Abstract
The Himalaya are among the youngest and highest mountains in the world, but the exact timing of their uplift and origins of their biodiversity are still in debate. The Himalayan region is a relatively small area but with exceptional diversity and endemism. One common hypothesis to explain the rich montane diversity is uplift-driven diversification—that orogeny creates conditions favoring rapid in situ speciation of resident lineages. We test this hypothesis in the Himalayan region using amphibians and reptiles, two environmentally sensitive vertebrate groups. In addition, analysis of diversification of the herpetofauna provides an independent source of information to test competing geological hypotheses of Himalayan orogenesis. We conclude that the origins of the Himalayan herpetofauna date to the early Paleocene, but that diversification of most groups was concentrated in the Miocene. There was an increase in both rates and modes of diversification during the early to middle Miocene, together with regional interchange (dispersal) between the Himalaya and adjacent regions. Our analyses support a recently proposed stepwise geological model of Himalayan uplift beginning in the Paleocene, with a subsequent rapid increase of uplifting during the Miocene, finally giving rise to the intensification of the modern South Asian Monsoon.
Keywords: orogenesis, monsoon system, biotic assembly, in situ diversification, amphibians and reptiles
INTRODUCTION
In the past 40 million years, there has been a sharp increase in global tectonic activity and associated orogeny [1]. These geological processes, in turn, have resulted in many climatic and environmental changes, which have strongly influenced regional biological diversification [2]. Among the global biodiversity hotspots, mountains surrounding the Qinghai–Tibetan Plateau are enigmatic and unusual [3], especially the Himalaya—the highest mountains in the world.
Geologically, the Himalaya are distinct from the Tibetan Plateau and the Hengduan Mountains (Fig. 1; definition of the Himalaya in the Supplementary Data), although all of these regions have often been considered as a greater and united Qinghai–Tibetan Plateau. The uplift of the Himalaya is suggested to be more important than the Tibetan Plateau in shaping Asian paleoclimate patterns [4]. This massive mountain range encompasses remarkable endemic diversity [5,6]. However, despite increasing interest, the overall biotic assembly remains poorly understood. In this study, we aim to better understand the origin of this remarkable biotic assembly in the Himalayan region. Time-calibrated analyses of patterns of diversification of biotic assembly can provide independent estimates or corroboration of the timing of geological processes such as mountain range formation and related climate changes.
Figure 1.
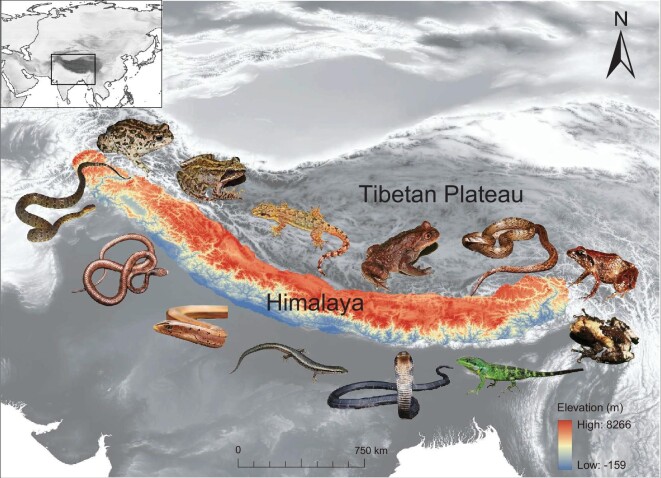
Map of the Himalaya geographic regions (highlighted by colors) used for analyses. The color scale on the bottom right indicates the elevations of the Himalaya. Representative species of the Himalaya are shown along the mountain range (from the upper left corner clockwise is Bufotes zamdaensis-Bufonidae, Nanorana rostandi-Dicroglossidae, Hemidactylus platyurus-Gekkonidae, Scutiger wuguanfui-Megophryidae, Oligodon lipipengi-Colubridae, Liurana medogensis-Ceratobatrachidae, Theloderma baibungense-Rhacophoridae, Mictopholis austeniana-Agamidae, Ophiophagus hannah-Elapidae, Asymblepharus himalayanus-Scincidae, Dopasia gracilis-Anguidae, Pareas monticola-Pareidae, Protobothrops himalayanus-Viperidae).
Geological studies about the Himalaya formation have made great progress recently, however, understanding the timing of the subsequent rise to current elevations has proved challenging and controversial [7]. Hypotheses about the geological history of the Himalaya largely differ in the timing and sequence of the uplift process (Fig. 2a and b). Ding et al. [8] proposed a stepwise model, which suggested that the Himalaya rose slowly from 1000–2500 m during the period of 56–23 Ma, with an additional rapid period of elevational increase to 4000 m from 23–19 Ma, and a final rise around 15 Ma to the current average elevations of about 5000 m. We label this model the ‘Stepwise hypothesis’ (Fig. 2a). Coincident with the rise of the highest elevations, the modern South Asian Monsoon began to intensify [4,8]. However, the discovery of fossil oaks (Quercus semecarpifolia) indicated that the Himalaya were the most recent component of the Tibet–Himalaya edifice to be elevated, and reached their current elevations during the Pliocene [9], although this has been questioned [7,10]. Recent hydrological and thermal evidence also supports that this region was probably not elevated to current elevations till the mid-Pliocene [11]. We label this view the ‘Late Orogeny hypothesis’ (Fig. 2b).
Figure 2.
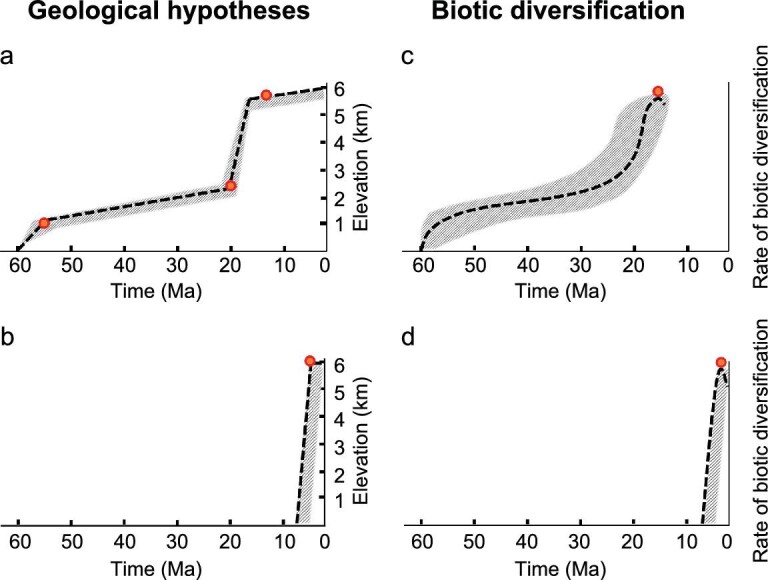
Schematic diagram of (a, b) two geological hypotheses regarding the uplift of the Himalaya to current elevations, and (c, d) the corresponding expected biotic assembly processes. The dashed lines show the general trends, with the range of consistent dates and elevations indicated by shading. The red solid circles indicate key time points. (a) The ‘Stepwise hypothesis’ refers to a model in which the Himalaya arose in a stepwise series of uplifts beginning in the Early Paleocene, but at a much faster rate during the Miocene (23–15 Ma). This diagram is adapted from Ding et al. [8]. (b) The ‘Late Orogeny hypothesis’ refers to a model in which the Himalaya started uplift during the Early Miocene and reached the current elevations much later (around 7.0–3.2 Ma). This diagram is drawn based on a synthesis of several studies [9,11]. (c) If the ‘Stepwise hypothesis’ is correct, the expected biotic diversification would begin in the Paleocene, but then exhibit a rapid increase during the early Miocene and hit the peak in the middle Miocene (ca. 15 Ma). (d) If the ‘Late Orogeny hypothesis’ is correct, the expected diversification of Himalayan biota would occur recently (after the Miocene), with a peak of diversification later than 7.0–3.2 Ma.
Orogenies create variable environmental conditions (such as varying climatic niches, new habitats and dispersal barriers) that increase the rate of speciation of organisms—a process termed uplift-driven diversification [12,13]. We thus expect an accelerated in situ diversification rate following the uplift of the Himalaya. Spicer [14] proposed that the rise of the Himalaya and the subsequent development of the South Asian Monsoon had major impacts on species diversification in this region and a recent study on the alpine flora diversification in the Himalaya supported this scenario [15]. We therefore expect a time-based record of biological processes to be informative about montane histories and environmental changes. Various hypotheses about Himalayan origins can be tested using phylogenetic information and estimates of the timing of biological speciation events. In Fig. 2, we show the predictions of biotic diversification that are associated with each of the two models discussed above (geological models: Fig. 2a and b; biotic diversification predictions, Fig. 2c and d). Trends of biotic predictions show the expectations of biological diversification under each model. We use ‘rate of biotic diversification’ in Fig. 2 to represent the net effect of biota assembly processes (e.g. all forms of diversification, minus extinction).
The effects of Himalayan orogeny and the subsequent monsoon system development on the biotic diversification rate are not clearly separable, because they may jointly provide ecological/evolutionary opportunity to accelerate the speciation rate. However, at larger scales, changes in the moisture load and strength of the monsoon systems during its intensification must have affected the availability of water throughout the Qinghai–Tibetan Plateau region, with the growing Himalayan rain shadow especially important [14]. Increased rains kept the south slope of the Himalaya wet and facilitated the establishment of rainforest, with a corridor connected with Southeast Asia [16]. Under the theoretical framework of phylogenetic niche conservatism, dispersal should be facilitated between similar environments inhabited by the source biota [17]. In contrast, by the time the Himalayan uplift reached almost 5000 m around 15 Ma [8], the aridity of central Asia and Tibet had been established [3]. Under this model, we could expect limited biotic interchanges between the Himalaya and either central Tibet or Central-West Asia, because of the reduced effectiveness of dispersal corridors between these areas.
Our understanding of the assembly processes of the Himalayan biota has been hindered by a lack of phylogenetic and diversification dating data. This lack of information results from the relative difficulty of sampling many Himalayan species. The Himalayan region encompasses multiple countries and has many access challenges, so sampling across the entire region is difficult, which has inhibited integrative studies of the origin of the Himalayan biota. Recently, new radiometric dates of paleontological data point to Himalayan high biodiversity originating in the Paleogene [14]. Another long-held view—the sink hypothesis—suggests that the Himalayan biota, due to its high connectivity, is largely comprised of elements from adjacent biotic realms, e.g. Western Asia, Southeast and East Asia [18,19]. Although there are a few existing phylogeographic studies on endemic Himalayan clades [20,21], a broad synthesis is lacking for most major groups, and there is little available information about broad biotic interchanges between the Himalaya and adjacent regions [3].
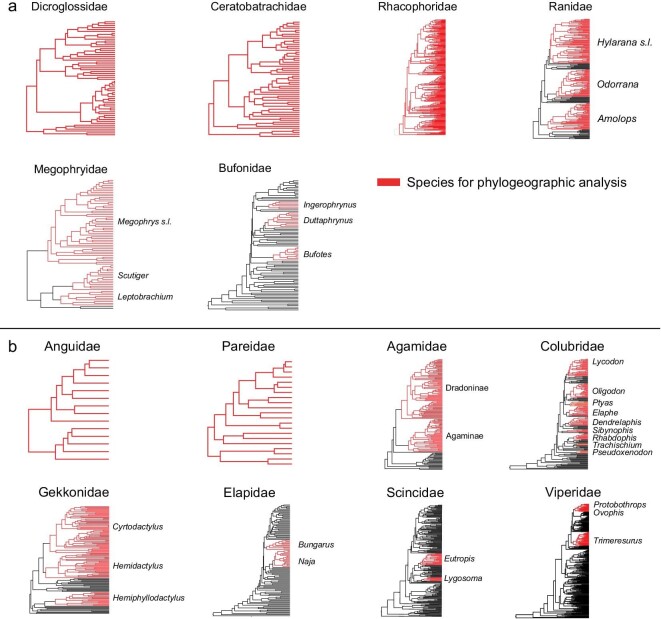
Amphibians and reptiles are ideal organisms for studying biogeographic relationships and they generally retain high-resolution signals of historical responses to environmental changes [22]. They are often used to test geological and climate hypotheses [23,24]. In this study, we reconstructed 14 time-calibrated phylogenies of Himalayan-associated amphibian and reptile families, from which we analyzed 35 well-sampled subclades, to explore the spatiotemporal evolution of Himalayan amphibians (Fig. 3a) and reptiles (Fig. 3b) using a maximal number of observed diversification events (MDE) [25]. We then tested the major competing geological hypotheses (as shown in Fig. 2), and considered the effects of the South Asian Monsoon system on the Himalayan biota.
Figure 3.
Overview of species used for our phylogeographic analysis. The 14 trees illustrate our family-level time trees: (a) for amphibians and (b) for reptiles.
RESULTS
Relative rates and ages of biogeographic events
A total of 14 independent time-calibrated phylogenies of Himalayan-associated amphibian and reptile families (Fig. 3) involving 85 genera and 1628 species (Ranidae, Rhacophoridae, Dicroglossidae, Ceratobatrachidae, Bufonidae, Megophryidae, Agamidae, Anguidae, Pareidae, Colubridae, Elapidae, Gekkonidae, Scincidae and Viperidae; Supplementary Data S1) were reconstructed from MCMCTREE [26]. Among these, we estimated times of divergence among 183 species that occur in the Himalaya. The average ages of major biogeographic events are presented in Table 1 (see Supplementary Data S2 for evidence that our results were not strongly influenced by our choice of priors).
Table 1.
Numbers and mean ages (with 95% confidence intervals) of different types of biogeographic events inferred in this study.
| Biogeographic event type | Number | Mean age (95% confidence interval) (Ma) |
|---|---|---|
| In situ diversificationa | 126 | 17.28 (23.10–12.36) |
| Dispersal | 87 | 19.18 (24.71–14.21) |
| Ambiguous events | 17 | 28.21 (35.76–21.05) |
| From Southeast Asia into the Himalaya | 49 | 19.95 (25.48–14.93) |
| From East Asia into the Himalaya | 2 | 5.08 (7.77–3.13) |
| From South Asia into the Himalaya | 8 | 19.99 (26.94–13.54) |
| From Central-West Asia into the Himalaya | 2 | 47.8 (56.65–37.45) |
aIn situ diversification events and dispersal events were summarized based on the result of BioGeoBEARS analysis (Supplementary Data S3). Detailed dispersal events were summarized from results of the BioGeoBEARS analysis (Supplementary Data S4).
We identified 230 biogeographic events related to the Himalayan species based on the detailed biogeographic analysis of 14 families (Supplementary Table S1), including 126 in situ diversification events, 87 dispersal events and 17 ambiguous events (see definition of these biogeographic events in methods and materials in Supplementary Data and graphically shown in Supplementary Fig. S1). In situ diversification events contributed more than half of the events that gave rise to the Himalayan biota (126/230 = 54.78%). The mean age of these biogeographic events consistently clusters at ∼20 Ma (Table 1). In other words, diversification events in the Himalaya were most densely concentrated in the Miocene.
Dynamics of in situ diversification and dispersal events
Our analysis revealed similar trends of in situ diversification and dispersal through time, estimated under an unconstrained biogeographic model (Fig. 4a). Both in situ diversification and dispersal events associated with the Himalaya began at approximately the same time around 70 Ma (Fig. 4a, Table 2). From 67–30 Ma (Paleocene to early Oligocene), the rate of both in situ diversification and dispersal was relatively low and increased slowly (Fig. 4a). During this period, the magnitude of in situ diversification was similar to dispersal. From 30 Ma (early Oligocene), in situ diversification and dispersal began to increase (Fig. 4a). Around 20 Ma (early Miocene), both processes (but especially in situ diversification) increased rapidly. Both rates peaked simultaneously around 13 Ma (middle Miocene) (Fig. 4a, Table 2). From each MDE curve, we detected two inflection points, indicating shifts in the diversification rate (Table 2). The dynamics of in situ diversification and dispersal rates remained essentially parallel across the Cenozoic. Both in situ diversification and dispersal rates showed a stepwise pattern of increases in the Paleocene and Miocene that best match the stepwise hypothesis for the formation of the Himalaya (Fig. 4a and Table 2). In contrast, our estimates of origination and peak diversification are not consistent with the late-uplift hypothesis.
Figure 4.
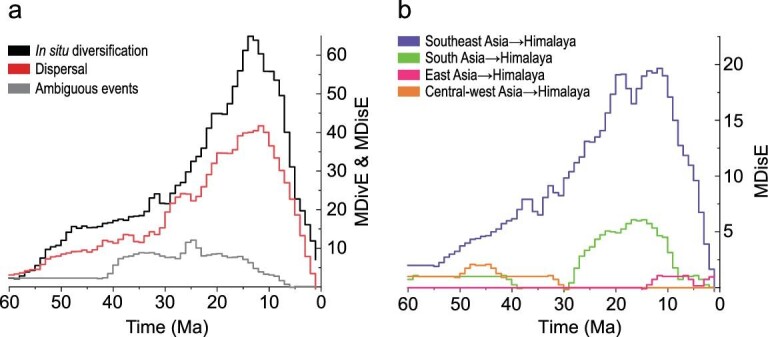
(a) The rates of in situ diversification and dispersal of the Himalayan herpetofauna through time (smoothed across 5 Ma windows). Dispersal indicates ‘dispersal into the Himalaya’. MDivE = maximal number of observed in situ diversification events per Ma. MDisE = maximal number of observed dispersal events per Ma. Ambiguous events are separately listed. (b) Dispersal events from adjacent regions into the Himalaya (smoothed across 5 Ma windows).
Table 2.
The empirical data and the predictions related to the biogeographic events under different geological models. 99% confidence intervals of the observation values are from 1000 bootstrap pseudoreplicates (see Supplementary Data S5).
| Predictionsa (Ma) | |||
|---|---|---|---|
| Features of the diversification dynamics | Observations (99% confidence interval) (Ma) | Stepwise hypothesis | Late Orogeny hypothesis |
| Origination of MDivE | 67 (67–54) | ||
| Origination of MDisE | 67 (67–54) | ||
| ∼60 | 23 | ||
| Peak of MDivE | 13.5 (14–11) | ||
| Peak of MDisE | 12 (15–13) | ||
| ∼15 | 5.3–2.5 | ||
| Inflection points of MDivE | 30 (35–11), 53 (56–27) | ||
| Inflection points of MDisE | 31 (39–11), 54 (58–27) | ||
| 21–19, 56 (58–54) | None mentioned | ||
aThe prediction values are consistent with data used in Fig. 2c and d.
Focusing on the interchange between adjacent regions, Southeast Asia is the area with the highest frequency of interactions with the Himalaya (Fig. 4b, see details in Supplementary Table S2). Southeast Asia shared interchanges with the Himalaya in 49 dispersal events (49/62 = 79% of all dispersal events), whereas there were many fewer exchanges with other areas (Table 1). The Himalayan interchanges with Southeast Asia began by 67 Ma and then peaked at around 19 Ma (Fig. 4b).
An analysis that examined the effect of including ‘potential Himalayan species’ (Supplementary Fig. S2a) produced virtually identical results. We also repeated our analyses using BEAST [27] instead of MCMCTREE [26], again with virtually identical results (Supplementary Fig. S2b). Prior and posterior probabilities from BEAST and MCMCTREE are compared in Supplementary Data S2, and indicate that our sequence data were informative.
DISCUSSION
Testing geological models for the origin of the Himalaya and subsequent monsoon intensification
Mountain building has long been viewed as an important direct driver of speciation [28]. Rapid uplift results in widespread isolation and speciation of ancestral populations. In addition, the origin of montane areas can create a new source and sink for dispersal from other montane areas. In our analyses of the herpetofauna (Fig. 4), both the in situ diversification rate, as well as the dispersal rate into the Himalaya, fit the Stepwise model [8] for the origin of this mountain range. In this model (Fig. 2a), the initial uplift of the Himalaya began in the Paleocene (∼60 Ma), coinciding with the 99% range of start of biota assembly process (67–54 Ma for in situ diversification and dispersal, Table 2). The Himalaya then grew slowly and gradually at first, but then rapidly increased in the Miocene until approximately 13 Ma, when they reached their current elevations (∼6000 m and still rising). Correspondingly, we detected significant inflection points in the rates of in situ diversification and dispersal that are broadly consistent with the expectations under the Stepwise model (Table 2). The early gradual uplift of the Himalaya was accompanied by a gradual increase in the diversification of the herpetofauna, as well as a gradual increase of dispersal events into the Himalaya. We also found a rapid increase of the diversification rate in the Miocene, consistent with a pulse of uplift-driven diversification (Fig. 2c).
In contrast, our results are inconsistent with the Late Orogeny hypothesis for Himalayan origins shown in Fig. 2b. For the Late Orogeny hypothesis, the expected origination of Himalayan biota diversification (23 Ma) is much later than we observed (∼60 Ma, Table 2). Furthermore, the occurrence of several inflection points in the MDE suggested non-gradual mountain building, contrasting with the predictions of the Late Orogeny hypothesis (Table 2). Therefore, our data broadly support the Stepwise model of Ding et al. [8], but not the Late Orogeny hypothesis.
In contrast to our findings of a rapid increase in diversification rates of the Himalayan herpetofauna in the Miocene, Xing and Ree [13] found no signal for rapid diversification of the Himalayan flora, which indicated that the Himalayan uplift may have been gradual rather than episodic. Interestingly, a recent study by Ding et al. [15] did detect acceleration of in situ speciation on alpine flora in the Himalaya during the Miocene. Generally, compared with plants, amphibians and reptiles are more sensitive to environmental changes and geographic isolation. Furthermore, long-distance pollen dispersal is more likely to slow vicariance of plant populations relative to that of amphibian and reptile populations [29]. Birds are also well-sampled across the Himalaya. However, the timescale for the diversification of present-day bird communities is largely limited to the Late Miocene [25,30], so diversification of these communities does not cover the scope of the competing geological models tested here.
Geological, climatological and biological changes are highly correlated [14]. The combination of complex topography and varying climates produces a biodiversity hotspot in the Himalaya [14]. The rapid Himalayan uplift and associated intensified South Asian Monsoon not only promoted a pulse of uplift-driven in situ diversification, but also affected the rates of biotic interchange (Fig. 4). As the Himalaya rose, the northward flow of moist air from the Indian Ocean was blocked by the high Himalaya and/or deflected to the east [14], and may have also given rise to the modern South Asian Monsoon [4,14,31,32]. These changes resulted in the aridification of central Asia starting from the Early Miocene [3], and ecological barriers in the eastern Himalaya since the Miocene [33]. Consequently, these changes may have limited dispersal between the Himalaya and Central-West Asia, South Asia and East Asia. In support of this scenario, we found much lower rates of dispersal of amphibians and reptiles between the Himalayan region and Central-West Asia, South Asia and East Asia, compared to the relatively high rates of dispersal between the Himalaya and Southeast Asia (Fig. 4b). Biotic interchange was likely restricted by the lack of a moist environment required by many reptiles and amphibians. In contrast, an expanded tropical forest belt is thought to have persisted between the Himalaya and Southeast Asia since the middle Miocene [16], which likely accounts for the high dispersal rates between these two regions (Fig. 4b).
In addition to the uplift of the Himalaya and the development of the monsoon system discussed above, global cooling may have contributed to extinction, which would have lowered the net diversification rates [34]. Although assembly patterns of the Himalayan herpetofauna do not closely match the global cooling trend, global cooling may have affected the diversification patterns we observed. For example, simulations [35] have shown that extinction caused by global cooling can potentially lead to patterns similar to the Miocene burst of Himalayan herpetofauna we observed. Many fossils have been found near Lunpola, central Tibet, which indicate this area experienced dramatic changes from a wet environment to the current dry environment [7,36]. However, there is no similar evidence of such a shift for the Himalaya.
How did the Himalayan fauna assemble?
Our analyses show a deep-rooted origin of Himalayan herpetofauna originating in the Paleocene, but with rapid diversification in the Miocene. These findings are broadly consistent with the hypothesis proposed by Spicer [14] to explain Himalayan biodiversity. In a recent critical review, Renner [37] argued that numerous phylogenetic studies incorrectly attributed relatively young ages (Miocene and later) for lineage divergence due to the uplift of the Qinghai–Tibetan Plateau. The references discussed by Renner [37] often combined different regions as the Qinghai–Tibetan Plateau and did not differentiate them. Following the advice of Renner [37], here we focus on the biota of Himalaya, which has a very different history from other parts of the Qinghai–Tibetan Plateau.
Our analyses quantify the relative contributions of in situ diversification and dispersal, which requires extensive sampling of both regions and taxa. Prior studies that focused on groups with high dispersal ability, or only on local endemics, have led to the conclusion that the Himalaya are a dispersal sink [18,19]. For example, cases of long-distance dispersal from the mountains of China–Indochina along the southern slope of the Himalayan chains have been reported, associated with very little in situ speciation [38]. However, the sink hypothesis is not sufficient to explain the origins of groups with lower dispersal ability, such as reptiles and amphibians. We found both in situ speciation and long-term dispersal were important processes for assembly of the herpetofauna. In groups with low vagility, in situ speciation is generally considered to be a more likely explanation for faunal assembly [20]. Our finding that most biotic dispersal of the Himalayan herpetofauna has been between the Himalaya and Southeast Asia has important conservation implications. Long-term maintenance of biotic diversity in the Himalaya likely depends on the preservation of a dispersal corridor between these two areas. Therefore, protection of this dispersal corridor should be an international conservation priority.
MATERIALS AND METHODS
Please refer to the Supplementary data online.
Supplementary Material
Acknowledgements
We would like to thank the government of the Xizang (Tibet) Autonomous Region, the Forestry Department and nature reserves for great help with field work. We are grateful to Lin Ding, Robert A. Spicer, Tao Su, Yao-Wu Xing and Fei-Xiang Wu for discussions of geological studies. We also thank De-Chun Jiang for suggestions on using MDE. We extend our gratitude to Field Museum of Natural History, USA, California Academy of Sciences, USA, Royal Ontario Museum, Canada for their assistance and support in facilitating specimen and tissue loans.
Contributor Information
Wei Xu, State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, China; Kunming College of Life Science, University of Chinese Academy of Sciences, Kunming 650204, China.
Wen-Jie Dong, State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, China; Kunming College of Life Science, University of Chinese Academy of Sciences, Kunming 650204, China.
Ting-Ting Fu, State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, China; Kunming College of Life Science, University of Chinese Academy of Sciences, Kunming 650204, China.
Wei Gao, State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, China; Kunming College of Life Science, University of Chinese Academy of Sciences, Kunming 650204, China.
Chen-Qi Lu, State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, China; Kunming College of Life Science, University of Chinese Academy of Sciences, Kunming 650204, China.
Fang Yan, State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, China.
Yun-He Wu, State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, China; Kunming College of Life Science, University of Chinese Academy of Sciences, Kunming 650204, China.
Ke Jiang, State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, China.
Jie-Qiong Jin, State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, China.
Hong-Man Chen, State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, China.
Ya-Ping Zhang, State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, China; Center for Excellence in Animal Evolution and Genetics, Chinese Academy of Sciences, Kunming 650223, China.
David M Hillis, Department of Integrative Biology and Biodiversity Center, University of Texas at Austin, Austin, TX 78712, USA.
Jing Che, State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, China; Center for Excellence in Animal Evolution and Genetics, Chinese Academy of Sciences, Kunming 650223, China.
FUNDING
This work was supported by the Second Tibetan Plateau Scientific Expedition and Research (STEP) program (2019QZKK0501), the Strategic Priority Research Program of the Chinese Academy of Sciences (CAS) (XDA20050201), the Youth Innovation Promotion Association of CAS, the Animal Branch of the Germplasm Bank of Wild Species of CAS (the Large Research Infrastructure Funding), the CAS Scholarship of [(2018) 8] to J.C., and the CAS President's International Fellowship Initiative (2018VBA0039) to D.M.H.
AUTHOR CONTRIBUTIONS
Y.-P.Z., D.M.H. and J.C. designed the research and managed the project; W.X. and C.-Q.L. collected data for phylogenetic analyses. W.X., W.-J.D., T.-T.F. and W.G. analyzed data; F.Y., Y.-H.W., K.J., J.-Q.J. and H.-M.C. did the field work and performed DNA experiments; W.X., D.M.H. and J.C. discussed and wrote the manuscript.
Conflict of interest statement. None declared.
REFERENCES
- 1.Pinter N, Brandon MT. How erosion builds mountains. Sci Am 2005; 15: 74–81. 10.1038/scientificamerican0497-74 [DOI] [Google Scholar]
- 2.Rahbek C, Borregaard MK, Antonelli Aet al. Building mountain biodiversity: geological and evolutionary processes. Science 2019; 365: 1114–9. 10.1126/science.aax0151 [DOI] [PubMed] [Google Scholar]
- 3.Favre A, Päckert M, Pauls SUet al. The role of the uplift of the Qinghai–Tibetan Plateau for the evolution of Tibetan biotas. Biol Rev 2015; 90: 236–53. 10.1111/brv.12107 [DOI] [PubMed] [Google Scholar]
- 4.Boos WR, Kuang Z. Dominant control of the South Asian monsoon by orographic insulation versus plateau heating. Nature 2010; 463: 218–23. 10.1038/nature08707 [DOI] [PubMed] [Google Scholar]
- 5.Kumar M, Maharaj KP. Geophysical upheavals and evolutionary diversification of plant species in the Himalaya. PeerJ 2018; 6: e5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Che J, Jiang K, Yan Fet al. Amphibians and Reptiles in Tibet—Diversity and Evolution. Beijing: Science Press, 2020. [Google Scholar]
- 7.Spicer RA, Tao S, Valdes PJet al. Why the ‘Uplift of the Tibetan Plateau’ is a myth. Natl Sci Rev 2021; 8: nwaa091. 10.1093/nsr/nwaa091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding L, Spicer RA, Yang Jet al. Quantifying the rise of the Himalaya orogen and implications for the South Asian monsoon. Geology 2017; 45: 215–8. 10.1130/G38583.1 [DOI] [Google Scholar]
- 9.Xu R, Tao JR, Sun XJ. On the discovery of a Quercus semicarpifolia bed in Mount Shisha Pangma and its significance in botany and geology. Acta Bot Sin 1973; 15: 103–19. [Google Scholar]
- 10.Guo SX, Spicer RA, Widdowson Met al. The composition of the middle Miocene (15 Ma) Namling paleoflora, South Central Tibet, in the context of other Tibetan and Himalayan Floras. Rev Palaeobot Palynol 2019; 271: 104088. [Google Scholar]
- 11.Chen C, Bai Y, Fang Xet al. Lower-altitude of the Himalayas before the mid-Pliocene as constrained by hydrological and thermal conditions. Earth Planet Sci Lett 2020; 545: 116422. 10.1016/j.epsl.2020.116422 [DOI] [Google Scholar]
- 12.Hoorn C, Mosbrugger V, Mulch Aet al. Biodiversity from mountain building. Nat Geosci 2013; 6: 154. 10.1038/ngeo1742 [DOI] [Google Scholar]
- 13.Xing Y, Ree RH. Uplift-driven diversification in the Hengduan Mountains, a temperate biodiversity hotspot. Proc Natl Acad Sci U S A 2017; 114: E3444–51. 10.1073/pnas.1616063114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spicer RA.Tibet, the Himalaya, Asian monsoons and biodiversity—in what ways are they related? Plant Diversity 2017; 39: 233–44. 10.1016/j.pld.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding WN, Ree RH, Spicer RAet al. Ancient orogenic and monsoon-driven assembly of the world's richest temperate alpine flora. Science 2020; 369: 578–81. 10.1126/science.abb4484 [DOI] [PubMed] [Google Scholar]
- 16.Jacques FMB, Shi G, Sua Tet al. A tropical forest of the middle Miocene of Fujian (SE China) reveals Sino-Indian biogeographic affinities. Rev Palaeobot Palynol 2015; 216: 76–91. 10.1016/j.revpalbo.2015.02.001 [DOI] [Google Scholar]
- 17.Crisp MD, Cook LG. Phylogenetic niche conservatism: what are the underlying evolutionary and ecological causes? New Phytol 2012; 196: 681–94. 10.1111/j.1469-8137.2012.04298.x [DOI] [PubMed] [Google Scholar]
- 18.Johansson US, Alstrom P, Olsson Uet al. Build-up of the Himalayan avifauna through immigration: a biogeographical analysis of the Phylloscopus and Seicercus warblers. Evolution 2007; 61: 324–33. 10.1111/j.1558-5646.2007.00024.x [DOI] [PubMed] [Google Scholar]
- 19.Martens J, Tietze DT, Päckert M. Phylogeny, biodiversity, and species limits of passerine birds in the Sino–Himalayan region—a critical review. Ornithol Monogr 2011; 70: 64–94. 10.1525/om.2011.70.1.64 [DOI] [Google Scholar]
- 20.Schmidt J, Opgenoorth L, Höll Set al. Into the Himalayan exile: the phylogeography of the ground Beetle Ethira clade supports the Tibetan origin of forest-dwelling Himalayan species groups. PLoS One 2012; 7: e45482. 10.1371/journal.pone.0045482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann S, Stöck M, Zheng YCet al. Molecular phylogenies indicate a Paleo-Tibetan origin of Himalayan lazy toads (Scutiger). Sci Rep 2017; 7: 3308. 10.1038/s41598-017-03395-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaffer HB, Gidis M, McCartney-Melstad Eet al. Conservation genetics and genomics of amphibians and reptiles. Annu Rev Anim Biosci 2015; 3: 113–38. 10.1146/annurev-animal-022114-110920 [DOI] [PubMed] [Google Scholar]
- 23.Che J, Zhou WW, Hu JSet al. Spiny frogs (Paini) illuminate the history of the Himalayan region and Southeast Asia. Proc Natl Acad Sci U S A 2010; 107: 13765–70. 10.1073/pnas.1008415107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esquerré D, Brennan IG, Catullo RAet al. How mountains shape biodiversity: the role of the Andes in biogeography, diversification, and reproductive biology in South America's most species-rich lizard radiation (Squamata: Liolaemidae). Evolution 2018; 73: 214–30. 10.1111/evo.13657 [DOI] [PubMed] [Google Scholar]
- 25.Klaus S, Morley RJ, Plath Met al. Biotic interchange between the Indian subcontinent and mainland Asia through time. Nat Commun 2016; 7: 12132. 10.1038/ncomms12132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z.PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 2017; 24: 1586–91. [DOI] [PubMed] [Google Scholar]
- 27.Suchard MA, Lemey P, Baele Get al. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol 2018; 4: vey016. 10.1093/ve/vey016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Hammen T, Werner JH, van Dommelen H. Palynological record of the upheaval of the Northern Andes: a study of the Pliocene and Lower Quaternary of the Colombian Eastern Cordillera and the early evolution of its High-Andean biota. Rev Palaeobot Palynol 1973; 16: 1–42, 47,–81, 84–122. 10.1016/0034-6667(73)90031-6 [DOI] [Google Scholar]
- 29.Elleouet JS, Aitken SN.. Long-distance pollen dispersal during recent colonization favors a rapid but partial recovery of genetic diversity in Picae sitchensis. New Phytol 2019; 222: 1088–100. 10.1111/nph.15615 [DOI] [PubMed] [Google Scholar]
- 30.Price TD, Hooper DM, Buchanan CDet al. Niche filling slows the diversification of Himalayan songbirds. Nature 2014; 509: 222–5. 10.1038/nature13272 [DOI] [PubMed] [Google Scholar]
- 31.Farnsworth A, Lunt DJ, Robinson SAet al. Past East Asian monsoon evolution controlled by paleogeography, not CO2. Sci Adv 2019; 5: eaax1697. 10.1126/sciadv.aax1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Acosta RP, Huber M.. Competing topographic mechanisms for the summer Indo-Asian Monsoon. Geophys Res Lett 2020; 47: e2019GL085112. 10.1029/2019GL085112 [DOI] [Google Scholar]
- 33.Li DB, Ou XK, Zhao JLet al. An ecological barrier between the Himalayas and the Hengduan Mountains maintains the disjunct distribution of Roscoea. J Biogeogr 2020; 47: 326–41. 10.1111/jbi.13729 [DOI] [Google Scholar]
- 34.Davis KE, Hill J, Astrop TIet al. Global cooling as a driver of diversification in a major marine clade. Nat Commun 2016; 7: 13003. 10.1038/ncomms13003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harvey PH, Nee MS. Phylogenies without fossils. Evolution 1994; 48: 523–9. 10.1111/j.1558-5646.1994.tb01341.x [DOI] [PubMed] [Google Scholar]
- 36.Su T, Farnsworth A, Spicer RAet al. No high Tibetan Plateau until the Neogene. Sci Adv 2019; 5: eaav2189. 10.1126/sciadv.aav2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renner SS.Available data point to a 4-km-high Tibetan Plateau by 40 Ma, but 100 molecular-clock papers have linked supposed recent uplift to young node ages. J Biogeogr 2016; 43: 1479–87. 10.1111/jbi.12755 [DOI] [Google Scholar]
- 38.Liu J, Möller M, Provan J. Geological and ecological factors drive cryptic speciation of yews in a biodiversity hotspot. New Phytol 2013; 199: 1093–108. 10.1111/nph.12336 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.