Abstract
Objectives
To determine whether anticoagulation therapy improves outcomes in patients with coronavirus disease 2019 (COVID-19) in Japan given their lower risk of thrombosis compared with Western cohorts.
Methods
The efficacy of anticoagulation therapy in hospitalized patients with COVID-19 was evaluated using a nationwide registry: the COVID-19 Registry Japan. The inverse probability of weight treatment method was used to adjust for baseline confounders in the anticoagulation and non-anticoagulation groups.
Results
Of the 1748 patients included, anticoagulants were used in 367 patients (treatment group). The patients in the anticoagulant group were older, predominantly male, and often presented with obesity, hyperlipidaemia, hypertension, diabetes and elevated D-dimer levels. Twenty-nine-day mortality was 7.6% in the whole cohort (treatment group, 11.2%; no treatment group, 6.6%), 6% in patients who were not treated with steroids (treatment group, 12.3%; no treatment group, 5.2%), and 11.2% in patients treated with steroids (treatment group, 10.5%; no treatment group, 11.8%). Mortality in the whole cohort was similar between the treatment and no treatment groups (P=0.99), and an insignificant decreasing trend in mortality was observed in patients treated with steroids (P=0.075).
Conclusions
Anticoagulants may be beneficial in Asians, in whom comorbidities and risk of thrombosis may differ from other ethnic groups.
Keywords: Anticoagulant therapy, Steroids, Coronavirus disease, Thrombosis, Asia
Introduction
Globally, coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has affected more than 120 million individuals and caused 2.7 million deaths (Roser et al., 2021). As of 23 March 2021, there have been 457,754 cases and 8861 deaths in Japan (Ministry of Health, Labour and Welfare, 2021), which is lower than the number of cases and deaths reported in other countries with outbreaks of COVID-19 (Roser et al., 2021).
Thromboembolism, in addition to inflammation, has been reported to be associated with severe SARS-CoV-2 infection (McBane et al., 2020). Despite controversy regarding appropriate dosing (i.e. prophylactic vs treatment dosing), several studies have shown that the use of anticoagulants, such as heparin, could cause a reduction in mortality and intubation in patients hospitalized for COVID-19 (Rentsch et al., 2019; Hanif et al., 2020; Nadkarni et al., 2020), leading to recommendations for their use in treatment guidelines (Cuker et al., 2021; National Institutes of Health 2021a).
In contrast, previous studies have shown that patients with COVID-19 in Japan have a lower prevalence of underlying diseases, such as diabetes and obesity, which are associated with the severity of COVID-19, compared with patients in Western countries (Matsunaga et al., 2020). In addition, the risk of developing venous thromboembolism is lower in Asians than in Caucasians due to genetic differences (Nicole Tran and Klatsky, 2019).
There is a need to investigate whether anticoagulants have the same effect on COVID-19 in Japanese patients as in other ethnic groups. However, to the best of the authors’ knowledge, there have been no large-scale reports on this topic. As such, this study was undertaken to investigate the efficacy of anticoagulants in reducing mortality using COVID-Registry Japan (COVIREGI-JP), a nationwide cohort of hospitalized patients.
Methods
Study design and data
This study used data from COVIREGI-JP (Matsunaga et al., 2020). The inclusion criteria for enrolment were: (1) a positive SARS-CoV-2 test result; and (2) inpatient treatment at a healthcare facility.
The case report form of the International Severe Acute Respiratory and Emerging Infection Consortium was modified for the collection of clinical epidemiological information and treatment data in Japan (ISARIC, 2021). Information on the use of anticoagulation therapy, including unfractionated heparin, low-molecular-weight heparin, fondaparinux and oral anticoagulants [warfarin, direct oral anticoagulants (dabigatran, rivaroxaban, apixaban and edoxaban)] during hospitalization was collected. This study did not distinguish between prophylactic and therapeutic administration for thromboembolism.
Study data were collected and managed using Research Electronic Data Capture, a secure, web-based data capture application hosted at the JCRAC data centre of the National Centre for Global Health and Medicine (Harris et al., 2009).
Data used were from cases that contained information on all of the major items, as of 2 November 2020, as described in a previous report (Matsunaga et al., 2020).
Population for analysis
Among all patients registered as COVID-19 cases in COVIREGI-JP, the following were excluded:
-
•
those who received antiplatelet and/or anticoagulation therapy prior to the study (the new user approach was employed to avoid bias introduced by the inclusion of prevalent users in the study cohort);
-
•
those who died within 4 days of admission to hospital (to exclude those who were already in a severe condition, to facilitate effective evaluation of treatment efficacy); and
-
•
those who were categorized as ‘severe’ (i.e. invasive or non-invasive mechanical ventilation, requiring supplemental oxygen, SpO2 ≤94% on room air or tachypnoea (respiratory rate ≥24 breaths per min)] at the time of admission (to exclude patients who were already severely ill at admission, and thus were less likely to show clinical benefit from anticoagulation therapy thereafter) (Beigel et al., 2020; Matsunaga et al., 2020).
Statistical analyses
The inverse probability of treatment weight (IPTW) method was used to adjust for baseline confounders. IPTW creates a pseudopopulation in which all participants are considered conditionally exchangeable by achieving a balance between the treated and non-treated groups on the baseline covariates. The weight for each participant is defined as the inverse of the probability of receiving the observed treatment conditional upon the baseline covariate. That is, the weight of each patient receiving the anticoagulant drug is the inverse of the probability of receiving the drug [propensity score (PS)], whereas the weight of a patient not receiving the anticoagulant drug is the inverse of 1-PS. PS was estimated using multi-variable logistic regression models, including the baseline variables in the model, which are listed in Table 1 . The association between anticoagulant drug administration and 29-day mortality was estimated using the IPTW of the marginal structural Cox model. Similarly, the associations between the administration of an anticoagulant drug and overall death were estimated for patients who received steroid treatment and those who did not receive steroid treatment during admission. The subgroup-specific PS model was used to account for the differences between the steroid and no steroid treatment groups. Time-varying confounding factors were not adjusted because the timing of anticoagulant prescription was not observed. Missing values were imputed using the mean values for continuous variables and median values for categorical variables. All statistical analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC, USA).
Table 1.
Characteristics of patients with or without anticoagulation treatment during hospitalization.
| No treatment (n=1381) | Treatment (n=367) | OR (95% CI) | P-valuea | Adjusted OR (95% CI) | P-valuea | ||
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | ||||||
| Age, years | Mean (SD) | 59.3 (21.7) | 65.3 (14.1) | ||||
| Median (IQR) | 62 (48–75) | 67 (56–76) | 1.02 (1.01–1.02) | <0.0001 | 1.02 (1.01–1.03) | <0.0001 | |
| Sex | Male | 876 (63.4%) | 264 (71.9%) | 1.48 (1.15–1.9) | 0.0025 | 1.62 (1.2–2.19) | 0.0017 |
| BMI, % | Mean (SD) | 24.5 (4.7) | 26 (5.1) | ||||
| Median (IQR) | 24.9 (21.9–26.3) | 26 (23.1–28.0) | 1.06 (1.04–1.09) | <0.0001 | 1.05 (1.02–1.08) | 0.0004 | |
| D-dimer | Mean (SD) | 0.9 (2.4) | 1.6 (3) | ||||
| Median (IQR) | 0.56 (0.00–0.56) | 0.7 (0.0016–1.4) | 1.10 (1.05–1.14) | <0.0001 | 1.07 (1.03–1.12) | 0.001 | |
| Days from disease onset | Mean (SD) | 6.4 (6.9) | 7.4 (6.4) | ||||
| Median (IQR) | 6 (3–9) | 7 (4, 9.5) | 1.02 (1.00–1.04) | 0.0297 | 1.01 (1–1.03) | 0.1136 | |
| Smoking history | Current/past smoker | 537 (38.9%) | 158 (43.1%) | 1.19 (0.94–1.5) | 0.1474 | 0.99 (0.76–1.28) | 0.9142 |
| Drinking alcohol | Yes | 898 (65%) | 247 (67.3%) | 1.11 (0.87–1.41) | 0.4148 | 0.96 (0.73–1.26) | 0.7593 |
| Myocardial infarction | Yes | 8 (0.6%) | 2 (0.5%) | 0.94 (0.2–4.45) | 0.9393 | ||
| Congestive heart failure | Yes | 44 (3.2%) | 10 (2.7%) | 0.85 (0.42–1.71) | 0.6502 | ||
| Myocardial infarction/congestive heart failure | Yes | 49 (3.5%) | 12 (3.3%) | 0.92 (0.48–1.75) | 0.7962 | 0.72 (0.36–1.42) | 0.3393 |
| Peripheral vascular disease | Yes | 11 (0.8%) | 4 (1.1%) | 1.37 (0.43–4.34) | 0.5894 | 0.99 (0.29–3.45) | 0.9929 |
| Cerebrovascular disease | Yes | 67 (4.9%) | 19 (5.2%) | 1.07 (0.63–1.81) | 0.7977 | 1.1 (0.61–1.97) | 0.7599 |
| Paralysis | Yes | 16 (1.2%) | 4 (1.1%) | 0.94 (0.31–2.83) | 0.914 | 1.05 (0.32–3.42) | 0.9409 |
| Dementia | Yes | 111 (8%) | 19 (5.2%) | 0.62 (0.38–1.03) | 0.0656 | 0.52 (0.3–0.91) | 0.0217 |
| COPD | Yes | 57 (4.1%) | 20 (5.4%) | 1.34 (0.79–2.26) | 0.2739 | ||
| Chronic lung disease (excluding COPD) | Yes | 42 (3%) | 11 (3%) | 0.99 (0.5–1.93) | 0.9653 | ||
| Bronchial asthma | Yes | 80 (5.8%) | 15 (4.1%) | 0.69 (0.39–1.22) | 0.2029 | ||
| COPD/chronic lung disease/bronchial asthma | Yes | 166 (12%) | 46 (12.5%) | 1.05 (0.74–1.49) | 0.7887 | 0.97 (0.67–1.41) | 0.889 |
| Mild liver disease | Yes | 39 (2.8%) | 11 (3%) | 1.06 (0.54–2.1) | 0.8596 | ||
| Moderate-to-severe liver dysfunction | Yes | 2 (0.1%) | 2 (0.5%) | 3.78 (0.53–26.91) | 0.1845 | ||
| Mild liver disease/moderate-to-severe liver dysfunction | Yes | 41 (3%) | 13 (3.5%) | 1.2 (0.64–2.26) | 0.573 | 0.99 (0.51–1.92) | 0.9824 |
| Peptic ulcer | Yes | 15 (1.1%) | 3 (0.8%) | 0.75 (0.22–2.61) | 0.6516 | 0.67 (0.19–2.43) | 0.5452 |
| Hypertension | Yes | 392 (28.4%) | 166 (45.2%) | 2.08 (1.64–2.64) | <0.0001 | 1.48 (1.1–1.99) | 0.0101 |
| Hyperlipidaemia | Yes | 174 (12.6%) | 68 (18.5%) | 1.58 (1.16–2.15) | 0.0036 | 1.01 (0.71–1.42) | 0.9763 |
| Diabetes without complications | Yes | 229 (16.6%) | 114 (31.1%) | 2.27 (1.74–2.95) | <0.0001 | ||
| Diabetes with complications | Yes | 32 (2.3%) | 17 (4.6%) | 2.05 (1.12–3.73) | 0.0192 | ||
| Diabetes (with or without complications) | Yes | 260 (18.8%) | 131 (35.7%) | 2.39 (1.86–3.08) | <0.0001 | 1.65 (1.26–2.18) | 0.0003 |
| Obesity (physicians' diagnosis) | Yes | 90 (6.5%) | 43 (11.7%) | 1.9 (1.3–2.79) | 0.001 | 1.28 (0.82–2.02) | 0.2795 |
| Moderate-to-severe renal dysfunction | Yes | 10 (0.7%) | 2 (0.5%) | 0.75 (0.16–3.44) | 0.7129 | ||
| Haemodialysis before admission | Yes | 4 (0.3%) | 2 (0.5%) | 1.89 (0.34–10.35) | 0.4633 | ||
| Moderate-to-severe renal dysfunction/haemodialysis before admission | Yes | 12 (0.9%) | 3 (0.8%) | 0.94 (0.26–3.35) | 0.9256 | 0.82 (0.21–3.13) | 0.7684 |
| Solid tumour | Yes | 53 (3.8%) | 15 (4.1%) | 1.07 (0.59–1.92) | 0.8262 | ||
| Metastatic solid tumour | Yes | 18 (1.3%) | 1 (0.3%) | 0.21 (0.03–1.55) | 0.1258 | ||
| Solid tumour/metastatic solid tumour | Yes | 70 (5.1%) | 16 (4.4%) | 0.85 (0.49–1.49) | 0.577 | 0.73 (0.4–1.33) | 0.3037 |
| Leukaemia | Yes | 3 (0.2%) | 1 (0.3%) | 1.26 (0.13–12.1) | 0.8442 | ||
| Lymphoma | Yes | 8 (0.6%) | 1 (0.3%) | 0.47 (0.06–3.76) | 0.4759 | ||
| Leukaemia/lymphoma | Yes | 11 (0.8%) | 2 (0.5%) | 0.68 (0.15–3.09) | 0.6206 | 0.73 (0.15–3.51) | 0.6954 |
| Collagen disease | Yes | 15 (1.1%) | 6 (1.6%) | 1.51 (0.58–3.93) | 0.3938 | 1.66 (0.58–4.73) | 0.3418 |
| Immunosuppression | Yes | 39 (2.8%) | 12 (3.3%) | 1.16 (0.6–2.24) | 0.6523 | 1.18 (0.56–2.51) | 0.658 |
| ACEI | Yes | 23 (1.7%) | 11 (3%) | 1.82 (0.88–3.78) | 0.1055 | 1.45 (0.68–3.08) | 0.3407 |
| ARB | Yes | 198 (14.3%) | 85 (23.2%) | 1.8 (1.35–2.4) | <0.0001 | 0.99 (0.7–1.4) | 0.961 |
SD, standard deviation; IQR, interquartile range; OR, odds ratio; CI, confidence interval; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Chi-squared test between the treatment and no treatment groups.
Ethical approval
This study was approved by NCGM Ethics Review Board (NCGM-G-003494-0).
Results
Of the 8912 patients, 1748 patients did not meet the exclusion criteria and were included in the study (anticoagulation treatment group, n=367; non-treatment group, n=1381). Table 1 shows the differences in background characteristics according to whether or not the patients were treated with anticoagulants during hospitalization. The patients in the treated group were older, predominantly male, had a higher body mass index (BMI), and had a higher D-dimer level at admission. Hypertension, hyperlipidaemia, diabetes and obesity (as diagnosed by a physician) were more common in the treatment group than in the non-treatment group. The use of angiotensin II receptor blockers (ARBs) before hospitalization was more common in the treatment group. After adjustment for multi-variate models to generate PS, most of these variables were still significantly different between the two groups, although the differences in obesity and use of ARBs disappeared. A significant difference in dementia was observed after adjustment.
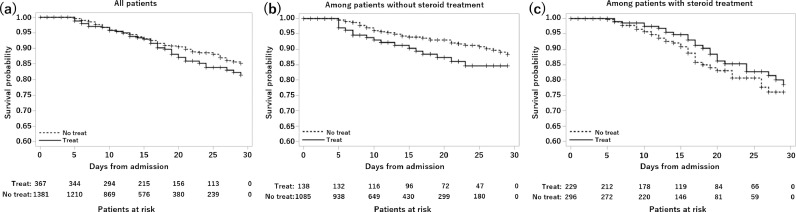
Figure 1 summarizes the survival probability by day 29 in patients who received and did not receive anticoagulation therapy during hospitalization. The results are presented for three groups: whole cohort (Figure 1a), patients who did not receive steroids (Figure 1b), and patients who received steroids (Figure 1c).
Figure 1.
Survival probability by day 29 in patients who did and did not receive anticoagulation therapy during hospitalization. The results are presented for the following three groups: whole cohort (a); patients who did not receive steroids (b); and patients who received steroids (c).
In the whole cohort, the survival probability tended to decrease more in the anticoagulant group after approximately 15 days of hospitalization. A stratified analysis according to the presence or absence of steroid use during hospitalization showed that the survival probability among patients who did not receive steroids in the anticoagulant group tended to be lower than that in the non-anticoagulant group from day 5 after hospitalization, and this trend continued until day 29. In contrast, in patients who received steroids, the survival probability in the non-anticoagulant group tended to be lower from approximately 1 week after admission compared with that in the anticoagulant group; this trend continued until day 29.
Table 2 shows a comparison of 29-day mortality between patients who received anticoagulation therapy and patients who did not receive anticoagulation therapy. In the whole cohort, the hazard ratio (HR) for 29-day mortality was slightly higher in the anticoagulant group than in the non-anticoagulant group, without any significant difference observed [HR 1.25; 95% confidence interval (CI) 0.86–1.81; P=0.242]. The IPTW-adjusted HR was 1.02 (95% CI 0.80–1.29; P=0.99). In patients who did not receive steroids, the crude and adjusted HRs were 1.62 (95% CI 0.94–2.79; P=0.084) and 1.31 (95% CI 0.97–1.78; P=0.082), respectively. In patients who received steroids, the crude and adjusted HRs were 0.76 (95% CI 0.45–1.29; P=0.311) and 0.72 (95% CI 0.5–1.03; P=0.075), respectively. When the interaction effect of steroid treatment and anticoagulation was included in the model, a P-value of 0.008 was observed, suggesting that the drug effect was different between patients who received and did not receive steroids. The missing data were complemented with the MCMC method of multiple imputation, and sensitivity analysis was performed. Interestingly, there was almost no difference in the results [adjusted HR or whole cohort: 1.00 (95% CI 0.79–1.27; P=1.00); no steroid therapy: 1.34 (95% CI 0.99–1.82; P=0.057); steroid therapy: 0.71 (95% CI 0.49–1.02; P=0.060)]. Table S1 (see online supplementary material) summarizes the distribution of PS. There was no extreme weighting by PS, and the IPTW method was considered acceptable.
Table 2.
Comparison of 29-day mortality between patients who received and those who did not receive anticoagulation treatment.
| Survivor | Non-survivor | Total number | HRa | 95% CI | P-value | aHRb | 95% CI | P-value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude cohort | |||||||||||
| Whole cohort | 1616 | 92.40% | 132 | 7.60% | 1748 | ||||||
| No treatment | 1290 | 93.4% | 91 | 6.6% | 1381 | ||||||
| Treatment | 326 | 88.8% | 41 | 11.2% | 367 | 1.25 | (0.86–1.81) | 0.242 | 1.02 | (0.80–1.29) | 0.99 |
| No steroid therapy | 1150 | 94% | 73 | 6% | 1223 | ||||||
| No treatment | 1029 | 94.8% | 56 | 5.2% | 1085 | ||||||
| Treatment | 121 | 87.7% | 17 | 12.3% | 138 | 1.62 | (0.94–2.79) | 0.084 | 1.31 | (0.97–1.78) | 0.082 |
| Steroid therapy | 466 | 88.80% | 59 | 11.20% | 525 | ||||||
| No treatment | 261 | 88.2% | 35 | 11.8% | 296 | ||||||
| Treatment | 205 | 89.5% | 24 | 10.5% | 229 | 0.76 | (0.45–1.29) | 0.311 | 0.72 | (0.50–1.03) | 0.075 |
HR, hazard ratio; aHR, adjusted hazard ratio; CI, confidence interval; IPTW, inverse probability of treatment weight.
HR for mortality in the treatment group compared with that in the no treatment group.
IPTW-aHR.
The characteristics of patients with or without anticoagulation treatment during hospitalization in the weighted population were analysed further. The results are presented in Table S2 (see online supplementary material). Insufficient adjustment for age and dementia was observed. Therefore, in addition to the IPTW analysis, another analysis was performed in which age and dementia were directly included in the Cox proportional hazard model.
The adjusted HRs were as follows: whole cohort: 1.18 (95% CI 0.94–1.50; P=0.16); no steroid therapy: 1.62 (95% CI 1.19–2.20; P=0.0023); and steroid therapy: 0.78 (95% CI 0.54–1.11; P=0.17).
Table 3 shows the complications during hospitalization in patients who received anticoagulation therapy and patients who did not receive anticoagulation therapy. Overall, complications were more frequently observed in the anticoagulant group than in the non-anticoagulant group.
Table 4.
Complications during hospitalization in patients with or without anticoagulation therapy.
| No treatment (n=1381) | Treatment (n=367) | |
|---|---|---|
| ARDS | 108 (7.8%) | 149 (40.6%) |
| Cerebral infarction or hemorrhage | 5 (0.4%) | 4 (1.1%) |
| Bloody sputum/haemoptysis | 16 (1.2%) | 5 (1.4%) |
| Deep vein thrombosis | 2 (0.1%) | 19 (5.2%) |
| Myocardial ischaemia | 2 (0.1%) | 5 (1.4%) |
| Gastrointestinal bleeding | 13 (0.9%) | 8 (2.2%) |
| Pulmonary thromboembolism | 1 (0.1%) | 8 (2.2%) |
ARDS, acute respiratory distress syndrome.
Table 3.
Anticoagulation treatment and respiratory supporta during hospitalization.
| No oxygen | Oxygen | IMV/ECMO | Total number | ||||
|---|---|---|---|---|---|---|---|
| Whole cohort | 494 | 28.30% | 943 | 54% | 310 | 17.70% | 1747 |
| No treatment | 479 | 34.7% | 771 | 55.9% | 130 | 9.4% | 1380 |
| Treatment | 15 | 4.1% | 172 | 46.9% | 180 | 49.0% | 367 |
| No steroid therapy | 459 | 37.6% | 621 | 50.8% | 142 | 11.6% | 1222 |
| No treatment | 452 | 41.7% | 555 | 51.2% | 77 | 7.1% | 1084 |
| Treatment | 7 | 5.1% | 66 | 47.8% | 65 | 47.1% | 138 |
| Steroid therapy | 35 | 6.7% | 322 | 61.3% | 168 | 32.0% | 525 |
| No treatment | 27 | 9.1% | 216 | 73.0% | 53 | 17.9% | 296 |
| Treatment | 8 | 3.5% | 106 | 46.3% | 115 | 50.2% | 229 |
IMV/ECMO, invasive mechanical ventilation/extracorporeal membrane oxygenation.
Definitions are as reported previously (Matsunaga et al., 2020).
Discussion
To the best of the authors’ knowledge, this is the largest study to evaluate the efficacy of anticoagulants in reducing mortality in patients hospitalized for COVID-19 in Japan. After PS IPTW adjustment, no clear effect of anticoagulant use or non-use on mortality was found in the entire cohort; however, a trend towards lower mortality in the steroid use group was identified.
Past studies on the use of anticoagulants in other countries have reported their effectiveness against severe illness and death in hospitalized patients with COVID-19 (Rentsch et al., 2019; Hanif et al., 2020; Nadkarni et al., 2020). In the present study, the trend towards anticoagulation benefit was found in the steroid use group alone, which may be attributed to several reasons. First, in most previous studies, anticoagulation therapy was initiated 24–48 h after admission (Rentsch et al., 2019; Nadkarni et al., 2020). Unfortunately, COVIREGI-JP does not collect data concerning the timing of anticoagulation therapy initiation or the length of treatment. In addition, the treatment may have been interrupted. Although patients who were critically ill on admission were not included in this study, it is possible that the study included a population in whom anticoagulation therapy was initiated too late. Notably, there were significantly more patients in the anticoagulant group on invasive mechanical ventilation/extracorporeal membrane oxygenation (IMV/ECMO) during hospitalization compared with the non-anticoagulant group (49% vs 9.4%), indicating a higher number of critically ill patients in the anticoagulant group. As the PS used for adjustment was based on factors at the time of admission (e.g. patient background, D-dimer, etc.), it is possible that it was not entirely accurate as it did not account for other conditions, including severity of illness at the time of anticoagulant initiation.
Second, this study may not have found a benefit for the whole cohort because the included patients with COVID-19 had fewer thrombotic events, comorbidities associated with severe disease, and severity of disease compared with other studies. The median D-dimer level at admission in the study participants was lower than that reported in a previous study (Nadkarni et al., 2020). There were few episodes of deep vein thrombosis and pulmonary embolism in the present study, although they may have been under-reported. Overall, 28% of patients did not receive oxygen during hospitalization, and although the mortality rate in the anticoagulant group was similar to that reported in a previous study (Rentsch et al., 2019), the corresponding rate in the non-anticoagulant group was considerably lower than reported previously (Rentsch et al., 2019; Nadkarni et al., 2020). The frequency of comorbidities (such as diabetes and high BMI) that can lead to serious illness was also lower in the present study compared with previous cohort studies (Rentsch et al., 2019; Nadkarni et al., 2020).
Since June 2020, steroids have been used actively in Japan to reduce mortality (Horby et al., 2021). This study was novel in that the use of steroids was not included in the PS model, but was analysed in a stratified manner to assess the benefit of anticoagulation more accurately. In patients who did not receive steroids, the non-anticoagulant group included many mildly ill patients (i.e. more than 40% did not use oxygen), which may have contributed to the failure to prove the efficacy of anticoagulants in patients in this stratum or in the whole cohort (including patients in this stratum).
The steroid population, which included more severely ill patients compared with the overall cohort, still had a higher rate of IMV/ECMO use in the anticoagulant group than in the non-anticoagulant group; however, the difference was narrowed compared with that in the whole cohort. Assuming that all the patients who died in this study cohort were treated with IMV/ECMO, the fatality rates among intubated patients would be as follows: patients who received steroids [56/77 (72.7%) in the non-anticoagulant group; 17/65 (26.2%) in the anticoagulant group] and patients who did not receive steroids [35/53 (67.9%) in the non-anticoagulant group; 24/115 (67.9%) in the anticoagulant group]. Notably, more patients (62.1%) in the steroid use group were enrolled in COVIREGI-JP after June 2020 than patients in the non-steroid use group (35.2%). As novel evidence of COVID-19 emerges over time, it is necessary to consider the impact of improved management other than steroid use. This point may, at least in part, explain the finding that the IPTW-adjusted HR, adjusting age and dementia by including those in the Cox model, showed that anticoagulation therapy may have been more harmful to the patients who did not receive steroid therapy.
The involvement of thrombosis in the severity of COVID-19 has been highlighted since the early stages of the pandemic, and an algorithm for anticoagulation was issued by Mount Sinai Hospital in April 2020 (Mount Sinai Health System, 2021). While direct oral anticoagulants (DOACs) have been used in other countries, not all DOACs have been approved for thromboprophylaxis in Japan. The use of warfarin is also considered suboptimal because of the difficulty in controlling thrombosis. Although the authors issued a recommendation for the subcutaneous administration of unfractionated heparin or low-molecular-weight heparin for hospitalized patients (Sato et al., 2020), this occurred later than the recommendations in overseas reports; therefore, the use of anticoagulants did not become a standard practice in Japan immediately. The rate of anticoagulant use was low in the present study cohort [367/1748 (21%)] of hospitalized patients with COVID-19.
In addition to the points discussed thus far, there are several caveats to the interpretation of the results of this study. As this was an observational study using registry data, it is subject to limitations as described previously (Matsunaga et al., 2020), such as bias from the overall inpatient population in Japan and future data updates. Although the COVIREGI-JP data provided information on the indications for anticoagulant use (e.g. therapeutic or prophylactic), there were cases in which it was difficult to make a strict distinction because the doses of anticoagulants were not collected. Therefore, the authors did not distinguish between the two. This is an area where there is still insufficient evidence on the appropriate target population, and the superiority of prophylactic or therapeutic dosing (National Institutes of Health 2021b; Sadeghipour et al., 2021).
In conclusion, this study found that anticoagulation therapy tended to reduce 29-day mortality in hospitalized patients with COVID-19 in Japan who were also treated with steroids. These results suggest that anticoagulants would be beneficial in Asians, in whom comorbidities and risk of thrombosis may differ from other ethnic groups, and provide a rationale for promoting anticoagulation therapy in hospitalized patients in Asian countries, including Japan. Further studies are needed to determine the appropriate target population and treatment initiation.
Declaration of Competing Interest
H. Ohtsu reports personal fees as a statistician and as an external consultant for clinical trials from EPS International, outside the submitted work. S. Saito reports grants from Shionogi, outside the submitted work. The other authors report no conflicts of interest.
Acknowledgments
Acknowledgements
The authors wish to thank all the participating facilities for their care of patients with COVID-19 and their cooperation with data entry to the registry.
Funding
This research was funded by the Health and Labor Sciences Research Grant, ‘Research for risk assessment and implementation of crisis management functions for emerging and re-emerging infectious diseases’ (Grant No. 19HA100).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.09.014.
Appendix. Supplementary materials
References
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19 – final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuker A, Tseng EK, Nieuwlaat R, Angchaisuksiri P, Blair C, Dane K, et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5:872–888. doi: 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanif A, Khan S, Mantri N, Hanif S, Saleh M, Alla Y, et al. Thrombotic complications and anticoagulation in COVID-19 pneumonia: a New York City hospital experience. Ann Hematol. 2020;99:2323–2328. doi: 10.1007/s00277-020-04216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISARIC. Clinical Data Collection – The COVID-19 case report forms (CRFs). 2021. Available at: https://isaric.org/research/covid-19-clinical-research-resources/covid-19-crf/(Accessed 25 March 2021).
- Matsunaga N, Hayakawa K, Terada M, Ohtsu H, Asai Y, Tsuzuki S, et al. Clinical epidemiology of hospitalized patients with COVID-19 in Japan: report of the COVID-19 Registry Japan. Clin Infect Dis. 2020:ciaa1470. doi: 10.1093/cid/ciaa1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBane RD, 2nd, Torres Roldan VD, Niven AS, Pruthi RK, Franco PM, Linderbaum JA, et al. Anticoagulation in COVID-19: a systematic review, meta-analysis, and rapid guidance from Mayo Clinic. Mayo Clin Proc. 2020;95:2467–2486. doi: 10.1016/j.mayocp.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health, Labour and Welfare. Current status of new coronavirus infections and the Ministry of Health, Labour and Welfare's response. 2021. Available at: https://www.mhlw.go.jp/stf/newpage_17547.html (Accessed 25 March 2021).
- Mount Sinai Health System. Mount Sinai COVID-19 anticoagulation algorithm. 2021. Available at: https://emergencymedicinecases.com/wp-content/uploads/2020/04/COVID-19-Anticoagulation-Algorithm-version_final_1.1.pdf (Accessed 25 March 2021).
- Nadkarni GN, Lala A, Bagiella E, Chang HL, Moreno PR, Pujadas E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. ACTIV trial of blood thinners pauses enrollment of critically ill COVID-19 patients. 2021a. Available at: https://www.nih.gov/news-events/news-releases/nih-activ-trial-blood-thinners-pauses-enrollment-critically-ill-covid-19-patients (Accessed 21 March 2021).
- National Institutes of Health. Antithrombotic therapy in patients with COVID-19. 2021b. Available at: https://www.covid19treatmentguidelines.nih.gov/antithrombotic-therapy/ (Accessed 25 March 2021).
- Nicole Tran H, Klatsky AL. Lower risk of venous thromboembolism in multiple Asian ethnic groups. Prev Med Rep. 2019;13:268–269. doi: 10.1016/j.pmedr.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentsch CT, Beckman JA, Tomlinson L, Gellad WF, Alcorn C, Kidwai-Khan F, et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. 2019;372:n311. doi: 10.1136/bmj.n311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roser M, Ritchie H, Ortiz-Ospina E, Hasell J. Coronavirus pandemic (COVID-19). 2021. Available at: https://ourworldindata.org/coronavirus (Accessed 25 March 2021).
- Sadeghipour P, Talasaz AH, Rashidi F, Sharif-Kashani B, Beigmohammadi MT, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato R, Ishikane M, Kinoshita N, Suzuki T, Nakamoto T, Hayakawa K, et al. A new challenge of unfractionated heparin anticoagulation treatment for moderate to severe COVID-19 in Japan. Glob Health Med. 2020;2:190–192. doi: 10.35772/ghm.2020.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.