Abstract
Background: Emergency medicine is acuity-based and focuses on time-sensitive treatments for life-threatening diseases. Prolonged time in the emergency department, however, is associated with higher mortality in critically ill patients. Thus, we explored management after an acuity-based intervention, which we call perpetuity, as a potential mechanism for increased risk. To explore this concept, we evaluated the impact of each hour above a lung-protective tidal volume on risk of mortality. Methods: This cohort analysis includes all critically ill, non-trauma, adult patients admitted to two academic EDs between 1 November 2013 and 30 April 2017. Cox models with time-varying covariates were developed with time in perpetuity as a time-varying covariate, defined as hours above 8 mL/kg ideal body weight, adjusted for covariates. The primary outcome was the time to in-hospital death. Results: Our analysis included 2025 patients, 321 (16%) of whom had at least 1 h of perpetuity time. A partial likelihood-ratio test comparing models with and without hours in perpetuity was statistically significant (χ2(3) = 13.83, p = 0.0031). There was an interaction between age and perpetuity (Relative risk (RR) 0.9995; 95% Confidence interval (CI95): 0.9991–0.9998). For example, for each hour above 8 mL/kg ideal body weight, a 20-year-old with 90% oxygen saturation has a relative risk of death of 1.02, but a 40-year-old with 90% oxygen saturation has a relative risk of 1.01. Conclusions: Perpetuity, illustrated through the lens of mechanical ventilation, may represent a target for improving outcomes in critically ill patients, starting in the emergency department. Research is needed to evaluate the types of patients and interventions in which perpetuity plays a role.
Keywords: perpetuity, acuity, emergency department, critical care, critically ill, mechanical ventilation, intubation
1. Introduction
Emergency department (ED) visits in the United States totaled over 146 million in 2016, a 12% increase over five years [1,2]. The percentage of those patients requiring critical care, time providing critical care, and admissions to the intensive care unit (ICU) has increased disproportionately, despite decreasing ED- and ICU-bed availability [3,4,5]. This prolongs ED boarding and creates difficult ICU triage decisions that can lead to delayed or inappropriate care and poorer outcomes [6,7,8,9,10,11,12]. A recent study reported improved outcomes with a dedicated ICU-level area in the ED [13], suggesting a need to identify contributing factors and targets for interventions to reduce this risk.
Conceptually, the flow of time-related events in ED care is one potential factor. Patients are treated based on triage acuity and time-sensitive interventions for high-acuity processes (e.g., sepsis, stroke, myocardial infarction, and cardiac arrest) are performed to attenuate, or even reverse, the propagation of disease. There is not, however, enough dependable data on how outcomes are shaped by the care that follows such interventions. Data are increasingly important as the transition between the ED and ICU becomes blurred.
Acute respiratory failure represents one such paradox. Most patients with acute respiratory failure present to the ED. In many of these patients, early intubation is associated with improved outcomes [14], and is performed earlier with higher acuity. Mechanical ventilation after intubation, however, is often suboptimal [15,16,17,18], persists into the ICU, and is closely associated with outcomes [18,19,20,21].
We conceptualized care provided after an acuity-based intervention as the “perpetuity” of disease, where suboptimal or even injurious management perpetuates risk from critical illness, and hypothesized that it may play a role in mortality. We aimed to explore the concept of perpetuity by evaluating one type of management known to be associated with affecting outcomes after a defined acuity-based intervention: ventilator management after intubation.
2. Methods
2.1. Study Setting
This study was conducted at two academic medical centers where the EDs are staffed with emergency medicine faculty and residents, and the ICUs are staffed with pulmonary and critical care faculty, fellows, and internal medicine residents. The Banner–University Medical Center Tucson (BUMCT) is a Level 1 trauma center with an annual ED census of 85,000. The Banner–University Medical Center South (BUMCS) has an annual ED census of 54,000. Neither hospital operates a formal ED–ICU. After the decision is made to transition care from the ED to the ICU, the ICU team is responsible for the patient’s care, including the remaining time that the patient is boarded in the ED.
All ED patients requiring ICU care are incorporated into a quality improvement registry, which is maintained by data downloaded from the hospitals’ electronic medical record (EPIC systems, Verona, WI, USA). This project adhered to the STROBE reporting guidelines and Patient-Centered Outcomes Research Institute (PCORI) standards for registry studies [22], and it was granted exemption from informed consent and approved by the University of Arizona Institutional Review Board (#1607695679).
2.2. Study Design
This is a retrospective cohort analysis of adult patients (>18 years), intubated in the prehospital setting or ED, and admitted to the ICU for non-traumatic critical illness at two academic hospitals between 1 November 2013 and 30 April 2017 and followed until death or hospital exit (whichever came first). We excluded patients intubated in the ICU (see Appendix A: Methods). We defined a period of time as contributing to perpetuity if the patient was charted as receiving tidal volumes > 8 mL/kg predicted body weight over that period of time after intubation. Tidal volumes are documented at the initiation of mechanical ventilation and then semi-regularly (with an hourly goal) by the respiratory therapy staff. We followed the patient until death or hospital exit (whichever came first).
2.3. Calculation of Time in Perpetuity
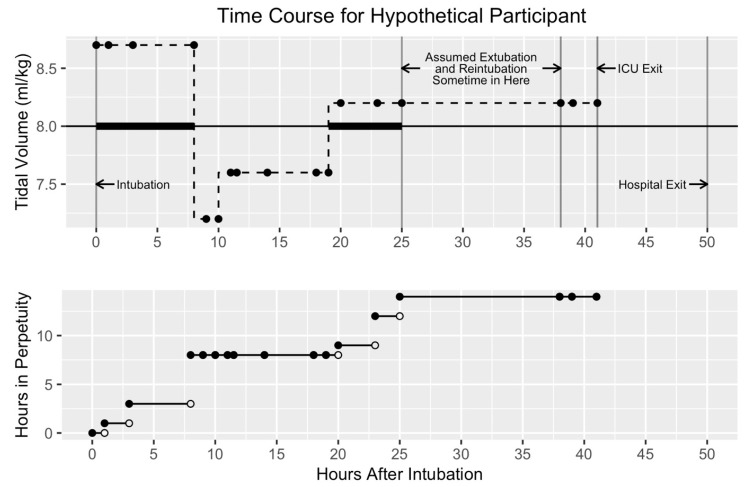
The exact amount of time each patient spent in perpetuity cannot be known because we do not have continuous tidal volume readings. We thus approximated time in perpetuity as follows: at every time stamp, we determined whether or not the tidal volume was >8 mL/kg. If it was, we added the period of time between the previous and current time stamps to the previous total time in perpetuity. The new total time in perpetuity was designated as the value of perpetuity at that time stamp. Thus, time in perpetuity either remained the same or increased at every time stamp, depending on whether the observed tidal volume at that time stamp was ≤8 mL/kg or >8 mL/kg, respectively. The accumulation of perpetuity began at intubation or ED arrival, whichever came last. It stopped accumulating at ICU exit, death, or hypothesized extubation, whichever came first (Figure 1). The primary outcome was time to in-hospital death.
Figure 1.
Time course for a hypothetical patient.
Figure 1 Legend: This figure demonstrates how perpetuity is calculated for a hypothetical patient given the preprocessing assumptions. The upper panel shows the tidal volumes (y-axis) and the lower panel shows the hours accumulated in perpetuity (y-axis) over time (x-axis). Time zero is intubation time, and intervals between charted tidal volumes (solid dots) accumulate perpetuity time if they are >8 mL/kg. For this patient, each tidal volume charted for the first 6 h was >8 mL/kg and accumulated time in perpetuity. However, no time was accumulated after that until 18–25 h, when tidal volumes again crossed the threshold. The large gap between 25 h and the next charted tidal volume exceeds the cutoff threshold for assumed extubation and reintubation and thus does not contribute to perpetuity time.
2.4. Hypothesized Extubation
To mitigate potential issues with inflating time in perpetuity, we made assumptions regarding long durations between successively charted tidal volumes. Specifically, we were concerned that long intervals could have resulted from undocumented extubations. After an analysis of time intervals, and accounting for typical staff shift hours, we chose two durations (eight and twelve hours) between charted tidal volumes as cut-offs (see Appendix A). No periods of time over the 8 mL/kg threshold after the first interval of greater than eight or twelve hours were included in the perpetuity calculation. We did this for two primary reasons: (a) we did not want to include tidal volumes possibly resulting from re-intubations, and (b) we did not want to include long periods of unintubated time in the total time in perpetuity (Figure 1). Each cut-off choice resulted in the creation of a different data set.
2.5. Sensitivity Analysis
We conducted a sensitivity analysis to better explore the effects of our preprocessing decisions, specifically those related to hypothesized extubation and the calculation of time in perpetuity. These preprocessing choices could affect later inference; thus we examined the data sets for the two choices. We explored the effect of the choices of eight and twelve hours as cut-offs by allowing the cut-offs to be four, five, six, seven, and twenty-four hours. We explored the effect of disallowing further incrementation of hours in perpetuity after the first cut-off interval by (a) allowing intervals of all sizes at any point in time to increment the time in perpetuity (no adjustment data set) and (b) allowing only intervals less than six hours long to increment the time in perpetuity (gaps ≥ 6 h removed data set). The key difference between the “gaps ≥ 6 h removed” data set and the six-hour cut-off dataset is that in the first, but not the second, small intervals after the first long interval are still allowed to increment the time in perpetuity. We examined the effect of assuming a constant charted tidal volume in large intervals by instead interpolating linearly between the first and second tidal volumes in intervals greater than or equal to six hours (linear interpolation in gaps ≥ 6 h data set). Only the portion of the intervals predicted to be over the 8 mL/kg threshold was added to the time in perpetuity. We explored the effect of propagating charted tidal volumes backward by instead assuming the last charted tidal volume carried forward (last-one-forward data sets), see Appendix A.
2.6. Data Analysis
Cox models with time-varying covariates were fit to the eight- and twelve-hour cut-off data. The models had identical specifications. The outcome variable was time to death during the hospital stay. Patients were censored at hospital discharge. The primary predictor of interest was hours in perpetuity, which was treated as a time-varying covariate. Other predictors that are potential confounders or indicate severity of illness were chosen, and included age, initial oxygen saturation at triage, hours in acuity, mode of arrival (ground transport, air transport, private vehicle, and other), hospital campus (BUMCT or BUMCS), whether or not the patient was on non-invasive positive pressure ventilation prior to intubation, ideal body weight, body mass index, and the Emergency Severity Index score at ED triage. Heart rate, systolic blood pressure, and mean arterial pressure were included as static covariates set to their first recorded values. Two interactions we thought were clinically important were included a priori as well: the interaction of hours in perpetuity with age and the interaction of hours in perpetuity with oxygen saturation.
Penalized splines were used to handle nonlinearity of continuous predictors [23]. To decide which predictors were best included as nonlinear, we fit a separate model per continuous predictor. In each of these models, we included a single predictor as nonlinear and allowed the optimal degrees of freedom for the nonlinear portion to be estimated with Akaike’s Information Criterion [24]. All other predictors in these models were left as linear. We tested whether that nonlinear portion of the spline predictor was statistically significant, at a 5% false positive rate, using the Wald χ2 test. We next fit a model that included, as nonlinear, all predictors indicated by the previous step. For each spline term, we set the degrees of freedom to be the previously identified optimal degrees of freedom. The interactions of hours in perpetuity with age and oxygen saturation were included in this model as well. As a final check on the form of the nonlinear predictors, we again tested the statistical significance of the nonlinear portion of each spline term. Only those spline terms with nonlinear portions statistically significant at a 5% false positive rate (hours in acuity and heart rate) were included as nonlinear in the final model.
An additional (reduced) model was fit to each data set that did not include any of the terms involving time in perpetuity (the main effect of hours in perpetuity and both interactions with hours in perpetuity). The full and reduced models for a given data set were compared using a partial likelihood-ratio test in order to test whether the inclusion of perpetuity in its three forms resulted in a meaningfully better model fit. For the sensitivity analysis, only the final full and reduced models described above were fit to each data set.
Data processing and statistical analyses were conducted in the R statistical computing language [25]. Data manipulation was handled with the tidyr and dplyr packages [26,27], plots were created with the ggplot2 package [28], and results tables were constructed with the xtable package [29]. The Cox models with time-varying covariates were fit with the coxph function in the survival package [30,31].
We conducted a simple power calculation in order to gain insight into the number of participants needed to detect various hazard ratios corresponding to a one-hour increase in perpetuity time. Our approach follows van Belle in assuming survival times are exponentially distributed [32]. We assume we have two groups of participants, one with one hour longer in perpetuity than the other. Then, with 1000 participants per group (2000 participants in total) and a 5% significance level, we will have ≥80% power to detect a hazard ratio less than 0.89 or greater than 1.13. As (a) hazard ratios of 0.89 and 1.13 are fairly small, and (b) we collected data on around 2000 participants, we are confident that the study is well-powered.
3. Results
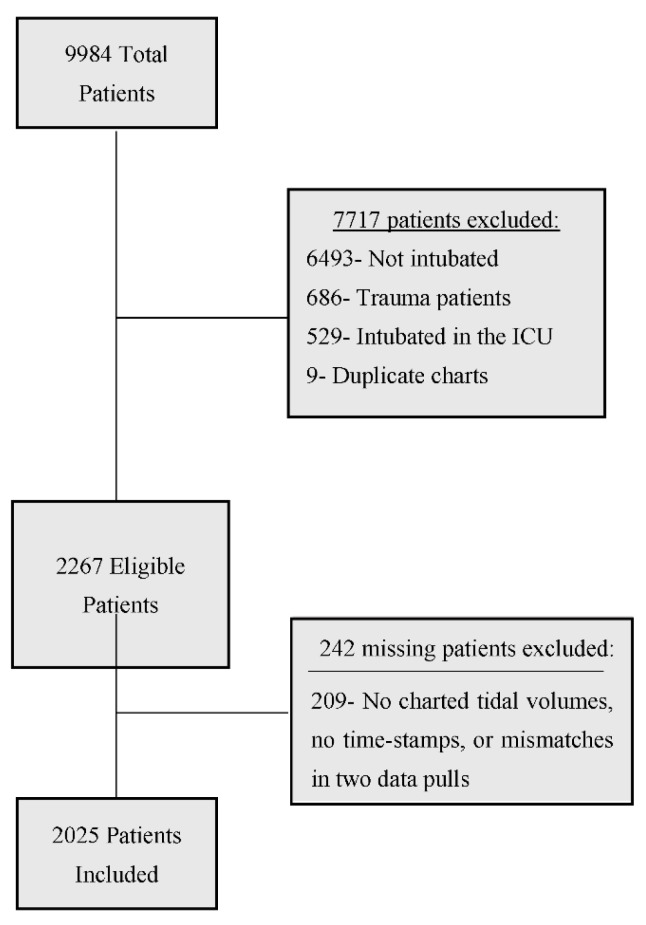
Our final analyses were based on 2025 participants (Figure 2). Demographic data are found in Table 1. Sixteen percent (321) patients had at least one hour of perpetuity time. For both the eight- and twelve-hour cut-off models, the following were statistically significant (all p < 0.05): the interactions of age/perpetuity and oxygen saturation/perpetuity as well as the main effects of perpetuity, age, oxygen saturation, hours in acuity (the nonlinear component), campus, non-invasive positive pressure ventilation, heart rate (the nonlinear component), and triage score, see Table 2 and Table 3 for estimated coefficients. Note that systolic blood pressure and mean arterial pressure were highly correlated ( = 0.92), and in models that left out either predictor, the other was statistically significant (p < 0.05). For both models, a partial likelihood-ratio test indicated that the inclusion of perpetuity significantly improved model fit (eight-hour model: χ2 = 13.83, df = 3, p = 0.0031; twelve-hour model: χ2 = 8.61, df = 3, p = 0.0348—Appendix B Table A2 and Table A4).
Figure 2.
Patient flow chart. Other includes: missing or clearly incorrect height, weight, systolic blood pressure, mean arterial blood pressure, questionable death status, having a documented hospital discharge before ICU discharge, and charted tidal volumes that occurred after ICU discharge.
Table 1.
Demographics.
| Characteristic | Number (Total n = 2025) | Percent or Mean (SD) |
|---|---|---|
| Demographics | ||
| Age, mean (SD) | -- | 56 (18) |
| Male | 1198 | 59% |
| Height (cm) | -- | 170.5 (10.2) |
| Weight (kg) | -- | 82.2 (26.8) |
| Ideal Body Weight | -- | 64.6 (10.6) |
| Body Mass Index | -- | 28.3 (9.2) |
| Mode of arrival | ||
| EMS Ground | 1621 | 80% |
| Private Vehicle | 251 | 12% |
| EMS Air | 83 | 4% |
| Other | 70 | 3% |
| Campus | ||
| BUMCT * | 1282 | 63% |
| BUMCS * | 743 | 37% |
| Hospital Shift at ED Arrival | ||
| Day Shift (7 a.m.–7 p.m.) | 1146 | 57% |
| Night Shift (7 p.m.–7 a.m.) | 879 | 43% |
| Admitting Diagnosis | ||
| Neurologic/psychiatric | 557 | 28% |
| Respiratory, all causes | 525 | 26% |
| Cardiac arrest | 204 | 10% |
| Infectious/Allergic/Immunologic | 203 | 8% |
| Toxin/Toxidrome | 156 | 7% |
| Cardiovascular | 134 | 6% |
| GI | 112 | 2% |
| Renal/GU | 39 | 2% |
| Endocrine | 33 | 1% |
| Hematologic/Oncologic | 29 | 1% |
| Musculoskeletal | 15 | 1% |
| Trauma | 13 | 1% |
| No Diagnosis Given | 5 | 0% |
| Location of Intubation | ||
| Prehospital | 269 | 13% |
| Emergency Department | 1756 | 87% |
| Noninvasive ventilation use | 315 | 16% |
| Emergency Severity Index | ||
| 1 | 940 | 46% |
| 2 | 985 | 49% |
| 3 | 98 | 5% |
| 4 | 2 | <1% |
| Vital Signs | ||
| Heart Rate | -- | 105 (28.5) |
| Systolic Blood Pressure | -- | 133.2 (36.4) |
| Diastolic Blood Pressure | -- | 98.4 (24.7) |
| Mean Arterial pressure | -- | 96.5 (27.1) |
| Oxygen Saturation | -- | 93.3 (10.5) |
| Acuity # Minutes | -- | 99.9 (167.7) |
| Acuity Hours | -- | 1.7 (2.8) |
| Outcomes | ||
| Discharged Alive | 1570 | 78% |
| In-hospital death | 455 | 22% |
Standard deviation [SD]; Emergency medical services [EMS]; * BUMCT (Banner–University Medical Center Tucson); BUMCS (Banner University Medical Center South); Emergency Department [ED]; Gastrointestinal [GI]; Genitourinary [GU]. # Acuity is the time between ED arrival and the time of intubation.
Table 2.
8 h Cut-Off Model: Cox model with hours in perpetuity as a time-varying covariate.
| Estimated Coefficient (95% CI) | Relative Risk (95% CI) | χ2 | df | p Value | |
|---|---|---|---|---|---|
| Hours in Perpetuity (>8 mL/kg) | 0.1558 (0.0737, 0.2379) | 1.1686 (1.0765, 1.2686) | 13.8442 | 1 | 0.0002 |
| Age | 0.028 (0.0219, 0.0342) | 1.0284 (1.0221, 1.0348) | 79.0050 | 1 | 0.0000 |
| O2 | −0.0135 (−0.02, −0.0071) | 0.9865 (0.9802, 0.993) | 16.7289 | 1 | 0.0000 |
| Hours in Acuity (Linear) | 0.0148 (−0.042, 0.0717) | 1.015 (0.9589, 1.0743) | 0.2621 | 1 | 0.6087 |
| Hours in Acuity (Nonlinear) | 9.3494 | 3 | 0.0250 | ||
| Arrival by EMS Ground | 0.0645 (−0.4077, 0.5367) | 1.0666 (0.6652, 1.7104) | 0.0717 | 1 | 0.7889 |
| Other Mode of Arrival | 0.0459 (−0.6304, 0.7222) | 1.047 (0.5324, 2.059) | 0.0177 | 1 | 0.8942 |
| POV Mode of Arrival | −0.2503 (−0.8365, 0.3359) | 0.7786 (0.4332, 1.3992) | 0.7004 | 1 | 0.4026 |
| BUMCS | 0.4478 (0.248, 0.6476) | 1.5648 (1.2814, 1.9109) | 19.2972 | 1 | 0.0000 |
| NIPPV prior to intubation | −0.9528 (−1.2801, −0.6255) | 0.3857 (0.278, 0.535) | 32.5512 | 1 | 0.0000 |
| Ideal Body Weight | −0.0016 (−0.0111, 0.0078) | 0.9984 (0.9889, 1.0079) | 0.1161 | 1 | 0.7333 |
| BMI | 0.0015 (−0.0087, 0.0117) | 1.0015 (0.9913, 1.0117) | 0.0788 | 1 | 0.7789 |
| Heart Rate (Linear) | 7 × 10−4 (−0.0022, 0.0036) | 1.0007 (0.9978, 1.0036) | 0.2327 | 1 | 0.6296 |
| Heart Rate (Nonlinear) | 11.6439 | 2 | 0.0033 | ||
| Systolic Blood Pressure | −0.0024 (−0.0089, 0.0042) | 0.9976 (0.9911, 1.0042) | 0.4916 | 1 | 0.4832 |
| Mean Arterial Pressure | −0.0052 (−0.014, 0.0037) | 0.9949 (0.9861, 1.0037) | 1.2898 | 1 | 0.2561 |
| Acuity Score | −0.4362 (−0.6355, −0.2368) | 0.6465 (0.5297, 0.7891) | 18.3887 | 1 | 0.0000 |
| Hours in Perpetuity × Age | −5 × 10−4 (−9 × 10−4, −2 × 10−4) | 0.9995 (0.9991, 0.9998) | 7.4002 | 1 | 0.0065 |
| Hours in Perpetuity × O2 | −0.0014 (−0.0023, −6 × 10−4) | 0.9986 (0.9977, 0.9994) | 10.8938 | 1 | 0.0010 |
Confidence interval [CI]; Noninvasive positive-pressure ventilation [NIPPV]; Privately-owned vehicle [POV]; Body mass index [BMI]. The estimated coefficient is on the log(hazard rate) scale. A relative risk over one indicates that having the associated covariate (or each unit of the associated covariate) increases the hazard of dying in the hospital. The nonlinear portion of the spline terms for hours in acuity and heart rate do not have single estimated coefficients. Thus, only χ2 tests and the associated p values are given for these rows. 2025 patients are included in this model.
Table 3.
12 h Cut-Off Model: Cox model with hours in perpetuity as a time-varying covariate.
| Estimated Coefficient (95% CI) | Relative Risk (95% CI) | χ2 | df | p Value | |
|---|---|---|---|---|---|
| Hours in Perpetuity (>8 mL/kg) | 0.1042 (0.0254, 0.183) | 1.1099 (1.0257, 1.2009) | 6.7183 | 1 | 0.0095 |
| Age | 0.0279 (0.0217, 0.0341) | 1.0283 (1.022, 1.0347) | 78.1274 | 1 | 0.0000 |
| O2 | −0.014 (−0.0205, −0.0076) | 0.9861 (0.9797, 0.9924) | 18.2653 | 1 | 0.0000 |
| Hours in Acuity (Linear) | 0.0145 (−0.0423, 0.0712) | 1.0146 (0.9586, 1.0738) | 0.2491 | 1 | 0.6177 |
| Hours in Acuity (Nonlinear) | 9.4794 | 3 | 0.0236 | ||
| Arrival by EMS Ground | 0.0743 (−0.3989, 0.5475) | 1.0771 (0.671, 1.729) | 0.0947 | 1 | 0.7583 |
| Other Mode of Arrival | 0.0663 (−0.6111, 0.7437) | 1.0685 (0.5427, 2.1037) | 0.0368 | 1 | 0.8479 |
| POV Mode of Arrival | −0.2338 (−0.8186, 0.351) | 0.7915 (0.4411, 1.4205) | 0.6140 | 1 | 0.4333 |
| BUMCS | 0.4415 (0.242, 0.6409) | 1.555 (1.2738, 1.8983) | 18.8148 | 1 | 0.0000 |
| NIPPV prior to intubation | −0.94 (−1.2658, −0.6142) | 0.3906 (0.282, 0.5411) | 31.9737 | 1 | 0.0000 |
| Ideal Body Weight | −6 × 10−4 (−0.01, 0.0089) | 0.9994 (0.99, 1.0089) | 0.0141 | 1 | 0.9054 |
| BMI | 0.0011 (−0.009, 0.0112) | 1.0011 (0.991, 1.0113) | 0.0442 | 1 | 0.8334 |
| Heart Rate (Linear) | 7 × 10−4 (−0.0021, 0.0036) | 1.0007 (0.9979, 1.0036) | 0.2398 | 1 | 0.6244 |
| Heart Rate (Nonlinear) | 11.4993 | 2 | 0.0035 | ||
| Systolic Blood Pressure | −0.0025 (−0.0091, 0.0041) | 0.9975 (0.991, 1.0041) | 0.5474 | 1 | 0.4594 |
| Mean Arterial Pressure | −0.0049 (−0.0137, 0.004) | 0.9951 (0.9863, 1.004) | 1.1622 | 1 | 0.2810 |
| Acuity Score | −0.4323 (−0.6313, −0.2332) | 0.649 (0.5319, 0.792) | 18.1107 | 1 | 0.0000 |
| Hours in Perpetuity × Age | −4 × 10−4 (−7 × 10−4, 0) | 0.9996 (0.9993, 1) | 4.4328 | 1 | 0.0353 |
| Hours in Perpetuity × O2 | −9 × 10−4 (−0.0017, −1 × 10−4) | 0.9991 (0.9983, 0.9999) | 5.1694 | 1 | 0.0230 |
The estimated coefficient is on the log(hazard rate) scale. A relative risk over one indicates that having the associated covariate (or each unit of the associated covariate) increases the hazard of dying in the hospital. The nonlinear portion of the spline terms for hours in acuity and heart rate do not have single estimated coefficients. Thus, only χ2 tests and the associated p values are given for these rows. 2025 patients are included in this model.
Because perpetuity occurs in the models in two interactions and as a main effect, it is easiest to see its impact by fixing age and oxygen saturation and then examining the hazard ratio associated with a one-hour increase in perpetuity for those fixed values.
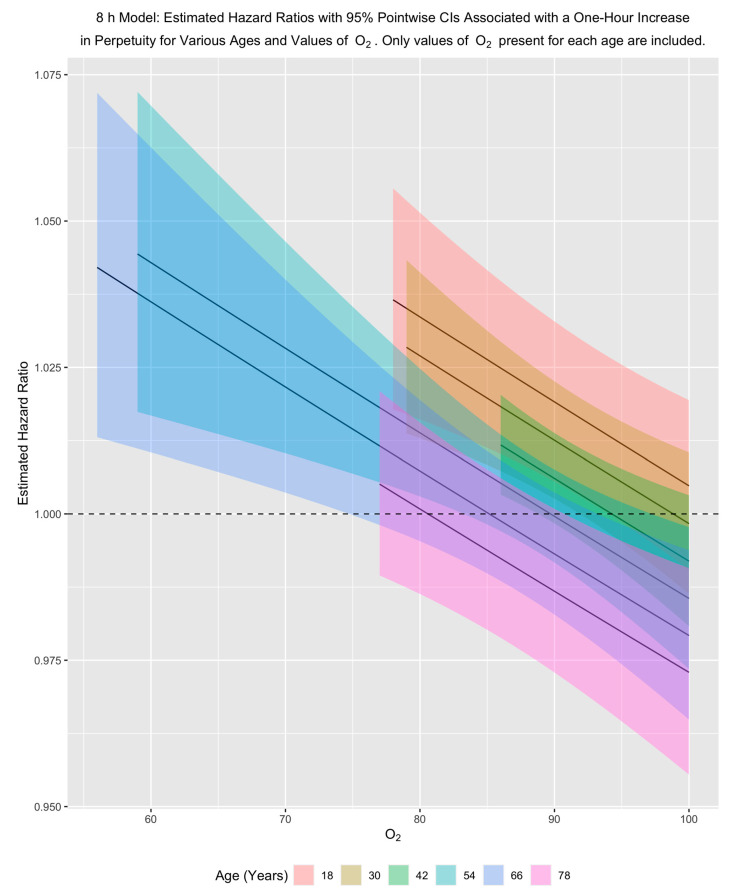
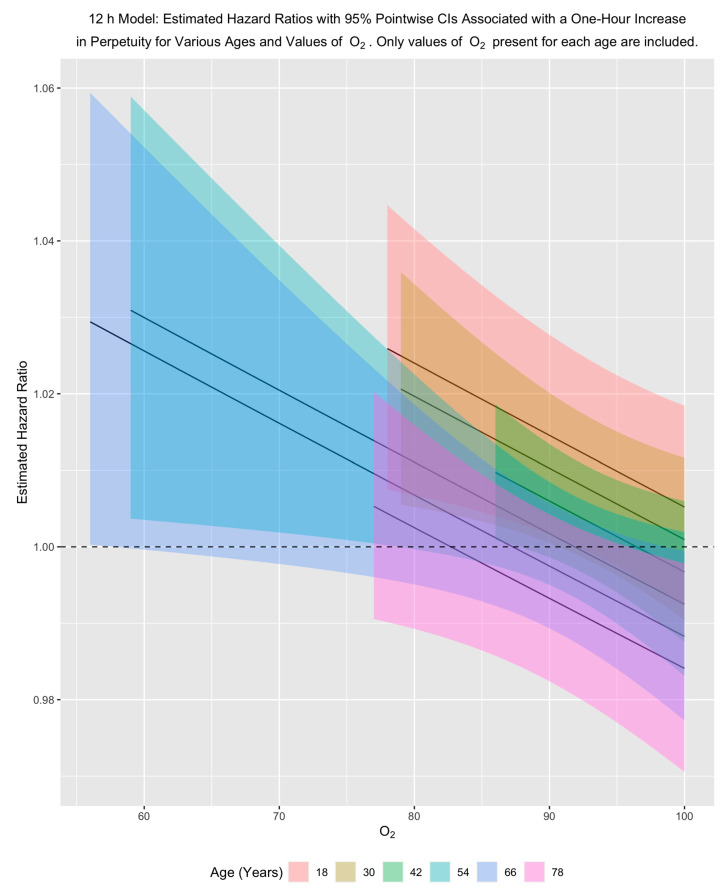
Our results show that for a fixed age, the hazard of dying in the hospital decreases with an increase in oxygen saturation and decreases with increasing age for a fixed oxygen saturation. In examining the effect of perpetuity, these patterns indicate that for younger people and for older people with low/moderate oxygen saturation, a one-hour increase in perpetuity is associated with an increased hazard of dying in the hospital. This pattern holds across both primary models, although it is stronger for the eight-hour model (Figure 3 and Figure 4).
Figure 3.
8 h model: Estimated adjusted hazard ratios with 95% pointwise confidence intervals associated with a one-hour increase in perpetuity for various ages and values of O2. Only values of O2 present for each age are included in the plots. O2 is triage oxygen saturation.
Figure 4.
12 h model: estimated adjusted hazard ratios with 95% pointwise confidence intervals associated with a one-hour increase in perpetuity for various ages and values of O2. Only values of O2 present for each age are included in the plots. O2 is triage oxygen saturation.
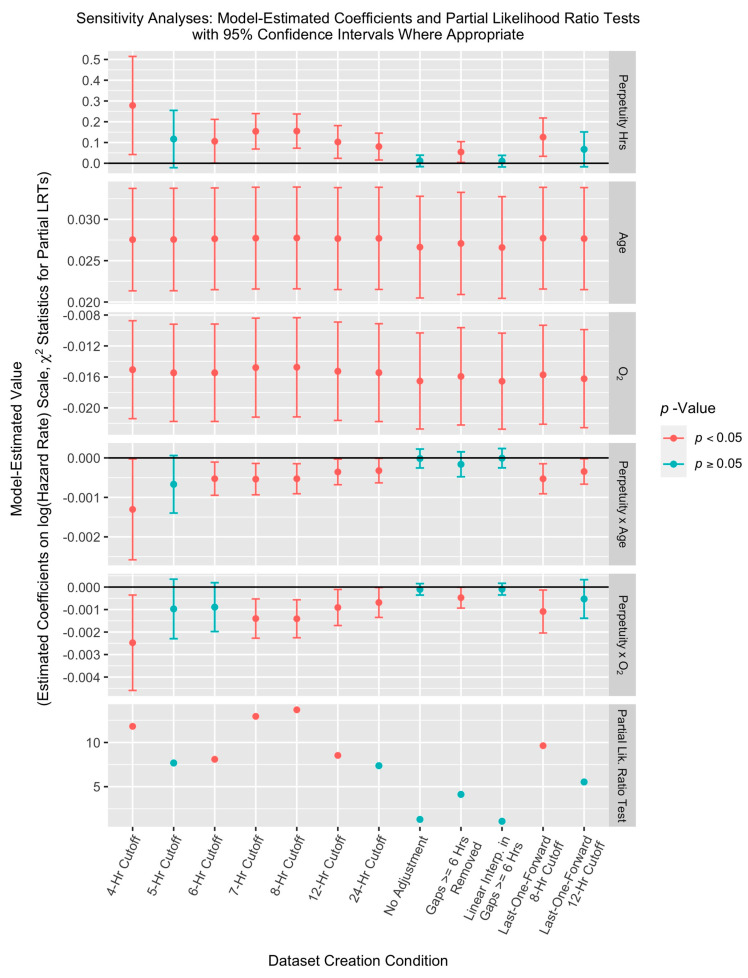
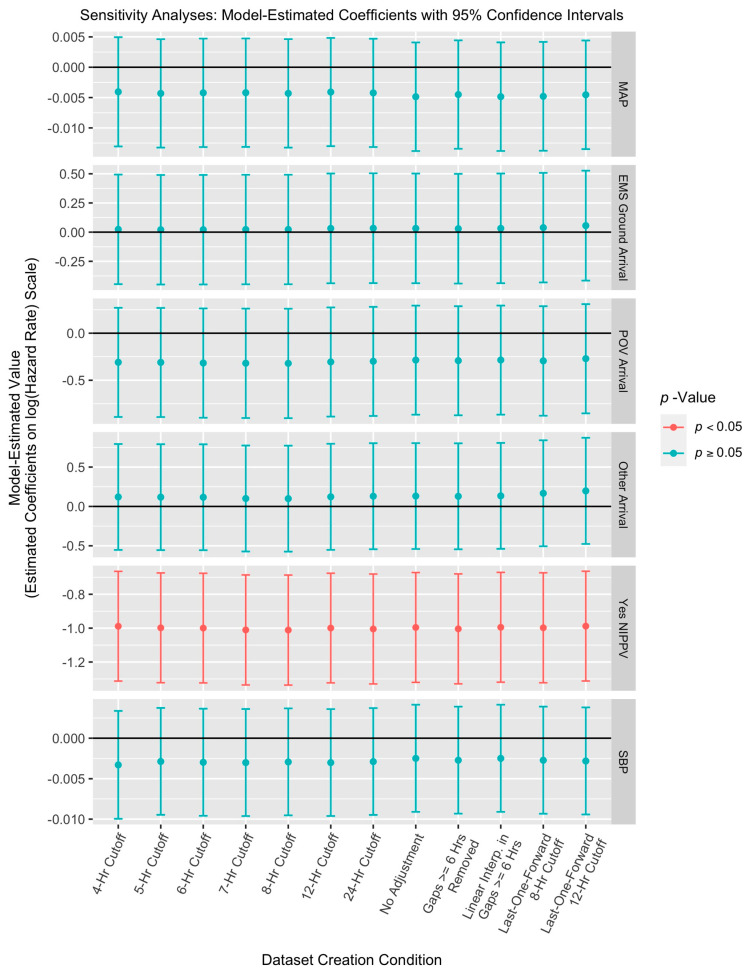
The sensitivity analyses showed that the inclusion of hours in perpetuity resulted in a statistically significant improvement in the model fit for the four-, six-, and seven-hour cut-off models and in the eight-hour last-one-forward model (Appendix B). However, perpetuity did not significantly improve the model fit for the five- and twenty-four-hour cut-off models, the no-adjustment model, the model with intervals ≥ six hours removed from perpetuity, the model with linear interpolation in intervals ≥ six hours, and the twelve-hour last-one-forward model.
4. Discussion
The aim of this study was to explore the concept of perpetuity using an aspect of mechanical ventilation widely considered to be injurious (high tidal volumes) that can only occur after an acuity-based intervention (intubation). Our results support the concept of perpetuity as a contributing factor to risk after acuity-based interventions, where risk accumulates from inappropriate, inadequate, or injurious management after that intervention and is indefinite unless something changes. To our knowledge, only two other studies have shown an increase in risk of death per unit of time receiving injurious mechanical ventilation; one with tidal volumes >8 mL/kg for >24 h [33], and the other with injurious driving pressure or mechanical power per day [34]. Our results extend this knowledge to include accumulated risk per hour of injurious mechanical ventilation, even when discontinuous.
Interestingly, we found that younger age and more severe hypoxemia poses more risk from time in perpetuity. We hypothesize that those patients may have respiratory failure etiologies (e.g., acute respiratory distress syndrome [ARDS]) that tend to reduce lung compliance and thus increase susceptibility to perpetuity-based injury (i.e., ventilator-induced lung injury [VILI]).
Given the concerning data on mechanical ventilation in the ED as well as the debate about its management, we used intubation as our acuity-based intervention and mechanical ventilation as our perpetuity-based management [35,36]. Patients require intubation for many etiologies, and the higher the risk of respiratory arrest, the sooner the patient will be intubated (i.e., acuity). However, mechanical ventilation in the ED has proved to be troublesome. Lung protective ventilation is used infrequently and some patients progress to ARDS shortly after hospital admission, indicating VILI may contribute [15,16,17]. A recent study showed that most patients do not receive lung protective ventilation while in the ED and are less likely to have ventilator adjustments during times of ED strain [18]. Inertia dictates that management strategies in the ED often carry over into the ICU for significant periods of time [18,19,20].
High tidal volume is an attractive option for evaluating perpetuity as volutrauma is known to be injurious regardless of the precipitating requirement for intubation [37,38,39]. While tidal volumes may only be injurious in proportion to reductions in lung compliance, and mechanical power research is evolving our understanding of ventilator-induced lung injury [40,41,42,43], large tidal volumes are still widely considered to be injurious. Unfortunately, PEEP values were not available in our dataset. Given the observed interaction with age and degree of hypoxemia, it is possible that our association is an underestimation of the risk of perpetuity. Future research should evaluate this concept using driving pressure and mechanical power, adjusted for severity of lung injury.
Another example of perpetuity is antibiotic timing in sepsis. The data on timing of antibiotics have some limitations, but generally indicated earlier appropriate antibiotics improves outcomes. Thus, initiatives mainly focus on initial antibiotic timing. However, the second dose is delayed in one third of patients, which worsens outcomes irrespective of the timing of the first dose and, paradoxically, more commonly with optimal first-dose timing [44]. Studies are needed to explore what other interventions and in what other diseases, perpetuity may play a role in accumulated risk over time.
Our sensitivity analyses showed that the inclusion of hours in perpetuity resulted in a statistically significant improvement in the model fit for the four-hour, six-hour, and seven-hour cut-off models and the last-one-forward eight-hour cut-off model, but not for the five- and twenty-four-hour and no-adjustment models, the model where intervals ≥ six hours were removed from perpetuity, the model with linear interpolation in intervals ≥ six hours, and the last-one-forward 12 h cut-off model. The first conclusion that can be reached is that while the results from the eight- and twelve-hour models are not due to finely tuned preprocessing choices; preprocessing does have the ability to affect inference. Second, the no-adjustment model results are not entirely unexpected. Assuming that long intervals between successive tidal volumes are indicative of extubation and possible reintubation, the no-adjustment data set leaves possibly large periods of extubation in some perpetuity calculations. This could result in substantial noise in the data.
The five- and twenty-four-hour cut-off results initially appear to be outliers given that all the other basic cut-off models resulted in the same inference. However, the χ2 values for the five-, six-, twelve-, and twenty-four-hour cut-off models are all close to the test’s critical value. Thus, while the inferences for these tests differ, the results are not substantially different.
The results for the remaining data sets are more challenging to explain. However, they highlight the need for documentation of (1) extubation and reintubation, (2) changes to the ordered tidal volume, and (3) the use of spontaneous breathing mode. Having these three types of information could dramatically reduce the noise in the data by giving more information on the patients’ charted tidal volumes at each point in time. This could lead to more accurate estimates of the time in perpetuity and its effect on the risk of dying in the hospital.
The time to ICU-level of care, not the time to the ICU, improves outcomes, and delaying perpetuity-focused care until ICU admission is a missed opportunity [13,45]. Gunnerson and colleagues found that an ED-based ICU program reduced 30-day mortality and ICU admissions [13]. These findings, along with our results, and those by Sjoding [33], and Leisman [44], suggest that targeted interventions focused on reducing perpetuity are opportunities to improve outcomes for patients regardless of their physical location.
Clinical and philosophical implications of perpetuity are potentially immense. As the critical care requirements of ED patients increases, there is substantial debate about what the response to this burden should entail. EDs provide an increasing majority of hospital associated medical care [46], and are under pressure to transfer care to admitting services after completing time-sensitive goals. The American College of Emergency Physicians (ACEP) has a policy statement that “the ED should not be utilized as an extension of the ICU and other inpatient units for admitted patients because this practice adversely affects patient safety, quality, and access to care” [47]. Similarly, the American Academy of Emergency Medicine (AAEM) has a policy statement that critically ill patients should be transferred to the ICU within six hours of arrival to the ED, as “further delay can deplete the ED of resources” [48]. While ACEP believes that hospitals have the responsibility to provide the appropriate inpatient beds and staffing [47], critically ill patients remain at high risk of delayed critical care delivery, and our data suggest perpetuity-based injury contributes to poor outcomes. More than one third of ICU patients spend more than six hours in the ED, which is not based on physiologic or outcome parameters, rather it is the mean boarding time for critical care beds in overcrowded hospitals [9]. Some intubated patients may have shorter stays in the ED [49], but time to ICU admission is associated with mortality even when boarding time is significantly fewer than six hours [50].
Our study has several limitations. We evaluated the effect of duration in perpetuity (time receiving tidal volumes > 8 mL/kg) on mortality, rather than the effect of the intervention itself (incidence of tidal volumes > 8 mL/kg or the magnitude of tidal volumes) or the magnitude of excess tidal volumes. Given the potential influence of the magnitude of tidal volumes in addition to time, we fit a model where the time in perpetuity is weighted by the tidal volume’s percent over the 8 mL/kg threshold (Appendix B, Table A3 and Table A5). Results for these models parallel results for the last-one-forward eight- and twelve-hour models on which they are based, with relative risk of weighted time in perpetuity of 3.7723 (1.2652, 11.2468) in the 8 h cutoff model.
Our results could be influenced by the intervention itself, and most importantly, by the limitations of time-stamped data and the assumptions made in preprocessing. Each assumption was made in the context of the most likely clinical explanation and biased against the hypothesis. We performed several sensitivity analyses to ensure that our assumptions did not inadvertently affect the results. Sensitivity results indicated that pre-processing does affect inference but that many preprocessing choices lead to the conclusion that time in perpetuity impacts the risk of in-hospital mortality. Regardless, even digitally time-stamped data should be interpreted with caution, and the sensitivity analyses may not have adequately detected concerns with the assumptions.
Furthermore, time in perpetuity was accumulated at any point during the initial mechanical ventilation period and it is unclear if early perpetuity is more, or less, injurious than perpetuity that occurs later in the course. Missing data presents another limitation. Eleven percent of patients (242/2267) meeting our inclusion criteria were missing data. Of those, 176 patients died shortly after arrival in the ED (median time 24 min); and no charted tidal volumes were available on those patients. These patients could have biased our results as they were not included in our analysis.
In summary, our results from this large dataset of critically ill patients suggest the presence of a time-based risk from high tidal volume ventilation that varies based on age and degree of hypoxemia. This time-dependent risk, which we term perpetuity, is a potential target in emergency and critical care research design as well as clinical care to improve outcomes.
This work was presented at the National Foundation of Emergency Medicine annual meeting at the SAEM meeting in Las Vegas, Nevada, in May 2019.
Appendix A. Methods
Appendix A.1. Target Population
The primary goal of this study is to better understand the impact of time in perpetuity while in the emergency department (ED) or intensive care unit (ICU) on the time to dying in the hospital. However, identifying the endpoint of acuity (equally, the beginning of perpetuity) for all ED patients is challenging because of the wide range of conditions with which patients present upon arriving at the ED. Thus, we chose to isolate a more uniform sub-population of all ED patients in which to investigate the role of perpetuity. This population is composed of people for whom intubation marks the point at which their most immediate medical needs have been met. In other words, we consider here people for whom intubation is the endpoint of acuity and the beginning of perpetuity.
To pull from this target sub-population, of all ED patients admitted to the Banner University Medical Center-Tucson and South EDs between 1 November 2013 and 30 April 2017, we only include in the analyses those who were at least 18 years old, were intubated either before arriving at the hospital or while in the ED and were classified as medical admissions (not trauma). Note that we exclude patients intubated in the ICU. We do this because intubation in the ICU could have occurred for two reasons: (1) intubation is delayed and should have been completed earlier and (2) intubation is only necessary as the result of a worsening condition in the ED and/or ICU and does not represent the endpoint of acuity. While the first group of people is part of our sub-population, the second group is not. There is not enough information in the data to separate these two groups of patients, and thus they must all be either included in or excluded from our sample. Because we feel it preferable to exclude part of the sub-population than to include people who are not part of it, we exclude from analysis patients intubated in the ICU.
Appendix A.2. Data Pre-Processing
Our data consist of a variety of pieces of demographic and medical information including, for example, time of arrival at the ED, time of intubation, charted tidal volumes and their corresponding time stamps, ICU entry time, ICU exit time, hospital exit time, and death status. Pre-processing included:
All nonsensical (e.g., single-digit tidal volumes, abnormally large volumes) and missing values were manually checked and corrected if possible.
For all patients intubated before reaching the hospital, their intubation time was changed to be their hospital arrival time.
Some charted tidal volumes appeared to occur before intubation (an impossible scenario). For these people, we moved their intubation time back so that it aligned with their first charted tidal volume time.
For some of the people marked as intubated in the ED, their intubation time was recorded as being prior to their ED arrival. For these people, we moved their ED arrival time back so that it aligned with their intubation time. This change was made after the previous change.
An additional issue that arose with the data set related to the time between successively charted tidal volumes. While some of these intervals were short (e.g., a few minutes), others were quite long (e.g., a few days). This is not inherently unusual or problematic outside of the context of this data set. However, the concern here was that long intervals between successive tidal volumes were likely indicative of unmarked extubation then reintubation sometime before the interval’s latter endpoint. Because of this, we adjusted the calculation of time in perpetuity as follows: if a patient had a period of time over eight hours between any two tidal volume observations, we stopped incrementing the time in perpetuity at the beginning of that interval. Thus, time in perpetuity stopped changing after the beginning of the first interval over eight hours in length. In order to confirm final results, we also set the cut-off to twelve hours with the assumption that twelve hours between charted values would cross more than one staff shift and would be unlikely to be a mistake, oversight, or due to being busy on shift and not able to keep up with charting.
The period of time between initial intubation and first charting was not checked for length (see previous bullet point) because this interval looked like it was artificially inflated for some people by recording only a date and not a time at intubation. For these people, a default time at the start of that day was set by the system. Checking this interval for the extubation cut-off would have resulted in unnecessary removal of too many patients from the data set. This data anomaly (having an intubation date but not an intubation time) affected 178 people, and many of them were consequently affected by the pre-processing decisions made in C and D. In order to challenge the default intubation time and subsequent pre-processing for these people, we constructed two additional data sets. In the first, for all 178 people, we moved their intubation time to align with their ED arrival time. In the second, we moved their intubation time one day later (the end of the day they were intubated rather than the beginning). In the analysis of these two new data sets, we only fit the two primary models as determined in the analyses of the original data sets. For both data sets, no inference changed for the 8-h cutoff model. However, the partial likelihood ratio test comparing nested models with and without perpetuity terms was not statistically significant at a 5% false positive rate in either data set’s 12 h model.
Calculating time spent in perpetuity also required pre-processing. The exact amount of time each patient spent in perpetuity cannot be known because we do not have continuous tidal volume readings. We thus approximated time in perpetuity as follows: for any two consecutive charted tidal volumes, if the latter tidal volume exceeded the patient’s threshold, the entire interval was considered to be spent in perpetuity. Moreover, because our research question is on the role of time in perpetuity while in the ED or ICU on the risk of death in the hospital, and because the time in perpetuity changes over the course of each patient’s hospital stay, we constructed an analysis dataset that updated each patient’s cumulative time in perpetuity at each of that patient’s charted tidal volumes. Note that as per our research question, time in perpetuity was not allowed to increase past ICU exit. If a patient had a charted tidal volume that exceeded threshold after ICU exit, the only portion of the interval of time straddling the ICU exit that was added to perpetuity was the portion before the ICU exit. We continued to follow the patient until death or hospital exit (whichever came first).
Appendix A.3. Sensitivity Analyses
In addition to the primary analysis, we conducted a sensitivity analysis to better explore both (a) the effect of the choices of eight and twelve hours as cut-offs; (b) the effect of disallowing further incrementation of hours in perpetuity after the first cut-off interval; and (c) the effect of the specific calculation of hours in perpetuity (at least in large intervals). We examined data sets that made different preprocessing choices to account for the effect of these choices on inferences. The specific data sets used in the sensitivity analysis are described below.
To explore the effect of cut-off, we constructed data sets with cut-offs of four, five, six, seven, and 24 h. We constructed a data set with no cut-off (thus removing the assumption that intervals of time over a particular threshold were indicative of extubation and reintubation). To explore the effect of disallowing perpetuity incrementation after cut-off, we constructed a data set in which no periods of time between successive tidal volume chartings that were greater than or equal to six hours were allowed to increment time in perpetuity. However, smaller intervals, even after the first interval greater than or equal to six hours, were allowed to increase time in perpetuity if appropriate. To explore the perpetuity calculation in large intervals, we constructed a data set with the following property: for tidal volume intervals greater than or equal to six hours, we assume the tidal volume changed linearly from the first endpoint to the second. Using this linear interpolation, we found the portion of the interval predicted to be over threshold. We incremented the total time in perpetuity by only that portion. Note that we only use linear interpolation for gaps greater than or equal to six hours. For smaller gaps, we use the tidal volume at the right endpoint of the interval to determine whether or not to add the interval into the perpetuity summation. Additionally, in both of the previous data sets, the interval between intubation and the first charted tidal volume was not considered when searching for gaps greater than or equal to six hours. To explore the decision to assign the tidal volume charted at the latter endpoint of each interval to that interval, we constructed two data sets that instead assigned the tidal volume charted at the beginning of each interval to that interval. In these data sets, because we assumed that the tidal volume at the start of each interval carried forward, we allowed perpetuity to increment every minute when the tidal volume was over 8 mL/kg. Note that we continued to implement the eight- and twelve-hour cutoffs with these last-one-forward data sets.
For each dataset, we fit both the full and reduced models described above. The only difference was that no spline predictors were included in any of the models. For each data set, the full and reduced models were compared with a partial likelihood ratio test to determine whether or not perpetuity in all its forms makes a statistically significant impact on the model fit.
Appendix A.4. Other Models
In addition to the full, adjusted models, we also fit two other models per primary data set. The first was an unadjusted model. This model included hours in perpetuity as the only predictor. The second was a model that weighted each interval in perpetuity by how far it was above the 8 mL/kg threshold (as a percentage of the threshold). For example, if an interval of one hour in length was 10% over the threshold and an interval of two hours in length was 30% over the threshold, the total time in perpetuity for those two intervals was 1 h*0.1 + 2 h*0.3. Note that weighted perpetuity models are based on the last-one-forward models. Fitted model estimates and partial likelihood ratio test results (for the second type of model) are given in the section below.
Appendix B. Results
Appendix B.1. Non-Linearity of Hours in Acuity and Heart Rate
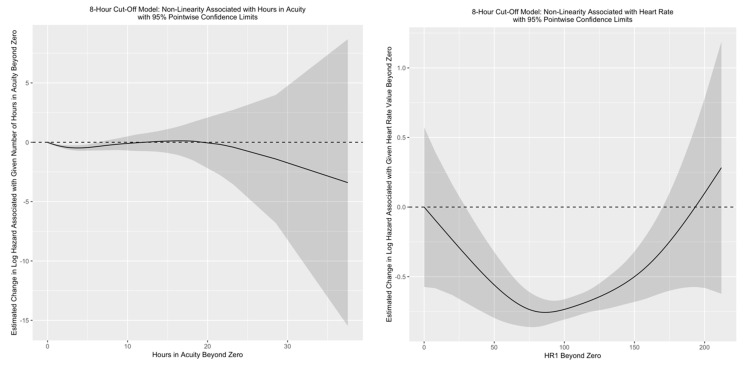
Figure A1.
The effect of hours in acuity and heart rate are complex and depend upon the starting number of acuity hours or starting heart rate. The association is illustrated below for the 8 h model.
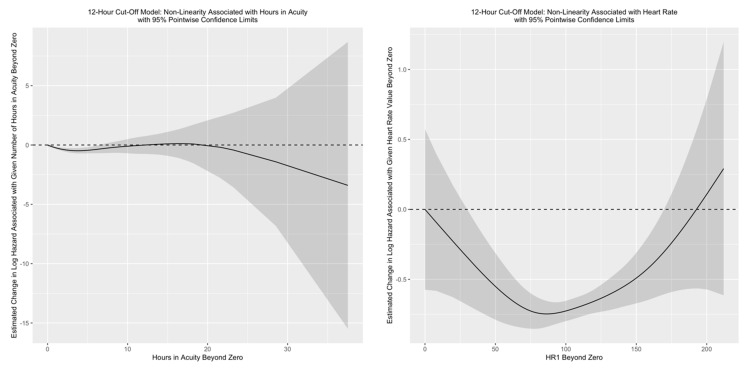
Figure A2.
For the 12 h model.
Appendix B.2. Unadjusted Model Results
Table A1.
8 h Cut-Off.
| Estimated Coefficient (95% CI) | Relative Risk (95% CI) | χ2 | df | p Value | |
|---|---|---|---|---|---|
| Hours in Perpetuity | - | 0 | 0.1855 | 1 | 0.6667 |
Table A1: 8 h Cut-Off, Unadjusted Model: Cox model with hours in perpetuity as a time-varying covariate. The estimated coefficient is on the log(hazard rate) scale. A relative risk over 1 indicates that having the associated covariate (or each unit of the associated covariate) increases the hazard of dying in the hospital. The non-linear portion of the spline terms for hours in acuity and heart rate do not have single estimated coefficients. Thus, only χ2 tests and the associated p values are given for these rows. 2025 patients are included in this model.
Table A2.
12-h Cut-Off.
| Estimated Coefficient (95% CI) | Relative Risk (95% CI) | χ2 | df | p Value | |
|---|---|---|---|---|---|
| Hours in Perpetuity | −0.0012 (−0.0069, 0.0045) | 0.9988 (0.9932, 1.0045) | 0.1703 | 1 | 0.6798 |
Table A2: 12 h Cut-Off, 8 mL/kg Threshold Unadjusted Model: Cox model with hours in perpetuity as a time-varying covariate. The estimated coefficient is on the log(hazard rate) scale. A relative risk over 1 indicates that having the associated covariate (or each unit of the associated covariate) increases the hazard of dying in the hospital. The non-linear portion of the spline terms for hours in acuity and heart rate do not have single estimated coefficients. Thus, only χ2 tests and the associated p values are given for these rows. 2025 patients are included in this model.
Appendix B.3. Results from Models with Perpetuity Weighted by the Magnitude of the Charted Tidal Volume
Table A3.
8 h Cut-Off Model.
| Estimated Coefficient (95% CI) | Relative Risk (95% CI) | χ2 | df | p Value | |
|---|---|---|---|---|---|
| Weighted Hours in Perpetuity | 1.3277 (0.2353, 2.4201) | 3.7723 (1.2652, 11.2468) | 5.6743 | 1 | 0.0172 |
| Age | 0.0279 (0.0217, 0.0342) | 1.0283 (1.0219, 1.0348) | 77.1117 | 1 | 0.0000 |
| O2 | −0.0144 (−0.0208, −0.0079) | 0.9857 (0.9794, 0.9921) | 18.9938 | 1 | 0.0000 |
| Hours in Acuity (Linear) | 0.014 (−0.0428, 0.0708) | 1.0141 (0.9581, 1.0734) | 0.2333 | 1 | 0.6291 |
| Hours in Acuity (Non-Linear) | 14.4508 | 3 | 0.0024 | ||
| Arrival by EMS Ground | 0.0921 (−0.3801, 0.5644) | 1.0965 (0.6838, 1.7583) | 0.1462 | 1 | 0.7022 |
| Other Mode of Arrival | 0.1327 (−0.5432, 0.8085) | 1.1419 (0.5809, 2.2446) | 0.1480 | 1 | 0.7004 |
| POV Mode of Arrival | −0.1992 (−0.7843, 0.3858) | 0.8194 (0.4564, 1.4708) | 0.4455 | 1 | 0.5045 |
| BUMCS | 0.4127 (0.2129, 0.6125) | 1.5109 (1.2373, 1.8451) | 16.3948 | 1 | 0.0001 |
| Had NIPPV | −0.9321 (−1.2587, −0.6056) | 0.3937 (0.284, 0.5458) | 31.2938 | 1 | 0.0000 |
| Ideal Body Weight | −0.0022 (−0.0116, 0.0073) | 0.9978 (0.9885, 1.0073) | 0.2005 | 1 | 0.6543 |
| BMI | 0.003 (−0.0072, 0.0132) | 1.003 (0.9928, 1.0133) | 0.3312 | 1 | 0.5649 |
| Heart Rate (Linear) | 7× 10−4 (−0.0022, 0.0035) | 1.0007 (0.9978, 1.0035) | 0.2235 | 1 | 0.6364 |
| Heart Rate (Non-Linear) | 11.0571 | 2 | 0.0044 | ||
| Systolic Blood Pressure | −0.0024 (−0.009, 0.0042) | 0.9976 (0.991, 1.0042) | 0.5168 | 1 | 0.4722 |
| Mean Arterial Pressure | −0.0052 (−0.0141, 0.0037) | 0.9948 (0.986, 1.0037) | 1.3198 | 1 | 0.2506 |
| Acuity Score | −0.3774 (−0.579, −0.1758) | 0.6856 (0.5605, 0.8388) | 13.4658 | 1 | 0.0002 |
| Weighted Hours in Perpetuity × Age | −0.0056 (−0.0121, 8 × 10−4) | 0.9944 (0.988, 1.0008) | 2.9704 | 1 | 0.0848 |
| Weighted Hours in Perpetuity × O2 | −0.0116 (−0.0224, −8 × 10−4) | 0.9885 (0.9778, 0.9992) | 4.3987 | 1 | 0.0360 |
Table A3: 8 h Cut-Off, Last-One-Forward, Minute-Increment Model with Hours in Perpetuity Weighted by the Charted Tidal Volume’s Percent Over the 8 mL/kg Threshold: Cox model with hours in perpetuity as a time-varying covariate. The estimated coefficient is on the log(hazard rate) scale. A relative risk over 1 indicates that having the associated covariate (or each unit of the associated covariate) increases the hazard of dying in the hospital. The non-linear portion of the spline terms for hours in acuity and heart rate do not have single estimated coefficients. Thus, only χ2 tests and the associated p values are given for these rows. 2025 patients are included in this model.
Table A4.
8 h Cut-Off.
| Loglik | Chisq | Df | p (>|Chi|) | |
|---|---|---|---|---|
| 1 | −3051.34 | |||
| 2 | −3055.74 | 8.8 | 3 | 0.0321 |
Table A4: 8 h Cut-Off, Last-One-Forward, Minute-Increment Model with Hours in Perpetuity Weighted by the Charted Tidal Volume’s Percent Over the 8 mL/kg Threshold: Partial likelihood ratio test comparing models with and without any terms involving perpetuity. Model with perpetuity has main effect of perpetuity, interaction of perpetuity and age, interaction of perpetuity and O2, and other covariates. Reduced model removes the three named terms but still contains the other covariates. Both models incorporate non-linear effects of hours in acuity and HR1.
Table A5.
12-h Cut-Off Model.
| Estimated Coefficient (95% CI) | Relative Risk (95% CI) | χ2 | df | p Value | |
|---|---|---|---|---|---|
| Weighted Hours in Perpetuity | 0.5459 (−0.3614, 1.4532) | 1.7261 (0.6967, 4.2766) | 1.3906 | 1 | 0.2383 |
| Age | 0.0279 (0.0217, 0.0341) | 1.0283 (1.0219, 1.0347) | 76.8969 | 1 | 0.0000 |
| O2 | −0.015 (−0.0214, −0.0086) | 0.9851 (0.9789, 0.9914) | 21.2007 | 1 | 0.0000 |
| Hours in Acuity (Linear) | 0.0133 (−0.0435, 0.0701) | 1.0134 (0.9575, 1.0726) | 0.2112 | 1 | 0.6458 |
| Hours in Acuity (Non-Linear) | 14.4563 | 3 | 0.0024 | ||
| Arrival by EMS Ground | 0.0982 (−0.3742, 0.5706) | 1.1032 (0.6878, 1.7693) | 0.1660 | 1 | 0.6837 |
| Other Mode of Arrival | 0.1441 (−0.5321, 0.8202) | 1.1549 (0.5874, 2.2709) | 0.1744 | 1 | 0.6762 |
| POV Mode of Arrival | −0.1916 (−0.7764, 0.3933) | 0.8257 (0.46, 1.4819) | 0.4121 | 1 | 0.5209 |
| BUMCS | 0.4114 (0.2118, 0.611) | 1.5089 (1.2359, 1.8423) | 16.3148 | 1 | 0.0001 |
| Had NIPPV | −0.9255 (−1.2518, −0.5992) | 0.3963 (0.286, 0.5493) | 30.9002 | 1 | 0.0000 |
| Ideal Body Weight | −0.0012 (−0.0106, 0.0083) | 0.9988 (0.9895, 1.0083) | 0.0580 | 1 | 0.8096 |
| BMI | 0.0032 (−0.0069, 0.0132) | 1.0032 (0.9931, 1.0133) | 0.3758 | 1 | 0.5398 |
| Heart Rate (Linear) | 7 × 10−4 (−0.0021, 0.0036) | 1.0007 (0.9979, 1.0036) | 0.2488 | 1 | 0.6179 |
| Heart Rate (Non-Linear) | 10.9098 | 2 | 0.0047 | ||
| Systolic Blood Pressure | −0.0023 (−0.0089, 0.0043) | 0.9977 (0.9911, 1.0043) | 0.4831 | 1 | 0.4870 |
| Mean Arterial Pressure | −0.0053 (−0.0142, 0.0036) | 0.9947 (0.9859, 1.0037) | 1.3410 | 1 | 0.2468 |
| Acuity Score | −0.3801 (−0.5816, −0.1787) | 0.6838 (0.559, 0.8364) | 13.6775 | 1 | 0.0002 |
| Weighted Hours in Perpetuity × Age | −0.0038 (−0.0089, 0.0013) | 0.9962 (0.9912, 1.0013) | 2.1370 | 1 | 0.1438 |
| Weighted Hours in Perpetuity × O2 | −0.0039 (−0.0128, 0.005) | 0.9961 (0.9873, 1.005) | 0.7305 | 1 | 0.3927 |
Table A5: 12 h Cut-Off, Last-One-Forward, Minute-Increment Model with Hours in Perpetuity Weighted by the Charted Tidal Volume’s Percent Over the 8 mL/kg Threshold: Cox model with hours in perpetuity as a time-varying covariate. The estimated coefficient is on the log(hazard rate) scale. A relative risk over 1 indicates that having the associated covariate (or each unit of the associated covariate) increases the hazard of dying in the hospital. The non-linear portion of the spline terms for hours in acuity and heart rate do not have single estimated coefficients. Thus, only χ2 tests and the associated p values are given for these rows. 2025 patients are included in this model.
Table A6.
12 h Cut-Off.
| Loglik | Chisq | df | p (>|Chi|) | |
|---|---|---|---|---|
| 1 | −3053.17 | |||
| 2 | −3055.74 | 5.14 | 3 | 0.1615 |
Table A6: 12 h Cut-Off, 8 mL/kg Threshold Last-One-Forward, Minute-Increment Model with Hours in Perpetuity Weighted by the Charted Tidal Volume’s Percent Over the 8 mL/kg Threshold: Partial likelihood ratio test comparing models with and without any terms involving perpetuity. Model with perpetuity has main effect of perpetuity, interaction of perpetuity and age, interaction of perpetuity and O2, and other covariates. Reduced model removes the three named terms but still contains the other covariates. Both models incorporate non-linear effects of hours in acuity and HR1.
Appendix B.4. Sensitivity Results
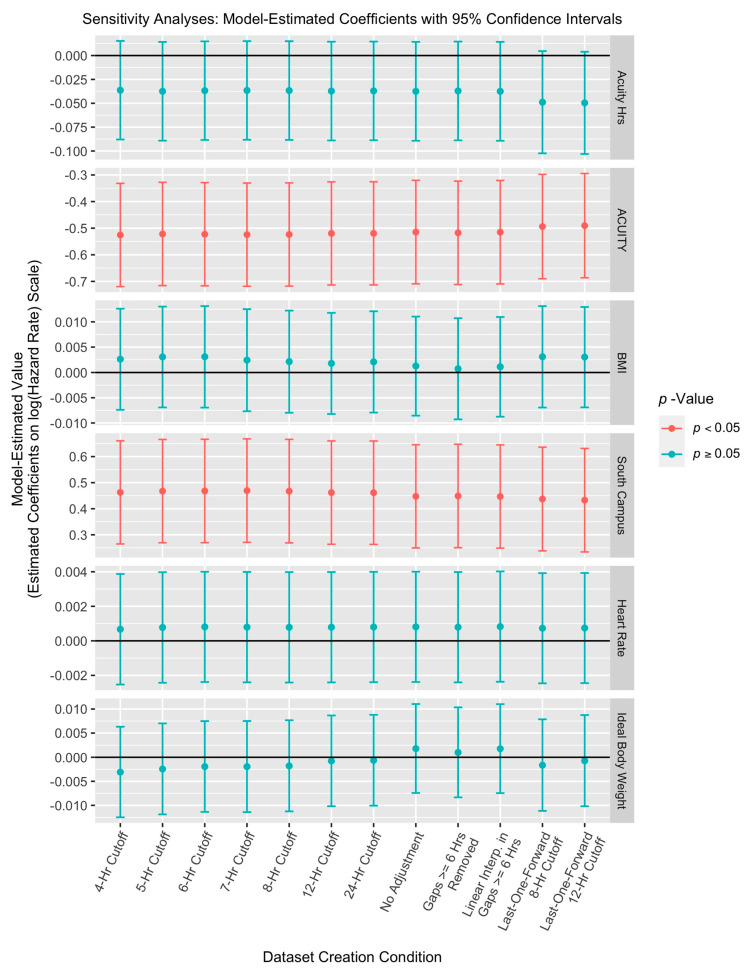
The plots below present the coefficient estimates and partial likelihood ratio test statistics from the sensitivity analyses. The y-axis for the coefficient estimates is on the log hazard rate scale. The y-axis for the partial likelihood ratio test statistics is on the scale of the χ2 test statistic. For the partial likelihood ratio test sections of the plots, a large χ2 test statistic and corresponding small p-value indicates that the presence of perpetuity in the model statistically significantly improves the model fit. In general, color corresponds to the p value of the associated Wald χ2 test (for coefficient estimates) or the partial likelihood ratio χ2 test (for model comparisons). Finally, note that all these models include data from 2025 patients.
Figure A3.
Sensitivity of inferential results to pre-processing choices. ßEstimated coefficients on the log(hazard rate) scale with 95% confidence intervals and partial likelihood ratio test χ2 test statistics for each model are shown. Plotted values are colored based on their associated p-value.
Figure A4.
Sensitivity of inferential results to pre-processing choices. Estimated coefficients on the log(hazard rate) scale with 95% confidence intervals for each model are shown. Plotted values are colored based on their associated p-value.
Figure A5.
Sensitivity of inferential results to pre-processing choices. Estimated coefficients on the log(hazard rate) scale with 95% confidence intervals for each model are shown. Plotted values are colored based on their associated p-value.
Author Contributions
Conceptualization, J.M.M., J.M.F., C.D.H., E.J.B. and C.B.C.; Data curation, J.M.M., J.M.F.; Formal analysis, J.M.M. and J.M.F.; Methodology, J.M.M., J.M.F., E.J.B. and K.L.; Project administration, J.M.M. and C.B.C.; Supervision, J.M.M., E.J.B. and C.B.C.; Writing—original draft, J.M.M. and J.M.F.; Writing—review & editing, J.M.M., J.M.F., C.D.H., E.S.C. and K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the National Foundation of Emergency Medicine.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the University of Arizona (protocol code 1607695679 on 12 July 2016).
Informed Consent Statement
The Institutional Review Board determined that the requirements for obtaining informed consent could be waived for this study given the retrospective analysis of a de-identified pre-existing clinical quality database.
Data Availability Statement
The University of Arizona clinical quality improvement database is not publicly accessible.
Conflicts of Interest
J.M.M. reports no conflict of interest; J.M.F. reports no conflict of interest; E.J.B. reports no conflict of interest; C.D.H. reports no conflict of interest; E.S.C. reports no conflict of interest; K.L. reports no conflict of interest; C.B.C. reports no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Control C.F.D. National Hospital Ambulatory Medical Care Survey: 2011 Emergency Department Summary Tables. [(accessed on 5 August 2021)]; Available online: http://www.cdc.gov/nchs/ahcd.htm.
- 2.Rui P., Kang K., Ashman J.J. National Hospital Ambulatory Medical Care Survey: 2016 Emergency Department Summary Tables. [(accessed on 5 August 2021)];2016 Available online: https://www.cdc.gov/nchs/data/nhamcs/web_tables/2016_ed_web_tables.pdf.
- 3.Mullins P.M., Goyal M., Pines J.M. National growth in intensive care unit admissions from emergency departments in the United States from 2002 to 2009. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2013;20:479–486. doi: 10.1111/acem.12134. [DOI] [PubMed] [Google Scholar]
- 4.Herring A.A., Ginde A.A., Fahimi J., Alter H.J., Maselli J.H., Espinola J.A., Sullivan A.F., Camargo C.A., Jr. Increasing critical care admissions from U.S. emergency departments, 2001–2009. Crit. Care Med. 2013;41:1197–1204. doi: 10.1097/CCM.0b013e31827c086f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medicine I.O. The National Academies of Sciences Engineering Medicine. The National Academies Press; Washington, DC, USA: 2006. Hospital-Based Emergency Care: At the Breaking Point. [Google Scholar]
- 6.Mathews K.S., Durst M.S., Vargas-Torres C., Olson A.D., Mazumdar M., Richardson L.D. Effect of Emergency Department and ICU Occupancy on Admission Decisions and Outcomes for Critically Ill Patients. Crit. Care Med. 2018;46:720–727. doi: 10.1097/CCM.0000000000002993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer A.J., Thode H.C., Jr., Viccellio P., Pines J.M. The association between length of emergency department boarding and mortality. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2011;18:1324–1329. doi: 10.1111/j.1553-2712.2011.01236.x. [DOI] [PubMed] [Google Scholar]
- 8.Intas G., Stergiannis P., Chalari E., Tsoumakas K., Fildissis G. The impact of ED boarding time, severity of illness, and discharge destination on outcomes of critically ill ED patients. Adv. Emerg. Nurs. J. 2012;34:164–169. doi: 10.1097/TME.0b013e318251515f. [DOI] [PubMed] [Google Scholar]
- 9.Chalfin D.B., Trzeciak S., Likourezos A., Baumann B.M., Dellinger R.P., Delay-Ed Study Group Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit. Care Med. 2007;35:1477–1483. doi: 10.1097/01.CCM.0000266585.74905.5A. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso L.T., Grion C.M., Matsuo T., Anami E.H., Kauss I.A., Seko L., Bonametti A.M. Impact of delayed admission to intensive care units on mortality of critically ill patients: A cohort study. Crit. Care. 2011;15:R28. doi: 10.1186/cc9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cline S.D., Schertz R.A., Feucht E.C. Expedited admission of patients decreases duration of mechanical ventilation and shortens ICU stay. Am. J. Emerg. Med. 2009;27:843–846. doi: 10.1016/j.ajem.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Duke G., Green J., Briedis J. Survival of critically ill medical patients is time-critical. Crit. Care Resusc. 2004;6:261–267. [PubMed] [Google Scholar]
- 13.Gunnerson K.J., Bassin B.S., Havey R.A., Haas N.L., Sozener C.B., Medlin R.P., Jr., Gegenheimer-Holmes J.A., Laurinec S.L., Boyd C., Cranford J.A., et al. Association of an Emergency Department-Based Intensive Care Unit With Survival and Inpatient Intensive Care Unit Admissions. JAMA Netw. Open. 2019;2:e197584. doi: 10.1001/jamanetworkopen.2019.7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kangelaris K.N., Ware L.B., Wang C.Y., Janz D.R., Zhuo H., Matthay M.A., Calfee C.S. Timing of Intubation and Clinical Outcomes in Adults With Acute Respiratory Distress Syndrome. Crit. Care Med. 2016;44:120–129. doi: 10.1097/CCM.0000000000001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuller B.M., Mohr N.M., Dettmer M., Kennedy S., Cullison K., Bavolek R., Rathert N., McCammon C. Mechanical ventilation and acute lung injury in emergency department patients with severe sepsis and septic shock: An observational study. Acad. Emerg. Med. 2013;20:659–669. doi: 10.1111/acem.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuller B.M., Mohr N.M., Miller C.N., Deitchman A.R., Levine B.J., Castagno N., Hassebroek E.C., Dhedhi A., Scott-Wittenborn N., Grace E., et al. Mechanical Ventilation and ARDS in the ED: A Multicenter, Observational, Prospective, Cross-sectional Study. Chest. 2015;148:365–374. doi: 10.1378/chest.14-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilcox S.R., Richards J.B., Fisher D.F., Sankoff J., Seigel T.A. Initial mechanical ventilator settings and lung protective ventilation in the ED. Am. J. Emerg. Med. 2016;34:1446–1451. doi: 10.1016/j.ajem.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 18.Owyang C.G., Kim J.L., Loo G., Ranginwala S., Mathews K.S. The effect of emergency department crowding on lung-protective ventilation utilization for critically ill patients. J. Crit. Care. 2019;52:40–47. doi: 10.1016/j.jcrc.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuller B.M., Roberts B.W., Mohr N.M., Knight W.A.T., Adeoye O., Pappal R.D., Marshall S., Alunday R., Dettmer M., Goyal M., et al. The ED-SED Study: A Multicenter, Prospective Cohort Study of Practice Patterns and Clinical Outcomes Associated With Emergency Department SEDation for Mechanically Ventilated Patients. Crit. Care Med. 2019;47:1539. doi: 10.1097/CCM.0000000000003928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuller B.M., Ferguson I.T., Mohr N.M., Drewry A.M., Palmer C., Wessman B.T., Ablordeppey E., Keeperman J., Stephens R.J., Briscoe C.C., et al. A Quasi-Experimental, Before-After Trial Examining the Impact of an Emergency Department Mechanical Ventilator Protocol on Clinical Outcomes and Lung-Protective Ventilation in Acute Respiratory Distress Syndrome. Crit. Care Med. 2017;45:645–652. doi: 10.1097/CCM.0000000000002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuller B.M., Ferguson I.T., Mohr N.M., Drewry A.M., Palmer C., Wessman B.T., Ablordeppey E., Keeperman J., Stephens R.J., Briscoe C.C., et al. Lung-Protective Ventilation Initiated in the Emergency Department (LOV-ED): A Quasi-Experimental, Before-After Trial. Ann. Emerg. Med. 2017;70:406–418. doi: 10.1016/j.annemergmed.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gliklich R.E., Dreyer N.A., Leavy M.B., Valentgas P., Khurana L. Standards in the Conduct of Registry Studies for Patient-Centered Outcomes Research: A Guidance Document for the Patient-Centered Outcomes Research Institute. Patient-Centered Outcomes Research Institute; Washington, DC, USA: 2012. p. 58. [Google Scholar]
- 23.Eilers P.H.C., Marx B.D. Flexible Smoothing with B-Splines and Penalties. Stat. Sci. 1996;11:89–102. doi: 10.1214/ss/1038425655. [DOI] [Google Scholar]
- 24.Hurvich C.M., Simonof J.S., Tsai C.L. Smoothing parameter selection in nonparametic regression using an improved Akaike information criterion. J. R. Stat. Soc. Ser. B Stat. Methol. 1998;60:271–293. doi: 10.1111/1467-9868.00125. [DOI] [Google Scholar]
- 25.Team R.C. R: A language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2020. [Google Scholar]
- 26.Wickham H., Franois R. Dplyr: A Grammar of Data Manipulation. R Foundation for Statistical Computing; Vienna, Austria: 2020. R Package Version 0.8.5. [Google Scholar]
- 27.Wickham H., Henry L. Tidyr: Tidy Messy Data. R Foundation for Statistical Computing; Vienna, Austria: 2020. R Package Version 1.1.0. [Google Scholar]
- 28.Wickham H. Ggplot2: Elegant Graphics for Data Analysis. 1st ed. Springer; New York, NY, USA: 2009. p. 213. [Google Scholar]
- 29.Dahl D.B., Scott D., Roosen C., Magnusson A., Swinton J. Xtable: Export Tables to LaTeX or HTML. R Foundation for Statistical Computing; Vienna, Austria: 2019. R Package Version 1.8-4. [Google Scholar]
- 30.Therneau T. A Package for Survival Analysis in R. R Foundation for Statistical Computing; Vienna, Austria: 2020. R Package Version 3.1-12. [Google Scholar]
- 31.Therneau T., Grambsch P. Modeling Survival Data: Extending the Cox Model. 1st ed. Springer; New York, NY, USA: 2000. p. 350. [DOI] [Google Scholar]
- 32.Van Belle G. Statistical Rules of Thumb. John Wiley & Sons; New York, NY, USA: 2011. [Google Scholar]
- 33.Sjoding M.W., Gong M.N., Haas C.F., Iwashyna T.J. Evaluating Delivery of Low Tidal Volume Ventilation in Six ICUs Using Electronic Health Record Data. Crit. Care Med. 2019;47:56–61. doi: 10.1097/CCM.0000000000003469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urner M., Juni P., Hansen B., Wettstein M.S., Ferguson N.D., Fan E. Time-varying intensity of mechanical ventilation and mortality in patients with acute respiratory failure: A registry-based, prospective cohort study. Lancet Respir. Med. 2020;8:905–913. doi: 10.1016/S2213-2600(20)30325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Handel D.A. Early Goal-Directed Ventilation: Serving the Greater Good? Ann. Emerg. Med. 2015;66:542–543. doi: 10.1016/j.annemergmed.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Mosier J.M., Hypes C., Joshi R., Whitmore S., Parthasarathy S., Cairns C.B. Ventilator Strategies and Rescue Therapies for Management of Acute Respiratory Failure in the Emergency Department. Ann. Emerg. Med. 2015;66:529–541. doi: 10.1016/j.annemergmed.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 37.Acute Respiratory Distress Syndrome N., Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B.T., Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 38.Amato M.B., Barbas C.S., Medeiros D.M., Magaldi R.B., Schettino G.P., Lorenzi-Filho G., Kairalla R.A., Deheinzelin D., Munoz C., Oliveira R., et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N. Engl. J. Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 39.Serpa Neto A., Cardoso S.O., Manetta J.A., Pereira V.G., Esposito D.C., Pasqualucci Mde O., Damasceno M.C., Schultz M.J. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: A meta-analysis. JAMA J. Am. Med. Assoc. 2012;308:1651–1659. doi: 10.1001/jama.2012.13730. [DOI] [PubMed] [Google Scholar]
- 40.Cressoni M., Gotti M., Chiurazzi C., Massari D., Algieri I., Amini M., Cammaroto A., Brioni M., Montaruli C., Nikolla K., et al. Mechanical Power and Development of Ventilator-induced Lung Injury. Anesthesiology. 2016;124:1100–1108. doi: 10.1097/ALN.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 41.Gattinoni L., Tonetti T., Cressoni M., Cadringher P., Herrmann P., Moerer O., Protti A., Gotti M., Chiurazzi C., Carlesso E., et al. Ventilator-related causes of lung injury: The mechanical power. Intensive Care Med. 2016;42:1567–1575. doi: 10.1007/s00134-016-4505-2. [DOI] [PubMed] [Google Scholar]
- 42.Tonetti T., Vasques F., Rapetti F., Maiolo G., Collino F., Romitti F., Camporota L., Cressoni M., Cadringher P., Quintel M., et al. Driving pressure and mechanical power: New targets for VILI prevention. Ann. Transl. Med. 2017;5:286. doi: 10.21037/atm.2017.07.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collino F., Rapetti F., Vasques F., Maiolo G., Tonetti T., Romitti F., Niewenhuys J., Behnemann T., Camporota L., Hahn G., et al. Positive End-expiratory Pressure and Mechanical Power. Anesthesiology. 2019;130:119–130. doi: 10.1097/ALN.0000000000002458. [DOI] [PubMed] [Google Scholar]
- 44.Leisman D., Huang V., Zhou Q., Gribben J., Bianculli A., Bernshteyn M., Ward M.F., Schneider S.M. Delayed Second Dose Antibiotics for Patients Admitted From the Emergency Department With Sepsis: Prevalence, Risk Factors, and Outcomes. Crit. Care Med. 2017;45:956–965. doi: 10.1097/CCM.0000000000002377. [DOI] [PubMed] [Google Scholar]
- 45.Vincent J.L., Hall J.B., Slutsky A.S. Ten big mistakes in intensive care medicine. Intensive Care Med. 2015;41:505–507. doi: 10.1007/s00134-014-3570-7. [DOI] [PubMed] [Google Scholar]
- 46.Marcozzi D., Carr B., Liferidge A., Baehr N., Browne B. Trends in the Contribution of Emergency Departments to the Provision of Hospital-Associated Health Care in the USA. Int. J. Health Serv. 2018;48:267–288. doi: 10.1177/0020731417734498. [DOI] [PubMed] [Google Scholar]
- 47.American College of Emergency P. Boarding of admitted and intensive care patients in the emergency department. Policy statement. Ann. Emerg. Med. 2011;58:110. doi: 10.1016/j.annemergmed.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 48.Perkins J., Motov S. American Academy of Emergency Medicine Clinical Practice Committee Statement. Mortality Related to Delay of ICU Patient Transfer from the Emergency Department. [(accessed on 29 October 2015)]; Available online: http://www.aaem.org/UserFiles/file/icu_transfer_statement.pdf.
- 49.Montgomery P., Godfrey M., Mossey S., Conlon M., Bailey P. Emergency department boarding times for patients admitted to intensive care unit: Patient and organizational influences. Int. Emerg. Nurs. 2014;22:105–111. doi: 10.1016/j.ienj.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Groenland C.N.L., Termorshuizen F., Rietdijk W.J.R., van den Brule J., Dongelmans D.A., de Jonge E., de Lange D.W., de Smet A., de Keizer N.F., Weigel J.D., et al. Emergency Department to ICU Time Is Associated With Hospital Mortality: A Registry Analysis of 14,788 Patients From Six University Hospitals in The Netherlands. Crit. Care Med. 2019;47:1564. doi: 10.1097/CCM.0000000000003957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The University of Arizona clinical quality improvement database is not publicly accessible.