Abstract
COVID-19 is an ongoing public health emergency that has affected millions of people worldwide and is still a threat to many more. One of the pathophysiological features of COVID-19 is associated with the activation of vascular endothelial cells (ECs) leading to the disruption of vascular integrity, coagulation and inflammation. An interlink mechanism between coagulation and inflammatory pathways has been reported in COVID-19. Multiple components are involved in these pathological pathways. Out of all, Von Willebrand Factor (VWF) is one of the primary components of coagulation pathway and also a mediator of vascular inflammation that plays an important role in thrombo-inflammation that further leads to acute respiratory distress syndrome (ARDS). The thrombo-inflammatory co-morbidities such as hyper-coagulation, thrombosis, ARDS etc. have become the major cause of mortality in the patients of COVID-19 admitted to the ICU. Thus, VWF can be explored as a potential target to manage COVID-19 associated co-morbidities. Supporting this hypothesis, there are literature reports which disclose previous attempts to target VWF for the management of thrombo-inflammation in other pathological conditions. The current report summarizes emerging insights into the pathophysiology, mechanism(s), diagnosis, management and foundations for research on this less explored clinically relevant glycoprotein as coagulation biomarker in COVID-19.
Keywords: COVID-19, SARS-CoV-2, Von willebrand factor, Thrombo-inflammatory complications, Pulmonary embolism
Graphical abstract
1. Introduction
A new outbreak with pneumonia like symptoms originated from Hubei province of Wuhan, China, in December 2019 [1,2]. On 12th December 2019, Wuhan Municipal Health Commission (WMHC) reported 27 cases of viral pneumonia with seven critically ill patients [3]. A new type of virus called novel coronavirus (2019-nCoV) was identified as a root cause of this pneumonia. Perceiving the increased health risk, World Health Organization (WHO) pronounced the situation as public health emergency of international concern (PHEIC) on 30 January 2020. On 11 February 2020, WHO officially named this pneumonia outbreak as COVID-19 and designated 2019-nCoV as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [4,5]. By 11 march 2020, director general of WHO declared the COVID-19 outbreak a global pandemic [6].
Many patients with COVID-19 get seriously ill, developing life-threatening complications with a high mortality rate. Some of the leading causes of mortality in patients with COVID-19 are thrombo-inflammatory co-morbidities such as hyper-coagulation, thrombosis and respiratory failure due to acute respiratory distress syndrome (ARDS) [7,8]. Endothelial cells (ECs) are the main center of these complications, where all the thrombo-inflammatory events takes place in response to the pathogenic attack [9]. To date, the activation of ECs have been largely overlooked as a therapeutic target in COVID-19, yet emerging evidence suggests that these cells contribute to the initiation and propagation of ARDS by vascular leakage, coagulation and inflammation [10]. Among these cellular events, coagulation cascade is one the established features of COVID-19 and there is an established interlink between the coagulation cascade and inflammatory pathways through thrombin and plasmin-mediated activation [11]. This interlinked mechanism is recently proposed to induce coagulopathies such as thrombosis in COVID-19 [12,13]. There are various inflammatory coagulation biomarkers such fibrin, D-dimer, P-selectin, platelets, Von Willebrand factor (VWF), coagulation factor VIII etc, which are clinically relevant biomarkers of coagulopathies associated with COVID-19 [14]. Specialized receptors present on the ECs are the main site of action of these hemostasis biomarkers [15]. Among these biomarkers, VWF has a leading role in thrombo-inflammation and other coagulopathies caused due to SARS-CoV-2 infection [16]. Briefly, VWF is a multifunctional glycoprotein that plays important roles in primary and secondary hemostasis. It serves as a mediator to anchor the platelet to the subendothelial collagen as well as a carrier for coagulation factor VIII in plasma. VWF is synthesized in EC and megakaryocytes and is present in cell, plasma and subendothelial collagen. Cellular VWF is present in vascular EC and in platelets, stored in Weibel Palade bodies (WPB) and in alpha granules, respectively, and released upon stimulation. On the other hand, VWF present in plasma circulates as a large globular protein and derived almost entirely from EC release. In subendothelial collagen, VWF arises from abluminal release of EC and is bound to the molecules of the extracellular matrix (ECM) and, endothelial and vascular smooth muscle cell (VSMC) surface receptors [17]. Critically, evidences suggest that the elevated levels of VWF may contribute to coagulopathies in various diseases including cancer [18], traumatic brain injury [19], liver diseases [20], atherosclerotic cardiovascular disease [21], thrombosis [22] etc. Several reports discuss the potential benefits of targeting VWF as a novel therapy in coagulopathies associated with these pathological conditions [[22], [23], [24], [25], [26], [27]].
Researchers have recently suggested that the severe cases of COVID-19 could also be linked to the raised level of VWF in blood [28,29]. In this context, we have attempted to discuss the mechanism of how VWF interact with the ECs and platelets, and how its level increases in the blood during SARS-CoV-2 infection. We further discuss the pathology regarding how this factor is involved in the thrombo-inflammatory co-morbidities such as ARDS and thrombosis, associated with COVID-19. The possible treatment options are also provided in this report that may build the foundation of the future research for the management of different co-morbidities raised due to VWF in COVID-19 patients.
2. Factors playing key role in COVID-19 associated coagulopathy (thrombosis)
Coagulopathy is one of the most prevalent complications occurring in both ambulatory as well as hospitalized patients of COVID-19 and is characterized by increased thrombotic and microvascular complications. Various pro-inflammatory and coagulation factors play an important role in these complications by activating ECs, platelets and macrophages. Briefly, coagulopathy shows elevated VWF, fibrinogen, factor VIII, elevated D‐dimers, with minimal change in prothrombin time (PT), activated partial thromboplastin time (aPTT), and platelet count in early stages of infection.
One study suggests that tissue factor (TF) in association with coagulation factor VII triggers the extrinsic coagulation. The signaling pathway in which TF is overexpressed entails the activation of NADPH oxidase that in turn activates NF-kappaB transcription factor, responsible for the transcription of TF gene. When a single stranded RNA virus enters the ECs, it activates the NADPH oxidase through RNA responsive toll-like receptor-7 (TLR-7). Similarly, in SARS-CoV-2 infection, it is hypothesized that the entry of this virus in ECs leads to the over-expression of TF that exist if only NADPH oxidase get activated [30]. Angiotensin converting enzyme 2 (ACE2) receptor, the entry point of SARS-CoV-2, is another factor that is majorly expressed in ECs and contribute to unwarranted coagulation by abnormal activation of renin-angiotensin system (RAS) [31]. Thus, the over-activated ACE2 may, directly or indirectly, lead to coagulopathies in COVID-19 patients. As per a recent report, high level of serum ferritin is also detected in COVID-19 patients, which is associated with a major complication called “hyperferritinemic syndrome” [32], which leads to macrophage activation that further contributes to different coagulopathies in these patients. Besides, the changes in the hemostatic biomarkers such as increase in D‐dimer and fibrin/fibrinogen degradation products indicate the essence of coagulopathy in the patients of COVID-19 [33]. Han et al. examined hemostatic parameters from 94 SARS-CoV‐2‐infected patients and reported the prothrombin time (PT) activity was found to be lower in the patients compared with healthy controls (81% vs. 97%; P < .001) [34]. Similarly, Guan et al. performed a study on 1099 patients and reported higher levels of D‐dimer in 46.4% cases. Notably, the increase in D‐dimer value was more significant in critically ill patients [35]. There are numbers of reports that suggest significantly higher D‐dimer and Fibrinogen degradation Products (FDP) levels, longer PT and prolonged activated partial thromboplastin time (aPTT) in non-survivors as compared to the survivors [[36], [37], [38]]. On the other hand, low levels of platelets i.e. thrombocytopenia is also considered as the most important indicator of disseminated intravascular coagulation (DIC) in the patients of COVID-19. Apparently, a low platelet count is associated with increased risk of disease severity and mortality in these patients. Importantly, the dysregulated proinflammatory cytokines such as interleukin (IL)‐1β and IL‐6 cause “cytokine storm” that in turn leads to proliferation of the megakaryocytes and ultimately cause thrombocytosis [39].
3. What is VWF?
VWF is one of the main components of the blood coagulation system and is synthesized in EC and platelets. The main function of this protein is to form a framework for platelet binding [40]. Briefly, VWF is a blood glycoprotein that is required for normal hemostasis, and deficiency of VWF leads to von Willebrand disease (VWD), a common inherited bleeding disorder. VWF mediates the adhesion of platelets to sites of vascular damage by binding to specific platelet membrane glycoproteins and to constituents of exposed connective tissue. These activities appear to be regulated by allosteric mechanisms and possibly by hydrodynamic shear forces. VWF is also a carrier protein for blood clotting factor VIII, and this interaction is required for normal factor 8 survivals in the circulation [41]. VWF is stored in vascular EC in special organelles and in α-granules of platelets where it secrets in the form of multimers [42]. The assembly of this complex multimeric protein requires many separate steps, each of which may be disrupted by mutations. The largest VWF multimers are hemostatically active, whereas small multimers are not. Various X-ray crystallographic structures of VWF, co-crystallized with corresponding ligands, are available in the protein data bank (PDB) at URL http://www.rscb.org/pdb. The structural data of all the PDBs available for this glycoprotein from Homo sapiens is summarized in Table 1 .
Table 1.
X-ray crystallographic structural data of VWF from Homo sapiens.
| S. No. | PDB ID | Domain | Ligand | Resolution (Å) | Reference |
|---|---|---|---|---|---|
| 1 | 1AO3 | A3 | No ligand | 2.2 | [43] |
| 2 | 1AUQ | A1 | No ligand | 2.3 | [44] |
| 3 | 1ATZ | A3 | No ligand | 1.8 | [45] |
| 4 | 1FE8 | A3 | RU5 | 2.0 | [46] |
| 5 | 1IJK | A1 | Botrocetin | 2.6 | [47] |
| 6 | 1OAK | A1 | NMC-4 | 2.2 | [48] |
| 7 | 1UEX | A1 | Bitiscetin | 2.8 | [49] |
| 8 | 2ADF | A3 | 82D6A3 | 1.9 | [50] |
| 9 | 3HXO | A1 | ARC1172 | 2.4 | [51] |
| 10 | 3HXQ | A1 | ARC1172 | 2.6 | [51] |
| 11 | 3GXB | A2 | No ligand | 1.9 | [52] |
| 12 | 3ZQK | A2 | No ligand | 1.7 | [53] |
| 13 | 4NT5 | CTCK | No ligand | 3.2 | [54] |
| 14 | 6N29 | D’D3 | No ligand | 2.5 | [55] |
| 15 | 7EOW | A1 | Caplacizumab | 1.6 | [56] |
| 16 | 7F49 | A1 | BT-100 | 2.1 | [57] |
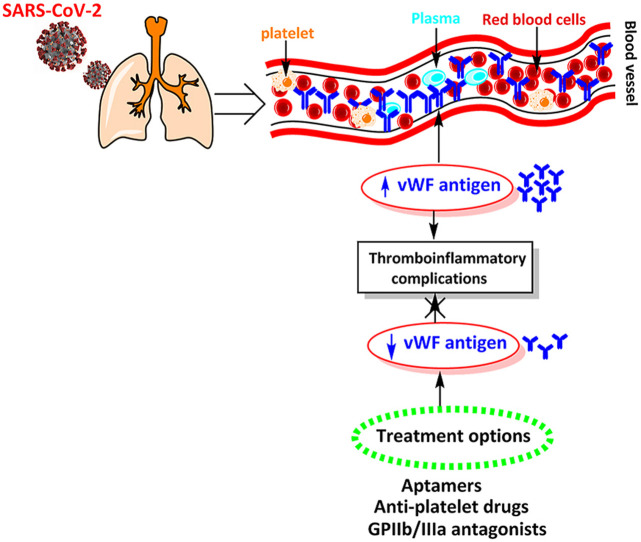
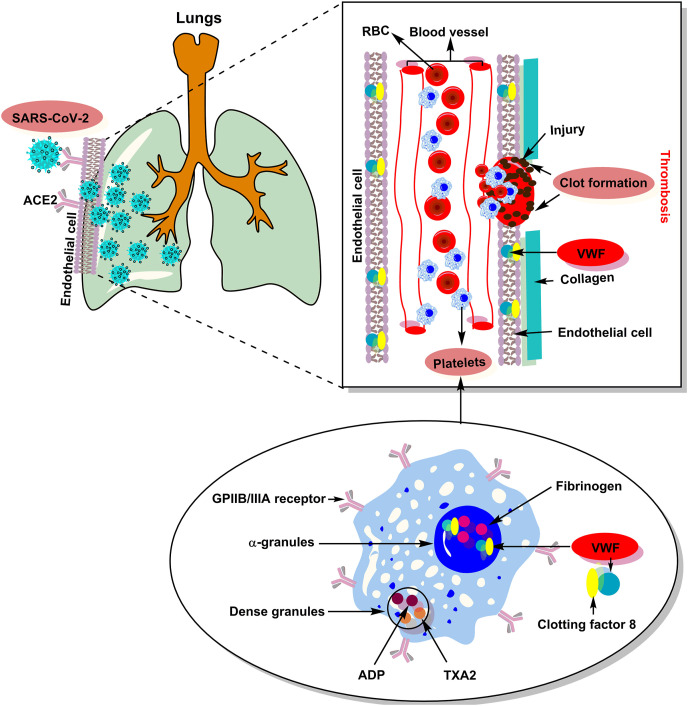
As soon as any damage occurs on the blood vessel, a rapid mechanism of blood coagulability is launched. The level and activity of VWF in the blood vary from person to person. The lowest levels are linked to VWD, which is a hereditary blood disorder that is characterized by spontaneous bleeding [58]. Recent literature reports suggest that viral infections can cause local inflammation in the walls of blood vessels and capillaries [59,60]. This leads to the release of VWF into the blood that eventually causes clotting. Fig. 1 demonstrates the role of VWF and possible pathophysiological mechanism of thrombosis in SARS-CoV-2 infected COVID-19 patients. Essentially, the SARS-CoV-2 virus replication leads to damaged vessels walls. As a response to the damage, the body tries to patch the possible holes by releasing VWF into the blood. As a result, the risks of blood clotting increase. A significant number of deaths from COVID-19 are linked to clotting [61,62]. COVID-19 manifests in different people in completely different ways. One of the possible causes of this phenomenon may be a different level of VWF in blood of patients [63].
Fig. 1.
Proposed pathophysiology and role of VWF in thrombosis associated with SARS-CoV-2 infection.
The researchers suggest that the level and activity of VWF might be important predictors of COVID-19 related mortality. Various clinical reports suggest that an elevated level of VWF in the blood of COVID-19 patients may lead to different coagulopathies such as endotheliopathy, thrombotic thrombocytopenic purpura, thrombocytopenia, pulmonary embolism, DIC, thrombophilia, thrombotic microangiopathy etc [64].
4. Role of VWF in COVID-19 associated thrombo-inflammatory complications
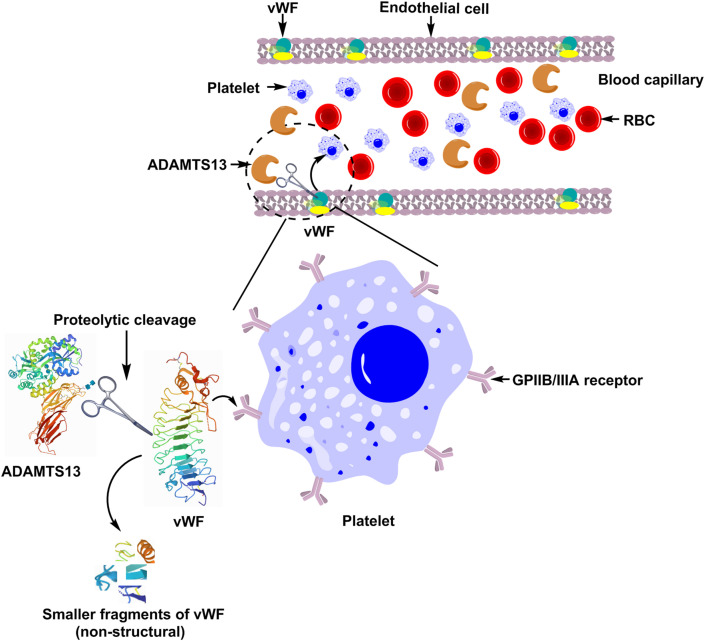
Acute COVID-19 conditions are hyper-inflammatory and prothrombotic in nature and therefore negatively affect the levels of several thrombo-inflammatory markers. VWF is a multimeric protein that is released into the circulation from endothelial stores in a highly thrombogenic form, characterized by the presence of ultralarge multimers. Under normal circumstances, these ultralarge multimers are cleaved by the protease ADAMTS-13 (A Disintegrin and Metalloproteinase with a ThromboSpondin type 1 motif, member 13) leading to subsequent decrease in the high thrombogenicity of released VWF (Fig. 2 ). ADAMTS-13 is a zinc containing protease, also known as VWF cleaving protease which cleaves VWF between Tyr1605 and Met1606 in the A2 domain (i.e. the proteolysis site). It is well reported that congenital or autoantibody-induced deficiency of ADAMTS-13 leads to the conditions that are characterized with increased incidence of thrombotic complications, which can be severe and potentially fatal, as in thrombotic thrombocytopenic purpura (TTP). Acquired ADAMTS-13 deficiency is associated with systemic disorders, including severe inflammatory diseases and sepsis. It is plausible that COVID-19 being a severe inflammatory disease, is associated with acquired ADAMTS-13 deficiency and, thereby increased VWF thrombogenicity [65]. The main risk behind the progression of complicated stage of COVID-19 appears to be the pre-existing “inflamed” plasma membranes or the so-called “activated endothelium” due to several reasons. The hallmark of endothelial activation is elevated plasma level of VWF. Moreover, VWF appears to be an important component of the “clot” in COVID-19. Helms et al. analyzed patients with the occurrence of thromboembolic events and noted that VWF activity and VWF antigen (VWF:Ag) were considerably increased, as was factor VIII. Pro-inflammatory cytokines IL-8 and TNF-α released in cytokine storm of COVID-19 upregulate endothelial derived ultra large VWF multimers (eULVWF) release from endothelial weibel palade bodies (WPB) and cytokine IL-6 inhibits cleavage of eULVWF by ADAMTS-13 enzyme, hence increasing release of VWF. Histone which is a damage associated molecular patterns (DAMP) released from NETs also stimulates VWF secretion from WPB. In fact, hypoxia upregulates and activates VWF and potentially causes thrombosis by increasing blood viscosity and through a hypoxia-inducible transcription factor-dependent signaling pathway in the lung endothelial cells and in megakaryocytes. Megakaryocytes usually known as the “producers of platelets” are an anti-viral alarm system of the body. Lung is the major organ where megakaryocytes reside making it a major site for the production of platelets contributing for about 50% of the total production rendering it a highly prothrombotic or “platelet rich plasma” environment. Autopsy findings suggest in severe COVID-19, megakaryocytes are reported to be actively producing platelets in the lung and are involved in vascular thrombosis. Megakaryocytes can be activated to produce platelets by rolling on VWF-GPIb under high shear stress and increased WPB expression in the pulmonary vascular endothelial cells. As VWF multimers are also secreted into the subendothelial space by WPB hence, extravascular megakaryocytes that are present in the lungs may also get activated. It has been observed that the alveolar macrophages may easily be infected by SARS-CoV-2 (may be before even entering the alveolar cells) and initiate the cytokine storm. In complicated COVID-19, pulmonary artery circulation carries blood with markedly lower oxygen content which in turn upregulates and activates VWF. As lungs are the “filter” of the pulmonary circulation, therefore VWF/platelet/NETs/fibrin strings excluded from the endothelial cells of the venous system results in pulmonary embolism [66]. A cohort study among COVID-19 patients suggest increased thrombotic tendency. Approximately one third of patients had CT scan evidence of pulmonary embolism (PE). In fact, two-thirds of the patients without PE also had elevated D-dimers with a higher cut off value of 2660 μg/L being more predictive of PE. A Dutch study of severely ill COVID-19 patients admitted to ICU similarly identified a 31% incidence of thrombotic events including PE, deep vein thrombosis (DVT) and ischemic strokes. A retrospective study of COVID-19 patients revealed that significantly elevated VWF levels (VWF: antigen-555%, VWF: activity-520%) and factor VIII (clotting activity of 369%) and higher D-dimer and prolonged prothrombin time (PT) were associated with a higher probability of mortality. Taken together, these findings strongly support the existence of a syndrome of COVID-19-associated Coagulopathy (CAC) characterized by derangements in clotting tests (PT and APTT), elevated D-dimer and an increased thrombotic tendency [67]. VWF is located on the macrophage surface and is removed by macrophages and hepatocytes from the circulation. Although macrophages are present in all tissues, these are predominantly located in the liver sinusoids (called Kupffer cells) and lung capillaries. As VWF is cleared by macrophages, it has been postulated that the increased VWF load in circulation may contribute to macrophage over activation and hence MAS (macrophage activation syndrome). In the pulmonary microvasculature, local endothelial cell dysfunction also appears to play a major role in the thrombo-inflammatory processes. The cytokine storm and MAS together could activate the expression of active tissue factor (TF) within the lungs, further triggering the coagulation cascade. In this context, acute pulmonary embolism (PE) has arisen as a potential severe complication of COVID-19 infection. Autopsy findings suggested that PE or lung thrombosis may represent a frequent cause of death in COVID-19 patients [68]. Marked endothelial activation subsequent to the inflammatory state could elevate the amount of VWF, which could then overload the VWF-cleaving protease, ADAMTS-13, leading to platelet aggregates that could also contribute to microthrombi. Formation of thrombi in the microvasculature is a part of the physiological attempt to restrict the viral invasion. Indeed, viral invasion causes severe inflammation of the lungs which in turn induces a local activation of hemostasis driven by the interactions between platelets and endothelium. It has been hypothesized that generation of microthrombi during COVID-19 is a potential cornerstone associated with endothelial cells dysfunction. Interestingly, in COVID-19, this process is limited to the lungs [69].
Fig. 2.
ADAMTS-13 based regulation of thrombogenic function of VWF.
In addition to VWF elevation, several other factors like endothelial dysfunction, activation of toll-like receptor, and tissue-factor pathway may cause pro-inflammatory and procoagulant effects, resulting in a dysregulation of the coagulation cascade with the consequent formation of intra-alveolar or systemic fibrin clots [70]. These severe pulmonary changes eventually lead to multiorgan failure involving especially the kidneys and, less frequently, the liver [71].
5. VWF in critical and non-critical patients
Recently a study was performed by Goshua et al., 2020, on hospitalized adult (≥18 years) patients with laboratory-confirmed COVID-19 [72]. They assessed markers of endothelial cell and platelet activation, including VWF antigen, soluble thrombomodulin, soluble P-selectin, and soluble CD40 ligand, as well as coagulation factors, endogenous anticoagulants, and fibrinolytic enzymes in both critical as well as non-critical patients. In this single center cross-sectional study, it was observed that markers of endothelial cell and platelet activation including VWF antigen and soluble P-selectin were significantly elevated in the critical patients. Surprisingly, VWF antigen concentrations were also elevated above the normal range in non-critical patients. It is evident from this study that upregulation of VWF is a constant feature in both critical and non-critical patients of the COVID-19 infection and should be focused-on to develop a holistic therapy for the management and treatment of COVID-19.
6. Targeting VWF for the management of co-morbidities in COVID-19 patients
On the basis of critical role of VWF in various thrombo-inflammatory complications, several approaches to balance the levels of VWF have been reported. In the following sections, some therapeutic options that may lower the blood VWF levels and may be helpful in the management of co-morbidities in the SARS-CoV-2 infected patients are presented.
6.1. Aptamers
Nucleic acid aptamers are single stranded DNA or RNA molecules which may form 3D structures capable of binding specifically to proteins or other cellular targets. It has been recently shown that inhibiting VWF activity by blocking its A1 domain acts as a promising therapeutic target for arterial thromboses. For this purpose, caplacizumab was evaluated for its anti-thrombotic effects in a phase 2 clinical trial (the TITAN study) and a phase 3 clinical trial (the HERCULES study). These studies reported a therapeutic effect of caplacizumab on aTTP, including faster recovery of platelet counts, fewer plasma exchange sessions, and shorter hospital stays. Bleeding event was reported as a common adverse event in patients treated with caplacizumab compared with patients without it. Subsequently, caplacizumab, an anti-VWF A1 domain nanobody, was approved for aTTP in Europe and the United States. ARC1779, an aptamer to the VWF A1 domain, was evaluated in a clinical trial for acquired thrombotic thrombocytopenic purpura (aTTP). The trial showed no serious adverse events such as bleeding, even in patients with aTTP who had severe thrombocytopenia. Recently a novel DNA aptamer, TAGX-0004, was developed that targets the VWF A1 domain. Being a Ds base containing DNA aptamer, TAGX-0004 exhibits high affinity for VWF. Sakai et al. compared TAGX-0004, ARC1779, and caplacizumab in terms of their inhibitory effects on VWF activity, disclosing that the minimum concentration of TAGX-0004 necessary for inhibition was significantly lower than that of ARC1779. Platelet aggregation test PAT analysis revealed that TAGX-0004 could block VWF function via the A1 domain at least 10 times more strongly than ARC1779. Total thrombus formation analysis system T-TAS also revealed that TAGX-0004 was superior to ARC1779 at inhibiting VWF function. It was confirmed from the in vitro analysis that TAGX-0004 has the potency to prevent platelet thrombus formation. In comparison with caplacizumab, TAGX-0004 displayed equally potent inhibition against thrombosis formation under different blood flow conditions. As described above regarding the adverse effects of caplacizumab treatment, there are still concerns regarding bleeding caused by anti-VWF agents. Although anti-VWF agents have demonstrated superior safety profiles in comparison to anti-platelet agents, there is need to prepare measures to quickly treat bleeding that occurs during aptamer treatment [73].
6.2. Anti-GPIIb/IIIa drugs
Blocking the GPIIb/IIIa receptor can prevent platelet aggregation mediated by activating factors. Once platelet aggregation is blocked, generation of platelet thrombi cannot occur. Many GPIIb/IIIa antagonists have been developed, such as abciximab, eptifibatide and tirofiban as the anti-platelet agents. Pharmacodynamic studies on these three agents have reported their ability to develop and sustain a platelet aggregation inhibition of >80%. Abciximab is the first GPIIb/IIIa receptor antagonist used in clinical practice. This drug is the fragment of recombinant human-mouse monoclonal chimeric antibody, which inhibits the GPIIb/IIIa receptors in a dose dependent manner. Abciximab also inhibits platelet receptors aIIb/b3 (for VWF), and thus inhibits platelet aggregation via fibrinogen. However, abciximab has the drawbacks of immunogenicity, drug reaction irreversibility, and high cost. Therefore, micromolecular antagonists of GPIIb/IIIa receptor (e.g., eptifibatide and tirofiban) have been developed. The interaction between platelets and collagen is the key cause of platelet adhesion, aggregation, and activation. This interaction has become the potential target for the development of new anti-platelet therapeutics. There are at least three kinds of receptors present on the surfaces of platelets that can combine with collagen, including GPIb/IX (functions with the VWF), GPIa-IIa (mediates platelet adhesion), and GPVI (the main receptor in platelet activation). Platelets adhesion to the damaged vessel wall is the initial trigger for arterial haemostasis and thrombosis. VWF mediates platelets adherence to the sub-endothelium which forms a bridge between collagens within the damaged blood vessel. Another, 6B4-antigen-binding fragment (Fab) is a murine monoclonal antibody that targets the human platelet GPIb alpha and inhibits the VWF binding. The anti-thrombotic effects of 6B4-Fab on acute platelet-mediated thrombosis have been tested in baboons. It has been proven as an effective anti-thrombotic agent without the side effects of bleeding or thrombocytopenia. Hence, H6B4-Fab can be further developed. Abciximab, on the other hand, causes long (24–48 h) functional inhibition of GPIIb/IIIa receptors, owing to its high affinity [74,75].
7. Conclusion
There is growing evidence supporting the idea that vascular occlusions in pulmonary and systemic circulation are among the most severe and common causes of poor outcome in COVID-19 patients. These vascular events appear to be mostly caused by local formation of thrombi, rather than by venous thromboembolism, which likely form as a consequence of a thrombo-inflammatory process, triggered by SARS-CoV-2 infection-induced endothelial damage and cytokine storm. Among the several players implicated in thrombo-inflammation, VWF has one of the leading roles. VWF plays two important roles in normal hemostasis: it carries factor VIII and mediates platelet-vessel wall and platelet-to-platelet interaction, especially at high shear, through its binding to the platelet membrane glycoprotein (GP) Ib and GPIIb/IIIa.1. The recent clinical reports document an elevated level of VWF antigen in the plasma samples of critically ill patients of COVID-19 admitted to ICU. This hemostatic factor contributes to an increased risk of thrombo-inflammatory co-morbidities in these patients. Besides, an imbalance between the VWF antigen and ADAMTS13 activity is also correlated with the severity of COVID-19 infection. Essentially, VWF/ADAMTS13 ratio is considered as a prognostic marker in COVID-19. Overall, the treatment options like VWF antibodies, anti-platelet drugs, aptamers, etc. that reduces the level of VWF may offer new therapeutic directions in thrombo-inflammatory co-morbidities of COVID-19. Further trials are needed to maintain the level of VWF in the blood of SARS-CoV-2 infected patients in order to choose the best treatment option.
Author contributions
S. C. and P.K.S. conceived the idea and structured the review; K. S. and S. C., contributed in the review of literature and preparation of the first draft; P. K. S. revised the manuscript to final version.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zu Z.Y., Jiang M.D., Xu P.P., Chen W., Ni Q.Q., Lu G.M., Zhang L.J. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 2020:200490. doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh P., Pathania S., Rawal R. Exploring RdRp–remdesivir interactions to screen RdRp inhibitors for the management of novel coronavirus 2019-nCoV. SAR QSAR Environ. Res. 2020;31:857–867. doi: 10.1080/1062936X.2020.1825014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Z., Shan J. Novel coronavirus: where we are and what we know. Infection. 2019;48(2):155–163. doi: 10.1007/s15010-020-01401-y. [Internet] 2020 Apr [cited 2020 Apr 30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Organization W.H. World Health Organization; Geneva: 2020. WHO Director-General’s Remarks at the Media Briefing on 2019-nCoV on 11 February 2020.https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 Available via. Accessed, 10. [Google Scholar]
- 5.Gorbalenya A., Baker S., Baric R., de Groot R., Drosten C., Gulyaeva A., Haagmans B., Lauber C., Leontovich A., Neuman B. The species severe acute respiratory syndrome related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed.: Atenei Parmensis. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson P.G., Qin L., Puah S.H. COVID‐19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre‐COVID‐19 ARDS. Med. J. Aust. 2020 doi: 10.5694/mja2.50674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribes A., Vardon-Bounes F., Mémier V., Poette M., Au-Duong J., Garcia C., Minville V., Sié P., Bura-Rivière A., Voisin S. Thromboembolic events and covid-19. Advances in Biological Regulation. 2020:100735. doi: 10.1016/j.jbior.2020.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guglielmetti G., Quaglia M., Sainaghi P.P., Castello L.M., Vaschetto R., Pirisi M., Della Corte F., Avanzi G.C., Stratta P., Cantaluppi V. “War to the knife” against thromboinflammation to protect endothelial function of COVID-19 patients. Crit. Care. 2020;24:1–4. doi: 10.1186/s13054-020-03060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teuwen L.-A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020:1–3. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. The Lancet Respiratory Medicine. 2020 doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cynthia M., Mulvey J., Berlin D., Nuovo G. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020 doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell C.M., Kahwash R. Will complement inhibition Be the new target in treating COVID-19–related systemic thrombosis? Circulation. 2020;141:1739–1741. doi: 10.1161/CIRCULATIONAHA.120.047419. [DOI] [PubMed] [Google Scholar]
- 14.Escher R., Breakey N., Lämmle B. Thrombosis Research; 2020. ADAMTS13 activity, von Willebrand factor, factor VIII and D-dimers in COVID-19 inpatients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grobler C., Bredenkamp J., Grobbelaar M., Maphumulo S., Laubscher J., Steenkamp J., Kell D., Pretorius E. Platelets and Erythrocytes; 2020. COVID-19: The Rollercoaster of Fibrin (ogen), D-dimer, von Willebrand Factor, P-selectin and Their Interactions with Endothelial Cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morici N., Bottiroli M., Fumagalli R., Marini C., Cattaneo M. Role of von Willebrand factor and ADAMTS-13 in the pathogenesis of thrombi in SARS-CoV-2 infection: time to rethink. Thromb. Haemostasis. 2020;120 doi: 10.1055/s-0040-1713400. [DOI] [PubMed] [Google Scholar]
- 17.Randi A.M., Laffan M.A. Von Willebrand factor and angiogenesis: basic and applied issues. J. Thromb. Haemostasis. 2017;15:13–20. doi: 10.1111/jth.13551. [DOI] [PubMed] [Google Scholar]
- 18.Patmore S., Dhami S.P.S., O'Sullivan J.M. Von Willebrand factor and cancer; metastasis and coagulopathies. J. Thromb. Haemostasis. 2020;18:2444–2456. doi: 10.1111/jth.14976. [DOI] [PubMed] [Google Scholar]
- 19.Xu X., Kozar R., Zhang J., Dong J.f. Diverse activities of von Willebrand factor in traumatic brain injury and associated coagulopathy. J. Thromb. Haemostasis. 2020;18:3154–3162. doi: 10.1111/jth.15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groeneveld D.J., Poole L.G., Luyendyk J.P. Targeting von Willebrand factor in liver diseases: a novel therapeutic strategy? J. Thromb. Haemostasis. 2021 doi: 10.1111/jth.15312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montoro-García S., Shantsila E., Lip G.Y. Potential value of targeting von Willebrand factor in atherosclerotic cardiovascular disease. Expert Opin. Ther. Targets. 2014;18:43–53. doi: 10.1517/14728222.2013.840585. [DOI] [PubMed] [Google Scholar]
- 22.Bae O.-N. Targeting von Willebrand factor as a novel anti-platelet therapy; application of ARC1779, an anti-vWF aptamer, against thrombotic risk. Arch Pharm. Res. (Seoul) 2012;35:1693–1699. doi: 10.1007/s12272-012-1000-3. [DOI] [PubMed] [Google Scholar]
- 23.Qi Y., Chen W., Liang X., Xu K., Gu X., Wu F., Fan X., Ren S., Liu J., Zhang J. Novel antibodies against GPIbα inhibit pulmonary metastasis by affecting vWF-GPIbα interaction. J. Hematol. Oncol. 2018;11:1–17. doi: 10.1186/s13045-018-0659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Firbas C., Siller-Matula J.M., Jilma B. Targeting von Willebrand factor and platelet glycoprotein Ib receptor. Expet Rev. Cardiovasc. Ther. 2010;8:1689–1701. doi: 10.1586/erc.10.154. [DOI] [PubMed] [Google Scholar]
- 25.Xu X., Wang C., Wu Y., Houck K., Hilton T., Zhou A., Wu X., Han C., Yang M., Yang W. Conformation-dependent blockage of activated VWF improves outcomes of traumatic brain injury in mice, Blood. The Journal of the American Society of Hematology. 2021;137:544–555. doi: 10.1182/blood.2020007364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eerenberg E.S., Levi M. Seminars in Thrombosis and Hemostasis. Thieme Medical Publishers; 2014. The potential therapeutic benefit of targeting ADAMTS13 activity; pp. 28–33. [DOI] [PubMed] [Google Scholar]
- 27.Diener J., Daniel Lagasse H., Duerschmied D., Merhi Y., Tanguay J.F., Hutabarat R., Gilbert J., Wagner D., Schaub R. Inhibition of von Willebrand factor‐mediated platelet activation and thrombosis by the anti‐von Willebrand factor A1‐domain aptamer ARC1779. J. Thromb. Haemostasis. 2009;7:1155–1162. doi: 10.1111/j.1538-7836.2009.03459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ladikou E.E., Sivaloganathan H., Milne K.M., Arter W.E., Ramasamy R., Saad R., Stoneham S.M., Phillips B., Eziefula A.C., Chevassut T. Von Willebrand Factor (vWF): marker of endothelial damage and thrombotic risk in COVID-19? Clin. Med. 2020 doi: 10.7861/clinmed.2020-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zachariah U., Nair S., Goel A., Balasubramanian K., Mackie I., Elias E., Eapen C. Targeting raised von Willebrand factor levels and macrophage activation in severe COVID-19: consider low volume plasma exchange and low dose steroid. Thromb. Res. 2020;192:2. doi: 10.1016/j.thromres.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiNicolantonio J.J., McCarty M. Thrombotic complications of COVID-19 may reflect an upregulation of endothelial tissue factor expression that is contingent on activation of endosomal NADPH oxidase. Open Heart. 2020;7 doi: 10.1136/openhrt-2020-001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J., Saguner A.M., An J., Ning Y., Yan Y., Li G. Dysfunctional coagulation in COVID-19: from cell to bedside. Adv. Ther. 2020:1. doi: 10.1007/s12325-020-01399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Z., Long F., Yang Y., Chen X., Xu L., Yang M. Serum ferritin as an independent risk factor for severity in COVID-19 patients. J. Infect. 2020;81:647–679. doi: 10.1016/j.jinf.2020.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iba T., Levy J.H., Levi M., Thachil J. Coagulopathy in COVID‐19. J. Thromb. Haemostasis. 2020;18:2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han H. ClinChemLabMed; 2020. Prominent Changes in Blood coagulationofpatientswithSARS-CoV-2infection. [Google Scholar]
- 35.Guan W.-j., Ni Z.-y., Hu Y., Liang W.-h., Ou C.-q., He J.-x., Liu L., Shan H., Lei C.-l., Hui D.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin. Chim. Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadler J.E. Biochemistry and genetics of von Willebrand factor. Annu. Rev. Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 41.Koster T., Vandenbroucke J., Rosendaal F., Briët E., Blann A. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet. 1995;345:152–155. doi: 10.1016/s0140-6736(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 42.Hayward C., Kelton J. Multimerin: a multimeric protein stored in platelet alpha-granules. Platelets. 1995;6:1–10. doi: 10.3109/09537109509013255. [DOI] [PubMed] [Google Scholar]
- 43.Bienkowska J., Cruz M., Atiemo A., Handin R., Liddington R. The von Willebrand factor A3 domain does not contain a metal ion-dependent adhesion site motif. J. Biol. Chem. 1997;272:25162–25167. doi: 10.1074/jbc.272.40.25162. [DOI] [PubMed] [Google Scholar]
- 44.Emsley J., Cruz M., Handin R., Liddington R. Crystal structure of the von Willebrand Factor A1 domain and implications for the binding of platelet glycoprotein Ib. J. Biol. Chem. 1998;273:10396–10401. doi: 10.1074/jbc.273.17.10396. [DOI] [PubMed] [Google Scholar]
- 45.Huizinga E.G., Van Der Plas R.M., Kroon J., Sixma J.J., Gros P. Crystal structure of the A3 domain of human von Willebrand factor: implications for collagen binding. Structure. 1997;5:1147–1156. doi: 10.1016/s0969-2126(97)00266-9. [DOI] [PubMed] [Google Scholar]
- 46.Romijn R.A., Bouma B., Wuyster W., Gros P., Kroon J., Sixma J.J., Huizinga E.G. Identification of the collagen-binding site of the von Willebrand factor A3-domain. J. Biol. Chem. 2001;276:9985–9991. doi: 10.1074/jbc.M006548200. [DOI] [PubMed] [Google Scholar]
- 47.Fukuda K., Doggett T.A., Bankston L.A., Cruz M.A., Diacovo T.G., Liddington R.C. Structural basis of von Willebrand factor activation by the snake toxin botrocetin. Structure. 2002;10:943–950. doi: 10.1016/s0969-2126(02)00787-6. [DOI] [PubMed] [Google Scholar]
- 48.Celikel R., Varughese K.I., Yoshioka A., Ware J., Ruggeri Z.M. Crystal structure of the von Willebrand factor A1 domain in complex with the function blocking NMC-4 Fab. Nat. Struct. Biol. 1998;5:189–194. doi: 10.1038/nsb0398-189. [DOI] [PubMed] [Google Scholar]
- 49.Maita N., Nishio K., Nishimoto E., Matsui T., Shikamoto Y., Morita T., Sadler J.E., Mizuno H. Crystal structure of von Willebrand factor A1 domain complexed with snake venom, bitiscetin: insight into glycoprotein Ibα binding mechanism induced by snake venom proteins. J. Biol. Chem. 2003;278:37777–37781. doi: 10.1074/jbc.M305566200. [DOI] [PubMed] [Google Scholar]
- 50.Staelens S., Hadders M.A., Vauterin S., Platteau C., De Maeyer M., Vanhoorelbeke K., Huizinga E.G., Deckmyn H. Paratope determination of the antithrombotic antibody 82D6A3 based on the crystal structure of its complex with the von Willebrand factor A3-domain. J. Biol. Chem. 2006;281:2225–2231. doi: 10.1074/jbc.M508191200. [DOI] [PubMed] [Google Scholar]
- 51.Huang R.-H., Fremont D.H., Diener J.L., Schaub R.G., Sadler J.E. A structural explanation for the antithrombotic activity of ARC1172, a DNA aptamer that binds von Willebrand factor domain A1, Structure. 2009;17:1476–1484. doi: 10.1016/j.str.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Q., Zhou Y.-F., Zhang C.-Z., Zhang X., Lu C., Springer T.A. Structural specializations of A2, a force-sensing domain in the ultralarge vascular protein von Willebrand factor. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106:9226–9231. doi: 10.1073/pnas.0903679106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jakobi A.J., Mashaghi A., Tans S.J., Huizinga E.G. Calcium modulates force sensing by the von Willebrand factor A2 domain. Nat. Commun. 2011;2:1–9. doi: 10.1038/ncomms1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Y.-F., Springer T.A. Highly reinforced structure of a C-terminal dimerization domain in von Willebrand factor, Blood. The Journal of the American Society of Hematology. 2014;123:1785–1793. doi: 10.1182/blood-2013-11-523639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong X., Leksa N.C., Chhabra E.S., Arndt J.W., Lu Q., Knockenhauer K.E., Peters R.T., Springer T.A. The von Willebrand factor D′ D3 assembly and structural principles for factor VIII binding and concatemer biogenesis, Blood. The Journal of the American Society of Hematology. 2019;133:1523–1533. doi: 10.1182/blood-2018-10-876300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee H.T., Park U.B., Jeong T.J., Gu N., Lee S.H., Kim Y., Heo Y.-S. High-resolution structure of the vWF A1 domain in complex with caplacizumab, the first nanobody-based medicine for treating acquired TTP. Biochem. Biophys. Res. Commun. 2021;567:49–55. doi: 10.1016/j.bbrc.2021.06.030. [DOI] [PubMed] [Google Scholar]
- 57.Zhu S., Gilbert J.C., Hatala P., Harvey W., Liang Z., Gao S., Kang D., Jilma B. The development and characterization of a long acting anti‐thrombotic von Willebrand factor (VWF) aptamer. J. Thromb. Haemostasis. 2020;18:1113–1123. doi: 10.1111/jth.14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fogarty H., Doherty D., O'Donnell J.S. New developments in von Willebrand disease. Br. J. Haematol. 2020;191:329–339. doi: 10.1111/bjh.16681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Østergaard L. SARS CoV‐2 related microvascular damage and symptoms during and after COVID‐19: consequences of capillary transit‐time changes, tissue hypoxia and inflammation. Physiological reports. 2021;9 doi: 10.14814/phy2.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borczuk A.C., Salvatore S.P., Seshan S.V., Patel S.S., Bussel J.B., Mostyka M., Elsoukkary S., He B., Del Vecchio C., Fortarezza F. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Mod. Pathol. 2020;33:2156–2168. doi: 10.1038/s41379-020-00661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Escher R., Breakey N., Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goshua G., Pine A.B., Meizlish M.L., Chang C.-H., Zhang H., Bahel P., Baluha A., Bar N., Bona R.D., Burns A.J. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. The Lancet Haematology. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ladikou E.E., Sivaloganathan H., Milne K.M., Arter W.E., Ramasamy R., Saad R., Stoneham S.M., Philips B., Eziefula A.C., Chevassut T. Von Willebrand factor (vWF): marker of endothelial damage and thrombotic risk in COVID-19? Clin. Med. 2020;20:e178. doi: 10.7861/clinmed.2020-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Becker R.C. COVID-19 update: covid-19-associated coagulopathy. J. Thromb. Thrombolysis. 2020;50:54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morici N., Bottiroli M., Fumagalli R., Marini C., Cattaneo M. Role of von Willebrand factor and ADAMTS-13 in the pathogenesis of thrombi in SARS-CoV-2 infection: time to rethink. Thromb. Haemostasis. 2020;120:1339–1342. doi: 10.1055/s-0040-1713400. [DOI] [PubMed] [Google Scholar]
- 66.Varatharajah N. Emperipolesis, Megakaryocytes,“Self-Association” of Von Willebrand Factor and beyond. 2020. COVID-19 clot: what is it? Why in the lungs? Extracellular Histone,“Auto-activation” of prothrombin. [Google Scholar]
- 67.Agbuduwe C., Basu S. Haematological manifestations of COVID‐19: from cytopenia to coagulopathy. Eur. J. Haematol. 2020;105:540–546. doi: 10.1111/ejh.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alexander V., Zachariah U., Goel A., Kandasamy S., Chacko B., Punitha J.V., Nair S., David V., Prabhu S., Balasubramanian K. Low-volume plasma exchange and low-dose steroid to treat secondary hemophagocytic lymphohistiocytosis: a potential treatment for severe COVID-19? Current Medical Issues. 2020;18:77. [Google Scholar]
- 69.Thachil J., Srivastava A. Seminars in Thrombosis and Hemostasis. Thieme Medical Publishers; 2020. SARS-2 coronavirus–associated hemostatic lung abnormality in COVID-19: is it pulmonary thrombosis or pulmonary embolism? pp. 777–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sakr Y., Giovini M., Leone M., Pizzilli G., Kortgen A., Bauer M., Tonetti T., Duclos G., Zieleskiewicz L., Buschbeck S. Pulmonary embolism in patients with coronavirus disease-2019 (COVID-19) pneumonia: a narrative review. Ann. Intensive Care. 2020;10:1–13. doi: 10.1186/s13613-020-00741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lax S.F., Skok K., Zechner P., Kessler H.H., Kaufmann N., Koelblinger C., Vander K., Bargfrieder U., Trauner M. Annals of Internal Medicine. 2020. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goshua G., Pine A.B., Meizlish M.L., Chang C.-H., Zhang H., Bahel P., Baluha A., Bar N., Bona R.D., Burns A.J. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. The Lancet Haematology. 2020 doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sakai K., Someya T., Harada K., Yagi H., Matsui T., Matsumoto M. Novel aptamer to von Willebrand factor A1 domain (TAGX-0004) shows total inhibition of thrombus formation superior to ARC1779 and comparable to caplacizumab. Haematologica. 2020;105 doi: 10.3324/haematol.2019.235549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ji X., Hou M. Novel agents for anti-platelet therapy. J. Hematol. Oncol. 2011;4:44. doi: 10.1186/1756-8722-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cox D. Anti‐platelet agents: past, present and future. ISBT Sci. Ser. 2020;15:131–141. [Google Scholar]