Abbreviations
- MSM
sex with men
- PrEP
pre‐exposure prophylaxis
- HCV
hepatitis C virus infection
- PY
person‐years
1. INTRODUCTION
In general, HIV‐negative men who have sex with men (MSM) have been considered to be at low risk for hepatitis C virus (HCV) infection.1, 2 HIV‐negative MSM who access pre‐exposure prophylaxis (PrEP) have reported sexual behaviours that could place them at high risk of HCV, including high partner numbers, chemsex and injecting drug use.3, 4 Early diagnosis of HCV infection allows early linkage to treatment and care, and reduction in onward transmission of infection.5 Data from European PrEP trials and cohort studies of PrEP users have reported high baseline HCV prevalence and incidence during follow‐up.6, 7, 8 In contrast, studies from North America have generally found low levels of HCV endemicity among PrEP users.9, 10, 11
The PROUD study was an open‐label trial of HIV PrEP among 544 HIV‐negative MSM.12, 13 As there were no data on HCV incidence in HIV‐negative MSM using PrEP in the UK, we implemented routine quarterly screening during the long‐term follow‐up phase when all participants had access to PrEP. We report HCV prevalence and incidence among participants in the PROUD study.
2. MATERIALS AND METHODS
PROUD was an open‐label, wait‐list trial design that randomized MSM attending participating sexual health centres in England to receive HIV PrEP immediately or after a deferral period of 1 year (the deferred phase). Five hundred and forty‐four participants were recruited between November 2012 and April 2014, and follow‐up continued to October 2016. The protocol was modified in November 2014 following an interim analysis which showed PrEP to be highly effective, resulting in some participants in the deferred arm being offered PrEP earlier than one year (N = 163).13
The initial PROUD protocol followed national guidelines on HCV testing, with screening 'on indication'. Screening at enrolment was not mandated. The tests used during the trial varied by site, and the use of antibody, antigen or viral load tests depending on whether the participant had a prior history of HCV. Information on HCV was collected in a number of ways. At enrolment, participants self‐reported a diagnosis with HCV in the previous 12 months, and the clinician reported whether the participant had ever been screened for, and if so, diagnosed with HCV. At each visit, the number of HCV screens and positive screens (although not distinguishing the type of test) since the previous visit was recorded. For all new infections, clinics were asked to provide detailed clinical and laboratory information, including dates of last positive and last negative test (for all assays), HCV viral load test results, liver function test results and history of injecting drug use. In March 2015, additional funding was acquired which allowed screening at every quarterly study visit; at this time, all participants had access to PrEP and the trial was closed to further recruitment.
If the first HCV antibody (anti‐HCV) test was negative, the participant was assumed to be seronegative at enrolment. If the first test was positive, the Trial Management Group determined whether infection was most likely acquired before enrolment (and thus contributed to the seroprevalence analysis) or after enrolment (and thus contributed to the incidence analysis) based on alanine transaminase and HCV viral load measurements, in relation to time since randomization.
2.1. Statistical analysis
The cumulative incidence of HCV infection (time to diagnosis) was estimated using Kaplan‐Meier analysis and randomized groups compared with the log‐rank test, censoring at the time of the last screen for HCV. Estimation of incidence by calendar year was complicated by the highly variable time between the last negative test and the first positive test which, in some cases, could span adjacent calendar years. To address this, the date of infection was imputed 1000 times assuming a uniform distribution, and calculating the incidence for each calendar year within each imputed dataset.14 Estimates were obtained by averaging across the imputed datasets, and confidence intervals derived using Rubin's rule.15 All analyses were done in STATA version 15.1.
3. RESULTS
Characteristics of the 544 study participants have been previously described.13 Figure 1 illustrates the hepatitis C screening and infection among study participants. One hundred and thirty‐three (24.4%) participants were screened for HCV at enrolment, and 499 (91.7%) were tested at least once during follow‐up. Nine participants were only screened at baseline and therefore could not contribute to an incidence analysis. A HCV screen was conducted at 54.0% (3213/5946) of visits (higher during the phase of routine quarterly screening [80.6%] compared to the earlier phase of testing on indication [34.9%]), with a median of 6 (IQR: 3‐8) screens per participant. Of the 45 participants who were never tested, 14 participants were also missing information on HCV history collected at enrolment.
Figure 1.
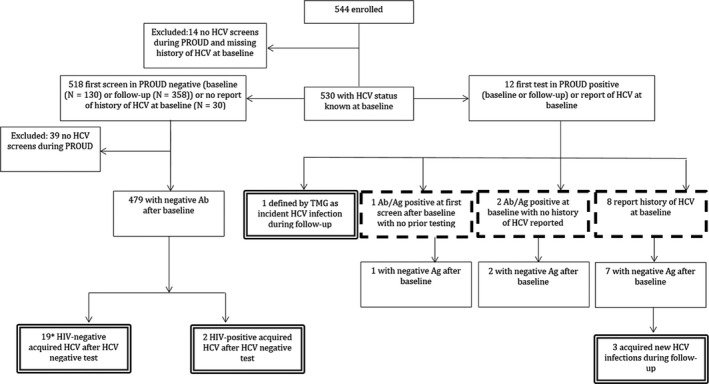
Hepatitis C screening and infection consort diagram. Seroprevalent infections indicated by dashed box. Incidence infections indicated by double‐lined box. TMG, Trial Management Group
3.1. Hepatitis C seroprevalence at enrolment
The seroprevalence at enrolment was 2.1% (11/530; 95% CI: 1.0%‐3.7%). The 11 cases were identified as follows: eight participants were reported by the clinician at enrolment to have had a previous diagnosis of HCV; two were diagnosed with HCV a few days before enrolment; two participants had HCV viraemia detected at their first post‐enrolment test (they were not tested at enrolment), of which one was judged to have acquired infection before enrolment.
3.2. Hepatitis C incidence
HCV incidence is based on the 490 participants who were considered HCV seronegative at enrolment, or who had previously cleared HCV infection prior to enrolment, and had at least one post‐enrolment HCV test. Table 1 presents baseline characteristics of these participants and indicates a cohort with high‐risk behaviours: 226 (47.6%) reported use of chemsex‐associated drugs in the past three months, 282 (57.6%) were diagnosed with any STI in the past 12 months, 170 (36.9%) reported using post‐exposure prophylaxis in the past 12 months, and the median number of partners in the past three months was 10.
Table 1.
Baseline characteristics of PROUD participants included in HCV incidence analysis
| Characteristic |
N (%) (Total = 490) |
|---|---|
| Age (median) | 35 (29‐42) |
| Ethnicity | |
| White | 395 (81.4%) |
| Black | 17 (3.5%) |
| Asian | 28 (5.8%) |
| Other | 45 (9.3%) |
| University degree | 303 (62.3%) |
| Full‐time employment | 351 (72.4%) |
| Born in the UK | 292 (60.1%) |
| Relationship status | |
| Partner, living together | 145 (29.8%) |
| Partner, living separately | 74 (15.2%) |
| Single | 267 (54.9%) |
| Circumcised | 142 (29.4%) |
| Chemsex‐associated drugs* | 226 (47.6%) |
| STI diagnosed in past 12 mo | |
| Any** | 282 (57.6%) |
| Rectal*** | 162 (34.8%) |
| Rectal*** or syphilis | 183 (39.3%) |
| Number of sexual partners in past 3 mo, median | 10 (5‐20) |
| Number of ncRAI partners in past 3 mo, median | 2 (1‐5) |
| Number of HIV tests in past 12 mo | 3 (2‐4) |
| Use of PEP in past 12 mo | 170 (36.9%) |
Data are median (IQR) or n (%). Number of participants missing baseline data: ethnicity, 5; university degree, 4; full‐time employment, 5; relationship status, 4; chemsex, 15; STI, 24; total number of partners, 8; ncRAI partners, 25; HIV test, 17; PEP, 29.
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; ncRAI, receptive anal intercourse without a condom; PEP, post‐exposure prophylaxis; STI, sexually transmitted infection; UK, United Kingdom.
Methamphetamine, GHB, mephedrone or ketamine.
Chlamydia, gonorrhoea, or syphilis.
Rectal chlamydia or rectal gonorrhoea.
The median follow‐up (enrolment to last HCV test) was 2.6 (IQR: 2.1‐3.0) years, with a total follow‐up of 1188.8 person‐years (PY). Overall, 25 participants had a new HCV infection, yielding an incidence rate of 2.1 per 100 PY (25/1188.8; 95% CI: 1.4‐3.1). Three of these were re‐infections (the previous infections had cleared spontaneously or with treatment before enrolment), and one participant experienced two infections during follow‐up (only the first infection was included in the incidence calculation). Two HCV infections were acquired after diagnosis of HIV whilst the participant was no longer on PrEP but was being actively followed up. Excluding these cases from the HCV incidence calculation reduced the estimate only slightly (1.9 per 100 PY [95% CI: 1.2‐2.9]). Use of nonprescribed injected drugs was reported by 11 participants at the suspected time of HCV infection, was denied by 12 and was unknown for two.
Figure 2 shows the cumulative incidence of time to a new HCV diagnosis, stratified by randomized arm (P‐value log‐rank test = 0.87). As only three infections (one immediate, two deferred) were observed during the deferred phase of the study (ie the period before the DEF arm had access to PrEP), the trial essentially provides no randomized information of whether access to PrEP affects the risk of acquiring HCV infection. Accounting for uncertainty in the time of acquisition of HCV infection, HCV incidence appeared to increase over calendar time (Table 2), reaching an estimated 4.0 per 100 PY in 2016 (95% CI: 2.0‐8.1, P‐value for trend = 0.09).
Figure 2.
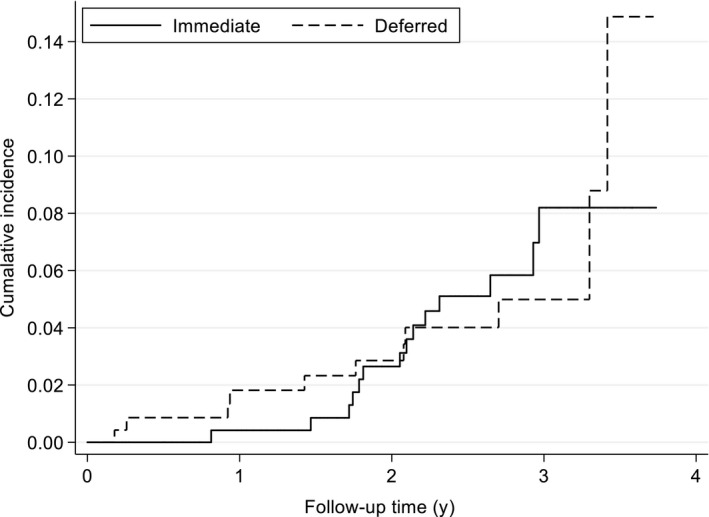
Cumulative incidence of HCV infection (time from enrolment to diagnosis). For illustrative purposes, one participant (IMM) diagnosed 4 d before the end of the trial is excluded. (P‐value for log‐rank test = 0.87)
Table 2.
HCV incidence stratified by calendar year
| Year | Person‐years follow‐up | Estimated no. of HCV infectionsa | Rate per 100 PY (95% CI)* |
|---|---|---|---|
| 2013 | 154.8 | 2.8 | 1.8 (0.5‐6.2) |
| 2014 | 431.4 | 5.7 | 1.3 (0.5‐3.3) |
| 2015 | 393.2 | 8.2 | 2.1 (1.0‐4.4) |
| 2016 | 205.1 | 8.2 | 4.0 (2.0‐8.1) |
Accounting for infection interval over calendar years.
P‐value for trend = 0.09.
4. DISCUSSION
This analysis demonstrates a high overall incidence of hepatitis C infection (2.1 per 100 PY) in the PROUD trial. This is higher than, although compatible with, the incidence reported in other contemporaneous PrEP studies in Europe. In the AmPrEP study, HCV incidence during follow‐up was 1.9 per 100 PY (95% CI: 1.1‐3.4). The incidence rate of primary infection was 1.0 per 100 PY (95% CI: 0.5‐2.2) and of re‐infection 25.5 per 100 PY (95% CI: 11.5‐56.8).6 In the ANRS IPERGAY study of on‐demand PrEP, HCV incidence was estimated to be 1.4 per 100 PY (95% CI: 0.7‐2.4).7 A large French cohort estimated HCV incidence to be 1.2 per 100 PY for HIV‐negative MSM PrEP users.8
In contrast, demonstration and implementation cohorts of PrEP users in North America have reported lower incidence of HCV infection. An incidence of 0.7 per 100 PY (95% CI: 0.08‐2.4) was reported from a San Francisco clinic.10 A retrospective cohort study in a Montreal sexual health clinic that compared the incidence of STIs prior to, and in 12 months following, the prescription of PrEP in 109 MSM found no incident cases of HCV during either time period.9 The disparity in HCV incidence seen between European and North American PrEP studies could be due to a different risk of exposure to the virus (ie variation in the prevalence of active infection among MSM) or differences in the extent of high‐risk sexual behaviours. PROUD participants were at the far end of this spectrum, reporting an average of 10 sexual partners in the three months before enrolment and 37% having used post‐exposure prophylaxis in the previous 12 months (Table 1).
Few studies have estimated HCV incidence in general HIV‐negative MSM populations. A meta‐analysis by Ghisla et al estimated incidence to be 0.43 per 1000 PY (95% CI: 0.01‐0.86).2 A cohort in Amsterdam did not observe any HCV infections in HIV‐negative participants during 7808 PY of follow‐up (0 per 1000 PY, 95% CI: 0.0‐0.5).16 In a UK (Brighton) hospital, HCV incidence was estimated at 0.15 per 100 PY (95% CI: 0.05‐0.35)17 among 57% of eligible participants who were tested. The difference in HCV incidence between HIV‐negative MSM in general and those seeking PrEP is likely to be explained by the lower sexual risk behaviours and lower testing rates in the former group.
Although the increase in HCV incidence over calendar timein PROUD was not conventionally statistically significant, the low seroprevalence at enrolment (2.1%, coincidently identical to the overall incidence of 2.1% per year) suggests that the increasing incidence is genuine. This increase occurred despite an overall decline in HCV prevalence in England in recent years.18 Phylogenetic analysis of HCV infections in the AmPrEP study and a cohort of HIV‐negative and HIV‐positive MSM in a French clinic19, 20 suggest that there was substantial sexual mixing between HIV‐negative and HIV‐positive populations, who have high HCV prevalence21 and incidence,2, 22 and it is likely that PrEP facilitates this mixing. Although the frequency of HCV testing increased part way through the study and probably resulted in more rapid diagnosis of infections, we used an imputation method that should correct for any bias arising from this.
The 2.1% seroprevalence of HCV in PROUD participants at enrolment was considerably lower than that reported in the AmPrEP demonstration project (4.8%).19 The IPERGAY study reported only one HCV infection at enrolment.7 However, HCV seroprevalence changes rapidly in the context of a high incidence and comparisons between studies will be affected by the calendar time over which seroprevalence was calculated.
Our study has two major limitations. First, the small number of incident HCV infections limited our ability to examine risk factors for the acquisition of HCV, including geographical region. The second limitation is around the generalizability of our findings. PROUD participants were at much higher risk of acquiring HIV infection than other MSM attending sexual health clinics in the same time period, and the same may apply to HCV infection. The use of nonprescribed injected drugs was reported by 11 of the 25 incident cases, and this cannot be excluded as a possible route of transmission. However, qualitative research indicates that sharing of needles is uncommon among MSM injecting chemsex drugs in the UK, with high awareness of the risks of doing so.23 Even if these 11 cases are discounted, the estimated incidence is still high, suggesting that HCV is highly transmissible through sexual contact, with the risk of epidemic spread of the infection in certain populations.
Regular care for PrEP provides an opportunity to screen for and provide early intervention for HCV. The increasing availability of HCV antigen/antibody testing makes screening in this population more feasible.24 The high incidence of HCV that we observed in PROUD supports the 2018 BHIVA/BASHH recommendation for quarterly HCV testing among HIV‐negative MSM using PrEP in the UK, in line with other STIs.25 Also, a recent modelling study has indicated that screening and treating PrEP users for HCV at least every 12 months can reduce HCV incidence by 67.3% (uncertainty range 52.7%‐79.2%).26 However, HCV incidence can vary markedly by location and time, and guidance in the UK and other countries27, 28 should be regularly reviewed in the light of local evidence.
CONFLICTS OF INTERESTS
The PROUD study was provided drug free of charge by Gilead Sciences plc. that also distributed it to participating clinics and provided funds for additional diagnostic tests for HCV and drug levels. EW university fees and stipend funded by Gilead Science plc. SM reports grants from the European Union H2020 scheme, EDCTP 2, the National Institute of Health Research and Gilead Sciences; other support from Gilead Sciences and the Population Council Microbicide Advisory Board; and is Chair of the Project Advisory Committee for USAID grant awarded to CONRAD to develop tenofovir‐based products for use by women (nonfinancial). DTD has received fees for participation on advisory boards and educational workshops from ViiV Healthcare and Gilead Sciences. AC has received consultancy fees from Gilead and ViiV/GSK, and travel bursaries to education events from Gilead. DP is an employee of Gilead Sciences. NV has received conference support from Gilead and Janssen. MD, DW, AS, MG, JF, CL and RG have no conflicts to declare.
ACKNOWLEDGEMENTS
Gilead Sciences awarded a grant to fund the increased quarterly screening of hepatitis C. The PROUD study was supported by ad hoc funding from the MRC Clinical Trials Unit at University College London and an innovations grant from Public Health England, and most clinics received support through the UK National Institute of Health Research Clinical Research Network. Gilead Sciences provided Truvada PrEP for the PROUD study. We thank the PROUD participants who recognized the need for the study design, the dedication of the clinic teams and the oversight that the governance committees provided as the study evolved.
Desai M, White E, Vora N, et al. High incidence of Hepatitis C virus infection observed in the PROUD study of HIV pre‐exposure prophylaxis. J Viral Hepat. 2020;27:852–857. 10.1111/jvh.13297
Desai and White are joint first authors with equal contribution.
REFERENCES
- 1.Jin F, Matthews GV, Grulich AE. Sexual transmission of hepatitis C virus among gay and bisexual men: a systematic review. Sex Health. 2017;14(1):28‐41. [DOI] [PubMed] [Google Scholar]
- 2.Ghisla V, Scherrer AU, Nicca D, Braun DL, Fehr JS. Incidence of hepatitis C in HIV positive and negative men who have sex with men 2000–2016: a systematic review and meta‐analysis. Infection. 2017;45(3):309‐321. [DOI] [PubMed] [Google Scholar]
- 3.Desai M, White E, Vora N, et al.Chemsex in the PROUD Study. PHE Conference; Warwick, UK.s2016.
- 4.Edmundson C, Heinsbroek E, Glass R, et al. Sexualised drug use in the United Kingdom (UK): A review of the literature. Int J Drug Policy. 2018;55:131‐148. [DOI] [PubMed] [Google Scholar]
- 5.Martin NK, Vickerman P, Dore GJ, Hickman M. The hepatitis C virus epidemics in key populations (including people who inject drugs, prisoners and MSM): the use of direct‐acting antivirals as treatment for prevention. Curr Opin HIV AIDS. 2015;10(5):374‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoornenborg ECL, Achterbergh RCA, Schim van der Loeff M, et al. on behalf of the Amsterdam PrEP Project Team in the HIV Transmission Elimination AMsterdam Initiative (H‐TEAM) . High incidence of hepatitis C virus (re‐)infections among PrEP users in the Netherlands: Implications for prevention, monitoring and treatment. AIDS conference. 2018, abstract 2682. http://programme.aids2018.org/Abstract/Abstract/2682
- 7.Gras J, Mahjoub N, Carreau I, et al.HCV RNA and Antigen Detection for Diagnosis of Acute Hepatitis C among MSM on PrEP. Conference on Retroviruses and Oppotunistic Infections; s2018.
- 8.Cotte L, Cua E, Reynes J, et al. Hepatitis C virus incidence in HIV‐infected and in preexposure prophylaxis (PrEP)‐using men having sex with men. Liver Int. 2018;38(10):1736‐1740. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS. 2018;32(4):523‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volk JE, Marcus JL, Phengrasamy T, Hare CB. Incident hepatitis C virus infections among users of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis. 2015;60(11):1728‐1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikati T, Jamison K, Borges CM, et al. Low prevalence of Hepatitis C Virus among NYC initiating PrEP and PEP, 2016‐2017. Conference on Retroviruses and Opportunistic Infections; 2018.
- 12.Dolling D, Desai M, Apea V,, et al.; on behalf of the PROUD study . Who accesses PrEP? An analysis of baseline data in the PROUD pilot. Third joint conference of BHIVA with BASHH; 4th April 2014; Liverpool 2014.
- 13.McCormack S, Dunn DT, Desai M, et al. Pre‐exposure prophylaxis to prevent the acquisition of HIV‐1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open‐label randomised trial. Lancet. 2016;387(10013):53–60. 10.1016/S0140-6736(15)00056-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandormael A, Dobra A, Barnighausen T, de Oliveira T, Tanser F. Incidence rate estimation, periodic testing and the limitations of the mid‐point imputation approach. Int J Epidemiol. 2018;47(1):236‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miles A. Obtaining predictions from models fit to multiply imputed data. Soc Methods Res. 2015;45(1):175‐185. [Google Scholar]
- 16.van de Laar TJ, van der Bij AK, Prins M, et al. Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission. J Infect Dis. 2007;196(2):230‐238. [DOI] [PubMed] [Google Scholar]
- 17.Richardson D, Fisher M, Sabin CA. Sexual transmission of hepatitis C in MSM may not be confined to those with HIV infection. J Infect Dis 2008;197(8):1213‐1214, author reply 4–5. [DOI] [PubMed] [Google Scholar]
- 18.Public Health England . Hepatitis C in England 2019: Working to eliminate hepatitis C as a major public health threat. 2019.
- 19.Hoornenborg E, Achterbergh RCA, Schim van der Loeff MF, et al. MSM starting preexposure prophylaxis are at risk of hepatitis C virus infection. AIDS. 2017;31(11):1603‐1610. [DOI] [PubMed] [Google Scholar]
- 20.Charre C, Cotte L, Kramer R, et al. Hepatitis C virus spread from HIV‐positive to HIV‐negative men who have sex with men. PLoS ONE. 2018;13(1):e0190340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin NK, Thornton A, Hickman M, et al. Can hepatitis C virus (HCV) direct‐acting antiviral treatment as prevention reverse the HCV epidemic among men who have sex with men in the United Kingdom? Epidemiological and modeling insights. Clin Infect Dis. 2016;62(9):1072‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yaphe S, Bozinoff N, Kyle R, Shivkumar S, Pai NP, Klein M. Incidence of acute hepatitis C virus infection among men who have sex with men with and without HIV infection: a systematic review. Sex Transm Infect. 2012;88(7):558‐564. [DOI] [PubMed] [Google Scholar]
- 23.Bourne A, Reid D, Hickson F, et al. "Chemsex" and harm reduction need among gay men in South London. Int J Drug Pol. 2015;26:1171‐1176. [DOI] [PubMed] [Google Scholar]
- 24.Tillmann HL. Hepatitis C virus core antigen testing: role in diagnosis, disease monitoring and treatment. World J Gastroenterol. 2014;20(22):6701‐6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brady MRA, on behalf of the PrEP guideline writing group . BHIVA/BASHH guidelines on the use of HIV pre‐exposure prophylaxis (PrEP). 2018.
- 26.McGregor L, Desai M, Martin N, et al. Scaling up screening and treatment for elimination of hepatitis C among men who have sex with men in the era of HIV pre‐exposure prophylaxis. EClinMed 2019;19:100217. online first. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centres for Disease Control, US Public Health Service . Preexposure prophylaxis for the prevention of HIV infection in the United States‐ 2017 Update. A cinical practice guide. 2018.
- 28.Wright E, Grulich A, Roy K, et al. Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine HIV pre‐exposure prophylaxis: clinical guidelines. Update April 2018. J Virus Erad 2018;4(3):143‐159. [PMC free article] [PubMed] [Google Scholar]


