ABSTRACT
Background
Calf circumference (CC) is used in geriatric studies as a simple and practical skeletal muscle (SM) marker for diagnosing low SM and sarcopenia. Currently applied CC cutoff points were developed in samples including older participants; values representative of the full adult lifespan are lacking.
Objectives
We aimed to develop CC cutoff points and to identify relevant confounding factors from the large and diverse NHANES 1999–2006 population sample.
Methods
Demographic, anthropometric, and imaging data (DXA, appendicular lean mass) from the adult (age ≥18 y) NHANES sample were partitioned into subgroups according to sex, age, ethnicity, and race. Adults aged 18–39 y and BMI (in kg/m2) 18.5–24.9 were set as a reference population; CC cutoff points were derived at 1 and 2 SDs below the mean.
Results
The sample included 17,789 participants, 51.3% males and 48.7% females, with respective ages (mean ± SD) of 43.3 ± 16.1 y and 45.5 ± 16.9 y. CC was strongly correlated with appendicular lean mass, r = 0.84 and 0.86 for males and females (both P < 0.001), respectively. Significant differences in mean CC were present across sex, ethnic, self-reported race, and BMI groups. Adjusting CC for adiposity using BMI revealed a decrease in CC beginning after the second decade in males and third decade in females. Rounded CC cutoff values for moderately and severely low CC were 34 cm and 32 cm (males), and 33 cm and 31 cm (females), respectively. Our findings support the use of BMI-adjusted CC values for participants outside the normal-weight BMI range (18–24.9).
Conclusions
This study defined CC values in a diverse population sample along with a BMI-adjustment approach that helps to remove the confounding effects of adiposity and thereby improves CC as a useful clinical estimate of SM mass.
Keywords: anthropometry, calf circumference, muscle mass, body composition, malnutrition, cutoff
See corresponding editorial on page 1398.
Introduction
Skeletal muscle (SM) is the largest body compartment in people who are not obese (1) and has a substantial reserve of available protein that can be metabolized during periods of negative nitrogen balance (2). Accordingly, SM mass and function are classic markers of nutritional status closely associated with clinical outcomes, including morbidity and mortality (3).
Over the past several decades, SM mass and function were additionally centered as the pathophysiological markers of sarcopenia (4) and its variants such as sarcopenic obesity (5). Muscle mass was also included as one of the phenotypic criteria for diagnosing malnutrition according to the Global Leadership Initiative on Malnutrition (6). Measuring regional and whole-body SM mass is now possible with computed tomography and MRI, methods available to investigators primarily working at research facilities. DXA is an alternative imaging method that provides estimates of appendicular lean soft tissue (ALST) or appendicular lean mass, a compartment highly correlated with appendicular skeletal muscle and whole-body muscle mass (7). All 3 of these imaging methods have served as references for developing other simpler, lower cost measures of SM mass such as anthropometry and bioelectrical impedance analysis. Nonetheless, there is a demand for simple tools that can be applied in routine clinical use that improve the nutritional status assessment in every patient, regardless of financial constraints (8).
One anthropometric approach gaining interest as a marker of SM mass in limited settings is calf circumference (CC) measurement. Values for CC are highly correlated with direct measurements of SM mass in cadavers (r = 0.90 in males and r = 0.77 in females) (9). Kawakami et al. (10) reported high correlations between CC and DXA ALST (r = 0.81 in males and r = 0.73 in females) and height-adjusted ALST (r = 0.80 in males and r = 0.69 in females). Santos et al. (11) confirmed these findings and reported prediction equations for DXA appendicular SM from CC using data from 15,293 adult participants in the NHANES 1999–2006. CC measurements are associated with grip strength (12), fat-free mass, and basal energy expenditure (13), rates of hospital readmission (14), insulin resistance, and carotid atherosclerosis (15).
CC measurements are largely used in geriatric studies as a muscle marker, and are the most used tool for muscle mass assessment in clinical practice for the diagnosis of sarcopenia in older people (16). Recently, CC has been recommended as a muscle marker for sarcopenia case finding by the Asian Working Group for Sarcopenia Consensus (17), as well as a component of sarcopenia screening tools such as that proposed by Ishii et al. (12) and the SARC-CalF score (18).
Although CC has many important qualities as a practical tool for evaluating the adequacy of SM mass in limited settings or in patients whose mobility is restricted, an important gap remains: values, appropriately adjusted for confounding factors, in a large and diverse sample are lacking. All of the CC cutoff points published so far came from studies in older persons (10, 18–22). With that in mind, the aim of the current study was to fill that gap by analyzing a large sample of healthy participants from the NHANES 1999–2006 database.
Methods
Study design
This was a cross-sectional study using the database developed from the NHANES survey data collected from 1999 to 2006. Participants in NHANES had body shape and composition evaluated using a combination of anthropometric and imaging methods. The NHANES protocol was approved by the Institutional Review Board of the National Center for Health Statistics, CDC. All participants provided written informed consent. The NHANES DXA dataset used in this study is accessible online at the CDC website (https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx).
Participants
Because random sampling is not feasible, the NHANES applies a complex multistage sampling strategy from the entire US population that includes subgroups of people who are institutionalized. In the current study, NHANES participants were excluded if they were aged <18 y, pregnant, or had missing data on our primary variable dataset. Sample data were partitioned into subgroups according to sex, age, ethnicity, and race. People self-identifying as Mexican American (MA) were coded as such in this phase of NHANES. People self-identifying as non-Hispanic white (NHW) or non-Hispanic black (NHB) were coded as separate race groups. Remaining participants were coded as “other” (OTHR), a smaller diverse sample that included other Hispanics and multiracial persons. Participants were grouped into 7 age categories, 18–19, ≥20 y in 10-y increments, and 70+.
DXA assessments
Body composition was evaluated at the Mobile Examination Center with a Hologic QDR 4500A fan beam X-ray bone densitometer (Hologic Inc., and Hologic Discovery software, version 12.1). The whole-body scans were performed under standard conditions and a detailed description can be found in the NHANES procedures manual (23). Exclusion criteria for DXA examination were weight >136 kg (300 lb), height >1.96 m (6 ft 5 in), or contrast-based radiological nuclear examinations in the prior 72 h. ALST was estimated as the sum of the lean soft tissue from the legs and arms and adjusted by height squared to obtain the appendicular lean mass index (ALMI, in kilograms per meter squared).
Anthropometric measures
Weight, height, and CC were measured at the Mobile Examination Center using the standard methodology described in detail in the NHANES procedures manual (23). BMI was estimated from weight and height and stratified according to WHO standards (24).
Participants clothed in underwear with disposable paper gowns and foam slippers had their weight measured with a Toledo digital scale (Mettler-Toledo Inc.) and height measured with a fixed stadiometer (Seca electronic stadiometer) with a vertical backboard and a movable headboard. The participants stood on the floor, with the heels of both feet together. The buttocks, shoulder blades, and the back of the head stayed contacting the vertical backboard. The participants’ heads were aligned in the Frankfurt horizontal plane. A nonflexible steel measuring tape was used for all of the anthropometric assessments.
CC was measured using the steel measuring tape while the participant remained in a seated position. The maximal CC was measured on a perpendicular plane to the long axis on the right calf, to the nearest 0.1 cm.
Statistical analysis
To increase the representativeness of the sample at the individual participant level, probability sampling weights were applied considering survey nonresponse, oversampling, poststratification, and sampling errors. The sample characteristics were described as absolute and relative frequencies (categorical variables) or mean and SDs (continuous variables). Outliers for CC measurements were identified and removed from the dataset based on the IQR method with a threshold of 3. Descriptive statistics, including CC mean and SD, median, 5th, 50th, and 95th percentiles, were calculated for established sex, ethnic, race, age, and BMI groups. The relation between ALMI and CC was tested by Pearson correlation coefficient, and its strength was classified according to r values as very high (r = 0.90–1.00), high (r = 0.70–0.90), moderate (0.50–0.70), low (0.30–0.50), or negligible (0.00–0.30) (25).
Significant differences for CC across race and ethnic groups were evaluated with variance and post hoc tests for each group by age, sex, ethnicity, and race. The Levene test was used to evaluate if each group sample had equal variances. We applied a 1-factor ANOVA and parametric Bonferroni test for equal variance, and Kruskal–Wallis and Dunn tests for unequal variances. Data from an individual age group (18–39 y) were explored to determine CC values in young adults with BMI 18.5–24.9 as the reference population. We used weighted linear regression models controlled by age to determine BMI adjustment factors for CC estimation and applied them in participants with BMIs different from 18.5–24.9. Significance was based on P < 0.05 for all tests.
All parameter estimates were obtained using Python 3.7.6 and its associated “Scipy” (Scientific Library for Python for free) and “Statsmodels,” a Python module that provides classes and functions for the estimation of many different statistical models, as well as for conducting statistical tests, and statistical data exploration. Graphs were made using the Stata 16.1 program (StataCorp.).
Results
A total sample of 17,789 participants (51.3% males and 48.7% females) was analyzed from 17,856 participants, after excluding outliers (n = 67) as described in Methods (Supplemental Figure 1). Tables 1 and 2 summarize the age and body composition characteristics of the sample according to sex, self-reported race, and ethnicity. From the total sample, race and ethnicity were distributed as follows: 46.7% NHW, 21.6% NHB, 23.8% MA, and 7.9% OTHR. The mean sample age was 43.3 ± 16.1 y for males and 45.5 ± 16.9 y for females. The mean sample BMI did not differ between males and females, 27.9 ± 5.3 and 28.0 ± 6.7, respectively, but there were some differences in BMI among race and ethnic groups. Males and females from OTHR had a lower BMI than their NHW peers, although NHB and MA females had a higher BMI than NHW females. NHW males had the highest fat mass among the ethnic and race groups, whereas NHB males showed the highest lean mass and ALMI. Among the females, NHBs showed the highest fat mass, lean mass, and ALMI mean values among ethnic and race groups.
TABLE 1.
Characteristics of the participants from NHANES 1999–2006: males1
| Male | Total (n = 9134) | NH white (n = 4298) | NH black (n = 1959) | Mexican American (n = 2191) | OTHR (n = 686) | P value |
|---|---|---|---|---|---|---|
| Age, y | 43.3 ± 16.1 | 45.0 ± 16.3 | 40.9 ± 15.32 | 36.3 ± 13.52 | 40.0 ± 14.92 | <0.001 |
| Weight, kg | 86.8 ± 18.5 | 88.3 ± 18.1 | 87.3 ± 20.5 | 80.6 ± 16.52 | 80.6 ± 18.12 | <0.001 |
| Height, cm | 176.3 ± 7.6 | 177.5 ± 7.2 | 177.2 ± 7.2 | 170.1 ± 7.12 | 171.8 ± 7.62 | <0.001 |
| BMI, kg/m2 | 27.9 ± 5.3 | 28.0 ± 5.3 | 27.8 ± 5.9 | 27.8 ± 4.9 | 27.2 ± 5.12 | <0.001 |
| Fat mass, kg | 25.1 ± 10.1 | 25.8 ± 10.1 | 23.3 ± 11.22 | 23.3 ± 9.02 | 22.9 ± 9.32 | <0.001 |
| Fat mass, % | 27.7 ± 6.2 | 28.1 ± 6.1 | 25.3 ± 6.82 | 27.8 ± 5.52 | 27.3 ± 5.92 | <0.001 |
| Lean mass, kg | 59.8 ± 9.7 | 60.5 ± 9.4 | 61.9 ± 10.72 | 55.6 ± 8.52 | 56.0 ± 10.12 | <0.001 |
| ALMI, kg/m2 | 8.6 ± 1.3 | 8.5 ± 1.2 | 9.3 ± 1.52 | 8.5 ± 1.1 | 8.4 ± 1.3 | <0.001 |
Values shown as mean ± SD; P value indicates significant differences in variables among ethnic and race groups. ALMI, appendicular lean mass index; NH, non-Hispanic; OTHR, other ethnic and race classification.
Significantly different compared with NH white using pairwise test for multiple comparisons of independent groups (P values adjusted by Bonferroni method: P < 0.001).
TABLE 2.
Characteristics of the participants from NHANES 1999–2006: females1
| Female | Total (n = 8655) | NH white (n = 4011) | NH black (n = 1891) | Mexican American (n = 2040) | OTHR (n = 713) | P value |
|---|---|---|---|---|---|---|
| Age, y | 45.5 ± 16.9 | 47.1 ± 17.1 | 42.8 ± 15.82 | 38.2 ± 14.62 | 42.6 ± 15.82 | <0.001 |
| Weight, kg | 73.8 ± 18.5 | 73.5 ± 18.1 | 82.1 ± 20.52 | 72.0 ± 16.52 | 68.3 ± 17.02 | <0.001 |
| Height, cm | 162.3 ± 6.8 | 163.2 ± 6.4 | 163.1 ± 6.5 | 157.8 ± 6.42 | 158.2 ± 6.72 | <0.001 |
| BMI, kg/m2 | 28.0 ± 6.7 | 27.6 ± 6.6 | 30.9 ± 7.42 | 28.9 ± 6.42 | 27.2 ± 6.12 | <0.001 |
| Fat mass, kg | 30.4 ± 12.0 | 30.2 ± 11.9 | 34.4 ± 13.42 | 30.2 ± 10.5 | 27.8 ± 10.72 | <0.001 |
| Fat mass, % | 39.6 ± 6.7 | 39.5 ± 6.9 | 40.3 ± 6.9 | 40.6 ± 5.82 | 39.2 ± 6.1 | <0.001 |
| Lean mass, kg | 41.9 ± 7.4 | 41.8 ± 7.1 | 46.0 ± 8.12 | 40.4 ± 6.82 | 39.2 ± 7.02 | <0.001 |
| ALMI, kg/m2 | 6.7 ± 1.3 | 6.6 ± 1.2 | 7.8 ± 1.52 | 6.7 ± 1.2 | 6.6 ± 1.2 | <0.001 |
Values shown as mean ± SD; P value indicates significant differences in variables among ethnic and race groups. ALMI, appendicular lean mass index; NH, non-Hispanic; OTHR, other ethnic and race classification.
Significantly different compared with NH white using pairwise test for multiple comparisons of independent groups (P values adjusted by Bonferroni method: P < 0.001).
The correlation scatterplot between CC and ALMI is presented in Figure 1. There was a high positive correlation for both sexes (Pearson correlation r = 0.80 for males and females), and CC alone explained 64% and 65% of ALMI variability for males and females, respectively. These correlations varied with age, with the highest values in participants aged <20 y (r = 0.84 for males and r = 0.86 for females) and the lowest values in participants aged >70 y (r = 0.75 for males and r = 0.71 for females) (Supplemental Table 1).
FIGURE 1.
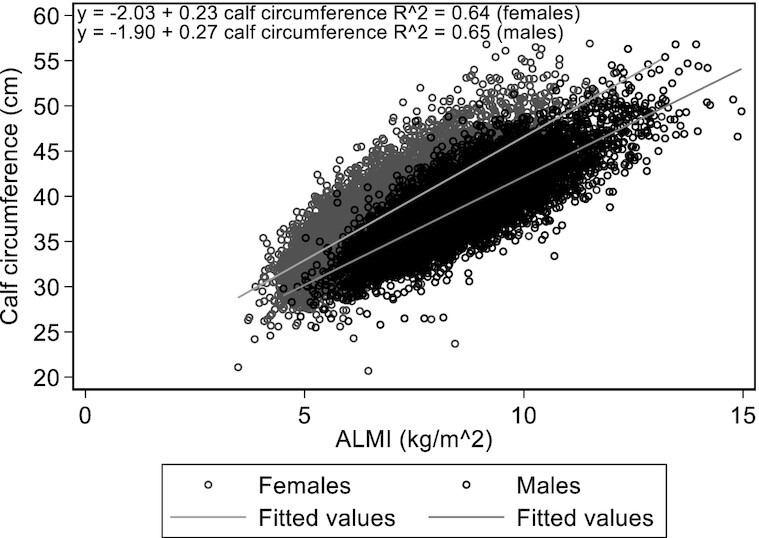
Correlation, linear regression, and coefficient of determination (R^2) between appendicular lean mass index (ALMI) and calf circumference in males (n = 9134, black dots) and females (n = 8655, gray dots). Pearson correlation coefficient = 0.80 (males and females).
Table 3 shows CC values by sex, ethnicity, and race. There were significant differences not only between males and females, as expected, but also among ethnic and race groups. Males had higher CC mean values than females for all ethnic and race groups except NHBs, whereas females had higher CC mean values than males. All CC mean values differed among the ethnic and race groups. In males, the lowest CC was found in the MA group, whereas in females, the lowest CC mean values were present in the OTHR group. The ethnic and race group with the highest CC also differed between males and females: in males, NHWs had the highest mean values (39.6 ± 3.8 cm), whereas in females, the highest mean values were found in NHBs (39.4 ± 4.7 cm).
TABLE 3.
Mean ± SD values for calf circumference according to sex, ethnicity, and race (n = 17,789)1
| Males (n = 9134) | Females (n = 8655) | |
|---|---|---|
| Total | 39.3 ± 3.9 | 38.2 ± 4.5 |
| Non-Hispanic white (n = 8309) | 39.6 ± 3.8 | 38.3 ± 4.5 |
| Non-Hispanic black (n = 3850) | 39.2 ± 4.2 | 39.4 ± 4.7 |
| Mexican American (n = 4231) | 37.8 ± 3.5 | 37.1 ± 4.2 |
| Other ethnicity and race (n = 1399) | 38.5 ± 3.9 | 37.0 ± 4.3 |
All values for males and females are significantly different according to ethnicity and race: non-Hispanic white > non-Hispanic black > Other ethnicity and race > Mexican American for males; non-Hispanic black > non-Hispanic white > Mexican American > Other ethnicity and race for females (P < 0.001 from pairwise test for multiple comparisons of independent groups: Dunn test).
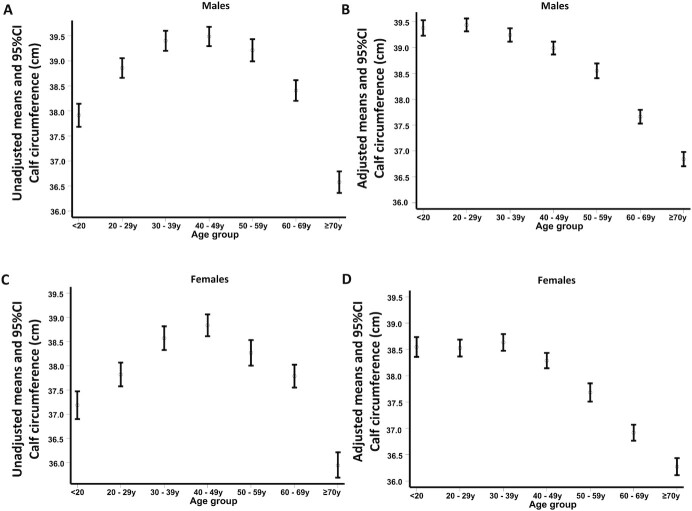
Figure 2 shows the variation of CC according to age and ethnicity and race for males and females. For all of the ethnic and race groups, in males and females, CC remained almost stable until age 60 y and decreased slowly after this age. The differences were more remarkable among ethnic and race groups for males in the fourth decade, when NHW males began to show higher CC mean values than the other ethnic and race groups. In females, NHBs showed higher CC values than all of the other ethnic and race groups until the seventh decade, and from this age on, their values were similar to those found for NHW females. Across all age groups, MA and OTHR females showed comparable values, consistently lower than NHB and NHW females. This variation with age changed when CC values were adjusted by BMI. Figure 3 shows the unadjusted and BMI-adjusted CC mean values according to age group. Unadjusted CC mean values increased progressively until the fourth decade, and then decreased slowly, with a marked decrease after the sixth decade for males and females. A different result was observed when CC mean values were adjusted by BMI: the CC peak was present in the second decade for males and in the third decade for females, with a progressive decline after these ages.
FIGURE 2.
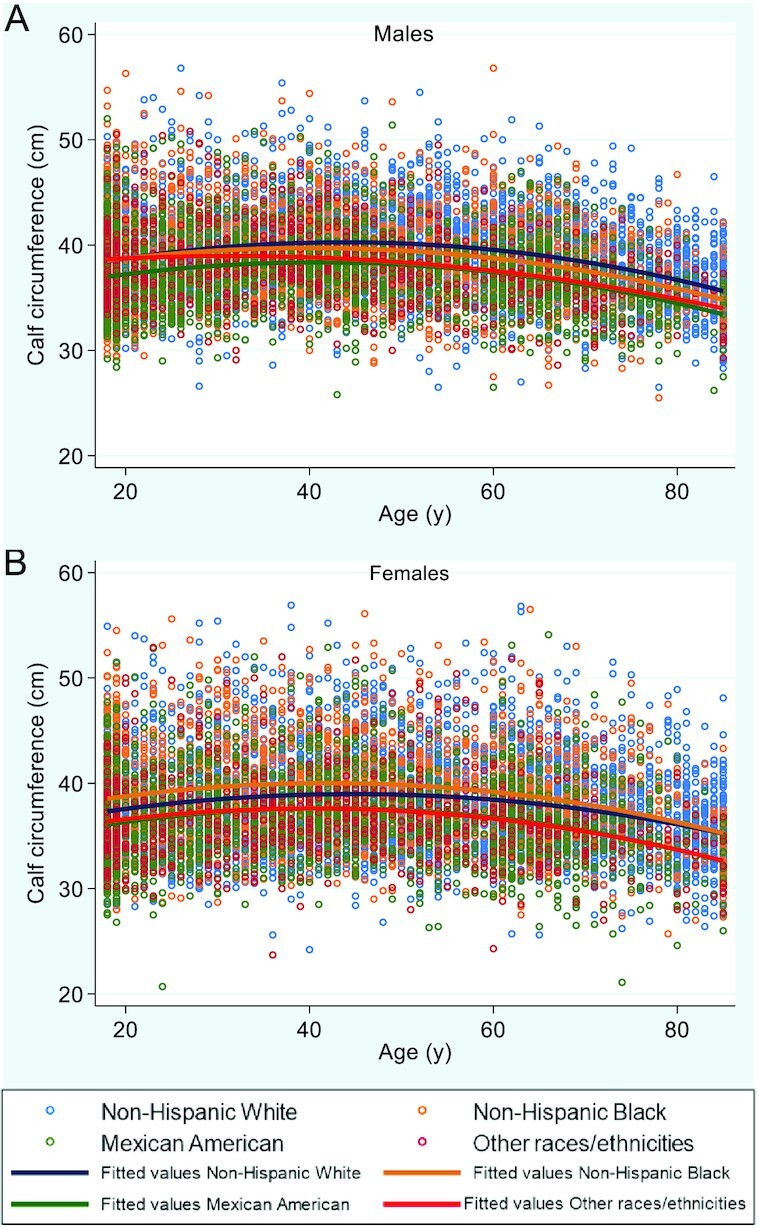
Distribution of calf circumference values according to age, ethnicity, and race, for (A) males (n = 9134) and (B) females (n = 8655).
FIGURE 3.
Unadjusted and BMI-adjusted calf circumference (mean and limits of 95% confidence interval) according to age for males (n = 9134) and females (n = 8655). (A) unadjusted males, (B) BMI-adjusted males, (C) unadjusted females, and (D) BMI-adjusted females.
As shown in Figure 4, CC also varied among BMI groups. Across age groups, there was an evident difference in CC values between BMI categories. For males and females in all age groups, CC values were lower in participants with BMI <18.5 and higher in participants with overweight or any level of obesity than in participants with normal BMI.
FIGURE 4.
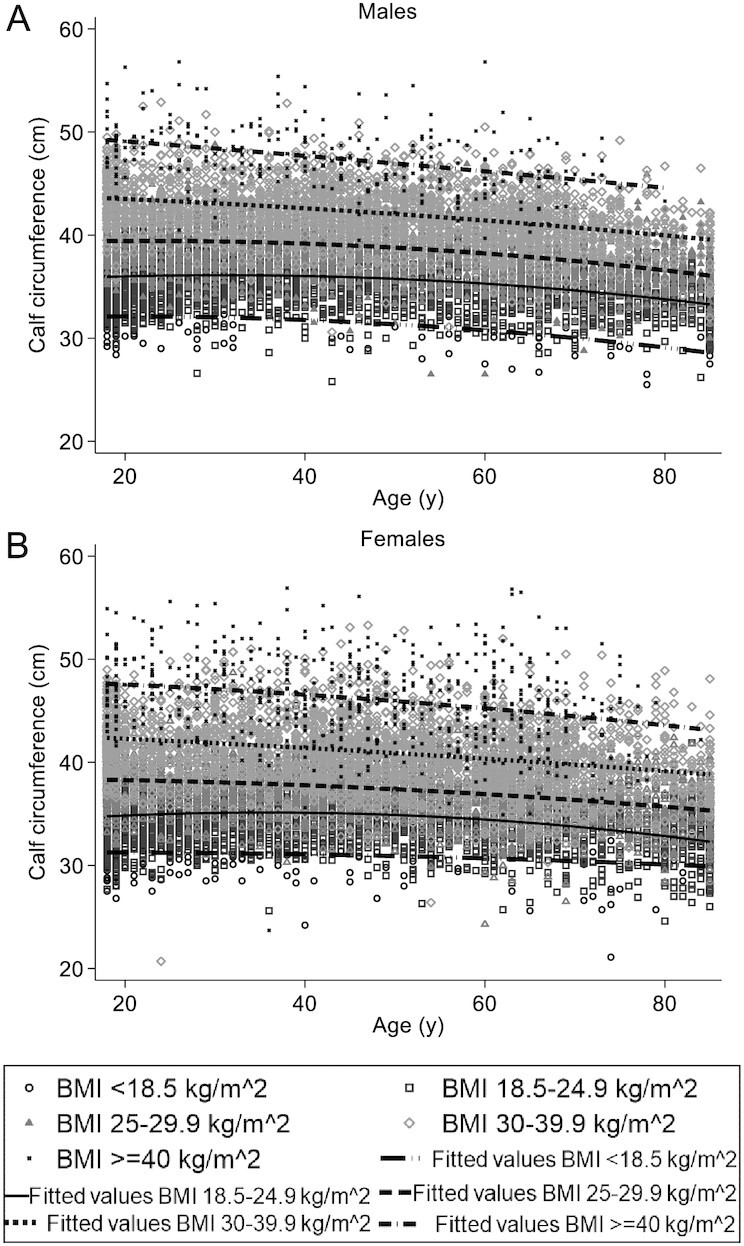
Distribution of calf circumference values according to age and BMI categories, for (A) males (n = 9134) and (B) females (n = 8655).
The weighted CC percentiles, means, and SDs according to sex, ethnicity, race, age, and BMI categories are shown as Supplemental Tables 2–5 (males) and Supplemental Tables 6–9 (females).
A subsample of 3104 participants aged 18–39 y and with normal BMI (18.5–24.9) was defined as the reference population. Their CC mean values with SDs are presented in Table 4. The respective cutoff values were generated using 1 or 2 SDs below each mean to define, respectively, moderately low or severely low CC values. The generated cutoff values for the total sample, NHWs, and NHBs are remarkably similar, and only MAs and OTHRs had significantly smaller values than the other ethnic and race groups. The use of rounded figures for cutoff values was suggested in the updated European Working Group on Sarcopenia in Older People (EWGSOP2) Consensus (4) to facilitate their clinical practice use. Accordingly, rounded cutoff values for males are 34 and 32 cm (using, respectively, 1 and 2 SDs) for the total sample and all ethnic and race groups—except for MAs (33 and 31 cm). For females, the rounded cutoff values are 33 and 31 cm (1 and 2 SDs, respectively) for the total sample, NHWs, and NHBs. For MA and OTHR females, the values would be lower: 32 and 30 cm, using, respectively, 1 and 2 SDs.
TABLE 4.
Reference1 and cutoff values2 for calf circumference according to sex, ethnicity, and race, from participants with normal BMI3
| Males | Females | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. values1 | Cutoff values2 | Ref. values1 | Cutoff values2 | |||||||||
| n | Mean ± SD | −1 SD | −1 SD RV | −2 SD | −2 SD RV | n | Mean ± SD | −1 SD | −1 SD RV | −2 SD | −2 SD RV | |
| Total | 1639 | 36.3 ± 2.2 | 34.1 | 34 | 31.9 | 32 | 1465 | 35.3 ± 2.3 | 33.0 | 33 | 30.7 | 31 |
| Non-Hispanic white | 633 | 36.6 ± 2.2 | 34.4 | 34 | 32.2 | 32 | 656 | 35.6 ± 2.2 | 33.4 | 33 | 31.2 | 31 |
| Non-Hispanic black | 429 | 36.4 ± 2.2 | 34.2 | 34 | 32.0 | 32 | 279 | 35.3 ± 2.2 | 33.1 | 33 | 30.9 | 31 |
| Mexican American | 428 | 34.9 ± 2.1 | 32.8 | 33 | 30.7 | 31 | 378 | 33.9 ± 2.3 | 31.6 | 32 | 29.3 | 29 |
| Other ethnicity and race group | 149 | 36.0 ± 2.1 | 33.9 | 34 | 31.8 | 32 | 152 | 34.6 ± 2.2 | 32.4 | 32 | 30.2 | 30 |
Reference values defined as mean values from participants aged 18–39 y.
Cutoff values defined as 1 or 2 SDs below the mean values.
Normal BMI: 18.5–24.9 kg/m2; for other BMI groups, use the adjusting factors for correction of calf circumference (Table 5). RV: rounded value.
Considering that CC varied among different BMI groups, we propose CC adjustment factors for participants with BMI values outside the 18–24.9 range, using age-adjusted linear regression models. This approach enables the use of the suggested cutoff values in participants with any BMI. The total sample's adjustment factors and stratified by sex, ethnicity, and race are presented in Tables 5 and 6.
TABLE 5.
BMI adjustment factors for calf circumference for males outside the 18.5–24.9 BMI range1
| Total | ||
|---|---|---|
| BMI group | Adjustment factor, cm | Rounded value |
| <18.5 | +4.3 | +4.0 |
| 25–29.9 | −3.4 | −3.0 |
| 30–39.9 | −6.8 | −7.0 |
| ≥40 | −12.0 | −12.0 |
| Non-Hispanic white | ||
| BMI group | Adjustment factor, cm | Rounded value |
| <18.5 | +4.7 | +5.0 |
| 25–29.9 | −3.4 | −3.0 |
| 30–39.9 | −6.7 | −7.0 |
| ≥40 | −11.9 | −12.0 |
| Non-Hispanic black | ||
| BMI group | Adjustment factor, cm | Rounded value |
| <18.5 | +4.2 | +4.0 |
| 25–29.9 | −3.4 | −3.0 |
| 30–39.9 | −7.2 | −7.0 |
| ≥40 | −12.0 | −12.0 |
| Mexican American | ||
| BMI group | Adjustment factor, cm | Rounded value |
| <18.5 | +4.0 | +4.0 |
| 25–29.9 | −3.1 | −3.0 |
| 30–39.9 | −6.4 | −6.0 |
| ≥40 | −12.1 | −12.0 |
| Other ethnicity and race | ||
| BMI group | Adjustment factor, cm | Rounded value |
| <18.5 | +3.4 | +3.0 |
| 25–29.9 | −3.5 | −4.0 |
| 30–39.9 | −6.9 | −7.0 |
| ≥40 | −12.2 | −12.0 |
Adjustment factor from linear regression for calf circumference, adjusted by age, for BMI outside the 18.5–24.9 range. All BMI ranges given in units of kg/m2.
TABLE 6.
BMI adjustment factors for calf circumference for females outside the 18.5–24.9 BMI range1
| Total | ||
|---|---|---|
| BMI group | Adjustment factor, cm | Rounded value |
| <18.5 | +3.9 | +4.0 |
| 25–29.9 | −3.0 | −3.0 |
| 30–39.9 | −6.7 | −7.0 |
| ≥40 | −11.6 | −12.0 |
| Non-Hispanic white | ||
| BMI group | Adjustment factor, cm | Rounded value |
| <18.5 | +4.0 | +4.0 |
| 25–29.9 | −3.2 | −3.0 |
| 30–39.9 | −6.9 | −7.0 |
| ≥40 | −11.8 | −12.0 |
| Non-Hispanic black | ||
| BMI group | Adjustment factor, cm | Rounded value |
| <18.5 | +4.3 | +4.0 |
| 25–29.9 | −3.2 | −3.0 |
| 30–39.9 | −6.9 | −7.0 |
| ≥40 | −11.7 | −12.0 |
| Mexican American | ||
| BMI group | Adjustment factor, cm | Rounded value |
| <18.5 | +4.1 | +4.0 |
| 25–29.9 | −2.9 | −3.0 |
| 30–39.9 | −6.3 | −6.0 |
| ≥40 | −11.6 | −12.0 |
| Other ethnicity and race | ||
| BMI group | Adjustment factor, cm | Rounded value |
| <18.5 | +3.7 | +4.0 |
| 25–29.9 | −2.8 | −3.0 |
| 30–39.9 | −6.7 | −7.0 |
| ≥40 | −10.8 | −11.0 |
Adjustment factor from linear regression for calf circumference, adjusted by age, for BMI outside the 18.5–24.9 range. All BMI ranges given in units of kg/m2.
We estimated the prevalence of low CC by 2 approaches: using the adjustment factors and cutoff values developed for the total sample (CVall), and using the ethnicity and race-specific adjustment factors and cutoff values (CVrac), as demonstrated in Tables 4, 5, and 6. The moderately low and severely low CC prevalences using CVall were 22.4% and 9.4%, respectively, in the whole sample. The moderately low and severely low CC prevalences were slightly lower using CVrac: 20.2% and 8.0%, respectively.
We also assessed the prevalence of low CC according to BMI, using the ethnicity and race-specific adjustment factor and the raw CC values for the whole sample (Figure 5). The moderately low CC prevalence varied from 11.6% (BMI <18.5) to 22.0% (BMI 30–39.9). The severely low CC prevalence varied from 3.0% (BMI <18.5) to 16.2% (BMI ≥40). When the raw CC values were used, without the adjustment factor, the prevalences of moderately low and severely low CC were 42.0% and 44.8%, respectively, in participants with BMI <18.5, and the presence of low CC in participants with BMI ≥30 was found in only a minimal number of participants (<0.5%).
FIGURE 5.
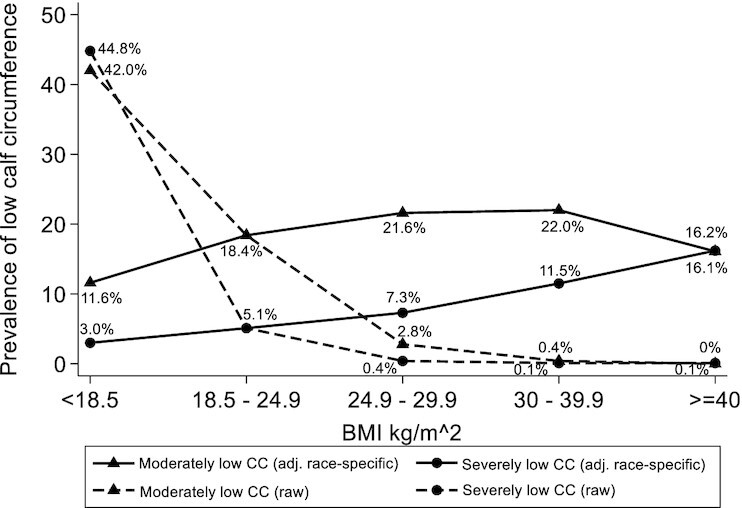
Low calf circumference (CC) prevalence according to BMI. Moderately or severely low CC: 1 (triangles) and 2 (circles) SDs below the mean from the reference population, respectively. Solid lines: using ethnicity and race-specific BMI adjustment factors and cutoff values. Dotted lines: using CC raw values and ethnicity and race-specific cutoff values. adj., adjusted.
Discussion
In this study, to the best of our knowledge, we show for the first time CC values from a large sample of healthy NHANES participants varying in age, ethnicity, and self-reported race. Until now, all published CC cutpoints were developed from people aged >40 y and were defined by statistical methods (receiver operating characteristic) as the best CC value to identify low muscle mass as measured by DXA or bioelectrical impedance analysis for diagnosing sarcopenia (10, 19–22, 26–28).
In all of these studies, except for Bahat et al. (19), CC cutpoints were smaller for females than males. In our study, the rounded cutoff values using 1 SD below the mean were similar to those previously published: 33–34 cm for males (4 of 6 studies) (10, 19–20, 26) and 32–33 cm for females (5 of 8 studies) (10, 19–21, 26). However, we found smaller rounded cutoff values using 2 SDs below the mean (severely low CC): 31–32 cm for males and 29–31 cm for females. Considering that the previously published CC cutoffs were defined to diagnose sarcopenia in older people, our findings suggest that, for older adults, a moderately low CC (1 SD below the mean) can be adequate for sarcopenia diagnosis/screening.
We found a strong correlation between CC and ALMI measured by DXA in all age groups, although the correlations were smaller in the older age groups. In the other noted published studies, this correlation was strong only when participants were aged <60 y (10, 27, 28), being moderate in people aged ≥70 y (21, 22). This smaller correlation between CC and ALMI in older people could be explained by changes in body composition with aging: for the same CC, the nonmuscular components of the calf, not detected by the circumference measurement (subcutaneous adipose tissue and intramuscular adipose tissue), will be larger in older persons (29). Nevertheless, Asai et al. (30) found a strong correlation (r = 0.91 for males and r = 0.90 for females) between CC and calf muscle mass measured by MRI in people aged >60 y. Considering the higher nonmuscular component of the CC in older people, and the risk of muscle mass overestimation, we suggest the cutoff point obtained using 1 SD below the mean (from a reference young population) to detect low muscle mass in adults aged >65 y.
Confirming previous studies using different tools to evaluate body composition, CC values also differ among the ethnic and race groups. Ethnic and race differences in muscularity have been reported across these sociodemographic groups, as demonstrated by other studies (31, 32). Notwithstanding, at the same BMI, these differences are smaller (by only decimal points), with no clinical practice significance when we use rounded CC values as suggested in the recent Consensus (4). Only MAs (females and males) and females from OTHRs have a different suggested cutoff. The differences in CC measurements appear related to the difference in BMI among the ethnic and race groups (adipose tissue component from CC) rather than the muscle component itself.
The age-related changes in muscle mass were also observed in this study. Our results for unadjusted mean CC values are similar to those found by Landi et al. (33), being almost stable until the fifth decade and decreasing slightly after this age. It is interesting to note that the CC variation across the age groups changed after BMI adjustment: the peak values for males and females are in the second and third decade, respectively, with a steeper decrease after the fourth decade, comparable to muscle strength variation with age. It appears as if BMI confounds the effect of age on CC; after this adjustment, CC improves as a muscle mass marker and could also have stronger correlations with function.
As expected, mean CC values are significantly higher in persons with overweight/obesity, and smaller in persons with BMI <18.5, regardless of age, ethnicity, or race. This observation can be explained by a larger amount of adipose and intermuscular adipose tissue in the calves, as demonstrated by Hilton et al. (34). Obesity is considered one of the limitations of CC diagnostic performance as a muscle mass marker (35), and BMI, as a marker of adiposity/obesity, is an independent associated factor for predicting calf muscle mass measured by MRI, in addition to CC per se (30). We also show in this study the influence of BMI on age and ethnic and race CC variation. To overcome this important effect, we suggested adjustment factors for BMIs beyond the 18.5–24.9 range. Using raw CC measurements would underestimate the prevalence of low CC in people with BMI ≥25, and overestimate it in those with BMI <18.5. In our sample, using the raw CC values, almost 87% of the participants with BMI <18.5 would be classified as having a low CC, and almost no participant with BMI >30 would have low CC. We do not believe that these prevalence estimates represent the actual frequency of low muscle mass in the general population. The prevalence of low muscle mass is more realistic using the adjusted CC values, identifying low CC even in the presence of obesity. Age might explain the lower prevalence of low CC values in people with BMI <18.5 when we use the adjustment factor: these individuals are significantly younger than those in the other BMI categories. The statistical approach suggested in this study (BMI adjustment factors) is easier than using the CC corrected by skinfold thickness, suggested by some authors (36), especially in people with overweight and obesity. The adjusted CC is easily obtained by adding 4 cm (BMI <18.5) or subtracting 3, 7, or 12 cm (BMI 25–29, 30–39, and ≥40, respectively) from the CC measure. The BMI adjustment factors would also allow the correct identification of low CC under any BMI, using the cutoff values developed from the reference population.
One limitation of this study is the use of BMI as a marker of CC's adiposity component. We are aware of the modest association between BMI and percentage fat mass in this same sample (11). Notwithstanding, BMI as an adiposity marker can be used in any clinical setting where CC measurement is intended to be used. Another limitation is using cutpoint values derived from a reference (healthy and young) population in older persons. As observed with other body composition tools, such as DXA, an older person's CC muscle component is smaller than a younger person's, even for the same CC measurement and BMI. Nonetheless, our cutoff values are remarkably similar to those generated from studies with older participants.
In conclusion, this study proposes CC values that can be used as a marker for the muscle mass assessment. The BMI adjustment factors should be used to obtain a better estimation of the muscle compartment in persons with BMI different from the normal range. This approach enables the use of CC in clinical practice, helping in the identification of low muscle mass. Further clinical studies could show the improvement of the role of CC as a muscle marker and its association with functional or adverse outcomes with this new approach.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—MCG, SBH: designed and conducted the research, contributed to the analysis and interpretation of data, and drafted, critically revised, and gave the final approval for the manuscript; AM, NR: performed the statistical analysis, critically revised, and gave the final approval for the manuscript; TGB-S: contributed to the interpretation of data, drafted, critically revised, and gave the final approval for the manuscript; and all authors: read and approved the final manuscript.
The authors report no conflicts of interest.
Notes
This work was partially supported by NIH NORC Center Grants P30DK072476, Pennington/Louisiana; and P30DK040561, Harvard; and R01DK109008, Shape UP! Adults.
Supplemental Tables 1–9 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ALMI, appendicular lean mass index; ALST, appendicular lean soft tissue; CC, calf circumference; CVall, cutoff value total sample; CVrac, ethnicity and race-specific cutoff values; NHB, non-Hispanic black; NHW, non-Hispanic white; MA, Mexican American; OTHR, other ethnicity and race; SM, skeletal muscle.
Contributor Information
Maria Cristina Gonzalez, Post-Graduate Program in Health and Behavior, Catholic University of Pelotas, Pelotas, Rio Grande do Sul, Brazil; Pennington Biomedical Research Center, LSU System, Baton Rouge, LA, USA.
Ali Mehrnezhad, Louisiana State University, Baton Rouge, LA, USA.
Nariman Razaviarab, Louisiana State University, Baton Rouge, LA, USA.
Thiago G Barbosa-Silva, Federal University of Pelotas, Pelotas, Rio Grande do Sul, Brazil.
Steven B Heymsfield, Pennington Biomedical Research Center, LSU System, Baton Rouge, LA, USA.
Data Availability
Data described in the manuscript and code book are publicly and freely available without restriction at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
References
- 1.Later W, Bosy-Westphal A, Kossel E, Gluer CC, Heller M, Muller MJ. Is the 1975 Reference Man still a suitable reference?. Eur J Clin Nutr. 2010;64:1035–42. [DOI] [PubMed] [Google Scholar]
- 2.Argiles JM, Campos N, Lopez-Pedrosa JM, Rueda R, Rodriguez-Manas L. Skeletal muscle regulates metabolism via interorgan crosstalk: roles in health and disease. J Am Med Dir Assoc. 2016;17:789–96. [DOI] [PubMed] [Google Scholar]
- 3.Deutz NEP, Ashurst I, Ballesteros MD, Bear DE, Cruz-Jentoft AJ, Genton L, Landi F, Laviano A, Norman K, Prado CM. The underappreciated role of low muscle mass in the management of malnutrition. J Am Med Dir Assoc. 2019;20:22–7. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donini LM, Busetto L, Bauer JM, Bischoff S, Boirie Y, Cederholm T, Cruz-Jentoft AJ, Dicker D, Fruhbeck G, Giustina A et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr. 2020;39:2368–88. [DOI] [PubMed] [Google Scholar]
- 6.Jensen GL, Cederholm T, Correia M, Gonzalez MC, Fukushima R, Higashiguchi T, de Baptista GA, Barazzoni R, Blaauw R, Coats AJS et al. GLIM criteria for the diagnosis of malnutrition: a consensus report from the global clinical nutrition community. JPEN J Parenter Enteral Nutr. 2019;43:32–40. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z, Wang Z, Lohman T, Heymsfield SB, Outwater E, Nicholas JS, Bassford T, LaCroix A, Sherrill D, Punyanitya M et al. Dual-energy X-ray absorptiometry is a valid tool for assessing skeletal muscle mass in older women. J Nutr. 2007;137:2775–80. [DOI] [PubMed] [Google Scholar]
- 8.Bistrian BR, Mogensen KM, Christopher KB. Plea for reapplication of some of the older nutrition assessment techniques. JPEN J Parenter Enteral Nutr. 2020;44:391–4. [DOI] [PubMed] [Google Scholar]
- 9.Tresignie J, Scafoglieri A, Pieter Clarys J, Cattrysse E. Reliability of standard circumferences in domain-related constitutional applications. Am J Hum Biol. 2013;25:637–42. [DOI] [PubMed] [Google Scholar]
- 10.Kawakami R, Murakami H, Sanada K, Tanaka N, Sawada SS, Tabata I, Higuchi M, Miyachi M. Calf circumference as a surrogate marker of muscle mass for diagnosing sarcopenia in Japanese men and women. Geriatr Gerontol Int. 2015;15:969–76. [DOI] [PubMed] [Google Scholar]
- 11.Santos LP, Gonzalez MC, Orlandi SP, Bielemann RM, Barbosa-Silva TG, Heymsfield SB, Group CS. New prediction equations to estimate appendicular skeletal muscle mass using calf circumference: results from NHANES 1999–2006. JPEN J Parenter Enteral Nutr. 2019;43:998–1007. [DOI] [PubMed] [Google Scholar]
- 12.Ishii S, Tanaka T, Shibasaki K, Ouchi Y, Kikutani T, Higashiguchi T, Obuchi SP, Ishikawa-Takata K, Hirano H, Kawai H et al. Development of a simple screening test for sarcopenia in older adults. Geriatr Gerontol Int. 2014;14(Suppl 1):93–101. [DOI] [PubMed] [Google Scholar]
- 13.Isobe Y, Sakurai M, Kita Y, Takeshita Y, Misu H, Kaneko S, Takamura T. Fat-free mass and calf circumference as body composition indices to determine non-exercise activity thermogenesis in patients with diabetes. J Diabetes Investig. 2016;7:352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Real GG, Fruhauf IR, Sedrez JHK, Dall'Aqua EJF, Gonzalez MC. Calf circumference: a marker of muscle mass as a predictor of hospital readmission. JPEN J Parenter Enteral Nutr. 2018;42:1272–9. [DOI] [PubMed] [Google Scholar]
- 15.Park JS, Cho MH, Ahn CW, Kim KR, Huh KB. The association of insulin resistance and carotid atherosclerosis with thigh and calf circumference in patients with type 2 diabetes. Cardiovasc Diabetol. 2012;11:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruyère O, Beaudart C, Reginster J-Y, Buckinx F, Schoene D, Hirani V, Cooper C, Kanis JA, Rizzoli R, McCloskey E. Assessment of muscle mass, muscle strength and physical performance in clinical practice: an international survey. Eur Geriatr Med. 2016;7:243–6. [Google Scholar]
- 17.Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300. [DOI] [PubMed] [Google Scholar]
- 18.Barbosa-Silva TG, Menezes AM, Bielemann RM, Malmstrom TK, Gonzalez MC, Grupo de Estudos em Composição Corporal e Nutrição. Enhancing SARC-F: improving sarcopenia screening in the clinical practice. J Am Med Dir Assoc. 2016;17:1136–41. [DOI] [PubMed] [Google Scholar]
- 19.Bahat G, Tufan A, Tufan F, Kilic C, Akpinar TS, Kose M, Erten N, Karan MA, Cruz-Jentoft AJ. Cut-off points to identify sarcopenia according to European Working Group on Sarcopenia in Older People (EWGSOP) definition. Clin Nutr. 2016;35:1557–63. [DOI] [PubMed] [Google Scholar]
- 20.Hwang AC, Liu LK, Lee WJ, Peng LN, Chen LK. Calf circumference as a screening instrument for appendicular muscle mass measurement. J Am Med Dir Assoc. 2018;19:182–4. [DOI] [PubMed] [Google Scholar]
- 21.Kim S, Kim M, Lee Y, Kim B, Yoon TY, Won CW. Calf circumference as a simple screening marker for diagnosing sarcopenia in older Korean adults: the Korean Frailty and Aging Cohort Study (KFACS). J Korean Med Sci. 2018;33:e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rolland Y, Lauwers-Cances V, Cournot M, Nourhashemi F, Reynish W, Riviere D, Vellas B, Grandjean H. Sarcopenia, calf circumference, and physical function of elderly women: a cross-sectional study. J Am Geriatr Soc. 2003;51:1120–4. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. National Center for Health Statistics (NCHS) . National Health and Nutrition Examination Survey questionnaire (or examination protocol, or laboratory protocol). 2006; [cited 10 October, 2020] [Internet]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/1999-2000/manuals/bm.pdf. [Google Scholar]
- 24.World Health Organization . Obesity: preventing and managing the global epidemic. Geneva, Switzerland: World Health Organization; 2000. [PubMed] [Google Scholar]
- 25.Mukaka MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24:69–71. [PMC free article] [PubMed] [Google Scholar]
- 26.Barbosa-Silva TG, Bielemann RM, Gonzalez MC, Menezes AM. Prevalence of sarcopenia among community-dwelling elderly of a medium-sized South American city: results of the COMO VAI? study. J Cachexia Sarcopenia Muscle. 2016;7:136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawakami R, Miyachi M, Sawada SS, Torii S, Midorikawa T, Tanisawa K, Ito T, Usui C, Ishii K, Suzuki K et al. Cut-offs for calf circumference as a screening tool for low muscle mass: WASEDA'S Health Study. Geriatr Gerontol Int. 2020;20:943–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ukegbu PO, Kruger HS, Meyer JD, Nienaber-Rousseau C, Botha-Ravyse C, Moss SJ, Kruger MI. The association between calf circumference and appendicular skeletal muscle mass index of black urban women in Tlokwe City. J Endocrinol Metab Diabetes S Afr. 2018;23:86–90. [Google Scholar]
- 29.Roubenoff R. Sarcopenia: effects on body composition and function. J Gerontol A Biol Sci Med Sci. 2003;58:1012–17. [DOI] [PubMed] [Google Scholar]
- 30.Asai C, Akao K, Adachi T, Iwatsu K, Fukuyama A, Ikeda M, Yamada S. Maximal calf circumference reflects calf muscle mass measured using magnetic resonance imaging. Arch Gerontol Geriatr. 2019;83:175–8. [DOI] [PubMed] [Google Scholar]
- 31.He Q, Heo M, Heshka S, Wang J, Pierson RN Jr, Albu J, Wang Z, Heymsfield SB, Gallagher D. Total body potassium differs by sex and race across the adult age span. Am J Clin Nutr. 2003;78:72–7. [DOI] [PubMed] [Google Scholar]
- 32.Silva AM, Shen W, Heo M, Gallagher D, Wang Z, Sardinha LB, Heymsfield SB. Ethnicity-related skeletal muscle differences across the lifespan. Am J Hum Biol. 2010;22:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landi F, Calvani R, Tosato M, Martone AM, Fusco D, Sisto A, Ortolani E, Savera G, Salini S, Marzetti E. Age-related variations of muscle mass, strength, and physical performance in community-dwellers: results from the Milan EXPO survey. J Am Med Dir Assoc. 2017;18:88.e17. [DOI] [PubMed] [Google Scholar]
- 34.Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther. 2008;88:1336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim WS, Lim JP, Chew J, Tan AWK. Calf circumference as a case-finding tool for sarcopenia: influence of obesity on diagnostic performance. J Am Med Dir Assoc. 2020;21:1359–61. [DOI] [PubMed] [Google Scholar]
- 36.Stewart AD, Stewart A, Reid DM. Correcting calf girth discriminates the incidence of falling but not bone mass by broadband ultrasound attenuation in elderly female subjects. Bone. 2002;31:195–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript and code book are publicly and freely available without restriction at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.