Abstract
Bruton’s tyrosine kinase (Btk) is required for normal B-cell development, as defects in Btk lead to X-linked immunodeficiency (xid) in mice and X-linked agammaglobulinemia (XLA) in humans. Here we demonstrate a functional interaction between the multifunctional transcription factor TFII-I and Btk. Ectopic expression of wild-type Btk enhances TFII-I-mediated transcriptional activation and its tyrosine phosphorylation in transient-transfection assays. Mutation of Btk in either the PH domain (R28C, as in the murine xid mutation) or the kinase domain (K430E) compromises its ability to enhance both the tyrosine phosphorylation and the transcriptional activity of TFII-I. TFII-I associates constitutively in vivo with wild-type Btk and kinase-inactive Btk but not xid Btk. However, membrane immunoglobulin M cross-linking in B cells leads to dissociation of TFII-I from Btk. We further show that while TFII-I is found in both the nucleus and cytoplasm of wild-type and xid primary resting B cells, nuclear TFII-I is greater in xid B cells. Most strikingly, receptor cross-linking of wild-type (but not xid) B cells results in increased nuclear import of TFII-I. Taken together, these data suggest that although the PH domain of Btk is primarily responsible for its physical interaction with TFII-I, an intact kinase domain of Btk is required to enhance transcriptional activity of TFII-I in the nucleus. Thus, mutations impairing the physical and/or functional association between TFII-I and Btk may result in diminished TFII-I-dependent transcription and contribute to defective B-cell development and/or function.
The B-cell antigen receptor (BCR) complex consists of membrane immunoglobulin (Ig) and the Igα/β heterodimer. The cytoplasmic tails of the Igα and Igβ polypeptides contain immunoreceptor tyrosine activation motifs that are critical for signaling (47). Surface engagement of the BCR leads to tyrosine phosphorylation of the immunoreceptor tyrosine activation motifs. This is correlated with activation and recruitment of nonreceptor tyrosine kinases, including Syk (49) and various members of the Src family (10). Cross-linking of the BCR also leads to the activation of the nonreceptor molecule Bruton’s tyrosine kinase (Btk) (5, 12, 55).
Btk is the target of multiple mutations in humans, each of which results in X-linked agammaglobulinemia (XLA) (67, 69). A spontaneous mutation in mice (R28C) produces X-linked immunodeficiency (xid) (46, 66). In XLA, B-cell development is arrested at the pre-B-cell stage, resulting in a near absence of B cells and a failure to produce serum Ig. The xid phenotype is characterized by a less severe defect in which B cells are generated, but only to around 50% of normal, and only certain istotypes of serum Ig (IgM and IgG3) are drastically diminished. In xid mice, the B-1 population is largely absent and conventional B cells (B-2 or B-0) are functionally compromised such that they fail to proliferate in response to stimulation via the BCR or CD38 (24, 77) and are hyporesponsive to CD40L (19), interleukin-5 (23, 33), interleukin-10 (16), and lipopolysaccharide (2, 25). Thus, Btk appears to be critical for multiple signaling pathways important for B-cell differentiation and proliferation. In addition, Btk is an effector of FcERI in mast cells (27). The basis for the difference in the phenotypic manifestations of mutation of murine and human Btk is not well understood. An R28C mutation in humans results in the full XLA phenotype (71). Conversely, deletional mutation of the mouse Btk gene produces the typical xid mouse (28, 29). However, coexpression of xid and a mutation (nude) that results in the absence of T cells or deletional mutation of CD40 produces a phenotype resembling that of XLA (30, 43, 75). Together, these results suggest that there is a redundant B-cell developmental pathway in the mouse that is not present in humans.
Btk belongs to the Tec family of nonreceptor tyrosine kinases that includes TecI (39), TecII (40), Itk (60), Bmx/Etk (45, 64), and DSrc28C (found in drosophila) (17). Members of this family contain SH1, SH2, and SH3 domains but lack the typical myristylation site and negative regulatory tyrosine of src family members (39). A distinctive feature of these kinases is the presence of a pleckstrin homology (PH) domain in the N-terminal region, followed by a unique Tec homology (TH) domain. The TH domain contains a region that may be involved in metal binding (70) and is followed by a proline-rich region that is likely to mediate protein-protein binding (reviewed in reference 11).
Although PH domains have been described in many proteins involved in membrane localization, signal transduction, and cytoskeletal structure (21, 26, 34, 58), the precise function of the PH domain in Btk may not be fully understood. As with several other PH domains, it binds with high affinity to phosphatidylinositol[3,4,5]triphosphate (PIP3) in an R28C-sensitive manner (54). Thus, the generation of PIP3 by phosphatidylinositol 3-kinase (PI3K) following cross-linking of the BCR can lead to PH domain-mediated membrane localization of Btk and potentiation of its activation (36). In parallel, phosphorylation of tyrosine 551 by a src family kinase induces the autophosphorylation of tyrosine 223 and augmented kinase activity (22, 38). There is evidence that Btk, by this regulated association with the cellular membrane, is able to modulate activation of phospholipase C (PLC) and, subsequently, calcium release and influx, resulting in the generation of a sustained stimulatory signal (48, 63, 76). Thus, Btk may function at a critical juncture of events regulating calcium signaling by inositol trisphosphate and depletion of intracellular stored calcium (14). Moreover, increasing PIP3 by genetic depletion of the SH2-containing inositol polyphosphate phosphatase leads to increased association of Btk with the membrane and hyperresponsiveness to BCR stimulation (6, 56). However, it is not clear that Btk function is limited to a role in PLC activation. For instance, Btk is necessary for B-cell activation via CD38, a pathway that does not involve release of Ca2+ stores (32).
Despite the biological importance of Btk in B-cell differentiation and its important role in calcium signaling and phospholipid metabolism, the precise function of this kinase remains to be determined at the biochemical level. Therefore, it is of considerable interest to dissect the Btk-dependent pathway(s) and identify functional targets and downstream consequences of Btk action. Recently, a protein called BAP-135 was shown to interact with Btk in vivo via the TH and PH domains (78). BAP-135 was tyrosine phosphorylated by Btk in vitro and transiently tyrosine phosphorylated after IgM stimulation of B cells in vivo, suggesting that BAP-135 is a potential physiological target of Btk (78). However, these studies failed to assign any direct function to BAP-135. Sequence comparison (database search) revealed that BAP-135 is identical to TFII-I (18, 53), a ubiquitously expressed multifunctional transcription factor that is capable of binding to several promoter elements, including initiator (Inr) elements (50–52). TFII-I has also been cloned as a functional gene that is deleted in Williams-Beuren syndrome (44). Functional studies demonstrated that TFII-I is capable of transcriptionally activating model promoters in vitro and in vivo, including the murine T-cell receptor-derived Vβ 5.2 promoter (9, 41). Recent cDNA cloning and functional expression experiments demonstrated that TFII-I functions as a basal factor through the Inr element (53), as well as an activator through upstream promoter elements in the absence of a functional Inr (18, 31, 53). TFII-I is phosphorylated on serine/threonine, as well as tyrosine, residues in various cell types and appears to be downstream of several signal transduction pathways (31, 42). Together, these observations suggest that TFII-I links signal responses to transcription of several eukaryotic genes (18, 31, 42, 53). Here we examined whether TFII-I is a functional target of Btk. Our findings have led us to suggest a novel pathway through which B-cell-specific signals mediated by Btk might communicate with target genes via TFII-I.
MATERIALS AND METHODS
Mice and cells.
A colony of BALB/cAnN-Xid mice derived from breeding pairs originally supplied to us by Carl Hansen of the National Institutes of Health Genetic Resource Center is maintained at Tufts University. BALB/cBy mice were either obtained directly from The Jackson Laboratory or bred from this stock at Tufts University. The Ramos, COS7, and BAL-17 cell lines were obtained from David Thorley-Lawson (Tufts University), Brent Cochran (Tufts University), and Ranjan Sen (Brandeis University), respectively.
Constructs.
The eukaryotic expression vector pEBGII-I (pEBG containing TFII-I cDNA) is derived from pEBG (65) in which the human EF-1a promoter drives the expression of protein fused to glutathione S-transferase (GST) (p146) (9). The hemagglutinin HA-tagged wild-type murine Btk construct in the pGD backbone (8) was generously provided by Genhong Cheng (University of California Los Angeles) and was used exclusively as the source of wild-type Btk for all experiments. Two different lines of mutant Btk constructs were used. R28C and K430E mutant constructs (see Fig. 2 and 4) were generated by site-directed mutagenesis of the murine Btk construct. Mutant constructs derived from different constructs were also used (see Fig. 1 and 3). These were prepared as follows. A cDNA encoding wild-type human Btk was amplified by PCR from a human Epstein-Barr virus-transformed cell line. The cDNA was sequenced, and a K430E mutant construct was generated by site-directed mutagenesis. This was cloned into the BamHI site of the pCMV-HA vector. A cDNA encoding an xid mutant form of Btk was obtained by amplification of first-strand cDNA derived from spleen RNA from an xid mouse. The cDNA was sequenced and inserted into the BamHI site of the pCMV-HA vector. In physical-interaction studies (see Fig. 3 and 4), no apparent differences were observed. A reporter construct containing the murine T-cell receptor Vβ 5.2 basal promoter (−100 to +9) and the firefly luciferase gene (pGL3; Promega) was previously described (9). A vector containing the herpes simplex virus thymidine kinase promoter and the Renilla luciferase gene (pRL-TK; Promega) has also been described (9).
FIG. 2.
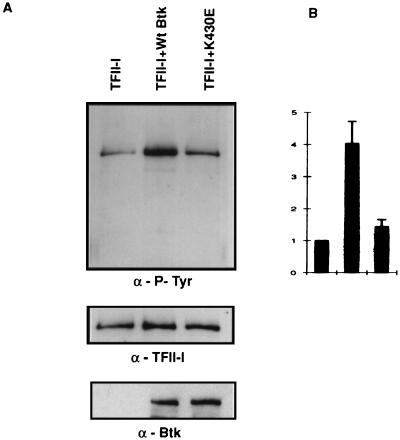
Ectopic expression of wild-type, but not K430E mutant, Btk leads to enhanced tyrosine phosphorylation of TFII-I. (A) TFII-I and either wild-type or K430E mutant Btk was coexpressed in COS cells, and TFII-I was pulled down by GST-agarose beads and probed with anti-P-Tyr (α-P-Tyr) antibody 4G10 in a Western blot analysis. The blot was stripped and reprobed with anti-TFII-I (α-TFII-I) antibody. The lysates were also tested for the expression of wild-type and K430E Btks. (B) For quantitation, these experiments were performed three times and the results are represented as graphs with error bars.
FIG. 4.
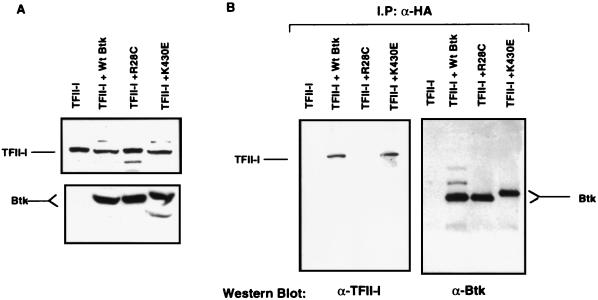
TFII-I and Btk associate in the cytoplasm. Wild-type Btk (WtBtk) or R28C or K430E mutant Btk was ectopically expressed in COS7 cells. Cytoplasmic extracts (normalized by total protein concentration) were prepared, the ectopically expressed Btk was immunoprecipitated by anti-HA antibody, and the coimmunoprecipitated endogenous TFII-I was visualized by anti-TFII-I (α-TFII-I) antibody. The blot was stripped and reprobed with anti-Btk (α-Btk) antibody.
FIG. 1.
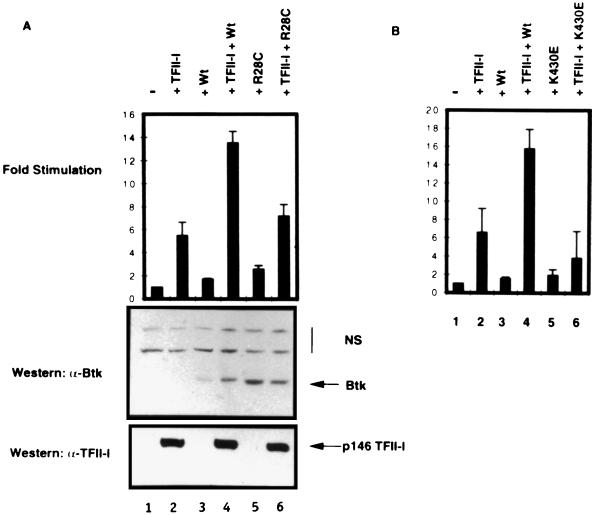
Wild-type Btk, but not mutant Btks, potentiates TFII-I-dependent transcriptional stimulation of Vβ 5.2 in COS7 cells. (A) Transient transfection of COS7 cells. Shown are basal-level expression of the Vβ 5.2 promoter (−, lane 1) and expression in the presence of ectopic TFII-I alone (+TFII-I, lane 2), wild-type Btk (+Wt, lane 3), or xid mutant Btk (+R28C, lane 5). Cotransfection of wild-type Btk with TFII-I (TFII-I + Wt, lane 4), but not xid mutant Btk with TFII-I (+TFII-I + R28C, lane 6), further potentiates TFII-I-mediated activation of the Vβ 5.2 reporter. Western blotting of transfection extracts with an anti-Btk antibody (α-Btk) or an anti-TFII-I antibody (α-TFII-I) demonstrates equivalent levels of ectopic TFII-I expression in the indicated lanes. NS, nonspecific bands. (B) Wild-type Btk, but not kinase-deficient (K430E) Btk, potentiates TFII-I-mediated stimulation of the Vβ 5.2 promoter. The Vβ 5.2 promoter basal expression (−, lane 1) is stimulated by TFII-I (+TFII-I, lane 2). Neither wild-type (+Wt, lane 3) nor K430E mutant (+K430E, lane 5) Btk affects Vβ 5.2 promoter expression independently. Cotransfection of TFII-I with wild-type Btk (TFII-I + Wt, lane 4) but not kinase-deficient Btk (+TFII-I + K430E, lane 6) further potentiates TFII-I-mediated activation of the Vβ 5.2 promoter.
FIG. 3.
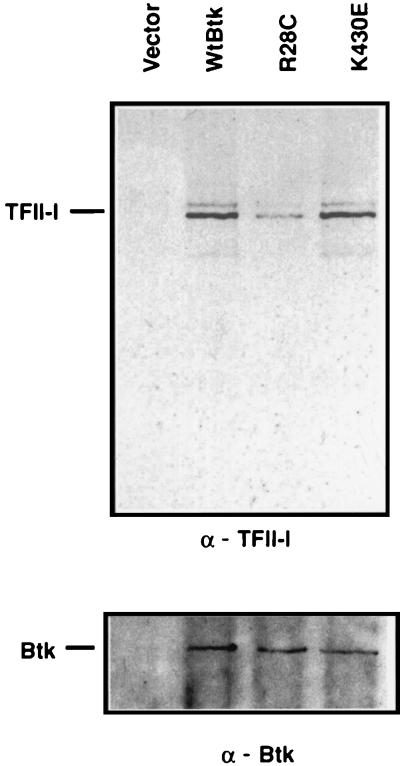
TFII-I interacts with both wild-type and K430E mutant Btks but not with R28C mutant Btk. (A) Normalization of extracts expressing ectopic TFII-I and Btk proteins. COS7 cells ectopically expressing either TFII-I alone (TFII-I) or together with HA-tagged wild-type Btk (TFII-I + Wt Btk), xid mutant Btk (TFII-I + R28C), or kinase-deficient Btk (TFII-I + K430E) were Western blotted with an anti-Btk antibody (Btk). The blot was then stripped and reprobed with an anti-TFII-I antibody (TFII-I). (B) Normalized extracts from panel A were employed for immunoprecipitation (I.P.) studies with an anti-HA (α-HA) antibody and probed either with an anti-TFII-I (α-TFII-I) antibody (left) or with an anti-Btk (α-Btk) antibody (right). The α-TFII-I blot (left) was stripped of immune complexes and reprobed with an anti-Btk antibody (Btk; right). Comparable amounts of Btk protein were precipitated with the anti-HA antibody from extracts ectopically expressing either wild-type or mutant Btk.
Transient transfections.
Transfections were carried out with Lipofectamine in accordance with the manufacturer’s (GIBCO BRL) protocol. A 10-μg sample of expression plasmid pEBGII-I was used for transient transfection per 100-mm-diameter plate. The pGL3-Vβ 5.2 reporter (200 ng) and pTK-RL were transfected either alone or with wild-type Btk (1 μg), R28C Btk (100 ng), K430E Btk (750 ng), or p146 (350 ng) as previously described (9). Total transfected DNA was normalized through the use of empty vectors pEBB (65) and pGD (8).
Briefly, COS7 cells were grown in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) to approximately 50% confluency in a six-well plate (Costar). The medium was changed 3 h prior to transfection. The indicated DNA constructs were added to 100 μl of DMEM in tube A, and 15 μl Lipofectamine was added to 85 μl of DMEM in tube B. Tubes A and B were mixed and incubated for 45 min at room temperature. During this incubation period, COS7 cells were washed twice in 2 ml of Dulbecco phosphate-buffered saline (PBS). After the incubation, 1 ml of DMEM was added to the A-and-B mixture, which was then added to the washed COS7 cells. After 12 h of incubation, 1.2 ml of DMEM containing 20% FBS was added and the mixture was incubated for an additional 12 h. After this period, the medium was aspirated and 2 ml of DMEM containing 10% FBS was added to the plates and the cultures were incubated for an additional 12 to 24 h. For reporter assays, luciferase activity was assessed as previously described (Promega). All raw values obtained from firefly luciferase were adjusted relative to Renilla luciferase values. The results reported here are averages of three experiments.
Preparation and normalization of whole-cell lysates.
For coimmunoprecipitation assays with COS7 cells, the lysates were prepared as follows. Because the various expression constructs are driven by different promoters, we adjusted the amounts of transfected DNA according to protein expression. Therefore, wild-type Btk (45 μg) and R28C Btk (1.5 μg) were transfected with 10 μg of p146 while K430E Btk (6 μg) was transfected with 3.5 μg of p146. When transfected without a Btk-containing construct, p146 was transfected at 10 μg. Total amounts of transfected DNA were normalized through the use of an empty vector as described above. COS7 cells were washed on the plates twice with 10 ml of PBS per plate. Three plates were used for each expression. The cells were collected by scraping in PBS into a final volume of 50 ml. The scraped cells were pelleted, and supernatants were aspirated. At 36 h posttransfection, cells were harvested, washed twice in PBS buffer, and lysed in lysis buffer (25 mM Tris [pH 8.0, 25°C], 150 mM NaCl, 1.2% Nonidet P-40, 5 mM NaF, 2 mM Na3VO4) containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride; 1% aprotinin; and leupeptin, antipain, and soybean trypsin inhibitor at 10 μg/ml each). The lysate was clarified by centrifugation for 30 min at 12,000 rpm (Eppendorf microcentrifuge 5415C) at 4°C. Supernatants were collected, and the total protein concentrations in each case were measured (Bio-Rad). In order to normalize for p146 expression, Western analysis with an anti-TFII-I antibody (41) was performed. Similarly, Btk expression was normalized by Western analysis with an anti-Btk antibody (Santa Cruz). Levels of ectopic Btk and TFII-I were calculated by densitometry of the autoradiographs derived from Western analysis for the immunoprecipitation studies.
Coimmunoprecipitation. (i) COS7 cells.
Protein A Sepharose beads containing an anti-HA antibody were prepared by addition of 50 μl of protein A Sepharose (1:1 slurry) to 50 μl of culture supernatants of the B-cell hybridoma 12CA5 expressing an anti-HA monoclonal antibody. The suspension was incubated for 2 h with rocking at 4°C. Antibody-coupled beads were microcentrifuged at 9,000 rpm at 4°C, and the supernatant was aspirated. Pellets were washed in 1 ml of PBS by vortexing for 5 s and incubation on ice for 5 min. The beads were microcentrifuged and washed four times. Matched whole-cell extracts (described above; 50 μg of each) or cytoplasmic extracts (150 μg) were added to the anti-HA antibody-coupled and washed beads in the presence of protease inhibitors (10 mM pepstatin A, 10 mM iodoacetamide, 10 mM leupeptin, soy bean trypsin inhibitor at 10 μg/ml, and 1 mM EDTA). This suspension was incubated for 12 h with rocking at 4°C. The beads were washed six times with lysis buffer containing protease inhibitors. Laemmli buffer was added to the bead pellets, and the lysate was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis.
B-cell lines.
Whole-cell lysates were prepared from human (Ramos) or murine (BAL-17) B cells and subjected to immunoprecipitation with an anti-Btk (PharMingen) or a control antibody. Whole-cell lysates (Ramos, 800 μg; BAL-17, 1 mg) or cytoplasmic lysates (unstimulated or anti-Ig antibody stimulated; 200 μg of each) were added to the anti-Btk or control antibody in lysis buffer with protease inhibitors (antipain at 1 mg/ml, 10 mM pepstatin, 0.5 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride, aprotinin at 1 μg/ml, 10 mM leupeptin, and soy bean trypsin inhibitor at 10 μg/ml) and incubated for 2 h with rocking at 4°C. Protein A Sepharose (50 ml; 1:1 slurry) was added, and the incubation was continued for 2 h at 4°C with rocking. The reaction mixture was centrifuged for 2 min at 10,000 rpm (Eppendorf microcentrifuge 5415C) and 4°C, and the supernatant was aspirated. The beads were washed three times in 1 ml of lysis buffer, each time with 5 s of vortexing and 5 min of incubation on ice. After the final wash, the supernatant was aspirated, 50 μl of 2× Laemmli buffer was added, and the mixture was subjected to SDS-PAGE followed by Western blot analysis.
Western blot analysis.
Samples resuspended in Laemmli buffer were heated to 100°C for 5 min and subjected to SDS-PAGE. Wet transfer to nitrocellulose was accomplished by electrophoresis in buffer containing 0.025 M Tris (pH 8.0, 25°C), 0.192 M glycine, and 20% methanol for 3 h at a constant 0.43 A and 4°C. Transfer to nitrocellulose was accomplished in a semidry blotter (C.B.S. Scientific) in buffer containing 0.025 M Tris base (pH 8.0, 25°C), 0.192 M glycine, and 20% methanol for 45 min at 115 mA and room temperature. The nitrocellulose blot was blocked for 30 min in TBS (10 mM Tris [pH 8.0], 150 mM NaCl) containing 6% nonfat dry milk (Carnation). For phosphospecific blocking 5% bovine serum albumin (fraction 5; Sigma) was used. For TFII-I Western blotting, primary (anti-TFII-I, 1:2,500 dilution), anti-Btk (1:2,000 dilution; Santa Cruz), anti-phosphotyrosine monoclonal (4G10, 0.5 μg/ml; Upstate Biotechnology), and secondary (1:1,500 dilution; Zymed) anti-rabbit horseradish peroxidase-linked antibodies were incubated in TBS containing 0.05% Tween 20. All Western blots were developed by enhanced chemiluminescence (Amersham).
For removal of immune complexes from Western blot membranes, each blot was incubated with 62.3 mM Tris (pH 6.9)–2% SDS–100 mM β-mercaptoethanol for 2 h at 55°C with one exchange of buffer after 1 h. The membrane stripped of immune complexes was washed in TBS and reblocked as required for the next round of Western analysis.
Immunostaining.
Antibody staining of splenic B cells was performed essentially as previously described (61). The primary antibody, anti-TFII-I rabbit serum (IgG fraction, purified over Affigel Blue; Bio-Rad), or a nonspecific control (rabbit anti-human idiotype, generously provided by David Stollar, Tufts University School of Medicine) was diluted 1:10. The secondary antibody, fluorescein isothiocyanate (FITC)-conjugated AffiniPure F(ab′)2 donkey anti-rabbit IgG (Jackson ImmunoResearch), was used at a concentration of 10 μg/ml. After completion of the cell suspension staining procedure, 2 × 105 stained cells were cytospun (Shandon Cytospin 2) onto Superfrost Plus slides (Fisher) at 300 rpm for 5 min. A drop of mounting buffer (50% glycerol in PBS) with propidium iodide (35 ng/ml) was added, and a coverslip was placed on top and carefully sealed. These cells were visualized by using the Noran Odyssey XL laser scanning confocal microscope with a 60× Nikon objective (numerical aperture, 1.4). Images were obtained with Noran Intervision 2D Image Analysis modules and averaged 16 times to improve image quality. A composite image of fluorescein and propidium iodide stains was generated. Nuclear TFII-I (FITC) staining was quantitated by calculating the number of FITC pixels in the propidium iodide staining region.
Anti-IgM antibody stimulation of B cells.
For anti-IgM antibody activation, splenic B cells were purified from BALB/cByJ and BALB/c.xid mice and placed into complete RPMI medium (107/ml) as previously described (74). For stimulation, (Fab′)2 goat anti-mouse IgM (Jackson ImmunoResearch) at 10 μg/ml was used with 107 splenic B cells. The primary B cells were incubated either with medium alone or with anti-IgM antibody for 10 min at 37°C and then pelleted. Cell pellets were washed three times in Dulbecco PBS, and whole-cell or nuclear extracts were prepared (13).
For anti-IgM antibody stimulation of Ramos cells, they were cultured in complete RPMI 1640 medium. The Ramos cells (107) were treated either with medium alone or with mouse anti-human IgM antibody (HB57) at 10 μg/ml for 10 min at 37°C. Cells were pelleted and washed three times in PBS, and cytoplasmic and nuclear extracts were prepared (13).
RESULTS
Wild-type, but not R28C or K430E, Btk augments TFII-I-dependent transcriptional activation in COS7 cells.
To gain insight into the functional significance of Btk–TFII-I interactions while avoiding complications due to the presence of high endogenous levels of TFII-I and Btk in B cells, we chose to express Btk and TFII-I ectopically. We did this in COS7 cells because these cells have a low level of TFII-I (9) and do not express endogenous Btk. The TFII-I construct expressed a 146-kDa GST–TFII-I fusion protein (9) that allowed us to distinguish it from the endogenous protein, while the Btk constructs were HA tagged.
The described constitutive interaction between Btk and TFII-I/BAP-135 (78) suggested that Btk might regulate the transcriptional activity of TFII-I in vivo. To test this hypothesis, TFII-I (146 kDa) and either the wild-type Btk or the R28C mutant Btk expression plasmid were cotransfected into COS7 cells with the luciferase reporter cassette driven by the TFII-I-responsive Vβ 5.2 basal promoter (9, 42). Consistent with the low abundance of endogenous TFII-I in COS7 cells, the basal activity of this model Vβ 5.2 promoter was low. Transfection of TFII-I increased the expression of the Vβ 5.2 construct sixfold (Fig. 1A, top; compare lanes 2 and 1). As a control for TFII-I specificity, we employed a Vβ 5.2 promoter in which the Inr element is mutated. This mutated promoter (which shows very low basal activity) did not respond to ectopic TFII-I (9 and data not shown). As expected, given the low abundance of endogenous TFII-I, transfection of wild-type Btk in the absence of ectopic TFII-I did not significantly alter the Vβ 5.2 promoter activity (lane 3). However, cotransfection of wild-type Btk with TFII-I stimulated the Vβ 5.2 promoter activity (lane 4) to nearly triple the level seen with TFII-I only (compare lanes 2 with 4). Importantly, R28C mutant Btk did not significantly alter either the basal (lane 5) or the TFII-I-stimulated (lane 6) activity of the Vβ 5.2 promoter, although there was a marginal increase in reporter activity when both TFII-I and R28C mutant Btk were coexpressed. The expression of the wild-type and mutant Btk proteins in these transfectants was shown to be comparable by a Western blot of the extracts probed with an anti-Btk antibody (Fig. 1A, middle). In addition, and more importantly, the levels of ectopic TFII-I expression were nearly identical in all cases (Fig. 1A, bottom). Together, these data suggest that the transcriptional activity of TFII-I is functionally modulated by Btk.
Given the observation that Btk could tyrosine phosphorylate BAP-135/TFII-I in vitro (78), we tested the importance of the tyrosine kinase activity of Btk for the transcriptional function of TFII-I by employing the K430E mutant form of Btk that is deficient in catalytic activity (35). While wild-type Btk enhanced the TFII-I-mediated activity of the Vβ 5.2 basal promoter (compare lanes 2 and 4 in Fig. 1B), the K430E mutant had no such effect (lanes 2 and 6). The significance, if any, of the slight inhibition of the transcriptional activity of the ectopically expressed TFII-I in the presence of K430E is not known. Once again, the differences in transcriptional activity were not due to differences in the expression level of either Btk or TFII-I, since Western blot assays showed that similar amounts of each protein were expressed (data not shown). Thus, an intact kinase domain of Btk is required for its transcriptional effects on TFII-I. We also demonstrated that under our transfection conditions, tyrosine phosphorylation of TFII-I was enhanced by wild-type Btk type but not by K430E mutant Btk (Fig. 2A). Thus, cotransfection of wild-type Btk and TFII-I resulted in fourfold enhancement of tyrosine phosphorylation of TFII-I (Fig. 2B). In contrast, cotransfection of K430E mutant Btk and TFII-I resulted in no significant enhancement of TFII-I tyrosine phosphorylation (Fig. 2B). Basal tyrosine phosphorylation of TFII-I is known to occur and accounts for its basal transcriptional activity (42). Although these data, together with the earlier observations (78), are consistent with the idea that TFII-I is a direct substrate of Btk, an indirect mechanism involving another kinase cannot be ruled out. Regardless of whether Btk directly or indirectly tyrosine phosphorylates TFII-I, these data suggest that both a functional PH domain and an intact kinase domain of Btk may be required to augment the transcriptional activation of TFII-I.
Wild-type Btk and K430E Btk, but not R28C Btk, interact with TFII-I.
The functional data led us to characterize further the physical association of TFII-I with various forms of Btk. Whole-cell extracts derived from COS7 cells cotransfected with TFII-I and HA-tagged Btk were subjected to immunoprecipitation with an anti-HA monoclonal antibody (Fig. 3A). First, we demonstrated that under our conditions, TFII-I (p146) protein was expressed at similar levels when transfected alone or in conjunction with either wild-type Btk or mutant Btk constructs (Fig. 3A, top). Additionally, the levels of Btk protein were also similar under these conditions (Fig. 3A, bottom). The reason for the anomalous migration of K430E mutant Btk is unclear, but it could be that the increase in net negative charge alters SDS binding and therefore mobility on SDS-PAGE. As expected, the ectopically expressed TFII-I was not precipitated by an anti-HA antibody, but when coexpressed with wild-type Btk, it was coimmunoprecipitated by an anti-HA antibody (Fig. 3B, left). In the same experiment, TFII-I failed to coimmunoprecipitate with R28C mutant Btk (Fig. 3B). In contrast, coimmunoprecipitation of TFII-I with K430E mutant Btk occurred as with wild-type Btk. The same blot, when stripped and reprobed with an anti-Btk antibody, revealed that almost identical quantities of wild-type Btk and mutant Btk were immunoprecipitated by the anti-HA antibody under these conditions (Fig. 3B, right). Together, these data indicate that TFII-I physically interacted with wild-type Btk and K430E Btk with equal efficiency but associated poorly (fivefold less) with the R28C (xid) mutant form of Btk. Although Btk appeared to be quantitatively precipitated under these conditions, approximately 20% of the ectopically expressed TFII-I associated with wild-type Btk and K430E Btk compared to the input.
Because these experiments utilized whole-cell extracts, we were concerned that during lysis, the cytoplasmic and nuclear components contacted each other artifactually. Another concern was that overexpression of TFII-I might result in artificially high cytoplasmic TFII-I that might not be observed otherwise. To rule out these possibilities, only Btk (either wild-type or mutant Btk) was transfected in COS7 cells and cytoplasmic extracts were prepared. Endogenous TFII-I was immunoprecipitated from these cytoplasmic extracts by using an anti-HA antibody. The purity of the cytoplasmic extracts was checked by Western blot analysis with an anti-TBP antibody, and they were found to be free of nuclear contamination within the limits of detection (data not shown). As can be seen in Fig. 4, wild-type Btk and K430E mutant Btk coprecipitated endogenous TFII-I to similar extents, while R28C mutant Btk coprecipitated much less TFII-I. Equal amounts of Btk were precipitated in all of the lanes (Fig. 4, bottom). Hence, we concluded that TFII-I and Btk interact in the cytoplasm.
TFII-I constitutively interacts with Btk in B-cell lines.
These data provided a mechanistic insight into a novel Btk-mediated pathway involving transcription factor TFII-I. However, they did not immediately indicate whether the difference in the interactions of wild-type Btk and xid Btk with TFII-I observed in COS7 cells reflected the situation in B cells. Therefore, we analyzed the interactions between Btk and TFII-I in B cells. Although Btk was shown to be associated with TFII-I in human B cells (78), the situation in murine B cells was unknown. We found that endogenous TFII-I and Btk were constitutively associated in murine BAL-17 whole-cell extracts (Fig. 5). We also noted that both of the two previously described forms of TFII-I (with apparent molecular masses of 120 and 128 kDa) (41) were coprecipitated by an anti-Btk, but not a control, antibody. Although both forms were specifically coprecipitated, the relative abundance of these two forms differed substantially between the two species. We have not determined the basis for this difference. Compared to the lysate-only lane, which is one-fifth of the immunoprecipitation input lane, it appeared that only about 15% of endogenous TFII-I constitutively associated with Btk in unstimulated B-cell lines.
FIG. 5.
TFII-I is constitutively associated with Btk in both human and murine B cells but dissociates from Btk upon anti-Ig antibody stimulation. (A) Whole-cell lysates prepared from human (Ramos) and murine (BAL-17) B cells were subjected to immunoprecipitation (IP) with an anti-Btk (α-Btk) antibody. A highly purified preparation of native TFII-I was used as a positive control. An anti-TFII-I (α-TFII-I) antibody recognizes two immunoreactive forms of endogenous TFII-I in each cell line, although in different ratios. Neither form is coprecipitated with a control antibody (Control), but both forms of endogenous TFII-I are coprecipitated by an anti-Btk antibody. (B) Cytoplasmic extracts were prepared from Ramos cells treated either with medium alone (−) or with anti-Ig (α-Ig) antibody, and endogenous TFII-I was coimmunoprecipitated with an anti-Btk antibody and visualized with an anti-TFII-I antibody. The blot was stripped and reprobed with anti-Btk antibody. (C) Cytoplasmic lysates from each treatment were analyzed by Western blot analysis for total cytoplasmic TFII-I and represent half of the amount used in panel B.
Given the constitutive association between TFII-I and Btk in unstimulated B cells, we wanted to test whether Btk would be associated with TFII-I when B cells were stimulated with anti-Ig antibody. Cytoplasmic extracts were prepared from Ramos cells treated either with medium or with anti-Ig antibody for 10 min. TFII-I was immunoprecipitated from these extracts with anti-Btk antibody and probed with anti-TFII-I antibody by Western analysis (Fig. 5B). While significant amounts of total cellular TFII-I (∼15%) were associated with Btk in unstimulated cells, fourfold less TFII-I was associated with Btk in cells stimulated with anti-Ig antibody (Fig. 5B). The blot was stripped and reprobed with anti-Btk antibody to show that equivalent amounts of Btk were precipitated in both lanes. Surprisingly, control Western blot analysis showed that cytoplasmic TFII-I levels in unstimulated and stimulated cells were similar, suggesting that the dissociated TFII-I remained in the cytoplasm of these cells under our assay conditions (Fig. 5C). Nevertheless, taken together, these data suggest that although TFII-I is associated constitutively with Btk in the cytoplasm of unstimulated B cells, they dissociate upon anti-Ig antibody stimulation.
Subcellular localization of TFII-I is different in wild-type versus xid-derived primary B cells.
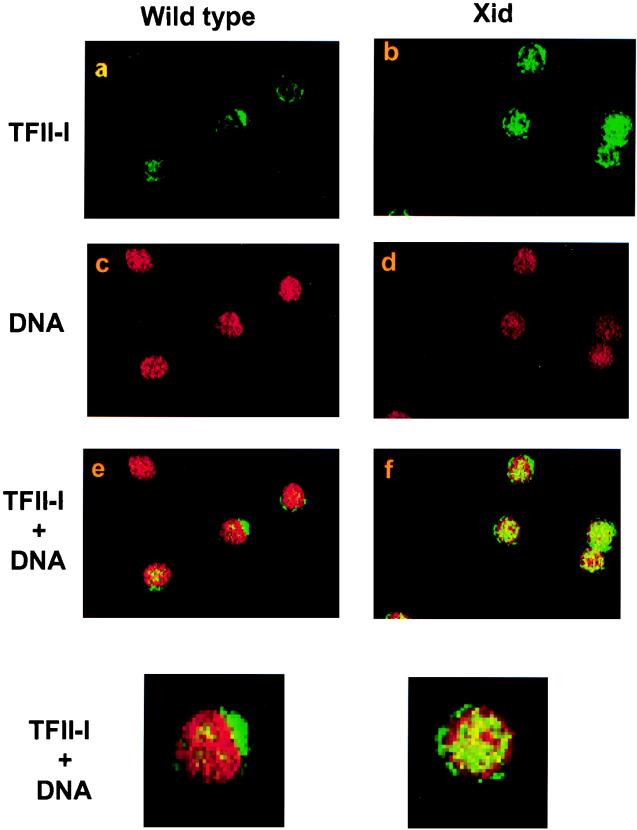
Given the constitutive association between TFII-I and wild-type Btk in resting Ramos B cells and their dissociation upon anti-Ig antibody stimulation, we examined whether the distribution and/or expression of TFII-I in splenic B cells derived from wild-type and xid mice might differ. Immunohistochemical analysis using an anti-TFII-I antibody revealed TFII-I in wild-type and xid primary splenic B cells (Fig. 6). An intense green cytoplasmic fluorescence is seen together with some scattered nuclear staining in wild-type cells (panel a). This distribution is brought out when the propidium iodide stain of DNA (red in panel c) is superimposed on the green TFII-I strain. Scattered discrete yellow spots indicate nuclear TFII-I in wild-type cells (panel e). In contrast, resting xid B cells show marked nuclear TFII-I staining (panel b and f). Counts by different observers (ignorant of the source of cells under examination) showed that 73% of the wild-type B cells had predominant cytoplasmic staining (27% nuclear). In comparison, only 34% of the xid B cells had predominant cytoplasmic staining (66% nuclear dominance). Furthermore, pixel counting by confocal microscopy revealed twice as much FITC–TFII-I in xid than wild-type nuclei (Fig. 6). The control for immunostaining was performed with a control antibody that did not produce any fluorescent staining.
FIG. 6.
Localization of TFII-I in wild-type and xid mutant primary B cells. Localization of TFII-I in primary B cells by fluorescent staining and confocal microscopy. Wild-type (a) and xid mutant (b) splenic B cells were stained with an anti-TFII-I rabbit serum. Nuclear DNA was revealed by red propidium iodide staining (c and d). The two images were superimposed to generate panels e (wild type) and f (xid). In wild-type cells, TFII-I was predominantly cytoplasmic with some scattered nuclear staining (green in a and yellow in e). In xid B cells, the larger amount of nuclear green (b) and intense yellow (pseudocoloring showing concordance of red and green staining) in the superimposed image (f) indicated that these cells have more nuclear TFII-I. In three experiments, pixel counting revealed that the amount of nuclear TFII-I was 2.2-, 1.5-, and 2.3-fold greater in xid B cells than in wild-type B cells. The bottom panels are enlarged images of the central cells of panels e and f.
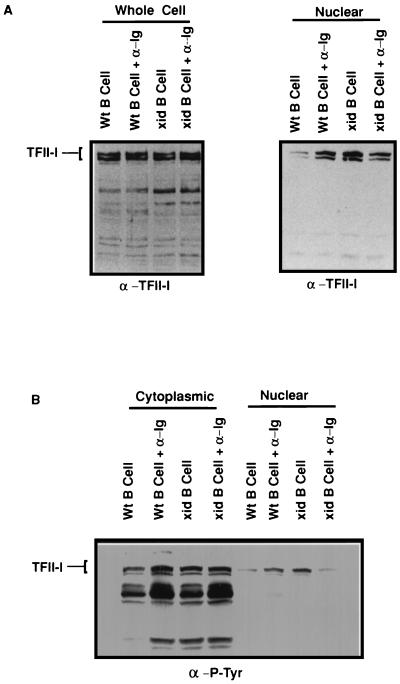
We then used a biochemical approach to assess the potential difference(s) in expression and/or subcellular localization of TFII-I after stimulation via the BCR. Whole-cell extracts prepared from splenic B cells derived from wild-type and xid mice [after incubation for 10 min in medium alone or with F(ab′)2 anti-IgM antibody] were analyzed by Western blot analysis using an anti-TFII-I antibody (Fig. 7A, left). These data indicated that the total TFII-I levels in both extracts were roughly equivalent in all cases, suggesting that there is no apparent difference in TFII-I expression between wild-type and xid-derived B cells. Nuclear extracts were then prepared from wild-type and xid B cells that had been incubated for 10 min in medium alone or with F(ab′)2 anti-IgM antibody. Western blotting with an anti-TFII-I antibody (Fig. 7A, right) indicated nearly threefold more TFII-I in the nuclei of unstimulated xid than wild-type B cells (consistent with the immunohistochemical data above). Anti-IgM antibody stimulation of resting wild-type B cells resulted in approximately a 2.7-fold increase in nuclear TFII-I (compare lanes 1 and 2). In contrast, anti-IgM activation of resting xid B cells produced no increase in nuclear TFII-I (perhaps there was even a small decrease; compare lanes 3 and 4). The precise reason(s) for this apparent decrease is unknown. Importantly, the differences in nuclear levels of TFII-I were not due to differential loading because roughly equivalent levels of nuclear protein were seen by Coomassie staining of the membrane stripped of immune complexes (data not shown).
FIG. 7.
Nuclear distribution and tyrosine phosphorylation of TFII-I in wild-type (Wt) and xid mutant primary B cells. (A) Nuclear distribution of TFII-I in wild-type and xid mutant primary B cells. Shown is SDS-PAGE of whole-cell lysates (Whole Cell) derived from wild-type and xid mutant primary B cells, loaded according to equivalent cell numbers, and subsequent Western blotting with an anti-TFII-I (α-TFII-I) antibody (left) either in the absence or in the presence of anti-IgM (α-Ig) antibody stimulation. Also shown are nuclear extracts (Nuclear) from resting wild-type and xid mutant primary B cells (right) in the absence or presence of IgM (α-Ig) antibody activation. (B) Cytoplasmic and nuclear extracts from panel A were subjected to Western blot analysis with anti-P-Tyr (α-P-Tyr) antibody. The position of TFII-I was determined by running a purified preparation of native authentic TFII-I (data not shown).
In order to determine the phosphorylation status of TFII-I in primary B cells before and after anti-Ig antibody stimulation, lysates were probed with anti-P-Tyr antibodies. As seen in Fig. 7B, tyrosine phosphorylation of TFII-I was increased upon anti-Ig antibody stimulation in the cytoplasm of wild-type B cells but remained unchanged in the cytoplasm of xid B cells. As seen with Ramos cells, Western blot analysis of cytoplasmic extracts from primary B cells also did not show any appreciable differences in the TFII-I protein levels in these lanes (data not shown). Also, the cytoplasmic extracts were tested for nuclear contamination by using an anti-TBP antibody and found to be free of TBP within the limits of detection (data not shown). Although the tyrosine phosphorylation of nuclear TFII-I appears to increase upon anti-Ig antibody stimulation in wild-type B cells compared to xid B cells, a corresponding change in the absolute amounts of nuclear TFII-I (Fig. 7A, right) under similar conditions makes this less substantial.
DISCUSSION
The consequences of BCR-mediated activation of wild-type and xid B cells are dramatically different. Wild-type B cells enter and transit the cell cycle, while xid B cells undergo apoptosis before they can synthesize a significant amount of DNA (3, 57, 59, 62). The mechanistic basis for these phenotypic differences is not fully established. In both cell types, Btk can be phosphorylated and kinase activity is present (46, 66). Early activation events, such as the elevation of major histocompatibility complex class II, proceed normally in xid cells (20). However, the induced activation of PLC and the influx of Ca2+ are dampened (48) with a much-diminished decrease in the sustained elevation of cytoplasmic Ca2+ (76).
Full activation of Btk appears to depend on both transphosphorylation by a src (or Syk) family kinase (63) and membrane localization (1, 38). Membrane localization of Btk and other members of the Tec family of cytosolic tyrosine kinases can be effected by the binding of their N-terminal PH domains to phospholipid moieties (35). Specifically, the PH domain of Btk has high affinity for PIP3 (54), which is typically generated by activated PI3K (reviewed in reference 7). Activation of Btk appears to be downstream of PI3K, as it is inhibited by wortmannin and enhanced by ectopic expression of the p110 subunit of PI3K (72). Additional support for the importance of this mechanism derives from the observation that Btk activation is inhibited by SH2-containing inositol polyphosphate phosphatase (6, 56), an enzyme that hydrolyzes PIP3 to PIP2. Recent evidence indicates that PLC is a downstream effector of Btk (6, 56, 63). Thus, acting through PLC, activated Btk can bring about a Ca2+ influx (14). Interestingly, Itk, a Tec family member that is expressed only in T cells, is downstream of PI3K and upstream of both inositol (1, 4, 5)-triphosphate generation and Ca2+ influx (37).
Mutations in the PH domain of Btk might affect function by altering affinity for inositol phosphates (15, 54), resulting in reduced (80) or increased (36) membrane localization. The Btk PH domain has also been implicated as a protein interaction domain (68, 79). The PH domain of Itk appears to function as an intramolecular binding site (4). Indeed, Yang and Desiderio provided evidence that the PH domain participates in the association of Btk and BAP-135/TFII-I (78). Therefore, in Tec family members, PH domain functions may not be limited to membrane binding. Our data add further support to this view. We show that, as in human B cells, Btk and TFII-I are constitutively associated both in the murine B-cell line BAL-17 and primary splenic B cells from wild-type mice. We further show that the R28C mutation of xid mice decreases the in vivo association of Btk and TFII-I, providing a plausible explanation for the diminished ability of R28C Btk to phosphorylate TFII-I (78). While this suggests a dual function for the PH domain of Btk, there is no a priori reason to rule this possibility out.
TFII-I is a transcription initiation factor (50–53) that is tyrosine phosphorylated transiently shortly (10 min) after IgM antibody stimulation of B cells (78). TFII-I can function both as a basal factor through the Inr element (50–53) and as an activator in the absence of a functional Inr element (18, 31, 53). Thus, TFII-I is likely to participate in the regulation of the transcription of several eukaryotic genes (18, 31, 53). TFII-I is basally phosphorylated on serine/threonine, as well as tyrosine, residues in various cell types and undergoes induced tyrosine phosphorylation upon signaling via several growth factor receptors (31, 42). Moreover, transcriptional activation of the c-fos promoter by TFII-I in serum-stimulated NIH 3T3 cells requires an intact Ras pathway (31). Taken together, these data suggest that TFII-I is a novel factor that links signal transduction to transcriptional events in different cell types. Our current data are consistent with this role of TFII-I in B cells.
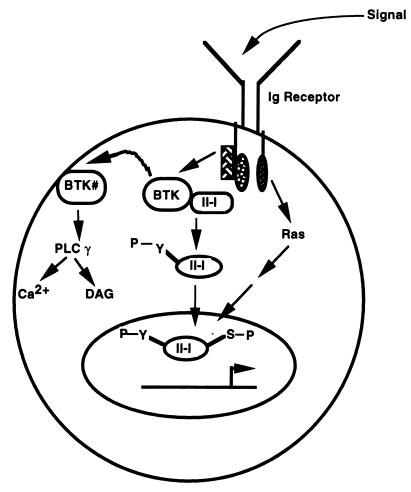
Western blot analysis and immunohistochemical analysis reveal that although the total amounts of TFII-I are essentially equivalent in wild-type and xid splenic B cells, there is less nuclear TFII-I in wild-type than xid resting B cells. Activation of wild-type B cells through the BCR increased nuclear TFII-I but failed to do so in xid cells. Our explanation for these observations is that activation of Btk both induces the tyrosine phosphorylation of TFII-I (either directly or indirectly) and reduces the constitutive association of Btk and TFII-I (Fig. 8). Dissociated TFII-I is then able to translocate to the nucleus at some point in an active tyrosine-phosphorylated form. In contrast, in xid B cells, the mutated Btk is unable to associate effectively with TFII-I with the result that a larger fraction of TFII-I is free to translocate while its Btk-dependent tyrosine phosphorylation would be reduced. Our experiment with the Ramos B-cell line is also consistent with this model, although the amount of cytoplasmic TFII-I appears to be much smaller. However, this could also reflect the way these extracts are prepared, since, in contrast to the immunohistochemical analysis, there appears to be significantly less cytoplasmic TFII-I in primary B-cell extracts (data not shown). It is also interesting that because the total amounts of cytoplasmic TFII-I in the absence and in the presence of anti-Ig antibody stimulation remain similar in Ramos cells, the cytoplasmic TFII-I that is dissociated from Btk upon anti-Ig antibody stimulation may not immediately translocate to the nucleus. Furthermore, it is worth noting that only one time point (10 min) for anti-IgM antibody stimulation has been tested. It remains likely that the kinetics of TFII-I translocation (nuclear import and export) are complex and that several time points need to be looked at. Regardless of the potential difference between the primary murine B cells and the human B-cell line, our model (Fig. 8) suggests that phosphorylation of TFII-I on tyrosine (by Btk) would not be necessary for translocation, nor would the kinase activity of Btk be necessary for the association of Btk with TFII-I. Indeed, we found that kinase-inactive Btk is able to associate with TFII-I in vivo.
FIG. 8.
Model for Btk-dependent TFII-I function. A fraction of TFII-I is constitutively associated with Btk in the cytoplasm of wild-type resting B cells. Upon signaling through the Ig receptor, Btk is activated (Btk#), leading to tyrosine phosphorylation of TFII-I either directly or indirectly. While Btk# may localize to the plasma membrane to bind phospholipids, trigger calcium signaling, and activate diacyl glycerol (DAG), tyrosine-phosphorylated TFII-I is released from Btk# and translocates to the nucleus, where it is serine phosphorylated, perhaps through a Ras-dependent pathway. Although tyrosine phosphorylation of TFII-I may not be necessary for its nuclear import, it may be required for its maximal transcriptional activity. In xid B cells, TFII-I may not be constitutively associated with Btk and thus, increased amounts are in the nucleus.
We also demonstrated in a heterologous expression system that wild-type Btk, but not R28C or kinase-inactive Btk, augments the transcriptional activity of ectopically expressed TFII-I. It was previously reported that the in vitro kinase activity of R28C mutant Btk is not compromised (46, 66). Our data suggest that the kinase-deficient mutant form of Btk interacts with and presumably retains TFII-I in the cytoplasm but cannot phosphorylate it. In contrast, the xid mutant form of Btk, which fails to interact with TFII-I, allows TFII-I to diffuse to the nucleus. This suggests that both kinase activity and the ability to directly associate with TFII-I are necessary for Btk to function as an upstream positive regulator of TFII-I transcriptional activity. Taken together, these data suggest that the association of Btk and TFII-I links BCR activation and transcriptional events mediated by TFII-I (Fig. 8). Note, however, that the tyrosine phosphorylation status of nuclear TFII-I in resting xid B cells has not been explained and may involve either (i) different sites of phosphorylation or (ii) Btk-independent phosphorylation. Given the facts that TFII-I is ubiquitously expressed and only a fraction of TFII-I appears to be associated with Btk, the latter explanation could be reasonable. What is also of interest is the fact that the extent of tyrosine phosphorylation of cytoplasmic TFII-I is much greater than that of the corresponding nuclear TFII-I, suggesting that prior to nuclear entry, it undergoes dephosphorylation. This observation is consistent with the previously published observation that TFII-I undergoes rapid but transient tyrosine phosphorylation upon BCR cross-linking (78).
A question that has remained unclear is how Btk links upstream signals (that originate due to BCR engagement and activation of Src family kinases) to downstream events (e.g., induction of Bcl-xL expression). One link could arise via Btk regulation of PLC and the consequent activation of protein kinase C and calcineurin. TFII-I might establish an additional link between Btk-mediated signaling and transcription because TFII-I possesses Inr-dependent transcription properties and because many of the genes that are important for normal B-cell development are Inr-containing genes (e.g., λ5, VpreB, TdT, and possibly RAG, CD5, Bcl-2, and Bcl-xL); a subset of these might be potentially TFII-I responsive. One tantalizing possibility in the latter two examples links defective Btk activity to apoptosis (3, 73). Activation of normal B cells with anti-IgM antibody correlated with increased Bcl-xL expression, whereas activation of xid B cells failed to induce Bcl-xL and did increase apoptosis (3). It is possible that TFII-I links Btk-mediated signaling events to downstream gene activation since TFII-I is downstream of the Ras pathway and because the Bcl-xL gene is potentially TFII-I responsive. Indeed, our preliminary data strongly indicate that Bcl-xL is transcriptionally regulated by TFII-I in transient-transfection assays. However, it is important to note that the transcriptional activity of TFII-I is not necessarily restricted to Inr-containing promoters, as it can activate the c-fos promoter through upstream promoter elements. Even if a subset of these genes are TFII-I responsive in B cells, a compromised interaction between Btk and TFII-I might lead to aberrant expression of these genes and might contribute to the XLA and xid phenotypes. Further analysis of TFII-I function will shed new light upon this novel regulatory pathway of gene expression.
ACKNOWLEDGMENTS
We thank Ranjan Sen and Brigitte Huber for many helpful discussions and for critically reading the manuscript. We thank Robert Wilson for help with photography of confocal images and Genhong Chen for the wild-type HA-tagged Btk construct.
This work was supported in part by grants from NIH to S.P. (AI 33507 and CA 69618), H.H.W. (AI 15803), and A.L.R. (AI 41147) and from the American Cancer Society (RPG-98-104-01-TBE) to A.L.R.
REFERENCES
- 1.Afar D E, Park H, Howell B W, Rawlings D J, Cooper J, Witte O N. Regulation of Btk by Src family tyrosine kinases. Mol Cell Biol. 1996;16:3465–3471. doi: 10.1128/mcb.16.7.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amsbaugh D F, Hansen C T, Prescot B, Stashak P W, Barthold D R, Parker P J. Genetic control of the antibody response to type III pneumonococcal polysaccharides in mice. I. Evidence that an X-linked gene plays a decisive role in determining responsiveness. J Exp Med. 1972;136:931–936. doi: 10.1084/jem.136.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson J S, Teutsch M, Dong Z, Wortis H H. An essential role for Bruton’s tyrosine kinase in the regulation of B-cell apoptosis. Proc Natl Acad Sci USA. 1996;93:10966–10971. doi: 10.1073/pnas.93.20.10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreotti A H, Bunnell S C, Feng S, Berg L J, Schreiber S L. Regulatory intramolecular association in a tyrosine kinase of the Tec family. Nature. 1997;385:93–97. doi: 10.1038/385093a0. [DOI] [PubMed] [Google Scholar]
- 5.Aoki Y, Isselbacher K, Pillai S. Bruton’s tyrosine kinase is tyrosine phosphorylated and activated in pre-B lymphocytes and receptor-ligated B cells. Proc Natl Acad Sci USA. 1994;91:10606–10609. doi: 10.1073/pnas.91.22.10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolland S, Pearse R N, Kurosaki T, Ravetch J V. SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity. 1998;8:509–516. doi: 10.1016/s1074-7613(00)80555-5. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter C L, Cantley L C. Phosphoinositide kinases. Curr Opin Cell Biol. 1996;8:153–158. doi: 10.1016/s0955-0674(96)80060-3. [DOI] [PubMed] [Google Scholar]
- 8.Cheng G, Ye Z S, Baltimore D. Binding of Bruton’s tyrosine kinase to Fyn, Lyn, or Hck through a Src homology 3 domain-mediated interaction. Proc Natl Acad Sci USA. 1994;91:8152–8155. doi: 10.1073/pnas.91.17.8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheriyath V, Novina C D, Roy A L. TFII-I regulates Vβ promoter activity through an initiator element. Mol Cell Biol. 1998;18:4444–4454. doi: 10.1128/mcb.18.8.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark M R, Johnson S A, Cambier J C. Analysis of Igα-tyrosine kinase interaction reveals two levels of binding specificity and tyrosine phosphorylated Igα stimulation of Fyn activity. EMBO J. 1994;13:1911–1919. doi: 10.1002/j.1460-2075.1994.tb06460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desiderio S. Role of Btk in B cell development and signaling. Curr Opin Immunol. 1997;9:534–540. doi: 10.1016/s0952-7915(97)80107-0. [DOI] [PubMed] [Google Scholar]
- 12.de Weers M, Brouns G S, Hinshelwood S, Kinnon C, Schuurman R K, Hendriks R W, Borst J. B-cell antigen receptor stimulation activates the human Bruton’s tyrosine kinase, which is deficient in x-linked agammaglobulinemia. J Biol Chem. 1994;269:23857–23860. [PubMed] [Google Scholar]
- 13.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluable extract from isolated mammalian nuclei. Methods Enzymol. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fluckiger A-C, Li Z, Kato R M, Wahl M I, Ochs H D, Longnecker R, Kinet J-P, Witte O N, Scharenberg A M, Rawlings D J. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J. 1998;17:1973–1985. doi: 10.1093/emboj/17.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuda M, Kojima T, Kabayama H, Mikoshiba K. Mutation of the pleckstrin homology domain of Bruton’s tyrosine kinase in immunodeficiency impaired inositol 1,3,4,5-tetrakisphosphate binding capacity. J Biol Chem. 1996;271:30303–30306. doi: 10.1074/jbc.271.48.30303. [DOI] [PubMed] [Google Scholar]
- 16.Go N F, Castle B E, Barret R, Kastelein R, Dang W, Mosmann T R, Moore K W, Howard M. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Exp Med. 1990;172:1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregory R J, Kammermeyer K L, Vincent III W S, Wadsworth S G. Primary sequence and developmental expression of a novel Drosophila melanogaster src gene. Mol Cell Biol. 1987;7:2119–2127. doi: 10.1128/mcb.7.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grueneberg D A, Henry R W, Brauer A, Novina C D, Cheriyath V, Roy A L, Gilman M. A multifunctional DNA-binding protein that promotes the formation of serum response factor/homeodomain complexes: identity to TFII-I. Genes Dev. 1997;11:2482–2493. doi: 10.1101/gad.11.19.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasbold J, Klaus G G. B cells from CBA/N mice do not proliferate following ligation of CD40. Eur J Immunol. 1994;24:152–157. doi: 10.1002/eji.1830240123. [DOI] [PubMed] [Google Scholar]
- 20.Hawrylowicz C M, Keeler K D, Klaus G G. Activation and proliferation signals in mouse B cells. I. Comparison of the capacity of anti-Ig antibodies or phorbol myristic acetate to activate B cells from CBA/N or normal mice into G1. Eur J Immunol. 1984;14:244–250. doi: 10.1002/eji.1830140308. [DOI] [PubMed] [Google Scholar]
- 21.Hemmings B A. PH domains—a universal membrane adapter. Science. 1997;275:1899. doi: 10.1126/science.275.5308.1899. [DOI] [PubMed] [Google Scholar]
- 22.Hinshelwood S, Lovering R C, Genevier H C, Levinsky R J, Kinnon C. The protein defective in X-linked agammaglobulinemia, Bruton’s tyrosine kinase, shows increased autophosphorylation activity in vitro when isolated from cells in which the B cell receptor has been cross-linked. Eur J Immunol. 1995;25:1113–1116. doi: 10.1002/eji.1830250439. [DOI] [PubMed] [Google Scholar]
- 23.Hitoshi Y, Sonoda E, Kikuchi Y, Yonehara S, Nakauchi H, Takatsu K. IL-5 receptor positive B cells, but not eosinoophils, are functionally and numerically influenced in mice carrying the X-linked immune defect. Int Immunol. 1993;5:1183–1190. doi: 10.1093/intimm/5.9.1183. [DOI] [PubMed] [Google Scholar]
- 24.Howard M, Grimaldi J C, Bazan J F, Lund F E, Santos-Argumedo L, Parkhouse R M, Walseth T F, Lee H C. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993;262:1056–1059. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- 25.Huber B, Melchers F. Frequencies of mitogen reactive B cells in the mouse: lipopolysaccharide, lipoprotein, and Nocardia mitogen reactive B cells in CBA/N mice. Eur J Immunol. 1979;9:827–829. doi: 10.1002/eji.1830091016. [DOI] [PubMed] [Google Scholar]
- 26.Hyvonen M, Saraste M. Structure of the PH domain and Btk motif from Bruton’s tyrosine kinase: molecular explanations for X-linked agammaglobulinemia. EMBO J. 1997;16:3396–3404. doi: 10.1093/emboj/16.12.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawakami Y, Yao L, Miura T, Tsukada S, Witte O N, Kawakami T. Tyrosine phosphorylation and activation of Bruton tyrosine kinase upon Fc epsilon RI cross-linking. Mol Cell Biol. 1994;14:5108–5113. doi: 10.1128/mcb.14.8.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerner J D, Appleby M W, Mohr R N, Chien S, Rawlings D J, Maliszewski C R, Witte O N, Perlmutter R M. Impaired expansion of mouse B cell progenitors lacking Btk. Immunity. 1995;3:301–312. doi: 10.1016/1074-7613(95)90115-9. [DOI] [PubMed] [Google Scholar]
- 29.Khan W N, Alt F W, Gerstein R M, Malynn B A, Larsson I, Rathbun G, Davidson L, Muller S, Kantor A B, Herzenberg L A, Rosen F S, Sideras P. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 30.Khan W N, Nilsson A, Mizoguchi E, Castigli E, Forsell J, Bhan A K, Geha R, Sideras P, Alt F W. Impaired B cell maturation in mice lacking Bruton’s tyrosine kinase (Btk) and CD40. Intl Immunol. 1997;9:395–405. doi: 10.1093/intimm/9.3.395. [DOI] [PubMed] [Google Scholar]
- 31.Kim D-W, Cheriyath V, Roy A L, Cochran B H. TFII-I enhances activation of the c-fos promoter through interactions with upstream elements. Mol Cell Biol. 1998;18:3310–3320. doi: 10.1128/mcb.18.6.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirkham P A, Santos-Argumedo L, Harnett M M, Parkhouse R M. Murine B-cell activation via CD38 and protein tyrosine phosphorylation. Immunology. 1994;83:513–516. [PMC free article] [PubMed] [Google Scholar]
- 33.Koike M, Kikuchi Y, Tominage A, Takaki S, Akagi K, Miyazaki J-I, Yamamura K-I, Takatsu K. Defect of IL-5-receptor-mediated signaling in B cells of X-linked immunodeficient (xid) mice. Int Immunol. 1995;7:21–30. doi: 10.1093/intimm/7.1.21. [DOI] [PubMed] [Google Scholar]
- 34.Lemmon M A, Ferguson K M, Schlessinger J. PH domains: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell. 1996;85:621–624. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]
- 35.Li T, Tsukada S, Satterthwaite A, Havlik M H, Park H, Takatsu K, Witte O N. Activation of Bruton’s tyrosine kinase (BTK) by a point mutation in its pleckstrin homology (PH) domain. Immunity. 1995;2:451–460. doi: 10.1016/1074-7613(95)90026-8. [DOI] [PubMed] [Google Scholar]
- 36.Li T, Rawlings D J, Park H, Kato R M, Witte O N, Satterthwaite A B. Constitutive membrane association potentiates activation of Bruton tyrosine kinase. Oncogene. 1997;15:1375–1383. doi: 10.1038/sj.onc.1201308. [DOI] [PubMed] [Google Scholar]
- 37.Liu K-Q, Bunnell S C, Gurnial C B, Berg L. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J Exp Med. 1998;187:1721–1727. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahajan S, Fargnoli J, Burkhardt A L, Kut S A, Saouaf S J, Bolen J B. Src family protein tyrosine kinases induce autoactivation of Bruton’s tyrosine kinase. Mol Cell Biol. 1995;15:5304–5311. doi: 10.1128/mcb.15.10.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mano H, Ishikawa F, Nishida J, Hirai H, Takaku F. A novel protein-tyrosine kinase, tec, is preferentially expressed in liver. Oncogene. 1990;5:1781–1786. [PubMed] [Google Scholar]
- 40.Mano H, Mano K, Tang B, Koehler M, Yi T, Gilbert D J, Jenkins N A, Copeland N G, Ihle J N. Expression of a novel form of tec kinase in hematopoietic cells and mapping of the gene to chromosome 5 near Kit. Oncogene. 1993;8:417–424. [PubMed] [Google Scholar]
- 41.Manzano-Winkler B, Novina C D, Roy A L. TFII-I is required for transcription of the naturally TATA-less but initiator-containing Vβ promoter. J Biol Chem. 1996;271:12076–12081. doi: 10.1074/jbc.271.20.12076. [DOI] [PubMed] [Google Scholar]
- 42.Novina C D, Cheriyath V, Roy A L. Regulation of TFII-I activity by phosphorylation. J Biol Chem. 1998;273:33443–33448. doi: 10.1074/jbc.273.50.33443. [DOI] [PubMed] [Google Scholar]
- 43.Oka Y, Rolink A G, Andersson J, Kamanaka M, Uchida J, Yasui T, Kishimoto T, Kikutani H, Melchers F. Profound reduction of mature B cell numbers, reactivities and serum Ig levels in mice which simultaneously carry the XID and CD40 deficiency genes. Int Immunol. 1996;8:1675–1685. doi: 10.1093/intimm/8.11.1675. [DOI] [PubMed] [Google Scholar]
- 44.Perez Juardo L A, Wang Y-K, Peoples R, Coloma A, Cruces J, Franke U. A duplicated gene in the breakpoint regions of the 7q11.23 Williams-Beuren syndrome deletion encodes the initiator binding protein TFII-I and BAP-135, a phosphorylation target of Btk. Hum Mol Genet. 1998;7:325–334. doi: 10.1093/hmg/7.3.325. [DOI] [PubMed] [Google Scholar]
- 45.Qui Y, Robinson D, Pretlow T G, Kung H J. Etk/Bmx, a tyrosine kinase with a pleckstrin-homology domain, is an effector of phosphatidylinositol 3′-kinase and is involved in interleukin 6-induced neuroendocrine differentiation of prostate cancer cells. Proc Natl Acad Sci USA. 1998;95:3644–3649. doi: 10.1073/pnas.95.7.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rawlings D J, Saffran D C, Tsukada S, Largaespada D A, Grimaldi J C, Cohen L, Mohr R N, Bazan J F, Howard M, Copeland N G, Jenkins N A, Witte O N. Mutation of the unique region of Bruton’s tyrosine kinase in immunodeficient XID mice. Science. 1993;261:358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 47.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- 48.Rigley K P, Harnett M M, Phillips R J, Klaus G B. Analysis of signaling via surface immunoglobulin receptors on B cells from CBA/N mice. Eur J Immunol. 1989;19:2081–2086. doi: 10.1002/eji.1830191117. [DOI] [PubMed] [Google Scholar]
- 49.Rowley R B, Burkhardt A L, Chao H G, Matsueda G R, Bolen J B. Syk protein-tyrosine kinase is regulated by tyrosine phosphorylated Igα/Igβ immunoreceptor tyrosine activation motif binding and autophosphorylation. J Biol Chem. 1995;270:11590–11594. doi: 10.1074/jbc.270.19.11590. [DOI] [PubMed] [Google Scholar]
- 50.Roy A L, Meisterernst M, Pognonec P, Roeder R G. Cooperative interaction of an initiator-binding transcription initiation factor and the helix-loop-helix activator USF. Nature. 1991;354:245–248. doi: 10.1038/354245a0. [DOI] [PubMed] [Google Scholar]
- 51.Roy A L, Malik S, Meisterernst M, Roeder R G. An alternative pathway for transcription initiation involving TFII-I. Nature. 1993;365:355–359. doi: 10.1038/365355a0. [DOI] [PubMed] [Google Scholar]
- 52.Roy A L, Carruthers C, Gutjahr T, Roeder R G. Direct role for MYC in transcription initiation mediated by interactions with TFII-I. Nature. 1993;365:359–361. doi: 10.1038/365359a0. [DOI] [PubMed] [Google Scholar]
- 53.Roy A L, Du H, Gregor P D, Novina C D, Martinez E, Roeder R G. Cloning of an Inr- and E-box binding protein, TFII-I, that interacts physically and functionally with USF1. EMBO J. 1997;16:7091–7104. doi: 10.1093/emboj/16.23.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salim K, Bottomley M J, Querfurth E, Zvelebil M J, Gout I, Scaife R, Margolis R L, Gigg R, Smith C I, Driscoll P C, Waterfield M D, Panayotou G. Distinct specificities in the recognition of phosphoinositides by the pleckstrin homology domains of dynamin and Bruton’s tyrosine kinase. EMBO J. 1996;15:6241–6250. [PMC free article] [PubMed] [Google Scholar]
- 55.Saouaf S J, Mahajan S, Rowley R B, Kut S A, Fargnoli J, Burkhardt A L, Tsukada S, Witte O N, Bolen J B. Temporal differences in the activation of three classes of non-transmembrane protein tyrosine kinases following B-cell antigen receptor surface engagement. Proc Natl Acad Sci USA. 1994;91:9524–9528. doi: 10.1073/pnas.91.20.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scharenberg A M, El-Hillal O, Fruman D A, Beitz L O, Li Z, Lin S, Gout I, Cantley L C, Rawlings D J, Kinet J-P. Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase-dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. EMBO J. 1998;17:1961–1972. doi: 10.1093/emboj/17.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scher I. The CBA/N mouse strain: an experimental model illustrating the influence of the X-chromosome on immunity. Adv Immunol. 1982;33:1–71. doi: 10.1016/s0065-2776(08)60834-2. [DOI] [PubMed] [Google Scholar]
- 58.Shaw G. The pleckstrin homology domain: an intriguing multifunctional protein module. Bioessays. 1996;18:35–46. doi: 10.1002/bies.950180109. [DOI] [PubMed] [Google Scholar]
- 59.Sieckmann D G, Finkelman F D, Thompson C B, Scher I. Activation of mouse lymphocytes by anti-immunoglobulin. IV. Stimulation with soluble heterologous anti-delta antibodies. Cell Immunol. 1984;85:1–14. doi: 10.1016/0008-8749(84)90272-7. [DOI] [PubMed] [Google Scholar]
- 60.Siliciano J D, Morrow T A, Desiderio S V. Itk, a T-cell-specific tyrosine kinase gene inducible by interleukin 2. Proc Natl Acad Sci USA. 1992;89:11194–11198. doi: 10.1073/pnas.89.23.11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sloan-Lancaster J, Zhang W, Presley J, Williams B L, Abraham R T, Lippincott-Schwartz J, Samelson L E. Regulation of ZAP-70 intrecellular localization: visualization with the green fluorescent protein. J Exp Med. 1997;186:1713–1724. doi: 10.1084/jem.186.10.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Solvason N, Wu W W, Kabra N, Lund-Johansen F, Roncarolo M G, Behrens T W, Grillot D A M, Nunez G, Lees E, Howard M. Transgene expression of bcl-xL permits anti-immunoglobulin (Ig)-induced proliferation in xid B cells. J Exp Med. 1998;187:1081–1091. doi: 10.1084/jem.187.7.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takata M, Kurosaki T. A role for Bruton’s tyrosine kinase in B cell antigen receptor-mediated activation of phospholipase C-gamma 2. J Exp Med. 1996;184:31–40. doi: 10.1084/jem.184.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tamagnone L, Lahtinen I, Mustonen T, Virtaneva K, Francis F, Muscatelli F, Alitalo R, Smith C I, Larsson C, Alitalo K. BMX, a novel nonreceptor tyrosine kinase gene of the BTK/ITK/TEC/TXK family located in chromosome Xp22.2. Oncogene. 1994;9:3683–3688. [PubMed] [Google Scholar]
- 65.Tanaka M, Gupta R, Mayer B J. Differential inhibition of signaling pathways by dominant-negative SH2/SH3 adapter proteins. Mol Cell Biol. 1995;15:6829–6837. doi: 10.1128/mcb.15.12.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomas J D, Sideras P, Smith C I E, Vorechovsky I, Chapman V, Paul W E. Colocalization of X-linked agammaglobulinemia and X-linked immunodeficiency genes. Science. 1993;261:355–358. doi: 10.1126/science.8332900. [DOI] [PubMed] [Google Scholar]
- 67.Tsukada S, Saffran D C, Rawlings D J, Parolini O, Allen R C, Klisak I, Sparkes R S, Kubagawa H, Mohandas T, Quan S, Belmont J W, Cooper M D, Conley M E, Witte O N. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72:279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 68.Tsukada S, Simon M I, Witte O N, Katz A. Binding of δγ subunits of heterotrimeric G proteins to the PH domain of Bruton’s tyrosine kinase. Proc Natl Acad Sci USA. 1994;91:11256–11260. doi: 10.1073/pnas.91.23.11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, Hammarstrom L, Kinnon C, Levinsky R, Bobrow M, Smith C I, Bentley D R. The gene involved in X-linked agammaglobulinemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 70.Vihinen M, Nilsson L, Smith C I. Tec homology (TH) adjacent to the PH domain. FEBS Lett. 1994;350:263–265. doi: 10.1016/0014-5793(94)00783-7. [DOI] [PubMed] [Google Scholar]
- 71.Vihinen M, Belohardsky B H, Haire R N, Holinski-Feder E, Kwan S P, Lappalainen I, Lehvaslaiho H, Lester T, Meindl A, Ochs H D, Ollilila J, Vorechovsky I, Weiss M, Smith C I. BTKbase, mutation database for X-linked agammaglobulinemia (XLA) Nucleic Acids Res. 1997;25:166–171. doi: 10.1093/nar/25.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wahl M I, Fluckiger A C, Kato R M, Park H, Witte O N, Rawlings D J. Phosphorylation of two regulatory tyrosine residues in the activation of Bruton’s tyrosine kinase via alternative receptors. Proc Natl Acad Sci USA. 1997;94:11526–11533. doi: 10.1073/pnas.94.21.11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woodland R T, Schmidt M R, Korsmeyer S J, Gravel K A. Regulation of B cell survival in xid mice by the proto-oncogene bcl-2. J Immunol. 1996;156:2143–2154. [PubMed] [Google Scholar]
- 74.Wortis H H, Teutsch M, Higer M, Zheng J, Parker D C. B-cell activation by crosslinking of surface IgM or ligation of CD40 involves alternative signal pathways and results in different B-cell phenotypes. Proc Natl Acad Sci USA. 1995;92:3348–3352. doi: 10.1073/pnas.92.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wortis H H, Burkly L, Hughes D, Roschelle S, Waneck G. Lack of mature B cells in nude mice with X-linked immune deficiency. J Exp Med. 1982;155:903–913. doi: 10.1084/jem.155.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamada H, June C H, Finkelman F, Brunswick M, Ring M S, Lees A, Mond J J. Persistent calcium elevation correlates with the induction of surface immunoglobulin-mediated B cell DNA synthesis. J Exp Med. 1993;177:1613–1621. doi: 10.1084/jem.177.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamashita Y, Miyake K, Kikuchi Y, Takatsu K, Noda T, Kosugi A, Kimoto M. A monoclonal antibody against murine CD38 homologue delivers a signal into B cells for prolongation of survival and protection against apoptosis in vitro: unresponsiveness of XID B cells to mAb. Immunology. 1995;85:248–255. [PMC free article] [PubMed] [Google Scholar]
- 78.Yang W, Desiderio S. BAP-135, a target for Bruton’s tyrosine kinase in response to B cell receptor engagement. Proc Natl Acad Sci USA. 1997;94:604–609. doi: 10.1073/pnas.94.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yao L, Kawakami Y, Kawakami T. The pleckstrin homology domain of Bruton’s tyrosine kinase interacts with protein kinase C. Proc Natl Acad Sci USA. 1994;91:9175–9179. doi: 10.1073/pnas.91.19.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang, K., and H. H. Wortis. Unpublished data.