Abstract
Background:
Fecal microbiota transplantation (FMT) has been studied for the treatment of metabolic syndrome with varying success. However, the possibility of utilizing FMT to prevent metabolic syndrome is to date unknown.
Methods:
Secondary analysis of a double-blind, randomized, placebo-controlled pilot trial of FMT in obese metabolically healthy patients was conducted. Post-prandial glucose and insulin levels were measured.
Results:
A total of 22 patients were enrolled, 11 in each arm. There were no baseline differences in the area under the curve (AUC) of glucose or insulin in the FMT group compared to placebo. There was a significant change in glucose AUC at week 12 compared to baseline, and in the insulin AUC at week 6 compared to baseline in the FMT group vs. placebo (change in glucose AUC (mg/dl x 60min): 579 vs 1978, p=0.03) (change in insulin AUC (μU/ml x 60min): 137 vs 2728, p=0.01).
Conclusions:
These data suggest that FMT may have a potential role in preventing the development of metabolic syndrome in patients with obesity.
Keywords: Obesity, Diabetes, Metabolic syndrome, FMT
Introduction:
Obesity continues to be a major health burden and public health concern, and is associated with chronic diseases such as diabetes mellitus and cardiovascular disease.[1] Given the lack of effective medical therapy for obesity and its consequences, fecal microbiota transplantation (FMT) has been studied for the treatment of metabolic syndrome with varying success.[2–4] However, the possibility of utilizing FMT to prevent metabolic syndrome is thus far unknown. Data are emerging suggesting that obese metabolically healthy patients may have metabolic alterations that confer potential future risk.[5] It is possible that preventative therapies initiated prior to metabolic derangement may be critical for obese patients. Clinical and microbial results from a pilot randomized-controlled trial evaluating FMT in obese metabolically healthy patients, utilizing a single lean donor stool, have previously been reported.[6] While FMT did not induce clinical weight loss in this pilot trial at an early timepoint, we did not assess the potential body weight-independent effects of FMT on blood glucose regulation. Herein, we report a secondary analysis of the impact of FMT on post-prandial glucose regulation.
Methods:
Secondary analysis was conducted of a single-center, double-blind, randomized, placebo-controlled pilot trial of FMT in obese metabolically healthy patients (body mass index [BMI], 35kg/m2 or higher without diabetes, metabolic syndrome, or non-alcoholic fatty liver disease).[6] Participants were randomized 1:1 to receive treatment (an induction dose of 30 FMT capsules followed by two maintenance doses of 12 capsules at week 4 and week 8), or an identical placebo capsule. A single healthy lean donor (BMI, 17.5 kg/m2) was used. Patients were assessed with a mixed meal tolerance test (MMT) at baseline prior to FMT, at week 6, and at week 12 post-FMT, with sampling at −15, 0, 15, 30, 45, and 60 min, in which glucose and insulin levels were measured. Insulin was measured by ELISA (Millipore, Burlington, MA, USA), and glucose was measured by colorimetric assay (ThermoFisher Scientific, Waltham, MA, USA). Data are presented as median (min, max) box plots. All statistical analyses were performed using GraphPad Prism 6. Data were analyzed by the Mann-Whitney test. Differences were considered significant at p <0.05.
Results:
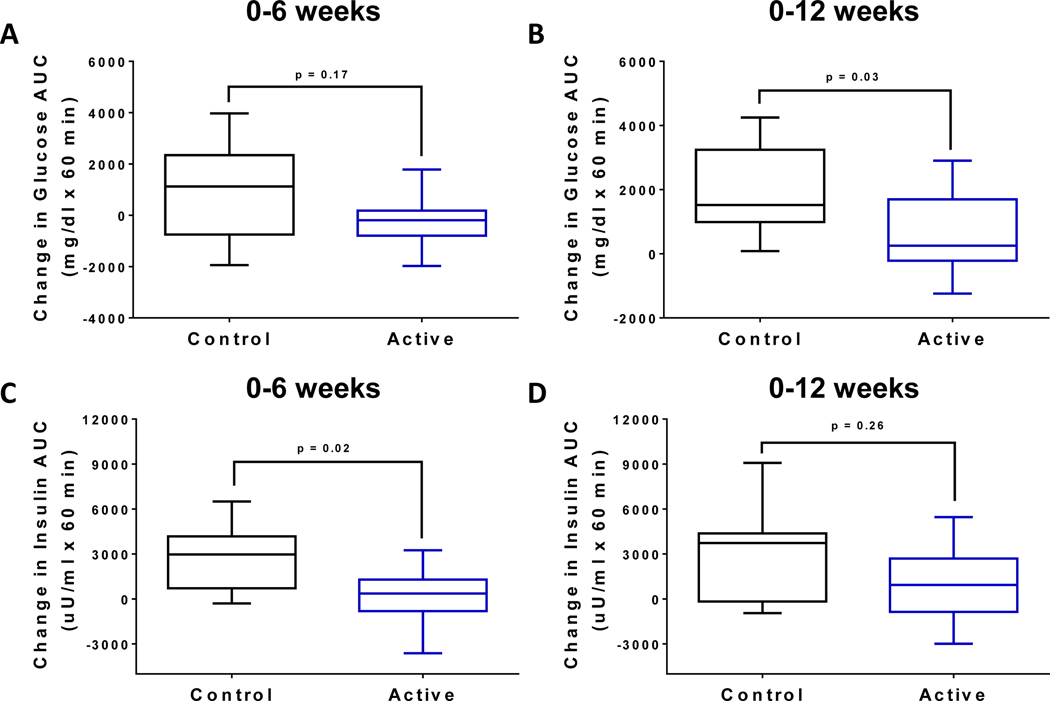
We enrolled 22 patients, 11 in each arm, primarily female (10 in each group). There were no significant baseline clinical differences between the FMT and placebo groups: baseline median BMI 40.8 (min 35, max 51.7) vs. 39.1 (min 36, max 49.1), p=0.73; age 44.5± 14.4 vs 43.3± 12.8, p=0.84. Two patients in the placebo group withdrew prior to week 12 and were excluded from the analysis. There were no statistically significant baseline differences in the area under the curve (AUC) of glucose or insulin in the FMT group compared to placebo (glucose AUC (mg/dl x 60min): 10389 vs. 10008, p=0.63; insulin AUC (μU/ml x 60min): 5668 vs 8008, p =0.07). Glucose and insulin excursions during the MMT remained stable in patients receiving FMT compared with placebo throughout the study. Overall, there was a significantly lower change in glucose AUC at week 12 compared to baseline in the FMT group compared to placebo (glucose AUC (mg/dl x 60min): 579 vs. 1978, p=0.03) (Fig. 1A–B). A similar trend was observed when comparing the baseline to week 6 glucose AUC; however, this did not reach significance (−262 vs 862, p=0.17). Similarly, there was a significantly lower change in insulin AUC at week 6 compared to baseline in the FMT group compared to placebo (insulin AUC (μU/ml x 60min): 137 vs. 2728, p=0.02) (Fig. 1C–D). There were no significant weight changes observed in either group.
Fig. 1:
Impact of FMT on plasma glucose and insulin excursions during a mixed meal tolerance test. (A) Change in the AUC of plasma glucose levels at (A) week 6, and (B) week 12 relative to baseline in the active treatment arm compared to placebo. Change in the AUC of plasma insulin levels at (C) week 6, and (D) week 12 relative to baseline in the active treatment arm compared to placebo. Data are presented as median (min, max) box plots.
Conclusion:
To our knowledge, this is the first trial to assess the efficacy of FMT treatment in metabolically healthy patients with obesity. Studies have shown that metabolically healthy patients with obesity have altered metabolic profiling which may confer future cardiac risk, and that there is clearly a role for preventative therapies.[5] There has been variable response in insulin measurement post FMT for patients with metabolic syndrome. A recent study using FMT to treat metabolic syndrome among patients with obesity and insulin resistance did not report improvement in insulin sensitivity, as assessed by hyperinsulinemic euglycemic clamp.[4] In this trial, patients notably did not have evidence of baseline metabolic derangement. Patients in the FMT arm were found to have a decrease in the change in AUC of both glucose and insulin levels compared to baseline after FMT. Although there are limitations to this analysis, including the fact that a hyperinsulinemic euglycemic clamp measurement of insulin sensitivity was not performed as this was not the primary outcome, these preliminary data suggest that FMT may have the potential to serve a role in preventing the development of metabolic syndrome in patients with obesity. Further studies to assess this outcome are needed in view of the gravity of the obesity epidemic and the current lack of effective medical therapies.
Acknowledgments
Funding: This study was funded by an internal Brigham and Women’s Hospital grant for the Junior faculty award to JRA. BHM is the recipient of a Medical Research Council Clinical Research Training Fellowship (grant reference: MR/R000875/1). The Division of Integrative Systems Medicine and Digestive Disease at Imperial College, London, UK, receives financial support from the National Institute for Health Research (NIHR) Imperial Biomedical Research CenFuntre (BRC) based at Imperial College Healthcare NHS Trust and Imperial College London, UK. BPC received funding from Nutricia (award # 2018–55).
Conflicts of interest: JRA consults for and has research support from Finch Therapeutics Group. ZK is an employee/shareholder of Finch Therapeutics Group.
CCT: Apollo Endosurgery – Consultant/Research Support (Consulting fees/Institutional Research Grants), Aspire Bariatrics – Research Support (Institutional Research Grant), BlueFlame Healthcare Venture Fund – General Partner, Boston Scientific – Consultant (Consulting fees)/Research Support (Institutional Research Grant), Covidien/Medtronic – Consultant (Consulting Fees)
Fractyl – Consultant/Advisory Board Member (Consulting Fees), GI Dynamics – Consultant (Consulting Fees)/ Research Support (Institutional Research Grant), GI Windows – Ownership interest
Olympus/Spiration – Consultant (Consulting Fees)/Research Support (Equipment Loans), Spatz – Research Support (Institutional Research Grant), USGI Medical – Consultant (Consulting Fees)/Advisory Board Member (Consulting fees)/Research Support (Research Grant)
Footnotes
JH, BHM, JRM, AC and BPC have no relevant conflicts to disclose.
References:
- 1.Wadden TA, Webb VL, Moran CH, Bailer BA (2012) Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. Circulation 125 (9):1157–1170. doi: 10.1161/CIRCULATIONAHA.111.039453 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M (2012) Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143 (4):913–916.e917. doi: 10.1053/j.gastro.2012.06.031 [doi] [DOI] [PubMed] [Google Scholar]
- 3.Kootte RS, Levin E, Salojarvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, Bouter KE, Koopen AM, Holst JJ, Knop FK, Blaak EE, Zhao J, Smidt H, Harms AC, Hankemeijer T, Bergman J, Romijn HA, Schaap FG, Olde Damink SWM, Ackermans MT, Dallinga-Thie GM, Zoetendal E, de Vos WM, Serlie MJ, Stroes ESG, Groen AK, Nieuwdorp M (2017) Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab 26 (4):611–619 e616. doi: 10.1016/j.cmet.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 4.Yu EW, Gao L, Stastka P, Cheney MC, Mahabamunuge J, Torres Soto M, Ford CB, Bryant JA, Henn MR, Hohmann EL (2020) Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med 17 (3):e1003051. doi: 10.1371/journal.pmed.1003051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Telle-Hansen VH, Christensen JJ, Formo GA, Holven KB, Ulven SM (2020) A comprehensive metabolic profiling of the metabolically healthy obesity phenotype. Lipids Health Dis 19 (1):90. doi: 10.1186/s12944-020-01273-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allegretti JR, Kassam Z, Mullish BH, Chiang A, Carrellas M, Hurtado J, Marchesi JR, McDonald JAK, Pechlivanis A, Barker GF, Miguens Blanco J, Garcia-Perez I, Wong WF, Gerardin Y, Silverstein M, Kennedy K, Thompson C (2020) Effects of Fecal Microbiota Transplantation With Oral Capsules in Obese Patients. Clin Gastroenterol Hepatol 18 (4):855–863 e852. doi: 10.1016/j.cgh.2019.07.006 [DOI] [PubMed] [Google Scholar]