Abstract
Purpose:
Although infection with high-risk human papillomavirus (HPV) is a prerequisite for cervical cancer development, HPV infection is not sufficient to promote cancer in the majority of infected women. We tested the hypothesis that human herpesviruses might cooperate with HPV to promote the development of cervical dysplasia, an early indicator of cervical cancer development.
Methods:
This study used archived specimens from a cohort of human immunodeficiency virus (HIV)-seropositive women seeking gynecological care at the Medical Center of New Orleans, Louisiana. Viral DNA was detected by PCR amplification and risk of abnormal cervical cytology was determined in relation to virus test results.
Results:
Consensus human herpesvirus PCR with herpes speciation by restriction endonuclease digestion revealed Epstein-Barr virus (EBV) to be the most prevalent herpesvirus in cervicovaginal lavage specimens. Further analysis using an EBV-specific PCR assay and cervical swab specimens demonstrated an approximately four-fold increased risk of abnormal cervical cytology in women testing positive for cervical EBV and high-risk HPV compared to women testing positive for high-risk HPV alone. This relationship was independent of markers of advancing HIV disease.
Conclusion:
Cervical shedding of EBV appears to predict a greater risk of cervical dysplasia in HIV-infected women with a high-risk HPV infection.
Keywords: human papillomavirus (HPV), Epstein-Barr virus (EBV), human immunodeficiency virus (HIV), cervix, dysplasia
INTRODUCTION
Infection with high-oncogenic risk human papillomavirus (hrHPV) is a prerequisite for development of cervical cancer, and lifetime risk of acquiring HPV infection exceeds 80% in women in the United States [1]. While HPV infection remains highly prevalent, it is estimated that only two in ten HPV-infected women will develop cervical dysplasia within five years [2,3]. Of these women, less than 10% will develop cervical cancer in situ, and less than 2% will develop invasive cervical cancer [4]. This discrepancy between the high prevalence of HPV infection and the much lower prevalence of cervical abnormalities, together with the long lag time between HPV acquisition and cervical cancer development, suggests that cervical cancer development is a complex process in which HPV plays a significant role but is assisted by other cofactors. A wide range of cofactors have been proposed, with evidence supporting a role for smoking, long-term use of oral contraceptives, and increasing parity (reviewed in [5]). Infection with HIV clearly increases risk of HPV infection and HPV-associated cervical neoplasia, emphasizing the role of immunity in determining the outcome of HPV exposure.
The female genital tract is frequently a reservoir of infection for members of the human herpesvirus family. Transient shedding of nearly all members of the family have been reported, with the exception of varicella zoster virus. Classical genital herpes lesions result from reactivation of persistent herpes simplex virus (HSV)-1 or HSV-2 infection, while shedding of cytomegalovirus, Epstein-Barr virus, human herpesvirus-6, human herpesvirus-7 and Kaposi’s Sarcoma herpesvirus has been described but is rarely symptomatic [6-10]. The lack of acute symptoms associated with genital herpesvirus shedding does not preclude a role for these viruses in establishing a form of chronic cervical inflammation, which may in turn contribute to carcinogenesis. Perhaps even more importantly, members of the herpesvirus family have been implicated in the etiology of human epithelial and endothelial cancers, including nasopharyngeal carcinoma (EBV), Kaposi’s sarcoma (KSHV), and a subset of gastric carcinomas (EBV). We postulated that genital human herpesvirus infections might cooperate with HPV to promote cervical dysplasia. We report prevalent cervical shedding of herpesviruses in the genital tract of HIV-infected women seeking routine gynecological care in New Orleans, Louisiana. In this cohort, women with concurrent detection of high-risk HPV and EBV were more likely to have cervical dysplasia than women who were HPV positive but negative for EBV in the genital tract.
METHODS
Study Participants
Study participants were women enrolled into one of two research studies conducted at the Medical Center of Louisiana, New Orleans HIV Outpatient (HOP) Clinic, New Orleans, Louisiana, between 1999 and 2004. The first study investigated the local immune response to HPV infection and the second study investigated the natural history of HPV infection and HPV-related disease in high-risk populations. Both studies were open to adult women infected with HIV who were undergoing routine gynecological screening. Exclusion criteria for both studies were similar and included pregnancy, hysterectomy, and chronic illness. Protocols for both studies were approved by local Institutional Review Boards. Written informed consent was obtained from all women prior to study enrollment. Women in both studies completed a demographic and sexual history survey. Further details of both studies are published elsewhere [11,12].
Clinical Specimens
Details of the clinical specimens collected from women enrolling in the two research studies have been reported elsewhere [11,12]. Two specimens were utilized for viral DNA detection in this report. For the first cohort of women, cervicovaginal lavages (CVLs) were collected by bathing the cervix in 5cc of sterile phosphate-buffered saline for 30 seconds and aspirating the fluid. For the second cohort of women, cervical swabs were collected by inserting a cotton-tipped swab into the cervical os, rotating the swab several times, and inserting the swab in specimen transport media (Digene). Specimens were kept on ice until processed. Cellular DNA was extracted from the cervical swabs and cell pellets obtained from the CVLs. Women in both studies received a clinician-administered pelvic exam and Papanicolaou (Pap) smear prior to collection of samples for viral DNA testing. Pap smears were evaluated by a Pathologist and graded according to the Bethesda recommendations (1991). For analysis, cytology was categorized as SIL (inclusive of low- and high-grade squamous intraepithelial lesions, SIL) or abnormal cytology (inclusive of any SIL and atypical squamous cells of undetermined significance, ASCUS). Results of recent (typically within 30 days of study enrollment) HIV peripheral viral load assay and CD4+ T cell counts were extracted from the participant’s clinical charts.
HPV DNA Detection
Presence of HPV DNA in CVL and cervical swab cell extracts was determined using the Roche PGMY09/11 L1-specific consensus primer PCR [13] according to the manufacturer’s protocol. Amplification of cellular β-globin (housekeeping) gene was performed simultaneously to ensure adequacy of the sample for PCR analysis. Specimens were considered HPV positive if both the 250 base-pair (bp) human β-globin and 450 bp HPV L1 amplicons were observed by ethidium bromide-stained agarose gel electrophoresis. The genotype of HPV was determined by subjecting the PCR products to the Linear Array assay (Roche) per the suggested protocol. For this analysis, HPV genotypes 6, 11, 40, 42, 53, 54, 66, and 84 (MM8) were considered to be low-oncogenic risk and genotypes 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 55, 56, 58, 59, 68, 82 (MM4), 83 (MM7), and 73 (MM9) were considered to be high-oncogenic risk.
Human Herpesvirus Detection
In order to detect human herpesviruses in DNA extracts from CVL specimens, we adapted a PCR assay first published by Johnson, et. al [14]. The assay targets a region of the viral DNA polymerase gene that is widely conserved across the human herpesvirus family. Five microliters (5μL) of DNA extract was amplified in a PCR reaction consisting of 1μM forward (HSV-P1) and reverse (HSV-P2) primers, 250 μM dNTP mix, 1.5 mM MgCl2, and 2.5 units AmpliTaq Gold (Roche Molecular Systems). Reactions were conducted at 94°C for one minute, 60°C for 30 seconds, and 72°C for one minute, for a total of 40 cycles. Amplicons were digested with BamH1 and BstU1 restriction endonucleases and visualized by agarose gel electrophoresis with ethidium-bromide staining. The specific human herpesvirus detected was determined based on unique banding patterns as described in Johnson, et. al [14].
Epstein-Barr Virus (EBV) Detection
Epstein-Barr virus was detected by PCR assay targeting the BamH1-W repeat region of the EBV genome [15]. Five microliters (5μL) of DNA extract was amplified in a PCR reaction consisting of 1μM forward (EBV-P1) and reverse (EBV-P2) primers, 250 μM dNTP mix, 2.5 mM MgCl2, and 2.5 units AmpliTaq Gold (Roche Molecular Systems). Reactions were conducted at 95°C for one minute, 55°C for 45 seconds, and 72°C for one minute, for a total of 40 cycles. Amplification products were visualized on an ethidium bromide-stained agarose gel.
Statistical Analysis
Fisher’s exact tests were used to analyze contingency tables for categorical variables. Continuous variables with approximately normal distribution were compared using the student’s t-test, and when continuous variable data violated the normality assumption, the Wilcoxon rank-sum test was used. A value of p<0.05 was considered statistically significant. Odds Ratios (OR) were calculated to determine associations between cervical virus infection(s) and cervical cytology. Multinomial logistic regression models were used to evaluate the independent contributions of factors associated with increased risk of a woman having SIL or ASCUS cytology vs normal cytology. Statistical package software used included SAS, SPSS, R statistical software and GraphPad Prism.
RESULTS
Characteristics of the study population.
Archived cervicovaginal lavage (CVL) specimens were used in this study. Subjects were predominantly African-American (85.6%) women with a mean age of 36.8 years (Table 1). The majority were unmarried (88.1%) mothers (85.6%). Roughly half of the subjects had a history of smoking, and nearly half reported a history of an abnormal Pap smear. Based on peripheral blood CD4+ T cell count, 21.6% were immune suppressed (less than 200 cells/ml) at the time of sample collection. Women were evenly distributed across three peripheral blood HIV viral load strata, with 31.4% of subjects having low viral loads (<400 copies/ml), 32.6% having intermediate viral loads (400-10,000 copies/ml), and 34.3% having high viral loads (>10,000 copies/ml).
Table 1.
Univariate analysis of associations between cohort demographics, clinical factors, cervicovaginal virus detection and squamous intraepithelial lesion (SIL) cytology diagnosis.
| Overall (n = 236) |
SIL Positivea (n = 73) |
p-valueb | |
|---|---|---|---|
| No. (%) | No. (%) | ||
| Race/Ethnicity | 0.447 | ||
| Caucasian | 32 (13.6) | 8 (25.0) | |
| African-American | 202 (85.6) | 64 (31.7) | |
| Hispanic | 2 (0.8) | 1 (50.0) | |
| Age (yrs) | 0.069 | ||
| 20-30 | 59 (25.0) | 24 (40.7) | |
| 31-40 | 97 (41.1) | 31 (40.0) | |
| >40 | 80 (33.9) | 18 (22.5) | |
| Mean | 36.8 | ||
| Marital status | 0.050 | ||
| Married/co-habitant partner | 28 (11.9) | 4 (14.3) | |
| Single/divorced/widowed | 208 (88.1) | 69 (33.2) | |
| Education | 0.670 | ||
| 6-11 years | 34 (14.4) | 12 (35.3) | |
| High School graduate | 172 (72.9) | 54 (31.4) | |
| College graduate or above | 21 (8.9) | 5 (23.8) | |
| Smoking history | 0.153 | ||
| Never | 100 (42.4) | 37 (37.0) | |
| Ever | 131 (55.5) | 36 (27.5) | |
| Parity | 1.000 | ||
| Nulliparous | 22 (9.3) | 7 (31.8) | |
| ≥1 | 202 (85.6) | 63 (31.2) | |
| Male sex partners, past year | 0.911 | ||
| None | 36 (15.3) | 10 (27.8) | |
| 1 | 111 (47.0) | 35 (31.5) | |
| 2 or more | 40 (17.0) | 12 (30.0) | |
| Recall history of abnormal Pap smear | 0.004 | ||
| Yes | 112 (47.5) | 49 (43.8) | |
| No | 85 (36.0) | 20 (23.5) | |
| HPV infection | <0.001 | ||
| Positive | 115 (48.7) | 56 (48.7) | |
| Negative | 121 (51.3) | 17 (14.0) | |
| hrHPV infection | <0.001 | ||
| Positive | 102 (43.2) | 52 (51.0) | |
| Negative | 134 (56.8) | 21 (15.7) | |
| HHV infection | 0.077 | ||
| Positive | 47 (19.9) | 20 (42.6) | |
| Negative | 189 (80.1) | 53 (28.0) | |
| CD4+ T cell count | 0.002 | ||
| <200 cells/ml | 51 (21.6) | 25 (49.0) | |
| 200-500 cells/ml | 92 (39.0) | 30 (32.6) | |
| >500 cells/ml | 89 (37.7) | 18 (20.2) | |
| Mean cells/ml | 442.8 | ||
| Peripheral HIV viral load | 0.144 | ||
| <400 copies/ml | 74 (31.4) | 19 (25.7) | |
| 400-10,000 copies/ml | 77 (32.6) | 22 (28.6) | |
| >10,000 copies/ml | 81 (34.3) | 32 (39.5) | |
| Mean copies/ml | 62,653.9 |
SIL positive included all cases of squamous intraepithelial lesion diagnosed on cytology smear (low-grade and high-grade). Missing data (participant did not respond, marked ‘unknown’, or information was otherwise unavailable): education, n = 9; smoking history, n = 5; parity, n = 4; Recall history of abnormal Pap smear, n = 39; male sex partners in past year, n = 49; CD4+ T cell count and HIV viral load, n = 4.
Probability (p) values were calculated using Chi-square analysis or Fisher’s Exact test, as appropriate.
Abbreviations: HPV, human papillomavirus. hrHPV, high-risk human papillomavirus. HHV, human herpesvirus. HIV, human immunodeficiency virus.
Prevalence of HPV and human herpesviruses.
Infection with HIV increases risk of acquisition and persistence of HPV infection. Accordingly, prevalence of genital HPV infection in this population was 48.7%. The prevalence of low-risk HPV genotypes, high-risk HPV genotypes, infection with multiple HPV genotypes, and HPV-16 are given in Table 2. We tested these same specimens for presence of human herpesviruses using a PCR assay targeting a consensus sequence of the viral DNA polymerase gene of related human herpesviruses known to infect the female genital tract (herpes simplex viruses 1 and 2, Epstein-Barr virus, cytomegalovirus, and Kaposi’s Sarcoma herpesvirus). One in five women were positive for a human herpesvirus. The most prevalent herpesvirus detected was Epstein-Barr virus (12.7%), with only 6.8% of the women having DNA evidence of infection with herpes simplex viruses, the herpesviruses typically associated with genital herpes. No association between herpesvirus DNA detection and high-risk HPV DNA detection was observed. An association was seen between infection with specific HPV genotypes (6 and 35) and detection of genital EBV (Online Resource 1).
Table 2.
Prevalence of HPV and human herpesviruses in cervicovaginal specimens of HIV-infected women.
| Virus detection | Overall (n = 236) |
hrHPV negative (n = 134) |
hrHPV positive (n = 102) |
p-valuec |
|---|---|---|---|---|
| HPV | No. (%) | No. (%) | No. (%) | |
| Any | 115 (48.7) | |||
| Low-riska | 53 (22.5) | |||
| High-riskb | 102 (43.2) | |||
| Multiple | 57 (24.2) | |||
| HPV-16 | 4 (1.7) | |||
| HHV | ||||
| Any | 47 (19.9) | 24 (17.9) | 23 (22.5) | .41 |
| HSV-1 (HHV1) | 1 (0.4) | 1 (0.7) | 0 (0) | 1.00 |
| HSV-2 (HHV2) | 15 (6.4) | 7 (5.2) | 8 (7.8) | .43 |
| EBV (HHV4) | 30 (12.7) | 16 (11.9) | 14 (13.7) | 0.70 |
| CMV (HHV5) | 0 (0) | 0 (0) | 0 (0) | N/A |
| KSHV (HHV8) | 0 (0) | 0 (0) | 0 (0) | N/A |
Low-risk: HPV genotypes (6, 11, 40, 42, 53, 54, 66, 84) that are rarely associated with cervical cancer.
High-risk: HPV genotypes (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 55, 56, 57, 58, 59, 68, 73, 82, 83) that have been associated with cervical cancer.
Fisher’s Exact Test p-value is presented.
Abbreviations: hrHPV, high-risk HPV. HPV, human papillomavirus. HHV, human herpesvirus. HSV-1, herpes simplex virus-1. HSV-2, herpes simplex virus-2. EBV, Epstein-Barr virus. CMV, cytomegalovirus. KSHV, Kaposi’s sarcoma herpesvirus.
Univariate analysis of risk factors for cervical squamous intraepithelial lesions (SIL).
We performed univariate analysis to determine the demographic, clinical and viral variables associated with squamous intraepithelial lesions (SIL) in this HIV-positive cohort (Table 1). Age, race, and socioeconomic status were not significantly associated with cervical cytology. Markers of sexual activity such as parity and number of male sex partners in the past year were also not predictive of cervical cytology findings. As expected, women were more likely to have SIL if they were HPV positive or high-risk HPV positive (p<0.001), if they recalled having an abnormal Pap smear diagnosis (p=0.004), and if they were immune suppressed based on reduced CD4+ T cell counts (p=0.002). Similar associations were seen when risk of any abnormal cytology (including atypical squamous cells of undetermined significance [ASCUS] cytology, low-grade and high-grade SIL) was considered (data not shown). A significant association (p<0.05) was also seen between detection of any HHV and abnormal cervical cytology (ASCUS included, data not shown), but this association was attenuated in risk analysis for SIL (p=0.077, Table 1).
Squamous intraepithelial lesions (SIL) in HIV-infected women with genital detection of high-risk human papillomaviruses and human herpesviruses
Next, we ascertained the contribution of human herpesviruses to the risk of cervical SIL (top portion of Table 3). As expected, detection of hrHPV in the absence of herpesvirus infection conferred 6-fold increased risk of SIL, while detection of a herpesvirus, in absence of hrHPV infection, was not associated with increased risk of SIL. Concurrent detection of hrHPV and herpesvirus raised the risk of SIL to 10-fold over that of virus-negative women; however, the independent contribution of herpesvirus infection was not statistically significant when risk of SIL in hrHPV-herpesvirus dual-positive women was compared to risk in hrHPV positive, herpesvirus negative women.
Table 3.
Impact of genital high-risk HPV and human herpesvirus detection on cervical squamous intraepithelial lesions (SIL) in HIV-infected women.
| Genital hrHPV |
Genital HHV |
SIL-Negative No. (%) |
SIL-Positive No. (%) |
Odds Ratio (95% CI) |
p-valueb |
|---|---|---|---|---|---|
| Any HHV | |||||
| − | − | 95 (86.4) | 15 (13.6) | Reference | |
| + | − | 41 (51.9) | 38 (48.1) | 5.87 (2.91-11.83) | <.0001 |
| − | + | 18 (75.0) | 6 (25.0) | 2.11 (0.72-6.17) | .14 |
| + | + | 9 (39.1) | 14 (60.9) | 9.85 (3.63-26.76) | <.0001 |
| + | − | 41 (51.9) | 38 (48.1) | Reference | |
| + | + | 9 (39.1) | 14 (60.9) | 1.68 (0.65-4.33)a | .20 |
| EBV | |||||
| − | − | 100 (84.7) | 18 (15.3) | Reference | |
| + | − | 46 (52.3) | 42 (47.7) | 5.07 (2.64-9.75) | <.0001 |
| − | + | 13 (81.3) | 3 (18.7) | 1.28 (0.33-4.96) | .48 |
| + | + | 4 (28.6) | 10 (71.4) | 13.89 (3.94-49.15) | <.0001 |
| + | − | 46 (52.3) | 42 (47.7) | Reference | |
| + | + | 4 (28.6) | 10 (71.4) | 2.74 (0.80-9.40)a | .09 |
Odds ratio of SIL in women with both hrHPV and HHV (top of table) or EBV (bottom of table) compared to women with hrHPV but no HHV or EBV, respectively.
Fisher’s Exact Test p-value is presented.
Abbreviations: hrHPV, high-risk human papillomavirus. HHV, human herpesvirus. SIL, squamous intraepithelial lesion. CI, confidence interval. EBV, Epstein-Barr virus.
Since Epstein-Barr virus infection was the predominant herpesvirus detected in our population, we investigated the effect of cervical EBV detection on risk of SIL (bottom portion of Table 3). Detection of hrHPV infection remained significantly associated with SIL in the absence of EBV infection (5-fold increased risk), while EBV infection in the absence of hrHPV was not significantly associated with SIL. Risk of SIL increased to 14-fold in dual-virus positive women compared to uninfected women. The contribution of EBV to this association demonstrated a trend towards significance when compared to hrHPV-positive, EBV negative women (OR [95% CI], 2.74 [0.80, 9.40]). Strikingly, 71.4% of women with both hrHPV and EBV had SIL while less than half (47.7%) of women with hrHPV infection (without EBV) had SIL. In this analysis, the category ‘SIL-negative’ included women with abnormal cervical cytology classified as ASCUS. Similar trends in dual-virus positive women (compared to hrHPV positive, EBV negative women) were observed when ASCUS was included with SIL as abnormal cytology (OR [95% CI], 4.35 [0.92, 20.63]) and when women with ASCUS were excluded from the analysis (OR [95% CI], 4.41 [0.91, 21.42]), indicating that this heterogeneous diagnosis does not significantly alter the associations (data not shown).
Univariate analysis of risk factors associated with genital detection of EBV among high-risk HPV-positive women
The marked increase in prevalence of SIL in women with both EBV and hrHPV compared to women with hrHPV alone prompted us to examine the variables associated with EBV detection (Table 4). In analysis restricted to women with hrHPV infection, EBV detection was not associated with race, age, or HIV disease (peripheral blood CD4+ T cell counts or HIV viral load). In fact, the only variable evaluated that was significantly (p<0.05) associated with EBV detection in hrHPV-infected women was self-reported history of an abnormal Pap smear.
Table 4.
Risk factorsa for EBV detection in cervicovaginal lavage of HIV-positive women who are also hrHPV-positive (n=102).
| EBV Negative (n = 88) |
EBV Positive (n = 14) |
p-valueb | |
|---|---|---|---|
| Race/Ethnicity | .693 | ||
| Caucasian | 10 (11.4) | 1 (7.7) | |
| African-American | 78 (88.6) | 12 (92.3) | |
| Hispanic | (N/A) | (N/A) | |
| Age | .298 | ||
| 20-30 | 31 (35.2) | 2 (14.3) | |
| 31-40 | 33 (37.5) | 7 (50.0) | |
| >40 | 23 (26.1) | 5 (35.7) | |
| Mean | 34.8 | 37.8 | .195 |
| Marital status | .917 | ||
| Married/co-habitant partner | 7 (8.0) | 1 (7.1) | |
| Single/divorced/widowed | 81 (92.0) | 13 (92.9) | |
| Education level | .254 | ||
| 6-11 years | 8 (9.5) | 3 (21.4) | |
| High School graduate | 68 (80.9) | 8 (57.1) | |
| College graduate or above | 8 (9.5) | 3 (21.4) | |
| Smoking history | .939 | ||
| Never | 45 (51.1) | 7 (50.0) | |
| Ever | 43 (48.9) | 7 (50.0) | |
| Parity | .298 | ||
| Nulliparous | 6 (7.3) | 0 (0) | |
| ≥ 1 | 76 (92.7) | 14 (100) | |
| History of abnormal Pap smear | .012 | ||
| Yes | 49 (65.3) | 11 (78.6) | |
| No | 26 (34.7) | 3 (21.4) | |
| Male sex partners in past year | .644 | ||
| None | 9 (13.0) | 2 (22.2) | |
| 1 | 41 (59.4) | 4 (44.4) | |
| 2 or more | 19 (27.5) | 3 (33.4) | |
| Male sex partners in past month | .712 | ||
| None | 16 (27.6) | 3 (37.5) | |
| 1 | 39 (67.2) | 5 (62.5) | |
| 2 or more | 3 (5.2) | 0 (0) | |
| CD4+ T cell count | .980 | ||
| <200 | 24 (27.9) | 4 (28.6) | |
| 200-500 | 33 (38.3) | 5 (35.7) | |
| >500 | 29 (33.7) | 5 (35.7) | |
| Mean | 398 | 401 | .673 |
| Peripheral HIV viral load | .895 | ||
| <400 | 24 (27.9) | 2 (14.3) | |
| 400-10,000 | 25 (29.0) | 5 (35.7) | |
| >10,000 | 37 (43.0) | 7 (50.0) | |
| Mean | 95,408 | 53,384 | .450 |
All data is self-reported on demographic survey, except CD4+ T cell counts and peripheral HIV viral load which were extracted from the clinical chart.
Probability (p) was derived from Chi square analysis, or Fisher Exact test as appropriate.
Abbreviations: HIV, human immunodeficiency virus. EBV, Epstein-Barr virus. hrHPV, high-risk HPV.
Multinomial logistic regression model of abnormal cytology risk among women testing positive for hrHPV
To determine if EBV infection was a covariate of known risk factors for cervical SIL diagnosis, we conducted multinomial logistic regression analysis for the categories SIL, ASCUS and normal cytology with covariates including age, race, CD4+ T cell count, and EBV status in the model and restricting analysis to only those women who had hrHPV infection (n=99). The resulting multinomial logistic regression model coefficients, covariate test statistics and p-values are reported in Table 5. In the model, older age and increased CD4+ T cell count were independently associated with a decreased risk of SIL (p=0.021 and 0.011, respectively), while detection of EBV was independently associated with an increased risk of SIL (p=0.034, OR [95% CI], 6.46 [1.15, 36.30]). Performing a logistic regression for abnormal cytology (ASCUS included with SIL) did not appreciably change the results of the analysis, with the exception that African-American race was significantly independently associated with a decreased risk of abnormal cytology (p=0.027). The associations also did not change when HIV viral load was included in the model, likely because HIV viral load covaries with CD4+ T cell counts. Figure 1 displays the predicted probability of an abnormal pap smear as a function of CD4 count at the mean age of 35.3 years old and as a function of age at the mean CD4 count of 401.2 for African Americans in the study using the model described in Table 5.
Table 5.
Multinomial regression model predicting abnormal cytology for women who had high-risk HPV infection.
| Model for ASCUS vs Normal Cytology | |||
|---|---|---|---|
| Variable | Coefficient | Test Statistic | p-value |
| Intercept | 1.472 | 1.256 | .209 |
| Age | −.437 | −1.126 | .260 |
| African American Race | −3.319 | −2.660 | .008 |
| CD4+ T Cell Count | .001 | .004 | .997 |
| EBV | 1.816 | 1.569 | .116 |
| Model for SIL vs Normal Cytology | |||
| Variable | Coefficient | Test Statistic | p-value |
| Intercept | 2.089 | 1.844 | .065 |
| EBV | 1.866 | 2.121 | .034 |
| CD4+ T Cell Count | −.671 | −2.532 | .011 |
| Age | −.580 | −2.309 | .021 |
| African American Race | −2.113 | −1.817 | .069 |
Abbreviations: HPV, human papillomavirus. EBV, Epstein-Barr virus. SIL, squamous intraepithelial lesion (includes low-grade and high-grade SIL).
Fig. 1.

Predicted probability of an abnormal cytology result as (a) a function of CD4+ T cell count at the mean age of 35.3 years old and (b) as a function of age at the mean CD4+ T cell count of 401.2 for high-risk HPV positive African American women in the study, using the model described in table 7.
Association of SIL with high-risk HPV and EBV detection in cervical swab specimens from a second cohort of HIV-infected women
The intriguing findings of our initial study prompted us to investigate a second population of HIV-infected women for whom cervical swabs were available for analysis (total n, 295 women). We reasoned that cervical swabs would more directly reflect the state of the cervix, compared to the cervicovaginal lavages analyzed in the initial study that collect cells from throughout the vaginal canal. We also improved detection of EBV by using a more sensitive and more specific PCR assay that amplifies the BamH1-W repeat region of the EBV genome [15]. In this cohort (presented in Table 6), high-risk HPV prevalence remained high (38.6%). Prevalence of EBV was much higher in this cohort (44.7%) compared to the initial cohort (12.7%), likely reflecting the improved sensitivity and specificity of the EBV detection assay. There was no association between hrHPV infection and detection of cervical EBV, suggesting that the viruses themselves are independent (data not shown). Interestingly, among women who were EBV negative, hrHPV was no longer significantly associated with SIL; likewise, EBV detection in the absence of hrHPV clearly did not increase risk of SIL. Risk of SIL was only significantly increased in women who were positive for both hrHPV and EBV at the cervix (OR [95% CI], 7.17 [3.42, 15.04]). The contribution of EBV was ~4-fold (OR [95% CI], 4.40 [1.98, 9.76]) in women with both viruses at the cervix compared to women with hrHPV alone. When virus detection was plotted according to cervical cytology result, the prevalence of dual cervical virus positivity increased with increasing cervical pathology grade (Figure 2).
Table 6.
Association of cervical high-risk HPV and EBV detection with cervical squamous intraepithelial lesions (SIL) in HIV-infected women.
| Cervical hrHPVa |
Cervical EBV |
SIL-Negative No. (%) |
SIL-Positive No. (%) |
Odds Ratio (95% CI) |
p-valueb |
|---|---|---|---|---|---|
| − | − | 86 (82.7) | 18 (17.3) | Reference | |
| + | − | 44 (74.6) | 15 (25.4) | 1.63 (0.75-3.54) | .15 |
| − | + | 63 (81.8) | 14 (18.2) | 1.06 (0.49-2.29) | .52 |
| + | + | 22 (40.0) | 33 (60.0) | 7.17 (3.42-15.04) | <.0001 |
| + | − | 44 (74.6) | 15 (25.4) | Reference | |
| + | + | 22 (40.0) | 33 (60.0) | 4.40 (1.98-9.76)c | .0002 |
Epstein-Barr virus and high-risk HPV were detected in cervical swabs collected from HIV-positive women enrolled in cohort 2.
Fisher’s Exact test p-value is presented.
Odds ratio of SIL in women with both hrHPV and EBV compared to women with hrHPV but no EBV.
Abbreviations: HIV, human immunodeficiency virus. hrHPV, high-risk human papillomavirus. EBV, Epstein-Barr virus. SIL, squamous intraepithelial lesion. CI, confidence interval.
Fig. 2.
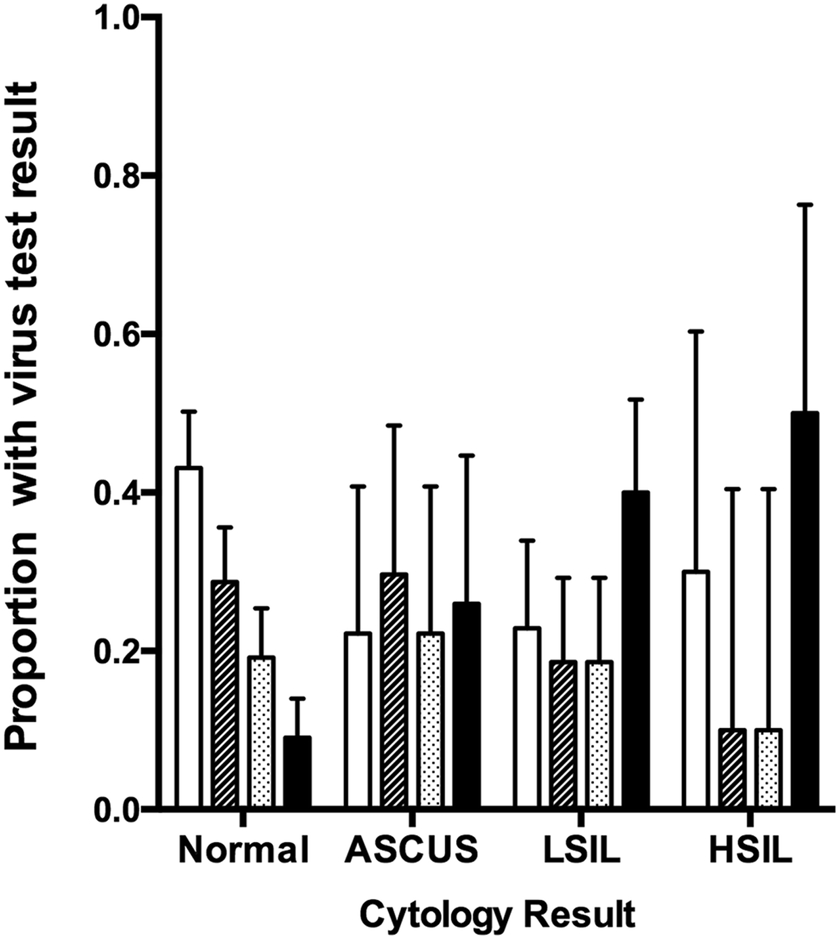
Prevalence of concurrent high-risk HPV and EBV in cervical swab specimens according to cytology result. Error bars represent 95% confidence interval. ASCUS, atypical squamous cells of undetermined significance. LSIL, low grade squamous intraepithelial lesion. HSIL, high grade squamous intraepithelial lesion.
DISCUSSION
The notion that herpesviruses could play a role in cervical carcinogenesis in cooperation with HPV was first proposed by Nobel laureate Harald zur Hausen [16]. We have presented compelling evidence that HIV-infected, high-risk HPV-positive women are at increased risk of abnormal cervical cytology if they are also positive for Epstein-Barr virus in the genital tract. In contrast, cervical EBV infection was not a significant predictor of SIL in HIV-seropositive Italian women [17]. The rate of EBV detection (45%, using the sensitive EBV-specific PCR assay in the cohort receiving cervical swabs) was much higher in the New Orleans cohort than in the Italian cohort (10%), suggesting population-based and/or assay-dependent differences between these two cohorts. In the Italian study, HPV infection was associated with high HIV viral loads and low CD4+ T cell counts, suggesting that compromised immunity was playing a substantial role in HPV-mediated SIL in this population. This association may have masked the contribution of EBV to HPV-mediated SIL. Multinomial logistic regression analysis of our data demonstrated that the association between EBV infection and SIL cervical cytology was independent of CD4+ T cell count in the high-risk HPV positive women in our cohort.
It is interesting to note that HPV-16, widely believed to be the most oncogenic HPV genotype, only accounted for a small percentage (3.5%) of the high-risk HPV infections identified in our study. It is possible that the relatively high rates of non-HPV-16 infections, which are predicted to require more assistance from carcinogenic co-factors, might have revealed the relationship between EBV, HPV and cervical dysplasia in our cohort. This relationship may be masked in populations in which the highly potent HPV-16 is the dominant high-risk genotype.
We cannot rule out the possibility that the association seen between EBV, HPV and cervical dysplasia is a cohort effect unique to our population; however, there are reports that suggest similar trends in other populations. Silver et al. reported a significantly higher EBV prevalence in Indian women with an abnormal Pap smear compared to women with a normal Pap smear (29.7% and 16.8%, respectively) [18]. In this population, 69% of women with biopsy-confirmed cervical intraepithelial neoplasia (CIN) were EBV positive. Similarly, concurrent detection of high-risk HPV and EBV in cervical specimens was associated with abnormal cytology in a population of commercial sex workers in Nairobi, Kenya [19]. Interestingly, women in this cohort testing positive for EBV at baseline appeared to experience delayed clearance of baseline HPV infection, even when accounting for HIV infection. Studies to explore the relationship between EBV, HPV and cervical abnormalities in a variety of populations, with and without HIV infection, are warranted.
Prevalence of HSV-1, HSV-2, CMV, and KSHV herpesvirus infections of the genital tract was low (0.4%, 6.4%, 0% and 0% respectively) in our study. This is somewhat surprising, given the HIV status of the population under investigation. We suspect that the relative insensitivity of the consensus DNA-polymerase PCR assay led to under-representation of these herpesviruses in our population. We performed assay sensitivity testing on BCBL-1 cells (lymphoma cells known to contain KSHV), as well as clinical specimens known to be infected with HSV-1, HSV-2, or CMV. In each instance, the consensus PCR assay was able to amplify herpesvirus sequence from the positive control specimens; however, testing of standard dilutions indicated a decreased sensitivity for amplification of CMV (data not shown). This likely explains the lack of detection of CMV in our study. The study by Silver et al. reported higher rates of genital CMV infection (26%) than EBV infection (20%) [18]. We cannot rule out a role for CMV as a co-factor for HPV based on the results of our study, as the consensus PCR assay was likely inadequate for assessing CMV status; however, the study by Silver et al. found no association between CMV detection and cervical cytology.
While viral infection with either high-risk HPV or EBV and low peripheral CD4+ T cell counts were associated with SIL, low CD4+ T cell counts did not predict EBV status among high-risk HPV positive women. There was also no association between EBV infection and high-risk HPV infection in our study. Together these results suggest that EBV is not merely a marker of compromised immunity or high-risk HPV infection; however, the use of viral genomic DNA-targeted PCR assays to detect EBV limits our ability to interpret the role EBV may be playing in HPV-mediated cervical dysplasia. The assays do not distinguish between true infection and transient viral deposition, nor do they differentiate between replicating (lytic) infection and latent/quiescent infection. Further, our clinical sampling methods do not allow for definitive identification of the nature of detected EBV genomic sequence (cell-associated or cell-free virus), nor can we precisely pinpoint the cell type infected with EBV (infiltrating lymphocytes versus squamous epithelial cells). Understanding the biology of EBV in the female genital tract will reveal the potential mechanisms of this interaction between high-risk HPV and EBV. The detection of EBV at the cervix may have clinical utility as a triage test in high-risk HPV-positive populations regardless of the biological role EBV might be playing in HPV-associated neoplasms.
Supplementary Material
ACKNOWLEDGEMENTS AND DISCLOSURES
Funding:
This work was supported by the National Cancer Institute of the National Institutes of Health (NIH) [R01CA121979 to MEH]. Archived clinical specimens used in this study were collected during the course of previous studies supported by the Doris Duke Foundation and the Louisiana Health Excellence Fund (awards to MEH). JEC has received support from the National Institute of General Medical Sciences of the NIH through the Center of Biomedical Research Excellence (P20 GM103501) and the Louisiana Clinical and Translational Science (LA CaTS) Center (U54 GM104940). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the sponsoring agencies and affiliate institutions.
Footnotes
Potential conflicts of interest: MEH has received compensation from Merck Pharmaceuticals as a medical consultant (05/2007-04/2009) and has served on an advisory panel for Napo Pharmaceuticals (04/2018). These activities were unrelated to the work described in this manuscript. All other authors have nothing to disclose.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Institutional Review Board approval of the protocol was secured prior to study initiation.
Informed consent: Informed written consent was obtained from all study participants prior to participation.
REFERENCES
- 1.Chesson HW, Dunne EF, Hariri S, Markowitz LE (2014) The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis 41 (11):660–664. doi: 10.1097/OLQ.0000000000000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castle PE, Wacholder S, Sherman ME, Lorincz AT, Glass AG, Scott DR, Rush BB, Demuth F, Schiffman M (2002) Absolute risk of a subsequent abnormal pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer 95 (10):2145–2151. doi: 10.1002/cncr.10927 [DOI] [PubMed] [Google Scholar]
- 3.Kjaer S, Hogdall E, Frederiksen K, Munk C, van den Brule A, Svare E, Meijer C, Lorincz A, Iftner T (2006) The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res 66 (21):10630–10636. doi: 10.1158/0008-5472.CAN-06-1057 [DOI] [PubMed] [Google Scholar]
- 4.Scheurer ME, Tortolero-Luna G, Adler-Storthz K (2005) Human papillomavirus infection: biology, epidemiology, and prevention. Int J Gynecol Cancer 15 (5):727–746. doi: 10.1111/j.1525-1438.2005.00246.x [DOI] [PubMed] [Google Scholar]
- 5.Castellsague X, Munoz N (2003) Chapter 3: Cofactors in human papillomavirus carcinogenesis--role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr (31):20–28 [PubMed] [Google Scholar]
- 6.Knox GE, Pass RF, Reynolds DW, Stagno S, Alford CA (1979) Comparative prevalence of subclinical cytomegalovirus and herpes simplex virus infections in the genital and urinary tracts of low-income, urban women. J Infect Dis 140 (3):419–422 [DOI] [PubMed] [Google Scholar]
- 7.Sixbey JW, Lemon SM, Pagano JS (1986) A second site for Epstein-Barr virus shedding: the uterine cervix. Lancet 2 (8516):1122–1124 [DOI] [PubMed] [Google Scholar]
- 8.Leach CT, Newton ER, McParlin S, Jenson HB (1994) Human herpesvirus 6 infection of the female genital tract. J Infect Dis 169 (6):1281–1283 [DOI] [PubMed] [Google Scholar]
- 9.Okuno T, Oishi H, Hayashi K, Nonogaki M, Tanaka K, Yamanishi K (1995) Human herpesviruses 6 and 7 in cervixes of pregnant women. J Clin Microbiol 33 (7):1968–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calabro ML, Fiore JR, Favero A, Lepera A, Saracino A, Angarano G, Schulz TF, Chieco-Bianchi L (1999) Detection of human herpesvirus 8 in cervicovaginal secretions and seroprevalence in human immunodeficiency virus type 1-seropositive and -seronegative women. J Infect Dis 179 (6):1534–1537. doi: 10.1086/314765 [DOI] [PubMed] [Google Scholar]
- 11.Chaturvedi AK, Dumestre J, Gaffga AM, Mire KM, Clark RA, Braly PS, Dunlap K, Beckel TE, Hammons AF, Kissinger PJ, Hagensee ME (2005) Prevalence of human papillomavirus genotypes in women from three clinical settings. J Med Virol 75 (1):105–113. doi: 10.1002/jmv.20244 [DOI] [PubMed] [Google Scholar]
- 12.Chaturvedi AK, Myers L, Hammons AF, Clark RA, Dunlap K, Kissinger PJ, Hagensee ME (2005) Prevalence and clustering patterns of human papillomavirus genotypes in multiple infections. Cancer Epidemiol Biomarkers Prev 14 (10):2439–2445. doi: 10.1158/1055-9965.EPI-05-0465 [DOI] [PubMed] [Google Scholar]
- 13.Gravitt PE, Peyton CL, Apple RJ, Wheeler CM (1998) Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol 36 (10):3020–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson G, Nelson S, Petric M, Tellier R (2000) Comprehensive PCR-based assay for detection and species identification of human herpesviruses. J Clin Microbiol 38 (9):3274–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong KY, Collins RJ, Srivastava G, Pittaluga S, Cheung AN, Wong LC (1993) Epstein Barr virus in carcinoma of the cervix. Int J Gynecol Pathol 12 (3):224–227 [DOI] [PubMed] [Google Scholar]
- 16.zur Hausen H (1982) Human genital cancer: synergism between two virus infections or synergism between a virus infection and initiating events? Lancet 2 (8312):1370–1372 [DOI] [PubMed] [Google Scholar]
- 17.Ammatuna P, Giovannelli L, Giambelluca D, Mancuso S, Rubino E, Colletti P, Mazzola G, Belfiore P, Lima R (2000) Presence of human papillomavirus and Epstein-Barr virus in the cervix of women infected with the human immunodeficiency virus. J Med Virol 62 (4):410–415 [DOI] [PubMed] [Google Scholar]
- 18.Silver MI, Paul P, Sowjanya P, Ramakrishna G, Vedantham H, Kalpana B, Shah KV, Gravitt PE (2011) Shedding of Epstein-Barr virus and cytomegalovirus from the genital tract of women in a periurban community in Andhra Pradesh, India. J Clin Microbiol 49 (7):2435–2439. doi: 10.1128/JCM.02206-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cameron JE, Rositch AF, Vielot NA, Mugo NR, Kwatampora JKL, Waweru W, Gilliland AE, Hagensee ME, Smith JS (2018) Epstein-Barr virus, high-risk human papillomavirus and abnormal cervical cytology in a prospective cohort of African female sex workers. Sex Transm Dis 45 (10):666–672. doi: 10.1097/OLQ.0000000000000857 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


