Excessive complement activation contributes to lung disease and adverse patient outcomes in COVID-19 (see the related research articles by Yan et al. and Ma et al.).
Abstract
Excessive complement activation contributes to lung disease and adverse patient outcomes in COVID-19 (see the related research articles by Yan et al. and Ma et al.).
The complement system is an integral part of innate immune defense. It consists of about 50 proteins in plasma, on cell surfaces, and inside host cells. The traditional view is that complement proteins guard the local extracellular spaces and systemic bloodstream against invading pathogens. Loss-of-function mutations resulting in terminal complement pathway deficiencies are associated with a 10,000-fold higher risk for life-threatening meningococcal infections in humans. Surprisingly, the complement system is redundant for defense against most pathogens except encapsulated bacteria. Recent concepts embrace the view that complement factors mediate functions inside cells either directly or through surface receptors. Complement activity fine-tunes homeostasis, metabolism, and biogenesis. On the other hand, uncontrolled complement activation causes disease and can even worsen the outcome of infections. Toxic complement effectors mediate tissue destruction and organ injury during inflammatory diseases. Acute respiratory distress syndrome (ARDS) and sepsis are frequent and severe complications of acute infections and notorious for excessive complement consumption. The three pathways of complement activation are designed for immune sensing of nonself surfaces and foreign antigens. The mannose-binding lectin (MBL)/ficolin pathway starts with soluble pathogen pattern recognition receptors as sensors for foreign carbohydrate motifs (Fig. 1). The alternative pathway is fueled by a spontaneous “smoldering” hydrolysis of C3 targeting all surfaces, unless these surfaces present complement inhibitory proteins (CD46, CD55, and CD59) as a protective self-signal. This C3 “tick-over” is sustained by the high concentrations of C3 in plasma (1 to 2 g/liter), the highest level of all complement factors. The classical pathway is initiated by antigen-antibody complexes that are recognized by the multimeric C1 complex. As a safeguard, IgG antibodies bound in clusters or pentameric IgM are required to surpass the activation threshold. All complement pathways converge on C3 convertase complexes leading to C3 cleavage into the larger C3b and the smaller anaphylactic C3a peptides. C3b is essential for the formation of C5 convertase for cleavage of C5 into C5b and the anaphylatoxin C5a. C5b is the starting point of the pore-forming membrane attack complex (MAC) consisting of C5b-C9 with a channel diameter of ~100 Å. The C3/C5 hub represents a gigantic amplification loop. The alternative C3bBb convertase (half-life of ~3 min) cleaves additional C3, resulting in more C3bBb and so on and so forth. This enzymatic chain reaction can deposit millions of C3b molecules on target surfaces in a few seconds. It is no surprise that such explosive events need to be tightly regulated to maintain the delicate balance of effective and justified pathogen attack, while avoiding damage of innocent bystander cells.
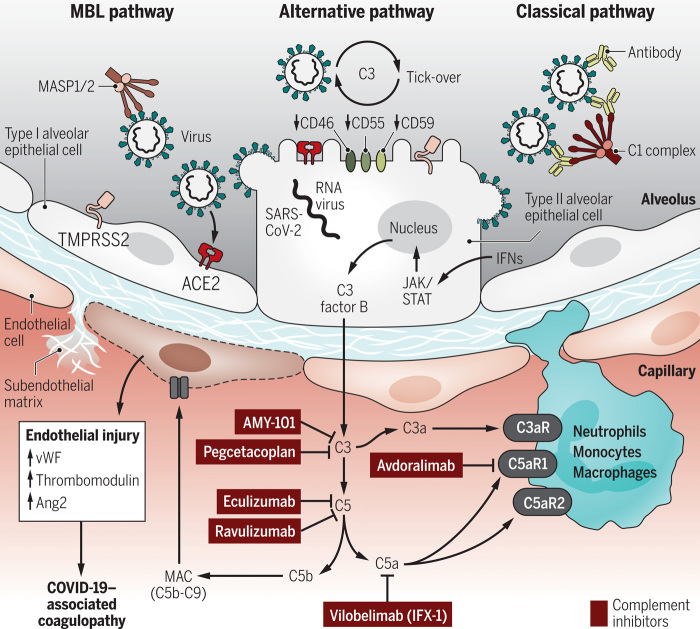
Fig. 1. Current concepts of complement activation and potential therapeutic interventions in severe COVID-19.
SARS-CoV-2 infection of alveolar epithelial cells (type II and type I) and subsequent interferon-dependent JAK1/2-STAT1–induced expression of C3 and factor B culminates in nontraditional intracellular processing of complement proteins. In the extracellular spaces, SARS-CoV-2 activates complement via the MBL/ficolin pathway, which senses glycosylated S and N proteins. The C3 tick-over of the alternative pathway is accelerated by a lack of complement inhibitors (CD46, CD55, and CD59) on infected host cells and virions. The classical pathway is activated by antibodies against viral antigens and COVID-19–associated autoantibodies. All complement activation mechanisms converge on the C3/C5 hub to form the MAC (C5b-C9) and generate anaphylatoxins (C3a and C5a). These effectors recruit myeloid cells and cause endothelial activation, endothelial injury, coagulopathy, and hyperinflammation. The figure shows the molecular targets of several complement-inhibiting drugs proposed as COVID-19 treatments: Eculizumab and ravulizumab block C5 conversion, AMY-101 and pegcetacoplan antagonize C3 activation, the IFX-1 monoclonal antibody inhibits C5a, and avdoralimab blocks the C5aR1 receptor. vWF, von Willebrand factor; Ang2, angiopoietin-2; C3aR, C3a receptor; C5aR1, C5a receptor 1; C5aR2, C5a receptor 2; MASP1/2, mannan-binding lectin serine proteases 1 and 2; ACE2, angiotensin-converting enzyme 2; TMPRSS2, transmembrane protease, serine 2.
CREDIT: KELLIE HOLOSKI/SCIENCE IMMUNOLOGY
In the April and May issues of Science Immunology, two articles highlight the importance of complement activation in severe COVID-19. Ma et al. (1) report an observational clinical study of a total of n = 259 participants classified in subcohorts of critically ill patients with SARS-CoV-2 (from two clinical centers) compared with patients with influenza or acute respiratory failure of other causes requiring invasive mechanical ventilation. Higher complement activation products in blood correlated with and predicted increased disease severity. The concentrations of soluble MAC (sC5b-C9) were higher (P < 0.0001) in patients with COVID-19, as compared with patients with influenza. SARS-CoV-2–associated acute respiratory failure showed increased sC5b-C9 compared with ARDS from non–COVID-19 etiologies. The plasma levels of sC5b-C9 and C5a correlated as expected, because both analytes arise together from C5 cleavage, although C5a has an ultrashort plasma half-life (<5 min). C5a is rapidly inactivated by plasma carboxypeptidase N and B, which remove the C-terminal arginine residue from the polypeptide chain. The degradation product C5adesArg has low affinity for the C5aR1 receptor but retains binding to the homologous C5aR2 receptor. Complement diagnostics remains a technically challenging field and not only because of the volatile nature of activation products. Antibody-based tests must selectively recognize the neoepitopes of complement proteins that are created by proteolytic cleavage. Cross-reactivity of anti-C5a antibodies with C5 or C5b can be a serious impediment.
Ma et al. (1) also addressed which of the three complement activation pathways is engaged. The authors found higher concentrations of factor B and Ba in patients with severe COVID-19 who required intensive care treatment and in nonsurvivors. Factor B is a zymogen in the alternative pathway. SARS-CoV-2 infection down-regulates expression of CD46, CD55, and CD59 (2), which may contribute to increased alternative pathway activation. It is worth mentioning that the C3bBb amplification loop is also triggered by the MBL/ficolin and classical pathways. The SARS-CoV-2 S protein contains numerous N-acetylglucosamine moieties (3). N-acetylglucosamine is recognized by ficolins. SARS-CoV-2 N protein is likewise glycosylated and directly binds to MASP2 of the MBL/ficolin pathway. The classical pathway may be activated in the later phases of infection by virus-specific antibodies (Fig. 1) and COVID-19–associated autoantibodies. The latter could fuel tissue injury. Last, Ma et al. (1) describe the statistical correlation of factor D (which cleaves factor B in the alternative pathway) with markers of endothelial cell injury (Ang2) and coagulation such as von Willebrand factor (vWF), which is released by activated platelets and endothelial cells. The cross-talk between coagulation and complement could contribute to COVID-19–associated coagulopathy and thromboembolic events. Thrombin can directly cleave C5 to bypass C3 (4). C5a ligation with C5aR1 and C5aR2 induces extracellular trap formation and appearance of externalized histones (5), which are procoagulant and provoke lung injury.
In the second article, Yan et al. (6) identify that the transcriptomic signatures of SARS-CoV-2–infected human lung epithelial cells were enriched for changes in complement gene expression. SARS-CoV-2–induced factor B and C3 as the central players of the alternative pathway, but also up-regulated other complement genes such as C1r and C1s. The expression levels of C3 correlated with viral loads. In scRNA-seq datasets from biopsies and lavages of patients with COVID-19, C3 was up-regulated in type II alveolar epithelial cells (AT2s), and the C3aR receptor was induced in macrophages and monocytes. It is appreciated that extrahepatic production of complement factors occurs at proximal sites of pathogen encounters such as in organs with mucosal surfaces and in leukocytes. However, not all lung cell types produce complement factors, and no single-cell type expresses all complement genes in relevant quantities. Yan et al. (6) found that C3 protein was processed to its active form in SARS-CoV-2–infected lung epithelial cells (Calu-3 and iPSC-derived AT2s). C3a was detected in the lung epithelial cells and existed in direct linear correlation with SARS-CoV-2 N protein. The C3 activation was prevented by a cell-permeable factor B inhibitor. The authors interpret their findings in support of the concept of a nontraditional intracellular complement activation mechanism. Cathepsin L can process C3 into biologically active C3a and C3b (7). Interestingly, cathepsin L expression is increased by SARS-CoV-2 pseudovirus infection, primes S protein, and enhances virus entry (8). In addition, invading pathogens can carry bound C3 into the cytosol (9). The intracellular C3 flags viruses for proteasomal degradation and contributes to the activation of the viral RNA-sensing MAVS signaling pathway (9). Interestingly, several viruses have evolved evasion strategies to cleave C3 with viral proteases (9).
Yan et al. (6) further characterize that SARS-CoV-2–induced expression of C3 and Factor B was dependent on type I interferons, the interferon-activated JAK1/2-STAT1 signaling pathway and NF-κB RelA. The JAK1/2 inhibitor ruxolitinib reduced generation of C3a, presumably by shutting off C3 and factor B production during SARS-CoV-2 infection.
In conclusion, complement activation is a predominant feature of severe COVID-19. Several approaches are conceivable to control complement activation as a therapeutic principle in COVID-19 (10). A number of complement blockers are either readily available for drug repurposing or in advanced stages of drug development (Fig. 1). Eculizumab is a monoclonal antibody to block C5 conversion and the first drug of its class. Eculizumab improves the life expectancy of patients with paroxysmal nocturnal hemoglobinuria (an acquired deficiency in glycosylphosphatidylinositol-anchored CD55 and CD59) and is effective in treating atypical hemolytic uremic syndrome (aHUS) and neuromyelitis optica spectrum disorder (NMOSD). C3 inhibition can be achieved by AMY-101 and pegcetacoplan. It is too early to predict which complement blocker may have the best efficacy and a favorable risk profile to selectively suppress hyperinflammation while sparing the protective antiviral activities of complement. Clinical trials to control complement activation and improve patient outcomes of COVID-19 are underway.
Acknowledgments
The author thanks C. O’Neal for reading the manuscript, A. Jayaraman for assistance, and the Evans Center for Interdisciplinary Biomedical Research at Boston University School of Medicine for their support of the Affinity Research Collaborative on “Respiratory viruses: A focus on COVID-19.” Funding: The author’s research is supported by the National Institutes of Health (1R01HL141513, 1R01HL139641, 1R01AI153613, and 1UL1TR001430) and the Deutsche Forschungsgemeinschaft (BO3482/3-3). Competing interests: The author declares that he has no competing interests.
REFERENCES AND NOTES
- 1.Ma L., Sahu S. K., Cano M., Kuppuswamy V., Bajwa J., McPhatter J., Pine A., Meizlish M. L., Goshua G., Chang C.-H., Zhang H., Price C., Bahel P., Rinder H., Lei T., Day A., Reynolds D., Wu X., Schriefer R., Rauseo A. M., Goss C. W., O’Halloran J. A., Presti R. M., Kim A. H., Gelman A. E., Dela Cruz C. S., Lee A. I., Mudd P. A., Chun H. J., Atkinson J. P., Kulkarni H. S., Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. Sci. Immunol. 6, eabh2259 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang J., Hume A. J., Abo K. M., Werder R. B., Villacorta-Martin C., Alysandratos K. D., Beermann M. L., Simone-Roach C., Lindstrom-Vautrin J., Olejnik J., Suder E. L., Bullitt E., Hinds A., Sharma A., Bosmann M., Wang R., Hawkins F., Burks E. J., Saeed M., Wilson A. A., Mühlberger E., Kotton D. N., SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. Cell Stem Cell 27, 962–973.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wintjens R., Bifani A. M., Bifani P., Impact of glycan cloud on the B-cell epitope prediction of SARS-CoV-2 Spike protein. NPJ Vaccines 5, 81 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huber-Lang M., Sarma J. V., Zetoune F. S., Rittirsch D., Neff T. A., McGuire S. R., Lambris J. D., Warner R. L., Flierl M. A., Hoesel L. M., Gebhard F., Younger J. G., Drouin S. M., Wetsel R. A., Ward P. A., Generation of C5a in the absence of C3: A new complement activation pathway. Nat. Med. 12, 682–687 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Bosmann M., Grailer J. J., Ruemmler R., Russkamp N. F., Zetoune F. S., Sarma J. V., Standiford T. J., Ward P. A., Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 27, 5010–5021 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan B., Freiwald T., Chauss D., Wang L., West E., Mirabelli C., Zhang C. J., Nichols E.-M., Malik N., Gregory R., Bantscheff M., Ghidelli-Disse S., Kolev M., Frum T., Spence J. R., Sexton J. Z., Alysandratos K. D., Kotton D. N., Pittaluga S., Bibby J., Niyonzima N., Olson M. R., Kordasti S., Portilla D., Wobus C. E., Laurence A., Lionakis M. S., Kemper C., Afzali B., Kazemian M., SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation. Sci. Immunol. 6, eabg0833 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liszewski M. K., Kolev M., Le Friec G., Leung M., Bertram P. G., Fara A. F., Subias M., Pickering M. C., Drouet C., Meri S., Arstila T. P., Pekkarinen P. T., Ma M., Cope A., Reinheckel T., Rodriguez de Cordoba S., Afzali B., Atkinson J. P., Kemper C., Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity 39, 1143–1157 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao M.-M., Yang W.-L., Yang F.-Y., Zhang L., Huang W.-J., Hou W., Fan C.-F., Jin R.-H., Feng Y.-M., Wang Y.-C., Yang J.-K., Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct. Target. Ther. 6, 134 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tam J. C. H., Bidgood S. R., McEwan W. A., James L. C., Intracellular sensing of complement C3 activates cell autonomous immunity. Science 345, 1256070 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Risitano A. M., Mastellos D. C., Huber-Lang M., Yancopoulou D., Garlanda C., Ciceri F., Lambris J. D., Complement as a target in COVID-19? Nat. Rev. Immunol. 20, 448 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]