Abstract
Purpose
Dental evaluation and management prior to hematopoietic stem cell transplant (HSCT) plays a vital role in identifying and treating infections that may be life-threatening. The purpose of this study is to describe the dental management of patients undergoing pre-HSCT examination with the Dental Service at Memorial Sloan-Kettering Cancer Center (MSKCC) and to report on odontogenic complications.
Methods
Patients referred for evaluation as part of the standard preparation for HSCT were included. Following clinical and radiological examination, patients were assigned to one of three groups based on risk of odontogenic infection and treatment was provided as indicated. Patients were followed, and their medical records were reviewed for odontogenic complications during the transplant admission.
Results
Of the 375 patients evaluated, 350 patients underwent HSCT: Allogeneic 143 (40.9%), Autologous 207 (59.1%). The distribution of primary cancer diagnosis was as follows: multiple myeloma 104 (29.7%), leukemias 95 (27.1%), Hodgkin’s lymphoma 28 (8.0%), Non-Hodgkin’s Lymphoma 99 (28.3%), and other conditions 24 (6.9%). The median time from dental evaluation to transplant was 29 days. The median Decayed, Missing, Filled Teeth Index was 17. The median Community Periodontal Index was 1. Based on dental status, 145 patients (41.4%) were classified as low-risk, 133 (38%) as moderate-risk and 72 (20.6%) as high-risk of odontogenic infection. 114 patients (32.6%) required dental treatment prior to HSCT and 100 of these (28.6%) completed treatment. 2 (0.57%) patients had odontogenic complications.
Conclusions
With conservative pre-HSCT dental treatment based on an infection risk classification system, a low odontogenic complication rate was observed.
Keywords: Hematopoietic stem cell transplant, dental evaluation and management, oral infection, oral complications, supportive care, immunosuppression
Introduction
Hematopoietic stem cell transplant (HSCT) is widely used in the management of a variety of malignant and non-malignant conditions for the purpose of immune system reconstitution following myeloablation and immunosuppression secondary to cytotoxic chemotherapy conditioning regimens. These regimens, in addition to the underlying disease and the transplant itself, can render patients severely immunocompromised prior to, during and for some time after HSCT [1,2]. Technological advances have expanded the use of HSCT, with more than 50,000 transplants performed annually worldwide[3].
The oral cavity is a known reservoir of bacteria with pathogenic potential in the immunocompromised host [4]. Patients treated with high dose chemotherapy frequently develop oral mucositis and dryness, which, coupled with neutropenia, can increase the susceptibility to local infection. Increased susceptibility to local infection is associated with the potential for systemic spread, which can endanger and complicate the course of patients’ medical treatments [5,6]. Although not common, odontogenic infections can become severe enough to contribute to significant morbidity and sometimes mortality of patients [7–9]. Poor dental health has been shown to increase the risk of bacteremia from oral sources in HSCT recipients [10]. The National Cancer Institute recommends that “dental foci are potential sources of systemic infections that need to be eliminated or ameliorated before commencement of anticancer therapy” [8] and the Centers for Disease Control and Prevention recommend that “dental consults be obtained for all HSCT candidates to assess their state of oral health, and perform any needed dental procedures to decrease the risk of oral infections after transplant” [11]. More recently, the joint task force of the Multinational Association for Supportive Care in Cancer with the International Society of Oral Oncology and the European Society for Blood and Marrow Transplantation have recommended that it is the standard of care for patients to receive a comprehensive dental and oral evaluation prior to undergoing high-dose chemotherapy / HSCT, in order to eliminate both odontogenic and non-odontogenic sources of infection. A thorough oral evaluation with appropriate treatment can further serve the purpose of ensuring oral health and function and minimizing any oral symptoms, which ultimately contributes to improvement of quality of life [9,6].
Lack of a formal detailed consensus as to the extent of necessary pre-HSCT dental treatment to avoid odontogenic complications has led to treatment strategies ranging from limited conservative treatment to aggressive dental treatment [8,9]. This can result in some confusion for dental and medical practitioners when treatment planning the pre-HSCT patient: under-treatment of odontogenic disease can potentially result in infections complicating the course of HSCT, while over-treatment of dental conditions can result in a delay of necessary medical treatment and a potential for dental treatment-related morbidity. In this study, we propose a simple classification system based on patients’ overall burden of dental disease, which classifies patients into one of three risk categories (Table 1). With the use of this system, we aim to facilitate stratification of treatment needs and enhance communication among treating practitioners, thereby efficiently optimizing patients in preparation for HSCT.
Table 1:
Proposed risk classification based on dental status
| Class I – Low Risk | Class II – Moderate Risk | Class III – High Risk | |
|---|---|---|---|
| Caries |
Evaluation • No caries • Incipient caries – enamel or early dentin lesions AND • Asymptomatic |
Evaluation • Dentinal caries with no pulp involvement AND • Asymptomatic • No periapical radiolucency |
Evaluation • Gross caries near pulp/pulpal involvement AND • Symptomatic • +/− Periapical radiolucency |
|
Treatment • No treatment or observe |
Treatment • Restore or observe |
Treatment • Endodontic therapy • Extraction |
|
| Periapical Status |
Evaluation • No periapical radiolucency • Asymptomatic |
Evaluation • Periapical radiolucency • Asymptomatic |
Evaluation • Periapical radiolucency • Symptomatic |
|
Treatment • No treatment |
Treatment • Based on case by case clinical judgment |
Treatment • Endodontic therapy • Extraction |
|
| Third Molar Status |
Evaluation • Extracted or erupted AND • Asymptomatic |
Evaluation • Partially erupted, unerupted or impacted AND • Asymptomatic |
Evaluation • Partially erupted, unerupted or impacted AND • Symptomatic |
|
Treatment • No treatment |
Treatment • Prophylaxis • Extra soft brushes • Irrigation syringes |
Treatment • Extraction • Local treatment – Prophylaxis, extra soft brushes, Chlorhexidine 0.12% irrigation, systemic antibiotic operculectomy |
|
| Periodontal Status |
Evaluation • Oral hygiene excellent or good • No or mild gingivitis • No or mild periodontitis • Asymptomatic |
Evaluation • Oral hygiene fair • Mild or moderate gingivitis • Moderate periodontitis • Asymptomatic |
Evaluation • Oral hygiene poor • Severe gingivitis • Advanced periodontitis • Symptomatic |
|
Treatment • No treatment or prophylaxis • Extra soft brushes |
Treatment • Prophylaxis • Extra soft brushes |
Treatment • Extraction • Prophylaxis / SRP • Extra soft brushes |
(SRP: Scaling and root planing)
Materials and Methods
This prospective study was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board and was conducted in line with the principles of the Declaration of Helsinki. Patients referred to the Dental Service at Memorial Sloan Kettering Cancer Center (MSKCC) for oral evaluation as part of the standard preparation for HSCT were included in this study.
Patients underwent a standard radiographic examination consisting of a digital panoramic radiograph. When indicated, selected digital periapical radiographs were taken to further evaluate third molar status, osseous pathology, periapical pathologies, existing endodontic therapies, periodontal attachment levels and caries.
The clinical intraoral examination consisted of three standard components [12]:
An examination of the oral and oropharyngeal mucosa.
A periodontal examination including periodontal charting of clinical attachment levels, furcation involvements, tooth mobility and notation of bleeding, plaque and calculus.
A hard tissue examination charting the number of decayed, missing and filled teeth.
Plaque accumulation was recorded using the Simplified Oral Hygiene Index (OHI-S) and dental status was recorded using the Decayed, Missing, Filled Teeth (DMFT) index. The World Health Organization Community Periodontal Index (CPI) was used to assess the presence/absence of active periodontal disease [12]. The CPI is calculated from the following clinical measurements:
Probing pocket measurements performed with a standard periodontal probe measuring the depth in millimeters of the periodontal pocket (distance from the free gingival margin to the periodontal attachment apparatus or base of the gingival sulcus or periodontal pocket).
Bleeding on probing of the gingiva recorded as present or absent.
Calculus recorded as present or absent.
Presence of acute odontogenic infection was recorded according to the observed signs of infection: pain, swelling, erythema, increased temperature, and impaired function in the area of infection.
Based on the clinical and radiographic evaluation, patients were assigned to one of three risk categories with corresponding treatment recommendations: low risk / no treatment necessary, moderate risk / treat or observe depending on medical status and time to transplant, and high risk / treatment necessary prior to transplant (Table 1). Patients were treated with caries control, endodontic therapy, periodontal therapy (dental prophylaxis and scaling and root planing) and tooth extraction as indicated.
Following the pre-HSCT oral evaluation and completion of any necessary dental treatment, patients who completed HSCT were followed during their admission for any clinical evidence of odontogenic complication in the form of acute odontogenic infection. Data was obtained from the patients’ medical records and by examination when requested by transplant team.
Results
A total of 375 patients presented to the Dental Service for pre-HSCT evaluation during a 2-year period; 350 of these patients subsequently underwent HSCT and were included in this study. Of these patients 207 (59.1%) underwent autologous HSCT and 143 (40.9%) underwent allogeneic HSCT. See Table 2 for disease distribution and patient characteristics.
Table 2:
Patient characteristics
| Total number of patients who completed HSCT | 350 |
| Age (median) | 54 (range 8-75) |
| Sex (male/female) | 207 (59.1%) / 143 (40.9%) |
| Primary disease - Multiple Myeloma - Non-Hodgkin’s Lymphoma - Hodgkin’s Lymphoma - Leukemia - Other |
104 (29.7%) 99 (28.3%) 28 (8.0%) 95 (27.1%) 24 (6.9%) |
| Transplant type - Allogeneic - Autoloaous |
143 (40.9%) 207 (59.1%) |
(HSCT: Hematopoietic stem cell transplant)
The median time between dental evaluation and admission for HSCT was 29 days with a range of 4-301 days. 145 patients (41.4%) were classified as dental risk class I, 133 (38.0%) as dental risk class II and 72 (20.6%) as dental risk class III. The median DMFT was 17. Advanced dental caries was determined to be present in 67 patients (19.1%) and 46 of these patients (68.7%) were treated with dental restorations prior to HSCT. 68 patients (19.4%) were determined to have periapical pathology and 29 of these patients (42.6%) were treated with extraction or endodontics prior to HSCT. Of 54 patients (15.4%) with impacted third molars, only one was treated with laser operculectomy prior to HSCT. With regards to clinical periodontal status, the median plaque index was 0.83, the median calculus index was 0 and the median Community Periodontal index was 1. Radiographic analysis of attachment levels showed 221 patients (63.1%) with normal attachment levels, 69 patients (19.7%) with mild attachment loss, 45 patients (12.9%) with moderate attachment loss and 15 patients (4.3%) with severe attachment loss. Of the 60 patients with moderate or severe marginal periodontitis, 24 (40.0%) received treatment prior to HSCT. Overall, 114 patients (32.6%) required / were recommended to have dental treatment prior to HSCT (extraction, restoration, endodontic therapy, periodontal therapy) and 100 (28.6%) completed treatment at MSKCC or were referred to a local dentist for treatment. The dental characteristics are displayed in Table 3.
Table 3:
Dental characteristics
| Observed (n=350) | Treated (n=100) | |
|---|---|---|
| Median time from dental evaluation to transplant admission | 29 days (range: 4-301 days) | |
| Dental risk classification - I - II - III |
145 (41.4%) 133 (38.0%) 72 (20.6%) |
|
| DMFT (median) | 17 (range 0-32) | |
| Advanced caries | 67 (19.1%) | 46/67 (68.7%) |
| Periapical disease - Post-endodontic |
68 (19.4%) - 31/68 (45.6%) |
29/68 (42.6%) |
| Third molars - Impacted |
54 (15.4%) |
1 (2.9%) |
| Periodontal status - Clinical periodontal status - OHI-S: PI (median) CI (median) - CPI (median) - Radiographic periodontal attachment loss: Normal Mild Moderate Severe |
0.83 (range 0-2.66) 0 (range 0-2.66) 1 (range 0-4) 221 (63.1%) 69 (19.7%) 45 (12.9%) 15 (4.3%) |
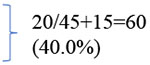
|
(DMFT: Decayed Missing Filled Teeth; OHI-S: Simplified Oral Hygiene Index; PI: Plaque index; CI: Calculus index; CPI: Community Periodontal Index)
Two patients (0.57%) developed odontogenic complications during their transplant admissions. Both patients had been classified as dental risk class II prior to transplant and were not treated due to lack of symptoms. Both complications involved endodontically treated teeth with periapical radiolucencies that developed acute apical abscesses, and both resolved with antibiotic therapy. See Figure 1.
Figure 1:
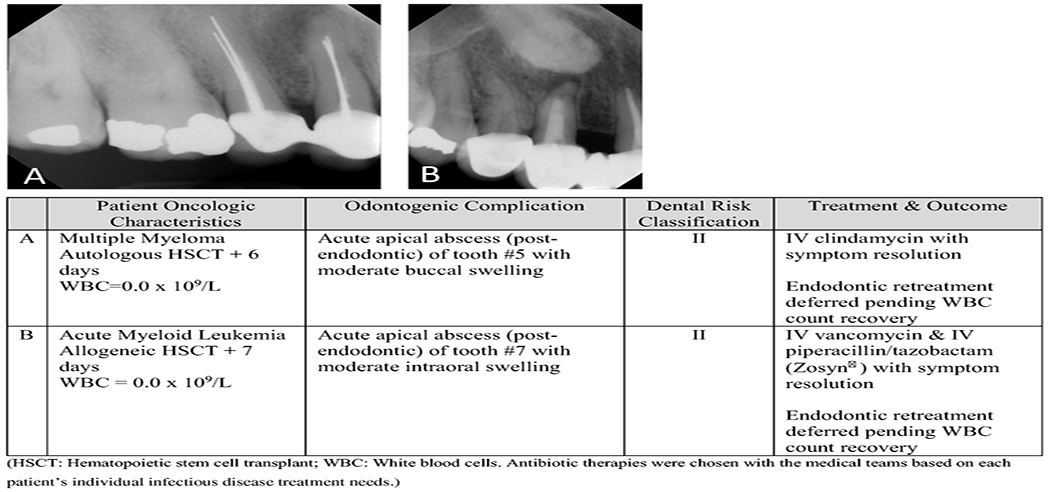
Odontogenic complications
Discussion
Immunosuppression before, during and after HSCT can be severe and, depending on the underlying disease and the treatment regimen, long lasting. In patients who undergo allogeneic transplant, factors like stem cell source, histocompatibility antigen (HLA) matching and graft versus host disease (GVHD) can prolong immunosuppression and thereby the period where it is difficult for patients to receive routine dental care. Local oral factors such as hyposalivation, dysgeusia, oral pain and oral GVHD, all common oral complications of cancer therapy, can make maintaining dental health in the setting of prolonged immunosuppression challenging. These considerations add importance to the provision of adequate pre-HSCT dental evaluation and treatment, as this have the potential to not only prevent oral complications during transplant, but also after.
Access to oral care can be a barrier that influences the timely delivery of pre-HSCT dental treatment [13–15], and delivering adequate dental treatment in preparation for HSCT despite time constraints and in the setting of cytopenias can be challenging for the dental practitioner. Ideally, treatment of all dental pathology would be completed prior to transplant, but this is often not possible. Knowledge of oncologic diseases, treatment modalities, and their toxicities is essential, as is communication with the medical team to determine proper timing and necessity of dental treatment prior to HSCT. To simplify communication, Akashi et al proposed a myelosuppression grading scale as a tool to facilitate communication of the myelosuppression status of patients undergoing chemotherapy [16]. It is widely accepted that at least two weeks should be allowed between surgical dental treatment and chemotherapy administration to allow for soft tissue healing [17]. In our study, the median time between dental evaluation and transplant admission was 29 days, which, for most patients, is sufficient time to complete the recommended pre-HSCT dental treatment. A specialized dental clinic within a cancer center can improve access to care and allow for easy communication with the medical specialties, thereby facilitating efficient, timely, and medically appropriate dental treatment.
Several studies have attempted to clarify the relationship between dental disease and infectious complications during transplant. Some authors have hypothesized that a less aggressive approach to eliminating dental disease is acceptable, with no adverse effects on HSCT outcomes. They have supported minimal intervention protocols based on low complication rates and a low probability of infection. Toljanic et al reported on forty-eight patients observed pre- and post-chemotherapy with an odontogenic infection rate of 4% [18]. Melkos et al reported on fifty-eight patients with no significant difference in infection rate between treatment and non-treatment groups [19]. Yamagata et al discussed forty-one patients evaluated and treated conservatively pre-HSCT with no peri-HSCT complications [20] and Peters et al looked at twenty-three postendodontic periapical radiolucencies with no difference in transplant outcomes between treated and non-treated groups, suggesting that there is no need to treat asymptomatic lesions [21]. Schuurhuis et al studied 64 patients undergoing intensive chemotherapy for various hematological malignancies and concluded that their low complication rate of 4% supports their hypothesis that oral foci of infection can be left untreated without an increase in infectious complications during intensive chemotherapy [22]. Recently, Sultan et al published on the risk of bacteremia from oral sources in AML patients and found that oral health status did not increase the risk of bacteremia during chemotherapy or allogeneic HSCT [23].
Somewhat in contrast to these studies, Elad et al, in a decision analysis to determine the effect of dental treatment prior to chemotherapy on patient survival, recommends that dental treatment prior to chemotherapy is the preferred strategy based on data showing that 1.8 of every 1000 HSCT patients will die secondary to oral infection [9]. This finding suggests that minimal intervention may not always be adequate to control oral complications during immunosuppression. Elad [24] and other authors [25,15] have also reported that there is typically a heavy burden of dental disease in the HSCT patient population and our findings support this with a median DMFT of 17 and the majority of our patients classified as moderate or high risk based on their dental status. Few studies have looked specifically at DMFT and transplant outcomes; Ertas et al found that DMFT scores increased after HSCT [26] and Dobr et al found an association between caries and certain HLA types [27].
Studies evaluating periodontal disease in HSCT patients have supported a link between periodontal and systemic infection and recommend periodontal treatment prior to HSCT [28,29]. Akintoye et al however, found no association between radiographic attachment loss and septicemia in HSCT patients [30]. In our cohort we saw no complications related to marginal periodontitis, even with conservative periodontal treatment numbers.
Two studies have looked at partially impacted third molars during HSCT; Yamagata et al had no complications in 35 patients treated based on symptoms [31] and Öhman et al reported only local infections in a group of 22 patients [32].
In this study, we employ a risk classification system based on the clinical severity of overall dental disease and the correlating risk of development of acute infection. Through the use of this system we have found that we are able to efficiently treatment plan and manage dental conditions in preparation for HSCT. Our classification system supports a conservative treatment approach, individualized to each patient, and based on the estimated risk of acute odontogenic infection, and we feel that our low complication rate of 0.57% supports this strategy. The complications that developed in our cohort consisted of two teeth with existing endodontic therapy, that became acutely infected during transplant conditioning. Both patients were febrile, bacteremic and were managed successfully with the administration of broad-spectrum antibiotics. The two patients were classified pre-HSCT as moderate risk (class II) making the rate of complication in this group 1.5%. Class I and III had a 0% complication rate in our cohort, which is not surprising given the nature of odontogenic disease in these groups: no / mild disease with low risk of infection and no treatment necessary, and severe disease with high risk of infection and treatment necessary. The two complications in our cohort suggest that one cannot completely rely on clinical symptoms or radiographic presentation alone to predict the pathogenicity of periapical lesions, and the distribution of complications within the groups may suggest that attention should be given to asymptomatic odontogenic disease, especially periapical disease, if time and patient condition permit.
A limitation of this clinical study is the lack of long term dental follow up after transplant. MSKCC is a tertiary referral center with patients coming for treatment sometimes from long distances. For this reason, most patients see their general dentists for post-HSCT dental care and follow up for this study was therefore limited to the period of hospital admission. This follow up was in turn limited to consultations requested by the medical teams and medical record review, and for this reason there may have been an underreporting of odontogenic complications. Furthermore, in the severely neutropenic patient, the signs and symptoms of inflammation and infection can present atypically, and oral or odontogenic infection may therefore be missed. A future study might look at untreated dental pathologies to determine whether there is an association between these and systemic signs of infection such as fever, bacteremia or other infectious complications.
Our study of 350 patients is a large cohort including patients with different primary oncologic diseases. Patients were treated with different chemotherapy and transplant regimens, and prophylactic regimens for infections would therefore also vary. It is therefore difficult to differentiate between odontogenic risk in specific disease populations, however our low complication rate supports the importance of a thorough dental evaluation in preventing odontogenic complications for all HSCT patients. A future study direction would be to investigate individual risk prediction based on odontogenic disease characteristics and oncologic disease and treatment related factors such as primary disease, transplant type, conditioning regimen toxicity / degree of myelosuppression, and risk of or presence of GVHD.
Conclusion
Involvement of dental providers with specialty knowledge within the field of Oral Oncology can improve access to and coordination of dental and oral care to ensure timely and appropriate pre-HSCT dental treatment. Dental treatment planning of the pre-HSCT patient can be complicated by time constraints and the medical complexity of the patients, and a system to help guide decision making based on the risk of odontogenic infection is helpful when comprehensive treatment of the entire dentition is not possible. Contradicting treatment philosophies, such as minimal vs aggressive intervention, have been reported in the literature, and there continues to be disagreement as to the appropriate amount of dental treatment necessary for the pre-HSCT patient. We have found that a risk classification based on dental status has facilitated an efficient and individualized evaluation and treatment of our pre-HSCT patients. Even with conservative treatment, we experience a low complication rate indicating that, when necessary, treatment deferment of low to moderate risk, asymptomatic caries and apical and marginal periodontitis does not increase the incidence of odontogenic infectious complications during HSCT.
Acknowledgements
The authors would like to thank Kant Wu for his efforts and contributions to this project.
Funding
This research was performed as part of standard clinical protocol and therefore did not require funding.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest / competing interests
The authors declare that they have no conflict of interest.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the ethics committee of Memorial Sloan Kettering Cancer Center.
Consent to participate
An exemption and waiver of informed consent was granted for this study by the Institutional Review Board of Memorial Sloan Kettering Cancer Center.
Consent for publication
Not applicable.
Code availability
Not applicable.
Disclosure of conflicts of interest
The authors declare that they have no conflicts of interest.
The authors confirm that they have full control of all primary data and this is available for review if requested.
Availability of data and material (data transparency)
The authors affirm that this manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
References
- 1.Epstein JB, Raber-Drulacher JE, Wilkins A, Chavarria MG, Myint H (2009) Advances in hematologic stem cell transplant: An update for oral health care providers. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology 107 (3):301–312 [DOI] [PubMed] [Google Scholar]
- 2.Copelan EA (2006) Hematopoietic stem-cell transplantation. New England Journal of Medicine 354 (17):1813–1826 [DOI] [PubMed] [Google Scholar]
- 3.Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A, Szer J, Lipton J, Schwendener A, Gratwohl M, Frauendorfer K, Niederwieser D, Horowitz M, Kodera Y (2010) Hematopoietic stem cell transplantation: a global perspective. Jama 303 (16):1617–1624. doi: 10.1001/jama.2010.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg MS, Cohen SG, McKitrick JC, Cassileth PA (1982) The oral flor as a source of septicemia in patients with acute leukemia. Oral surgery, oral medicine, and oral pathology 53 (1):32–36 [DOI] [PubMed] [Google Scholar]
- 5.Heimdahl A, Mattsson T, Dahllof G, Lonnquist B, Ringden O (1989) The oral cavity as a port of entry for early infections in patients treated with bone marrow transplantation. Oral Surgery Oral Medicine and Oral Pathology 68 (6):711–716 [DOI] [PubMed] [Google Scholar]
- 6.Elad S, Raber-Durlacher JE, Brennan MT, Saunders DP, Mank AP, Zadik Y, Quinn B, Epstein JB, Blijlevens NM, Waltimo T, Passweg JR, Correa ME, Dahllof G, Garming-Legert KU, Logan RM, Potting CM, Shapira MY, Soga Y, Stringer J, Stokman MA, Vokurka S, Wallhult E, Yarom N, Jensen SB (2015) Basic oral care for hematology-oncology patients and hematopoietic stem cell transplantation recipients: a position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT). Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 23 (1):223–236. doi: 10.1007/s00520-014-2378-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heimdahl A (1999) Prevention and management of oral infections in cancer patients. Supportive Care in Cancer 7 (4):224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Consensus statement: Oral complications of cancer therapies (1990). NCI Monographs (9):3–8 [PubMed] [Google Scholar]
- 9.Elad S, Thierer T, Bitan M, Shapira MY, Meyerowitz C (2008) A decision analysis: The dental management of patients prior to hematology cytotoxic therapy or hematopoietic stem cell transplantation. Oral Oncology 44 (1):37–42 [DOI] [PubMed] [Google Scholar]
- 10.Graber CJ, De Almeida KNF, Atkinson JC, Javaheri D, Fukuda CD, Gill VJ, Barrett AJ, Bennett JE (2001) Dental health and viridans streptococcal bacteremia in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplantation 27 (5):537–542 [DOI] [PubMed] [Google Scholar]
- 11.CDC (2000) Guidelines for Preventing Opportunistic Infections Among Hematopoietic Stem Cell Transplant Recipients. Recommendations of CDC, the Infectious Disease Society of America, and the American Society of Blood and Marrow Transplantation. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr4910a1.htm. [DOI] [PMC free article] [PubMed]
- 12.WHO (2013) World Health Organization Oral Health Surveys Basic Methods. http://www.who.int/oral_health/publications/9789241548649/en/.
- 13.Nuernberg MA, Nabhan SK, Bonfim CM, Funke VA, Torres-Pereira CC (2016) Access to oral care before hematopoietic stem cell transplantation: understand to improve. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 24 (8):3307–3313. doi: 10.1007/s00520-016-3142-1 [DOI] [PubMed] [Google Scholar]
- 14.Epstein JB, Guneri P, Barasch A (2014) Appropriate and necessary oral care for people with cancer: guidance to obtain the right oral and dental care at the right time. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 22 (7):1981–1988. doi: 10.1007/s00520-014-2228-x [DOI] [PubMed] [Google Scholar]
- 15.Durey K, Patterson H, Gordon K (2009) Dental assessment prior to stem cell transplant: Treatment need and barriers to care. British Dental Journal 206 (9) [DOI] [PubMed] [Google Scholar]
- 16.Akashi M, Shibuya Y, Kusumoto J, Furudoi S, Inui Y, Yakushijin K, Okamura A, Matsuoka H, Komori T (2013) Myelosuppression grading of chemotherapies for hematologic malignancies to facilitate communication between medical and dental staff: lessons from two cases experienced odontogenic septicemia. BMC oral health 13:41. doi: 10.1186/1472-6831-13-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sculean A, Gruber R, Bosshardt DD (2014) Soft tissue wound healing around teeth and dental implants. Journal of clinical periodontology 41 Suppl 15:S6–22. doi: 10.1111/jcpe.12206 [DOI] [PubMed] [Google Scholar]
- 18.Toljanic JA, Bedard JF, Larson RA, Fox JP (1999) A prospective pilot study to evaluate a new dental assessment and treatment paradigm for patients scheduled to undergo intensive chemotherapy for cancer. Cancer 85 (8):1843–1848 [PubMed] [Google Scholar]
- 19.Melkos AB, Massenkeil G, Arnold R, Reichart PA (2003) Dental treatment prior to stem cell transplantation and its influence on the posttransplantation outcome. Clinical oral investigations 7 (2) :113–115 [DOI] [PubMed] [Google Scholar]
- 20.Yamagata K, Onizawa K, Yanagawa T, Hasegawa Y, Kojima H, Nagasawa T, Yoshida H (2006) A prospective study to evaluate a new dental management protocol before hematopoietic stem cell transplantation. Bone Marrow Transplantation 38 (3):237–242 [DOI] [PubMed] [Google Scholar]
- 21.Peters E, Monopoli M, Woo SB, Sonis S (1993) Assessment of the need for treatment of postendodontic asymptomatic periapical radiolucencies in bone marrow transplant recipients. Oral Surgery Oral Medicine and Oral Pathology 76 (1):45–48 [DOI] [PubMed] [Google Scholar]
- 22.Schuurhuis JM, Span LF, Stokman MA, van Winkelhoff AJ, Vissink A, Spijkervet FK (2016) Effect of leaving chronic oral foci untreated on infectious complications during intensive chemotherapy. British journal of cancer 114 (9):972–978. doi: 10.1038/bjc.2016.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sultan AS, Zimering Y, Petruzziello G, Alyea EP 3rd, Antin JH, Soiffer RJ, Ho VT, Sonis ST, Woo SB, Marty FM, Treister NS (2017) Oral health status and risk of bacteremia following allogeneic hematopoietic cell transplantation. Oral surgery, oral medicine, oral pathology and oral radiology 124 (3):253–260. doi: 10.1016/j.oooo.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 24.Elad S, Garfunkel AA, Or R, Michaeli E, Shapira MY, Galili D (2003) Time limitations and the challenge of providing infection-preventing dental care to hematopoietic stem-cell transplantation patients. Supportive Care in Cancer 11 (10):674–677 [DOI] [PubMed] [Google Scholar]
- 25.Fernandes LL, Torres SR, Garnica M, de Souza Goncalves L, Junior AS, de Vasconcellos AC, Cavalcanti W, Maiolino A, de Barros Torres MC (2014) Oral status of patients submitted to autologous hematopoietic stem cell transplantation. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 22 (1):15–21. doi: 10.1007/s00520-013-1940-2 [DOI] [PubMed] [Google Scholar]
- 26.Ertas ET, Kurnaz F, Zorba YO, Kocyigit I, Sisman Y, Kaynar L, Sekerci AE, Ertas H, Cetin M (2014) Comparison of chemotherapy and hematopoietic stem cell transplantation pre and postterm DMFT scores: a preliminary study. Nigerian journal of clinical practice 17 (1):32–37. doi: 10.4103/1119-3077.122831 [DOI] [PubMed] [Google Scholar]
- 27.Dobr T, Passweg J, Weber C, Tichelli A, Heim D, Meyer J, Gratwohl A, Waltimo T (2007) Oral health risks associated with HLA-types of patients undergoing hematopoietic stem cell transplantation. European journal of haematology 78 (6):495–499. doi: 10.1111/j.1600-0609.2007.00841.x [DOI] [PubMed] [Google Scholar]
- 28.Raber-Durlacher JE, Laheij AM, Epstein JB, Epstein M, Geerligs GM, Wolffe GN, Blijlevens NM, Donnelly JP (2013) Periodontal status and bacteremia with oral viridans streptococci and coagulase negative staphylococci in allogeneic hematopoietic stem cell transplantation recipients: a prospective observational study. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 21 (6):1621–1627. doi: 10.1007/s00520-012-1706-2 [DOI] [PubMed] [Google Scholar]
- 29.Raber-Durlacher JE, Epstein JB, Raber J, van Dissel JT, van Winkelhoff AJ, Guiot HF, van der Velden U (2002) Periodontal infection in cancer patients treated with high-dose chemotherapy. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 10 (6):466–473. doi: 10.1007/s00520-002-0346-3 [DOI] [PubMed] [Google Scholar]
- 30.Akintoye SO, Brennan MT, Graber CJ, McKinney BE, Rams TE, Barrett AJ, Atkinson JC (2002) A retrospective investigation of advanced periodontal disease as a risk factor for septicemia in hematopoietic stem cell and bone marrow transplant recipients. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 94 (5):581–588 [DOI] [PubMed] [Google Scholar]
- 31.Yamagata K, Onizawa K, Yanagawa T, Takeuchi Y, Hasegawa Y, Chiba S, Bukawa H (2011) Prospective study establishing a management plan for impacted third molar in patients undergoing hematopoietic stem cell transplantation. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics 111 (2):146–152. doi: 10.1016/j.tripleo.2010.09.006 [DOI] [PubMed] [Google Scholar]
- 32.Öhman D, Björk Y, Bratel J, Kristiansson C, Johansson P, Johansson JE, Brune M, Hasséus B (2010) Partially erupted third molars as a potential source of infection in patients receiving peripheral stem cell transplantation for malignant diseases: A retrospective study. European Journal of Oral Sciences 118 (1):53–58 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors affirm that this manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.


