Abstract
Alexia is common in the context of aphasia. It is widely agreed that damage to phonological and semantic systems not specific to reading causes co-morbid alexia and aphasia. Studies of alexia to date have only examined phonology and semantics as singular processes or axes of impairment, typically in the context of stereotyped alexia syndromes. However, phonology, in particular, is known to rely on subprocesses, including sensory-phonological processing, motor-phonological processing, and sensory-motor integration. Moreover, many people with stroke aphasia demonstrate mild or mixed patterns of reading impairment that do not fit neatly with one syndrome. This cross-sectional study tested whether the hallmark symptom of phonological reading impairment, the lexicality effect, emerges from damage to a specific subprocess of phonology in stroke patients not selected for alexia syndromes. Participants were 30 subjects with left-hemispheric stroke and 37 age- and education-matched controls. A logistic mixed-effects model tested whether post-stroke impairments in sensory phonology, motor phonology, or sensory-motor integration modulated the effect of item lexicality on patient accuracy in reading aloud. Support vector regression voxel-based lesion-symptom mapping localized brain regions necessary for reading and non-orthographic phonological processing. Additionally, a novel support vector regression structural connectome-symptom mapping method identified the contribution of both lesioned and spared but disconnected, brain regions to reading accuracy and non-orthographic phonological processing. Specifically, we derived whole-brain structural connectomes using constrained spherical deconvolution-based probabilistic tractography and identified lesioned connections based on comparisons between patients and controls. Logistic mixed-effects regression revealed that only greater motor-phonological impairment related to lower accuracy reading aloud pseudowords versus words. Impaired sensory-motor integration was related to lower overall accuracy in reading aloud. No relationship was identified between sensory-phonological impairment and reading accuracy. Voxel-based and structural connectome lesion-symptom mapping revealed that lesioned and disconnected left ventral precentral gyrus related to both greater motor-phonological impairment and lower sublexical reading accuracy. In contrast, lesioned and disconnected left temporoparietal cortex is related to both impaired sensory-motor integration and reduced overall reading accuracy. These results clarify that at least two dissociable phonological processes contribute to the pattern of reading impairment in aphasia. First, impaired sensory-motor integration, caused by lesions disrupting the left temporoparietal cortex and its structural connections, non-selectively reduces accuracy in reading aloud. Second, impaired motor-phonological processing, caused at least partially by lesions disrupting left ventral premotor cortex and structural connections, selectively reduces sublexical reading accuracy. These results motivate a revised cognitive model of reading aloud that incorporates a sensory-motor phonological circuit.
Keywords: stroke, aphasia, alexia, lesion-symptom mapping, structural connectivity
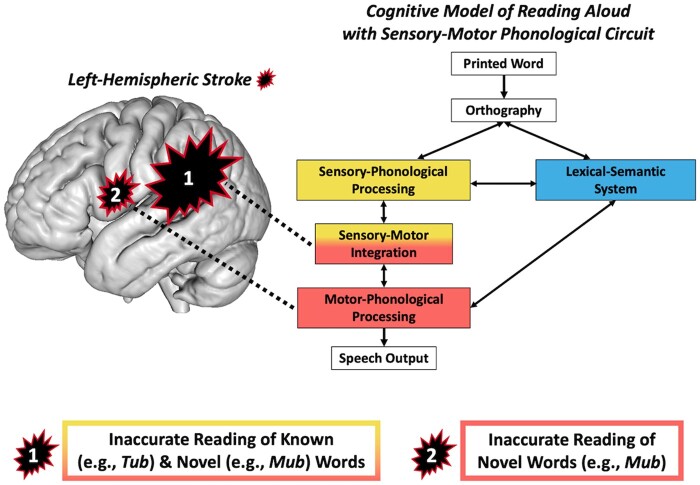
Reading models do not incorporate known sensory-motor subprocesses of speech processing. Dickens et al. report two types of acquired reading impairment: posterior frontal lesions impair motor speech and novel word reading, whereas temporoparietal lesions impair sensory-motor integration and known and novel word reading.
Graphical Abstract
Graphical Abstract.
Introduction
Most people with stroke aphasia have a co-occurring reading impairment, known as alexia.1,2 Accurate reading aloud of known and novel words requires the cooperation of at least two cognitive processes (Fig. 1A).7,8 The first is a lexical process that enables the computation of known pronunciations and meanings from print. The second is a sublexical process that enables the phonological decoding of print. Knowledge of the most frequent or probable spelling-to-sound mappings underlies sublexical reading.3,9,10 Sublexical reading thus enables the reading of novel words. Acquired impairments of the lexical and sublexical reading processes result in dissociable deficits observed in alexia syndromes.11–16 In stroke aphasia, the most common co-morbid alexia is an acquired impairment of the sublexical reading process.1 The hallmark symptom in patients with a sublexical reading deficit is a lexicality effect in which the ability to read pronounceable non-words aloud (e.g. pseudowords such as ‘mub’) is selectively impaired relative to reading real words aloud (e.g. ‘tub’). ‘Phonological alexia’ refers to a symptom complex related to sublexical reading impairment, which includes this lexicality effect.17
Figure 1.
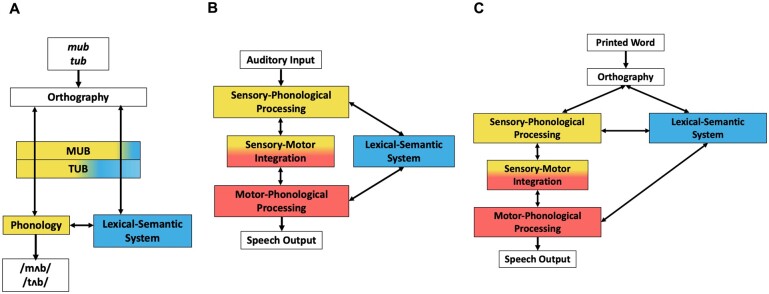
Cognitive models of reading aloud and speech processing. (A) Schematic of a two-route cognitive model of reading aloud. Pseudowords (mub) depend on phonological decoding (yellow) for accurate reading aloud. Both phonological decoding and lexical-semantic processing (blue) contribute to reading known words (tub). The relative proportions of yellow and blue in the text boxes visualize the relative contributions of phonological decoding and lexical-semantic processing to reading a pseudoword or a word aloud. This cognitive model is agnostic to the specific computational implementation (e.g. distributed versus symbolic) but is most analogous to the connectionist triangle model of reading.3,4 (B) Schematic of a cognitive model of speech processing. Speech comprehension is enabled by the route from sensory-phonological processing (yellow) to the lexical-semantic system (blue). Speech production is enabled by the routes from the lexical-semantic system to sensory-phonological processing and motor-phonological processing (red). Speech repetition is enabled by the sensory-motor integration circuit (yellow and red). This schematic is an adaptation of the semantic-lexical-auditory-motor (SLAM) model of speech production,5 which is a computational implementation of Hierarchical State Feedback Control theory.6 Within the SLAM model, the dominant processing route for speech production proceeds from lexical-semantics to sensory phonology to motor phonology, which reflects the hypothesized central role of sensory-phonological targets in speech production. (C) Schematic of a preliminary integrated model of reading and speech processing that combines the architectures of model A and model B. The present study tests whether sublexical reading is particularly dependent on intact motor-phonological processing, which would support a cognitive model of reading aloud that incorporates a sensory-motor phonological circuit (model C).
The aetiology of sublexical reading impairment in aphasia is not fully understood. Phonological alexia was originally described as alexia without comparable aphasia17 and has been argued to result from a reading-specific impairment, at least in some patients.9,18–20 However, a co-morbid phonological deficit that is not specific to reading is evident in most documented cases of phonological alexia (e.g. impaired speech repetition).21–24 The common association between phonological alexia and a general phonological impairment motivated researchers to propose the primary systems hypothesis of alexia,25,26 which asserts that phonological alexia is just one manifestation of a general phonological impairment. Reading is a cultural acquisition that is learned through extensive instruction and practice.27 Reading aloud is thus thought to depend on more evolutionarily old, or ‘primary’, neurocognitive systems, such as phonology, rather than reading-specific neurocognitive systems. In keeping with long-standing linguistic tradition, contemporary cognitive and computational models of reading and phonological alexia treat phonology as an abstract, unitary system of form representations.3,7,10,18,28 Within the primary systems account, damage to this phonological system results in symptoms that vary according to task difficulty and according to the severity of phonological impairment.29 We refer to the current primary systems notion that sublexical reading depends on a functionally unitary phonological system as the ‘undifferentiated phonological reading hypothesis’. Consistent with this hypothesis, pseudoword reading impairment has been associated with lesions throughout left perisylvian regions that are thought to constitute a distributed phonological network.14,16,22,30–32
Despite the dominant proposition that reading relies at least partially on more general language components such as phonology, models of reading have largely been developed separately from models of speech processing (Fig. 1B). Unlike computational models of reading, contemporary computational and neural models of speech processing differentiate sensory-phonological processing and motor-phonological processing, with a sensory-motor integration process linking the two.6,33–35 Sensory-phonological processing relies on auditory forms of speech sounds and words, which are represented in the superior temporal gyrus and sulcus.36,37 These auditory forms enable speech perception and comprehension, in addition to providing auditory targets for speech production.38 Impaired sensory-phonological processing thus impairs auditory comprehension39–42 and may contribute to post-stroke anomia.43,44 Motor-phonological processing relies mainly on the left ventral precentral gyrus and inferior frontal gyrus, which are thought to represent motor plans for phonemes and syllables.6,45,46 Anterior insula has also been implicated in impaired motor-phonological processing47,48 (but see Basilakos et al.49). Impaired motor-phonological processing underlies apraxia of speech,50,51 which is characterized by impaired articulatory planning (i.e. ambiguous or distorted phonemes, slow speech rate, pauses and prolongations and abnormal prosody52). Sensory-motor integration, a process associated with left posterior planum temporale and supramarginal gyrus,33,34 refers to the translation from auditory representations to motor representations. Impaired sensory-motor integration is thought to be especially detrimental to speech repetition ability.44,53 Different patterns of speech and language deficits thus result from impairment of these three phonological subprocesses. In this way, models of speech processing conceptualize phonology as relying on both sensory and motor phonological codes, whereas the current primary systems account of phonological alexia relies on the traditional linguistic notion of phonology as a unitary system.
It has been speculated that subtypes of phonological impairment may underlie phonological alexia.20,29 However, it remains unknown whether sublexical reading relies disproportionately on specific phonological subprocesses and, by extension, the brain regions subserving these subprocesses. Our previous voxel-based lesion-symptom mapping (VLSM) study found that strokes involving left ventral precentral gyrus (lvPCG) selectively impaired pseudoword reading relative to word reading,54 suggesting that intact motor-phonological processing may be particularly important for sublexical reading. Other lesion studies have also found associations between frontal lesions and impaired pseudoword reading.16,22,24,30,31 We refer to the possibility of at least partially dissociable contributions of primary phonological subsystems to reading aloud as the ‘differentiated phonological reading hypothesis’. A necessary step towards developing an integrated model of reading and language (e.g. Fig. 1C) is testing whether incorporating the architectural details of models of speech processing into a model of reading aloud adds explanatory value in accounting for sublexical reading impairment.
This study utilizes behavioural and neuroanatomical evidence to determine the explanatory value of integrating the architectural details of contemporary neurocognitive models of speech processing into a model of reading aloud. We tested competing predictions of the undifferentiated and differentiated phonological reading hypotheses. First, we tested whether post-stroke impairments of sensory-phonological processing, motor-phonological processing, and sensory-motor integration differentially relate to sublexical reading impairment. The undifferentiated phonological reading hypothesis predicts no preferential associations between the three phonological subprocesses and sublexical reading accuracy. In contrast, our differentiated phonological reading hypothesis predicts that only a motor-phonological processing deficit selectively impairs sublexical reading accuracy, as evidenced by a greater post-stroke lexicality effect. Second, we applied both VLSM and a novel structural connectome lesion-symptom mapping (CLSM) approach to identify the anatomical networks subserving sublexical reading and phonological subprocesses. Despite the widely-held notion that phonological processing relies on a distributed left-perisylvian network, no prior study has examined the role of structural connectivity in acquired phonological reading impairment. By quantifying patterns of brain damage using VLSM and patterns of structural disconnection between processors using CLSM, we aimed to reveal how network-level disruptions lead to co-morbid deficits in phonological subprocesses and reading. The differentiated phonological reading hypothesis predicts that lesioned or disconnected lvPCG underlies selective impairments in both motor-phonological processing and sublexical reading. Our findings support this hypothesis, clarifying the neurocognitive bases of phonological reading impairments in aphasia, and motivating a cognitive model of reading aloud that incorporates a sensory-motor phonological circuit.
Materials and methods
Participants
Participants were 30 adults with left-hemisphere stroke and 37 age- and education-matched controls (Table 1; see Supplementary material for additional recruitment information). The Georgetown University Institutional Review Board approved the study protocol, and all participants gave written informed consent in accordance with the Declaration of Helsinki.
Table 1.
Stroke and control group demographics and stroke group clinical data.
| Cohort | Age | Education | Race | Sex | Chronicity | Lesion size | WAB AQ |
|---|---|---|---|---|---|---|---|
| Stroke | 62.90 (9.84) | 17.03 (2.74) | 9 African American | 12 F, 18 M | 45 (58) | 77 288 (71 444) | 81.73 (17.05) |
| n = 30 | |||||||
| 21 Caucasian | |||||||
| Control | 59.42 (12.46) | 16.59 (2.50) | 11 African American | 19 F, 18 M | |||
|
n = 37 | |||||||
| 26 Caucasian |
Values are shown as mean (standard deviation). Age and education are in years. Stroke chronicity is in months. Lesion size is in mm3. WAB AQ represents the Aphasia Quotient from the ‘Western Aphasia Battery—Revised’,109 which measures aphasia severity: 0–25 = very severe, 26–50 = severe, 51–75 = moderate, 76+ = mild.
Cognitive assessments
Oral reading assessment
Participants completed an oral reading assessment that quantified sublexical reading impairment. Specifically, participants read aloud a list of 20 pseudowords followed by a list of 20 words that differed by 1 initial consonant (e.g. ‘tub’ versus ‘mub’). In order to minimize articulatory complexity as a potential confound, all stimuli were monosyllabic, 3–4 letters long, and did not contain consonant cluster onsets. The real words had highly consistent (predictable) pronunciations (see Supplementary Tables 1 and 2 for stimuli). The first reading attempt of each trial was scored for accuracy. Responses on pseudowords were coded as incorrect if they contained spelling-to-sound mappings that do not occur in American English (see Supplementary material for task administration details). Errors reading the words and pseudowords were coded as a (i) orthographically related word error (orthographic error; ‘mub’ > ‘mud’), (ii) orthographically unrelated word error (e.g. ‘mub’ > ‘cat’), (iii) non-word neologisms (e.g. ‘mub’ > ‘muz’) and (iv) omissions. A real word error was classified as orthographically related to the target if it shared at least 1 letter (for 3–4 letter targets) or 2 letters (for 5+ letter targets) with the target.
Phonological assessments
The stroke cohort completed a battery of non-orthographic phonological assessments. Phonological subprocesses assessed included sensory phonology, sensory-motor integration and motor phonology (Fig. 1). A sensory phonology score was computed as the average of accuracies on syllable counting and rhyme judgment tasks of words presented auditorily via headphones.55 For syllable counting, the participant heard 30 pre-recorded words presented simultaneously with a picture of the target and was instructed to select the number of syllables contained within the word (1, 2 or 3). For rhyme judgment, the participant heard 40 pairs of pre-recorded monosyllabic words and was asked to indicate whether the words rhymed (yes/no). One stroke subject did not complete the syllable counting task, so their sensory phonology score incorporated only the accuracy on the rhyme judgment task. A sensory-motor integration score was measured as accuracy on pseudoword repetition.55 Sixty pre-recorded pseudowords (1–3 syllables long) were presented once via headphones, and the subject was instructed to repeat aloud what they heard. Accuracy on the first attempt was scored. A motor phonology score was computed based on a motor speech evaluation conducted by speech-language pathologists (S.F.S. and C.M.V.) with additional reference to videos of spontaneous speech, repetition, and naming. Specifically, motor phonology was calculated as the total score (0–52 points) on the Apraxia of Speech Rating Scale-3 (ASRS-3),56 which measures the presence, prominence and severity of phonetic, prosodic and other features associated with apraxia of speech. The ASRS-3 Total Score was multiplied by −1 so that a lower score indicates greater motor-phonological impairment. A patient’s overall aphasia severity was assessed through the Western Aphasia Battery—Revised.57
Neuroimaging
Image acquisition
Brain images were acquired via Georgetown’s 3T Siemens MAGNETOM Prisma scanner, including a T1-weighted magnetization prepared rapid gradient echo (MPRAGE) sequence (1 mm3 voxels), a fluid-attenuated inversion recovery (FLAIR) sequence (1 mm3 voxels), and a high angular resolution diffusion imaging (HARDI) sequence (81 directions at b = 3000, 40 at b = 1200, 7 at b = 0; 2 mm3 voxels). See Supplementary material for imaging acquisition and preprocessing details.
Lesion segmentation
Native space stroke lesions were manually traced on the MPRAGE by author P.E.T. using ITK-SNAP (http://www.itksnap.org/)58 with reference to the FLAIR image. Native space MPRAGEs and lesion masks were warped to the Clinical Toolbox Older Adult Template59 via a custom pipeline (see Dickens et al.54 for details).
Structural connectome construction
Structural connectomes were constructed from the preprocessed HARDI data through MRtrix 3.0.60 Voxelwise fibre orientation distributions were computed using multi-shell, multi-tissue constrained spherical deconvolution.61 Structural connectivity was quantified through 15 million streamlines generated by probabilistic anatomically constrained tractography62 on the white matter fibre orientation distributions in native space (algorithm = iFOD2, step = 1, min/max length = 10/300, angle = 45, backtracking allowed, dynamic seeding, streamlines cropped at grey matter-white matter interface). Edges of the structural connectome were generated by assigning streamlines to parcels of the Lausanne atlas at scale 125 (https://github.com/mattcieslak/easy_lausanne).63 Finally, connectome edge values were binarized such that a connection between brain areas were quantified as either present (1) or absent (0). See Supplementary Material for additional details on brain parcellation and connectome construction.
Statistical analyses
Behavioural analyses
Behavioural association between phonological impairments and sublexical reading impairment was determined through a logistic mixed-effects model using the ‘lme4’ function ‘glmer’64 in R.65 The model tested whether subject impairments in sensory phonology, sensory-motor integration and motor phonology modulated the effect of item lexicality on patient accuracy reading aloud. The maximal random effects structure, justified by the experimental design and the data, was first identified via backward elimination.66–68 Fitting of fixed effects was then performed through multiple regression with backward elimination in order to test all possible two-way interactions between item lexicality and subject phonological abilities (sensory phonology score, sensory-motor integration score and motor phonology score). The initial, maximal fixed effects structure specified all possible two-way interactions between item lexicality and subject phonological abilities. Stepwise removal of higher-order random effects and non-significant fixed effects was assessed through likelihood ratio tests (P > 0.05). Covariates (lesion volume, stroke chronicity, age and education) were tested for inclusion after all non-significant higher-order fixed effects were removed. To permit interpretation of fixed effects against the grand mean, continuous covariates were Z-scored, and item lexicality was deviation-contrast coded (−1/2 = ‘pseudoword’ and +1/2 = ‘word’). Results are reported as odds ratios (ORs) and associated 95% confidence intervals (CIs), with statistical significance of fixed effects determined through the Wald Z-statistic and associated P-value (α = 0.05, two-tailed). Variance inflation factors were examined to identify any problematic multicollinearity, as indicated by a variance inflation factor >10.69 Overall, the final multiple regression model identified independent relations between the three phonological scores and reading accuracy.
Voxel-based lesion-symptom mapping
To identify the brain regions subserving reading and phonological subprocesses, we conducted support-vector regression VLSM (SVR-VLSM).70 The SVR-VLSM analyses were run using a MATLAB toolbox developed by our group (https://github.com/atdemarco/svrlsmgui/).71 Analyses were limited to voxels lesioned in at least five patients. Covariates (lesion volume, age and education) were regressed out of both the lesion and behavioural data prior to modelling.71 Significance was determined using a permutation-based approach in which behavioural scores were randomly reassigned to participants. SVR β-maps were generated for each of 10 000 permutations and catalogued on a voxel-wise basis [P < 0.005, one-tailed (negative)].72 Multiple comparisons correction was achieved via a cluster size threshold [family-wise error rate (FWER) P < 0.05]. We conducted five SVR-VLSM analyses. First, we aimed to identify brain regions that are generally important for reading through SVR-VLSM of overall accuracy on the matched words and pseudowords. Next, SVR-VLSM was conducted with pseudoword reading accuracy as the dependent variable and matched word reading accuracy as a covariate. Including matched real-word reading accuracy as covariate controls for the contributions of shared processes that are not selectively important for pseudoword reading (e.g. vision, articulation). This approach thus isolates the neural substrates that selectively enable sublexical reading. The final three models identified brain regions important for each of three phonological subprocesses: the sensory phonology score, the sensory-motor integration score and the motor phonology score.
Structural connectome lesion-symptom mapping
Our SVR-CLSM approach is an extension of SVR-VLSM. SVR-CLSM quantifies lesion through anatomical disconnection and thus identifies parcel-based neuroanatomical networks, as opposed to voxel-based clusters. The SVR-CLSM analyses complement the SVR-VLSM analyses by identifying the necessary contributions of both lesioned and spared, but disconnected, brain regions to reading and phonology. Our approach differs from a prior implementation of CLSM42,73 in that we define lesioned connections on a normative basis by comparing stroke and control connectomes rather than relying on differences in streamline counts between stroke connectomes. Additionally, we do not limit analyses to connections between a sub-set of left-hemispheric regions-of-interest. Specifically, a connection between brain parcels (i.e. an edge) was considered as lesioned if it was present in 100% of control subjects’ connectomes, but absent in a stroke subject’s connectome. Lesion tracings were not used to exclude connections because individual parcels are frequently only partially lesioned by a stroke and because it is impossible to know whether a tract passing near or through areas of the lesion is functional. Connections not present in 100% of control subjects were excluded from analyses in order to reduce Type I error. The reliability of disconnection-behaviour associations was increased by limiting analyses to left-hemisphere and inter-hemispheric connections lesioned in at least 10% of stroke subjects, and by regressing lesion volume, age and education out of both the connectome edge values and behavioural data.71 Statistical significance of the SVR-CLSM β-maps was determined via a permutation-based FWER correction [10 000 permutations, FWER P < 0.05, one-tailed (negative)]72 and was evaluated at both the edge-level (disconnection between pairs of brain parcels) and parcel-level (disconnection of a single brain parcel, including all anatomical endpoints). Five SVR-CLSM analyses were conducted with the same combination of dependent variables and covariates as in the SVR-VLSM analyses.
Data availability
Data will be made available upon request.
Results
Behavioural analyses
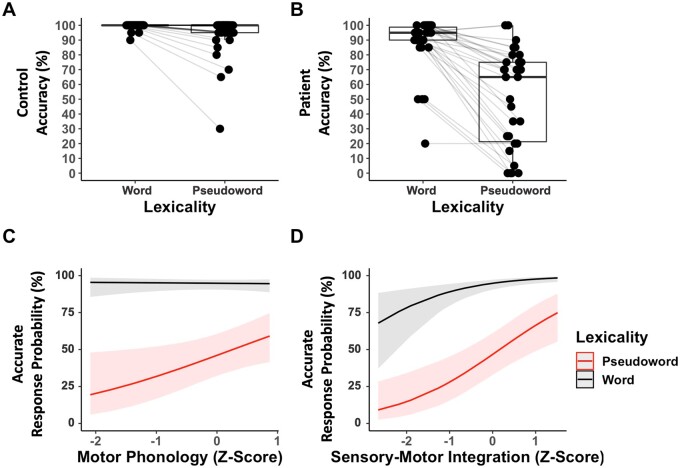
Stroke subjects varied in how item lexicality related to reading accuracy and in the degree of impairment on the phonological tasks (Table 2; Fig. 2B; see Supplementary Table 3 for correlations between the language and reading scores). If motor phonology is especially critical for sublexical reading accuracy, then greater motor-phonological impairment should selectively relate to a greater lexicality effect in reading accuracy. Consistent with this hypothesis, a logistic mixed-effects regression revealed that only the motor phonology score modulated the magnitude of the lexicality effect in reading aloud (Fig. 2C; see Supplementary Table 4 for full model estimates). Specifically, a lower motor phonology score selectively related to lower pseudoword reading accuracy [lexicality × motor phonology interaction: Z = −1.965, P = 0.049, OR = 0.51, 95% CI = 0.264–0.998]. The interactions of lexicality and the sensory phonology score and of lexicality and the sensory-motor integration score were not significant and were consequently eliminated from the model (P > 0.10). A lower sensory-motor integration score related to lower overall accuracy on the matched words and pseudowords (Fig. 2D; sensory-motor integration main effect: Z = 3.509, P < 0.001, OR = 2.25, 95% CI = 1.43 to 3.55). The sensory phonology score was not a significant predictor of reading accuracy and was therefore eliminated from the model (P > 0.10). Lesion volume was the only significant covariate (P < 0.001). Variance inflation factors for the final fitted model were all < 1.5, indicating no problematic multicollinearity.69
Table 2.
Summary of participant accuracy (%) and error proportions on reading tasks (top) and performance on language tasks (bottom).
| Group | Pseudowords | Words | |
|---|---|---|---|
| Control | |||
| Range | 30–100 | 90–100 | |
| Mean (SD) | 93.65 (13.52) | 99.46 (1.97) | |
| Orthographic error | 0.43 (0.42) | 0.67 (0.52) | |
| Non-word error | 0.57 (0.42) | 0.33 (0.52) | |
| Omission error | 0 (0) | 0 (0) | |
| Unrelated word error | 0 (0) | 0 (0) | |
| Stroke | |||
| Range | 0–100 | 20–100 | |
| Mean (SD) | 51.00 (32.97) | 87.17 (19.06) | |
| Mean Z (SD) | −3.15 (2.44) | −6.25 (9.69) | |
| Orthographic error | 0.42 (0.21) | 0.72 (0.37) | |
| Non-word error | 0.53 (0.25) | 0.19 (0.31) | |
| Omission error | 0.01 (0.06) | 0.07 (0.16) | |
| Unrelated word error | 0.03 (0.06) | 0.02 (0.07) | |
|
| |||
| Stroke | Motor Phonology Score | Sensory-Motor Integration Score | Sensory Phonology Score |
|
| |||
| Range | −26 to 0 | 0–100 | 65.00–98.75 |
| Mean (SD) | −7.30 (8.85) | 64.00 (24.00) | 88.00 (11.00) |
Z-scores are relative to control performance on the reading assessment. Motor phonology score = the Total Score on the Apraxia of Speech Rating Scale 3. Sensory-motor integration score = accuracy on pseudoword repetition. Sensory phonology score = the average of accuracies on auditory syllable counting and auditory rhyme judgment. The motor-phonology score was multiplied by −1 so that a lower score indicates greater impairment. All scores except for motor phonology are expressed as accuracy (%). Error frequencies were calculated as a proportion out of total errors for each subject, and are summarized in the table as a by-subject mean and standard deviation (excluding subjects who performed at ceiling). Four incorrect patient responses on the reading task were unable to be fully transcribed and categorized due to lack of speech intelligibility.
Figure 2.
Relationship between patient phonological abilities and reading accuracy. (A) Control accuracy reading aloud the matched words and pseudowords. (B) Patient accuracy reading aloud the matched words and pseudowords. Grey lines connect paired observations within a subject. (C) A logistic mixed-effects model of patient accuracy reading aloud the matched pseudowords and words (30 subjects, 40 items, 1200 observations) revealed that lower motor-phonological processing scores (ASRS-3 Total Score) selectively related to inaccurate pseudoword reading [lexicality × motor phonology interaction: Z = −1.965, P = 0.049, odds ratio (OR) = 0.51, 95% confidence interval (CI) = 0.264–0.998]. Covariates tested for inclusion: lesion volume, stroke chronicity, age and education. (D) The same logistic mixed-effects model revealed that lower sensory-motor integration scores related to lower overall accuracy reading aloud the matched pseudowords and words (sensory-motor integration main effect: Z = 3.509, P < 0.001, OR = 2.25, 95% CI = 1.43–3.55). The sensory-phonological processing score did not independently relate to patient reading accuracy. See Supplementary Table 4 for logistic mixed model estimates.
Correlational analyses of z-scored error type frequencies were conducted to clarify how impairments in motor phonology and sensory-motor integration drive inaccurate reading aloud. Only orthographic and non-word errors were frequent enough for analysis (Table 2). Consistent with the interaction identified by the mixed-effects model, greater motor phonological impairment correlated with a higher incidence of orthographically related word errors on pseudowords [r(28) = −0.49, P = 0.006], but not on words (P = 0.106). Motor phonological impairment did not correlate with the incidence of non-word errors on either pseudowords (P = 0.965) or words (P = 0.337). Consistent with the main effect identified by the mixed-effects model, greater sensory-motor integration impairment correlated with a higher incidence of orthographically related word errors on both pseudowords [r(28) = −0.62, P < 0.001] and words [r(28) = −0.61, P < 0.001]. Additionally, greater sensory-motor integration impairment correlated with more non-word errors on words [r(28) = −0.42, P = 0.020], but not on pseudowords (P = 0.269).
Lesion-symptom mapping analyses
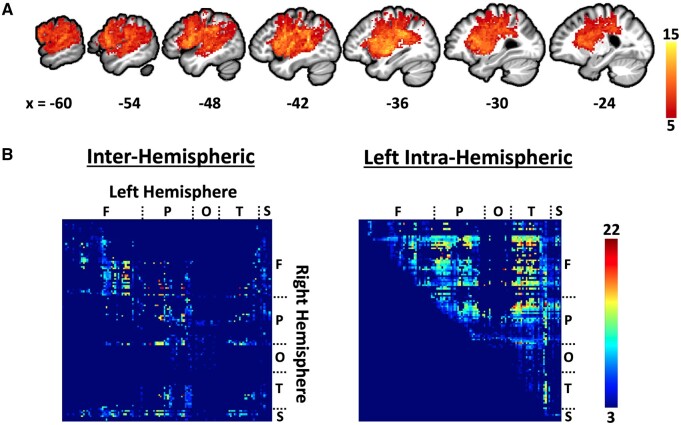
Voxel-wise overlap of the patients’ lesions demonstrates predominantly perisylvian lesion coverage typical of left middle cerebral artery strokes (Fig. 3A). The structural connectome lesion overlap map reveals the expected loss of interhemispheric connections in addition to the loss of connections within the left hemisphere (Fig. 3B).
Figure 3.
Lesion overlap maps. (A) Voxel-wise lesion overlap map with x-axis MNI coordinates of sagittal slices. (B) Edge-wise lesion overlap within the structural connectome after excluding edges not present in 100% of controls. An edge consists of the connection between two parcels of the Lausanne atlas scale 12562 and is represented by a cell within the matrices at the location at which the parcels intersect on the x and y axes. Each row and column within the matrices correspond to a parcel (brain region) of Lausanne atlas scale 125. An edge (i.e. connection) was classified as lesioned if it was present in 100% of control subjects, but missing in the patient. Parcel locations are labeled as follows: F = Frontal, P = Parietal, O = Occipital, T = Temporal, S = Subcortical. Only inter-hemispheric connections and left intra-hemispheric connections were considered in SVR-CLSM analyses. Only the upper right half of the left intra-hemispheric connectome is shown because the matrix is symmetrical along the diagonal.
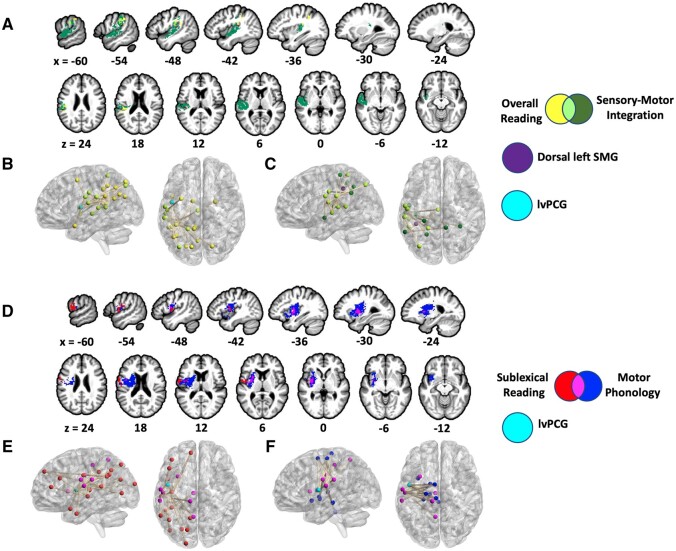
We conducted SVR-VLSM and SVR-CLSM analyses to identify the anatomical networks that subserve reading accuracy and phonological subprocesses. Lesions involving left supramarginal gyrus (SMG), parietal operculum, and intraparietal sulcus related to reduced overall reading accuracy (both words and pseudowords), but this result did not reach cluster-wise significance (SVR-VLSM cluster-wise P = 0.056; cluster size: 3343 mm3; centre-of-mass MNI coordinates: −47.1, −35.4, 30.9; Fig. 4A). Sixteen disconnections primarily involving left posterior perisylvian cortex related to lower overall reading accuracy, with three disconnections involving lvPCG (SVR-CLSM edge-wise FWER P < 0.05; Fig. 4B; Table 3). Consistent with the behavioural association between a lower sensory-motor integration score and lower overall reading accuracy, SVR-VLSM and SVR-CLSM of the sensory-motor integration score yielded significant results that overlap with the results for overall reading accuracy. Specifically, lesions involving left supramarginal gyrus, parietal operculum, superior temporal gyrus (STG), and ventral postcentral gyrus related to a lower sensory-motor integration score (SVR-VLSM cluster-wise P = 0.003; cluster size: 17 172 mm3; centre-of-mass: −51.1, −20.2, 9.5; Fig. 4A), overlapping the SVR-VLSM cluster for overall reading accuracy in SMG and parietal operculum. A lower sensory-motor integration score related to disconnections of dorsal SMG regardless of anatomical endpoint (SVR-CLSM parcel-wise P < 0.05) and 16 disconnections involving posterior temporal and/or inferior parietal cortex (SVR-CLSM edge-wise P < 0.05), with no frontal involvement (Fig. 4C; Table 3). Brain regions implicated in the SVR-CLSM analyses of both overall reading accuracy and sensory-motor integration score include anterior SMG, mid-posterior STG, planum temporale, ventral postcentral gyrus, parietal operculum, angular gyrus, dorsal superior temporal sulcus, posterior cingulate, and superior parietal cortex. Edge-wise overlap between the SVR-CLSM results for overall reading accuracy and sensory-motor integration included the disconnection between anterior SMG and angular gyrus. Overall, these results revealed that lesioned or disconnected temporoparietal cortex relates to both reduced overall reading accuracy and a reduced sensory-motor integration score.
Figure 4.
Results of SVR-VLSM and SVR-CLSM analyses. (A) SVR-VLSM results (voxel-wise P < 0.005, cluster-wise FWER P < 0.05) for both overall reading accuracy (yellow; cluster-wise P = 0.056) and sensory-motor integration score (dark green; cluster-wise P = 0.003). (B) SVR-CLSM results (edge-wise FWER P < 0.05) for overall reading accuracy (yellow). (C) SVR-CLSM results (edge-wise FWER P < 0.05) for sensory-motor integration score (dark green). Disconnected dorsal SMG (purple) related to worse sensory-motor integration ability regardless of anatomical endpoint (parcel-wise FWER P < 0.05). (D) SVR-VLSM results (voxel-wise P < 0.005, cluster-wise FWER P < 0.05) for both sublexical reading accuracy (red; cluster-wise P = 0.030) and motor phonology score (blue; cluster-wise P = 0.0004). (E) SVR-CLSM results (edge-wise FWER P < 0.05) for sublexical reading accuracy (red). (F) SVR-CLSM results (edge-wise FWER P < 0.05) for motor phonology score (blue). For B, E, and F, lvPCG is marked in cyan for visualization. B, C, E, and F each display a left sagittal and dorsal view of the brain. Lesion size, age, and education were regressed out of the behavioural and lesion/connectome data in all analyses. SVR-VLSM and SVR-CLSM results were visualized with Mango (http://ric.uthscsa.edu/mango/index.html) and BrainNet,73 respectively.
Table 3.
SVR-CLSM results for overall reading accuracy and sensory-motor integration (Edge-wise FWER P < 0.05).
| Task | Edge | SVR-β | MN1 1 | MNI 2 |
|---|---|---|---|---|
| Overall Reading Accuracy | Left ventral postcentral gyrus/parietal operculum <> Left angular gyrus | 9.65 | −54, −14, 16 | −39, −56, 23 |
| Left ventral postcentral gyrus/parietal operculum <> Left precuneus | 9.41 | −54, −14, 16 | −5, −64, 30 | |
| Left ventral postcentral gyrus <> Left angular gyrus | 9.41 | −58, −9, 26 | −39, −56, 23 | |
| Left posterior cingulate <> Left dorsal superior temporal sulcus | 9.41 | −5, −15, 33 | −52, −42, 9 | |
| Left superior parietal cortex <> Left planum temporale | 9.28 | −21, −45, 60 | −52, −30, 8 | |
| Left ventral postcentral gyrus <> Left anterior STG a | 9.27 | −58, −9, 26 | −46, 8, −21 | |
| Left anterior SMG <> Left angular gyrus b | 9.18 | −59, −27, 26 | −35, −74, 44 | |
| Left ventral precentral gyrus/frontal operculum <> Left superior parietal cortex a | 9.16 | −47, −2, 9 | −15, −74, 50 | |
| Left angular gyrus <> Left caudate | 8.96 | −44, −58, 41 | −13, 0, 9 | |
| Left ventral precentral gyrus/frontal operculum <> Left intraparietal sulcus a | 8.89 | −47, −2, 9 | −24, −73, 26 | |
| Left isthmus cingulate <> left anterior SMG | 8.73 | −9, −42, 16 | −59, −27, 26 | |
| Left ventral precentral gyrus/frontal operculum <> Left angular gyrus a | 8.67 | −47, −2, 9 | −44, −58, 41 | |
| Left dorsal SMG <> Left precuneus | 8.48 | −40, −38, 38 | −11, −45, 44 | |
| Right putamen <> Left posterior SMG | 8.07 | 23, −1, −2 | −47, −41, 27 | |
| Left caudal middle frontal cortex <> Left ventral postcentral gyrus/parietal operculum | 7.96 | −32, 4, 53 | −54, −14, 16 | |
| Right superior parietal cortex <> Left mid-posterior STG | 7.66 | 18, −73, 47 | −57, −20, −1 | |
|
| ||||
| Sensory-Motor Integration Score | Left ventral postcentral gyrus/parietal operculum <> Left mid-posterior STG | 10 | −54, −14, 16 | −57, −20, −1 |
| Left ventral postcentral gyrus <> Left mid-anterior STG | 9.55 | −58, −9, 26 | −51, −8, −9 | |
| Left superior parietal cortex <> Left mid-posterior STG | 9.38 | −21, −45, 60 | −57, −20, −1 | |
| Left anterior SMG <> Left mid-posterior STG | 9.29 | −59, −27, 26 | −57, −20, −1 | |
| Left ventral postcentral gyrus <> Left mid-posterior STG | 9.24 | −58, −9, 26 | −57, −20, −1 | |
| Left posterior cingulate <> Left angular gyrus | 9.21 | −5, −15, 33 | −58, −51, 31 | |
| Right precuneus <> Left angular gyrus | 8.97 | 8, −44, 49 | −58, −51, 31 | |
| Left dorsomedial postcentral gyrus <> Left mid-posterior STG | 8.90 | −27, −33, 60 | −57, −20, −1 | |
| Left ventral postcentral gyrus/parietal operculum <> Left intraparietal sulcus | 8.74 | −54, −14, 16 | −23, −55, 41 | |
| Left anterior SMG <> Left dorsal superior temporal sulcus | 8.74 | −59, −27, 26 | −52, −42, 9 | |
| Left anterior SMG <> Left planum temporale | 8.74 | −59, −27, 26 | −52, −30, 8 | |
| Left anterior SMG <> Left Heschl’s gyri | 8.74 | −59, −27, 26 | −41, −23, 9 | |
| Right superior parietal cortex <> Left mid-posterior STG | 8.63 | 31, −42, 47 | −57, −20, −1 | |
| Left anterior SMG <> Left angular gyrus b | 8.63 | −59, −27, 26 | −58, −51, 31 | |
| Left posterior cingulate <> Left mid-posterior STG | 8.57 | −5, −15, 33 | −57, −20, −1 | |
| Left anterior SMG <> Left angular gyrus | 8.34 | −59, −27, 26 | −35, −74, 44 | |
SVR-β = support vector regression beta coefficient. MNI 1 and 2 represent the Montreal Neurologic Institute coordinates for parcel centroids to the left and right of the ‘<>’. P = 0.0001 for all edges. An edge consists of the connection between two Lausanne Atlas Scale 125 parcels.
Edge that is also significant in the analysis of sublexical reading accuracy (see Table 4).
Edge that is significant for both tasks.
As predicted by the differentiated phonological reading hypothesis, SVR-VLSM and SVR-CLSM confirmed that lesions and disconnections involving lvPCG related to both reduced sublexical reading accuracy and a reduced motor-phonology score (Fig. 4D). SVR-VLSM replicated the association between lesioned lvPCG and reduced pseudoword reading accuracy relative to word reading accuracy (see Supplementary Fig. 1 for comparison with a cluster from Dickens et al.54). The identified cluster centres on the left frontal operculum (MNI centre of mass: −43.9, −2.6, 7.5) and spans lvPCG, ventral postcentral gyrus, insula (superior precentral gyrus and inferior long anterior gyrus), extreme capsule, putamen, and the internal capsule (cluster-wise P = 0.030; cluster size: 4734 mm3). A lower motor phonology score related to a more extensive cluster centred at the extreme capsule (MNI centre of mass: −32.5, −2.2, 8.2) and spanning lvPCG/frontal operculum, ventral postcentral gyrus, posterior and anterior insula, putamen, caudate, and subcortical white matter (SVR-VLSM cluster-wise P = 0.0004; cluster size: 20 672 mm3; Fig. 4D). Voxel-wise overlap between the clusters for sublexical reading accuracy and motor phonology score included lvPCG/frontal operculum, insula (superior precentral gyrus and inferior long anterior gyrus), subcortical white matter, and putamen. Lower pseudoword reading accuracy relative to word reading accuracy related to twenty-two disconnections primarily involving left dorsal perisylvian cortex (SVR-CLSM edge-wise FWER P < 0.05; Table 4; Fig. 4E). Consistent with the SVR-VLSM results, seven of these twenty-two edges connected lvPCG and posterior regions, including SMG, angular gyrus, intraparietal sulcus, superior parietal cortex, precuneus, and lateral occipital cortex. Notably, these results revealed that the three lvPCG disconnections identified in the analysis of overall reading accuracy (Table 3) relate to lower pseudoword reading accuracy relative to word reading accuracy, thus confirming the preferential association between disconnected lvPCG and less accurate sublexical reading. A lower motor phonology score related to twenty left intra-hemispheric disconnections involving the frontal cortex, parietal cortex, insula, and deep structures, with five disconnections involving lvPCG (Fig. 4F; Table 4). In addition to lvPCG, brain regions implicated in the SVR-CLSM analyses of both sublexical reading and motor phonology include dorsal anterior insula, anterior SMG, ventral postcentral gyrus, parietal operculum, paracentral lobule, posterior cingulate, and precuneus. There was no edge-wise overlap between the SVR-CLSM results of sublexical reading and motor phonology (i.e. the exact connections were different). Overall, these SVR-VLSM and SVR-CLSM results confirm that lesions and disconnections of lvPCG preferentially relate to both reduced sublexical reading accuracy and a reduced motor phonology score, as predicted by the differentiated phonological reading hypothesis.
Table 4.
SVR-CLSM results for sublexical reading and motor phonology (Edge-wise FWER P < 0.05)
| Task | Edge | SVR-β | MNI 1 | MNI 1 |
|---|---|---|---|---|
| Sublexical Reading Accuracy | Left superior frontal gyrus <> Left angular gyrus | 10 | −9, 50, 39 | −58, −51, 31 |
| Left ventral postcentral gyrus/parietal operculum <> Left lateral occipital cortex | 9.57 | −54, −14, 16 | −43, −75, 4 | |
| Left ventral postcentral gyrus <> Left anterior middle temporal gyrus | 9.42 | −58, −9, 26 | −53, −2, −29 | |
| Left paracentral lobule <> Left mid-posterior STG | 9.34 | −10, −28, 49 | −57, −20, −1 | |
| Left rostral middle frontal gyrus <> Left mid-posterior STG | 9.31 | −44, 25, 28 | −57, −20, −1 | |
| Left rostral middle frontal gyrus <> Left mid-posterior STG | 9.31 | −40, 39, 14 | −57, −20, −1 | |
| Left ventral precentral gyrus/frontal operculum <> Left intraparietal sulcus a | 9.25 | −47, −2, 9 | −24, −73, 26 | |
| Left ventral precentral gyrus/frontal operculum <> Left angular gyrus a | 9.18 | −47, −2, 9 | −44, −58, 41 | |
| Left ventral precentral gyrus/frontal operculum <> Left superior parietal cortex a | 9.06 | −47, −2, 9 | −15, −74, 50 | |
| Left primary motor cortex <> Left Heschl’s gyri | 9.04 | −40, −13, 33 | −41, −23, 9 | |
| Left anterior SMG <> Left angular gyrus | 8.91 | −59, −27, 26 | −58, −51, 31 | |
| Left ventral precentral gyrus/frontal operculum <> Left precuneus | 8.89 | −47, −2, 9 | −5, −53, 58 | |
| Left ventral postcentral gyrus/parietal operculum <> Left precuneus | 8.82 | −54, −14, 16 | −10, −55, 34 | |
| Left ventral precentral gyrus/frontal operculum <> Left dorsal SMG | 8.78 | −47, −2, 9 | −40, −38, 38 | |
| Left ventral precentral gyrus/frontal operculum <> Left anterior SMG | 8.76 | −47, −2, 9 | −59, −27, 26 | |
| Left ventral precentral gyrus/frontal operculum <> Left lateral occipital cortex | 8.74 | −47, −2, 9 | −17, −96, 19 | |
| Left primary motor cortex <> Left mid-posterior STG | 8.67 | −40, −13, 33 | −57, −20, −1 | |
| Left posterior STG <> Left dorsal anterior insula | 8.49 | −50, −42, 15 | −30, 12, 7 | |
| Left posterior cingulate cortex <> Left angular gyrus | 8.48 | −5, −15, 33 | −58, −51, 31 | |
| Left ventral postcentral gyrus <> Left Heschl's gyri | 8.46 | −58, −9, 26 | −41, −23, 9 | |
| Left ventral postcentral gyrus <> Left anterior STG a | 8.44 | −58, −9, 26 | −46, 8, −21 | |
| Left superior frontal gyrus <> Left posterior STG | 8.41 | −9, 50, 39 | −50, −42, 15 | |
|
| ||||
| Motor Phonology Score | Left superior frontal gyrus <> Left ventral precentral gyrus/frontal operculum | 10 | −47, −2, 9 | −4, −23, 68 |
| Left dorsomedial precentral gyrus <> Left ventral precentral gyrus/frontal operculum | 10 | −47, −2, 9 | −10, −28, 49 | |
| Left ventral precentral gyrus/frontal operculum <> Left paracentral lobule | 9.35 | −6, −1, 60 | −54, −14, 16 | |
| Left ventral precentral gyrus/frontal operculum <> Left paracentral lobule | 9.35 | −6, −1, 60 | −59, −27, 26 | |
| Left ventral precentral gyrus/frontal operculum <> Left posterior cingulate | 9.31 | −58, −9, 26 | −14, −17, 6 | |
| Left superior frontal gyrus <> Left ventral postcentral gyrus/parietal operculum | 9.31 | −58, −9, 26 | −23, −3, −2 | |
| Left dorsomedial precentral gyrus <> Left ventral postcentral gyrus/parietal operculum | 9.31 | −58, −9, 26 | −19, −4, −5 | |
| Left paracentral lobule <> Left ventral postcentral gyrus/parietal operculum | 9.31 | −54, −14, 16 | −24, −25, −11 | |
| Left paracentral lobule <> Left ventral postcentral gyrus/parietal operculum | 9.31 | −58, −9, 26 | 0, −29, −26 | |
| Left superior frontal gyrus <> Left anterior SMG | 8.95 | −54, −14, 16 | −13, 0, 9 | |
| Left ventral postcentral gyrus/parietal operculum <> Left precuneus | 8.95 | −59, −27, 26 | −13, 0, 9 | |
| Left ventral anterior insula <> Left dorsal anterior insula | 8.95 | −58, −9, 26 | −24, −25, −11 | |
| Left ventral postcentral gyrus <> Left thalamus | 8.88 | −47, −2, 9 | −5, −15, 33 | |
| Left ventral postcentral gyrus/parietal operculum <> Left caudate | 8.79 | −6, −1, 60 | −47, −2, 9 | |
| Left anterior SMG <> Left caudate | 8.53 | −33, 9, −8 | −30, 12, 7 | |
| Left ventral postcentral gyrus <> Left putamen | 8.36 | −20, −16, 60 | −47, −2, 9 | |
| Left ventral postcentral gyrus <> Left pallidum | 8.01 | −20, −16, 60 | −54, −14, 16 | |
| Left ventral postcentral gyrus <> Left hippocampus | 8.01 | −4, −23, 68 | −54, −14, 16 | |
| Left ventral postcentral gyrus/parietal operculum <> Left hippocampus | 8.01 | −10, −28, 49 | −54, −14, 16 | |
| Left ventral postcentral gyrus <> Left brainstem | 8.01 | −54, −14, 16 | −5, −53, 58 | |
SVR-β = support vector regression beta coefficient. MNI 1 and 2 represent the Montreal Neurologic Institute coordinates for parcel centroids to the left and right of the ‘<>’. P = 0.0001 for all edges. An edge consists of the connection between two Lausanne Atlas Scale 125 parcels.
Edge that is also significant in the analysis of overall reading accuracy (see Table 3).
SVR-VLSM found that a lower sensory phonology score related to a cluster spanning medial postcentral gyrus, superior parietal cortex, and intraparietal sulcus (cluster-wise P = 0.034; cluster size: 4906 mm3; MNI centre-of-mass: −31.7, −36.6, 46; Supplementary Fig. 2). Consistent with the lack of a behavioural association in the logistic mixed-effects model of reading accuracy (Fig. 2), this cluster did not overlap with the clusters for either overall reading accuracy or sublexical reading accuracy. SVR-CLSM did not reveal any significant association between the sensory phonology score and structural disconnections.
Discussion
The primary aim of this study was to isolate the contributions of phonological subprocesses to reading. In support of the differentiated phonological reading hypothesis, our mixed-effects regression and multivariate lesion-symptom mapping analyses isolated differential contributions of phonological subprocesses to reading. Our results provide evidence for two types of phonological reading impairment in stroke aphasia. Lesions to and structural disconnections of the left temporoparietal cortex result in a sensory-motor integration deficit that impairs the reading aloud of both words and pseudowords. In contrast, lesions to and structural disconnections of lvPCG result in a motor-phonological deficit that preferentially impairs sublexical reading. Our results clarify the neuroanatomical bases of phonological processing in reading and language and motivate the development of an integrated cognitive model of print and speech processing in which an orthographic processing system interacts with a sensory-motor phonological circuit (Fig. 1C).
Sensory-motor integration in reading
Sensory-motor integration in speech processing involves the translation from auditory input representations to motor output representations. Impaired sensory-motor integration thus results in impaired speech repetition, which is a hallmark symptom of conduction aphasia.44,75 The relative importance of specific cortex and white matter connections to sensory-motor integration is unsettled (see Ardila76 and Berthier et al.77 for reviews). According to the classical view,78,79 which persists today (e.g. see Jones et al.80), sensory-motor integration is enabled by the arcuate fasciculus, which connects auditory representations in the posterior superior temporal cortex to motor representations in the frontal cortex. This classical account has been under increasing scrutiny over the last several decades, with researchers arguing that lesioned posterior perisylvian cortex accounts for conduction aphasia.44,81,82 Moreover, contemporary dual-stream models of speech processing attribute sensory-motor integration to a patch of temporoparietal cortex, typically either left posterior planum temporale6,33 or left SMG.34,35 Our SVR-VLSM and SVR-CLSM results for pseudoword repetition support left superior temporal (particularly mid-posterior STG) and inferior parietal (particularly ventral postcentral gyrus and SMG) cortex and connections as critical substrates for sensory-motor integration in speech processing. Within models of speech motor control,6,83 accurate speech production depends on auditory and somatosensory targets provided by STG and ventral postcentral gyrus, respectively. Intact mid-posterior STG and ventral postcentral gyrus may thus enable the interaction of auditory and somatosensory processing prior to and during speech repetition, with SMG enabling integration with motor processing. Our SVR-CLSM results also suggest that interactions between the left angular gyrus and other components of the default mode network84 (left posterior cingulate cortex and right precuneus) may support accurate sensory-motor integration, perhaps by contributing to speech action-perception awareness.85 Thus, in contrast to the classical account, our findings suggest that sensory-motor integration depends on intact cortical processes not mediated by white matter projections to the frontal cortex. Our SVR-CLSM results do not rule out the necessity of other regions for intact sensory-motor integration. Indeed, sensory-motor integration is a computation that likely involves dynamic, parallel interactions across many cortical zones.86,87 However, our results indicate that left temporoparietal circuits are required for intact sensory-motor integration.
Pseudoword repetition accuracy has frequently served as an index of the general phonological impairment in phonological alexia cases.20,88 Pseudoword repetition differs from other phonological tasks (e.g. rhyme judgment) in that it emphasizes the transformation of input phonology into output phonology (i.e. sensory-motor integration). Our results suggest that impaired sensory-motor integration does not selectively relate to inaccurate sublexical reading. Specifically, our mixed-effects regression analysis (Fig. 2), which controlled for sensory- and motor-phonological processing, demonstrated a relationship between inaccurate pseudoword repetition and inaccurate reading aloud regardless of item lexicality. In line with this behavioural association, our SVR-VLSM and SVR-CLSM results suggest that sensory-motor integration and reading rely on shared neural substrates. Specifically, reduced pseudoword repetition accuracy and overall reading accuracy were both related to lesioned or disconnected superior temporal and inferior parietal cortex. The exact patterns of temporoparietal disconnections associated with inaccurate reading and speech repetition were largely different, which may reflect differing task demands. Unlike pseudoword repetition, reading requires the subject to generate the phonological code from print. It is thus unsurprising that reduced overall reading accuracy related to disconnections of cortex that support phonological processing (STG, SMG, lvPCG, and ventral postcentral gyrus), visual processing (lateral occipital cortex), and multi-modal or executive processing (angular gyrus and prefrontal cortex). Overall, our results demonstrate that impaired sensory-motor integration and alexia are linked, but impaired sensory-motor integration leads to a general reading impairment, not to a selective sublexical reading impairment.
We propose that temporoparietal circuits provide the sensory-motor integration mechanisms and sensory targets necessary for phonological contributions to both reading and speech processing. Prior lesion studies have consistently implicated lesioned superior temporal and inferior parietal cortex as causing alexia14,16,22,30,31,54,89 and impaired speech repetition.90–92 Notably, a disturbance in reading aloud is frequently evident in cases of conduction aphasia.75 Moreover, fMRI evidence suggests that print and speech processing converge in the left superior temporal cortex93–95 and that SMG supports both spelling-to-sound translation96,97 and non-orthographic phonological processing.98,98 Developmental dyslexia has been associated with structural and functional differences in the temporoparietal cortex.99 Together with this extant literature, our finding that impaired sensory-motor integration relates to reduced overall reading accuracy suggests that all words, whether known or novel, rely on shared sensory-motor integration processes and sensory-phonological targets for accurate phonological processing during reading. Consistent with this interpretation, lower accuracy on pseudoword repetition correlated with more orthographically related word errors on both pseudowords and words, as well as more non-word neologisms on words. Thus, impaired sensory-motor integration correlates with both greater reliance on spared knowledge of known phonological forms as well as disrupted computation of known phonological forms. This result is expected in the context of a motor-phonological system operating without access to a full repertoire of intact sensory-phonological targets. Disruption of this temporoparietal circuit, therefore, does not result in a selective pseudoword reading deficit in reading aloud. Rather, the pattern of reading performance in the context of a sensory-motor integration deficit reflects pre-morbid task difficulty. Pseudowords are harder to read than words, as evidenced by the fact that even our control subjects usually demonstrated a measurable lexicality effect in reading aloud (Table 2). A lexicality effect may therefore be evident in patients despite there being no selective impairment of pseudoword reading. The contribution of spared semantic knowledge to the computation of phonological codes is unlikely to compensate for the sensory-motor integration impairment, at least in the context of speeded reading aloud. Indeed, reading aloud is arguably a predominantly phonological task,101 given that the mapping from print to sound is more systematic than the mapping from print to meaning. The lack of an independent relationship between the sensory phonology score and reading accuracy may reflect the nature of the tasks used to measure sensory phonology. Impaired auditory rhyme judgement and syllable counting may have been related to a working memory deficit rather than a linguistic deficit in our sample, as evidenced by the association between lesioned superior parietal cortex and a lower sensory-phonology score. However, our SVR-CLSM analyses implicated disconnected superior temporal gyrus, a region that supports sensory-phonological processing,6,36 with inaccurate pseudoword repetition and reading aloud. Overall, we suggest that damage to temporoparietal networks results in a sensory-motor integration deficit that impairs speech production and reading aloud of both words and pseudowords.
Motor-phonological processing in reading
Motor-phonological processing relies on abstract motor speech programmes that ensure fluent and accurate speech articulation. Apraxia of speech is a disorder of speech motor programming with unclear neural bases (see Miller and Guenther46 and Ogar et al.102 for reviews). Neural correlates of apraxia of speech include lesions involving the anterior insula,47,48 the basal ganglia,103 inferior frontal gyrus,51 precentral gyrus,49,52,104 and parietal operculum.49 Our SVR-VLSM and SVR-CLSM results suggest that impaired speech motor programming results from disruption of a distributed network of the frontal cortex, parietal cortex, posterior/anterior insula, and subcortical structures. Notably, for the goals of the present study, disconnections involving lvPCG were implicated as participating in the network underlying motor speech impairment. This result supports the notion that lvPCG is a neural seat for motor-phonological programmes.6,46 Additionally, our finding of a role for somatosensory cortex in motor-phonological processing, instead of auditory cortex, supports somatosensory targets being particularly critical for the fluent articulation of phonemes, as proposed by the Hierarchical State Feedback Control model.6 Overall, we suggest that impaired speech motor programming results from disruptions of both perisylvian phonological circuits and subcortical motor circuits and is thus not reducible to a single lesion site. Importantly, our results speak to the neural bases of impaired speech motor programming, rather than syndromic cases of apraxia of speech. While chronic post-stroke apraxia of speech seems to be associated with large frontal lesions suggestive of non-focal network disruption,50 different patterns of symptoms likely result from damage to different components of cortical and subcortical networks.56
Our behavioural and lesion-symptom mapping results provide strong evidence that a motor-phonological deficit leads to a preferential impairment of sublexical reading. Mixed-effects regression confirmed our prediction that greater motor-phonological impairment relates to less accurate reading of pseudowords relative to words. Lesions to and disconnections of lvPCG related to both motor-phonological impairment and inaccurate pseudoword reading relative to word reading, thus demonstrating a neural link. These results align with our previous SVR-VLSM study, which found that lesions involving lvPCG preferentially impaired pseudoword reading relative to word reading.54 The consistent shared involvement of lvPCG in both sublexical reading and motor-phonological processing across our SVR-VLSM and SVR-CLSM analyses suggests that lesioned and/or disconnected lvPCG drives the relationship between inaccurate sublexical reading and speech motor programming deficits. Consistent with this conclusion, neuroimaging and lesion evidence supports the role of lvPCG in both language and motor speech processing.45,46,99 Our results also implicate lesioned or disconnected mid/anterior insula as causing inaccurate sublexical reading, which could relate to impaired speech motor planning.47 Overall, these results demonstrate that motor-phonological impairment and sublexical reading impairment are behaviourally and neuroanatomically linked.
We propose that lvPCG provides motor-phonological targets that are important for both sublexical reading and motor speech programming. Pseudowords, given that they have never been seen, heard, or pronounced before, lack support from long-term orthographic, auditory, and motor memory. Disruption of motor-phonological programmes may thus impair the normal sensory-motor interactions that enable the online construction of a plausible, novel phonological code. Without a motor target, any phonological code constructed based on orthographic input is less likely to be translated into plausible, novel phonological output. The fact that the relationship between motor-phonological impairment and pseudoword reading impairment was driven by real word orthographic errors indicates that the loss of motor speech programmes demands greater reliance on spared lexical knowledge in reading aloud. The relative preservation of word reading may also reflect that known word motor programmes are over-learned in partially redundant cortical and subcortical motor networks.46 Consistent with this proposal, people with apraxia of speech often articulate low-frequency syllables less accurately than high-frequency syllables.104 The fact that the motor phonology score did not relate to overall reading accuracy confirms that the underlying deficit is not a low-level motor deficit, particularly dysarthria. Specifically, dysarthric speech results from motor weakness or paralysis and therefore would affect the reading aloud of all letter strings whose pronunciation involves the dysarthric articulators, regardless of lexicality. Overall, we propose that intact motor-phonological programmes are partially responsible for accurate sublexical reading.
Alternative interpretations
Our results demonstrate that phonological contributions to reading and speech processing are behaviourally and neuroanatomically linked. However, it should be noted that our results cannot rule out co-localization of separable neural substrates or computations for phonological processing in reading and language. Indeed, some researchers assert that sublexical reading is accomplished through a reading-specific computation (e.g. see Coltheart et al.9). Our finding of two types of dissociable phonological contributions to reading, however, supports the idea that reading and language rely on shared sensory-motor circuits, at a minimum.
The extent to which the results of our analyses of reading aloud are generalizable to silent reading is an open question. Relative to silent reading, reading aloud emphasizes the computation of a complete and accurate phonological code. However, phonological processing certainly occurs in silent reading.106 Moreover, fMRI evidence indicates that silent and oral reading depend on a shared neurocognitive architecture,95,107 with differences in activation reflecting task demands.108 A recent eye-tracking study found a correlation between ocular dynamics in silent reading of infrequent letter strings and articulatory dynamics in reading aloud the same letter strings, suggesting the involvement of motor representations in the silent reading of unfamiliar letter strings.109 Future research should examine the relationship between impaired motor-phonological processing and performance on reading tasks that do not require overt speech production. It is also notable that we contrasted performance reading pronounceable pseudowords versus regular words. While this contrast directly isolates the lexicality effect without introducing difficulties due to phonotactic violations or knowledge of irregular words, future research should determine the extent to which our findings are generalizable to other letter strings.
Conclusion
Phonological processing is a fundamental language component that contributes to reading.26,100,101 Contemporary accounts of reading and alexia refer to phonological processing in only the broadest of terms.22,26 In contrast, neurocognitive models of speech processing explicitly appreciate that phonological processing is not monolithic.6,33,34 By addressing this incongruity between models of reading and speech processing, we identified dissociable contributions of sensory-motor integration and motor-phonological processing to reading, as predicted by our differentiated phonological reading hypothesis. These results provide evidence for a more nuanced account of the neurocognitive bases and behavioural symptoms of phonological reading impairment in aphasia. Namely, at least two types of phonological impairment account for variation in reading abilities in stroke aphasia. Patients with lesions affecting the left temporoparietal cortex and its structural connections can be expected to demonstrate a sensory-motor integration deficit that disturbs the reading aloud of words and pseudowords. In contrast, patients with left frontal lesions affecting the ventral premotor cortex and its structural connections can be expected to demonstrate a motor-phonological impairment that preferentially disturbs sublexical reading. These findings underscore how integrating models of speech processing and reading can reveal insights into the shared computational architecture underlying reading and language.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
We thank our participants for their dedication to research on the neurobiology of reading and language.
Funding
This work was supported by the United States of America’s National Institute on Deafness and Other Communication Disorders grants F30DC018215 (J.V.D.) and R01DC014960 (P.E.T.), National Institute of Neurological Disorders and Stroke grant U10NS086513 (A.T.D.), and Eunice Kennedy Shriver National Institute of Child Health and Human Development grant K12HD093427 (A.T.D.).
Competing interests
The authors report no competing interests.
Glossary
- ASRS-3
Apraxia of Speech Rating Scale-3
- CI
confidence interval
- FLAIR
fluid-attenuated inversion recovery
- HARDI
high angular resolution diffusion imaging
- lvPCG
left ventral precentral gyrus
- MNI
Montreal Neurological Institute
- MPRAGE
magnetization prepared rapid gradient echo
- OR
odds ratio
- SMG
supramarginal gyrus
- STG
superior temporal gyrus
- SVR-CLSM
support vector regression structural connectome lesion-symptom mapping
- SVR-VLSM
support vector regression voxel-based lesion-symptom mapping
References
- 1.Brookshire CE, Wilson JP, Nadeau SE, Gonzalez Rothi LJ, Kendall DL.. Frequency, nature, and predictors of alexia in a convenience sample of individuals with chronic aphasia. Aphasiology. 2014;28(12):1464–1480. [Google Scholar]
- 2.Webb WG, Love RJ.. Reading problems in chronic aphasia. J Speech Hear Disord. 1983;48(2):164–171. [DOI] [PubMed] [Google Scholar]
- 3.Plaut DC, McClelland JL, Seidenberg MS, Patterson K.. Understanding normal and impaired word reading: Computational principles in quasi-regular domains. Psychol Rev. 1996;103(1):56–115. [DOI] [PubMed] [Google Scholar]
- 4.Plaut DC.Structure and function in the lexical system: Insights from distributed models of word reading and lexical decision. Lang Cogn Process. 1997;12(5-6):765–806. [Google Scholar]
- 5.Walker GM, Hickok G.. Bridging computational approaches to speech production: The semantic–lexical–auditory–motor model (SLAM). Psychon Bull Rev. 2016;23(2):339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hickok G.Computational neuroanatomy of speech production. Nat Rev Neurosci. 2012;13(2):135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harm MW, Seidenberg MS.. Computing the meanings of words in reading: Cooperative division of labor between visual and phonological processes. Psychol Rev. 2004;111(3):662–720. [DOI] [PubMed] [Google Scholar]
- 8.Marshall JC, Newcombe F.. Patterns of paralexia: A psycholinguistic approach. J Psycholinguist Res. 1973;2(3):175–199. [DOI] [PubMed] [Google Scholar]
- 9.Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J.. DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychol Rev. 2001;108(1):204–256. [DOI] [PubMed] [Google Scholar]
- 10.Perry C, Ziegler JC, Zorzi M.. Nested incremental modeling in the development of computational theories: The CDP+ model of reading aloud. Psychol Rev. 2007;114(2):273–315. [DOI] [PubMed] [Google Scholar]
- 11.Coslett HB, Turkeltaub PE.. Acquired dyslexia. In: Hickok G, Small S, eds. Neurobiology of Language. Waltham, MA: Academic Press; 2015:791–804. [Google Scholar]
- 12.Woollams AM, Plaut DC, Lambon Ralph MA, Patterson K.. SD-Squared: On the association between semantic dementia and surface dyslexia. Psychol Rev. 2007;114(2):316–339. [DOI] [PubMed] [Google Scholar]
- 13.Wilson SM, Brambati SM, Henry RG, et al. The neural basis of surface dyslexia in semantic dementia. Brain. 2009;132(1):71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brambati SM, Ogar J, Neuhaus J, Miller B, Gorno-Tempini M.. Reading disorders in primary progressive aphasia: A behavioral and neuroimaging study. Neuropsychologia. 2009;47(8-9):1893–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borghesani V, Hinkley LBN, Ranasinghe KG, et al. Taking the sublexical route: Brain dynamics of reading in the semantic variant of primary progressive aphasia. Brain. 2020;143(8):2545–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomasino B, Ius T, Skrap M, Luzzatti C.. Phonological and surface dyslexia in individuals with brain tumors: Performance pre-, intra-, immediately post-surgery and at follow-up. Hum Brain Mapp. 2020;41(17):5015–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beauvois MF, Dérouesné J.. Phonological dyslexia: Three dissociations. J Neurosurg Psychiatry. 1979;42(12):1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nickels L, Biedermann B, Coltheart M, Saunders S, Tree JJ.. Computational modelling of phonological dyslexia: How does the DRC model fare? Cogn Neuropsychol. 2008;25(2):165–193. [DOI] [PubMed] [Google Scholar]
- 19.Tree JJ, Kay J.. Phonological dyslexia and phonological impairment: An exception to the rule? Cogn Neuropsychol. 2006;44(14):2861–2873. [DOI] [PubMed] [Google Scholar]
- 20.Friedman RB.Two types of phonological alexia. Cortex. 1995;31(2):397–403. [DOI] [PubMed] [Google Scholar]
- 21.Lambon Ralph MA, Graham NL.. Acquired phonological and deep dyslexia. Neurocase. 2000;6(2):141–178. [Google Scholar]
- 22.Rapcsak SZ, Beeson PM, Henry ML, et al. Phonological dyslexia and dysgraphia: Cognitive mechanisms and neural substrates. Cortex. 2009;45(5):575–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coltheart M. (Ed.). Phonological dyslexia: a special issue of the journal cognitive neuropsychology. Hove, UK: Psychology Press; 1996. [Google Scholar]
- 24.Fiez JA, Tranel D, Seager-Frerichs D, Damasio H.. Specific reading and phonological processing deficits are associated with damage to the left frontal operculum. Cortex. 2006;42(4):624–643. [DOI] [PubMed] [Google Scholar]
- 25.Patterson K, Ralph MA.. Selective disorders of reading? Curr Opin Neurobiol. 1999;9(2):235–239. [DOI] [PubMed] [Google Scholar]
- 26.Woollams AM.Connectionist neuropsychology: Uncovering ultimate causes of acquired dyslexia. Philos Trans R Soc B Biol Sci. 2014;369(1634):20120398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dehaene S, Cohen L.. Cultural recycling of cortical maps. Neuron. 2007;56(2):384–398. [DOI] [PubMed] [Google Scholar]
- 28.Welbourne SR, Woollams AM, Crisp J, Ralph MAL.. The role of plasticity-related functional reorganization in the explanation of central dyslexias. Cogn Neuropsychol. 2011;28(2):65–108. [DOI] [PubMed] [Google Scholar]
- 29.Farah MJ.Phonological dyslexia: Loss of a reading-specific component of the cognitive architecture? Cogn Neuropsychol. 1996;13(6):849–868. [Google Scholar]
- 30.Ripamonti E, Aggujaro S, Molteni F, Zonca G, Frustaci M, Luzzatti C.. The anatomical foundations of acquired reading disorders: A neuropsychological verification of the dual-route model of reading. Brain Lang. 2014;134:44–67. [DOI] [PubMed] [Google Scholar]
- 31.Woollams AM, Halai A, Lambon MA.. Mapping the intersection of language and reading: The neural bases of the primary systems hypothesis. Brain Struct Funct. 2018;223(8):3769–3786. [DOI] [PubMed] [Google Scholar]
- 32.Boukrina O, Barrett AM, Alexander EJ, Yao B, Graves WW.. Neurally dissociable cognitive components of reading deficits in subacute stroke. Front Hum Neurosci. 2015;9:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hickok G, Poeppel D.. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8(5):393–402. [DOI] [PubMed] [Google Scholar]
- 34.Rauschecker JP, Scott SK.. Maps and streams in the auditory cortex: Nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12(6):718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueno T, Saito S, Rogers TT, Ralph MAL.. Lichtheim 2: Synthesizing aphasia and the neural basis of language in a neurocomputational model of the dual dorsal-ventral language pathways. Neuron. 2011;72(2):385–396. [DOI] [PubMed] [Google Scholar]
- 36.Turkeltaub PE, Coslett HB.. Localization of sublexical speech perception components. Brain Lang. 2010;114(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dewitt I, Rauschecker JP.. Phoneme and word recognition in the auditory ventral stream. Proc Natl Acad Sci. 2012;109(8):E505–E514. [22308358] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hickok G, Houde J, Rong F.. Sensorimotor integration in speech processing: Computational basis and neural organization. Neuron. 2011;69(3):407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pillay SB, Binder JR, Humphries C, Gross WL, Book DS.. Lesion localization of speech comprehension deficits in chronic aphasia. Neurology. 2017;88(10):970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bates E, Wilson SM, Saygin AP, et al. Voxel-based lesion–symptom mapping. Nat Neurosci. 2003;6(5):448–450. [DOI] [PubMed] [Google Scholar]
- 41.Dronkers NF, Wilkins DP, Valin RD, Van Redfern BB, Jaeger JJ.. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92(1-2):145–177. [DOI] [PubMed] [Google Scholar]
- 42.Fridriksson J, Den Ouden DB, Hillis AE, et al. Anatomy of aphasia revisited. Brain. 2018;141(3):848–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Axer H, von Keyserlingk AG, Berks G, von Keyserlingk DG.. Supra- and infrasylvian conduction aphasia. Brain Lang. 2001;76(3):317–331. [11247647] [DOI] [PubMed] [Google Scholar]
- 44.Buchsbaum BR, Baldo J, Okada K, et al. Conduction aphasia, sensory-motor integration, and phonological short-term memory - an aggregate analysis of lesion and fMRI data. Brain Lang. 2011;119(3):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Indefrey P, Levelt WJM.. The spatial and temporal signatures of word production components. Cognition. 2004;92(1-2):101–144. [DOI] [PubMed] [Google Scholar]
- 46.Miller HE, Guenther FH.. Modelling speech motor programming and apraxia of speech in the DIVA/GODIVA neurocomputational framework. Aphasiology. 2021;35(4):424–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dronkers NF.A new brain region for coordinating speech articulation. Nature. 1996;384(6605):159–161. [DOI] [PubMed] [Google Scholar]
- 48.Ogar JM, Willock S, Baldo J, Wilkins D, Ludy C, Dronkers N.. Clinical and anatomical correlates of apraxia of speech. Brain Lang. 2006;97(3):343–350. [DOI] [PubMed] [Google Scholar]
- 49.Basilakos A, Rorden C, Bonilha L, Moser D, Fridriksson J.. Patterns of poststroke brain damage that predict speech production errors in apraxia of speech and aphasia dissociate. Stroke. 2015;46(6):1561–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trupe LA, Varma DD, Gomez Y, et al. Chronic apraxia of speech and Broca’s area. Stroke. 2013;44(3):740–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K.. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127(7):1479–1487. [DOI] [PubMed] [Google Scholar]
- 52.Graff-Radford J, Jones DT, Strand EA, Rabinstein AA, Duffy JR, Josephs KA.. The neuroanatomy of pure apraxia of speech in stroke. Brain Lang. 2014;129:43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hickok G, Okada K, Serences JT.. Area Spt in the human planum temporale supports sensory-motor integration for speech processing. J Neurophysiol. 2009;101(5):2725–2732. [DOI] [PubMed] [Google Scholar]
- 54.Dickens JV, Fama ME, DeMarco AT, Lacey EH, Friedman RB, Turkeltaub PE.. Localization of phonological and semantic contributions to reading. J Neurosci. 2019;39(27):5361-5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fama ME, Henderson MP, Snider SF, Hayward W, Friedman RB, Turkeltaub PE.. Self-reported inner speech relates to phonological retrieval ability in people with aphasia. Conscious Cogn. 2019;71:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Utianski RL, Duffy JR, Clark HM, et al. Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain Lang. 2018;184:54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kertesz A. Western Aphasia Battery - Revised. San Antonio, T X: Pearson; 2006. [Google Scholar]
- 58.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128. [DOI] [PubMed] [Google Scholar]
- 59.Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO.. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61(4):957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tournier JD, Smith R, Raffelt D, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202:116137. [DOI] [PubMed] [Google Scholar]
- 61.Jeurissen B, Tournier J, Dhollander T, Connelly A, Sijbers J.. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage. 2014;103:411–426. [DOI] [PubMed] [Google Scholar]
- 62.Smith RE, Tournier JD, Calamante F, Connelly A.. Anatomically-constrained tractography: Improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage. 2012;62(3):1924–1938. [DOI] [PubMed] [Google Scholar]
- 63.Daducci A, Gerhard S, Griffa A, et al. The connectome mapper: An open-source processing pipeline to map connectomes with MRI. PLoS One. 2012;7(12):e48121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bates D, Maechler M, Bolker B, et al. Linear mixed-effects models using “Eigen” and S4. R Package Version 1.1-21. 2019. http://dk.archive.ubuntu.com/pub/pub/cran/web/packages/lme4/lme4.pdf. Accessed June 25, 2020
- 65.R Core Team. R: A language and environment for statistical computing. 2019. https://www.r-project.org/. Accessed June 25, 2020.
- 66.Barr DJ, Levy R, Scheepers C, Tily HJ.. Random effects structure for confirmatory hypothesis testing: Keep it maximal. J Mem Lang. 2013;68(3):255–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brauer M, Curtin JJ.. Linear mixed-effects models and the analysis of nonindependent data: A unified framework to analyze categorical and continuous independent variables that vary within-subjects and/or within-items. Psychol Methods. 2018;23(3):389–411. [DOI] [PubMed] [Google Scholar]
- 68.Meteyard L, Davies RAI.. Best practice guidance for linear mixed-effects models in psychological science. J Mem Lang. 2020;112:104092. [Google Scholar]
- 69.Kutner MH, Nachtsheim CJ, Neter J, Li W.. Applied linear statistical models. Boston, MA: McGraw-Hill Irwin; 2005. [Google Scholar]
- 70.Zhang Y, Kimberg DY, Coslett HB, Schwartz MF, Wang Z.. Multivariate lesion-symptom mapping using support vector regression. Hum Brain Mapp. 2014;35(12):5861–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DeMarco AT, Turkeltaub PE.. A multivariate lesion symptom mapping toolbox and examination of lesion-volume biases and correction methods in lesion-symptom mapping. Hum Brain Mapp. 2018;39(11):4169–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kimberg DY, Coslett HB, Schwartz MF.. Power in voxel-based lesion – symptom mapping. J Cogn Neurosci. 2007;19(7):1067–1080. [DOI] [PubMed] [Google Scholar]
- 73.Yourganov G, Fridriksson J, Rorden C, Gleichgerrcht E, Bonilha L.. Multivariate connectome-based symptom mapping in post-stroke patients: Networks supporting language and speech. J Neurosci. 2016;36(25):6668–6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xia M, Wang J, He Y.. BrainNet viewer: A network visualization tool for human brain connectomics. PLoS One. 2013;8(7):e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Benson DF, Sheremata WA, Bouchard R, Segarra JM, Price D, Geschwind N.. Conduction aphasia: A clinicopathological study. Arch Neurol. 1973;28(5):339–346. [DOI] [PubMed] [Google Scholar]
- 76.Ardila A.A review of conduction aphasia. Curr Neurol Neurosci Rep. 2010;10(6):499–503. [DOI] [PubMed] [Google Scholar]
- 77.Berthier ML, Ralph MAL, Pujol J, Green C.. Arcuate fasciculus variability and repetition: The left sometimes can be right. Cortex. 2012;48(2):133–143. [DOI] [PubMed] [Google Scholar]
- 78.Geschwind N.Disconnexion syndromes in animals and man. In: Selected papers on language and the brain. New York, NY: Springer; 1974:105–236. [Google Scholar]
- 79.Wernicke C.Der Aphasische Symptomenkomplex. Breslau, Poland: Cohn & Weigert; 1874. [Google Scholar]
- 80.Jones P, Prejawa S, Hope TMH, et al. Sensory-to-motor integration during auditory repetition: A combined fMRI and lesion study. Front Hum Neurosci. 2014;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palumbo C, Alexander MP, Naeser MA.. CT scan lesion sites associated with conduction aphasia. In: Kohn SE, ed. Conduction aphasia. Mahwah, NJ: Lawrence Erlbaum Associates; 1992:51–76. [Google Scholar]
- 82.Dronkers NF.The pursuit of brain–language relationships. Brain Lang. 2000;71(1):59–61. [DOI] [PubMed] [Google Scholar]
- 83.Guenther FH.Cortical interactions underlying the production of speech sounds. J Commun Disord. 2006;39(5):350–365. [DOI] [PubMed] [Google Scholar]
- 84.Raichle ME.The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–447. [DOI] [PubMed] [Google Scholar]
- 85.van Kemenade BM, Arikan BE, Kircher T, Straube B.. The angular gyrus is a supramodal comparator area in action–outcome monitoring. Brain Struct Funct. 2017;222(8):3691–3703. [DOI] [PubMed] [Google Scholar]
- 86.Leonard MK, Cai R, Babiak MC, Ren A, Chang EF.. The peri-Sylvian cortical network underlying single word repetition revealed by electrocortical stimulation and direct neural recordings. Brain Lang. 2019;193:58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cogan GB, Thesen T, Carlson C, Doyle W, Devinsky O, Pesaran B.. Sensory-motor transformations for speech occur bilaterally. Nature. 2014;507(7490):94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tree JJ.Two types of phonological dyslexia - a contemporary review. Cortex. 2008;44(6):698–706. [DOI] [PubMed] [Google Scholar]
- 89.Aguilar OM, Kerry SJ, Crinion JT, Callaghan MF, Woodhead ZVJ, Leff AP.. Dorsal and ventral visual stream contributions to preserved reading ability in patients with central alexia. Cortex. 2018;106:200–212. [DOI] [PubMed] [Google Scholar]
- 90.Baldo JV, Katseff S, Dronkers NF.. Brain regions underlying repetition and auditory-verbal short-term memory deficits in aphasia: Evidence from voxel-based lesion symptom mapping. Aphasiology. 2012;26(3-4):338–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rogalsky C, Poppa T, Chen K, et al. Speech repetition as a window on the neurobiology of auditory – motor integration for speech: A voxel-based lesion symptom mapping study. Neuropsychologia. 2015;71:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fridriksson J, Kjartansson O, Morgan PS, et al. Impaired speech repetition and left parietal lobe damage. J Neurosci. 2010;30(33):11057–11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wilson SM, Bautista A, Mccarron A.. Convergence of spoken and written language processing in the superior temporal sulcus. Neuroimage. 2018;171:62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Atteveldt N, Formisano E, Goebel R, Blomert L.. Integration of letters and speech sounds in the human brain. Neuron. 2004;43(2):271–282. [DOI] [PubMed] [Google Scholar]
- 95.Richardson FM, Seghier ML, Leff AP, Thomas MSC, Price CJ.. Multiple routes from occipital to temporal cortices during reading. J Neurosci. 2011;31(22):8239–8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taylor JSH, Rastle K, Davis MH.. Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychol Bull. 2013;139(4):766–791. [DOI] [PubMed] [Google Scholar]
- 97.Jobard G, Crivello F, Tzourio-Mazoyer N.. Evaluation of the dual route theory of reading: A metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20(2):693–712. [DOI] [PubMed] [Google Scholar]
- 98.Oberhuber M, Hope TMH, Seghier ML, et al. Four functionally distinct regions in the left supramarginal gyrus support word processing. Cereb Cortex. 2016;26(11):4212–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Price CJ.A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62(2):816–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eden GF, Olulade OA, Evans TM, Krafnick AJ, Alkire DR.. Developmental dyslexia. In: Hickok G, Small S, eds. Neurobiology of language. Waltham, MA: Academic Press; 2015:815–826. [Google Scholar]
- 101.Frost R.Toward a strong phonological theory of visual word recognition: True issues and false trails. Psychol Bull. 1998;123(1):71–99. [DOI] [PubMed] [Google Scholar]
- 102.Ogar JM, Slama H, Dronkers NF, Amici S, Gorno-Tempini ML.. Apraxia of speech: An overview. Neurocase. 2005;11(6):427–432. [DOI] [PubMed] [Google Scholar]
- 103.Kertesz A.Subcortical lesions and verbal apraxia. In: Rosenbek J, McNeil M, Aronson A, eds. Apraxia of speech: physiology, acoustics, linguistics, management. London, UK: College-Hill Press; 1984:73–90. [Google Scholar]
- 104.Itabashi R, Nishio Y, Kataoka Y, et al. Damage to the left precentral gyrus is associated with apraxia of speech in acute stroke. Stroke. 2016;47(1):31–36. [DOI] [PubMed] [Google Scholar]
- 105.Aichert I, Ziegler W.. Syllable frequency and syllable structure in apraxia of speech. Brain Lang. 2004;88(1):148–159. [DOI] [PubMed] [Google Scholar]
- 106.Leinenger M.Phonological coding during reading. Psychol Bull. 2014;140(6):1534–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dietz NAE, Jones KM, Gareau L, Zeffiro TA, Eden GF.. Phonological decoding involves left posterior fusiform gyrus. Hum Brain Mapp. 2005;26(2):81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carreiras M, Mechelli A, Este A, Price CJ.. Brain activation for lexical decision and reading aloud: Two sides of the same coin? J Cogn Neurosci. 2007;19(3):433–444. [DOI] [PubMed] [Google Scholar]
- 109.Taitz A, Assaneo MF, Shalom DE, Trevisan MA.. Motor representations underlie the reading of unfamiliar letter combinations. Sci Rep. 2020;10(1):3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon request.