Abstract
Background
Posttraumatic stress disorder (PTSD) is twice as prevalent among females as compared to males following potentially traumatic events. While there is evidence for aberrant functional connectivity between hubs of the central executive network (CEN), salience network (SN), and the default mode network (DMN) in PTSD, little is known regarding sex-specificity of this connectivity. The current study aims to directly examine sex-specific resting-state functional connectivity (rs-FC) in trauma exposed males and females, with and without PTSD.
Methods
One hundred and seventy-eight individuals underwent functional magnetic resonance imaging (fMRI) at rest, of them 85 females (45 with PTSD) and 93 males (57 with PTSD). We conducted whole-brain seed-based analysis using CEN (lateral prefrontal cortex [lPFC]), SN (anterior cingulate cortex [ACC], insula, amygdala [AMG]), and DMN (medial prefrontal cortex [mPFC], posterior parietal cortex [PCC], and hippocampus [HIP]) hubs as seed regions. Group-by-Sex ANOVA was conducted.
Results
The amygdala-precuneus, ACC-precuneus, and hippocampus-precuneus pathways exhibited significant group-by-sex interaction effects, with females with PTSD consistently differing in connectivity patterns from males with PTSD and from trauma-exposed healthy females.
Conclusions
Sex-specific neural connectivity patterns were found within and between key nodes of the CEN, DMN, and the SN, suggesting opposite patterns of connectivity in PTSD and trauma-exposed controls as a function of sex as a biological variable (SABV). This may point to mechanistic sex differences in adaptation following trauma and may inform differential neural targets for treatment of females and males with PTSD.
Keywords: Gender, Trauma, Neurobiology, Pathophysiology, SABV, Mechanism
1. Introduction
Posttraumatic stress disorder (PTSD) is a prevalent and debilitating disorder, associated with significant burden (Gradus, 2017). This disorder disproportionately affects women, who have twofold the prevalence relative to men (Breslau et al., 1997). Previous studies seeking to understand this discrepancy have identified increased rates of exposure to interpersonal trauma, particularly gender-based violence, as possible contributors to increased risk (Olff, 2017). Additional studies have identified cognitive features such ruminative coping following trauma being more prevalent in females and increasing risk for PTSD (Su and Chen, 2018).
Recently, much attention has been turned to behavioral processes (e.g., emotional regulation fear processing (Bolea-Alamanac et al., 2018; Hammoud et al., 2019; Pineles et al., 2020), and reward processing (Aupperle et al., 2012; Dreher et al., 2007; Etkin and Wager, 2007; Helpman et al., 2016; Olson et al., 2018; Wang et al., 2020) associated with PTSD, and their underlying neural substrates. These substrates include functional circuits (e.g., limbic system, prefrontal cortex, striatal regions, as well as the hippocampus) and larger systems in which they are organized (salience network, the default mode network, and the central executive network; Menon, 2011). Both processes and underlying neural function appear to be, at least in part, regulated by steroid hormones such as estrogen (Lebron-Milad et al., 2012), progesterone (Sharma et al., 2021), and allopregnalone (Rasmusson and Pineles, 2018). Therefore, the function of these neural circuits and systems may be key in understanding sex-dependent bias in PTSD.
Ample evidence has been presented for the importance of sex as a biological variable (SABV) in the neural circuitry involved in the neurobiological model of stress response and neuropsychiatric disorders, as well as in healthy individuals, including: (a) non-human data directly demonstrating sex-specific patterns (Horovitz et al., 2014); (b) vast human data demonstrating sex-specific neurobiological substrates of stress response in healthy subjects (Bale and Epperson, 2015; Bangasser and Wicks, 2017); (c) sex differences in neural connectivity patterns in healthy individuals (Fisher et al., 2016), particularly of amygdala (Alarcón et al., 2015; Kogler et al., 2014; Wu et al., 2016); and (d) initial evidence for sex-specific neural patterns in neuropsychiatric disorders other than PTSD, such as depression (Filippi et al., 2013). Nevertheless, little evidence has been presented as to the role of sex-specific patterns in the neurobehavioral model of PTSD (see Helpman et al., 2017; Seligowski et al., 2020, for a review), despite a surge in neurobiological research exploring this model, independent of sex.
Indeed, dysregulated connectivity within and between three major neural networks has been identified in connection with PTSD. The three networks are, the central executive network (CEN: lateral prefrontal cortex [lPFC]); the salience network (SN: anterior cingulate cortex [ACC], insula, amygdala [AMG]); and the default mode network (DMN: medial prefrontal cortex [mPFC], posterior parietal cortex [PCC], hippocampus [HIP]; Akiki et al., 2017) Findings support increased activity and internal connectivity of the SN in PTSD, possibly reflecting heightened threat-detection associated with PTSD, and poor regulation of this network by the CEN and DMN, which demonstrate reduced connectivity in PTSD. Cognitive deficits manifesting in intrusion and dissociation symptoms found in individuals with PTSD, are associated with poor DMN function (Akiki et al., 2017).
Yet, while studies in healthy humans suggest sex-specific rs-FC patterns including lower rs-FC in the SN-CEN, DMN-SN, and within DMN and CEN in females compared to males (Ernst et al., 2019; Kogler et al., 2016b), research to date, that has focused on the role the DMN, SN and CEN may play in the pathophysiology of PTSD, have commonly treated sex as a potential confounder, rather neglecting to examine sex-dependent patterns of rs-FC in PTSD.
The current study aims to address this gap by unpacking sex-specific patterns in the neurobiology of PTSD by comparing resting-state functional connectivity (rs-FC) within and between DMN, CEN, and SN in a sample of trauma exposed males and females, with and without PTSD. Our aims are threefold:
-
1.
Explore the sex-dependent patterns of connectivity in individuals who were exposed to trauma and developed PTSD, and in those who did not develop PTSD. We expect patterns of within- and between-network connectivity of CEN, SN, and DMN, to discern trauma-exposed individuals who developed PTSD from those who did not, and to differ between males and females.
-
2.
Replicate previous findings regarding sex differences in within- and between-network connectivity. We expect lower SN-CEN and DMN-SN and within DMN and CEN connectivity among females as compared to males, as previously found (Ernst et al., 2019; Kogler et al., 2016b).
-
3.
Replicate previous findings regarding diagnostic group difference in connectivity within and between SN, DMN, and CEN in trauma exposed individuals. We expect lowered connectivity between the CEN-SN, CEN-DMN, within CEN, and within DMN connectivity among individuals with PTSD than in trauma-exposed healthy controls, as previously found (Akiki et al., 2017).
2. Materials and methods
2.1. Sample
To construct a sufficiently large sample to test sex-related patterns among trauma-exposed subjects with and without PTSD, three studies were combined enrolling 181 individuals (N = 106 PTSD, N = 75 healthy controls) with a history of trauma exposure (106 PTSD), 86 (47 PTSD) females and 95 (59 PTSD) males. Participants took part in three neuroimaging studies at the New York State Psychiatric Institute (NYSPI). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects/patients were approved by the NYSPI Institutional Review Board, and all participants provided written informed consent after receiving an explanation of the procedures (see Table 1 for demographic details).
Table 1.
Demographics and clinical variables by sex.
| Statistical test (between groups) |
|||||||
|---|---|---|---|---|---|---|---|
| Females (N = 85) | Males (N = 93) | χ2 | Df | p | |||
| Group | PTSD (N,%) | 45 (52.94%) | 57 (61.29%) | 1.27 | 1 | .26 | |
| Race | White (N,%) | 29 (34.12%) | 35 (37.66%) | 10.70 | 6 | .10 | |
| Black (N,%) | 27 (31.76%) | 33 (35.48%) | |||||
| Hispanic (N,%) | 21 (24.71%) | 9 (9.68%) | |||||
| Asian/Pacific Islander (N,%) | 0 (0%) | 1 (1.08%) | |||||
| Other (N,%) | 4 (4.7%) | 10 (10.75%) | |||||
| Age at Trauma (adult vs. child) | Adult | 66 (77.65%) | 60 (64.52%) | .32 | 1 | .57 | |
| Trauma Type | Military | 3 (3.53%) | 16 (17.2%) | 17.65 | 2 | <.001 | |
| Interpersonal non-military | 58 (68.23%) | 39 (41.94%) | |||||
| Non-interpersonal, non-military | 19 (22.35%) | 37 (39.79%) | |||||
| F | Df | p | |||||
| Age | Mean (SD) | 36.63 (13.91) | 46.08 (14.50) | 19.60 | 2,177 | <.001 | |
| CAPS | Mean (SD) | 38.39 (35.68) | 35.43 (29.19) | .64 | 1,172 | .42 | |
| HRSD | 11.33 (8.91) | 9.80 (8.03) | 1.40 | 1,173 | .24 | ||
Note: CAPS=Clinician assessment of posttraumatic symptoms; HRSD=Hamilton Rating Scale for Depression.
2.2. Procedure
Detailed inclusion and exclusion criteria for each study appear in Table S1 in the supplementary materials. Briefly, all participants met DSM-IV-TR (“Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text ... - American Psychiatric Association - Google Books,” n.d.) criteria A1 and A2 or DSM-5 (Association, 2013) PTSD criterion A for adult traumatic events. Clinical evaluators administered the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (First and Gibbon, 2004) and the Clinician-Administered PTSD Scale (CAPS) (Weathers et al., 2001) to establish psychiatric diagnoses and assess PTSD severity. Exclusion criteria for participants in the trauma-exposed, healthy control group (control) consisted of current or past Axis I disorders (apart from past depression), including substance use disorders. Exclusion criteria for all groups included any condition that would rule out MRI administration, or use of psychotropic medication. MRI collection and preprocessing protocols are included in the supplementary materials.
2.3. Seed-based functional connectivity analyses
Resting-state functional connectivity analyses were carried out using a seed-based approach, implemented in the CONN-fMRI Functional Connectivity toolbox v13. Before correlation analysis, band-pass filtering with a frequency window of 0.01–0.09 Hz was performed. Outlier detection was carried out with artifact detection tools (ART) implemented in CONN. The principal component-based noise-correction method, “CompCor,” implemented in this toolbox, was used for additional control of physiological noise and head motion effects. Outlier volumes in each participant were identified as having large spiking artifacts (i.e., volumes >3 standard deviations from the mean image intensity), or large motion (i.e., 0.5 mm for scan-to-scan head-motion composite changes in the x, y, or z direction). Anatomical images were segmented into grey matter, white matter, and cerebrospinal fluid (CSF) regions. Covariates corresponding to head motion (6 realignment parameters and their derivatives), outliers (one covariate per outlier consisting of 0s everywhere and a 1 for the outlier time point), and the BOLD time series from the subject-specific white matter and CSF masks were used in the connectivity analysis as predictors of no interest and were removed from the BOLD functional time series using linear regression.
From the original sample, three individuals with PTSD were excluded from further analysis because of movement exceeding ±1.5 mm and because more than 20% of their data points have been detected as outliers. Their demographic information can be found in the supplementary materials. Consequently, the final analysis included 45 females with PTSD, 40 trauma exposed, healthy females, 57 males with PTSD, and 38 trauma-exposed, healthy males. The sum of root mean square (RMS) of 6 relative head motion parameters (movement from this time point to the next one) was calculated for each participant in all groups (trauma-exposed healthy controls, PTSD). No significant difference in head motion was found between each pair of groups (p > .5).
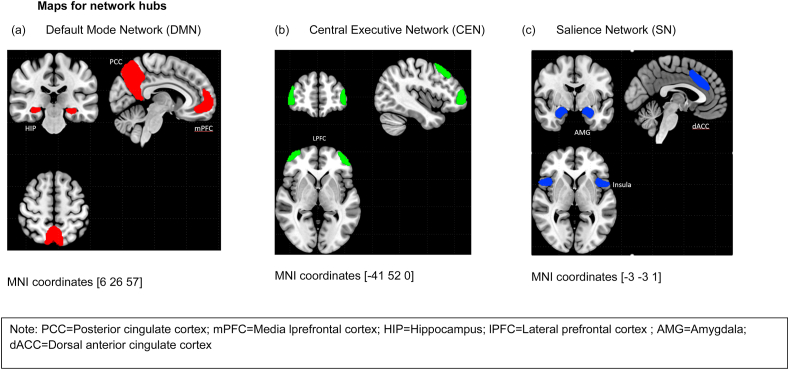
ROI-to-voxel whole-brain connectivity analysis was performed using ROIs identified as key nodes in CEN (lateral prefrontal cortex [lPFC]), SN (anterior cingulate cortex [ACC], insula, amygdala [AMG]) and DMN (medial prefrontal cortex [mPFC], posterior parietal cortex [PCC], and hippocampus [HIP]) hubs as seed regions (see Fig. 1). All ROIs were defined based on the CONN ICA analyses of the HCP dataset of 497 participants. Bivariate regression analyses were used to determine the linear association of the BOLD time series between each seed ROI and all other voxels in the brain, for each subject. Both positive and negative correlations were examined. The resulting correlation coefficients were transformed into z-scores, using Fisher's transformation to satisfy normality assumptions. Age and sites were regressed out in all individuals as covariates of no interest as they provided possible confounds (see Table S2).
Fig. 1.
Maps for network hubs.
Note: PCC=Posterior cingulate cortex; mPFC = Media lprefrontal cortex; HIP=Hippocampus; lPFC = Lateral prefrontal cortex; AMG = Amygdala; dACC = Dorsal anterior cingulate cortex.
2.4. Statistical analyses
To disentangle sex, group, and interaction effects, we conducted a two-by-two group (PTSD, trauma exposed healthy control) by sex (male, female) ANOVA. We used a threshold of PFWE<0.05 whole-brain voxel-wise, family-wise-error-rate (FWE)-corrected with a minimum of 20 voxels. Next, we extracted mean cluster values, using the MarsBaR function (http://marsbar.sourceforge.net), and subjected them to post hoc tests. To test the direction of the group by sex interaction, we used post hoc independent t-tests in SPSS 22 for detecting sex difference in PTSD and controls.
3. Results
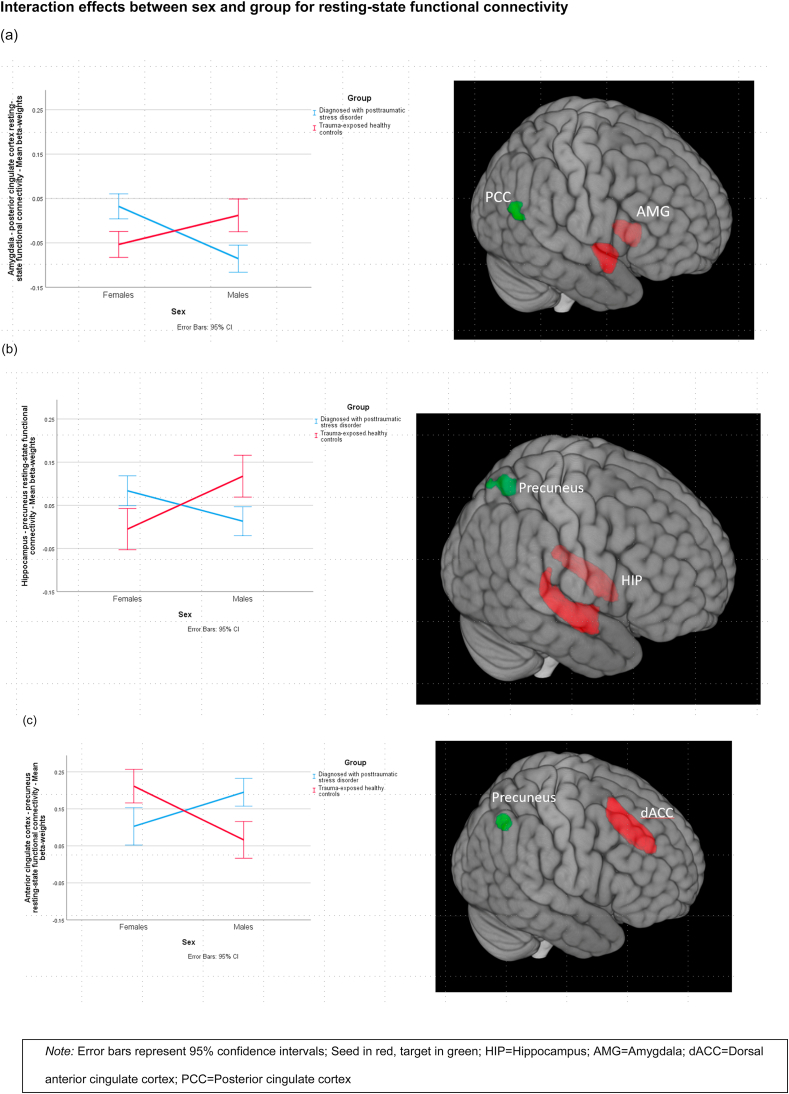
Three pathways with group-by-sex interaction effects survived multiple corrections: amygdala-PCC, ACC-precuneus, and hippocampus-precuneus (angular gyrus portion) (for all pathways surviving corrections, please see Table 2). In order to ensure that these effects were not attributable to trauma type, which differed by sex, we conducted ANOVAs on these pathways including type of trauma as a covariate, with the group-by-sex effect remaining intact (see Supplementary materials). Then, to examine the direction of the interaction effects, we conducted post hoc tests for these three pathways. The results indicate that within PTSD, females differed from males, however, in TEHC, the difference between females and males was reversed. Specifically, females with PTSD significantly differed from males with PTSD and from healthy trauma-exposed females, but not from healthy trauma exposed males, in these pathways. For the amygdala and posterior cingulate cortex (p < .001), and for the precuneus and hippocampus (p = .01), females with PTSD showed weaker rs-FC than PTSD males and healthy trauma-exposed females. For precuneus and anterior cingulate cortex (p < .001), females with PTSD had stronger rs-FC than did males with PTSD and healthy trauma-exposed females (see Fig. 2 for a complete account; additional subgroup analyses by scanner and TR can be found in Fig. S2 in the supplement) (see Table 3 for complete post-hoc tests of the three pathways).
Table 2.
Group by Sex ANOVA test.
| Network | Effect type | Seed | p FWE-corr | Cluster size | T | z | X | y | z | Target region | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SN | ||||||||||||
| Group | ||||||||||||
| AMG | 0.0090 | 119 | 4.78 | 4.62 | 6 | 52 | 14 | BA10/mPFC | DMN | PTSD < CONTROL | ||
| ACC | 0.0000 | 213 | 5.5 | 5.27 | −10 | −58 | 44 | BA31/PCC | DMN | PTSD > CONTROL | ||
| Insula (left) | 0.0470 | 93 | 4.57 | 4.43 | −54 | −46 | 22 | BA39/Angular Gyrus | DMN | PTSD < CONTROL | ||
| Insula (right) | 0.0210 | 108 | 4.03 | 3.93 | −66 | −42 | 16 | BA22/STG/Wernicke | PTSD < CONTROL | |||
| Sex | ||||||||||||
| Insula (left) | 0.0000 | 229 | 4.65 | 4.5 | 52 | 14 | 4 | BA44/IFG parsopecularis | CEN | F < M | ||
| 0.0030 | 156 | 4.05 | 3.95 | 4 | 2 | 66 | BA6/Premotor/SMA | F < M | ||||
| 0.0060 | 141 | 4.04 | 3.94 | −42 | 8 | 2 | BA44/IFG parsopecularis | CEN | F < M | |||
| Insula (right) | 0.0010 | 195 | 4.75 | 4.6 | 46 | 8 | 2 | BA44/IFG parsopecularis | CEN | F < M | ||
| Group x Sex | ||||||||||||
| AMG | 0.0380 | 90 | 4.66 | 4.51 | 30 | −68 | 12 | BA7/Precuneus | DMN | See Fig. 2 | ||
| ACC | 0.0390 | 98 | 5.24 | 5.04 | 16 | −66 | 38 | BA7/Precuneus | DMN | See Fig. 2 | ||
| CEN | ||||||||||||
| Group | ||||||||||||
| lPFC (left) | 0.0020 | 170 | 5.57 | 5.53 | 4 | 28 | 50 | BA8/dlPFC | CEN | PTSD < CONTROL | ||
| 0.0040 | 151 | 4.28 | 4.17 | −62 | −30 | −16 | BA21/MTG | PTSD < CONTROL | ||||
| PPC (left) | 0.0330 | 100 | 4.25 | 4.14 | −48 | 28 | 22 | BA46/dlPFC | CEN | PTSD < CONTROL | ||
| PPC (right) | 0.0250 | 104 | 4.7 | 4.55 | −2 | −76 | 34 | BA18/Visual association Cortex | PTSD < CONTROL | |||
| Sex | ||||||||||||
| lPFC (left) | 0.0250 | 106 | 4.19 | 4.08 | −52 | 20 | −10 | BA47/vlPFC | SN | F < M | ||
| PPC (right) | 0.0070 | 133 | 4.88 | 4.72 | −38 | −54 | 34 | BA39/Angular Gyrus | DMN | F < M | ||
| 0.0400 | 94 | 4.3 | 4.18 | 48 | −40 | 38 | BA40/Supramarginal | CEN | F < M | |||
| DMN | ||||||||||||
| Group | ||||||||||||
| mPFC | 0.0000 | 255 | 4.93 | 4.76 | −14 | 48 | 46 | BA8/dlPFC | CEN | PTSD < CONTROL | ||
| PCC | 0.0390 | 98 | 5.23 | 5.03 | −38 | 10 | 42 | BA8/dlPFC | CEN | PTSD < CONTROL | ||
| 0.0020 | 173 | 4.69 | 4.55 | 56 | −68 | 30 | BA39/Angular Gyrus | DMN | PTSD < CONTROL | |||
| Sex | ||||||||||||
| PCC | 0.0000 | 217 | 4.71 | 4.56 | −10 | −78 | 32 | BA18/Visual association Cortex | F < M | |||
| mPFC | 0.0020 | 174 | 4.56 | 4.42 | −48 | −4 | −2 | BA22/STG/Wernicke | F < M | |||
| 0.0170 | 116 | 4.55 | 4.42 | −32 | −44 | 70 | BA7/Precuneus | DMN | F < M | |||
| HIP | 0.0050 | 139 | 4.28 | 4.17 | −28 | −44 | 66 | BA7/Precuneus | DMN | F < M | ||
| Group x Sex | ||||||||||||
| HIP | 0.0020 | 158 | 5.23 | 5.03 | −38 | −78 | 36 | BA39/Angular Gyrus | DMN | See Fig. 2 | ||
Note: SN=Salience network, CEN=Central executive network, DMN = Default mode network, CONTROL = Trauma exposed healthy controls, AMG = Amygdala, PCC=Posterior cingulate cortex, HIP=Hippocampus, ACC = Anterior cingulate cortex, mPFC = medial prefrontal cortex. lPFC = lateral prefrontal cortex, dlPFC = dorsolateral prefrontal cortex, vlPFC = ventrolateral prefrontal cortex, PPC=Posterior parietal cortex, BA=Brodmann's area, MTG = Medial temporal gyrus, STG=Superior temporal gyrus, SMA=Supplementary motor area, IFG=Inferior frontal gyrus, FWE-corr = Family wise error correction.
Fig. 2.
Interaction effects between sex and group for resting-state functional connectivity.
Note: Error bars represent 95% confidence intervals; Seed in red, target in green; HIP=Hippocampus; AMG = Amygdala; dACC = Dorsal anterior cingulate cortex; PCC=Posterior cingulate cortex. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 3.
Post-hoc tests on pathways evincing interaction effects.
| Pathway | Index group | Comparison group | M | SE | p |
|---|---|---|---|---|---|
| AMG-PCC | CONTROL Females | PTSD Females | −0.09* | 0.02 | 0.00 |
| CONTROL Males | −0.07* | 0.02 | 0.01 | ||
| PTSD Males | 0.03 | 0.02 | 0.14 | ||
| PTSD Females | CONTROL Males | 0.02 | 0.02 | 0.38 | |
| PTSD Males | 0.12* | 0.02 | 0.00 | ||
| CONTROL Males | PTSD Males | 0.10* | 0.02 | 0.00 | |
| HIP-Precuneus | CONTROL Females | PTSD Females | −0.09* | 0.03 | 0.00 |
| CONTROL Males | −0.12* | 0.03 | 0.00 | ||
| PTSD Males | −0.02 | 0.03 | 0.50 | ||
| PTSD Females | CONTROL Males | −0.03 | 0.03 | 0.26 | |
| PTSD Males | 0.07* | 0.03 | 0.01 | ||
| CONTROL Males | PTSD Males | 0.10* | 0.03 | 0.00 | |
| ACC-Precuneus | CONTROL Females | PTSD Females | 0.11* | 0.03 | 0.00 |
| CONTROL Males | 0.15* | 0.03 | 0.00 | ||
| PTSD Males | 0.02 | 0.03 | 0.60 | ||
| PTSD Females | CONTROL Males | 0.04 | 0.03 | 0.28 | |
| PTSD Males | −0.09* | 0.03 | 0.00 | ||
| CONTROL Males | PTSD Males | −0.13* | 0.03 | 0.00 |
Note: CONTROL = Trauma exposed healthy controls, AMG = Amygdala, PCC=Posterior cingulate cortex, HIP=Hippocampus, ACC = Anterior cingulate cortex; * = The mean difference is significant at the 0.01 level.
The ANOVA revealed a significant main effect of the group, with expected lowered connectivity between the CEN-SN, CEN-DMN, within CEN, and within DMN connectivity among individuals with PTSD than in trauma-exposed healthy controls (all p's < .05, see Table 2 for group differences in all pathways). Additionally, the ACC-PCC pathway evinced higher connectivity among those with PTSD than in those without (p < .001). The main effects of sex indicated that overall females evinced weaker CEN-SN and CEN-DMN connectivity, as well as, within CEN and DMN connectivity when compared to males (all p's < 0.05, please see Table 2 for sex differences in all pathways).
4. Discussion
The present findings are the first, to our knowledge, to demonstrate sex-specific patterns of neural functioning among trauma-exposed individuals with and without PTSD. The findings suggest that the patterns of connectivity distinguishing trauma-exposed individuals who develop PTSD from those who do not are reversed for males and for females. For males, weaker DMN and DMN-SN (AMG-PCC, HIP-precuneus) connectivity was found in those with PTSD compared to those without PTSD, and stronger SN-DMN (ACC-precuneus) connectivity in those with PTSD compared to those without PTSD. For females, the opposite pattern emerged: stronger connectivity was found among individuals with PTSD than in trauma-exposed healthy individuals, for AMG-PCC and HIP-precuneus, and weaker connectivity for ACC-precuneus among individuals with PTSD than in trauma-exposed healthy controls, for the third. The reliability of our findings is bolstered by main effects for sex (|e.g., weaker connectivity within CEN, within DMN, and between CEN-SN and CEN-DMN among females as compared to males) and PTSD diagnosis (weaker CEN-SN, CEN-DMN, CEN, and DMN connectivity among individuals with PTSD than in controls), which are consistent with those documented so far in the literature (Akiki et al., 2017; Alarcón et al., 2015; Fisher et al., 2016; Kogler et al., 2016a; Wu et al., 2016). The present findings have important potential implications for understanding the role of SABV in the pathophysiology of PTSD. The inherent heterogeneity of PTSD, both clinically and, possibly, neurobiologically, has been cited as one of the major challenges in understanding and treating this disorder, warranting the study of “more homogeneous, biologically defined subgroups… improving diagnosis and treatment.“ (Maron-Katz et al., 2020).
To date, the literature has focused separately either on the effect of PTSD (vs. controls) or on the effect of SABV, regarding other variable at most as noise to control for, rather than the focus of analyses. Such focus on each piece of the whole picture separately detracts from the ability to disentangle the PTSD heterogeneity (Maron-Katz et al., 2020). The present study points to the importance of taking into account the interaction between PTSD and SABV when examining the neural markings of trauma and PTSD. Further elucidating this complex relationship can shed light on potential distinct pathways contributing to the development of PTSD in females vs. males, creating the opposite patterns of abnormalities that we found. If the mechanisms contributing to the emergence of PTSD differ as a function of SABV, the treatments targeting the mechanism should differ as well.
Our sex-dependent, inverse findings regarding DMN-SN connectivity (AMG-PCC and ACC-precuneus) for individuals with and without PTSD may involve the contribution of SABV to the mechanisms underlying clinical symptoms. AMG-PCC connectivity is implicated in the symptomatology of both PTSD (Lanius et al., 2010; Zhou et al., 2012) and anxiety disorders (Hamm et al., 2014; Makovac et al., 2016), and in underlying mechanisms such as stress response (Veer et al., 2011) and emotional regulation (Li et al., 2016). In females, stronger coupling in the AMG-PCC pathway has been found in those with postpartum depression than in healthy postpartum women (Chase et al., 2014), suggesting enhanced connectivity in this pathway in females with psychopathology, beyond PTSD. The opposite patterns found in the present study are also consistent with previous studies suggesting an inverse, sex-dependent association between rs-FC of the amygdala (SN), and cortisol, a steroid hormone related to stress response: a negative correlation was found in healthy females, and a positive one in healthy males (Kogler et al., 2016a). The PCC (DMN), was found to be involved in self-referential processing and autobiographical memory (Kim, 2012; Menon, 2011), and the amygdala was found to be involved in emotional processing and response (Sah et al., 2003). This may indicate sex-dependent, maladaptive responses to stressful events, including appropriate coding of emotional information about the self in a stressful situation. This pathway, therefore, may also be implicated in the dysfunctional memory formation associated with posttraumatic reactions. Focusing on the ACC-precuneus, rs-FC has been positively correlated with PTSD symptom severity (Makovac et al., 2016), depression (Connolly et al., 2013), panic disorder (Shin et al., 2013), and negatively with ADHD (Castellanos et al., 2008), suggesting a more general contribution to psychopathology perhaps through associated attentional processes. Although we found no sex-group interactions within SN and in CEN rs-FC, we did reveal main effects for sex. Similarly, opposing patterns of rs-FC within the SN and between the SN and CEN have been previously found to be associated with emotional regulation, a mechanism intimately involved in PTSD (Wu et al., 2016). Thus, even though the findings reported here are novel, they are consistent with current knowledge about the associations between SABV and the underlying mechanisms at the basis of PTSD, such as sex-disparate underpinnings of emotional regulation and response to stress.
We also found sex-dependent, opposite patterns of within DMN connectivity (HIP-precuneus) for individuals with and without PTSD. HIP-precuneus connectivity has been previously found to differentiate controls from PTSD (Lazarov et al., 2017), and has been associated with memory problems and cognitive impairment (Apple et al., 2018; Xue et al., 2019). Within DMN connectivity has also previously been implicated in sex-specific, opposite association with pubertal maturation (Ernst et al., 2019), suggesting the involvement of sex hormones in the onset of this disparity.
Post hoc explanations of the intriguing findings in the present study suggest that distinct developmental pathways in females and males may contribute to the distinct pathophysiology for SABV. Epidemiological findings suggest disproportionate internalizing psychopathology in females and in males, including PTSD. This discrepancy has been tied to hormonal changes in puberty. During pubertal maturation, weaker FC within DMN was found in females and stronger FC in males, with lower connectivity of the ACC, a major DMN hub. This pattern of FC was also able to predict higher internalizing symptoms at 2-year follow-up (Ernst et al., 2019). Therefore, hormonal changes may bring about neural changes, sensitizing females to the development of internalizing symptomatology. Our findings similarly point at weaker within DMN rs-FC associated with PTSD in females specifically, but at an inverse relationship in men. This suggests that men may develop PTSD by a different pathway of risk. In fact, distinct, and even opposing, neural underpinnings of the similar mechanisms (e.g., stress response, emotional regulation) may differentially confer risk: Integrating the present findings with the available literature may suggest that developmental changes, such as steroid hormone surges during puberty, may be involved in a cascade of neural changes. These may result in differential sensitization of circuitry, possibly through a differential response to the further introduction of steroid hormones (e.g., cortisol). This compounded effect may enhance risk for affective and stress-related disorders in women (Ravi et al., 2019).
Sex- and gender-specific patterns of adaptive biological stress response have been demonstrated repeatedly and shown to associate not only with biological factors such as gonadal steroids (Shansky et al., 2010) but also as psychosocial factors such as the type of stressor (Lee et al., 2014) and gender role of the individual (Manigault et al., 2021). It therefore appears that adaptation in face of stress and trauma may involve not only biologically sensitized sex-dependent neural underpinnings (McLaughlin et al., 2015) but a psychosocially moderated response. This response may be differentially employed in compensation of both developmentally distinct neural underpinnings and of specific situational and individual differences. The development of sex-and-gender-specific coping and regulation strategies in the face of stress has long been demonstrated, and theoretically grounded in socio-evolutionary factors (Taylor et al., 2000). Therefore, our findings may demonstrate implications of these on adaptation in the face of trauma, which may thus involve a complex array of interactions between biological, psychological, and social factors.
The main limitation of the present study lies in its exploratory nature. The study proposes pioneering findings that should be more elaborately examined in future studies. Our study is further limited in its inability to directly address the issue of endocrine correlates of SABV in PTSD. We did not collect endocrine data or data on the endocrine status (e.g., time in cycle, menopause, etc.) that may have further elucidated the mechanism underlying these differences. We have similarly not been able to analyze the finer resolutions of trauma type, nor have we collected data regarding psychosocial aspects of gender role and identity of participants, so that we are unable to comment on the associations of such variables with our findings. Additional limitations include a small sample size and a lack of a non-trauma- exposed sample, which could enable us to discern sex-related patterns in trauma-related and PTSD-related circuitries. Future research should include SABV in studies of clinical populations, involving multi-level measurement, to begin untangling the complex bio-psycho-social network involved in the clinically significant response to stress and trauma.
5. Conclusions
The present results demonstrate opposite patterns of neural connectivity in PTSD and healthy, trauma-exposed controls as a function of SABV. This may point to mechanistic differences in adaptation following trauma, dependent on sex. The different mechanisms may require interventions based on sex-dependent targeting. These findings are an important step toward further multilevel, mechanistic studies, necessary for the development of personalized, mechanism-informed treatment of PTSD and stress-related disorders.
Statement of interests and role of funding sources
Dr Helpman has no conflicts of interest to report. Her work on early stages of data collection was funded by NIMH T32 Fellowship (T32MH096724; Wainberg & Oquendo). She wishes to acknowledge the work of Michal Tevet, who assisted with technical editing of the manuscript.
Dr. Zhu has no conflicts of interest to report. Her work on the manuscript was supported by K01MH122774 and Brain and Behavior Research Foundation Grant.
Dr Suarez-Jimenez has no conflicts of interest to report.
Dr. Zilcha-Mano has no conflicts of interest to report.
Dr. Lazarov has no conflicts of interest to report.
Dr. Ruthererfod has no conflicts of interest to disclose. Data collection in his Lab was funded by NIMH R01 MH111596 (Rutherford).
Dr. Neria has no conflicts of interest to disclose. Data collection in his Lab was funded by NIMH R01 grants (R01MH072833 and R01MH105355; Neria).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100389.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data that is sharable (privacy etc.) will be made available upon request.
References
- Akiki T.J., Averill C.L., Abdallah C.G. A network-based neurobiological model of PTSD: evidence from structural and functional neuroimaging studies. Curr. Psychiatr. Rep. 2017 doi: 10.1007/s11920-017-0840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón G., Cservenka A., Rudolph M.D., Fair D.A., Nagel B.J. Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage. 2015;115:235–244. doi: 10.1016/j.neuroimage.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apple A.C., Schroeder M.P., Ryals A.J., Wagner L.I., Cella D., Shih P.A., Reilly J., Penedo F.J., Voss J.L., Wang L. Hippocampal functional connectivity is related to self-reported cognitive concerns in breast cancer patients undergoing adjuvant therapy. NeuroImage Clin. 2018;20:110–118. doi: 10.1016/j.nicl.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association A.P. fifth ed. American Psychiatric Publishing; 2013. Diagnostic and Statistical Manual of Mental Disorders. [DOI] [Google Scholar]
- Aupperle R.L., Melrose A.J., Stein M.B., Paulus M.P. Executive function and PTSD: disengaging from trauma. Neuropharmacology. 2012;62:686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T.L., Epperson C.N. Sex differences and stress across the lifespan. Nat. Neurosci. 2015;18:1413–1420. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Wicks B. Sex-specific mechanisms for responding to stress. J. Neurosci. Res. 2017;95:75–82. doi: 10.1002/jnr.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolea-Alamanac B., Bailey S.J., Lovick T.A., Scheele D., Valentino R. Female psychopharmacology matters! towards a sex-specific psychopharmacology. J. Psychopharmacol. 2018;1 doi: 10.1177/0269881117747578. 026988111774757–026988111774757. [DOI] [PubMed] [Google Scholar]
- Breslau N., Davis G.C., Andreski P., Peterson E.L., Schultz L.R. Sex differences in posttraumatic stress disorder. Arch. Gen. Psychiatr. 1997;54:1044–1048. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Margulies D.S., Kelly C., Uddin L.Q., Ghaffari M., Kirsch A., Shaw D., Shehzad Z., Di Martino A., Biswal B., Sonuga-Barke E.J.S., Rotrosen J., Adler L.A., Milham M.P. Cingulate-precuneus interactions: a New locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol. Psychiatr. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase H.W., Moses-Kolko E.L., Zevallos C., Wisner K.L., Phillips M.L. Disrupted posterior cingulate–amygdala connectivity in postpartum depressed women as measured with resting BOLD fMRI. Soc. Cognit. Affect Neurosci. 2014;9:1069–1075. doi: 10.1093/scan/nst083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly C.G., Wu J., Ho T.C., Hoeft F., Wolkowitz O., Eisendrath S., Frank G., Hendren R., Max J.E., Paulus M.P., Tapert S.F., Banerjee D., Simmons A.N., Yang T.T. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol. Psychiatr. 2013;74:898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders, fourth ed., Text ... - American psychiatric association - Google Books [WWW Document], n.d. URL https://books.google.co.il/books/about/Diagnostic_and_Statistical_Manual_of_Men.html?id=_w5-BgAAQBAJ&redir_esc=y (accessed 11.12.20).
- Dreher J.-C., Schmidt P.J., Kohn P., Furman D., Rubinow D., Berman K.F. Menstrual cycle phase modulates reward-related neural function in women. Proc. Natl. Acad. Sci. U. S. A. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Benson B., Artiges E., Gorka A.X., Lemaitre H., Lago T., Miranda R., Banaschewski T., Bokde A.L.W., Bromberg U., Brühl Rüdiger, Büchel C., Cattrell A., Conrod P., Desrivières S., Fadai T., Flor H., Grigis A., Gallinat J., Garavan H., Gowland P., Grimmer Y., Heinz A., Kappel V., Nees F., Papadopoulos-Orfanos D., Penttilä J., Poustka L., Smolka M.N., Stringaris A., Struve M., van Noort B.M., Walter H., Whelan R., Schumann G., Grillon C., Martinot M.L.P., Martinot J.L., Dalley J., Subramaniam N., Theobald D., Bach C., Barker G.J., Fauth-Bühler M., Millenet S., Spanagel R., Albrecht L., Ivanov N., Rapp M., Reuter J., Strache N., Ströhle A., Poline J.B., Schwartz Y., Thyreau B., Ireland J., Rogers J., Bordas N., Bricaud Z., Filippi I., Galinowski A., Gollier-Briant F., Hall D., Havatzias S., Jia T., Mallik C., Nymberg C., Ruggeri B., Smith L., Stueber K., Topper L., Werts H., Brühl R., Ihlenfeld A., Walaszek B., Hübner T., Müller K., Paus T., Ripke S., Mennigen E., Schmidt D., Vetter N.C., Ziesch V., Carter D., Connolly C., Nugent S., Jones J., Yacubian J., Schneider S., Head K., Heym N., Newman C., Pausova Z., Tahmasebi A., Stephens D. Pubertal maturation and sex effects on the default-mode network connectivity implicated in mood dysregulation. Transl. Psychiatry. 2019;9 doi: 10.1038/s41398-019-0433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatr. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M., Valsasina P., Misci P., Falini A., Comi G., Rocca M.A. The organization of intrinsic brain activity differs between genders: a resting-state FMRI study in a large cohort of young healthy subjects massimo. Hum. Brain Mapp. 2013;34:1330–1343. doi: 10.1002/hbm.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Gibbon M. In: Comprehensive Handbook of Psychological Assessment. Hilsenroth M.J., Segal D.L., editors. vol. 2. John Wiley & Sons Inc; Hoboken, NJ: 2004. The structured clinical Interview for DSM-IV Axis I disorders (SCID-I) and the structured clinical Interview for DSM-IV Axis II disorders (SCID-II) pp. 134–143. Personality Assessment. [Google Scholar]
- Fisher P.M., Larsen C.C., Beliveau V., Henningsson S., Pinborg A., Holst K.K., Jensen P.S., Svarer C., Siebner H.R., Knudsen G.M., Frokjaer V.G. Pharmacologically induced sex-hormone fluctuation effects on resting-state functional connectivity in a risk model for depression: a randomised trial. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradus J.L. Prevalence and prognosis of stress disorders: a review of the epidemiologic literature. Clin. Epidemiol. 2017;9:251–260. doi: 10.2147/CLEP.S106250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm L.L., Jacobs R.H., Johnson M.W., Fitzgerald D.A., Fitzgerald K.D., Langenecker S.A., Monk C.S., Phan K.L. Aberrant amygdala functional connectivity at rest in pediatric anxiety disorders. Biol. Mood Anxiety Disord. 2014;4:15. doi: 10.1186/s13587-014-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud M.Z., Foa E.B., Milad M.R. Estradiol, threat conditioning and extinction, posttraumatic stress disorder, and prolonged exposure therapy: a common link. J. Neuroendocrinol. 2019 doi: 10.1111/jne.12800. [DOI] [PubMed] [Google Scholar]
- Helpman L., Marin M.-F.M.-F., Papini S., Zhu X., Sullivan G.M.G.M., Schneier F., Neria M., Shvil E., Malaga Aragon M.J.M.J., Markowitz J.C.J.C., Lindquist M.A.M.A., Wager T.D., Milad M.R., Neria Y. Neural changes in extinction recall following prolonged exposure treatment for PTSD: a longitudinal fMRI study. NeuroImage Clin. 2016;12:715–723. doi: 10.1016/j.nicl.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helpman L., Penso J., Zagoory-Sharon O., Feldman R., Gilboa-Schechtman E. Endocrine and emotional response to exclusion among women and men; cortisol, salivary alpha amylase, and mood. Hist. Philos. Logic. 2017;30 doi: 10.1080/10615806.2016.1269323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz O., Tsoory M.M., Yovell Y., Richter-Levin G. A rat model of pre-puberty (Juvenile) stress-induced predisposition to stress-related disorders: sex similarities and sex differences in effects and symptoms. World J. Biol. Psychiatr. 2014;15:36–48. doi: 10.3109/15622975.2012.745604. [DOI] [PubMed] [Google Scholar]
- Kim H. A dual-subsystem model of the brain's default network: self-referential processing, memory retrieval processes, and autobiographical memory retrieval. Neuroimage. 2012;61:966–977. doi: 10.1016/j.neuroimage.2012.03.025. [DOI] [PubMed] [Google Scholar]
- Kogler L., Gur R.C., Derntl B. Sex differences in cognitive regulation of psychosocial achievement stress: brain and behavior. Hum. Brain Mapp. 2014 doi: 10.1002/hbm.22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler L., Müller V.I., Seidel E.-M., Boubela R., Kalcher K., Moser E., Habel U., Gur R.C., Eickhoff S.B., Derntl B. Sex differences in the functional connectivity of the amygdalae in association with cortisol. Neuroimage. 2016;134:410–423. doi: 10.1016/j.neuroimage.2016.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler L., Müller V.I., Seidel E.-M., Boubela R., Kalcher K., Moser E., Habel U., Gur R.C., Eickhoff S.B., Derntl B., Mueller V.I., Seidel E.-M., Boubela R., Kalcher K., Moser E., Habel U., Gur R.C., Eickhoff S.B., Derntl B. Sex differences in the functional connectivity of the amygdalae in association with cortisol. Neuroimage. 2016;134:410–423. doi: 10.1016/j.neuroimage.2016.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius R.A., Bluhm R.L., Coupland N.J., Hegadoren K.M., Rowe B., Théberge J., Neufeld R.W.J., Williamson P.C., Brimson M. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr. Scand. 2010;121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Lazarov A., Zhu X., Suarez-Jimenez B., Rutherford B.R., Neria Y. Resting-state functional connectivity of anterior and posterior hippocampus in posttraumatic stress disorder. J. Psychiatr. Res. 2017;94:15–22. doi: 10.1016/j.jpsychires.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron-Milad K., Graham B.M., Milad M.R. Low estradiol levels: a vulnerability factor for the development of posttraumatic stress disorder. Biol. Psychiatr. 2012;72:6–7. doi: 10.1016/j.biopsych.2012.04.029. [DOI] [PubMed] [Google Scholar]
- Lee M.R., Cacic K., Demers C.H., Haroon M., Heishman S., Hommer D.W., Epstein D.H., Ross T.J., Stein E.a., Heilig M., Salmeron B.J. Gender differences in neural-behavioral response to self-observation during a novel fMRI social stress task. Neuropsychologia. 2014;53:257–263. doi: 10.1016/j.neuropsychologia.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Tong L., Guan M., He W., Wang L., Bu H., Shi D., Yan B. Altered resting-state amygdala functional connectivity after real-time fMRI emotion self-regulation training. BioMed Res. Int. 2016:1–8. doi: 10.1155/2016/2719895. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makovac E., Watson D.R., Meeten F., Garfinkel S.N., Cercignani M., Critchley H.D., Ottaviani C. Amygdala functional connectivity as a longitudinal biomarker of symptom changes in generalized anxiety. Soc. Cognit. Affect Neurosci. 2016;11:1719–1728. doi: 10.1093/scan/nsw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manigault A.W., Shorey R.C., Appelmann H., Hamilton K.R., Scanlin M.C., Juster R.P., Zoccola P.M. 2021. Gender Roles Are Related to Cortisol Habituation to Repeated Social Evaluative Stressors in Adults: Secondary Analyses from a Randomized Controlled Trial. Stress. [DOI] [PubMed] [Google Scholar]
- Maron-Katz A., Zhang Y., Narayan M., Wu W., Toll R.T., Naparstek S., De Los Angeles C., Longwell P., Shpigel E., Newman J., Abu-Amara D., Marmar C., Etkin A. Individual patterns of abnormality in resting-state functional connectivity reveal two data-driven PTSD subgroups. Am. J. Psychiatr. 2020;177:244–253. doi: 10.1176/appi.ajp.2019.19010060. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Gold A.L., Duys A., Lambert H.K., Peverill M., Heleniak C., Shechner T., Wojcieszak Z., Pine D.S. Maltreatment exposure, brain structure, and fear conditioning in children and adolescents. Neuropsychopharmacology. 2015:1–30. doi: 10.1038/npp.2015.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cognit. Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Olff M. Sex and gender differences in post-traumatic stress disorder: an update. Eur. J. Psychotraumatol. 2017;8:1351204. doi: 10.1080/20008198.2017.1351204. [DOI] [Google Scholar]
- Olson E.A., Kaiser R.H., Pizzagalli D.A., Rauch S.L., Rosso I.M. Anhedonia in trauma-exposed individuals: functional connectivity and decision-making correlates. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2018;3:959–967. doi: 10.1016/j.bpsc.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineles S.L., Nillni Y.I., Pinna G., Webb A., Arditte Hall K.A., Fonda J.R., Irvine J., King M.W., Hauger R.L., Resick P.A., Orr S.P., Rasmusson A.M. Associations between PTSD-Related extinction retention deficits in women and plasma steroids that modulate brain GABAA and NMDA receptor activity. Neurobiol. Stress. 2020;13 doi: 10.1016/j.ynstr.2020.100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson A.M., Pineles S.L. Neurotransmitter, peptide, and steroid hormone abnormalities in PTSD: biological endophenotypes relevant to treatment. Curr. Psychiatr. Rep. 2018;20 doi: 10.1007/s11920-018-0908-9. [DOI] [PubMed] [Google Scholar]
- Ravi M., Stevens J.S., Michopoulos V. Neuroendocrine pathways underlying risk and resilience to PTSD in women. Front. Neuroendocrinol. 2019 doi: 10.1016/j.yfrne.2019.100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P., Faber E.S.L., Lopez De Armentia M., Power J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Seligowski, A. V, Harnett, N.G., Merker, J.B., Ressler, K.J., n.d. Nervous and endocrine system dysfunction in posttraumatic stress disorder: an overview and consideration of sex as a biological variable. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 5, 381–391. 10.1016/j.bpsc.2019.12.006. [DOI] [PMC free article] [PubMed]
- Shansky R.M., Hamo C., Hof P.R., Lou W., McEwen B.S., Morrison J.H. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cerebr. Cortex. 2010;20:2560–2567. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Cameron A., Fang Z., Ismail N., Smith A. The regulatory roles of progesterone and estradiol on emotion processing in women. Cognit. Affect Behav. Neurosci. 2021:1–13. doi: 10.3758/s13415-021-00908-7. [DOI] [PubMed] [Google Scholar]
- Shin Y.W., Dzemidzic M., Jo H.J., Long Z., Medlock C., Dydak U., Goddard A.W. Increased resting-state functional connectivity between the anterior cingulate cortex and the precuneus in panic disorder: resting-state connectivity in panic disorder. J. Affect. Disord. 2013;150:1091–1095. doi: 10.1016/j.jad.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y.J., Chen S.H. Negative cognitions prior to trauma predict acute posttraumatic stress disorder symptomatology. J. Trauma Stress. 2018 doi: 10.1002/jts.22255. [DOI] [PubMed] [Google Scholar]
- Taylor S.E., Klein L.C., Lewis B.P., Gruenewald T.L., Gurung R.a.R., Updegraff J.a. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol. Rev. 2000;107:411–429. doi: 10.1037//0033-295X.107.3.411. [DOI] [PubMed] [Google Scholar]
- Veer I.M., Oei N.Y.L., Spinhoven P., van Buchem M.A., Elzinga B.M., Rombouts S.A.R.B. Beyond acute social stress: increased functional connectivity between amygdala and cortical midline structures. Neuroimage. 2011;57:1534–1541. doi: 10.1016/j.neuroimage.2011.05.074. [DOI] [PubMed] [Google Scholar]
- Wang J.X., Zhuang J.Y., Fu L., Lei Q., Fan M., Zhang W. How ovarian hormones influence the behavioral activation and inhibition system through the dopamine pathway. PLoS One. 2020;15 doi: 10.1371/journal.pone.0237032. e0237032–e0237032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers F.W., Keane T.M., Davidson J.R.T. Clinician-administered PTSD scale: a review of the first ten years of research. Depress. Anxiety. 2001 doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Wu Y., Li H., Zhou Y., Yu J., Zhang Y., Song M., Qin W., Yu C., Jiang T. Sex-specific neural circuits of emotion regulation in the centromedial amygdala. Sci. Rep. 2016;6 doi: 10.1038/srep23112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J., Guo H., Gao Y., Wang X., Cui H., Chen Z., Wang B., Xiang J. Altered directed functional connectivity of the Hippocampus in mild cognitive impairment and Alzheimer's disease: a resting-state fMRI study. Front. Aging Neurosci. 2019;11:326. doi: 10.3389/fnagi.2019.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Wang Z., Qin L., Wan J., Sun Y., Su S., Ding W., Xu J. Early altered resting-state functional connectivity predicts the severity of post-traumatic stress disorder symptoms in acutely traumatized subjects. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046833. e46833–e46833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data that is sharable (privacy etc.) will be made available upon request.