Abstract
Pili torti is a rare condition characterized by the presence of the hair shaft, which is flattened at irregular intervals and twisted 180° along its long axis. It is a form of hair shaft disorder with increased fragility. The condition is classified into inherited and acquired. Inherited forms may be either isolated or associated with numerous genetic diseases or syndromes (e.g., Menkes disease, Björnstad syndrome, Netherton syndrome, and Bazex-Dupré-Christol syndrome). Moreover, pili torti may be a feature of various ectodermal dysplasias (such as Rapp-Hodgkin syndrome and Ankyloblepharon-ectodermal defects-cleft lip/palate syndrome). Acquired pili torti was described in numerous forms of alopecia (e.g., lichen planopilaris, discoid lupus erythematosus, dissecting cellulitis, folliculitis decalvans, alopecia areata) as well as neoplastic and systemic diseases (such as cutaneous T-cell lymphoma, scalp metastasis of breast cancer, anorexia nervosa, malnutrition, cataracts, and chronic graft-vs.-host disease). The condition may also be induced by several drugs (epidermal growth factor receptor inhibitors, oral retinoids, sodium valproate, and carbamide perhydrate). The diagnosis of pili torti is based on trichoscopic or microscopic examination. As pili torti is a marker of numerous congenital and acquired disorders, in every case, the search for the signs of underlying conditions is recommended.
Keywords: pili torti, trichoscopy, hair shaft abnormalities, hair shaft disorder, hair disease, twisted hair
1. Introduction
Pili torti, also known as “twisted hair”, was first described by Galewsky, and, independently, by Ronchese in 1932 [1,2]. It is characterized by the presence of the hair shaft, flattened at irregular intervals and twisted 180° along its long axis, with each twist being 0.4 to 0.9 mm wide and occurring in groups of 3 to 10 (Figure 1) [3].
Figure 1.
Pili torti, characterized by the presence of the hair shaft flattened and twisted 180° along its long axis. Reproduced with permission from J. Taczała, MSc.
Pili torti is a form of hair shaft disorder with increased fragility [4]. It is classified into inherited or acquired. A wide range of changes associated with pili torti suggest specific pathophysiological mechanisms [5]. In inherited forms, the twisting of the hair is caused by an unequal development of the outer root sheath cells. Cell vacuolation and the irregular thickness of the outer root sheath at the suprabulbar level induce an uneven molding of the inner root sheath and hair shaft [6]. In acquired forms, a perifollicular inflammation followed by fibrosis generates rotational forces and deforms the hair follicle [7]. The cross-sectional area of pili torti hair is significantly smaller than a normal hair sample (2210 ± 1090 vs. 3370 ± 821 (µm2); p < 0.001) and the tensile strength of pili torti is 2.1 times lower than that of normal hair [8]. No abnormalities in the hair cortex keratin within pili torti axis are observed [5].
Clinically, patients with pili torti have fragile, brittle, dry, and coarse hair. Patchy alopecia may develop. The scalp hair, especially in the occipital and temporal areas, is most commonly affected [9]. However, the eyebrows, eyelashes, axillary, and pubic hair may also be involved [9]. Usually, not all hair is affected by pili torti, and only a part of hair length may be changed [10]. Isolated pili torti may be occasionally found in the normal scalp. However, it may be associated with numerous local and systemic conditions [11,12].
In this review, we analyse current data about the possible causes of pili tori and discuss the underlying conditions. Available data on the management of pili torti are presented.
2. Inherited Pili Torti
Inherited pili torti may be categorized as: classic early onset (Ronchese type), late onset (Beare type), and pili torti associated with genetic diseases or syndromes [2].
2.1. Isolated Pili Torti
2.1.1. Early Onset (Ronchese) Type
The classic (Ronchese) form is an autosomal dominant or recessive condition, beginning in the early childhood [2,13]. The disease onset is between the third month and third year of life. Girls with blond hair are most commonly affected. In early onset pili torti, clusters of twists of hair are usually detected. The condition often improves with age, especially after puberty [2,13].
2.1.2. Late Onset (Beare) Type
Late onset type is an autosomal dominant disorder, typically occurring after puberty. It is more commonly observed in individuals with dark hair. Contrary to the early onset type, the twists of hair are usually single in the late onset type [2,13].
2.2. Pili Torti in Genetic Diseases or Syndromes
The list of genetic diseases and syndromes associated with pili torti is presented in Table 1. The most common conditions are described below.
Table 1.
Pili torti in genetic diseases and syndromes.
| Disease/Syndrome | Genetic Defect, Inheritance | Other Clinical Findings | Other Hair Shaft Abnormalities |
|---|---|---|---|
| Abnormal hair, joint laxity, and developmental delay [14,15] | HEPHL1 gene (AR) | growth and developmental delay, joint laxity, neurologic abnormalities | trichorrhexis nodosa |
| Acrofacial dysostosis, Palagonia type [16] |
NK (AD) | short stature, vertebral anomalies, syndactyly, oligodontia, cleft lip | - |
| Arginosuccinic aciduria [17] | ASL gene (AR) | lethargy, vomiting, seizures, cerebral edema, hepatomegaly | trichorrhexis nodosa |
| Autosomal recessive ichthyosis with hypotrichosis [18] | ST14 gene (AR) | lamellar ichthyosis, follicular atrophoderma, hypohidrosis | dysplastic hair, pili bifurcati |
| Bazex-Dupre-Christol syndrome [19] | ACTRT1 gene (XD) | follicular atrophoderma, multiple basal cell carcinomas, milia, hypohidrosis | trichorrhexis nodosa |
| Björnstad syndrome [20,21] | BCS1L gene (AR, AD) | sensorineural hearing loss | - |
| Citrullinemia [22] | ASS1 gene (AR) | hyperammonemia, lethargy, poor feeding, vomiting, high intracranial pressure, scaly skin eruption | trichorrhexis nodosa |
| Congenital disorder of glycosylation, type Ia [23] |
PMM2 gene (AR) | hypotonia, strabismus, cerebellar hypoplasia, seizures, mental and physical retardation, hepatomegaly, liver fibrosis, fat pads, ‘orange peel’ skin | trichorrhexis nodosa |
| Congenital erythropoietic porphyria [24] | UROS gene (AR) | severe skin photosensitivity (scarring, blistering), erythrodontia, reddish-colored urine, anemia |
- |
| Congenital hypotrichosis with juvenile macular dystrophy [25] | CDH3 gene (AR) | retinal degeneration | - |
| Conradi-Hünermann syndrome [26] | EBP gene (XD) | short stature, asymmetric short limbs, vertebral malformations, hip dysplasia, chondrodysplasia punctata, follicular atrophoderma, abnormal nails, craniofacial anomalies, cataracts | - |
| Crandall syndrome [27] | NK (AR) | neurosensory deafness, hypogonadism with decreased levels of luteinizing hormone and growth hormone |
- |
| Giant axonal neuropathy [28] | GAN gene (AR) | progressive sensorimotor peripheral neuropathy, axonal loss, optic atrophy, ophthalmoplegia, skeletal deformations | trichorrhexis nodosa |
| Hypotrichosis 6 [29,30] | DSG4 gene (AR) | hyperkeratotic follicular papules, erythema, scaling, dry skin |
monilethrix-like hair, trichoschisis, trichorrhexis nodosa-like defects, tapered hair |
| Laron syndrome [31] | GHR gene (AR) | short stature, obesity, facial dysmorphism, hypogenitalism, elevated serum growth hormone, undetectable or low serum insulin-like growth factor 1 | - |
| Marie Unna hypotrichosis [32] | U2HR gene (AD) | - | - |
| McCune-Albright syndrome [33] | GNAS1 gene (not inherited) | polyostotic fibrous dysplasia, cafe-au-lait skin pigmentation, multiple endocrine dysfunction | - |
| Menkes disease [5] | ATP7A gene (XR) | growth retardation, vascular, neurological, and skeletal abnormalities, pale skin | trichoclasis, trichorrhexis nodosa, trichoptilosis |
| Mitochondrial diseases [34] | all modes of inheritance can be expected | mental retardation, failure to thrive, hypotonia, hypoparathyroidism | longitudinal grooving with cuticle loss |
| Netherton syndrome [35,36] | SPINK5 gene (AR) | congenital erythroderma, atopic manifestations, increased IgE level | trichorrhexis I vaginata, trichorrhexis nodosa, trichoschisis, trichoptilosis |
| Occipital horn syndrome [37] | ATP7A gene (XR) | tallness, pectus excavatum, dorsal kyphosis, occipital horn exostoses, joint laxity, loose skin, decreased serum copper and ceruloplasmin | - |
| Olmsted syndrome [38] | TRPV3 gene (AD) | constriction of digits (‘pseudoainhum’), mutilating palmoplantar keratoderma, onychodystrophy, periorificial keratotic plaques | trichorrhexis nodosa |
| Peeling skin syndrome [39] | CDSN gene (AR) | superficial patchy peeling of the entire skin, erythroderma, atopy, nail anomalies | trichorrhexis invaginata-like changes, monili-form hair shaft diameter reductions, irregular hair shaft torsions |
| Salti-Salem syndrome [40] | NK (AD) | hypogonadotropic hypogonadism | - |
| Steatocystoma multiplex [41] | KRT17 gene (AD) | subcutaneous cysts | pili canaliculi |
| Tricho-hepato-enteric syndrome [42] | TTC37, SKIV2L genes (AR) | low birth weight, failure to thrive, facial dysmorphism, diarrhoea, liver disease | trichorrhexis nodosa, aniso- and poilkilotrichosis |
| Trichothiodystrophy, photosensitive [43,44] | ERCC2, XPD genes (AR) | mental and physical retardation, short stature, facial dysmorphism, ichthyosis, photosensitivity, ocular abnormalities | Pili annulati (‘tiger-tail’ hair), trichoschisis, trichorrhexis nodosa |
AD, autosomal dominant; AR, autosomal recessive; NK, not known; XD, X-linked dominant; XR, X-linked recessive.
2.2.1. Menkes Disease
Menkes disease, also known as kinky hair disease, is a rare neurodegenerative disease with X-linked recessive inheritance [45]. It is caused by different mutations in the ATPase Copper Transporting Alpha (ATP7A) gene, that result in the failure of copper absorption in the intestines followed by copper deficiency [46].
Pili torti is the most characteristic hair shaft defect in Menkes disease. The twists of hair shaft are probably due to the low activity of copper-dependent enzymes, which are important in creating disulfide bonds in hair keratin. Other hair shaft abnormalities are trichoclasis, trichorrhexis nodosa, and trichoptilosis [5].
In patients with Menkes disease, neurodegenerative signs, including seizures, feeding difficulties, hypotonia, and psychomotor retardation usually occur at 2–3 months of age [5]. Other common features are a jowly face with fair complexion, skin and joint laxity, arterial abnormalities, skeletal changes, and urinary tract infections caused by the diverticula of the bladder [5].
In therapeutic management, copper supplementation is the most important [5].
2.2.2. Björnstad Syndrome
Björnstad syndrome is an autosomal recessive or dominant condition caused by a missense mutation in the ubiquinol-cytochrome c reductase complex chaperone (BCS1L) gene [47]. It is characterized by the presence of pili torti and sensorineural hearing loss [47].
In Björnstad syndrome, pili torti develops during the first two years of life [20,21]. It usually affects exclusively the scalp hair. Eyebrows and eyelashes remain unaffected [20,21]. A positive correlation between severity of hair shaft abnormalities and hearing loss has been suggested [20].
2.2.3. Netherton Syndrome
Netherton syndrome is a rare autosomal recessive disorder characterized by a triad of symptoms: congenital ichthyosiform erythroderma, hair shaft abnormalities, and atopic diathesis [48,49]. It is caused by a mutation in the serine protease inhibitor Kazal type 5 (SPINK5) gene, which encodes lymphoepithelial Kazal-type-related inhibitor (LEKTI) [48,49].
Hair shaft abnormalities in Netherton syndrome are probably caused by disturbances in the keratinization process [50]. Pili torti is frequently observed, however trichorrhexis invaginata (“bamboo hair”) is a pathognomonic feature. Other hair shaft defects, such as trichorrhexis nodosa, trichoschisis, and trichoptilosis may also be detected [35,36]. Hair shaft abnormalities in Netherton syndrome tend to improve with age. Complete resolution may occur [51,52]. The presence of hair shaft abnormalities is one of diagnostic criteria of Netherton syndrome, which emphasizes their importance in the diagnostic process [53].
2.2.4. Bazex-Dupré-Christol Syndrome
Bazex-Dupré-Christol syndrome is an X-linked semidominant disorder characterized by follicular atrophoderma, multiple milia, hypotrichosis, hypohidrosis, and an early development of basal cell carcinomas [54,55]. It is caused by mutations in the actin-related protein T1 (ACTRT1) gene [56].
Hair shaft abnormalities may be an initial presentation of Bazex-Dupré-Christol syndrome, and may precede the development of basal cell carcinomas [57]. They are reported in 85% of cases, and include pili torti and trichorrhexis nodosa [58]. Hypotrichosis is usually diffuse in boys, whereas abnormal hair is admixed with normal hair in girls. Eyebrows may also be affected [57].
2.3. Pili Torti in Ectodermal Dysplasias
Ectodermal dysplasias include a heterogenous group of inherited disorders, characterized by congenital defects in one or more ectodermal structures and their appendages (hair, teeth, nails, and sweat glands) [59]. Pili torti has been reported in numerous ectodermal dysplasias (Table 2). The selected conditions are described below.
Table 2.
Pili torti in ectodermal dysplasias.
| Disease/Syndrome | Genetic Defect, Inheritance | Other Clinical Findings | Other Hair Shaft Abnormalities |
|---|---|---|---|
| Ankyloblepharon-ectodermal defects-cleft lip and palate syndrome [60] | TP63 gene (AD) | hypoplastic maxilla, palmoplantar hyperkeratosis, dystrophic nails, cleft lip/palate, dental anomalies, ankyloblepharon, lacrimal duct atresia, auricular abnormalities | pili canaliculi, trichoclasis, trichorrhexis nodosa, pili annulati, pili triangulati |
| Basan syndrome [61] | SMARCAD1 gene (AD) | neonatal blisters and milia, adermatoglyphia, traumatic blistering and fissuring, hypohidrosis | - |
| Cleft lip/palate-ectodermal dysplasia syndrome [25] | PVRL1 gene (AR) | mental retardation, facial dysmorphism (protruding and malformed ears, micrognathia, bilateral cleft lip/palate), syndactyly, palmoplantar keratoderma, hypohidrosis, teeth, and nail anomalies | - |
| Ectodermal dysplasia 4, hair/nail type [62] | KRT85, KRT74, HOXC13 genes (AR) | congenital nail dystrophy | - |
| Ectodermal dysplasia with corkscrew hairs [26,63] | NK (AR) | facial dysmorphism, cleft lip/palate, scalp keloids, follicular plugging, keratosis pilaris, xerosis, eczema, palmoplantar keratodermia, cutaneous syndactyly, onychodysplasia, teeth abnormalities | - |
| Ectodermal dysplasia with syndactyly [25] | PVRL-4 gene (AR) | highly arched palate, teeth abnormalities, syndactyly, hypoplastic nails, dry skin with hyperkeratosis | - |
| Ectrodactyly, ectodermal dysplasia, and cleft lip/palate syndrome 3 [64] | EEC3 gene (AD) | hearing loss, cleft lip/palate, dysplastic teeth, ectrodactyly, syndactyly, nail dystrophy, hypopigmentated skin, hyperkeratosis, skin atrophy, genitourinary anomalies | pseudomoniletrix, pili canaliculi, longitudinal grooving, trichothiodystrophy |
| Goltz syndrome [65] | PORCN gene (XD) | cleft palate, syndactyly, polydactyly, skin atrophy, telangiectasia, herniation of fat, papillomas, nail and teeth anomalies, ocular anomalies (coloboma of iris and choroid, strabismus, microphthalmia) | atrophic hair with reduced diameters, flattened hair shafts, trichorrhexis nodosa, pili trianguli et canaliculi |
| Hidrotic ectodermal dysplasia [66,67,68] | GJB6 gene (AD) | short stature, clubbed digits, palmoplantar hyperkeratosis, hyperpigmentation, nail dystrophy, cataract, photophobia, strabismus | trichorrhexis nodosa, trichoptilosis, pili bifurcati, variable diameter, damaged cuticles, irregular helical twists, pili canaliculi |
| Hypohidrotic Ectodermal Dysplasia [12] | EDA1/EDAR, EDARADD, WNT10A genes (XR, AR, AD) | facial dysmorphism (prominent forehead, thick lips, flattened nasal bridge), teeth abnormalities, hypohidrosis | trichorrhexis nodosa, pili bifurcati, variable shaft thickness |
| Hypotrichosis-osteolysis-periodontitis-palmoplantar keratoderma syndrome [25] | NK gene (AD) | onychogryphosis, acroosteolysis, linear or reticular palmoplantar keratoderma and erythematous, psoriasis-like skin lesions, periodontitis, premature teeth loss, lingua plicata, ventricular tachycardia | pili annulati |
| Oculo-dento-digital syndrome [69,70] | GJA1 gene (AD, AR) | facial dysmorphism (narrow, pinched nose, hypoplastic alae nasi, prominent columella, narrow nasal bridge), microphthalmia, microdontia, syndactyly, camptodactyly, clinodactyly, brittle nails | “tiger tail” aspect, monilethrix, pili annulati |
| Pachyonychia congenita-2 [71,72,73] | KRT17 gene (AD) | palmoplantar hyperkeratosis, nail dystrophy, hyperhidrosis, cystic lesions (steatocystoma multiplex, pilosebaceous cysts), folliculitis, natal teeth | - |
| Rapp-Hodgkin syndrome [74,75] | TP63 gene (AD) | short stature, hypohidrosis, facial dysmorphism (narrow nose, small mouth, cleft lip, hypoplastic maxilla, prominent, malformed auricles), dysplastic nails, teeth abnormalities, chronic epiphora | pili canaliculi |
| Reeds syndrome [76] | NK (AD) | lobster claw deformity, nasolacrimal obstruction, cleft lip/palate, teeth abnormalities | - |
| Salamon syndrome [77] | NK (AR) | everted lower lip, teeth abnormalities, protruding ears | - |
| Schöpf-Schulz-Passarge syndrome [78] | WNT10A gene (AR) | Palmoplantar keratoderma, nail dystrophy, hypodontia, eyelid cysts | - |
| Trichodysplasia-xeroderma [79] | NK (AD) | dry skin | trichorrhexis nodosa |
AD, autosomal dominant; AR, autosomal recessive; NK, not known; XD, X-linked dominant; XR, X-linked recessive.
2.3.1. Rapp-Hodgkin Syndrome
Rapp-Hodgkin syndrome is a form of ectodermal dysplasia inherited as an autosomal dominant trait and characterized by anhidrotic ectodermal dysplasia, cleft lip, and cleft palate [80]. It is caused by the mutations in the Tumor Protein P63 (TP63) gene encoding p63 transcription factor [81].
It was hypothesized that hypoplastic adnexal structures, hypoplastic epidermis, folliculitis, atopy, and immunological disturbances in Rapp-Hodgkin syndrome contribute to scalp dermatitis, alopecia, and hair shaft abnormalities [82]. Hair shaft abnormalities in the disease include pili torti and pili canaliculi [74,75]. Scalp hair, eyebrows, eyelashes, and other body hair are affected. Alopecia usually does not affect the occipital and temporal areas [83,84]. Pili torti is one of the most distinctive clinical features in patients with Rapp-Hodgkin syndrome. Thus, it helps to discern it from other types of ectodermal dysplasia with dental abnormalities [85].
Other features of the Rapp-Hodgkin syndrome are narrow nose, small mouth, oligodontia or anodontia, conical teeth, anonychia, hyponychia, narrow or dystrophic nails, ear and ear canal abnormalities, lacrimal duct abnormalities, and genitourinary abnormalities [86,87].
2.3.2. Ankyloblepharon-Ectodermal Defects-Cleft Lip/Palate Syndrome
Ankyloblepharon-ectodermal defects-cleft lip/palate syndrome is a form of ectodermal dysplasia inherited in an autosomal dominant fashion caused by mutations in TP63 gene [60].
Pili torti is a common finding in the disease, observed in up to 59% of cases [60]. Pili trianguli et canaliculi, and irregular indentation and shallow grooves are also commonly detected [60].
Other clinical features in ankyloblepharon-ectodermal defects-cleft lip/palate syndrome include abnormal fibrous strands of tissue that can partially or completely fuse the upper and lower eyelids (ankyloblepharon), mild to severe skin erosions, and cleft palate and/or cleft lip. Scalp erosions are considered as a main cause of morbidity in infants with ankyloblepharon-ectodermal defects-cleft lip/palate syndrome [88].
3. Acquired Pili Torti
Acquired pili torti may be associated with numerous dermatological and systemic conditions (Table 3) or may be drug-induced (Table 4).
Table 3.
Conditions associated with acquired pili torti.
| Conditions Associated with Acquired Pili Torti |
|---|
| lichen planopilaris [89]; |
| frontal fibrosing alopecia [89]; |
| alopecia areata [90]; |
| central centrifugal cicatricial alopecia [91]; |
| discoid lupus erythematosus [89,92]; |
| dissecting cellulitis [89]; |
| folliculitis decalvans [89]; |
| pseudopelade of Brocq [89]; |
| traction alopecia [93]; |
| linear scleroderma en coup de sabre [94,95,96]; |
| repetitive trauma [9]; |
| scalp metastasis of breast cancer [97]; |
| cutaneous T-cell lymphoma [98]; |
| acne conglobate [99]; |
| anorexia nervosa [100,101]; |
| graft-vs.-host disease [102]; |
| hair transplantation [9]; |
| malnutrition [103]; |
| systemic sclerosis [24]; |
| cataracts [99]. |
Table 4.
Drugs related to pili torti formation.
3.1. Pili Torti Associated with Cicatricial Alopecias
The term cicatricial alopecia corresponds to a heterogeneous group of disorders characterized by irreversible destruction of hair follicles with subsequent scarring [109]. It results in abnormal hair shaft formation with the presence of pili torti [7]. To date, data considering pili torti in cicatricial alopecias are limited. However, it may be suggested that the number of pili torti correlates with the severity and duration of inflammatory/fibrosis process.
The clinical and trichoscopic characteristics of cicatricial alopecias associated with pili torti are presented in Table 5.
Table 5.
The clinical and trichoscopic characteristic of cicatricial alopecias associated with pili torti.
| Disease | Epidemiology | Clinical Features | Trichoscopy |
|---|---|---|---|
| Lichen Planopilaris | women 40–60 years of age | multifocal, confluent areas of hair loss with perifollicular hyperkeratosis and erythema at the periphery; the vertex and the parietal area are most commonly affected |
perifollicular scaling, hair casts, perifollicular erythema, white dots, white and milky red areas, loss of follicular openings |
| Frontal Fibrosing Alopecia | post-menopausal women | recession of the frontotemporal hairline, eyebrow loss | perifollicular erythema, perifollicular scaling, hair casts, white areas, loss of follicular openings |
| Discoid Lupus Erythematosus | women 20–40 years of age | well-demarcated annular or oval plaques with follicular plugging, erythema, telangiectasia, scaling, dyspigmentation | follicular red dots, large yellow or yellow-brown dots, “red spiders on yellow dots”, scattered brown discoloration, white and milky red areas, loss of follicular openings |
| Pseudopelade of Brocq | middle-aged white women | asymptomatic, asymmetrical, white, or porcelain-white patches involving the vertex or parietal area | white dots, white and milky-red areas, loss of follicular openings, variations in hair diameter |
| Folliculitis Decalvans |
young to middle-aged adult men of African descent | tender, recurrent papulo-pustular lesions on the vertex and occipital area | hair tufts consisting of 5–20 hair surrounded by yellowish tubular scaling, starburst sign, coiled capillary loops, white and milky-red areas, loss of follicular openings |
| Dissecting Cellulitis |
young men of African descent | perifollicular pustules, painful nodules, abscesses with sinus tracts involving the vertex and occipital area | 3D yellow dots, yellow structureless areas, black dots, pinpoint-like vessels with whitish halo, white areas, loss of follicular openings |
| Central Centrifugal Cicatricial Alopecia |
middle-aged women of African descent | scarring hair loss initially involving the vertex or crown of the scalp and slowly progressing peripherally | peripilar gray/white halo, perifollicular scaling, loss of follicular openings |
| Traction Alopecia |
women and children of African descent | hair loss and thinning, pustules, inflammatory papules; may progress to scarring alopecia |
perifollicular erythema, hair thinning, focal decrease in hair density, honeycomb pattern, pinpoint white dots, irregular white patches |
| Linear Scleroderma en Coup de Sabre | Children and women within the first two decades of life | single erythematous or violaceous linear indurated plaque, progressing to hyperpigmented or hypopigmented streak on the forehead |
scattered black dots, broken hairs, short thick linear and branching tortuous vessels on the periphery of the lesion, white areas, loss of follicular openings |
3.1.1. Pili Torti Associated with Primary Cicatricial Alopecias
Lichen Planopilaris
The classic variant of lichen planopilaris is one of the most common forms of primary cicatricial alopecia [110]. The pathogenesis of the disease has not been fully elucidated [109]. However, a misdirected cellular immune response to an unknown antigen in the basement membrane zone, leading to the destruction of follicular stem cells in the bulge region of the hair follicle has mainly been suggested [109].
Pili torti is identified in 31.82–51.9% of patients with lichen planopilaris [89,111]. It is usually present in long-lasting disease [89,111]. Other characteristic trichoscopic features of lichen planopilaris are perifollicular scaling and perifollicular erythema [112].
Frontal Fibrosing Alopecia
Frontal fibrosing alopecia is considered as a variant of lichen planopilaris [113,114]. The etiology and the pathogenesis of the disease are still unknown [114]. However, the role of sexual hormones in the development of frontal fibrosing alopecia has been proposed [114].
Pili torti is observed in 71.4% of patients with frontal fibrosing alopecia [89]. Its presence has been also described in the eyebrow area [115]. Similar to the classic variant of lichen planopilaris, perifollicular erythema and perifollicular scaling are the most characteristic trichoscopic features of frontal fibrosing alopecia [116].
Discoid Lupus Erythematosus
Discoid lupus erythematosus is the most common subtype of chronic cutaneous lupus erythematosus [114].
Pili torti is present in 7.3–14.3% of cases [89,92]. Other characteristic trichoscopic findings of discoid lupus erythematosus include follicular red dots, large yellow dots, and thick arborizing vessels [2].
Pseudopelade of Brocq
Pseudopelade of Brocq is a form of lymphocytic primary scarring alopecia. There is still no clear consensus whether the disease is a distinct entity or represents the end stage of any given cicatricial scalp disorder [109,114].
Pili torti is observed in 40% of patients with pseudopelade of Brocq [89]. Other trichoscopic findings of the disease are non-specific [89,117,118,119].
Folliculitis Decalvans
Folliculitis decalvans is a form of primary neutrophilic cicatricial alopecia [114]. The frequent association with Staphylococcus aureus infections suggests that the disease is caused by an excessive inflammatory response to staphylococcal antigens [109].
Pili torti is identified in 47.1% of patients with folliculitis decalvans, regardless of the severity and stage of the disease [89,120]. Other characteristic trichoscopic features of the disease include hair tufts consisting of 5–20 hairs surrounded by yellowish tubular scaling [117].
Dissecting Cellulitis
Dissecting cellulitis is an infrequent form of primary neutrophilic cicatricial alopecia characterized by the occlusion of follicular openings [121].
Pili torti is present in 16.7% of cases [89]. The most characteristic trichoscopic findings of dissecting cellulitis are 3D (soap bubble) yellow dots [117,122].
Central Centrifugal Cicatricial Alopecia
Central centrifugal cicatricial alopecia is a form of lymphocyte-predominant cicatricial alopecia [123]. The pathogenesis of the disease has not been fully described. The role of genetic predisposition, use of chemical straighteners, traction hairstyles, bacterial or fungal infections of the scalp have been suggested [109].
Pili torti was described in a few cases of central centrifugal cicatricial alopecia [91]. The most specific trichoscopic feature of the disease is peripilar gray/white halo [124].
3.1.2. Pili Torti Associated with Secondary Cicatricial Alopecias
Traction Alopecia
Traction alopecia is a form of acquired hair loss that results from persistent, pulling forces on the hair follicles associated with traction-inducing hairstyles [125]. In its early phase, areas of non-scarring hair loss are present. Subsequently, the disease may progress to scarring alopecia [125].
Pili torti is reported in 56% of patients with traction alopecia, and is present during the scarring stage of the disease [93]. Other trichoscopic findings of traction alopecia are non-specific [93].
Linear Scleroderma en Coup de Sabre
Linear scleroderma “en coup de sabre”, a form of linear morphea, is one of the cause of secondary cicatricial alopecia.
In linear scleroderma “en coup de sabre”, diffuse distribution of pili torti is observed [95]. Moreover, trichoscopy reveals scattered black dots, broken hairs, and short thick linear and branching tortuous vessels on the periphery of the lesion [95].
3.2. Pili Torti in Non-Cicatricial Alopecias
Pili Torti in Alopecia Areata
Alopecia areata is an autoimmune form of non-scarring hair loss that may affect any hair-bearing area. It is caused by the infiltration of T helper cells, cytolytic T cells, natural killer cells, and plasmacytoid dendritic cells around the lower part of the hair bulb during the anagen phase, which induces the collapse of the hair follicle immune privilege and hair loss [126].
The pathogenesis of pili torti in alopecia areata has not been described [90]. It may be hypothesized that it results from perifollicular infiltrates that induce pressure on the epithelium and disrupt hair shaft formation. Pili torti in alopecia areata was reported in one study conducted by Park et al. [90] with the frequency 6% and 2% of patients with localized and diffuse hair loss, respectively. The most characteristic trichoscopic features of alopecia areata are exclamation mark hairs, while yellow dots and vellus hairs [2].
3.3. Pili Torti in Malignancies
Pili torti was described as the most common trichoscopic feature of the erythrodermic variants of cutaneous T-cell lymphoma present in 81% of cases [98]. It is characterized by high sensitivity and specificity of 81% and 93%, respectively [98]. It was hypothesized that in cutaneous lymphomas, pili torti results from folliculotropic inflammation without or with the mucinous degeneration of the hair follicle, which induces pressure on the epithelium, and, thus, affects hair shaft formation [98].
Moreover, one case report described a patient with scalp metastases of breast cancer, with the presence of pili torti on trichoscopic examination [97].
3.4. Drug-Induced Pili Torti
Associations between pili torti and numerous drugs were described in the literature [104,105,106,107,108].
Pili torti is most commonly described after the use of epidermal growth factor receptor inhibitors. In an animal model, it was reported that epidermal growth factor receptor inhibitors impair DNA integrity and lead to apoptosis of both keratinocytes (interfollicular epidermis, outer root sheath, and matrix) and non-proliferative, differentiated cells of the hair shaft [127]. Inhibition of epidermal growth factor receptor pathways also interferes with transcription factor expression. Thus, it impairs proper differentiation cues for hair shaft cells, resulting in loss of the medulla layer. Moreover, in mice harboring a disruption of the epidermal growth factor receptor-allele it was showed that hair follicles fail to enter catagen, and remain in an aberrant anagen state [104]. Subsequently, the thinning or loss of the outer and inner root sheaths, together with perifollicular inflammation and fibrosis are detected. In a case series of patients treated with erlotinib, histological examination of the scalp showed irregular thinning of the outer root sheath and disintegration of the inner root sheath [104].
Other drugs which may induce pili torti are oral retinoids, sodium valproate, and carbamide perhydrate.
Retinoic acid plays an important role in hair follicle formation and patterning through the regulation of the homeobox genes [128]. It was suggested that retinoids influence the keratinization of the inner root sheaths of the hair follicle in the anagen phase, which leads to pili torti formation [129].
The mechanism of pili torti formation after valproate use is unclear. It may be associated with valproate chelating properties (copper, zinc, and magnesium) as well as the inhibition of metalloproteins [130].
Carbamide peroxide is a source of hydrogen peroxide, which affects the hair shaft by oxidation and decomposition of cysteine (which accounts for 20% of the amino acids of the hair keratin) as well as the formation of cysteic acid. Moreover, carbamide peroxide destroys the majority of the disulfide bridges of hair keratin [108].
3.5. Other Secondary Causes of Pili Torti
Pili torti was also described in other systemic conditions, such as anorexia nervosa [100,101], malnutrition [103], cataracts [99], and chronic graft-vs.-host disease [102].
4. Diagnosis
The diagnosis of pili torti is based on trichoscopic and microscopic examination.
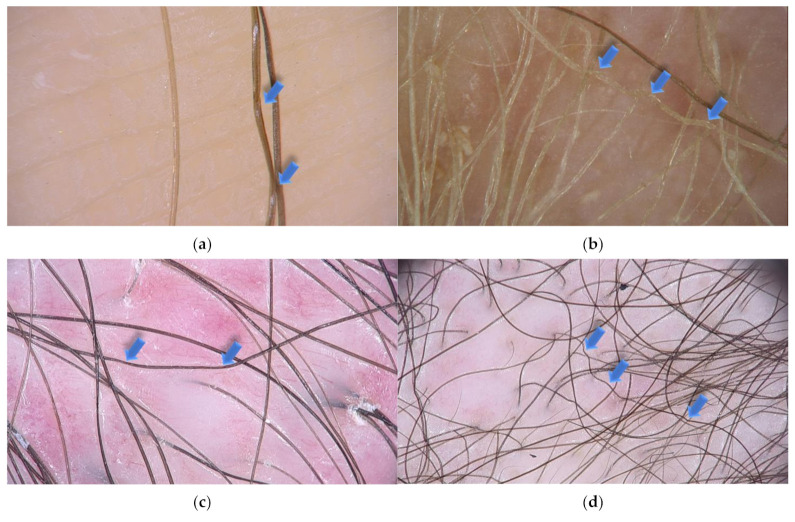
Trichoscopy, hair and scalp dermoscopy, is a rapid technique that is useful in the diagnosis of scalp and hair diseases as well as genetic disorders, including ectodermal dysplasias [10,131]. It can be performed with a manual dermoscope (10 magnification) or a videodermoscope (20–1000 magnification) [132]. This noninvasive method replaced light microscopy, which required pulling of multiple hairs for investigation. This is particularly burdensome in cases, where only few hairs might be affected [9]. In pili torti, low magnification trichoscopy reveals the hair shafts bent at different angles and at irregular intervals. Regular twists of the hair shaft along the long axis are observed at high magnification (Figure 2).
Figure 2.
Trichoscopy shows pili torti (blue arrows) in various local and systemic conditions. (a) Pili torti in patient with late onset (Beare) type with single twists of hair (×70); (b) pili torti in patient with ectodermal dysplasia with the presence of multiple twist of hair (×50); (c) pili torti in patient with lichen planopilaris. Perifollicular scaling and milky-red areas are also presented (×20); (d) numerous pili tori in patient with mycosis fungoides (×20).
Microscopic examination shows groups of three or four regularly spaced twists at irregular intervals along the shaft [10].
Genetic diseases and syndromes should be excluded in every patient with pili torti. The search of other signs of underlying conditions should be performed in the case of acquired pili torti.
5. Treatment
There is no specific treatment of pili torti. The avoidance of trauma to the hair is recommended. Other forms of management include sleeping on a satin pillowcase, avoiding excessive grooming, braiding, heat treatments, and dying. Gentle shampoos may be beneficial [104,133].
Congenital pili torti may improve spontaneously after puberty. Drug-induced cases tend to resolve after the discontinuation of the offending agent [105,106]. In regard to acquired pili torti, the treatment of the underlying condition is most important.
Efficacy of pharmacological treatment in pili torti is limited [26]. Topical minoxidil has been suggested as a beneficial therapeutic option for patients with hair shaft abnormalities with increased fragility. However, it only has an impact on hair density and does not induce a causal treatment.
6. Conclusions
Pili torti is a rare condition, which may be associated with numerous congenital or acquired conditions. In every case of pili torti, the identification of the underlying disorder determines the therapeutic approach and prognosis.
Acknowledgments
Authors are indebted to J. Taczała, for preparation of the figure of pili torti.
Author Contributions
Conceptualization, A.W.-B. and L.R.; methodology, A.W.-B., A.H. and M.G.; investigation, A.H., J.Ż. and L.B.; data curation, A.H., J.Ż. and A.R.; writing—original draft preparation, A.H., A.W.-B. and M.O.; writing—review and editing, L.R., J.Ż., L.B., M.G. and A.R.; supervision, L.R. and M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ronchese F. Twisted Hairs (Pili Torti) Arch. Dermatol. Syphilol. 1932;26:98–109. doi: 10.1001/archderm.1932.01450030101015. [DOI] [Google Scholar]
- 2.Rudnicka L., Olszewska M., Rakowska A. Atlas of Trichoscopy. 1st ed. Springer; London, UK: 2012. [Google Scholar]
- 3.Mirmirani P., Samimi S.S., Mostow E. Pili torti: Clinical findings, associated disorders, and new insights into mechanisms of hair twisting. Cutis. 2009;84:143–147. [PubMed] [Google Scholar]
- 4.Mirmirani P., Huang K.P., Price V.H. A practical, algorithmic approach to diagnosing hair shaft disorders. Int. J. Dermatol. 2011;50:1–12. doi: 10.1111/j.1365-4632.2010.04768.x. [DOI] [PubMed] [Google Scholar]
- 5.Yang J.J., Cade K.V., Rezende F.C., Pereira J.M., Pegas J.R. Clinical presentation of pili torti--Case report. An. Bras. Dermatol. 2015;90:29–31. doi: 10.1590/abd1806-4841.20153540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maruyama T., Toyoda M., Kanei A., Morohashi M. Pathogenesis in pili torti: Morphological study. J. Dermatol. Sci. 1994;7:S5–S12. doi: 10.1016/0923-1811(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 7.Whiting D.A. Hair shaft defects. In: Olsen E.A., editor. Disorders of Hair Growth: Diagnosis and Treatment. 2nd ed. McGraw Hill; New York, NY, USA: 2003. pp. 123–175. [Google Scholar]
- 8.Marubashi Y., Yanagishita T., Muto J., Taguchi N., Sugiura K., Kawamoto Y., Akiyama M., Watanabe D. Morphological analyses in fragility of pili torti with Björnstad syndrome. J. Dermatol. 2017;44:455–458. doi: 10.1111/1346-8138.13700. [DOI] [PubMed] [Google Scholar]
- 9.Rudnicka L., Olszewska M., Waśkiel A., Rakowska A. Trichoscopy in Hair Shaft Disorders. Dermatol. Clin. 2018;36:421–430. doi: 10.1016/j.det.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Rakowska A., Slowinska M., Kowalska-Oledzka E., Rudnicka L. Trichoscopy in genetic hair shaft abnormalities. J. Dermatol. Case Rep. 2008;2:14–20. doi: 10.3315/jdcr.2008.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tosti A. Dermoscopy of the Hair and Nails. 2nd ed. CRC Press; Boca Raton, FL, USA: 2016. [Google Scholar]
- 12.Rouse C., Siegfried E., Breer W., Nahass G. Hair and sweat glands in families with hypohidrotic ectodermal dysplasia: Further characterization. Arch. Dermatol. 2004;140:850–855. doi: 10.1001/archderm.140.7.850. [DOI] [PubMed] [Google Scholar]
- 13.Rogers M. Hair shaft abnormalities: Part I. Australas J. Dermatol. 1995;36:179–184. 176–185. doi: 10.1111/j.1440-0960.1995.tb00969.x. quiz. [DOI] [PubMed] [Google Scholar]
- 14.Shapira S.K., Neish A.S., Pober B.R. Unknown syndrome in sibs: Pili torti, growth delay, developmental delay, and mild neurological abnormalities. J. Med. Genet. 1992;29:509–510. [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma P., Reichert M., Lu Y., Markello T.C., Adams D.R., Steinbach P.J., Fuqua B.K., Parisi X., Kaler S.G., Vulpe C.D., et al. Biallelic HEPHL1 variants impair ferroxidase activity and cause an abnormal hair phenotype. PLoS Genet. 2019;15:e1008143. doi: 10.1371/journal.pgen.1008143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorge G., Pavone L., Polizzi A., Mauceri L., Leonardi R.M., Tripi T., Opitz J.M. Another “new” form, the palagonia type of acrofacial dysostosis in a Sicilian family. Am. J. Med. Genet. 1997;69:388–394. doi: 10.1002/(SICI)1096-8628(19970414)69:4<388::AID-AJMG10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 17.Phillips M.E., Barrie H., Cream J.J. Arginosuccinic aciduria with pili torti. J. R. Soc. Med. 1981;74:221–222. doi: 10.1177/014107688107400314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basel-Vanagaite L., Attia R., Ishida-Yamamoto A., Rainshtein L., Ben Amitai D., Lurie R., Pasmanik-Chor M., Indelman M., Zvulunov A., Saban S., et al. Autosomal recessive ichthyosis with hypotrichosis caused by a mutation in ST14, encoding type II transmembrane serine protease matriptase. Am. J. Hum. Genet. 2007;80:467–477. doi: 10.1086/512487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yung A., Newton-Bishop J.A. A case of Bazex-Dupre-Christol syndrome associated with multiple genital trichoepitheliomas. Br. J. Dermatol. 2005;153:682–684. doi: 10.1111/j.1365-2133.2005.06819.x. [DOI] [PubMed] [Google Scholar]
- 20.Richards K.A., Mancini A.J. Three members of a family with pili torti and sensorineural hearing loss: The Bjornstad syndrome. J. Am. Acad. Dermatol. 2002;46:301–303. doi: 10.1067/mjd.2002.107969. [DOI] [PubMed] [Google Scholar]
- 21.Petit A., Dontenwille M.M., Bardon C.B., Civatte J. Pili torti with congenital deafness (Bjornstad’s syndrome)--report of three cases in one family, suggesting autosomal dominant transmission. Clin. Exp. Dermatol. 1993;18:94–95. doi: 10.1111/j.1365-2230.1993.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 22.Patel H.P., Unis M.E. Pili torti in association with citrullinemia. J. Am. Acad. Dermatol. 1985;12:203–206. doi: 10.1016/S0190-9622(85)80018-9. [DOI] [PubMed] [Google Scholar]
- 23.Silengo M., Valenzise M., Pagliardini S., Spada M. Hair changes in congenital disorders of glycosylation (CDG type 1) Eur. J. Pediatric. 2003;162:114–115. doi: 10.1007/s00431-002-1054-1. [DOI] [PubMed] [Google Scholar]
- 24.Kurwa A.R., Abdel-Aziz A.H. Pili torti-congenital and acquired. Acta Dermatol. Venereol. 1973;53:385–392. [PubMed] [Google Scholar]
- 25.Hoeger P., Kinsler V., Yan A., Harper J., Oranje A., Bodemer C., Larralde M., Luk D., Mendiratta V., Purvis D. Harper’s Textbook of Pediatric Dermatology. 1st ed. Wiley-Blackwell; Oxford, UK: 2019. [Google Scholar]
- 26.McMichael A.J., Hordinsky M.K. Hair and Scalp Disorders: Medical, Surgical, and Cosmetic Treatments. 2nd ed. CRC Press; London, UK: 2018. p. 325. [Google Scholar]
- 27.Crandall B.F., Samec L., Sparkes R.S., Wright S.W. A familial syndrome of deafness, alopecia, and hypogonadism. J. Pediatric. 1973;82:461–465. doi: 10.1016/S0022-3476(73)80121-0. [DOI] [PubMed] [Google Scholar]
- 28.Rybojad M., Moraillon I., Bonafé J.L., Cambon L., Evrard P. Pilar dysplasia: An early marker of giant axonal neuropathy. Ann. Dermatol. Venereol. 1998;125:892–893. [PubMed] [Google Scholar]
- 29.Schaffer J.V., Bazzi H., Vitebsky A., Witkiewicz A., Kovich O.I., Kamino H., Shapiro L.S., Amin S.P., Orlow S.J., Christiano A.M. Mutations in the desmoglein 4 gene underlie localized autosomal recessive hypotrichosis with monilethrix hairs and congenital scalp erosions. J. Investig. Dermatol. 2006;126:1286–1291. doi: 10.1038/sj.jid.5700237. [DOI] [PubMed] [Google Scholar]
- 30.Zlotogorski A., Marek D., Horev L., Abu A., Ben-Amitai D., Gerad L., Ingber A., Frydman M., Reznik-Wolf H., Vardy D.A., et al. An autosomal recessive form of monilethrix is caused by mutations in DSG4: Clinical overlap with localized autosomal recessive hypotrichosis. J. Investig. Dermatol. 2006;126:1292–1296. doi: 10.1038/sj.jid.5700251. [DOI] [PubMed] [Google Scholar]
- 31.Lurie R., Ben-Amitai D., Laron Z. Laron syndrome (primary growth hormone insensitivity): A unique model to explore the effect of insulin-like growth factor 1 deficiency on human hair. Dermatology. 2004;208:314–318. doi: 10.1159/000077839. [DOI] [PubMed] [Google Scholar]
- 32.Spiegl B., Hundeiker M. Congenital hereditary hypotrychosis. Generalized autosomal dominant hypotrichosis with pili torti (hypotrichosis congenita hereditaria Marie Unna) Fortschr. Med. 1979;97:2018–2022. [PubMed] [Google Scholar]
- 33.Pierini A.M., Ortonne J.P., Floret D. Cutaneous manifestations of McCune-Albright syndrome: Report of a case (author’s transl) Ann. Dermatol. Venereol. 1981;108:969–976. [PubMed] [Google Scholar]
- 34.Bodemer C., Rotig A., Rustin P., Cormier V., Niaudet P., Saudubray J.M., Rabier D., Munnich A., de Prost Y. Hair and skin disorders as signs of mitochondrial disease. Pediatrics. 1999;103:428–433. doi: 10.1542/peds.103.2.428. [DOI] [PubMed] [Google Scholar]
- 35.Srinivas S.M., Hiremagalore R., Suryanarayan S., Budamakuntala L. Netherton syndrome with pili torti. Int. J. Trichol. 2013;5:225–226. doi: 10.4103/0974-7753.130424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kharge P., Shanmukhappa A., Shivaram B., Budamakuntala L. Comèl–Netherton’s syndrome in siblings. Indian J. Paediatr. Dermatol. 2016;17 doi: 10.4103/2319-7250.184332. [DOI] [Google Scholar]
- 37.Ronce N., Moizard M.P., Robb L., Toutain A., Villard L., Moraine C. A C2055T transition in exon 8 of the ATP7A gene is associated with exon skipping in an occipital horn syndrome family. Am. J. Hum. Genet. 1997;61:233–238. doi: 10.1016/S0002-9297(07)64297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mevorah B., Goldberg I., Sprecher E., Bergman R., Metzker A., Luria R., Gat A., Brenner S. Olmsted syndrome: Mutilating palmoplantar keratoderma with periorificial keratotic plaques. J. Am. Acad. Dermatol. 2005;53:S266–S272. doi: 10.1016/j.jaad.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 39.Mevorah B., Orion E., De Viragh P., Bergman R., Gat A., Legume C., Van Neste D.J.J., Brenner S. Peeling skin syndrome with hair changes. Dermatology. 1998;197:373–376. doi: 10.1159/000018034. [DOI] [PubMed] [Google Scholar]
- 40.Miteva M., Tosti A. Dermatoscopy of hair shaft disorders. J. Am. Acad. Dermatol. 2013;68:473–481. doi: 10.1016/j.jaad.2012.06.041. [DOI] [PubMed] [Google Scholar]
- 41.Pietrzak A., Bartosinska J., Filip A.A., Rakowska A., Adamczyk M., Szumilo J., Kanitakis J. Steatocystoma multiplex with hair shaft abnormalities. J. Dermatol. 2015;42:521–523. doi: 10.1111/1346-8138.12837. [DOI] [PubMed] [Google Scholar]
- 42.Goulet O., Vinson C., Roquelaure B., Brousse N., Bodemer C., Cezard J.P. Syndromic (phenotypic) diarrhea in early infancy. Orphanet J. Rare Dis. 2008;3:6. doi: 10.1186/1750-1172-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tay C.H. Ichthyosiform erythroderma, hair shaft abnormalities, and mental and growth retardation. A new recessive disorder. Arch. Dermatol. 1971;104:4–13. doi: 10.1001/archderm.1971.04000190006002. [DOI] [PubMed] [Google Scholar]
- 44.Botta E., Nardo T., Broughton B.C., Marinoni S., Lehmann A.R., Stefanini M. Analysis of mutations in the XPD gene in Italian patients with trichothiodystrophy: Site of mutation correlates with repair deficiency, but gene dosage appears to determine clinical severity. Am. J. Hum. Genet. 1998;63:1036–1048. doi: 10.1086/302063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tonnesen T., Kleijer W.J., Horn N. Incidence of Menkes disease. Hum. Genet. 1991;86:408–410. doi: 10.1007/BF00201846. [DOI] [PubMed] [Google Scholar]
- 46.Horn N., Wittung-Stafshede P. ATP7A-Regulated Enzyme Metalation and Trafficking in the Menkes Disease Puzzle. Biomedicines. 2021;9:391. doi: 10.3390/biomedicines9040391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinson J.T., Fantin V.R., Schönberger J., Breivik N., Siem G., McDonough B., Sharma P., Keogh I., Godinho R., Santos F., et al. Missense mutations in the BCS1L gene as a cause of the Björnstad syndrome. N. Engl. J. Med. 2007;356:809–819. doi: 10.1056/NEJMoa055262. [DOI] [PubMed] [Google Scholar]
- 48.Chavanas S., Bodemer C., Rochat A., Hamel-Teillac D., Ali M., Irvine A.D., Bonafe J.L., Wilkinson J., Taieb A., Barrandon Y., et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat. Genet. 2000;25:141–142. doi: 10.1038/75977. [DOI] [PubMed] [Google Scholar]
- 49.Sprecher E., Chavanas S., DiGiovanna J.J., Amin S., Nielsen K., Prendiville J.S., Silverman R., Esterly N.B., Spraker M.K., Guelig E., et al. The spectrum of pathogenic mutations in SPINK5 in 19 families with Netherton syndrome: Implications for mutation detection and first case of prenatal diagnosis. J. Investig. Dermatol. 2001;117:179–187. doi: 10.1046/j.1523-1747.2001.01389.x. [DOI] [PubMed] [Google Scholar]
- 50.Krafchik B.R., Toole J.W. What is Netherton’s syndrome? Int. J. Dermatol. 1983;22:459–462. doi: 10.1111/j.1365-4362.1983.tb02173.x. [DOI] [PubMed] [Google Scholar]
- 51.Smith D.L., Smith J.G., Wong S.W., deShazo R.D. Netherton’s syndrome: A syndrome of elevated IgE and characteristic skin and hair findings. J. Allergy Clin. Immunol. 1995;95:116–123. doi: 10.1016/S0091-6749(95)70159-1. [DOI] [PubMed] [Google Scholar]
- 52.Ancuta N., Persa G., Caius S. Netherton syndrome—A small series study. Is there a correlation between atopy manifestations and the presence of multiple hair shaft dystrophies? RoJCED. 2017;4:28–32. [Google Scholar]
- 53.Schmuth M., Martinz V., Janecke A.R., Fauth C., Schossig A., Zschocke J., Gruber R. Inherited ichthyoses/generalized Mendelian disorders of cornification. Eur. J. Hum. Genet. 2013;21:123–133. doi: 10.1038/ejhg.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bazex A., Dupre A., Christol B. Follicular atrophoderma, baso-cellular proliferations and hypotrichosis. Ann. Dermatol. Syphiligr. 1966;93:241–254. [PubMed] [Google Scholar]
- 55.Viksnins P., Berlin A. Follicular atrophoderma and basal cell carcinomas: The Bazex syndrome. Arch. Dermatol. 1977;113:948–951. doi: 10.1001/archderm.1977.01640070082013. [DOI] [PubMed] [Google Scholar]
- 56.Bal E., Park H.S., Belaid-Choucair Z., Kayserili H., Naville M., Madrange M., Chiticariu E., Hadj-Rabia S., Cagnard N., Kuonen F., et al. Mutations in ACTRT1 and its enhancer RNA elements lead to aberrant activation of Hedgehog signaling in inherited and sporadic basal cell carcinomas. Nat. Med. 2017;23:1226–1233. doi: 10.1038/nm.4368. [DOI] [PubMed] [Google Scholar]
- 57.Moreau-Cabarrot A., Bonafe J.L., Hachich N., Jalby B.C., Aubert G., Rolland M., Bazex J. Follicular atrophoderma, basal cell proliferation and hypotrichosis (Bazex-Dupre-Christol syndrome). A study in 2 families. Ann. Dermatol. Venereol. 1994;121:297–301. [PubMed] [Google Scholar]
- 58.Tiodorovic-Zivkovic D., Zalaudek I., Ferrara G., Giorgio C.M., Di Nola K., Procaccini E.M., Argenziano G. Clinical and dermatoscopic findings in Bazex-Dupre-Christol and Gorlin-Goltz syndromes. J. Am. Acad. Dermatol. 2010;63:722–724. doi: 10.1016/j.jaad.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 59.Itin P.H. Etiology and pathogenesis of ectodermal dysplasias. Am. J. Med. Genet. A. 2014;164A:2472–2477. doi: 10.1002/ajmg.a.36550. [DOI] [PubMed] [Google Scholar]
- 60.Dishop M.K., Bree A.F., Hicks M.J. Pathologic changes of skin and hair in ankyloblepharon-ectodermal defects-cleft lip/palate (AEC) syndrome. Am. J. Med. Genet. A. 2009;149a:1935–1941. doi: 10.1002/ajmg.a.32826. [DOI] [PubMed] [Google Scholar]
- 61.Campos-Domínguez M., Feito-Rodríguez M., Molina-López I., Lucas-Laguna R.D., Martínez-Glez V., Suárez-Fernández R. A newmutation of smarcad1 in a case of basan syndrome (congenital milia and lack of fingerprints); Proceedings of the 13th World Congress of Pediatric Dermatology; Chicago, IL, USA. 6–9 July 2017; p. S18. [Google Scholar]
- 62.Calzavara-Pinton P., Carlino A., Benetti A., De Panfilis G. Pili torti and onychodysplasia. Report of a previously undescribed hidrotic ectodermal dysplasia. Dermatologica. 1991;182:184–187. doi: 10.1159/000247779. [DOI] [PubMed] [Google Scholar]
- 63.Abramovits-Ackerman W., Bustos T., Simosa-Leon V., Fernandez L., Ramella M. Cutaneous findings in a new syndrome of autosomal recessive ectodermal dysplasia with corkscrew hairs. J. Am. Acad. Dermatol. 1992;27:917–921. doi: 10.1016/0190-9622(92)70287-P. [DOI] [PubMed] [Google Scholar]
- 64.Wawrzycki B., Pietrzak A., Chodorowska G., Filip A.A., Petit V., Rudnicka L., Dybiec E., Rakowska A., Sobczynska-Tomaszewska A., Kanitakis J. Ectrodactyly-ectodermal dysplasia-clefting syndrome with unusual cutaneous vitiligoid and psoriasiform lesions due to a novel single point TP63 gene mutation. Postepy Dermatol. Alergol. 2019;36:358–364. doi: 10.5114/ada.2018.73437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bree A.F., Grange D.K., Hicks M.J., Goltz R.W. Dermatologic findings of focal dermal hypoplasia (Goltz syndrome) Am. J. Med. Genet. C Semin. Med. Genet. 2016;172c:44–51. doi: 10.1002/ajmg.c.31472. [DOI] [PubMed] [Google Scholar]
- 66.Kantaputra P., Intachai W., Kawasaki K., Ohazama A., Carlson B., Quarto N., Pruksachatkun C., Chuamanochan M. Clouston syndrome with pili canaliculi, pili torti, overgrown hyponychium, onycholysis, taurodontism and absence of palmoplantar keratoderma. J. Dermatol. 2020;47:e230–e232. doi: 10.1111/1346-8138.15333. [DOI] [PubMed] [Google Scholar]
- 67.Hirano S.A., Mason A.R., Salkey K., Williams J.V., Pariser D.M. Light microscopic hair shaft analysis in ectodermal dysplasia syndromes. Pediatr Dermatol. 2012;29:414–420. doi: 10.1111/j.1525-1470.2011.01606.x. [DOI] [PubMed] [Google Scholar]
- 68.Sanches S., Rebellato P.R.O., Fabre A.B., Campos G.L.M. Do you know this syndrome? Clouston syndrome. An. Bras. Dermatol. 2017;92:417–418. doi: 10.1590/abd1806-4841.20175716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thoden C.J., Ryoppy S., Kuitunen P. Oculodentodigital dysplasia syndrome. Report of four cases. Acta Paediatr. Scand. 1977;66:635–638. doi: 10.1111/j.1651-2227.1977.tb07960.x. [DOI] [PubMed] [Google Scholar]
- 70.Adamski H., Chevrant-Breton J., Odent S., Patoux-Pibouin M., Le Marec B., Laudren A., Urvoy M. Hair shaft dysplasia in oculo-dento-digital syndrome. Report of a mother-daughter case. Ann. Dermatol. Venereol. 1994;121:694–699. [PubMed] [Google Scholar]
- 71.Leachman S.A., Kaspar R.L., Fleckman P., Florell S.R., Smith F.J., McLean W.H., Lunny D.P., Milstone L.M., van Steensel M.A., Munro C.S., et al. Clinical and pathological features of pachyonychia congenita. J. Investig. Dermatol. Symp. Proc. 2005;10:3–17. doi: 10.1111/j.1087-0024.2005.10202.x. [DOI] [PubMed] [Google Scholar]
- 72.Irvine A.D., McLean W.H. Human keratin diseases: The increasing spectrum of disease and subtlety of the phenotype-genotype correlation. Br. J. Dermatol. 1999;140:815–828. doi: 10.1046/j.1365-2133.1999.02810.x. [DOI] [PubMed] [Google Scholar]
- 73.Munro C.S. Pachyonychia congenita: Mutations and clinical presentations. Br. J. Dermatol. 2001;144:929–930. doi: 10.1046/j.1365-2133.2001.04216.x. [DOI] [PubMed] [Google Scholar]
- 74.Silengo M.C., Davi G.F., Bianco R., Costa M., DeMarco A., Verona R., Franceschini P. Distinctive hair changes (pili torti) in Rapp-Hodgkin ectodermal dysplasia syndrome. Clin. Genet. 1982;21:297–300. doi: 10.1111/j.1399-0004.1982.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 75.Salinas C.F., Montes G.M. Rapp-Hodgkin syndrome: Observations on ten cases and characteristic hair changes (pili canaliculi) Birth Defects Orig. Artic. Ser. 1988;24:149–168. [PubMed] [Google Scholar]
- 76.Reed W.B., Brown A.C., Sugarman G.I., Schlesinger L. The REEDS syndrome. Birth Defects Orig. Artic. Ser. 1975;11:61–73. [PubMed] [Google Scholar]
- 77.Giorgini S., Battini M.L., Martinelli C., Melli M.C., Farella V., Policarpi F. Salamon syndrome: A case report. Ann. Ital. Dermatol. Clin. E Sper. 1992;46:217–220. [Google Scholar]
- 78.Szepetiuk G., Vanhooteghem O., Muller G., Stene J.J., Nikkels A.F. Schöpf-Schulz-Passarge syndrome with pili torti: A new association? Eur. J. Dermatol. 2009;19:517–518. doi: 10.1684/ejd.2009.0743. [DOI] [PubMed] [Google Scholar]
- 79.Pinheiro M., Freire-Maia N. Trichodysplasia-xeroderma: An autosomal dominant condition. Clin. Genet. 1987;31:337–342. doi: 10.1111/j.1399-0004.1987.tb02818.x. [DOI] [PubMed] [Google Scholar]
- 80.Rapp R.S., Hodgkin W.E. Anhidrotic ectodermal dysplasia: Autosomal dominant inheritance with palate and lip anomalies. J. Med. Genet. 1968;5:269–272. doi: 10.1136/jmg.5.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vera-Carbonell A., Moya-Quiles M.R., Ballesta-Martinez M., Lopez-Gonzalez V., Bafalliu J.A., Guillen-Navarro E., Lopez-Exposito I. Rapp-Hodgkin syndrome and SHFM1 patients: Delineating the p63-Dlx5/Dlx6 pathway. Gene. 2012;497:292–297. doi: 10.1016/j.gene.2012.01.088. [DOI] [PubMed] [Google Scholar]
- 82.Park S.W., Yong S.L., Martinka M., Shapiro J. Rapp-Hodgkin syndrome: A review of the aspects of hair and hair color. J. Am. Acad. Dermatol. 2005;53:729–735. doi: 10.1016/j.jaad.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 83.Moerman P., Fryns J.P. Ectodermal dysplasia, Rapp-Hodgkin type in a mother and severe ectrodactyly-ectodermal dysplasia-clefting syndrome (EEC) in her child. Am. J. Med. Genet. 1996;63:479–481. doi: 10.1002/(SICI)1096-8628(19960614)63:3<479::AID-AJMG12>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 84.Breslau-Siderius E.J., Lavrijsen A.P., Otten F.W., van der Schroeff J.G., Swart J.G. The Rapp-Hodgkin syndrome. Am. J. Med. Genet. 1991;38:107–110. doi: 10.1002/ajmg.1320380124. [DOI] [PubMed] [Google Scholar]
- 85.Crawford P.J.M., Aldred M.J., Clarke A., Tso M.S.Y. Rapp-Hodgkin syndrome: An ectodermal dysplasia involving the teeth, hair, nails, and palate. Oral Surg. Oral Med. Oral Pathol. 1989;67:50–62. doi: 10.1016/0030-4220(89)90302-2. [DOI] [PubMed] [Google Scholar]
- 86.Witkop C.J., Jr., Brearley L.J., Gentry W.C., Jr. Hypoplastic enamel, onycholysis, and hypohidrosis inherited as an autosomal dominant trait. A review of ectodermal dysplasia syndromes. Oral Surg. Oral Med. Oral Pathol. 1975;39:71–86. doi: 10.1016/0030-4220(75)90398-9. [DOI] [PubMed] [Google Scholar]
- 87.Tosun G., Elbay U. Rapp-Hodgkin syndrome: Clinical and dental findings. J. Clin. Pediatric Dent. 2009;34:71–75. doi: 10.17796/jcpd.34.1.kr015833p1qg6873. [DOI] [PubMed] [Google Scholar]
- 88.Fete M., vanBokhoven H., Clements S.E., McKeon F., Roop D.R., Koster M.I., Missero C., Attardi L.D., Lombillo V.A., Ratovitski E., et al. International Research Symposium on Ankyloblepharon-Ectodermal Defects-Cleft Lip/Palate (AEC) syndrome. Am. J. Med. Genet. A. 2009;149A:1885–1893. doi: 10.1002/ajmg.a.32761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karadag Kose O., Gulec A.T. Evaluation of a Handheld Dermatoscope in Clinical Diagnosis of Primary Cicatricial Alopecias. Dermatol. Ther. 2019;9:525–535. doi: 10.1007/s13555-019-0304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park J., Kim J.I., Kim H.U., Yun S.K., Kim S.J. Trichoscopic Findings of Hair Loss in Koreans. Ann. Dermatol. 2015;27:539–550. doi: 10.5021/ad.2015.27.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaur A., Batrani M., Kubba A., Kubba R. Central centrifugal cicatricial alopecia in Asian scalp: Beyond boundaries and race; Proceedings of the American Academy of Dermatology 2019 Annual Meeting; Washington, DC, USA. 1–5 March 2019; p. AB179. [Google Scholar]
- 92.Gomez-Quispe H., Elena de Las Heras-Alonso M., Lobato-Berezo A., Velasco-Tamariz V., Pindado-Ortega C., Moreno-Arrones O.M., Vano-Galvan S., Saceda-Corralo D. Trichoscopic findings of discoid lupus erythematosus alopecia: A cross-sectional study. J. Am. Acad. Dermatol. 2021;84:804–806. doi: 10.1016/j.jaad.2020.05.144. [DOI] [PubMed] [Google Scholar]
- 93.Karadağ Köse Ö., Borlu M. Evaluation of trichoscopic findings of tractional alopecia. Türkiye Klin. Derm. Derg. 2019;29:7–14. doi: 10.5336/dermato.2019-64693. [DOI] [Google Scholar]
- 94.Montoya C.L., Calvache N. Linear Morphea Alopecia: New Trichoscopy Findings. Int. J. Trichol. 2017;9:92–93. doi: 10.4103/ijt.ijt_34_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saceda-Corralo D., Tosti A. Trichoscopic Features of Linear Morphea on the Scalp. Ski. Appendage Disord. 2018;4:31–33. doi: 10.1159/000478022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Svigos K., Criscito M., Marji J., Brinster N.K., Lo Sicco K. Linear morphea with evidence of hair regrowth; Proceedings of the SID 2020 Annual Meeting; Virtual Meeting. 13–16 May 2020; p. S9. [Google Scholar]
- 97.Gajda P., Rakowska A., Czuwara J., Samochocki Z. Ogniska łysienia jako pierwszy objaw wznowy raka sutka? Dermatol. Rev. 2018;105:682–683. doi: 10.5114/dr.2018.79515. [DOI] [Google Scholar]
- 98.Rakowska A., Jasińska M., Sikora M., Czuwara J., Gajda-Mróz P., Warszawik-Hendzel O., Kwiatkowska M., Waśkiel-Burnat A., Olszewska M., Rudnicka L. Cutaneous T-cell lymphoma in erythrodermic cases may be suspected on the basis of scalp examination with dermoscopy. Sci. Rep. 2021;11:282. doi: 10.1038/s41598-020-78233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gold S.C., Delaney T.J. Familial acne conglobata, hidradenitis suppurativa, pili torti and cataracts*. Br. J. Dermatol. 1974;91:54–57. doi: 10.1111/j.1365-2133.1974.tb12514.x. [DOI] [Google Scholar]
- 100.Lurie R., Danziger Y., Kaplan Y., Sulkes J., Abramson E., Mimouni M. Acquired pili torti—A structural hair shaft defect in anorexia nervosa. Cutis. 1996;57:151–156. [PubMed] [Google Scholar]
- 101.Strumia R., Borghi A., Colombo E., Manzato E., Gualandi M. Low prevalence of twisted hair in anorexia nervosa. Clin. Exp. Dermatol. 2005;30:349–350. doi: 10.1111/j.1365-2230.2005.01745.x. [DOI] [PubMed] [Google Scholar]
- 102.Penzi L.R., Saavedra A., Senna M.M. Long-standing pili torti in 2 patients with chronic graft-vs.-host disease. Jaad Case Rep. 2018;4:44–46. doi: 10.1016/j.jdcr.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Evans J.B., Hastings J.G., Kaffenberger B.H. Acquired Pili Torti. Jama Dermatol. 2019;155:488. doi: 10.1001/jamadermatol.2018.4677. [DOI] [PubMed] [Google Scholar]
- 104.Kremer N., Martinez H., Leshem Y.A., Hodak E., Zer A., Brenner B., Amitay-Laish I. The trichoscopic features of hair shaft anomalies induced by epidermal growth factor receptor inhibitors: A case series. J. Am. Acad. Dermatol. 2020 doi: 10.1016/j.jaad.2020.03.055. (in press) [DOI] [PubMed] [Google Scholar]
- 105.Pirmez R., Piñeiro-Maceira J., Gonzalez C.G., Miteva M. Loose Anchoring of Anagen Hairs and Pili Torti due to Erlotinib. Int. J. Trichol. 2016;8:186–187. doi: 10.4103/ijt.ijt_16_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hays S.B., Camisa C. Acquired pili torti in two patients treated with synthetic retinoids. Cutis. 1985;35:466–468. [PubMed] [Google Scholar]
- 107.Caneppele S., Mazereeuw-Hautier J., Bonafé J.L. Sodium valproate-induced kinky hair syndrome. Ann. Dermatol. Venereol. 2001;128:134–135. [PubMed] [Google Scholar]
- 108.Bolck F., Ziegler V., Sieler H. Bleaching of hair by carbamide perhydrate. Contact Dermat. 1977;3:214–215. doi: 10.1111/j.1600-0536.1977.tb03655.x. [DOI] [PubMed] [Google Scholar]
- 109.Kanti V., Röwert-Huber J., Vogt A., Blume-Peytavi U. Cicatricial alopecia. Jddg J. Dtsch. Dermatol. Ges. 2018;16:435–461. doi: 10.1111/ddg.13498. [DOI] [PubMed] [Google Scholar]
- 110.Errichetti E., Figini M., Croatto M., Stinco G. Therapeutic management of classic lichen planopilaris: A systematic review. Clin. Cosmet. Investig. Dermatol. 2018;11:91–102. doi: 10.2147/CCID.S137870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eftekhari H., Azimi S.Z., Rafiei R., Darjani A., Alizadeh N., Rafiei E., Ghadarjani R., Gharaei Nejad K. Dermoscopic features of lichen planopilaris in Northern Iran: A prospective observational study. Int. J. Dermatol. 2019;58:1406–1414. doi: 10.1111/ijd.14589. [DOI] [PubMed] [Google Scholar]
- 112.Waśkiel A., Rakowska A., Sikora M., Olszewska M., Rudnicka L. Obraz trichoskopowy liszaja płaskiego mieszkowego. Dermatol. Rev. 2018;105:63–75. doi: 10.5114/dr.2018.74167. [DOI] [Google Scholar]
- 113.Kossard S. Postmenopausal frontal fibrosing alopecia. Scarring alopecia in a pattern distribution. Arch. Dermatol. 1994;130:770–774. doi: 10.1001/archderm.1994.01690060100013. [DOI] [PubMed] [Google Scholar]
- 114.Fanti P.A., Baraldi C., Misciali C., Piraccini B.M. Cicatricial alopecia. G Ital. Dermatol. Venereol. 2018;153:230–242. doi: 10.23736/s0392-0488.18.05889-3. [DOI] [PubMed] [Google Scholar]
- 115.Ferrari B., Vincenzi C., Tosti A. Pili Torti as a Sign of Eyebrow Involvement in Frontal Fibrosing Alopecia. Ski. Appendage Disord. 2019;5:393–395. doi: 10.1159/000502059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Inui S., Nakajima T., Shono F., Itami S. Dermoscopic findings in frontal fibrosing alopecia: Report of four cases. Int. J. Dermatol. 2008;47:796–799. doi: 10.1111/j.1365-4632.2008.03681.x. [DOI] [PubMed] [Google Scholar]
- 117.Rudnicka L., Olszewska M., Rakowska A., Slowinska M. Trichoscopy update 2011. J. Dermatol. Case Rep. 2011;5:82–88. doi: 10.3315/jdcr.2011.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qi S., Zhao Y., Zhang X., Li S., Cao H., Zhang X. Clinical features of primary cicatricial alopecia in Chinese patients. Indian J. Dermatol. Venereol. Leprol. 2014;80:306–312. doi: 10.4103/0378-6323.136833. [DOI] [PubMed] [Google Scholar]
- 119.Mathur M., Acharya P. Trichoscopy of primary cicatricial alopecias: An updated review. J. Eur. Acad. Dermatol. Venereol. 2020;34:473–484. doi: 10.1111/jdv.15974. [DOI] [PubMed] [Google Scholar]
- 120.Saceda-Corralo D., Moreno-Arrones O.M., Rodrigues-Barata R., Rubio-Lombrana M., Mir-Bonafe J.F., Morales-Raya C., Miguel-Gomez L., Hermosa-Gelbard A., Jaen-Olasolo P., Vano-Galvan S. Trichoscopy activity scale for folliculitis decalvans. J. Eur. Acad. Dermatol. Venereol. 2020;34:e55–e57. doi: 10.1111/jdv.15900. [DOI] [PubMed] [Google Scholar]
- 121.Segurado-Miravalles G., Camacho-Martinez F.M., Arias-Santiago S., Serrano-Falcon C., Serrano-Ortega S., Rodrigues-Barata R., Jaen Olasolo P., Vano-Galvan S. Epidemiology, clinical presentation and therapeutic approach in a multicentre series of dissecting cellulitis of the scalp. J. Eur. Acad. Dermatol. Venereol. 2017;31:e199–e200. doi: 10.1111/jdv.13948. [DOI] [PubMed] [Google Scholar]
- 122.Abedini R., Kamyab Hesari K., Daneshpazhooh M., Ansari M.S., Tohidinik H.R., Ansari M. Validity of trichoscopy in the diagnosis of primary cicatricial alopecias. Int. J. Dermatol. 2016;55:1106–1114. doi: 10.1111/ijd.13304. [DOI] [PubMed] [Google Scholar]
- 123.Ogunleye T.A., McMichael A., Olsen E.A. Central centrifugal cicatricial alopecia: What has been achieved, current clues for future research. Dermatol. Clin. 2014;32:173–181. doi: 10.1016/j.det.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 124.Herskovitz I., Miteva M. Central centrifugal cicatricial alopecia:Challenges and solutions. Clin. Cosmet. Investig. Dermatol. 2016;9:175–181. doi: 10.2147/CCID.S100816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Munoz Moreno-Arrones O., Vano-Galvan S. Bitemporal hair loss related to traction alopecia. Dermatol. Online J. 2016;22:16. doi: 10.5070/D3229032511. [DOI] [PubMed] [Google Scholar]
- 126.Simakou T., Butcher J.P., Reid S., Henriquez F.L. Alopecia areata: A multifactorial autoimmune condition. J. Autoimmun. 2019;98:74–85. doi: 10.1016/j.jaut.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 127.Amberg N., Sotiropoulou P.A., Heller G., Lichtenberger B.M., Holcmann M., Camurdanoglu B., Baykuscheva-Gentscheva T., Blanpain C., Sibilia M. EGFR Controls Hair Shaft Differentiation in a p53-Independent Manner. iScience. 2019;15:243–256. doi: 10.1016/j.isci.2019.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Duverger O., Morasso M.I. Role of homeobox genes in the patterning, specification, and differentiation of ectodermal appendages in mammals. J. Cell. Physiol. 2008;216:337–346. doi: 10.1002/jcp.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alting K., van Hunsel F. Curling of Hair in Two Female Patients Taking Alitretinoin. Drug Saf. Case Rep. 2018;5:26. doi: 10.1007/s40800-018-0092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yasemin G. Curly Hair Induced by Valproate in Bipolar Disorder. Clin. Psychopharmacol. Neurosci. 2016;14:114. doi: 10.9758/cpn.2016.14.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rakowska A., Górska R., Rudnicka L., Zadurska M. Trichoscopic Hair Evaluation in Patients with Ectodermal Dysplasia. J. Pediatric. 2015;167:193–195. doi: 10.1016/j.jpeds.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 132.Waśkiel A., Rakowska A., Sikora M., Olszewska M., Rudnicka L. Trichoscopy of alopecia areata: An update. J. Dermatol. 2018;45:692–700. doi: 10.1111/1346-8138.14283. [DOI] [PubMed] [Google Scholar]
- 133.Gelles L.N. Picture of the month. Pili torti. Arch. Pediatric Adolesc. Med. 1999;153:647–648. doi: 10.1001/archpedi.153.6.647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.