Abstract
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its new variants reported in different countries have posed a serious threat to human health and social fabrics worldwide. In addition, these new variants hindered the efforts of vaccines and other therapeutic developments. In this review article, we explained the emergence of new variants of SARS-CoV-2, their transmission risk, mortality rate, and, more importantly, the impact of each new variant on the efficacy of the developed vaccines reported in different literature and findings. The literature reported that with the emergence of new variants, the efficacy of different vaccines is declined, hospitalization and the risk of reinfection is increased. The reports concluded that the emergence of a variant that entirely evades the immune response triggered by the vaccine is improbable. The emergence of new variants and reports of re-infections are creating a more distressing situation and therefore demands further investigation to formulate an effective therapeutic strategy.
Keywords: SARS-CoV-2, New Variants, Vaccine, Pathogenesis, Therapeutics, Infectivity
1. Introduction
Coronaviruses (CoVs), from ++RNA lineage, are known to cause respiratory illness in mammals, birds, and humans [1], with disease severity ranging from mild to lethal [2]. The CoV disease 2019 (COVID-19), caused by severe acute respiratory syndrome CoV 2 (SARS-CoV-2) ( Fig. 1), was first reported in Wuhan, China, in December 2019, causing an epidemic of rare viral pneumonia [3]. SARS-CoV-2 was provisionally named 2019 novel CoV or 2019-nCoV [4]. The unprecedented rate of interhuman transmission of the virus enabled its worldwide penetration. The novel beta-CoV named SARS-CoV-2 [5] has significantly devastated human health worldwide due to upper respiratory complexities resulting in severe pneumonia and bronchiolitis [6], [7]. Consequently, On March 11, 2020, the World Health Organization (WHO) declared COVID-19 as a global pandemic [8].
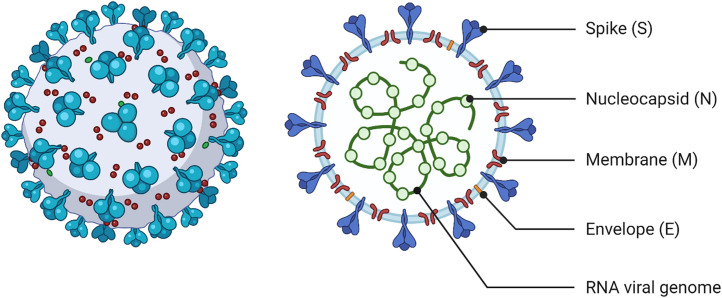
Fig. 1.
Graphical representation of SARS-CoV-2 structure. Interspersed genomic regions are also shown in the right panel (created with biorender.com).
SARS-CoV-2 reportedly has a zoonotic source and is considered as a successor of SARS-CoV-1 [9]. Comparative genomics investigation unveiled that SARS-CoV-2 is 89% identical with bat SARS-CoVZXC21 and only 82% with SARS [10]. Furthermore, it has been genetically linked to the bat CoVs; however, the genetic diversity suggests that the virus spillover occurred in late 2019 and an intermediate host is possibly involved [11], [12], [13]. Nonetheless, a mutational study clarified all these doubts and discovered additional information on SARS-CoV-2 transmission and assisted to trace its propagation [14], [15]. As an intermediary host for SARS-CoV-2 spread to humans, pangolins have been elusively reported to play a significant role [16].
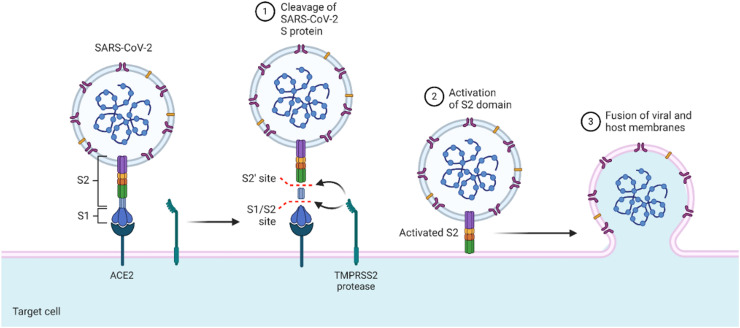
SARS-CoV-2, like other coronaviruses, also uses the same S protein for attachment to ACE2 and ingress to the host cell ( Fig. 2) [17]. The NTD domain of the S1 subunit (comprised of the NTD and CTD domains) of the S protein helps the virus to attach, while the S2 subunit mediates the viral membrane integration at the CTD [18]. A basic amino acid-enriched furin cleavage site (S2′) is obtained upon the cleavage of the S1 and S2 subunits. During viral endocytosis, the S2′ site acts as a fusion peptide after the S2 subunit is cleaved. The presence of the S2′ site is a distinguishable feature of SARS-CoV-2, which makes it unique in origin from the others. This S2′ site via various irreversible conformations stimulates the protein for the fusion of the viral and host membranes, rendering the penetration of SARS-CoV-2 into the prone cells [19], [20]. Due to the significant likeness with SARS at the receptor-binding site (RBD), both viruses exhibit a similar binding mechanism to the ACE2 [21], [22], [23]. However, the distinguished feature, the S2′ site, makes SARS-CoV-2 have enhanced pathogenicity and transmissibility [24], [25]. Structural data have indicated that the availability of key hotspots (i.e., Glu31 and Lys353) facilitates binding in a claw-like structure of ACE2 [26]. Residues such as Glu31 and Lys353 possess greater compatibility for Leu455, Phe486, Glu493, Ser494, and Asp501, ultimately enhancing the binding with the host receptor [27]. The super affinity and super efficiency of the entry are associated with tissue tropism and the host range [26], [28]. Upon the binding of the S1 subunit, the fusion is further assisted by the S2 subunit by decreasing the distance between the host cell and viral spike protein [29]. Next, the fusion peptide starts acting and completes the other necessary steps, such as deformation and membrane attachment [30]. Either the endosomal or non-endosomal pathway, or both, could be selected for entry into the host cell [31]. Alternative approaches may be used based on the pH of the cell. In the case of low pH inside the cell, the endosomal pathway is utilized, assisted by cysteine protease cathepsin [32]. At the same time, for the non-endosomal pathway, TMPRSS2 and TMPTESS11D facilitate the S1/S2 cleavage to stimulate the S protein for induction. In this regard, the host membrane fluidity is stimulated by the IFITM3 to halt the SARS-CoV-2 invasion into the cell [33], [34].
Fig. 2.
The attachment of spike protein to the ACE2 of human cells for infection. The S1, S2 and cleavage site of S protein is depicted to clearly show the early stage of infection (created with biorender.com).
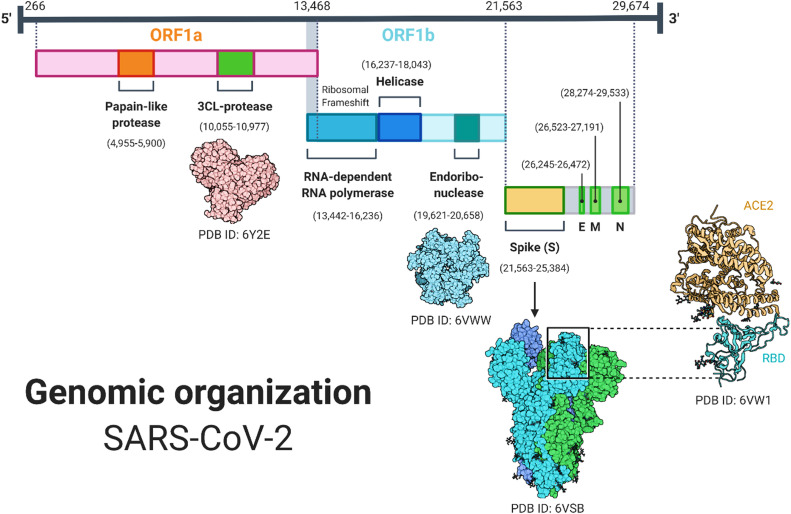
The life cycle of SARS-CoV-2 inside the human cell mainly relies on the nonstructural proteins (NSPs), which are necessary for entry to the cell glycoprotein (spike glycoprotein)[35]. SARS-CoV-2 shares a similar genomic arrangement with related beta-coronaviruses. The positive-sense, single-stranded, ~ 30 kb RNA genome codes six functionally unique open reading frames (ORFs) in 5–3′ sequence: ORF1a/ORF1b (replicase), envelope (E) protein, spike protein, nucleocapsid (N), and membrane (M). Additionally, the accessory proteins are encoded by seven ORFs, which are sprinkled among the structural genes ( Fig. 3) [36].
Fig. 3.
Graphical illustration of the genomic regions of SARS-CoV-2. The two open reading frames (ORF1a and ORF1B), their nucleotides dimension, and the protein encoded by a specific region is given in detail (created with biorender.com).
The ongoing pandemic is a continuous threat to public health, and still, no proven effective anti-COVID19 drugs, vaccines or any other therapeutics have shown potential health benefits. The age factor, ecological factors, gender differences and rapid evolution of the SARS-CoV-2 is making it difficult for researchers to develop a comprehensive therapeutic strategy. The ongoing clinical trials of different therapies are far away from reality. The race for a final magic drug still continues and according to the August 17, 2021 updates data provided by the COVID-19 vaccine and therapeutics tracker, 408 clinical trials of 138 vaccine candidates are under consideration and are in different clinical phases (https://covid19.trackvaccines.org/). However, with the emergence of new variants i.e. B.1.1.7, B.1.351, B.1.617, P.1, B.1.618 and many others have posed serious threat to the efficacy of the already developed vaccines. Many studies revealed demonstrated the efficacy of different vaccine against different variants. To understand the impact of different variants on the efficacy of different vaccine, the current review provides information on the genomic features, specific substitutions of amino acids, targeted protein, origin and epidemiology. This review also provides knowledge on the impact of different variants on the efficacy of different vaccines i.e. Moderna, AstraZeneca, Sinopharm, CanSino, Sinovac and Johnson Johnson.
1.1. Transmission and disease behaviour of SARS-CoV-2
The preliminary route for SARS-CoV-2 transmission is close contact with an infected person [37], [38]. Secondary events such as small tiny droplets or aerosols cleared by an infected person either by sneezing, speaking, or coughing helps the virus to spread to another person [39], [40], [41]. The eyes and nose are alternate routes of transmission for the virus [42]. In addition, contaminated surfaces may also enable the spread of the virus. However, further investigations are required to confirm the fast transmission routes of SARS-CoV-2 [43].
Variable symptoms such as loss of smell, fatigue, fever, breathing hitches, cough, and loss of taste and asymptomatic cases have been reported [44], [45]. These symptoms take approximately 1–14 days after exposure to develop [46]. Among patients with noticeable symptoms, approximately 81% of them developed mild pneumonia [47]; 14% developed severe symptoms such as severe hypoxia and dyspnea [48]; and 5% developed fatal symptoms such as respiratory disruption, shock, and multi-organ defects. One-third of the infected people showed no noticeable symptoms [49]. On the contrary, some patients reportedly experienced symptoms for weeks or months after their initial recovery from COVID-19 infection, referred to as “Long COVID.” These patients are more sensitive to organ impairment [50]. Nonetheless, the long-term impact of COVID-19 infection needs further investigation [51].
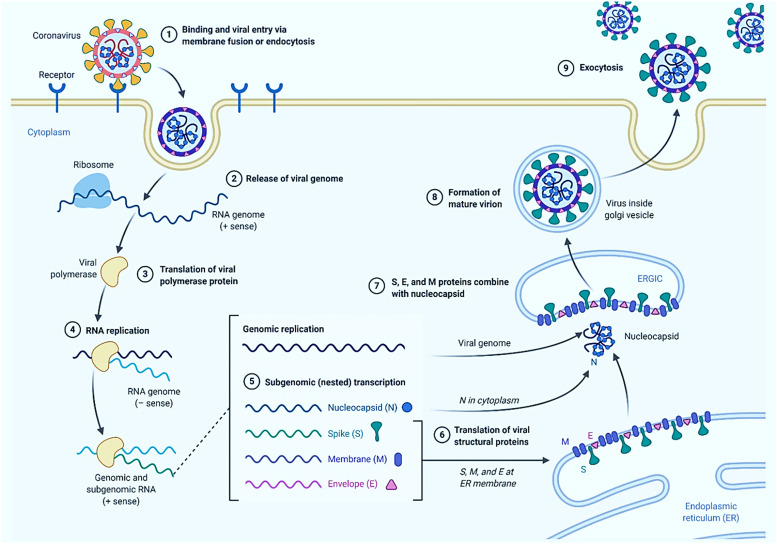
COVID-19 primarily and rapidly affects the upper respiratory (nose, sinuses, and throat) and lower respiratory (lungs and windpipe) tracts [52], [53]. The abundant expression of angiotensin-converting enzyme 2 (ACE2) on the surface of type II alveolar cells enables the virus to damage the lungs significantly [54]. Moreover, the severity of organ damage is directly correlated with ACE2 density [55], [56]. Therefore, most deaths in SARS-CoV-2 infection could be attributed to densely infected lungs [57]. Furthermore, although no association between the central nervous system (CNS) involvement and COVID-19 has been reported, low levels of SARS-CoV-2 have been detected in the brains of patients who died due to COVID-19 [58]. It has been speculated that the virus may have entered the bloodstream, evading the blood–brain barrier to pass into the CNS, although the exact mechanism needs further research [59], [60]. In addition, ACE2 expression in the gastrointestinal organs may affect different tissues [61]. Acute myocardial injury, cardiovascular complications, thrombosis, venous thromboembolism, pulmonary embolism, ischemic events, vasoconstrictive responses, and injury to the olfactory bulbs have been reported in patients who died from COVID-19 [62], [63], [64], [65]. Lymphocyte-containing inflammatory infiltrates and diffused alveolar damage also contribute to death risk in COVID-19 [66], [67]. Conclusively, the pathophysiology of COVID-19 is haphazard and may need further research to reveal the diversity in affected organs [68]. The whole pathogenesis cascade of SARS-CoV-2 is illustrated in Fig. 4.
Fig. 4.
SARS-CoV-2 genome replication pathway and life cycle in the host cell (created with www.biorender.com).
1.1.1. SARS-CoV-2 vaccine designing
To enforce a more robust lifelong immunity against the infection of SARS-CoV-2, immunization is an efficient approach. To date, 242 vaccines are under trial for clinical evaluation. From peptides to nucleic acids and from lipid-encoated to adenovirus-based anti-COVID-19 vaccines, they are under investigation. Among the others, lipid-encoated mRNA and live or attenuated vaccines have also been part of this fight. Of 138 vaccine candidates, only 21 vaccine candidates are approved.; A total of 31 reached the stage-III performance evaluation; and others are still in different stages (Phase I, 36 vaccine candidates Phase II, 65 candidates (https://covid19.trackvaccines.org/vaccines/#progress-meter)). Vector-based S protein vaccine designed by CanSino Biologics with double-blinded efficacy evaluation on 603 volunteers resulted in safe strong humoral immune responses [69]. On the contrary, using Chimpanzee vector-based vaccine ChAd0×1 by the University of Oxford produced neutralizing antibodies when evaluated in 1077 individuals [70]. This vaccine, however, is still under the Phase-II clinical stage and reported that it works in a dose-dependent manner. A lipid nanoparticle-encoated mRNA-based vaccine known as mRNA-1273, co-manufactured by Moderna and NIAID, also triggered a robust immune response against the SARS-CoV-2 [71]. Increased antibodies production was observed after the injection of the second dose. Furthermore, inactivated and whole vaccines are under investigation and 320 participants developed effective neutralizing antibodies [72]. Approximately 4.74 billion doses as of August 17, 2021, have been administered worldwide [73]. A list of approved vaccine are given in Table 1.
Table 1.
represent the WHO approved list of vaccines against SARS-CoV-2.
| S.No | Vaccine name | Type of vaccine | Country | Status | Manufacturer |
|---|---|---|---|---|---|
| 1 | (CIGB): CIGB-66 | Protein Subunit | Cuba | Approved | Center for Genetic Engineering and Biotechnology |
| 2 | EpiVacCorona | Protein Subunit | Russian Federation | Approved | FBRI |
| 3 | Covishield | Non Replicating Viral Vector | India | Approved | Serum Institute of India |
| 4 | Ad5-nCoV | Non Replicating Viral Vector | China | Approved | CanSino |
| 5 | Sputnik Light | Non Replicating Viral Vector | Russian Federation | Approved | Gamaleya |
| 6 | Ad26. COV | Non Replicating Viral Vector | USA | Approved | Janssen (Johnson & Johnson) |
| 7 | COVID-19 Inactivated Vaccine | Inactivated | Iran | Approved | Shifa Pharmed Industrial Co |
| 8 | Inactivated (Vero Cells) | Inactivated | China | Approved | Sinopharm (Wuhan) |
| 9 | Covaxin | Inactivated | India | Approved | Bharat Biotech |
| 10 | QazVac | Inactivated | Kazakhstan | Approved | Kazakhstan RIBSP |
| 11 | SARS-CoV-2 Vaccine (Vero Cells) | Inactivated | China | Approved | Minhai Biotechnology Co |
| 12 | BNT162b1 | RNA | Germany | Approved | Pfizer/BioNTech |
| 13 | mRNA-1273.211 | RNA | USA | Approved | Moderna |
1.2. Evolution of SARS-CoV-2 new variants
The natural selection process is liable for the selection of mutations and the fate of emerging variants. Mutations that facilitated the survival, replication, and fitness of an organism were maintained in the population and the deleterious ones were purged. A strong correlation between the selection process and chance events played a significant role in the evolution of the new variants. Variations in the genomics sequences were referred to as new variants, while those that induced changes in the characteristic properties, i.e., virulence or transmissibility, were tagged as new strains. Understanding the viral nomenclature helps in controlling the infection and better surveillance. Classification of viruses into large groups known as clades and the species of SARS-CoV-2 were further classified into many groups based on the genetic variability when compared to the reference sequence. Many variants after the reference strain were reported and their potential features were explored to understand their role in the transmissibility and vaccine effectiveness. The following sections will discuss the notable variants reported during this pandemic.
1.2.1. D614G variant
The replacement of a polar charged amino acid–aspartic acid by a nonpolar aliphatic amino acid glycine at position 614 reported in early 2020 played an essential role in the conformational changes in the S1 and S2 domains of the spike protein. This mutation made it easier for the viral spike protein to connect to the ACE2 receptor, consequently enhancing the risk of infection [74]. Spike D614G variation became universally prevalent a few months after perception of the parental strain, probably due to better ability to interact with human ACE2 receptor [75]. In both human and animal models, the D614G mutation resulted in increased replication capacity and susceptibility. The disease severity of the patients infected with the D614G variant was comparable with the wild type; however, viral load in the nasopharyngeal was reported to be comparatively high. In addition, loss of smell was more dominant in infections with this strain due to its robust interaction with host ACE2 in the olfactory epithelium [76]. Furthermore, an investigation of animal models infected with this strain reported a higher level of neutralizing antibodies than the zero strain. Structural modeling study of furin binding to the wild type and D614G revealed that the binding of furin was enhanced by the mutation and thus potentially increases the infectivity [77]. Consequently, this showed that strain had no significant impact on the vaccine efficacy or any other therapeutics agents [78]. The D614G in the furin binding was a notable frequent mutation reported in almost all the new variants [79].
1.2.2. Lineage B.1.1.7 (Alpha)
The evolution of the SARS-CoV-2 new variant, B.1.1.7, also known as Variant of Concern (VOC), is reported to be 70% more transmissible than the former one and is rapidly diffusing worldwide. There is an instant need to discover how the new variant interacts with the host receptor (ACE2). Recent reports from England regarding the origin of novel contagious strain (B.1.1.7) of SARS-CoV-2 have further exacerbated the situation [80]. The novel evolved substitutions in the S protein of B.1.1.7 (deletion 69–70, 144, and substitutions P681H, E484K, A570D, T716I, N501Y, S982A, D1118H, and many others) might have altered the SARS-CoV-2 ability to transmit and infect. Consequently, the currently available vaccines against COVID-19 might not be effective against B.1.1.7 lineage [81]. The substitution mutations N501Y, E484K, and others within the receptor-binding domain (RBD) of the United Kingdom (UK) and South African SARS-CoV-2 strains are now spreading unchecked [82]. The number of 501Y mutation-associated cases increased from 0.1% in early October to 49.7% in late November in the UK. The mutation N501Y co-occurs with other mutations in the N, orf8, orf1a, and S glycoprotein in 501, involving two deletions—Δ69 and Δ70 [83]. Multiple amino acid alterations in the spike protein have been linked to this lineage, such as deletions at 69/70, ORF8 mutations, P681H, N501Y, and E484K mutations [84]. The N501Y mutation, which is a replacement of asparagine by tyrosine in the receptor-binding motif is of major concern [85]. Structural modeling analysis revealed that the orientation of aromatic ring and establishment of additional hydrogen bonds with the ACE2 receptor increases the binding affinity and consequently the infection. Moreover, increasing specificity towards the ACE2 receptor by this mutation was also observed. The hospitalization and mortality rates were reported to increase by 52% and 59%, respectively [86]. Minimal reduction in the efficacy of natural and vaccination-triggered antibodies was observed [80]. Computational analysis of the binding of the N501Y variant of RBD to the host receptor ACE2 revealed enhanced binding and variations in the inter-hydrogen bonding network [87]. They also revealed the structural basis for B.1.351, P.1, and B.1.617, which concluded that the binding affinity was enhanced substantially and structural dynamic properties were also altered [88], [89], [90].
Furthermore, among the notable variations that emerged included P681H substitution in the Furin binding motif 682–685. This was also reported in Nigeria (eta) and was classified as lineage B.1.1.207 [80]. In the Nigerian variant along with E484K, another mutation, F888L, in the spike protein was also identified. This mutation was reported to alter the biological efficiency of SARS-CoV-2 by performing hydrolysis by TMPRSS2 and augment viral invasion [91]. In addition, it is possible that this crucial alteration enabled the virus’ escape from the host’s immune system. This is accomplished by avoiding neutralizing antibodies, perhaps resulting in concerns regarding the effectiveness of vaccinations presently being delivered to the general public [91], [92]. Furthermore, the two deletions 69/70 were also reported to help the virus to escape the host immune system, while the mutations in ORF8 were believed to act as inactivating mutations, thus increasing the susceptibility of mutations inductions in other regions [93]. The UK variant with E484K mutation was also reported and named as VOCs 21FEB-02 [94].
1.2.3. Lineage B.1.351 (Beta)
The beta variant from South Africa, which was reported in December 2020, carries E484K mutations amongst others. A total of 12 variations and one deletion were reported in this lineage [95]. Young individuals with comorbidities are more likely to be infected by this variant, and it causes serious disease more frequently than other variants in similar situations. The new variants from both UK and South Africa appear to be more contagious; however, mutations in the UK variants are unlikely to impede the effectiveness of the developed vaccines, though the South African variants (501. V2) may interfere with it to some extent, particularly because of K417N and E484K mutations [96]. In this regard, the lack of empirical data is the major challenge to predict which one of the recently emerged strains of SARS-CoV-2 is more lethal [97]. The transmissibility of this variant has been reported to increase up to 52%, while the hospitalization of patients remains under investigation. Moreover, the mortality rate due to the infection of this variant is increased. The risk of reinfection with this variation has reportedly reduced because the T cell response provoked by the D614G variant remains effective against it. More importantly, the efficacy of the developed vaccine is reduced for many. Furthermore, conformational changes in the flexible loop site of S RBD induced by the E484K mutation confers a critical role in immune evasion, viral receptor binding affinity, and infectivity.
1.2.4. Cluster 5 variant
In Denmark, ΔFVI-spike, characterized as cluster 5 strain and identified in minks, exacerbated the situation and may further aggravate it. As of November 2020, 205 active mink-mediated corona cases have been confirmed [98]. Infection with this strain may reduce viral neutralization sensitivity, resulting in a reduction in the durability of immune protections offered by vaccination and the natural process of infection. According to WHO, the cluster 5 variant moderately lessened sensitivity to counteracting antibodies. Because viral development in mink reservoirs may lead to the recurring risk of human infection from minks, the adaptability of this variant in minks remains a huge health concern in the future [99].
1.2.5. Lineage B.1.258∆
This variant is placed within the clade B.1.258 and was detected in Czech Republic and Slovakia in late 2020 [100]. This variant carries an N439K mutation in the RBD, with similar deletions 69–70 at the terminal regions of the spike glycoprotein. It has been reported to increase the viral load and escape the immune response triggered by a previous infection. H69/V70 deletion is a tolerant mutation in which the antigenic peptides in the amino-terminal region (variable loops) are modified, resulting in resistance to neutralization by convalescent sera and vaccination [100], [101].
1.2.6. P.1 or 20J/501Y.V3
Lineage P.1 was characterized as a new variant with 17 mutations in total and 11 in spike protein. Since December 2020, 42% of the samples were annotated with P.1 lineage infection [102]. The N501Y and E484K mutations were primarily detected. This mutant has the most spike protein variations, and all of these changes have major consequences for infectivity, reinfection incidence, and antibody-mediated immune evasion. The WHO named it the Gamma variant with the most important three mutations, K417T, E484K, and N501Y, in the RBD domain [103]. With increased hospitalization, this variant reportedly increased the infection rate by + 161% and mortality rate by + 50%. The reinfection is reduced, while the immunity with natural or vaccine-mediated is retained. P.2 lineage cases were later reported in Brazil, harboring the E484K substitution only [104], [105]. Interestingly, the P.1 and P.2 (zeta) lineages were not correlated. According to research, an infection caused by the Gamma variant can yield roughly 10 times more viral load than those infected with one of the other lineages discovered in Brazil (B.1.1.28 or B.1.195) [106]. The Gamma variant had 2.2 times higher transmissibility with the capacity to infect both adults and elderly people, indicating that P.1 and P.1-like clades were more proficient at attacking younger individuals, regardless of gender. The Gamma variant is 1.4–2.2 fold more communicable, according to a report of samples taken in Manaus between November 2020 and January 2021. It was also shown to be capable of evading 25–61% of acquired immunity from subsequent CoV diseases, referring to the possibility of re-infection after recovery from an earlier COVID-19 infection. In terms of fatality ratios, Gamma infections were shown to be 10–80% more fatal [107]. People who have been completely vaccinated with Pfizer or Moderna had a considerably reduced neutralization effect against the Gamma variant, according to research, although the influence on the disease’s course remains unknown [108]. According to a pre-print research published by the Oswaldo Cruz Foundation in early April, patients who received the first dosage of Sinovac’s Coronavac vaccine had a 50% effectiveness rate in real-world situations. After the second dose, the effectiveness rate was expected to be higher. However, the research remains underway as of July 2021. The Oxford–AstraZeneca vaccine appears to be effective against the Gamma variant, according to an initial report from two tests, albeit the specific level of efficiency has not yet been published. CoronaVac appears to be effective against the Gamma form as well, according to preliminary results from an Instituto Butantan research, which has yet to be extended to obtain conclusive data as of July 2021[107].
1.2.7. Lineage B.1.617 and B.1.617.2
Also known as VUI‑21APR‑01, lineage B.1.617 emerged in India in October 2020 with three prominent substitutions L452R, E484Q, and P681R in the spike protein. The two mutations in the RBD domain and one adjacent to the Furin binding site accelerates the transmission of this variant [109]. The infection rate, hospitalization, and mortality remain under investigation, whereas the reinfection, vaccine, and natural immunity have slightly reduced. On the contrary, the B.1.617.2 variant owns L452R, T478K, and P681R substitutions, which increases the transmissibility and hospitalization by + 64% and + 85%, respectively [110]. Moreover, the reinfection probability is decreased, while the vaccine efficacy is also compromised [111].
1.2.8. Lineage B.1.168
With mutations E484K and D618G and two amino acid deletions, Tyr145 and His146, in the spike protein, this variant was reported in West Bengal, India. It was reported as the key immune evading variant due to its ability to escape from multiple monoclonal antibodies and convalescent plasma [109], [146].
1.2.9. Other notable variants
Besides the aforementioned variants, other variants have been reported in different countries. For instance, Los Angeles reported lineage B.1.429 or the Epsilon variant in 50% of the samples, exhibiting different mutations in ORF1ab and spike protein. This variant with D1183Y and I4205V mutations in the ORF1ab and S13I, W152C, and L452R mutations in the spike protein is also known as CAL 0.20C, 20C/S:452R, CA VUI, or 21C. Variants B.1.429 and B.1.427 were classified as VOCs by the CDC[112].
The Epsilon (CAL 0.20C) variant was first detected in California in a total of 1230 samples during the COVID-19 pandemic [113]. This variant disappeared; however, it was detected again in November 2020 in California. By January 2021, the Epsilon variant was reported in 36% of the total samples processed in California, which then increased to 50% in the following months. In addition, this variant was discovered in various regions in Northern California, according to a joint press statement released by the University of Southern California and Santa Clara Public Health Department. The prevalence of the variant in sequenced samples from Northern California increased from 3% to 25% between November and December 2020. In a research focusing on Southern California, CAL 0.20C was defined as belonging to clade 20C and responsible for around 36% of the infection, whereas an evolving mutant from the 20G clade accounts for approximately 24% of the infection. Consequently, as of January 2021, the 20G clade prevailed in the United States (US) overall. Following the rise of Epsilon cases in California, the variant has been found in variable percentages in most states in the US. Furthermore, small populations have been found infected with this variant in other North American nations, as well as Europe, Asia, and Australia. However, its frequency swiftly decreased after an initial spike in February 2021, as it was outbred by the more contagious Alpha variant. Nonetheless, as of April 2021, the Epsilon variant remains prevalent in portions of northern California. It had almost vanished from the southern portions of the state and had not been successful in establishing a stronghold beyond. Only 3.2% of all cases in the US were infected with the Epsilon variant, while more than 2/3 were infected with the Alpha variant. As of July 2021, the WHO does not recognize the Epsilon variant as a VOC [113], [114], [115].
The Philippines’ Department of Health verified the identification of two COVID-19 mutations in Central Visayas on February 18, 2021: E484K and N501Y. The mutations were found in 37 of 50 samples, with both mutations co-occurring in 29 of them, thus creating a variant named lineage P.3 or the Theta variant. The variant was also detected in Japan, UK, and Malaysia. As of July 2021, the Theta variant is no longer regarded as VOC by the WHO [116], [117].
Japan reported a new variant known as R.1 and was designated as VOC by the National Institute of Infectious Diseases in Japan [117], [118]. It harbors E484K mutation on the RBD of the spike protein and W152L mutation on the N-terminal domain. The mutations may potentially have implications for immune evasion. Moreover, an estimated 6999 infections have been reported in 30 other countries and 1244 cases in the US. In a limited study conducted in the US, the Pfizer–BioNtech vaccine was shown to be 94% effective in preventing R.1 hospitalization and mortality during an epidemic. As the Alpha followed by the Delta variant increased in Japan, R.1 instances became increasingly rare [119], [120].
Linage B.1.620, also known as Lithuanian strain, was found in Lithuania in March 2021. The variant could be found in both Central Africa and North America. Other European nations, such as France and Belgium, have also discovered the occurrence of this variation. In comparison to the reference variant, this lineage exhibits 23 alterations and deletions, most of which are distinct mutations. There is an E484K mutation in this lineage. This variant has the D614G mutation, which was seen in the majority of the circulating strains. P681H and S477N are two more noteworthy variants [121], [122], [123].
In October 2020, lineage B.1.618 was discovered for the first time. It shares the E484K mutation with many other variations and exhibited considerable expansion in West Bengal, India, in April 2021. The PANGOLIN database revealed 135 sequences discovered in India as of April 23, 2021, with single-digit counts in each of the other eight nations [124], [125].
On February 24, 2021, Public Health England recognized Lineage B.1.1.318 as a Variant Under Investigation (VUI) (VUI-21FEB-04, previously VUI-202102/04). It has been found in 16 individuals in the UK [126]. Although lineage B.1.1.317 is not a variant of concern, it is notable; the Queensland Health ordered two individuals undergoing hotel quarantine in Brisbane, Australia, to spend an additional 5 days in quarantine on top of the statutory 14 days when it was proven they were infected with the variant.
1.3. SARS-CoV-2 new variants and the fate of COVID-19 vaccine
The rapid availability of vaccinations has altered the natural interaction between SARS-CoV-2 and its human hosts [127]. The emergence of SARS-CoV-2 variants that are partly or completely resistant to the antibody response induced by COVID-19 vaccinations needs further evaluation [128]. According to a study, several vaccines produced for the initial strain had lesser efficacy against the newly emerged variants [129]. However, the US Food and Drug Administration declared all FDA-approved vaccinations still effective against circulating SARS-CoV-2 strains as of February 2021 [128].
1.3.1. Vaccine effectiveness against Alpha (Lineage B.1.1.7)
Several primary studies examined by the WHO revealed that Pfizer–BioNTech, Oxford–AstraZeneca, and Novavax vaccines were effective against COVID-19 caused by the Alpha variant, with no data for other vaccines yet. In addition, the WHO demonstrated that the most widely dispersed immunizations retained antibody neutralization against the Alpha variant, which is relevant to how vaccines, including Pfizer–BioNTech, Sputnik V, Moderna, BBIBP-CorV, CoronaVac, and Covaxin, can control the pandemic by preventing asymptomatic infections. In the case of the Oxford–AstraZeneca vaccine, there is a low to moderate reduction, and there is no data for other vaccines to date [130]. Early reports suggested that Moderna and Pfizer–BioNTechvaccines offer safety against the variants [131], [132]. According to a trial, the Oxford–AstraZeneca vaccine exhibited a 42–89% efficiency against the Alpha variant as compared to 71–91% against other variants. Similarly, initial findings from clinical data also revealed that the Novavax vaccine is 96% efficient against the original strain and 86% effective against the Alpha variant [133].
1.3.2. Vaccine effectiveness against Beta (Lineage B.1.351)
Preliminary findings reviewed by the WHO indicated that the Oxford–AstraZeneca vaccine has shown a considerable reduction of new infections caused by the beta variant, Novavax vaccine demonstrated a moderate protective effect against this variant, Pfizer–BioNTech and Johnson & Johnson vaccines have lessened efficacy against the Beta lineage, with no data for other vaccines yet. In addition, the WHO revealed that most widely circulated vaccines may have reduced antibody neutralization against the Beta variant, which is relevant to how vaccines can control the pandemic by reducing asymptomatic infections. BBIBP-CorV and CoronaVac have shown minimal to modest reductions in the effectiveness against the Beta lineage, while Johnson & Johnson, Oxford–AstraZeneca, Pfizer–BioNTech, Sputnik V, Moderna, and Novavax resulted in nominal to considerable decline against this variant [130]. Moderna has begun testing its vaccine against the Beta variant, also known as lineage B.1.351. [134]. Pfizer said on February 17, 2021, that neutralizing activity for this version had been lowered by two-thirds, but no entitlements regarding the vaccine’s efficacy in preventing infection from this variant could yet be made [135]. Several studies eventually verified that sera from patients inoculated with the Moderna and Pfizer–BioNTech vaccines against the Beta variant had lower neutralizing activity [104]. On April 1, 2021, an assessment on a Pfizer–BioNTech South African vaccination study indicated that the vaccine was a 100% effective thus far. Beta variants were found in 6/9 infections in the placebo control group [136]. Johnson & Johnson developed vaccine, Ad26. COV2. S, was tested in South Africa and claimed a 72% effectiveness against COVID-19 (moderate to severe) in the US and 57% in South Africa as of January 2021 [137]. The Financial Times reported on February 6, 2021, that data from preliminary trials of a study conducted by the University of the Witwatersrand (South Africa), in collaboration with Oxford University, revealed that the Oxford–AstraZeneca vaccine was less effective against this variant. The study indicated that the AZD1222 vaccine provided only “limited protection” in all cases, except the most severe instances of COVID-19 in a sample size of 2000 people [138]. The South African Minister of Health delayed the scheduled deployment of around a million doses of the vaccine on February 7, 2021, while they evaluate the data and expect guidance on how to proceed [138], [139]. The “preliminary efficacy” of the Novavax vaccine (NVX-CoV2373) was reported as 51% against the Beta lineage. The disease condition for this trial, conducted in March 2021, ranged from mild to severe COVID-19 [140].
1.3.3. Vaccine effectiveness against Gamma (Lineage P.1)
Multiple exploratory trials assessed by the WHO showed that CoronaVac and BBIBP-CorV were effective against COVID-19 caused by the Gamma variant of SARS-CoV-2, with no data for additional vaccinations yet. In addition, the WHO found that Oxford–AstraZeneca and CoronaVac preserved antibody neutralization against the Gamma linage (no to minimal reduction), whereas Pfizer–BioNTech and Moderna had a relatively low neutralization (minimal to moderate reduction), with no information for other vaccines to date. This is important because vaccines can control the pandemic by preventing asymptomatic infections [141]. The Gamma variant, first discovered in Brazil and also known as 20J/501Y.V3, may partially evade vaccination, specifically the Pfizer–BioNTech vaccine [104].
1.3.4. Vaccine effectiveness against Delta (Lineage B.1.617)
Several studies examined by the WHO indicated that Oxford–AstraZeneca and Pfizer–BioNTech were effective against COVID-19 caused by the delta variant of SARS-CoV-2, with no data for other vaccines. In addition, the WHO showed reduced antibody neutralization against the Delta variant with Oxford–AstraZeneca (significant reduction), Pfizer–BioNTech, and Covaxin (small to moderate reduction), with no findings for other vaccines yet [130]. Spike mutations D111D (synonymous), G142D, P681R, E484Q [142], and L452R [143] are among the 15 characteristic mutations, of which the last two are synergistic for antibody invasion.
The advent of SARS-CoV-2 new variants has further aggravated the situation, and the efficacy of the developed vaccines may or may not be compromised. Preliminary reports from Pfizer revealed that the new variants slightly reduced the efficacy of the mRNA-based vaccine [144], [145]. Scientists also believed that new variants completely evading the immune responses triggered by vaccines are very unlikely. Furthermore, it is believed that the E484K variant may compromise the effectiveness of the current vaccine. The emergence of new variants and reports of reinfection are creating a more distressing situation and therefore demands further investigation to formulate an effective therapeutic strategy.
2. Conclusion
Researchers, private firms, educational institutes, ministries, and people from all walks of life are focusing on eliminating COVID-19, which has posed a serious health risk to human life. Preliminary data generated from experiments are available for secondary research. In this regard, computational methods and recent machine learning algorithms have exponentially increased research on SARS-CoV-2; from virtual drug screening to molecular mechanism and from vaccine designing to COVID-19 platforms development, computational approaches have been of great interest. They have significantly accelerated the comprehension of genomic patterns, proteomics, structure determination, mutation stability, and function correlation, as well as tracing. However, our knowledge concerning the virus remains limited. To the best of our knowledge, there are no effective anti-COVID-19 strategies formulated yet, and further research is needed to be warranted to increase our understanding of SARS-CoV-2 and forestall this infection in a living host. Despite the increased interest to explore COVID-19, insightful understanding of the disease is needed. Specifically, the evolution of SARS-CoV to SARS-CoV-2 with altered virus-host interactions in terms of enhanced binding potentially led to higher levels of pathogenicity. Thus prompted understanding of the associated viral mutations and viral evolution is needed. Although, only few polymorphic sites are identified for the SARS-CoV-2 variants, the spectrum of variants specific intra-host variant interactions and dynamics related to the evolution of this virus remains unknown. A genomic level mechanistic surveillance of the virus may help to manage the potential spread of novel coronaviruses affecting humans. Apart from this, the already developed vaccines and drugs are also challenged by the emerging novel variants of the virus. Herein, advanced studies are needed to explore the phenotypic relation during virus-host interactions and assessing the impact of environmental changes in relation to genomic sequences, more systematic methods are needed to grasp the pathogenicity of COVID-19 associated infections. Furthermore, vigorous methods for drugs screening such as artificial intelligence (AI) assisted discovery and its laboratory tests are needed to hamper the coronaviruses evolution. Further, mutational landscape of spike protein to predict further lethal variant may inform the potential risk of emerging variants.
Funding
Dong-Qing Wei is supported by grants from the Key Research Area Grant 2016YFA0501703 of the Ministry of Science and Technology of China, the National Natural Science Foundation of China (Grant No. 32070662, 61832019, 32030063), the Science and Technology Commission of Shanghai Municipality (Grant No.: 19430750600), the Natural Science Foundation of Henan Province (162300410060), as well as SJTU JiRLMDS Joint Research Fund and Joint Research Funds for Medical and Engineering and Scientific Research at Shanghai Jiao Tong University (YG2017ZD14).
CRediT authorship contribution statement
All the authors were involved in data collection, drafting, graphics, final manuscript. Dr. Anwar Mohammad revised the whole manuscript, worked the new figures, critically improved the text. Dong-Qing Wei supervised, provided resources and wrote the final draft.
Conflict of interest statement
Declared None.
References
- 1.Lefkowitz E.J., Dempsey D.M., Hendrickson R.C., Orton R.J., Siddell S.G., Smith D.B. Virus taxonomy: the database of the International Committee on Taxonomy of Viruses (ICTV) Nucleic Acids Res. 2018;46:D708–D717. doi: 10.1093/nar/gkx932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee A., Kulcsar K., Misra V., Frieman M., Mossman K. Bats and coronaviruses. Viruses. 2019;11:41. doi: 10.3390/v11010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. New Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorbalenya Alexander E., Baker Susan, Baric Ralph, de Groot Raoul J. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xydakis M.S., Dehgani-Mobaraki P., Holbrook E.H., Geisthoff U.W., Bauer C., Hautefort C., Herman P., Manley G.T., Lyon D.M., Hopkins C. Smell and taste dysfunction in patients with COVID-19. Lancet Infect. Dis. 2020;20:1015–1016. doi: 10.1016/S1473-3099(20)30293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019–nCoV) in Wuhan, China. J. Med. Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estola T. Coronaviruses, a new group of animal RNA viruses. Avian Dis. 1970;14:330–336. doi: 10.2307/1588476. [DOI] [PubMed] [Google Scholar]
- 10.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. Addendum: a pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;588:E6. doi: 10.1038/s41586-020-2951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perlman S. Another decade, another coronavirus. New Engl. J. Med. 2020;382:760–762. doi: 10.1056/NEJMe2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benvenuto D., Giovanetti M., Ciccozzi A., Spoto S., Angeletti S., Ciccozzi M. The 2019-new coronavirus epidemic: evidence for virus evolution. J. Med. Virol. 2020;92:455–459. doi: 10.1002/jmv.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acter T., Uddin N., Das J., Akhter A., Choudhury T.R., Kim S. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: a global health emergency. Sci. Total Environ. 2020;730 doi: 10.1016/j.scitotenv.2020.138996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilal El Idrissi H. COVID-19: what you need to know. Gene Rep. 2020;20 doi: 10.1016/j.genrep.2020.100756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popa A., Genger J.-W., Nicholson M.D., Penz T., Schmid D., Aberle S.W., Agerer B., Lercher A., Endler L., Colaço H., Smyth M., Schuster M., Grau M.L., Martínez-Jiménez F., Pich O., Borena W., Pawelka E., Keszei Z., Senekowitsch M., Laine J., Aberle J.H., Redlberger-Fritz M., Karolyi M., Zoufaly A., Maritschnik S., Borkovec M., Hufnagl P., Nairz M., Weiss G., Wolfinger M.T., von Laer D., Superti-Furga G., Lopez-Bigas N., Puchhammer-Stöckl E., Allerberger F., Michor F., Bock C., Bergthaler A. Genomic epidemiology of superspreading events in Austria reveals mutational dynamics and transmission properties of SARS-CoV-2. Sci. Transl. Med. 2020;12:eabe2555. doi: 10.1126/scitranslmed.abe2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang T., Wu Q., Zhang Z. Probable Pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020;30:1578. doi: 10.1016/j.cub.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tortorici M.A., Veesler D. Structural insights into coronavirus entry. Adv. Virus Res. 2019;105:93–116. doi: 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4:1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burkard C., Verheije M.H., Wicht O., van Kasteren S.I., van Kuppeveld F.J., Haagmans B.L., Pelkmans L., Rottier P.J., Bosch B.J., de Haan C.A. Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walls A.C., Tortorici M.A., Bosch B.-J., Frenz B., Rottier P., DiMaio F., Rey F.A., Veesler D. Cryo-electron microscopy structure of a coronavirus spike glycoprotein trimer. Nature. 2016;531:114–117. doi: 10.1038/nature16988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(281–292):281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li F. Receptor recognition mechanisms of coronaviruses: a decade of structural studies. J. Virol. 2015;89:1954–1964. doi: 10.1128/JVI.02615-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W., Zhang C., Sui J., Kuhn J.H., Moore M.J., Luo S., Wong S.K., Huang I.C., Xu K., Vasilieva N., Murakami A., He Y., Marasco W.A., Guan Y., Choe H., Farzan M. Receptor and viral determinants of SARS‐coronavirus adaptation to human ACE2. EMBO J. 2005;24:1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belouzard S., Chu V.C., Whittaker G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. USA. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirchdoerfer R.N., Cottrell C.A., Wang N., Pallesen J., Yassine H.M., Turner H.L., Corbett K.S., Graham B.S., McLellan J.S., Ward A.B. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531:118–121. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qu X.-X., Hao P., Song X.-J., Jiang S.M., Liu Y.X., Wang P.G., Rao X., Song H.D., Wang S.Y., Zuo Y., Zheng A.H., Luo M., Wang H.L., Deng F., Wang H.Z., Hu Z.H., Ding M.X., Zhao G.P., Deng H.K. Identification of two critical amino acid residues of the severe acute respiratory syndrome coronavirus spike protein for its variation in zoonotic tropism transition via a double substitution strategy. J. Biol. Chem. 2005;280:29588–29595. doi: 10.1074/jbc.M500662200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qu X.X., Hao P., Song X.J., Jiang S.M., Liu Y.X., Wang P.G., Rao X., Song H.D., Wang S.Y., Zuo Y., Zheng A.H., Luo M., Wang H.L., Deng F., Wang H.Z., Hu Z.H., Ding M.X., Zhao G.P., Deng H.K. Identification of two critical amino acid residues of the severe acute respiratory syndrome coronavirus spike protein for its variation in zoonotic tropism transition via a double substitution strategy. J. Biol. Chem. 2005;280:29588–29595. doi: 10.1074/jbc.M500662200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore M.J., Dorfman T., Li W., Wong S.K., Li Y., Kuhn J.H., Coderre J., Vasilieva N., Han Z., Greenough T.C., Farzan M., Choe H. Retroviruses pseudotyped with the severe acute respiratory syndrome coronavirus spike protein efficiently infect cells expressing angiotensin-converting enzyme 2. J. Virol. 2004;78:10628–10635. doi: 10.1128/JVI.78.19.10628-10635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du L., Yang Y., Zhou Y., Lu L., Li F., Jiang S. MERS-CoV spike protein: a key target for antivirals. Expert Opin. Ther. Targets. 2017;21:131–143. doi: 10.1080/14728222.2017.1271415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alsaadi E.A., Jones I.M. Membrane binding proteins of coronaviruses. Future Virol. 2019;14:275–286. doi: 10.2217/fvl-2018-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosch B.J., Bartelink W., Rottier P.J. Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J. Virol. 2008;82:8887–8890. doi: 10.1128/JVI.00415-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millet J.K., Whittaker G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc. Natl. Acad. Sci. USA. 2014;111:15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li K., Markosyan R.M., Zheng Y.-M., Golfetto O., Bungart B., Li M., Ding S., He Y., Liang C., Lee J.C., Gratton E., Cohen F.S., Liu S.L. IFITM proteins restrict viral membrane hemifusion. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim Y.X., Ng Y.L., Tam J.P., Liu D.X. Human coronaviruses: a review of virus–host interactions. Diseases. 2016;4:26. doi: 10.3390/diseases4030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haque S.M., Ashwaq O., Sarief A., Azad John Mohamed A.K. A comprehensive review about SARS-CoV-2. Future Virol. 2020;15:625–648. doi: 10.2217/fvl-2020-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei Z.Y., Li H.S., Zhou J.Y., Han C., Dong H., Wu Y.Z., He W.F., Tian Y., Luo G.X. Mechanism of transcriptional regulation of Meox1 by transforming growth factor β (1) and its effect on cell migration of adult human dermal fibroblasts. Zhonghua shao shang za zhi = Zhonghua shaoshang zazhi = Chin. J. Burns. 2020;36:224–233. doi: 10.3760/cma.j.cn501120-20200109-00014. [DOI] [PubMed] [Google Scholar]
- 37.Chan J.F., Yuan S., Kok K.-H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C., Poon R.W., Tsoi H.W., Lo S.K., Chan K.H., Poon V.K., Chan W.M., Ip J.D., Cai J.P., Cheng V.C., Chen H., Hui C.K., Yuen K.Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J.-Y., You Z., Wang Q., Zhou Z.J., Qiu Y., Luo R., Ge X.Y. The epidemic of 2019-novel-coronavirus (2019-nCoV) pneumonia and insights for emerging infectious diseases in the future. Microbes Infect. 2020;22:80–85. doi: 10.1016/j.micinf.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klompas M., Baker M.A., Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA. 2020;324:441–442. doi: 10.1001/jama.2020.12458. [DOI] [PubMed] [Google Scholar]
- 40.Stadnytskyi V., Bax C.E., Bax A., Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. USA. 2020;117:11875–11877. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morawska L., Milton D.K. It is time to address airborne transmission of Coronavirus Disease 2019 (COVID-19) Clin. Infect. Dis. 2020;71:2311–2313. doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahman H.S., Aziz M.S., Hussein R.H., Othman H.H., Salih Omer S.H., Khalid E.S., Abdulrahman N.A., Amin K., Abdullah R. The transmission modes and sources of COVID-19: a systematic review. Int. J. Surg. Open. 2020;26:125–136. doi: 10.1016/j.ijso.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prentiss M., Chu A., Berggren K.K. Superspreading events without superspreaders: using high attack rate events to estimate No for airborne transmission of COVID-19. medRxiv. 2020;2020 doi: 10.1101/2020.10.21.20216895. (2010.2021.20216895.) [DOI] [Google Scholar]
- 44.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 45.Xydakis M.S., Dehgani-Mobaraki P., Holbrook E.H., Geisthoff U.W., Bauer C., Hautefort C., Herman P., Manley G.T., Lyon D.M., Hopkins C. Smell and taste dysfunction in patients with COVID-19. Lancet Infect. Dis. 2020;20:1015–1016. doi: 10.1016/s1473-3099(20)30293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Backer J.A., Klinkenberg D., Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20-28 January 2020. Eur. Surveill. 2020;25 doi: 10.2807/1560-7917.es.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Z., McGoogan J.M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 48.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oran D.P., Topol E.J. The proportion of SARS-CoV-2 infections that are asymptomatic. Ann. Intern. Med. 2021;174:655–662. doi: 10.7326/M20-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rabi F.A., Al Zoubi M.S., Kasasbeh G.A., Salameh D.M., Al-Nasser A.D. SARS-CoV-2 and Coronavirus Disease 2019: what we know so far. Pathogens. 2020;9:9. doi: 10.3390/pathogens9030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S., for the China Medical Treatment Expert Group for Covid-19 Clinical characteristics of Coronavirus Disease 2019 in China. New Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collange O., Tacquard C., Delabranche X., Leonard-Lorant I., Ohana M., Onea M., Anheim M., Solis M., Sauer A., Baloglu S., Pessaux P., Ohlmann P., Kaeuffer C., Oulehri W., Kremer S., Mertes P.M. Coronavirus Disease 2019: associated multiple organ damage. Open Forum Infect. Dis. 2020;7:249. doi: 10.1093/ofid/ofaa249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mokhtari T., Hassani F., Ghaffari N., Ebrahimi B., Yarahmadi A., Hassanzadeh G. COVID-19 and multiorgan failure: a narrative review on potential mechanisms. J. Mol. Histol. 2020;51:613–628. doi: 10.1007/s10735-020-09915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moldoveanu B., Otmishi P., Jani P., Walker J., Sarmiento X., Guardiola J., Saad M., Yu J. Inflammatory mechanisms in the lung. J. Inflamm. Res. 2009;2:1–11. [PMC free article] [PubMed] [Google Scholar]
- 55.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sherren P.B., Ostermann M., Agarwal S., Meadows C.I.S., Ioannou N., Camporota L. COVID-19-related organ dysfunction and management strategies on the intensive care unit: a narrative review. Br. J. Anaesth. 2020;125:912–925. doi: 10.1016/j.bja.2020.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robba C., Battaglini D., Pelosi P., Rocco P.R.M. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev. Respir. Med. 2020;14:865–868. doi: 10.1080/17476348.2020.1778470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paniz-Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J., Sordillo E.M., Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J. Med. Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garg R.K. Spectrum of neurological manifestations in Covid-19: a review. Neurol. India. 2020;68:560–572. doi: 10.4103/0028-3886.289000. [DOI] [PubMed] [Google Scholar]
- 61.Cheung K.S., Hung I.F.N., Chan P.P.Y., Lung K.C., Tso E., Liu R., Ng Y.Y., Chu M.Y., Chung T., Tam A.R., Yip C., Leung K.H., Fung A.Y., Zhang R.R., Lin Y., Cheng H.M., Zhang A., To K., Chan K.H., Yuen K.Y., Leung W.K. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong Cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bénézit F., Le Turnier P., Declerck C., Paillé C., Revest M., Dubée V., Tattevin P., RAN COVID Study G. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect. Dis. 2020;20:1014–1015. doi: 10.1016/S1473-3099(20)30297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pérez-Bermejo J.A., Kang S., Rockwood S.J., Simoneau C.R., Joy D.A., Ramadoss G.N., Silva A.C., Flanigan W.R., Li H., Nakamura K., Whitman J.D., Ott M., Conklin B.R., McDevitt T.C. SARS-CoV-2 infection of human iPSC-derived cardiac cells predicts novel cytopathic features in hearts of COVID-19 patients. bioRxiv: Prepr. Serv. Biol. 2020 doi: 10.1101/2020.08.25.265561. (2020.2008.2025.265561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maccio U., Zinkernagel A.S., Shambat S.M., Zeng X., Cathomas G., Ruschitzka F., Schuepbach R.A., Moch H., Varga Z. SARS-CoV-2 leads to a small vessel endotheliitis in the heart. EBioMedicine. 2021;63 doi: 10.1016/j.ebiom.2020.103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao X., Chen D., Szabla R., Zheng M., Li G., Du P., Zheng S., Li X., Song C., Li R., Guo J.T., Junop M., Zeng H., Lin H. Broad and differential animal angiotensin-converting enzyme 2 receptor usage by SARS-CoV-2. J. Virol. 2020;94:94. doi: 10.1128/jvi.00940-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Konopka K.E., Nguyen T., Jentzen J.M., Rayes O., Schmidt C.J., Wilson A.M., Farver C.F., Myers J.L. Diffuse alveolar damage (DAD) resulting from coronavirus disease 2019 infection is morphologically indistinguishable from Other Causes of DAD. Histopathology. 2020;77:570–578. doi: 10.1111/his.14180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adachi T., Chong J.M., Nakajima N., Sano M., Yamazaki J., Miyamoto I., Nishioka H., Akita H., Sato Y., Kataoka M., Katano H., Tobiume M., Sekizuka T., Itokawa K., Kuroda M., Suzuki T. Clinicopathologic and immunohistochemical findings from autopsy of patient with COVID-19, Japan. Emerg. Infect. Dis. 2020;26:2157–2161. doi: 10.3201/eid2609.201353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.González Pessolani T., Muñóz Fernández de Legaria M., Elices Apellániz M., Salinas Moreno S., Lorido Cortés MdM, García Sánchez S. Multi-organ pathological findings associated with COVID-19 in postmortem needle core biopsies in four patients and a review of the current literature. Revista Española de Patología. 〈 10.1016/j.patol.2020.09.003〉. [DOI] [PMC free article] [PubMed]
- 69.Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., Li J.X., Yang B.F., Wang L., Wang W.J., Wu S.P., Wang Z., Wu X.H., Xu J.J., Zhang Z., Jia S.Y., Wang B.S., Hu Y., Liu J.J., Zhang J., Qian X.A., Li Q., Pan H.X., Jiang H.D., Deng P., Gou J.B., Wang X.W., Wang X.H., Chen W. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/s0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A., Dold C., Faust S.N., Finn A., Flaxman A.L., Hallis B., Heath P., Jenkin D., Lazarus R., Makinson R., Minassian A.M., Pollock K.M., Ramasamy M., Robinson H., Snape M., Tarrant R., Voysey M., Green C., Douglas A.D., Hill A., Lambe T., Gilbert S.C., Pollard A.J., on behalf of theOxford COVID Vaccine Trial Group Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/s0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A.B., Flach B., Lin B.C., Doria-Rose N.A., O’Dell S., Schmidt S.D., Corbett K.S., Swanson P.A., Padilla M., Neuzil K.M., Bennett H., Leav B., Makowski M., Albert J., Cross K., Edara V.V., Floyd K., Suthar M.S., Martinez D.R., Baric R., Buchanan W., Luke C.J., Phadke V.K., Rostad C.A., Ledgerwood J.E., Graham B.S., Beigel J.H., mRNA-Study G. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. New Engl. J. Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xia S., Duan K., Zhang Y., Zhao D., Zhang H., Xie Z., Li X., Peng C., Zhang Y., Zhang W., Yang Y., Chen W., Gao X., You W., Wang X., Wang Z., Shi Z., Wang Y., Yang X., Zhang L., Huang L., Wang Q., Lu J., Yang Y., Guo J., Zhou W., Wan X., Wu C., Wang W., Huang S., Du J., Meng Z., Pan A., Yuan Z., Shen S., Guo W., Yang X. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang D., Comish P., Kang R. The hallmarks of COVID-19 disease. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yurkovetskiy L., Wang X., Pascal K.E., Tomkins-Tinch C., Nyalile T.P., Wang Y., Baum A., Diehl W.E., Dauphin A., Carbone C., Veinotte K., Egri S.B., Schaffner S.F., Lemieux J.E., Munro J.B., Rafique A., Barve A., Sabeti P.C., Kyratsous C.A., Dudkina N.V., Shen K., Luban J., Yurkovetskiy L., Wang X., Pascal K.E., Tomkins-Tinch C., Nyalile T., Wang Y., Baum A., Diehl W.E., Dauphin A., Carbone C., Veinotte K., Egri S., Schaffner S., Lemieux J.E., Munro J., Rafique A., Barve A., Sabeti P.C., Kyratsous C.A., Dudkina N., Shen K., Luban J., Yurkovetskiy L., Wang X., Pascal K.E., Tomkins-Tinch C., Nyalile T., Wang Y., Baum A., Diehl W.E., Dauphin A., Carbone C., Veinotte K., Egri S.B., Schaffner S.F., Lemieux J.E., Munro J., Rafique A., Barve A., Sabeti P.C., Kyratsous C.A., Dudkina N., Shen K., Luban J. Structural and functional analysis of the D614G SARS-CoV-2 spike protein variant. Cell. 2020;183(739–751):739–751.e8. doi: 10.1016/j.cell.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou B., Thao T.T.N., Hoffmann D., Taddeo A., Ebert N., Labroussaa F., Pohlmann A., King J., Portmann J., Halwe N.J., Ulrich L., Trüeb B.S., Kelly J.N., Fan X., Hoffmann B., Steiner S., Wang L., Thomann L., Lin X., Stalder H., Pozzi B., de Brot S., Jiang N., Cui D., Hossain J., Wilson M., Keller M., Stark T.J., Barnes J.R., Dijkman R., Jores J., Benarafa C., Wentworth D.E., Thiel V., Beer M. SARS-CoV-2 spike D614G variant confers enhanced replication and transmissibility. bioRxiv: Prepr. Serv. Biol. 2020 [Google Scholar]
- 76.Zhang L., Jackson C.B., Mou H., Ojha A., Rangarajan E.S., Izard T., Farzan M., Choe H. The D614G mutation in the SARS-CoV-2 spike protein reduces S1 shedding and increases infectivity. BioRxiv. 2020 [Google Scholar]
- 77.Mohammad A., Alshawaf E., Marafie S.K., Abu-Farha M., Abubaker J., Al-Mulla F. Higher binding affinity of furin for SARS-CoV-2 spike (S) protein D614G mutant could be associated with higher SARS-CoV-2 infectivity. Int. J. Infect. Dis. 2021;103:611–616. doi: 10.1016/j.ijid.2020.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Groves D.C., Rowland-Jones S.L., Angyal A. The D614G mutations in the SARS-CoV-2 spike protein: implications for viral infectivity, disease severity and vaccine design. Biochem. Biophys. Res. Commun. 2021;538:104–107. doi: 10.1016/j.bbrc.2020.10.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tu H., Avenarius M.R., Kubatko L., Hunt M., Pan X., Ru P., Garee J., Thomas K., Mohler P., Pancholi P., Jones D. Distinct patterns of emergence of SARS-CoV-2 spike variants including N501Y in clinical samples in Columbus Ohio. bioRxiv. 2021 doi: 10.1101/2021.01.12.426407. (2021.2001.2012.426407) [DOI] [Google Scholar]
- 80.Leung K., Shum M.H., Leung G.M., Lam T.T., Wu J.T. Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Eur. Surveill.: Bull. Eur. sur les Mal. Transm. = Eur. Commun. Dis. Bull. 2021;26 doi: 10.2807/1560-7917.ES.2020.26.1.2002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harrington D., Kele B., Pereira S., Couto-Parada X., Riddell A., Forbes S., Dobbie H., Cutino-Moguel T. Confirmed reinfection with SARS-CoV-2 variant VOC-202012/01. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2021 doi: 10.1093/cid/ciab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fiorentini S., Messali S., Zani A., Caccuri F., Giovanetti M., Ciccozzi M., Caruso A. First detection of SARS-CoV-2 spike protein N501 mutation in Italy in August, 2020. Lancet Infect. Dis. 2021;21:147. doi: 10.1016/S1473-3099(21)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pater A.A., Bosmeny M.S., Barkau C.L., et al. Emergence and evolution of a prevalent new SARS-CoV-2 variant in the United States. bioRxiv. 2021 [Google Scholar]
- 84.Galloway S.E., Paul P., MacCannell D.R., Johansson M.A., Brooks J.T., MacNeil A., Slayton R.B., Tong S., Silk B.J., Armstrong G.L., Biggerstaff M., Dugan V.G. Emergence of SARS-CoV-2 B.1.1. 7 lineage—united states, december 29, 2020–january 12, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:95–99. doi: 10.15585/mmwr.mm7003e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raskin S. Genetics of COVID-19. J. Pediatr. 2021;97:378–386. doi: 10.1016/j.jped.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Su S., Wang Q., Jiang S. Facing the challenge of viral mutations in the age of pandemic: developing highly potent, broad‐spectrum, and safe COVID‐19 vaccines and therapeutics. Clin. Transl. Med. 2021;11:284. doi: 10.1002/ctm2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khan A., Zia T., Suleman M., Khan T., Ali S.S., Abbasi A.A., Mohammad A., Wei D.Q. Higher infectivity of the SARS-CoV-2 new variants is associated with K417N/T, E484K, and N501Y mutants: an insight from structural data. J. Cell. Physiol. 2021;236:7045–7057. doi: 10.1002/jcp.30367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hussain I., Pervaiz N., Khan A., Saleem S., Shireen H., Wei D.Q., Labrie V., Bao Y., Abbasi A.A. Evolutionary and structural analysis of SARS-CoV-2 specific evasion of host immunity. Genes Immun. 2020;21:409–419. doi: 10.1038/s41435-020-00120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khan A., Khan S., Saleem S., Nizam-Uddin N., Mohammad A., Khan T., Ahmad S., Arshad M., Ali S.S., Suleman M., Wei D.Q. Immunogenomics guided design of immunomodulatory multi-epitope subunit vaccine against the SARS-CoV-2 new variants, and its validation through in silico cloning and immune simulation. Comput. Biol. Med. 2021;133 doi: 10.1016/j.compbiomed.2021.104420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khan A., Wei D.-Q., Kousar K., et al. Preliminary structural data revealed that the SARS-CoV-2 B.1.617 variant’s RBD binds to ACE2 receptor stronger than the wild type to enhance the infectivity. ChemBioChem. 2021 doi: 10.1002/cbic.202100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maison D.P., Ching L.L., Shikuma C.M., Nerurkar V.R. Genetic characteristics and phylogeny of 969-bp S gene sequence of SARS-CoV-2 from Hawai’i Reveals the Worldwide Emerging P681H Mutation. Hawai’i J. Health Soc. Welf. 2021;80:52–61. [PMC free article] [PubMed] [Google Scholar]
- 92.Lubinski B., Tang T., Daniel S., Jaimes J.A., Whittaker G. Functional evaluation of proteolytic activation for the SARS-CoV-2 variant B.1.1. bioRxiv: Prepr. Serv. Biol. 2021 doi: 10.1016/j.isci.2021.103589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang P., Casner R.G., Nair M.S., Wang M., Yu J., Cerutti G., Liu L., Kwong P.D., Huang Y., Shapiro L., Ho D.D. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe. 2021;29(747–751):747–751. doi: 10.1016/j.chom.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rahimi F., Abadi A.T.B. Implications of the emergence of a new variant of SARS-CoV-2, VUI-202012/01. Arch. Med. Res. 2021;52:569–571. doi: 10.1016/j.arcmed.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang J.W., Toovey O.T., Harvey K.N., Hui D.D. Introduction of the South African SARS-CoV-2 variant 501Y.V2 into the UK. J. Infect. 2021;82:8. doi: 10.1016/j.jinf.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Davies M.A. HIV and risk of COVID-19 death: a population cohort study from the Western Cape Province, South Africa. medRxiv. 2020 [Google Scholar]
- 97.Cheng M.H., Krieger J.M., Kaynak B., Arditi M.A., Bahar I. Genome-scale metabolic modeling reveals SARS-CoV-2-induced host metabolic reprogramming and identifies metabolic antiviral targets. bioRxiv. 2021 [Google Scholar]
- 98.Voloch C.M., Silva F R., de Almeida L.G.P., Cardoso C.C., Brustolini O.J., Gerber A.L., Guimarães A.P.C., Mariani D., Costa R.M., Ferreira O.C., Cavalcanti A.C., Frauches T.S., de Mello C.M.B., Galliez R.M., Faffe D.S., Castiñeiras T.M.P.P., Tanuri A., de Vasconcelos A.T.R. Genomic characterization of a novel SARS-CoV-2 lineage from Rio de Janeiro, Brazil. medRxiv. 2020 doi: 10.1101/2020.12.23.20248598. (2020.2012.2023.20248598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lassaunière R., Fonager J., Rasmussen M., Frische A., Polacek C., Rasmussen T.B., Lohse L., Belsham G.J., Underwood A., Winckelmann A.A., Bollerup S., Bukh J., Weis N., Sækmose S.G., Aagaard B., Alfaro-Núñez A., Mølbak K., Bøtner A., Fomsgaard A. In vitro characterization of fitness and convalescent antibody neutralization of SARS-CoV-2 Cluster 5 variant emerging in mink at Danish Farms. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.698944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brejová B., Hodorová V., Boršová K., et al. B.1.258Δ, a SARS-CoV-2 variant with ΔH69/ΔV70 in the Spike protein circulating in the Czech Republic and Slovakia. arXiv Prepr. 2021 (arXiv:2102.04689 2021) [Google Scholar]
- 101.Focosi D., Tuccori M., Baj A., Maggi F. Multidisciplinary Digital Publishing Institute; 2021. SARS-CoV-2 Variants: A Synopsis of In Vitro Efficacy Data of Convalescent Plasma, Currently Marketed Vaccines, and Monoclonal Antibodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Faria N.R., Mellan T.A., Whittaker C., Claro I.M., Candido D., Mishra S., Crispim M., Sales F., Hawryluk I., McCrone J.T., Hulswit R., Franco L., Ramundo M.S., de Jesus J.G., Andrade P.S., Coletti T.M., Ferreira G.M., Silva C., Manuli E.R., Pereira R., Peixoto P.S., Kraemer M., Gaburo N Jr, Camilo C., Hoeltgebaum H., Souza W.M., Rocha E.C., de Souza L.M., de Pinho M.C., Araujo L., Malta F., de Lima A.B., Silva J., Zauli D., Ferreira A., Schnekenberg R.P., Laydon D.J., Walker P., Schlüter H.M., Dos Santos A., Vidal M.S., Del Caro V.S., Filho R., Dos Santos H.M., Aguiar R.S., Proença-Modena J.L., Nelson B., Hay J.A., Monod M., Miscouridou X., Coupland H., Sonabend R., Vollmer M., Gandy A., Prete CA Jr, Nascimento V.H., Suchard M.A., Bowden T.A., Pond S., Wu C.H., Ratmann O., Ferguson N.M., Dye C., Loman N.J., Lemey P., Rambaut A., Fraiji N.A., Carvalho M., Pybus O.G., Flaxman S., Bhatt S., Sabino E.C. Genomics and epidemiology of the P. 1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021;372:815–821. doi: 10.1126/science.abh2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maggi F., Novazzi F., Genoni A., Baj A., Spezia P.G., Focosi D., Zago C., Colombo A., Cassani G., Pasciuta R., Tamborini A., Rossi A., Prestia M., Capuano R., Azzi L., Donadini A., Catanoso G., Grossi P.A., Maffioli L., Bonelli G. Imported SARS-CoV-2 variant P.1 in traveler returning from Brazil to Italy. Emerg. Infect. Dis. 2021;27:1249–1251. doi: 10.3201/eid2704.210183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hoffmann M., Arora P., Groß R., Seidel A., Hörnich B.F., Hahn A.S., Krüger N., Graichen L., Hofmann-Winkler H., Kempf A., Winkler M.S., Schulz S., Jäck H.M., Jahrsdörfer B., Schrezenmeier H., Müller M., Kleger A., Münch J., Pöhlmann S. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021;184(2384–2393):2384–2393. doi: 10.1016/j.cell.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hirotsu Y., Omata M. Discovery of a SARS-CoV-2 variant from the P.1 lineage harboring K417T/E484K/N501Y mutations in Kofu, Japan. J. Infect. 2021;82:276–316. doi: 10.1016/j.jinf.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Annavajhala M.K., Mohri H., Wang P., Nair M., Zucker J.E., Sheng Z., Gomez-Simmonds A., Kelley A.L., Tagliavia M., Huang Y., Bedford T., Ho D.D., Uhlemann A.C. A novel and expanding SARS-CoV-2 variant, B.1.526, identified in New York. medRxiv. 2021 doi: 10.1038/s41586-021-03908-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garcia-Beltran W.F., Lam E.C., St Denis K., Nitido A.D., Garcia Z.H., Hauser B.M., Feldman J., Pavlovic M.N., Gregory D.J., Poznansky M.C., Sigal A., Schmidt A.G., Iafrate A.J., Naranbhai V., Balazs A.B. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. medRxiv. 2021 doi: 10.1101/2021.02.14.21251704. (2021.2002.2014.21251704.) [DOI] [Google Scholar]
- 108.Wall E.C., Wu M., Harvey R., Kelly G., Warchal S., Sawyer C., Daniels R., Hobson P., Hatipoglu E., Ngai Y., Hussain S., Nicod J., Goldstone R., Ambrose K., Hindmarsh S., Beale R., Riddell A., Gamblin S., Howell M., Kassiotis G., Libri V., Williams B., Swanton C., Gandhi S., Bauer D.L. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397:2331–2333. doi: 10.1016/S0140-6736(21)01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dholariya S., Parchwani D.N., Singh R., Sonagra A., Motiani A., Patel D. Notable and emerging variants of SARS-CoV-2 virus: a quick glance. Indian J. Clin. Biochem.: IJCB. 2021:1–8. doi: 10.1007/s12291-021-00991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yadav P., Sapkal G.N., Abraham P., et al. Neutralization of variant under investigation B.1.617 with sera of BBV152 vaccinees. bioRxiv. 2021 doi: 10.1093/cid/ciab411. [DOI] [PubMed] [Google Scholar]
- 111.Planas D., Veyer D., Baidaliuk A., et al. Reduced sensitivity of infectious SARS-CoV-2 variant B.1.617.2 to monoclonal antibodies and sera from convalescent and vaccinated individuals. bioRxiv. 2021 [Google Scholar]
- 112.Shen X., Tang H., Pajon R., Smith G., Glenn G.M., Shi W., Korber B., Montefiori D.C. Neutralization of SARS-CoV-2 Variants B.1.429 and B.1.351. New Engl. J. Med. 2021;384:2352–2354. doi: 10.1056/NEJMc2103740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shen X., Tang H., Pajon R., Smith G., Glenn G.M., Shi W., Korber B., Montefiori D.C. Neutralization of SARS-CoV-2 variants B.1.429 and B.1.351. New Engl. J. Med. 2021;384:2352–2354. doi: 10.1056/NEJMc2103740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McCallum M., Bassi J., De Marco A., et al. SARS-CoV-2 immune evasion by variant B.1.427/B.1.429. BioRxiv. 2021 doi: 10.1126/science.abi7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McCallum M., Bassi J., De Marco A., Chen A., Walls A.C., Di Iulio J., Tortorici M.A., Navarro M.J., Silacci-Fregni C., Saliba C., Sprouse K.R., Agostini M., Pinto D., Culap K., Bianchi S., Jaconi S., Cameroni E., Bowen J.E., Tilles S.W., Pizzuto M.S., Guastalla S.B., Bona G., Pellanda A.F., Garzoni C., Van Voorhis W.C., Rosen L.E., Snell G., Telenti A., Virgin H.W., Piccoli L., Corti D., Veesler D. SARS-CoV-2 immune evasion by the B.1.427/B.1.429 variant of concern. Science. 2021;373:648–654. doi: 10.1126/science.abi7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Parums D.V. Revised World Health Organization (WHO) Terminology for Variants of Concern and Variants of Interest of SARS-CoV-2. Med. Sci. Monit. 2021:27. doi: 10.12659/MSM.933622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barona-Gómez F., Delaye L., Díaz-Valenzuela E., et al. Phylogenomics and population genomics of SARS-CoV-2 in Mexico during the pre-vaccination stage reveals variants of interest B.1.1. 28.4, B.1.1. 222 or B.1.1. 519 and B.1.243 with mutations in the Spike protein and the Nucleocapsid. medRxiv. 2021 doi: 10.1099/mgen.0.000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nagano K., Tani-Sassa C., Iwasaki Y., et al. SARS-CoV-2 R. 1 lineage variants prevailed in Tokyo in March 2021. medRxiv. 2021 doi: 10.1002/jmv.27240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cavanaugh A.M., Fortier S., Lewis P., Arora V., Johnson M., George K., Tobias J., Lunn S., Miller T., Thoroughman D., Spicer K.B. COVID-19 outbreak associated with a SARS-CoV-2 R. 1 lineage variant in a skilled nursing facility after vaccination program—Kentucky, March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:639–643. doi: 10.15585/mmwr.mm7017e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hirotsu Y., Omata M. Detection of R. 1 lineage severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with spike protein W152L/E484K/G769V mutations in Japan. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dudas G., Hong S.L., Potter B., et al. Travel-driven emergence and spread of SARS-CoV-2 lineage B.1.620 with multiple VOC-like mutations and deletions in Europe. medRxiv. 2021 doi: 10.1038/s41467-021-26055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kustin T., Harel N., Finkel U., et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat. Med. 2021:1–6. doi: 10.1038/s41591-021-01413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tragni V., Preziusi F., Laera L., et al. A modular molecular framework for quickly estimating the binding affinity of the spike protein of SARS-CoV-2 variants for ACE2, in presence of mutations at the spike receptor binding domain. bioRxiv. 2021 [Google Scholar]
- 124.Tada T., Zhou H., Dcosta B.M., Samanovic M.I., Mulligan M.J., Landau N.R. The spike proteins of SARS-CoV-2 B.1.617 and B.1.618 variants identified in india provide partial resistance to vaccine-elicited and therapeutic monoclonal antibodies. BioRxiv. 2021 [Google Scholar]
- 125.Liu J., Liu Y., Xia H., Zou J., Weaver S.C., Swanson K.A., Cai H., Cutler M., Cooper D., Muik A., Jansen K.U., Sahin U., Xie X., Dormitzer P.R., Shi P.Y. BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants. Nature. 2021;596 doi: 10.1038/s41586-021-03693-y. 273-5. [DOI] [PubMed] [Google Scholar]
- 126.Tegally H., Ramuth M., Amoaka D., et al. A novel and expanding SARS-CoV-2 Variant, B.1.1. 318, dominates infections in Mauritius. medRxiv. 2021 [Google Scholar]
- 127.Burioni R., Topol E.J. Has SARS-CoV-2 reached peak fitness? Nat. Med. 2021;27:1323–1324. doi: 10.1038/s41591-021-01421-7. 1-1. [DOI] [PubMed] [Google Scholar]
- 128.Food U., Administration D. Coronavirus (COVID-19) update: FDA issues policies to guide medical product developers addressing virus variants. FDA Febr. 2021;23:2021. [Google Scholar]
- 129.Mahase E. Covid-19: where are we on vaccines and variants? Br. Med. J. Publ. Group. 2021 doi: 10.1136/bmj.n597. [DOI] [PubMed] [Google Scholar]
- 130.Organization WH. COVID-19 weekly epidemiological update, edition 45, 22, June 2021.
- 131.Muik A., Wallisch A.-K., Sänger B., Swanson K.A., Mühl J., Chen W., Cai H., Maurus D., Sarkar R., Türeci Ö., Dormitzer P.R., Şahin U. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine–elicited human sera. Science. 2021;371:1152–1153. doi: 10.1126/science.abg6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., Wang M., Yu J., Zhang B., Kwong P.D., Graham B.S., Mascola J.R., Chang J.Y., Yin M.T., Sobieszczyk M., Kyratsous C.A., Shapiro L., Sheng Z., Huang Y., Ho D.D. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 133.Mahase E. Covid-19: novavax vaccine efficacy is 86% against UK variant and 60% against South African variant. Br. Med. J. Publ. Group. 2021 doi: 10.1136/bmj.n296. [DOI] [PubMed] [Google Scholar]
- 134.Tanne J.H. Covid-19: moderna plans booster doses to counter variants. BMJ. 2021;372:232. doi: 10.1136/bmj.n232. [DOI] [PubMed] [Google Scholar]
- 135.Liu Y., Liu J., Xia H., Zhang X., Fontes-Garfias C.R., Swanson K.A., Cai H., Sarkar R., Chen W., Cutler M., Cooper D., Weaver S.C., Muik A., Sahin U., Jansen K.U., Xie X., Dormitzer P.R., Shi P.Y. Neutralizing activity of BNT162b2-elicited serum. New Engl. J. Med. 2021;384:1466–1468. doi: 10.1056/NEJMc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Doshi P. Covid-19 vaccines: in the rush for regulatory approval, do we need more data? BMJ. 2021;373:1244. doi: 10.1136/bmj.n1244. [DOI] [PubMed] [Google Scholar]
- 137.Johnson Johnson. Argo-two decades: global oceanography, revolutionized. Annu. Rev. Mar. Sci. 2021 doi: 10.1146/annurev-marine-022521-102008. [DOI] [PubMed] [Google Scholar]
- 138.Cohen J. South Africa suspends use of AstraZeneca’s COVID-19 vaccine after it fails to clearly stop virus variant. Science. 2021:10. [Google Scholar]
- 139.Su S., Shao Y., Jiang S. Human challenge trials to assess the efficacy of currently approved COVID-19 vaccines against SARS-CoV-2 variants. Emerg. Microbes Infect. 2021;10:439–441. doi: 10.1080/22221751.2021.1896956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., Plested J.S., Zhu M., Cloney-Clark S., Zhou H., Smith G., Patel N., Frieman M.B., Haupt R.E., Logue J., McGrath M., Weston S., Piedra P.A., Desai C., Callahan K., Lewis M., Price-Abbott P., Formica N., Shinde V., Fries L., Lickliter J.D., Griffin P., Wilkinson B., Glenn G.M. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. New Engl. J. Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Organization WH, Weekly epidemiological update on COVID-19-1, June 2021.
- 142.Ives A.R., Bozzuto C. Estimating and explaining the spread of COVID-19 at the county level in the USA. Commun. Biol. 2021;4:1–9. doi: 10.1038/s42003-020-01609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Padma T. India’s COVID-vaccine woes–by the numbers. Nature. 2021;592:500–501. doi: 10.1038/d41586-021-00996-y. [DOI] [PubMed] [Google Scholar]
- 144.Muik A., Wallisch A.-K., Sänger B., Swanson K.A., Mühl J., Chen W., Cai H., Maurus D., Sarkar R., Türeci Ö., Dormitzer P.R., Şahin U. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine–elicited human sera. Science. 2021;371:1152–1153. doi: 10.1126/science.abg6105. (eabg6105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Collier D.A., De Marco A., Ferreira I., Meng B., Datir R., Walls A.C., Kemp S S.A., Bassi J., Pinto D., Fregni C.S., Bianchi S., Tortorici M.A., Bowen J., Culap K., Jaconi S., Cameroni E., Snell G., Pizzuto M.S., Pellanda A.F., Garzoni C., Riva A., CITIID-NIHR BioResource COVID- C., Elmer A., Kingston N., Graves B., McCoy L.E., Smith K.G., Bradley J.R., Temperton N., Ceron-Gutierrez L L., Barcenas-Morales G., COVID- Genomics UK (COG-UK) c, Harvey W., Virgin H.W., Lanzavecchia A., Piccoli L., Doffinger R., Wills M., Veesler D., Corti D., Gupta R.K. SARS-CoV-2 B.1.1.7 sensitivity to mRNA vaccine-elicited, convalescent and monoclonal antibodies. medRxiv. 2021 doi: 10.1101/2021.01.19.21249840. (2021.2001.2019.21249840.) [DOI] [Google Scholar]
- 146.Khan Abbas, et al. The SARS-CoV-2 B.1.618 variant slightly alters the spike RBD–ACE2 binding affinity and is an antibody escaping variant: a computational structural perspective. RSC Advances. 2021:30132–30147. doi: 10.1039/D1RA04694B. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]