Abstract
A total of 122 Streptococcus suis serotype 2 strains were characterized thoroughly by comparing clinical and pathological observations, ribotype profiles, and antimicrobial resistance. Twenty-one different ribotype profiles were found and compared by cluster analysis, resulting in the identification of three ribotype clusters. A total of 58% of all strains investigated were of two ribotypes belonging to different ribotype clusters. A remarkable relationship existed between the observed ribotype profiles and the clinical-pathological observations because strains of one of the two dominant ribotypes were almost exclusively isolated from pigs with meningitis, while strains of the other dominant ribotype were never associated with meningitis. This second ribotype was isolated only from pigs with pneumonia, endocarditis, pericarditis, or septicemia. Cluster analysis revealed that strains belonging to the same ribotype cluster as one of the dominant ribotypes came from pigs that showed clinical signs similar to those of pigs infected with strains with the respective dominant ribotype profiles. Furthermore, strains belonging to different ribotype clusters had totally different patterns of resistance to antibiotics because strains isolated from pigs with meningitis were resistant to sulfamethazoxazole and strains isolated from pigs with pneumonia, endocarditis, pericarditis, or septicemia were resistant to tetracycline.
The gram-positive bacterium Streptococcus suis is an organism of increasing importance in pig production all over the world. Today 35 different serotypes of S. suis are described (9, 10, 13, 19). Most of the serotypes have been detected in healthy pigs, but a few of the serotypes are very often associated with clinical infections such as meningitis, septicemia, pneumonia, endocarditis, pericarditis, and arthritis (6). S. suis serotype 2 has widely been reported to be the most pathogenic serotype, but in Denmark the most prevalent pathogenic S. suis serotype was, until recently, serotype 7 (19). However, since 1982 serotype 2 has been isolated more and more frequently from diseased pigs, and today, S. suis serotype 2 is the dominant pathogenic serotype in Denmark (1, 8).
Along with the increasing importance of S. suis serotype 2 infections in pig production, an intensive search for virulence factors has been carried out (11, 14, 20, 21, 25, 28). Unfortunately, this work has so far not revealed any true virulence factors (22). Genotypic characterization of S. suis in general has primarily been done by restriction endonuclease analysis (3, 16) or ribotyping (4, 12, 18, 23, 24) and has revealed differences and homologies at the serotype and the subserotype levels. However, a thorough characterization of S. suis serotype 2 strains has never been performed.
In this study, four different collections of S. suis serotype 2 strains were investigated. The first collection consisted of 5 well-characterized avirulent strains, the second consisted of 18 strains isolated from Danish pigs from 1967 to 1982, the third consisted of 16 strains isolated from humans from 1966 to 1989, and the fourth consisted of 83 stains isolated from Danish pigs from 1995 to 1997. In all, 122 strains were investigated by ribotyping and for antimicrobial resistance, and possible associations with clinical-pathological observations were examined.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Five well-characterized avirulent S. suis serotype 2 strains, strains 12, 18, 25, and T15 (4, 27, 29, 31) and strain 89-1591 (21a), were donated by Hilde Smidt, DLO-Institute for Animal Science and Health, Lelystad, The Netherlands. Eighteen S. suis serotype 2 strains isolated from pigs from 1967 to 1982 and 16 S. suis strains isolated from humans from 1966 to 1989 were kindly donated by Susanne Sauer and Helle B. Konradsen, Statens Serum Institut, Copenhagen, Denmark.
The remaining 83 strains were isolated from diseased pigs at the Danish Veterinary Laboratory from 1995 to 1997 and were identified as S. suis.
Pathology and clinical disease.
Detailed information about porcine-derived strains collected during the period from 1967 to 1982 were, unfortunately, not available.
The 83 strains isolated over the period from 1995 to 1997 were isolated from pigs received at the Danish Veterinary Laboratory for routine diagnostic investigation. The observed pathological findings were compared with information on the clinical symptoms presented by the veterinarians on standard forms following submission of the material to the laboratory. Pigs with a history of central nervous system symptoms were routinely investigated by histopathology. The most significant pathological changes were registered as meningitis, pneumonia, pericarditis, endocarditis, arthritis, and septicemia. Septicemia was diagnosed only when S. suis serotype 2 strains were isolated from at least two different organs and the anamnestic information was in accordance with septicemia.
Data for pigs for which there was insufficient material (e.g., only one organ) and for which very limited information was available from the veterinarian were excluded because no conclusion could be made concerning significant pathology, history, or cause of death.
Isolation, identification, serotyping, and antimicrobial susceptibility testing.
Isolation, identification, serotyping, and antimicrobial susceptibility testing were done as described previously (1, 2).
Extraction of DNA.
All concentrations given below in parentheses are the final concentrations. A total of 125 ml of Todd-Hewitt broth plus 5% horse serum that had been inoculated and incubated overnight was centrifuged, and the pellet was resuspended in 0.6 ml of 10% sucrose–50 mM Tris (pH 8.0). After the addition of lysozyme (1.67 mg/ml) and EDTA (0.1 M), the suspension was incubated at 20°C for 30 min. The cell membrane was lysed by the addition of sodium dodecyl sulfate (1.67%), and the liberated protein was degraded during a 2-h incubation at 60°C with proteinase K (0.26 mg/ml). Nucleic acids were extracted with phenol-chloroform and precipitated with ethanol.
Ribotyping.
Approximately 15 μg of DNA was digested with 30 U of restriction endonuclease at 37°C for 3 h. The DNA fragments were separated on a 0.7% agarose gel. The gels were run overnight at 1.4 V/cm. The separated DNA fragments were blotted onto a nylon membrane (Hybond; Amersham) by traditional Southern blotting and fixed with UV light.
Hybridization was done overnight in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at 60°C. As probe, a sequence complementary to a conserved region of the 16S gene (31) randomly labeled with digoxigenin (DIG) was used. After hybridization the membrane was washed stringently with 0.1× SSC at 60°C. The addition of anti-DIG–alkaline phosphatase conjugate and substrate reaction with 5-bromo-4-chloro-3-indolyllphosphate toluidinium salt and Nitro Blue Tetrazolium were done with the DIG Wash and Block Buffer Set as described by the manufacturer (Boehringer-Mannheim).
Cluster analysis was done with GelCompar software, version 4.0 (Applied Maths, Kortrijk, Belgium).
RESULTS
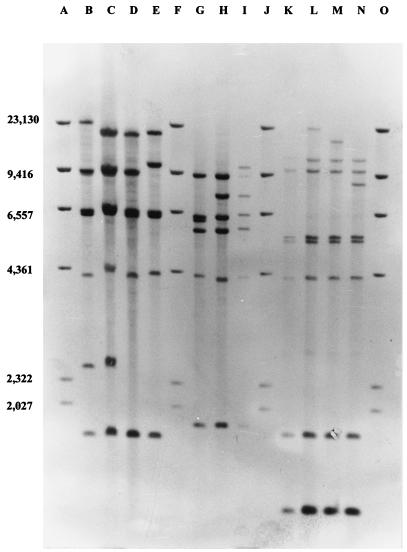
During the initial experiments the restriction endonucleases BamHI, BglII, EcoRI, HaeIII, HindIII, and SmaI were used to generate DNA fragment patterns. It was found that EcoRI gave highly heterogeneous band patterns, facilitating segregation of the strains analyzed. All strains were also analyzed by using HaeIII for restriction endonuclease analysis (data not shown). On the basis of an investigation of all 122 isolates, 21 different ribotype profiles were detected, and 11 of these were found repeatedly (Fig. 1). Of these 11 ribotype profiles, 2 (ribotypes 1 and 11) accounted for 58% of all isolates analyzed.
FIG. 1.
Hybridization patterns of DIG-labeled 16S rRNA gene probes with Southern-blotted EcoRI restriction fragments of DNA from strains of all 11 repeatedly observed ribotype profiles. DIG-labeled HindIII restriction fragments were used as markers. Lanes B, C, D, and E, ribotype group I, ribotype profiles 1, 2, 3, and 4, respectively; lanes G, H, and I, ribotype group II, ribotype profiles 5, 6, and 7, respectively; lanes K, L, M, and N, ribotype group III, ribotype profiles 8, 9, 10, and 11, respectively; lanes A, F, J, and O, marker DNA. The numbers on the left are in base pairs.
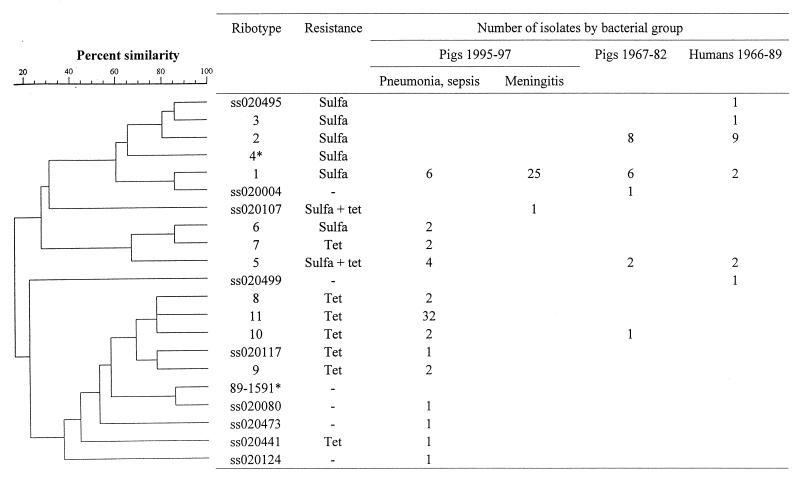
On the basis of cluster analysis, all identified ribotype profiles could be separated into two major clusters and one intermediate cluster (Fig. 2). Cluster I consisted of four refound ribotypes (ribotypes 1, 2, 3, and 4 in Fig. 1) and three unique ribotypes. Cluster II consisted of three refound ribotypes (ribotypes 5, 6, and 7 in Fig. 1), while cluster III consisted of four refound ribotypes (ribotypes 8, 9, 10, and 11 in Fig. 1) and seven unique ribotypes.
FIG. 2.
Dendrogram obtained from cluster analysis of EcoRI ribotype profiles among 117 S. suis isolates. The isolates included strains from pigs with different clinical infections from 1967 to 1982 and 1995 to 1997, strains isolated from humans from 1960 to 1989, and avirulent strains. Ribotype nos. corresponds to the respective ribotype profiles given in the legend to Fig. 1, and the remaining ribotype profiles (ss02 and 89-1591) are the ribotypes profiles observed only once. The antimicrobial resistance expressed by more than 50% of the strains in a group are mentioned. Abbreviations: Tet, tetracycline; Sulfa, sulfamethoxazole. *, avirulent isolate. (The correlation is by number of bands, using Jaccard coefficients with a maximum tolerance of 0.8% and a minimum surface of 0.0%. A total of 4 to 396 zones were used. Clustering was done by the unweighted pair group method with arithmetic means.
Avirulent strains.
When analyzing the collection of strains previously described as avirulent (4, 27, 29, 31), three ribotype profiles were observed. Strains 12, 18, and T15 all had the ribotype 4 profile, strain 25 had the ribotype 3 profile, and strain 89-1591 had a unique profile.
Strains isolated from pigs from 1967 to 1982 and from humans from 1966 to 1989.
Among the 34 strains isolated from humans and pigs, ribotype profiles were compared by species of origin and MICs (Fig. 2). In total, eight ribotype profiles were identified. Determination of the antimicrobial resistance patterns revealed that a large fraction of the strains were resistant to sulfamethoxazole. Two ribotype profiles were dominant, because 74% (25 of 34) of the strains in these collections were of ribotype profiles 1 and 2. Sixteen strains were isolated from humans with clinical meningitis. Of these, 56% (9 of 16) had the ribotype 2 profile and 13% (2 of 16) had the ribotype 1 profile, while the remaining strains had four different ribotype profiles. The strains originating from pigs had five different ribotype profiles. Among these strains, 44% (8 of 18) shared the ribotype 2 profile and 33% (6 of 18) shared the ribotype 1 profile. Of all the strains originating from humans or pigs and isolated before 1989, 50% (17 of 34) shared the ribotype 2 profile. Ribotype 2 was seen only in the collections of strains isolated before 1989.
Strains isolated from pigs from 1995 to 1997.
For the 83 S. suis serotype 2 strains isolated at the Danish Veterinary Laboratory, the ribotype profiles, MICs, and clinical-pathological observations were compared (Fig. 2). A total of 14 ribotype profiles were identified. Two of the ribotype profiles (profile 1, belonging to ribotype cluster I, and profile 11, belonging to ribotype cluster III) were dominant because 76% (70 of 92) of the strains had one of these two ribotype profiles. A total of 81% (26 of 32) of the strains in ribotype cluster I were isolated from pigs with meningitis. None of the strains belonging to ribotype cluster III originated from pigs with meningitis. All strains in ribotype cluster III were isolated from pigs with clinical pneumonia, pericarditis, endocarditis, or septicemia.
The MIC determinations indicated two patterns of resistance. First, a few strains that were evenly distributed among all ribotype profiles were resistant to multiple antibiotics (data not shown). Detailed data on the antimicrobial resistance of all strains will be reported elsewhere (2). Second, major differences were identified when the antibiotic resistance was compared with the ribotype clusters. A vast majority of the strains in ribotype cluster I were resistant to sulfamethoxazole, while most strains in ribotype cluster III were resistant to tetracycline.
DISCUSSION
S. suis is a very common infectious agent in pigs, and carriage rates of up to 100% among all pig herds have been reported (17). However, because only a few of these infections result in clinical disease (7), a majority of the S. suis strains normally isolated from pigs are expected to be less virulent. Furthermore, the existence of avirulent S. suis serotype 2 strains has been demonstrated (30). Because the high carriage rates complicate efficient control of S. suis, a test discriminating between avirulent and virulent S. suis serotype 2 strains would be of high value. Ribotyping has been proposed as such a test since the ribotype profiles of avirulent and virulent S. suis serotype 2 strains were claimed to be easily distinguished due to the presence of specific bands (18).
During this study, five well-characterized avirulent strains were analyzed by ribotyping. Surprisingly, the ribotype profiles of these strains were found to be closely related to the ribotype profiles of strains from diseased animals. Four of the avirulent strains originated from The Netherlands, and three of these strains had the ribotype 4 profile. Ribotype profile 4 mapped in the middle of ribotype cluster I. The fourth of the avirulent Dutch strains had the ribotype 3 profile, which was identical to the ribotype profile of a strain isolated from a human patient with clinical meningitis. The fifth avirulent strain, strain 89-1591, was the only strain originating from Canada; thus, the unique ribotype profile of this geographically distant strain was not unexpected. In summary, the avirulent strains had ribotype profiles very closely related to the ribotype profiles of virulent strains, and one of the avirulent strains had a profile identical to the profiles of virulent strains. Thus, this study did not find that avirulent strains could easily be discriminated from virulent strains by examination of the ribotype profiles.
When analyzing the collections of strains isolated before 1989, eight ribotype profiles were identified. Two of the ribotype profiles, profiles 1 and 2, represented 74% of the strains in these collections. These two ribotype profiles have recently been described as ribotype profiles A and B, respectively (23). In the same paper ribotype profile A was described as the profile for strains expressing the muramidase-released protein (MRP) and the elongation factor (EF) protein, which were formerly believed to be virulence factors (22, 28), while ribotype profile B was described as the profile for strains expressing the proteins MRP and EF* (a high-molecular-mass version of EF [29]). Strains with the MRP EF phenotype are believed to be highly virulent, and strains with the MRP EF* phenotype are believed to be moderately virulent.
It has previously been proposed that strains with ribotype profile 2 (or ribotype profile B) are particularly infective for humans (20). In this study, 16 strains isolated from humans were analyzed, and 9 of these 16 strains had ribotype profile 2. However, because ribotype profile 2 was also observed for 8 of 18 strains isolated from pigs, the rate of occurrence of strains of human origin with ribotype profile 2 is not significantly above the expected rate. Thus, this study does not support the hypothesis that ribotype profile 2 (or ribotype profile B) is more common among humans than among pigs.
When the strains isolated from diseased pigs from 1995 to 1997 were analyzed, it was observed that the Danish pig herds are infected with at least 14 different S. suis serotype 2 ribotypes. Furthermore, when the ribotype profiles were grouped into different ribotype clusters (Fig. 2), a segregation of the S. suis serotype 2 strains presently infecting Danish pigs became obvious. A total of 76% of the strains had ribotype profiles 1 and 11, which belonged to ribotype clusters I and III, respectively. Furthermore, there is a clear association between ribotype and clinical-pathological observations because 25 of the 31 strains with ribotype profile 1 came from pigs with meningitis. From the remaining six pigs only lungs were received for autopsy. Thus, the complete pathological status of these pigs is unknown. A strikingly different pattern was seen when the strains with the ribotype 11 profile were analyzed, because none of these strains were isolated from pigs with meningitis. All strains with the ribotype 11 profile came from pigs with pneumonia, endocarditis, pericarditis, or septicemia. A similar association between ribotype and clinical-pathological observations has previously been described for Streptococcus pyogenes. The reason for this was found to be virulence factors (15, 26) or resistance to treatment with antibiotics (5). Furthermore, a correlation between restriction fragment analysis and clinical signs has been suggested for S. suis (16). Whether the association described here for S. suis is caused by yet unknown virulence factors remains to be investigated.
The segregation of Danish S. suis serotype 2 strains into two major ribotype clusters that were clearly associated with different clinical diseases became even more obvious when MIC data were included. This also indicated that the Danish pig herds are presently infected with two major ribotypes belonging to different ribotype clusters. Nearly all strains in ribotype cluster I were resistant to sulfamethoxazole and sensitive to tetracycline, while strains in ribotype cluster III were resistant to tetracycline. Some strains in ribotype cluster II were resistant to tetracycline, while others were resistant to sulfamethoxazole.
The observation that one of the dominant ribotypes, ribotype 1, was sensitive to tetracycline was quite unexpected since tetracyclines have been used extensively in Danish pig production. From 1988 to 1994 the use of active tetracyclines increased from 3 to 31 tons per year and then decreased to approximately 8 tons per year from 1995 to 1996. Despite this level of use of tetracyclines in Denmark, ribotype 1 strains are isolated very frequently and create severe problems in Danish pig herds; thus, one could hypothesize that ribotype 1 strains have a reservoir different from that of the other dominant ribotype, ribotype 11, which is tetracycline resistant. Furthermore, the presence of different reservoirs could indirectly cause the observed associations between ribotype profiles and clinical-pathological observations because the clinical-pathological observations could partly be a result of different infection routes. However, this hypothesis must be supported by further work.
When the pig-derived strains isolated from 1967 to 1982 and 1995 to 1997 were compared, a progression from the prevalence of one ribotype cluster to the prevalence of another was observed. Among the strains isolated from 1967 to 1982, strains of ribotype cluster I predominated because 83% of all strains originating from pigs belonged to ribotype cluster I. At the same time, strains belonging to ribotype cluster II and ribotype cluster III were only rarely isolated. Exactly the opposite was seen for the collection of strains isolated from 1995 to 1997. Among the strains in that collection 39% were of ribotype cluster I and 52% were of ribotype cluster III.
The prevalence of S. suis serotype 2 strains belonging to ribotype cluster III has thus increased remarkably since 1989. While only two isolates obtained before 1989 belonged to ribotype cluster III, 59% of the S. suis serotype 2 strains isolated from pigs from 1995 to 1997 belonged to ribotype cluster II. However, because the anamnestic data for strains isolated from 1967 to 1982 are limited, a one-sided representation of strains in this collection cannot be ruled out.
On the basis of the results of this study, it is concluded that (i) virulent and avirulent S. suis serotype 2 strains can be very closely related, (ii) most S. suis infections in Denmark are caused by two dominant S. suis serotype 2 strains belonging to different ribotype clusters, (iii) a clear relation between the ribotype profile and antimicrobial resistance exists, and (iv) in Denmark, ribotype profiles for S. suis strains are clearly associated with clinical-pathological observations.
It is thus believed that ribotyping as described here can be used very efficiently during epidemiological investigations. Furthermore, on the basis of the ribotype profiles of infecting S. suis serotype 2 strains found in pig herds, the types of clinical problems that may occur can be predicted.
ACKNOWLEDGMENTS
We thank Helena Christensen and Lis B. Hansen for outstanding technical assistance.
This study was supported by a grant from the Federation of Danish Pigproducers and Slaughterhouses.
REFERENCES
- 1.Aarestrup F M, Jorsal S E, Jensen N E. Serological characterization and antimicrobial susceptibility of Streptococcus suis isolates from diagnostic samples in Denmark during 1995 and 1996. Vet Microbiol. 1998;60:59–66. doi: 10.1016/s0378-1135(98)00147-3. [DOI] [PubMed] [Google Scholar]
- 2.Aarestrup F M, Rasmussen S R, Artursson K, Jensen N E. Trends in the resistance to antimicrobial agents of Streptococcus suis isolates from Denmark and Sweden. Vet Microbiol. 1998;63:71–80. doi: 10.1016/s0378-1135(98)00228-4. [DOI] [PubMed] [Google Scholar]
- 3.Amass S F, SanMiguel P, Clarck L K. Demonstration of vertical transmission of Streptococcus suis in swine by genomic fingerprinting. J Clin Microbiol. 1997;35:1595–1596. doi: 10.1128/jcm.35.6.1595-1596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaudoin M, Harel J, Higgins R, Gottschalk M, Frenette M, McInnes J I. Molecular analysis of isolates of Streptococcus suis capsular type 2 by restriction-endonuclease-digested DNA separated on SDS-PAGE and by hybridization with an rDNA probe. J Gen Microbiol. 1992;138:2639. doi: 10.1099/00221287-138-12-2639. [DOI] [PubMed] [Google Scholar]
- 5.Bingen E, Denamur E, Lambert-Zechovsky N, Braimi N, El Lakany M, Elion J. DNA restriction fragment length polymorphism differentiates recurrence from relapse in treatment failures of Streptococcus pyogenes pharyngitis. J Med Microbiol. 1992;37:162–164. doi: 10.1099/00222615-37-3-162. [DOI] [PubMed] [Google Scholar]
- 6.Clifton-Hadley F A. Streptococcus suis type 2 infections. Br Vet J. 1983;139:1–5. doi: 10.1016/s0007-1935(17)30581-x. [DOI] [PubMed] [Google Scholar]
- 7.Clifton-Hadley F A. Proceedings of the American Association of Swine Practitioners. Perry, Iowa: American Association of Swine Practitioners; 1986. The epidemiology, diagnosis, treatment, and control of Streptococcus suis type 2 infections; pp. 471–491. [Google Scholar]
- 8.Danish Veterinary Laboratory and Danish Veterinary Institute for Virus Research. The 1996 annual report. Copenhagen, Denmark: DVL and DVIV; 1996. [Google Scholar]
- 9.Gottschalk M G, Higgins R, Jaques M, Mittal K R, Hendrichen J. Description of 14 new capsular typees of Streptococcus suis. J Clin Microbiol. 1989;27:2633–2636. doi: 10.1128/jcm.27.12.2633-2636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottschalk M G, Higgins R, Jaques M, Beaudoin M, Hendrichen J. Characterization of six new capsular types (23 through 28) of Streptococcus suis. J Clin Microbiol. 1991;29:2590–2594. doi: 10.1128/jcm.29.11.2590-2594.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottschalk M G, Higgins R, Jaques M, Dubreil D. Production and characterization of two Streptococcus suis capsular type 2 mutants. Vet Microbiol. 1992;30:59–71. doi: 10.1016/0378-1135(92)90094-a. [DOI] [PubMed] [Google Scholar]
- 12.Harel J, Higgins R, Gottschalk M G, Bigras-Ppoulin M. Genomic relatedness among reference strains of different Streptococcus suis serotypes. Can J Vet Res. 1994;58:259–262. [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins R, Gottschalk M, Boudreau M, Lebrun A, Henrichsen J. Description of six new capsular types (29-34) of Streptococcus suis. J Vet Invest. 1995;7:405–406. doi: 10.1177/104063879500700322. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs A A C, Loeffen P L W, van den Berg A J G, Storm P K. Identification, purification and characterization of a thiol-activated hemolysis (suilysin) of Streptococcus suis. Infect Immun. 1994;62:1742–1748. doi: 10.1093/benz/9780199773787.article.b00034458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masuyama T, Ishii E, Muraoka K, Honjo S, Yamaguchi H, Hara T, Shimazaki K, Koga T, Moriya K, Ide M, Miyazaki S. Outbreak of acute glomerulonephritis in children: observed association with the T1 subtype of group A streptococcal infection in Northern Kyushu, Japan. Acta Paediatr Jpn. 1996;38:128–131. doi: 10.1111/j.1442-200x.1996.tb03454.x. [DOI] [PubMed] [Google Scholar]
- 16.Mogollon J D, Pijoan C, Murtaugh M P, Kaplan E L, Collins J E, Cleary P P. Characterization of prototype and clinically defined strains of Streptococcus suis by genomic fingerprinting. J Clin Microbiol. 1990;28:2462–2466. doi: 10.1128/jcm.28.11.2462-2466.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mwaniki C G, Robertson I D, Trott D J, Atyeo R F, Lee B J, Hampson D J. Clonal analysis and virulence of Australian isolates of Streptococcus suis type. Epidemiol Infect. 1994;113:321–334. doi: 10.1017/s095026880005175x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okwumabua O, Staats J, Chengappa M M. Detection of genomic heterogeneity in Streptococcus suis isolates by DNA restriction fragment length polymorphisms of rRNA genes (ribotyping) J Clin Microbiol. 1995;33:968–972. doi: 10.1128/jcm.33.4.968-972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perch B, Pedersen K B, Henrichsen J. Serology of capsulated streptococci pathogenic for pigs: six new serotypes of Streptococcus suis. J Clin Microbiol. 1983;17:993–996. doi: 10.1128/jcm.17.6.993-996.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quessy S, Dubreuil J D, Hacques M, Malouin F, Higgins R. Increase of capsular material thickness following in vivo growth of virulent Streptococcus suis serotype 2 strains. FEMS Microbiol Lett. 1994;115:19–26. doi: 10.1111/j.1574-6968.1994.tb06608.x. [DOI] [PubMed] [Google Scholar]
- 21.Salasia S I O, Lämmler C, Herrmann G. Properties of a Streptococcus suis isolate of serotype 2 and two capsular mutants. Vet Microbiol. 1995;45:151–156. doi: 10.1016/0378-1135(95)00036-a. [DOI] [PubMed] [Google Scholar]
- 21a.Smidt, H. (ID-DLO, Lelystad, The Netherlands). Personal communication.
- 22.Smith H E, Vecht U, Wisselink H J, Stockhofe-Zurwieden N. Proceedings of the 14th IPVS Congress. 1996. Streptococcus suis mutants in which the genes muramidase-released protein (MRP) and extracellular protein factor (EF) are inactivated are virulent for pigs. [Google Scholar]
- 23.Smith H E, Rijnsburger M, Stockhofe-Zurwieden N, Wisselink H J, Vecht U, Smits M A. Virulent strains of Streptococcus suis serotype 2 and highly virulent strains of Streptococcus suis serotype 1 can be recognized by a unique ribotype profile. J Clin Microbiol. 1997;35:1049–1053. doi: 10.1128/jcm.35.5.1049-1053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staats J J, Plattner B L, Nietfeld J, Dritz S, Chengappa M M. Use of ribotyping and hemolysin activity to identify highly virulent Streptococcus suis type 2 isolates. J Clin Microbiol. 1998;36:15–19. doi: 10.1128/jcm.36.1.15-19.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tikkanen K, Haataja S, Finne J. The galactosyl-(1-4)-galactose-binding adhesin of Streptococcus suis: occurrence in strains of different hemagglutination activities and induction of opsonic antibodies. Infect Immun. 1996;64:3659–3665. doi: 10.1128/iai.64.9.3659-3665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Upton M, Carter P E, Morgan M, Edwards G F, Pennington T H. Clonal structure of invasive Streptococcus pyogenes in northern Scotland. Epidemiol Infect. 1995;115:231–241. doi: 10.1017/s0950268800058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vecht U, Arends J P, van der Molen E J, van Leengoed L A M. Differences in virulence between two strains of Streptococcus suis type 2 after experimentally induced infection of newborn germ-free pigs. Am J Vet Res. 1989;50:1037–1043. [PubMed] [Google Scholar]
- 28.Vecht U, Wisselink H J, Jellema M L, Smith H D. Identification of two proteins associated with virulence of Streptococcus suis type 2. Infect Immun. 1991;59:3156–3162. doi: 10.1128/iai.59.9.3156-3162.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vecht U, Wisselink H J, Stockhofe-Zurwieden N, Smith H E. Characterization of virulence of the Streptococcus suis serotype 2 reference strain Henrichsen S 735 in newborn gnotobiotic pigs. Vet Microbiol. 1996;51:125–136. doi: 10.1016/0378-1135(96)00028-4. [DOI] [PubMed] [Google Scholar]
- 30.Vecht U, Stockhofe-Zurwieden N, Tetenburg B J, Wisselink H J, Smith H E. Virulence of Streptococcus suis type 2 for mice and pigs appeared host-specific. Vet Microbiol. 1997;58:53–60. doi: 10.1016/s0378-1135(97)00131-4. [DOI] [PubMed] [Google Scholar]
- 31.Weisburg W G, Barns S D, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]