Abstract
The maternal recognition of pregnancy is a necessary prerequisite for gestation maintenance through prolonging the corpus luteum lifespan and ensuring progesterone production. In addition to pituitary prolactin and placental lactogens, decidual derived prolactin family members have been presumed to possess luteotropic effect. However, there was a lack of convincing evidence to support this hypothesis. Here, we unveiled an essential role of uterine Notch2 in pregnancy recognition and corpus luteum maintenance. Uterine-specific deletion of Notch2 did not affect female fertility. Nevertheless, the expression of decidual Prl8a2, a member of the prolactin family, was downregulated due to Notch2 ablation. Subsequently, we interrupted pituitary prolactin function to determine the luteotropic role of the decidua by employing the lipopolysaccharide-induced prolactin resistance model, or blocking the prolactin signaling by prolactin receptor-Fc fusion protein, or repressing pituitary prolactin release by dopamine receptor agonist bromocriptine, and found that Notch2-deficient females were more sensitive to these stresses and ended up in pregnancy loss resulting from abnormal corpus luteum function and insufficient serum progesterone level. Overexpression of Prl8a2 in Notch2 knockout mice rescued lipopolysaccharide-induced abortion, highlighting its luteotropic function. Further investigation adopting Rbpj knockout and DNMAML overexpression mouse models along with chromatin immunoprecipitation assay and luciferase analysis confirmed that Prl8a2 was regulated by the canonical Notch signaling. Collectively, our findings demonstrated that decidual prolactin members, under the control of uterine Notch signaling, assisted pituitary prolactin to sustain corpus luteum function and serum progesterone level during post-implantation phase, which was conducive to pregnancy recognition and maintenance.
Author summary
Progesterone secreted from the corpus luteum in the ovary is indispensable to pregnancy maintenance in both rodents and humans. Therefore, prolonged corpus luteum lifespan and sustainable progesterone production is a prerequisite for a successful pregnancy. In rodents, in addition to pituitary prolactin and placental lactogens, decidual derived factors have been presumed to possess luteotropic effects during the post-implantation stage. In this study, utilizing a mouse model with uterine specific deletion of Notch2, which displayed decreased level of decidual prolactin member Prl8a2, combined with multiple approaches to interrupt the pituitary prolactin signal, we demonstrated that decidual derived Prl8a2 assisted pituitary prolactin to sustain corpus luteum function and serum progesterone level during post-implantation phase, which was conducive to pregnancy recognition and maintenance. In addition, the expression of decidual Prl8a2 was under the direct control of the canonical Notch pathway. Together, we herein provide convincing evidence that decidual produced Prl8a2, modulated by uterine canonical Notch signaling, exhibits luteotropic functions and contributes to pregnancy maintenance.
Introduction
The successful establishment and maintenance of pregnancy relies on the elaborately regulated fetal-maternal crosstalk in the uterus as well as the coordinated adaptation of other maternal organs. Sustainable corpus luteum (CL) function and continuous progesterone (P4) secretion, which is pivotal to gestation maintenance, necessitates the maternal recognition of pregnancy as a prerequisite [1–3].
Mechanisms underlying pregnancy recognition vary considerably among species. In rodents, granulosa cells transform into morphologically and functionally distinct CL after ovulation. In the absence of mating, the CL regresses rapidly so that the next estrus cycle proceeds. If mating occurs, the cervical stimulation evokes the release of prolactin (Prl) from the anterior pituitary, which is able to support the CL for 10–12 days [4]. The further extension of CL lifespan throughout gestation requires the presence of conceptus [5]. With embryonic development and placental formation, various lactogenic hormones secreted by placental trophoblast cells replace pituitary-derived Prl to support CL function until the final parturition [6]. Apart from pituitary Prl and trophoblast lactogens, decidual cells also release factors belonging to the Prl family [7]. Upon embryo implantation in mice, uterine stromal cells experience decidualization and express decidual-specific Prl family members, e.g. Prl8a2 and Prl3c1 [8,9], which have been speculated contributive to CL maintenance and P4 production [10]. However, the functional relationship between pituitary Prl and these decidual-derived lactogens during pregnancy recognition and maintenance remains unclear.
Prl exerts its effects through the Prl receptor (Prlr) expressed in CL cells and activates downstream Stat5 [11,12]. The Prl signaling directly regulates genes related to P4 synthesis [13]. Mice with ablation of Prl or Prlr are infertile [14,15], while supplementation of exogenous P4 rescues early pregnancy failure in Prlr-deficient females [16,17], further reinforcing the essential role of Prl signaling in the maternal recognition of pregnancy in mice.
The Notch signaling is an evolutionarily conserved pathway that enables intercellular communication in short distance. Since its first discovery in Drosophila [18], the Notch pathway has been reported to affect a variety of fundamental biological aspects including cell proliferation, differentiation, death and fate decision [19]. In mammals, there are five ligands comprising three delta-like ligands (Dll1, Dll3, Dll4) and two jagged ligands (Jag1 and Jag2), all belonging to the Delta/Serrate/LAG-2 (DSL) family, and four receptors, Notch1-4 [20]. The ligand-receptor binding elicits sequential cleavages of the Notch receptor by gamma-secretases and subsequent release of the Notch intracellular domain (NICD) from the cell membrane [21,22]. The NICD then translocates into the nucleus, interacts with the CBF-1/Su(H)/LAG1 (CSL)/Rbpj transcription factor and recruits the transcriptional co-activator Mastermind-like (MAML) in place of transcriptional repressors to activate the transcription of target genes including the Hes and Hey family members [23,24].
Physiological roles of the Notch signaling in female reproduction have been widely investigated. All four Notch receptors as well as the Jagged1 and Dll4 ligand are expressed in the human endometrium [25], among which Notch1 modulates the progression of stromal-decidual transformation [26]. Similarly in mice, uterine-specific deletion of Notch1 hampers decidualization by repressing cell cycle progression and triggering stromal cell apoptosis [27]. On the other hand, aberrant activation of the Notch1 pathway in the uterus leads to DNA hypermethylation of the progesterone receptor (PR) and thus compromises the P4 signaling and results in uterine developmental defects and infertility [28]. Notably, Notch proteins and ligands are expressed abundantly in endothelial cells and mural cells in the peri-implantation uterus [29], and Dll4 inhibition impairs angiogenesis during decidualization [30]. Meanwhile, our previous work has demonstrated the crucial role of Rbpj during early pregnancy. Specifically, Rbpj guides embryonic-uterine orientation and promotes decidual remodeling via Notch-independent and -dependent mechanisms, respectively [31].
In the present study, we intended to investigate the potential role of the Notch2 receptor in the uterus during peri-implantation period. Employing mice with uterine-specific deficiency of Notch2, we unraveled an unexpected involvement of uterine Notch2 in extending CL lifespan, guaranteeing P4 secretion and maintaining gestation via activating decidual Prl8a2 expression, and provided convincing proofs that decidual derived Prl family members contributed to pregnancy recognition and maintenance together with pituitary Prl.
Results
Notch2 was efficiently ablated in the uterus
We first examined the spatiotemporal expression pattern of active Notch2 (NICD2) in the uterus during early pregnancy. Upon embryo implantation on day 5 of pregnancy, NICD2 appeared in epithelial and stromal cells surrounding the blastocyst. With the progression of decidualization from day 6 to day 8, the expression of NICD2 became more extensive throughout the secondary decidual zone (SDZ) rather than the primary decidual zone (PDZ) (Fig 1A). The localization of NICD2 was highly consistent with that of Rbpj [31], implying a potential role of Notch2-mediated canonical Notch signaling in early pregnancy events.
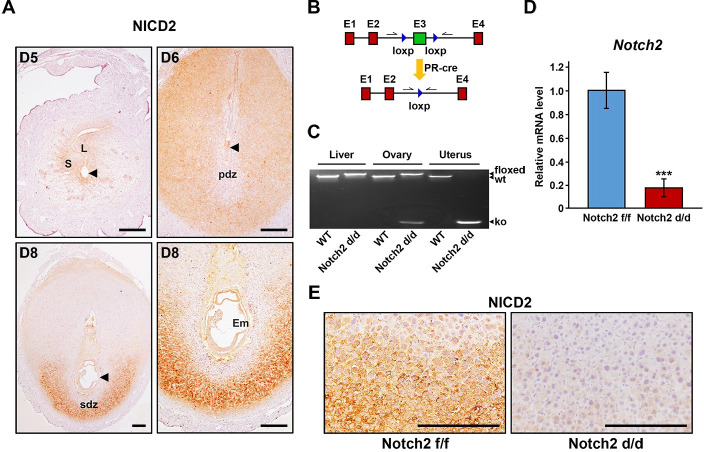
Fig 1. Notch2 was efficiently deleted in the uterus.
(A) The spatiotemporal expression pattern of NICD2 in the uterus during early pregnancy was revealed by immunohistochemistry. Arrowheads indicate the developing embryo. L, luminal epithelium; S, stroma; pdz, primary decidual zone; sdz, secondary decidual zone; Em, embryo. Scale bar: 100μm. (B) The scheme shows the strategy of uterine-conditional Notch2 ablation. Arrows indicate primers used for the examination of knockout efficiency. (C) Genotyping analysis was carried out to validate knockout efficiency at DNA level in the liver, ovary and uterus. (D) Knockout efficiency at mRNA level in the uterus was further confirmed by QRT-PCR. Data are presented as mean±SEM. (E) Immunohistochemistry staining of NICD2 revealed that Notch2 was efficiently ablated at protein level in the uterus. Scale bar: 100μm. ***p<0.001.
Considering that systemic Notch2 knockout results in embryonic lethality [32], we introduced transgenic mice with Notch2 exon3 flanked by loxp sites (hereafter referred to as Notch2 f/f), and crossed them with the PR-driven cre recombinase (PR-cre) mouse line to specifically delete Notch2 in the uterus (hereafter referred to as Notch2 d/d) (Fig 1B). According to genotyping analysis (Fig 1C), quantitative real-time polymerase chain reaction (QRT-PCR) (Fig 1D) and immunohistochemistry (IHC) (Fig 1E), the knockout efficiency in the uterus was confirmed at DNA, mRNA and protein level, respectively. Notably, the ovary exhibited partial cre activity (Fig 1C) due to PR expression in the CL [33]. However, since Notch2 was not detected in the CL (S1A Fig), the level of NICD2 protein in the ovary was not affected despite the deletion of Notch2 in the CL at DNA level.
Uterine Notch2 deficiency exerted no obvious influence on fertility
In order to uncover the involvement of Notch2 in early pregnancy events, we surveyed pregnancy status of Notch2 f/f and Notch2 d/d females at different timepoints. On day 5, embryo implantation occurred normally in both Notch2 f/f and Notch2 d/d mice, visualized by the blue dye reaction (Fig 2A), and the number of implantation sites (IS) was comparable (Fig 2B). In addition, Cox2 staining, a marker for embryo attachment [34], revealed successful implantation in Notch2 d/d mice (Fig 2C). In response to implantation, uterine stromal cells underwent decidual transformation to support embryonic development before placentation. Here, we also observed proper embryo development upon Notch2 deficiency on day 6 and day 8 (Fig 2D). The average weight of IS in Notch2 d/d group rivaled that of Notch2 f/f group on day 8 (Fig 2E), suggesting no obvious defects during the period of decidualization. Furthermore, we compared the litter size between Notch2 f/f and Notch2 d/d mice, and found that uterine ablation of Notch2 did not affect female fertility (Fig 2F).
Fig 2. Uterine Notch2 deficiency did not affect implantation, decidualization and fertility.
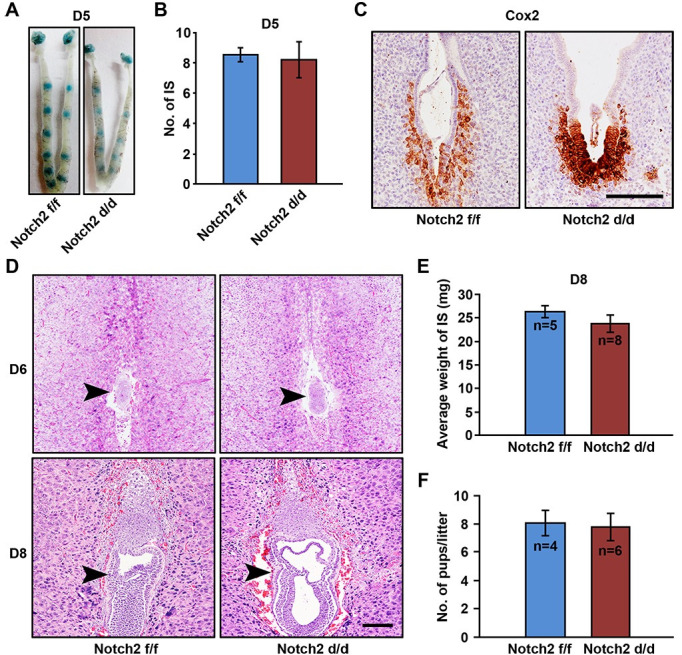
(A) Implantation sites were visualized by intravenous injection of blue dye on day 5 in Notch2 f/f and Notch2 d/d mice. (B) The number of implantation sites on day 5 was comparable between Notch2 f/f and Notch2 d/d mice. IS, implantation sites. (C) Embryo attachment was detected on day 5 in Notch2 f/f and Notch2 d/d mice by immunohistochemistry staining for Cox2. Scale bar: 50μm. (D) H&E staining displayed normal embryo development on day 6 and 8 in Notch2 f/f and Notch2 d/d females. Arrowheads indicate the embryo. Scale bar: 50μm. (E) The average weight of implantation sites was measured on day 8 in Notch2 f/f and Notch2 d/d groups. (F) The litter size was compared between Notch2 f/f and Notch2 d/d females. Data in (B), (E) and (F) are presented as mean±SEM.
Notch2 d/d females were more sensitive to lipopolysaccharide (LPS)-induced Prl resistance
Despite the fact that uterine Notch2 deficiency did not hamper implantation and decidualization, we indeed noticed that the expression of Prl8a2, a member belonging to the Prl family that was specifically produced by decidual cells, was significantly decreased in the Notch2 d/d uterus at both mRNA and protein level (Fig 3A and 3B). Meanwhile, the level of Prl per se remained unchanged (Fig 3A). Decidual Prl family members were speculated involved in sustaining P4 production during pregnancy together with pituitary Prl [10]. Oil-induced decidualization in pseudopregnant mice led to elevated serum P4 level (S2A Fig), and treating cultured CL cells with the supernatant of decidualized uterus promoted P4 production (S2B Fig). We measured the level of serum P4 in Notch2 f/f and Notch2 d/d females, but did not find a significant difference between two groups (Fig 3C), possibly because pituitary derived Prl was sufficient to support CL function in Notch2 d/d mice. Notably, the expression of Prlr in CL was comparable between Notch2 f/f and Notch2 d/d mice (S1B Fig). We therefore attempted to interfere the Prl effect to investigate the contribution of decidual-specific Prl members to pregnancy maintenance.
Fig 3. Notch2 d/d females were less tolerant to LPS-induced Prl resistance.
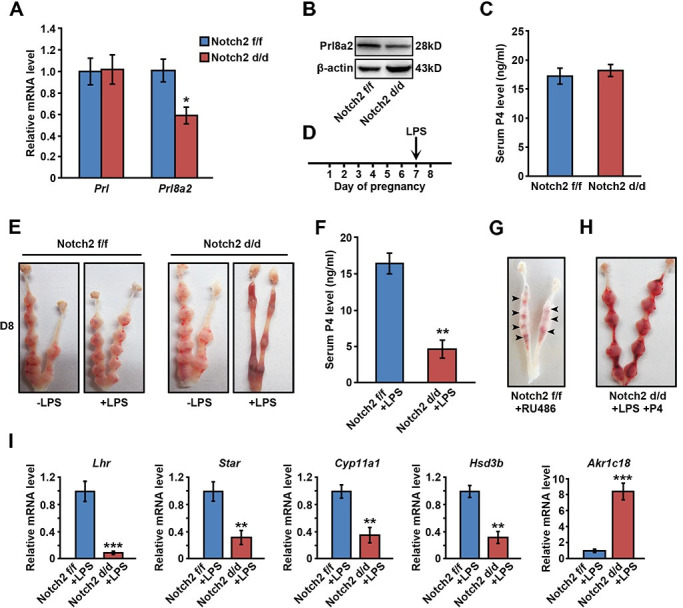
(A) The relative mRNA level of Prl and Prl8a2 in Notch2 f/f and Notch2 d/d decidua was examined by QRT-PCR. (B) Western blot analysis revealed the protein level of Prl8a2 in Notch2 f/f and Notch2 d/d decidua. (C) Notch2 f/f and Notch2 d/d females exhibited similar serum P4 level. (D) The scheme shows the LPS treatment schedule. (E) Notch2 d/d mice treated with low-dose LPS experienced pregnancy loss on day 8. (F) The serum P4 level was measured in LPS-treated Notch2 f/f and Notch2 d/d females. (G) RU486 injection resulted in abortion in Notch2 f/f mice. (H) Pregnancy failure in LPS-treated Notch2 d/d females was rescued by exogenous P4 supplementation. (I) The relative mRNA level of genes related to P4 synthesis or degradation was detected by QRT-PCR in ovaries of LPS-treated Notch2 f/f and Notch2 d/d females. Data in (A), (C), (F) and (I) represents mean±SEM. *p<0.5, **p<0.01, ***p<0.001.
It has been reported that LPS stimuli induces the ovarian resistance to Prl and therefore results in abnormal CL function, inadequate P4 production and pregnancy failure [35]. Here, we established a slight Prl resistance model by administrating low-dose LPS on day 7 (Fig 3D). In the Notch2 f/f group, pregnancy was not compromised on day 8, suggesting that Notch2 f/f females were tolerant to slight Prl resistance. However, Notch2 deficiency led to early pregnancy loss in response to low-dose LPS treatment (Fig 3E). In the subsequent analysis, we unraveled that serum P4 level in Notch2 d/d mice was declined after LPS stimulation (Fig 3F), while exogenous P4 supplementation rescued abortion in LPS-treated Notch2 d/d mice (Fig 3H). Meanwhile, treatment of RU486, a progesterone receptor antagonist, gave rise to similar miscarriage in Notch2 f/f mice (Fig 3G), further indicating that the pregnancy failure of Notch2 d/d females in response to LPS was attributed to insufficient P4 secretion. In addition, we examined the expression of genes associated with P4 synthesis and degradation in ovaries. P4 synthesis related genes including Lhr, Star, Cyp11a1 and Hsd3b were dramatically downregulated in Notch2 d/d ovaries, while the level of P4 degradative gene Akr1c18 was significantly increased (Fig 3I). These findings suggested that females with uterine Notch2 ablation showed less tolerance to LPS-induced Prl resistance due to abnormal CL function and insufficient P4 supply.
Notch2 d/d females suffered from pregnancy failure when pituitary Prl signaling was blocked
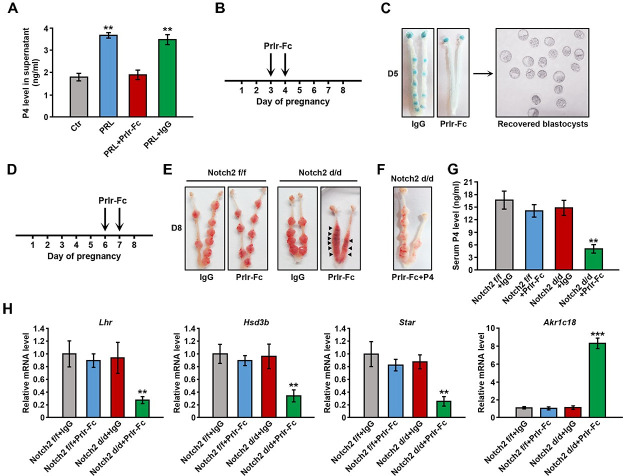
Decidual produced Prl family members differ greatly from pituitary Prl in structure, and do not share the canonical Prlr. We therefore blocked the pituitary Prl signaling using the Prlr-Fc fusion protein to solely explore the contribution of decidual Prl members. The Prlr-Fc fusion protein comprised the ligand-binding extracellular domain of Prlr and the Fc fragment of IgG, and could compete with Prlr for ligand binding without transmitting downstream signaling. We first validated the efficiency of Prlr-Fc in the in vitro CL culture system. Prl stimulated the production of P4 by CL cells as expected, which was effectively reversed by the addition of Prlr-Fc (Fig 4A). Furthermore, we tested the effectiveness of Prlr-Fc in vivo. P4 secretion by CL starting from day 3 of pregnancy, which depended on the Prl signaling, was essential to uterine receptivity and embryo implantation. We treated pregnant mice with Prlr-Fc on day 3 and 4, and then checked embryo implantation on day 5 (Fig 4B). After Prlr-Fc administration, females failed in implantation despite the presence of well-developed blastocysts (Fig 4C), implying that the Prlr-Fc fusion protein could successfully interrupt the Prl signaling in vivo.
Fig 4. Notch2 d/d females ended up in abortion when Prl signaling was blocked.
(A) P4 level in the supernatant of cultured CL cells was measured upon PRL, PRL+Prlr-Fc or PRL+IgG treatment. (B) The scheme shows the schedule of Prlr-Fc treatment. (C) Females treated with Prlr-Fc failed in implantation on day 5 despite the presence of morphologically normal blastocysts. (D) The schedule of Prlr-Fc treatment is shown. (E) Notch2 d/d females with Prlr-Fc administration suffered from pregnancy loss. Arrowheads indicate absorbed implantation sites. (F) Exogenous P4 supplementation alleviated abortion in Notch2 d/d females with Prlr-Fc administration. (G) The serum P4 level was measured in Notch2 f/f and Notch2 d/d females after Prlr-Fc treatment. (H) The relative mRNA level of genes related to P4 synthesis or degradation was examined by QRT-PCR analysis in ovaries of Notch2 f/f and Notch2 d/d females with Prlr-Fc administration. Mice with IgG administration were used as control. Data in (A), (G) and (H) represents mean±SEM. **p<0.01, ***p<0.001.
Confirming the efficiency of the Prlr-Fc fusion protein, we next treated Notch2 f/f and Notch2 d/d females with Prlr-Fc on day 6 and day 7, and then examined the uterus on day 8 (Fig 4D). Prlr-Fc injection led to severe abortion in Notch2 d/d mice, but did not exerted obviously adverse effects on Notch2 f/f females (Fig 4E). Supplementation of exogenous P4 could rescue the pregnancy failure in Prlr-Fc treated Notch2 d/d mice (Fig 4F). In addition, serum P4 level was significantly decreased in Notch2 d/d mice after Prlr-Fc treatment (Fig 4G). Consistently, P4 synthesis-related genes were downregulated, while P4 degradation-related gene was upregulated (Fig 4H).
Pituitary release of Prl is precisely regulated by the dopamine system. The activation of the dopamine signaling inhibits Prl secretion [36], which was further proven by the phenomenon that dopamine receptor agonist bromocriptine-pretreated females exhibited compromised embryo implantation (S3A Fig). In the subsequent study, we repressed pituitary Prl release by bromocriptine to investigate the luteotropic role of decidual Prl members. Similar with Prlr-Fc, bromocriptine treatment resulted in decreased weight of IS on day 8 and declined serum P4 level (S3B, S3C and S3D Fig). These findings indicated that mice with uterine Notch2 deficiency ended up in pregnancy failure in response to aberrant pituitary Prl signaling, owing to inadequate P4 production.
Prl8a2 overexpression rescued LPS-induced abortion in Notch2 d/d mice by promoting P4 production
Since the expression of decidual Prl8a2 was suppressed upon Notch2 ablation, we wondered whether overexpression of Prl8a2 could rescue the abovementioned defect in the Prl resistance model. We implemented lentivirus-mediated Prl8a2 overexpression (S4A and S4B Fig). Prl8a2 overexpression remarkably augmented P4 secretion by cultured CL cells (Fig 5A). We then intravenously injected the virus into Notch2 d/d mice on day 5 and 6, followed by low-dose LPS treatment on day 7, and checked the uterus on day 8 (Fig 5B). Females pre-treated with the control virus ended up in abortion after LPS stimulation, while mice with Prl8a2 overexpression exhibited normal embryo development in most cases, except for embryo retardation in several IS (Fig 5C and 5D). In addition, Prl8a2 overexpression resulted in elevated serum P4 level (Fig 5E), upregulation of P4 synthesis-related genes as well as downregulation of P4 degradation-associated gene (Fig 5F). These findings provided convincing evidence that decidual Prl8a2 contributed to the maintenance of CL function during post-implantation period in mice.
Fig 5. Prl8a2 overexpression rescued pregnancy failure in LPS-treated Notch2 d/d mice.
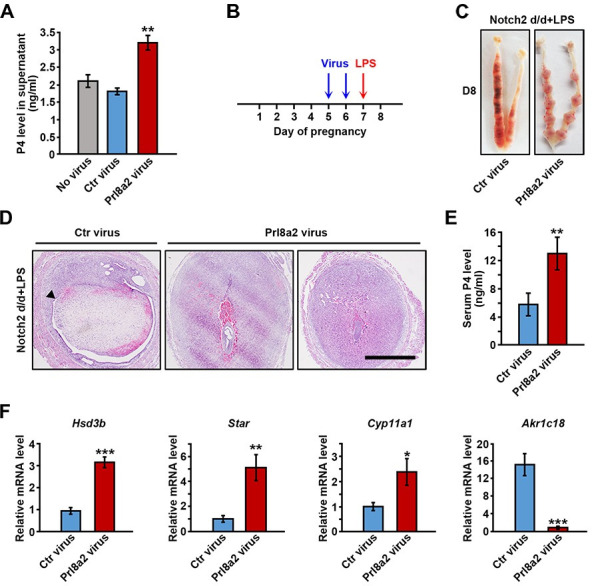
(A) Lentivirus-mediated Prl8a2 overexpression prompted P4 secretion by cultured CL cells. (B) Virus injection and LPS treatment was carried out as illustrated. (C) Overexpression of Prl8a2 in Notch2 d/d mice rescued LPS-induced pregnancy failure. (D) H&E staining revealed normal embryo development in most implantation sites of LPS-treated Notch2 d/d mice after Prl8a2 overexpression. Arrowhead indicates abortion-induced decidual shedding. Scale bar: 300μm. (E) The serum P4 level was compared in LPS-treated Notch2 d/d mice with or without Prl8a2 overexpression. (F) The relative mRNA level of genes related to P4 synthesis or degradation in ovaries of LPS-treated Notch2 d/d mice with or without Prl8a2 overexpression. Data in (A), (E) and (F) are presented as mean±SEM. *p<0.5, **p<0.01, ***p<0.001.
Decidual Prl8a2 was modulated by the canonical Notch signaling pathway
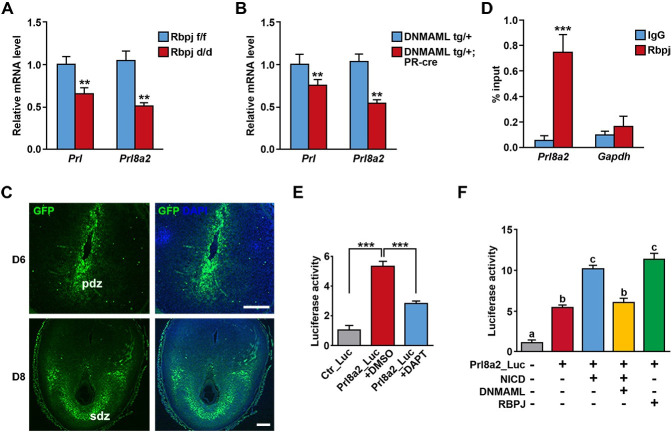
Given that uterine Notch2 deficiency led to downregulation of decidual Prl8a2, we next investigated the possibility that Prl8a2 was regulated by the canonical Notch pathway. We adopted two mouse lines with either deletion of Rbpj or overexpression of DNMAML in the uterus. DNMAML only contains the N terminus of MAML, which is capable of binding NICD without recruiting transcriptional co-activators and thus interferes endogenous MAML function [37]. In both mouse models, the expression level of decidual Prl and Prl8a2 was significantly declined (Fig 6A and 6B), implying that decidual Prl members might be under the control of the canonical Notch pathway. Meanwhile, employing mouse model carrying green fluorescent protein (GFP) reporter driven by Rbpj responsive element, we observed activation of the canonical Notch signaling in decidual cells (Fig 6C), in accord with the expression pattern of NICD2 (Fig 1A), further indicating the potential participation of canonical Notch pathway in decidual transformation and decidual Prl members expression.
Fig 6. Prl8a2 was under the control of canonical Notch signaling.
The relative mRNA level of Prl and Prl8a2 was examined in Rbpj f/f versus Rbpj d/d decidua (A) and DNMAML tg/+ versus DNMAML tg/+;PR-cre decidua (B). (C) GFP signals in implantation sites of mice carrying Rbpj responsive element-driven GFP on day 6 and 8. Nuclei were stained by DAPI. pdz, primary decidual zone; sdz, secondary decidual zone. Scale bar: 50μm and 100μm. (D) Chromatin immunoprecipitation assay showed the direct binding of Rbpj on Prl8a2 promoter. IgG was served as isotype control. Gapdh was served as negative control. (E) Luciferase analysis revealed Prl8a2 promoter activity in the presence of DAPT or DMSO. (F) Prl8a2 promoter activity was enhanced by NICD and RBPJ, which was reversed by DNMAML. Data in (A), (B) (D) and (E) are presented as mean±SEM. **p<0.01, ***p<0.001, a vs b and b vs c: P < 0.01, a vs c: P < 0.001.
We screened the promoter region of Prl8a2 and sought out a conserved Rbpj binding sequence 3000bp upstream the transcription start site. Chromatin immunoprecipitation (ChIP) assay demonstrated the direct binding of Rbpj at this site (Fig 6D). Furthermore, we cloned this site into the luciferase reporter system, and found that DAPT, which inhibited the gamma-secretase complex and the release of NICD, could obviously repress Prl8a2 promoter activity (Fig 6E). In the subsequent analysis, we revealed that NICD could remarkably activate the Prl8a2 promoter, which was reversed by simultaneous overexpression of DNMAML. In addition, Rbpj alone significantly enhanced the activity of Prl8a2 promoter (Fig 6F). These findings further confirmed the direct regulation of Prl8a2 transcription by canonical Notch signaling pathway.
Discussion
The establishment and maintenance of gestation necessitates not only the intimate interactions between the conceptus and the uterus, but also the adaptive changes in other maternal systems and organs. Sexual intercourse ignites a neuroendocrine reflex, prompting Prl surges released from the anterior pituitary, which extends the CL lifespan and guarantees P4 provision during early pregnancy [4]. In the present study, we uncovered an unexpected role of uterine Notch2 in pregnancy maintenance via upregulating decidual Prl8a2, a member belonging to the Prl family, and provided explicit evidence that decidual Prl8a2 facilitated CL maintenance and P4 production along with Prl released from the pituitary gland (Fig 7).
Fig 7. Working model.
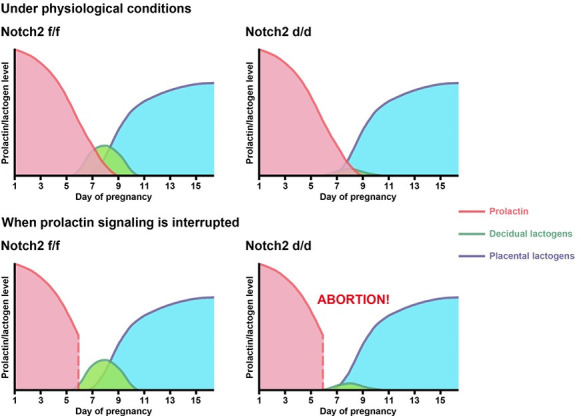
Under normal physiological circumstances, pituitary prolactin sustains CL function until mid-gestation, which is then taken over by placental lactogens. Decidual lactogens assist pituitary prolactin in pregnancy maintenance, while the latter plays the predominant role. Therefore, no obvious defects in fertility were noticed in Notch2 d/d mice. However, when the prolactin signaling is blocked, decidual lactogens were capable of ensuring progesterone production in Notch2 f/f mice, but Notch2 d/d mice experienced pregnancy loss due to insufficient level of decidual lactogens.
In mammals, embryonic development takes place in the maternal uterus. In response to blastocyst implantation, the endometrium experiences stromal-decidual transformation to provide an appropriate environment for the growing conceptus [38,39]. Plentiful researches have been carried out regarding the involvement of Notch signaling in the uterus during early pregnancy [27–29,40–42]. In our previous study, we uncovered dual roles of Rbpj, the nuclear transducer of the Notch signaling pathway, in blastocyst orientation and stromal decidualization via Notch-independent and -dependent mechanisms, respectively [31]. Considering the Notch-dependent participation of Rbpj during the period of decidual remodeling, we attempted to excavate the upstream Notch receptor that might be involved. We screened several Notch receptors and eventually put emphasis on Notch2, since its spatiotemporal expression pattern was highly consistent with that of Rbpj particularly during decidualization (Fig 1A), and its role in early pregnancy events had not been explored. Owing to embryonic lethality resulting from systemic Notch2 deficiency [32], we specifically deleted Notch2 in the uterus by employing the PR-Cre mouse model. To our surprise, we did not find obvious defects in female fertility upon Notch2 ablation (Fig 2), which might be attributed to the compensatory effect by other Notch receptors.
In the further detailed analysis, we noticed that the expression of Prl family members, e.g. Prl8a2, was decreased in the Notch2 d/d decidua. The physiological roles of these decidual Prl members were unclear. It had been hypothesized that decidual derived Prl family members share similar function with the pituitary Prl in pregnancy recognition and maintenance [10]. In Notch2 d/d females, the CL production of P4 and pregnancy maintenance were not compromised despite the declined level of decidual Prl members. We speculated that this might be due to the predominant function of Prl released from the pituitary gland. Therefore, several models were established to weaken the leading role of pituitary Prl, including the LPS-induced Prl resistance model, blocking the Prl signaling using the Prlr-Fc fusion protein, as well as inhibiting pituitary Prl release using the dopamine receptor agonist bromocriptine.
However, it is hard to deny that each model has its limitations. In the LPS-induced Prl resistance model, although low-dose LPS indeed enforced the ovarian resistance to the Prl signaling and impaired pregnancy in Notch2 d/d mice (Fig 3), these defects might also arose from the inflammatory reaction in the endometrium elicited by LPS, since LPS-induced endometrial inflammation is detrimental to embryo survival [43–47]. In the case of interrupting Prl signaling with Prlr-Fc, the Prlr-Fc fusion protein efficiently blocked the Prl signaling and disturbed CL function (Fig 4). However, Prlr is expressed in the uterus as well as the developing embryo in addition to the CL [17], implying the existence of other targets of the Prl signaling. As a matter of fact, Prl could also target the uterus and repress the production of factors that were harmful to gestation maintenance [48]. Therefore, the pregnancy loss in Prlr-Fc treated Notch2 d/d females might also be attributed to the dysregulated Prl signaling in the uterus. In bromocriptine-treated Notch2 d/d mice, the phenotype was not as severe as that in the other two models (S3 Fig), which might be owing to the method of drug delivery. The release of Prl form the pituitary is fluctuant, so the timing of bromocriptine administration might directly influence the consequences [49]. In spite of the respective flaw in each model, the combination of these three models provided convincing evidence that decidual Prl family members facilitated pituitary Prl to sustain the CL function and P4 secretion during gestation.
Our study highlighted the importance of decidual Prl8a2 in supporting CL function. Prl8a2 exhibits abundant and specific expression in decidualized stromal cells [50–52], and thus serves as a widely-accepted marker for decidualization, but little is known with respect to its physiological functions. In the present study, our in vivo and in vitro experiments confirmed that Prl8a2 promoted P4 secretion from the CL (Fig 5), which was the first time to our knowledge that the luteotropic role of Prl8a2 has been demonstrated. It has been reported that Prl8a2 knockout female mice displayed no obvious abnormality during pregnancy under normal circumstances, which consisted with the phenotype of Notch2 d/d mice, but were more sensitive to hypoxia stimuli [53]. However, it is unclear whether this was attributed to insufficient CL function and P4 secretion. Given that Prl8a2 does not share the same receptor with Prl, the specific receptor that mediates Prl8a2 effects warrants further identification.
In human, Prl is expressed abundantly by decidualized stromal cells. The decidual Prl gene possesses a different promoter from that of the pituitary Prl, which results from the insertion of transposon element during evolution [54]. This inserted region is enriched with binding sites for multiple transcription factors [55], implying a complicated regulatory mechanism. However, the molecular mechanism by which the expression of Prl8a2 is regulated remains elusive. We noticed that in mice with uterine-conditional deletion of Rbpj or overexpression of DNMAML, the expression of Prl8a2 displayed similar downregulation. In addition, a conserved Rbpj binding site was found upstream of the Prl8a2 promoter, and ChIP assay together with luciferase analysis confirmed that Prl8a2 expression was controlled by the canonical Notch pathway (Fig 6). Whether the expression of Prl8a2 is modulated by other signaling pathways or transcription factors requires more detailed investigations.
Materials and methods
Ethics statement
All mice used in the research were housed in the Animal Care Facility of Xiamen University, according to guidelines for the use and care of laboratory animals. All experimental procedures were approved by the Animal Welfare Committee of Research Organization (X200811), Xiamen University.
Animals and treatments
Notch2 f/f mice were purchased from Jackson Laboratory. Rbpj f/f and DNMAML tg/+ mice were generated as previously described [56,57]. Uterine-specific mutant mice were generated by crossing Notch2 f/f or Rbpj f/f or DNMAML tg/+ mice with PR-Cre mice [33].
Female mice at the age of 6–8 weeks were mated with fertile males to induce pregnancy (day 1 represented the day when vaginal plug was seen). Pregnant females were sacrificed at different time points. On day 5 of pregnancy, implantation sites were visualized by intravenous injection of 100μl Chicago blue (Sigma, C8679), and the number of implantation sites was recorded. On day8, the weight of implantation sites was recorded. Implantation sites, ovaries and blood samples were collected.
For LPS stimulation, 1mg LPS (Sigma, L2880) dissolved in 100μl saline was intraperitoneally injected on day 7. For Prlr-Fc fusion protein treatment, 1mg Prlr-Fc (Provided by Ziqiang Li (Beijing VDJBio Co. LTD)) dissolved in 100μl phosphate buffered saline (PBS) was intraperitoneally injected on day 6 and 7. For bromocriptine administration, pregnant females received a gavage of 100μg bromocriptine mesylate (Sigma, Y0000677) on day 6 and 7. For exogenous progesterone supplementation, 2mg progesterone (Sigma, P0130) dissolved in 100μl sesame oil (Sigma, S3547) was subcutaneously injected. For RU486 administration, 300ug RU486 (Sigma, M8046) dissolved in 100μl PBS was intraperitoneally injected. For Prl8a2 overexpression, 0.1 ml concentrated lentivirus was intravenously injected at indicated time points.
For oil-induced pseudopregnancy model, females were mated with vasectomized males, and sesame oil was infused into the uterine lumen on day 4, to mimic blastocysts transferring into the uterus.
P4 level measurement
Blood samples and CL cell culture supernatants were collected and the P4 level was measured by radioimmunoassay (RIA).
Hematoxylin and eosin staining
Collected implantation sites were fixed in 10% neutral buffered formalin (NBF) overnight at room temperature (RT), dehydrated in ethanol and xylene, and then embedded in paraffin. 5μm paraffin sections were deparaffinized and rehydrated using xylene and ethanol. Hematoxylin and eosin staining was performed according to manufacturer’s instructions (Solarbio, G1120).
Immunohistochemistry
Implantation site and ovary samples were fixed in 10% NBF overnight at RT, dehydrated using ethanol and xylene, and embedded in paraffin. 5μm paraffin sections were deparaffinized and rehydrated in xylene and ethanol. Rehydrated sections were then subjected to heat-mediated epitope retrieval for 15min at 120°C in sodium citrate buffer, treated with 3% hydrogen peroxide/methanol solution for 10min at RT. Antigen blocking was performed using 0.5% bovine serum albumin (BSA) for 1h at RT. Subsequently, sections were incubated with primary antibody overnight at 4°C, washed by phosphate buffered saline (PBS) for 3 times, and incubated with horse radish peroxidase (HRP)-conjuncted secondary antibody (NeoBioscience, ANR02-2, dilution: 1:200) for 1h at RT, and developing was performed using the diaminobenzidine (DAB) kit (ZSGB-BIO, ZLI-9018). Antibodies against NICD2 (Abcam, ab52302, dilution: 1:200) and Cox2 (Servicebio, GB11077-1, dilution: 1:200) were used in the research.
In situ hybridization
For probe construction, target gene cDNAs were amplified by PCR, and digoxin (DIG)-labeled probes were generated by in vitro transcription using the DIG RNA labeling mix (Roche, 11277073910). Sequences of primers used for probe production were: 5’ ACTTGCCTGCCAAAGAGAGAA 3’ (forward) and 5’ TGACTGTCCATTCAGGAGGC 3’ (reverse). For in situ hybridization, frozen sections were fixed in 4% paraformaldehyde (PFA) for 1h at RT, treated with acetic anhydride-containing triethanolamine solution for 10min at RT, and then subjected to probe incubation overnight at 60°C. Subsequently, sections were treated with RNase for 30min at 37°C, and incubated with anti-DIG antibody (Roche, 11214667001, dilution: 1:3000) overnight at 4°C. Developing was performed using nitroblue tetrazolium (NBT, Gentihold, N1332) and 5-bromo-4-chloro-3-indolyl-phosphate (BCIP, Gentihold, B1360) after 10min levamisole treatment.
RNA extraction and QRT-PCR
For RNA extraction, frozen tissues were ground in Trizol and subjected to chloroform extraction, isopropanol precipitation and ethanol washing. Reverse transcription and QRT-PCR were performed according to manufacturer’s instructions (Takara, RR047A & RR820A). Primer sequences used in QRT-PCR analysis were listed in S1 Table.
Protein extraction and Western blot
For protein extraction, cells were lysed in ice-cold RIPA buffer for 1h and subjected to centrifugation (20000g, 30min, 4°C). Supernatant was collected. Protein samples were subjected to electrophoresis, electrotransfer, membrane blocking using 5% skim milk for 1h at RT and primary antibody incubation overnight at 4°C. Home-made rabbit polyclonal primary antibody against mouse Prl8a2 was used at a dilution of 1:1000. The membrane was washed by Tris buffered saline Tween (TBST) for 3 times, and incubated with HRP-conjuncted anti-rabbit secondary antibody (NeoBioscience, ANR02-2, dilution: 1:5000) for 1h at RT, and developing was performed using the SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher, 34578).
Chromatin immunoprecipitation
Cultured decidualized stromal cells were crosslinked in 1% formaldehyde/PBS solution for 20min at RT, which was terminated by 0.125M glycine for 10min on ice. Cells were collected after PBS washing, and subjected to lysis and sonication until the chromatin was sheared to an average length of 200-400bp. 1% of chromatin fragments was served as input, while the rest was divided equally, and incubated with antibody against Rbpj (CST, 5313, dilution: 1:50) and IgG (CST, 3900, dilution: 1:50) overnight at 4°C. The DNA-protein-antibody complexes were incubated with protein A magnetic beads for 3-4h at 4°C. Beads together with the DNA-protein-antibody complexes were collected and washed in the order of low salt buffer (containing 150mM NaCl), high salt buffer (containing 500mM NaCl), LiCl buffer (containing 250mM LiCl) and TE buffer. Precipitated DNA-protein complexes were eluted from beads and subjected to crosslink reversal in 5M NaCl for 8h at 65°C. DNA was purified by phenol chloroform isoamyl alcohol extraction and ethanol precipitation. Primers used in the subsequent QRT analysis were designed targeting 3000bp upstream Prl8a2 transcription start site: 5’ CCTGTAGATAGATTGCTGGGGC 3’ (forward) and 5’ TAATCGCCTCCCAACAGACTTC 3’ (reverse).
In vitro corpus luteum cell culture
Isolated ovaries from D4 pregnant females were placed in the DMEM-F12 medium (Sigma-Aldrich, D6434). CL was separated and digested by collagenase (Gibco, 17018–029) for 20min at 37°C. Digested CL cells were collected by centrifugation and cultured in DMEM-F12 supplemented with 10% charcoal-stripped fetal bovine serum (CS-FBS) (Biological Industries, 04-201-1A), 88μg/ml sodium pyruvate and 292μg/ml L-glutamine.
Luciferase reporter analysis
The conserved Rbpj binding sequence upstream the transcription start site of Prl8a2 was cloned into the pGL3-Basic vector (Promega). The sequences of primers used in vector construction are listed in S2 Table. The pRL-TK plasmid (Promega) was used as internal control. Plasmids were transfected into 293T cells. Cells were collected 48h after transfection, and fluorescence was measured using the dual luciferase reporter system (Promega).
Lentivirus vector construction and packaging
The coding sequence of Prl8a2 were amplified by PCR, and inserted into the pLVX-IRES-ZsGreen1 vector (Clontec). The sequences of primers used in vector construction are listed in S2 Table. The modified vector was transfected into 293T, and viruses were collected from the supernatant 3 days after transfection.
Statistics
All data were presented as mean±S.E.M. Comparation between two groups was analyzed with t tests, while one-way ANOVA was performed for comparation between more than two groups. P<0.05 was considered statistically significant.
Supporting information
(A) The expression of NICD2 was detected by immunohistochemistry in Notch2 f/f and Notch2 d/d ovaries. (B) In situ hybridization analysis revealed Prlr expression in Notch2 f/f and Notch2 d/d ovaries. CL, corpus luteum. Scale bar: 100 μm and 200 μm.
(TIF)
(A) The serum P4 level in pseudopregnant females with or without oil-induced decidualization was measured at indicated time points. (B) The supernatant of decidualized uterus promoted P4 secretion by cultured CL cells compared to undecidualized uterus. Data represent mean±SEM. *p<0.5, **p<0.01.
(TIF)
(A) Females pretreated with bromocriptine exhibited impaired implantation despite the presence of morphologically normal blastocysts. (B) The size of implantation sites in Notch2 d/d mice pretreated with bromocriptine was smaller compared to Notch2 f/f mice, and the average weight of implantation sites was declined according to (C). (D) The serum P4 level was detected in Notch2 f/f and Notch2 d/d mice pretreated with bromocriptine. Data in (C) and (D) represent mean±SEM. **p<0.01.
(TIF)
(A) GFP signals in 293T cells transfected with Prl8a2 overexpression vectors. (B) Western blot analysis revealed Prl8a2 expression in 293T cells after transfection. NS, non-specific band.
(TIF)
(DOCX)
(DOCX)
Acknowledgments
We are grateful to Ziqiang Li (Beijing VDJBio Co. LTD) for providing Prlr-Fc recombinant protein.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work is supported by the National Key R&D Program of China (2017YFC1001402 to W.H.; 2018YFC1004401 to K.S.), the National Natural Science Foundation (81830045 and 82030040 to W.H., 81971388 and 81601285 to K.S., 82071652 to D.L.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bazer FW. History of Maternal Recognition of Pregnancy. Adv Anat Embryol Cell Biol. 2015;216:5–25. doi: 10.1007/978-3-319-15856-3_2 . [DOI] [PubMed] [Google Scholar]
- 2.Ben-Jonathan N, LaPensee CR, LaPensee EW. What can we learn from rodents about prolactin in humans? Endocr Rev. 2008;29(1):1–41. doi: 10.1210/er.2007-0017 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28(1):117–49. doi: 10.1210/er.2006-0022 . [DOI] [PubMed] [Google Scholar]
- 4.Freeman ME, Neill JD. The pattern of prolactin secretion during pseudopregnancy in the rat: a daily nocturnal surge. Endocrinology. 1972;90(5):1292–4. doi: 10.1210/endo-90-5-1292 . [DOI] [PubMed] [Google Scholar]
- 5.Morishige WK, Pepe GJ, Rothchild I. Serum luteinizing hormone, prolactin and progesterone levels during pregnancy in the rat. Endocrinology. 1973;92(5):1527–30. doi: 10.1210/endo-92-5-1527 . [DOI] [PubMed] [Google Scholar]
- 6.Soares MJ, Faria TN, Roby KF, Deb S. Pregnancy and the prolactin family of hormones: coordination of anterior pituitary, uterine, and placental expression. Endocr Rev. 1991;12(4):402–23. doi: 10.1210/edrv-12-4-402 . [DOI] [PubMed] [Google Scholar]
- 7.Simmons DG, Rawn S, Davies A, Hughes M, Cross JC. Spatial and temporal expression of the 23 murine Prolactin/Placental Lactogen-related genes is not associated with their position in the locus. BMC Genomics. 2008;9:352. doi: 10.1186/1471-2164-9-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prigent-Tessier A, Tessier C, Hirosawa-Takamori M, Boyer C, Ferguson-Gottschall S, Gibori G. Rat decidual prolactin. Identification, molecular cloning, and characterization. J Biol Chem. 1999;274(53):37982–9. doi: 10.1074/jbc.274.53.37982 . [DOI] [PubMed] [Google Scholar]
- 9.Roby KF, Deb S, Gibori G, Szpirer C, Levan G, Kwok SC, et al. Decidual prolactin-related protein. Identification, molecular cloning, and characterization. J Biol Chem. 1993;268(5):3136–42. . [PubMed] [Google Scholar]
- 10.Gibori G, Rothchild I, Pepe GJ, Morishige WK, Lam P. Luteotrophic action of decidual tissue in the rat. Endocrinology. 1974;95(4):1113–8. doi: 10.1210/endo-95-4-1113 . [DOI] [PubMed] [Google Scholar]
- 11.Ruff SJ, Leers-Sucheta S, Melner MH, Cohen S. Induction and activation of Stat 5 in the ovaries of pseudopregnant rats. Endocrinology. 1996;137(10):4095–9. doi: 10.1210/endo.137.10.8828462 . [DOI] [PubMed] [Google Scholar]
- 12.Russell DL, Norman RL, Dajee M, Liu X, Hennighausen L, Richards JS. Prolactin-induced activation and binding of stat proteins to the IL-6RE of the alpha 2-macroglobulin (alpha 2M) promoter: relation to the expression of alpha 2M in the rat ovary. Biol Reprod. 1996;55(5):1029–38. doi: 10.1095/biolreprod55.5.1029 . [DOI] [PubMed] [Google Scholar]
- 13.Feltus FA, Groner B, Melner MH. Stat5-mediated regulation of the human type II 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene: activation by prolactin. Mol Endocrinol. 1999;13(7):1084–93. doi: 10.1210/mend.13.7.0314 . [DOI] [PubMed] [Google Scholar]
- 14.Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, et al. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 1997;16(23):6926–35. doi: 10.1093/emboj/16.23.6926 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11(2):167–78. doi: 10.1101/gad.11.2.167 . [DOI] [PubMed] [Google Scholar]
- 16.Binart N, Helloco C, Ormandy CJ, Barra J, Clement-Lacroix P, Baran N, et al. Rescue of preimplantatory egg development and embryo implantation in prolactin receptor-deficient mice after progesterone administration. Endocrinology. 2000;141(7):2691–7. doi: 10.1210/endo.141.7.7568 . [DOI] [PubMed] [Google Scholar]
- 17.Reese J, Binart N, Brown N, Ma WG, Paria BC, Das SK, et al. Implantation and decidualization defects in prolactin receptor (PRLR)-deficient mice are mediated by ovarian but not uterine PRLR. Endocrinology. 2000;141(5):1872–81. doi: 10.1210/endo.141.5.7464 . [DOI] [PubMed] [Google Scholar]
- 18.Artavanis-Tsakonas S, Muskavitch MA, Yedvobnick B. Molecular cloning of Notch, a locus affecting neurogenesis in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1983;80(7):1977–81. doi: 10.1073/pnas.80.7.1977 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137(2):216–33. doi: 10.1016/j.cell.2009.03.045 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meurette O, Mehlen P. Notch Signaling in the Tumor Microenvironment. Cancer Cell. 2018;34(4):536–48. doi: 10.1016/j.ccell.2018.07.009 . [DOI] [PubMed] [Google Scholar]
- 21.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398(6727):518–22. doi: 10.1038/19083 . [DOI] [PubMed] [Google Scholar]
- 22.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393(6683):382–6. doi: 10.1038/30756 . [DOI] [PubMed] [Google Scholar]
- 23.Kovall RA. More complicated than it looks: assembly of Notch pathway transcription complexes. Oncogene. 2008;27(38):5099–109. doi: 10.1038/onc.2008.223 . [DOI] [PubMed] [Google Scholar]
- 24.Lu FM, Lux SE. Constitutively active human Notch1 binds to the transcription factor CBF1 and stimulates transcription through a promoter containing a CBF1-responsive element. Proc Natl Acad Sci U S A. 1996;93(11):5663–7. doi: 10.1073/pnas.93.11.5663 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuman C, Menkhorst E, Winship A, Van Sinderen M, Osianlis T, Rombauts LJ, et al. Fetal-maternal communication: the role of Notch signalling in embryo implantation. Reproduction. 2014;147(3):R75–86. doi: 10.1530/REP-13-0474 . [DOI] [PubMed] [Google Scholar]
- 26.Afshar Y, Miele L, Fazleabas AT. Notch1 is regulated by chorionic gonadotropin and progesterone in endometrial stromal cells and modulates decidualization in primates. Endocrinology. 2012;153(6):2884–96. doi: 10.1210/en.2011-2122 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afshar Y, Jeong JW, Roqueiro D, DeMayo F, Lydon J, Radtke F, et al. Notch1 mediates uterine stromal differentiation and is critical for complete decidualization in the mouse. FASEB J. 2012;26(1):282–94. doi: 10.1096/fj.11-184663 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su RW, Strug MR, Jeong JW, Miele L, Fazleabas AT. Aberrant activation of canonical Notch1 signaling in the mouse uterus decreases progesterone receptor by hypermethylation and leads to infertility. Proc Natl Acad Sci U S A. 2016;113(8):2300–5. doi: 10.1073/pnas.1520441113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shawber CJ, Lin L, Gnarra M, Sauer MV, Papaioannou VE, Kitajewski JK, et al. Vascular Notch proteins and Notch signaling in the peri-implantation mouse uterus. Vasc Cell. 2015;7:9. doi: 10.1186/s13221-015-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Pascual CM, Ferrero H, Zimmermann RC, Simon C, Pellicer A, Gomez R. Inhibition of Delta-like 4 mediated signaling induces abortion in mice due to deregulation of decidual angiogenesis. Placenta. 2014;35(7):501–8. doi: 10.1016/j.placenta.2014.03.019 . [DOI] [PubMed] [Google Scholar]
- 31.Zhang S, Kong S, Wang B, Cheng X, Chen Y, Wu W, et al. Uterine Rbpj is required for embryonic-uterine orientation and decidual remodeling via Notch pathway-independent and -dependent mechanisms. Cell Res. 2014;24(8):925–42. doi: 10.1038/cr.2014.82 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamada Y, Hiroe T, Suzuki Y, Oda M, Tsujimoto Y, Coleman JR, et al. Notch2 is required for formation of the placental circulatory system, but not for cell-type specification in the developing mouse placenta. Differentiation. 2007;75(3):268–78. doi: 10.1111/j.1432-0436.2006.00137.x . [DOI] [PubMed] [Google Scholar]
- 33.Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41(2):58–66. doi: 10.1002/gene.20098 . [DOI] [PubMed] [Google Scholar]
- 34.Chakraborty I, Das SK, Wang J, Dey SK. Developmental expression of the cyclo-oxygenase-1 and cyclo-oxygenase-2 genes in the peri-implantation mouse uterus and their differential regulation by the blastocyst and ovarian steroids. J Mol Endocrinol. 1996;16(2):107–22. doi: 10.1677/jme.0.0160107 . [DOI] [PubMed] [Google Scholar]
- 35.Erlebacher A, Zhang D, Parlow AF, Glimcher LH. Ovarian insufficiency and early pregnancy loss induced by activation of the innate immune system. J Clin Invest. 2004;114(1):39–48. doi: 10.1172/JCI20645 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhodes SJ, DiMattia GE, Rosenfeld MG. Transcriptional mechanisms in anterior pituitary cell differentiation. Curr Opin Genet Dev. 1994;4(5):709–17. doi: 10.1016/0959-437x(94)90138-s . [DOI] [PubMed] [Google Scholar]
- 37.Weng AP, Nam Y, Wolfe MS, Pear WS, Griffin JD, Blacklow SC, et al. Growth suppression of pre-T acute lymphoblastic leukemia cells by inhibition of notch signaling. Mol Cell Biol. 2003;23(2):655–64. doi: 10.1128/MCB.23.2.655-664.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7(3):185–99. doi: 10.1038/nrg1808 [DOI] [PubMed] [Google Scholar]
- 39.Zhang S, Lin H, Kong S, Wang S, Wang H, Wang H, et al. Physiological and molecular determinants of embryo implantation. Mol Aspects Med. 2013;34(5):939–80. doi: 10.1016/j.mam.2012.12.011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lustgarten Guahmich N, Farber G, Shafiei S, McNally D, Redmond D, Kallinos E, et al. Endothelial deletion of ADAM10, a key regulator of Notch signaling, causes impaired decidualization and reduced fertility in female mice. Angiogenesis. 2020;23(3):443–58. doi: 10.1007/s10456-020-09723-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchetto NM, Begum S, Wu T, O’Besso V, Yarborough CC, Valero-Pacheco N, et al. Endothelial Jagged1 Antagonizes Dll4/Notch Signaling in Decidual Angiogenesis during Early Mouse Pregnancy. Int J Mol Sci. 2020;21(18). doi: 10.3390/ijms21186477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otti GR, Saleh L, Velicky P, Fiala C, Pollheimer J, Knofler M. Notch2 controls prolactin and insulin-like growth factor binding protein-1 expression in decidualizing human stromal cells of early pregnancy. PLoS One. 2014;9(11):e112723. doi: 10.1371/journal.pone.0112723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cha J, Bartos A, Egashira M, Haraguchi H, Saito-Fujita T, Leishman E, et al. Combinatory approaches prevent preterm birth profoundly exacerbated by gene-environment interactions. J Clin Invest. 2013;123(9):4063–75. doi: 10.1172/JCI70098 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li L, Shi L, Yang X, Ren L, Yang J, Lin Y. Role of invariant natural killer T cells in lipopolysaccharide-induced pregnancy loss. Cell Immunol. 2013;286(1–2):1–10. doi: 10.1016/j.cellimm.2013.10.007 . [DOI] [PubMed] [Google Scholar]
- 45.Presicce P, Park CW, Senthamaraikannan P, Bhattacharyya S, Jackson C, Kong F, et al. IL-1 signaling mediates intrauterine inflammation and chorio-decidua neutrophil recruitment and activation. JCI Insight. 2018;3(6). doi: 10.1172/jci.insight.98306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang S, Cao C, Piao H, Li Y, Tao Y, Zhang X, et al. Tim-3 protects decidual stromal cells from toll-like receptor-mediated apoptosis and inflammatory reactions and promotes Th2 bias at the maternal-fetal interface. Sci Rep. 2015;5:9013. doi: 10.1038/srep09013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng J, Li Y, Sang Y, Xu L, Jin X, Tao Y, et al. Pigment epithelium-derived factor, a novel decidual natural killer cells-derived factor, protects decidual stromal cells via anti-inflammation and anti-apoptosis in early pregnancy. Hum Reprod. 2020;35(7):1537–52. doi: 10.1093/humrep/deaa118 . [DOI] [PubMed] [Google Scholar]
- 48.Bao L, Tessier C, Prigent-Tessier A, Li F, Buzzio OL, Callegari EA, et al. Decidual prolactin silences the expression of genes detrimental to pregnancy. Endocrinology. 2007;148(5):2326–34. doi: 10.1210/en.2006-1643 . [DOI] [PubMed] [Google Scholar]
- 49.Larsen CM, Grattan DR. Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology. 2010;151(8):3805–14. doi: 10.1210/en.2009-1385 . [DOI] [PubMed] [Google Scholar]
- 50.Gu Y, Soares MJ, Srivastava RK, Gibori G. Expression of decidual prolactin-related protein in the rat decidua. Endocrinology. 1994;135(4):1422–7. doi: 10.1210/endo.135.4.7925104 . [DOI] [PubMed] [Google Scholar]
- 51.Orwig KE, Ishimura R, Muller H, Liu B, Soares MJ. Identification and characterization of a mouse homolog for decidual/trophoblast prolactin-related protein. Endocrinology. 1997;138(12):5511–7. doi: 10.1210/endo.138.12.5628 . [DOI] [PubMed] [Google Scholar]
- 52.Orwig KE, Soares MJ. Transcriptional activation of the decidual/trophoblast prolactin-related protein gene. Endocrinology. 1999;140(9):4032–9. doi: 10.1210/endo.140.9.6954 . [DOI] [PubMed] [Google Scholar]
- 53.Alam SM, Konno T, Dai G, Lu L, Wang D, Dunmore JH, et al. A uterine decidual cell cytokine ensures pregnancy-dependent adaptations to a physiological stressor. Development. 2007;134(2):407–15. doi: 10.1242/dev.02743 . [DOI] [PubMed] [Google Scholar]
- 54.Gerlo S, Davis JR, Mager DL, Kooijman R. Prolactin in man: a tale of two promoters. Bioessays. 2006;28(10):1051–5. doi: 10.1002/bies.20468 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lynch VJ, Brayer K, Gellersen B, Wagner GP. HoxA-11 and FOXO1A cooperate to regulate decidual prolactin expression: towards inferring the core transcriptional regulators of decidual genes. PLoS One. 2009;4(9):e6845. doi: 10.1371/journal.pone.0006845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, et al. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol. 2002;14(6):637–45. doi: 10.1093/intimm/dxf030 . [DOI] [PubMed] [Google Scholar]
- 57.Tu L, Fang TC, Artis D, Shestova O, Pross SE, Maillard I, et al. Notch signaling is an important regulator of type 2 immunity. J Exp Med. 2005;202(8):1037–42. doi: 10.1084/jem.20050923 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The expression of NICD2 was detected by immunohistochemistry in Notch2 f/f and Notch2 d/d ovaries. (B) In situ hybridization analysis revealed Prlr expression in Notch2 f/f and Notch2 d/d ovaries. CL, corpus luteum. Scale bar: 100 μm and 200 μm.
(TIF)
(A) The serum P4 level in pseudopregnant females with or without oil-induced decidualization was measured at indicated time points. (B) The supernatant of decidualized uterus promoted P4 secretion by cultured CL cells compared to undecidualized uterus. Data represent mean±SEM. *p<0.5, **p<0.01.
(TIF)
(A) Females pretreated with bromocriptine exhibited impaired implantation despite the presence of morphologically normal blastocysts. (B) The size of implantation sites in Notch2 d/d mice pretreated with bromocriptine was smaller compared to Notch2 f/f mice, and the average weight of implantation sites was declined according to (C). (D) The serum P4 level was detected in Notch2 f/f and Notch2 d/d mice pretreated with bromocriptine. Data in (C) and (D) represent mean±SEM. **p<0.01.
(TIF)
(A) GFP signals in 293T cells transfected with Prl8a2 overexpression vectors. (B) Western blot analysis revealed Prl8a2 expression in 293T cells after transfection. NS, non-specific band.
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.