Abstract
Various surgical strategies have been developed to alleviate elevated intraspinal pressure (ISP) following acute traumatic spinal cord injury (tSCI). Surgical decompression of either the dural (durotomy) or the dural and pial (myelotomy) lining of the spinal cord has been proposed. However, a direct comparison of these two strategies is lacking. Here, we compare the histological and functional effects of durotomy alone and durotomy plus myelotomy in a rodent model of acute thoracic tSCI. Our results indicate that tSCI causes local tissue edema and significantly elevates ISP (7.4 ± 0.3 mmHg) compared with physiological ISP (1.7 ± 0.4 mmHg; p < 0.001). Both durotomy alone and durotomy plus myelotomy effectively mitigate elevated local ISP (p < 0.001). Histological examination at 10 weeks after tSCI revealed that durotomy plus myelotomy promoted spinal tissue sparing by 13.7% compared with durotomy alone, and by 25.9% compared with tSCI-only (p < 0.0001). Both types of decompression surgeries elicited a significant beneficial impact on gray matter sparing (p < 0.01). Impressively, durotomy plus myelotomy surgery increased preservation of motor neurons by 174.3% compared with tSCI-only (p < 0.05). Durotomy plus myelotomy surgery also significantly promoted recovery of hindlimb locomotor function in an open-field test (p < 0.001). Interestingly, only durotomy alone resulted in favorable recovery of bladder and Ladder Walk performance. Combined, our data suggest that durotomy plus myelotomy following acute tSCI facilitates tissue sparing and recovery of locomotor function. In the future, biomarkers identifying spinal cord injuries that can benefit from either durotomy alone or durotomy plus myelotomy need to be developed.
Keywords: decompression, functional recovery, intraspinal pressure, spinal cord injury, tissue sparing
Introduction
Traumatic spinal cord injury (tSCI) is a devastating event that can leave patients with motor and sensory loss and frequently with lifelong chronic pain. Unfortunately, there is currently no treatment strategy to restore spinal cord neurons and circuits after tSCI. With an estimated 18,000 new cases each year and >300,000 patients living with functional tSCI sequelae in the United States alone,1 there is a tremendous need to develop therapeutic strategies for patients with acute SCI. The cellular and tissue damage after SCI is thought to occur in two phases: the primary phase in which cells and tissue are destroyed immediately as a result of the primary insult, and the secondary phase of the injury in which multiple cellular and molecular sequelae of the primary injury result in the expansion of the primary injury site over a more protracted time frame (hours to days).2,3 Hemorrhage and edema lead to swelling of the contused spinal cord within the thecal sac and may result in subarachnoid occlusion.4 The swollen, injured cord is compressed against the stiff dural sleeve,5 leading to elevated intraspinal pressure (ISP). This has been documented in rats,6 pigs,7 and humans,8 and is referred to as “spinal compartment syndrome.”9 Increased ISP contributes to a loss of vascular autoregulation and inefficient heterogeneous perfusion of the contused spinal cord, which exacerbates oxidative stress and secondary injury.7 Recently, elevated ISP has been shown to be associated with negative neurological outcomes in patients with SCI,10–13 and careful management of increased ISP after SCI appears to be associated with improved functional outcomes in some patients.14,15 Current surgical practice attempts decompression of the bony canal with laminectomies, realignment of deformity, and stabilization of the spinal column within 24 h following injury.16 However, this type of surgery does not alleviate elevated ISP, which is seen in approximately half of patients with tSCI.8 In order to efficiently lower acutely elevated ISP acutely following tSCI, the dural lining of the spinal cord needs to be opened (durotomy).6 Additional mitigation of elevated ISP may be obtained by opening the pial lining of the spinal cord (myelotomy) and gentle debridement of the necrotic injury core.6
The possible effect of these interventions on counteracting secondary injury and tissue loss remains controversial.17–21 Several studies suggest a trend toward positive effects of durotomy on functional recovery in rodents22,23 and human patients.15 Although clinical studies on the effect of myelotomy are lacking, a recent meta-analysis of rodent studies suggested a significant improvement of locomotor function in moderate severity injuries following myelotomy.24 To date, a comparison of the effectiveness of durotomy compared with myelotomy for halting secondary tissue loss and promoting functional recovery has been lacking.
The current report investigates the impact of durotomy alone versus durotomy plus myelotomy surgery on secondary tissue loss and functional recovery in a rodent model of moderate tSCI.
Methods
SCI
All animal work described here was performed in accordance the guidelines from Office of Animal Welfare from the National Institutes of Health, and all procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
Animals were randomly assigned to different treatment groups, and researchers were blinded to their status during the behavioral studies and analyses. Female Long Evans rats (n = 41, 8–12 weeks old; Envigo Labs; also see Table 1) were anesthetized using isoflurane (5% to induce and 2.5% to maintain) and shaved, and the area overlying the T6–T11 vertebrae was cleaned and sterilized. A ∼2.5 cm longitudinal incision centered over T7–T10 was made before subperiostal dissection of paraspinal muscles. A laminectomy was performed to expose the spinal cord at T8. To produce a contusion type lesion at T8, a third generation Ohio State University spinal cord contusion device with a 2 mm diameter probe tip was used. During the contusion, the probe induced a 0.9 mm displacement of the spinal cord resulting in a moderate injury.25,26 Tissue displacement and peak force data were collected and were used as indicators of injury consistency and severity between animals (see Table 1). Variations of spinal cord displacement were <20% in our animal cohort.
Table 1.
In Vivo Study Groups and Data for Contusion SCI in Rats
| Animal group | n | Mean displacement (mm) ± SD | Mean peak force (N) ± SD |
|---|---|---|---|
| Laminectomy | 7 | - | - |
| SCI | 12 | 0.79 ± 0.02 | 40.60 ± 4.28 |
| SCI + durotomy alone | 7 | 0.75 ± 0.01 | 41.24 ± 3.69 |
| SCI + durotomy + myelotomy | 15 | 0.79 ± 0.03 | 40.43 ± 3.18 |
SCI, spinal cord injury; SD, standard deviation.
ISP measurements and decompression
Before and after tSCI, ISP measurements were obtained using previously described methods.6 Briefly, ISP was measured using a 1F Millar Mikro-Tip pressure probe (tip diameter = 330 μm). For implantation, a small opening was made in the dural membrane using the tip of a 21-gauge needle, and ISP was recorded pre-injury and immediately after injury (∼ 15 min after impact). The probe was inserted into the spinal cord ∼4 mm caudal to the injury center. Surgical decompression was performed along the length of the exposed spinal cord, with the ISP probe positioned at the injury center. Durotomy was produced by incising the thecal sack with a #11 blade, and then gently expanding the dural opening using two Dumond #5 forceps. For myelotomy surgeries, a longitudinal pial incision was made along the posterior median sulcus with a #15 blade after the durotomy. The injury core was then gently irrigated with a physiological saline solution. ISP recordings were also made after durotomy alone and after durotomy plus myelotomy surgeries. All pressure measurements were collected using a digitizer and iox2 software from Emka Technologies (Falls Church, VA). The data collected were then transferred to Microsoft Excel to obtain ISP over time. A total of 11 animals were included in the ISP recordings.
After injury and decompression, animals intended for survival studies were returned to the surgical area for closure, with some receiving durotomy or durotomy plus myelotomy prior to closure. To reduce excessive adhesion, a small piece of adipose tissue was positioned on top of the spinal cord. Muscle and skin were sutured in layers to close the wound. In our animal cohort, there were no peri- or post-operative complications related to either durotomy alone or durotomy combined with myelotomy procedures. Animals were treated with lactated Ringer's solution (subcutaneous; 5–10 mL), and analgesics (buprenorphine; 0.03 mg/kg) every 12 h for 48 h after surgery. Subcutaneous injections of gentamicin (5 mg/kg) were given once daily for 5 days post- injury. Animals were allowed to recover in warmed cages and manual bladder expression was performed twice daily until normal void response recovered (up to 7–10 days post-injury).
Water content analysis
A total of seven animals were subjected to surgical spinal cord injuries and then randomly assigned to either a 4-h (n = 4) or 72-h (n = 3) survival group. The animals were treated post-operatively, as described, including post-operative analgesia and bladder expression. At the time of euthanasia, rats were given an intraperitoneal injection of 0.8 mL Beuthanasia–D (390 mg/mL pentobarbital sodium, 50 mg/mL phenytoin sodium). Spinal cords were extracted and cut into ∼5 mm segments centered over the injury center. Cord segments were placed into pre-weighed Eppendorf tubes and weighed on an analytical scale. The cord segments were placed in a 75°C oven for 72 h and then weighed again to determine water loss. The percent water content was calculated using the following formula:
Urine volume data collection and analysis
After thoracic SCI, all animals required manual bladder expression. As part of record keeping, every bladder expression was characterized by size; observations ranged from empty to very large. Animals typically regained independent bladder control within 7–14 days. Urine from the first bladder expression of the day was collected in a weight boat and measured (grams, n = 45 measurements) on days 3, 7, and 14 post-injury. Using the quantitative values measured during this test, an observed-value number scale was designed. The bladder volume was given a score between 0 and 4; corresponding to empty, very small, small, medium, and large, respectively (calculated, empty <0.04 mL, very small <0.33 mL, small <1.7 mL, medium <3.25 mL, large <4.45 mL). The observed scores from every bladder expression were then averaged over time in order to determine the relative bladder function recovery time for each experimental group.
Behavioral analysis
In the weeks prior to surgery, the animals were acclimated to the various pieces of behavioral equipment that they would be tested on after surgery. In the instances of the Basso, Beattie, Bresnahan (BBB) locomotor test and the Ladder Walk (LW) test, the animals were administered a pre-injury baseline test.
Open-field locomotor test – BBB
The BBB locomotor rating scale is a sensitive, observer-scored test of hindlimb locomotor behavior. The test animals were placed into a ∼100 cm diameter arena with 19 cm walls (a children's play pool) that had a lightly textured, uniform floor. For 4 min, the animal was permitted to explore the arena while two observers recorded locomotor actions on a score sheet live, according to the BBB locomotor rating scale. The test was also video recorded. The observers compared scoring, which was done live, and, if necessary, came to a consensus on the final left and right hindlimb scores. The BBB test was repeated every week for 8 weeks.
Ladder Walk test
The LW test requires no pre-training. The apparatus consists of two clear plexiglass walls ∼90 cm long and ∼15 cm tall that are ∼10 cm wide, creating a corridor. A series of 1 mm diameter stainless steel rungs were intermittently spaced (gaps range between 1 and 3 cm) to create a ladder-type floor. The entire apparatus was elevated slightly to create a space below for the animal's leg to fall. The home cage was placed at one end of the apparatus. For each trial, the animal was placed at the end opposite to the home cage and allowed to walk the length of the apparatus to return to the home cage. After each successful run, the placement of the rungs was changed to prevent the animals from memorizing a specific rung pattern. The test consisted of 10 trials and was recorded on video. The videos were later reviewed at half speed; the total number of attempted steps and the number of slips or misses (unsuccessful grasps of the rung) were counted for each hindlimb. The percentage of errors was recorded. The animals were tested prior to injury to establish a baseline error percentage, then again at weeks 6, 8, and 10 post-injury.
Von Frey test
Prior to testing, the rats were habituated to the arena: a four-sided acrylic box with a wire mesh (5.5 × 5.5 mm squares) floor (40 cm high walls) on 20 cm aluminum legs. Because the rats were required to remain still during testing, they were provided with cereal treats to eat while in the arena. After habituation, during the testing, the sensitivity of the plantar surface of the hind paw was measured with Von Frey filaments: nylon hairs of varying diameter that were pressed gently into the plantar paw surface until the point of buckling. The point of buckling for each filament corresponds to a known force. The filaments were applied to the paws through the mesh floor in order of ascending diameter; the mechanical withdrawal threshold of each rat was defined as the minimum diameter filament that elicits a paw withdrawal reaction at least three times out of five trials. The test was performed weekly up to 8 weeks post-injury. A simplified up-down method was used to score the results from the Von Frey test as previously described.27
Histological staining and analysis: Cresyl violet and myelin stain
Sections were collected and mounted onto slides (250 μm apart). Slides were then fixed with 4% paraformaldehyde (Macron Chemicals, Center Valley, PA) to ensure tissue adherence. Sections then underwent a series of alcohol incubations for dehydration beginning with 4 min in deionized-water, followed by 5 min periods in 70%, 95%, and 100% ethanol followed by a xylene incubation for 10 min and then back through the ethanol series. Sections were then incubated for 10 min in an aqueous myelin dye (Eriochrome Cyanine R; Fluka, St. Louis, MO) and differentiated for 1 min in 1% ammonium hydroxide (Fisher Scientific) after being washed in water. Sections were then briefly rinsed in water and incubated in Cresyl violet stain (Sigma-Aldrich, St. Louis, MO) for 3 min. Following this step, sections were rinsed with deionized water 10 times and then quickly dehydrated in a stepwise manner for 4 min each in 70% and 95% alcohol. The Cresyl violet was then briefly differentiated in 250 mL of 95% alcohol containing five drops of glacial acetic acid (Fisher Scientific). Once differentiation was sufficient, sections continued through a stepwise dehydration process consisting of 5 min each in 95% and 100% ethanol, followed by final 10 min incubation in xylene. Slides were then cover slipped using Permount.
Immunohistochemistry
Rats were perfused and spinal cords were sectioned as described previously. Free floating 25 μm sections were kept in cryo-protectant solution (30% sucrose, 30% Ethylene glycol, 0.1 M phosphate buffer, 1% polyvinyl-pyrrolidone [PVP-40]) at 4˚C. On the day of the experiment, free-floating sections, 250 μm apart, from each spinal cord were washed with phosphate-buffered saline (PBS) (pH 7.2). Antigen retrieval was performed by incubating the slices in sodium citrate buffer, at 95˚C, pH 6, for 10 min. Slices were then blocked and permeabilized with 1% goat serum and 0.1% Triton X-100 in PBS for 1 h. Next, slices were incubated with primary antibody (Neuronal Nuclei, MAB 377, EMD Millipore Corp, USA, 1/200) at 37˚C for 1 h. Following washes, the slices were incubated with goat anti-mouse secondary antibody (Alexa Fluor 488 goat anti-mouse IgG, Life technologies, USA, 1/500) for 1 h at 37˚C. After washing with 1 × PBS, the slices were incubated 4ˊ,6-diamidino-2-phenylindole (DAPI; Life Technologies, USA, 1/1000) to label all nuclei. Finally, the slices were mounted onto microscope slides with mounting medium (glycerol and p-phenylenediamine in PBS, pH 9.0). The same procedure was used to label cells Mouse Anti-Glial Fibrillary Acidic Protein (GFAP; MAB3402, EMD Millipore Corporation, USA, 1:1500) to detect astrocytes.
Fluorescent images were captured using an Axio Zoom V.16 (Zeiss) fluorescent microscope equipped with an Apotome.2 structured illumination device for optical sectioning. All images were analyzed using ImageJ software. All NeuN positive cells in the ventral horn of the spinal cord were counted using ImageJ software. Confocal microscopy was performed using a Leica SP8X confocal microscope. All confocal images were acquired with a dry 10 × objective.
Statistical analysis
Continuous variables are presented as means ± standard error of the mean (SEM). GraphPad Prism 7 (GraphPad Software, Inc., San Diego, CA) was used for all statistical analyses. All significant findings reported were obtained with one- or two-way analysis of variance (ANOVA) with α set at p < 0.05 and Fisher least significant difference (LSD) post-hoc test.
Results
SCI causes tissue edema and elevated ISP
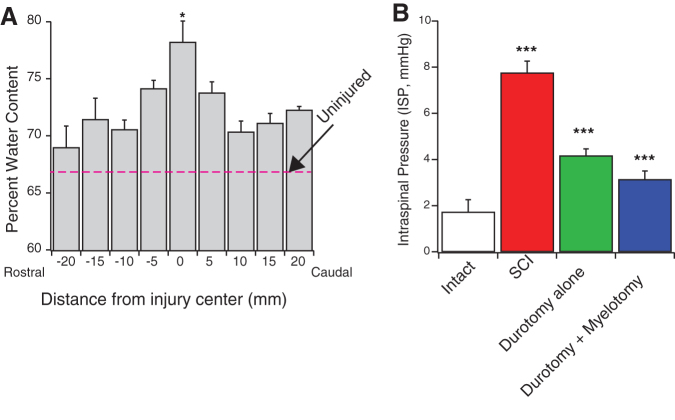
Intact rodent thoracic spinal cords had average water content of 66.7 ± 0.01% (See dotted line in Fig. 1A). SCI gave rise to significant tissue edema most pronounced within a 10 mm segment centered over the lesion center. Four hours after injury, the water content of the lesion center had increased by ∼12% to an average of 74.6 ± 0.02%. The water content of the lesion center (10 mm segment) was significantly higher than spinal cord tissue in the periphery (71.6 ± 0.7%; p < 0.01). Tissue edema at the lesion center remained elevated up to 3 days after injury (78.21 ± 1.85%; p < 0.05, Fig. 1A). To determine ISP at the injury center, we implanted a micro-sensor into the contused spinal cord. We detected a significantly increased ISP at 4 h following injury (7.4 ± 0.3 mmHg) compared with 1.7 ± 0.4 mmHg measured in the intact spinal cord (p < 0.001). Intraspinal pressure remained elevated up to 3 days after injury (7.9 ± 0.6 mmHg). ISP was effectively attenuated by both durotomy alone (4.17 ± 0.3 mmHg; p < 0.001) and durotomy + myelotomy (3.14 ± 0.4 mmHg; p < 0.001; Fig. 1B).
FIG. 1.
Contusion spinal cord injury (SCI) results in edema and elevated intraspinal pressure (ISP). (A) Intact rodent thoracic spinal cords have average water content of 66.7 ± 0.01%. (Red dotted line). At 3 days after SCI, a significant increase in water content (78.21 ± 1.85%; p < 0.05, n = 3) was detected. Water content was measured within 40 mm of spinal cord centered over the injury site. Spinal cords segments (5 mm) were collected and the wet and dry weight of the cord segments were collected to determine water content of the spinal cord. (B) Three days after injury, the ISP was significantly increased (7.9 ± 0.6 mmHg) compared with control animals (1.84 ± 0.5 mmHg; p < 0.001). Durotomy alone (4.17 ± 0.3 mmHg; p < 0.001) and durotomy plus myelotomy (3.14 ± 0.4 mmHg; p < 0.001) resulted in significantly decreased ISP compared with SCI alone group. R = rostral, C = caudal directions. ***p < 0.001. Color image is available online.
Decompression of the spinal cord promotes tissue sparing
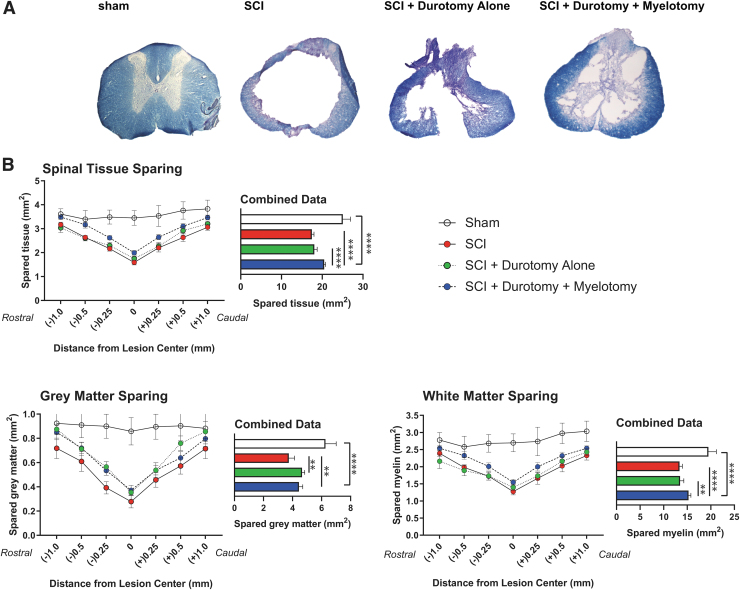
Analysis of spared spinal cord tissue following a moderate thoracic SCI was conducted 10 weeks after injury. Spinal cord cross-sections of 7 mm long spinal segments centered over the lesion epicenter were examined using Eriochrome Cyanine R and Cresyl violet staining (Fig. 2A). SCI resulted in significant atrophy of neural tissue at the injury site compared with intact animals (1.58 mm2 vs. 3.45 mm2). Durotomy alone increased the average area of spared spinal cord tissue at the injury center by 10.8% (1.75 mm2); however, this did not reach statistical significance. Durotomy plus myelotomy surgery promoted tissue sparing at the injury center by 25.9% (1.99 mm2) compared with injury-only animals. Durotomy plus myelotomy resulted in significantly increased spinal tissue sparing compared with either durotomy alone or tSCI-only treated animals (p < 0.0001; Fig. 2B). Tissue sparing achieved by durotomy plus myelotomy was most pronounced in the lesion center (spanning 1 mm rostral to 1 mm caudal) compared with tSCI-only animals (p < 0.05).
FIG. 2.
Surgical decompression promotes tissue sparing after spinal cord injury (SCI). (A) Axial spinal cord cross sections of the lesion epicenter stained with eriochrome cyanine R and Cresyl violet were examined at 250-μm intervals. (B) Histological analysis of spared tissue showed that durotomy plus myelotomy resulted in significantly increased neural tissue sparing overall compared with the SCI alone group (p < 0.0001), while the effect of durotomy alone did not reach statistical significance (p > 0.05). Analysis of spared gray matter revealed that both durotomy alone and durotomy plus myelotomy resulted in significantly increased sparing compared with traumatic SCI (tSCI) only (p < 0.01). Analysis of spared white matter tracts showed that durotomy plus myelotomy significantly increased sparing compared with durotomy alone (p < 0.01) and control animals (p < 0.0001). **p < 0.01, ****p < 0.0001. Color image is available online.
We then analyzed the effect of decompression surgery on the amount of spared white matter tracts. Durotomy alone did not lead to a significant increase of white matter sparing compared with tSCI only (1.39 mm2 vs. 1.27 mm2, respectively). Durotomy plus myelotomy resulted in an 21.3% increase of spared white matter at the injury center compared with tSCI-only (1.54 mm2 vs. SCI 1.27 mm2). Enhanced tissue sparing was observed throughout the rostral-caudal extent of the lesion area, with the most pronounced effects in the periphery of the lesion area ∼1 mm away from the injury center. Durotomy plus myelotomy significantly increased white matter sparing compared with durotomy alone (p < 0.01) and control SCI animals (p < 0.0001; Fig. 2B).
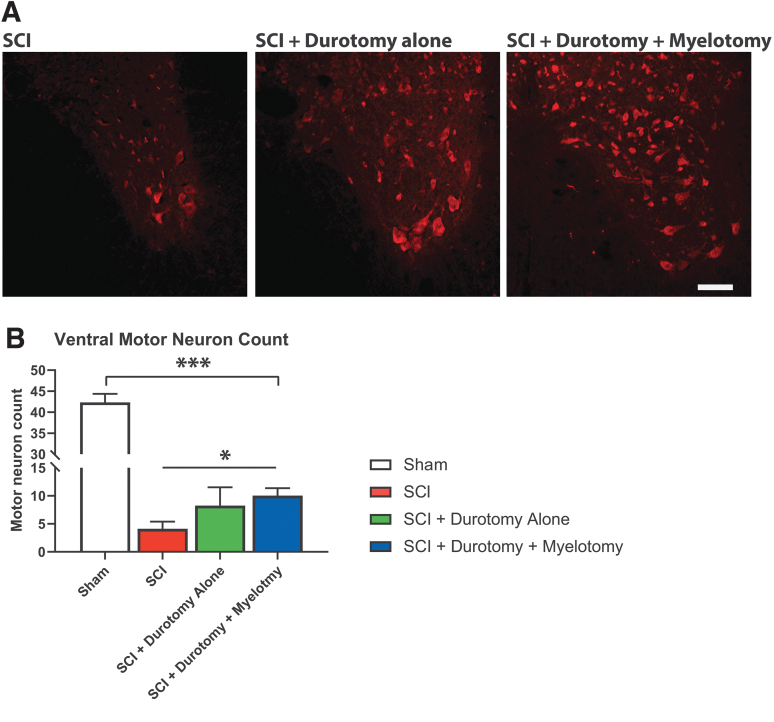
Analysis of gray matter sparing revealed that myelotomy increased gray matter sparing by 25.0% compared with durotomy alone and by 32.1% compared with tSCI-only (0.37 mm2, 0.35 mm2, 0.28 mm2; p < 0.01 for both comparisons). Enhanced sparing of gray matter appeared to be most pronounced in areas rostral to the injury center (Fig. 2B). Histological analysis of NeuN immunoreactive motor neurons revealed that thoracic contusion SCI resulted in a significant loss of ventral horn motor neurons at the injury center (1 mm segment) 12 weeks post-injury (Fig. 3). Durotomy alone increased the number of spared motor neurons at the injury center by 100.0%. However, given the high variability, this difference was not statistically significant. Durotomy plus myelotomy surgery led to a statistically significant increase in preservation of motor neurons by 143.4% compared with tSCI-only (p < 0.05).
FIG. 3.
Effect of surgical decompression on local motor neurons. (A) Representative examples of NeuN stained axial histological slides. (B) Histological analysis revealed that thoracic contusion spinal cord injury resulted in a significant decrease in ventral horn motor neuron counts at the injury center 8 weeks after injury (p < 0.001). Surgical decompression of the spinal cord, either by durotomy alone or durotomy plus myelotomy led to a trend toward an increase in the number of spared ventral horn motor neurons, although this was only significant following durotomy plus myelotomy (p < 0.05). ***p < 0.001, *p < 0.05. Color image is available online.
Decompression results in improved bladder function
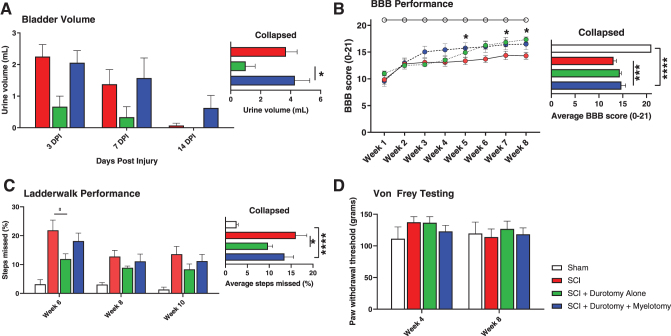
Inability of rats to voluntarily void the bladder of urine after injury is a common feature of thoracic contusion models. With moderate SCI such as the one examined in this study, rats typically regain the ability to empty their bladder after 10–14 days post-injury. We measured residual urine volumes for all animals after sham, SCI, SCI plus durotomy alone, and SCI plus durotomy plus myelotomy surgeries over the first 2 weeks (Fig. 4A). Analysis of our data demonstrated that durotomy alone promoted faster bladder recovery compared with durotomy plus myelotomy (p < 0.05).
FIG. 4.
Surgical decompression promotes functional outcome after spinal cord injury (SCI). (A) Durotomy alone resulted in earlier bladder reflex recovery than durotomy plus myelotomy (p < 0.05). (B) As expected, all injured animals showed some degree of spontaneous recovery of their hindlimb open-field locomotion. Durotomy plus myelotomy led to significantly better Basso, Beattie, Bresnahan (BBB) scores overall compared with SCI control treatment (p < 0.001). Durotomy plus myelotomy animals showed accelerated locomotor recovery compared with durotomy alone animals. From 5 weeks onwards, durotomy plus myelotomy treated animals performed better than SCI control treated animals (p < 0.05). At 8-week follow-up, durotomy animals performed better than SCI control treated animals (p < 0.05). (C) The Ladder Walk test was administered to examine the sensory-induced hindlimb motor function. Durotomy alone resulted in a significant overall decrease in Ladder Walk error rate compared with control animals (p < 0.05). (D) Von Frey testing was performed to determine decompression-related changes of nociception in our study animals. No significant differences were found. *p < 0.05, ***p < 0.001, ****p < 0.0001. Color image is available online.
Decompression results in improved locomotor recovery
Thoracic contusion SCIs in rodents led to significant functional hindlimb deficits, resulting in uncoordinated gait, difficulty in weight bearing, and, in many cases, complete hindlimb paralysis. Hindlimb locomotor testing using the BBB grading score confirmed significant impairment of hindlimb function in animals following contusion SCI followed by partial recovery over the ensuing 4–6 weeks (Fig. 4B). Durotomy plus myelotomy resulted in significantly improved BBB scores compared with tSCI-only controls (p < 0.001) from 5 weeks post-injury. Durotomy alone also resulted in higher BBB scores than tSCI-only, albeit at later follow-up (p < 0.05).
The LW test provides a quantitative measurement of skilled aspects of locomotion, with sensory components.28 Because rung placements are constantly altered, rodents are unable to learn a specific gait pattern to navigate the apparatus. Therefore, comparisons of LW scores reveal differences in sensing the location of the rung, stepping, and grasping skill, and not simply differences in learning ability. As expected, SCI rats at 6, 8, and 10 weeks post-injury had vastly increased error rates compared with sham animals (Fig. 4C). Surgical decompression by durotomy alone resulted in a significant overall decrease in LW error rate compared with SCI alone (p < 0.05), but no statistically significant effect was seen for durotomy plus myelotomy animals.
The Von Frey assay is a test of paw sensitivity. Filaments of increasing stiffness are applied to a rodent's hind paw until a filament is found that reliably produces a withdrawal response. In the present study, neither SCI-only nor SCI with decompression (durotomy alone or durotomy plus myelotomy) significantly altered hind paw sensitivity (Fig. 4D).
Discussion
Our current study suggests that durotomy combined with myelotomy more efficiently counteracts secondary tissue loss following acute tSCI than durotomy alone. Both types of decompression surgeries enhance recovery of open field locomotion. Recovery of LW and bladder function were in particular facilitated by durotomy alone.
Demyelination and loss of white matter tissue within the spinal cord in both pre-clinical models and humans are significant contributors to loss of function after tSCI.29–33 In the current study, we aimed to determine the distinct effects of durotomy alone versus durotomy plus myelotomy on neural tissue sparing in the spinal segment of the injury. We found that durotomy alone had only a modest effect whereas myelotomy resulted in a significant increase in white matter sparing. A comparison of these two interventions has been lacking, because previous studies have mainly focused on the effect of durotomy plus myelotomy on myelin sparing following SCI. There is a paucity of data regarding the effect of durotomy alone on tissue sparing following tSCI. Jalan and colleagues reported increased white matter sparing in a few animals that underwent durotomy combined with duraplasty, compared with control animals.17 However, half of the animal cohort was excluded from the analysis because they had spinal compression caused by the dural closure with hydrogel sealant. More data are available regarding the effect of durotomy plus myelotomy on counteracting local secondary tissue loss. In one study, durotomy plus myelotomy within 24 h following a rodent thoracic contusion SCI resulted in enhanced white matter sparing detected histologically 42 days post-trauma.18 The beneficial effect of durotomy plus myelotomy on secondary white matter loss has been further corroborated by several other studies.19–21 Our previous study revealed that durotomy plus myelotomy also reduced the size of the lesion cavity.21 The effects of meningeal decompression on local gray matter sparing have not been addressed by previous studies. Interestingly, in our current study, we report a significant increase in gray matter sparing following both durotomy alone and durotomy plus myelotomy, compared with our control cohort.
In the current study, we measured increased tissue edema and intraspinal pressures at the lesion center acutely after a contusion tSCI. We hypothesize that superior spinal cord tissue sparing seen following durotomy combined with myelotomy was in part caused by more effective mitigation of trauma-related increases in ISP. We have previously shown that durotomy combined with myelotomy results in superior treatment of elevated ISP compared with durotomy only when performed within the first 24 h after tSCI.6 Elevated ISP impairs vascular autoregulation and heterogenous perfusion of the contused spinal cord.34 Ensuing tissue ischemia propagates secondary injury of the tissue that was spared by the primary tissue insult. Experiments in a porcine SCI model revealed that sustained spinal cord compression leads to a dramatic increase of the lactate-pyruvate (L/P) ratio, which is a marker of tissue hypoxia.35 Accordingly, durotomy and expansile duroplasty in patients with tSCI improves spinal cord pressure reactivity and spinal cord perfusion pressure.15 Removal of the hemorrhage at the injury center might have also contributed to enhanced tissue sparing seen following durotomy combined with myelotomy. The hematoma at the injury exerts a mass effect on the adjacent spinal cord tissue, as evidenced by the displacement of the intrinsic spinal cord vasculature.36 Hemorrhage causes direct neural tissue damage by thrombin and hemoglobin. Thrombin in high doses leads to motor neuron cell death, neurite retraction, and astrogliosis via activation of the protease activated receptor (PAR)-1 in vitro.37 In vivo application of thrombin exacerbates brain edema38 and contributes to neurological deficits.39 Hemoglobin is the other component of the blood clot that contributes to secondary injury. Infusion of lysed erythrocytes into the brain results in edema, disruption of the blood–brain barrier, and damage of the neural tissue by oxidative stress.39 In summary, we hypothesize that myelotomy can limit local hemorrhage, resulting in additional tissue sparing by mitigating elevated ISP and reduction of blood clot mediated toxicity. This treatment effect would be important in cervical SCI, because even a small number of spared motor neurons may allow for recruitment of additional muscle groups and thereby increase upper extremity function. Importantly, tetraplegic patients regard upper extremity function as a major determinant for quality of life.40 Our recent research suggests that spared tissue may play a critical role in novel therapeutic approaches such as transcutaneous stimulation, which may activate dormant circuitry within the cervical spine.41
To date, the specific effects of durotomy alone compared with durotomy and myelotomy on functional recovery have not been investigated in experimental studies. Mixed results have been reported regarding the impact of durotomy on functional outcome. Several studies have reported positive effects on functional outcome,22,23 while other studies found no significant benefits.42 A rodent study by Smith and colleagues investigated the role of durotomy and duraplasty in cervical contusion injury.23 Following injury, the animals underwent a durotomy and in some animals, the dural opening was repaired with a dural allograft and fibrin sealant. These animals displayed significantly improved forelimb grip strength compared with animals that underwent contusion only or durotomy only. They also displayed a smaller lesion volume than the durotomy-only group. Similar results were reported by Zhang and colleagues, who performed epidural decompression (laminectomy) or durotomy following acute weight drop injury in the thoracic spine.22 They found that durotomy alone significantly promoted behavioral recovery and an earlier downregulation of ED-1, indicating that durotomy may reduce the post-traumatic inflammatory response. Somewhat inconclusive results were presented in a study by Jalan and colleagues utilizing a T10 contusion injury.17 After injury, the animals underwent either a 3 or a 5 level durotomy with or without duraplasty. Catwalk analysis revealed a significant decrease of the regularity index after a 3 or a 5 level durotomy without dural closure, but none of the interventions had a significant impact on general locomotor performance of the hind paws. However, in these experiments, dural closure was performed with the use of a hydrogel sealant, which caused severe spinal cord compression in approximately half of the animals. Omitting these animals for analysis revealed significantly improved behavioral outcomes in rodents that underwent durotomy in combination with dural closure. Camlar and colleagues investigated the role of acute (6–8 h after injury) and delayed (24 h) durotomy following a thoracic weight drop injury.42 In this study, no significant functional benefit of durotomy was noted. However, the authors used the Tarlov classification of motor function,43 which allows for assessment of only four levels of function, and may not have been sensitive enough to detect small differences in recovery between groups. In conclusion, the available literature supports the findings of our current report; that dural decompression enhances functional recovery following traumatic SCI.
Few studies have investigated the role of durotomy in human SCI. An initial case series of six patients by Perkins and Dean suggested that durotomy in addition to bony decompression and stabilization might promote functional recovery.44 A recent open label prospective clinical trial further investigated the role of dural decompression.15 A total of 21 patients ranging between 19 and 68 years in age with traumatic cervical or thoracic SCI underwent either bony decompression (laminectomy) or durotomy and duraplasty. Importantly, the ISP at the injury site was measured during the acute phase after surgery. The study showed that durotomy with duraplasty allowed for a superior decompression of the contused spinal cord, resulting in improved spinal cord perfusion pressure and vascular reactivity. Follow-up data suggested a tendency toward better improvement of American Spinal Injury Association (ASIA) scores, walking, and bladder function in patients who underwent durotomy and duraplasty compared with patients with laminectomy only.
Myelotomy as a treatment of acute SCI has been the topic of several animal studies. Most studies have shown that myelotomy results in favorable functional outcomes,18–20,45 while some do not support this hypothesis.46 Yang and colleagues investigated the optimal time window for myelotomy in a thoracic weight drop injury model.18 This study concluded that durotomy plus myelotomy performed within 24 h following injury results in significantly increased white matter sparing at the injury center and better functional recovery compared with control animals. Guizar-Sahagun explored the impact of the extent of the bony decompression (number of laminectomies), durotomy plus myelotomy, and intraspinal debridement on functional outcomes following a thoracic weight drop injury.19 The authors report that more extensive longitudinal opening of the spinal cord and debridement exacerbated intraspinal hemorrhage. In contrast, a more minimal debridement resulted in increased myelin sparing, in particular in the lesion penumbra. There was also a tendency toward improved recovery of hindlimb motor function. A meta-analysis of several studies18–20,45 revealed that durotomy plus myelotomy results in improved outcomes, in particular following moderate severity contusion injuries.24 A study by Qin and coworkers24 concluded that at 6 weeks post-injury, animals that underwent durotomy plus myelotomy displayed a BBB hindlimb locomotor score 3.27 points higher than control animals. A similar beneficial effect of myelotomy was found in the current study, in which animals with durotomy plus myelotomy surgeries had a BBB score of 16.5 compared with 14.3 in control SCI-only animals. To date, only a few case reports describe myelotomy performed in human patients.47–49
In the current study, durotomy combined with myelotomy resulted in an accelerated improvement of open field locomotion compared with durotomy alone. This difference may be explained by the immediate and more efficient relief of elevated ISP achieved by myelotomy compared with durotomy when performed within 24 h following injury.6 Myelotomy decompresses the pial lining, which contributes ∼50% of the increased ISP acutely following injury.6 Later, during the subacute post-traumatic phase, release of inflammatory cytokines including metalloproteinases weakens and damages the extracellular matrix50 and leads to a slow expansion of the pial linings following traumatic injury.4 A more protracted rate of locomotor recovery seen following durotomy alone appears to be in line with this gradual decompression of the spinal cord.
An interesting finding from this study was that durotomy alone led to increased recovery of bladder function compared with durotomy plus myelotomy, which runs contrary to the otherwise superior histological and behavioral outcomes seen following durotomy plus myelotomy. One possible explanation for this result is that surgical opening of the pia with an additional disruption of the injury core resulted in irritation of the spinal cord, leading to transient urinary retention in animals that received durotomy plus myelotomy. Transient urinary retention is commonly seen in patients who undergo myelotomy for treatment of intractable pain or resection of intraspinal tumors.51 These patients typically require an indwelling urinary catheter for the first days after surgery before bladder function returns. This, however, highlights the importance of carefully weighing the potential benefits of surgical decompression against the potential complications. Typically, the rate of surgical complications increases with the degree of invasiveness of the procedure.52,53 Accordingly, the complication rate of durotomy following acute tSCI was acceptable, with only 1 out of 10 patients developing a cerebrospinal fluid (CSF) leak.15 Moreover, disruption of the dural integrity may promote an inflammatory reaction and increase scar formation after SCI.23,54 Although expansile duraplasty is a standard clinical procedure with a sewed-in alloderm graft, this procedure is not standardized in the experimental SCI literature. Expansile duraplasty with primary dural closure in rats, although technically feasible, is typically not performed. The current report placed a fat pad as an epidural graft, similar to the dural closure procedure used in pituitary surgery.55 Other rodent experiments have used fibrin sealant, allograft,23,54 or collagen matrix.56 Some of these dural closures have been associated with complications in rodent experiments; for example, Jalan and colleagues report that utilization of commercially available dural sealant can lead to compression of the spinal cord in almost half of their animals.17 It is important to emphasize that most patients with SCI have polytrauma and are frequently hemodynamically unstable at presentation to the hospital. Additional surgical decompression of the spinal cord adds complexity and time to the surgical procedures. Although clinical data are lacking, mortality appears to be increased in animals that undergo myelotomy (13.9%) compared with control animals (6.3%).22 In another rodent study, extensive myelotomy was associated with a 12.5% rate of hemorrhage.19 The authors did not report on the outcomes of these animals but suggested a trend toward improved functional outcome in animals undergoing a less extensive spinal decompression. It is important to note that in our study, we did not encounter additional complications after either durotomy alone or durotomy plus myelotomy surgeries.
Conclusion
In summary, emerging evidence suggests that decompression surgery of the contused spinal cord may promote functional recovery. However, injuries that may benefit from this intervention are not yet defined, and possible procedure-related complications need to be carefully studied. Eventually, biomarkers identifying injuries that may benefit from the surgical decompression procedures need to be developed.
Funding Information
This study was funded by the Craig H. Neilsen Foundation (CHNF), the Department of Defense (DoD) Congressionally Directed Medical Research Programs (CDMRP; W81XWH-18-1-0753), and the Raisbeck Foundation, as well as the W. M. Keck Microscopy Center at the University of Washington for the use of a Leica SP8X confocal microscope (originally funded by National Institutes of Health [NIH] S10 OD016240 and the UW Student Technology Fee).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.INFO-Pages, S. Spinal Cord Injury Facts & Statistics. https://www.sci-info-pages.com/spinal-cord-injury-facts-and-statistics/ (Last accessed December13, 2020)
- 2.Hansebout, R.R., Tanner, J.A., and Romero-Sierra, C. (1984). Current status of spinal cord cooling in the treatment of acute spinal cord injury. Spine (Phila Pa 1976) 9, 508–511 [DOI] [PubMed] [Google Scholar]
- 3.Karsy, M., and Hawryluk, G. (2019). Modern medical management of spinal cord injury. Curr. Neurol. Neurosci. Rep. 19, 65. [DOI] [PubMed] [Google Scholar]
- 4.Jones, C.F., Cripton, P.A., and Kwon, B.K. (2012). Gross morphological changes of the spinal cord immediately after surgical decompression in a large animal model of traumatic spinal cord injury. Spine (Phila Pa 1976) 37, E890–899 [DOI] [PubMed] [Google Scholar]
- 5.Maikos, J.T., Elias, R.A., and Shreiber, D.I. (2008). Mechanical properties of dura mater from the rat brain and spinal cord. J. Neurotrauma 25, 38–51 [DOI] [PubMed] [Google Scholar]
- 6.Khaing, Z.Z., Cates, L.N., Fischedick, A.E., McClintic, A.M., Mourad, P.D., and Hofstetter, C.P. (2017). Temporal and spatial evolution of raised intraspinal pressure after traumatic spinal cord injury. J. Neurotrauma 34, 645–651 [DOI] [PubMed] [Google Scholar]
- 7.Streijger, F., So, K., Manouchehri, N., Tigchelaar, S., Lee, J.H.T., Okon, E.B., Shortt, K., Kim, S.E., McInnes, K., Cripton, P., and Kwon, B.K. (2017). Changes in pressure, hemodynamics, and metabolism within the spinal cord during the first 7 days after injury using a porcine model. J. Neurotrauma 34, 3336–3350 [DOI] [PubMed] [Google Scholar]
- 8.Werndle, M.C., Saadoun, S., Phang, I., Czosnyka, M., Varsos, G.V., Czosnyka, Z.H., Smielewski, P., Jamous, A., Bell, B.A., Zoumprouli, A., and Papadopoulos, M.C. (2014). Monitoring of spinal cord perfusion pressure in acute spinal cord injury: initial findings of the injured spinal cord pressure evaluation study. Crit. Care Med. 42, 646–655 [DOI] [PubMed] [Google Scholar]
- 9.Cao, X.J., Feng, S.Q., Fu, C.F., Gao, K., Guo, J.S., He, X.J., and Huang, Z.W. (2015). Repair, protection and regeneration of spinal cord injury. Neural Regen. Res. 10, 1953–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varsos, G.V., Werndle, M.C., Czosnyka, Z.H., Smielewski, P., Kolias, A.G., Phang, I., Saadoun, S., Bell, B.A., Zoumprouli, A., Papadopoulos, M.C., and Czosnyka, M. (2015). Intraspinal pressure and spinal cord perfusion pressure after spinal cord injury: an observational study. J. Neurosurg. Spine 23, 763–771 [DOI] [PubMed] [Google Scholar]
- 11.Phang, I., and Papadopoulos, M.C. (2015). Intraspinal pressure monitoring in a patient with spinal cord injury reveals different intradural compartments: Injured Spinal Cord Pressure Evaluation (ISCoPE) Study. Neurocrit. Care 23, 414–418 [DOI] [PubMed] [Google Scholar]
- 12.Hogg, F.R.A., Gallagher, M.J., Chen, S., Zoumprouli, A., Papadopoulos, M.C., and Saadoun, S. (2019). Predictors of intraspinal pressure and optimal cord perfusion pressure after traumatic spinal cord injury. Neurocrit. Care 30, 421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, S., Gallagher, M.J., Hogg, F., Papadopoulos, M.C., and Saadoun, S. (2018). Visibility graph analysis of intraspinal pressure signal predicts functional outcome in spinal cord injured patients. J. Neurotrauma 35, 2947–2956 [DOI] [PubMed] [Google Scholar]
- 14.Zhu, F., Yao, S., Ren, Z., Telemacque, D., Qu, Y., Chen, K., Yang, F., Zeng, L., and Guo, X. (2019). Early durotomy with duroplasty for severe adult spinal cord injury without radiographic abnormality: a novel concept and method of surgical decompression. Eur. Spine J 28, 2275–2282 [DOI] [PubMed] [Google Scholar]
- 15.Phang, I., Werndle, M.C., Saadoun, S., Varsos, G.V., Czosnyka, M., Zoumprouli, A., and Papadopoulos, M.C. (2015). Expansion duroplasty improves intraspinal pressure, spinal cord perfusion pressure and vascular pressure reactivity index in patients with traumatic spinal cord injury. J. Neurotrauma 32, 865–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson, J.R., Tetreault, L.A., Kwon, B.K., Arnold, P.M., Mroz, T.E., Shaffrey, C., Harrop, J.S., Chapman, J.R., Casha, S., Skelly, A.C., Holmer, H.K., Brodt, E.D., and Fehlings, M.G. (2017). Timing of decompression in patients with acute spinal cord injury: a systematic review. Global Spine J. 7, 95S–115S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jalan, D., Saini, N., Zaidi, M., Pallottie, A., Elkabes, S., and Heary, R.F. (2017). Effects of early surgical decompression on functional and histological outcomes after severe experimental thoracic spinal cord injury. J. Neurosurg. Spine 26, 62–75 [DOI] [PubMed] [Google Scholar]
- 18.Yang, D.G., Li, J.J., Gu, R., Yang, M.L., Zhang, X., Du, L.J., Sun, W., Gao, F., Hu, A.M., Wu, Y.Y., He, J.G., Feng, Y.T., and Chu, H.Y. (2013). Optimal time window of myelotomy in rats with acute traumatic spinal cord injury: a preliminary study. Spinal Cord 51, 673–678 [DOI] [PubMed] [Google Scholar]
- 19.Guizar-Sahagun, G., Martinez-Cruz, A., Franco-Bourland, R.E., Cruz-Garcia, E., Corona-Juarez, A., Diaz-Ruiz, A., Grijalva, I., Reyes-Alva, H.J., and Madrazo, I. (2017). Creation of an intramedullary cavity by hemorrhagic necrosis removal 24 h after spinal cord contusion in rats for eventual intralesional implantation of restorative materials. PLoS One 12, e0176105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalderon, N., Muruganandham, M., Koutcher, J.A., and Potuzak, M. (2007). Therapeutic strategy for acute spinal cord contusion injury: cell elimination combined with microsurgical intervention. PLoS One 2, e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guest, J.D., Moore, S.W., Aimetti, A.A., Kutikov, A.B., Santamaria, A.J., Hofstetter, C.P., Ropper, A.E., Theodore, N., Ulich, T.R., and Layer, R.T. (2018). Internal decompression of the acutely contused spinal cord: differential effects of irrigation only versus biodegradable scaffold implantation. Biomaterials 185, 284–300 [DOI] [PubMed] [Google Scholar]
- 22.Zhang, J., Wang, H., Zhang, C., and Li, W. (2016). Intrathecal decompression versus epidural decompression in the treatment of severe spinal cord injury in rat model: a randomized, controlled preclinical research. J. Orthop. Surg. Res. 11, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith, J.S., Anderson, R., Pham, T., Bhatia, N., Steward, O, and Gupta, R. (2010). Role of early surgical decompression of the intradural space after cervical spinal cord injury in an animal model. J. Bone Joint Surg. Am. 92, 1206–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin, C., Zhang, W.H., Yang, D.G., Yang, M.L., Du, L.J., and Li, J.J. (2018). Myelotomy promotes locomotor recovery in rats subjected to spinal cord injury: A meta-analysis of six randomized controlled trials. Neural. Regen. Res. 13, 1096–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somerson, S.K., and Stokes, B.T. (1987). Functional analysis of an electromechanical spinal cord injury device. Exp. Neurol. 96, 82–96 [DOI] [PubMed] [Google Scholar]
- 26.Behrmann, D.L., Bresnahan, J.C., Beattie, M.S., and Shah, B.R. (1992). Spinal cord injury produced by consistent mechanical displacement of the cord in rats: behavioral and histologic analysis. J. Neurotrauma 9, 197–217 [DOI] [PubMed] [Google Scholar]
- 27.Bonin, R.P., Bories, C. and De Koninck, Y. (2014). A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol. Pain 10, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metz, G.A. and Whishaw, I.Q. (2009). The ladder rung walking task: a scoring system and its practical application. J. Vis. Exp. 28, 1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guest, J.D., Hiester, E.D., and Bunge, R.P. (2005). Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp. Neurol. 192, 384–393 [DOI] [PubMed] [Google Scholar]
- 30.Bunge, R.P., Puckett, W.R., Becerra, J.L., Marcillo, A., and Quencer, R.M. (1993). Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv. Neurol. 59, 75–89 [PubMed] [Google Scholar]
- 31.Bunge, R.P., Bunge, M.B., and Rish (1960). Electron microscopic study of demyelination in an experimentally induced lesion in adult cat spinal cord. J. Biophys. Biochem. Cytol. 7, 685–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bunge, M.B., Bunge, R.P., and Ris, H. (1961). Ultrastructural study of remyelination in an experimental lesion in adult cat spinal cord. J. Biophys. Biochem. Cytol. 10, 67–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blight, A.R. (1983). Cellular morphology of chronic spinal cord injury in the cat: analysis of myelinated axons by line-sampling. Neuroscience 10, 521–543 [DOI] [PubMed] [Google Scholar]
- 34.Gallagher, M.J., Hogg, F.R.A., Zoumprouli, A., Papadopoulos, M.C., and Saadoun, S. (2019). Spinal cord blood flow in patients with acute spinal cord injuries. J. Neurotrauma 36, 919–929 [DOI] [PubMed] [Google Scholar]
- 35.Okon, E.B., Streijger, F., Lee, J.H., Anderson, L.M., Russell, A.K., and Kwon, B.K. (2013). Intraparenchymal microdialysis after acute spinal cord injury reveals differential metabolic responses to contusive versus compressive mechanisms of injury. J. Neurotrauma 30, 1564–1576 [DOI] [PubMed] [Google Scholar]
- 36.Khaing, Z.Z., Cates, L.N., Hyde, J., DeWees, D.M., Hammond, R., Bruce, M., and Hofstetter, C.P. (2020). Contrast-enhanced ultrasound for assessment of local hemodynamic changes following a rodent contusion spinal cord injury. Mil. Med. 185, 470–475 [DOI] [PubMed] [Google Scholar]
- 37.Striggow, F., Riek, M., Breder, J., Henrich-Noack, P., Reymann, K.G., and Reiser, G. (2000). The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proc. Natl. Acad. Sci. U. S. A. 97, 2264–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee, K.R., Colon, G.P., Betz, A.L., Keep, R.F., Kim, S., and Hoff, J.T. (1996). Edema from intracerebral hemorrhage: the role of thrombin. J. Neurosurg. 84, 91–96 [DOI] [PubMed] [Google Scholar]
- 39.Hua, Y., Schallert, T., Keep, R.F., Wu, J., Hoff, J.T., and Xi, G. (2002). Behavioral tests after intracerebral hemorrhage in the rat. Stroke 33, 2478–2484 [DOI] [PubMed] [Google Scholar]
- 40.Snoek, G.J., MJ, I.J., Hermens, H.J., Maxwell, D., and Biering-Sorensen, F. (2004). Survey of the needs of patients with spinal cord injury: impact and priority for improvement in hand function in tetraplegics. Spinal Cord 42, 526–532 [DOI] [PubMed] [Google Scholar]
- 41.Inanici, F., Samejima, S., Gad, P., Edgerton, V.R., Hofstetter, C.P., and Moritz, C.T. (2018). Transcutaneous electrical spinal stimulation promotes long-term recovery of upper extremity function in chronic tetraplegia. IEEE Trans. Neural Syst. Rehabil. Eng. 26, 1272–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camlar, M., Turk, C., Buhur, A., Oltulu, F., Oren, M., Senoglu, M., and Ozer, F. (2019). Does decompressive duraplasty have a neuroprotective effect on spinal trauma? An experimental study. World Neurosurg. 126, e288–e294 [DOI] [PubMed] [Google Scholar]
- 43.Tarlov, I.M. (1972). Acute spinal cord compression paralysis. J. Neurosurg. 36, 10–20 [DOI] [PubMed] [Google Scholar]
- 44.Perkins, P.G., and Deane, R.H. (1988). Long-term follow-up of six patients with acute spinal injury following dural decompression. Injury 19, 397–401 [DOI] [PubMed] [Google Scholar]
- 45.Hu, A.M., Li, J.J., Sun, W., Yang, D.G., Yang, M.L., Du, L.J., Gu, R., Gao, F., Li, J., Chu, H.Y., Zhang, X., and Gao, L.J. (2015). Myelotomy reduces spinal cord edema and inhibits aquaporin-4 and aquaporin-9 expression in rats with spinal cord injury. Spinal Cord 53, 98–102 [DOI] [PubMed] [Google Scholar]
- 46.Meyer, C., Bendella, H., Rink, S., Gensch, R., Seitz, R., Stein, G., Manthou, M., Papamitsou, T., Nakamura, M., Bouillon, B., Galea, M., Batchelor, P., Dunlop, S., and Angelov, D. (2018). The effect of myelotomy following low thoracic spinal cord compression injury in rats. Exp. Neurol. 306, 10–21 [DOI] [PubMed] [Google Scholar]
- 47.Zhu, H., Feng, Y.P., Young, W., You, S.W., Shen, X.F., Liu, Y.S., and Ju, G. (2008). Early neurosurgical intervention of spinal cord contusion: an analysis of 30 cases. Chin. Med. J. (Engl) 121, 2473–2478 [PubMed] [Google Scholar]
- 48.Koyanagi, I., Iwasaki, Y., Isu, T., Akino, M., Abe, H., Mitsumori, K., Nakagawa, T., Sakuragi, M., Saitoh, H., and Nomura, M. (1989). Myelotomy for acute cervical cord injury. Report of four cases [in Japanese]. Neurol. Med. Chir. (Tokyo) 29, 302–306 [DOI] [PubMed] [Google Scholar]
- 49.Tachibana, S., Okada, K., Ohwada, T,. and Yada, K. (1984). Posterior longitudinal myelotomy as a surgical treatment of acute cervical spinal cord injury [in Japanese]. No Shinkei Geka 12, 183–188 [PubMed] [Google Scholar]
- 50.Gaudet, A.D., and Popovich, P.G. (2014). Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp. Neurol. 258, 24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alizada, O., Kemerdere, R., Ulu, M.O., Akgun, M.Y., Isler, C., Kizilkilic, O., and Hanci, M.M. (2020). Surgical management of spinal intramedullary tumors: ten-year experience in a single institution. J. Clin. Neurosci. 73, 201–208 [DOI] [PubMed] [Google Scholar]
- 52.Hasan, S., Hartl, R., and Hofstetter, C.P. (2019). The benefit zone of full-endoscopic spine surgery. J. Spine Surg. 5, S41–S56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee, M.J., Konodi, M.A., Cizik, A.M., Bransford, R.J., Bellabarba, C., and Chapman, J.R. (2012). Risk factors for medical complication after spine surgery: a multivariate analysis of 1,591 patients. Spine J. 12, 197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iannotti, C., Zhang, Y.P., Shields, L.B., Han, Y., Burke, D.A., Xu, X.M., and Shields, C.B. (2006). Dural repair reduces connective tissue scar invasion and cystic cavity formation after acute spinal cord laceration injury in adult rats. J. Neurotrauma 23, 853–865 [DOI] [PubMed] [Google Scholar]
- 55.Hofstetter, C.P., Mannaa, R.H., Mubita, L., Anand, V.K., Kennedy, J.W., Dehdashti, A.R., and Schwartz, T.H. (2010). Endoscopic endonasal transsphenoidal surgery for growth hormone-secreting pituitary adenomas. Neurosurg. Focus 29, E6. [DOI] [PubMed] [Google Scholar]
- 56.Neulen, A., Gutenberg, A., Takacs, I., Weber, G., Wegmann, J., Schulz-Schaeffer, W., and Giese, A. (2011). Evaluation of efficacy and biocompatibility of a novel semisynthetic collagen matrix as a dural onlay graft in a large animal model. Acta Neurochir. (Wien) 153, 2241–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]