Abstract
Introduction:
Management of retroperitoneal and lateral pelvic lymph nodes (RLPN) in rectal cancer remains unclear. With total neoadjuvant therapy (TNT), more patients have radiologic complete clinical response (rCR). We sought to evaluate the impact of radiographic persistent RLPN after neoadjuvant therapy on survival.
Materials and Methods:
Patients with rectal adenocarcinoma with isolated RLPN metastasis, who received neoadjuvant therapy before surgery were included from the United States Rectal Cancer Consortium database. Primary outcomes were recurrence-free survival (RFS) and overall survival (OS).
Results:
Of 77 patients, all received neoadjuvant therapy, with 35 (46%) receiving TNT. Posttreatment, 33 (43%) had rCR while 44 (57%) had radiographic persistent RLPN. Median number of radiographic positive RLPN was 1 (IQR 1–2).
Receipt of TNT was associated with radiographic RLPN rCR (OR 4.77, 95% CI 1.81–12.60, p < .01). However, there was no difference in RFS and OS between patients who achieved rCR or with persistent RLPN (all p > .05).
Conclusions:
Radiographic persistence of RLPN was not associated with worse survival in well-selected patients and may not be a reliable indicator of pathological response. TNT may be the preferred management strategy to select patients given its association with rCR. Radiographic persistence of RLPN after preoperative therapy should not necessarily preclude surgery.
Keywords: neoadjuvant therapy, rectal cancer, retroperitoneal lateral pelvic lymph nodes, total neoadjuvant therapy
1 ∣. INTRODUCTION
In the United States, colorectal cancer is the third most common cause of cancer-related deaths.1 While 15%–20% of rectal cancer cases have evidence of retroperitoneal lateral pelvic lymph node (RLPN) disease, the clinical significance and optimal management strategy remains unknown.2,3
Eastern countries, such as Japan and Korea, consider RLPN metastasis as regional disease and protocols recommend total mesorectal excision (TME) and RLPN lymphadenectomy without neoadjuvant therapy.4 In contrast, Western countries, including the United States, consider RLPN metastasis as systemic disease that portends a worse prognosis, and the use of preoperative therapy is well-established.5 Furthermore, with the widespread application of a total neoadjuvant therapy (TNT) strategy, or administration of chemoradiation before surgery, more patients have a RLPN clinical response on imaging.6 However, the prognostic significance of persistent RLPN remains unclear. Given the low incidence of isolated RLPN metastases and the different treatment approach to RLPN disease based on geography, the optimal management strategy for these patients remains non-standardized. Thus, the aim of this study was to evaluate the impact of radiographic persistent RLPN after neoadjuvant therapy on oncologic outcomes.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Data source and study cohort selection
In a retrospective manner, patients were identified from the United States Rectal Cancer Consortium (USRCC) database, a multi-institutional consortium of six high-volume tertiary referral centers including Emory University, University of Michigan, University of Pittsburgh Medical Center, The Ohio State University Wexner Medical Center, Vanderbilt University Medical Center, and Washington University School of Medicine in St. Louis. With a series of objectives determined a priori, the database was created using a standardized data form. Data were collected at each institution and combined into a shared, common database. This study was one of these predetermined objectives. Before data collection, Institutional Review Board approval was obtained at each site.
All patients with primary rectal adenocarcinoma with isolated RLPN metastasis on radiographic imaging who received neoadjuvant therapy before undergoing low anterior resection (LAR) or abdominoperineal resection (APR) without RLPN resection were included from 2007 to 2017. Patients with unknown RLPN status, who did not receive neoadjuvant therapy, who underwent an emergent or palliative operation, or who received a resection margin with macroscopic evidence of tumor (R2) were excluded.
2.2 ∣. Study variables and outcomes
Demographic, perioperative, pathologic, and long-term outcomes data were collected via electronic medical record (EMR) review. Staging was recorded with the contemporary American Joint Committee on Cancer (AJCC) 8th edition criteria. RLPN were considered positive retroperitoneal, periaortic, presacral, and/or lateral pelvic lymph nodes along the iliac and/or obturator vessels visualized on computed tomography (CT), magnetic resonance imaging (MRI), endorectal ultrasound (ERUS), or positron emission tomography (PET) as documented in the original radiology report. Variability in the imaging modality selected was due to institution-specific protocols. Further, depending on imaging modality used, positive nodes before or after neoadjuvant therapy were determined by each institution's radiologist based on size criteria, changes in morphology, differences in dynamic gadolinium enhancement on MRI, and/or increased FDG uptake on PET/CT scans. Neoadjuvant therapy was defined as receiving chemotherapy, chemoradiation, or a TNT program before surgery. Disappearing RLPN was defined strictly as radiologic complete clinical response (rCR) to therapy in RLPN only and was not reflective of response in the primary or mesorectal nodes. Primary outcomes were recurrence-free survival (RFS) and overall survival (OS).
2.3 ∣. Statistical analysis
Statistical analysis was conducted using SPSS 26.0 software (IBM Inc.). Descriptive statistics for each variable of interest were reported. Statistical significance was defined as a significance level of p < .05. For discrete variables, a χ2 test or Fisher's exact test was used. A student's t-test or a Mann-Whitney test was performed for continuous variables. Univariate and multivariable logistic regression analysis were performed to determine the association between clinicopathologic variables and radiographic persistence of RLPN. Kaplan-Meier analysis, log-rank tests, univariate, and multivariable Cox proportional hazard analysis were performed to evaluate the association of radiographic persistence stent of RLPN and survival. Statistically significant and clinically relevant covariate(s) were included in the multivariable models.
3 ∣. RESULTS
3.1 ∣. Study cohort characteristics
Of the 1881 patients in the USRCC database, 77 met the inclusion criteria. Demographic and clinicopathologic data are listed in Table 1. 1804 patients were excluded for recurrent disease (n = 1), stage IV disease (n = 26), receiving an emergent surgery for palliative intent (n = 1), non-LAR/non-APR operations (n = 2), a R2 resection (n = 8), non-adenocarcinoma histology (n = 4), negative preoperative RLPN status (n = 370), missing neoadjuvant therapy data (n = 15), and missing histopathologic data, including the specific number of positive RLPN (n = 1377). Given the imaging modality selected was dependent on institution-specific protocols, 20 (26%) patients were not diagnosed with MRI, but rather CT, ERUS, and/or PET. The median age at diagnosis was 56 years (interquartile range [IQR] 49–63). Rectal tumors were predominantly located in the lower or middle rectum 68 (94%). The majority of patients had clinical stage III disease before neoadjuvant therapy 50 (96%). Median number of positive RLPN on pretreatment imaging was 1 (IQR 1–2). Thirty-five (47%) received TNT, 40 (52%) received neoadjuvant chemoradiation, and 2 (5%) received neoadjuvant chemotherapy. Posttreatment, 33 (43%) had rCR while 44 (57%) had radiographic persistence of RLPN. Median number of positive RLPN on re-staging imaging was 1 (IQR 1–2).
TABLE 1.
Demographic and clinicopathologic factors of the study population
| All patients n = 77 (%) |
RP/pelvic LN disappearance n = 33 (43) |
RP/Pelvic LN persistence n = 44 (57) |
p value | |
|---|---|---|---|---|
| Age at diagnosis (median, IQR) | 56 (49–63) | 57 (49–63) | 54 (47–63) | .40 |
| Sex | ||||
| Male | 35 (45) | 16 (48) | 19 (43) | .64 |
| Female | 42 (55) | 17 (52) | 25 (57) | |
| Race | ||||
| White | 63 (82) | 24 (72) | 39 (89) | .18 |
| Black | 12 (16) | 8 (24) | 4 (9) | |
| Other | 2 (2) | 1 (3) | 1 (2) | |
| Functional status | ||||
| Independent | 75 (98) | 32 (100) | 43 (98) | .35 |
| Partially dependent | 1 (2) | 0 (0) | 1 (2) | |
| Pretreatment stage (AJCC 8th ed) | ||||
| I | 2 (2) | 1 (5) | 0 (0) | .31 |
| II | 2 (2) | 1 (5) | 0 (0) | |
| III | 50 (96) | 20 (90) | 30 (100) | |
| Pretreatment tumor location | ||||
| Lower rectum | 34 (47) | 12 (41) | 22 (51) | .70 |
| Middle rectum | 34 (47) | 15 (52) | 19 (44) | |
| Upper rectum | 4 (6) | 2 (7) | 2 (5) | |
| Neoadjuvant chemotherapy | ||||
| No | 39 (95) | 11 (100) | 28 (93) | .95 |
| Yes | 2 (5) | 0 (0) | 2 (7) | |
| Neoadjuvant chemoradiation | ||||
| No | 0 (0) | 0 (0) | 2 (6) | .97 |
| Yes | 40 (52) | 11 (100) | 29 (94) | |
| Total neoadjuvant therapy | ||||
| No | 42 (55) | 11 (33) | 31 (71) | <.01 |
| Yes | 35 (46) | 22 (67) | 13 (29) | |
| Operation type | ||||
| LAR | 47 (61) | 22 (67) | 25 (57) | .52 |
| APR | 30 (39) | 11 (33) | 19 (43) | |
| Approach | ||||
| Open | 32 (42) | 14 (42) | 18 (41) | .89 |
| Minimally invasive | 45 (58) | 19 (58) | 26 (59) | |
| # Positive mesorectal LN, pretreatment (median, IQR) | 2 (1–5) | |||
| # Positive RP LN, pretreatment (median, IQR) | 0 (0–1) | |||
| # Positive pelvic LN, pretreatment (median, IQR) | 1 (1–2) | |||
| # Positive RP + pelvic LN, pretreatment (median, IQR) | 1 (1–2) | |||
| # Positive mesorectal LN, posttreatment (median, IQR) | 1 (0–2) | |||
| # Positive RP LN, posttreatment (median, IQR) | 0 (0) | |||
| # Positive pelvic LN, posttreatment (median, IQR) | 1 (0–2) | |||
| # Positive RP + pelvic LN, posttreatment (median, IQR) | 1 (1–2) | |||
| Adjuvant chemotherapy | ||||
| No | 9 (22) | 2 (20) | 7 (23) | .99 |
| Yes | 32 (78) | 8 (80) | 24 (77) | |
| Adjuvant chemoradiation | ||||
| No | 39 (95) | 10 (100) | 29 (94) | .99 |
| Yes | 2 (5) | 0 (0) | 2 (6) | |
| Tumor differentiation | ||||
| Well | 1 (2) | 1 (4) | 0 (0) | .35 |
| Moderate | 50 (85) | 21 (84) | 29 (85) | |
| Poor | 7 (12) | 2 (8) | 5 (15) | |
| Undifferentiated | 1 (2) | 1 (4) | 0 (0) | |
| Pathologic response after NAT | ||||
| No response | 11 (15) | 4 (13) | 7 (17) | .10 |
| Partial response | 50 (70) | 19 (61) | 31 (76) | |
| Complete response | 11 (15) | 8 (26) | 3 (7) | |
| Pathologic stage (AJCC 8th ed) | ||||
| 0 | 11 (14) | 9 (26) | 2 (5) | .10 |
| I | 14 (20) | 6 (19) | 8 (20) | |
| II | 17 (30) | 7 (23) | 10 (26) | |
| III | 29 (41) | 10 (32) | 19 (49) | |
| Final margin status | ||||
| R0 | 71 (92) | 32 (97) | 39 (88) | .35 |
| R1 | 6 (8) | 1 (3) | 5 (11) | |
| LVI | ||||
| No | 60 (85) | 25 (81) | 35 (88) | .67 |
| Yes | 11 (15) | 6 (19) | 5 (12) | |
| PNI | ||||
| No | 51 (81) | 21 (81) | 30 (81) | .85 |
| Yes | 12 (19) | 5 (19) | 7 (19) | |
| Follow-up in months (median, IQR) | 19 (12–45) | 16 (12–34) | 23 (15–55) | .50 |
Abbreviations: AJCC, American Joint Committee on Cancer; LAR, low anterior resection; APR, abdominoperineal resection; IQR, interquartile range; LN, lymph node; LVI, lymphovascular invasion; PNI, perineural invasion; RP, retroperitoneum.
Compared to patients with RLPN disappearance, patients with radiographic persistent RLPN were less likely to receive TNT (29% vs. 67%, p < .01). Median follow-up was 19 months (IQR 12–45).
3.2 ∣. Radiographic persistence of RLPN
On univariate analysis, receipt of TNT was associated with a decreased odds of radiographic persistence of RLPN (OR 0.21, 95% CI 0.08–0.55, p = .01) (Table 2).
TABLE 2.
Association of clinicopathologic factors with retroperitoneal and/or pelvic LN radiographic persistence
| Univariate regression | ||
|---|---|---|
| OR (95% CI) | p value | |
| Age at diagnosis (median, IQR) | 0.98 (0.85–1.02) | .40 |
| Sex | ||
| Male | Reference | |
| Female | 1.23 (0.50–3.07) | .64 |
| Race | ||
| White | Reference | |
| Black | 0.31 (0.08–1.13) | .08 |
| Other | 0.52 (0.04–10.30) | .74 |
| Functional status | ||
| Independent | Reference | |
| Partially dependent | - | |
| Pretreatment stage (AJCC 8th ed) | ||
| I | Reference | |
| II | - | |
| III | - | |
| Pretreatment tumor location | ||
| Lower rectum | Reference | |
| Middle rectum | 0.69 (0.26–1.83) | .46 |
| Upper rectum | 0.55 (0.07–4.38) | .57 |
| Neoadjuvant chemotherapy | ||
| No | Reference | |
| Yes | - | |
| Neoadjuvant chemoradiation | ||
| No | Reference | |
| Yes | - | |
| Total neoadjuvant therapy | ||
| No | Reference | |
| Yes | 0.21 (0.08–0.55) | <.01 |
Abbreviations: AJCC, American Joint Committee on Cancer; LAR, low anterior resection; CI, confidence interval; IQR, interquartile range; LN, lymph node; OR, odds ratio.
3.3 ∣. Oncologic outcomes
3.3.1 ∣. Recurrence-free survival
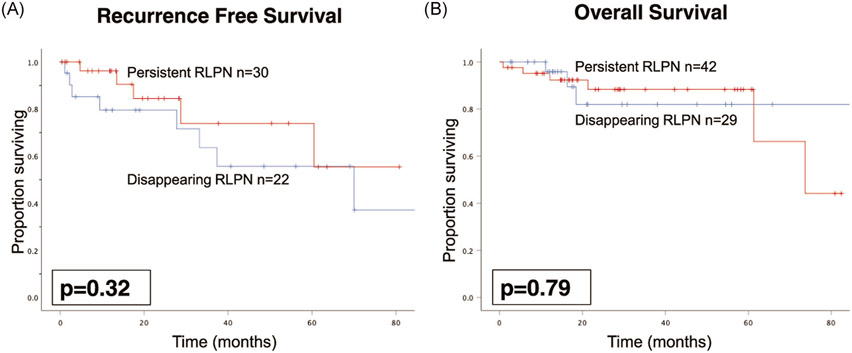
While receipt of TNT was associated with RLPN rCR, 5-year RFS for patients with rCR compared to radiographic persistence of RLPN was similar at 46% and 55%, respectively (p = .32), on Kaplan-Meier analysis (Figure 1A). On univariate analysis, radiographic persistence of RLPN was not associated with worse RFS (HR 0.57, 95% CI 0.18–1.77, p = .33) (Table 3). Accounting for TNT, radiographic persistence of RLPN was not associated with worse RFS (HR 0.44, 95% CI 0.12–1.54, p = .20).
FIGURE 1.
Kaplan-Meier curves comparing RFS (A) and OS (B) for disappearing (blue) versus persistent (red) RLPN after neoadjuvant therapy. RFS, recurrence-free survival; RLPN, retroperitoneal and lateral pelvic lymph nodes; OS, overall survival
TABLE 3.
Association of retroperitoneal and pelvic LN radiographic persistence with oncologic outcomes
| Univariate regression |
Multivariable regression |
|||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) |
p value | |
| RFS | ||||
| Age at diagnosis (median, IQR) | 0.98 (0.95–1.02) | .39 | ||
| Sex | ||||
| Male | Reference | |||
| Female | 1.00 (0.31–3.24) | .99 | ||
| Race | ||||
| White | Reference | |||
| Black | 2.11 (0.65–6.89) | .22 | ||
| Other | - | |||
| Functional status | ||||
| Independent | Reference | |||
| Partially dependent | - | |||
| Pretreatment stage (AJCC 8th ed) | ||||
| I | Reference | |||
| II | - | |||
| III | - | |||
| Pretreatment tumor location | ||||
| Lower rectum | Reference | |||
| Middle rectum | 0.61 (0.18–2.03) | .42 | ||
| Upper rectum | - | - | ||
| Neoadjuvant chemotherapy | ||||
| No | Reference | |||
| Yes | - | |||
| Neoadjuvant chemoradiation | ||||
| No | Reference | |||
| Yes | - | |||
| Total neoadjuvant therapy | ||||
| No | Reference | Reference | ||
| Yes | 0.86 (0.28–2.65) | .80 | 0.58 (0.17–2.01) | .39 |
| Operation type | ||||
| LAR | Reference | |||
| APR | 1.33 (0.44–4.01) | .61 | ||
| Approach | ||||
| Open | Reference | |||
| Minimally invasive | 0.32 (0.09–1.15) | .08 | ||
| Final margin status | ||||
| R0 | Reference | |||
| R1 | 2.2 (0.49–10.27) | .30 | ||
| LVI | ||||
| No | Reference | |||
| Yes | 1.75 (0.53–5.76) | .36 | ||
| PNI | ||||
| No | Reference | |||
| Yes | 1.76 (0.50–6.20) | .38 | ||
| Posttreatment LN disappearance/radiographic RLPN persistence | ||||
| 0 LN | Reference | Reference | ||
| ≥1 LN | 0.57 (0.18–1.77) | .33 | 0.44 (0.12–1.54) | .20 |
| OS | ||||
| Age at diagnosis (median, IQR) | 1.01 (0.96–1.07) | |||
| Sex | ||||
| Male | Reference | |||
| Female | 0.44 (0.11–1.77) | .25 | ||
| Race | ||||
| White | Reference | |||
| Black | 1.80 (0.36–9.95) | .47 | ||
| Other | - | |||
| Functional status | ||||
| Independent | Reference | |||
| Partially Independent | - | |||
| Pretreatment stage (AJCC 8th ed) | ||||
| I | Reference | |||
| II | - | |||
| III | - | |||
| Pretreatment tumor location | ||||
| Lower rectum | Reference | |||
| Middle rectum | 0.14 (0.02–1.15) | .07 | ||
| Upper rectum | - | |||
| Neoadjuvant chemotherapy | ||||
| No | Reference | |||
| Yes | - | - | ||
| Neoadjuvant chemoradiation | ||||
| No | Reference | |||
| Yes | 0.22 (0.03–1.99) | .18 | ||
| Total neoadjuvant therapy | ||||
| No | Reference | Reference | ||
| Yes | 0.49 (0.10–2.34) | .37 | 0.49 (0.10–2.45) | .38 |
| Operation type | ||||
| LAR | Reference | |||
| APR | 1.23 (0.30–4.95) | .77 | ||
| Approach | ||||
| Open | Reference | |||
| Minimally invasive | 0.56 (0.13–2.45) | .44 | ||
| Final margin status | ||||
| R0 | Reference | |||
| R1 | 4.19 (0.93–21.14) | .08 | ||
| LVI | ||||
| No | Reference | |||
| Yes | 5.62 (1.40–22.63) | .02 | ||
| PNI | ||||
| No | Reference | |||
| Yes | 1.2 (0.14–10.27) | .87 | ||
| Posttreatment LN disappearance/radiographic RLPN Persistence | ||||
| 0 LN | Reference | Reference | ||
| ≥1 LN | 1.20 (0.30–4.84) | .80 | 1.02 (0.25–4.26) | .97 |
Abbreviations: CI, confidence interval; APR, abdominoperineal resection; HR, hazard ratio; IQR, interquartile range; LAR, low anterior resection; LN, lymph node; LVI, lymphovascular invasion PNI, perineural invasion; RFS, recurrence-free survival; RLPN, retroperitoneal and lateral pelvic lymph nodes.
3.3.2 ∣. Overall survival
Similar to RFS, there was no difference in 5-year OS between patients with rCR (82%) or persistence of RLPN (88%) (p = .79) (Figure 1B). On univariate analysis, radiographic persistence of RLPN was not associated with worse OS (HR 1.20, 95% CI 0.30–4.84, p = .80) (Table 3). Accounting for TNT, radiographic persistence of RLPN was not associated with worse OS (HR 1.02, 95% CI 0.25-4.26, p = .97).
4 ∣. DISCUSSION
Currently, the optimal treatment paradigm for the management of isolated RLPN disease is still debated with differing approaches favored in Eastern and Western countries. Our analyses of a large, multi-institutional database of patients with rectal cancer in the United States reveal radiographic persistence of RLPN was not associated with worse RFS or OS in well-selected patients.
In patients who received preoperative chemoradiation and TME with mesorectal lymphadenectomy, locoregional recurrence rates have previously been reported as 4.6%–7.9% with 67.4%–82.7% of patients recurring in RLPN.7,8 While presence of RLPN on clinical staging may indicate an increased risk for local recurrence, existing literature on its impact on long-term oncologic outcomes is inconsistent.9,10 As a result, clinical management and the decision to proceed with surgery remains controversial, particularly in patients with isolated, persistent RLPN disease who received neoadjuvant therapy.
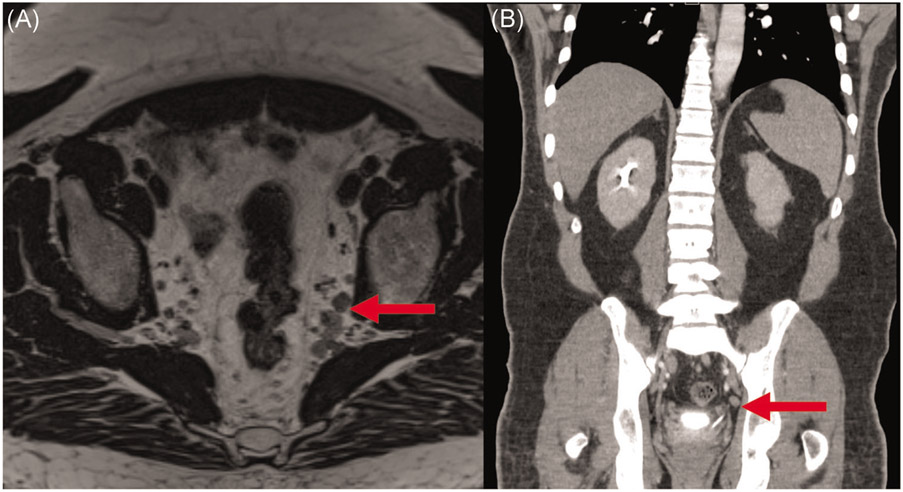
In the context of the Western neoadjuvant therapy approach, it is important to note that specifically for suspicious RLPN, radiographic imaging may not be a reliable indicator of pathologic response. In our surgical patient population, the median positive RLPN count pretreatment was 1 (IQR 1–2). After neoadjuvant therapy, the median positive RLPN count remained unchanged. Currently, magnetic resonance imaging (MRI) serves as the mainstay for determining N stage. After patients receive neoadjuvant therapy, MRI has a reported accuracy rates of 75% and 71% for T stage and N stage, respectively, and 63%–68% for the detection of RLPN (Figure 2).11-13 The inability to definitively characterize RLPN status is predominantly due to radiation-associated tissue changes, including fibrosis, peritumoral infiltration of inflammatory cells, and vascular proliferation.14 Thus, there is a tendency to overestimate the extent of nodal involvement. Given the discordance between imaging findings after neoadjuvant therapy and pathologic N staging, uniform criteria for the radiographic characterization (including size, margin, morphology, enhancement, and signal intensity) of RLPN is critical, particularly in patients with a low burden of RLPN disease who go on to receive neoadjuvant therapy.15,16
FIGURE 2.
Axial T2 SPACE MRI (A) and coronal venous CT (B) of suspicious pelvic lymph nodes after neoadjuvant therapy. CT, computed tomography; MRI, magnetic resonance imaging
Eastern countries have adopted a lateral pelvic lymph node dissection (LPLD) or extended lymphadenectomy (EL) approach to excise RLPN, specifically along the obturator muscle, common iliac artery, internal iliac artery, and external iliac artery in addition to removing the traditional mesorectal lymph nodes. Ishiguro et al.17 demonstrated the presence of RLPN in 93 patients with T4 rectal cancer was associated with worse OS (HR 2.09, 95% CI 1.06–4.10, p = .03) and RFS (HR 2.61, 95% CI 1.38–4.92, p = .01), thus advocating for RLPN removal at the time of surgery. In 2017, a randomized controlled trial for 701 patients with clinical stage II/III rectal cancer by Fujita et al.18 demonstrated lower locoregional recurrence rates with TME and EL compared to TME alone at 7.4% versus 12.6%, respectively. However, it is worth noting the study participants did not receive neoadjuvant therapy and results from the long-term follow-up of this trial did not support routine application of TME with EL in all patients, and may be more beneficial in patients with clinical stage III disease.19
In contrast to the Eastern approach, TME with mesorectal lymphadenectomy in the setting of a multimodality treatment paradigm has become the preferred treatment strategy for rectal cancer surgery in Western countries, namely Europe and the United States, given RLPN is considered a marker of metastatic disease.20 A neoadjuvant treatment strategy, including TNT, chemoradiation, and short and long course radiation have shown to be effective in improving locoregional recurrence and rCR rates in several randomized controlled trials, including the Dutch Rectal Cancer Trial and German CAO/ARO/AIO-94 Trial.21-25 Based on our data, a TNT approach may be the preferred management strategy to select patients for surgery, given its association with rCR. In the persistent RLPN group, there was no progression of disease as the RLPN number remained the same. Furthermore, while the pathologic complete response rate was higher in patients who achieved rCR, long-term oncologic outcomes were similar between the rCR and persistent RLPN groups, which may suggest a high rate of false positives for suspicious RLPN on diagnostic imaging. Thus, we believe persistent RLPN after TNT should not preclude proctectomy with TME. The similarity between oncologic outcomes may be related to a small sample size, relatively short median follow-up (19 months), and/or false positives in the persistent RLPN group.
When comparing rectal cancer patients who either received preoperative radiation therapy and TME alone to patients who underwent TME with EL, Watanabe and colleagues determined there was no significant difference in 5-year disease-free survival rates.26 In a randomized controlled trial of 51 patients by Nagawa et al.27 neoadjuvant radiation before surgery with EL did not improve disease-free survival or recurrence rates, supporting neoadjuvant therapy can be employed as an alternative to EL. It is likely both a neoadjuvant treatment strategy with TME and TME plus EL either sterilizes or removes RLPN disease to clear the circumferential resection margin, which is supported by their equivocal oncologic outcomes.28
Importantly, EL also carries a significant risk for morbidity. In a meta-analysis by Georgiou et al.29 comparing EL to standard rectal resection, EL resulted in an increased risk of postoperative complications, including sexual and urinary dysfunction, in addition to not conferring an improvement in 5-year OS (HR 1.09, 95% CI 0.78–1.50, p = .62).17 The adverse impact of EL on quality-of-life due to sacrificing the pelvic autonomic nerves required for an oncologic resection is a major concern and has been well-documented.30,31 Without a clear survival benefit and risk of morbidity associated with extensive dissection of pelvic autonomic nerve plexus, a Western approach thereby avoids the unnecessary risk of sexual and urinary complications, though it is worth noting radiation therapy is not without its own risk for sexual dysfunction and incontinence.32,33
In the preoperative setting, it is imperative RLPN are accurately staged, especially given the desmoplasic reaction associated with neoadjuvant chemoradiation or radiation therapy. In a 2019 retrospective, multi-center cohort study of 1216 patients with low rectal cancer, Ogura et al.34 reported lateral lymph nodes ≥7mm on staging MRI which persisted after neoadjuvant chemoradiation therapy at >4 mm had a 52.3% 5-year risk local recurrence in the internal iliac compartment, which is comparable to our study findings. With the bias to overestimate suspicious lymph nodes, universal criteria is critical to differentiate non versus complete responders to better inform the extent of resection and long-term surveillance. As new modalities and techniques such diffusion-weighted imaging MRI and lymph node-specific contrast agents show promise in their reliability in predicting nodal status after neoadjuvant therapy, accurate preoperative diagnosis is imperative.35,36
Limitations of this study include those inherent to a retrospective design, including missing data. Second, different imaging modalities were used to determine RLPN involvement, thus introducing variability in diagnostic accuracy rates. Third, there is no standardized definition for “suspicious” lymph nodes across institutions given the imaging approaches employed and the emergence of new modalities and techniques. Fourth, there is practice pattern variability for chemotherapy regimen, radiation therapy dose, and time from neoadjuvant chemoradiation or TNT until surgery. Fifth, we were not able to obtain histopathologic confirmation of RLPN given RLPN lymphadenectomy is not routinely performed in the United States. Lastly, this patient population represents a well-selected surgical patient population, though a large multi-institutional database allows for generalizability.
5 ∣. CONCLUSIONS
While a TNT is associated with the rCR of isolated and unresected RLPN, the persistence of RLPN on imaging was not associated with worse RFS or OS in well-selected patients. TNT may be the preferred management strategy to best select patients for surgery. Moreover, radiographic imaging of RLPN may not be a reliable indicator of pathological response. Thus, uniform radiologic diagnostic criteria are needed and radiographic persistence of RLPN after preoperative therapy should not necessarily preclude proctectomy with TME. A prospective randomized controlled trial comparing neoadjuvant therapy and TME to TME with EL is needed to validate these findings.
ACKNOWLEDGMENT
This study is supported in part by the Katz Foundation and the National Center for Advancing Translational Science (Grant/Award Number: UL1TR002378/TL1TR002382).
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–164. [DOI] [PubMed] [Google Scholar]
- 2.Nagasaki T, Akiyoshi T, Fujimoto Y, et al. Preoperative chemoradiotherapy might improve the prognosis of patients with locally advanced low rectal cancer and lateral pelvic lymph node metastases. World J Surg. 2017;41(3):876–883. [DOI] [PubMed] [Google Scholar]
- 3.Ishihara S, Kawai K, Tanaka T, et al. Oncological outcomes of lateral pelvic lymph node metastasis in rectal cancer treated with preoperative chemoradiotherapy. Dis Colon Rectum. 2017;60(5):469–476. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20(2):207–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Wang J, Ma X, et al. A review of neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Int J Biol Sci. 2016;12(8):1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludmir EB, Palta M, Willett CG, Czito BG. Total neoadjuvant therapy for rectal cancer: an emerging option. Cancer. 2017;123(9):1497–1506. [DOI] [PubMed] [Google Scholar]
- 7.Kim TH, Jeong SY, Choi DH, et al. Lateral lymph node metastasis is a major cause of locoregional recurrence in rectal cancer treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol. 2008;15(3):729–737. [DOI] [PubMed] [Google Scholar]
- 8.Kusters M, Marijnen CA, van de Velde CJ, et al. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol. 2010;36(5):470–476. [DOI] [PubMed] [Google Scholar]
- 9.Akiyoshi T, Toda S, Tominaga T, et al. Prognostic impact of residual lateral lymph node metastasis after neoadjuvant (chemo)radiotherapy in patients with advanced low rectal cancer. BJS Open. 2019;3(6):822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson JS, Quyn AJ, Sagar PM. Rectal cancer lateral pelvic sidewall lymph nodes: a review of controversies and management. Br J Surg. 2020;107(12):1562–1569. [DOI] [PubMed] [Google Scholar]
- 11.Kim NK, Kim MJ, Yun SH, Sohn SK, Min JS. Comparative study of transrectal ultrasonography, pelvic computerized tomography, and magnetic resonance imaging in preoperative staging of rectal cancer. Dis Colon Rectum. 1999;42(6):770–775. [DOI] [PubMed] [Google Scholar]
- 12.Chen CC, Lee RC, Lin JK, Wang LW, Yang SH. How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy? Dis Colon Rectum. 2005;48(4):722–728. [DOI] [PubMed] [Google Scholar]
- 13.Al-Sukhni E, Milot L, Fruitman M, et al. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19(7):2212–2223. [DOI] [PubMed] [Google Scholar]
- 14.Kuo L-J, Chern MC, Tsou MH, et al. Interpretation of magnetic resonance imaging for locally advanced rectal carcinoma after preoperative chemoradiation therapy. Dis Colon Rectum. 2005;48(1):23–28. [DOI] [PubMed] [Google Scholar]
- 15.Grubnic S, Vinnicombe SJ, Norman AR, Husband JE. MR evaluation of normal retroperitoneal and pelvic lymph nodes. Clin Radiol. 2002;57(3):193–200, discussion 201-214. [DOI] [PubMed] [Google Scholar]
- 16.Kim MJ, Hur BY, Lee ES, et al. Prediction of lateral pelvic lymph node metastasis in patients with locally advanced rectal cancer with preoperative chemoradiotherapy: focus on MR imaging findings. PLOS One. 2018;13(4):e0195815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishiguro S, Akasu T, Fujita S, Yamamoto S, Kusters M, Moriya Y. Pelvic exenteration for clinical T4 rectal cancer: oncologic outcome in 93 patients at a single institution over a 30-year period. Surgery. 2009;145(2):189–195. [DOI] [PubMed] [Google Scholar]
- 18.Fujita S, Mizusawa J, Kanemitsu Y, et al. Mesorectal excision with or without lateral lymph node dissection for clinical stage II/III lower rectal cancer (JCOG0212): a multicenter, randomized controlled, noninferiority trial. Ann Surg. 2017;266(2):201–207. [DOI] [PubMed] [Google Scholar]
- 19.Tsukamoto S, Fujita S, Ota M, et al. Long-term follow-up of the randomized trial of mesorectal excision with or without lateral lymph node dissection in rectal cancer (JCOG0212). Br J Surg. 2020;107(5):586–594. [DOI] [PubMed] [Google Scholar]
- 20.Weiser MR. AJCC 8th Edition: Colorectal Cancer. Ann Surg Oncol. 2018;25(6):1454–1455. [DOI] [PubMed] [Google Scholar]
- 21.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345(9):638–646. [DOI] [PubMed] [Google Scholar]
- 22.Iqbal A, George TJ. Randomized clinical trials in colon and rectal cancer. Surg Oncol Clin N Am. 2017;26(4):689–704. [DOI] [PubMed] [Google Scholar]
- 23.Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373(9666):811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12(6):575–582. [DOI] [PubMed] [Google Scholar]
- 25.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–1933. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe T, Tsurita G, Muto T, et al. Extended lymphadenectomy and preoperative radiotherapy for lower rectal cancers. Surgery. 2002;132(1):27–33. [DOI] [PubMed] [Google Scholar]
- 27.Nagawa H, Muto T, Sunouchi K, et al. Randomized, controlled trial of lateral node dissectionvs. nerve-preserving resection in patients with rectal cancer after preoperative radiotherapy. Dis Colon Rectum. 2001;44(9):1274–1280. [DOI] [PubMed] [Google Scholar]
- 28.Atef Y, Koedam TW, van Oostendorp SE, Bonjer HJ, Wijsmuller AR, Tuynman JB. Lateral pelvic lymph node metastases in rectal cancer: a systematic review. World J Surg. 2019;43(12):3198–3206. [DOI] [PubMed] [Google Scholar]
- 29.Georgiou P, Tan E, Gouvas N, et al. Extended lymphadenectomy versus conventional surgery for rectal cancer: a meta-analysis. Lancet Oncol. 2009;10(11):1053–1062. [DOI] [PubMed] [Google Scholar]
- 30.Cöl C, Hasdemir O, Yalcin E, et al. The assessment of urinary function following extended lymph node dissection for colorectal cancer. Eur J Surg Oncol. 2005;31(3):237–241. [DOI] [PubMed] [Google Scholar]
- 31.Hojo K, Sawada T, Moriya Y. An analysis of survival and voiding, sexual function after wide iliopelvic lymphadenectomy in patients with carcinoma of the rectum, compared with conventional lymphadenectomy. Dis Colon Rectum. 1989;32(2):128–133. [DOI] [PubMed] [Google Scholar]
- 32.Marijnen CA, van de Velde CJ, Putter H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2005;23(9):1847–1858. [DOI] [PubMed] [Google Scholar]
- 33.Kusters M, Beets GL, van de Velde CJ, et al. A comparison between the treatment of low rectal cancer in Japan and the Netherlands, focusing on the patterns of local recurrence. Ann Surg. 2009;249(2):229–235. [DOI] [PubMed] [Google Scholar]
- 34.Ogura A, Konishi T, Beets GL, et al. Lateral nodal features on restaging magnetic resonance imaging associated with lateral local recurrence in low rectal cancer after neoadjuvant chemoradiotherapy or radiotherapy. JAMA Surg. 2019;154(9):e192172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koh DM, George C, Temple L, et al. Diagnostic accuracy of nodal enhancement pattern of rectal cancer at MRI enhanced with ultrasmall superparamagnetic iron oxide: findings in pathologically matched mesorectal lymph nodes. Am J Roentgenol. 2010;194(6):W505–W513. [DOI] [PubMed] [Google Scholar]
- 36.van Heeswijk MM, Lambregts DM, Palm WM, et al. DWI for assessment of rectal cancer nodes after chemoradiotherapy: is the absence of nodes at DWI proof of a negative nodal status? Am J Roentgenol. 2017;208(3):W79–W84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.