Abstract
Caribbean Women’s Health and Transnational Ethnobotany. Immigrants from the Dominican Republic (DR) and Haiti are among the top foreign–born communities in New York City (NYC). As people migrate to new countries, they bring their ethnomedical beliefs and practices, and adapt their plant pharmacopoeias. Haiti and the DR share a flora on the island of Hispaniola. In NYC, the flora is limited to what is available in the city. We selected plants for future laboratory research based on ethnobotanical data from two surveys among Dominicans in the DR and NYC, and a Haitian literature review. In both Dominican datasets, gynecological infections were the top women’s health condition treated with plants. We identified 10 species for this purpose reported by Dominicans that are also known medicines in Haitian culture, although not yet documented for women’s health. Plants for gynecological infections potentially cause dysbiosis of the vaginal microbiota, and may increase rather than prevent disease. There is a public health need to assess traditional medicines for their ability to inhibit pathogenic bacteria, while causing minimal disruption to the vaginal flora. Several species are known antibacterials, but remain to be tested for their efficacy. These results also provide a foundation for a planned ethnobotanical survey among NYC Haitian women.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12231-021-09526-3.
Keywords: Urban ethnobotany, Ethnomedicine, Traditional knowledge, Gynecological infections, New York City, Haiti, Dominican Republic, Medicinal plants
Introduction
There are over 3.1 million immigrants living in New York City (NYC), the largest number in the city’s history (New York City Mayor’s Office of Immigrant Affairs 2020). As people migrate to other countries, they bring and adapt their plant pharmacopeias to new environments even if previously known plant species are unavailable (Vandebroek and Balick 2012). Over 500,000 foreign–born immigrants in NYC are from the island of Hispaniola, consisting of the nations of Haiti and the Dominican Republic (DR) (New York City Mayor’s Office of Immigrant Affairs 2020). There exists a rich history of traditional herbal medicine (hereafter referred to as ethnomedicine or traditional medicine, abbreviated TM) in both cultures within the Caribbean and the diaspora (Ososki et al. 2002; Quinlan 2010; Vandebroek et al. 2010; Volpato et al. 2009a, b; Weniger et al. 1986). Medicinal plants sold in botánicas (Latino and Caribbean healing shops) and prescribed by curanderos (healers) provide Caribbean and Latino immigrant communities in NYC sources of culturally significant remedies (Balick et al. 2000; Pieroni and Vandebroek 2007). Previous collaborations with the Dominican community in NYC have established how plant knowledge changes and adapts to urban environments (Vandebroek and Balick 2012). For example, food plants that are readily available in cities play an increased role in medicinal plant knowledge in NYC compared to in the DR (Vandebroek and Balick 2014). There have also been studies comparing and documenting medicinal plants for a select group of women’s health conditions (uterine fibroids, menorrhagia, endometriosis, and hot flashes) in Dominican TM literature and in practice in NYC (Ososki et al. 2002). Public health research indicates that TM involving medicinal plants is one of the primary healthcare options for women’s health in Haiti and continues to be a significant mode of healthcare delivery after migration to the United States (US) (Pan American Health Organization 2020; Seay et al. 2017a; TRAMIL 2020). On the island of Hispaniola, both Haiti and the DR have a shared flora and therefore a shared repository of medicinal plants (Carmona et al. 2010). In NYC, herbs are limited to what is available in grocery stores, botánicas, and markets. However, there has yet to be an ethnobotanical survey to document Haitian ethnomedicine in the United States.
In the literature, studies have used ethnobotany research to compare and demonstrate how two cultural groups living in close proximity with a shared flora can maintain their own unique TM practices reflective of their distinct histories and cultures (Jiang and Quave 2013; Pieroni et al. 2011). In urban environments, factors such as knowledge exchange with other communities, availability of plant species, or pressures to maintain a distinct cultural identity can shape cultural adaptation after migration (Ceuterick et al. 2008). Intracultural comparisons of TM practices within a cultural group, for example by comparing first generation immigrants with their peers living in the country of origin, can be used to study the resilience of TM (Ceuterick et al. 2011; Vandebroek and Balick 2012). On the other hand, intercultural (cross–cultural) comparisons can identify what is shared between different cultural groups in terms of plant species used, but also address health disparities that influence the use of these plants (Vandebroek 2016). Observing how two distinct cultures adapt their plant knowledge in shared environments can provide insights into specific community health needs. This is especially relevant for immigrants as politically and culturally distinct communities are often indiscriminately lumped together by census and public health records (Ceuterick et al. 2011). Comparative urban ethnobotany research in Atlanta, Georgia, evaluated differences in TM knowledge between Taiwanese and Chinese immigrants, two groups that are often considered broadly Chinese by census data (Jiang and Quave 2013). The authors of this study argued that intercultural comparisons can be applied to inform public health policy and improve access to healthcare for underserved populations by providing cultural sensitivity training for healthcare professionals.
In this paper, we set out to select plant species for laboratory analysis and to lay the groundwork for a future ethnobotanical survey with Haitian women in NYC. First, using two existing datasets (Vandebroek and Balick 2012), we made an intracultural comparison (between Dominicans living in the DR and NYC) of medicinal plants used to treat women’s health concerns. Next, we compared these plant species with those recorded as medicines in Haitian ethnobotanical literature, adding an intercultural perspective. Our hypothesis was three–fold: (1) since both groups are island–born, there likely exist similarities in women’s health concerns of Dominicans in both locations; but (2) we expect that different plant species are used in the DR and NYC for women’s health, based upon their availability in both locations; and (3) plant species important to Dominicans for women’s health will also have records as traditional medicines in Haitian ethnobotanical literature.
Methods
Two of the datasets used in this analysis originated as part of a larger ethnomedicine survey conducted in NYC and the DR between 2005–2006 (NIH grant #R21 AT001889; PI Michael J. Balick). Our methodological approach is highlighted in Fig. 1. Intracultural comparison of these two datasets aimed to identify the most salient women’s health conditions treated with medicinal plants before and after migration. For these conditions, we assumed that plant species that were used in NYC by the Dominican community must have cultural importance for other Caribbean diaspora communities and are therefore the best candidate species for future ethnopharmacology studies. We further narrowed down the selection of candidate species for laboratory analysis through a cross–cultural (Dominican–Haitian) comparison. A review of the Haitian ethnobotanical literature generated a list of overlapping species used in both Dominican and Haitian cultures as medicines. These shared species were next reviewed in the biomedical literature for existing evidence of overall antimicrobial activity (Fig. 1).
Fig. 1.
Flipped (reverse) methodology for medical ethnobotany research in response to the COVID–19 pandemic based upon the three datasets described in this paper: Two ethnobotanical Dominican datasets from New York City (NYC) and the Dominican Republic (DR), and a Haitian ethnobotanical literature review. Comparisons of these three datasets resulted in a list of species hypothesized to be relevant in both Dominican and Haitian traditional medicine (TM) for the main women’s health condition treated with medicinal plants. These plants will be prioritized in future laboratory studies investigating their bioactivity. Future ethnobotanical fieldwork with the Haitian community in NYC will further explore the role of these species, and will either disprove or confirm their relevance in urban Haitian TM. Fieldwork will likely also record new species not previously identified by our cross–cultural data comparison, and thus additional candidate species for laboratory studies
For the ethnomedicine survey, participants were recruited through convenience sampling, including snowball sampling (Tongco 2007). All participants in both survey locations met the following criteria: They were 18 years or older, born in the DR, had self–reported familiarity with medicinal plants, and confirmed a willingness to be interviewed. In NYC, 174 participants (110 women and 64 men) were surveyed in primarily Dominican communities in Washington Heights and the Bronx. In the DR, 145 participants (87 women and 58 men) were interviewed from four provinces: 1) Distrito Nacional (Santo Domingo); 2) San Pedro de Macoris; 3) Santiago; and 4) La Vega. Study areas selected in the DR corresponded to regions where a majority of Dominicans living in NYC originated (Fig. 2). More than half of all participants (60% in the DR and 63% in NYC) were female in both locations. The City University of New York (CUNY) Institutional Review Board (IRB) granted permission for this study (IRB #04–06-94 0599).
Fig. 2.
A. Study areas highlighted in grey in the Dominican Republic (provinces where surveys were conducted); B. New York City (zip codes where participants lived)
Study participants were lay persons who had a general knowledge of medicinal plants for self-care at home, except for 9 (NYC) and 17 (DR) persons who were specialists or healers. The average age of participants reporting women’s health conditions was 52 ± 12.5 years in NYC and 52 ± 16.4 years in the DR. The majority of participants in both locations received health insurance through government–funded programs, although significantly more Dominican participants living in NYC (83%) had government–funded healthcare compared to their peers in the DR (65%) (z–test with Yates correction; z = 3.02; p = 0.003) (Table 1).
Table 1.
Health insurance coverage of participants in New York City (NYC) and the Dominican Republic (DR)
| Type of Insurance | NYC Participants | DR Participants |
|---|---|---|
| Government–funded | 104 | 72 |
| Private | 10 | 18 |
| Combination | 0 | 6 |
| No insurance | 11 | 15 |
Each participant received an ID to maintain anonymity. Questionnaires were administered verbally in Spanish, and responses were recorded on printed forms. Interviews were also tape–recorded with participant permission. The questionnaire included quantitative and qualitative elements to assess plant knowledge for 30 pre–selected common health conditions chosen based on estimated prevalence in both populations. Plants used for each condition were recorded as their common Spanish names. At the end of the questionnaire, participants were asked to freelist any other conditions not named as part of the survey that they had familiarity with treating with medicinal plants.
Reported plants were collected and vouchered through field collections in the DR and by purchasing plant material in botánicas in NYC, then identified through reference works and taxonomists at the New York Botanical Garden (NYBG). Voucher specimens were deposited at the NY Steere Herbarium and the Jardin Botanico Nacional in Santo Domingo, DR. Scientific plant names followed the Catalogue of Life (https://www.catalogueoflife.org) and plant family names followed APG IV (http://www.mobot.org/MOBOT/research/APweb/).
Interview data from both survey locations were stored in separate Microsoft Access databases. Databases were exported to Microsoft Excel for further analysis. Each record represented a plant–use report that included the health condition, the common name of the plant used, and information on preparation and administration in Spanish and English. Information on the reporting participant (including ID, age, and sex) for each plant–use report was also listed. For this analysis, plant–use reports were screened for relevance to women’s health conditions. This reduced the number of participants for this paper to 126 and 121 for NYC and the DR, respectively. Male participants contributed a significant share of plant knowledge related to women’s health (88 use reports in NYC and 135 in the DR, representing 20% and 31% of all use–reports, respectively) and therefore their data were included in the analysis. No obvious gender differences were found in the number or kind of plants, or specific women’s health conditions, and the data were subsequently pooled. Reports that could apply to reproductive health in men and women (STDs) were also included. Popular plant species and health conditions were defined as those reported five times or more by participants.
The third dataset in this analysis was derived from Haitian ethnomedicine literature searches conducted in 2020 using Web of Science and Google Scholar to locate primary literature. Combinations of search terms included: [Haiti, ethnobotany], [Haiti, ethnopharmacology], [Haiti, medicinal plants], [Haiti, ethnobotany, women], and [Haiti, women’s health, plants]. Titles and abstracts were screened for relevance. Due to the lack of ethnobotanical research in Haiti, articles relating to plant use in Haitian diaspora were also included. Additionally, the TRAMILibrary database from the “Program of Applied Research to Popular Medicine in the Caribbean” was queried using the search term “Haiti” to collect additional information on common names and uses of plants with cultural significance (TRAMIL 2020). As one of the primary literature papers (Weniger et al. 1986) was supported by TRAMIL, there were some duplicate entries that were eliminated before analysis. Only 15 primary literature articles were located, demonstrating the need for further documentation of Haitian ethnomedicine practices.
A preliminary biomedical literature search related to the use of plants for gynecological infections was conducted using the Web of Science database. Search terms for each plant included the species Latin name (including all synonyms listed on the Catalogue of Life) and combinations of [microbiome], [vagina], [vaginal infection], [antibacterial], [lactobacillus], [women’s health], and [STI]/[STD]. Titles and abstracts were screened for relevance and 162 were retained for full reading.
Results
Of all plant–use reports in NYC, 11% were for women’s health conditions compared to 13% in the DR (Table 2).
Table 2.
Total plant-use reports (for all illnesses) in the Dominican Republic (DR) and New York City (NYC) compared to plant-use reports for women’s health
| Women’s health reports | Reports for all illnesses | |
|---|---|---|
| NYC | 438 | 4,179 |
| DR | 432 | 3,324 |
In both the DR and NYC, the top three women’s health conditions treated with medicinal plants coincided. These were gynecological infections (including vaginal and sexually transmitted infections), dysmenorrhea, and conditions related to birth and aftercare (puerperium) (Table 3). There were also similar numbers of popular species in NYC and the DR (50 versus 67 species). Of the top species, there were 15 species in NYC and 32 in the DR that were uniquely mentioned in each location. Caesalpinia coriaria (Jacq.) Willd. (guatapanál), Bixa orellana L. (bija), Pinus occidentalis Morelet (cuaba), and Beta vulgaris L. (remolacha or beets) were all reported at least 10 times by participants in NYC, but never or rarely mentioned in DR interviews. Similarly, Ambrosia artemisiifolia L. (altamisa) and Senna italica Mill. (sen) were reported 10 times or more in DR interviews but rarely by participants in NYC (Electronic Supplementary Material [ESM] Appendix 1).
Table 3.
Top reported women’s health conditions by 121 participants in the Dominican Republic (DR) and 126 participants in New York City (NYC). Only health conditions reported five times or more are listed
| Top Conditions | Number of plant-use reports in NYC | Number of plant-use reports in the DR |
|---|---|---|
| Gynecological infections | 87 | 103 |
| Dysmenorrhea (menstrual cramps) | 62 | 65 |
| Birth/puerperium | 58 | 54 |
| Infertility | 46 | 36 |
| Contraception | 32 | 30 |
| Abortion | 26 | 39 |
| Feminine Cleansing | 13 | 6 |
| Menstruation | 9 | 8 |
| AIDS | 5 | 0 |
| Gynecological cysts | 5 | 7 |
| Pregnancy | 0 | 5 |
The method of administration was also analyzed for plant–use reports of gynecological infections and feminine cleansing. Approximately one–fourth (26%) of preparations used for these purposes in NYC were applied externally (Table 4). Botellas are a popular Dominican preparation and consist of multiple plant and non–plant ingredients. Previous work evaluating botellas in NYC has established that one of the main uses for botellas in Dominican ethnomedicine is to treat reproductive and genitourinary health conditions (Vandebroek et al. 2010).
Table 4.
Method of administration of plant preparations for gynecological infections and feminine cleansing by Dominicans in New York City (NYC) (n = 76) and the Dominican Republic (DR) (n = 89)
| Method of administration | Number of plant-use reports in NYC |
Number of plant-use reports in the DR |
|---|---|---|
| Applied | 6 | 18 |
| Bath/Wash | 20 | 6 |
| Botella | 21 | 30 |
| Intravaginal douche | 6 | 3 |
| Drink | 30 | 9 |
| Steam bath | 2 | 0 |
| Tea | 45 | 64 |
| Unspecified | 5 | 1 |
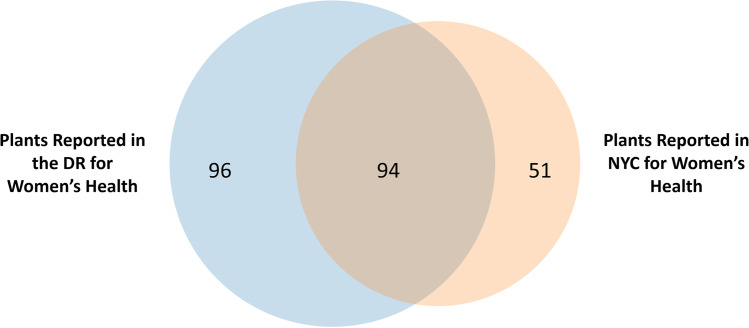
Comparison of plant species reported by Dominicans in NYC and the DR showed that almost twice as many unique species were recorded in the DR (96 species vs. 51 unique species in NYC) (Fig. 3). An approximate 40% overlap existed in species used for women’s health conditions in both locations. Plants used for women’s health in NYC included a larger number of reports for well–known food plants (e.g., Beta vulgaris and Zea mays L.) than in the DR.
Fig. 3.
Intracultural comparison of plant species reported for women’s health by Dominicans in NYC and DR
A cross–cultural comparison of plants used to treat women’s health conditions in NYC by Dominicans and those recorded in Haitian ethnomedicine literature identified 58 overlapping species. Of the 18 species used to treat gynecological infections, which was the top–reported women’s health concern for Dominicans in both populations, 10 species were shared with Haitian ethnomedicine according to the literature (Fig. 4).
Fig. 4.
Cross–cultural comparison of plant species for women’s health. Colored circles represent the following data: Number of plant species reported by Dominicans in NYC for any women’s health condition (red, green, purple, and gray colors; total of 143 species); subset of plant species only reported for gynecological infections in NYC (purple and gray, 18 species); and plant species identified as medicines by Haitian literature review for treating any kind of illness (blue, green, and gray; total of 293 species). The purple intersection represents eight species reported for gynecological infections in NYC that did not intersect with the Haitian ethnomedicine literature review. The gray intersection represents 10 species that were shared by overlapping circles and these plants are listed by their scientific names in the in–lay table. These species are of high priority for future ethnopharmacological research
We reviewed overlapping species selected from this cross–cultural comparison in the biomedical literature (ESM Appendix 2). Aloe vera (L.) Burm.f., a well–known medicinal plant globally, had well–documented antibacterial activity. However, other species such as Tradescantia spathacea Sw. or Petiveria alliacea L. had little representation in the biomedical literature. Species with reported antibacterial properties had been evaluated against bacteria such as Staphylococcus aureus and Escherichia coli that are associated with gynecological infections amongst other diseases (Blando et al. 2019; Kargaran et al. 2017; Saddiq and Al-Ghamdi 2018; Samsudin et al. 2018). However, this search did not identify a study where these species had been tested against specific gynecological pathogens or the vaginal microbiota. There were studies indicating that plants, such as Aloe vera, Plantago major L., and Opuntia ficus–indica (L.) Mill., have prebiotic activity for gut Lactobacilli (Guevara-Arauza et al. 2012; Lukova et al. 2018; Paz Quezada et al. 2017). This may also be relevant to the treatment of gynecological infections.
Discussion
We acknowledge the limitations of making cross–cultural comparisons between our ethnobotanical fieldwork data and a review of the Haitian literature, since these comparisons are based on very different methodologies. We developed this approach in response to fieldwork restrictions during the COVID–19 pandemic. The main objective was to generate hypotheses for forthcoming laboratory (ethnopharmacology) and field (ethnobotany) studies. As opposed to the typical approach followed in ethnopharmacology research (original field surveys followed by laboratory studies), we used a flipped (reverse) methodology (analysis of data from previous fieldwork with a related community to select women’s health conditions and plants to study in the laboratory, followed by a future ethnobotanical field survey). This approach generated our next hypothesis, namely that the plant species selected here will likely be mentioned during forthcoming interviews with the Haitian community in NYC, given their previously established importance as Caribbean herbal medicines (Fig. 1).
The difference in the number of plant–use reports between Dominicans in NYC and the DR suggests a loss in plant knowledge associated with migration (Fig. 3). The lower number of unique plant species recorded in NYC was consistent with previous observations in the Dominican diaspora community since many medicinal plants are replaced by more accessible options in the city available in grocery stores and markets (Vandebroek and Balick 2014). Our current research and previous studies in the Dominican community in NYC have focused on ethnomedicine practices of first–generation immigrants. Subsequent generations may experience further loss of plant knowledge as the result of acculturation. Future ethnobotanical studies evaluating plant knowledge across generations would continue to shed light on long–term trends in medicinal plant use after migration. However, despite this observed change in plant knowledge after migration, the greatest women’s health concerns treated with medicinal plants remained consistent in both locations (Table 3)—reinforcing the need to focus laboratory and fieldwork to address these concerns.
Gynecological infections were identified as the top reported health condition in both Dominican populations. Although we grouped vaginal infections and STIs into one category, it is important to note the diversity of terms used to describe individual conditions in the plant–use reports. Gonorrhea and syphilis were specifically recorded as individual health conditions, while “enfermedades venéreas” (venereal diseases) or “enfermedades de la calle” (street diseases) referred to STIs generally. These were grouped together broadly as STIs. Vaginal infections were most frequently reported as symptoms such as “flujo vaginal” (vaginal discharge) and “comezón de vagina” (vaginal itching), rather than specific infections. This aligns with the nature of common infections, such as bacterial vaginosis, that share many of the same symptoms with other infections and cannot be self–diagnosed accurately (Amabebe and Anumba 2018). Although we grouped these conditions together for analysis, medical professionals treating Dominican women need to become familiar with these specific terms, in Spanish, in order to provide the best care. Along with plants used for feminine cleansing, preparations for gynecological infections are often applied directly to the vagina for treatment. Intravaginal cleansing practices are standard across the broader Caribbean region to tighten, clean, and dry the vagina (van Andel et al. 2008). Women’s healthcare research in Miami supports that medicinal plants used for vaginal infections and intravaginal cleansing are also significant in the Haitian community in the United States (Seay et al. 2017a). In NYC, research focused on women’s health concerns and products sold for women’s health in botánicas showed that douching, among other methods that involve applying herbs to the vagina, are used for more than intravaginal cleansing and gynecological infections. Preparations applied vaginally are also used to address other issues such as infertility, menopause, abortion, as well as social and spiritual issues (Anderson et al. 2008). Since preparations of these plants are applied directly to the vagina, they can potentially disrupt the protective vaginal microbiota (Seay et al. 2017a).
The vaginal microbiota is primarily composed of Lactobacillus species and promotes a naturally acidic environment that aids in the prevention of infections (Amabebe and Anumba 2018). Vaginal infections and STIs, while generally considered treatable, can also increase the likelihood of more severe outcomes, such as human papillomavirus (HPV), pelvic inflammatory disease (PID), reduced fertility, pre–term birth, spontaneous abortion, and cervical cancer (Seay et al. 2017b). The likelihood of these negative outcomes increases with recurrent infections that are directly linked to disruption of the vaginal microbiota. Women treated with over–the–counter medications for bacterial vaginosis, the most common vaginal infection in women of reproductive age globally, experience a 50% rate of recurrent infection as result of the disruption of the vaginal microbiota by broad–spectrum antibiotics (Huang et al. 2014). There is also evidence that medicinal plants used for intravaginal cleansing and gynecological infections may negatively impact the vaginal microbiota and cause disease (Halperin 1999; Seay et al. 2017a; van Andel et al. 2008).
However, none of the plants selected from our cross–cultural comparison for gynecological infections had literature documenting their bioactivity related to women’s health. It is crucial for women’s health that the plants used for these purposes inhibit pathogenic bacteria, while causing minimal disruption to the vaginal microbiota. Previous studies documenting the effects of natural products on vaginal bacteria have accomplished this in a laboratory setting by comparing minimal inhibitory concentrations (MIC) in microbiological assays to determine whether extracts had higher antibacterial effects (lower MIC values) for pathogenic bacteria than beneficial microbes (higher MIC values) (Machado et al. 2017; Mandim et al. 2019). However, these studies did not focus on medicinal plants prepared according to their traditional methods of preparation. Different plant parts and extraction methods can alter the chemistry and medicinal properties (Gu et al. 2019; Kulakowski et al. 2015). Plant–use reports for gynecological infections in the Dominican community in NYC included different forms of preparations of each species used for the same purpose. For example, the leaves of Bixa orellana are prepared either as a tea or botella for gynecological infections. In order to provide relevant information about the biomedical effectiveness of these species to women who use these plants, there needs to be laboratory research on the antibacterial effects and chemistry of the plant as it is traditionally prepared.
Our cross–cultural comparison and literature review also revealed the need to further document Haitian TM through fieldwork. Our search only found 15 scientific articles focused on different aspects of Haitian ethnobotany (Albuquerque et al. 2012; Albuquerque et al. 2013; Farmer 1988; Paul and Akers 2000; Paul and Cox 1995; Pawlus and Kinghorn 2007; Quinlan 2010; Safford 1916; Tarter 2015; Weniger et al. 1982; Weniger et al. 1986). Several of these articles were not based in Haiti, but in Cuba or Miami and focused on the Haitian diaspora (Hodges and Bennett 2006; Volpato and Godínez 2004; Volpato et al. 2009a,b). The most comprehensive ethnobotanical survey was conducted several decades ago in 1986 as part of TRAMIL–funded ethnobotany research (Weniger et al. 1986). This author also published the only article that focused on medicinal plants used for women’s health in Haiti (Weniger et al. 1982), which documented plants used as antifertility agents. Although this work also provided a review of biomedical literature to support the use of several species and further laboratory studies, this review is several decades old and would benefit from updating. Lack of documentation of traditional plant use for women’s health is not unique to Haitian ethnobotany. Other authors have noted that many ethnobotanical studies aggregate all women’s health conditions together under vague terms such as “reproductive disorders” or they omit plants used for women’s health altogether, especially those used as abortifacients and other controversial uses (van Andel et al. 2014). The consequences of overlooking TM for women’s health in ethnobotanical research is far reaching globally. Without proper documentation of the preparations used, there cannot be further research on the efficacy and safety of use for the women who use these plants.
There is a significant need to document current Haitian ethnobotany with a focus on women’s health to identify further species for laboratory study. Haitian women, living in Haiti and abroad, have the highest incidence of cervical cancer in the Western Hemisphere (Seay et al. 2017b). Women in Haiti also suffer from one of the highest rates of maternal mortality (521 deaths/100,000 births) in the New World (WHO et al. 2019). These high numbers can be linked, in part, to the prevalence of gynecological, including puerperal, infections (Gibson et al. 2013; Seay et al. 2017b). In Haiti, this is primarily due to lack of access to medical services and clean water. Public health research indicates that traditional medicine is not only culturally important, but one of the only options available for the treatment of women’s health conditions in Haiti (Pan American Health Organization 2020). As Haitian women migrate to the United States, these plants remain an important part of their cultural tradition as well as a way to deal with continued health disparities that contribute to illness (Menard et al. 2010). Haitian immigrants in NYC are one of the foreign–born immigrant groups least likely to receive necessary Medicaid assistance although they are also considered one of the lowest income immigrant groups (New York City Mayor’s Office of Immigrant Affairs 2020). According to public health research, language barriers, lack of acculturation, limited duration of residence in the United States, unfamiliarity with the health care system, and undocumented immigration status correlate with lower access to Medicaid in the U.S. Haitian community (Saint-Jean and Crandall 2005). Evaluation of medicinal plants for women’s health, especially those used for gynecological infections, will provide scientific information to support culturally competent women’s healthcare through outreach to community members and medical professionals alike (Vandebroek 2013).
Conclusion
Medicinal plants can provide low–cost and effective healthcare when other means are not readily available within a population. The World Health Organization recommends that medicinal plants are assessed in laboratory studies. However, we would like to acknowledge that this view can be controversial among ethnobiologists and that the call for scientific validation of TM and medicinal plants is not universally accepted. There is a significant research gap in Haitian ethnomedicine both in Haiti and abroad, especially for women’s health conditions. Intra– and intercultural comparisons, as we made using ethnobotanical fieldwork data from the transnational Dominican community and a Haitian literature review, has provided preliminary data to begin to fill this gap in knowledge. Gynecological infections were the greatest women’s health concern for Dominican women, but plants used for these conditions were understudied in the biomedical literature in the context of women’s health. Cross–cultural comparisons with existing Haitian literature have identified plant species that will be prioritized for laboratory analysis to provide evidence–based feedback to Caribbean women who use these plants. Future ethnobotanical fieldwork will expand the scope of laboratory studies and provide further cross–cultural comparisons. These approaches will generate information that will be returned to the Haitian community through outreach and help promote culturally competent community health.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The maps of New York City and the Dominican Republic were made by Liz Gjieli, Geographical Information Manager at The New York Botanical Garden. The authors thank all interviewees for participating in interviews. The transnational Dominican community holds sole ownership over their traditional knowledge and biocultural heritage.
Literature Cited
- Abbaszadegan A, Sahebi S, Gholami A, Delroba A, Kiani A, Iraji A, Abbott PV. Time–dependent antibacterial effects of Aloe vera and Zataria multiflora plant essential oils compared to calcium hydroxide in teeth infected with Enterococcus faecalis. Journal of Investigative and Clinical Dentistry. 2016;7(1):93–101. doi: 10.1111/jicd.12123. [DOI] [PubMed] [Google Scholar]
- Adomi PO. Screening of the leaves of three Nigerian medicinal plants for antibacterial activity. African Journal of Biotechnology. 2008;7(15):2540–2542. [Google Scholar]
- Aidah N, Abdullah N, Oskoueian E, Sieo CC, Saad WZ. Membrane–active antibacterial compounds in methanolic extracts of Jatropha curcas and their mode of action against Staphylococcus aureus S1434 and Escherichia coli E216. International Journal of Agriculture and Biology. 2014;16(4):723–730. [Google Scholar]
- Akinpelu DA, Aiyegoro OA, Okoh AI. The bioactive potentials of two medicinal plants commonly used as folklore remedies among some tribes in West Africa. African Journal of Biotechnology. 2009;8(8):1660–1664. [Google Scholar]
- Akinyele TA, Igbinosa EO, Akinpelu DA, Okoh AI. In vitro assessment of the synergism between extracts of Cocos nucifera husk and some standard antibiotics. Asian Pacific Journal of Tropical Biomedicine. 2017;7(4):306–313. doi: 10.1016/j.apjtb.2016.12.022. [DOI] [Google Scholar]
- ———, O. O. Okoh, D. A. Akinpelu, and A. I. Okoh 2011. In vitro antibacterial properties of crude aqueous and n–hexane extracts of the husk of Cocos nucifera. Molecules 16(3):2135–2145. 10.3390/molecules16032135. [DOI] [PMC free article] [PubMed]
- Albuquerque, U. P., J. G. Melo, M. F. Medeiros, I. R. Menezes, G. J. Moura, A. C. Asfora El–Deir, R. R. Nóbrega Alves, P. Muniz de Medeiros, T. A. Araújo, M. A. Ramos, R. R. Silva, A. L. Almeida, and C. F. Almeida. 2012. Natural products from ethnodirected studies: Revisiting the ethnobiology of the zombie poison. Evidence–Based Complementary and Alternative Medicine 2012. [DOI] [PMC free article] [PubMed]
- ———, J. S. Silva, J. L. A. Campos, R. S. Sousa, T. C. Silva, and R. R. N. Alves. 2013. The current status of ethnobiological research in Latin America: Gaps and perspectives. Journal of Ethnobiology and Ethnomedicine 9(1):1–9. [DOI] [PMC free article] [PubMed]
- Alemdar S, Agaoglu S. Investigation of in vitro antimicrobial activity of Aloe vera juice. Journal of Animal and Veterinary Advances. 2009;8(1):99–102. [Google Scholar]
- Ali SW, Purwar R, Joshi M, Rajendran S. Antibacterial properties of Aloe vera gel–finished cotton fabric. Cellulose. 2014;21(3):2063–2072. doi: 10.1007/s10570-014-0175-9. [DOI] [Google Scholar]
- Alviano WS, Alviano DS, Diniz CG, Antoniolli AR, Alviano CS, Farias LM, Carvalho MAR, Souza MMG, Bolognese AM. In vitro antioxidant potential of medicinal plant extracts and their activities against oral bacteria based on Brazilian folk medicine. Archives of Oral Biology. 2008;53(6):545–552. doi: 10.1016/j.archoralbio.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Amabebe E, Anumba DO. The vaginal microenvironment: The physiologic role of lactobacilli. Frontiers in Medicine. 2018;5:181. doi: 10.3389/fmed.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amjed, S., K. Junaid, J. Jafar, T. Amjad, W. Maqsood, N. Mukhtar, K. Tariq, M. Sharif, S. J. Awan, and F. Ansari. 2017. Detection of antibacterial activities of Miswak, Kalonji and Aloe vera against oral pathogens and anti–proliferative activity against cancer cell line. BMC Complementary and Alternative Medicine 17. 10.1186/s12906-017-1778-0. [DOI] [PMC free article] [PubMed]
- Ammar I, Bardaa S, Mzid M, Sahnoun Z, Rebaii T, Attia H, Ennouri M. Antioxidant, antibacterial and in vivo dermal wound healing effects of Opuntia flower extracts. International Journal of Biological Macromolecules. 2015;81:483–490. doi: 10.1016/j.ijbiomac.2015.08.039. [DOI] [PubMed] [Google Scholar]
- Anderson MR, Mckee D, Yukes J, Alvarez A, Karasz A. An investigation of douching practices in the botánicas of the Bronx. Culture, Health and Sexuality. 2008;10(1):1–11. doi: 10.1080/13691050701516363. [DOI] [PubMed] [Google Scholar]
- Andleeb S, Alsalme A, Al-Zaqri N, Warad I, Alkahtani J, Bukhari SM. In –vitro antibacterial and antifungal properties of the organic solvent extract of Argemone mexicana L. Journal of King Saud University Science. 2020;32(3):2053–2058. doi: 10.1016/j.jksus.2020.01.044. [DOI] [Google Scholar]
- Anwar S, Zubair M, Rashid U, Rizwan K, Shahid M, Bukhari IH, Jamil M, Ahmad VU, Rasool N. Antimicrobial potential of methanolic extract and various fractions of Jatropha curcas roots. Asian Journal of Chemistry. 2013;25(13, A):7477–7480. doi: 10.14233/ajchem.2013.14901. [DOI] [Google Scholar]
- Arbia L, Chikhi-Chorfi N, Betatache I, Pham-Huy C, Zenia S, Mameri N, Drouiche N, Lounici H. Antimicrobial activity of aqueous extracts from four plants on bacterial isolates from periodontitis patients. Environmental Science and Pollution Research. 2017;24(15):13394–13404. doi: 10.1007/s11356-017-8942-4. [DOI] [PubMed] [Google Scholar]
- Aruwa CE, Amoo SO, Kudanga T. Extractable and macromolecular antioxidants of Opuntia ficus–indica cladodes: Phytochemical profiling, antioxidant, and antibacterial activities. South African Journal of Botany. 2019;125:402–410. doi: 10.1016/j.sajb.2019.08.007. [DOI] [Google Scholar]
- ———, S. Amoo, and T. Kudanga. 2019b. Phenolic compound profile and biological activities of Southern African Opuntia ficus–indica fruit pulp and peels. LWT–food Science and Technology 111:337–344. 10.1016/j.lwt.2019.05.028.
- Ayyoob M, Khurshid MF, Asad M, Shah SNH. Assessment of eco–friendly natural antimicrobial textile finish extracted from Aloe Vera and neem plants. Fibres and Textiles in Eastern Europe. 2015;23(6):120–123. doi: 10.5604/12303666.1172176. [DOI] [Google Scholar]
- Babahmad RA, Aghraz A, Boutafda A, Papazoglou EG, Tarantilis PA, Kanakis C, Hafidi M, Ouhdouch Y, Outzourhit A, Ouhammou A. Chemical composition of essential oil of Jatropha curcas L. leaves and its antioxidant and antimicrobial activities. Industrial Crops and Products. 2018;121:405–410. doi: 10.1016/j.indcrop.2018.05.030. [DOI] [Google Scholar]
- Babaji P, Jagtap K, Lau H, Bansal N, Thajuraj S, Sondhi P. Comparative evaluation of antimicrobial effect of herbal root canal irrigants (Morinda citrifolia, Azadirachta indica, Aloe vera) with sodium hypochlorite: An in vitro study. Journal of International Society of Preventive and Community Dentistry. 2016;6(3):196–199. doi: 10.4103/2231-0762.183104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachabi F, Togbe CE, Odjo T, Were EO, Sere Y, Gumedzoe YMD. Laboratory assessment of the antibacterial effect of Moringa oleifera Lam., Azadirachta indica L. and Jatropha curcas L. on Pantoea spp and Sphingomonas spp in rice seed in Benin. Tropical Agriculture. 2018;95(2):106–114. [Google Scholar]
- Balick MJ, Fronenberg F, Ososki AL, Reiff M, Fugh-Berman A, Roble M, Lohr P, Atha D. Medicinal plants used by Latino healers for women’s health conditions. Economic Botany. 2000;54(3):344–357. doi: 10.1007/BF02864786. [DOI] [Google Scholar]
- Banadkoki AZ, Kouhsari E, Amirmozafari N, Roudbary M, Nasrabadi MRB. Antibacterial, antifungal, and cytotoxic activities of some medicinal plants against multidrug resistance pathogens. Reviews in Medical Microbiology. 2018;29(4):182–188. doi: 10.1097/MRM.0000000000000146. [DOI] [Google Scholar]
- Barragan–Menendez, C., D. Galvez–Lopez, R. Rosas–Quijano, M. Salvador–Figueroa, I. Ovando–Medina, and A. Vazquez–Ovando. 2020. Films of chitosan and Aloe vera for maintaining the viability and antifungal activity of Lactobacillus paracasei TEP6. Coatings 10(3). 10.3390/coatings10030259.
- Basannavar S, Pothuraju R, Sharma RK. Effect of Aloe vera (Aloe barbadensis Miller) on survivability, extent of proteolysis and ACE inhibition of potential probiotic cultures in fermented milk. Journal of the Science of Food and Agriculture. 2014;94(13):2712–2717. doi: 10.1002/jsfa.6615. [DOI] [PubMed] [Google Scholar]
- Bashir A, Saeed B, Mujahid TY, Jehan N. Comparative study of antimicrobial activities of Aloe vera extracts and antibiotics against isolates from skin infections. African Journal of Biotechnology. 2011;10(19):3835–3840. [Google Scholar]
- Bawankar R, Deepti VC, Singh P, Subashkumar R, Vivekanandhan G, Babu S. Evaluation of bioactive potential of an Aloe vera sterol extract. Phytotherapy Research. 2013;27(6):864–868. doi: 10.1002/ptr.4827. [DOI] [PubMed] [Google Scholar]
- Benzidia B, Barbouchi M, Hammouch H, Belahbib N, Zouarhi M, Erramli H, Daoud NA, Badrane N, Hajjaji N. Chemical composition and antioxidant activity of tannins extract from green rind of Aloe vera (L.) Burm. F. Journal of King Saud University Science. 2019;31(4):1175–1181. doi: 10.1016/j.jksus.2018.05.022. [DOI] [Google Scholar]
- Bhattacharjee I, Chatterjee SK, Chandra G. Isolation and identification of antibacterial components in seed extracts of Argemone mexicana L. (Papaveraceae) Asian Pacific Journal of Tropical Medicine. 2010;3(7):547–551. doi: 10.1016/S1995-7645(10)60132-0. [DOI] [PubMed] [Google Scholar]
- Blando, F., R. Russo, C. Negro, L. De Bellis, and S. Frassinetti. 2019. Antimicrobial and antibiofilm activity against Staphylococcus aureus of Opuntia ficus–indica (L.) Mill. cladode polyphenolic extracts. Antioxidants 8(5). 10.3390/antiox8050117. [DOI] [PMC free article] [PubMed]
- Brahmi, F., S. Haddad, K. Bouamara, D. Yalaoui–Guellal, E. Prost–Camus, J. P. P. De Barros, M. Prost, A. G. Atanasov, K. Madani, L. Boulekbache–Makhlouf, and G. Lizard. 2020. Comparison of chemical composition and biological activities of Algerian seed oils of Pistacia lentiscus L., Opuntia ficus indica (L.) Mill. and Argania spinosa L. Skeels. Industrial Crops and Products 151. 10.1016/j.indcrop.2020.112456.
- Canche–Escamilla, G., P. Colli–Acevedo, R. Borges–Argaez, P. Quintana–Owen, J. Fernando May–Crespo, M. Caceres–Farfan, J. A. Yam Puc, P. Sansores–Peraza, and B. Marina Vera–Ku. 2019. Extraction of phenolic components from an Aloe vera (Aloe barbadensis Miller) crop and their potential as antimicrobials and textile dyes. Sustainable Chemistry and Pharmacy 14. 10.1016/j.scp.2019.100168.
- Carlos Guevara-Arauza J, de Jesus Ornelas-Paz J, Jaqueline Pimentel-Gonzalez D, Rosales Mendoza S, Soria Guerra RE, Paz Maldonado LMT. Prebiotic effect of mucilage and pectic–derived oligosaccharides from nopal (Opuntia ficus–indica) Food Science and Biotechnology. 2012;21(4):997–1003. doi: 10.1007/s10068-012-0130-1. [DOI] [Google Scholar]
- Carmona EC, Ramírez AV, Cano-Ortiz A. Contribution to the biogeography of the Hispaniola (Dominican Republic, Haiti) Acta Botanica Gallica. 2010;157(4):581–598. doi: 10.1080/12538078.2010.10516233. [DOI] [Google Scholar]
- Ceja–Medina, L. I., R. I. Ortiz–Basurto, L. Medina–Torres, F. Calderas, M. J. Bernad–Bernad, R. F. Gonzalez–Laredo, J. A. Ragazzo–Sanchez, M. Calderon–Santoyo, M. Gonzalez–avila, I. Andrade–Gonzalez, and O. Manero. 2020. Microencapsulation of Lactobacillus plantarum by spray drying with mixtures of Aloe vera mucilage and Agave fructans as wall materials. Journal of Food Process Engineering 43(8). 10.1111/jfpe.13436.
- Cellini L, Di Bartolomeo S, Di Campli E, Genovese S, Locatelli M, Di Giulio M. In vitro activity of Aloe vera inner gel against Helicobacter pylori strains. Letters in Applied Microbiology. 2014;59(1):43–48. doi: 10.1111/lam.12241. [DOI] [PubMed] [Google Scholar]
- Ceuterick M, Vandebroek I, Pieroni A. Resilience of Andean urban ethnobotanies: A comparison of medicinal plant use among Bolivian and Peruvian migrants in the United Kingdom and in their countries of origin. Journal of Ethnopharmacology. 2011;136(1):27–54. doi: 10.1016/j.jep.2011.03.038. [DOI] [PubMed] [Google Scholar]
- ———, I. Vandebroek, B. Torry, and A. Pieroni. 2008. Cross–cultural adaptation in urban ethnobotany: The Colombian folk pharmacopoeia in London. Journal of Ethnopharmacology 120(3):342–359. [DOI] [PubMed]
- Chandra G, Bhattacharjee I, Chatterjee S. Bacillus cereus infection in stinging catfish, Heteropneustes fossilis (Siluriformes: Heteropneustidae) and their recovery by Argemone mexicana seed extract. Iranian Journal of Fisheries Sciences. 2015;14(3):741–753. [Google Scholar]
- Chugh CA, Mehta S, Dua H. Phytochemical screening and evaluation of biological activities of some medicinal plants of Phagwara. Punjab. Asian Journal of Chemistry. 2012;24(12):5903–5905. [Google Scholar]
- Chumpol J, Siri S. Electrospun cellulose acetate membrane for size separating and antibacterial screening of crude polysaccharides. IET Nanobiotechnology. 2016;10(6):405–410. doi: 10.1049/iet-nbt.2015.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogo LL, Bastos Monteiro CL, Miguel MD, Miguel OG, Cunico MM, Ribeiro ML, de Camargo ER, Botao Kussen GM, Nogueira KdaS, Dalla Costa LM. Anti-Helicobacter pylori activity of plant extracts traditionally used for the treatment of gastrointestinal disorders. Brazilian Journal of Microbiology. 2010;41(2):304–309. doi: 10.1590/S1517-83822010000200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuong TV, Chin KB. Effects of Annatto (Bixa orellana L.) seeds powder on physicochemical properties, antioxidant, and antimicrobial activities of pork patties during refrigerated storage. Korean Journal for Food Science of Animal Resources. 2016;36(4):476–486. doi: 10.5851/kosfa.2016.36.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahiya P, Purkayastha S. Phytochemical screening and antimicrobial activity of some medicinal plants against multi–drug resistant bacteria from clinical isolates. Indian Journal of Pharmaceutical Sciences. 2012;74(5):443–450. doi: 10.4103/0250-474X.108420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabighane B, Zarei A, Shahneh AZ. The effects of different levels of Aloe vera gel on ileum microflora population and immune response in broilers: A comparison to antibiotic effects. Journal of Applied Animal Research. 2012;40(1):31–36. doi: 10.1080/09712119.2011.620435. [DOI] [Google Scholar]
- Das, B., N. Salvanna, P. R. Reddy, J. Paramesh, and R. Das. 2018. Our phytochemical research on Jatropha species. ARKIVOC 1, SI: 114–133. 10.24820/ark.5550190.p010.285.
- de los Angeles Ortega-Ortega M, del Socorro Cruz-Cansino N, Alanis-Garcia E, Delgado-Olivares L, Alberto Ariza-Ortega J, Ramirez-Moreno E, de Jesus Manriquez-Torres J. Optimization of ultrasound extraction of cactus pear (Opuntia ficus indica) seed oil based on antioxidant activity and evaluation of its antimicrobial activity. Journal of Food Quality. 2017 doi: 10.1155/2017/9315360. [DOI] [Google Scholar]
- Donkor, A. M., M. N. Donkor, and N. Kuubabongnaa. 2020. Evaluation of anti–infective potencies of formulated aloin A ointment and aloin an isolated from Aloe barbadensis Miller. BMC Chemistry 14(1). 10.1186/s13065-020-0659-7. [DOI] [PMC free article] [PubMed]
- El Mannoubi I. Effect of extraction solvent on phenolic composition, antioxidant and antibacterial activities of skin and pulp of Tunisian red and yellow–orange Opuntia ficus indica fruits. Journal of Food Measurement and Characterization. 2020 doi: 10.1007/s11694-020-00673-0. [DOI] [Google Scholar]
- El-Beltagi HS, Mohamed HI, Elmelegy AA, Eldesoky SE, Safwat G. Phytochemical screening, antimicrobial, antioxidant, anticancer activities, and nutritional values of Cactus (Opuntia ficus indica) pulp and peel. Fresenius Environmental Bulletin. 2019;28(2A):1545–1562. [Google Scholar]
- Ennouri M, Ammar I, Khemakhem B, Attia H. Chemical composition and antibacterial activity of Opuntia ficus–indica F. inermis (Cactus Pear) flowers. Journal of Medicinal Food. 2014;17(8):908–914. doi: 10.1089/jmf.2013.0089. [DOI] [PubMed] [Google Scholar]
- Erhabor JO, Idu M. In vitro antibacterial, antioxidant, cytogenotoxic and nutritional value of Aloe barbadensis Mill. (Asphodelaceae) Journal of Microbiology Biotechnology and Food Sciences. 2020;10(1):12–21. doi: 10.15414/jmbfs.2020.10.1.12-21. [DOI] [Google Scholar]
- Farmer P. Bad blood, spoiled milk: Bodily fluids as moral barometers in rural Haiti. American Ethnologist. 1988;15(1):62–83. doi: 10.1525/ae.1988.15.1.02a00050. [DOI] [Google Scholar]
- Fit IN, Rapuntean G, Rapuntean S, Chirila F, Nadas GC. Antibacterial effect of essential vegetal extracts on Staphylococcus aureus compared to antibiotics. Notulae Botanicae Horti Agrobotanici Cluj-napoca. 2009;37(2):117–123. [Google Scholar]
- Ganesan P, Karthik T. Analysis of colour strength, colour fastness and antimicrobial properties of silk fabric dyed with natural dye from red prickly pear fruit. Journal of the Textile Institute. 2017;108(7):1173–1179. doi: 10.1080/00405000.2016.1222862. [DOI] [Google Scholar]
- Ganesh MS, John NAA, Radhika J. A comparative study of antibacterial potential of various extracts of different parts of Jatropha curcas Linn against bacterial strains. International Journal of Life Science And Pharma Research. 2020;10(2):L83–L87. doi: 10.22376/ijpbs/lpr.2020.10.2.L83-87. [DOI] [Google Scholar]
- García-Varela R, García-García RM, Barba-Dávila BA, Fajardo-Ramírez OR, Serna-Saldívar SO, Cardineau GA. Antimicrobial activity of Rhoeo discolor phenolic rich extracts determined by flow cytometry. Molecules. 2015;20(10):18685–18703. doi: 10.3390/molecules201018685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam CVS, Rekha M, Mourya P, Sukanya S, Unissa H. Evaluation of antibacterial and antifungal activity of Aloe vera gel. Indo American Journal of Pharmaceutical Sciences. 2017;4(4):834–839. doi: 10.5281/zenodo.546665. [DOI] [Google Scholar]
- Ghayempour S, Montazer M, Rad MM. Simultaneous encapsulation and stabilization of Aloe vera extract on cotton fabric for wound dressing application. RSC Advances. 2016;6(113):111895–111902. doi: 10.1039/c6ra22485g. [DOI] [Google Scholar]
- Gibson M, Carlson Bowles B, Jansen L, Leach J. Childbirth education in rural Haiti: Reviving low–tech teaching strategies. The Journal of Perinatal Education. 2013;22(2):93–102. doi: 10.1891/1058-1243.22.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokulan, K., P. Kolluru, C. E. Cerniglia, and S. Khare. 2019. Dose–dependent effects of aloin on the intestinal bacterial community structure, short chain fatty acids metabolism and intestinal epithelial cell permeability. Frontiers in Microbiology 10. 10.3389/fmicb.2019.00474. [DOI] [PMC free article] [PubMed]
- Gonzalez BA, Dominguez-Espinosa R, Alcocer BR. Use of Aloe vera juice as substrate for growth of Lactobacillus plantarum and L. casei. Ciencia Y Tecnologia Alimentaria. 2008;6(2):152–157. doi: 10.1080/11358120809487640. [DOI] [Google Scholar]
- Goud, S., S. Aravelli, S. Dronamraju, G. Cherukuri, and P. Morishetty. 2018. Comparative evaluation of the antibacterial efficacy of the Aloe Vera, 3% sodium hypochlorite, and 2% chlorhexidine gluconate against Enterococcus faecalis: an in vitro study. Cureus 10(10). 10.7759/cureus.3480. [DOI] [PMC free article] [PubMed]
- Gu RH, Morcol T, Liu B, Shi MJ, Kennelly EJ, Long CL. GC–MS, UPLC–QTOF–MS, and bioactivity characterization of Acer truncatum seeds. Industrial Crops and Products. 2019;138:111480. doi: 10.1016/j.indcrop.2019.111480. [DOI] [Google Scholar]
- Guedes RCM, Nogueira NGP, Fusco-Almeida AM, Souza CRF, Oliveira WP. Antimicrobial activity of crude extracts of Petiveria alliacea L. Latin American Journal of Pharmacy. 2009;28(4):520–524. [Google Scholar]
- Guevara-Arauza JC, de Jesus Ornelas-Paz J, Jaqueline Pimentel-Gonzalez D, Rosales Mendoza S, Soria Guerra RE, Paz Maldonado LMT. Prebiotic effect of mucilage and pectic–derived oligosaccharides from nopal (Opuntia ficus–indica) Food Science and Biotechnology. 2012;21(4):997–1003. doi: 10.1007/s10068-012-0130-1. [DOI] [Google Scholar]
- Habibi G, Arjomandzadegan M, Tayeboon M, Didgar F, Sarmadiani H, Sadrnia M, Mirhosseini F, Geravand S, Abdoli M. Comparison of antibacterial effects of a carrier produced in microemulsion system from aqueous extract of Aloe vera with selected antibiotics on Enterobacteriacea. Iranian Journal of Microbiology. 2018;10(5):334–341. [PMC free article] [PubMed] [Google Scholar]
- Hai, Z., Y. Ren, J. Hu, H. Wang, Q. Qin, and T. Chen. 2019. Evaluation of the treatment effect of Aloe vera fermentation in burn injury healing using a rat model. Mediators of Inflammation 2019. 10.1155/2019/2020858. [DOI] [PMC free article] [PubMed]
- Halperin DT. Dry sex practices and HIV infection in the Dominican Republic and Haiti. Sexually Transmitted Infections. 1999;75(6):445. doi: 10.1136/sti.75.6.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq, A., M. Siddiqi, S. Z. Batool, A. Islam, A. Khan, D. Khan, S. Khan, H. Khan, A. A. Shah, F. Hasan, S. Ahmed, and M. Badshah. 2019. Comprehensive investigation on the synergistic antibacterial activities of Jatropha curcas pressed cake and seed oil in combination with antibiotics. AMB Express 9. 10.1186/s13568-019-0793-6. [DOI] [PMC free article] [PubMed]
- He CL, Fu BD, Shen HQ, Jiang XL, Wei XB. Fumaric acid, an antibacterial component of Aloe vera L. African Journal of Biotechnology. 2011;10(15):2973–2977. doi: 10.5897/AJB10.1497. [DOI] [Google Scholar]
- Hodges S, Bennett BC. The ethnobotany of Pluchea carolinensis (Jacq.) G. Don (Asteraceae) in the botánicas of Miami, Florida. Economic Botany. 2006;60(1):75–84. doi: 10.1663/0013-0001(2006)60[75:TEOPCJ]2.0.CO;2. [DOI] [Google Scholar]
- Hovorkova P, Lalouckova K, Skrivanova E. Determination of in vitro antibacterial activity of plant oils containing medium–chain fatty acids against gram–positive pathogenic and gut commensal bacteria. Czech Journal of Animal Science. 2018;63(3):119–125. doi: 10.17221/70/2017-CJAS. [DOI] [Google Scholar]
- Hrishikesh, V. and P. Meena. 2011. Antimicrobial activity of some selected medicinal plants on skin pathogens. Research Journal of Biotechnology 6(3):67–72. https://www.paho.org/salud-en-las-americas-2017/?p=4110.
- Huang H, Song L, Zhao W. Effects of probiotics for the treatment of bacterial vaginosis in adult women: A meta-analysis of randomized clinical trials. Archives of Gynecology and Obstetrics. 2014;289(6):1225–1234. doi: 10.1007/s00404-013-3117-0. [DOI] [PubMed] [Google Scholar]
- Ibrahim NA, Abd El-Ghany NA, Eid BM, Mabrouk EM. Green options for imparting antibacterial functionality to cotton fabrics. International Journal of Biological Macromolecules. 2018;111:526–533. doi: 10.1016/j.ijbiomac.2018.01.013. [DOI] [PubMed] [Google Scholar]
- Imran H, Sohail T, Atiq-ur-Rehman IW, Fatima N, Shakir M. A comparative study of four indigenous medicinal plants of Pakistan against some oral pathogens. Bangladesh Journal of Medical Science. 2020;19(2):284–290. doi: 10.3329/bjms.v19i2.45009. [DOI] [Google Scholar]
- Jain S, Rathod N, Nagi R, Sur J, Laheji A, Gupta N, Agarwal P, Prasad S. Antibacterial effect of Aloe vera gel against oral pathogens: An in vitro study. Journal of Clinical and Diagnostic Research. 2016;10(11):ZC41–ZC44. doi: 10.7860/JCDR/2016/21450.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessenia Pesantes–Sangay, S., R. Damaso Calla–Poma, M. Fe Requena–Mendizabal, M. Isabel Alvino–Vales, and P. Alejandro Millones–Gomez. 2020. Chemical composition and antibacterial effect of Plantago major extract on periodontal pathogens. Pesquisa Brasileira Em Odontopediatria E Clinica Integrada 20. 10.1590/pboci.2020.100.
- Jiang, L., T. Yi, Z. Shen, Z. Teng, and J. Wang. 2019. Aloe–emodin attenuates Staphylococcus aureus pathogenicity by interfering with the oligomerization of alpha–toxin. Frontiers in Cellular and Infection Microbiology 9. 10.3389/fcimb.2019.00157. [DOI] [PMC free article] [PubMed]
- Jiang, M., K. Deng, C. Jiang, M. Fu, C. Guo, X. Wang, X. Wang, F. Meng, S. Yang, K. Deng, T. Chen, and H. Xin. 2016. Evaluation of the antioxidative, antibacterial, and anti–inflammatory effects of the Aloe fermentation supernatant containing Lactobacillus plantarum HM218749.1. Mediators of Inflammation 2016. 10.1155/2016/2945650. [DOI] [PMC free article] [PubMed]
- Jiang S, Quave CL. A comparison of traditional food and health strategies among Taiwanese and Chinese immigrants in Atlanta, Georgia, USA. Journal of Ethnobiology and Ethnomedicine. 2013;9(1):61. doi: 10.1186/1746-4269-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose Gallardo–Vasquez, G., J. Elvira Chavez–Flores, and M. Contreras–Torvisco. 2019. Evaluation of the antibacterial effect of latex Jatropha curcas ‘pinon’ in front of Staphylococcus aureus. DUAZARY 16(1):105–114. 10.21676/2389783X.2533.
- Karakas FP, Yildirim A, Turker A. Biological screening of various medicinal plant extracts for antibacterial and antitumor activities. Turkish Journal of Biology. 2012;36(6):641–652. doi: 10.3906/biy-1203-16. [DOI] [Google Scholar]
- Kargaran, M., A. R. Moradabadi, M. Arjomandzadegan, H. Hosseini, G. Habibi, M. Tayeboon, H. Karami, and A. Akbari. 2017. Effects of the aqueous extract of Aloe vera on the morphological and physiological properties of E. coli. Iranian Red Crescent Medical Journal 19(2). 10.5812/ircmj.23896.
- Kerdudo, A., V. Gonnot, E. N. Ellong, L. Boyer, T. Michel, S. Adenet, K. Rochefort, and X. Fernandez. 2015. Essential oil composition and biological activities of Petiveria alliacea L. from Martinique. Journal of Essential Oil Research 27(3):186–196. 10.1080/10412905.2015.1014118.
- ———, V. Gonot, E. N. Ellong, K. Rochefort, L. Boyer, T. Michel, and X. Fernandez. 2014. New antibacterial compounds from plant biodiversity. Planta Medica 80(16):1462–1463.
- Khan AM, Bhadauria S. Analysis of medicinally important phytocompounds from Argemone mexicana. Journal of King Saud University Science. 2019;31(4):1020–1026. doi: 10.1016/j.jksus.2018.05.009. [DOI] [Google Scholar]
- Kim, Y. W., Y. J. Jeong, A. Y. Kim, H. H. Son, J. A. Lee, C. H. Jung, C. H. Kim, and J. Kim. 2014. Lactobacillus brevis strains from fermented Aloe vera survive gastroduodenal environment and suppress common food borne enteropathogens. PLOS One 9(3). 10.1371/journal.pone.0090866. [DOI] [PMC free article] [PubMed]
- Kulakowski D, Kitalong C, Negrin A, Tadao VR, Balick MJ, Kennelly EJ. Traditional preparation of Phaleria nisidai, a Palauan tea, reduces exposure to toxic daphnane–type diterpene esters while maintaining immunomodulatory activity. Journal of Ethnopharmacology. 2015;173:273–279. doi: 10.1016/j.jep.2015.06.023. [DOI] [PubMed] [Google Scholar]
- Kumar D, Kashyap SK, Maherchandani S. Antibacterial activity of some plant extracts. Veterinary Practitioner. 2009;10(2):148–151. [Google Scholar]
- Kumar S, Budhwar L, Yadav A, Yadav M, Yadav JP. Phytochemical screening and antibacterial activity of Aloe vera collected from different climatic regions of India. Natural Products Journal. 2016;6(1):73–82. doi: 10.2174/2210315506666151208213012. [DOI] [Google Scholar]
- Kusuma SAF, Erika IH, Annisa GP. Antibacterial activity of ethanol extract of Jatropha leaves against Pseudomonas aeruginosa ATCC 27853. Research Journal of Pharmaceutical Biological and Chemical Sciences. 2017;8(S):243–248. [Google Scholar]
- Lagnika L, Anago E, Sanni A. Screening for antibacterial, antioxidant activity and toxicity of some medicinal plants used in Benin folkloric medicine. Journal of Medicinal Plants Research. 2011;5(5):773–777. [Google Scholar]
- Lasalita-Zapico FC, Aguilar CHM, Madas JB, Eroy MN. Chemical composition, antimicrobial properties and toxicity of Jatropha curcas provenances from diverse origins. International Journal of Agriculture and Biology. 2012;14(4):625–628. [Google Scholar]
- Lawrence R, Tripathi P, Jeyakumar E. Isolation, purification, and evaluation of antibacterial agents from Aloe vera. Brazilian Journal of Microbiology. 2009;40(4):906–915. doi: 10.1590/S1517-83822009000400023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Cho S, Paik HD, Choi CW, Nam KT, Hwang SG, Kim SK. Investigation on antibacterial and antioxidant activities, phenolic and flavonoid contents of some Thai edible plants as an alternative for antibiotics. Asian-Australasian Journal of Animal Sciences. 2014;27(10):1461–1468. doi: 10.5713/ajas.2013.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ST, Ariffin A, Son R, Ghazali HM. Effect of lipase hydrolysis on the antibacterial activity of coconut oil, palm mesocarp oil and selected seed oils against several pathogenic bacteria. International Food Research Journal. 2015;22(1):46–54. [Google Scholar]
- Leontiev, R., N. Hohaus, C. Jacob, M. C. H. Gruhlke, and A. J. Slusarenko. 2018. A comparison of the antibacterial and antifungal activities of thiosulfinate analogues of allicin. Scientific Report 8. 10.1038/s41598-018-25154-9. [DOI] [PMC free article] [PubMed]
- Li, J., M. Qin, C. Liu, W. Ma, X. Zeng, and Y. Ji. 2020. Antimicrobial photodynamic therapy against multidrug–resistant Acinetobacter baumannii clinical isolates mediated by aloe–emodin: An in vitro study. Photodiagnosis and Photodynamic Therapy 29. 10.1016/j.pdpdt.2019.101632. [DOI] [PubMed]
- Luciano–Montalvo, C., I. Boulogne, and J. Gavillan–Suarez. 2013. A screening for antimicrobial activities of Caribbean herbal remedies. BMC Complementary and Alternative Medicine 13. 10.1186/1472-6882-13-126. [DOI] [PMC free article] [PubMed]
- Lukova P, Karcheva-Bahchevanska D, Mollova D, Nikolova M, Mladenov R, Iliev I. Study of prebiotic potential and antioxidant activity in Plantago spp. leaves after enzymatic hydrolysis with hemicellulase and xylanase. Engineering in Life Sciences. 2018;18(11):831–839. doi: 10.1002/elsc.201800071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———, M. Nikolova, E. Petit, R. Elboutachfaiti, T. Vasileva, P. Katsarov, H. Manev, C. Gardarin, G. Pierre, P. Michaud, I. Iliev, and C. Delattre. 2020. Prebiotic activity of poly–and oligosaccharides obtained from Plantago major L. leaves. Applied Sciences (Switzerland) 10(8). 10.3390/APP10082648.
- Machado D, Gaspar C, Palmeira-de-liveira A, Cavaleiro C, Salgueiro L, Martinez-de-Oliveira J, Cerca N. Thymbra capitata essential oil as potential therapeutic agent against Gardnerella vaginalis biofilm–related infections. Future Microbiology. 2017;12(5):407–416. doi: 10.2217/fmb-2016-0184. [DOI] [PubMed] [Google Scholar]
- Mahajan, T. 2020. Comparative evaluation of disinfecting alginate using Aloe vera and 0.2% chlorhexidine digluconate by internal disinfection method – an in vivo study. Journal of Evolution of Medical and Dental Sciences 9(27):1944–1947. 10.14260/jemds/2020/423.
- Mandal SM, Dey S, Mandal M, Sarkar S, Maria-Neto S, Franco OL. Identification and structural insights of three novel antimicrobial peptides isolated from green coconut water. Peptides. 2009;30(4):633–637. doi: 10.1016/j.peptides.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Mandim F, Barros L, Calhelha RC, Abreu RM, Pinela J, Alves MJ, Heleno S, Santos PF, Ferreira IC. Calluna vulgaris (L.) Hull: Chemical characterization, evaluation of its bioactive properties and effect on the vaginal microbiota. Food and Function. 2019;10(1):78–89. doi: 10.1039/C8FO01910J. [DOI] [PubMed] [Google Scholar]
- Maragatham C, Panneerselvam A. Etiological study of diabetic foot ulcer infection and anti–foot ulcer activity of Aloe barbadensis Mill. Journal of Pure and Applied Microbiology. 2010;4(2):853–857. [Google Scholar]
- Mazhari M, Esmaeilipour O, Mirmahmoudi R, Badakhshan Y. Comparison of antibiotic, probiotic, and great plantain (Plantago major L.) on growth performance, serum metabolites, immune response, and ileal microbial population of broilers. Poultry Science Journal. 2016;4(2):97–105. doi: 10.22069/PSJ.2016.10041.1164. [DOI] [Google Scholar]
- Mbanga J, Mangoma N, Saidi B. An evaluation of the antimicrobial activities of Aloe barbadensis, A. chabaudii and A. arborescens leaf extracts used in folklore veterinary medicine in Zimbabwe. Journal of Animal and Veterinary Advances. 2010;9(3):2918–2923. [Google Scholar]
- Mbega ER, Mortensen CN, Mabagala RB, Wulff EG. The effect of plant extracts as seed treatments to control bacterial leaf spot of tomato in Tanzania. Journal of General Plant Pathology. 2012;78(4):277–286. doi: 10.1007/s10327-012-0380-z. [DOI] [Google Scholar]
- Medina-Flores D, Ulloa-Urizar G, Camere-Colarossi R, Caballero-Garcia S, Mayta-Tovalino F, del Valle-Mendoza J. Antibacterial activity of Bixa orellana L. (achiote) against Streptococcus mutans and Streptococcus sanguinis. Asian Pacific Journal of Tropical Biomedicine. 2016;6(5):400–403. doi: 10.1016/j.apjtb.2016.03.005. [DOI] [Google Scholar]
- Mehrotra S, Srivastava AK, Nandi SP. Comparative antimicrobial activities of neem, amla, aloe, assam tea and clove extracts against Vibrio cholerae, Staphylococcus aureus and Pseudomonas aeruginosa. Journal of Medicinal Plants Research. 2010;4(22):2393–2398. [Google Scholar]
- Mehta R, Dhruv S, Kaushik V, Sen KK, Khan NS, Abhishek A, Dixit AK, Tripathi VN. A comparative study of antibacterial and antifungal activities of extracts from four indigenous plants. Bioinformation. 2020;16(3):267–272. doi: 10.6026/97320630016267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard J, Kobetz E, Maldonado JC, Barton B, Blanco J, Diem J. Barriers to cervical cancer screening among Haitian immigrant women in Little Haiti. Miami. Journal of Cancer Education. 2010;25(4):602–608. doi: 10.1007/s13187-010-0089-7. [DOI] [PubMed] [Google Scholar]
- Mendez L, Rojas-Vera J, Contreras-Moreno B, Celis MT, Rosenzweig-Levy P. Phytochemical screening of Jatropha curcas L. leaves and roots collected from Merida-Venezuela. Ciencia E Ingenieria. 2020;41(1):75–80. [Google Scholar]
- ———, J. Rojas, B. Contreras–Moreno, J. Velasco, P. Rosezweig Levy, and M. T. Celis. 2018. Biological activities analyzed in Jatropha curcas Linn. extracts. Ciencia E Ingenieria 39(2):153–160.
- Mendez M, Rodriguez R, Ruiz J, Morales-Adame D, Castillo F, Hernandez-Castillo FD, Aguilar CN. Antibacterial activity of plant extracts obtained with alternative organics solvents against food–borne pathogen bacteria. Industrial Crops and Products. 2012;37(1):445–450. doi: 10.1016/j.indcrop.2011.07.017. [DOI] [Google Scholar]
- Metiner K, Özkan O, Seyyal AK. Antibacterial effects of ethanol and acetone extract of Plantago major L. on gram positive and gram–negative bacteria. Kafkas Universitesi Veteriner Fakultesi Dergisi. 2012;18(3):503–505. [Google Scholar]
- More NV, Kharat KR, Kharat AS. Berberine from Argemone mexicana L. exhibits a broadspectrum antibacterial activity. ACTA Biochimica Polonica. 2017;64(4):653–660. doi: 10.18388/abp.2017_1621. [DOI] [PubMed] [Google Scholar]
- Muddassir M, Ansari F, Ahmed M, Butt F. Biological activities of extracts obtained from natural origin. Pakistan Journal of Medical and Health Sciences. 2018;12(4):1599–1603. [Google Scholar]
- Namuli A, Abdullah N, Sieo CC, Zuhainis SW, Oskoueian E. Phytochemical compounds and antibacterial activity of Jatropha curcas Linn. extracts. Journal of Medicinal Plants Research. 2011;5(16):3982–3990. [Google Scholar]
- Negahdari, S., H. Galehdari, M. Kesmati, A. Rezaie, and G. Shariati. 2017. Wound healing activity of extracts and formulations of Aloe vera, henna, Adiantum capillus–veneris, and myrrh on mouse dermal fibroblast cells. International Journal of Preventive Medicine 8. 10.4103/ijpvm.IJPVM_338_16. [DOI] [PMC free article] [PubMed]
- New York City Mayor’s Office of Immigrant Affairs. 2020. State of our immigrant city. https://www1.nyc.gov/assets/immigrants/downloads/pdf/MOIA-Annual-Report-for-2019.pdf.
- Ochoa Pacheco A, Marin Moran J, Gonzalez Giro Z, Hidalgo Rodriguez A, Juliet Mujawimana R, Tamayo Gonzalez K, Sariego Frometa S. In vitro antimicrobial activity of total extracts of the leaves of Petiveria alliacea L. (Anamu) Brazilian Journal of Pharmaceutical Sciences. 2013;49(2):241–250. doi: 10.1590/S1984-82502013000200006. [DOI] [Google Scholar]
- Oghenemaro EF, Johnson J, Itohan IM, Richard SO, Michael O. Antimicrobial activity of Aloe vera gel and honey against bacteria isolates from wound aspirates. International Journal of Pharmaceutical Sciences and Research. 2018;9(11):4890–4893. doi: 10.13040/IJPSR.0975-8232.9(11).4890-93. [DOI] [Google Scholar]
- Okmen AS. Antioxidant and antibacterial activities of different plants extracts against Staphylococcus aureus isolated from soccer player’s shoes and knowledge and applications about foot hygiene of the soccer players. African Journal of Traditional, Complementary and Alternative Medicines. 2015;12(3):143–149. doi: 10.4314/ajtcam.v12i3.18. [DOI] [Google Scholar]
- Oluwasina, O. O., I. V. Ezenwosu, C. O. Ogidi, and V. O. Oyetayo. 2019. Antimicrobial potential of toothpaste formulated from extracts of Syzygium aromaticum, Dennettia tripetala and Jatropha curcas latex against some oral pathogenic microorganisms. AMB Express 9. 10.1186/s13568-019-0744-2. [DOI] [PMC free article] [PubMed]
- Ososki AL, Lohr P, Reiff M, Balick MJ, Fronenberg F, Fugh-Berman A, O’Connor B. Ethnobotanical literature survey of medicinal plants in the Dominican Republic used for women’s health conditions. Journal of Ethnopharmacology. 2002;79(3):285–298. doi: 10.1016/S0378-8741(01)00376-2. [DOI] [PubMed] [Google Scholar]
- Palmeri R, Parafati L, Restuccia C, Fallico B. Application of prickly pear fruit extract to improve domestic shelf life, quality, and microbial safety of sliced beef. Food and Chemical Toxicology. 2018;118:355–360. doi: 10.1016/j.fct.2018.05.044. [DOI] [PubMed] [Google Scholar]
- ———, L. Parafati, E. Arena, E. Grassenio, C. Restuccia, and B. Fallico. 2020. Antioxidant and antimicrobial properties of semi–processed frozen prickly pear juice as affected by cultivar and harvest time. Foods 9(2). 10.3390/foods9020235. [DOI] [PMC free article] [PubMed]
- Pan American Health Organization. (2020). Haiti. Health in the Americas. https://www.paho.org/salud-en-las-americas-2017/?p=4110 (December 2020).
- Pandey R, Mishra A. Antibacterial activities of crude extract of Aloe barbadensis to clinically isolated bacterial pathogens. Applied Biochemistry and Biotechnology. 2010;160(5):1356–1361. doi: 10.1007/s12010-009-8577-0. [DOI] [PubMed] [Google Scholar]
- Passafiume, R., R. Gaglio, G. Sortino, and V. Farina. 2020. Effect of three different Aloe vera gel–based edible coatings on the quality of fresh–cut “Hayward” kiwifruits. Foods 9(7). 10.3390/foods9070939. [DOI] [PMC free article] [PubMed]
- Patri G, Sahu A. Role of herbal agents – tea tree oil and Aloe vera as cavity disinfectant adjuncts in minimally invasive dentistry – an in vivo comparative study. Journal of Clinical and Diagnostic Research. 2017;11(7):CD05–CD09. doi: 10.7860/JCDR/2017/27598.10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, A. and B. Akers. 2000. Use of Psathyrella cf. hymenocephala (Coprinaceae) as a spice in Haiti. Mycologist 14(4):161–164.
- ——— and P. A. Cox. 1995. An ethnobotanical survey of the uses for Citrus aurantium (Rutaceae) in Haiti. Economic Botany 49(3):249–256.
- Pawlus AD, Kinghorn AD. Review of the ethnobotany, chemistry, biological activity, and safety of the botanical dietary supplement Morinda citrifolia (noni) Journal of Pharmacy and Pharmacology. 2007;59(12):1587–1609. doi: 10.1211/jpp.59.12.0001. [DOI] [PubMed] [Google Scholar]
- Paz Quezada M, Salinas C, Gotteland M, Cardemil L. Acemannan and fructans from Aloe vera (Aloe barbadensis Miller) plants as novel prebiotics. Journal of Agricultural and Food Chemistry. 2017;65(46):10029–10039. doi: 10.1021/acs.jafc.7b04100. [DOI] [PubMed] [Google Scholar]
- Pieroni A, Giusti ME, Quave CL. Cross–cultural ethnobiology in the Western Balkans: Medical ethnobotany and ethnozoology among Albanians and Serbs in the Pešter Plateau, Sandžak. South-Western Serbia. Human Ecology. 2011;39(3):333. doi: 10.1007/s10745-011-9401-3. [DOI] [Google Scholar]
- ——— and I. Vandebroek, eds. 2007. Traveling cultures and plants: The ethnobiology and ethnopharmacology of migrations, vol. 7. New York: Berghahn Books.
- Prabhakaran D, Rajeshkanna A, Senthamilselvi MM. Antimicrobial activity of Argemone mexicana Linn (flowers) Indo American Journal of Pharmaceutical Sciences. 2016;3(5):450–454. [Google Scholar]
- Prakoso, Y. A., C. S. Rini, and R. Wirjaatmadja. 2018. Efficacy of Aloe vera, Ananas comosus, and Sansevieria masoniana cream on the skin wound infected with MRSA. Advances in Pharmacological Sciences 2018. 10.1155/2018/4670569. [DOI] [PMC free article] [PubMed]
- Prastiyanto, M. E., P. D. Tama, N. Ananda, W. Wilson, and A. H. Mukaromah. 2020. Antibacterial potential of Jatropha sp. latex against multidrug–resistant bacteria. International Journal of Microbiology 2020. 10.1155/2020/8509650. [DOI] [PMC free article] [PubMed]
- Quinlan MB. Ethnomedicine and ethnobotany of fright, a Caribbean culture–bound psychiatric syndrome. Journal of Ethnobiology and Ethnomedicine. 2010;6(1):1–18. doi: 10.1186/1746-4269-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R’bia O, Chkioua C, Hellal R, Herchi W, Smiti SA. Antioxidant and antibacterial activities of Opuntia ficus indica seed oil fractions and their bioactive compounds identification. Turkish Journal of Biochemistry-turk Biyokimya Dergisi. 2017;42(4):481–491. doi: 10.1515/tjb-2016-0200. [DOI] [Google Scholar]
- Raga DD, Espiritu RA, Shen CC, Ragasa CY. A bioactive sesquiterpene from Bixa orellana. Journal of Natural Medicines. 2011;65(1):206–211. doi: 10.1007/s11418-010-0459-9. [DOI] [PubMed] [Google Scholar]
- Raina SM, Hassan MD. Screening of phytochemical properties and antimicrobial activity of Malaysian medicinal plants against aquatic bacteria. Malaysian Journal of Microbiology. 2016;12(4):284–290. doi: 10.21161/mjm.83816. [DOI] [Google Scholar]
- Ramirez–Moreno, E., R. Carino–Cortes, N. del Socorro Cruz–Cansino, L. Delgado–Olivares, J. Alberto Ariza–Ortega, V. Yelina Montanez–Izquierdo, M. Manuela Hernandez–Herrero, and T. Filardo–Kerstupp. 2017. Antioxidant and antimicrobial properties of cactus pear (Opuntia) seed oils. Journal of Food Quality, Special Issue 2017. 10.1155/2017/3075907.
- Ranjani M, Rajan S, Brindha P. Antioxidant and antibacterial potentials of Aloe vera juice extract against wound isolates. Journal of Pure and Applied Microbiology. 2010;4(2):733–739. [Google Scholar]
- Ranjbar, R., M. Arjomandzadegan, and H. Hosseiny. 2017. Evaluation of antioxidant activity and growth control properties of nanoscale structure produced from Aloe vera var. littoralis extract on clinical isolates of Salmonella. Scientia Pharmaceutica 85(3). 10.3390/scipharm85030028. [DOI] [PMC free article] [PubMed]
- Reddy BU. Enumeration of antibacterial activity of few medicinal plants by bioassay method. E-journal of Chemistry. 2010;7(4):1449–1453. doi: 10.1155/2010/687182. [DOI] [Google Scholar]
- Rosas-Pinon Y, Mejia A, Diaz-Ruiz G, Isabel Aguilar M, Sanchez-Nieto S, Fausto Rivero-Cruz J. Ethnobotanical survey and antibacterial activity of plants used in the Altiplane region of Mexico for the treatment of oral cavity infections. Journal of Ethnopharmacology. 2012;141(3):860–865. doi: 10.1016/j.jep.2012.03.020. [DOI] [PubMed] [Google Scholar]
- Rukmini JN, Manasa S, Rohini C, Sireesha LP, Ritu S, Umashankar GK. Antibacterial efficacy of tender coconut water (Cocos nucifera L) on Streptococcus mutans: An in vitro study. Journal of International Society of Preventive and Community Dentistry. 2017;7(2):130–134. doi: 10.4103/jispcd.JISPCD_275_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddiq AA, Al-Ghamdi H. Aloe vera extract: A novel antimicrobial and antibiofilm against methicillin resistant Staphylococcus aureus strains. Pakistan Journal of Pharmaceutical Sciences. 2018;31(5, S):2123–2130. [PubMed] [Google Scholar]
- Safford WE. Identity of cohoba, the narcotic snuff of ancient Haiti. Journal of the Washington Academy of Sciences. 1916;6(15):547–562. [Google Scholar]
- Saint-Jean G, Crandall LA. Sources and barriers to health care coverage for Haitian immigrants in Miami-Dade County, Florida. Journal of Health Care for the Poor and Underserved. 2005;16(1):29–41. doi: 10.1353/hpu.2005.0016. [DOI] [PubMed] [Google Scholar]
- Salah F, El Ghoul Y, Alminderej FM, El Golli-Bennour E, Ouanes Z, Maciejak O, Jarroux N, Majdoub H, Sakli F. Development, characterization, and biological assessment of biocompatible cellulosic wound dressing grafted Aloe vera bioactive polysaccharide. Cellulose. 2019;26(8):4957–4970. doi: 10.1007/s10570-019-02419-8. [DOI] [Google Scholar]
- ———, Y. El Ghoul, A. Mandhi, H. Majdoub, N. Jarroux, and F. Sakli. 2017. Effect of the deacetylation degree on the antibacterial and antibiofilm activity of acemannan from Aloe vera. Industrial Crops and Products 103:13–18. 10.1016/j.indcrop.2017.03.031.
- Samsudin NIP, Lee HY, Chern PE, Ng CT, Panneerselvam L, Phang SY, Tan WT, Mahyudin NA. In vitro antibacterial activity of crude medicinal plant extracts against ampicillin plus penicillin–resistant Staphylococcus aureus. International Food Research Journal. 2018;25(2):573–579. [Google Scholar]
- Sanchez E, Davila-Avina J, Castillo SL, Heredia N, Vazquez-Alvarado R, Garcia S. Antibacterial and antioxidant activities in extracts of fully grown cladodes of 8 cultivars of cactus pear. Journal of Food Science. 2014;79(4):M659–M664. doi: 10.1111/1750-3841.12416. [DOI] [PubMed] [Google Scholar]
- ———, C. Rivas Morales, S. Castillo, C. Leos–Rivas, L. Garcia–Becerra, and D. M. Ortiz Martinez. 2016. Antibacterial and antibiofilm activity of methanolic plant extracts against nosocomial microorganisms. Evidence–based Complementary and Alternative Medicine 2016. 10.1155/2016/1572697. [DOI] [PMC free article] [PubMed]
- Saosoong K, Ruangviriyachai C. Antimicrobial activity and chemical constituents of the extract from Jatropha curcas fruit. Oriental Journal of Chemistry. 2016;32(2):1163–1169. doi: 10.13005/ojc/320242. [DOI] [Google Scholar]
- Sarkar, K. K., T. Mitra, R. N. Acharyya, and S. K. Sadhu. 2019. Phytochemical screening and evaluation of the pharmacological activities of ethanolic extract of Argemone mexicana Linn. aerial parts. Oriental Pharmacy and Experimental Medicine 19(1):91–106. 10.1007/s13596-018-0357-3.
- Schmidt C, Fronza M, Goettert M, Geller F, Luik S, Flores EM, Bittencourt CF, Zanetti GD, Heinzmann BM, Laufer S, Merfort I. Biological studies on Brazilian plants used in wound healing. Journal of Ethnopharmacology. 2009;122(3):523–532. doi: 10.1016/j.jep.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Seay JS, Mandigo M, Kish J, Menard J, Marsh S, Kobetz E. Intravaginal practices are associated with greater odds of high–risk HPV infection in Haitian women. Ethnicity and Health. 2017;22(3):257–265. doi: 10.1080/13557858.2016.1246423. [DOI] [PubMed] [Google Scholar]
- Seay J, Mandigo M, Hew K, Kobetz E. Vaginal infections in Haitian immigrant women living in Miami, Florida. Journal of Health Care for the Poor and Underserved. 2017;28(3):1141–1150. doi: 10.1353/hpu.2017.0102. [DOI] [PubMed] [Google Scholar]
- Sharanappa R, Vidyasagar GM. Preliminary screening of ethnomedicinal plants for antibacterial activity. International Journal of Pharmaceutical Sciences and Research. 2015;6(9):3928–3935. doi: 10.13040/IJPSR.0975-8232.6(9).3928-35. [DOI] [Google Scholar]
- Sharma AK, Gangwar M, Kumar D, Nath G, Sinha ASK, Tripathi YB. Phytochemical characterization, antimicrobial activity and reducing potential of seed oil, latex, machine oil and presscake of Jatropha curcas. Avicenna Journal of Phytomedicine. 2016;6(4):366–375. [PMC free article] [PubMed] [Google Scholar]
- Shen X, Chen W, Zheng Y, Lei X, Tang M, M., H. Wang, and F. Song. Chemical composition, antibacterial and antioxidant activities of hydrosols from different parts of Areca catechu L. and Cocos nucifera L. Industrial Crops and Products. 2017;96:110–119. doi: 10.1016/j.indcrop.2016.11.053. [DOI] [Google Scholar]
- Shigematsu E, Dorta C, Santos DN, Ferreira KA, Goes-Favoni SP, Oshiiwa M, Mauro MA. Edible coating with coconut water to preserve probiotic strains and sensory characteristics of minimally processed carrots. International Food Research Journal. 2019;26(4):1285–1292. [Google Scholar]
- Silva SS, Popa EG, Gomes ME, Cerqueira M, Marques AP, Caridade SG, Teixeira P, Sousa C, Mano JF, Reis RL. An investigation of the potential application of chitosan/aloe–based membranes for regenerative medicine. ACTA Biomaterialia. 2013;9(6):6790–6797. doi: 10.1016/j.actbio.2013.02.027. [DOI] [PubMed] [Google Scholar]
- Singh SK, Pandey VD, Singh A, Singh C. Antibacterial activity of seed extracts of Argemone mexicana L. on some pathogenic bacterial strains. African Journal of Biotechnology. 2009;8(24):7077–7081. [Google Scholar]
- Sriprang S, Sriprang N, Sumpradit T, Shimbhu D. Antibacterial activities of crude extracts from physic nut (Jatropha curcas) seed residues. Scienceasia. 2010;36(4):346–348. doi: 10.2306/scienceasia1513-1874.2010.36.346. [DOI] [Google Scholar]
- Suhaili Z, Yeo CC, Yasin HN, Badaludin NA, Zakaria ZA. Antibacterial profile of Jatropha curcas latex extracts against selected human pathogenic bacteria. African Journal of Microbiology Research. 2011;5(29):5147–5154. doi: 10.5897/AJMR11.663. [DOI] [Google Scholar]
- Sujatha T, Abhinaya S, Sunder J, Thangapandian M, Kundu A. Efficacy of early chick nutrition with Aloe vera and Azadirachta indica on gut health and histomorphometry in chicks. Veterinary World. 2017;10(6):569–573. doi: 10.14202/vetworld.2017.569-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamine M, Nancib A, Nancib N, Boudrant J. Prickly pear cactus as a raw material for lactic acid production by Lactococcus lactis subsp. lactis. Malaysian Journal of Microbiology. 2018;14(1):16–24. [Google Scholar]
- Tan JBL, Lim YY, Lee SM. Antioxidant and antibacterial activity of Rhoeo spathacea (Swartz) Stearn leaves. Journal of Food Science and Technology. 2015;52(4):2394–2400. doi: 10.1007/s13197-013-1236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariq, H., M. Zia, Ihsan-ul-Haq, S. A. Muhammad, S. A. Khan, N. Fatima, A. Mannan, A. M. Abbasi, and M. Zhang. 2019. Antioxidant, antimicrobial, cytotoxic, and protein kinase inhibition potential in Aloe vera L. Biomed Research International 2019. 10.1155/2019/6478187. [DOI] [PMC free article] [PubMed]
- Tarter A. Trees in Vodou: An arbori–cultural exploration. Journal for the Study of Religion Nature and Culture. 2015;9(1):87–112. doi: 10.1558/jsrnc.v9i1.19582. [DOI] [Google Scholar]
- Tinpun K, Nakpheng T, Padmavathi AR, Srichana T. In vitro studies of Jatropha curcas L. latex spray formulation for wound healing applications. Turkish Journal of Pharmaceutical Sciences. 2020;17(3):271–279. doi: 10.4274/tjps.galenos.2019.69875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongco MDC. Purposive sampling as a tool for informant selection. Ethnobotany Research and Applications. 2007;5:147–158. doi: 10.17348/era.5.0.147-158. [DOI] [Google Scholar]
- Tongpoothorn W, Chanthai S, Sriuttha M, Saosang K, Ruangviriyachai C. Bioactive properties and chemical constituents of methanolic extract and its fractions from Jatropha curcas oil. Industrial Crops and Products. 2012;36(1):437–444. doi: 10.1016/j.indcrop.2011.10.011. [DOI] [Google Scholar]
- Tornero–Martinez, A., R. Cruz–Ortiz, M. Eugenia Jaramillo–Flores, P. Osorio–Diaz, S. Victoria Avila–Reyes, G. Monserrat Alvarado–Jasso, and R. Mora–Escobedo. 2019. In vitro fermentation of polysaccharides from Aloe vera and the evaluation of antioxidant activity and production of short chain fatty acids. Molecules 24(19). 10.3390/molecules24193605. [DOI] [PMC free article] [PubMed]
- TRAMIL. 2020. TRAMILibrary: Program of Applied Research to Popular Medicine in the Caribbean. http://www.tramil.net/ (June 2020).
- Usha G, Ravi D, Parthasarathy R. Effect of Probiotics along with selected prebiotics against respiratory tract infecting pathogens. Research Journal of Biotechnology. 2013;8(11):19–24. [Google Scholar]
- Vaghasiya YK, Parekh JP, Shukla VJ, Chanda SV. Antimicrobial and anti–inflammatory screening of four Indian medicinal plants. Latin American Journal of Pharmacy. 2011;30(4):661–666. [Google Scholar]
- Valera MC, Maekawa LE, de Oliveira LD, Cardoso Jorge AO, Shygei E, Talge Carvalho CA. In vitro antimicrobial activity of auxiliary chemical substances and natural extracts on Candida albicans and Enterococcus faecalis in root canals. Journal of Applied Oral Science. 2013;21(2):118–123. doi: 10.1590/1678-7757201302135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Andel T, de Boer HJ, Barnes J, Vandebroek I. Medicinal plants used for menstrual disorders in Latin America, the Caribbean, sub–Saharan Africa, South and Southeast Asia and their uterine properties: A review. Journal of Ethnopharmacology. 2014;155(2):992–1000. doi: 10.1016/j.jep.2014.06.049. [DOI] [PubMed] [Google Scholar]
- ———, S. de Korte, D. Koopmans, J. Behari–Ramdas, and S. Ruysschaert. 2008. Dry sex in Suriname. Journal of Ethnopharmacology 116(1):84–88. [DOI] [PubMed]
- Vandebroek I. Intercultural health and ethnobotany: How to improve healthcare for underserved and minority communities? Journal of Ethnopharmacology. 2013;148(3):746–754. doi: 10.1016/j.jep.2013.05.039. [DOI] [PubMed] [Google Scholar]
- ———. 2016. Cultural comparisons in ethnobiological research. In: Introduction to Ethnobiology, eds., R. R. Nobrega Alves and U. P. Albuquerque, 265–271. Basel, Switzerland: Springer.
- ——— and M. J. Balick. 2012. Globalization and loss of plant knowledge: Challenging the paradigm. PloS one 7(5):e37643. [DOI] [PMC free article] [PubMed]
- ——— and M. J. Balick. 2014. Lime for chest congestion, bitter orange for diabetes: Foods as medicines in the Dominican community in New York City. Economic Botany 68(2):177–189.
- ———, M. J. Balick, A. Ososki, F. Kronenberg, J. Yukes, C. Wade, F. Jiménez, B. Peguero, and D. Castillo. 2010. The importance of botellas and other plant mixtures in Dominican traditional medicine. Journal of Ethnopharmacology 128(1):20–41. [DOI] [PMC free article] [PubMed]
- Viju N, Satheesh S, Vincent SGP. Antibiofilm activity of coconut (Cocos nucifera Linn.) husk fibre extract. Saudi Journal of Biological Sciences. 2013;20(1):85–91. doi: 10.1016/j.sjbs.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viuda-Martos M, Ciro-Gomez GL, Ruiz-Navajas Y, Zapata-Montoya JE, Sendra E, Perez-Alvarez JA, Fernandez-Lopez J. In vitro antioxidant and antibacterial activities of extracts from Annatto (Bixa orellana L.) leaves and seeds. Journal of Food Safety. 2012;32(4):399–406. doi: 10.1111/j.1745-4565.2012.00393.x. [DOI] [Google Scholar]
- Vladimir Trejo-Flores J, Luna-Gonzalez A, Alvarez-Ruiz P, Escamilla-Montes R, Peraza-Gomez V, Diarte-Plata G, Esparza-Leal HM, Campa-Cordova AI, Gamez-Jimenez C, Rubio-Castro A. Protective effect of Aloe vera in Litopenaeus vannamei challenged with Vibrio parahaemolyticus and white spot syndrome virus. Aquaculture. 2016;465:60–64. doi: 10.1016/j.aquaculture.2016.08.033. [DOI] [Google Scholar]
- Volpato G, Godínez D. Ethnobotany of pru, a traditional Cuban refreshment. Economic Botany. 2004;58(3):381–395. doi: 10.1663/0013-0001(2004)058[0381:EOPATC]2.0.CO;2. [DOI] [Google Scholar]
- ———, D. Godínez, A. Beyra, and A. Barreto. 2009a. Uses of medicinal plants by Haitian immigrants and their descendants in the Province of Camagüey, Cuba. Journal of Ethnobiology and Ethnomedicine 5(1):16. [DOI] [PMC free article] [PubMed]
- ———, D. Godínez, and A. Beyra. 2009b. Migration and ethnobotanical practices: The case of tifey among Haitian immigrants in Cuba. Human Ecology 37(1):43–53.
- Wei L, Zhang W, Yin L, Yan F, Xu Y, Chen F. Extraction optimization of total triterpenoids from Jatropha curcas leaves using response surface methodology and evaluations of their antimicrobial and antioxidant capacities. Electronic Journal of Biotechnology. 2015;18(2):88–95. doi: 10.1016/j.ejbt.2014.12.005. [DOI] [Google Scholar]
- Weniger B, Haag-Berruier M, Anton R. Plants of Haiti used as antifertility agents. Journal of Ethnopharmacology. 1982;6(1):67–84. doi: 10.1016/0378-8741(82)90072-1. [DOI] [PubMed] [Google Scholar]
- ———, M. Rouzier, R. Daguilh, D. Henrys, J. H. Henrys, and R. Anton. 1986. Traditional medicine in the Central Plateau of Haiti. Journal of Ethnopharmacology 17(1):13–30. [DOI] [PubMed]
- WHO, Unicef, World Bank Group and United Nations Population Division . Trends in Maternal Mortality: 2000–2017. Geneva: World Health Organization; 2019. [Google Scholar]
- Yasmeen R, Hashmi AS, Anjum AA, Saeed S, Muhammad K. Antibacterial activity of indigenous herbal extracts against urease producing bacteria. Journal of Animal and Plant Sciences. 2012;22(2):416–419. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.