Abstract
SARS-CoV-2 affects mainly the lungs, however, other manifestations, including neurological manifestations, have also been described during the disease. Some of the neurological findings have involved intracerebral or subarachnoid hemorrhage, strokes, and other thrombotic/hemorrhagic conditions. Nevertheless, the gross pathology of hemorrhagic lesions in the central nervous system has not been previously described in Brazilian autopsy cases. This study aimed to describe gross and microscopic central nervous system (CNS) pathology findings from the autopsies and correlate them with the clinical and laboratory characteristics of forty-five patients with COVID-19 from Manaus, Amazonas, Brazil. Forty-four patients were autopsied of which thirty-eight of these (86.36%) were positive by RT-PCR for COVID-19, and six (13.3%) were positive by the serological rapid test. Clinical and radiological findings were compatible with the infection. The patients were classified in two groups: presence (those who had hemorrhagic and/or thrombotic manifestations in the CNS) and absence (those who did not present hemorrhagic and/or thrombotic manifestations in the CNS). For risk assessment, relative risk and respective confidence intervals were estimated. Macroscopic or microscopic hemorrhages were found in twenty-three cases (52,27%). The postmortem gross examination of the brain revealed a broad spectrum of hemorrhages, from spots to large and confluent areas and, under microscopy, we observed mainly perivascular discharge. The association analyses showed that the use of corticosteroid, anticoagulant and antibiotic had no statistical significance with a risk of nervous system hemorrhagic manifestations. However, it is possible to infer a statistical tendency that indicates that individuals with diabetes had a higher risk for the same outcome (RR = 1.320, 95% CI = 0.7375 to 2.416, p = 0.3743), which was not observed in relation to other comorbidities. It is unknown whether the new variants of the virus can cause different clinical manifestations, such as those observed or indeed others. As a result, more studies are necessary to define clinical and radiologic monitoring protocols and strategic interventions for patients at risk of adverse and fatal events, such as the extensive hemorrhaging described here. It is imperative that clinicians must be aware of comorbidities and the drugs used to treat patients with COVID-19 to prevent CNS hemorrhagic and thrombotic events.
Introduction
The coronavirus disease (COVID-19) is caused by a new type of beta coronavirus that is a positive-sense single-stranded RNA virus, and belongs to the Coronaviridae family. This virus has a genome similar to the viruses of the severe acute respiratory syndrome (SARS-CoV-1) and the Middle East respiratory syndrome (MERS-CoV), both categorized as severe respiratory syndromes [1,2]. The infection was first reported in December 2019 in the city of Wuhan, Hubei province, China, and was officially named COVID-19 on February 11th, 2020, by the World Health Organization (WHO) [3].
The disease affects mainly the lungs, however, different types of neurological manifestations, including headache, paresthesia, and impaired consciousness have been described during the disease [4,5]. The anosmia and/or ageusia reported in 88% of patients suggest neuroinvasion by the virus in the central nervous system (CNS) through the olfactory route [6]. In the first published cohort of cases with neurological manifestations, of 214 patients examined, 78 (36.4%) had neurologic manifestations. The authors reported that patients with severe infection were more likely to develop acute cerebrovascular disease, impaired consciousness, and skeletal muscle injury [7]. Other published cases also reported meningitis/encephalitis, stroke, cerebral venous sinus thrombosis, and acute hemorrhagic necrotizing encephalopathy [8–11].
SARS-CoV-2 (severe acute respiratory syndrome-related coronavirus 2) infections induce the systemic inflammatory response and may cause an imbalance between procoagulant and anticoagulant homeostatic mechanisms. According to a study of 388 patients from Italy, which describes the rates and characteristics of venous and arterial thromboembolic complications, such events occurred in 21% of the patients and included venous thromboembolism, ischemic stroke, and acute coronary syndrome [12]. In addition, high levels of D-dimer and severe platelet reduction have been reported as indicators of acute cerebrovascular events in patients with severe infection [13]. However, the mechanism that causes the hypercoagulable or hemorrhagic state and the frequency of fatal complications related to this event are unknown. In fatal cases of COVID-19, the best method for identifying the cause of death is still through a complete autopsy. Autopsies represent the ultimate diagnostic test, typically the gold standard, with 100% sensitivity and specificity for finding/excluding the causes of death [14].
The aim of this study is to describe gross and microscopic cerebrovascular pathology findings from the autopsies and correlate them with the clinical and laboratory characteristics of the COVID-19 patients. It is an extensive series of patients from the Brazilian Amazon with infection by SARS-CoV-2 and cerebrovascular disease, documented by complete autopsies, with extensive gross pathology documentation, and microscopic evidence of hemorrhages.
Material and methods
This observational study ran between April 3rd and July 24th, 2020, to describe the autopsy findings in hospitalized patients with suspected SARS-CoV-2 infection. All the tests and autopsies were performed at the Delphina Aziz Hospital, a tertiary hospital for the treatment of coronavirus patients in Manaus, Western Brazilian Amazon, and the largest public reference unit dedicated exclusively to the treatment of severe COVID-19 cases in the state, with an intensive care unit (ICU) capacity of 100 beds. At the beginning of the study, autochthonous SARS-CoV-2 transmission had already been recorded in Manaus, and the city became a major site of SARS-CoV-2 transmission in Brazil within a few weeks.
Participants
Hospitalized patients were included if they had clinical and/or radiological suspicion of COVID-19. Suspicion of COVID-19 was defined by the presence or history of fever and any respiratory symptom, e.g., cough or dyspnea and/or ground-glass opacity or pulmonary consolidation observed on a computed tomography [CT] scan). Patients 18 years of age or older at the time of inclusion and either had SpO2 ≤ 94% with room air, or required supplementary oxygen, or required invasive mechanical ventilation were included. Children under 18 years of age were not included due to their known lower morbidity/mortality from COVID-19 [15]. Patients were enrolled before laboratory confirmation of COVID-19 to avoid treatment delays.
Clinical and laboratory data
The hospital has all source documents registered online in an electronic medical recording system (Medview). Clinical analyses, laboratory examinations, and routine computed tomography scanning are also available on site.
According to the manufacturer’s recommendations, two nasopharyngeal swabs or one oropharyngeal swab (per institutional protocol) were used to extract viral RNA with the QIAamp Viral RNA mini kit. Subsequently, all swab specimens were tested for SARS-CoV-2 using the one-step multiplex RT-qPCR kit (Instituto de Biologia Molecular do Paraná, Curitiba, Brazil), following the manufacturer’s recommendations and targeting the virus nucleocapsid (N) (HEX) and ORF-1ab (FAM) genes and an endogenous human gene as the internal control (ROX). For all assays, specimens were considered positive if both viral targets, N1 and N2, showed cycle thresholds (CT) lower than 40.0.
Autopsy and histologic examination
In all, forty-four patients were examined using the standard autopsy procedure. Autopsies were performed within 12 hours of death and, before the autopsy procedure, the body was embalmed with 10% formalin. After the opening of the cranial cavity, the brain and cerebellum were fixed with 20% formalin. According to adapted protocols, tissue sampling was performed systematically in six fragments, and 1–2 for gross lesions [16,17]. The fragments selected were fixed in 10% neutral buffered formalin, embedded in a paraffin block, 5-μm sections, and submitted to standard processing with hematoxylin and eosin staining. Special stains for mycobacterium bacilli or fungi were made when necessary (Wade or Grocott-Gomori, respectively). Evaluation of hematoxylin and eosin sections was performed by two pathologists who described the main pathologic findings, and agreement was by consensus.
Statistical analysis
The patients were classified in two groups defined by the presence (1) or not (0) of hemorrhagic and/or thrombotic manifestations in the CNS. For descriptive analysis, variables with normal distribution were expressed as means and standard deviation, and those not normally distributed were expressed as median and interquartile ranges. Variables were compared between groups using the Mann-Whitney or T-tests when appropriate. To assess factors associated (Relative Risk) to nervous system hemorrhagic manifestations, variables were previously screened by simple log-binomial generalized linear regression, considering p<0.2 as a selection criterion for the multiple regression models, for which p<0.05 was considered as statistically significant. Stata® software (version 13) was used for statistical analyses.
Ethical considerations
This study was conducted in accordance with the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonization. The protocol was approved by the Brazilian Committee of Ethics in Human Research and was authorized by the National Research Ethics Committee accordingly (CAAE: 30152620.1.0000.0005). Signed informed consent was obtained from the legal representatives in all cases.
Results
Clinical and laboratory data
Of the forty-four patients that were autopsied, thirty-eight (86.4%) tested positive by RT-PCR for COVID-19, and six (13.6%) tested positive by the serological rapid test. Clinical and radiological findings were compatible with the infection. Macroscopic or microscopic hemorrhages were found in 23 of the 44 cases (52.3%). In this group, the mean age was 67, and most patients (n = 17; 73.9%) were male. Among the comorbidities in this group, arterial hypertension had the highest incidence (50%), followed by diabetes (54.55%) and obesity (23.81%) (Tables 1 and 2).
Table 1. Assessment of general data between study groups.
| General Data | Presence Frequency (%) | Absence Frequency (%) | Relative Risk | 95% CI | p |
|---|---|---|---|---|---|
| #Age | 67 (20–84) | 61 (35–83) | - | - | 0.6541 |
| #Hospitalization days | 11 (02–31) | 05 (01–36) | - | - | 0.1192 |
| #Body mass index | 26.1 (21.5–34.6) | 27.7 (25–34.1) | - | - | 0.1948 |
| #SpO2 | 95 (69–99) | 96 (92–100) | - | - | 0.2078 |
| #PaO2/FiO2 | 163.3 (87.1–716) | 163.3 (55–472.5) | - | - | 0.9170 |
| Gender (male) | 17/23 (73.91) | 15/21 (71.43) | 1.063 | 0.5982 to 2.218 | >0.9999 |
| Race (brown) | 16/23 (69.57) | 18/21 (85.71) | 0.6723 | 0.4071 to 1.281 | 0.2869 |
| Invasive mechanical ventilation | 22/23 (95.65) | 16/21 (76.19) | 3.474 | 0.9679 to 19.52 | 0.0883 |
#median (range)–Mann-Whitney test.
Table 2. Assessment of clinical data between study groups.
| Clinical Data | Presence Frequency (%) | Absence Frequency (%) | Relative Risk | 95% CI | p |
|---|---|---|---|---|---|
| Comorbidities | |||||
| Arterial hypertension | 11/22 (50.00) | 09/20 (45.00) | 1.100 | 0.6094 to 1.983 | 0.7675 |
| Cardiovascular disease | 04/22 (18.18) | 05/20 (25.00) | 0.8148 | 0.3315 to 1.554 | 0.7139 |
| Diabetes Mellitus | 12/22 (54.55) | 08/20 (40.00) | 1.320 | 0.7375 to 2.416 | 0.3743 |
| HIV | 01/23 (4.35) | 00/20 | 1.909 | 0.3878 to 2.547 | >0.9999 |
| Neurological disease | 02/23 (8.70) | 02/20 (10.00) | 0.9286 | 0.2716 to 1.816 | >0.9999 |
| Obesity | 05/21 (23.81) | 05/17 (29.41) | 0.875 | 0.3935 to 1.588 | 0.7268 |
| Pulmonary disease | 01/20 (5.00) | 01/17 (5.88) | 0.9211 | 0.1712 to 1.952 | >0.9999 |
| Renal chronic | 03/22 (13.64) | 01/16 (6.25) | 1.342 | 0.5193 to 2.145 | 0.6245 |
| Hospitalization therapy | |||||
| Antibiotic | 22/23 (95.65) | 19/21 (90.48) | 1.61 | 0.6124 to 8.857 | 0.5988 |
| Corticoid | 08/23 (34.78) | 03/21 (14.29) | 1.6 | 0.8737 to 2.642 | 0.1685 |
| Anticoagulant | 21/23 (91.30) | 17/21 (80.95) | 1.658 | 0.7154 to 5.85 | 0.4029 |
Pulmonary disease: Asthma; Cardiovascular disease: Cardiac insufficiency and arrhythmia; Neurological disease: Ischemic stroke and Parkinson’s.
Among the patients in the group with hemorrhagic and/or thrombotic lesions in the CNS (HTM; hemorrhagic and thrombotic manifestations), most of the cases were hospitalized for 10 days before death. Although neurological manifestations of COVID-19 have been described, none of these were observed at the time of hospital admission. Corticosteroids, anticoagulants, and antibiotics were used to treat 34.78%, 91.3%, and 95.65% of the affected group during hospitalization. The initial gasometry revealed a critical PaO2/FiO2 ratio (median = 163.3) in both groups. The laboratory tests evidenced slightly higher levels of D-dimer (N = 6; median = 1237 g/L [983.5–3144]) in the affected group, whereas the lactate dehydrogenase (N = 4; Median = 829 u/L [619.5–3061]) was higher in the group with no hemorrhagic and/or thrombotic lesions. Both groups presented an increase in C-reactive protein and ferritin levels with a median of 92.3 and 1449, respectively, in the affected group, and 78.9 and 1495, respectively, in the other group. The nitrogen waste was elevated in both groups, and the creatinine level (median 1.81 mg/dL) was 30% higher in the group with hemorrhagic and/or thrombotic lesions, while the urea levels were similar in both groups, approximately 40% above the laboratory reference. Blood tests also revealed anemia, in which the moderate form (hemoglobin level: 10.2–13.4g/dL) was more prevalent in the patients with no hemorrhagic/thrombotic events (n = 13), while the severe form (hemoglobin level <8 g/dL) had the same prevalence in both groups (n = 2). Refer to Table 3 for further laboratory data.
Table 3. Laboratory tests and analysis of data between study groups.
| Laboratorytests (Normal values in adults) | Presence | Absence | p |
|---|---|---|---|
| Hematological data | Median (25 and 75 interquartile) | ||
| Hemoglobin (11.0–16.0g/dL) | 11.4 (10.2; 13.4) | 11 (10.2; 12.8) | 0.5487 |
| Platelet (100–300×103/uL) | 220 (183; 318) | 249 (134.5; 363.5) | >0.9999 |
| WBC (4–10×109/L) | 10.9 (7.65; 15.4) | 12.6 (9.5; 17.32) | 0.2089 |
| Lymphocyte (1–4.8 x103/uL) | 5.6 (2.8; 10.1) | 4.1 (2.3; 8.8) | 0.3298 |
| Neutrophil (% of WBC) | 90.9 (85.9; 93.1) | 90.9 (86.4; 93.8) | 0.8115 |
| Biochemistryparameters | Median (25 and 75 interquartile) | ||
| D-dimer (up until 500ug/L) | 1237 (983.5; 3144) | 942.8 (414; 2811) | 0.3543 |
| DHL (240–480 U/L) | 555 (402; 1073) | 829.5 (619.5; 3061) | 0.2571 |
| INR (up until 1.00) | 1.2 (1.09; 1.28) | 1.2 (1.11; 1.31) | 0.5569 |
| C-reactive protein (0.3 to 10 mg/L) | 92.3 (57.1; 211.2) | 78.9 (67; 117.2) | 0.2476 |
| Ferritin (24 to 336 mg/L men) | 1449 (576.3; 2885) | 1495 (807.8; 3240) | 0.9143 |
| Glucose (45 to 96 mg/dL) | 186.5 (146; 272.8) | 159 (133.3; 291.8) | 0.7394 |
| Sodium (130–145 mmol/L) | 141 (136.5; 143.5) | 142.9 (138.8; 148) | 0.1584 |
| Potassium (3.6–5.0 mmol/L) | 4.39 (4.2; 5.2) | 4.34 (3.9; 4.6) | 0.3231 |
| Alanine aminotransferase (<36 U/L) | 52.3 (43.8; 153) | 41.8 (22.1; 75.6) | 0.2496 |
| Aspartate aminotransferase (<35 U/L) | 44 (36.2; 78.3) | 45.9 (38.4; 74) | 0.9829 |
| Direct Bilirubin (<0.3 mg/dL) | 0.23 (0.22; 0.52) | 0.43 (0.25; 0.78) | 0.4240 |
| Indirect bilirubin (0.2 to 1.2 mg/dL) | 0.2 (0.08; 0.38) | 0.31 (0.14; 0.48) | 0.1717 |
| Urea (7 to 20 mg/dL) | 63.9 (38.4; 104) | 62.1 (42.1; 139.2) | 0.5490 |
| Creatinine (0.6 to 1.2 mg/dL men) | 1.81 (0.95; 3.6) | 1.4 (0.92; 2.95) | 0.5030 |
| CK (55–170 U/L men) | 136 (74; 267.1) | 244.9 (81.3; 640.3) | 0.6239 |
| CKMB (0 to 4.9 ng/mL) | 28.8 (15.6; 47.6) | 46.8 (38.5; 107) | 0.4000 |
The association analyses showed that the use of corticosteroid, anticoagulant and antibiotic had no statistical significance with a risk hemorrhagic manifestations in the nervous system. However, it is possible to note a statistical tendency that indicates that individuals with diabetes had a higher risk for the hemorrhagic manifestations (RR = 1.320, 95% CI = 0.7375 to 2.416, p = 0.3743) and this was not observed with the other comorbidities.
Autopsy
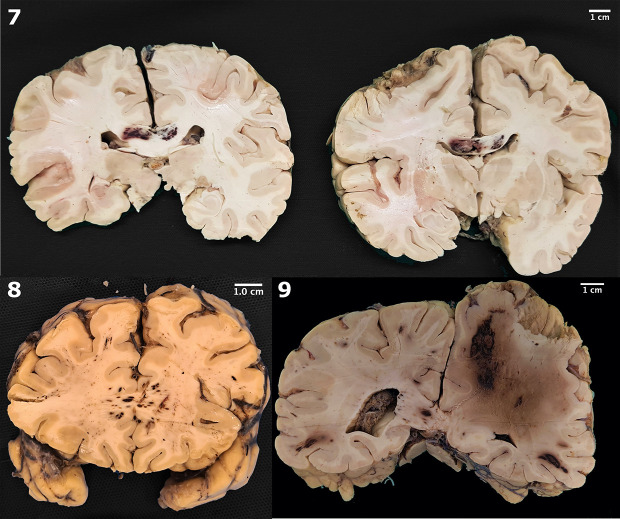
The gross postmortem examination of the brain revealed a large spectrum of hemorrhages, from spots to large and confluent areas in nine cases (20.4%) (Figs 1 and 2). The most common vascular territory identified affected the middle cerebral artery (three cases). Other cases affected were (one case, each): anterior cerebral, anterior inferior cerebellar, striatum, and basilar arteries (Table 4).
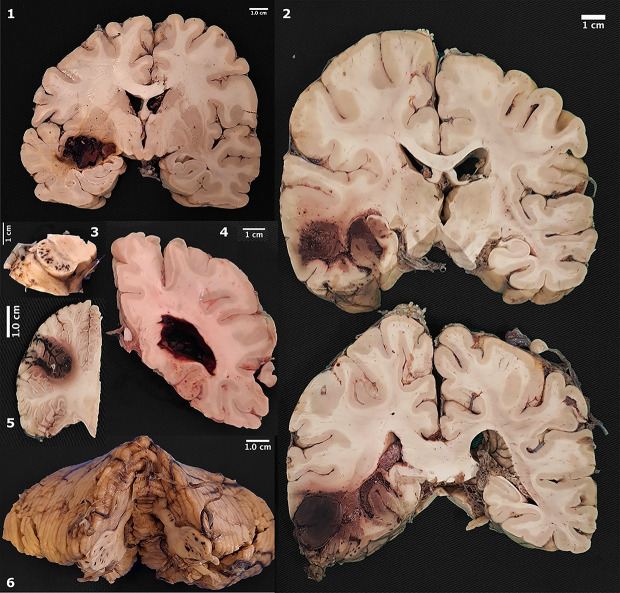
Fig 1. Gross pathology of macroscopic hemorrhages.
1. Hemorrhage in the putamen; 2. Extensive hemorrhage in temporal lobe; 3. Punctate hemorrhages in cerebral peduncles; 4. Lateral ventricle filled by blood; 5. Focal hemorrhage in cerebellar cortex; 6. Punctate hemorrhages in middle cerebellar peduncles.
Fig 2. Gross pathology of macroscopic hemorrhages.
7. Hemorrhage in corpus callosum; 8. Hemorrhages in forceps major (occipitalis); 9. Extensive hemispheric hemorrhage, with intense deviation from the midline.
Table 4. Distribution of macroscopic hemorrhages and vascular impairment.
| Topography of hemorrhage | Vascular territory | Laterality | Pattern |
|---|---|---|---|
| Hemorrhage in corpus callosum | Anterior cerebral | Bilateral | Focal |
| Focal hemorrhage in cerebellar cortex | Anterior inferior cerebelar | Unilateral | Focal |
| Extensive hemorrhage in temporal lobe | Middle cerebral | Unilateral | Focal |
| Hemoventriculum | Intraventricular hemorrhage | Bilateral | Diffuse |
| Extensive hemorrhage from temporal lobe to the occipital lobe | Middle cerebral | Unilateral | Diffuse |
| Hemorrhage in temporal lobe, putamen, and globus pallidus. Lateral ventricle filled with blood | Middle cerebral Lenticulostriate arteries |
Unilateral | Focal |
| Large hemorrhage in claustrum, putamen e globus pallidus. Punctate hemorrhages in cerebral peduncles and middle cerebellar peduncles |
Lenticulostriate arteries Basilar Posterior cerebral Anterior inferior cerebelar |
Bilateral | Diffuse |
| Petechial hemorrhage in pons | Basilar | Unilateral | Focal |
| Lateral ventricle filled with blood | - | Bilateral | Diffuse |
The histological examination of these lesions revealed erythrocytes in perivascular space and/or microhemorrhages (21; 47.7%) and thrombi in small-caliber vessels (3; 6.8%). Intraventricular hemorrhage was observed in three cases (6.8%).
In some cases, we observed mild brain swelling and hemorrhage, which was secondary to vascular damage. In one case, the vascular thrombotic disease prompted a systemic impairment, affected the pulmonary vascular bed and caused tissue infarction. Microthrombi affected the cerebral vessel and macrophages were present at the periphery of the lesions of the genu of the corpus callosum (Figs 3 and 4). The cut-off considered for perivascular cuffing was 12 lymphocytes, but up to 43 lymphocytes were observed around the vessels in 17 cases (38.6%) (Table 5).
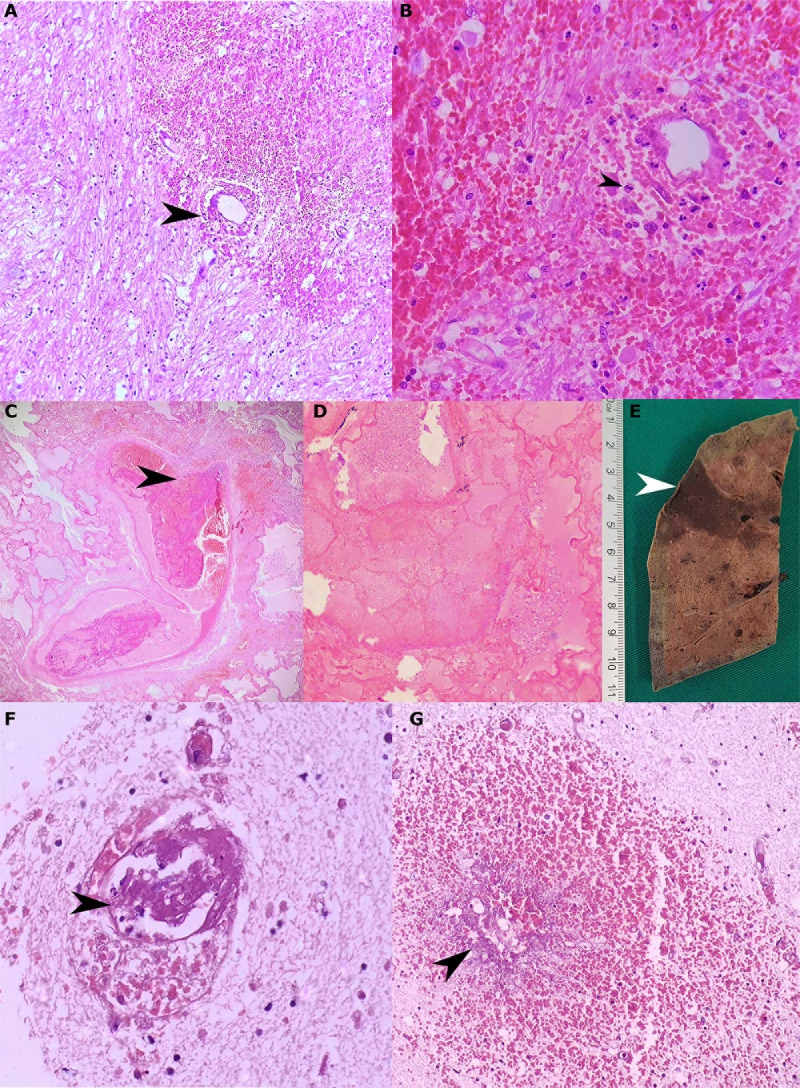
Fig 3. Microscopic thrombo-hemorrhagic alterations and systemic complications.
Intraparenchymal hemorrhage due to rupture of the vascular wall (black arrowhead, A). Intraparenchymal hemorrhage with discrete inflammatory infiltrates of neutrophils (black arrowhead), lymphocytes and histiocytes (B). Thrombi in a large vessel (C), causing pulmonary infarct (D and E), with microthrombi in cerebral parenchyma (black arrowhead, F). Petechial hemorrhage with wall vessel necrosis (G). (hematoxylin and eosin. A: 100x, B: 200x, C: 100x, D: 200x, F: 400x, G: 200x).
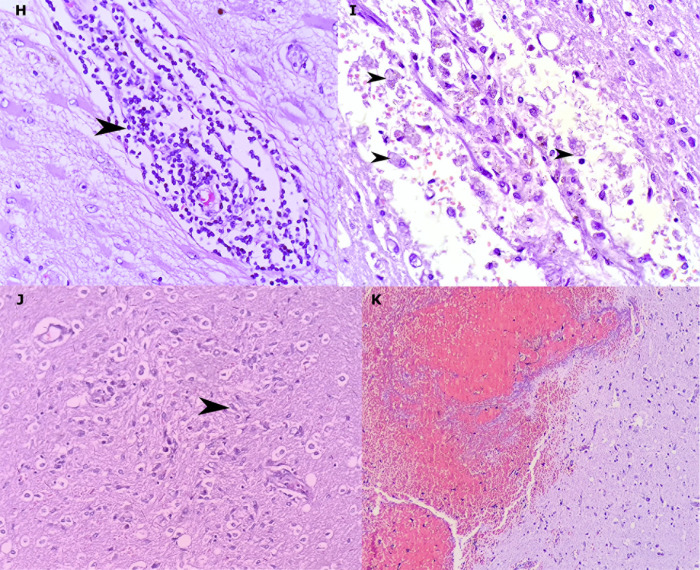
Fig 4. Inflammatory cells and reaction processes in the examined brains.
Lymphocytes (arrowhead) forming bulky perivascular cuffing (H). Macrophages (arrowhead), red cell diapedesis and edema around blood vessels (I). Microglial nodule (microglia cell, arrowhead) (J). Occurrence of intraparenchymal hemorrhagic phenomenon without association with inflammatory process (K). (hematoxylin and eosin. H: 400x, I: 200x, K: 200x).
Table 5. Hemorrhagic and thrombotic manifestations: Patterns and distributions.
| Frequency (%) | |
|---|---|
| Patterns | |
| Macroscopic alterations | 9 (39.1) |
| Focal | 5 (55.5) |
| Diffuse | 4 (44.4) |
| Unilateral | 5 (55.5) |
| Bilateral | 4 (44.4) |
| Hemorrhagic infarct | 1 (11.1) |
| Only microscopic alterations | 14 (60.9) |
| Acute (predominance of erythrocytes) | 23 (100) |
| Macroscopic topography of hemorrhages (n = 9) | |
| White matter | 5 (55.5) |
| Gray matter | 3 (33.3) |
| Basal ganglia | 2 (22.2) |
| Brainstem | 1 (11.1) |
| Cerebellum | 1 (11.1) |
| Subarachnoid | 1 (11.1) |
| Intraventricular | 3 (33.3) |
| Microscopic alterations (included all cases evaluated—n = 44) | |
| Congestion and/or edema | 23 (52.2) |
| Erythrocytes in perivascular space and/or microhemorrhages | 21 (47.7) |
| Lymphocyte cuffing | 17 (38.6) |
| Demyelination foci | 5 (11.4) |
| Microglial nodules | 5 (11.4) |
| Thrombi in small vessels | 3 (6.8) |
| Hemorrhagic infarct | 3 (6.8) |
| Ischemic infarct | 2 (4.5) |
| Vasculitis | 2 (4.5) |
| Subarachnoid hemorrhage | 1 (2.2) |
Discussion
In the severe acute respiratory syndrome described in March 2003 (SARS-CoV-1), thrombotic events were identified in hospitalized patients [18]. Neurological manifestations caused by SARS-CoV-2 have affected 36.7% of patients [7]. This autopsy study showed a frequency of 9/44 (20.4%) macroscopic and 14/44 (31.8%) microscopic hemorrhages or thrombotic phenomena in the nervous system. Tang et al. [19] suggest that hemostatic abnormalities, such as high D-dimer and fibrin degradation product (FDP) levels, longer prothrombin time, and activated partial thromboplastin time (aPTT), affect COVID-19 patients and are more frequent in severe disease when compared to survivors on admission [19]. Similarly, our patients with hemorrhages presented a trend for higher aPTT and D-dimer.
In COVID-19, some factors in thrombotic or hemorrhagic events should be discussed. Several drugs have been investigated for treatment of severe SARS CoV-2, but these medications may have adverse interactions with antiplatelet agents and anticoagulants. Some agents, such as bevacizumab and fingolimod, have been associated with excess or reduced risk of thrombotic events or thrombocytopenia in prior studies of non-COVID-19 populations [20]. In our study, the association between the use of corticoids, anticoagulants, or antibiotics with hemorrhagic/thrombotic events was not statistically significant.
Neurological manifestations, such as acute cerebrovascular diseases, impaired consciousness, and skeletal muscle injury, were more common in severe COVID-19 and presented a frequency of 36.4% in hospitalized patients [7,21]. Clinical and radiologic data on neurological conditions were limited in our study because of disease severity since patients required orotracheal intubation and sedation. Comparing the groups in our case series, these patients presenting central hemorrhagic lesions were older (mean of 67 years of age) than the non-affected group (mean of 61 years of age), which was also observed by Ling Mao et al. [7] in patients with neurological symptoms and COVID-19 in Wuhan, China. In other studies, the neurological manifestations were due to subarachnoid hemorrhage [9], ischemic strokes [22–24], acute hemorrhagic necrotizing encephalopathy [11], vascular and acute disseminated encephalomyelitis‑like pathology [25], encephalitis, and meningitis [8,26]. In our autopsies, we described macroscopic and microscopic hemorrhages, and we focused only on these manifestations, without exploring encephalitis, meningitis, or inflammatory alterations, since these will be reported in another study.
A pronounced CNS involvement with pan-encephalitis, meningitis, and brainstem neuronal cell damage in six autopsied patients was described by Weyhern et al. (2020) in patients from Germany. Petechial bleeding was found in four patients (8.8%), and only two of them were younger than 60 years old. In our patients, erythrocytes in perivascular space and/or microhemorrhage were found in 21/44 (47.7%). In a small series of COVID-19 patients younger than 50 years old, the vascular territory most affected was the left middle cerebral artery (3/5 patients), and three patients presented elevated levels of D-dimer (up to 500 ng per milliliter) [27]. In a study of 14 COVID-19 patients conducted by Bradley et al. (2020), only five patients were submitted to a brain examination, and one showed neuropathological alterations, with a scattered punctate subarachnoid hemorrhage and a rare microhemorrhage in the brainstem [28]. In other autopsy studies, samples from the brain are limited [29] or unavailable for gross pathology or histopathological evaluation [30]. In the postmortem case series of Matschke et al. (2020), which involved 43 autopsies, there was no evidence of cerebral bleeding or small vessel thrombosis [31]. In our patients, we frequently found microscopic petechial hemorrhages, similar to what is described in cerebral malaria [32]. The absence of large autopsy series describing the neuropathology associated with COVID-19 is notable, and the small amount of published data is also contradictory; therefore, any published data must be carefully reviewed so as not to increase the current disparity [33].
Intracranial hemorrhage can occur spontaneously in hypertensive or atherosclerotic patients and is secondary to the rupture of a vessel (due to high blood pressure or secondary to a spasmodic softening), rupture of capillaries or venules (due to fluctuating blood pressure or disorders of venous circulation), and due to diapedesis of red blood cells. In a series of cases described by Mutlu, Berry, and Alpers (1963), massive cerebral hemorrhage was secondary to diapedesis in 40% of cases, wherein rupture of capillaries or venules results in an effusion of erythrocytes. Due to the lack of clinical, radiological, and angiographic data, the etiology of the bleeding could not be determined in our patients. There are no extensive studies in the literature about the etiology of intracerebral hemorrhage in patients with COVID-19.
In our patients, there were no unequivocal signs that were suggestive of aneurysmal ruptures, or vascular malformations, which are findings that are consistent with hypertensive cerebrovascular disease or other macro or microscopic changes that indicate the significant etiology of hemorrhagic manifestations. However, current data on the pathophysiology of COVID-19, especially on damage to the central nervous system, cannot, with certainty, establish a causal relationship between a viral infection and hemorrhagic manifestations. Despite the absence of data, endotheliosis [34], larger vessel strokes [27], thrombotic and hemorrhagic complications have been described in COVID-19 [35].
The elevation of D-dimer levels, also observed in other case series [22,36,37], is directly related to the severity of the disease and can be used as one of the indicators of COVID-19 progression [7]. High levels of D-dimer suggest hypercoagulability, but do not establish the causality between cardiovascular disease (CVD) and COVID-19 [24].
Because of their potent anti-inflammatory properties, corticosteroids have been used for decades to treat many diseases. However, the literature has shown that the use of corticosteroids can cause lower coagulation factors [38–40]. It is known that glucocorticoid may induce hyperglycemia and has significant clinical implications in patients with and without diabetes mellitus [41,42]. The toxicity of hyperglycemia in critically ill patients can be explained by cellular glucose overload. This imbalance leads to excessive glycolysis and oxidative phosphorylation and increases reactive oxygen species (ROS) production. The ROS excess surpasses and/or compromises the cellular detoxification pathway and ultimately induces apoptosis [43].
The use of corticosteroids has also been associated with an increased risk of venous thromboembolism [44,45]. Majoor et al. (2016) investigated whether a 10-day prednisolone burst therapy would activate hemostasis in healthy individuals [46]. The authors concluded that oral prednisolone induces a procoagulant state, suggesting that corticosteroid treatment may increase the thromboembolic risk in patients with inflammatory diseases. The evidence presented by the authors on the greater risk associated with the repositioning of the medication in question is notorious (3.06; 2.77–3.38). A severely low platelet count is also an important manifestation of critical SARS-CoV-2 infection, as well as an independent risk factor for acute cerebrovascular events. The ACE2 protein has been identified as a functional receptor for SARS-CoV-2, and is expressed in several human organs and tissues, such as endothelial cells [47]. ACE2 performs an important role in, but is not limited to, the cardiovascular and renal functions, including blood pressure regulation. Once SARS-CoV-2 reaches the circulation and binds to the ACE2 receptor, it may increase blood pressure, and augment the risk of intracranial hemorrhage, especially in previously hypertensive patients [48]. Platelets are considered critical mediators of inflammatory processes and can be indicators of infectious agents. Activation and interactions among them and macrophages, monocytes, endothelial cells and lymphocytes play a critical role in the procoagulant effect [49]. A study of 191 patients with COVID-19 suggested coagulopathy as a potential life-threatening factor since 50% of those who died had some clotting disorder compared to 7% of survivors [50]. Despite that, in our cohort, only one patient had severe thrombocytopenia.
The limitations of our study include the absence of radiological data and clinical signs and symptoms, which was due to the sedation and the very severe clinical condition of the affected patients. We cannot safely establish the relationship between hemorrhagic manifestations and infection with the new coronavirus; however, autopsies in patients in this study reveal, in loco, the severity and potential lethality of these manifestations.
Autopsies in patients with COVID-19 help to elucidate the natural history of the disease and may aid the development of ideal therapeutic schemes when correlated with the clinical features of infected individuals. They can lead to better clinical practice by helping to understand the etiopathogenic mechanisms of little-known diseases and elucidating impairments hitherto not considered for evaluation by health care teams. Macroscopic and microscopic findings, which are essential for understanding the mechanism of death in patients, can also reveal other undiagnosed clinical conditions in vivo. In our study, complete autopsies provided valuable information about the neuropathological parainfectious processes in COVID-19, such as hemorrhagic damage. Thus, further studies are needed to define clinical and radiological monitoring protocols and strategic interventions for patients at risk of adverse and fatal events, such as the extensive hemorrhage described here. It is imperative that doctors are aware of the comorbidities and medications used to treat patients with COVID-19 to prevent CNS hemorrhagic and thrombotic events.
Supporting information
(XLSX)
(XLSX)
Acknowledgments
We would like to thank the families who, in times of grief, allowed the autopsies to be conducted and thus provide further insight into COVID-19. Also, we thank all the personnel from the CloroCovid research team, Hospital e Pronto Socorro Delphina Rinaldi Abdel Aziz, RB Patologia, and Departamento de Patologia e Medicina Legal (Universidade Federal do Amazonas) for their assistance with data collection and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—PROCAD-AM (Programa Nacional de Cooperação Acadêmica na Amazônia): 88887.321243/2019-00. Thanks go to Luís Felipe Jacinto Rêgo, Victor Medeiros Braga and Israel Dantas de Brito for their editing of the images. We would like to thank (in memoriam) the pathologist José de Ribamar Araújo, a great enthusiast of autopsies performed in Amazonas, whose hard work collaborated with the training of many doctors and pathologists.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was funded by the Government of the Amazonas State, Farmanguinhos - Fundação Oswaldo Cruz (Fiocruz), Superintendência da Zona Franca de Manaus (SUFRAMA), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Amazonas - FAPEAM (PAPAC 005/2019, PRO-STATE and POSGRAD calls) (Resolução N. 006/2020 - POSGRAD 2020) and Brazilian Ministry of Health (proposal no. 733781/19-035). ML and WM are research fellows from CNPq (National Council for Scientific and Technological Development). The funders had no role in the study design, data collection and analysis, or the decision to publish or prepare the manuscript.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JE, Heo JH, Kim HO, Song SH, Park SS, Park TH, et al. Neurological Complications during Treatment of Middle East Respiratory Syndrome. J Clin Neurol. 2017Jul;13(3):227–33. doi: 10.3988/jcn.2017.13.3.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao L, Wang M, Chen S, He Q, Chang J, Hong C, et al. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: A Retrospective Case Series Study. SSRN Electron J. 2020. [Google Scholar]
- 5.Ng Kee Kwong KC, Mehta PR, Shukla G, Mehta AR. COVID-19, SARS and MERS: A neurological perspective. J Clin Neurosci [Internet]. 2020;77:13–6. Available from: 10.1016/j.jocn.2020.04.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vonck K, Garrez I, De Herdt V, Hemelsoet D, Laureys G, Raedt R, et al. Neurological manifestations and neuro-invasive mechanisms of the severe acute respiratory syndrome coronavirus type 2. Eur J Neurol. 2020May. doi: 10.1111/ene.14329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020Apr. doi: 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis [Internet]. 2020;94:55–8. Available from: 10.1016/j.ijid.2020.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al Saiegh F, Ghosh R, Leibold A, Avery MB, Schmidt RF, Theofanis T, et al. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J Neurol Neurosurg Psychiatry. 2020;91(8):846–8. doi: 10.1136/jnnp-2020-323522 [DOI] [PubMed] [Google Scholar]
- 10.Hughes C, Nichols T, Pike M, Subbe C, Elghenzai S. Cerebral Venous Sinus Thrombosis as a Presentation of COVID-19. Eur J case reports Intern Med. 2020;7(5):001691. doi: 10.12890/2020_001691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19–associated Acute Hemorrhagic Necrotizing Encephalopathy: Imaging Features. Radiology. 2020Aug;296(2):E119–20. doi: 10.1148/radiol.2020201187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020Jul;191:9–14. doi: 10.1016/j.thromres.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020Jun;92(6):568–76. doi: 10.1002/jmv.25748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman L. Autopsy 2018. Circulation [Internet]. 2018. Jun 19;137(25):2686–8. Available from: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.118.033236 [DOI] [PubMed] [Google Scholar]
- 15.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 Infection in Children. N Engl J Med [Internet]. 2020. Apr 23;382(17):1663–5. Available from: http://www.nejm.org/doi/10.1056/NEJMc2005073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis FU. Autópsia em Patologia. 1st ed. Roca, editor. 2005. 392 p. [Google Scholar]
- 17.Powers JM. Practice guidelines for autopsy pathology: Autopsy procedures for brain, spinal cord, and neuromuscular system. Arch Pathol Lab Med. 1995;119(9):777–83. [PubMed] [Google Scholar]
- 18.Love S. Post mortem sampling of the brain and other tissues in neurodegenerative disease. Histopathology. 2004;44(4):309–17. doi: 10.1111/j.1365-2559.2004.01794.x [DOI] [PubMed] [Google Scholar]
- 19.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bikdeli B, Madhavan M V., Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up. J Am Coll Cardiol. 2020Jun;75(23):2950–73. doi: 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Wang L, Yan S, Yang F, Xiang L, Zhu J, et al. Clinical characteristics of 25 death cases with COVID-19: A retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis [Internet]. 2020;94:128–32. Available from: 10.1016/j.ijid.2020.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. The COVID-19 resource centre is hosted on Elsevier Connect, the company ‘ s public news and information. 2020;(January).
- 23.Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020. doi: 10.1515/cclm-2020-0369 [DOI] [PubMed] [Google Scholar]
- 24.Reddy ST, Reddy ST, Garg T, Shah C, Nascimento FA, Imran R, et al. Cerebrovascular Disease in Patients with COVID-19: A Review of the Literature and Case Series. Case Rep Neurol. 2020;77030:199–209. doi: 10.1159/000508958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol [Internet]. 2020;140(1):1–6. Available from: 10.1007/s00401-020-02166-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Weyhern CH, Kaufmann I, Neff F, Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet [Internet]. 2020;395(10241):e109. Available from: 10.1016/S0140-6736(20)31282-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020May;382(20):e60. doi: 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet [Internet]. 2020. Aug;396(10247):320–32. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673620313052. doi: 10.1016/S0140-6736(20)31305-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duarte-Neto AN, Monteiro RAA, Silva LFF, Malheiros DMAC, Oliveira EP, Theodoro-Filho J, et al. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology. 2020Aug;77(2):186–97. doi: 10.1111/his.14160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martines RB, Ritter JM, Matkovic E, Gary J, Bollweg BC, Bullock H, et al. Pathology and Pathogenesis of SARS-CoV-2 Associated with Fatal Coronavirus Disease, United States. Emerg Infect Dis [Internet]. 2020. Sep;26(9):2005–15. Available from: http://wwwnc.cdc.gov/eid/article/26/9/20-2095_article.htm. doi: 10.3201/eid2609.202095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020Nov;19(11):919–29. doi: 10.1016/S1474-4422(20)30308-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prapansilp P, Medana I, Mai NTH, Day NP, Phu NH, Yeo TW, et al. A clinicopathological correlation of the expression of the angiopoietin-Tie-2 receptor pathway in the brain of adults with Plasmodium falciparum malaria. Malar J. 2013Dec;12(1):50. doi: 10.1186/1475-2875-12-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glatzel M. Neuropathology of COVID-19: where are the neuropathologists? Brain Pathol. 2020;30(4):729. doi: 10.1111/bpa.12871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Correspondence Endothelial cell infection and endotheliitis in. Lancet [Internet]. 2020;6736(20):19–20. Available from: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sweid A, Hammoud B, Bekelis K, Missios S, Tjoumakaris SI, Gooch MR, et al. Cerebral ischemic and hemorrhagic complications of coronavirus disease 2019. Int J Stroke. 2020;0(0):1–10. doi: 10.1177/1747493020937189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldberg MF, Goldberg MF. Cerebrovascular Disease in COVID-19. 2020;1–3. [Google Scholar]
- 37.Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020Aug;91(8):889–91. doi: 10.1136/jnnp-2020-323586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLawhorn AS, Beathe J, YaDeau J, Buschiazzo V, Purdue PE, Ma Y, et al. Effects of steroids on thrombogenic markers in patients undergoing unilateral total knee arthroplasty: A prospective, double-blind, randomized controlled trial. J Orthop Res. 2015Mar;33(3):412–6. doi: 10.1002/jor.22776 [DOI] [PubMed] [Google Scholar]
- 39.Kagawa H, Okubo S, Yasunaga K. Plasma coagulation and fibrinolysis parameters in patients with collagen diseases, and analysis of the multimeric structure of von Willebrand factor (vWF). Int J Hematol. 1991Oct;54(5):429–39. [PubMed] [Google Scholar]
- 40.Inoh M, Tokuda M, Kiuchi H, Kurata N, Takahara J. Evaluating systemic lupus erythematosus disease activity using molecular markers of hemostasis. Arthritis Rheum. 1996Feb;39(2):287–91. doi: 10.1002/art.1780390217 [DOI] [PubMed] [Google Scholar]
- 41.Clore J, Thurby-Hay L. Glucocorticoid-Induced Hyperglycemia. Endocr Pract. 2009Jul;15(5):469–74. doi: 10.4158/EP08331.RAR [DOI] [PubMed] [Google Scholar]
- 42.Frontera JA, Fernandez A, Claassen J, Schmidt M, Schumacher HC, Wartenberg K, et al. Hyperglycemia After SAH. Stroke. 2006Jan;37(1):199–203. doi: 10.1161/01.STR.0000194960.73883.0f [DOI] [PubMed] [Google Scholar]
- 43.Van den Berghe G. How does blood glucose control with insulin save lives in intensive care? J Clin Invest. 2004Nov;114(9):1187–95. doi: 10.1172/JCI23506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huerta C. Risk Factors and Short-term Mortality of Venous Thromboembolism Diagnosed in the Primary Care Setting in the United Kingdom. Arch Intern Med. 2007May;167(9):935. doi: 10.1001/archinte.167.9.935 [DOI] [PubMed] [Google Scholar]
- 45.Polderman JA, Farhang-Razi V, Van Dieren S, Kranke P, DeVries JH, Hollmann MW, et al. Adverse side effects of dexamethasone in surgical patients. Cochrane Database Syst Rev. 2018Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Majoor CJ, Sneeboer MMS, de Kievit A, Meijers JCM, van der Poll T, Lutter R, et al. The influence of corticosteroids on hemostasis in healthy subjects. J Thromb Haemost. 2016;14(4):716–23. [DOI] [PubMed] [Google Scholar]
- 47.Hamming I, Timens W, Bulthuis M, Lely A, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004Jun;203(2):631–7. doi: 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Geng X, Tan Y, Li Q, Xu C, Xu J, et al. New understanding of the damage of SARS-CoV-2 infection outside the respiratory system. Biomed Pharmacother. 2020Jul;127:110195. doi: 10.1016/j.biopha.2020.110195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Gorp ECM, Suharti C, ten Cate H, Dolmans WM V., van der Meer JWM, ten Cate JW, et al. Review: Infectious Diseases and Coagulation Disorders. J Infect Dis. 1999Jul;180(1):176–86. doi: 10.1086/314829 [DOI] [PubMed] [Google Scholar]
- 50.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020Mar. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.