Abstract
Papillary fibroelastomas (PFE) are rare primary cardiac tumors characterized by non-malignant, pedunculated, endocardial lesions with a significant risk of embolic potential and death. With improvements in the imaging quality and availability of transthoracic echocardiograms (TTE), the diagnosis of PFE has become more common in the last 2 decades. PFE is changing from a rare “zebra” diagnosis to one that community providers will encounter in their practice and must appropriately treat to prevent morbidity and mortality. Data shows that there are significant survival and morbidity benefit associated with surgical excision over non-operative management, with the benefit of anticoagulation remaining unclear at this time. We report a case describing the diagnostic workup and management of a 58-year-old woman who presented with an unidentified endocardial mass determined to be a PFE. Based on current literature, we favor a strategy of early surgical excision of PFE for an optimal reduction in mortality and thromboembolic sequelae associated with this pathology.
Keywords: Papillary Fibroelastoma, Primary Cardiac Tumor, Anticoagulation, Aortic Valve Mass
Introduction
Primary cardiac tumors are rare, only occurring in 0.02% of autopsies, based on the data from 22 autopsy series.1 Fortunately, 75% are non-malignant tumors.2 They are primarily diagnosed in older patients, with a mean age of 60 years old at the time of diagnosis.3 Fibroelastomas are the most common valvular tumor, with diagnosis becoming more common in the past 2 decades due to advances in resolution and availability in echocardiography.3,4 Histopathologically, PFE is a small, highly papillary, pedunculated, avascular tumor covered by a single layer of endothelium. The tumor consists of a hyaline stroma with variable amounts of elastic fibrils.1 The size of PFE can vary, though most are in the range of 8 mm to 16 mm on the valvular surfaces and 22 mm on the non-valvular right heart endocardium.5 Although the pathogenesis of PFE is not well understood; it is hypothesized that microscopic endocardial damage and subsequent dysregulated endothelial repair leads to the excessive formation of basal membrane material and formation of PFE.1 Risk factors for the development of PFE include a history of endocardial surgery, thoracic radiation therapy, history of rheumatic heart disease, and cardiac valvular disease causing trans-valvular pressure gradient.3,6 Associations have also been found with other comorbidities to include hypertension, hyperlipidemia, diabetes, and chronic obstructive lung disease.7,8 Morphologically, PFE most commonly occurs at valvular surfaces, with the aortic valve being the most common location, followed by the mitral, tricuspid, and pulmonary valves. Outside of valvular surfaces, the left ventricle is the most common endocardial surface of occurrence.5
The clinical presentation of papillary fibroelastoma varies. When discovered early, papillary fibroelastomas can be asymptomatic. Still, sequelae range from disruption of cardiac valve function causing dyspnea or clinical heart failure syndrome to severe embolic complications such as ischemic stroke.3,9 In thromboembolic cases, the cerebral and retinal arteries are typically affected.10 With these possible sequelae in mind, we herein describe a case of a 58-year-old female with a history significant for mediastinal radiation therapy and moderate aortic regurgitation, who presented with an initially uncharacterized aortic valve mass later diagnosed as a fibroelastoma on transesophageal echocardiogram.
Case Description
A 58-year-old female with a past medical history significant for mediastinal radiation therapy in 1983 for a diagnosis of Hodgkin's lymphoma and moderate aortic regurgitation (American Heart Association [AHA] Stage B) underwent her routine yearly surveillance echocardiogram for moderate aortic regurgitation.11 Significantly, TTE performed one year ago did not detect evidence of a PFE. The current TTE revealed a new, calcified, 9-mm mobile density on the non-coronary cusp of the aortic valve. She denied any symptoms of recent fevers, dyspnea, headaches, vision changes, or focal neurological deficits. She denied any risk factors for infective endocarditis, which can mimic the appearance of PFE, including a history of intravenous drug use, chronic infusion therapy, or hemodialysis. Laboratory evaluation revealed no evidence of significant abnormalities, and 3 sets of blood cultures from multiple sites were negative for any evidence of bacteremia. She was admitted for further imaging and differentiation of her newly discovered cardiac mass. A repeat TTE was not able to discern the etiology of the mass. Therefore, she underwent further evaluation with a transesophageal echocardiogram (TEE). The TEE revealed good visualization of a tricuspid aortic valve with a 9-mm mobile, pedunculated, non-obstructing, calcified mass on the non-coronary cusp consistent with a calcified PFE with no other masses observed on the remaining valves or visible endocardial surfaces (Figure 1 and Figure 2). Given clear visualization of the PFE with TEE, further imaging studies were not pursued. The patient was counseled that elective surgical removal is the definitive management of PFE to reduce the risk of thromboembolism and was given the option for surgical referral. However, the patient desired to delay surgical intervention for a second opinion with her primary outpatient cardiologist. Given her election for nonsurgical management at that time, as well as the lack of other comorbidities, including heart failure, hypertension, previous embolic events, diabetes, and vascular disease, she was started on a low dose aspirin 81 milligrams (mg) by mouth daily and discharged home with yearly TTE follow-up. The patient ultimately decided to continue pursuing medical management of her PFE with aspirin and continued yearly TTE studies. She remains compliant on aspirin 81 mg daily and asymptomatic to date.
Figure 1.
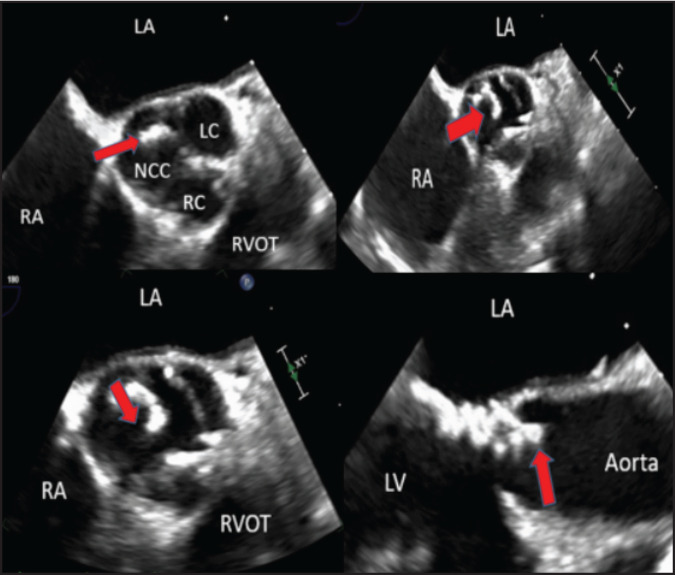
Scans of Transesophageal Echocardiogram. Top Left, Top Right, and Bottom Left: Transesophageal echocardiogram (TEE) 2-dimensional image on the mid esophageal short axial view of the patient's papillary fibroelastoma (red arrow). Bottom Right: TEE paraesophageal long view of the papillary fibroelastoma described as the left coronary cusp (LC), right coronary cusp (RC), right ventricular outflow tract (RVOT), left atrium (LA), right atrium (RA).
Figure 2.

3-Dimensional Transesophageal Echocardiogram of Papillary Fibroelastoma. A scan of the papillary fibroelastoma is shown (red arrow) on the non-coronary cusp using a 3-dimensional transesophageal echocardiogram.
Discussion
This case illustrates the recognition of a PFE in a patient with aortic valve regurgitation and a history of thoracic radiation therapy. The patient's chronic valvular regurgitation is a risk factor for the development of PFE by causing turbulent transvalvular blood flow resulting in microscopic valvular injury.3 The patient's history of mediastinal radiation may also have been a predisposing factor by inducing cardiac endothelial damage.6 When a cardiac valvular mass is initially incidentally visualized on imaging, other etiologies of valvular mass, based on clinical history and morphology, should also be considered. TTE evaluation should be followed with TEE evaluation to elucidate the mass both descriptively and quantitatively.12 TEE is the imaging modality of choice, and cardiac magnetic resonance (CMR) imaging is not required for diagnosis.13 In our case, the TEE adequately characterized the size and morphology of the PFE, so CMR was not necessary. However, CMR and positron emission tomography (PET) have been described in the literature as other viable modalities to investigate cardiac masses.14 CMR demonstrates high accuracy in differentiating cardiac thrombi from other tumors.15 PET imaging may also be used to distinguish between benign cardiac mass from malignant masses via evaluation of metabolic activity.16 Furthermore, additional studies have also shown that CMR may detect intra-cardiac masses missed on initial echocardiography.15,16,17
Patients with PFE are at significantly elevated risk for death when compared to age-matched healthy controls.5 Significantly, a retrospective review by Tamin et al showed that all-cause mortality at 5 years is twice as high in PFE patients as in age and sex-matched controls. Patients with PFE are at significantly higher risk of cerebrovascular accident (CVA) than age and sex-matched counterparts, even with medical and surgical treatment. Tamin and colleagues reported that the rate of observed CVA at 10 years was 2.4 times and 3.4 times higher in matched PFE patients who were treated with surgery and who were treated medically, respectively.5 Tumor mobility has also been shown as an independent predictor of PFE-related death and non-fatal embolization.1
Surgical resection is the gold standard of treatment for papillary fibroelastoma, even for those patients who are asymptomatic. In surgical removal, the roof of the pedicle and full thickness of endocardium is removed. The resulting defect may be closed by either primary closure or pericardial patch.18,19 Recurrence of PFE after surgical excision is rare, noted in 0.04% of PFE cases.5,20,21 Patients who undergo surgical excision have 30% higher overall survival rates in the first 7 years post-procedure than those who opt for non-operative management.5 Furthermore, among those who underwent surgical excision, only 8% experienced neurologic sequelae at 5-year follow-up, versus 13% in a pooled group of those who did not undergo surgical excision, regardless of anticoagulation decision.5 However, the optimal medical management of PFE is unknown. There are currently no randomized controlled trials directly comparing anticoagulation versus antiplatelet treatment versus surgical excision. In 2 separate retrospective reviews, Tamin et al and Gowda et al describe anticoagulation regimens with one of the following regimens: (1) warfarin, (2) heparin, (3) aspirin and clopidogrel, (4) aspirin alone, or (5) clopidogrel alone.1,5 There are currently no large-scale studies describing anticoagulation with direct oral anticoagulants. Even within cohorts, the decision to anticoagulate and the results of treatment were variable. In a case series of 725 patients identified via literature search, Gowda et al described only 57 patients anticoagulated with either heparin or warfarin.1 Tamin et al described a group of 121 PFE patients who experienced neurologic events despite being on anticoagulant therapy, of whom 27 were on warfarin (22%), 57 on aspirin (47%), 1 on clopidogrel (1%), and 2 on dual antiplatelet therapy (2%).5 This report was not sufficiently powered to detect a difference in outcomes between the different treatment modalities.5 Therefore, optimal anticoagulation regimen remains unclear, although non-operative management is inferior to surgical excision of PFE.
For those patients who undergo medical therapy alone, current literature agrees that serial TTE should be performed for surveillance. However, there is no clear consensus in recommendations for imaging intervals. Imaging intervals in case reports have ranged from every 2 months to more than 1 year. Mutlu et al pursued a close interval follow-up with TTE at 2-month intervals until 6 months, and then biannually after that for a PFE patient who presented with dyspnea.22 Others have followed asymptomatic patients at closer intervals. Seol et al pursued TTE at 2-month intervals for 3 years for asymptomatic patients.23 In contrast, Ayabe et al followed patients with less than 1 TTE a year to average 7 over 10 years. In our case, we considered the patient's AHA Stage B aortic regurgitation when proposing a surveillance schedule. We chose to pursue a yearly TTE surveillance schedule to adequately screen for both progression of her PFE and progression in her aortic regurgitation as recommended by the American College of Cardiology.
We conclude that all patients with PFE should be considered for surgical excision of the tumor as a first-line intervention. This procedure should be done regardless of symptoms to reduce incidence of thromboembolic events or death. If the patient is not a surgical candidate, medical management of PFE should focus on therapeutic anticoagulation or antiplatelet therapy. Clinical factors known to increase the risk of arterial thromboembolism, age, sex, and pertinent past medical history such as heart failure, hypertension, previous embolic events, diabetes, and history of coronary artery disease should be taken into consideration when crafting an individual risk-benefit assessment.24 It may be reasonable for patients with elevated risk factors for thromboembolism to undergo systemic anticoagulation with heparin or warfarin.
Conversely, those with less risk factors may warrant only aspirin or clopidogrel alone, as in our patient. Any decision to initiate systemic anticoagulation should be weighed against potentially fatal bleeding. Clinical risk factors for bleeding include hypertension, renal disease, liver disease, history of hemorrhagic stroke, prior bleeding, age, and concomitant anticoagulant use.25 These should be evaluated to assess a risk-benefit analysis before anticoagulation.
Papillary fibroelastoma is a histologically benign tumor that causes significant morbidity and mortality from thromboembolic events. Patients should undergo surgical excision of the fibroelastoma for survival benefit. If patients decline surgery and opt for medical management with anticoagulation therapy alone, the optimal regimen is unclear. The choice between administration of heparin, warfarin, aspirin, or clopidogrel should be made with consideration of thrombotic risk factors and factors for major bleeding events. In the future, more research is needed to compare varying regimens of anticoagulation treatment, especially the use of direct oral anticoagulants, as well as more thorough comparison between the long-term outcomes of anticoagulation instead of surgical excision in the treatment of PFE.
Abbreviations and Acronyms
- AHA
American Heart Association
- CMR
cardiac magnetic resonance
- CVA
cerebrovascular accident
- PET
positron emission tomography
- PFE
papillary fibroelastoma
- TEE
transesophageal echocardiogram
- TTE
transthoracic echocardiogram
Conflict of Interest
None of the authors identify a conflict of interest.
References
- 1.Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J. 2003;146((3)):404–410. doi: 10.1016/S0002-8703(03)00249-7. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors—diagnosis and surgical treatment. Dtsch Arztebl Int. 2014;111((12)):205–211. doi: 10.3238/arztebl.2014.02053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darvishian F, Farmer P. Papillary fibroelastoma of the heart: report of two cases and review of the literature. Ann Clin Lab Sci. 2001;31((3)):291–296. [PubMed] [Google Scholar]
- 4.Boodhwani M, Veinot JP, Hendry PJ. Surgical approach to cardiac papillary fibroelastomas. Can J Cardiol. 2007;23((4)):301–302. doi: 10.1016/S0828-282X(07)70759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamin SS, Maleszewski JJ, Scott CG, et al. Prognostic and bioepidemiologic implications of papillary fibroelastomas. J Am Coll Cardiol. 2015;65((22)):2420–2429. doi: 10.1016/j.jacc.2015.03.569. [DOI] [PubMed] [Google Scholar]
- 6.Fine NM, Foley DA, Breen JF, Maleszewski JJ. Multimodality imaging of a giant aortic valve papillary fibroelastoma. Case Rep Med. 2013;2013. 10.1155/2013/705101 doi: 10.1155/2013/705101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu Saleh WK, Al Jabbari O, Ramlawi B, Reardon MJ. Cardiac papillary fibroelastoma: single-institution experience with 14 surgical patients. Tex Heart Inst J. 2016;43((2)):148–151. doi: 10.14503/THIJ-14-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mkalaluh S, Szczechowicz M, Torabi S, et al. Surgery for cardiac papillary fibroelastoma: a 12-year single institution experience. Med Sci Monit Basic Res. 2017;23((1)):258–263. doi: 10.12659/MSMBR.904881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Wu L, Ni F, Ji W, Zhou C, Wu J. Critical illness polyneuropathy and myopathy: a systematic review. Neural Regen Res. 2014;9((1)):101. doi: 10.4103/1673-5374.125337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baikoussis N, Dedeilias P, Argiriou M, et al. Cardiac papillary fibroelastoma; when, how, why? Ann Card Anaesth. 2016;19((1)):162. doi: 10.4103/0971-9784.173040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. 2014;63((22)):e57–e185. doi: 10.1016/j.jacc.2014.02.536. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues JD, Ferreira J, Almeida J, Campelo M, Maciel MJ, Pinho P. Cardiac papillary fibroelastoma: report of a surgical series. Rev Port Cardiol. 2018;37((12)):981–986. doi: 10.1016/j.repc.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Hoey ET, Shahid M, Ganeshan A, Baijal S, Simpson H, Watkin RW. MRI assessment of cardiac tumours: part 1, multiparametric imaging protocols and spectrum of appearances of histologically benign lesions. Quant Imaging Med Surg. 2014;4((6)):478–488. doi: 10.3978/j.issn.2223-4292.2014.11.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hundley WG, Bluemke DA, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation task force on expert consensus documents. Circulation. 2010;121((22)):2462–2508. doi: 10.1161/CIR.0b013e3181d44a8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slonimsky, Slonimsky E, Konen O, Di Segni E, Konen E, Goitein O. Cardiac MRI: A useful tool for differentiating cardiac thrombi from tumors. Isr Med Assoc J. 2018;20((8)):472–475. [PubMed] [Google Scholar]
- 16.Nensa F, Tezgah E, Poeppel TD, et al. Integrated 18F-FDG PET/MR imaging in the assessment of cardiac masses: a pilot study. J Nucl Med. 2015;56((2)):255–260. doi: 10.2967/jnumed.114.147744. [DOI] [PubMed] [Google Scholar]
- 17.Gulati, Gulati G, Sharma S, Kothari SS, Juneja R, Saxena A, Talwar KK. Comparison of echo and MRI in the imaging evaluation of intracardiac masses. Cardiovasc Intervent Radiol. 2004;27((5)):459–469. doi: 10.1007/s00270-004-0123-4. [DOI] [PubMed] [Google Scholar]
- 18.Gopaldas RR, Atluri PV, Blaustein AS, Bakaeen FG, Huh J, Chu D. Papillary fibroelastoma of the aortic valve: operative approaches upon incidental discovery. Tex Heart Inst J. 2009;36((2)):160–163. [PMC free article] [PubMed] [Google Scholar]
- 19.Ngaage DL, Mullany CJ, Daly RC, et al. Surgical treatment of cardiac papillary fibroelastoma: a single center experience with eighty-eight patients. Ann Thorac Surg. 2005;80((5)):1712–1718. doi: 10.1016/j.athoracsur.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 20.Sun JP, Asher CR, Yang XS, et al. Clinical and echocardiographic characteristics of papillary fibroelastomas: a retrospective and prospective study in 162 patients. Circulation. 2001;103((22)):2687–2693. doi: 10.1161/01.CIR.103.22.2687. [DOI] [PubMed] [Google Scholar]
- 21.Haider I, Siddiqui M. Non-valvular right sided papillary fibroelastoma. J Am Coll Cardiol. 2014;63((12)):A715. doi: 10.1016/S0735-1097(14)715-4. [DOI] [Google Scholar]
- 22.Mutlu H, Demir IE, Leppo J, Levy WK. Nonsurgical management of a left ventricular pedunculated papillary fibroelastoma: a case report. J Am Soc Echocardiogr. 2008;21((7)):877.e4–877.e7. doi: 10.1016/j.echo.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Seol S-H, Kim D-S, Han Y-C, et al. Nonsurgical management of a tricuspid valvular pedunculated papillary fibroelastoma. Cardiovasc Ultrasound. 2009;7((1)):44. doi: 10.1186/1476-7120-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J-T, Wang S-L, Chu Y-J, et al. CHADS2 and CHA2DS2-VASc scores predict the risk of ischemic stroke outcome in patients with interatrial block without atrial fibrillation. J Atheroscler Thromb. 2017;24((2)):176–184. doi: 10.5551/jat.34900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shoeb M, Fang MC. Assessing bleeding risk in patients taking anticoagulants. J Thromb Thrombolysis. 2013;35((3)):312–319. doi: 10.1007/s11239-013-0899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


