Abstract
The manipulation of cells and particles suspended in viscoelastic fluids in microchannels has drawn increasing attention, in part due to the ability for single-stream three-dimensional focusing in simple channel geometries. Improvement in the understanding of non-Newtonian effects on particle dynamics has led to expanding exploration of focusing and sorting particles and cells using viscoelastic microfluidics. Multiple factors, such as the driving forces arising from fluid elasticity and inertia, the effect of fluid rheology, the physical properties of particles and cells, and channel geometry, actively interact and compete together to govern the intricate migration behavior of particles and cells in microchannels. Here, we review the viscoelastic fluid physics and the hydrodynamic forces in such flows and identify three pairs of competing forces/effects that collectively govern viscoelastic migration. We discuss migration dynamics, focusing positions, numerical simulations, and recent progress in viscoelastic microfluidic applications as well as the remaining challenges. Finally, we hope that an improved understanding of viscoelastic flows in microfluidics can lead to increased sophistication of microfluidic platforms in clinical diagnostics and biomedical research.
Keywords: Viscoelastic flow, Elastic and inertial force, Microfluidics, Numerical modeling, Particle separation and cell sorting, 3D focusing
Subject terms: Physics, Engineering
Modeling microscale manipulation of biofluids
Insights into the dynamic behavior of biological fluids on the microscale are enabling more efficient analysis of cells, bacteria, and other small biological particles for research and clinical diagnostics. Blood, saliva and other biofluids have viscoelastic properties, which means that they exhibit both viscous and elastic behaviors depending on the forces to which they are subjected. These properties shape the migration behaviors of suspended bioparticles in unique ways. Jian Zhou and Ian Papautsky of the University of Illinois at Chicago have reviewed current progress in understanding the migration behaviors in such fluids within microfluidic devices. The authors discuss design principles that have enabled the development of microfluidic systems capable of separating and purifying cells, bacteria, and small vesicles from highly heterogeneous biological specimens, and highlight future challenges that need to be addressed.
Introduction
The emergence of microfluidics has triggered an increased interest in biological and healthcare applications1–3 and fueled the development of approaches to the sorting and isolation of synthetic and biological microparticles, including beads4,5, cells6,7, bacteria8,9, and extracellular vesicles (EVs)10,11. Numerous active and passive platforms12 have been demonstrated for precise and high-throughput manipulation of such microparticles. Magnetic13–15, electrical16–18, acoustic19–23 and optical24–26 forces are the most common principles for active microfluidic devices. These platforms generally offer precise, on-demand control of spatial distribution but require control of the forces used as well as sophisticated device architecture. Conversely, passive microfluidic platforms rely on biophysical properties, such as the size, density, shape and deformability of cells or particles. Some of the most prominent passive techniques are deterministic lateral displacement (DLD)27, pinched flow fractionation (PFF)28, hydrodynamic filtration (HDF)29, cross-flow filtration (CFF)30, shear-induced diffusion (SID)31–33 and inertial microfluidics (iMF)6,34–43.
While the majority of microfluidic systems are aimed at biological and clinical applications, most operate based on Newtonian fluid behavior. This is because in these systems, biological samples are diluted 5–100× and thus are no longer non-Newtonian. However, most unmodified biological samples, such as blood31, saliva44 and cytoplasm45, are viscoelastic in nature, making separation of cellular components within them challenging. Viscoelastic fluids are non-Newtonian and are generally macromolecular or feature complex microstructures, giving rise to unique phenomena such as bread dough climbing up a rotating rod (Weissenberg effect46). Neutrally buoyant particles suspended in such fluids migrate laterally in confined shear flows47, subject to imbalanced normal stresses that are strongly dependent on particle size.
When viscoelastic migration met microfluidics nearly a decade ago48, it triggered burgeoning interest in both the fundamental investigations of particle dynamics and the applications using size-based lateral migration. The ability to fabricate long and narrow microchannels (the ratio of channel downstream length to its characteristic length such as hydraulic diameter can easily exceed 1000× in microfluidic channels) enables experimental observation and probing of particle dynamics in viscoelastic flows, which otherwise mostly relies on numerical simulations47. Recent investigations have experimentally confirmed many of the complex interactions predicted by simulations, such as the inward driving force due to fluid elasticity48–51, outward directing effect of shear thinning52,53, and particle motion following secondary flows in straight square channels54,55. Improvements in the understanding of particle dynamics have resulted in the expansion of applications based on viscoelastic migration, such as the focusing and separation of bioparticles in viscoelastic microflows. Recent manipulations of macromolecules such as DNA56 and separation of submicron exosomes57 in viscoelastic microchannels push the limits of passive microfluidics and expand the dynamic range of microfluidic separations even further.
In this review, we aim to discuss the progress in viscoelastic microfluidics, bridging the physics of conventional viscoelastic fluids and the characteristics of microfluidic flows toward a broad range of applications based on particle cross-stream migration. The increasing number of publications in this burgeoning field has led to several review papers that include discussion of particle manipulation in viscoelastic flows. Lu et al.58 and by Yuan et al.59 reviewed applications of cell focusing and separation in microfluidic devices, while D’Avino and colleagues47,60 discussed the current understanding of particle dynamics in viscoelastic fluid, but mainly in a broad context of non-Newtonian fluids. We hope that this review will offer a more practical understanding of viscoelastic microfluidics and their applications in cell separations. Thus, we first discuss the basic underlying principles of viscoelastic microfluidics, including fluid elasticity, inertia, shear thinning, particle blockage ratio and secondary flow, as they collectively govern the particle dynamics in microflows. We will then examine the properties of viscoelastic fluids and their implications for suspended particles in confined shear flows to better understand particle dynamics in microfluidic channels. Particle migration in pressure-driven microchannels will be covered in inertialess viscoelastic flows (Re ≪ 1) as well as in more complicated situations when fluid inertia is nonnegligible (Re ≥ 1). We then discuss the three pairs of competing phenomena that dominate particle migration and the effects of channel geometry and particle properties. We will also provide a brief introduction to computational models used for predicting particle dynamics in viscoelastic flows. Throughout the review, we include boxes that describe fundamental and practical aspects of viscoelastic fluids. Finally, we will conclude the review with illustrative experimental results and applications of viscoelastic microfluidics, as well as the remaining challenges and outlook.
Viscoelastic fluids and suspended particles
Lateral migration of particles in viscoelastic flow stems from the interaction of these particles with their suspending viscoelastic fluid. Understanding fluid properties is thus critical to deciphering and predicting the migration of particles in viscoelastic flows. Although viscoelastic fluids are generally well studied on the macroscale, they are far less common in microfluidics. In this section, we discuss some of the remarkable behaviors of viscoelastic fluids, focusing on their differences from Newtonian fluids and their properties (e.g., first and second normal stress differences) that are behind such differences and are responsible for particle migration in viscoelastic flows.
Viscoelastic fluids
Viscoelastic fluids exhibit many differences in behavior from Newtonian fluids, such as the Weissenberg rod climbing effect46, which can occur even without rods61,62. Other pronounced phenomena in viscoelastic flows include extrudate swell62,63, large vortices and pressure drop when entering contraction geometry63,64, and melt fracture46,62. In microscale flows, viscoelastic fluids have been reported to exhibit (1) vortex formation at small Reynolds numbers (Re < 0.1) in a contraction microchannel65, (2) a longer required entrance length for full development of parabolic velocity profile and concave velocity profile observed during flow development66, (3) steady asymmetric flow patterns and unsteady 3D flow patterns in a T-junction microchannel67, and (4) suppression of vortex formation downstream obstructions in microchannels68.
The distinct behavior of viscoelastic fluids is attributed to their unique rheological properties (see Box 1). These fluids consist of both viscous and elastic components and thus behave like a viscous fluid in some circumstances and as an elastic solid in others46,63. Unlike the constant viscosity in Newtonian fluids (η, only dependent on temperature), the viscosity of viscoelastic fluids is typically a decreasing function of the shear rate due to the macromolecular nature62. This phenomenon is known as shear thinning and is found to drive outward migration of particles in viscoelastic flow52,69. The elastic behavior in these fluids is related to the normal stresses that are generated by the orientation and alignment of macromolecules along the flow direction63,70. It is the difference between these normal stresses that causes particle cross-stream migration in viscoelastic flow.
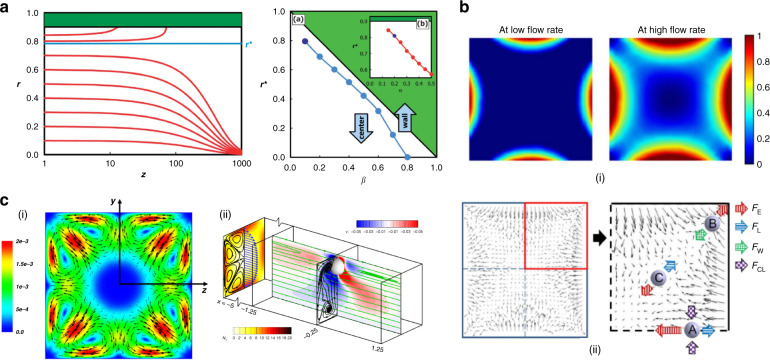
The normal stresses in viscoelastic flow are unequal, with two independent normal stress differences arising from the three normal stress components62. The first normal stress difference (N1) is defined as the difference between the streamwise normal stress (σxx) and the transverse normal stress (σyy)62,70. The coordinate system is set such that x is the downstream flow direction and y is in the direction of the gap (Fig. 1). Thus, N1 = σxx – σyy, which gives rise to the material property designated as the first normal stress difference coefficient: , where is the shear rate70,71. In a Newtonian fluid, N1 is zero, as normal stresses are the same in all directions. In viscoelastic fluids, N1 accounts for many aforementioned phenomena, including the Weissenberg rod climbing effect62, and it is one of the major forces responsible for particle migration in viscoelastic flows48.
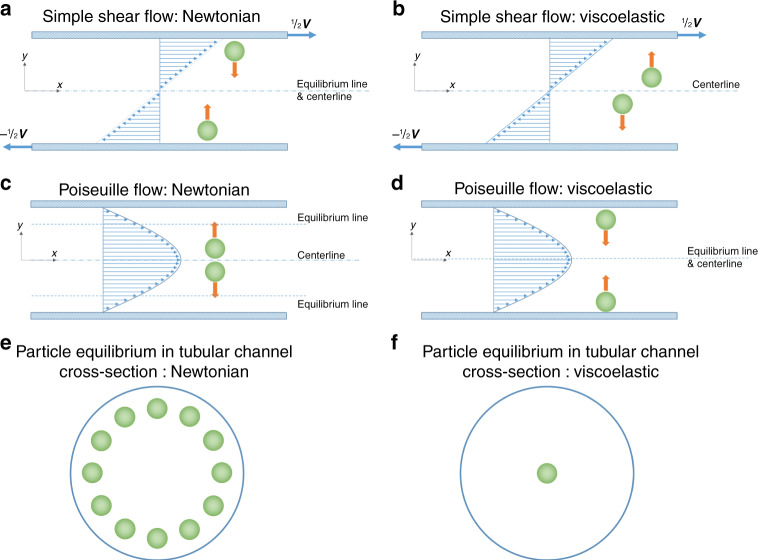
Fig. 1. Illustrations of particle dynamics in simple shear flow and Poiseuille flow.
In the case of simple shear flow, a particles migrate toward equilibrium positions at the centerline in Newtonian fluid dominated by inertial force86,87 but b migrate toward the walls in viscoelastic fluid84,85, regardless of particle initial position. c Particles laterally migrate to equilibrium positions near walls in Newtonian Poiseuille flow under the control of inertial forces86. d Particle laterally migrates to the centerline in viscoelastic Poiseuille flow undergoing elastic force47,69. e Particles equilibrate into an annulus near the sidewall of the circular channel in Newtonian flow undergoing inertial force. f Particles equilibrate into the center of the circular channel in viscoelastic flow dominated by elastic force
The other normal stress difference (N2) is defined as the difference between the transverse normal stresses (N2 = σyy – σzz), which can be viewed as a measure of the relative stretching of macromolecules in the velocity gradient direction (y direction) versus that in the z direction70. Similar to N1, N2 gives rise to another material property as the second normal stress difference coefficient: . Coefficients ψ2 and ψ2, together with η, are the viscometric functions that fully characterize a viscoelastic fluid in simple shear62,70. Although N2 is approximately an order of magnitude smaller than N1 and is much more difficult to measure62,72, it is responsible for the occurrence of secondary flow orthogonal to the axis flow in noncircular channels73–77. This secondary flow can impact particle focusing in viscoelastic flow54,55, which will be revisited in detail in the section “Particle migration in viscoelastic microfluidics”.
Additionally, fluid relaxation time (λ) is a key parameter required to evaluate the effect of fluid elasticity on particle migration in viscoelastic flow. The relaxation time is the characteristic time required for macromolecules of the fluid to relax from a deformed state to their equilibrium configuration78, and it cannot be directly measured79. There could be a spectrum of characteristic times in a real-world fluid79,80. In Newtonian fluids, λ = 0, as they do not exhibit any time-dependent effects70. A common method to derive the relaxation time is through a separate evaluation of fluid viscous and elastic responses to a shearing sinusoidal deformation, followed by observations of the intersection of the loss and the storage moduli79,81. Indirect determination of the relaxation time was also reported based on the particle migration in the microfluidic channel79.
Box 1. Newtonian and viscoelastic fluids in the context of particle migration.
Viscoelastic fluids exhibit many differences in behavior from Newtonian fluids, such as the Weissenberg rod climbing effect46. The distinct behavior of viscoelastic fluids is attributed to their unique rheological properties. These fluids consist of both viscous and elastic components, and thus behave as viscous fluid in some circumstances and as elastic solid in others46,63. While in Newtonian fluids the viscosity (η) is considered constant at any given temperature, in viscoelastic fluids viscosity is commonly a decreasing function of shear rate. This is known as shear thinning and is due to the macromolecular nature of viscoelastic fluids62. It is shear thinning that drives outward migration of particles in viscoelastic flow (see Box 3 for details)52,69.
The normal stresses in viscoelastic flow are unequal, which differs from Newtonian flows. Two independent normal stress differences (N1 and N2) can be formed from the three normal stress components62. The elastic force that drives particle lateral migration in viscoelastic flow mainly arises from the first normal stress difference (N1). N2 is known to cause secondary flow in noncircular cross-sections of microchannels, which tends to disperse (defocus) particles. A parameter named fluid relaxation time (λ) is required to evaluate the effect of fluid elasticity on particle migration in viscoelastic flow. This is the characteristic time required for the macromolecules of the fluid to relax from deformed state to their equilibrium configuration78. Key properties of viscoelastic and Newtonian fluids regarding particle migration are summarized in the table below.
| Newtonian fluid | Viscoelastic fluid | |
|---|---|---|
| Material function | η | η, ψ1, ψ2 |
| Normal stress differences | None | N1, N2 |
| Shear thinning | No | Yes (commonly) |
| Relaxation time (λ) | λ = 0 | λ > 0 |
| Driving factors responsible for particle lateral migration | Inertial force, drag force | Elastic force (N1), inertial force, shear thinning, N2-induced secondary flow, drag force |
Particles in viscoelastic fluid
Particles suspended in viscoelastic fluid migrate across the streamlines due to the imbalance of normal stresses. Both numerical simulations49,82–84 and experimental results84,85 have shown migration of particles driven by the fluid viscoelasticity in a simple shear flow (planar Couette flow). Particles near the centerplane migrate toward the closest wall, which is opposite of particle migration in Newtonian fluid (Fig. 1a, b)86,87. Particles migrate rapidly toward the wall before an abrupt decrease in migration velocity82,83. Normal stresses are responsible for the cross-streamline motion, and no migration was found in a purely viscous fluid when inertia is negligible (Re ≪ 1; see Box 2 for dimensionless numbers)83. Furthermore, wall confinement is necessary for migration83.
In planar Poiseuille flow, where curvature of velocity profile and shear rate gradient exist, the elastic force (Fe) stemming from the first normal stress difference (N1) drives particles toward the centerline where shear rate is lowest. This driving force depends strongly on particle size (Fe ~ a3). In contrast, fluid shear thinning forces particles to migrate toward the region of high-shear rate at the closest walls. The competition between the two factors suggests two stable equilibrium regions in the centerline and near wall. Lateral migration velocity, which is strongly dependent on particle size and fluid rheology, will also be discussed in this section.
Particle lateral migration has also been predicted and observed in Poiseuille flows (e.g., channel flow), which is more practical in the real world (Fig. 1c)69,88–91. Particle motion implicated by the imbalanced normal stresses is toward the low-shear-rate region within the flow47,88. In channel flow, the velocity gradient is not constant, and thus, the shear rate varies across the channel cross-section with the lowest in the channel centerline in a tube or in the central plane in a wide slit. As a result, particles are driven to the channel centerline or the centerplane under the control of fluid viscoelasticity. Tehrani91 confirmed that both the elastic property of the suspending fluid and the shear rate gradient are critical to the particle transverse motion. Despite the strong elasticity of the fluid, little migration in the flow with a central plug region or slip at the wall was observed in that work91.
When shear thinning of the viscoelastic fluid becomes relevant, particles are subjected to an additional tendency of moving to the high-shear rate region. Shear thinning causes particle migration in the opposite direction due to fluid elasticity. In channel flows, particles migrate toward channel walls instead of channel centerline if the shear-thinning effect is strong52,69. The migration behavior of particles is thus determined by the competing effects of fluid shear thinning and elasticity (Re ≪ 1). When shear thinning is excessively strong, particles migrate to the channel wall only; conversely, particles migrate to the channel centerline if elasticity is dominant47,69, such as in Boger fluids (elastic with constant viscosity)92. Sometimes, the term separatrix is employed to describe such competing effects on particle migration47,93. The opposing effects of fluid elasticity and shear thinning have been confirmed by direct numerical simulations in plane Poiseuille flow49,94 and demonstrated experimentally using polyvinyl pyrrolidone (PVP) and polyethylene oxide (PEO) solutions48,95. Nevertheless, shear thinning alone is insufficient to induce lateral displacement47. The simulation results by Huang and Joseph52 suggest that the shear-thinning effect is small when the inertia or shear rate is small.
Particle migration stemming from fluid viscoelasticity is strongly dependent on particle size48,91. Since the second normal stress difference (N2) is considerably smaller than N1, the driving elastic force (Fe) of particle migration can be assumed to mainly depend on N1. This elastic force is in the direction of the low-shear-rate region (e.g., channel center) and scales with the normal stress gradient as Fe ~ a3∇N148,91, where a is the particle diameter. By balancing this force with the Stokes drag (FD = 6πaηV) when shear thinning is negligible, the migration velocity (V) can be expressed as Further derivation51,96 shows that the migration velocity scales as
| 1 |
Consequently, the particle migration scales with the square of particle size and is dependent on the fluid rheological properties.
A similar expression considering both first and second normal stress differences shows dependence of the migration velocity on the particle size and fluid viscoelasticity. At a small Deborah number (e.g., De < 0.1) and small blockage ratio (e.g., β < 0.12; see Box 2 for dimensionless numbers), a dimensionless migration velocity (VM), which is the ratio of local migration velocity (V) to the mean flow velocity in the main flow (VM = Vr/V), can be derived as88–90,95,97
| 2 |
where ψ1,0 and ψ2,0 are respectively the first and second normal stress difference coefficients at zero shear rate, C ≅ 2, H is the low aspect ratio channel height and y is the vertical particle position (y = 0 is the central plane)47,88. Note that at large β, this expression may not hold, as Villone et al.93 found that all particles migrated toward the wall when β > 0.7. Although the second normal stress difference is generally small compared to N1, it induces recirculation orthogonal to the main flow in noncircular straight channels54,73–77,98–100. The intensity of such secondary flows in the channel cross-section is 2−3 orders of magnitude smaller than that of the main flow, but these flows may affect the particle migration54,55. The influence of the secondary flow will be revisited later in the context of microfluidic channel flow.
In this section, we have seen how viscoelastic fluids differ from classic Newtonian fluids. These differences are highlighted in Box 1. The normal stresses of viscoelastic fluids generate a set of complex viscometric parameters that fully describe these fluids in simple shear. These fluids and their flows can be characterized by nondimensional parameters, as shown in Box 2. All these can be directly applied to particles or cells suspended in such fluids. In the next section, we examine the impact of fluid viscoelasticity on particle migration.
Box 2 Dimensionless numbers in viscoelastic flows.
A group of nondimensional parameters used to characterize hydrodynamics and viscoelasticity of flows are summarized below. In addition to the commonly used Reynolds number (Re), the Deborah number (De) and the Weissenberg number (Wi) are used to characterize viscoelasticity of non-Newtonian flows. The former is the ratio of the fluid relaxation time to the time of observation (also denoted as fluid characteristic time); it describes the extent to which the response of a material to a deformation is viscoelastic rather than purely viscous181. The latter describes nonlinearity of rheological response and is the ratio of the elastic force (first normal stress difference) to the viscous force (viscous stress)47. For a given geometry, Wi and De are proportional to each other181 and are interchangeably used in some cases83,95,176. Note that both quantities are zero for Newtonian fluid as λ = 0. The elasticity number (El) indicates the relative importance of the elastic and inertial forces in shear flow46 and only depends on the fluid rheological properties and the characteristic length scale. When El ≫ 1, the fluid elastic force dominates, while the inertial stress is dominant when El ≪ 1. The two forces are considered comparable when 46. Additionally, blockage ratio is the ratio of particle size and channel characteristic length.
| Dimensionless parameter | Symbol | Definition | Expression |
|---|---|---|---|
| Reynolds number182 | Re | Ratio of inertial to viscous forces | |
| Weissenberg number47,181 | Wi | Ratio of first normal stress to shear stress/ratio of elastic to viscous force | |
| Deborah number47,181 | De | Ratio of fluid relaxation time to flow characteristic time | |
| Elasticity number46,144 | El | Ratio of Weissenberg number to Reynolds number/ratio of elastic over inertial forces | |
| Blockage ratio47 | β | Ratio between characteristic lengths of particle and channel |
ρ fluid density, V average flow velocity, Dh hydraulic diameter, Dh = 2WH/(W + H), where W and H are the channel width and height, respectively, η fluid dynamic viscosity, N1 first normal stress difference, λ fluid relaxation time, shear rate, tc flow characteristic time, a particle diameter
Particle migration in viscoelastic microfluidics
Particle migration in microfluidic channels has been exploited for flow focusing and sorting devices in the past decade due to the strong size dependence of particle migration in viscoelastic fluids, as indicated by expressions (1) and (2)48,50,56,95,101–103. Considering the relatively small lateral migration velocity of particles, which is generally 2−3 orders of magnitude slower than bulk flow47, dynamics of particle migration are readily observed in microfluidic devices due to their microscale characteristic lengths56. Factors that collectively determine particle migration and equilibration are discussed in this section, including the influence of fluid inertia, elasticity, rheology and channel geometry (see Box 3). Specifically, fluid inertia vs. elasticity, migration induced by the first normal stress difference (N1) vs. secondary flow induced by the second normal stress difference (N2), and elasticity vs. shear-thinning effect are identified as the three pairs of competitors that mainly govern the particle dynamics within microfluidic channel flows (Fig. 2). Thus, we next briefly discuss particle migration in the purely inertial flow before focusing on the two cases of viscoelastic fluid—migration in purely (inertialess) viscoelastic flows and migration in elasto-inertial flows.
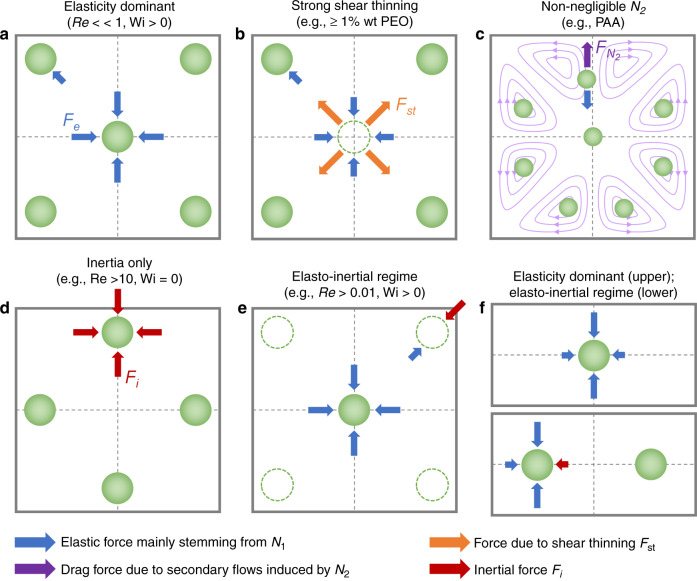
Fig. 2. Particle migration and focusing governed by forces stemming from fluid elasticity, inertia, shear thinning, and their interactions in channels with square and rectangular cross sections.
a Five focusing positions dominated by elastic force (Fe) in inertialess viscoelastic flows without the shear thinning effect (e.g., Re < 0.01 and Wi > 0). b Shear thinning of fluid leading to defocusing of particles in the center and leaving four focusing positions near the corners. Here, Fst represents the effect of shear thinning driving particles toward the walls. c Strong secondary flows induced by the second normal stress difference (N2) causing dispersion of small particles. Here, FN2 represents the drag force acting on particles due to secondary flows. The dispersion of particles due to secondary flows induced by N2 was reported experimentally55. However, positions in the centers of vortices were suggested only in simulations54 and have not been experimentally observed. d Four focusing positions resulted from the balance of inertial forces (grouped as Fi) in Newtonian inertial flows (e.g., 10 ≤ Re ≤ 100 and Wi = 0). e Interaction of elastic force (Fe) and inertial force (Fi) leading to elimination of the corner positions in viscoelastic flows with nonnegligible inertia (e.g., Re > 0.01 and Wi > 0). f Single focusing position and two positions in rectangular channels depending on the interaction of elastic and inertial forces. When the inertia is small (e.g., low flow rate), a single focusing position is present in the center; when the flow rate increases, two positions emerge as inertia becomes relevant
Box 3 Phenomena that govern particle migration in microfluidic channels.
Forces responsible for particle migration in microfluidic channels stem from fluid inertia, elasticity and rheology. In Newtonian channel flows, neutrally buoyant particles are known to migrate to their preferential positions near the channel wall, driven by inertial force (Fi)34. Shear-induced lift force (Fs), wall-induced lift force (Fw) and particle rotation-induced lift force (FΩ) are accounted for focusing of particles in inertial channel flows. In curved channels, Dean force (FD) due to secondary flow becomes significant. In viscoelastic flows, elastic force (Fe) which mainly stems from the first normal stress difference (N1) dominates particle migration when inertia is negligible (Re ≪ 1)48. Similar to Dean force, secondary flows induced by second normal stress difference (N2) give rise to a drag force (FN2) that causes particles to follow the secondary flows in channel cross-sections54,55. Shear thinning of fluid rheology results in reduced viscosity of the fluid at high-shear rate, which increases inertial force and drives particles toward channel wall. We denote shear thinning effect on particle migration as Fst. Shear thinning tends to defocus particles51. Characteristics of these forces on particle migration are listed in the table below.
| Source | Force or effect | Acting direction on particle migration |
|---|---|---|
| Fluid inertia | Shear-induced lift force (Fs) | Up shear rate gradient (toward wall) |
| Wall-induced lift force (Fw) | Away from wall | |
| Dean force (FD) | Follow the secondary flows in curved channels | |
| Fluid elasticity | First normal stress difference N1 (elastic force Fe) | Down shear rate gradient (away from wall) |
| Second normal stress difference N2 | Follow the secondary flows in noncircular cross-sections | |
| (inducing drag force FN2) | ||
| Fluid rheology | Shear thinning | |
| (inducing inertial force Fst) | Toward wall |
Inertial migration in Newtonian flows
Neutrally buoyant particles suspended in a Newtonian fluid are known to migrate to their preferential positions near the channel wall. Spontaneous formation of the particle annulus near the pipe wall (Fig. 1e) was first observed by Segré and Silberberg104,105 more than 50 years ago. Extensive investigations have been implemented to explore the mechanism of this intriguing phenomenon86,106–111. Similar to the particle migration in viscoelastic fluids, wall confinement and the shear gradient are among the key factors that induce inertial lift force responsible for such particle migration in Newtonian channel flows (Fig. 2d).
Zhou and Papautsky34 described a two-stage model for particle migration at moderate Reynolds numbers (10 ≤ Re ≤ 100), which predicts particle dynamics in channels with various cross-sectional geometries (Fig. 3a, b). The shear-induced lift force (Fs), wall-induced lift force (Fw) and particle rotation-induced lift force (FΩ) account for the particle migration in the model. The balance between the first two forces is responsible for the formation of the Segré–Silberberg annulus in a pipe flow (Fig. 1e), and it is also the premise to substantiate the importance of the third force, which is typically an order of magnitude smaller than Fs34. Particles undergoing Fs rapidly migrate toward the channel wall, where Fs is counteracted by the arising Fw. In radially asymmetric channels, such as square and rectangular channels, the small rotation-induced lift force thereafter drives the particles toward the centers of each wall. As a result, four stable equilibrium positions can be observed in the square microchannel (Fig. 2d). Similarly, two stable positions can be formed in the centers of long walls in rectangular channels due to the differentiated velocity profiles along the two cross-sectional axes. The unstable positions in the corners of the square channel and those in the rectangular channel become preferential at high Re112,113. Particle focusing in various inertial channels is summarized in the review by Martel and Toner39. It is clear that the shear-induced lift force acts on the opposite direction of the elastic force, whereas the wall-induced lift force is in the same direction as the elastic force if they are present in a viscoelastic flow.
Fig. 3. Two-stage migration of particles in inertia-dominant Newtonian flow and various focusing patterns in different inertial microchannels.
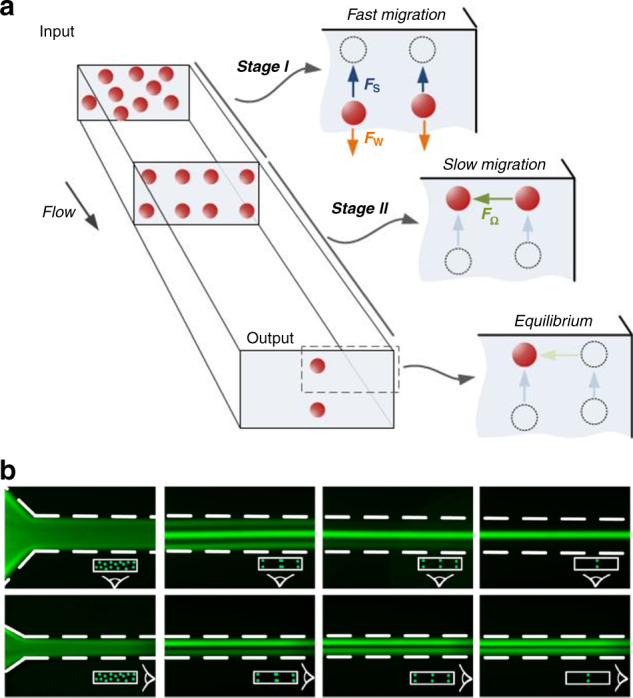
a Fast migration of particles toward channel walls in the first stage dominated by shear-induced lift force (Fs) and slow migration toward the stable positions in the second stage undergoing rotation-induced lift force (FΩ)34. Reproduced with permission from ref. 34. Copyright © Royal Society of Chemistry. b Fluorescent images of particle migration inside a rectangular channel validating the two-stage migration128. Reproduced with permissions from ref. 128. Copyright © AIP Publishing
As inertial forces are highly size-dependent and applicable at high flow rates, they have been widely exploited for particle and cell manipulation in microfluidic devices6,7,35,40,114–134. Many applications in a variety of areas, such as size-selective inertial sorting35,135, high-throughput cell filtration125,136, microfluidic flow cytometry137 and isolation of rare cells (e.g., circulating tumor cells)6,40,138, have been proposed and demonstrated in the literature. Many other properties of inertial manipulation systems, including the channel layout, cross-section geometry, particle shape and deformability, have also been explored for improved performance and diverse applications. More details on inertial mechanisms and applications can be found in reviews by Martel et al.39, Amini et al.38 and Zhang et al.42.
Particle migration in inertialess viscoelastic flows
At low Reynolds numbers (Re ≪ 1), inertial effects are decoupled, and it is possible to investigate particle migration under the influence of fluid elasticity alone (El ≫ 1). Indeed, the majority of the existing numerical simulation literature does this by assuming a negligible Reynolds number47,82,84,93,100,139. Experimentally, this is readily accessible, as viscoelastic fluids generally exhibit high viscosity, which yields small Reynolds numbers and limits fluid inertia effects. Under elastic force, particles migrate to the lower shear region of a microchannel cross-section, the axis of a circular channel or the centerplane in a wide rectangular channel48,56,84,95,101,140. Table 1 summarizes recent experimental studies that employ fluid elasticity for particle or cell manipulation within straight microfluidic channels. Leshansky et al.48 first observed the lateral migration and focusing of 8 µm diameter polystyrene particles in PVP solution (see Box 4 for common macromolecules used as elasticity enhancers in microfluidic systems and Box 5 for their rheological properties) toward the centerplane in their wide channel with 45 µm characteristic height (β = 18%). The focusing of particles becomes more pronounced with increased flow rate, which is in agreement with simulation results from planar Poiseuille viscoelastic flow69,93.
Table 1.
Summary of recent viscoelastic microfluidics for passive focusing and separation in straight channels (units are converted, and values not shown in original work are calculated based on available parameters. Not all channel geometries or particle sizes in original references are included in this table, but the optimal numbers may be selected here. Shear rates were calculated using in rectangular channels and in tubular channels (D is the diameter). All blockage ratios were calculated based on the available parameters, and only PS particles were used to calculate β whenever available. Asterisk marker (*) indicates that the value was used to calculate or was relevant to calculations of dimensionless numbers)
| Mode | Dimensionless numbers | Sample flow rate (µl/h) |
Shear rate (s−1) |
Channel dimensions | Target particle | Carrier fluid or elasticity enhancer | Remarks | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Re | Wi | El | β | Width (µm) | Height (µm) | Length (mm) | Type | Size (µm) | ||||||
| Elasto | 0.00158 | N/A | N/A | 0.11, 0.18 |
400–2000 100–2000 |
109–549 27.4–549 |
1000 | 45 | 20 | PS | 5, 8 |
PVP PAA |
Particle Re 5 × 10−5 |
48 |
| Elasto | ~0.001 | 0.008–0.8 | N/A | 0.08 | 0.45–45 | 2.55–255 | 50 µm (microtube inner diameter) | 45 | PS | 4 | PVP*, PEO |
Tube length 10 cm |
95 | |
| Elasto |
0.0037 0.016 |
0.36 0.4 |
98 25 |
0.11 0.10 |
60 534 |
267 297 |
50 100 |
50 100 |
50 | PS |
5.8 10 |
PVP | Optimal values | 101 |
| Elasto |
0.11–0.33 0.0015–0.015 |
178–533 0.18–1.8 |
1618–1615 120 |
0.02–0.2 |
5–15 N/A |
2.2 × 106−6.7 × 104 20–200 |
5 30 |
5 10 |
40 |
PS DNA |
0.1–1 0.69, 1.5 |
PEO | No focusing for 0.1 µm | 56 |
| Elasto | 1.74 | 263 | 151 | 0.16 | 300 | 1.5 × 104 | 30 | 50 | 10 | PS | 4.8 | PEO | High sheath flow rate | 162 |
| Elasto | 0.03–0.74 | 0.44–11 | 14.9 | 0.02–0.1 | 1200–30,000 | 31.4–786 | 300 (microtube inner diameter) | 300 | PS | 7, 15, 30 | PVP | Long channel | 51 | |
| Elasto |
0.035–2.3 0.018–7.1 |
7.9–507 4.0–1580 |
226–220 222–223 |
0.11 0.21 |
10–640 5–2000 |
57–3622 28−1.1 × 104 |
50 µm (microtube inner diameter) | 40 | PS |
5.8 10.5 |
DNA based |
Microtube, Sheath for chromatography |
163 | |
| Elasto | 3.57 × 10−6−1.78 × 10−3 | 0.002-0.93 | 522–560 | 0.11, 0.23, 0.35 | 0.06–30 | 0.533–267 | 43 µm (microtube inner diameter) | 30 |
PS RBCs |
5, 10, 15 N/A |
PVP | Two segments | 139 | |
| Elasto-deformability |
(1.2–9.9) × 10–3 (3.3–20) × 10−3 |
0.08–0.67 0.1–0.59 |
67.7 30.1 |
0.12 |
20–160 80–480 |
88.9–711 105–632 |
50 75 |
50 75 |
40 |
PS RBCs WBCs |
6 N/A N/A |
PVP | Deformability, separation purity >98% | 102 |
| PFF viscoelastic | 0.3–0.6 | N/A | N/A | 0.22, 0.35, 0.21 | 20 | 890–1740 | 66 | 63 | 20 | MV3, MCF, Hep G2 |
14 22 13 |
PBS/dextran PBS/RBC, whole blood |
Varying Sheath flow | 149 |
| Elasto-inertial PFF | 0.51−3.4 | 21.7−145 | 42.5* | 0.06−0.4 | 15−100 | 1968−13,125 | 50 | 40*,100, 25 | 20 | PS | 3.1, 9.9 | PEO | Sheath flow involved | 170 |
| Elasto-inertial PFF | 0.2−1.35 | 3.81−25.4 | 18.8* | 0.07−0.4 | 3−20 | 1120−7822 | 50 | 25*, 100, 40, 15 | 20 | PS | 4.18, 3.5−6 | PEO | Peanut-shaped | 161 |
| Elasto-inertial | 0.07−0.37 | 1.6−8.04 | 22.9-21.7 | 0.048, 0.12 | 40−200 | 178−1132 | 50 | 50 | 40 | PS | 2.4, 5.9 | PEO*, PVP | 3D in PEO | 50 |
| Elasto-inertial |
0.5 0.81 |
12.84 12.7 |
25.68 15.7 |
0.02, 0.1 | 270 | 1200 | 50 | 50 | 25 |
PS RBCs |
1, 5 8 |
PEO | Buffer for separation | 147 |
| Elasto-inertial |
0.69 4.17 |
152 146.7 |
220 35.2 |
0.1−0.3 |
100 3000 |
1.4 × 104 6.7 × 103 |
40 100 |
10 50 |
30 50 |
PS, E. coli, RBC PS, MCF, RBC |
1, 3 ~1 8 5, 15 18 8 |
PEO | Separation achieved; large by side/small in center | 103 |
| Elasto- inertial |
0.02−1.11 0.01−5.56 |
4.9−247 1.2−60 |
245−222 120−10 |
0.1, 0.17 | 1.2 × 103−6 × 104 | 24.7−1234 | 300 | 300 | 300 | PS | 30, 50 |
PVP PEO |
Shear thinning | 96 |
| Elasto-inertial |
0.21−8.55 0.07−9.97 0.06−10.16 |
2.43−97.7 0.45−63.7 0.29−52.7 |
11.6−11.4 6.43−6.39 5.2−4.8 |
0.04−0.2 |
60−4800 60−8400 60−10,800 |
267−21,333 133−18,666 89−16,000 |
50 100 150 |
50 | 40 | PS | 10, 5, 2 | PEO*, PVP | Multiple stream focusing | 143 |
| Elasto-inertial | 105−4422 | 17−566 | 0.16−0.13 | 0.01− 0.1 | 3.6 × 104−1.2 × 106 | 3.9 × 104−1.3 × 106 | 80 | 80 | 35 | PS | 1, 3, 6, 8 | HA | WBC deformed | 151 |
| Elasto-inertial | 2.5−34.5 | 1.5−12.2 | 0.35−0.60 | 0.08−0.4 | 3 × 103−2.4 × 104 | 5.9 × 103−4.7 × 104 |
25 70 |
250 | 40 |
PS, parasite, WBC |
2, 10 1.5−2 9−15 |
HA | Two stages; separation achieved | 171 |
| Elasto-inertial | 17−29 | 11−88 | 0.4−6.1 | 0.05−0.1 | 300 | 2.5 × 106−5.5 × 106 | 20 | 50 | 15 | PS, bacteria, platelets | 1−2 | PEO | Viscoelastic flow as buffer | 166 |
HA hyaluronic acid, PVP poly(vinyl pyrrolidone), PEO poly(ethylene oxide), PAA polyacrylamide, RBC red blood cell, WBC white blood cell, PS polystyrene particles, FFF field flow fractionation, PFF pinched flow fractionation, N/A not available or not able to be calculated
Particles migrate toward the center of the circular channel cross-section due to fluid elasticity when the fluid inertia is negligible. D’Avino et al.95 and Seo et al.51 demonstrated particle focusing in a stable equilibrium position in the tube axis with Re ≪ 1 and β ≤ 10% (Fig. 1f). Enhanced focusing quality was found with an increasing flow rate (higher Wi or De), as shown in Fig. 4a. Romeo et al.97 proposed a dimensionless parameter Θ that accounts for the fluid rheology, flow rate, channel geometrical parameters and particle size. They found that particle focusing occurred within tubular microchannels when Θ > 1. Their prediction using Θ was valid in the limit of low elasticity (De < 0.05), negligible inertia (Re < 0.0005) and a certain blockage ratio (0.01 ≤ β ≤ 0.3)97.
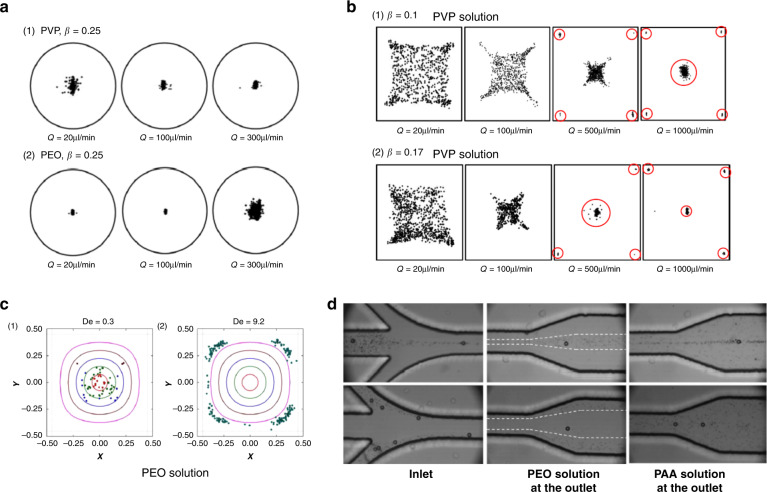
Fig. 4. Particle dynamics in viscoelastic microchannels with negligible inertia.
Particle focusing into the micropipe axis in PVP fluid with negligible inertia and particle defocusing in PEO solution due to the shear thinning effect at a high flow rate51. The channel length is 30 cm. Reproduced with permission from ref. 51. Copyright © Royal Society of Chemistry. b Four focusing positions near the corners in addition to channel axis in square microchannel and effect of particle blockage ratio on focusing in 8% PVP solution96. The channel length is 30 cm. Reproduced with permission from ref. 96. Copyright © AIP Publishing. c Four focusing positions near corners of a square channel due to strong shear thinning (1.6% wt PEO)53. The channel length is 8 cm. Reproduced with permission from ref. 53. Copyright © Springer Nature. d Effect of secondary flow on particle migration due to second normal stress difference in PAA solution (strong second normal stress difference N2) compared to the migration in PEO solution.55 Reproduced with permissions from ref. 55. Copyright © Springer Nature
Unlike the single focusing position in microtubes, multiple equilibrium positions exist within noncircular cross-sections of microchannels (Fig. 2). Since microfluidic channels with rectangular cross-sections are the most common, benefiting from soft-photolithography fabrication methods141,142, many researchers have probed particle migration dynamics in such channels50,56,96,101–103,143–145. As the driving force due to fluid elasticity (elastic force Fe) is directed down the shear rate gradient47, particles are expected to migrate to the centerplane of a rectangular channel with an excessively low aspect ratio (AR, the ratio of cross-sectional height and width). Particles were found to concentrate on the middle plane in a slit with a small aspect ratio (AR = 0.045)48. As the AR increases (e.g., 0.25 < AR < 0.33), the focused band shrinks as a result of the altered shear rate gradient along the width103,143. Kim et al.56 used a similar rectangular channel for focusing DNA molecules (AR = 0.33 and Re ≪ 1).
In channels with square cross-sections (AR = 1), five distinct focusing positions emerge. Four equilibrium positions in the corners were observed in addition to the single position in the center of the cross-section (Fig. 2a)50,56,96. The presence of these additional positions is the result of the reduced shear rate in the corners50. Therefore, the migration direction depends on the initial positions of particles within the cross-section54,100. Without inertia, shear thinning and secondary flow effects, particles initially closer to the corners are attracted to the four positions, while other particles are driven by the elastic force to the position in the center. Interestingly, Del Giudice et al.101 observed only a single focusing position at the center in their square channel. The authors attributed this discrepancy to the surface property of their channel, but it is likely a result of the limited number of events recorded, as Seo et al.96 found a small number of particles in the corners (Fig. 4b) where more events were analyzed. Nevertheless, corner positions can be eliminated by introducing inertial forces, which will be discussed later50.
Contrasting the effect of fluid elasticity on particle migration, shear thinning of the fluid causes particle migration toward the channel wall in viscoelastic flows (Fig. 2b). Simulation results suggest that this effect becomes more significant when fluid elasticity is stronger (e.g., De > 2.5)49,52. The competition between shear thinning and fluid elasticity leads to an unstable separatrix between the channel centerline and wall47,92. With negligible inertia, particles initially located between the separatrix and channel wall migrate toward and equilibrate near the wall; particles on the other side of the separatrix are focused into the centerline. A strong shear-thinning effect moves the separatrix toward the channel centerline, leading to more particles migrating laterally to the wall47,52. Therefore, in square channels, the stable position in the center may disappear, leaving only the four positions in the corners when shear thinning is strong (Fig. 2b)53. The separatrix is close to the wall at weak shear thinning, and all particles migrate to the centerline47,52. As a result, particles may migrate bidirectionally under the influence of shear thinning, leading to particle focusing in both the channel centerline and the wall. This pattern of particle focusing was observed in a microtubular flow with a shear-thinning PEO solution (β = 0.16, De = 0.03, 1% wt PEO)95.
Shear thinning can also cause particle defocusing from the position in the channel center. As the flow rate increases, particles were found to be focused tighter and underwent stronger elastic forces in a viscoelastic flow without shear thinning (PVP solution in Fig. 4a). In contrast, particles were dispersed from the focusing position in the cross-section center (Fig. 4a) as an increased flow rate led to the onset of shear thinning in the PEO solution51. Similar observations were obtained in a square microchannel. When shear thinning was weak (De = 0.13), particles were focused mostly in the center position with some in the corner positions53. However, all particles were near corners, and no particle in the center was observed when shear thinning was very strong (De = 9.2 in PEO solution, as shown in Fig. 4c)53. Additionally, when inertia becomes comparable to elastic force, shear thinning leads to defocusing of particles from the channel centerline, and particles tend to move to the regions near the center of each wall96. For the commonly used PEO solution, shear thinning is weak when the PEO concentration is less than 2500 ppm144.
Similar to the interplay between shear thinning and fluid elasticity, migration due to N2-induced secondary flow can compete for elastic migration due to N1, leading to altered focusing dynamics within microchannels54,55. In noncircular channels, the second normal stress difference N2 is known to induce secondary flow perpendicular to the main flow direction73,75. For instance, in a square microchannel, a nonnegligible N2 induces eight recirculating vortices in the cross-section (Fig. 2c), and various recirculation secondary flows can also be generated in a rectangular microchannel depending on its aspect ratio73,75. The recirculation goes from the high-shear region to the low-shear region along the wall. Particles in the microchannels with noncircular cross-sections may follow the secondary flows undergoing drag force (Fig. 2c).
While elastic force focuses particles into equilibrium positions, N2-induced secondary flow tends to disperse particles. In a 3D simulation with negligible inertia, N2-induced secondary flow was found to affect the particle migration dynamics in a complex way depending on the blockage ratio and Deborah number54. For small particles (e.g., β = 0.1) and a large Deborah number (De = 2), the particle migration velocity due to the secondary flow surmounts the velocity owing to the elastic force stemming from N1. Consequently, particles follow the cross-sectional vortices, suggesting additional possible focusing positions in the eight vortices (Fig. 2c)54,146. This effect diminishes when De decreases (e.g., De < 0.5) or the particle size increases (e.g., β = 0.15)54. For a large particle, the migration due to elastic force is dominant and involves focusing into the centerline. These analytical results have been mostly validated by recent experiments in a rectangular microchannel (AR = 0.5) using PEO and polyacrylamide (PAA) solutions (Fig. 4d)55. N2 is not present in the PEO solution, but it is not negligible in the PAA solution. According to the confocal and microscopic images, smaller particles (β = 0.02) followed the secondary flow and could not be focused after introducing from the channel centerline in PAA solution. Conversely, larger particles (β = 0.2) remained stable in the centerline. Particle dispersion was not observed in the PEO solution under similar conditions. Based on this observation, separation of these two particles was also demonstrated by injecting the mixture slightly offset from the channel centerline in PAA solution55.
Box 4 Common macromolecules used as elasticity enhancers.
Macromolecules commonly used in microfluidic systems are listed in the table below. All of these materials exhibit shear thinning behavior, with PEO exhibiting the strongest shear-thinning95. PAA has a nonnegligible second normal stress difference55. Additionally, DNA sometimes can also be used as elasticity enhancer163. In terms of fluid rheology, either stronger or weaker, shear thinning can be achieved by varying amount of these macromolecules53. In fact, Boger fluids can also be approximated by mixing a small amount of high-molecular-weight polymer into a fluid with a relatively high viscosity70; such fluids show minimal change in viscosity with respect to shear rate in extended range but simultaneously exhibit distinct normal stresses, suggesting elastic behavior. Apart from mixing with water, these macromolecules are also commonly mixed into glycerol−water medium, which increases viscosity and thus reduces the Reynolds number (see Box 5 for details).
| Macromolecule | Abbreviation | Molecule weight (Mw) | Typical concentration | Cells suspended |
|---|---|---|---|---|
| Poly(vinyl pyrrolidone) | PVP | 3.6 × 105 g/mol48,50,51,95,96,101–103,139,176 | 5−8% wt | hMSC, RBC, WBC |
| Poly(ethylene oxide) | PEO | 2 × 106 g/mol50,56,147,161,162,170 | 0.05−0.3% wt | MCF-7, RBC, E. Coli, DNA |
| 4 × 106 g/mol51,95,96,103 | ||||
| Polyacrylamide | PAA | N/A48 | 0.0045% wt | Microparticles |
| Hyaluronic acid | HA | 1.65 × 106 g/mol151 | 0.1% w/v | WBC |
| 0.357 × 106 g/mol151 |
RBC red blood cell, WBC white blood cell, hMSC human mesenchymal stem cell
Box 5 Rheological properties of common viscoelastic solutions used in microfluidics.
Polymer elasticity enhancers including PEO, PVP and PAA are usually mixed with water or glycerol. Glycerol is used to enhance the viscosity and thus reduce fluid inertia. Rheological properties of these prepared solutions are dependent on the concentration of the polymers used. Note that long time storage could reduce or eliminate shear thinning effect of some polymers. For example, little shear thinning was observed for PEO solution stored at room temperature without light for 3 months103. The table below shows the rheological properties of the aforementioned molecules with common concentrations at 20 °C. This table is reproduced with permission from ref. 183.
| Properties | PEO concentration (ppm) | PEO/glycerol (wt %) | PVP (wt %) | PAA (ppm) | |||
|---|---|---|---|---|---|---|---|
| 500 | 1000 | 2000 | 15 | 45 | 8 | 50 | |
| Density ρ (g/cm3) | 1.0 | 1.0 | 1.0 | 1.03 | 1.10 | 1.05 | 1.0 |
| Zero-shear viscosity η0 (mPa·s) | 1.8 | 2.3 | 4.1 | 2.96 | 9.03 | 140 | 1.8 |
| Effective relaxation time λe (ms) | 4.3 | 6.8 | 10.6 | 11.0 | 24.0 | 2.3 | 10 |
PEO Mw = 2 × 106 g/mol; PVP Mw = 3.6 × 105 g/mol; PAA Mw = 18 × 106 g/mol
Elasto-inertial migration in viscoelastic flows
Thus far, we have discussed the effects of fluid inertia and elasticity on particle migration as mutually exclusive conditions. Complex migration dynamics can be expected when both are present in a viscoelastic fluid, and termed elasto-inertial migration (e.g., Re > 0.01 and Wi > 0). Coupled with the effects of shear thinning, particle blockage ratio and channel cross-sectional geometry, inertial and elastic forces act on particles synergistically in some scenarios, while antagonistically in others, dictating lateral migration of particles within microfluidic channels.
The synergistic effect of fluid elasticity and inertia promotes particle lateral migration necessary to achieve a single position focusing in square microchannels (Fig. 2e)50,96,147. Although inertia is negligible at a small Reynolds number (Re ≪ 1), its effect on particle migration becomes pronounced and serves to eliminate the otherwise stable positions in the corners of a square microchannel when Re is increased to approximately unity (e.g., 0.01 < Re < 10). In particular, the wall-induced lift force (Fw) displaces particles away from the corner positions. Subsequently, elastic force (Fe) drives them toward the channel centerline where the only stable position exists (Fig. 2e). Although the shear-induced lift force acts in the opposite direction of Fw and Fe, the latter two are dominant when Re is small50. Considering the strong size dependence of the wall-induced lift force () near the wall region148 and the predominant elastic force at a high elasticity number (e.g., El = 21), large particles can be focused into the cross-section center undergoing the synergetic interaction of the two forces, which has been demonstrated in microfluidic devices (Fig. 5a)50,147.
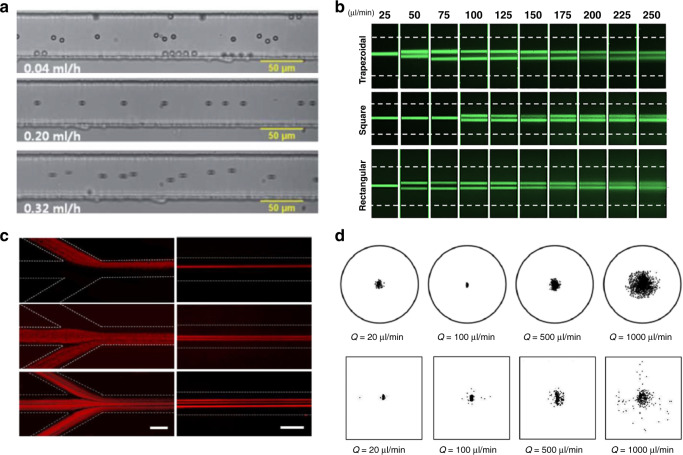
Fig. 5. Particle dynamics in viscoelastic microfluidics with effects of inertia and shear thinning.
a Inertial force necessary to eliminate the four corner positions in the square channel. A single stream of particles was observed at an increased flow rate where inertial force was sufficient to repel particles from four corners50. The channel length is 4 cm. Reproduced with permission from ref. 50. Copyright © Royal Society of Chemistry. b One and two streams observed in rectangular, square and trapezoid channels when both elastic and inertial effects are present145. The channel length is 5 cm. Reproduced with permission from ref. 145. Copyright © AIP Publishing. c One, two and three focused streams observed in the rectangular channel144. The channel length is 4 cm. Reproduced with permission from ref. 144. Copyright © 2017, American Chemical Society. d Defocusing of particles observed in both circular micropipes (β = 0.1)51 and square microchannels (β = 0.17)96 when the shear thinning effect was strong (1% wt PEO). The channel length is 30 cm. Reproduced with permission from ref. 96. Copyright © AIP Publishing
Unlike the synergetic interaction of Fw and Fe, the shear-induced lift force (Fs) counteracts the elastic force (Fe) when particles are away from the channel wall. This competing effect is responsible for the varying focusing positions observed in the centerplane of rectangular channels (AR < 1)103,144,145. Dissimilar to a wide band in the centerplane observed in inertialess viscoelastic flow, the band evolves into 1, 2 and 3 focused streams depending on the blockage ratio and channel AR when inertia is not negligible in PEO solutions (Fig. 5b, c)103,144,145. The shear-induced lift force drives particles in the centerplane horizontally toward the two sidewalls, where it is balanced by a horizontal elastic force due to the large shear rate gradient. The balance of these two forces gives rise to two focused positions in the centerplane but near sidewalls (Fig. 2f). Increasing the fluid elasticity (larger El) brings the two focused positions closer to each other144. A further increase in El leads to the merging of the two positions into a single position in the center144. The mechanism of the presence of three focused streams remains to be determined.
Although the PEO solution and its shear-thinning effect are commonly used to eliminate corner focusing positions in square channels, this effect leads to particle defocusing in the center position at a high-shear rate (Fig. 5d). Seo et al.51,96 observed that particles (β = 0.05 and β = 0.1) started to defocus in 1% (wt) PEO solution when the flow rate exceeded 100 μL/min in their 300 μm diameter microtube (). This is due to shear thinning becoming more pronounced when the shear rate is larger144, which displaces particles toward the wall in viscoelastic flows, as already discussed52,146. Shear thinning reduces viscosity (η ≈ 0.33 pa s at , and η ≈ 0.42 pa s at )51,96, leading to increased Re. Nonnegligible inertial forces at higher Re start competing with elastic force at high-shear rate. For the PEO solution, shear thinning is weak when the concentration is below 0.25% (wt) in water but becomes significant when the concentration is higher144. Furthermore, when inertia is relevant, strong shear thinning at a high flow rate reduces fluid viscosity and thus effectively amplifies the Reynolds number, which results in an increased inertial force (Fs) that drives particles away from the centerline.
The particle blockage ratio also influences the focusing pattern in a square channel96. Larger particles (β = 0.17 and β = 0.25) were found to show better resistance to the wall-pointed effect of shear thinning than smaller particles (β = 0.1)51,96. Interestingly, the focusing pattern observed for the smaller particles (β = 0.1) at a low flow rate is close to the combination of inertial focusing in a Newtonian fluid and elastic focusing with shear thinning, with four positions close to the centers of each wall and one position in the channel centerline96. The results from multiple simulations52,93 also suggest different migration dynamics for varying blockage ratios. However, the prediction that particles always migrate to the channel wall when the blockage ratio exceeds a critical value (β = 0.25 in the work by Huang et al.52) differs from experimental results that showed no particle focusing near the wall when β = 0.25 51. Similarly, particles139 and cells149 were found to migrate toward the channel centerline even when β = 0.35. Simulation results by Villone et al.93 suggest that the critical value should be ~0.75.
When the inertia and fluid elasticity are comparable (El ~ 1), elastic force is found to be dominant in particle focusing dynamics. Recent 2D simulations150 suggest that particle migration is dominated by fluid viscoelasticity in this case and that the inertia is negligible. The domination of elastic force has been confirmed experimentally by Lim et al.151 in a weakly viscoelastic flow at El ≈ 0.15. Particles with a diameter of 8 µm were focused at the centerline of a square microchannel in a hyaluronic (HA) solution at Re up to 4422 (Wi = 556). Nonetheless, the absence of particles in the corners implies that inertial forces are not negligible. Similar results can be found in the work by Del Giudice et al.53, where particle migration toward the centerline was observed at both El ≈ 0.4 and El ≈ 40. These results, along with simulations, suggest that particle migration is dominated by elastic force when . These results show the inability of using the elasticity number alone for the prediction of particle migration dynamics. The 2D simulations150 suggest that Wi has to be at least two orders of magnitude smaller than Re for the inertial forces to be competitive.
In this section, we have seen how particles migrate in purely (inertialess) viscoelastic flows and elasto-inertial flows. In an inertialess (Re ≪ 1) viscoelastic flow, without the effects of shear thinning and secondary flow, particles are driven to their stable focusing positions within channel cross-sections by elastic force, which is attributed mainly to the first normal stress difference (N1). The migration can be sped up either by increasing the fluidic elasticity or by a larger particle blockage ratio. The number of equilibrium positions varies with the cross-sectional geometry, with a single position in the channel centerline in circular channels, an additional four positions located in the corners of square channels, and various positions in the centerplane of rectangular channels. Additionally, shear thinning at a high-shear rate reverses the particle migration direction and displaces the particles toward the four corners of square channels, and the existence of secondary flow due to the second normal stress difference N2 causes particle migration following the recirculating flow orthogonal to the main flow, disrupting the 5-position focusing pattern. Smaller particles are found to be more susceptible to secondary flow.
Finally, the competition between the inertial and elastic forces in viscoelastic flow dictates particle focusing dynamics when inertia is not negligible (e.g., Re > 0.01). While the focusing dynamics remain the same as the inertialess viscoelastic flow in circular channels, a single focusing position in the center of square cross-sections is achieved with corner positions eliminated by wall-induced lift force at increased Re. The competition of shear-induced lift force and elastic force in the horizontal direction leads to varying focusing positions observed in low-AR rectangular microchannels103,144,145. Surprisingly, fluid elasticity remains dominant in determining particle migration dynamics even when inertial and elastic forces are comparable (). Nevertheless, the effect of inertial force is evident under this condition. Additionally, the shear-thinning effect and particle blockage ratio can modify migration dynamics. As a result, particles in viscoelastic flow show complex behavior that is dependent on the interactions among inertia, elasticity (N1), shear thinning, secondary flow (N2), particle blockage ratio and channel cross-sectional geometry. Two additional factors that contribute here are the overall channel geometry and the physical properties of particles, which we will discuss in the next section.
Channel geometry and particle physical properties
In addition to the cross-sectional geometry, the overall geometric layout of the microfluidic channel can significantly impact the particle migration dynamics. In Newtonian flow, the curvature of a microchannel is known to induce a pair of counterrotating secondary flows in its cross-section115,124. In a rectangular spiral channel, such recirculation reduces the number of focusing positions to only one, typically near the inner wall where the shear-induced lift force (Fs) is balanced with the Dean drag force (FD)123,124. Due to the high-throughput nature of spiral microchannels, they have been used for the isolation of rare cells40,152.
Similarly, the curvature of the channel is found to alter the focusing dynamics of particles in viscoelastic flows. Lee et al.153 showed that the strong Dean drag was counteracted by the elastic force instead of the shear-induced lift force, leading to a single focusing position near the center of the outer wall. This focusing position is on the opposite side of Newtonian flow focusing. Another work154 confirmed the distinctive equilibrium position and showed in detail the progressive evolution of focusing positions as the flow rate increased (Fig. 6a). Using a double-spiral channel, focusing of 100 nm diameter particles was achieved recently155. Instead of achieving single-position focusing in a spiral channel50, Cha et al.156 introduced expansions to the straight channel. The expansions lead to the formation of curved streamlines, which induce Hoop stress directed toward the channel center71, leading to single-position focusing in an inertialess viscoelastic flow. A similar effect of triangular expansions was observed in PEO solution157, and separation based on cell sizes was achieved recently158.
Fig. 6. Effects of channel geometry and particle deformability on lateral migration and focusing.
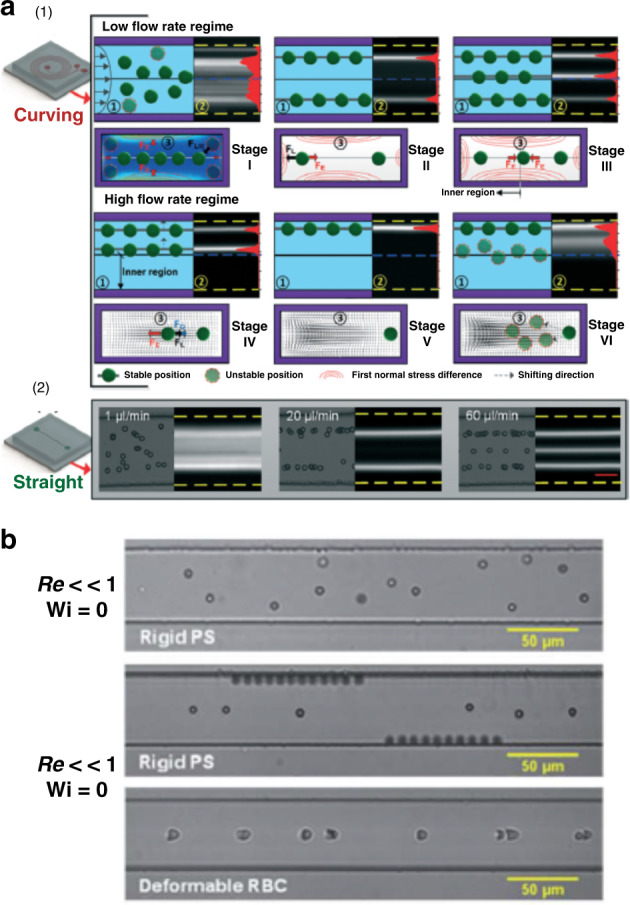
a Various focused streams observed both in spiral and straight rectangular microchannels when inertia was relevant154. Reproduced with permission from ref. 154. Copyright © Royal Society of Chemistry. b Single position focusing of red blood cells attributed to the interaction of the elastic force and deformability-induced lift force102. Reproduced with permission from ref. 102. Copyright © Royal Society of Chemistry
Particle physical properties such as deformability and shape can also significantly impact migration dynamics in viscoelastic flows. These properties are known markers for differential manipulation within microfluidic channels in Newtonian fluids159,160. Particle deformability in viscoelastic flow has been demonstrated in square microchannels using red blood cells (RBCs), as shown in Fig. 6b102. Deformable cells were found to experience an additional force that drives them away from the corners, leading to 3D focusing in an inertialess flow and thus better separation performance than in inertial flows (enrichment ratio 336 vs. 69)102. In terms of particle shape, peanut-shaped particles were found to migrate closer to the channel sidewalls than spherical particles in an elasto-inertial pinched flow fractionation (ei-PFF) device161. Other flow configurations, such as sheath flow, can be used to help manipulate particle migration in viscoelastic flow147,162,163. Ultimately, both the deformability and the shape of particles lead to distinct migration dynamics in viscoelastic flows since both cause changes in the effective particle size.
Numerical simulations
Numerical simulations are frequently used in conjunction with experimental studies when investigating particle migration in viscoelastic flow. Numerical simulations offer a convenient way to predict and visualize particle migration in 3D, which helps guide experimental investigations. The most common models used for numerically simulating viscoelastic flow include the Oldroyd-B, Giesekus and Phan-Thien Tanner models47,52,96,146. The upper-convected Maxwell (UCM) model, or Oldroyd-B model, predicts the first normal stress difference (N1) on particle migration without considering shear thinning (constant viscosity). The Giesekus and Phan-Thien Tanner models consider the shear thinning effect, with the former also predicting the second normal stress difference (N2)47,54. These models predict the motions of particles in viscoelastic flows, which help decipher the complex and often competing effects of fluid rheology on migration, such as the outward migration caused by shear thinning and inward migration due to N1 (Fig. 7a)52,54. However, significant skills in computational modeling are required. Some experimental researchers used the commercially available software COMSOL Multiphysics® to simulate the distribution of N1 and force vectors in the cross-sections of microfluidic channels (Fig. 7b)50,96. Particle motions and trajectories are not available using COMSOL Multiphysics®.
Fig. 7. Numerical simulations predicting particle migration and focusing in viscoelastic channel flows.
a Giesekus model used to predict particle downstream trajectories (red curves) and the dependence of migration direction on particle blockage ratio (β) in a micropipe flow95. Reproduced with permission from ref. 95. Copyright © Royal Society of Chemistry. b COMSOL Multiphysics® was used to predict the distribution of the first normal stress difference (N1) and the vector of lateral force in a square channel cross-section96. Reproduced with permission from ref. 96. Copyright © AIP Publishing. c Simulations of the effect of secondary flow induced by the second normal stress difference (N2) on particle migration in square microchannels using the Giesekus model54,146. Reproduced with permission from refs. 54,146. Copyright © Elsevier and copyright © Cambridge University Press, respectively
Motions of particles suspended in simple shear, planar Poiseuille and microfluidic channel flows have been simulated using the three models for deciphering the effects of N1, N2, shear thinning, and inertia. Many existing simulations report on particle migration in simple shear and Poiseuille flows, either in 2D or in 3D. Particles were found to migrate toward the closest wall in simple shear flow82,83,140. In planar Poiseuille flows, simulation results were consistent with experimental observations that particles migrate toward the centerline when fluid elasticity was dominant and inertia was negligible93,150. Those also extensively examined in Poiseuille flows include the competitions between inertial and elastic forces93,150 and between elastic force and shear thinning49,52. In channel flows, simulations were implemented in straight microchannels with circular95 or more commonly square50,53,54,145,146,164 cross-sections to investigate the effects of elasticity, inertia, shear thinning and secondary flow induced by N2. Simulation results found that secondary flow and shear thinning tend to move particles away from the channel center54,146. Additionally, recent simulation work165 suggests that particle deformability contrasts with migration due to elasticity (Fig. 7c). Simulation results are common compared with experimental observations, and discrepancies can sometimes be noted51,139,149. The discrepancies may be attributed to the limited channel length in the experiments and the deformation of the channel cross-section under fluid pressure.
Applications of viscoelastic microfluidics
Currently, the main applications of viscoelastic microfluidics are in the focusing and separation of particles and cells in microfluidic devices. Compared to inertial microfluidics, which have been widely used for these applications42, particle manipulation in viscoelastic flows is particularly intriguing due to its advantages, including focusing of submicron particles, 3D focusing and wide dynamic range of flow rate50. While inertial microfluidics are effective in manipulating particles or cells a few microns or above in size (a > 3 μm), viscoelastic microfluidics have shown reliable performance in focusing and separating smaller particles (1 μm or below), such as bacteria166, exosomes57, and DNA56. To date, most literature related to viscoelastic flow in microfluidic channels lies in the exploration of particle focusing and the underlying mechanisms. While single position focusing holds great potential for applications such as flow cytometry167, it might be deemed less useful in separation applications because it is not easy to differentiate particles due to a single focusing position. Examples of recent applications of viscoelastic microfluidics are highlighted in Fig. 8.
Fig. 8. Recent applications of viscoelastic microfluidics.
a Size-selective particle, cell and exosome separations based on elastic force achieved in microchannels with sheath flow in the center147. Reproduced with permission from ref. 147. Copyright © Royal Society of Chemistry. b Viscoelastic coflow for exosome separation57. Reproduced with permission from ref. 57. Copyright © 2017, American Chemical Society. c Tumor cell-line cell separation from blood using PEO core flow169. Reproduced with permission from ref. 169. Copyright © Royal Society of Chemistry. d Combination of viscoelasticity and inertia for pinched flow fractionation (eiPFF)170. Reproduced with permission from ref. 170. Copyright © 2015, American Chemical Society. e Shape-based separation of peanut particles in a viscoelastic PFF device161. f Sheathless separation of RBCs and E. coli in viscoelastic fluid flowing in a rectangular microchannel103. Reproduced with permission from ref. 161. Copyright © 2015, American Chemical Society. g Separation of fungus from blood in a rectangular viscoelastic channel174. Reproduced under a Creative Commons Attribution 4.0. h Microchannels consisting of two segments for sheathless particle separation139. Reproduced with permission from ref. 139. Copyright © Elsevier. i Separation of malaria parasites from WBCs in a two-segment channel171. Reproduced with permission from ref. 171. Copyright © Royal Society of Chemistry. j Filtration of particles in a square microchannel using both elastic and inertial forces175. Reproduced with permission from ref. 175. Copyright © Elsevier. k Single-stream focusing of cells in viscoelastic microflow used for measurements and monitoring of cell deformability176. Reproduced with permission from ref. 176. Copyright © 2012, American Chemical Society. l Focusing of DNA molecules in a low-AR rectangular microchannel based on the elastic force and flexibility-induced lift force56. Reproduced with permission from ref. 56. Copyright © 2012, American Chemical Society
Viscoelastic microfluidic devices are effective in sorting particles and cells ranging from tens of nanometers to tens of microns in size. The earliest such platform was proposed by Nam et al.147 who used buffer solution in the middle of a microchannel flanked by two sample flows (Fig. 8a). Due to the size-dependent migration toward the stable positions in the centerline (see expression (1)), separation of particles and blood cells was readily achieved with efficiency and purity both as high as 99%147. Since then, the same flow configuration has been used to separate exosomes (30−200 nm) from other EVs (Fig. 8b)57, bacteria (1 μm) from platelets166 and leukocytes168, and circulating tumor cells (CTCs) from blood (Fig. 8c)169. Particle migration due to viscoelasticity has also been coupled with pinched flow fractionation (ei-PFF) to achieve easy separation (Fig. 8d, e)161,170. However, the throughput of both approaches was mediocre (<90 µL/h).
Sheathless separation of particles can be achieved using either rectangular channels (Fig. 8f, g) or two-segment assembly of microchannels (Fig. 8h)139. Rectangular channels are capable of positioning particles or cells into different lateral positions based on size as a result of the interaction of inertial and elastic forces103. The two-segment configuration was modified to improve the throughput (Fig. 8i)171. Using HA instead of PEO solution, the throughput was increased to 400 µL/min, while the separation efficiency and purity remained competitive. The separation of malaria parasites from white blood cells (WBCs) was successfully demonstrated in this device, with a 94% recovery rate for parasites and 99% recovery rate for WBCs. The purity reached 99% for both parasites and WBCs. Size-selective separation of particles was also reported using ferrofluid for magnetophoresis after an initial 3D focusing in a similar two-segment channel172. Recently, Liu et al.103 showed the high-profile separation of MCF-7 cells from red blood cells (RBCs) and separation of E. coli from RBCs in low-AR rectangular channels (Fig. 8f). A similar design was used for the separation of microalgae and bacteria173 and for the filtration of fungi from WBCs (Fig. 8g)174. Additionally, particle separation was also reported in square microchannels by tuning the inertial forces175 and by an external magnetic field (negative magnetophoresis of the viscoelastic ferrofluid)172 (Fig. 8j). Separation performance in viscoelastic fluids is generally excellent in terms of efficiency and purity, although the throughput remains mediocre.
Other applications, including measurement of cell deformability and orientation control, have also been demonstrated in viscoelastic microchannels. Cell deformability itself can be a biomarker for cell sorting since it gives rise to the additional lift force that can eliminate the corner focusing positions, promoting cell focusing in the channel centerline102. Cha et al.176 used this phenomenon for measurements of cell deformability (Fig. 8k). A cross-slot channel was designed after the square focusing channel to allow cells to be stretched undergoing extensional flow in viscoelastic fluids. Changes in RBC deformability due to heat shocks were measured, and a decrease in deformability in human mesenchymal stem cells (hMSCs) due to nutrient starvation was monitored in their device. While the flow rate is far from impressive (160 µL/h), such an approach does not require a sophisticated design of channels and avoids the adverse influence of multiple focusing positions in inertial microfluidic channels. Other applications include focusing on macromolecules (e.g., DNA56), which is otherwise challenging in Newtonian inertial devices (Fig. 8l), and orientation control of nonspherical bioparticles (e.g., RBCs177,178). Additionally, relaxation time measurement of viscoelastic fluids was proposed based on particle focusing in a straight channel79 and the onset of instability in a serpentine channel179.
Concluding remarks and perspectives
The understanding of complex particle dynamics in viscoelastic flows has dramatically improved in recent years. The well-established knowledge of viscoelastic materials has promoted the advancement of research on particle interactions with surrounding fluids. For many years, investigations of such interactions had to rely mostly on numerical simulations, while experimental investigations were limited to simple shear or concentric Couette flows and planar Poiseuille flows. The existence of imbalanced normal stress differences not only results in distinct phenomena in viscoelastic fluids, such as the Weissenberg rod climbing effect, but also leads to the lateral migration of suspended particles47.
Applying microfluidic techniques to investigations of viscoelastic flows has extended the research frontier into the microscale as well as into the probing of the broad spectrum of particle dynamics. Advancements in microfabrication readily provide access to microchannels with a high ratio of channel length to characteristic length (e.g., Dh). These microchannels enable observation of full particle dynamics on a short time scale since the lateral migration is typically two to three orders of magnitude smaller than the downstream velocity47. The microscale characteristic length also permits an easy decoupling of the effect of fluid inertia (Re ≪ 1) to allow the inquiry of the sole effect of fluid viscoelasticity on particle dynamics. Elastic force stemming mainly from the first normal stress difference N1, the effect of fluid shear thinning, and the secondary flow induced by the second normal stress difference N2 collectively govern particle migration behavior and its equilibrium positions within channel cross-sections in inertialess flows (Re ≪ 1). As the Reynolds number increases, inertial forces become relevant and interact with the above factors, giving rise to distinct focusing dynamics of particles, such as single-position (3D) focusing in square channels50 and multiple-stream focusing in rectangular channels143,144. More intricate interactions and competitions can occur when channel geometry and particle properties (e.g., deformability) are involved. Nevertheless, constant effort in experimental and numerical studies has further unraveled the migration physics in viscoelastic flows.
Despite tremendous progress, a number of questions remain unanswered. First, although the elasticity number is commonly used for assessing the relative importance of fluid elasticity and inertia, this number alone is not sufficient. Recent experimental results53,151 showed particle focusing in the centers of square channels when inertial and elastic forces were thought to be comparable as El ~ 1. Inertial forces exhibited only auxiliary influence to eliminate the corner focusing positions, and the particle focusing pattern remained unchanged even when El < 1. This echoes a 2D direct simulation150 that suggests that “El < 0.01” is necessary for the inertial effect to be dominant in the viscoelastic flow. Currently, no work has been reported with experiments in such regimes to determine a more accurate criterion for assessing the relative importance of fluid elasticity and inertia on particle migration. Table 1 shows that the smallest El reported is 0.13. An enlarged channel characteristic length might be helpful to achieve a small El in viscoelastic microchannels.
Second, more work is still necessary to elucidate the shear-thinning effect in viscoelastic flow with nonnegligible inertia. According to the simulations52, most particles migrate to the core area of the channel centerline, while others form long chains and move along the walls. Although particle defocusing from the centerline was evident at a high-shear rate, the formation of long chains and particle annuli near walls was not observed in experiments96.
Third, the particle blockage ratio shows complex and sometimes contradictory effects on particle migration dynamics and focusing positions. While the elastic force is strongly dependent on particle size, direct simulations suggest the opposing effect of the blockage ratio: particle migration toward the centerline is generally dominated by the elastic force, but migration can reverse when the particle size exceeds a critical value49,93. Reversed migration due to large particle size has not been reported.
Furthermore, focusing dynamics in rectangular channels remains not fully understood, especially when inertia is relevant. Rectangular channels are the most popular channels for applications such as cell sorting. Laterally distinctive focusing positions for particles of different sizes enabled easy separation of cells and E. coli in a straight channel103. However, the force competitions that lead to multiple focusing positions in the centerplane remain unclear. While the balance between inertial force (shear-induced lift force) and elastic force explains the formation of two streams straddling the centerline, the current understanding does not explain the presence of a focusing position at the center when multiple positions exist.
Finally, many combinations of fluid composition, flow conditions, and microchannel geometry remain unexplored. The most commonly used channel geometry is a rectangular cross-section, and viscoelastic flows are frequently investigated in these microchannels either with or without inertia. Particle migration in viscoelastic flow with strong shear thinning but negligible inertia has been examined in square straight microchannels only53. Other geometries, such as circular and triangular microchannels, receive even less attention, as fabrication of such microchannels is not straightforward. Nonetheless, microchannels with triangular cross-sections can be interesting to explore for viscoelastic flow, as unexpected focusing positions of particles were found in Newtonian flows43,180. Similarly, the migration dynamics of particles in spiral channels are not fully explored in viscoelastic flow. When inertia is relevant, the focusing position of particles is generally near the outer wall instead of near the inner wall in inertial microfluidics. Two other conditions (inertialess and coupled with strong shear thinning) were not investigated, possibly due to negligible Dean vortices. Nevertheless, the change in the velocity profile under such conditions is likely to influence particle migration.
In terms of applications, focusing and separation are the main themes so far. Viscoelastic microfluidics has shown excellent performance, particularly in the separation of small particles and in precise spatial manipulation, such as 3D focusing, as well as a broad dynamic range suitable for various applications. The existing literature suggests the high (e.g., ≥99%) efficiency and purity of such devices. Nonetheless, the throughput of these systems is typically much lower than that of their inertial counterparts using Newtonian fluids. Owing to the high viscosity, viscoelastic microchannels require high driving pressures but generally manifest smaller Reynolds numbers. Based on the elasticity number, the practical way to improve the throughput seems to be tuning the fluid rheology and characteristic length, as shown in recent works51,151.
The current enthusiastic investigations into the viscoelastic manipulation of particles in microchannels lead to a new promising area of microfluidics, viscoelastic microfluidics. Considering the viscoelastic nature of many biological samples, such as blood, viscoelastic microfluidics suggest the possibility of handling biosamples with minimal preparation. More work into the particle dynamics in complex conditions will be imperative to achieve such perspective as sample dilution is currently required to avoid particle−particle interactions. Despite the promise in processing biosamples, a few disadvantages of this method for particle or cell separation need further attention. These include the low throughput despite the dynamic range being wide (theoretically), and the typical requirement of elasticity enhancers, which can contaminate samples. Due to the high viscosity of some viscoelastic flows, this method also necessitates a high pressure drop inside microfluidic channels, which can cause device failure in practice.
Another exciting advantage of viscoelastic microfluidics is its capability of handling submicrometer particles (e.g., exosomes) and macromolecules, such as DNA and proteins. Manipulation of EVs and macromolecules without sophisticated and time-consuming labeling can be ideal and is in high demand. Inertial microfluidics typically works well on the cell-size scale (a > 3 μm), but viscoelastic microfluidics empowers the manipulation of smaller sizes down to nanometers57,155. For instance, the secondary flow induced by the second normal stress difference is more effective in controlling dynamics of small particles rather than large ones.
Acknowledgements
We gratefully acknowledge the partial funding support from the Richard and Loan Hill Department of Bioengineering at the University of Illinois at Chicago and the University of Illinois Cancer Center.
Author contributions
J.Z. contributed to data analysis and manuscript writing. I.P. contributed to manuscript writing.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Alix-Panabieres C, Pantel K. Challenges in circulating tumour cell research. Nat. Rev. Cancer. 2014;14:623–631. doi: 10.1038/nrc3820. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe M, et al. Isolation and molecular analysis of circulating tumor cells from lung cancer patients using a microfluidic chip type cell sorter. Cancer Sci. 2018;109:2539–2548. doi: 10.1111/cas.13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiele J, Bethel K, Králíčková M, Kuhn P. Circulating tumor cells: fluid surrogates of solid tumors. Annu Rev. Pathol.-Mech. 2017;12:419–447. doi: 10.1146/annurev-pathol-052016-100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.J. Zhou, et al. Microfluidic separation of particles from whole blood using shear induced diffusion. In Proceedings of Microfluidics, BioMEMS, and Medical Microsystems XV, 100610X, https://www.spiedigitallibrary.org/conferenceproceedings-of-spie/10061/100610X/Microfluidic-separation-of-particles-from-whole-blood-using-shear-induced/10.1117/12.2255681.short?SSO=1.
- 5.Sajeesh P, Sen AK. Particle separation and sorting in microfluidic devices: a review. Microfluid. Nanofluid. 2014;17:1–52. [Google Scholar]
- 6.Zhou J, et al. Isolation of circulating tumor cells in non-small-cell-lung-cancer patients using a multi-flow microfluidic channel. Microsyst. Nanoeng. 2019;5:8. doi: 10.1038/s41378-019-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulasinghe A, Zhou J, Kenny L, Papautsky I, Punyadeera C. Capture of circulating tumour cell clusters using straight microfluidic chips. Cancers. 2019;11:89. doi: 10.3390/cancers11010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz J, Graells T, Walldén M, Hjort K. Inertial focusing with sub-micron resolution for separation of bacteria. Lab Chip. 2019;19:1257–1266. doi: 10.1039/c9lc00080a. [DOI] [PubMed] [Google Scholar]
- 9.Pitt WG, et al. Rapid separation of bacteria from blood—review and outlook. Biotechnol. Prog. 2016;32:823–839. doi: 10.1002/btpr.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gholizadeh S, et al. Microfluidic approaches for isolation, detection, and characterization of extracellular vesicles: current status and future directions. Biosens. Bioelectron. 2017;91:588–605. doi: 10.1016/j.bios.2016.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu R, et al. Extracellular vesicles in cancer—implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018;15:617. doi: 10.1038/s41571-018-0036-9. [DOI] [PubMed] [Google Scholar]
- 12.Yu ZT, Aw Yong KM, Fu J. Microfluidic blood cell sorting: now and beyond. Small. 2014;10:1687–1703. doi: 10.1002/smll.201302907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pamme N, Opin Curr. On-chip bioanalysis with magnetic particles. Chem. Biol. 2012;16:436–443. doi: 10.1016/j.cbpa.2012.05.181. [DOI] [PubMed] [Google Scholar]
- 14.He J, Huang M, Wang D, Zhang Z, Li G. Magnetic separation techniques in sample preparation for biological analysis: a review. J. Pharm. Biomed. Anal. 2014;101:84–101. doi: 10.1016/j.jpba.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Hejazian M, Li W, Nguyen N. Lab on a chip for continuous-flow magnetic cell separation. Lab Chip Miniaturisation Chem. Biol. 2015;15:959–970. doi: 10.1039/c4lc01422g. [DOI] [PubMed] [Google Scholar]
- 16.Jubery TZ, Srivastava SK, Dutta P. Dielectrophoretic separation of bioparticles in microdevices: A review. Electrophoresis. 2014;35:691–713. doi: 10.1002/elps.201300424. [DOI] [PubMed] [Google Scholar]
- 17.Alshareef M, et al. Separation of tumor cells with dielectrophoresis-based microfluidic chip. Biomicrofluidics. 2013;7:011803. doi: 10.1063/1.4774312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S, et al. High-throughput particle manipulation by hydrodynamic, electrokinetic, and dielectrophoretic effects in an integrated microfluidic chip. Biomicrofluidics. 2013;7:024106. doi: 10.1063/1.4795856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antfolk M, Laurell T. Continuous flow microfluidic separation and processing of rare cells and bioparticles found in blood–A review. Anal. Chim. Acta. 2017;965:9–35. doi: 10.1016/j.aca.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Wu M, et al. Acoustofluidic separation of cells and particles. Microsyst. Nanoeng. 2019;5:32. doi: 10.1038/s41378-019-0064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenshof A, Magnusson C, Laurell T. Acoustofluidics 8: Applications of acoustophoresis in continuous flow microsystems. Lab Chip. 2012;12:1210–1223. doi: 10.1039/c2lc21256k. [DOI] [PubMed] [Google Scholar]
- 22.Franke T, Braunmüller S, Schmid L, Wixforth A, Weitz D. Surface acoustic wave actuated cell sorting (SAWACS) Lab Chip. 2010;10:789–794. doi: 10.1039/b915522h. [DOI] [PubMed] [Google Scholar]
- 23.Schmid L, Weitz DA, Franke T. Sorting drops and cells with acoustics: acoustic microfluidic fluorescence-activated cell sorter. Lab Chip. 2014;14:3710–3718. doi: 10.1039/c4lc00588k. [DOI] [PubMed] [Google Scholar]
- 24.Li, Q., Li, J. & Hu, X. Microfluidic particle sorter based on optical tweezers array system. In Proceedings of IEEE NANOMED, Hong Kong, China, 21−25. https://ieeexplore.ieee.org/document/5749798 (2010).
- 25.Spencer D, Elliott G, Morgan H. A sheath-less combined optical and impedance micro-cytometer. Lab Chip. 2014;14:3064–3073. doi: 10.1039/c4lc00224e. [DOI] [PubMed] [Google Scholar]
- 26.Hu X, et al. Precise label-free leukocyte subpopulations separation using hybrid acoustic-optical chip. Lab Chip. 2018;18:3405–3412. doi: 10.1039/c8lc00911b. [DOI] [PubMed] [Google Scholar]
- 27.Huang LR, Cox EC, Austin RH, Sturm JC. Continuous particle separation through deterministic lateral displacement. Science. 2004;304:987–990. doi: 10.1126/science.1094567. [DOI] [PubMed] [Google Scholar]
- 28.Yamada M, Nakashima M, Seki M. Pinched flow fractionation: Continuous size separation of particles utilizing a laminar flow profile in a pinched microchannel. Anal. Chem. 2004;76:5465–5471. doi: 10.1021/ac049863r. [DOI] [PubMed] [Google Scholar]
- 29.Yamada M, Seki M. Hydrodynamic filtration for on-chip particle concentration and classification utilizing microfluidics. Lab Chip. 2005;5:1233–1239. doi: 10.1039/b509386d. [DOI] [PubMed] [Google Scholar]
- 30.Altmann J, Ripperger S. Particle deposition and layer formation at the crossflow microfiltration. J. Membr. Sci. 1997;124:119–128. [Google Scholar]
- 31.Zhou J, et al. Isolation of cells from whole blood using shear-induced diffusion. Sci. Rep. 2018;8:9411. doi: 10.1038/s41598-018-27779-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou J, Papautsky I. Size-dependent enrichment of leukocytes from undiluted whole blood using shear-induced diffusion. Lab Chip. 2019;19:3416. doi: 10.1039/c9lc00786e. [DOI] [PubMed] [Google Scholar]
- 33.Zhou J, et al. Label-free separation and culture of tumor cells in a microfluidic biochip. Analyst. 2020;145:1706–1715. doi: 10.1039/c9an02092f. [DOI] [PubMed] [Google Scholar]
- 34.Zhou J, Papautsky I. Fundamentals of inertial focusing in microchannels. Lab Chip. 2013;13:1121–1132. doi: 10.1039/c2lc41248a. [DOI] [PubMed] [Google Scholar]
- 35.Zhou J, Giridhar PV, Kasper S, Papautsky I. Modulation of aspect ratio for complete separation in an inertial microfluidic channel. Lab Chip. 2013;13:1919–1929. doi: 10.1039/c3lc50101a. [DOI] [PubMed] [Google Scholar]
- 36.Tu C, et al. A flexible cell concentrator using inertial focusing. Biomed. Microdevices. 2017;19:83. doi: 10.1007/s10544-017-0223-y. [DOI] [PubMed] [Google Scholar]
- 37.Li M, van Zee M, Goda K, Di Carlo D. Size-based sorting of hydrogel droplets using inertial microfluidics. Lab Chip. 2018;18:2575–2582. doi: 10.1039/c8lc00568k. [DOI] [PubMed] [Google Scholar]
- 38.Amini H, Lee W, Di Carlo D. Inertial microfluidic physics. Lab Chip. 2014;14:2739–2761. doi: 10.1039/c4lc00128a. [DOI] [PubMed] [Google Scholar]
- 39.Martel JM, Toner M. Inertial focusing in microfluidics. Annu. Rev. Biomed. Eng. 2014;16:371–396. doi: 10.1146/annurev-bioeng-121813-120704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warkiani ME, et al. Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics. Nat. Protoc. 2016;11:134–148. doi: 10.1038/nprot.2016.003. [DOI] [PubMed] [Google Scholar]
- 41.Hou HW, Bhattacharyya RP, Hung DT, Han J. Direct detection and drug-resistance profiling of bacteremias using inertial microfluidics. Lab Chip. 2015;15:2297–2307. doi: 10.1039/c5lc00311c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, et al. Fundamentals and applications of inertial microfluidics: A review. Lab Chip. 2016;16:10–34. doi: 10.1039/c5lc01159k. [DOI] [PubMed] [Google Scholar]
- 43.Kim J, Lee J, Je T, Jeon E, Lee W. Size-dependent inertial focusing position shift and particle separations in triangular microchannels. Anal. Chem. 2018;90:1827–1835. doi: 10.1021/acs.analchem.7b03851. [DOI] [PubMed] [Google Scholar]
- 44.Stokes JR, Davies GA. Viscoelasticity of human whole saliva collected after acid and mechanical stimulation. Biorheology. 2007;44:141–160. [PubMed] [Google Scholar]
- 45.Berret J. Local viscoelasticity of living cells measured by rotational magnetic spectroscopy. Nat. Commun. 2016;7:10134. doi: 10.1038/ncomms10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denn MM. Fifty years of non-Newtonian fluid dynamics. AIChE J. 2004;50:2335–2345. [Google Scholar]
- 47.D’Avino G, Maffettone P. Particle dynamics in viscoelastic liquids. J. Non-Newton. Fluid Mech. 2015;215:80–104. [Google Scholar]
- 48.Leshansky AM, Bransky A, Korin N, Dinnar U. Tunable nonlinear viscoelastic “focusing” in a microfluidic device. Phys. Rev. Lett. 2007;98:234501. doi: 10.1103/PhysRevLett.98.234501. [DOI] [PubMed] [Google Scholar]
- 49.Huang P, Feng J, Hu HH, Joseph DD. Direct simulation of the motion of solid particles in Couette and Poiseuille flows of viscoelastic fluids. J. Fluid Mech. 1997;343:73–94. [Google Scholar]
- 50.Yang S, Kim JY, Lee SJ, Lee SS, Kim JM. Sheathless elasto-inertial particle focusing and continuous separation in a straight rectangular microchannel. Lab Chip. 2011;11:266–273. doi: 10.1039/c0lc00102c. [DOI] [PubMed] [Google Scholar]
- 51.Seo KW, Byeon HJ, Huh HK, Lee SJ. Particle migration and single-line particle focusing in microscale pipe flow of viscoelastic fluids. RSC Adv. 2014;4:3512–3520. [Google Scholar]
- 52.Huang P, Joseph D. Effects of shear thinning on migration of neutrally buoyant particles in pressure driven flow of Newtonian and viscoelastic fluids. J. Non-Newton. Fluid Mech. 2000;90:159–185. [Google Scholar]
- 53.Del Giudice F, D’Avino G, Greco F, Netti PA, Maffettone PL. Effect of fluid rheology on particle migration in a square-shaped microchannel. Microfluid. Nanofluid. 2015;19:95–104. [Google Scholar]
- 54.Villone MM, D’Avino G, Hulsen MA, Greco F, Maffettone PL. Particle motion in square channel flow of a viscoelastic liquid: Migration vs. secondary flows. J. Non-Newton. Fluid Mech. 2013;195:1–8. [Google Scholar]
- 55.Lim H, Nam J, Shin S. Lateral migration of particles suspended in viscoelastic fluids in a microchannel flow. Microfluid. Nanofluid. 2014;17:683–692. [Google Scholar]
- 56.Kim JY, Ahn SW, Lee SS, Kim JM. Lateral migration and focusing of colloidal particles and DNA molecules under viscoelastic flow. Lab Chip. 2012;12:2807–2814. doi: 10.1039/c2lc40147a. [DOI] [PubMed] [Google Scholar]
- 57.Liu C, et al. Field-free isolation of exosomes from extracellular vesicles by microfluidic viscoelastic flows. ACS Nano. 2017;11:6968–6976. doi: 10.1021/acsnano.7b02277. [DOI] [PubMed] [Google Scholar]
- 58.Lu X, Liu C, Hu G, Xuan X. Particle manipulations in non-Newtonian microfluidics: A review. J. Colloid Interface Sci. 2017;500:182–201. doi: 10.1016/j.jcis.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 59.Yuan D, et al. Recent progress of particle migration in viscoelastic fluids. Lab Chip. 2018;18:551–567. doi: 10.1039/c7lc01076a. [DOI] [PubMed] [Google Scholar]
- 60.D’Avino G, Greco F, Maffettone PL. Particle migration due to viscoelasticity of the suspending liquid and its relevance in microfluidic devices. Annu. Rev. Fluid Mech. 2017;49:341–360. [Google Scholar]
- 61.Reiner M, Blair G, Hawley H. The Weissenberg effect in sweetened condensed milk. J. Soc. Chem. Ind. 1949;68:327–328. [Google Scholar]
- 62.Phan-Thien, N. Understanding Viscoelasticity: An Introduction to Rheology (Springer Science & Business Media, 2012).
- 63.Baird, D. G. Flow behavior of viscoelastic fluids. AccessScience. Retrieved 19 Nov 2020, 10.1036/1097-8542.YB110147 (2011).
- 64.Bagley E, Birks A. Flow of polyethylene into a capillary. J. Appl. Phys. 1960;31:556–561. [Google Scholar]
- 65.Lee D, Ahn KH. Time-Weissenberg number superposition in planar contraction microchannel flows. J. Non-Newton. Fluid Mech. 2014;210:41–46. [Google Scholar]
- 66.Li Z, Haward SJ. Viscoelastic flow development in planar microchannels. Microfluid. Nanofluid. 2015;19:1123–1137. [Google Scholar]
- 67.Soulages J, Oliveira MSN, Sousa PC, Alves MA, McKinley GH. Investigating the stability of viscoelastic stagnation flows in T-shaped microchannels. J. Non-Newton. Fluid Mech. 2009;163:9–24. [Google Scholar]
- 68.Ribeiro VM, Coelho PM, Pinho FT, Alves MA. Viscoelastic fluid flow past a confined cylinder: Three-dimensional effects and stability. Chem. Eng. Sci. 2014;111:364–380. [Google Scholar]
- 69.Jefri M, Zahed A. Elastic and viscous effects on particle migration in Plane‐Poiseuille flow. J. Rheol. 1978;1989(33):691–708. [Google Scholar]
- 70.Shaw, M. T. Introduction to polymer rheology (John Wiley & Sons, 2012).
- 71.Bird, R. B., Armstrong, R. C. & Hassager, O. Dynamics of polymeric liquids. Volume 1: Fluid Mechanics (John Wiley and Sons, New York, NY, 1987).
- 72.Magda J, Lou J, Baek S, DeVries K. Second normal stress difference of a Boger fluid. Polymer. 1991;32:2000–2009. [Google Scholar]
- 73.Yue P, Dooley J, Feng JJ. A general criterion for viscoelastic secondary flow in pipes of noncircular cross section. J. Rheol. 1978;2008:315–332. [Google Scholar]
- 74.Townsend P, Walters K, Waterhouse W. Waterhouse, Secondary flows in pipes of square cross-section and the measurement of the second normal stress difference. J. Non-Newton. Fluid Mech. 1976;1:107–123. [Google Scholar]
- 75.Dooley J, Hyun KS, Hughes K. An experimental study on the effect of polymer viscoelasticity on layer rearrangement in coextruded structures. Polym. Eng. Sci. 1998;38:1060–1071. [Google Scholar]
- 76.Giesekus H. Sekundärströmungen in viskoelastischen Flüssigkeiten bei stationärer und periodischer Bewegung. Rheol. Acta. 1965;4:85–101. [Google Scholar]
- 77.Dodson A, Townsend P, Walters K. Non-Newtonian flow in pipes of non-circular cross section. Comput. Fluids. 1974;2:317–338. [Google Scholar]
- 78.Li, D. Encyclopedia of microfluidics and nanofluidics (Springer Science & Business Media, 2008).
- 79.Del Giudice F, et al. Rheometry-on-a-chip: measuring the relaxation time of a viscoelastic liquid through particle migration in microchannel flows. Lab Chip. 2015;15:783–792. doi: 10.1039/c4lc01157k. [DOI] [PubMed] [Google Scholar]
- 80.Doraiswamy D. The origins of rheology: a short historical excursion. Rheol. Bull. 2002;71:1–9. [Google Scholar]
- 81.Macosko, C. W. Rheology: Principles, measurements, and applications. (VCH, New York, 1994).
- 82.D’Avino G, Tuccillo T, Maffettone P, Greco F, Hulsen M. Numerical simulations of particle migration in a viscoelastic fluid subjected to shear flow. Comput. Fluids. 2010;39:709–721. [Google Scholar]
- 83.D’Avino G, Maffettone P, Greco F, Hulsen M. Viscoelasticity-induced migration of a rigid sphere in confined shear flow. J. Non-Newton. Fluid Mech. 2010;165:466–474. [Google Scholar]
- 84.D’Avino G, et al. Migration of a sphere suspended in viscoelastic liquids in Couette flow: experiments and simulations. Rheol. Acta. 2012;51:215–234. [Google Scholar]
- 85.Karnis A, Mason S. Particle motions in sheared suspensions. XIX. Trans. Soc. Rheol. (1957−1977) 1966;10:571–592. [Google Scholar]
- 86.Ho BP, Leal LG. Inertial migration of rigid spheres in two-dimensional unidirectional flows. J. Fluid Mech. 1974;65:365–400. [Google Scholar]
- 87.Halow J, Wills G. Experimental observations of sphere migration in Couette systems. Ind. Eng. Chem. Fundamentals. 1970;9:603–607. [Google Scholar]
- 88.Ho BP, Leal LG. Migration of rigid spheres in a two-dimensional unidirectional shear flow of a second-order fluid. J. Fluid Mech. 1976;76:783–799. [Google Scholar]
- 89.Brunn P. The motion of rigid particles in viscoelastic fluids. J. Non-Newton. Fluid Mech. 1980;7:271–288. [Google Scholar]
- 90.Chan P, Leal L. A note on the motion of a spherical particle in a general quadratic flow of a second-order fluid. J. Fluid Mech. 1977;82:549–559. [Google Scholar]
- 91.Tehrani M, et al. An experimental study of particle migration in pipe flow of viscoelastic fluids. J. Rheol. 1996;40:1057–1077. [Google Scholar]
- 92.Lee EF, Koch DL, Joo YL. Cross-stream forces and velocities of fixed and freely suspended particles in viscoelastic Poiseuille flow: Perturbation and numerical analyses. J. Non-Newton. Fluid Mech. 2010;165:1309–1327. [Google Scholar]
- 93.Villone M, D’avino G, Hulsen M, Greco F, Maffettone P. Numerical simulations of particle migration in a viscoelastic fluid subjected to Poiseuille flow. Comput. Fluids. 2011;42:82–91. [Google Scholar]
- 94.Ko T, Patankar NA, Joseph DD. Lift and multiple equilibrium positions of a single particle in Newtonian and Oldroyd-B fluids. Comput. Fluids. 2006;35:121–146. [Google Scholar]
- 95.D’Avino G, et al. Single line particle focusing induced by viscoelasticity of the suspending liquid: Theory, experiments and simulations to design a micropipe flow-focuser. Lab Chip. 2012;12:1638–1645. doi: 10.1039/c2lc21154h. [DOI] [PubMed] [Google Scholar]
- 96.Seo KW, Kang YJ, Lee SJ. Lateral migration and focusing of microspheres in a microchannel flow of viscoelastic fluids. Phys. Fluids. 1994;2014:063301. [Google Scholar]
- 97.Romeo G, D’Avino G, Greco F, Netti PA, Maffettone PL. Viscoelastic flow-focusing in microchannels: Scaling properties of the particle radial distributions. Lab Chip. 2013;13:2802–2807. doi: 10.1039/c3lc50257k. [DOI] [PubMed] [Google Scholar]
- 98.Debbaut B, Avalosse T, Dooley J, Hughes K. On the development of secondary motions in straight channels induced by the second normal stress difference: experiments and simulations. J. Non-Newton. Fluid Mech. 1997;69:255–271. [Google Scholar]
- 99.Debbaut B, Dooley J. Secondary motions in straight and tapered channels: Experiments and three-dimensional finite element simulation with a multimode differential viscoelastic model. J. Rheol. 1999;43:1525–1545. [Google Scholar]
- 100.Villone MM, D’Avino G, Hulsen MA, Greco F, Maffettone PL. Simulations of viscoelasticity-induced focusing of particles in pressure-driven micro-slit flow. J. Non-Newton. Fluid Mech. 2011;166:1396–1405. [Google Scholar]
- 101.Del Giudice F, et al. Particle alignment in a viscoelastic liquid flowing in a square-shaped microchannel. Lab Chip. 2013;13:4263–4271. doi: 10.1039/c3lc50679g. [DOI] [PubMed] [Google Scholar]
- 102.Yang S, et al. Deformability-selective particle entrainment and separation in a rectangular microchannel using medium viscoelasticity. Soft Matter. 2012;8:5011–5019. [Google Scholar]
- 103.Liu C, et al. Size-based separation of particles and cells utilizing viscoelastic effects in straight microchannels. Anal. Chem. 2015;87:6041–6048. doi: 10.1021/acs.analchem.5b00516. [DOI] [PubMed] [Google Scholar]
- 104.Segré G, Silberberg A. Radial particle displacements in poiseuille flow of suspensions. Nature. 1961;189:209–210. [Google Scholar]
- 105.Segré G, Silberberg A. Behaviour of macroscopic rigid spheres in Poiseuille flow. J. Fluid Mech. 1962;14:136–157. [Google Scholar]
- 106.Rubinow SI, Keller JB. The transverse force on a spinning sphere moving in a viscous fluid. J. Fluid Mech. 1961;11:447–459. [Google Scholar]
- 107.Gotoh K. Migration of a neutrally buoyant particle in poiseuille flow: A Possible explanation. Nature. 1970;225:848–850. doi: 10.1038/225848a0. [DOI] [PubMed] [Google Scholar]
- 108.Matas JP, Morris JF, Guazzelli E. Lateral forces on a sphere. Oil Gas Sci. Technol. 2004;59:59–70. [Google Scholar]
- 109.Asmolov ES. The inertial lift on a spherical particle in a plane poiseuille flow at large channel Reynolds number. J. Fluid Mech. 1999;381:63–87. [Google Scholar]
- 110.Feng J, Hu HH, Joseph DD. Direct simulation of initial value problems for the motion of solid bodies in a Newtonian fluid. Part 2. Couette and Poiseuille flows. J. Fluid Mech. 1994;277:271–301. [Google Scholar]
- 111.Saffman PG. The lift on a small sphere in a slow shear flow. J. Fluid Mech. 1965;22:385–400. [Google Scholar]
- 112.Chun B, Ladd AJC. Inertial migration of neutrally buoyant particles in a square duct: An investigation of multiple equilibrium positions. Phys. Fluids. 2006;18:031704. [Google Scholar]
- 113.Hur SC, Mach AJ, Di Carlo D. High-throughput size-based rare cell enrichment using microscale vortices. Biomicrofluidics. 2011;5:022206. doi: 10.1063/1.3576780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang X, Zandi M, Ho C, Kaval N, Papautsky I. Single stream inertial focusing in a straight microchannel. Lab Chip. 2015;15:1812–1821. doi: 10.1039/c4lc01462f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Di Carlo D, Irimia D, Tompkins RG, Toner M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc. Natl Acad. Sci. USA. 2007;104:18892–18897. doi: 10.1073/pnas.0704958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Di Carlo D, Edd JF, Irimia D, Tompkins RG, Toner M. Equilibrium separation and filtration of particles using differential inertial focusing. Anal. Chem. 2008;80:2204–2211. doi: 10.1021/ac702283m. [DOI] [PubMed] [Google Scholar]
- 117.Hur SC, Kwong Tse HT, Di Carlo D. Sheathless inertial cell ordering for extreme throughput flow cytometry. Lab Chip. 2010;10:274–280. doi: 10.1039/b919495a. [DOI] [PubMed] [Google Scholar]
- 118.Mach AJ, Kim JH, Arshi A, Hur SC, Di Carlo D. Automated cellular sample preparation using a Centrifuge-on-a-Chip. Lab Chip. 2011;11:2827–2834. doi: 10.1039/c1lc20330d. [DOI] [PubMed] [Google Scholar]
- 119.Sollier E, et al. Size-selective collection of circulating tumor cells using Vortex technology. Lab Chip. 2014;14:63–77. doi: 10.1039/c3lc50689d. [DOI] [PubMed] [Google Scholar]
- 120.Kim J, et al. Inertial focusing in non-rectangular cross-section microchannels and manipulation of accessible focusing positions. Lab Chip. 2016;16:992–1001. doi: 10.1039/c5lc01100k. [DOI] [PubMed] [Google Scholar]
- 121.Bhagat AAS, Jothimuthu P, Papautsky I. Photodefinable polydimethylsiloxane (PDMS) for rapid lab-on-a-chip prototyping. Lab Chip. 2007;7:1192–7. doi: 10.1039/b704946c. [DOI] [PubMed] [Google Scholar]
- 122.Bhagat AAS, Kuntaegowdanahalli SS, Papautsky I. Enhanced particle filtration in straight microchannels using shear-modulated inertial migration. Phys. Fluids. 2008;20:101702. [Google Scholar]
- 123.Bhagat AAS, Kuntaegowdanahalli SS, Papautsky I. Continuous particle separation in spiral microchannels using Dean flows and differential migration. Lab Chip. 2008;8:1906–14. doi: 10.1039/b807107a. [DOI] [PubMed] [Google Scholar]
- 124.Kuntaegowdanahalli SS, Bhagat AAS, Kumar G, Papautsky I. Inertial microfluidics for continuous particle separation in spiral microchannels. Lab Chip. 2009;9:2973–80. doi: 10.1039/b908271a. [DOI] [PubMed] [Google Scholar]
- 125.Bhagat AAS, Kuntaegowdanahalli SS, Papautsky I. Inertial microfluidics for continuous particle filtration and extraction. Microfluid. Nanofluid. 2009;7:217–226. [Google Scholar]
- 126.Zhou J, Kasper S, Papautsky I. Enhanced size-dependent trapping of particles using microvortices. Microfluid. Nanofluid. 2013;15:611–623. doi: 10.1007/s10404-013-1176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang X, Zhou J, Papautsky I. Vortex-aided inertial microfluidic device for continuous particle separation with high size-selectivity, efficiency, and purity. Biomicrofluidics. 2013;7:044119. doi: 10.1063/1.4818906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou J, Giridhar PV, Kasper S, Papautsky I. Modulation of rotation-induced lift force for cell filtration in a low aspect ratio microchannel. Biomicrofluidics. 2014;8:044112. doi: 10.1063/1.4891599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang X, Papautsky I. Size-based microfluidic multimodal microparticle sorter. Lab Chip. 2015;15:1350–1359. doi: 10.1039/c4lc00803k. [DOI] [PubMed] [Google Scholar]
- 130.Myung JH, Hong S. Microfluidic devices to enrich and isolate circulating tumor cells. Lab Chip. 2015;15:4500–4511. doi: 10.1039/c5lc00947b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Warkiani ME, et al. Slanted spiral microfluidics for the ultra-fast, label-free isolation of circulating tumor cells. Lab Chip. 2014;14:128–137. doi: 10.1039/c3lc50617g. [DOI] [PubMed] [Google Scholar]
- 132.Martel JM, et al. Continuous flow microfluidic bioparticle concentrator. Sci. Rep. 2015;5:11300. doi: 10.1038/srep11300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ozkumur E, et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci. Transl. Med. 2013;5:1–11. doi: 10.1126/scitranslmed.3005616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Martel JM, Toner M. Inertial focusing dynamics in spiral microchannels. Phys. Fluids. 2012;24:032001. doi: 10.1063/1.3681228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang X, Liedert C, Liedert R, Papautsky I. A disposable, roll-to-roll hot-embossed inertial microfluidic device for size-based sorting of microbeads and cells. Lab Chip. 2016;16:1821–1830. doi: 10.1039/c6lc00215c. [DOI] [PubMed] [Google Scholar]
- 136.Mach AJ, Di Carlo D. Continuous scalable blood filtration device using inertial microfluidics. Biotechnol. Bioeng. 2010;107:302–311. doi: 10.1002/bit.22833. [DOI] [PubMed] [Google Scholar]
- 137.Bhagat AAS, Kuntaegowdanahalli SS, Kaval N, Seliskar CJ, Papautsky I. Inertial microfluidics for sheath-less high-throughput flow cytometry. Biomed. Microdevices. 2010;12:187–195. doi: 10.1007/s10544-009-9374-9. [DOI] [PubMed] [Google Scholar]
- 138.Shen S, et al. High-throughput rare cell separation from blood samples using steric hindrance and inertial microfluidics. Lab Chip. 2014;14:2525–2538. doi: 10.1039/c3lc51384j. [DOI] [PubMed] [Google Scholar]
- 139.Nam J, et al. Microfluidic device for sheathless particle focusing and separation using a viscoelastic fluid. J. Chromatogr. A. 2015;1406:244–250. doi: 10.1016/j.chroma.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 140.Caserta S, D’Avino G, Greco F, Guido S, Maffettone PL. Migration of a sphere in a viscoelastic fluid under planar shear flow: Experiments and numerical predictions. Soft Matter. 2011;7:1100–1106. [Google Scholar]
- 141.Xia Y, Whitesides GM. Soft lithography. Annu. Rev. Mater. Sci. 1998;28:153–184. [Google Scholar]
- 142.Mukherjee P, Nebuloni F, Gao H, Zhou J, Papautsky I. Rapid prototyping of soft lithography masters for microfluidic devices using dry film photoresist in a non-cleanroom setting. Micromachines. 2019;10:192. doi: 10.3390/mi10030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xiang N, Dai Q, Ni Z. Multi-train elasto-inertial particle focusing in straight microfluidic channels. Appl. Phys. Lett. 2016;109:134101. [Google Scholar]
- 144.Yang SH, Lee DJ, Youn JR, Song YS. Multiple-line particle focusing under viscoelastic flow in a microfluidic device. Anal. Chem. 2017;89:3639–3647. doi: 10.1021/acs.analchem.6b05052. [DOI] [PubMed] [Google Scholar]
- 145.Raoufi MA, et al. Experimental and numerical study of elasto-inertial focusing in straight channels. Biomicrofluidics. 2019;13:034103. doi: 10.1063/1.5093345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li G, McKinley GH, Ardekani AM. Dynamics of particle migration in channel flow of viscoelastic fluids. J. Fluid Mech. 2015;785:486–505. [Google Scholar]
- 147.Nam J, Lim H, Kim D, Jung H, Shin S. Continuous separation of microparticles in a microfluidic channel via the elasto-inertial effect of non-Newtonian fluid. Lab Chip. 2012;12:1347–1354. doi: 10.1039/c2lc21304d. [DOI] [PubMed] [Google Scholar]
- 148.Di Carlo D, Edd JF, Humphry KJ, Stone HA, Toner M. Particle segregation and dynamics in confined flows. Phys. Rev. Lett. 2009;102:094503. doi: 10.1103/PhysRevLett.102.094503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Geislinger TM, Stamp MEM, Wixforth A, Franke T. Hydrodynamic and label-free sorting of circulating tumor cells from whole blood. Appl. Phys. Lett. 2015;107:203702. [Google Scholar]
- 150.Trofa M, et al. Numerical simulations of the competition between the effects of inertia and viscoelasticity on particle migration in Poiseuille flow. Comput. Fluids. 2015;107:214–223. [Google Scholar]
- 151.Lim EJ, et al. Inertio-elastic focusing of bioparticles in microchannels at high throughput. Nat. Commun. 2014;5:4120. doi: 10.1038/ncomms5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu Z, Chen Y, Wang M, Chung AJ. Continuous inertial microparticle and blood cell separation in straight channels with local microstructures. Lab Chip. 2016;16:532–542. doi: 10.1039/c5lc01435b. [DOI] [PubMed] [Google Scholar]
- 153.Lee DJ, Brenner H, Youn JR, Song YS. Multiplex particle focusing via hydrodynamic force in viscoelastic fluids. Sci. Rep. 2013;3:3258. doi: 10.1038/srep03258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xiang N, et al. Fundamentals of elasto-inertial particle focusing in curved microfluidic channels. Lab Chip. 2016;16:2626–2635. doi: 10.1039/c6lc00376a. [DOI] [PubMed] [Google Scholar]
- 155.Liu C, et al. Sheathless focusing and separation of diverse nanoparticles in viscoelastic solutions with minimized shear thinning. Anal. Chem. 2016;88:12547–12553. doi: 10.1021/acs.analchem.6b04564. [DOI] [PubMed] [Google Scholar]
- 156.Cha S, et al. Hoop stress-assisted three-dimensional particle focusing under viscoelastic flow. Rheol. Acta. 2014;53:927–933. [Google Scholar]
- 157.Yuan D, et al. Dean-flow-coupled elasto-inertial three-dimensional particle focusing under viscoelastic flow in a straight channel with asymmetrical expansion–contraction cavity arrays. Biomicrofluidics. 2015;9:044108. doi: 10.1063/1.4927494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yuan D, et al. Sheathless Dean-flow-coupled elasto-inertial particle focusing and separation in viscoelastic fluid. RSC Adv. 2017;7:3461–3469. [Google Scholar]
- 159.Hur SC, Henderson-Maclennan NK, McCabe ERB, Di Carlo D. Deformability-based cell classification and enrichment using inertial microfluidics. Lab Chip. 2011;11:912–920. doi: 10.1039/c0lc00595a. [DOI] [PubMed] [Google Scholar]
- 160.Hou HW, et al. Deformability based cell margination–a simple microfluidic design for malaria-infected erythrocyte separation. Lab Chip. 2010;10:2605–2613. doi: 10.1039/c003873c. [DOI] [PubMed] [Google Scholar]
- 161.Lu X, Xuan X. Elasto-inertial pinched flow fractionation for continuous shape-based particle separation. Anal. Chem. 2015;87:11523–11530. doi: 10.1021/acs.analchem.5b03321. [DOI] [PubMed] [Google Scholar]
- 162.Yuan D, et al. Investigation of particle lateral migration in sample‐sheath flow of viscoelastic fluid and Newtonian fluid. Electrophoresis. 2016;37:2147–2155. doi: 10.1002/elps.201600102. [DOI] [PubMed] [Google Scholar]
- 163.Kang K, Lee SS, Hyun K, Lee SJ, Kim JM. DNA-based highly tunable particle focuser. Nat. Commun. 2013;4:2567. doi: 10.1038/ncomms3567. [DOI] [PubMed] [Google Scholar]
- 164.Raffiee AH, Ardekani AM, Dabiri S. Numerical investigation of elasto-inertial particle focusing patterns in viscoelastic microfluidic devices. J. Non-Newton. Fluid Mech. 2019;272:104166. [Google Scholar]
- 165.Villone MM, Greco F, Hulsen MA, Maffettone PL. Numerical simulations of deformable particle lateral migration in tube flow of Newtonian and viscoelastic media. J. Non-Newton. Fluid Mech. 2016;234:105–113. [Google Scholar]
- 166.Tian F, et al. Microfluidic co-flow of Newtonian and viscoelastic fluids for high-resolution separation of microparticles. Lab Chip. 2017;17:3078–3085. doi: 10.1039/c7lc00671c. [DOI] [PubMed] [Google Scholar]
- 167.Del Giudice F, D’Avino G, Greco F, Maffettone PL, Shen AQ. Fluid viscoelasticity drives self-assembly of particle trains in a straight microfluidic channel. Phys. Rev. Appl. 2018;10:064058. [Google Scholar]
- 168.Faridi MA, et al. Elasto-inertial microfluidics for bacteria separation from whole blood for sepsis diagnostics. J. Nanobiotechnol. 2017;15:3. doi: 10.1186/s12951-016-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tian F, et al. Label-free isolation of rare tumor cells from untreated whole blood by interfacial viscoelastic microfluidics. Lab Chip. 2018;18:3436–3445. doi: 10.1039/c8lc00700d. [DOI] [PubMed] [Google Scholar]
- 170.Lu X, Xuan X. Continuous microfluidic particle separation via elasto-inertial pinched flow fractionation. Anal. Chem. 2015;87:6389–6396. doi: 10.1021/acs.analchem.5b01432. [DOI] [PubMed] [Google Scholar]
- 171.Nam J, et al. High-throughput malaria parasite separation using a viscoelastic fluid for ultrasensitive PCR detection. Lab Chip. 2016;16:2086–2092. doi: 10.1039/c6lc00162a. [DOI] [PubMed] [Google Scholar]
- 172.Zhang J, et al. A novel viscoelastic-based ferrofluid for continuous sheathless microfluidic separation of nonmagnetic microparticles. Lab Chip. 2016;16:3947–3956. doi: 10.1039/c6lc01007e. [DOI] [PubMed] [Google Scholar]
- 173.Yuan D, et al. Sheathless separation of microalgae from bacteria using a simple straight channel based on viscoelastic microfluidics. Lab Chip. 2019;19:2811–2821. doi: 10.1039/c9lc00482c. [DOI] [PubMed] [Google Scholar]
- 174.Nam J, Jang WS, Hong DH, Lim CS. Viscoelastic Separation and Concentration of Fungi from Blood for Highly Sensitive Molecular Diagnostics. Sci. Rep. 2019;9:3067. doi: 10.1038/s41598-019-39175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ahn SW, Lee SS, Lee SJ, Kim JM. Microfluidic particle separator utilizing sheathless elasto-inertial focusing. Chem. Eng. Sci. 2015;126:237–243. [Google Scholar]
- 176.Cha S, et al. Cell stretching measurement utilizing viscoelastic particle focusing. Anal. Chem. 2012;84:10471–10477. doi: 10.1021/ac302763n. [DOI] [PubMed] [Google Scholar]
- 177.Won Seo K, Ha YR, Lee SJ. Vertical focusing and cell ordering in a microchannel via viscoelasticity: applications for cell monitoring using a digital holographic microscopy. Appl. Phys. Lett. 2014;104:213702. [Google Scholar]
- 178.Go T, Byeon H, Lee SJ. Focusing and alignment of erythrocytes in a viscoelastic medium. Sci. Rep. 2017;7:41162. doi: 10.1038/srep41162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zilz J, et al. Serpentine channels: Micro-rheometers for fluid relaxation times. Lab Chip. 2014;14:351–358. doi: 10.1039/c3lc50809a. [DOI] [PubMed] [Google Scholar]
- 180.Mukherjee P, Wang X, Zhou J, Papautsky I. Single stream inertial focusing in low aspect-ratio triangular microchannels. Lab Chip. 2019;19:147–157. doi: 10.1039/c8lc00973b. [DOI] [PubMed] [Google Scholar]
- 181.Dealy J. Weissenberg and Deborah numbers—their definition and use. Rheol. Bull. 2010;79:14–18. [Google Scholar]
- 182.Rott N. Note on the history of the Reynolds number. Fluid Mech. 1990;22:1–12. [Google Scholar]
- 183.Li D, Lu X, Xuan X. Viscoelastic separation of particles by size in straight rectangular microchannels: a parametric study for a refined understanding. Anal. Chem. 2016;88:12303–12309. doi: 10.1021/acs.analchem.6b03501. [DOI] [PubMed] [Google Scholar]