Abstract
Although emerging evidence suggests that schizophrenia (SZ) is associated with peripheral and central polyunsaturated fatty acid (PUFA) deficits, there is currently nothing known about the expression of genes that mediate PUFA biosynthesis in SZ patients. Here we determined Δ5 desaturase (FADS1), Δ6 desaturase (FADS2), elongase (HELO1 [ELOVL5]), peroxisomal (PEX19), and Δ9 desaturase (stearoyl-CoA desaturase, SCD) mRNA expression, and relevant fatty acid product:precursor ratios as estimates of enzyme activities, in the postmortem prefrontal cortex (PFC) of patients with SZ (n=20) and non-psychiatric controls (n=20). After correction for multiple comparisons, FADS2 mRNA expression was significantly greater in SZ patients relative to controls (+36%, p=0.002), and there was a positive trend found for FADS1 (+26%, p=0.15). No differences were found for HELO1 (+10%, p=0.44), PEX19 (+12%, p=0.44), or SCD (−6%, p=0.85). Both male (+34%, p=0.02) and female (+42%, p=0.02) SZ patients exhibited greater FADS2 mRNA expression relative to same-gender controls. Drug-free SZ patients (+37%, p=0.02), and SZ patients treated with typical (+40%, p=0.002) or atypical (+31%, p=0.04) antipsychotics, exhibited greater FADS2 mRNA expression relative to controls. Consistent with increased Δ6 desaturase activity, SZ patients exhibited a greater 20:3/18:2 ratio (+20%, p=0.03) and a positive trend was found for 20:4/18:2 (+13%, p=0.07). These data demonstrate abnormal, potentially compensatory, elevations in Δ6 desaturase (FADS2) expression in the PFC of SZ patients that are independent of gender and antipsychotic medications. Greater Δ6 desaturase expression and activity could have implications for central prostaglandin synthesis and proinflammatory signaling.
Keywords: Schizophrenia, Postmortem, Prefrontal cortex, Saturated fatty acids, Monounsaturated fatty acids, Polyunsaturated fatty acids, Stearoyl-CoA desaturase (SCD), Delta-5 desaturase (FADS1), Delta-6 desaturase (FADS2), Elongase (HELO1), Peroxisome (PEX19)
1. Introduction
Because mammals are incapable of synthesizing omega-6 and omega-3 fatty acids de novo, they are entirely dependent on dietary sources to procure and maintain adequate peripheral and central tissue concentrations. The principal polyunsaturated fatty acids (PUFA) in brain phospholipids are the omega-6 fatty acid arachidonic acid (AA, 20:4n-6) and the omega-3 fatty acid docosahexaenoic acid (DHA, 22:6n-3). The dietary omega-6 fatty acid precursor linoleic acid (LA, 18:2n-6) and omega-3 fatty acid precursor alpha-linolenic acid (ALA,18:3n-3) are converted to AA and DHA, respectively, through a series of common and competitive microsomal desaturation–elongation reactions, and DHA requires additional conversions within peroxisomes (reviewed in Sprecher and Chen, 1999). Principal human PUFA biosynthetic genes include FADS1 (Δ5 desaturase, Cho et al., 1999a), FADS2 (Δ6 desaturase, Cho et al., 1999b), HELO1 [ELOVL5] (elongase, Leonard et al., 2000), and genes required for peroxisome assembly including PEX19 (Götte et al., 1998). The principal enzyme mediating monounsaturated fatty acid biosynthesis from saturated fatty acids is Δ9 desaturase (stearoyl-CoA desaturase, SCD, Zhang et al., 1999). All of these enzymes are highly expressed in rat and human liver and brain, and their activities and expression are regulated by multiple factors including dietary PUFA composition (Cho et al., 1999a,b; Igarashi et al., 2007), gonadal hormones and insulin (reviewed in Brenner, 2003), as well as heritable genetic factors (Malerba et al., 2008; Nwankwo et al., 2003; Rzehak et al., 2008; Schaeffer et al., 2006; Williard et al., 2001).
An emerging body of evidence suggests that schizophrenia (SZ) is associated with peripheral and central PUFA deficits. Specifically, DHA and/or AA deficits are observed in postmortem brain tissue of SZ patients (Horrobin et al., 1991; McNamara et al., 2007; Yao et al., 2000) and in erythrocyte (red blood cell) membranes of antipsychotic-naive first-episode psychotic patients (Arvindakshan et al., 2003; Evans et al., 2003; Kale et al., 2008; Khan et al., 2002; Reddy et al., 2004). The etiology of PUFA deficits in SZ remains poorly understood, and could involve environmental factors, including dietary PUFA insufficiency (Brown et al., 1999; Henderson et al., 2006; Strassnig et al., 2005), elevated lipid peroxidation (Arvindakshan et al., 2003; Khan et al., 2002), and/or genetic factors including polymorphisms in PUFA biosynthetic genes (Malerba et al., 2008; Nwankwo et al., 2003; Rzehak et al., 2008; Schaeffer et al., 2006; Williard et al., 2001). Consistent with deficits in LA-AA and ALA-DHA biosynthesis, some studies (Evans et al., 2003; Kale et al., 2008; Reddy et al., 2004), but not all (Khan et al., 2002), have found that antipsychotic-naïve first- or early-episode psychotic patients exhibit normal erythrocyte ALA and LA levels but significantly lower DHA and AA levels. Furthermore, emerging evidence suggests that antipsychotic medications increase erythrocyte and cortical PUFA composition in SZ patients (Arvindakshan et al., 2003; Evans et al., 2003; Kaddurah-Daouk et al., 2007; Khan et al., 2002; McNamara et al., 2007), and preclinical (Fernø et al., 2005; McNamara et al., 2009) and clinical (Kaddurah-Daouk et al., 2007; Vik-Mo et al., 2008) studies suggest that atypical antipsychotic medications increase lipid biosynthetic enzyme activity and expression.
Together these findings support the hypothesis that impairments in PUFA biosynthesis contribute to the peripheral and central PUFA deficits observed in SZ patients, and that antipsychotic medications up-regulate PUFA biosynthesis. However, there is currently nothing known about the expression of genes that regulate PUFA biosynthesis in patients with SZ. As an initial evaluation, the present study determined elongase (HELO1 [ELOVL5]), Δ5 desaturase (FADS1), Δ6 desaturase (FADS2), peroxisome (PEX19), and stearoyl-CoA desaturase (SCD) mRNA expression in the postmortem prefrontal cortex (PFC, Brodmann area 10) of patients with SZ (n=20) and non-psychiatric controls (n=20). Enzyme activities were estimated using relevant fatty acid product:precursor ratios. We have previously reported that SZ patients exhibit lower DHA (−20%) and AA (−10%) levels in the postmortem PFC (BA 10), and that these deficits were greater in males and partially attenuated by antipsychotic medications (McNamara et al., 2007). Moreover, we have found that the age-related decline in DHA (−22%) and AA (−15%) compositions in the non-psychiatric human postmortem PFC (BA10) are associated with reciprocal, apparently compensatory, increases in FADS1, FADS2, and HELO1 mRNA expression (McNamara et al., 2008a). Based on these data, our specific prediction was that male and drug-free SZ patients would exhibit greater elongase and/or desaturase mRNA expression relative to non-psychiatric controls.
2. Methods
2.1. Postmortem brain tissues
Frozen, unfixed, postmortem PFC (BA10) from normal (no history of psychiatric illness) male and female controls (n=20) and male and female patients with DSM-IV defined SZ (n=20) were used. Brain tissue was generously provided by the Stanley Research Foundation Neuropathology Consortium (Torrey et al., 2000) and the Harvard Brain Tissue Resource Center. These tissues included samples used in our prior study (McNamara et al., 2007). Age at death (p=0.78), postmortem interval (p=0.41), brain weight (p=0.61), and brain pH (p=0.19) did not differ between controls and SZ patients (Table 1). At time of death, n=6 SZ patients were antipsychotic-free, n=6 SZ patients were receiving typical antipsychotic medications (fluphenazine, thiothixene, chlorpromazine, thioridazine, haloperidol), and n=8 SZ patients were receiving atypical antipsychotic medications (olanzapine, risperidone, clozapine). There were no significant differences in the age of controls, SZ-atypical, SZ-typical, and drug-free SZ subjects, F(3,39)=0.61, p=0.62. As a control for chronic atypical antipsychotic exposure, we also determined gene expression in a cohort (n=8) of similarly aged (p=0.49) patients with bipolar disorder receiving atypical antipsychotic medications (olanzapine, risperidone, quetiapine) at the time of death.
Table 1.
Comparison of subject and brain tissue characteristics.
| Control | SZ | p-valuea | |
|---|---|---|---|
| (n=20) | (n=20) | ||
| Patient characteristics | |||
| Age at death, mean±S.D. (range) | 45.7±11.7 (29–65) |
44.7±11.6 (25–65) |
0.78 |
| Gender | 12M,8F | 14M,6F | - |
| Raceb | 12C,8UN | 16C,4A | - |
| Cause of death | |||
| Suicide | 0 | 3 | |
| Cardiopulmonary | 11 | 9 | |
| Accident | 3 | 3 | |
| Other | 6 | 5 | |
| Tissue characteristics | |||
| Brain hemisphere | 12L/8R | 9L/11R | - |
| Brain mass (mean grams±S.D.) | 1456±162 | 1432±122 | 0.61 |
| Postmortem interval (mean hours±S.D.) | 23.4±7.9 | 26.1±11.9 | 0.41 |
| Tissue pH (mean±S.D.) | 6.3±0.3 | 6.2±0.3 | 0.19 |
Unpaired t-test (2-tail).
C=Caucasian, A=Asian, UN=Unknown.
2.2. RT-PCR
The real-time reverse transcriptase polymerase chain reaction (RT-PCR) procedure has been described in detail previously (McNamara et al., 2006). Primers and fluorogenic probes (Midland Certified Reagent Company, Midland, TX) were designed using Primer Express v.2.0 software (Applied Biosystems, Foster City, CA) based on the human mRNA sequence. Primer and probe sequences are presented in Table 2. Each probe was conjugated to a FAM reporter at the 5′ end and a TAMRA quencher at the 3′ end. The reverse primer for probes spanned an exon–intron junction to obviate genomic DNA contamination. Each primer pair yielded a single band on agarose gels for HELO1 (123 bp), FADS1 (80 bp), FADS2 (88 bp), PEX19 (159 bp), and SCD (150 bp). Reverse transcription was performed using the 9600 GeneAmp thermocycler (Perkin-Elmer, Norwalk, CT). The relative quantities for mRNA were normalized to GAPDH mRNA values obtained from the same tissue sample. All samples were processed by a technician blinded to illness state.
Table 2.
RT-PCR primer and probe sequences.
| Gene (accession number) | Primer and probe sequencesa | |
|---|---|---|
| HELO1 (NM_021814) | F | 5′-CACACTGCTGTCTCTGTATATGTTCTG-3′ |
| R | 5′-AGGACACGGATAATCTTCATATCTGAT-3′ | |
| P | 5′-TCAGGGCACACGCACCGCAG-3′ | |
| FADS1 (NM_013402) | F | 5′-TCCGCAAAGACCCAGACATC-3′ |
| R | 5′-CTGTTTCCCAAGCTCCACAGA-3′ | |
| P | 5′-TGCATCCCTTCTTCTTTGCCTTGGG-3′ | |
| FADS2 (NM_004265) | F | 5′-TTACAACATCACCAAATGGTCCAT-3′ |
| R | 5′-GAAGGCATCCGTTGCATCTT-3′ | |
| P | 5′-CCAGCGGGTCATCGGGCACTAC-3′ | |
| PEX19 (NM_002857) | F | 5′-GATCACAGAAAAGTATCCAGAATGGTT-3′ |
| R | 5′-CGAGCCTTTTGAGTGGTTTCAC-3′ | |
| P | 5′-CAGTTTGAGGCAGAGACCCCCACAGAC-3′ | |
| SCD (NM_005063) | F | 5′-CATAATTCCCGACGTGGCTTT-3′ |
| R | 5′-AGGTTTGTAGTACCTCCTCTGGAACA-3′ | |
| P | 5′-TTCTTCTCTCACGTGGGTTGGCTGCTT-3′ | |
| GAPDH (NM_002046) | F | 5′-CCACCCATGGCAAATTCC-3′ |
| R | 5′-TGGGATTTCCATTGATGACAAG-3′ | |
| P | 5′-CGTTCTCAGCCTTGACGGTGCCA-3′ |
Forward primer (F), Reverse primer (R), Probe (P).
2.3. Gas chromatography
The reliability and validity of the gas chromatography (GC) procedure has been described in detail previously (McNamara et al., 2008b). Briefly, total (triglyceride, phospholipid, and cholesteryl ester) fatty acid composition of frozen PFC samples (~100 mg, gray matter) was determined using the saponification and methylation methods originally described by Metcalfe et al. (1966). We have previously demonstrated that this method yields the same fatty acid composition as Folch (chloroform:methanol) extraction (McNamara et al., 2008b). Samples were analyzed with a Shimadzu GC-2014 equipped with an auto-injector (Shimadzu Scientific Instruments Inc., Columbia MD). The column was a DB-23 (123–2332): 30 m (length), I.D. (mm) 0.32 wide bore, film thickness of 0.25 μM (J&W Scientific, Folsom CA). The GC conditions were: column temperature ramping by holding at 120 °C for 1 min followed by an increase of 5 °C/min from 120–240 °C. The temperature of the injector and flame ionization detector was 250 °C. A split (8:1) injection mode was used. The carrier gas was helium with a column flow rate of 2.5 ml/min. Fatty acid identification was determined using retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). Analysis of fatty acid methyl esters is based on areas calculated with Shimadzu Class VP 4.3 software. Fatty acid composition data are expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids, wt.% total). We have previously demonstrated that wt.% total data are highly correlated with total mass data (nmol/g) (r=0.995, p≤0.0001) (McNamara et al., 2008b). All samples were processed by a technician blinded to illness state.
2.4. Statistical analysis
Analyses of variance (ANOVA) were performed using GBSTAT (Dynamic Microsystems, Inc., Silver Springs MD). The hypothesis that lipid biosynthetic gene expression (mRNA/GAPDH mRNA) differs by illness state was tested in a two-factor ANOVA, with Illness (Controls, SZ) and Gene (FADS1, FADS2, HELO1, PEX19, SCD) as main factors. Post-hoc tests (2-tailed) of simple effects were performed using the Bonferroni correction with a group-wise error rate of α=0.05 to evaluate illness state effects for the five different genes (α=0.05/5=0.01). For analysis of gender, a three factor ANOVA was used with Illness state (Controls, SZ), Gender (male, female), and Gene as main factors. A one factor ANOVA tested the effects of antipsychotic exposure among controls, and drug-free, typical antipsychotic-treated and atypical antipsychotic-treated SZ patients. Parametric linear regression analyses were performed to determine the interrelationship between relevant fatty acids ratios and gene expression. Exploratory analyses were conducted using Student’s t-tests (2-tailed, α=0.05), and effect size calculated using Cohen’s d, with small, medium, and large effect sizes being equivalent to d-values of 0.30, 0.50, and 0.80, respectively.
3. Results
3.1. Illness and gender effects
The two-factor ANOVA found significant main effects of Illness, F(1,196)=7.93, p=0.005, and Gene, F(4,196)=7.86, p≤0.0001, and the Illness×Fatty Acid Interaction, F(4,196)=1.34, p=0.254, was not significant. The significant main effect of illness is attributable to numerically greater expression of all PUFA biosynthetic genes, but not SCD, in SZ patients (Fig. 1A). After correction of multiple comparisons, FADS2 mRNA expression was significantly elevated in SZ patients relative to controls (+36%, p=0.002, d=1.1) and positive trends were found for FADS1 (+26%, p=0.15), HELO1 (+10%, p=0.44), and PEX19 (+12%, p=0.44) (Fig. 1A). SCD mRNA expression did not differ between groups (−6%, p=0.85). The three factor ANOVA found that the main effect of Gender, F(1,177)=2.14, p=0.15, the Illness×Gender interaction, F(4,177)=2.24, p=0.14, and the Illness×Gender×Gene Interaction, F(4,177)=0.49, p=0.99, were not significant. Both male (+34%, p=0.02, d=1.0) and female (+42%, p=0.02, d=1.4) SZ patients exhibited greater FADS2 mRNA expression relative to same-gender controls (Fig. 1B). Interestingly, female SZ patients exhibited numerically greater FADS1 (+27%, p=0.33) and FADS2 (+17%, p=0.32) mRNA expression relative to male SZ patients.
Fig. 1.
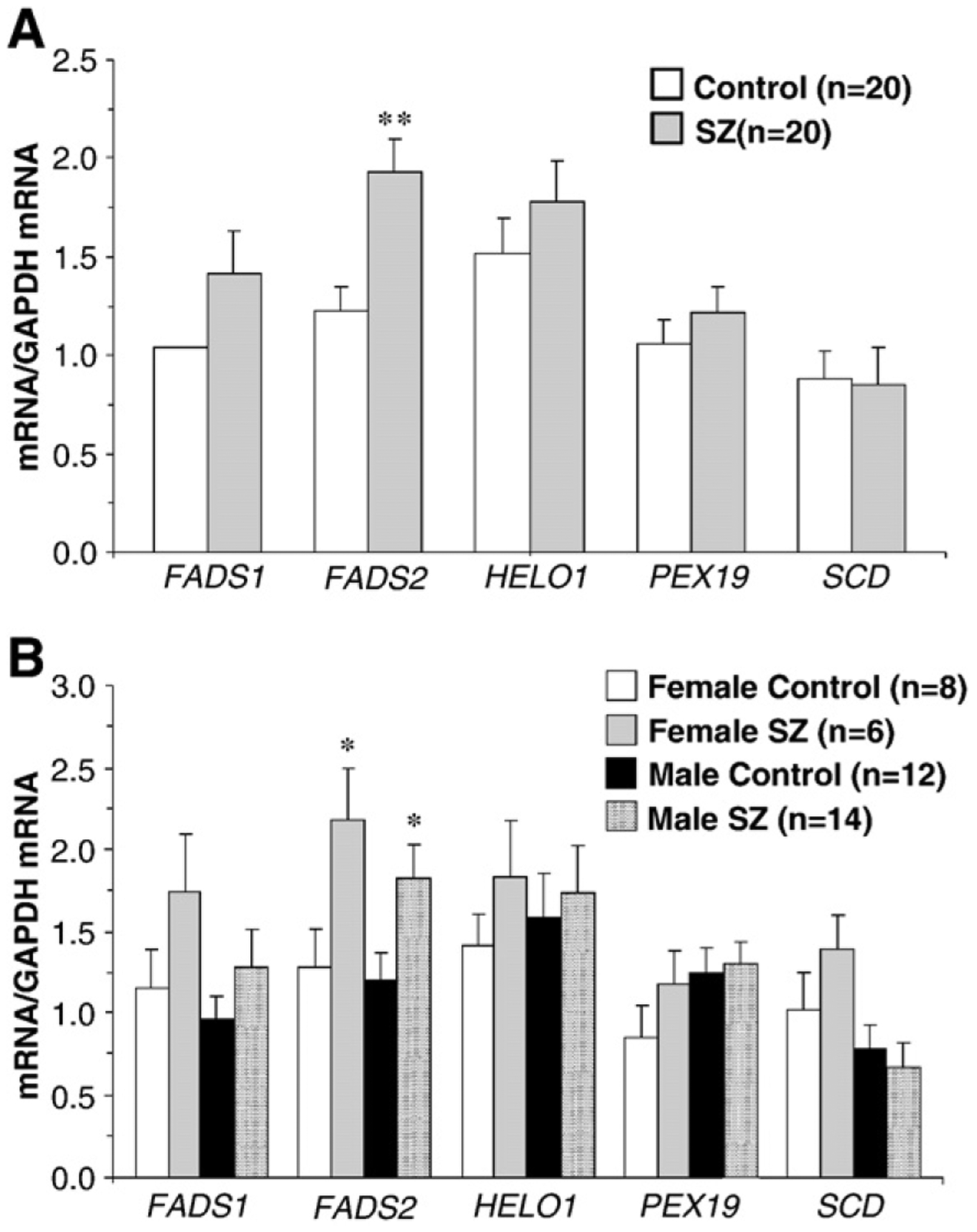
Lipid biosynthetic enzyme mRNA expression (mRNA/GAPDH mRNA) in the postmortem PFC (BA 10) of SZ patients (n=20) and non-psychiatric controls (n=20) before (A) and after (B) segregation by gender. FADS1 (Δ5 desaturase), FADS2 (Δ6 desaturase), HELO1 [ELOVL5] (elongase), PEX19 (peroxisome assembly), and SCD (Δ9 desaturase, stearoyl-CoA desaturase). Values are group mean±S.E.M. **p=0.002 vs. controls in (A), *P≤0.05 vs. same-gender controls in (B).
3.2. Antipsychotic medications
Analyses of antipsychotic treatment effects found a significant main effect for FADS2, F(3,39)=3.9, p=0.017. Relative to controls, FADS2 expression was greater in drug-free SZ patients (+37%, p=0.02, d=1.4) and in SZ patients treated with atypical (+31%, p=0.04, d=0.8) and typical (+40%, p=0.002, d=1.4) antipsychotic medications (Fig. 2A). FADS2 expression in bipolar patients receiving atypical antipsychotic medications did not differ from controls (−4%, p=0.81), and was numerically smaller than SZ patients treated with atypical antipsychotic medications (−35%, p=0.12) (Fig. 2B). Analyses for antipsychotic treatment did not find a significant main effect for FADS1, F(3,39)=1.9, p=0.15, HELO1, F(3,39)=0.6, p=0.59, PEX19, F(3,39)=0.3, p=0.86, or SCD, F(3,39)=1.1, p=0.37. Furthermore, FADS1, F(3,39)=0.03, p=0.96, HELO1, F(3,39)=0.3, p=0.76, PEX19, F(3,39)=0.2, p=0.79, and SCD, F(3,39)=1.9, p=0.17, expression did not differ among controls and SZ and bipolar patients treated with atypical antipsychotic medications (Fig. 2B).
Fig. 2.
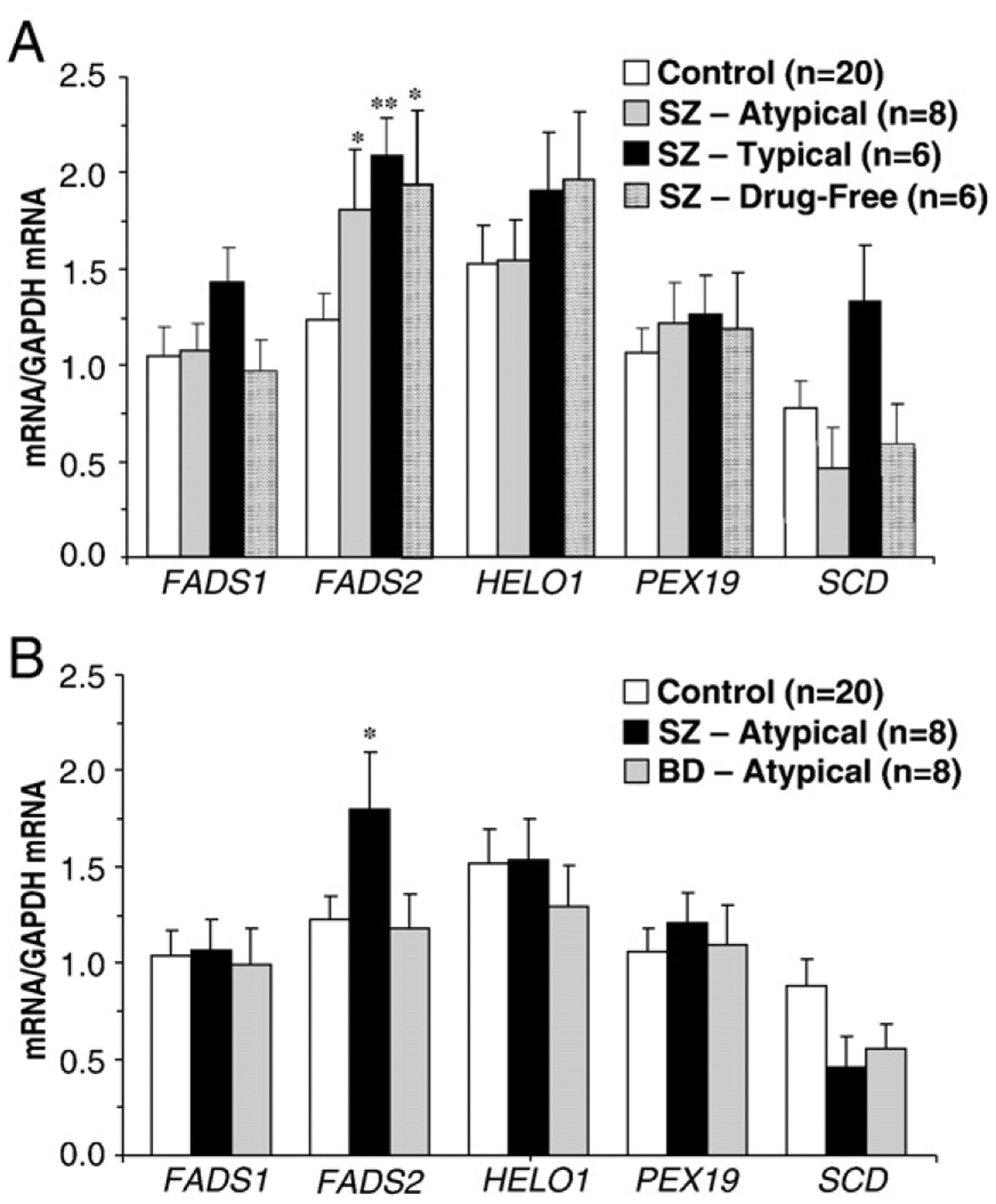
(A) Lipid biosynthetic enzyme mRNA expression (mRNA/GAPDH mRNA) in the postmortem PFC (BA 10) of non-psychiatric controls (n=20), SZ patients treated with atypical antipsychotics (n=8), SZ patients treated with typical antipsychotics (n=6), and drug-free SZ patients (n=6). (B) Lipid biosynthetic enzyme mRNA expression in non-psychiatric controls (n=20), SZ patients treated with atypical antipsychotics (n=8), and bipolar disorder (BD) patients treated with atypical antipsychotics (n=8). Values are group mean±S.E.M. *P≤0.05, **P≤0.01 vs. controls.
3.3. Fatty acid ratios
Consistent with increased Δ6 desaturase (FADS2) activity, SZ patients exhibited a greater 20:3/18:2 ratio (+20%, p=0.03) and a positive trend was observed for 20:4/18:2 (+13%, p=0.07) relative to controls (Table 3). There were no significant group differences for indices of Δ5 desaturase (FADS1) activity (20:4/20:3, −5%, p=0.46), HELO1 activity (22:4/20:4, +6%, p=0.37), or SCD activity (18:1/18:0, +4%, p=0.55; 16:1/16:0, +2%, p=0.69) (Table 3). Analysis by gender for the 20:3/18:2 ratio found a significant main effect of Illness, F(1,39)=4.15, p=0.049, and the main effect of Gender, F(1,39)=0.14, p=0.71, and the Illness×Gender interaction, F(1,39)=0.62, p=0.44, were not significant. The main effects of Illness and Gender, and the Illness×Gender interaction, were not significant for other fatty acid ratios. Analyses for antipsychotic treatment did not find a significant main effect for 20:3/18:2, F(3,39)=1.8, p=0.15, 20:4/18:2, F(3,39)=1.1, p=0.37, 20:4/20:3, F(3,39)=0.6, p=0.59, 22:4/20:4, F(3,39)=0.3, p=0.79, 18:1/18:0, F(3,39)=0.3, p=0.78, or 16:1/16:0, F(3,39)=0.3, p=0.83.
Table 3.
Fatty acid product: precursor ratios.
| Fatty acid ratioa | Control | SZ | Δ | d | p-valueb |
|---|---|---|---|---|---|
| (n=20) | (n=20) | ||||
| Δ5-desaturase (FADS1) | |||||
| 20:4/20:3 | 8.3±0.4 | 7.9±0.4 | −5% | 0.15 | 0.46 |
| Δ6-desaturase (FADS2) | |||||
| 20:3/18:2 | 1.4±0.1 | 1.7±0.1 | +20% | 0.64 | 0.03 |
| 20:4/18:2 | 11.4±0.6 | 13.1±0.7 | +13% | 0.42 | 0.07 |
| Elongase (HELO1) | |||||
| 22:4/20:4 | 0.67±0.0 | 0.71±0.0 | +6% | 0.29 | 0.37 |
| Δ9-desaturase (SCD) | |||||
| 18:1/18:0 | 0.93±0.0 | 0.97±0.0 | +4% | 0.20 | 0.55 |
| 16:1/16:0 | 0.03±.00 | 0.03±.00 | +2% | 0.13 | 0.69 |
Δ, Percent difference from controls.
d, Cohen’s d-values.
Values are group mean±S.E.M.
Unpaired t-test (two-tailed).
3.4. Correlations between mRNA expression and fatty acids
FADS2 mRNA expression was inversely correlated with 18:2n-6 composition in controls (r=−0.65, p=0.002) and a negative trend was observed in SZ patients (r=−0.27, p=0.25) (Fig. 3). FADS2 mRNA expression was not correlated with 20:4n-6 composition in controls (r=−0.26, p=0.26) or SZ patients (r=−0.41, p=0.06). FADS2 mRNA expression was positively correlated with the 20:4/18:2 ratio in controls (r=+0.68, p=0.0009) but not in SZ patients (r=+0.11, p=0.65) (Fig. 3). FADS2 mRNA expression was not correlated with the 20:3/18:2 ratio in either controls (r=+0.04, p=0.87) or SZ patients (r=+0.36, p=0.11). FADS1 mRNA expression was inversely correlated with DHA (22:6n-3) composition in SZ patients (r=−0.67, p=0.001) but not in controls (r=−0.24, p=0.31). FADS2 mRNA expression was inversely correlated with DHA composition in SZ patients (r=−0.45, p=0.04) but not in controls (r=−0.16, p=0.50). HELO1 mRNA expression was inversely correlated with DHA composition in SZ patients (r=−0.49, p=0.03) but not in controls (r=−0.29, p=0.21). PEX19 mRNA expression was not correlated with DHA composition in controls (r=+0.19, p=0.42) or SZ patients (r=+0.23, p=0.34).
Fig. 3.
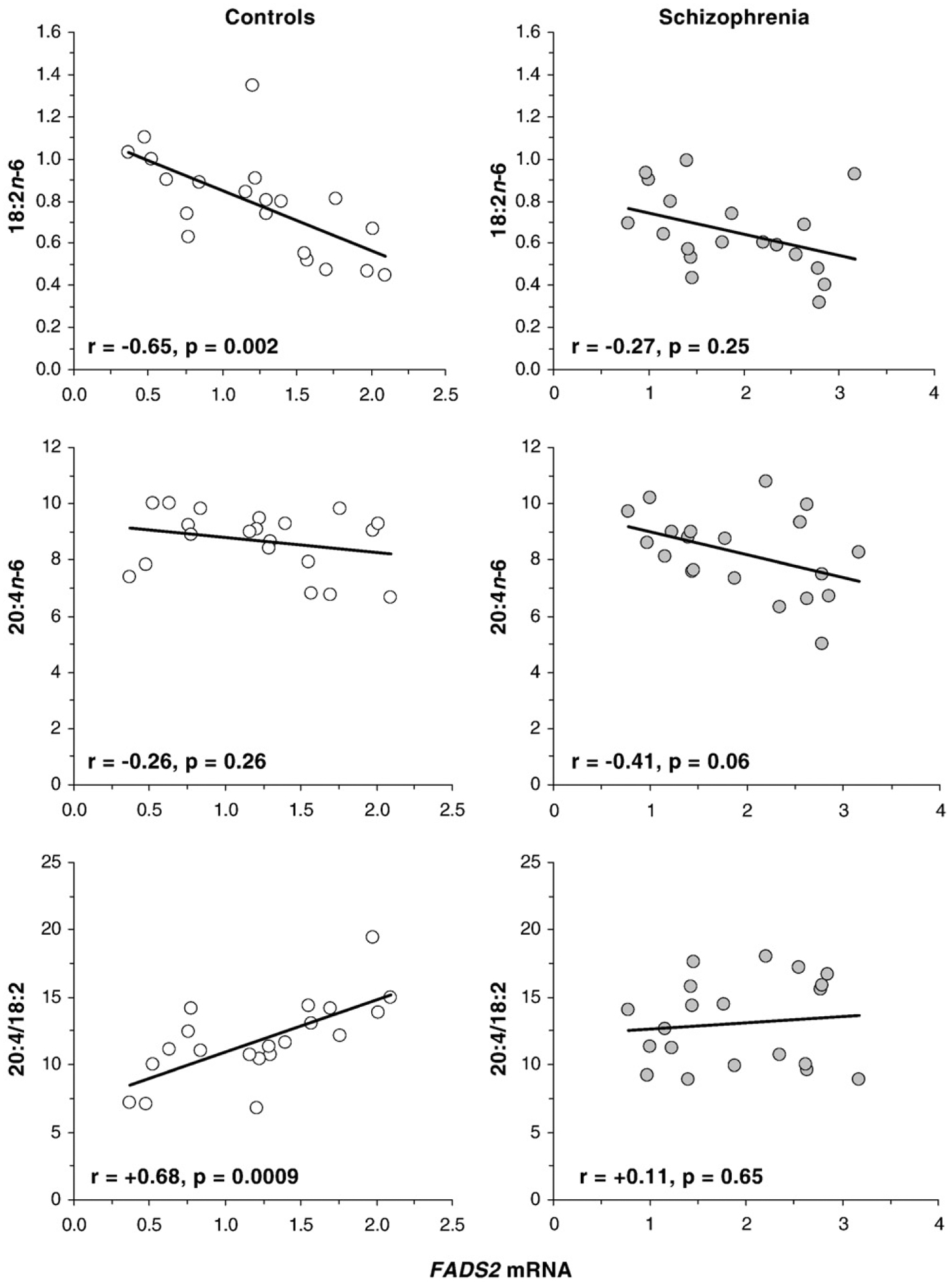
Linear regression analyses of FADS2 mRNA expression (mRNA/GAPDH mRNA) and 18:2n-6 composition (top panels), 20:4n-6 composition (middle panels), and the 20:4/18:2 ratio (bottom panels) in controls (n=20) and patients with SZ (n=20). Note that the 20:4/18:2 ratio is positively correlated with FADS2 mRNA expression in controls but not in SZ patients. Pearson correlation coefficients and associated p-values (two-tailed) are presented.
4. Discussion
Based on our prior postmortem studies finding apparently compensatory increases in PUFA biosynthetic gene expression in response to age-related decreases in PFC (BA 10) DHA and AA composition in normal controls (McNamara et al., 2008a), and gender-specific and antipsychotic-attenuated DHA and AA deficits in the PFC (BA10) of SZ patients (McNamara et al., 2007), it was hypothesized that male and drug-free SZ patients would exhibit compensatory increases in elongase and/or desaturase mRNA expression. Contrary to this hypothesis, we found that both male and female SZ patients exhibited greater FADS2 mRNA expression relative to same-gender controls, and that drug-free as well as antipsychotic-treated SZ patients exhibited greater FADS2 mRNA expression relative to controls. Consistent with elevated Δ6 desaturase (FADS2) activity, SZ patients exhibited a greater 20:3/18:2 ratio, and a positive trend was observed for the 20:4/18:2 ratio. However, the 20:4/18:2 ratio was positively correlated with FADS2 mRNA expression in controls, but not in SZ patients, suggesting a potential deficit in Δ6 desaturase enzyme activity. Although numerically greater FADS1 (+26%) and HELO1 (+10%) mRNA expression was observed in SZ patients, they did not differ significantly from controls. FADS1, FADS2 and HELO1 mRNA expression were all inversely correlated with DHA composition in SZ patients but not in controls. Collectively, these data demonstrate that SZ patients exhibit greater Δ6 desaturase (FADS2) mRNA expression in the postmortem PFC that is independent of gender and antipsychotic medications. Moreover, these data suggest that the central PUFA deficits observed in SZ patients cannot be attributed to deficits in PUFA biosynthetic gene expression.
This postmortem study has important limitations. Firstly, there were no data available regarding the PUFA composition of the diets of the subjects used in this study, and the PUFA content in diets of SZ patients is highly variable (Brown et al., 1999; Henderson et al., 2006; Strassnig et al., 2005). A second limitation is that fatty acid ratios represent a relatively crude index of enzyme activity. However, selective deletion (Stoffel et al., 2008; Williard et al., 2001) or pharmacological inhibition (Obukowicz et al., 1998) of Δ6 desaturase is associated with a larger membrane 20:4/18:2 ratio, and we found that FADS2 expression was positively correlated with the 20:4/18:2 ratio in controls. A third related limitation is that the immediate Δ6 desaturase product of 18:2n-6, 18:3n-6, was below the limit of reliable detection (<0.2%) in our samples, and we were therefore unable to calculate the 18:3/18:2 ratio which represents a more definitive index of Δ6 desaturase activity. Moreover, alpha-linolenic acid (18:3n-3) and eicosapentaenoic acid (20:5n-3) were below the limit of reliable detection (<0.2%), and we were therefore unable to calculate omega-3 fatty acid product:precursor ratios. Lastly, the small number of SZ patients in gender and drug subgroups may not be a representative sample in view of the heterogeneous clinical phenotype of SZ, the variety of different medications taken by SZ patients, and other potential clinical and demographic variables. Larger sample sizes will therefore be required to confirm the present findings.
The greater FADS2 mRNA expression observed in the postmortem PFC of SZ patients may reflect a compensatory increase in response to deficits in PFC PUFA composition. For example, we previously found that the age-related decline in DHA and AA compositions in normal human postmortem PFC (BA10) were associated with reciprocal increases in FADS1, FADS2, and HELO1 mRNA expression (McNamara et al., 2008a). In the present study, FADS1, FADS2, and HELO1 mRNA expression were all inversely correlated with DHA composition in SZ patients but not in controls, suggesting that deficits in DHA composition may contribute in part to the up-regulation of FADS2 mRNA expression in the PFC of SZ patients. However, this interpretation is not supported by a preclinical study finding that selective dietary-induced deficits in DHA composition were not associated with elevations in Δ6 desaturase mRNA expression in rat brain (Igarashi et al., 2007). This discrepancy may suggest that reductions in both DHA and AA may be required to up-regulate fatty acid biosynthetic gene expression in the mammalian brain. Indeed, both DHA and AA decrease FADS2 promoter activity in vitro (Nara et al., 2002). Nevertheless, the general pattern of greater FADS1, FADS2, and HELO1 mRNA expression in the PFC of SZ patients is consistent with a compensatory increase in response to deficits in PFC PUFA composition.
We have previously reported that SZ patients exhibit lower DHA (−20%) and AA (−10%) levels in the postmortem PFC (BA 10), and that these deficits were partially attenuated by antipsychotic medications (McNamara et al., 2007). Moreover, prior clinical (Arvindakshan et al., 2003; Evans et al., 2003; Kaddurah-Daouk et al., 2007; Khan et al., 2002; McNamara et al., 2007; Vik-Mo et al., 2008) and preclinical (Fernø et al., 2005; McNamara et al., 2009) studies suggest that antipsychotic medications increase peripheral and central PUFA composition in SZ patients by increasing PUFA biosynthetic enzyme expression and/or activities. In the present study, drug-free SZ patients exhibited greater FADS2 expression relative to controls, and bipolar patients being treated with atypical antipsychotics did not exhibit greater FADS2 mRNA expression. Together these data suggest that greater FADS2 mRNA expression observed on the PFC of SZ patients cannot be attributed to chronic antipsychotic medication exposure, though additional controlled studies will be required to confirm this.
The greater FADS2 mRNA expression found in the postmortem PFC of SZ patients was not associated with greater arachidonic acid (20:4n-6) composition, as would be predicted from the finding that reductions in FADS2 mRNA expression or activity decrease peripheral membrane 20:4n-6 composition (Obukowicz et al.,1998; Stoffel et al., 2008; Williard et al., 2001). Moreover, the 20:4/18:2 ratio was positively correlated with FADS2 mRNA expression in controls, but not in SZ patients, suggesting a potential deficit in FADS2 translation or Δ6 desaturase activity. However, because 20:4n-6 is a direct precursor for inflammatory eicosanoids, these data may have implications for cyclooxygenase- and/or lipoxygenase-mediated inflammatory eicosanoid synthesis. For example, pharmacological inhibition (Obukowicz et al., 1998) or deletion (Stoffel et al., 2008) of Δ6 desaturase activity is associated with reduced indices of inflammation and impaired eicosanoid synthesis in mice. Moreover, reductions in Δ6 desaturase activity secondary to polymorphisms in the FADS2 gene are associated with a lower prevalence of allergic rhinitis and atopic eczema (Schaeffer et al., 2006). It is relevant, therefore, that cyclooxygenase hyperactivity has been observed in platelets of drug-naïve SZ patients (Das and Khan, 1998), though evidence for elevated CSF eicosanoid levels in SZ remains controversial (Mathé et al., 1980; Nishino et al., 1998). Additionally, it will be of considerable interest to determine whether heritable genetic variants in the FADS2 gene (Malerba et al., 2008; Nwankwo et al., 2003; Rzehak et al., 2008; Schaeffer et al., 2006; Williard et al., 2001) contribute to elevated mRNA expression and membrane fatty acid abnormalities in SZ patients.
Although the peroxisomal assembly gene PEX19 has been found to be essential for peroxisomal formation and function (Götte et al., 1998), and peroxisomal formation disorders are associated with deficits in peripheral and central DHA and DPA composition (Martinez, 1992), in this study SZ patients did not exhibit deficits in PEX19 mRNA expression. However, it is possible that polymorphisms within the PEX19 gene, and/or alterations in the expression of other peroxisomal assembly genes, may contribute to impaired peroxisomal formation and function independent of mRNA expression (Matsuzono et al., 1999). In view of evidence for DPA and/or DHA deficits in erythrocytes (Arvindakshan et al., 2003; Evans et al., 2003; Kale et al., 2008; Khan et al., 2002; Reddy et al., 2004) and the postmortem PFC (McNamara et al., 2007) of SZ patients, a comprehensive interrogation for polymorphisms in peroxisomal genes in SZ patients appears warranted.
The synthesis of oleic acid (18:1n-9) and palmitioleic acid (16:1n-7) from stearic acid (18:0) and palmitic acid (16:0), respectively, is mediated by stearoyl-CoA desaturase (SCD) (Ntambi and Miyazaki, 2004), and we have previously found that SCD mRNA expression is positively correlated with indices of SCD activity (18:1/18:0, 16:1/16:0) in postmortem brain tissue (McNamara et al., 2008a). In the present study, SCD mRNA expression, and indices of SCD activity (18:1/18:0, 16:1/16:0), did not differ between SZ patients and controls. Interestingly, a recent clinical study found that SZ patients treated with olanzapine exhibited greater SCD mRNA expression in whole blood relative to untreated SZ patients (Vik-Mo et al., 2008). However, in the present study SCD mRNA expression in the PFC did not differ significantly between drug-free and antipsychotic-treated SZ patients. Indeed, negative trends were found for PFC SCD mRNA expression in SZ (−41%, p=0.13) and bipolar patients (−29%, p=0.26) treated with atypical antipsychotic medications. However, a positive trend was observed for SCD mRNA expression in SZ patients treated with typical antipsychotic medications (+42%, p=0.052).
In summary, the present study found greater Δ6 desaturase (FADS2) mRNA expression and indices of activity in the postmortem PFC of SZ patients. Greater FADS2 mRNA expression in SZ patients was not gender-specific and was not attenuated by antipsychotic medications, suggesting that the gender-specific and antipsychotic-attenuated DHA and AA deficits found in the postmortem PFC of SZ patients (McNamara et al., 2007) cannot be attributed to deficits in desaturase mRNA expression. Greater Δ6 desaturase expression and associated activity could potentially promote inflammatory eicosanoid synthesis (Obukowicz et al., 1998; Stoffel et al., 2008), as well as cardiovascular (Warensjö et al., 2008) and metabolic symptoms (Rimoldi et al., 2001) frequently observed in SZ patients. Remarkably, it was hypothesized over 25 years ago that elevated Δ6 desaturase-mediated omega-6 fatty acid biosynthesis and prostaglandin production could contribute to the pathoaetiology of SZ (Horrobin and Huang, 1983). It is also notable that FADS2 mRNA expression was inversely correlated with DHA composition in SZ patients but not in controls, suggesting that increasing PFC DHA composition may be an effective strategy to normalize central FADS2 expression in SZ patients. Nevertheless, more definitive isotope tracer studies are warranted to evaluate antemortem Δ6 desaturase activity in SZ patients prior to and following treatment with antipsychotic medications.
Acknowledgments
This work was supported in part by the National Institute of Health grants MH073704 and MH074858 to R.K.M., and DK59630 to P.T. Postmortem tissue was donated by The Stanley Medical Research Institute’s Brain Collection courtesy of Drs. Michael B. Knable, E. Fuller Torrey, Maree J. Webster, Serge Weis, and Robert H. Yolken, and the Harvard Brain Tissue Resource Center.
Role of funding source
Funding for this study was provided by NIMH Grants MH073704 and MH074858 to R.K.M.; the NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of interest statement
None of the authors have any actual or potential conflict of interest, including any financial, personal or other relationships with other people or organizations within three (3) years of beginning the work submitted that could inappropriately influence, or be perceived to influence, their work.
References
- Arvindakshan M, Sitasawad S, Debsikdar V, Ghate M, Evans D, Horrobin DF, Bennett C, Ranjekar PK, Mahadik SP, 2003. Essential polyunsaturated fatty acid and lipid peroxide levels in never-medicated and medicated schizophrenia patients. Biol. Psychiatry 53, 56–64. [DOI] [PubMed] [Google Scholar]
- Brenner RR, 2003. Hormonal modulation of delta6 and delta5 desaturases: case of diabetes. Prostaglandins. Leukot. Essent. Fat. Acids 68, 151–162. [DOI] [PubMed] [Google Scholar]
- Brown S, Birtwistle J, Roe L, Thompson C, 1999. The unhealthy lifestyle of people with schizophrenia. Psychol. Med 29, 697–701. [DOI] [PubMed] [Google Scholar]
- Cho HP, Nakamura M, Clarke SD, 1999a. Cloning, expression, and fatty acid regulation of the human delta-5 desaturase. J. Biol. Chem 274, 37335–37339. [DOI] [PubMed] [Google Scholar]
- Cho HP, Nakamura MT, Clarke SD, 1999b. Cloning, expression, and nutritional regulation of the mammalian delta-6 desaturase. J. Biol. Chem 274, 471–477. [DOI] [PubMed] [Google Scholar]
- Das I, Khan NS, 1998. Increased arachidonic acid induced platelet chem-iluminescence indicates cyclooxygenase overactivity in schizophrenic subjects. Prostaglandins Leukot. Essent. Fat. Acids 58, 165–168. [DOI] [PubMed] [Google Scholar]
- Evans DR, Parikh VV, Khan MM, Coussons C, Buckley PF, Mahadik SP, 2003. Red blood cell membrane essential fatty acid metabolism in early psychotic patients following antipsychotic drug treatment. Prostaglandins Leukot. Essent. Fat. Acids 69, 393–399. [DOI] [PubMed] [Google Scholar]
- Fernø J, Raeder MB, Vik-Mo AO, Skrede S, Glambek M, Tronstad KJ, Breilid H, Løvlie R, Berge RK, Stansberg C, Steen VM, 2005. Antipsychotic drugs activate SREBP-regulated expression of lipid biosynthetic genes in cultured human glioma cells: a novel mechanism of action? Pharmacogenomics J. 5, 298–304. [DOI] [PubMed] [Google Scholar]
- Götte K, Girzalsky W, Linkert M, Baumgart E, Kammerer S, Kunau WH, Erdmann R, 1998. Pex19p, a farnesylated protein essential for peroxisome biogenesis. Mol. Cell. Biol 18, 616–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DC, Borba CP, Daley TB, Boxill R, Nguyen DD, Culhane MA, Louie P, Cather C, Eden Evins A, Freudenreich O, Taber SM, Goff DC, 2006. Dietary intake profile of patients with schizophrenia. Ann. Clin. Psychiatry 18, 99–105. [DOI] [PubMed] [Google Scholar]
- Horrobin DF, Huang YS, 1983. Schizophrenia: the role of abnormal essential fatty acid and prostaglandin metabolism. Med. Hypotheses 10, 329–336. [DOI] [PubMed] [Google Scholar]
- Horrobin DF, Manku MS, Hillman H, Iain A, Glen M, 1991. Fatty acid levels in the brains of schizophrenics and normal controls. Biol. Psychiatry 30, 795–805. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI, 2007. Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J. Lipid. Res 48, 2463–2470. [DOI] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, McEvoy J, Baillie RA, Lee D, Yao JK, Doraiswamy PM, Krishnan KR, 2007. Metabolomic mapping of atypical antipsychotic effects in schizophrenia. Mol. Psychiatry 12, 934–945. [DOI] [PubMed] [Google Scholar]
- Kale A, Joshi S, Naphade N, Sapkale S, Raju MS, Pillai A, Nasrallah H, Mahadik SP, 2008. Opposite changes in predominantly docosahexaenoic acid (DHA) in cerebrospinal fluid and red blood cells from never-medicated first-episode psychotic patients. Schizophr. Res 98, 295–301. [DOI] [PubMed] [Google Scholar]
- Khan MM, Evans DR, Gunna V, Scheffer RE, Parikh VV, Mahadik SP, 2002. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr. Res 58, 1–10. [DOI] [PubMed] [Google Scholar]
- Leonard AE, Bobik EG, Dorado J, Kroeger PE, Chuang LT, Thurmond JM, Parker-Barnes JM, Das T, Huang YS, Mukerji P, 2000. Cloning of a human cDNA encoding a novel enzyme involved in the elongation of long-chain polyunsaturated fatty acids. Biochem. J 350, 765–770. [PMC free article] [PubMed] [Google Scholar]
- Malerba G, Schaeffer L, Xumerle L, Klopp N, Trabetti E, Biscuola M, Cavallari U, Galavotti R, Martinelli N, Guarini P, Girelli D, Olivieri O, Corrocher R, Heinrich J, Pignatti PF, Illig T, 2008. SNPs of the FADS gene cluster are associated with polyunsaturated fatty acids in a cohort of patients with cardiovascular disease. Lipids 43, 289–299. [DOI] [PubMed] [Google Scholar]
- Martinez M, 1992. Abnormal profiles of polyunsaturated fatty acids in the brain, liver, kidney and retina of patients with peroxisomal disorders. Brain Res. 583, 171–182. [DOI] [PubMed] [Google Scholar]
- Mathé AA, Sedvall G, Wiesel FA, Nybäck H, 1980. Increased content of immunoreactive prostaglandin E in cerebrospinal fluid of patients with schizophrenia. Lancet 1 (8158), 16–18. [DOI] [PubMed] [Google Scholar]
- Matsuzono Y, Kinoshita N, Tamura S, Shimozawa N, Hamasaki M, Ghaedi K, Wanders RJ, Suzuki Y, Kondo N, Fujiki Y, 1999. Human PEX19: cDNA cloning by functional complementation, mutation analysis in a patient with Zellweger syndrome, and potential role in peroxisomal membrane assembly. Proc. Natl. Acad. Sci. U. S. A 96, 2116–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Levant B, Taylor B, Ahlbrand R, Liu Y, Sullivan JR, Stanford K, Richtand NM, 2006. C57BL/6 J mice exhibit reduced dopamine D3 receptor-mediated locomotor-inhibitory function relative to DBA/2 J mice. Neuroscience 143, 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Hahn CG, Richtand NM, Stanford KE, 2007. Abnormalities in the fatty acid composition of the postmortem orbitofrontal cortex of schizophrenic patients: gender differences and partial normalization with antipsychotic medications. Schizophr. Res 91, 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Liu Y, Jandacek R, Rider T, Tso P, 2008a. The aging human orbitofrontal cortex: decreasing polyunsaturated fatty acid composition and associated increases in lipogenic gene expression and stearoyl-CoA desaturase activity. Prostaglandins. Leukot. Essent. Fat. Acids 78, 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Stanford KE, Hahn CG, Richtand NM, 2008b. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res. 160, 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Able JA, Jandacek R, Rider T, Tso P, 2009. Chronic risperidone treatment preferentially increases rat erythrocyte and prefrontal cortex omega-3 fatty acid composition: evidence for augmented biosynthesis. Schizophr. Res 107, 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe LD, Schmitz AA, Pelka JR, 1966. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal. Chem 38, 514–515. [Google Scholar]
- Nara TY, He WS, Tang C, Clarke SD, Nakamura MT, 2002. The E-box like sterol regulatory element mediates the suppression of human delta-6 desaturase gene by highly unsaturated fatty acids. Biochem. Biophys. Res. Commun 296, 111–117. [DOI] [PubMed] [Google Scholar]
- Nishino S, Mignot E, Benson KL, Zarcone VP, 1998. Cerebrospinal fluid prostaglandins and corticotropin releasing factor in schizophrenics and controls: relationship to sleep architecture. Psychiatry Res. 78, 141–150. [DOI] [PubMed] [Google Scholar]
- Ntambi JM, Miyazaki M, 2004. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res 43, 91–104. [DOI] [PubMed] [Google Scholar]
- Nwankwo JO, Spector AA, Domann FE, 2003. A nucleotide insertion in the transcriptional regulatory region of FADS2 gives rise to human fatty acid delta-6-desaturase deficiency. J. Lipid Res 44, 2311–2319. [DOI] [PubMed] [Google Scholar]
- Obukowicz MG, Welsch DJ, Salsgiver WJ, Martin-Berger CL, Chinn KS, Duffin KL, Raz A, Needleman P, 1998. Novel, selective delta6 or delta5 fatty acid desaturase inhibitors as antiinflammatory agents in mice. J. Pharmacol. Exp. Ther 287, 157–166. [PubMed] [Google Scholar]
- Reddy RD, Keshavan MS, Yao JK, 2004. Reduced red blood cell membrane essential polyunsaturated fatty acids in first episode schizophrenia at neuroleptic-naive baseline. Schizophr. Bull 30, 901–911. [DOI] [PubMed] [Google Scholar]
- Rimoldi OJ, Finarelli GS, Brenner RR, 2001. Effects of diabetes and insulin on hepatic delta6 desaturase gene expression. Biochem. Biophys. Res. Commun 283, 323–326. [DOI] [PubMed] [Google Scholar]
- Rzehak P, Heinrich J, Klopp N, Schaeffer L, Hoff S, Wolfram G, Illig T, Linseisen J, 2008. Evidence for an association between genetic variants of the fatty acid desaturase 1 fatty acid desaturase 2 (FADS1 FADS2) gene cluster and the fatty acid composition of erythrocyte membranes. Br. J. Nutr 15, 1–7. [DOI] [PubMed] [Google Scholar]
- Schaeffer L, Gohlke H, Müller M, Heid IM, Palmer LJ, Kompauer I, Demmelmair H, Illig T, Koletzko B, Heinrich J, 2006. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplo-types are associated with the fatty acid composition in phospholipids. Hum. Mol. Genet 15, 1745–1756. [DOI] [PubMed] [Google Scholar]
- Sprecher H, Chen Q, 1999. Polyunsaturated fatty acid biosynthesis: a microsomal–peroxisomal process. Prostaglandins Leukot. Essent. Fat. Acids 60, 317–321. [DOI] [PubMed] [Google Scholar]
- Stoffel W, Holz B, Jenke B, Binczek E, Günter RH, Kiss C, Karakesisoglou I, Thevis M, Weber AA, Arnhold S, Addicks K, 2008. Delta6-desaturase (FADS2) deficiency unveils the role of omega3- and omega6-polyunsaturated fatty acids. EMBO J. 27, 2281–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassnig M, Singh Brar J, Ganguli R, 2005. Dietary fatty acid and anti-oxidant intake in community-dwelling patients suffering from schizophrenia. Schizophr. Res 76, 343–351. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Webster M, Knable M, Johnston N, Yolken RH, 2000. The Stanley Foundation brain collection and Neuropathology Consortium. Schizophr. Res 44, 151–155. [DOI] [PubMed] [Google Scholar]
- Vik-Mo AO, Birkenaes AB, Fernø J, Jonsdottir H, Andreassen OA, Steen VM, 2008. Increased expression of lipid biosynthesis genes in peripheral blood cells of olanzapine-treated patients. Int. J. Neuropsychopharmacol 11, 679–684. [DOI] [PubMed] [Google Scholar]
- Warensjö E, Sundström J, Vessby B, Cederholm T, Risérus U, 2008. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am. J. Clin. Nutr 88, 203–209. [DOI] [PubMed] [Google Scholar]
- Williard DE, Nwankwo JO, Kaduce TL, Harmon SD, Irons M, Moser HW, Raymond GV, Spector AA, 2001. Identification of a fatty acid delta6-desaturase deficiency in human skin fibroblasts. J. Lipid Res 42, 501–508. [PubMed] [Google Scholar]
- Yao JK, Leonard S, Reddy RD, 2000. Membrane phospholipid abnormalities in postmortem brains from schizophrenic patients. Schizophr. Res 42, 7–17. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ge L, Parimoo S, Stenn K, Prouty SM, 1999. Human stearoyl-CoA desaturase: alternative transcripts generated from a single gene by usage of tandem polyadenylation sites. Biochem. J 340, 255–264. [PMC free article] [PubMed] [Google Scholar]


