Abstract
Objectives
Few studies have reported clinical COVID-19 sequelae six months (M6) after hospital discharge, but none has studied symptom severity.
Methods
Prevalence and severity of 7 symptoms were estimated until M6 using the self-administered influenza severity scale in COVID-19 hospitalized patients enrolled in the French COVID cohort. Factors associated with severity were assessed by logistic regression. Anxiety, depression and health-related quality of life (HRQL) were also assessed.
Results
At M6, among the 324 patients (median age 61 years, 63% men, 19% admitted to intensive care during the acute phase), 187/324 (58%) reported at least one symptom, mostly fatigue (47%) and myalgia (23%). Symptom severity was scored, at most, mild in 125 (67%), moderate in 44 (23%) and severe in 18 (10%). Female gender was the sole factor associated with moderate/severe symptom reporting (OR = 1.98, 95%CI=1.13-3.47). Among the 225 patients with psychological assessment, 24 (11%) had anxiety, 18 (8%) depressive symptoms, and their physical HRQL was significantly poorer than the general population (p=0.0005).
Conclusion
Even if 58% of patients reported ≥1 symptom at M6, less than 7% rated any symptom as severe. Assessing symptoms severity could be helpful to identify patients requiring appropriate medical care. Women may require special attention.
Keywords: COVID-19, Sequelae, Persistent symptoms, Risk factors
Introduction
Since the first cases of SARS-CoV-2 infection in December 2019, clinical presentation of COVID-19 in its acute phase has been largely described (Docherty et al., 2020; Richardson et al., 2020). However, long-term clinical sequelae of COVID-19 remain unclear. A few studies reported persistent symptoms 2 to 4 months post discharge (Carfì et al., 2020; Garrigues et al., 2020; Xiong et al., 2021). More recently, Huang et al. described the 6-months consequences of COVID-19 and reported presence of fatigue or myalgia in 63% of patients in an inpatients single-center cohort in China (Huang et al., 2021).
Here in a multicenter prospective cohort in France, we assessed self-reported symptoms 6 months after hospital admission for COVID-19, using the influenza severity scale and described the evolution following diagnosis. We also assessed anxiety, depression and health-related quality of life (HRQL) to measure the impact of these symptoms on patients’ global health.
Material and Methods
Study oversight
The French COVID cohort (NCT04262921) is a national prospective multi-center cohort study enrolling hospitalized patients with a RT-PCR virologically confirmed COVID-19 in 80 hospitals in France since January 24, 2020 (Yazdanpanah, 2021). Briefly, patients were followed-up from hospital admission (D1) throughout hospitalization for COVID-19 and at discharge, 2 to 4 weeks after discharge, month 3 (M3) and 6 (M6). The assessment of symptoms, anxiety, depression and health-related quality of life using self-administered questionnaires were proposed to the patients at each visit. All adult patients who fulfilled the self-administered symptoms questionnaire at months 3 (M3) and 6 (M6) by March 22nd, 2021 were included in the present analysis.
Procedures
The self-administered symptoms questionnaire recorded the presence and the severity of the 7 following symptoms: fatigue, myalgia, headache, cough, nasal obstruction, sore throat and feverishness. All symptoms were rated by the patient using a four-point scale (0, none; 1, mild; 2, moderate; 3, severe) using a self-administered questionnaire previously used in influenza infection (Duval et al., 2010; Hayden et al., 1997). For each date of evaluation, a total score ranging from 0 to 21 was obtained by summing the points attributed for each symptom. Based on the definition of symptom alleviation used in influenza, reporting of at least one moderate or severe symptom was considered to reflect an abnormal state of health. Four additional COVID-19 symptoms (joint pain, dyspnea, anosmia and ageusia) which were not included in the influenza questionnaire were also collected by the practitioner (thereafter referred to as “practitioner reported symptoms”) during M3 and M6 visits.
Anxiety and depression symptoms were assessed using the Hospital Anxiety and Depression scale (HADS), subdivided in the HADS-Anxiety (HADS-A) and the HADS-Depression (HADS-D) scales. Both scales contain 7 questions scored by the patients on a 4-point Likert scale (0–3) with higher scores indicating more severe anxiety/depression. The items scores were summed up separately for HADS-A and HADS-D, leading to two scores ranging between 0 and 21. Scores greater than or equal to 11 points indicated abnormal levels (Zigmond and Snaith, 1983).
Health-related quality of life (HRQL) was assessed using the SF-12 Health Survey (Gandek et al., 1998) including a Physical Component Summary (PCS) HRQL score and a Mental Component Summary (MCS) HRQL score. These scores range from 0 to 100, with a high value indicating good HRQL. A patient was defined as having an altered physical (or mental) HRQL if his PCS (or MCS) was lower than the 25th percentile of the score distribution in the general French population of the same age group and gender (Carrieri et al., 2003).
The self-administered questionnaires were collected using REDCap electronic data capture tools (Harris et al., 2009) with a secured personal access given to each patient after hospital discharge.
Statistical analyses
Categorical variables were summarized as counts (percentage) and frequency distributions were compared with the Chi-square, the Fisher exact or the McNemar for paired samples tests as appropriate. Continuous variables were expressed as median [interquartile range (IQR)] unless otherwise specified and compared with the Mann-Whitney U test. Prevalence of symptoms is given with their 95% confidence interval, estimated by using the exact Clopper-Pearson method.
To assess the representativeness of the population of patients who fulfilled the questionnaire, demographic characteristics, comorbidities and clinical data at hospital admission were compared between patients who fulfilled or not the 7-symptoms questionnaire at M3 and M6 using logistic multivariate regression models. The latter were adjusted on age, sex and ethnic group, in order to assess for confounding variables.
Associations between having at least one moderate or severe symptom at M6 and baseline characteristics were assessed through univariate logistic regressions. All variables were put in the multivariate models. Variable selection was then performed by starting with a model that included all covariates and then excluding those that did not improve the overall fit as measured by the likelihood ratio test (LRT). A P-value cut-off point of .05 was used as a stopping rule for this backward manual selection. Two-way interactions between risk factors kept in the multivariate analysis (including “ICU during the acute phase”) were tested. No imputation strategy was applied for missing data. Any case that had a missing value was discarded from the analysis.
All tests were 2-sided and p-values <.05 were considered significant. All statistical analyses were performed using R version 4.0.2.
Ethics and regulatory issues
The study was conducted with the understanding and the consent of each participant or its surrogate. The French Ethics Committee (CPP-Ile-de-France VI, ID RCB: 2020-A00256-33) approved the study protocol.
Results
Patient characteristics
Of 3,497 French COVID cohort adult participants enrolled between January 24th and September 22nd, 2020, in order to allow for a six-month follow-up, 392 died during initial hospitalization and 45 between hospital discharge and M6. Out of the 3,060 patients alive at M6, 324 patients fulfilled the 7-symptoms questionnaire at M3 and M6 and represented the study population (Figure S1). The median time interval between hospital admission and M6 assessment was 185 [182-191] days.
The main demographic comorbidities and characteristics at admission of these 324 patients are presented in Table 1 . The median age was 61 years [52-69], and 205 (63%) were men. The most common comorbidities were hypertension (n=110, 35%), chronic pulmonary disease (n=56, 18%), chronic cardiac disease (n=57, 18%), obesity (n=53, 17%) and diabetes (n=48, 15%). Fifty-four patients (19%) were admitted to intensive care unit (ICU) at any time during hospitalization.
Table 1.
Baseline characteristics in patients of the French COVID cohort who fully completed the 7-symptoms questionnaire 6 months after diagnostic confirmation.
| Category | n/ntot (%) N = 324 |
|---|---|
| Male Sex | 205/324 (63) |
| Ethnic group | |
| White | 212/264 (80) |
| Black | 25/264 (10) |
| Arab | 19/264 (7) |
| Asian | 4/264 (2) |
| Other | 4/264 (2) |
| Age | |
| Adult (18-64) | 200/324 (62) |
| Elders (>64) | 124/324 (38) |
| Smoking history | |
| Current smoker | 19/265 (7) |
| Never smoked | 171/265 (64) |
| Former smoker | 75/265 (28) |
| Health worker | 31/306 (10) |
| Intensive care unit | |
| At any time | 54/286 (19) |
| At admission | 33/302 (11) |
| Comorbidities | |
| Diabetes | 48/311 (15) |
| Hypertension | 110/311 (35) |
| Obesity | 53/304 (17) |
| Chronic cardiac disease | 57/311 (18) |
| Chronic pulmonary disease | 56/311 (18) |
| Chronic kidney disease | 16/311 (5) |
| Moderate or severe chronic liver disease | 3/311 (1) |
| Mild chronic liver disease | 4/311 (1) |
| Chronic neurological disorder | 20/311 (6) |
| Malignant neoplasm | 20/310 (6) |
| Chronic haematologic disease | 16/311 (5) |
| AIDS/HIV | 1/311 (0.3) |
| Dementia | 1/311 (0.3) |
| Rheumatologic disorder | 16/311 (5) |
| Number of comorbidities* | |
| 0 | 99/311 (32) |
| 1 | 94/311 (30) |
| 2 or more | 118/311 (38) |
* Comorbidities were defined using the Charlson comorbidity index, with the addition of clinician-defined obesity.
As compared to the 2,736 patients who did not complete the 7-symptoms questionnaire at M3 and M6, the 324 patients were younger, less likely to have diabetes, chronic kidney disease at admission, or to have been hospitalized in ICU (Table S1).
Symptoms at M6
At Month 6, 187 (58%) of the 324 patients who completed the self-administered questionnaire reported at least one persistent symptom: 80 (25%) reported one symptom, 48 (15%) two and 59 (18%) ≥ 3 symptoms. A total 7-symptoms score ≥ 3 was found in 73 (22.5%, 95% CI = [18.1%; 27.5%]) patients. Among these 187 patients, median [IQR] total 7-symptoms score was 2 [1-3].
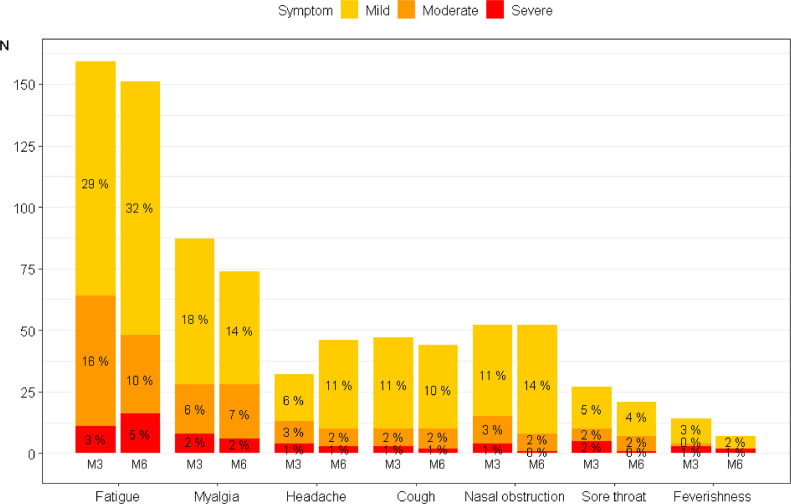
Among the 7 self-reported symptoms, the most frequent was fatigue (n=151, 47%) which was scored as mild in 103 (68%), moderate in 32 (21%) and severe in 16 (11%) (Figure 1 ). Myalgia was the second most frequent symptom (n=74, 23%), which was scored as mild in 46 (62%), moderate in 22 (30%) and severe in 6 (8%) patients. Either of the 2 symptoms was reported in 160 (49%) patients. Among the 187 patients with at least 1 symptom, the severity of the reported symptoms was scored at most mild in two thirds (n=125, 67%), moderate in 44 (23%) and severe in 18 (10%).
Figure 1.
Frequency and severity of self-reported symptoms in patients from the French COVID cohort at 3 and 6 months (M3 and M6) after hospital admission.
Barplot representing each symptom severity for the N = 324 patients who fully completed their 7-symptoms questionnaire at 3 and 6 months after diagnostic confirmation. The score for each symptom is given on a four-degree scale going from 0 to 3 (i.e. none, mild, moderate, severe). The corresponding percentages are given in each colored bar. Of note, patients presenting no symptom at all are not represented on this graph.
Reporting of a moderate or severe symptom at M6 was only associated with female gender (OR = 1.98, 95% CI = 1.13 - 3.47) in multivariate analysis after variable selection, while age (OR = 0.94, 95% CI = 0.52 - 1.65), hospitalization in ICU (OR = 0.67, 95% CI = 0.28 - 1.44), or having ≥2 comorbidities (OR=1.13, 95% CI = 0.62 – 2.03) were not (Table S2).
Among the 324 analyzed patients, 301 (93%) had practitioner examination available at M6: the practitioner reported joint pain in 53 (18%), dyspnea in 64 (21%), anosmia in 24 (8%), and ageusia in 21 (7%) patients.
HADS and HRQL at M6
HADS and SF-12 Health survey were completed in 225/324 (69%) patients at M6. Median HADS-Anxiety score was 4.0 [3.0; 7.0]; 24 (11%) patients had a HADS-Anxiety score above or equal to 11. Median HADS-Depression score was 2.0 [1.0; 5.0]; 18 (8%) patients had a HADS-Depression above or equal to 11.
Median Physical HRQL score was 50.2 [42.2 – 53.9]; 79 (35%, 95% CI [29 - 42%]) of the patients had physical HRQL lower than the 25th percentile of the distribution of the score in the general French population (p=0.0005); 66% of patients with a 7-symptoms score ≥ 3 at M6 also had an impaired physical HRQL.
Median mental HRQL was 51.2 [42.3 – 55.8]; 59 (26%, 95% CI [21 - 32%]) patients had a mental HRQL lower than the 25th percentile of the distribution of the score in the general French population, which was not statistically different (p=0.7).
Global burden of the disease at Month 6
The combinations of 7-symptoms score ≥ 3, anxiety, depression, and impaired physical and mental HRQL at M6 are presented in Figure S2; 116/225 (52%) patients presented at least one modality among total 7-symptoms score ≥3, HADS-Anxiety score ≥ 11, HADS-Depression score ≥ 11, impaired physical HRQL and impaired mental HRQL. Most frequent modality and combinations were impaired physical HRQL, total 7-symptoms score ≥3, and combination of impaired physical HRQL with total 7-symptoms score ≥3. Twenty-eight of the 135 (21%) patients who had professional activities before admission had not returned to work at M6.
M6 evaluation as compared to previous evaluations
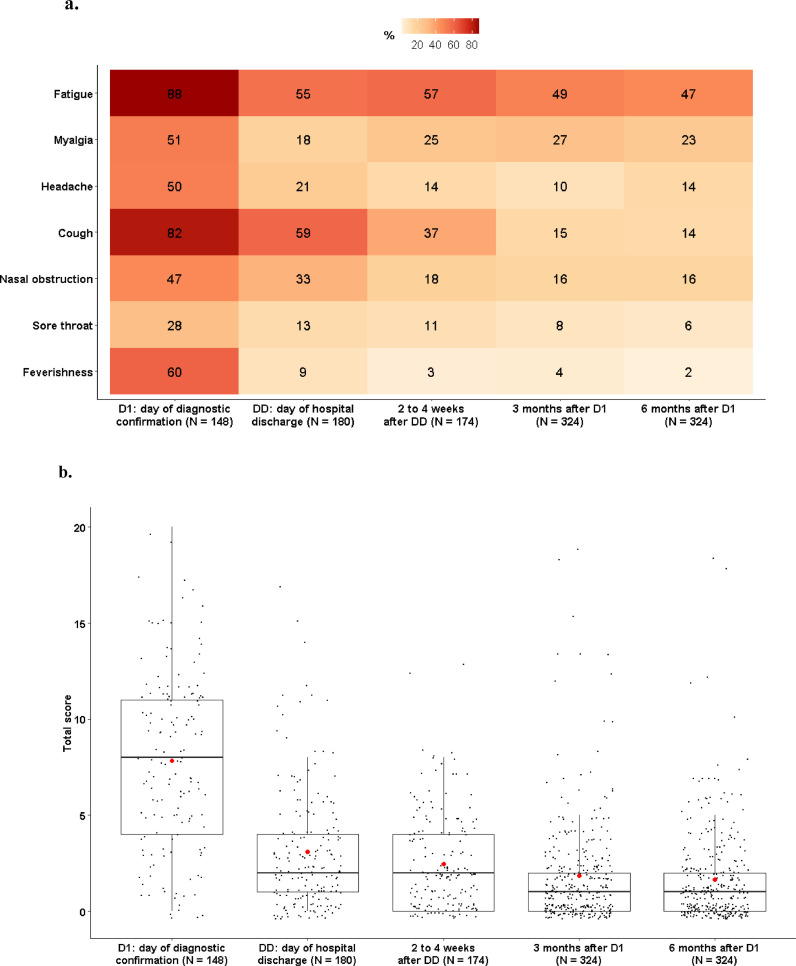
The proportions of patients who self-reported symptoms regardless of their severity at D1, discharge, 2 to 4 weeks after discharge, M3 and M6 are shown in Figure 2 a. Self-reporting of each symptom was not significantly different between M3 and M6. Similar results were obtained in a sensitivity analysis excluding patients who were admitted to ICU at any time during hospitalization (Figure S3). Reporting of each symptom according to its severity at M3 and M6 are represented in Figure 1.
Figure 2.
Evolution of 7-symptoms over time in patients from the French COVID cohort.
a. Heatmap of the 7 self-reported symptoms. For a given symptom at a given time-point, the box is colored according to the proportion of patients reporting this symptom, and the percentage is displayed in each box. P-values of McNemar test for paired samples comparing proportions of patients reporting symptoms at 3 and 6 months (M3 and M6) after hospital admission are: Fatigue, p=0.5; Myalgia, p=0.2; Headache, p=0.06; Cough, p=0.8; Nasal obstruction, p=1; Sore throat, p=0.3; Feverishness, p=0.2 b. Boxplots of the total score obtained by adding the scores obtained for each of the 7 self-reported symptoms. The score for each symptom is given on a four-degree scale going from 0 to 3, the total score is thus between 0 and 21. The red dots represent the mean values.
Evolution of 7-symptoms total score over time is shown in Figure 2b. Of note, median 7-symptoms score in patients with at least one symptom at M6 was 9 [5 – 11], 3 [1 -6], 2 [1 – 5], 2 [1 – 3], 2 [1 – 4] at D1, discharge, 2 to 4 weeks after discharge, M3 and M6, respectively (Figure S4).
Proportion of patients with a total 7-symptoms score ≥ 3 was not significantly different between M3 (24% [19-29%]) and M6 (23% [18-28%]) (p=0.8). Rates of practitioner-reported symptoms, anxiety, depression and physical and mental HRQL remained also stable between M3 and M6 (Table 2 ).
Table 2.
Practitioner-reported symptoms, Hospital Anxiety and Depression scale (HADS), and Health-related quality of life (HRQL) at 3 and 6 months (M3 and M6) after hospital admission.
| M3 | M6 | |
|---|---|---|
| Practitioner-reported symptoms N=301 | ||
| Joint pain | 51 (17%) | 53 (18%) |
| Dyspnea | 73 (24%) | 64 (21%) |
| Anosmia | 24 (8%) | 24 (8%) |
| Agueusia | 25 (8%) | 21 (7%) |
| At least 1 among above symptoms | 122 (41%) | 111 (37%) |
| HADS N= 225 | ||
| HADS-A ≥11 | 26 (12%) | 24 (11%) |
| HADS-D ≥ 11 | 18 (8%) | 18 (8%) |
| SF-12 N=225 | ||
| Impaired physical HRQL | 95 (42%) | 79 (35%) |
| Impaired mental HRQL | 62 (28%) | 59 (26%) |
Note: HADS is divided into an anxiety (HADS‐A) and depression (HADS‐D) subscale. Each HADS item was scored on a 4‐point Likert scale (0–3) with higher scores indicating more severe anxiety/depression. The items scores were summed up separately for HADS-A and HADS-D, leading to two scores ranging between 0 and 21. Scores between 11–21 points indicated abnormal levels. SF-12: an individual was defined as having an impaired physical (or mental) health-related quality of life if his Physical Component Summary (or Mental Component Summary) was lower than the 25th percentile of the distribution of the score in the general French population of the same age group and gender.
Discussion
To our knowledge, this is the first study assessing the severity of persistent symptoms 6 months after hospital admission for COVID-19 and their evolution since admission. In our population, 56% of patients still reported at least one symptom at M6, but less than 7% rated any symptom as severe. Except for female gender, we did not identify any other factor linked to high burden of symptoms at M6 that could prompt specific follow-up management options, and thus improve patients' outcome.
The French COVID cohort was launched at the very beginning of COVID-19 pandemic when the first cases were identified in France and recruited patients in all types of French hospitals, throughout France including overseas territories. The database extraction date of March 22, 2021 made it possible to assess the health status of patients included between January 24, 2020 and September 22, 2020. The younger age of the population who responded to the questionnaire compared to those who did not, was probably explained by a greater propensity of younger people to complete a questionnaire online. The lower age of the respondents was logically associated with a lower proportion of comorbidity and of ICU admission.
The proportion of patients (approximately one out of two) who reported at least one symptom at M6 using the 7-symptom influenza questionnaire was high, with most common reported symptoms being fatigue and myalgia; this is consistent with previous studies on mid-term follow up of SARS-CoV-2 (Carfì et al., 2020; Garrigues et al., 2020; Huang et al., 2021; Xiong et al., 2021) or long term follow-up of other SARS survivors (Lam et al., 2009; Lee et al., 2007; Tansey et al., 2007), although the range of values was wide. This large range of reported symptoms could be due to differences in the characteristics of patients included in the cohort or filling the forms, such as the age, the proportions of women or of patients with comorbidities (Huang et al., 2021).
Since we did not know patients’ symptoms before they experienced COVID, we were not able to assess to what extent the M6 reported symptoms were related to the COVID episode or to a pre-existing condition. For this reason, and taking into account the experience of self-questionnaires performed in influenza, we assessed the severity of the symptoms and defined symptom alleviation as seven symptoms were scored absent or only mild (Duval et al., 2010; Hayden et al., 1997). This led to a fifth of the patients being considered to have a poor M6 clinical outcome. Beyond the proportion attributable to COVID that cannot be precisely estimated, on one hand, the association between a score higher than three and a poor physical quality of life, and on the other hand, the significantly higher proportion of subjects with an impaired physical quality of life compared to a control population argue for the COVID's responsibility for the symptoms reported by patients. This is all the more to be taken into account as the persistence of symptoms as well as the alteration in quality of life at M3 and M6 did not suggest a rapid improvement and suggests to extend the patient follow-up beyond M6. Considering the persistence of altered quality of life between M3 and M6, especially regarding mental health, supportive care should be provided as early as 3 months after hospitalization in patients who need it.
Female gender was found to be the sole risk factor of persistence of moderate or severe symptoms, consistent with previous studies in SARS-CoV-2 survivors (Ghosn et al., 2021; Huang et al., 2021; Xiong et al., 2021), whereas women are prone to develop less severe acute COVID than men (Richardson et al., 2020; Yazdanpanah and French COVID cohort investigators and study group, 2021; Zhou et al., 2020). The pathophysiology mechanisms underlying this finding remain to be explored. Neither ICU admission, nor reporting of moderate or severe symptoms at admission which could be considered as a proxy of the disease severity at admission, were associated with poor clinical outcome at M6.
This study has several limitations. First, M3 and M6 self-administered questionnaires were not completed by all survivors. On one hand, as the population was younger and therefore less prone to have been admitted to ICU during hospitalization, our study might underestimate the proportion of COVID-19 survivors with poor clinical outcome at M6 post-hospitalization (Huang et al., 2021). On the other hand, one cannot rule out that the propensity to complete a questionnaire is higher among those with symptoms than those without. Second, M6 assessment took place between end of July, 2020 and March, 2021, a time period during which the health situation in France deteriorated with an alternation of curfews and lockdown measures, thus promoting an anxiety-provoking climate. Differences in the patient characteristics of those evaluated and those not, specific health care facilities bottlenecked at the time of follow-up, improvement of support of care strategies over the outbreak and evolution of virus characteristics did not allow us to extrapolate our results to all survivors of COVID-19. Third, the 7-symptoms questionnaire was previously used to assess symptom alleviation in the cute phase of influenza disease, but not long-term sequelae. However, when focusing on patients with a total score above or equal to 3 at M6, the same number of patients had a poor outcome and the determinants remained the same.
Identifying patients needing specific care after COVID-19 could be beneficial in terms of public health. The use of a simple web-based questionnaire with a scale of severity could be helpful to identify patients requiring appropriate medical care including psychological support within months following recovery, especially since close follow-up of all COVID-19 survivors after hospital discharge seems difficult to achieve in the context of successive epidemic waves and congestion in health-care facilities.
In conclusion, persistence of symptoms in more than half of the patients and impaired physical health-related quality of life at M6 promotes long-term follow-up beyond six months following recovery.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Sources of funding: This work was supported by the REACTing (REsearch & ACtion emergING infectious diseases) consortium and by a grant of the French Ministry of Health (PHRC n°20-0424).
Group Information: The members of the French COVID cohort study and investigators groups are provided in Supplementary Material.
Additional Information: The study included a scientific advisory board composed of Dominique COSTAGLIOLA, Astrid VABRET, Hervé RAOUL and Laurence WEISS.
Ethics and regulatory issues: The study was conducted with the understanding and the consent of each participant or its surrogate. The French Ethics Committee (CPP-Ile-de-France VI, ID RCB: 2020-A00256-33) has approved the study protocol.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.09.011.
Appendix. Supplementary materials
References
- Carfì A, Bernabei R, Landi F. for the Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020;324:603. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrieri P, Spire B, Duran S, Katlama C, Peyramond D, François C, et al. Health-Related Quality of Life After 1 Year of Highly Active Antiretroviral Therapy. JAIDS J Acquir Immune Defic Syndr. 2003;32:38–47. doi: 10.1097/00126334-200301010-00006. [DOI] [PubMed] [Google Scholar]
- Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval X, van der Werf S, Blanchon T, Mosnier A, Bouscambert-Duchamp M, Tibi A, et al. Efficacy of oseltamivir-zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo-controlled trial. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandek B, Ware JE, Aaronson NK, Alonso J, Apolone G, Bjorner J, et al. Tests of Data Quality, Scaling Assumptions, and Reliability of the SF-36 in Eleven Countries. J Clin Epidemiol. 1998;51:1149–1158. doi: 10.1016/S0895-4356(98)00106-1. [DOI] [PubMed] [Google Scholar]
- Garrigues E, Janvier P, Kherabi Y, Le Bot A, Hamon A, Gouze H, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosn J, Piroth L, Epaulard O, Le Turnier P, Mentré F, Bachelet D, et al. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2021;27 doi: 10.1016/j.cmi.2021.03.012. 1041.e1-1041.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden FG, Osterhaus ADME, Treanor JJ, Fleming DM, Aoki FY, Nicholson KG, et al. Efficacy and Safety of the Neuraminidase Inhibitor Zanamivir in the Treatment of Influenzavirus Infections. N Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- Huang C, Huang L, Yeming Wang, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet Lond Engl. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MH-B, Wing Y-K, Yu MW-M, Leung C-M, Ma RCW, Kong APS, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169:2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- Lee AM, Wong JGWS, McAlonan GM, Cheung V, Cheung C, Sham PC, et al. Stress and psychological distress among SARS survivors 1 year after the outbreak. Can J Psychiatry Rev Can Psychiatr. 2007;52:233–240. doi: 10.1177/070674370705200405. [DOI] [PubMed] [Google Scholar]
- Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey CM, Louie M, Loeb M, Gold WL, Muller MP, de Jager J, et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med. 2007;167:1312–1320. doi: 10.1001/archinte.167.12.1312. [DOI] [PubMed] [Google Scholar]
- Xiong Q, Xu M, Li J, Liu Y, Zhang J, Xu Y, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanpanah Y, French COVID cohort investigators and study group Impact on disease mortality of clinical, biological, and virological characteristics at hospital admission and overtime in COVID-19 patients. J Med Virol. 2021;93:2149–2159. doi: 10.1002/jmv.26601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.