Abstract
Tuberculosis (TB) is the leading cause of infectious mortality in the world, affecting mainly developing countries (DC), while diabetes (DM) is one of the most prevalent chronic diseases.
This review analyzes the fact that diabetes is currently an important risk factor for developing TB, also presenting more complicated TB, more relapses and higher mortality. The DCs and the fourth world of the large cities are those with the highest incidence of TB and an increase in DM, which will make it difficult to control tuberculosis disease. At the same time, the COVID-19 pandemic is complicating the management of both diseases due to the difficulty of access to control and treatment and the worsening of socioeconomic inequalities. It is necessary to establish a bidirectional screening for TB and DM and promote recommendations for the joint management of both diseases.
Keywords: Tuberculosis, Diabetes, Incidence, Risk factors, Progression
Abstract
La tuberculosis (TB) era la primera causa de mortalidad infecciosa mundial hasta la pandemia de COVID-19. Afecta sobre todo a los países en vías de desarrollo (PVD), mientras que la diabetes mellitus (DM) es una de las enfermedades crónicas más prevalentes.
En esta revisión se objetiva que la DM constituye actualmente un importante factor de riesgo para desarrollar TB, presentando además TB más complicadas, más recaídas y mayor letalidad. Los PVD y el cuarto mundo de las grandes ciudades son los que presentan mayor incidencia de TB y un incremento de la DM, lo que dificultará el control de la enfermedad tuberculosa. Paralelamente, la pandemia por COVID-19 está complicando el manejo de ambas enfermedades por la dificultad de acceso al control y tratamiento y por el empeoramiento de desigualdades socioeconómicas. Es necesario establecer un cribado bidireccional de TB y DM e impulsar recomendaciones para el manejo conjunto de ambas enfermedades.
Palabras clave: Tuberculosis, Diabetes, Incidencia, Factores de riesgo, Progresión
Introduction
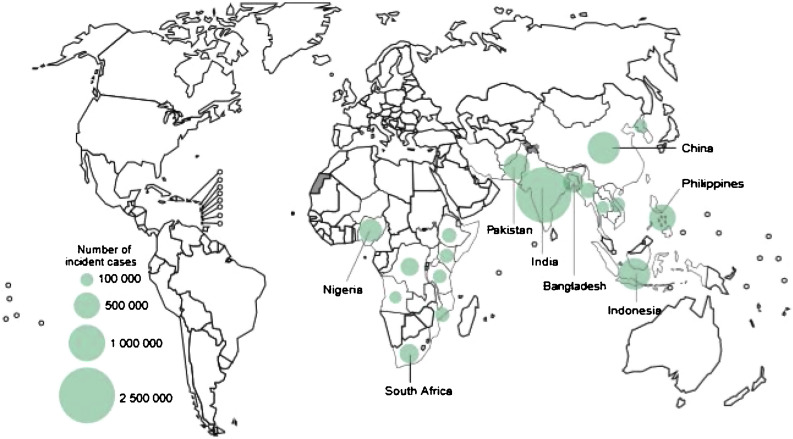
Tuberculosis (TB) is a disease that has been present in the world since the Palaeolithic age, 75,000 years ago1. It is the leading cause of mortality from an infectious disease worldwide, above HIV, although in 2020 it may be surpassed by COVID-192. It particularly affects the poorest countries and the most disadvantaged areas of the developed countries. The WHO estimates that in 2018 a total of 10 million people developed this disease which caused 1,200,000 deaths directly, plus another 250,000 deaths in HIV-positive people3. It is estimated that two thirds of TB cases worldwide are concentrated in eight countries: India (28%), China (9%), Indonesia (8%), Philippines (6%), Pakistan (6%), Nigeria (4%), Bangladesh (4%), and South Africa (3%) (Fig. 1 ). In Spain, the incidence rate of cases reported in Spain in 2019 was only 9.4 cases per 100,000 inhabitants3, although there is a significant underreporting4, 5.
Fig. 1.
Estimated incidence of TB in 2018, for countries with at least 100,000 incident cases. Source: Global Tuberculosis Report 2019. Geneva: World Health Organization; 2019. License: CC BY-NC-SA 3.0 IGO. Available in: https://www.who.int/tb/publications/global_report/en/.
Initially, Mycobacterium tuberculosis, the aetiological agent of TB, causes Latent Tuberculosis Infection (LTI) and only a small proportion of these patients (5–10%) will develop the disease in their lifetime. The WHO considers that the main risk factors for this are malnutrition, HIV infection, alcoholism, smoking and diabetes3. Other diseases or immunosuppressive treatments and advanced age could also play a role.
Over the last few years, migratory movements from countries with low incomes and high TB burdens to developed countries have changed the epidemiological pattern of the disease in recipient countries, concentrating in disadvantaged urban areas of large cities, where the migratory flow is more intense6.
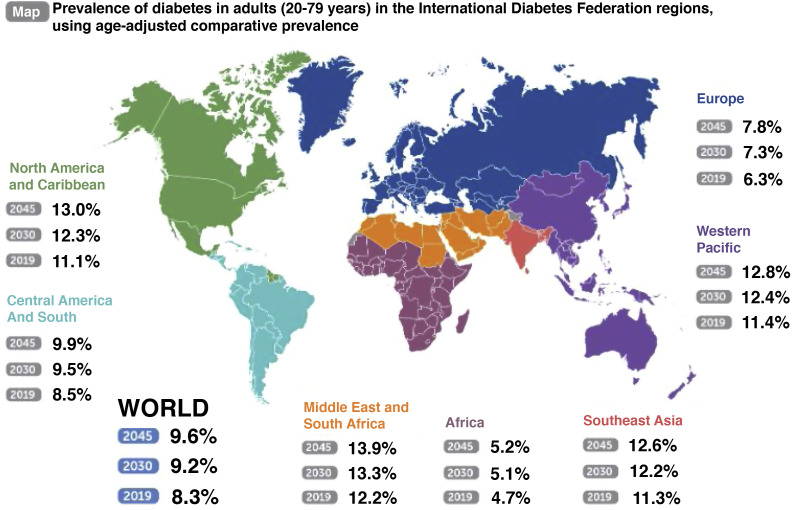
As for diabetes mellitus (DM), it is one of the most prevalent chronic diseases in our environment. A population study (Di@bet.es) carried out in Spain in 2012 showed that the registered prevalence of DM was around 13.8% of the population over 18 years of age, with 43.5% of cases being unknown (6% prevalence of ignored DM)7. The current estimate of the incidence in Spain annually is 11.6 cases per 1000 person-years8. Thanks to the use of large databases obtained from routine medical practice, the epidemiological study of this metabolic disease is becoming more and more accurate9. According to the International Diabetes Federation Diabetes Atlas (2019)10, about 463 million people worldwide suffer from this disease. The prevalence of DM worldwide is estimated at 9.3% in adults between 20 and 79 years of age (Fig. 2 ). Its impact varies considerably around the world and depends on factors such as diagnostic capacity, genetic inheritance, lifestyles, and the quality of health care that affected people receive, which is always lower in low-income countries.
Fig. 2.
Prevalence of diabetes in adults (20-79 years) in the International Diabetes Federation regions, using age-adjusted comparative prevalence. International Diabetes Federation. IDF Diabetes Atlas, 9th ed. Brussels, Belgium: International Diabetes Federation, 2019. Available in http://www.diabetesatlas.org.
Low- and middle-income countries are responsible for 80% of the global burden of DM (underdiagnosis, difficult access to treatment, greater number of complications and mortality). In fact, in these countries located mainly in the Middle East, East Pacific, Southeast Asia and Africa, a further increase in DM cases is expected in the coming years. The prevalence of DM in areas of North America, Mexico and the Caribbean is 11.1%, two points above the world average, and it is expected to increase to 13% in 204510. This increase is predicted as a consequence of the progressive changes in lifestyle and globalization, following the trend of greater consumption of inappropriate diets in these populations. And progressively improving access to DM diagnosis and increased life expectancy could also contribute. Consequently, it is estimated that the global incidence of DM will continue to increase, with the forecast for 2030 being 578 million affected (10.2% of the world population), directly influencing the increase in morbidity and mortality of this population10.
In this review, the relationships between TB and DM are considered, assuming that both diseases constitute a syndemic, which is being complicated by the COVID-19 pandemic. Some recommendations are also provided for improving clinical-epidemiological control.
Immunological mechanisms in the Mycobacterium tuberculosis infection and their impact on diabetes mellitus
There is evidence that DM is a risk factor for TB, and animal models have been used to study how hyperglycaemia could influence the immune response against M. tuberculosis, although a definitive explanation has not yet been reached.
A delay in the innate response of the alveolar macrophages was observed in hyperglycaemia-induced mice, which also impaired the phagocytic and antimicrobial functions against mycobacteria11. In addition, there would be a delay in the recruitment of myeloid cells to the infection site, as the hyperglycaemia would cause a defect in the expression of receptors related to antigen presentation and related to T cell activation. Mycobacteria would take longer to be delivered to the lymph nodes, and the antigen-specific T-cells activation, their proliferation and their migration to the infection site would also be delayed, since chemotaxis would also be affected. The neutrophil functions including migration to the place where they should act, their bactericidal activity and their killing abilities would also be impaired. There would also be a lower initial production of cytokines and IFN-y. This generalized delay would allow the bacilli to replicate for more days11.
In summary, hyperglycaemia in diabetic patients would alter cell activation, phagocytic capacity and microbicidal mechanisms, leukocyte transmigration and chemotaxis, and there would be a delay in antigenic presentation. While at the same time there would be a constant multiplication of bacilli12. These animal models have shown that once this adaptive immune response develops, it does so in a more pro-inflammatory environment where a large number of inflammatory cytokines have been generated and where there is already a high bacterial load, which is more difficult to contain11.
Diabetes as a risk factor for tuberculosis infection
Two cities with TB control programs have collected the risk factors associated with this infection for years. These have shown that the significance of DM is growing while some of the other risk factors are more controlled (HIV, drug addiction, etc.). Thus, in Barcelona in 2017, with an incidence of 16.5/100,000, 8.6% of the 265 patients detected had DM, while HIV infection only reached 7.9%. In New York, the impact of DM on TB is even more significant, since in 2019, with a TB incidence of 6.9/100,000, 23% of the 566 patients detected had DM, while HIV-infection was only present in 6%13, 14.
People with DM are at greater risk of presenting different types of infections, especially urinary, mucocutaneous and lower respiratory tract infections15, including TB16. In fact, the WHO considers DM as one of the main risk factors for TB3, since multiple studies have shown that between 5%–30% of patients with TB present concomitantly with DM developed years before17. The incidence of TB is triple in diabetics compared to non-diabetics18. In a cohort study carried out in the poorest district of Barcelona, a higher risk of developing TB was observed in diabetic patients compared to non-diabetic patients (HR 1.77; CI: 1.09–2.86) and especially in patients of Indian origin19.
It is considered that the prevalence of LTBI is approximately double in patients with DM compared to non-diabetics20, 21. A recent study estimates a somewhat lower prevalence, i.e. 1.59 times greater in diabetics from high TB incidence areas22. In a meta-analysis, an association between DM and LTBI was observed, although with a weak epidemiological association (OR = 1.18; 1.06–1.30)23. The prevalence of LTBI in diabetic patients was higher in those with poorer glycaemic control, with glycated haemoglobins greater than 7%24.
Additionally, patients with DM had a more severe clinical presentation25. It has been observed that patients diagnosed with TB and with a history of DM presented more cavitary patterns on chest x-rays, more adverse effects to anti-tuberculosis drugs, and a greater need for hospitalisation at the time of diagnosis26. The treatment guidelines indicated in patients with both pathologies do not differ from those indicated in non-diabetic patients27, despite the difficulty in handling this situation correctly.
Likewise, patients with DM have a greater risk of relapse after antituberculous treatment. A meta-analysis carried out in 2011 mentioned the probability as up to four times greater28, while another more recent study estimates this risk at 1.6429. It appears that in diabetic patients, the risk that the sputum cultures remain positive two to three months after initiation of treatment is approximately double. Probably, greater failure in the anti-tuberculosis treatment, a delay in the negativisation of the culture, and a higher relapse rate translates into greater infectivity: an increase in secondary transmission and a higher incidence of TB28, 29.
Diabetic patients have been seen as doubling the risk of having multidrug-resistant TB29, 30 and of presenting serious adverse reactions to the complicated treatment of multidrug-resistant TB31.
As with chronic complications of the disease, glycaemic control in diabetic TB patients most likely directly influences the prognosis of this infection. It has been seen that patients with DM and TB have poorer glycaemic control32, whereas good metabolic control would improve the functioning of the immune system and contribute to a better response to the treatment administered33. In fact, a recent study defines that a glycated haemoglobin equal to or greater than 7% would be a risk factor for the development of isoniazid-resistance and multidrug-resistance in patients with DM and TB34.
In a study on the long-term mortality of patients who had developed TB, DM was associated with lower survival at 5-years35. Another study observed that patients who present DM and TB simultaneously would have a 1.88 times higher risk of mortality29.
Tuberculosis as a risk factor for diabetes
It has been described that transient hyperglycaemia when TB is first diagnosed is related to an increased risk of progression to DM in some individuals. This is probably explained by the inflammation induced by the infection itself (physiological stress response to an infection27 and also due to the hyperglycaemic effect of some antituberculous drugs such as isoniazid36, especially in the case of isoniazid intoxication37, or due to the interaction of these with oral antidiabetics, as in the case of rifampicin with sulphonylureas and biguanides, reducing their plasma levels and making glycaemic control difficult27). Therefore, in patients with TB, some authors prefer hyperglycaemia treatment with insulin to avoid drug interactions27.
Some articles mention that TB itself could be a factor that could identify patients who would be more propense to future metabolic alterations38. Additionally, TB itself can cause tuberculous pancreatitis with the pancreatic endocrine hypofunction causing hyperglycemia27.
In another study carried out in Taiwan, a higher-than-expected incidence of DM, stroke and myocardial infarction was observed in patients with a history of TB, especially in those who had undergone a longer treatment39. In a recent meta-analysis, the mean prevalence of DM among people who had presented TB was 15.3%, while the prevalence in the general population was 8.8%. Thus, it was seen that the prevalence of DM in TB patients in each region studied approximately doubled the local prevalence, except for Central America and Europe, where the difference was not so marked40. Consequently, it would be convenient to confirm the new diagnoses of DM in this context, once the TB is resolved41, since TB is configured as a possible risk factor for DM.
The impact of the COVID-19 pandemic on diabetes and tuberculosis
The healthcare system has often been overwhelmed by the COVID-19 pandemic around the world. According to a WHO statement made from surveys from 163 countries, it is estimated that the healthcare of non-communicable diseases has been particularly affected. In the case of DM, 49% of the surveyed countries have seen how access to the treatment of this disease has been reduced, together with an increase in the secondary complications. Additionally, the pandemic is aggravating situations of socioeconomic inequality, and this fact directly affects the evolution of chronic diseases such as DM42.
In the case of COVID-19, some studies have already described DM as one of the most frequent comorbidities in infected patients treated in hospitals43, 44, although the prevalence of this disease among COVID-19 patients does not appear to be higher than that of the general population. However, they present worse disease progression, with more complications and even tripling the fatality of non-diabetics45. According to a study conducted in Wuhan (China), this higher fatality is associated with the higher risk of coagulopathy in patients with DM46. Therefore, DM would be a predictor of morbidity and mortality in patients with COVID-1947.
TB has also been identified as a possible risk factor for severity for COVID-1948. In fact, assuming the greater probability of poor progression in the case of developing both infections concomitantly and given that the initial symptoms may coincide (although they develop quicker in the case of COVID-19), in countries with a high incidence of TB, such as India, it is proposed to test patients with COVID-19 to rule out TB, and vice versa. This would be especially important when there is poor response to treatment, significant deterioration or atypical symptoms49.
Due to the extraordinary COVID-19 pandemic, it is estimated that the global detection of new TB cases will be 25% lower than the level of detection prior to the pandemic. As a result, an additional 190,000 TB deaths are estimated, that is, an increase of 13%. If this prediction comes true, it will mean a step backwards in the fight against TB, placing us at values similar to 2015. The WHO considers that TB surveillance and treatment centres should be considered as essential and kept active as a priority during the pandemic50.
Screenings and controls in patients with diabetes and/or tuberculosis
As described in this review, DM and TB behave like a syndemic, that is, a confluence of two highly prevalent diseases that act synergistically51. Therefore, their prevention and control should not be considered as separate entities. Bidirectional screening for both diseases is necessary, according to various studies and the WHO initiative «Collaborative framework for care and control of tuberculosis and diabetes» 52. It is considered that a better management of DM would help to control the burden of TB, especially in regions with a high incidence of this infection, and vice versa. Therefore, a key factor such as diet should also be taken into serious consideration in these regions.
Lee et al.23 detected a higher incidence of LTBI in diabetic patients and suggested that these data would be useful for future cost-effectiveness studies, to analyse the impact of LTBI screening in diabetic patients. However, the LTBI screening in patients with DM would be based on QuantiFERON or tuberculin tests and X-rays at least for those positive to one of the two tests mentioned. This has an affordable cost, and would imply prescribing LTBI treatments, which would prevent TB cases, or would directly detect some TB cases that would benefit from early diagnosis and treatment. This strategy would also make it possible to advance the study of contacts of TB cases, which would also be a step forward.
For patients with both diseases, good glycaemic control is recommended, thus reducing the chances of poor progression of both TB and DM. The joint approach of both pathologies represents a clinical challenge. The establishment of the correct antituberculous treatment and ensuring its compliance is a priority. Also, and especially when both diseases occur at the same time, cardiovascular risk factors should be controlled53. This control is based on the fact that the main causes of mortality in diabetic patients are cardiovascular diseases and that patients with TB also have a higher risk of presenting an acute coronary syndrome54, 55.
More clinical and epidemiological studies are needed that help us: to understand the impact of DM and its associated factors on the evolution of TB; to determine the most cost-effective interventions in the approach of the two pathologies29 and; to determine the Real World Data that could help clarify the potential interaction between both entities, and thus implement its prevention and control37.
Conclusions: DM and TB, a bidirectional relationship
There is already strong evidence that DM is a risk factor for TB, although the pathophysiological mechanisms have yet to be clarified. It is also suggested that TB may favour the presentation of DM in some patients. In addition, numerous studies have found that the progression of TB in patients with both diseases is slower27.
Both DM and TB are two of WHO’s main control targets. TB still causes the highest mortality from an infectious agent in the world or did until the onset of the COVID-19 pandemic, and DM has a high prevalence and greater growth in countries where the burden of TB is greatest. Consequently, the prevention and control of both diseases is a priority issue on a global public health level.
The COVID-19 pandemic is greatly complicating healthcare on a global scale. All patients, regardless of their pathology, are suffering significant delays in health care, and this fact has already been clearly demonstrated in TB56, 57 and in the DM58, and these delays complicate the natural history of both diseases.
Recommendations for the prevention and control of DM and TB in the COVID-19 era
In TB patients, it is advisable to carry out strict glycaemic control. Advantage should be taken of the laboratory tests (at the time of diagnosis and in months 1, 2, 4 and 6 of treatment in patients with a six-month treatment regimen59), with the aim of ruling out hyperglycaemia or the start-up of DM. This follow-up should continue for a longer time, to diagnose any possible incidence of DM in patients with sustained hyperglycaemia as soon as possible.
In patients with DM, LTBI and TB should be screened, since they are patients who could benefit from early LTBI or TB treatment.
In patients with TB and/or DM, SARS-CoV-2 infection should be ruled out since this infection can complicate the progression of these diseases. For this reason, these patients should constitute a priority group for vaccination against COVID-19.
Funding
This work has been partially funded by a FIS grant from the Carlos III Health Institute (Feder funds, ISCIII, file number PI16/01751), and by a grant awarded by IDIAP Jordi Gol i Gurina (Catalan Health Institute, PREDOC_ECO-19/2).
Conflict of interests
The authors declare that they have no conflict of interest.
Acknowledgements
To the working group of the DM and TB research project, made up of professionals from IDIAP (University Institute for Primary Health Care Research) Jordi Gol i Gurina, BASIQ and the Epidemiology Department of the Barcelona Public Health Agency.
Footnotes
Please cite this article as: Antonio-Arques V, Franch-Nadal J, Caylà JA. Diabetes y tuberculosis: una sindemia complicada por la COVID-19. Med Clin (Barc). 2021;157:288–293.
Representing the working group of the FIS PI16/01751 project. This group is made up of: Joan A. Caylà, Jose Luis del Val, Antonio Moreno, Àngels Orcau, Susana García, Josep Franch and Manel Mata.
References
- 1.Cardona P.J., Català M., Prats C. Origin of tuberculosis in the Paleolithic predicts unprecedented population growth and female resistance. Sci Rep. 2020;10:1–20. doi: 10.1038/s41598-019-56769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins University & Medicine. Available at: https://coronavirus.jhu.edu/. Última consulta 13 de enero de 2021.
- 3.WHO report. Globlal Tuberculosis Report 2019. Available at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports.
- 4.Gimenez-Duran J., Galmes-Truyols A., Gonzalez-Cortijo T., Portell-Arbona M., Bosch-Isabel C., Vanrell-Berga J.M., et al. Capture-recapture and anti-tuberculosis drug prescriptions, Balearic Islands, Spain, 2010-2012. Int J Tuberc Lung Dis. 2018;22:754–759. doi: 10.5588/ijtld.17.0303. [DOI] [PubMed] [Google Scholar]
- 5.Morales-García C., Rodrigo T., García-Clemente M.M., Muñoz A., Bermúdez P., Casas F., et al. Factors associated with unreported tuberculosis cases in Spanish hospitals. BMC Infect Dis. 2015;15:4–11. doi: 10.1186/s12879-015-1047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan K., Hirji M., Miniota J., Hu W., Wang J., Gardam M., et al. Domestic impact of tuberculosis screening among new immigrants to Ontario, Canada. CMAJ. 2018;187:473–481. doi: 10.1503/cmaj.150011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soriguer F., Goday A., Bosch-Comas, Bordiú E., Calle-Pascual A., Carmena R., et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: the Di@bet.es Study. Diabetologia. 2011;55:88–93. doi: 10.1157/13126836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rojo-Martínez G., Valdés S., Soriguer F., Vendrell J., Urrutia I., Pérez V., et al. Incidence of diabetes mellitus in Spain as results of the nation-wide cohort di@bet.es study. Sci Rep. 2020;10:1–9. doi: 10.1038/s41598-020-59643-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franch Nadal J., Mata Cases M., Mauricio Puente D. Epidemiología y control clínico de la diabetes mellitus tipo 2 y sus comorbilidades en España (estudio e-Control) Med Clin (Barc) 2016;147(Supl 1):1–7. doi: 10.1016/S0025-7753(17)30618-8. [DOI] [PubMed] [Google Scholar]
- 10.Internation Diabetes Federation . 9th edn. International Diabetes Federation; Brussels, Belgium: 2019. IDF Diabetes Atlas.http://www.diabetesatlas.org [Google Scholar]
- 11.Martinez N., Kornfeld H. Tuberculosis and diabetes: from bench to bedside and back. Int J Tuberc Lung Dis. 2019;23:669–677. doi: 10.5588/ijtld.18.0805. [DOI] [PubMed] [Google Scholar]
- 12.Ayelign B., Negash M., Genetu M., Wondmagegn T., Shibabaw T. Immunological impacts of diabetes on the susceptibility of Mycobacterium tuberculosis. J Immunol Res. 2019:1–8. doi: 10.1155/2019/6196532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Àngels Orcau i Palau, Carmen Gallego Cortés, Joan A. Caylà i Buqueras CR i G, Recull. La tuberculosi a Barcelona. Informe 2017. Available at: https://www.aspb.cat/wp-content/uploads/2020/10/tuberculosi-barcelona-2017.pdf.
- 14.New York City Health Department Annual Tuberculosis Summary, 2019. Available at: https://www1.nyc.gov/assets/doh/downloads/pdf/tb/tb2019.pdf.
- 15.Muller L.M.A.J., Gorter K.J., Hak E., Goudzwaard W.L., Schellevis F.G., Hoepelman A.I.M., et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41:281–288. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association Standards of Medical Care in Diabetes — 2018. Diabetes Care. 2018;41 [Google Scholar]
- 17.Ruslami R., Aarnoutse R.E., Alisjahbana B., Van Der Ven A.J.A.M., Van Crevel R. Implications of the global increase of diabetes for tuberculosis control and patient care. Trop Med Int Heal. 2010;15:1289–1299. doi: 10.1111/j.1365-3156.2010.02625.x. [DOI] [PubMed] [Google Scholar]
- 18.Jeon C.Y., Murray M.B. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:1091–1101. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonio-Arques V., Caylà J.A., Franch-Nadal J., Orcau À., Moreno A., Real J., et al. El papel de la diabetes en la incidencia de nuevos casos de tuberculosis en Ciutat Vella. Enfermedades Emergentes. 2020;19:180–185. http://enfermedadesemergentes.com/articulos/a758/taller_TBC2020_mesa_1.pdf Available at: [Google Scholar]
- 20.Barron M.M., Shaw K.M., Bullard K.M.K., Ali M.K., Magee M.J. Diabetes is associated with increased prevalence of latent tuberculosis infection: findings from the National Health and Nutrition Examination Survey, 2011–2012. Diabetes Res Clin Pract. 2018;139:366–379. doi: 10.1016/j.diabres.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Hensel R.L., Kempker R.R., Tapia J., Oladele A., Blumberg H.M., Magee M.J., et al. Increased risk of latent tuberculous infection among persons with pre-diabetes and diabetes mellitus HHS Public Access. Int J Tuberc Lung Dis. 2016;20:71–78. doi: 10.5588/ijtld.15.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin C.H., Kuo S.C., Hsieh M.C., Ho S.Y., Su I.J., Lin S.H., et al. Effect of diabetes mellitus on risk of latent TB infection in a high TB incidence area: a community-based study in Taiwan. BMJ Open. 2019;9:1–8. doi: 10.1136/bmjopen-2019-029948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee M.R., Huang Y.P., Kuo Y.T., Luo C.H., Shih Y.J., Shu C.C., et al. Diabetes mellitus and latent tuberculosis infection: a systemic review and metaanalysis. Clin Infect Dis. 2017;64:719–727. doi: 10.1093/cid/ciw836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martínez-Aguilar G., Serrano C.J., Castañeda-Delgado J.E., Macías-Segura N., Hernández-Delgadillo N., Enciso-Moreno L., et al. Associated risk factors for latent tuberculosis infection in subjects with diabetes. Arch Med Res. 2015;46:221–227. doi: 10.1016/j.arcmed.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Ugarte-Gil C., Alisjahbana B., Ronacher K., Riza A.L., Koesoemadinata R.C., Malherbe S.T., et al. Diabetes mellitus among pulmonary tuberculosis patients from 4 tuberculosis-endemic countries: the tandem study. Clin Infect Dis. 2020;70:780–788. doi: 10.1093/cid/ciz284. [DOI] [PubMed] [Google Scholar]
- 26.Moreno-Martínez A., Casals M., Orcau, Gorrindo P., Masdeu E., Caylà J.A. Factors associated with diabetes mellitus among adults with tuberculosis in a large European city, 2000-2013. Int J Tuberc Lung Dis. 2015;19:1507–1512. doi: 10.5588/ijtld.15.0102. [DOI] [PubMed] [Google Scholar]
- 27.Yorke E., Atiase Y., Akpalu J., Sarfo-Kantanka O., Boima V., Dey I.D. The bidirectional relationship between tuberculosis and diabetes. Tuberc Res Treat. 2017:1–6. doi: 10.1155/2017/1702578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker M.A., Harries A.D., Jeon C.Y., Hart J.E., Kapur A., Lönnroth K., et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huangfu P., Ugarte C., Pearson F., Golub J., Critchley J. The effects of diabetes on tuberculosis treatment outcomes: an updated systematic review and meta-analysis. J Epidemiol Community Health. 2016;70(Suppl 1) doi: 10.1136/jech-2016-208064.93. A50.2-A51. [DOI] [PubMed] [Google Scholar]
- 30.Salindri A.D., Kipiani M., Kempker R.R., Gandhi N.R., Darchia L., Tukvadze N., et al. Diabetes reduces the rate of sputum culture conversion in patients with newly diagnosed multidrug-resistant tuberculosis. Open Forum Infect Dis. 2016;3:1–10. doi: 10.1093/ofid/ofw126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muñoz-Torrico M., Caminero-Luna J., Migliori G.B., D’Ambrosio L., Carrillo-Alduenda J.L., Villareal-Velarde H., et al. La diabetes se asocia con reacciones adversas graves en la tuberculosis multirresistente. Arch Bronconeumol. 2017;53:245–250. doi: 10.1016/j.arbres.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 32.Kumpatla S., Sekar A., Achanta S., Sharath B.N., Kumar A.M.V., Harries A.D., et al. Characteristics of patients with diabetes screened for tuberculosis in a tertiary care hospital in South India. Public Heal Action. 2013;3:23–28. doi: 10.5588/pha.13.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song C., Xie W., Gong L., Ren M., Pan P., Luo B. The relationship between HbA1c control levels and antituberculosis treatment effects: a meta-analysis. J Chin Med Assoc. 2019;82:915–921. doi: 10.1097/JCMA.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 34.Lyu M., Wang D., Zhao J., Yang Z., Chong W., Zhao Z., et al. A novel risk factor for predicting anti-tuberculosis drug resistance in patients with tuberculosis complicated with type 2 diabetes mellitus. Int J Infect Dis. 2020;97:69–77. doi: 10.1016/j.ijid.2020.05.080. [DOI] [PubMed] [Google Scholar]
- 35.Ranzani O.T., Rodrigues L.C., Bombarda S., Minto C.M., Waldman E.A., Carvalho C.R.R. Long-term survival and cause-specific mortality of patients newly diagnosed with tuberculosis in São Paulo state, Brazil, 2010–15: a population-based, longitudinal study. Lancet Infect Dis. 2020;20:123–132. doi: 10.1016/S1473-3099(19)30518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Izzedine H., Launay-vacher V., Deybach C., Bourry E., Barrou B., Deray G. 2005. Drug-induced diabetes mellitus; pp. 1097–1109. [DOI] [PubMed] [Google Scholar]
- 37.Topcu I., Yentur E.A., Kefi A., Ekici N.Z., Sakarya M. Seizures, metabolic acidosis and coma resulting from acute isoniazid intoxication. Anaesth Intensive Care. 2005;33:518–520. doi: 10.1177/0310057x0503300416. [DOI] [PubMed] [Google Scholar]
- 38.Pearson F., Huangfu P., McNally R., Pearce M., Unwin N., Critchley J.A. Tuberculosis and diabetes: bidirectional association in a UK primary care data set. J Epidemiol Community Health. 2019;73:142–147. doi: 10.1136/jech-2018-211231. [DOI] [PubMed] [Google Scholar]
- 39.Salindria A.D., Wang J.Y., Linc H.H., Magee M. Post-tuberculosis incidence of diabetes, myocardial infarction, and stroke: retrospective cohort analysis of patients formerly treated for tuberculosis in Taiwan, 2002–2013. Int J Infect Dis. 2019;84:127–130. doi: 10.1016/j.ijid.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noubiap J.J., Nansseu J.R., Nyaga U.F., Nkeck J.R., Endomba F.T., Kaze A.D., et al. Global prevalence of diabetes in active tuberculosis: a systematic review and meta-analysis of data from 2·3 million patients with tuberculosis. Lancet Glob Heal. 2019;7:E448–E460. doi: 10.1016/S2214-109X(18)30487-X. [DOI] [PubMed] [Google Scholar]
- 41.Boillat-Blanco N., Ramaiya K.L., Mganga M., Minja L.T., Bovet P., Schindler C., et al. Transient hyperglycemia in patients with tuberculosis in Tanzania: implications for diabetes screening algorithms. J Infect Dis. 2016;213:1163–1172. doi: 10.1093/infdis/jiv568. [DOI] [PubMed] [Google Scholar]
- 42.UN INTERAGENCY TASK FORCE ON NCDs, OMS, PNUD. Hacer frente a las enfermedades no transmisibles durante la pandemia de COVID-19 y después de ella. Available at: http://apps.who.int/bookorders.
- 43.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Lancet. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katulanda P., Dissanayake H.A., Ranathunga I., Ratnasamy V., Wijewickrama P., Yogendranathan N., et al. Prevention and management of COVID-19 among patients with diabetes: an appraisal of the literature. Diabetologia. 2020;63:1440–1452. doi: 10.1007/s00125-020-05164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X., Chen Y., Wu C., Wei M., Xu J., Chao Y.C., et al. Coagulopathy is a major extrapulmonary risk factor for mortality in hospitalized patients with COVID-19 with type 2 diabetes. BMJ Open Diabetes Res Care. 2020;8(2):1–9. doi: 10.1136/bmjdrc-2020-001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hussain A., Bhowmik B., do Vale Moreira N.C. COVID-19 and diabetes: knowledge in progress. Diabetes Res Clin Pract. 2020;162:1–9. doi: 10.1016/j.diabres.2020.108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolff D., Nee S., Hickey N.S., Marschollek M. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection. 2020;49:15–28. doi: 10.1007/s15010-020-01509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jain V.K., Iyengar K.P., Samy D.A., Vaishya R. Tuberculosis in the era of COVID-19 in India. Diabetes Metab Syndr Clin Res Rev. 2020;14:1439–1443. doi: 10.1016/j.dsx.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glaziou P. Predicted impact of the COVID-19 pandemic on global tuberculosis deaths in 2020. medRxiv. 2020 doi: 10.1101/2020.04.28.20079582. [DOI] [Google Scholar]
- 51.Singer M. 1st ed. John Willey & Sons; Hoboken, New jersey, USA: 2009. Introduction to Syndemics: a critical systems approach to public and community health. [Google Scholar]
- 52.World Health Organization, International Union Against Tuberculosis and Lung Disease Collaborative framework for care and control of tuberculosis and diabetes. 2011. http://www.ncbi.nlm.nih.gov/pubmed/17158327 Available at: [PubMed]
- 53.Van Crevel R., Koesoemadinata R., Hill P.C., Harries A.D. Clinical management of combined tuberculosis and diabetes. Int J Tuberc Lung Dis. 2018;22:1404–1410. doi: 10.1101/2020.04.28.20079582. [DOI] [PubMed] [Google Scholar]
- 54.Chung W.S., Lin C.L., Hung C.T., Chu Y.H., Sung F.C., Kao C.H., et al. Tuberculosis increases the subsequent risk of acute coronary syndrome: a nationwide population-based cohort study. Int J Tuberc Lung Dis. 2014;18:79–83. doi: 10.5588/ijtld.13.0288. [DOI] [PubMed] [Google Scholar]
- 55.Huaman M.A., Kryscio R.J., Fichtenbaum C.J., Henson D., Salt E., Sterling T.R., et al. Tuberculosis and risk of acute myocardial infarction: a propensity score-matched analysis. Epidemiol Infect. 2017;145:1363–1367. doi: 10.1017/S0950268817000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Millet J.P., Orcau À. COVID y TB en Barcelona. Vol. 20. Enf Emerg. 2021;20:27–45. http://enfermedadesemergentes.com/articulos/a772/Jornada_TBC_2021_MESA2.pdf Available at: [Google Scholar]
- 57.Wingfield T., Karmadwala F., MacPherson P., Millington K.A., Walker N.F., Cuevas L.E., et al. Challenges and opportunities to end tuberculosis in the COVID-19 era. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(21)00161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holland D., Heald A.H., Stedman M., Green L., Scargill J., Duff C.J., et al. Impact of the UK COVID‐19 pandemic on HbA1c testing and its implications for diabetes diagnosis and management. Int J Clin Pract. 2021;75 doi: 10.1111/ijcp.13980. [DOI] [PubMed] [Google Scholar]
- 59.Ruiz-Manzano J., Blanquer R., Luis Calpe J., Caminero J.A., Caylà J., Domínguez J.A., et al. Diagnóstico y tratamiento de la tuberculosis. Arch Bronconeumol. 2008;44:551–566. doi: 10.1157/13126836. [DOI] [PubMed] [Google Scholar]