Significance
Many routine, inwardly focused behaviors require little attention and can be carried out automatically. However, environmental changes can necessitate a modification of routine behaviors, and this requires external focus. The default mode network (DMN) becomes active during the performance of routine tasks and is deactivated during new learning. Using an optogenetic approach, we show that activation of the ventral pallidum (VP), a subcortical node of the DMN, keeps animals in an inwardly focused state, severely compromising new learning. Conversely, VP inactivation allows escape from automatized behavior, conferring a learning advantage. We suggest that the VP plays a critical role in global brain state transitions between periods of outward focus and DMN states favoring behaviors reliant on learned internal schemata.
Keywords: default mode network, ventral pallidum, basal forebrain, anterior cingulate cortex, operant behavior
Abstract
Daily life requires transitions between performance of well-practiced, automatized behaviors reliant upon internalized representations and behaviors requiring external focus. Such transitions involve differential activation of the default mode network (DMN), a group of brain areas associated with inward focus. We asked how optogenetic modulation of the ventral pallidum (VP), a subcortical DMN node, impacts task switching between internally to externally guided lever-pressing behavior in the rat. Excitation of the VP dramatically compromised acquisition of an auditory discrimination task, trapping animals in a DMN state of automatized internally focused behavior and impairing their ability to direct attention to external sensory stimuli. VP inhibition, on the other hand, facilitated task acquisition, expediting escape from the DMN brain state, thereby allowing rats to incorporate the contingency changes associated with the auditory stimuli. We suggest that VP, instant by instant, regulates the DMN and plays a deterministic role in transitions between internally and externally guided behaviors.
A considerable amount of our time is spent performing automatic or habitual behaviors that are based on acquired knowledge about our environment. For example, we cross the road upon a green traffic light, we open the fridge to get a drink, or we shake hands when meeting a friend. These acquired action patterns are appropriate as long as the behavioral context remains stable, and they are beneficial because they permit fast, effective responses and free up cognitive resources for other purposes. However, when contingencies change, learned behaviors need to be modified. For example, the potential transmission of viruses makes physical contact undesirable, such that hand shaking must be suppressed. This necessitates adapting our routine response patterns, which in turn requires cognitive flexibility. Successful control of behavior thus involves both the maintenance of appropriate learned action patterns under stable environmental conditions as well as their flexible modification in response to changed environmental demands.
Recently, it has been suggested that the rapid performance of learned responses in a stable behavioral context is associated with activation of the default mode network (DMN) (1, 2). The DMN was originally described in humans as an interconnected set of brain regions that are active during the resting state in the absence of behavioral tasks (3), and early work has emphasized that internally directed processes, such as accessing autobiographical information or memory retrieval (4, 5), are associated with DMN activation. The work of Vatansever and colleagues adds a new perspective, as it implicates the DMN in the generation of automatized behaviors that, as the authors note, resembles an “autopilot” type of behavioral control. DMN-associated behaviors thus encompass not only internally orientated mental operations but also tasks involving actions that are reliant on internalized representations for which feedback from the environment is not a priority.
The DMN encompasses significant portions of the medial frontal and medial parietal cortex, but recent tractography work in humans has revealed that some subcortical brain structures also represent important DMN nodes (6). One of these noncortical DMN nodes is the basal forebrain (BF), which contains a collection of nuclei with substantial GABAergic and cholinergic corticopetal projections to DMN structures (7, 8). Previously, in primates, it has indeed been shown that BF inactivation leads to changes in global functional MRI signals involving the DMN (9), and we have recently provided evidence suggesting that the BF represents a subcortical DMN node in the rat (10), an idea that has gained recent support (6, 11, 12). In particular, we documented pronounced gamma oscillations in the ventral pallidum (VP) region of the BF during quiet wakefulness and self-grooming, behaviors compatible with DMN activation. Additionally, we demonstrated a directional influence of VP activity on the anterior cingulate cortex (ACC), an important cortical DMN region in rodents (10, 12–14), as well as a tight coupling between local field potential activity in the ACC and retrosplenial cortex (15), another node of the DMN, suggesting that the VP may be crucial for DMN-related transitions and state maintenance. Based on these considerations, we hypothesize here that VP activation, triggering a DMN-dominated brain state, should promote the execution of routine, learned responses in a stable behavioral context. We thus used an operant conditioning paradigm, in which rats lever-press on a variable interval (VI) reward schedule, as this is known to be particularly effective in producing highly stereotyped behavior that is driven by an acquired internal model (16, 17). Our task can be considered analogous to an “autopilot” mode of task performance in humans, as it has been used to formalize a dissociation between actions directed at achieving an outcome and behavioral responses elicited by a triggering stimulus (S-R). Early in training, lever pressing is variable, relatively inefficient, and sensitive to changes in reward value or contingency. That is, the animals’ actions are flexible to environmental exigencies and directed at a particular outcome. As training proceeds, the animals become experts at the task, and, as is the case for humans, this is evidenced by a decreasing variability and increasing efficiency in behavioral performance (18, 19). At the same time, the behavior becomes internalized and inflexible in that lever-press rates are now insensitive to environmental feedback such as changes to reward value or contingency (16, 17).
Convergent literature has already implicated the VP as an important regulator of behavioral participation in laboratory tasks (20–25). For example, pharmacological silencing of the VP leads to decreased lever pressing for preferred food in rats (25), an effect that the authors ascribe to the animals’ decreased willingness to expend effort to obtain a reward. More recently, it has been shown that task engagement depends on GABAergic VP neurons (26), with optogenetic silencing of this population decreasing response rate in a classical conditioning paradigm. However, these results are also compatible with an alternative interpretation, in which decreases in responding result not from motivational or appetitive effects but rather from a disengagement with automatic task performance and a redirection of attention to the external environment. A similar logic may also apply to studies showing that activation of GABAergic VP neurons leads to an increase in behavioral response rate such that the relative activity in these neurons may continuously modulate task participation. Consistent with the above findings, animals nose poke and exhibit place preference for optogenetic activation of GABAergic VP neurons (27). This latter study emphasizes a link between task participation and reward anticipation, supported also by synaptic coupling between the VP and the nucleus accumbens (NAcc) (28), where dopamine (DA) circuits play a major role in reward processing. The robust reciprocal connections between the NAcc and VP involving GABAergic and glutamatergic projections underscores the importance of the VP nucleus in reward processing through modulation of mesolimbic DA activity in the ventral tegmental area (29). In addition to this role in reward processing, the VP also projects to the cerebral cortex. A prominent indirect projection involves GABAergic and cholinergic projections to the medial dorsal nucleus of the thalamus (28), which in turn target the medial prefrontal cortical areas including the cingulate and prelimbic cortex in both rodents and primates (30, 31) that today are considered to be part of the DMN (32). The VP also harbors a direct cholinergic corticopetal projection that targets medial prefrontal cortical areas (28), although cholinergic neurons in the VP are far less numerous than in other BF nuclei such as the nearby nucleus basalis (33). In terms of connectivity, the VP could thus exert an influence on reward-related processing by acting on the mesolimbic DA system as well as contributing to global brain state regulation by acting on cortical DMN nodes. Convergent evidence suggests that the VP, particularly its GABAergic neuronal circuit, is a regulator of task participation or willingness to work for reward, and appropriate VP activity levels are important for successful adaptive behavior. These findings are compatible with a potential involvement of the VP in DMN regulation and indeed provide a neural pathway by which the VP could influence cortical state across the multiple interconnected regions making up the DMN.
While maintenance of action patterns is adaptive in a stable environment, learned actions must be modifiable when contingencies change, during which the DMN is thought to deactivate, allowing the dorsal attention network to assume control (34, 35). This leads to a transition from internally guided to externally guided behavior crucial to adapting behavioral strategies to sensory input. In other words, the brain must leave “autopilot” mode in order to integrate information from sensory and reward systems. Recent evidence suggests that the DMN may also be involved in these sorts of state transitions (36, 37). In our study, we decided to address this aspect of cognitive flexibility by employing task switching, transferring the rat from the VI operant schedule to an auditory discrimination paradigm. Here, lever presses during the presentation of specific auditory stimuli are rewarded, postive stimulus conditions or (S+), whereas rewards become unavailable for lever presses during the presentation of different auditory stimuli, negative stimulus conditions or (S−). Normally, rats stop lever pressing during the S− stimuli, adapting to the modified environmental contingency. We formulate two hypotheses concerning the influence of VP activity on success of auditory discrimination learning following task switching: We hypothesize that up-regulation of VP activity upon task switching should promote a DMN-dominated brain state, keeping the animals in a state of executing learned lever presses according to the VI schedule and failing to incorporate relevant auditory information from the environment. On the other hand, we anticipate that VP down-regulation upon task switching will tend to reduce the influence of DMN circuits on behavior, reducing the execution of learned responses while promoting acquisition of the auditory discrimination task. In the present study, we address both of these hypotheses using optogenetic activation and silencing of VP vcircuits.
Results
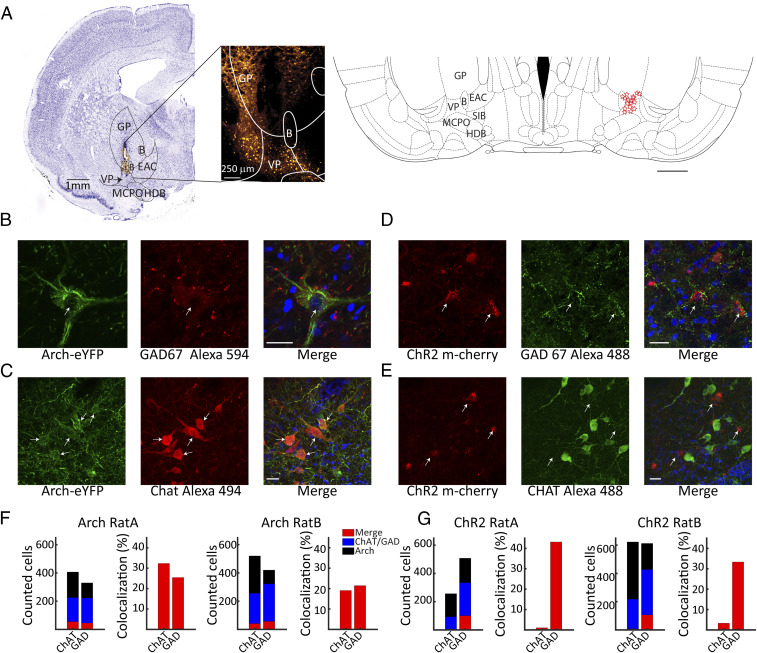
In order to activate or inhibit the VP, we expressed Channelrhodopsin (ChR2), AAV5-hSyn-ChR2(H134R)-mCherry, or Archaerhodopsin (Arch) (AAV5-hSyn-eArch3.0-EYFP) in the VP of wild-type Long–Evans rats (see Materials and Methods). We verified our viral injections and the location of our optic fiber using fluorescence and Nissl-stained sections, showing them to be within close proximity (<500 µm) to the VP (see Fig. 1A). Both viral constructs were well expressed in the VP, yet while the human synapsin promoter used in this study is not cell-type specific, differential transfection of distinct cell types cannot be a priori ruled out. We examined the coexpression of Arch and ChR2 with GABAergic and cholinergic neurons, the two main cell types of interest in the present study. Example immunohistochemical analyses for ChAT and GAD67 enzyme coexpression with Arch and ChR2 are shown in Fig. 1 B–E. For Arch, we found robust coexpression with both GAD67 and ChAT, as illustrated in Fig. 1 B and C, and cell counting in two animals yielded colocalization values in the range of 20 to 30% for these two enzymes (see Fig. 1F). This indicates viral transfection of Arch in both GABAergic and cholinergic cell populations within the VP. For ChR2, we found significant coexpression only for GAD67 (see Fig. 1D) and very little coexpression with ChAT despite good labeling of ChAT neurons (see Fig. 1E). This pattern was also confirmed by cell counting yielding ChR2-GAD67 coexpression in the range of 30 to 45% but under 5% for ChR2-ChAT (see Fig. 1G). Possible transfection of glutamatergic cells was not assessed in our study, but based on previous literature, glutamatergic cells make up only a minority of VP cells (28, 38). Taken together, our findings suggest that ChR2-mediated optogenetic activation of VP circuits will recruit largely inhibitory GABAergic cells, whereas Arch-mediated deactivation will inhibit GABAergic and cholinergic neurons.
Fig. 1.
Histology and opsin expression in the VP. (A) An example coronal section (Left) taken 0.96 mm posterior to bregma showing the optrode penetration in the VP. The area of interest is expanded in the fluorescence image to the right showing expression of ChR2 (mCherry) and with the optrode track visible in the VP. Schematic (Right) showing recording and stimulation sites (red stars) localized to the VP region from 17 rats; section taken 0.84 mm posterior to bregma. (B–E) Example colocalization of GAD- and ChAT-positive cells with Arch EYFP and ChR2 mCherry expression, respectively, and DAPI nuclear stain in blue. Note that there is no colocalization of ChAT+ neurons or ChR2 mCherry expression in E. (Scale bars: 25 mm.) (F and G) The number of counted cells positive for Arch EYFP or ChR2 mCherry (black) and GAD67 or ChAT (blue), as well as colocalization (Merge, red), are shown, respectively, on the left for two Arch F and two ChR2 G rats. The percentage of GAD- or ChAT-positive cells that were transfected by viral expression are shown at right. B, nucleus basalis; GP, globus pallidus; EAC, central extended amygdala; SIB, substantia innominata; HDB, horizontal limb diagonal band; MCPO, magnocellular preoptic nucleus.
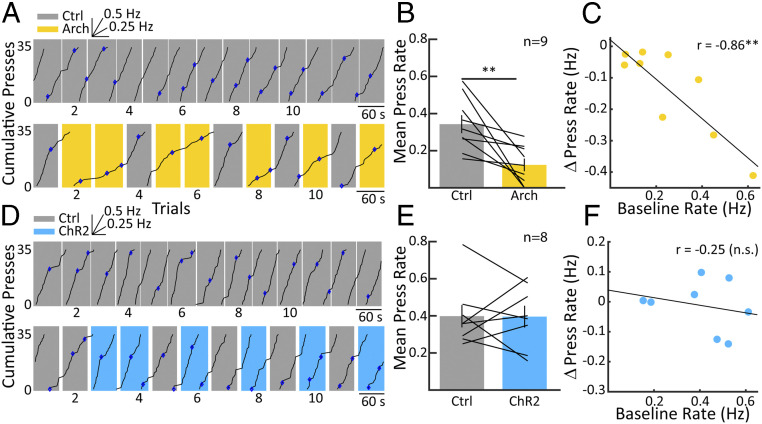
We first trained animals to reliably perform a habitual operant behavior in an established and highly stable behavioral context. Briefly, we trained Long–Evans rats to press a lever in order to receive a food reward and gradually advanced training through successive fixed ratio (FR) and VI schedules until rats exhibited stable responses on a VI-30 schedule. On a VI-30 schedule, rats tend to produce lever presses continuously, and a reward becomes available for a single lever press each time a random duration, in our case, 30 ± 5 s, has elapsed. Rats were kept in VI training until all rats participated in at least 13 sessions, making a minimum of 6,000 responses and receiving at least 600 rewards, ensuring that, at this point, their lever pressing had acquired a strong degree of automaticity according to established criteria (17). We then inhibited the VP using Arch stimulation in one group of animals and excited it using ChR2 in a separate group as the animals pressed a lever on a VI30 schedule of reinforcement. The top panel in Fig. 2A shows a cumulative record of lever pressing for an example animal. In this control condition, the animal emits highly stable lever presses throughout the duration of the session. Then, the same animal is transferred to the experimental condition (Fig. 2A, Bottom), in which random trials are paired with Arch stimulation. During Arch stimulation (yellow-shaded trials), the rate of lever pressing is strongly decreased and recovers on transition to control trials (gray shading). This was true across animals (Fig. 2B), in which Arch activation resulted in a significant decrease in lever-press rate (paired t test, n = 9, P < 0.01). Interestingly, animals that pressed at a higher rate during control conditions were affected more strongly by the Arch stimulation (Fig. 2C; n = 9, r = −0.86, P < 0.01). One might suspect that if inhibiting the VP causes a reduction in lever-press rate, then activating the VP would result in a rate increase. This, however, was not the case when ChR2 stimulation did essentially nothing to the animals’ lever-pressing behavior; Fig. 2D shows an example animal, and group results (Fig. 2E) revealed no significant change. Accordingly, no correlation was observed between the initial response rate under control conditions and the subsequent rate change during excitation of VP circuits (Fig. 2F). Taken together, inhibiting the VP strongly decreases lever pressing, whereas activation of the VP has no apparent effect on lever pressing during the VI component of the task.
Fig. 2.
Effects of VP inhibition and excitation on operant behavior. (A) A cumulative record displays responses over time on a VI-30 schedule (Top). Each uptick on the chart indicates a lever press, with steeper slopes indicating higher lever-pressing rates and vice versa. The inset above shows the slope for a 0.25- and 0.5-Hz response rate, and blue diamonds indicate lever presses that resulted in a reward. The record resets to zero at 35 responses. Note that the animal is responding at a steady slow rate as is typical for this reward schedule. Below is a cumulative record from the same animal but including trials with 594-nm laser stimulation of Archaerhodopsin in the VP (yellow shading). During trials with Arch-mediated VP inhibition, the rate of lever pressing slows and recovers almost immediately in the control trials (gray shading). (B) The bar chart shows mean response rates across nine animals in control versus Arch conditions, showing that, on average, lever-press rates during Arch-mediated VP inhibition were suppressed in comparisons to control trials. (C) The change in lever-press rate is plotted versus the baseline rate taken from control sessions as in A, Top. The higher the baseline lever-press rate was for individual animals, the greater the reduction in rate resulting from Arch-mediated inhibition. (D) As in A but for an example animal receiving ChR2-mediated VP excitation. Again, at the top, the animal is responding in a slow, stable fashion. Below, we can see that during trials with 473-nm laser stimulation of ChR2 (blue shading), there is no apparent change in the response rate versus the control trials (gray). This is summarized in E, in which there is indeed no significant difference in lever-press rates between ChR2 on and off conditions. F is as C, except with the absence of a significant correlation between baseline lever-press rate and changes in response rate during ChR2 activation. Error bars reflect SEM; **P < 0.01.
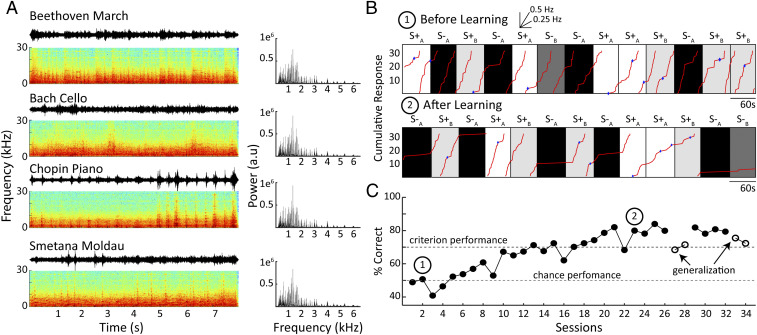
We then transferred animals from the VI-30 schedule to an auditory discrimination paradigm, allowing us to assess how VP inhibition and excitation impacted the animals’ ability to transition to the acquisition and performance of an auditory discrimination task. We utilized four auditory stimuli delivered continuously during the ∼60-s-duration trials. Animals were rewarded on the same VI-30 schedule for lever presses during presentation of two stimuli (S+A and S+B), while no reward was delivered during presentation of the other two stimuli (S−A and S−B). S+ and S− trials were randomized and equalized in terms of repetitions and duration such that animals spent an equal amount of time in the S+ and S− conditions. Auditory stimuli consisted of instrumental music segments, as these are spectrally rich and dynamic and likely to activate a broad range of auditory neurons. Music segments were up-sampled 3× for better correspondence to the auditory sensitivity of the rat and were spectrally normalized, which should minimize bias by limiting the rat’s ability to rely on differences in frequency power to solve the discrimination task (see Fig. 3A and Materials and Methods).
Fig. 3.
Auditory discrimination task. (A) Shows the four 8-s music pieces used in the auditory discrimination task at left. For each piece, we plot the waveform along with the associated spectrogram below it. Musical pieces were matched for their spectral power, as shown in the corresponding power spectral density plots to the right of the musical pieces. Two musical pieces were associated with reward (S + A, B), while the animal never received reward for lever presses during the other two (S − A, B). The separate music pieces for (S + A, B and S − A, B) were counterbalanced between animals to control for potential differences in discriminability. (B) The cumulative record of responses is shown for an animal on the second day of training (Top). Note that the animal is responding at roughly equal rates during the S± (light shading) and S− (darker shading) trials. Below it is a cumulative record of the same animal following 23 d of training on the task. Here, the animal is largely restricting its lever presses to trials during the auditory stimuli associated with reward (S + A, B), while responses during presentation of the unrewarded (S − A, B) stimuli are largely extinguished; blue diamonds indicate lever presses resulting in a reward. (C) Learning curve with discrimination performance over sessions for the same rat shown in B. Circled numbers represent sessions from which the cumulative records in B were taken. Following task acquisition, the animal participated in two separate generalization tests for 2 d using novel 8-s segments of the same classical music pieces (arrows). Note performance does not fall to chance following transfer.
An example behavioral record early in training (i.e., soon after the task switching to the auditory discrimination paradigm) illustrates that rats initially lever-press at similar rates during the presentation of S+ and S− stimuli (Fig. 3 B, Top). They thus continue to behave according to the established behavioral context of the VI-30 task despite changes to reward contingencies. After achieving criterion performance (70% correct responding), rats tended to press during S+ but not S− stimuli, demonstrated by a flattening of response rates during S− trials (Fig. 3 B, Bottom). An example learning curve (Fig. 3C) illustrates that animals are initially near chance performance (50% correct) and reach levels around 70% correct after 13 d of training. Since different stimulus pairings may be more or less discriminable, these pairings were counterbalanced across animals, and no significant differences were observed in the animals’ performance using the different auditory stimuli. Rats readily learned this task, achieving criterion performance in about 13 training days (n = 22; mean 13.3 ± 1.4 d); note that these analyses include also pilot animals used to validate our behavioral paradigm. To further characterize what animals learned, 19 animals were also tested on generalization to other segments of the same classical music pieces that they had never heard before. As evident in Fig. 3C, performance dropped slightly during the generalization test but remained well above chance for each of the two generalizations tested. This was confirmed across animals, with an average performance on the generalization test of 60.1 ± 1.5% (paired t test against 50%; n = 19, P < 0.001). The capacity for generalization suggests that rats extracted characteristic features from the music segments and formed a robust representation of the dynamic auditory structure. We consider it remarkable that rats are able to transfer learned knowledge to completely unfamiliar segments of four distinct classical-music styles.
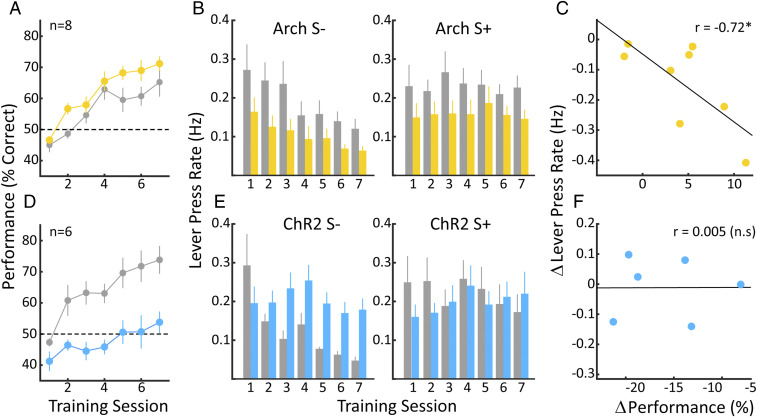
Having demonstrated that rats are able to task-switch from the VI-30 to our auditory task, we proceeded to examine the influence of VP circuit activity on this cognitively demanding process. Using two S+/S− stimulus pairs, we can examine how interventions in the VP circuit impact the transition from the internally guided VI task to an auditory discrimination task requiring attention to environmental exigencies. We coupled one pair of S+/S− stimuli with optogenetic intervention, leaving the second S+/S− stimulus set as a control. Thus, each animal serves as its own control, allowing for assessment of individual differences in task-related behaviors. To facilitate comparison across animals, we identified the first session in which behavioral performance exceeded 50%, in either the control or optogenetic stimulation condition, and designated the immediately preceding session as day 1 independently for each animal. Fig. 4A shows the average learning curves over the first 7 d of auditory discrimination for stimulus pairs coupled with Arch-mediated inhibition of the VP as well as for the control stimulus pairs. While rats learned the task under both conditions, Arch stimulation conferred a small but significant benefit in performance across animals. Two-way ANOVA for Arch and training day, [F(1.98) = 12.8; P < 0.001]; [F(6.98) = 15.8; P < 0.00001], with no interaction between training day and optogenetic stimulation (P > 0.5). Thus, Arch-mediated inhibition of the VP facilitated task-switching behavior, allowing rats to more effectively acquire the auditory discrimination task. In order to further characterize the beneficial impact of VP inhibition on behavioral performance, we compared lever-press rates for S+ and S− trials independently for control and Arch conditions. As seen in Fig. 4 B, Left, optogenetic inhibition of the VP consistently reduced the lever-press rate over the course of training during S− trials, consistent with the overall rate reduction during Arch stimulation under the VI-30 schedule prior to task switching. In addition, rates during S− systematically decreased over training days in both control and Arch conditions, as animals learned to discontinue lever presses in the nonrewarded context. A two-way ANOVA showed that both Arch and training day contributed rate reductions during the S− [F(1.98) = 19; P < 0.00001]; [F(6.98) = 3.2; P < 0.01], with no interaction between training day and optogenetic stimulation (P > 0.5). For the S+ trials (Fig. 4 B, Right), Arch stimulation also reduced rates, but in neither the control nor Arch conditions did these rates change over training days. Two-way ANOVA for Arch and training day, [F(1.98) = 13.3; P < 0.001]; [F(6.98) = 0.21; P > 0.5], with no interaction between training day and optogenetic stimulation (P > 0.5). In summary, increases in performance in both Arch and control conditions were driven by a decrease in lever-press rate during the S− as the animal abandons the VI-30 behavioral context and learns to attend to the auditory stimuli. The degree to which inhibition of the VP affected the lever-press rate on the VI-30 task was variable between animals, with individual animals showing rate reductions ranging from 0.05 to 0.4 Hz (see Fig. 1C). We therefore assessed how the magnitude of rate reductions correlated with performance on the auditory discrimination task (Fig. 4C). Indeed, the greater the rate reduction generated by inhibition of VP circuits, the greater the benefit to performance (r = −0.72; P < 0.05). In summary, Arch-mediated inhibition of the VP improved performance on the auditory discrimination task, and the degree to which it did so was related to the degree that it allowed for escape from the inwardly focused, automatized lever-press behavior as measured by lever-press rate reduction.
Fig. 4.
Up- and down-regulation of the VP has opposite effects on the performance of an auditory discrimination task. (A) Learning curves showing percent correct performance over days for control trials (gray) and trials with Arch-mediated inhibition of the VP (yellow). Note that Arch stimulation conferred a slight, but significant, increase in performance over control conditions over the seven training days. (B, Left) Average lever-press rates for the eight animals during unrewarded (S−) trials for control (gray) and Arch (yellow) conditions. Rates in the S− trials for both Arch and control conditions decrease over days consistent with acquisition of the task, while rates for Arch trials are consistently below control values. (Right) Average lever-press rates over training days for rewarded (S+) trials. No change in rate is observed over days in either the control or Arch conditions; however, lever-press rates in the Arch condition remain suppressed in relation to the control condition. (C) The change in the response rate is plotted against the change in performance for the individual animals. The greater the reduction in response rate, the greater the performance benefit. (D) As A but for animals receiving ChR2 activation of the VP. Here, ChR2 activation of the VP has a strong deleterious effect on acquisition and performance of the task. (E, Left) As B, except for ChR2-mediated excitation of the VP. While lever-press rates in the control S− trials decrease steadily over days, consistent with learning, lever-press rates in the ChR2 trials do not change over days. (E, Right) Lever-press rates in the S+) trials. Neither the control S+ rate nor the ChR2 S+ rate changes over days, nor are they different from one another. F is as C. No correlation between the decreased performance seen in ChR2 trials and the change in lever-press rate in the individual animals. Error bars represent ± SEM.
While ChR2-mediated excitation of VP circuits had no effect on the lever-press rate under the VI-30 schedule, it had a dramatic and detrimental impact on behavior during auditory discrimination following task switching. Fig. 4D shows the average learning curves across training days for stimuli pairs in VP activation and control conditions. As expected, animals display good acquisition and performance in discriminating the control stimuli. They are, however, grossly impaired when it comes to discriminating the stimuli paired with VP activation and, even at the end of training, are still hovering around chance performance. These differences were highly significant; two-way ANOVA for ChR2 and training day [F(1.70) = 81.16; P < 0.0001]; [F(6.70) = 7.58; P < 0.0001], with a significant interaction between ChR2 VP activation and training day (P < 0.05). It is conceivable that rats were actually learning the auditory discrimination during ChR2 stimulation but were unable to behaviorally express this, for example due to an inability to suppress lever pressing. We addressed this issue in additional experiments on a subset of the animals, in which we stopped ChR2 stimulation for an S+/S− pair that had previously been associated with laser stimulation and found that this was not the case (Performance ChrR2 On: 53.5 ± 4.3%; ChR2 Off: 52.7 ± 5.0%; t test: n = 3; P > 0.1). Using the same methodology as for the Arch animals, Fig. 4B, we find that the lever-press rate remained stable during the S− trials paired with ChR2 activation (Fig. 4 E, Left). During S− control trials, the lever-press rate did reduce systematically over training days; two-way ANOVA [F(1.70) = 17.7; P < 0.001]; [F(6.70) = 3.6; P < 0.005], with a significant interaction between ChR2 activation and training day (P < 0.05), consistent with successful learning of the auditory task under control conditions. As was the case for the Arch animals, response rates during the S+ trials did not change over the course of training, nor was there a difference in the lever-press rates during S+ trials paired with VP activation and the rates in the control S+ trials. Two-way ANOVA [F(1.70) = 0.7; P > 0.1]; [F(6.70) = 0.3; P > 0.5], and no significant interaction was observed (P > 0.5). ChR2 stimulation did not systematically affect lever pressing in the VI-30 task (Fig. 2E), and there was accordingly no significant correlation between ChR2 stimulation–induced changes in VI-30 lever pressing and performance on the auditory task (r = 0.07, P > 0.1; see Fig. 4F). Taken together, the rats largely failed to learn the auditory discrimination task during ChR2-mediated activation of VP circuits, apparently disregarding the auditory information present in the environment and instead persisting in the VI-30 behavior they had acquired prior to task switching.
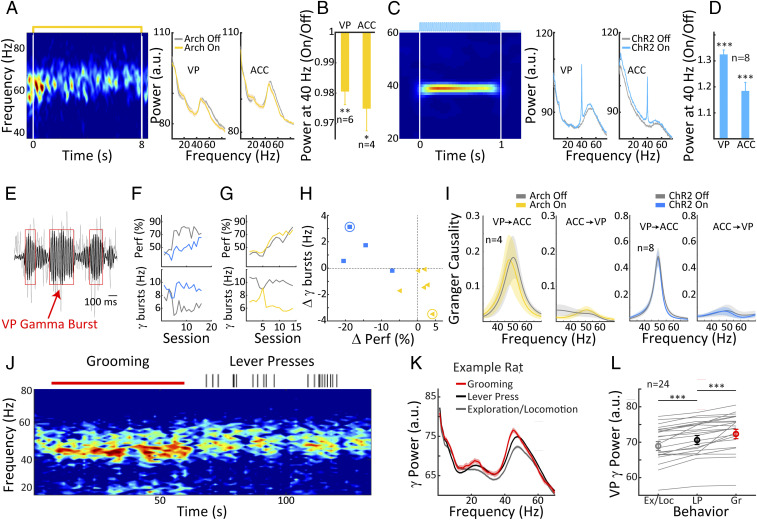
We made electrophysiological recordings from the VP and ACC in order to examine the effect of the optogenetic manipulations on neural circuit activity. Note that some of these recordings were made prior to the behavioral experiments and thus include subjects that did not move on to complete all of the behavioral stages. Eight animals in the ChR2 group had recordings of sufficient quality and free of line noise in both the VP and ACC, while in the Arch group, six animals had high-quality recordings in the VP and four animals in both the VP and ACC. We first focus on recordings in the home cage taken outside of task performance because, under these conditions, the VP displays pronounced gamma-band activation coupled to activity in the ACC, an important node of the DMN. As anticipated, we observed robust endogenous broad-band gamma oscillations in the VP, consistent with previous findings in the VP/nucleus basalis region (10, 39). Continuous Arch stimulation slightly reduced gamma-band activity in the VP and ACC as evidenced by the data shown for an example animal (Fig. 5 A and B).
Fig. 5.
DMN activity and its relationship to operant behavior and auditory discrimination learning. (A, Left) Example spectrogram of VP LFP recordings taken in the animals’ home cage with Arch stimulation (yellow line, Top) showing reduced gamma band activity during the Arch stimulation. Right panels show spectral power in the VP and the ACC with and without Arch stimulation (yellow and gray, respectively) for the same example rat. (B) Summary data over rats reveals a weak but significant suppression of gamma band activity at 40 to 60 Hz, both in the VP and the ACC. (C, Left) Spectrogram showing an example of 40-Hz entrainment by ChR2 40-Hz stimulation in the animals’ home cage (blue line, Top). (Right) Examples of spectral power in the VP and the ACC with and without ChR2 40-Hz stimulation. (D) Summary data over rats shows significant increases at 40 Hz power in both the VP and the ACC. (E) Example LFP trace from the VP showing gamma burst activity, gray raw data; black, 40- to 60-Hz band pass filtered. Red boxes mark detected gamma bursts. (F) Changes in burst frequency (Bottom) and discrimination performance (Top) over learning days; gray, control; blue, ChR2 activation. Note that ChR2 leads to an increased number of gamma bursts and a concomitant decrease in discrimination performance. (G) Same as F but for an Arch animal. A decrease in gamma burst frequency is associated with a slight increase in performance. (H) The correlation between changes in gamma burst frequency and changes in performance for both ChR2 (blue) and Arch (yellow) animals. Decreasing bursts in Arch animals are associated with better performance, and the opposite is true for the ChR2 animals. Blue and yellow circles denote the animals used for the examples in F and G, respectively. (I) Group mean of directional interactions computed during VP gamma bursts between the VP and the ACC for Arch, ChR2, and control. No differences between control trials and those paired with optogenetic activations were observed. (J) An example spectrogram of an LFP recording in the VP during a transition from grooming to lever pressing. Red bar above the spectrogram represents the time course of grooming, and vertical ticks mark lever presses. Note that pronounced gamma activity is present during both grooming and lever pressing. (K) Average VP gamma power for grooming, lever pressing, and exploratory/locomotion behavior; shaded areas represent SEM. (L) Statistical comparison of VP gamma (40 to 60 Hz) power for the three behaviors, exploration/locomotion (gray), lever press (black), and grooming (red). Note highest VP gamma power during grooming and intermediate up-regulation during lever-press behavior in contrast to exploratory/locomotion behavior. Error bars represent ± SEM.
Gamma power reductions were significant in both brain regions (VP: paired t test, n = 6, P < 0.01; ACC: paired t test, n = 4, P < 0.05), amounting to a decrease of about 2% on average (Fig. 5C). For ChR2 stimulation, we delivered 40 Hz laser pulse stimulation for 1-s epochs, as this has been shown to trigger activation of cortical BF target structures such as the ACC (10, 40). Optogenetic ChR2 activation resulted in strong entrainment of the local field potential (LFP) within the VP and ACC as shown in Fig. 5C for an example animal. The narrow-band entrainment at 40 Hz was significant within both regions across animals (Fig. 5F; VP and ACC: paired t tests against 1, P < 0.001). Our findings indicate that both Arch and ChR2 stimulation reliably modulate activity in local VP circuits as well in the ACC, which is a major node of the DMN and a cortical projection target of the VP.
Following task switching, we detected transient short-duration gamma bursts in the VP (see Materials and Methods) and analyzed how frequently they occurred during the acquisition of the auditory discrimination task. For ChR2 stimulation, we found that the pronounced impairment in discrimination performance relative to control trials was accompanied by a concurrent enhancement in gamma burst occurrence. Results for an example animal are summarized inFig. 5F, and the enhancement of gamma burst frequency was significant in three out of four animals tested comparing the last 7 d of task performance (paired t test: n = 7, P < 0.05). Arch stimulation had the opposite effect on gamma burst frequency, resulting in a suppression of gamma bursts that accompanied the slight advantage in discrimination performance associated with Arch stimulation. Results for an example animal are summarized in Fig. 5G, and gamma burst suppression was significant in four out of six animals tested (paired t test: n = 7, P < 0.05). In Fig. 5H, we summarize data from all available animals in the ChR2 and Arch stimulation conditions, plotting how behavioral performance and gamma burst frequency changed from the corresponding control trials as a result of optogenetic stimulation. Comparing ChR2 and Arch, we document a significant difference between these two optogenetic stimulation conditions on both gamma burst frequency (unpaired t test n = 10, P < 0.05) and behavioral performance (unpaired t test, n = 10, P < 0.001). Taken together, these findings suggest that ChR2 stimulation, by enhancing DMN activity, tends to keep animals in the default mode behavioral state with an inward focus. Under these conditions, they do not adequately process auditory information in the environment, hindering their auditory learning progress. Arch stimulation has the opposite effect, pushing animals away from the default mode behavioral state and toward an externally focused attentional state that confers a slight performance benefit relative to control trials. We have evidence compatible with the idea that the gamma burst effects described, Fig. 5 E–I, are not unique to the VP but are also broadcast to other DMN nodes. Analyses of directed coherence (i.e., Granger causality performed on the band pass filtered [45 to 60 Hz] gamma bursts) suggest that VP gamma band oscillations tend to drive activity in the ACC but not vice versa (see Fig. 5I). There was no difference between control and ChR2/Arch conditions, such that the nature of the coupling between the VP and the ACC was unaltered by optogenetic stimulation. However, as gamma bursts can be considered as coupling events, our data suggests that the change in occurrence of these coupling events in the VP has an impact on the ACC, a cortical node of the DMN.
In a previous study, we suggested that default mode behaviors in the rat, quiet wakefulness and self-grooming, were associated with particularly pronounced BF activation in the VP, whereas activity was suppressed during explorative behavior (10). Our present data suggests that lever pressing during the highly repetitive and stereotyped VI-30 task might also be considered a default mode behavior. To address this question, we examined LFP data from the VI-30 task during epochs of self-grooming, lever pressing, and locomotion/exploration of the operant chamber. An example spectrogram is shown in Fig. 5J, illustrating consecutive segments of grooming followed by lever pressing and showing reduced gamma power during the latter behavior. Spectral analysis of all available behavior-scored segments, shown in Fig. 5K, confirms that the highest values of broad-band VP gamma occurred during grooming, intermediate values during VI-30 lever pressing, and the lowest values during exploration. This effect was significant across the population of available animals (see Fig. 5L; n = 24, P < 0.001), suggesting that stereotyped lever pressing of the kind that occurs in highly trained animals on operant schedules is associated with pronounced VP gamma oscillations and thus could indeed be considered as a default mode behavior.
Discussion
Our previous results have implicated the VP as a key regulator of DMN activity such that the VP shows elevated activity during default mode behavioral states including quiet wakefulness and self-grooming (10, 39). Here, we tested the idea that up- and down-modulation of VP circuits might drive animals in and out of default mode behavioral states, thereby promoting execution of automatized behaviors according to learned schemata or allocation of attention to external stimuli, respectively. We first investigated the impact of VP modulation on lever-press rates under a VI-30 schedule of reinforcement. Based on previous literature (16, 17), lever pressing under these conditions becomes a highly stereotyped, automatized behavior, limiting attention to the external world and thus meeting the criteria for a default mode behavior (1, 2). Activation of the VP during automatized lever pressing had no effect on the animals’ behavior, as animals were already in a DMN-dominated brain state, and further up-regulation of the VP serves only to maintain that state. Conversely, inhibition of VP circuits strongly reduced lever pressing, a reduction that we suggest reflects a deactivation of the DMN and puts animals in an attentional state, disrupting the efficiency conferred by automatized task performance. Our observations are compatible with the idea that the VP regulates task participation, shown in operant and classical conditioning paradigms (21, 25–27). However, VP activation does not increase lever pressing despite the fact that lever-press rates on VI-30 are far below ceiling rates observed on other schedules (e.g., FR) (41). Our findings are therefore more consistent with a state switch in and out of a DMN brain state rather than graded regulation of task participation. Furthermore, they suggest that VP circuits are able to trigger a DMN brain state with little temporal lag in on- and offset, allowing instant-by-instant DMN regulation, which is conducive for adaptive behavior.
The key manipulation in our study is switching animals from an internally guided, DMN-dominated, VI-30 task to a discrimination task requiring attention to auditory stimuli. During one set of auditory stimuli, S+, rewards remain available on the same VI-30 schedule, whereas during another set of stimuli, S−, no rewards are available. Control animals learn this new contingency over a few training sessions, mainly by decreasing responses during S− and progressively mastering the auditory discrimination task. Task switching has been extensively used to examine the neural basis of cognitive control and flexibility (42, 43). Note that we examine a specific kind of task switching, namely, the switching from an internally to an externally guided task, which differs from more common forms such as reversal learning and set shifting (44, 45), in which externally directed attention is required throughout task performance. Frontal areas, including the lateral prefrontal and cingulate cortex, are known to play an important role in task switching (46–49), providing a potential common substrate for cognitive control and regulation of the DMN that also includes significant portions of the frontal cortex (3, 50). Compatible with this notion, separable populations of ACC neurons have been shown to participate in repetitive, memory-based (i.e., DMN-linked) and search-related (i.e., attention-linked) control of behavior (51). The effects of optogenetic up- and down-regulation of VP circuits on rats’ ability to acquire the auditory discrimination task following task switching were also highly compatible with a crucial role of the DMN. Upon switching to a task that necessitates attention to external stimuli, DMN activity is unhelpful, and task switching should therefore be facilitated by VP suppression. At the same time, augmentation of VP activity should keep the animals in a DMN-dominated brain state, jeopardizing direction of attention to auditory stimuli and hampering acquisition of the auditory discrimination task. We observed both of these effects. VP ChR2 stimulation caused a very pronounced deficit in auditory discrimination learning, as animals kept responding during S− trials, apparently oblivious to the auditory information instructing them that no reward was available. Failure to suppress responses on S− trials parallels previous results in macaques, showing elevated numbers of incomplete trials (52), as well as a disengagement from sensory-guided behavioral control and a concomitant increase in behavioral responses following pharmacological VP activation (52).
Our findings are also consistent with previous demonstrations linking operant responding to VP activation, particularly of the GABAergic cell population (26, 27), a population that we also target in our present study, as our optogenetic ChR2 stimulation specifically activates GABAergic VP neurons. Note that the learning deficits on the S− trials could also be explained by a failure of the animal to utilize the negative reward prediction error that occurs on these trials to update the behavioral response strategy. For example, GABAergic VP overactivation might interfere with the neural encoding of negative reward prediction errors that are necessary for adapting task contingencies following task switching. Consistent with this notion, reward prediction error signals have been demonstrated in VP neural responses in rats (53). We thus consider that this potential disengagement from reward-related feedback from the environment might, in addition to disengagement from sensory information, also play a role in our behavioral findings. Both effects are indeed compatible with a disengagement from the external environment and an internal focus that characterize DMN-dominated brain states. Potential modulations of reward-related processing might involve VP projections to the ventral tegmental area (VTA), which are indeed mostly GABAergic (28) and thus modulated in our task. Along these lines, pharmacological inhibition of the VP has been shown to result in an increase in VTA firing and a concomitant increase in tonic DA release in the NAcc (54). Dopaminergic activity in the VTA is also thought to play a central role in both the acquisition of goal-directed or action/outcome (A/O) behaviors through projections to the NAcc (55) as well as the development of automatic/S-R responding through a separate nigrostriatal pathway (56) but also potentially involving VTA (54). By contrast, Arch-mediated VP suppression, which, in our study, targeted both cholinergic and GABAergic VP neurons and slightly but significantly improved acquisition of the auditory task following task switching. This is compatible with previous work showing that pharmacological deactivation of the VP speeds up reaction times to low-value sensory cues, which can be considered as an enhancement in performance, potentially involving heightened engagement with sensory or reward-related aspects of the external environment (57), but see ref. 58. We attribute the improved learning rate to transient deactivation of the DMN, which reduces stereotyped responding, improves rats’ ability to attend to the auditory stimuli, and promotes more rapid task acquisition. This hypothesis is supported by the observation that pharmacological inactivation of the VP triggers exploratory foraging in the rat while at the same time suppressing stereotyped lever pressing (25). Our findings suggest that, in the Arch condition, animals are unimpaired in their utilization of negative prediction errors as they reliably learn to suppress responses on S− trials. The conjunction of reduced lever pressing and enhanced learning following task switching seems difficult to conceptualize based only on reward processing. We therefore consider that, while our results certainly do not rule out a VP role in task participation and reward processing, they are best explained by considering the VP as a promotor of DMN activation. Interestingly, noncholinergic and, putatively, GABAergic neurons in other BF structures (i.e., the nucleus basalis and horizontal diagonal band) show activity that is inversely correlated with reaction time (i.e., high firing rates are associated with shorter reaction times). It has been suggested that GABAergic activity in these nuclei is associated with allocation of attention or cues that predict reinforcement (59, 60), while neighboring cholinergic neurons seem to be more involved in signaling the reinforcement itself (60). These results are apparently at odds with our findings that activation of VP GABAergic neurons activates the DMN and withdraws resources from sensory processing. Taken together, however, these results may suggest that, while the VP and horizontal limb diagonal band/nucleus basalis harbor similar cell types and overall connectivity patterns, they might serve distinct functions related to the DMN and sensory processing–related cortical networks with which they are reciprocally connected.
While the DMN has traditionally been associated with inwardly focused and self-directed mental activity (3), some recent studies have suggested that it may also be active during task switching (36, 37, 61). This idea is compatible with an extensive literature in animal work, which has implicated the medial frontal cortex, including the ACC, in the control of transitions to and maintenance of automatized behaviors (62–68). We have previously demonstrated that pronounced gamma oscillations are present in the BF nuclei, including the magnocellular preoptic nucleus and the VP, as well as in cortical DMN targets, including the ACC, during DMN-associated behaviors of quiet wakefulness and self-grooming (10, 15). We show here that elevated gamma oscillations also occur during stereotyped lever pressing, reinforcing the notion of automatized behaviors occurring during DMN-dominated brain states, also in the rat. This is consistent with the observation that rats resort to their default response strategy during performance of an operant task employing auditory stimuli following electrical stimulation of the ACC (49). Crucially, we show that the degree to which bursts of VP gamma activity were modulated by optogenetic manipulations was correlated with learning of the externally focused auditory task. Animals with particularly large enhancement of VP gamma bursts suffered the largest learning impairment, whereas animals with the most pronounced VP gamma burst suppression benefited most in terms of learning. Gamma activations did not remain local to the VP but were broadcast to at least one cortical DMN node, as we demonstrate for the ACC, reinforcing the notion that the VP plays a key role in DMN regulation. Our findings extend the role of the VP, and more generally the BF, in DMN regulation beyond inward, self-focused behaviors to include stereotyped, automatized behaviors. We suggest that these nuclei play a critical role in global brain state transitions between periods of outward attentional focus and a DMN-dominated brain state that favors behaviors relying on learned internal schemata.
Materials and Methods
Details of the materials and methods can be found in the SI Appendix.
The local ethical committee on animal experimentation (Canton of Fribourg, Switzerland) approved all experimental procedures. All experiments were performed on adult male Long–Evans rats.
Supplementary Material
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2103642118/-/DCSupplemental.
Data Availability
Datasets have been deposited in the G-Node Open Data repository (DOI: 10.12751/g-node.zrkd6x) (69), and data have been deposited in the Collaborative Research in Computational Neuroscience (CRCNS) database (https://crcns.org/data-sets/bf). All other study data are included in the article and/or SI Appendix.
References
- 1.Vatansever D., Menon D. K., Stamatakis E. A., Default mode contributions to automated information processing. Proc. Natl. Acad. Sci. U.S.A. 114, 12821–12826 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shamloo F., Helie S., Changes in default mode network as automaticity develops in a categorization task. Behav. Brain Res. 313, 324–333 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Raichle M. E., The brain’s default mode network. Annu. Rev. Neurosci. 38, 433–447 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Philippi C. L., Tranel D., Duff M., Rudrauf D., Damage to the default mode network disrupts autobiographical memory retrieval. Soc. Cogn. Affect. Neurosci. 10, 318–326 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spreng R. N., Grady C. L., Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J. Cogn. Neurosci. 22, 1112–1123 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Alves P. N., et al., An improved neuroanatomical model of the default-mode network reconciles previous neuroimaging and neuropathological findings. Commun. Biol. 2, 370 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Do J. P., et al., Cell type-specific long-range connections of basal forebrain circuit. eLife 5, e13214 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaborszky L., et al., Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: An experimental study based on retrograde tracing and 3D reconstruction. Cereb. Cortex 25, 118–137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turchi J., et al., The basal forebrain regulates global resting-state fMRI Fluctuations. Neuron 97, 940–952.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair J., et al., Basal forebrain contributes to default mode network regulation. Proc. Natl. Acad. Sci. U.S.A. 115, 1352–1357 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peeters L. M., et al., Cholinergic modulation of the default mode like network in rats. iScience 23, 101455 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu W., Ma Z., Ma Y., Dopfel D., Zhang N., Suppressing anterior cingulate cortex modulates default mode network and behavior in awake rats. Cereb. Cortex 31, 312–323 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heilbronner S. R., Hayden B. Y., Dorsal anterior cingulate cortex: A bottom-up view. Annu. Rev. Neurosci. 39, 149–170 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jing W., et al., Reentrant information flow in electrophysiological rat default mode network. Front. Neurosci. 11, 93 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lozano-Montes L., et al., Optogenetic stimulation of basal forebrain parvalbumin neurons activates the default mode network and associated behaviors. Cell Rep. 33, 108359 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Colwill R. M., Rescorla R. A., Effect of reinforcer devaluation on discriminative control of instrumental behavior. J. Exp. Psychol. Anim. Behav. Process. 16, 40–47 (1990). [PubMed] [Google Scholar]
- 17.Dickinson A., Actions and habits: The development of behavioural autonomy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 308, 67–78 (1985). [Google Scholar]
- 18.Tang C., Pawlak A. P., Prokopenko V., West M. O., Changes in activity of the striatum during formation of a motor habit. Eur. J. Neurosci. 25, 1212–1227 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Carelli R. M., Wolske M., West M. O., Loss of lever press-related firing of rat striatal forelimb neurons after repeated sessions in a lever pressing task. J. Neurosci. 17, 1804–1814 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richard J. M., Stout N., Acs D., Janak P. H., Ventral pallidal encoding of reward-seeking behavior depends on the underlying associative structure. eLife 7, e33107 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang S. E., Smedley E. B., Stansfield K. J., Stott J. J., Smith K. S., Optogenetic inhibition of ventral pallidum neurons impairs context-driven salt seeking. J. Neurosci. 37, 5670–5680 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAlonan G. M., Robbins T. W., Everitt B. J., Effects of medial dorsal thalamic and ventral pallidal lesions on the acquisition of a conditioned place preference: Further evidence for the involvement of the ventral striatopallidal system in reward-related processes. Neuroscience 52, 605–620 (1993). [DOI] [PubMed] [Google Scholar]
- 23.Richard J. M., Ambroggi F., Janak P. H., Fields H. L., Ventral pallidum neurons encode incentive value and promote cue-elicited instrumental actions. Neuron 90, 1165–1173 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson P. I., Parente M. A., Stellar J. R., NMDA-induced lesions of the nucleus accumbens or the ventral pallidum increase the rewarding efficacy of food to deprived rats. Brain Res. 722, 109–117 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Farrar A. M., et al., Forebrain circuitry involved in effort-related choice: Injections of the GABAA agonist muscimol into ventral pallidum alter response allocation in food-seeking behavior. Neuroscience 152, 321–330 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephenson-Jones M., et al., Opposing contributions of GABAergic and glutamatergic ventral pallidal neurons to motivational behaviors. Neuron 105, 921–933.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faget L., et al., Opponent control of behavioral reinforcement by inhibitory and excitatory projections from the ventral pallidum. Nat. Commun. 9, 849 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Root D. H., Melendez R. I., Zaborszky L., Napier T. C., The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog. Neurobiol. 130, 29–70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith K. S., Tindell A. J., Aldridge J. W., Berridge K. C., Ventral pallidum roles in reward and motivation. Behav. Brain Res. 196, 155–167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray J. P., Price J. L., The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain-prefrontal cortex topography. J. Comp. Neurol. 323, 167–197 (1992). [DOI] [PubMed] [Google Scholar]
- 31.Ray J. P., Price J. L., The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 337, 1–31 (1993). [DOI] [PubMed] [Google Scholar]
- 32.Vives F., Mogenson G. J., Electrophysiological evidence that the mediodorsal nucleus of the thalamus is a relay between the ventral pallidum and the medial prefrontal cortex in the rat. Brain Res. 344, 329–337 (1985). [DOI] [PubMed] [Google Scholar]
- 33.Zaborszky L., Van den Pol A., Gyengesi E., “The basal forebrain cholinergic projection system in mice” in The Mouse Nervous System, Watson C., Paxino G., Puelles L., Eds. (Academic Press, 2012), p. 690. [Google Scholar]
- 34.Fransson P., How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia 44, 2836–2845 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Fox M. D., et al., The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U.S.A. 102, 9673–9678 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith V., Mitchell D. J., Duncan J., Role of the default mode network in cognitive transitions. Cereb. Cortex 28, 3685–3696 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crittenden B. M., Mitchell D. J., Duncan J., Recruitment of the default mode network during a demanding act of executive control. eLife 4, e06481 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prasad A. A., et al., Complementary roles for ventral pallidum cell types and their projections in relapse. J. Neurosci. 40, 880–893 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nair J., et al., Gamma band directional interactions between basal forebrain and visual cortex during wake and sleep states. J. Physiol. Paris 110, 19–28 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Kim T., et al., Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc. Natl. Acad. Sci. U.S.A. 112, 3535–3540 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferster C. B., Skinner B. F., Schedules of Reinforcement (Appleton-Century-Crofts, East Norwalk, CT, 1957). [Google Scholar]
- 42.Kiesel A., et al., Control and interference in task switching—A review. Psychol. Bull. 136, 849–874 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Monsell S., Task switching. Trends Cogn. Sci. 7, 134–140 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Izquierdo A., Brigman J. L., Radke A. K., Rudebeck P. H., Holmes A., The neural basis of reversal learning: An updated perspective. Neuroscience 345, 12–26 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tait D. S., Chase E. A., Brown V. J., Attentional set-shifting in rodents: A review of behavioural methods and pharmacological results. Curr. Pharm. Des. 20, 5046–5059 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Johnston K., Levin H. M., Koval M. J., Everling S., Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron 53, 453–462 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Rushworth M. F., Hadland K. A., Gaffan D., Passingham R. E., The effect of cingulate cortex lesions on task switching and working memory. J. Cogn. Neurosci. 15, 338–353 (2003). [DOI] [PubMed] [Google Scholar]
- 48.Asaad W. F., Rainer G., Miller E. K., Task-specific neural activity in the primate prefrontal cortex. J. Neurophysiol. 84, 451–459 (2000). [DOI] [PubMed] [Google Scholar]
- 49.Rodgers C. C., DeWeese M. R., Neural correlates of task switching in prefrontal cortex and primary auditory cortex in a novel stimulus selection task for rodents. Neuron 82, 1157–1170 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Buckner R. L., DiNicola L. M., The brain’s default network: Updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 20, 593–608 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Procyk E., Tanaka Y. L., Joseph J. P., Anterior cingulate activity during routine and non-routine sequential behaviors in macaques. Nat. Neurosci. 3, 502–508 (2000). [DOI] [PubMed] [Google Scholar]
- 52.Saga Y., et al., Ventral pallidum encodes contextual information and controls aversive behaviors. Cereb. Cortex 27, 2528–2543 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Ottenheimer D. J., et al., A quantitative reward prediction error signal in the ventral pallidum. Nat. Neurosci. 23, 1267–1276 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Floresco S. B., West A. R., Ash B., Moore H., Grace A. A., Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat. Neurosci. 6, 968–973 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Goto Y., Grace A. A., Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat. Neurosci. 8, 805–812 (2005). [DOI] [PubMed] [Google Scholar]
- 56.Faure A., Haberland U., Condé F., El Massioui N., Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J. Neurosci. 25, 2771–2780 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tachibana Y., Hikosaka O., The primate ventral pallidum encodes expected reward value and regulates motor action. Neuron 76, 826–837 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White J. K., et al., A neural network for information seeking. Nat. Commun. 10, 5168 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Avila I., Lin S. C., Motivational salience signal in the basal forebrain is coupled with faster and more precise decision speed. PLoS Biol. 12, e1001811 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hangya B., Ranade S. P., Lorenc M., Kepecs A., Central cholinergic neurons are rapidly recruited by reinforcement feedback. Cell 162, 1155–1168 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith V., Mitchell D. J., Duncan J., The effect of rule retrieval on activity in the default mode network. Neuroimage 202, 116088 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coutureau E., Marchand A. R., Di Scala G., Goal-directed responding is sensitive to lesions to the prelimbic cortex or basolateral nucleus of the amygdala but not to their disconnection. Behav. Neurosci. 123, 443–448 (2009). [DOI] [PubMed] [Google Scholar]
- 63.Killcross S., Coutureau E., Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb. Cortex 13, 400–408 (2003). [DOI] [PubMed] [Google Scholar]
- 64.Corbit L. H., Balleine B. W., The role of prelimbic cortex in instrumental conditioning. Behav. Brain Res. 146, 145–157 (2003). [DOI] [PubMed] [Google Scholar]
- 65.Tran-Tu-Yen D. A., Marchand A. R., Pape J. R., Di Scala G., Coutureau E., Transient role of the rat prelimbic cortex in goal-directed behaviour. Eur. J. Neurosci. 30, 464–471 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Coutureau E., Killcross S., Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats. Behav. Brain Res. 146, 167–174 (2003). [DOI] [PubMed] [Google Scholar]
- 67.Daw N. D., Niv Y., Dayan P., Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat. Neurosci. 8, 1704–1711 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Balleine B. W., Neural bases of food-seeking: Affect, arousal and reward in corticostriatolimbic circuits. Physiol. Behav. 86, 717–730 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Klaassen A., Heiniger A., Vaca Sánchez P., Harvey M., Rainer G., Ventral pallidum regulates the default mode network, controlling transitions between internally and externally guided behavior. G-Node. 10.12751/g-node.2i5788. Deposited 17 August 2021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets have been deposited in the G-Node Open Data repository (DOI: 10.12751/g-node.zrkd6x) (69), and data have been deposited in the Collaborative Research in Computational Neuroscience (CRCNS) database (https://crcns.org/data-sets/bf). All other study data are included in the article and/or SI Appendix.