Abstract
Purpose
Age-related cataract is the leading cause of blindness worldwide. Variants in the EPHA2 gene increase the disease risk, and its knockout in mice causes cataract. We investigated whether age, sex, and genetic background, risk factors for age-related cataract, and Epha2 genotype influence Epha2-related cataract development in mice.
Methods
Cataract development was monitored in Epha2+/+, Epha2+/−, and Epha2−/− mice (Epha2Gt(KST085)Byg) on C57BL/6J and FVB:C57BL/6J (50:50) backgrounds. Cellular architecture of lenses, endoplasmic reticulum (ER) stress, and redox state were determined using histological, molecular, and analytical techniques.
Results
Epha2−/− and Epha2+/− mice on C57BL/6J background developed severe cortical cataracts by 18 and 38 weeks of age, respectively, compared to development of similar cataract significantly later in Epha2−/− mice and no cataract in Epha2+/− mice in this strain on FVB background, which was previously reported. On FVB:C57BL/6J background, Epha2−/− mice developed severe cortical cataract by 38 weeks and Epha2+/− mice exhibited mild cortical cataract up to 64 weeks of age. Progression of cataract in Epha2−/− and Epha2+/− female mice on C57BL/6J and mixed background, respectively, was slower than in matched male mice. N-cadherin and β-catenin immunolabeling showed disorganized lens fiber cells and disruption of lens architecture in Epha2−/− and Epha2+/− lenses, coinciding with development of severe cataracts. EPHA2 immunolabeling showed intracellular accumulation of the mutant EPHA2-β-galactosidase fusion protein that induced a cytoprotective ER stress response and in Epha2+/− lenses was also accompanied by glutathione redox imbalance.
Conclusions
Both, Epha2−/− and Epha2+/− mice develop age-related cortical cataract; age as a function of Epha2 genotype, sex, and genetic background influence Epha2-related cataractogenesis in mice.
Keywords: Epha2, biological factors, mouse model, age-related cataract
Age-related cataract generally occurs after the age of 45 years due to progressive accumulation of insults in the ocular lens,1 and accounts for approximately 48% of blindness worldwide.2 It is a multifactorial disease with an interplay of environmental and genetic factors contributing to the disease risk. Older age, female gender, lifestyle choices, such as smoking and alcohol consumption, exposure to ultraviolet radiation from sunlight, corticosteroid use, and diseases, such as diabetes, pseudoexfoliation syndrome, and myopia, increase the risk of cataract.2–8 Depending upon the location of lens opacity, the disease presents as nuclear, cortical, and posterior subcapsular cataract, or mixed cataract involving more than one of these subtypes. Several epidemiological studies have shown ethnic differences in the prevalence of age-related cataract subtypes, likely due to differences in lifestyle, climatic environment, and genetic background.9–14 Genetic predisposition to cataract development has been evidenced through twin studies and clustering of the disease within families.15–17 Single nucleotide polymorphisms (SNPs) in multiple genes have been associated with increased susceptibility to age-related cataract.18–28
To date, genetic variation in the EPHA2 gene is the only genetic factor that has been reproducibly associated with the risk of age-related cataract in multiple geographically and ethnically diverse populations.29–32 SNPs in and around the EPHA2 gene are associated with the risk of all subtypes of the disease. In addition, a mis-sense coding variant in this gene causes Mendelian age-related cortical cataract with incomplete penetrance.33 Protein truncating and mis-sense mutations in the EPHA2 gene have been implicated in congenital cataract.33–36
EPHA2 encodes a membrane protein of the Eph tyrosine kinase receptor family that binds to membrane bound ephrin ligands on neighboring cells and leads to bidirectional signaling.37 EPHA2 signaling plays a role in epithelial homeostasis during development and adulthood.30,38,39 Interestingly, Epha2-null mice progressively develop cortical cataract with aging.30 The loss of EPHRIN-A5, a disputed ligand of EPHA2 in the lens, also leads to age-related cataract in mice.40,41 Thus, EPHA2 signaling is pivotal for mammalian lens development and for maintaining lens transparency throughout life.
Age-related cataract has been reported only in Epha2-null mice; mice carrying a single functional allele of Epha2, heterozygous-null, were not found to develop cataract up to 14 months of age.30 Whether Epha2 heterozygous-null mice develop cataract at a later age is not known. Interestingly, biological factors, such as age and female gender, that increase the risk of age-related cataract,6,8,42,43 also influence EPHA2 expression and/or signaling; EPHA2 expression reduces in mouse lens with age,30 and in cultured mammary epithelial cells, estrogen negatively regulates EPHA2 signaling.44 Hence, we hypothesized that biological factors that increase the risk of age-related cataract, particularly, age, genetic background, and sex, interact with EPHA2 signaling and influence cataract development, leading to cataract with increase in age in Epha2 heterozygous-null mice. In the present study, we tested this hypothesis in Epha2-knockout mice and found that age as a function of Epha2 genotype, genetic background, and sex significantly influence Epha2-related cataract development.
Materials and Methods
Animal Maintenance and Breeding
All experimental procedures on animals conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Animal Welfare Committee, Flinders University, South Australia.
The Epha2-knockout mice (B6; 129P2-Epha2Gt(KST085)Byg; KST085) on C57BL/6J background, generated using the secretory gene trapping method,45,46 were purchased from Mutant Mouse Regional Resources Centers, Missouri, USA. The anterior subcapsular cataract-causing deletion mutation in the Beaded filament structural protein 2 (Bfsp2) gene present in the 129P2 strain47,48 was eliminated by selecting no mutation carriers as breeders for colony maintenance, as identified by polymerase chain reaction (PCR)-based Bfsp2 genotyping in the first two generations. As the Epha2-knockout mice carry Epha2 gene-trapped allele/s, the symbols Epha2+/− and Epha2−/− used hereafter represent heterozygous and homozygous Epha2 gene-trap allele/s, respectively. To generate Epha2-knockout mice on FVB:C57BL/6J (50:50) mixed background, a homozygous Epha2-knockout (Epha2−/−) mouse on C57BL/6J background was crossed with a wild-type FVB/NJ mouse, and three pairs of resulting heterozygous Epha2-knockout (Epha2+/−) F2 mice on mixed background were interbred for colony maintenance. Epha2+/+, Epha2+/−, and Epha2−/− littermates from subsequent generations of these mice were used for experiments.
Genotyping
The FVB/NJ strain used for generation of Epha2-knockout mice on mixed background also carries the anterior subcapsular cataract causing mutation in the Bfsp2 gene.47,48 Hence, all mice on mixed background used in this study were genotyped for that and the Epha2 knockout mutation. Bfsp2 and Epha2 genotyping was performed as previously described,49 except for genotyping Bfsp2 mutation in some mice on mixed background mutant reverse primer 5′AGGGAGATCCTCTTGCTATCTAGCT was used, and PCR performed with annealing at 62°C for 40 cycles.
Ophthalmic Examination
Ophthalmic examination of the lens was performed in anesthetized mice on a photo-slit lamp biomicroscope (Topcon Medical Systems Inc., Oakland, NJ, USA) as previously described;49 cortical cataract was graded using Lens Opacities Classification System (LOCS) III in each eye of an animal.50 In mice on mixed background, anterior subcapsular cataract was also graded using the grading system similar to that used previously.49 The observations were recorded by digital photography at 40 × magnification and animals revived after examination as previously described.49
Histological Analysis
Mice were euthanized and whole eyes enucleated, fixed in 10% neutral buffer formalin, and paraffin embedded for histology. Histological analysis and imaging, using a 40 × objective, were performed as previously described.49,51
Immunolabeling
Antigens were retrieved by heat-induced epitope retrieval in 0.01 M citrate buffer with 0.05% Tween 20, pH 6 for N-cadherin and β-catenin and in Target Retrieval Solution, pH 9 (Dako Australia Pty Ltd., New South Wales, Australia) for EPHA2 labeling, as described elsewhere.51 Sections were blocked with 3% goat or donkey serum (Sigma-Aldrich Pty. Ltd., Sydney, Australia), incubated with mouse anti-N-cadherin (1:200; Life Technologies Australia Pty. Ltd., Victoria, Australia) or mouse anti-β-catenin (1:200; BD Transduction Laboratories, San Diego, CA, USA) or goat anti-mouse (m) EPHA2 (1:40; R&D Systems, Inc., Minneapolis, MN, USA) primary antibody followed by goat anti-mouse or donkey anti-goat IgG Alexa Fluor 488-conjugated (1:1000; Life Technologies Australia Pty. Ltd.) secondary antibody; control sections were incubated with equivalent amount of mouse or goat IgG. Labeled sections were mounted in Prolong AntiFade with DAPI (Life Technologies Australia Pty. Ltd.) and imaged as previously described.52
Western Blotting
Mice were euthanized and ocular lenses dissected and snap frozen for later protein extraction. For protein extraction, whole lens tissue was homogenized in 50 or 100 µl RIPA buffer,53 incubated on ice for 30 minutes, centrifuged to remove insoluble fraction, and clear lysate used for analysis. Forty microgram of lens protein per sample was size-separated by SDS-PAGE on 4 to 20% gradient Mini-PROTEAN TGX Stain-Free pre-cast gel (BioRad Laboratories Pty. Ltd., New South Wales, Australia), and proteins transferred onto polyvinylidene fluoride-low fluorescence (PVDF-LF) membrane in Trans-Blot Turbo buffer (BioRad Laboratories Pty. Ltd.) with 15% or 20% methanol on a Trans-Blot Turbo Transfer System (BioRad Laboratories Pty. Ltd.) as per the manufacturer's protocols. Protein gel and blot were imaged on a Chemi Doc Touch Imaging System (BioRad Laboratories Pty. Ltd.) to monitor protein transfer and document protein loading. Western blots were incubated with rabbit anti-β-galactosidase (1:500; Novus Biologicals, LLC, Centennial, CO, USA) or rabbit anti-BiP (1:1000; Cell Signaling Technology, Danvers, MA, USA) or rabbit anti-ATF6 (1:1000; Sigma-Aldrich) or mouse anti-CHOP (1:1000; Cell Signaling Technology) primary antibody followed by donkey anti-rabbit IgG-horseradish peroxidase conjugated (1:1000; Cat #711-035-152, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) or donkey anti-mouse IgG-horseradish peroxidase conjugated (1:1000; Cat #715-35-150, Jackson ImmunoResearch Laboratories) secondary antibody. Antibody binding was detected with Clarity Western ECL Substrate (BioRad Laboratories Pty. Ltd.) and imaged as described above and following the manufacturer's protocol.
Xbp1 mRNA Splicing
Total RNA from both lenses of a mouse was extracted, and cDNA reverse transcribed (RT) from 0.74 µg RNA, as previously described.49 Xbp1 transcript was amplified from cDNA by PCR using gene-specific primers54 and Hot Star Taq Plus DNA polymerase (Qiagen Pty. Ltd., Victoria, Australia) with Q solution. PCR conditions included enzyme activation at 95°C for 5 minutes, followed by 35 or 37 cycles of denaturation at 95°C, annealing at 62°C, and elongation at 72°C for 30 seconds each, and a final elongation at 72°C for 5 minutes. Amplicons were cleaned with Wizard SV Gel and PCR Clean-up System (Promega Australia, New South Wales, Australia), PstI digested and analyzed by agarose gel electrophoresis. Band intensities were quantified using the ImageJ software. Percent amount of spliced Xbp1 mRNA was assessed as described elsewhere.54 Specificity of amplicons was confirmed by sequencing.
Bip mRNA Expression
Total RNA was extracted, and cDNA synthesized as described above. From cDNA, Bip transcript was amplified by PCR using gene-specific primers 5′TGTGTGAGACCAGAACCGTC (forward) and 5′TCGCTGGGCATCATTGAAGT (reverse), and Hot Star Taq Plus DNA polymerase with Q solution. PCR was performed with enzyme activation at 95°C for 5 minutes, followed by 30 cycles of denaturation at 95°C for 30 seconds, annealing at 62°C for 30 seconds and elongation at 72°C for 45 seconds, and a final elongation at 72°C for 5 minutes. B2m transcript was amplified, to use as normalization control, as previously described,49 except amplification was performed for 39 cycles. Amplicons were analyzed by agarose gel electrophoresis and band intensities quantified using the ImageJ software. Specificity of amplicons was confirmed by sequencing.
Chromatography
Mouse lenses were immediately snap-frozen after dissection for later analysis. The lenses were homogenized as described in the Supplementary Methods. Free glutathione (GSH), glutathione disulfide (GSSG), and total soluble GSH were determined using the method described by Altes et al.55 with slight modification (i.e. inclusion of N-acetylcysteine as an internal standard). Concentration of protein-bound GSH (PSSG) was estimated as described in the Supplementary Methods. Protein concentration was determined using the Bradford method.56 The calibration curve used for determination of free GSH, GSSG, and total GSH is included in Supplementary Figure S1, and for determination of PSSG in Supplementary Figure S2. Levels of GSH and GSSG are reported as nmol/mg of protein, and of PSSG as nmol/mg of dried tissue.
Statistical Analysis
Statistical analyses of cataract grading data were performed using IBM Statistical Package for the Social Science (SPSS) 19. Anterior cortical cataract (ACC) grade in each animal was calculated as an average of cataract grades in the two eyes. Data for each group are presented as mean cortical cataract grade. Effect of Epha2 genotype at an age and of sex on cataract progression were analyzed using the Kruskal-Wallis test and significant differences analyzed pairwise using the Mann-Whitney U test. Effect of age on cataract progression in a genotype was analyzed using the Friedman test, and any significant differences further analyzed by the Wilcoxon Signed Ranks test. Statistical analyses of Xbp1 mRNA splicing and chromatography data were performed using GraphPad Prism 8 (version 8.4.0; GraphPad Software, San Diego, CA, USA). Differences in Xbp1 mRNA splicing between genotypes were compared by ANOVA with Dunnett's T test for multiple comparisons. Each parameter estimated by chromatography was compared between two genotypes by two-tailed t-test. Significance level was set at 0.05 or as indicated.
Results
Age and Genotype Influence Epha2-related Cataract Development
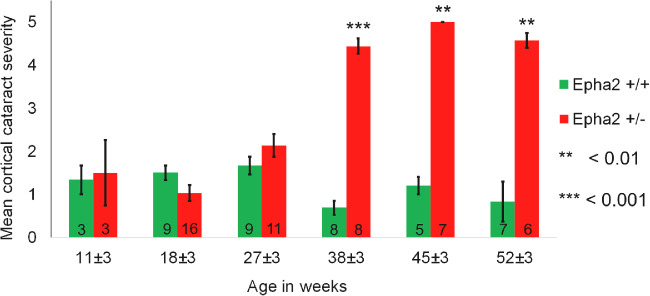
In order to determine whether Epha2 heterozygous-null mice develop cataract with increase in age, different groups of 11, 18, 27, 38, 45, and 52 weeks old Epha2 wild-type (Epha2+/+) and Epha2+/− mice on C57BL/6J background were examined for lens opacity. Epha2+/+ and Epha2+/− 11, 18, and 27-week-old mice exhibited similar mild anterior cortical lens opacity (Fig. 1, Supplementary Table S1) whereas 38, 45, and 52-week-old Epha2+/− mice exhibited significantly severe anterior cortical opacities (grade 4 or 5) than age-matched Epha2+/+ mice (38 weeks, P < 0.001; 45 and 52 weeks, P < 0.01; see Fig. 1, Supplementary Table S1), indicating development of ACC. As mild lens opacity seen in Epha2+/+ mice of all ages did not progress with age and was reversible, we concluded that it was transiently induced by anesthesia, as reported elsewhere.57
Figure 1.
Comparison of anterior cortical lens opacity in Epha2+/+ and Epha2+/− mice of different ages. Anterior cortical cataract was examined in groups of 11, 18, 27, 38, 45, and 52-week-old Epha2+/+ and Epha2+/− mice on C57BL/6J background. The bars represent mean cortical cataract grade in each age group. Data from each age group was compared by Mann-Whitney U test. Significant P values are indicated. The group sizes are indicated in the bars. Error bars represent standard error of the mean.
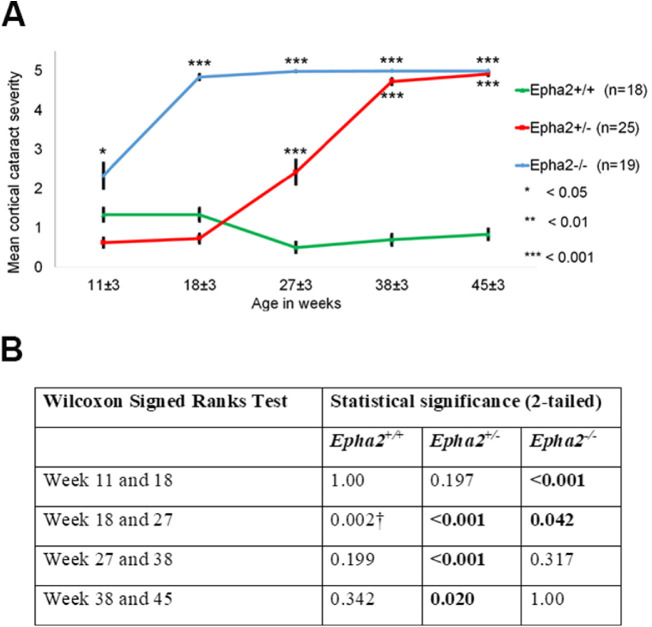
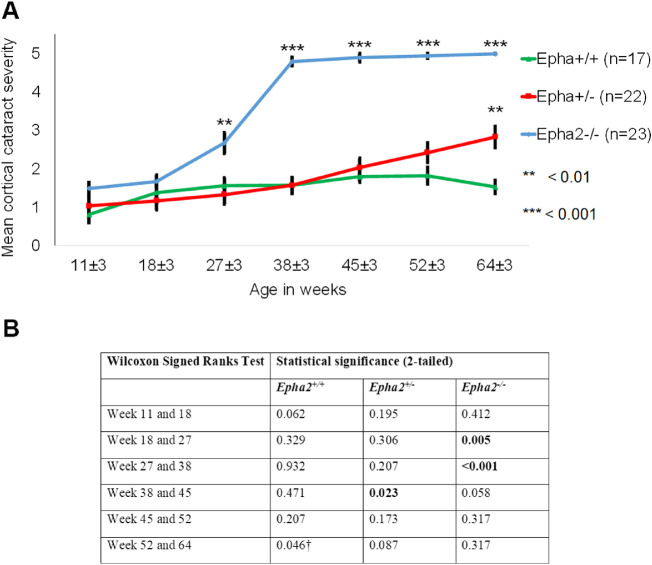
The phenotype of cataract observed in Epha2+/− mice was consistent with that reported in Epha2−/− mice in previous studies,30,39 and manifestation of severe cataract in 38-week-old and older mice supported our hypothesis. This finding is significantly different than no cataract reported in a previous study in Epha2+/− mice carrying the same knockout mutation on FVB background.30 Thus, to confirm this finding, in an independent experiment, we determined progression of cataract in cohorts of Epha2+/+, Epha2+/−, and Epha2−/− mice (n ≥ 18 per group) on C57BL/6J background from 11 to 45 weeks of age or until severe cataract developed (grade 4 to 5). Examinations were performed at approximately eight weekly intervals. Epha2+/+ mice exhibited anesthetic-induced mild ACC (grade < 2) up to 45 weeks of age. In comparison, Epha2+/− mice presented with mild ACC up to 18 weeks, moderate ACC by 27 weeks (grade >2, P < 0.001) and severe ACC by 38 or 45 weeks of age (grade 4 or 5, P < 0.001; Figs. 2A, 3, Supplementary Table S2). The differences in severity of cataract in Epha2+/− mice between different ages were statistically significant (Fig. 2B). In Epha2−/− mice, moderately severe ACC was observed earlier, at 11 weeks of age (grade >2, P < 0.05), and severe ACC by 18 or 27 weeks of age (grade 4 or 5, P < 0.001), compared to Epha2+/+ mice (see Figs. 2A, 3, Supplementary Table 2). The severity of cataract in these mice differed significantly between ages (see Fig. 2B). The observed differences in cataract severity between Epha2+/+, Epha2+/−, and Epha2−/− mice persisted up to 45 weeks of age (P < 0.001; see Fig. 2A). Overall, these experiments showed that Epha2+/− mice also develop age-related cataract and revealed a significant interactive effect of age and Epha2 genotype on cataract development in Epha2-knockout mice on C57BL/6J background.
Figure 2.
Progression of anterior cortical cataract in Epha2-knockout mice.Epha2+/+, Epha2+/−, and Epha2−/− mice on C57BL/6J background were monitored for cataract development from 11 ± 3 weeks to 45 ± 3 weeks of age. (A) Mean cortical cataract grade in each genotype of mice at each time point has been graphically represented. Epha2−/− mice developed severe cortical cataract at 18 ± 3 weeks of age and Epha2+/− mice at 38 ± 3 weeks of age. Epha2+/+ mice presented with very mild lens opacity up to 45 ± 3 weeks of age. The Mann Whitney U test P values from comparison with Epha2+/+ mice are indicated. Error bars represent standard error of the mean. After an animal exhibited a cataract grade of 4 or 5, the same grade was attributed to subsequent time points for data analysis, assuming no further increase in cataract severity occurred. Missing data at the first time point was substituted with data from the second time point assuming lesser cataract severity at the first than second time point. (B) The table shows Wilcoxon Signed Ranks test P values of comparison between time points indicating the effect of age on cataract progression in each genotype of mice. † Indicates likely artifact of anesthetic-induced cataract.
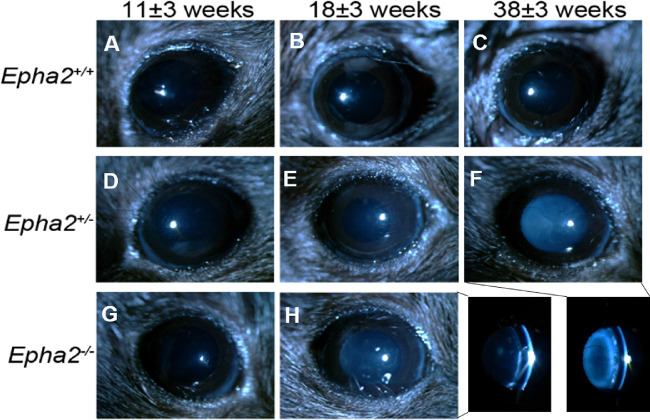
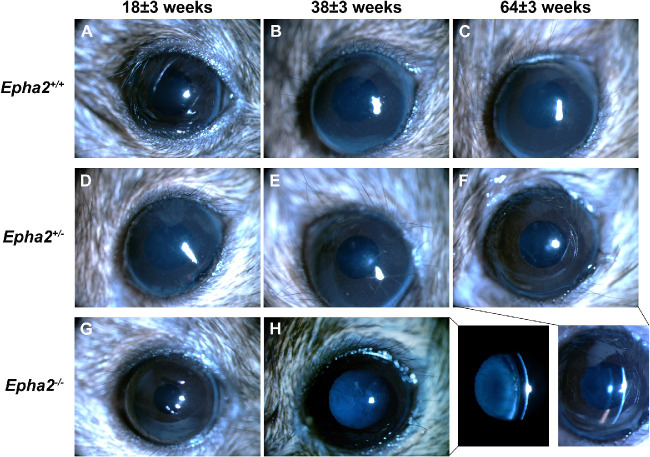
Figure 3.
Anterior cortical cataract developed in Epha2-knockout mice. Representative ophthalmic images of Epha2+/+ (A, B, C) and Epha2+/− (D, E, F) mice at 11 ± 3, 18 ± 3, and 38 ± 3 weeks of age and Epha2−/− (G, H) mice at 11 ± 3 and 18 ± 3 weeks of age, on C57BL/6J background, are shown. Epha2−/− mice progressively developed severe ACC by 18 ± 3 weeks of age, panel H, and Epha2+/− mice by 38 ± 3 weeks of age, panel F. The bottom right panel shows cross-section illumination images of cortical cataracts seen in panels H and F. Lenses of Epha2+/+ mice remained relatively clear up to 38 ± 3 weeks of age, panel C, and later (not shown). Magnification, times 40.
Lens Architecture Changes With Age and Genotype in Epha2-Knockout Mice
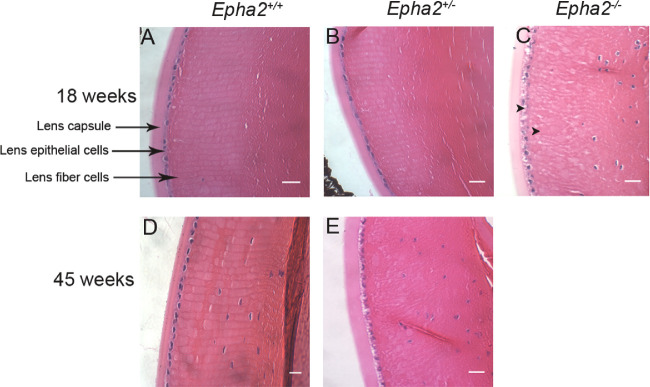
Histological analysis of 18-week-old Epha2+/+ and Epha2+/− lenses showed normal lens epithelial and fiber cell arrangement (Figs. 4A, 4B) whereas age-matched Epha2−/− lenses showed grossly disorganized, irregularly shaped, and swollen fiber cells and presence of vacuoles in the lens epithelium (arrowheads, Fig. 4C). Similar disruption of lens architecture was observed in 45-week-old Epha2+/− compared to age-matched Epha2+/+ lenses (Figs. 4D, 4E). The histological observations were consistent with ophthalmic observations in all three genotypes.
Figure 4.
Histological analysis showing disruption of lens architecture in Epha2-knockout mouse lenses. Representative images of hematoxylin and eosin-stained sections of lenses of 18-week-old Epha2+/+ (A), Epha2+/− (B), and Epha2−/− (C) mice and 45-week-old Epha2+/+ (D) and Epha2+/− (E) mice on C57BL/6J background showing nuclei (blue) and cytoplasm (pink) of lens epithelial and fiber cells as well as lens capsule. Disruption of fiber cell arrangement in the lens cortex can be seen in 18-week-old Epha2−/−, C, and 45-week-old Epha2+/−, E, mouse lenses as opposed to arrangement in meridional rows in Epha2+/+, A and D, and 18-week-old Epha2+/− lenses, B. Arrowheads show presence of vacuoles in the lens cortex and lens epithelial cells. Nucleated fiber cells are visible in those sections closer to the lens equator. Scale-bars 20 µm.
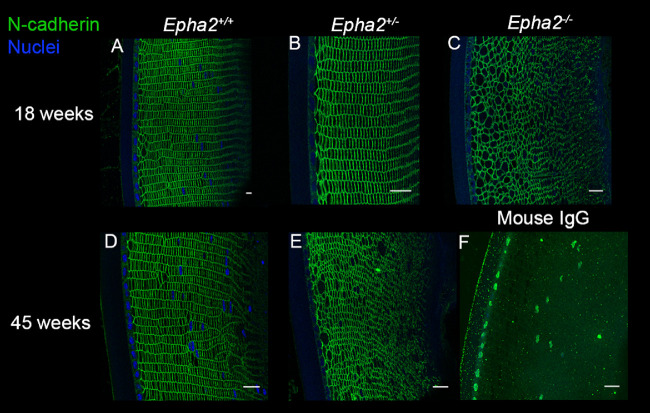
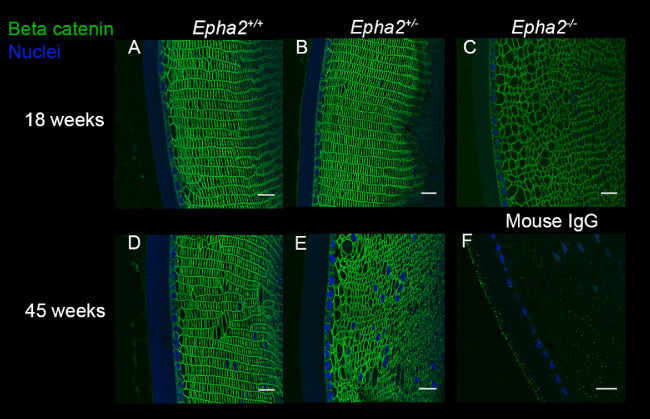
To determine the cellular organization and cell-cell junction integrity, lens sections of Epha2+/+, Epha2+/−, and Epha2−/− mice were labeled for adherens junction protein N-cadherin and adherens junction associated protein β-catenin58,59 at 18 and 45 weeks of age, when Epha2−/− and Epha2+/− mice, respectively, develop severe cataract. N-cadherin primarily showed peripheral localization in cortical lens fiber cells in lenses of all three genotypes of mice at both the ages (Fig. 5). In addition, it revealed regular arrangement of fiber cells in meridional rows in lenses of Epha2+/+ mice at both the ages and 18-week-old Epha2+/− mice (see Figs. 5A, 5B, 5D). However, disorganized, irregularly shaped, and sized fiber cells were observed in 18-week-old Epha2−/− and 45-week-old Epha2+/− mouse lenses (see Figs. 5C, 5E). Similar results were obtained upon immunolabeling of β-catenin (Fig. 6), demonstrating correlation between loss of lens fiber cell organization and development of severe cataract in both, Epha2−/− and Epha2+/− mice.
Figure 5.
N-cadherin immunolabeling showing disorganization of cellular architecture in Epha2-knockout mouse lenses. Representative confocal microscopy images of sections of lenses of 18-week-old Epha2+/+ (A), Epha2+/− (B), and Epha2−/− (C), and 45-week-old Epha2+/+ (D) and Epha2+/− (E) mice on C57BL/6J background immunolabeled with mouse anti-N-cadherin antibody (green) and DAPI (blue) to label the nuclei are shown. N-cadherin labeling delineated the cell boundary and showed well packed fiber cells arranged in meridional rows in 18, A, and 45-week-old Epha2+/+, D, and 18-week-old Epha2+/−, B, lenses. In contrast, it showed larger and disorganized fiber cells in 18-week-old Epha2−/−, C, and 45-week-old Epha2+/−, E, lenses. Sections probed with mouse IgG (negative control) revealed little or no non-specific labeling (F). Scale-bars 20 µm.
Figure 6.
β-Catenin labeling showing breakdown of lens architecture in Epha2-knockout mouse lenses. Representative confocal microscopy images of sections of lenses of 18-week-old Epha2+/+ (A), Epha2+/− (B), and Epha2−/− (C) and 45-week-old Epha2+/+ (D) and Epha2+/− (E) mice on C57BL/6J background immunolabeled with mouse anti-β-catenin antibody (green) and DAPI (blue) to stain the nuclei are shown. Similar to N-cadherin labeling, β-catenin labeling delineated meridional rows of fiber cells in 18, A, and 45-week-old Epha2+/+, D, and 18-week-old Epha2+/−, B, lenses. In contrast, gross disorganization of fiber cells in 18-week-old Epha2−/−, C, and 45-week-old Epha2+/−, E, lenses was observed. Sections probed with mouse IgG (negative control) revealed little or no non-specific labeling (F). Scale-bars 20 µm.
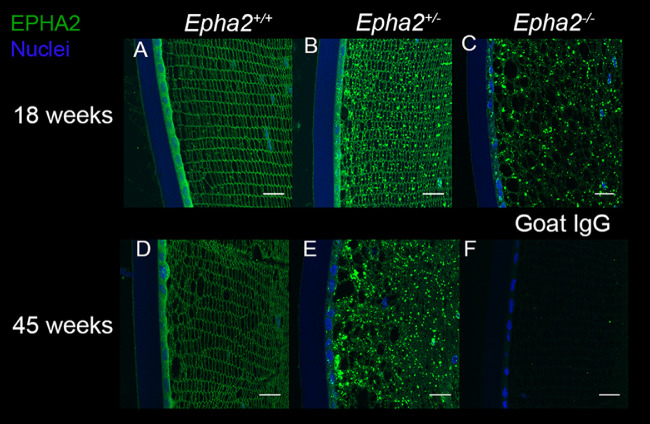
As the Epha2-knockout mouse strain used for this study was generated by secretory gene trapping approach and expresses partial EPHA2 ectodomain fused to β-galactosidase reporter protein that is potentially trapped in inclusion bodies inside the cell,45,46 we determined subcellular localization of EPHA2 (wild-type or fusion protein) at 18 and 45 weeks of age. Immunolabeling showed the presence of wild-type EPHA2 in the lens epithelium and at cell membrane in fiber cells in Epha2+/+ mice at both the ages (Figs. 7A, 7D), and in agreement with arrangement of lens fiber cells in meridional rows seen by N-cadherin and β-catenin labeling. The localization pattern was consistent with that reported previously30,38 and correlated with the role of EPHA2 in maintenance of inter-cellular contacts. Interestingly, in lenses of 18-week-old Epha2+/− mice, along with labeling of the cell membrane, EPHA2-positive labeling was observed in a granular pattern in lens epithelial and fiber cells (Fig. 7B). In lenses of 45-week-old Epha2+/− and 18-week-old Epha2−/− mice, mainly granular labeling pattern was observed (Figs. 7C, 7E). The granular labeling pattern revealed by an antibody that recognizes the EPHA2 ectodomain is consistent with intracellular trapping of the partial EPHA2-β-galactosidase fusion protein in the lens cortex. Similar granular labeling pattern was observed in lenses of 18-week-old Epha2−/− and 18 and 45-week-old Epha2+/− mice upon labeling of β-galactosidase reporter protein (Supplementary Fig. S3). However, fewer granules were present in 18-week-old compared to 45-week-old Epha2+/− lenses, as seen with EPHA2 labeling.
Figure 7.
Accumulation of mutant EPHA2 protein in Epha2-knockout mouse lenses detected by immunolabeling. Representative confocal microscopy images of lens sections of 18-week-old Epha2+/+ (A), Epha2+/− (B), and Epha2−/− (C) and 45-week-old Epha2+/+ (D) and Epha2+/− (E) mice on C57BL/6J background were immunolabeled with anti-mouse EPHA2 antibody (green) and DAPI (blue) to stain the nuclei. The lenses of 18, A, and 45-week-old Epha2+/+, D, showed the EPHA2 protein in lens epithelial cells and at the lens fiber cell periphery. Eighteen-week-old lenses of Epha2+/− mice, B, in addition to the protein in lens epithelium and lens fiber cell periphery, exhibited its presence in a granular pattern in cells. Lenses of 45-week-old Epha2+/−, E, and 18-week-old Epha2−/−, C, mice showed presence of the protein only in granular pattern likely indicating accumulation of the partial EPHA2-β-galactosidase fusion protein in lens cells. Similar labeling was absent in negative control sections incubated with goat IgG (F). Scale-bars 20 µm.
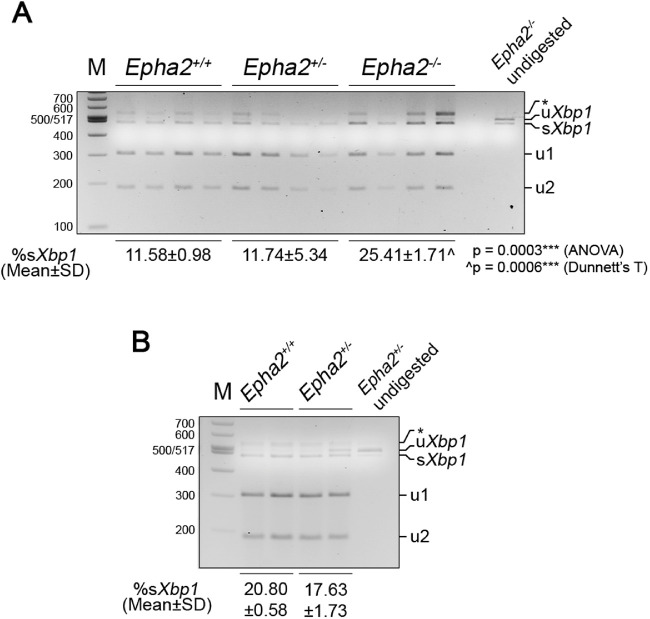
Next, to investigate if accumulation of the EPHA2-β-galactosidase fusion protein in lens cells led to endoplasmic reticulum (ER) stress and unfolded protein response (UPR), we examined expression of the ER stress chaperone, BiP.60 Upon analysis of lens proteins from 18, 27, and 44 week-old Epha2+/+, Epha2+/−, and Epha2−/− mice by Western blotting, BiP expression was not detected in any genotype at any age but was readily detected in HeLa cell lysate used as positive control (Supplementary Fig. S4A). Undetectable BiP expression in wild-type lenses is consistent with expression being restricted to the lens epithelium and newly differentiating fiber cells, as reported previously.61,62 In order to determine whether ER stress and UPR is induced earlier than 18 weeks, we examined expression of BiP, as well as of transcription factors XBP1 and ATF6, activated by ER stress, and CHOP, a downstream effector of UPR,60 in lenses of 8-week-old Epha2+/− and Epha2−/− and age-matched Epha2+/+ mice. Expression of BiP, ATF6, and CHOP was analyzed by Western blotting and of Xbp1 by RT-PCR; Bip expression was also analyzed by RT-PCR. Once again, expression of BiP protein was not detected in lenses of any genotype but was detected in the HeLa cell lysate (Supplementary Fig. S4B). However, Bip mRNA was detected in lenses of all genotypes, but expression levels were similar between genotypes (Supplementary Fig. S4C). Xbp1 mRNA splicing by IRE1, a sensor activated by ER stress, excises a 26-nucleotide long intron leading to production of XBP1 transcription factor.60 The levels of the spliced form of Xbp1 mRNA were significantly different between lenses of the three genotypes of mice (P = 0.0003), and significantly higher in Epha2−/− compared to Epha2+/+ lenses (P = 0.0006; Fig. 8A). However, its levels in Epha2+/− lenses were similar to those in Epha2+/+ lenses. As Epha2+/− mice exhibited a delay in development of severe cataract, we hypothesized that levels of the spliced form of Xbp1 mRNA may be increased in lenses of these mice at a later age. Thus, we further analyzed its levels in 14 to 19-week-old Epha2+/− and Epha2+/+ lenses. The levels were found to be lower rather than higher in Epha2+/− compared to Epha2+/+ lenses at that age (Fig. 8B). ATF6, another sensor of ER stress, upon translocation from the ER to golgi apparatus is proteolytically processed and the resulting processed protein, ATF6N, functions as a transcription factor.60 Expression of ATF6, predominantly the processed protein, was detected in lenses of all three genotypes of mice whereas both unprocessed and processed protein was detected in C2C12 cell lysate used as positive control (Supplementary Fig. S4B). The levels of the processed protein, ATF6N, were relatively higher (from ∼35 to 125%) in Epha2+/− and Epha2−/− than Epha2+/+ lenses (see Supplementary Fig. S4B, Graph). Expression of CHOP, a pro-apoptotic protein activated by severe ER stress, was not detected in any genotype of lenses but, as expected, was detected in thapsigargin treated C2C12 cell lysate used as positive control (see Supplementary Fig. S4B). These data suggest that accumulation of the partial EPHA2-β-galactosidase fusion protein is accompanied by activation of a moderate ER stress and UPR in the lens. Notably, the fusion protein was detected in the soluble protein fraction extracted in RIPA buffer but not in the insoluble protein fraction of 18-week-old Epha2+/− and Epha2−/− lenses (see Supplementary Fig. S5A, Fig. 7, and data not shown) nor of 43-week-old Epha2+/− lenses (Supplementary Fig. S5B), suggesting that the mutant protein is present in lens cells but does not form insoluble aggregates.
Figure 8.
Assessment of Xbp1 mRNA splicing in Epha2-knockout mouse lenses. Partial Xbp1 mRNA flanking the 26-nucleotide long intron was amplified from lenses (n = 4) of 8-week-old Epha2+/+, Epha2+/−, and Epha2−/− (A), and 14 to 19-week-old Epha2+/+ and Epha2+/− (B), mice on C57BL/6J background by RT-PCR using gene-specific primers. The PCR product was digested with PstI restriction enzyme and size separated on a 3% agarose gel. In B, each lane represents analysis of lenses of two mice of the same sex. PstI digestion of the unspliced variant (uXbp1) at the PstI site in the intron resulted in 301 bp (u1) and 193 bp (u2) fragments. The spliced variant (sXbp1) generated by excision of the intron, as expected, was not digested by PstI. Both unspliced and spliced variants were detected in lenses of all genotypes and at both the ages, A and B. Asterisks marks a hybrid of the two variants. The proportion of the spliced variant in a sample was calculated (sXbp1/(uXbp1+sXbp1+u1+u2)). Mean percent spliced variant in lenses of each genotype is indicated; SD = standard deviation. The data in A was statistically analyzed by ANOVA with Dunnett's T test for multiple comparisons. ***p < 0.001; ^ versus Epha2+/+.
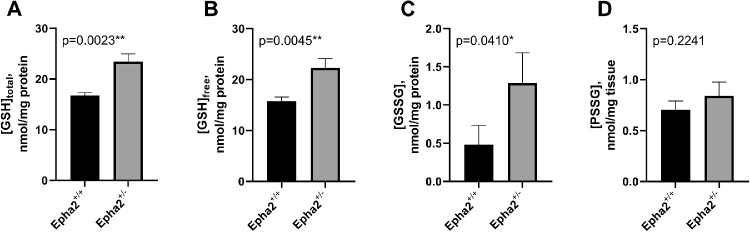
Furthermore, to examine the effect of partial and complete loss of EPHA2 on redox state of the lens, we determined the levels of lenticular glutathione (GSH), the main antioxidant in the lens.63 Levels were evaluated for both, the free, reduced form, and oxidized forms, including glutathione disulfide (GSSG) and protein-bound glutathione (PSSG). Glutathione levels in lenses of 22-week-old Epha2−/− and 43-week-old Epha2+/− mice were compared to those in age-matched Epha2+/+ mice. GSH/GSSG ratio, the metric useful for assessment of redox balance, was not used in this study because virtually undetectable levels of GSSG in several of the Epha2+/+ lenses led to artifactually high ratios. Lenses of 43-week-old Epha2+/− mice had significantly higher concentrations of total GSH (P < 0.005), free GSH (P < 0.005), and GSSG (P < 0.05) compared to those of age-matched Epha2+/+ mice (Figs. 9A–C), which indicated dysregulation of redox homeostasis in Epha2+/− lenses. However, concentrations of PSSG were not significantly different between lenses of the two genotypes. No significant difference in concentrations of total or free GSH, GSSG, or PSSG was noted between lenses of 22-week-old Epha2−/− and age-matched Epha2+/+ mice likely due to small sample size (Supplementary Fig. S6). Taken together, these data suggest that partial and complete loss of functional EPHA2 affect the morphology and arrangement of lens fiber cells in an age-dependent manner and partial loss of the protein also affects glutathione redox balance of the lens, which contributes to cataract development in mice.
Figure 9.
Comparison of glutathione levels in Epha2+/+ and Epha2+/− mouse lenses. Concentrations of total soluble GSH (A), GSH in its free, reduced form (B), and oxidized forms as soluble GSSG (C) and protein-bound PSSG (D) were compared between 43-week-old Epha2+/− and age-matched Epha2+/+ mouse lenses (n = 3), as described in Materials and Methods. Mean concentrations of total GSH, free GSH, and GSSG expressed as nmol/mg of lens protein and PSSG as nmol/mg dried tissue have been plotted. Two-tailed t-test P value of each comparison has been indicated. Significant P values are marked with asterisks; *P < 0.05, **P < 0.005. Error bars represent standard deviation from the mean.
Genetic Background Influences Epha2-Related Cataract Development
We hypothesized that the difference in timing of cataract progression in Epha2−/− mice found in our study and that reported by Jun et al.,30 and occurrence of cataract in Epha2+/− mice is due to difference in genetic backgrounds of Epha2-knockout mice used in the two studies. To test this hypothesis, we generated Epha2-knockout mice on mixed FVB:C57BL/6J (50:50) genetic background and determined cataract development in Epha2+/+ (n = 17), Epha2+/− (n = 22), and Epha2−/− (n = 23) mice from 11 to 64 weeks of age or until severe cataract developed.
FVB/NJ mice, used for generating Epha2-knockout mice on mixed background, carry a spontaneous mutation in the Bfsp2 gene64 that causes occasional anterior subcapsular and faint deeper lens opacities.47,64 To determine whether this mutation affects Epha2-related ACC development on mixed background, segregated analysis, by Bfsp2 genotype (wild-type or heterozygous/homozygous mutant), was performed. Epha2-related cataract progression in Epha2+/− and Epha2−/− mice carrying homozygous or heterozygous mutant Bfsp2, was similar to those carrying the wild-type Bfsp2 gene (see Supplementary Figs. S7, S8), suggesting that Bfsp2 genotype did not affect Epha2-related cataract development in mice on mixed background. Thus, combined analysis of data from all three Bfsp2 genotypes of mice is presented. The Bfsp2 mutation reportedly also does not affect the cataract phenotype related to Ephrin-A5 knockout.41
In Epha2+/+ mice on mixed background, only mild ACC (grade < 2) was observed up to 64 weeks of age (Figs. 10, 11, Supplementary Table S3) and attributed to anesthesia. Epha2+/− mice exhibited mild ACC up to 45 weeks of age and moderate ACC (grade >2) by 64 weeks of age compared to age-matched Epha2+/+ mice (P < 0.01; see Figs. 10A, 11, Supplementary Table S3). Epha2−/− mice, compared to Epha2+/+ mice, displayed mild ACC by 18 weeks that progressed to moderately severe ACC by 27 weeks (grade >2, P < 0.01) and severe ACC by 38 weeks of age (grade 4 or 5, P < 0.001; see Figs. 10A, 11, Supplementary Table S3). Increase in cataract severity between 18 and 27 weeks and 27 and 38 weeks of age in this genotype was significant (Fig. 10B).
Figure 10.
Progression of anterior cortical cataract in Epha2-knockout mice on FVB:C57BL/6J mixed background.Epha2+/+, Epha2+/−, and Epha2−/− mice on FVB:C57BL/6J (50:50) mixed background were monitored for cataract development from 11 ± 3 weeks to 64 ± 3 weeks of age. (A) Mean cortical cataract grade in each genotype of mice at each time point is graphically represented. Epha2−/− mice developed severe cataract by 38 ± 3 weeks of age whereas Epha2+/− mice developed only moderate cataract by 64 ± 3 weeks of age. Epha2+/+ mice presented with mild cataract up to 64 ± 3 weeks of age. The Mann Whitney U test P values from comparison with Epha2+/+ mice are indicated. Error bars represent standard error of the mean. After an animal exhibited a cataract grade of 4 or 5, the same grade was attributed to subsequent time points for data analysis assuming no further increase in cataract severity. (B) The table shows Wilcoxon Signed Ranks test P values of comparison between time points indicating the effect of age on cataract progression in each genotype of mice. † Indicates likely artifact of anesthetic-induced cataract.
Figure 11.
Anterior cortical cataract developed in Epha2-knockout mice on FVB:C57BL/6J mixed background. Representative ophthalmic images of Epha2+/+ (A, B, C) and Epha2+/− (D, E, F) mice at 18 ± 3, 38 ± 3 and 64 ± 3 weeks of age and Epha2−/− (G, H) mice at 18 ± 3 and 38 ± 3 weeks of age, on FVB:C57BL/6J (50:50) mixed background, are shown. No cataract was evident in Epha2+/+, C, or in Epha2+/−, F, mice up to 64 weeks of age. Epha2−/− mice progressively developed severe ACC by 38 ± 3 weeks of age, H. The bottom right panel shows cross-section illumination images of the Epha2−/− and Epha2+/− eyes seen in panels H and F, respectively. Magnification, times 40.
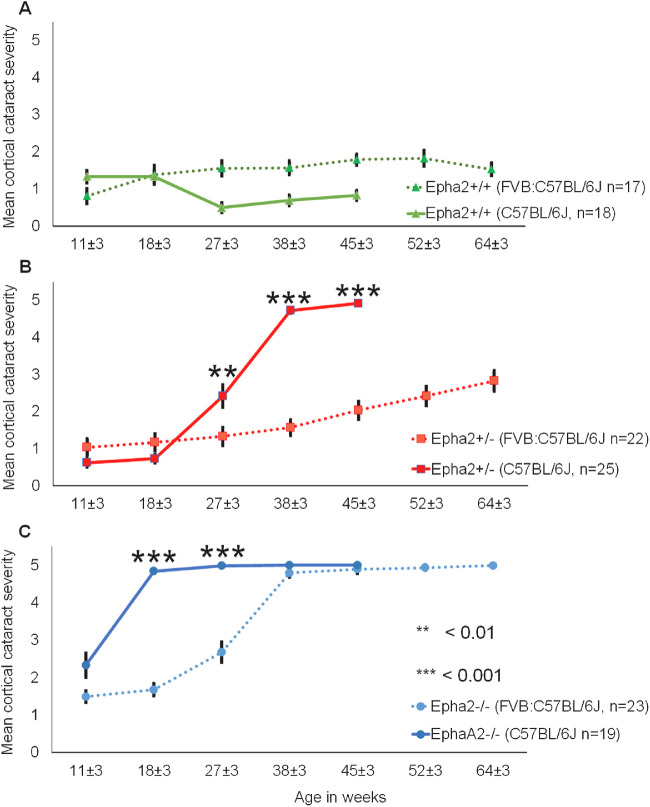
Next, we compared the effect of genetic background on cataract progression in each of the three genotypes of mice. Epha2+/+ mice on both genetic backgrounds exhibited only mild anesthetic-induced cataract at all time points (Fig. 12A); although statistically significant (P < 0.001), the difference is unlikely to be biologically relevant. On C57BL/6J background, Epha2+/− mice developed moderate cataract at 27 weeks (P < 0.01) and severe cataract by 38 and 45 weeks of age (P < 0.001; Fig. 12B); however, the mice on mixed background developed only moderately severe cataracts by 64 weeks of age. Similarly, Epha2−/− mice on C57BL/6J background developed significantly severe cataract by 18 and 27 weeks of age compared to those on mixed background (P < 0.001; Fig. 12C) that developed severe cataract much later, by 38 weeks of age. Together, these results suggest that Epha2-related cataract develops significantly faster on C57BL/6J than on FVB:C57BL/6J (50:50) mixed background. This also explains the reason for cataract development in Epha2+/− mice on C57BL/6J background discovered in this study.
Figure 12.
Comparison of progression of anterior cortical cataract in Epha2-knockout mice on C57BL/6J and FVB:C57BL/6J mixed backgrounds. Anterior cortical cataract severity was compared between C57BL/6J (solid lines) and FVB:C57BL/6J (50:50) mixed (dotted lines) backgrounds in Epha2+/+ (A), Epha2+/− (B), and Epha2−/− (C) mice, over time. Group sizes are indicated in each panel. Mean ACC grade in a genotype of mice on each background at each time point has been plotted. On both the genetic backgrounds, Epha2+/+ mice developed only mild cataracts up to 45 weeks of age that persisted in mice on the mixed background up to 64 ± 3 weeks of age, A. Epha2+/− mice on C57BL/6J background developed moderate cataracts by 27 weeks of age (P < 0.01) and severe cataracts by 38 or 45 weeks of age (P < 0.001) compared to mild cataracts in age-matched Epha2+/− mice on the mixed background; Epha2+/− mice on mixed background developed only moderate cataract up to 64 ± 3 weeks of age, B. Epha2−/− mice on C57BL/6J background developed severe cataracts by 18 ± 3 or 27 ± 3 weeks of age whereas those on the mixed background developed severe cataract by 38 ± 3 weeks of age, C. The latter mice had significantly less severe cataracts at 18 and 27 weeks of ages (P < 0.001). The p values from the Mann Whitney U test are indicated. Error bars represent standard error of the mean.
Sex Influences Epha2-Related Cataract Progression
Female subjects are at a higher risk of developing age-related cataract and some epidemiological studies suggest a protective effect of hormone replacement therapy in post-menopausal women.8,65 Moreover, estrogen reportedly affects EPHA2 signaling.44 Thus, we hypothesized that sex may influence Epha2-related cataract development. To determine this, we compared cataract grades between male and female Epha2+/− and Epha2−/− mice on the two genetic backgrounds.
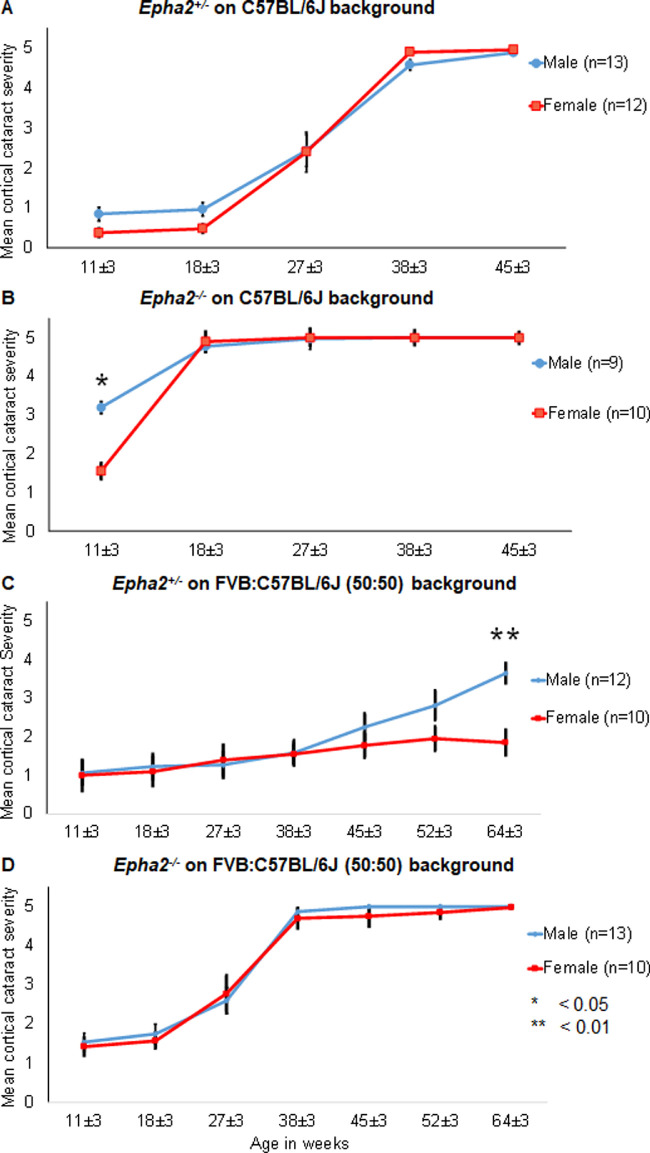
There was no difference in cataract severity between Epha2+/− male (n = 13) and female (n = 12) mice from 11 to 45 weeks of age, on C57BL/6J background (Fig. 13A). Whereas Epha2−/− male mice (n = 9) on C57BL/6J background displayed significantly higher cataract severity than female mice (n = 10) at 11 weeks of age (P < 0.05); both male and female mice progressed to develop severe cataract by 18 weeks of age (Fig. 13B).
Figure 13.
Comparison of progression of anterior cortical cataract between male and female Epha2-knockout mice on C57BL/6J and FVB:C57BL/6J mixed backgrounds. Anterior cortical cataract severity was compared between male (blue lines) and female (red lines) Epha2+/− and Epha2−/− mice on C57BL/6J and FVB:C57BL/6J (50:50) mixed genetic backgrounds. (A) Male and female Epha2+/− mice on C57BL/6J background showed similar progression of cataract from 11 ± 3 to 45 ± 3 weeks of age. (B) Female Epha2−/− mice on C57BL/6J background displayed milder cataract compared to male mice at 11 ± 3 weeks of age (P < 0.05) whereas both male and female mice exhibited similar severe cataracts at 18 ± 3 weeks of age that persisted subsequently. (C) Both male and female Epha2+/− mice on the mixed genetic background displayed mild cataract up to 45 ± 3 weeks of age. Male mice gradually developed moderately severe cataract by 64 ± 3 weeks of age whereas age-matched female mice exhibited significantly milder cataract up to that age (P < 0.01). (D) Male and female Epha2−/− mice on the mixed background exhibited similar rate of progression of cataract from 11 ± 3 to 64 ± 3 weeks of age. The P values from the Mann Whitney U test are indicated. Error bars represent standard error of the mean.
On mixed genetic background, both male (n = 12) and female (n = 10) Epha2+/− mice exhibited mild cataract up to 38 weeks of age, thereafter, male mice displayed higher grade cataracts than female mice and significantly higher at 64 weeks of age (P < 0.01; Fig. 13C). In contrast, there was no difference in cataract severity between male (n = 13) and female (n = 10) Epha2−/− mice on mixed background at any age (Fig. 13D). Overall, these results indicate that sex has a small effect on rate of progression of Epha2-related cataract and male mice start to develop cataract earlier than female mice.
Discussion
Involvement of the EPHA2 gene in cataract development in both humans and mice indicates a crucial role of EPHA2 signaling in the lens. In the present study, using an Epha2-knockout mouse model, we investigated the effect of various biological modifiers, Epha2 genotype, age, sex, and genetic background, on Epha2-related cataract development. In contrast to previous studies, this study shows that partial deficiency of functional Epha2 gene in mice also leads to ACC albeit at a later age than complete deficiency. Moreover, it shows the effect of genetic background and sex on progression of Epha2-related cataract.
The anterior cortical lens opacity observed in this study is similar to that reported by Jun et al. in null mice of this strain and another strain carrying a different Epha2-knockout mutation introduced using secretory gene trapping strategy.30 However, homozygous Epha2-knockout mice carrying an insertion mutation in the Epha2 gene and predicted to produce truncated protein (Jackson Laboratory strain #006028) generated by homologous recombination,66 on C57BL/6J background, develop mild nuclear cataract and exhibit smaller lenses with impaired refractive quality and suture formation; reports regarding development of age-related cataract in these mice are conflicting.38,39,67 The difference in lens phenotype between strains generated by secretory gene-trap approach and homologous recombination is likely due to differences in Epha2-knockout mutations and protein products produced, and is similar to differences in cataract phenotype observed in humans with different genetic mutations in EPHA2.33,34 Additionally, because in strain #006028 the mutant protein is absent in the lens38 initiation of compensatory mechanisms involving another EPH receptor may explain the subtle phenotype. However, as shown by our data, in the secretory gene-trap strains the mutant fusion-protein persists in the lens. This may prevent initiation of a compensatory mechanism or have a dominant negative effect, leading to a more severe phenotype. Further research is warranted to investigate these possibilities.
This study shows that disruption of shape, size, and packing of lens fiber cells underlies ACC development in the Epha2-knockout model used here and suggests that loss of functional EPHA2 receptor from the cell membrane is likely the primary cause of this cellular disorganization. Our data show that the partial EPHA2-β-galactosidase fusion protein produced in this strain accumulates in lens cells and is accompanied by induction of a moderate unfolded-protein response indicating some ER stress. Unfolded-protein response is activated through three stress sensing pathways - IRE1, ATF6, and PERK.60 Acute or mild ER stress triggers a cytoprotective UPR for restoring ER homeostasis by reducing un/misfolded protein burden and/or facilitating protein folding whereas chronic or severe ER stress leads to a cytotoxic response that results in cell death.60 Activation of XBP1/ IRE1 and ATF6 pathways in Epha2 null and ATF6 pathway in Epha2+/− mice in the absence of CHOP activation that leads to cell apoptosis suggests that the response is most probably cytoprotective and is induced for maintaining ER homeostasis. Consonant with this argument, the accumulated protein is detected in the soluble and not insoluble protein fraction of Epha2-knockout lenses. Hence, its aggregation or mis-folding is unlikely to underlie cataractogenesis in this model. The presence of a functional β-galactosidase reporter protein in this model30 also refutes aggregation of the fusion protein. Overexpression of unphosphorylated HSP25, the oligomeric form that acts as a chaperone, reported in lenses of Epha2 null mice30 may facilitate solubility of the fusion protein. Alternatively, it may be related to aging as HSP25 overexpression was also found in adult wild-type lenses, although to a lesser extent.30 In this study, lenses of Epha2+/− mice, despite accumulation of the fusion protein in cells, remained clear when wild-type EPHA2 protein produced by the normal allele was localized to the cell membrane (at 18 weeks of age), and developed severe cortical cataracts when it was lost from the cell membrane (at 45 weeks of age). Downregulation of Epha2 expression in the lens with age30 may explain the loss of wild-type protein from the cell membrane in older Epha2+/− lenses. Hence, loss of functional EPHA2 protein is the most likely cause of cataractogenesis in this model. However, the possibility that intracellular accumulation of the fusion protein contributes to scattering of light cannot be discounted.
Our data show an increase in oxidized glutathione (GSSG) in lenses of Epha2+/− mice at the age when they develop severe cortical cataracts, suggesting an increase in reactive oxygen and/or nitrogen species in these lenses. However, lenses of these mice also exhibited significantly higher levels of reduced (free) GSH than those of matched wild-type mice. GSH levels in the lens are maintained via a combination of de novo synthesis from constituent amino acids, recycling, and uptake from surrounding tissues.68 There is precedent for upregulation of one or more of these pathways as a protective response to oxidative stress69 that could explain the increased GSH levels in Epha2+/− lenses. Further research is required to elucidate the role of GSH and oxidative stress in development of cataract due to loss of functional EPHA2.
We found a temporal effect of Epha2 genotypes on development of cortical cataract in mice on two genetic backgrounds, an inbred and a mixed background. This demonstrates that age as a function of genotype has a significant effect on Epha2-related cataract development, which correlates with association of variants in the human EPHA2 gene with the risk of developing age-related cataract.29,30,32,70 The genetic backgrounds also conferred a temporal rather than a phenotypic effect on Epha2-related cataract. Genetic background is known to influence disease phenotype and severity in genetically and nongenetically induced animal models of diseases, for example, in animal models of epilepsy71 and cataract.40,41,72,73 An earlier development of cortical cataract in Epha2−/− and Epha2+/− mice on C57BL/6J background than on FVB:C57BL/6J (50:50) mixed background is most likely due to genetic modifiers and indicates the presence of susceptibility factors in the former and/or resistance factors in FVB/NJ background. Similarly, racial differences in susceptibility to cataract have been found in humans.74,75 Identification of the genetic modifiers in C57BL/6J and FVB/NJ backgrounds, may reveal additional risk factors for age-related cataract. An effect of genetic background on Epha2-related cataract in mice is consistent with differences in incidence, severity, and penetrance of cataract among individuals carrying mutations in the EPHA2 gene.33,76
Sex was found to have a significant, albeit small, effect on rate of progression of Epha2-related cataract in mice. Epha2−/− female mice on C57BL/6J background were protected from cataract at a younger age but progressed to develop severe cataract like male mice at a later age. A similar protective effect was seen in Epha2+/− female mice on mixed background. A protective effect was not evident in Epha2+/− female mice on C57BL/6J background and Epha2−/− female mice on mixed background, probably due to small sample size and intermediate rate of progression of cataract in these mice. Nevertheless, the effect of sex on cataract progression in mice is consistent with differences in prevalence of age-related cataract between male and female subjects reported in some epidemiological studies.2,8 Differences in estrogen levels between female and male mice likely underlie the protective effect of female gender during early stages of cataract development in Epha2-knockout mice and correlates with reduced susceptibility to age-related cataract in women on hormone replacement therapy.65 Further research is warranted to understand the role of estrogen in Epha2-related cataract.
In summary, this study, we believe for the first time, demonstrates that partial deficiency of the Epha2 gene is sufficient to cause cataract and shows an age-dependent effect of partial and complete deficiency of EPHA2 on cataract development in mice. It shows that female gender confers some protection and delays the onset, and genetic background has a major effect on rate of progression of Epha2-related cataract in mice. These findings reiterate that cataract is a multifactorial disease and suggest that interaction between genetic and other biological factors likely determines susceptibility to EPHA2-related cataract in humans.
Supplementary Material
Acknowledgments
Funded by the National Health and Medical Research Council (NHMRC), Australia (GNT11009955), the Flinders Medical Centre Foundation and the Flinders University, South Australia, Australia. J.E.C. and K.P.B. are recipients of NHMRC Practitioner and Senior Research Fellowships, respectively.
Disclosure: A. Dave, None; J.E. Craig, None; M. Alamein, None; K. Skrzypiec, None; J. Beltz, None; A. Pfaff, None; K.P. Burdon, None; N. Ercal, None; R.U. de Iongh, None; S. Sharma, None
References
- 1.Shiels A, Hejtmancik JF.. Genetic origins of cataract. Arch Ophthalmol. 2007; 125: 165–173. [DOI] [PubMed] [Google Scholar]
- 2.Abraham AG, Condon NG, West Gower E.. The new epidemiology of cataract. Ophthalmol Clin North Am. 2006; 19: 415–425. [DOI] [PubMed] [Google Scholar]
- 3.Pan CW, Cheng CY, Saw SM, Wang JJ, Wong TY.. Myopia and age-related cataract: a systematic review and meta-analysis. Am J Ophthalmol. 2013; 156: 1021–1033.e1021. [DOI] [PubMed] [Google Scholar]
- 4.Kanthan GL, Mitchell P, Burlutsky G, Rochtchina E, Wang JJ.. Pseudoexfoliation syndrome and the long-term incidence of cataract and cataract surgery: the blue mountains eye study. Am J Ophthalmol. 2013; 155: 83–88.e81. [DOI] [PubMed] [Google Scholar]
- 5.Jacques PF, Moeller SM, Hankinson SE, et al.. Weight status, abdominal adiposity, diabetes, and early age-related lens opacities. Am J Clin Nutr. 2003; 78: 400–405. [DOI] [PubMed] [Google Scholar]
- 6.Chang JR, Koo E, Agron E, et al.. Risk factors associated with incident cataracts and cataract surgery in the Age-related Eye Disease Study (AREDS): AREDS report number 32. Ophthalmology. 2011; 118: 2113–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunes A, Yasar C, Tok L, Tok O.. Prevalence of Pseudoexfoliation Syndrome in Turkish Patients with Senile Cataract. Seminars in Ophthalmology. 2017; 32: 297–301. [DOI] [PubMed] [Google Scholar]
- 8.West SK, Valmadrid CT.. Epidemiology of risk factors for age-related cataract. Surv Ophthalmol. 1995; 39: 323–334. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki K, Sasaki H, Jonasson F, Kojima M, Cheng HM.. Racial differences of lens transparency properties with aging and prevalence of age-related cataract applying a WHO classification system. Ophthalmic Res. 2004; 36: 332–340. [DOI] [PubMed] [Google Scholar]
- 10.Leske MC, Wu SY, Nemesure B, Hennis A.. Causes of visual loss and their risk factors: an incidence summary from the Barbados Eye Studies. Rev Panam Salud Publica. 2010; 27: 259–267. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JS, Xu L, Wang YX, You QS, Wang JD, Jonas JB.. Five-year incidence of age-related cataract and cataract surgery in the adult population of greater Beijing: the Beijing Eye Study. Ophthalmology. 2011; 118: 711–718. [DOI] [PubMed] [Google Scholar]
- 12.Rosman M, Zheng Y, Lamoureux E, et al.. Review of key findings from the Singapore Malay Eye Study (SiMES-1). Singapore Med J. 2012; 53: 82–87. [PubMed] [Google Scholar]
- 13.West SK, Duncan DD, Munoz B, et al.. Sunlight exposure and risk of lens opacities in a population-based study: the Salisbury Eye Evaluation project. JAMA. 1998; 280: 714–718. [DOI] [PubMed] [Google Scholar]
- 14.Vashist P, Talwar B, Gogoi M, et al.. Prevalence of cataract in an older population in India: the India study of age-related eye disease. Ophthalmology. 2011; 118: 272–278.e271–e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond CJ, Duncan DD, Snieder H, et al.. The heritability of age-related cortical cataract: the twin eye study. Invest Ophthalmol Vis Sci. 2001; 42: 601–605. [PubMed] [Google Scholar]
- 16.Iyengar SK, Klein BE, Klein R, et al.. Identification of a major locus for age-related cortical cataract on chromosome 6p12-q12 in the Beaver Dam Eye Study. Proc Natl Acad Sci USA. 2004; 101: 14485–14490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Congdon N, Broman KW, Lai H, et al.. Cortical, but not posterior subcapsular, cataract shows significant familial aggregation in an older population after adjustment for possible shared environmental factors. Ophthalmology. 2005; 112: 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Y, Shi X, Jin Y, et al.. Mutation screening of HSF4 in 150 age-related cataract patients. Mol Vis. 2008; 14: 1850–1855. [PMC free article] [PubMed] [Google Scholar]
- 19.Bhagyalaxmi SG, Srinivas P, Barton KA, et al.. A novel mutation (F71L) in alphaA-crystallin with defective chaperone-like function associated with age-related cataract. Biochim Biophys Acta. 2009; 1792: 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou P, Luo Y, Liu X, Fan L, Lu Y.. Down-regulation and CpG island hypermethylation of CRYAA in age-related nuclear cataract. FASEB J. 2012; 26: 4897–4902. [DOI] [PubMed] [Google Scholar]
- 21.Karas N, Gobec L, Pfeifer V, Mlinar B, Battelino T, Lukac-Bajalo J.. Mutations in galactose-1-phosphate uridyltransferase gene in patients with idiopathic presenile cataract. J Inherit Metab Dis. 2003; 26: 699–704. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Zhang L, Sun D, Li Z, Wang L, Liu P.. Genetic polymorphisms of superoxide dismutases, catalase, and glutathione peroxidase in age-related cataract. Mol Vis. 2011; 17: 2325–2332. [PMC free article] [PubMed] [Google Scholar]
- 23.Guven M, Unal M, Sarici A, Ozaydin A, Batar B, Devranoglu K.. Glutathione-S-transferase M1 and T1 genetic polymorphisms and the risk of cataract development: a study in the Turkish population. Curr Eye Res. 2007; 32: 447–454. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Zhong J, Bian Z, Fang X, Peng Y, Hu Y.. Association between polymorphisms of OGG1, EPHA2 and age-related cataract risk: a meta-analysis. BMC Ophthalmol. 2016; 16: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Q, Zhou N, Zhang N, Qi Y.. Mutational screening of EFNA5 in Chinese age-related cataract patients. Ophthalmic Res. 2014; 52: 124–129. [DOI] [PubMed] [Google Scholar]
- 26.Lin Q, Zhou N, Zhang N, et al.. Genetic variations and polymorphisms in the ezrin gene are associated with age-related cataract. Mol Vis. 2013; 19: 1572–1579. [PMC free article] [PubMed] [Google Scholar]
- 27.Shiels A, Bennett TM, Hejtmancik JF.. Cat-Map: putting cataract on the map. Mol Vis. 2010; 16: 2007–2015. [PMC free article] [PubMed] [Google Scholar]
- 28.Liao J, Su X, Chen P, et al.. Meta-analysis of genome-wide association studies in multiethnic Asians identifies two loci for age-related nuclear cataract. Hum Mol Genet. 2014; 23: 6119–6128. [DOI] [PubMed] [Google Scholar]
- 29.Shiels A, Bennett TM, Knopf HL, et al.. The EPHA2 gene is associated with cataracts linked to chromosome 1p. Mol Vis. 2008; 14: 2042–2055. [PMC free article] [PubMed] [Google Scholar]
- 30.Jun G, Guo H, Klein BE, et al.. EPHA2 is associated with age-related cortical cataract in mice and humans. PLoS Genet. 2009; 5: e1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan W, Hou S, Jiang Z, Hu Z, Yang P, Ye J.. Association of EPHA2 polymorphisms and age-related cortical cataract in a Han Chinese population. Mol Vis. 2011; 17: 1553–1558. [PMC free article] [PubMed] [Google Scholar]
- 32.Sundaresan P, Ravindran RD, Vashist P, et al.. EPHA2 Polymorphisms and Age-Related Cataract in India. PLoS One. 2012; 7: e33001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dave A, Laurie K, Staffieri SE, et al.. Mutations in the EPHA2 gene are a major contributor to inherited cataracts in South-Eastern Australia. PLoS One. 2013; 8: e72518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T, Hua R, Xiao W, et al.. Mutations of the EPHA2 receptor tyrosine kinase gene cause autosomal dominant congenital cataract. Hum Mutat. 2009; 30: E603–E611. [DOI] [PubMed] [Google Scholar]
- 35.Reis LM, Tyler RC, Semina EV.. Identification of a novel C-terminal extension mutation in EPHA2 in a family affected with congenital cataract. Mol Vis. 2014; 20: 836–842. [PMC free article] [PubMed] [Google Scholar]
- 36.Kaul H, Riazuddin SA, Shahid M, et al.. Autosomal recessive congenital cataract linked to EPHA2 in a consanguineous Pakistani family. Mol Vis. 2010; 16: 511–517. [PMC free article] [PubMed] [Google Scholar]
- 37.Pasquale EB.Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008; 133: 38–52. [DOI] [PubMed] [Google Scholar]
- 38.Shi Y, De Maria A, Bennett T, Shiels A, Bassnett S.. A Role for Epha2 in Cell Migration and Refractive Organization of the Ocular Lens. Invest Ophthalmol Vis Sci. 2012; 53: 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng C, Gong X.. Diverse Roles of Eph/ephrin Signaling in the Mouse Lens. PLoS One. 2011; 6: e28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper MA, Son AI, Komlos D, Sun Y, Kleiman NJ, Zhou R.. Loss of ephrin-A5 function disrupts lens fiber cell packing and leads to cataract. Proc Natl Acad Sci USA. 2008; 105: 16620–16625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Son AI, Cooper MA, Sheleg M, Sun Y, Kleiman NJ, Zhou R.. Further analysis of the lens of ephrin-A5-/- mice: development of postnatal defects. Mol Vis. 2013; 19: 254–266. [PMC free article] [PubMed] [Google Scholar]
- 42.Kanthan GL, Wang JJ, Rochtchina E, et al.. Ten-year incidence of age-related cataract and cataract surgery in an older Australian population. The Blue Mountains Eye Study. Ophthalmology. 2008; 115: 808–814.e801. [DOI] [PubMed] [Google Scholar]
- 43.Klein BE, Klein R, Lee KE, Gangnon RE.. Incidence of age-related cataract over a 15-year interval the Beaver Dam Eye Study. Ophthalmology. 2008; 115: 477–482. [DOI] [PubMed] [Google Scholar]
- 44.Zelinski DP, Zantek ND, Walker-Daniels J, Peters MA, Taparowsky EJ, Kinch MS.. Estrogen and Myc negatively regulate expression of the EphA2 tyrosine kinase. J Cell Biochem. 2002; 85: 714–720. [DOI] [PubMed] [Google Scholar]
- 45.Leighton PA, Mitchell KJ, Goodrich LV, et al.. Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature. 2001; 410: 174–179. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell KJ, Pinson KI, Kelly OG, et al.. Functional analysis of secreted and transmembrane proteins critical to mouse development. Nat Genet. 2001; 28: 241–249. [DOI] [PubMed] [Google Scholar]
- 47.Alizadeh A, Clark J, Seeberger T, Hess J, Blankenship T, FitzGerald PG.. Characterization of a mutation in the lens-specific CP49 in the 129 strain of mouse. Invest Ophthalmol Vis Sci. 2004; 45: 884–891. [DOI] [PubMed] [Google Scholar]
- 48.Sandilands A, Wang X, Hutcheson AM, et al.. Bfsp2 mutation found in mouse 129 strains causes the loss of CP49' and induces vimentin-dependent changes in the lens fibre cell cytoskeleton. Exp Eye Res. 2004; 78: 875–889. [DOI] [PubMed] [Google Scholar]
- 49.Dave A, Craig JE, Skrzypiec K, et al.. Epha2 genotype influences ultraviolet radiation induced cataract in mice. Exp Eye Res. 2019; 188: 107806. [DOI] [PubMed] [Google Scholar]
- 50.Chylack LT Jr, Wolfe JK, Singer DM, et al.. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993; 111: 831–836. [DOI] [PubMed] [Google Scholar]
- 51.Cain S, Martinez G, Kokkinos MI, et al.. Differential requirement for beta-catenin in epithelial and fiber cells during lens development. Dev Biol. 2008; 321: 420–433. [DOI] [PubMed] [Google Scholar]
- 52.Dave A, Craig JE, Sharma S.. The status of intercellular junctions in established lens epithelial cell lines. Mol Vis. 2012; 18: 2937–2946. [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma S, Koh KSY, Collin C, et al.. NHS-A isoform of the NHS gene is a novel interactor of ZO-1. Exp Cell Res. 2009; 315: 2358–2372. [DOI] [PubMed] [Google Scholar]
- 54.Thamsen M, Ghosh R, Auyeung VC, et al.. Small molecule inhibition of IRE1α kinase/RNase has anti-fibrotic effects in the lung. PLoS One. 2019; 14: e0209824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ates B, Ercal BC, Manda K, Abraham L, Ercal N. Determination of glutathione disulfide levels in biological samples using thiol-disulfide exchanging agent, dithiothreitol. Biomed Chromatogr. 2009; 23: 119–123. [DOI] [PubMed] [Google Scholar]
- 56.Bradford MM.A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 57.Ridder W 3rd, Nusinowitz S, Heckenlively JR.. Causes of cataract development in anesthetized mice. Exp Eye Res. 2002; 75: 365–370. [DOI] [PubMed] [Google Scholar]
- 58.Yap AS, Brieher WM, Gumbiner BM.. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997; 13: 119–146. [DOI] [PubMed] [Google Scholar]
- 59.Bagchi M, Katar M, Lewis J, Maisel H.. Associated proteins of lens adherens junction. J Cell Biochem. 2002; 86: 700–703. [DOI] [PubMed] [Google Scholar]
- 60.Bergmann TJ, Molinari M.. Three branches to rule them all? UPR signalling in response to chemically versus misfolded proteins-induced ER stress. Biol Cell. 2018; 110: 197–204. [DOI] [PubMed] [Google Scholar]
- 61.Watson GW, Andley UP.. Activation of the unfolded protein response by a cataract-associated alphaA-crystallin mutation. Biochem Biophys Res Commun. 2010; 401: 192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Firtina Z, Duncan MK.. Unfolded Protein Response (UPR) is activated during normal lens development. Gene Expr Patterns. 2011; 11: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giblin FJ.Glutathione: a vital lens antioxidant. J Ocular Pharmacol Ther. 2000; 16: 121–135. [DOI] [PubMed] [Google Scholar]
- 64.Simirskii VN, Lee RS, Wawrousek EF, Duncan MK.. Inbred FVB/N mice are mutant at the cp49/Bfsp2 locus and lack beaded filament proteins in the lens. Invest Ophthalmol Vis Sci. 2006; 47: 4931–4934. [DOI] [PubMed] [Google Scholar]
- 65.Aina FO, Smeeth L, Hubbard R, Hurt LS, Fletcher AE.. Hormone replacement therapy and cataract: a population-based case-control study. Eye (Lond). 2006; 20: 417–422. [DOI] [PubMed] [Google Scholar]
- 66.Brantley-Sieders DM, Caughron J, Hicks D, Pozzi A, Ruiz JC, Chen J.. EphA2 receptor tyrosine kinase regulates endothelial cell migration and vascular assembly through phosphoinositide 3-kinase-mediated Rac1 GTPase activation. J Cell Sci. 2004; 117: 2037–2049. [DOI] [PubMed] [Google Scholar]
- 67.Zhou Y, Shiels A.. Epha2 and Efna5 participate in lens cell pattern-formation. Differentiation. 2018; 102: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fan X, Monnier VM, Whitson J.. Lens glutathione homeostasis: Discrepancies and gaps in knowledge standing in the way of novel therapeutic approaches. Exp Eye Res. 2017; 156: 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krzywanski DM, Dickinson DA, Iles KE, et al.. Variable regulation of glutamate cysteine ligase subunit proteins affects glutathione biosynthesis in response to oxidative stress. Arch Biochem Biophys. 2004; 423: 116–125. [DOI] [PubMed] [Google Scholar]
- 70.Celojevic D, Abramsson A, Seibt Palmer M, et al.. EPHA2 Polymorphisms in Estonian Patients with Age-Related Cataract. Ophthalmic Genetics. 2016; 37: 14–18. [DOI] [PubMed] [Google Scholar]
- 71.Schauwecker PE.The relevance of individual genetic background and its role in animal models of epilepsy. Epilepsy Res. 2011; 97: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gerido DA, Sellitto C, Li L, White TW.. Genetic background influences cataractogenesis, but not lens growth deficiency, in Cx50-knockout mice. Invest Ophthalmol Vis Sci. 2003; 44: 2669–2674. [DOI] [PubMed] [Google Scholar]
- 73.Gong X, Agopian K, Kumar NM, Gilula NB.. Genetic factors influence cataract formation in alpha 3 connexin knockout mice. Dev Genet. 1999; 24: 27–32. [DOI] [PubMed] [Google Scholar]
- 74.West SK, Munoz B, Schein OD, Duncan DD, Rubin GS.. Racial differences in lens opacities: the Salisbury Eye Evaluation (SEE) project. Am J Epidemiol. 1998; 148: 1033–1039. [DOI] [PubMed] [Google Scholar]
- 75.Storey P, Munoz B, Friedman D, West S.. Racial differences in lens opacity incidence and progression: the Salisbury Eye Evaluation (SEE) study. Invest Ophthalmol Vis Sci. 2013; 54: 3010–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shentu XC, Zhao SJ, Zhang L, Miao Q.. A novel p.R890C mutation in EPHA2 gene associated with progressive childhood posterior cataract in a Chinese family. Int J Ophthalmol. 2013; 6: 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.