aggregate species) support the high promise of this resveratrol derivative as a powerful αS aggregation inhibitor. AM17 displays strong antioxidant properties [27] and is aggregation-inhibitory, independent of Cu2+, for Aβ [27] and αS (in the current study), further supporting high applicability of AM17 for multiple molecular events associated with various amyloid diseases. Interestingly, we observed that resveratrol inhibited αS aggregation depending on Cu2+. No such Cu2+dependency was observed with resveratrol for its inhibition of Aβ aggregation [27].
AM17 forms a 2:1 complex with Cu2+ (AM17/Cu2+) with Ka of ~4.0 × 1012 M−1 [27], whereas αS monomers bind to Cu2+ with Ka of 1.8 × 105 M−1 (Table S1). With these values, Cu2+ would bind to AM17 more preferentially than αS. Thus, the observed αS aggregation inhibition in presence of Cu2+ ions might occur via the AM17/Cu2+ complex. In addition, our results suggest that both AM17 and the AM17/Cu2+ complex display similar abilities to inhibit αS aggregation. The major Cu2+ binding sites on αS are located at its N-terminus (including Met1 [19, 34] and His 50 [35]), which readily interacts with its C-terminus [36]. Our result suggests no strong overlap between binding sites on αS for Cu2+ and AM17 (Table S1). As such, the Cu2+-independent effect of AM17 implies that AM17 might act on αS regions, which are critical in αS aggregation and distant from the two αS termini, such as the NAC domain or a part thereof [5, 6]. In contrast, αS aggregation-inhibitory potential of resveratrol depended on Cu2+. Given consideration of relatively weak binding between resveratrol and Cu2+ at a ratio of 2:1 (Ka ~ 5.0 × 106 M−1) [27], the dominant fraction of Cu2+ would bind to αS at its N-terminus in the presence of resveratrol. A computational study suggested binding of resveratrol to the αS C-terminus [37]. Thus, the co-presence of Cu2+ and resveratrol might alter αS aggregation behaviors by targeting the αS N- and C-termini, respectively. Though Cu2+ was previously shown to accelerate αS fibrilization [35, 38], no such effect was found in our study. The observed discrepancy is presumably due to differences in Cu2+ concentration or pH [35, 38], which may directly impact αS fibrillization kinetics [39].
Taken together, the present study shows strong αS aggregation inhibitory potential of AM17, acting on the three major αS conformers. Our study warrants testing of AM17’s therapeutic potential under biological contexts of PD.
Supplementary Material
HIGHLIGHTS.
A resveratrol derivative, AM17, inhibits aggregation of α-synuclein (αS) monomers
AM17 disaggregates αS oligomers and fibrils
AM17’s anti-αS aggregation effects are independent of a copper ion
Resveratrol inhibits αS aggregation depending on a copper ion
ACKNOWLEDGEMENTS
The authors thank Han Teng Li and Ego Wong for collection of preliminary data.
FUNDING
The authors thank supports from the NIH/NIA Grant R21AG049137 and the PSC-CUNY grant #66044-00 44.
Abbreviations:
- PD
Parkinson’s disease
- AD
Alzheimer’s disease
- αS
α-synuclein
- NAC
non-amyloid component
- Aβ
β-amyloid
- ThT
Thioflavin T
- TEM
transmission electron microscopy
- SDS
sodium dodecyl sulfate
- PAGE
polyacrylamide gel electrophoresis
- CD
circular dichroism
Footnotes
DECLARATIONS OF COMPETING INTEREST
None
SUPPLEMENTARY MATERIAL
Supplementary texts, Table S1, and Figs S1-S7 are available in Supplementary Material.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Johnson ME, Stecher B, Labrie V, Brundin L, Brundin P, Triggers, Facilitators, and Aggravators: Redefining Parkinson's Disease Pathogenesis, Trends Neurosci, 42 (2019) 4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Breydo L, Wu JW, Uversky VN, Alpha-synuclein misfolding and Parkinson's disease, Biochim Biophys Acta, 1822 (2012) 261–285. [DOI] [PubMed] [Google Scholar]
- [3].Irwin DJ, Lee VM, Trojanowski JQ, Parkinson's disease dementia: convergence of alpha-synuclein, tau and amyloid-beta pathologies, Nat Rev Neurosci, 14 (2013) 626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yoshimoto M, Iwai A, Kang D, Otero DA, Xia Y, Saitoh T, NACP, the precursor protein of the non-amyloid beta/A4 protein (A beta) component of Alzheimer disease amyloid, binds A beta and stimulates A beta aggregation, Proc Natl Acad Sci U S A, 92 (1995) 9141–9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Giasson BI, Murray IV, Trojanowski JQ, Lee VM, A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly, J Biol Chem, 276 (2001) 2380–2386. [DOI] [PubMed] [Google Scholar]
- [6].Hernandez M, Golbert S, Zhang LG, Kim JR, Creation of aggregation-defective alpha-synuclein variants by engineering the sequence connecting beta-strand-forming domains, Chembiochem, 12 (2011) 2630–2639. [DOI] [PubMed] [Google Scholar]
- [7].Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT Jr., NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded, Biochemistry, 35 (1996) 13709–13715. [DOI] [PubMed] [Google Scholar]
- [8].Eliezer D, Kutluay E, Bussell R Jr., Browne G, Conformational properties of alpha-synuclein in its free and lipid-associated states, J Mol Biol, 307 (2001) 1061–1073. [DOI] [PubMed] [Google Scholar]
- [9].Apetri MM, Maiti NC, Zagorski MG, Carey PR, Anderson VE, Secondary structure of alpha-synuclein oligomers: characterization by raman and atomic force microscopy, J Mol Biol, 355 (2006) 63–71. [DOI] [PubMed] [Google Scholar]
- [10].Zhou L, Kurouski D, Structural Characterization of Individual alpha-Synuclein Oligomers Formed at Different Stages of Protein Aggregation by Atomic Force Microscopy-Infrared Spectroscopy, Anal Chem, 92 (2020) 6806–6810. [DOI] [PubMed] [Google Scholar]
- [11].Celej MS, Sarroukh R, Goormaghtigh E, Fidelio GD, Ruysschaert JM, Raussens V, Toxic prefibrillar alpha-synuclein amyloid oligomers adopt a distinctive antiparallel beta-sheet structure, Biochem J, 443 (2012) 719–726. [DOI] [PubMed] [Google Scholar]
- [12].Vilar M, Chou HT, Luhrs T, Maji SK, Riek-Loher D, Verel R, Manning G, Stahlberg H, Riek R, The fold of alpha-synuclein fibrils, Proc Natl Acad Sci U S A, 105 (2008) 8637–8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG, Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis, Science, 300 (2003) 486–489. [DOI] [PubMed] [Google Scholar]
- [14].Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, Tzitzilonis C, Soragni A, Jessberger S, Mira H, Consiglio A, Pham E, Masliah E, Gage FH, Riek R, In vivo demonstration that alpha-synuclein oligomers are toxic, Proc Natl Acad Sci U S A, 108 (2011) 4194–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Alam P, Bousset L, Melki R, Otzen DE, alpha-synuclein oligomers and fibrils: a spectrum of species, a spectrum of toxicities, J Neurochem, 150 (2019) 522–534. [DOI] [PubMed] [Google Scholar]
- [16].Cascella R, Chen SW, Bigi A, Camino JD, Xu CK, Dobson CM, Chiti F, Cremades N, Cecchi C, The release of toxic oligomers from alpha-synuclein fibrils induces dysfunction in neuronal cells, Nat Commun, 12 (2021) 1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cristovao AC, Guhathakurta S, Bok E, Je G, Yoo SD, Choi DH, Kim YS, NADPH oxidase 1 mediates alpha-synucleinopathy in Parkinson's disease, J Neurosci, 32 (2012) 14465–14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sun F, Deng Y, Han X, Liu Q, Zhang P, Manzoor R, Ma H, A secret that underlies Parkinson's disease: The damaging cycle, Neurochem Int, 129 (2019) 104484. [DOI] [PubMed] [Google Scholar]
- [19].Wang C, Liu L, Zhang L, Peng Y, Zhou F, Redox reactions of the alpha-synuclein-Cu(2+) complex and their effects on neuronal cell viability, Biochemistry, 49 (2010) 8134–8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang X, Moualla D, Wright JA, Brown DR, Copper binding regulates intracellular alpha-synuclein localisation, aggregation and toxicity, J Neurochem, 113 (2010) 704–714. [DOI] [PubMed] [Google Scholar]
- [21].Brown DR, Metal binding to alpha-synuclein peptides and its contribution to toxicity, Biochem Biophys Res Commun, 380 (2009) 377–381. [DOI] [PubMed] [Google Scholar]
- [22].Okita Y, Rcom-H'cheo-Gauthier AN, Goulding M, Chung RS, Faller P, Pountney DL, Metallothionein, Copper and Alpha-Synuclein in Alpha-Synucleinopathies, Front Neurosci, 11 (2017) 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].El Gaamouch F, Liu K, Lin HY, Wu C, Wang J, Development of grape polyphenols as multi-targeting strategies for Alzheimer's disease, Neurochem Int, 147 (2021) 105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ladiwala AR, Lin JC, Bale SS, Marcelino-Cruz AM, Bhattacharya M, Dordick JS, Tessier PM, Resveratrol selectively remodels soluble oligomers and fibrils of amyloid Abeta into off-pathway conformers, J Biol Chem, 285 (2010) 24228–24237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, Shi X, Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses, Biochem Biophys Res Commun, 309 (2003) 1017–1026. [DOI] [PubMed] [Google Scholar]
- [26].Shayganfard M, Molecular and biological functions of resveratrol in psychiatric disorders: a review of recent evidence, Cell Biosci, 10 (2020) 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Martinez A, Alcendor R, Rahman T, Podgorny M, Sanogo I, McCurdy R, Ionophoric polyphenols selectively bind Cu(2+), display potent antioxidant and anti-amyloidogenic properties, and are non-toxic toward Tetrahymena thermophila, Bioorg Med Chem, 24 (2016) 3657–3670. [DOI] [PubMed] [Google Scholar]
- [28].Martinez A, Zahran M, Gomez M, Guevara J, Pichardo-Bueno R, Asim J, Ortiz G, Andoh Y, Shibutani S, Kaur B, Ionophoric polyphenols are permeable to the blood-brain barrier, interact with human serum albumin and Calf Thymus DNA, and inhibit AChE enzymatic activity, Med Chem Res, 29 (2020) 1956–1975. [Google Scholar]
- [29].Arbo BD, Andre-Miral C, Nasre-Nasser RG, Schimith LE, Santos MG, Costa-Silva D, Muccillo-Baisch AL, Hort MA, Resveratrol Derivatives as Potential Treatments for Alzheimer's and Parkinson's Disease, Front Aging Neurosci, 12 (2020) 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Singh A, Tripathi P, Yadawa AK, Singh S, Promising Polyphenols in Parkinson's Disease Therapeutics, Neurochem Res, 45 (2020) 1731–1745. [DOI] [PubMed] [Google Scholar]
- [31].Hernandez M, Hu Y, Kim JR, A conformation-switching fluorescent protein probe for detection of alpha synuclein oligomers, Chem Commun (Camb), 49 (2013) 10712–10714. [DOI] [PubMed] [Google Scholar]
- [32].Mach H, Volkin DB, Burke CJ, Middaugh CR, Ultraviolet absorption spectroscopy, Methods Mol Biol, 40 (1995) 91–114. [DOI] [PubMed] [Google Scholar]
- [33].Candreva J, Chau E, Rice ME, Kim JR, Interactions between Soluble Species of beta-Amyloid and alpha-Synuclein Promote Oligomerization while Inhibiting Fibrillization, Biochemistry, 59 (2020) 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jackson MS, Lee JC, Identification of the minimal copper(II)-binding alpha-synuclein sequence, Inorg Chem, 48 (2009) 9303–9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rasia RM, Bertoncini CW, Marsh D, Hoyer W, Cherny D, Zweckstetter M, Griesinger C, Jovin TM, Fernandez CO, Structural characterization of copper(II) binding to alpha-synuclein: Insights into the bioinorganic chemistry of Parkinson's disease, Proc Natl Acad Sci U S A, 102 (2005) 4294–4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ranjan P, Kumar A, Perturbation in Long-Range Contacts Modulates the Kinetics of Amyloid Formation in alpha-Synuclein Familial Mutants, ACS Chem Neurosci, 8 (2017) 2235–2246. [DOI] [PubMed] [Google Scholar]
- [37].Gautam S, Karmakar S, Batra R, Sharma P, Pradhan P, Singh J, Kundu B, Chowdhury PK, Polyphenols in combination with beta-cyclodextrin can inhibit and disaggregate alpha-synuclein amyloids under cell mimicking conditions: A promising therapeutic alternative, Biochim Biophys Acta Proteins Proteom, 1865 (2017) 589–603. [DOI] [PubMed] [Google Scholar]
- [38].Uversky VN, Li J, Fink AL, Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson's disease and heavy metal exposure, J Biol Chem, 276 (2001) 44284–44296. [DOI] [PubMed] [Google Scholar]
- [39].Uversky VN, Li J, Fink AL, Evidence for a partially folded intermediate in alpha-synuclein fibril formation, J Biol Chem, 276 (2001) 10737–10744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.