Significance
SNF2 family member ALC1 and poly(ADP-ribose) polymerase PARP1 work together in a coupled enzyme system to remodel chromatin in DNA repair and other nuclear processes. ALC1 is overexpressed in many cancers and can render tumors resistant to PARP inhibitors. Illuminating the function of the ALC1-PARP1 pathway will be important for understanding its role in tumorigenesis. Here, we define multiple contributions of PARP1 to ALC1–PARP1-mediated chromatin remodeling.
Keywords: SNF2 family ATPase, nucleosome remodeling, poly(ADP-ribose) synthesis, nucleosome binding, CHD1L
Abstract
The SNF2 family ATPase Amplified in Liver Cancer 1 (ALC1) is the only chromatin remodeling enzyme with a poly(ADP-ribose) (PAR) binding macrodomain. ALC1 functions together with poly(ADP-ribose) polymerase PARP1 to remodel nucleosomes. Activation of ALC1 cryptic ATPase activity and the subsequent nucleosome remodeling requires binding of its macrodomain to PAR chains synthesized by PARP1 and NAD+. A key question is whether PARP1 has a role(s) in ALC1-dependent nucleosome remodeling beyond simply synthesizing the PAR chains needed to activate the ALC1 ATPase. Here, we identify PARP1 separation-of-function mutants that activate ALC1 ATPase but do not support nucleosome remodeling by ALC1. Investigation of these mutants has revealed multiple functions for PARP1 in ALC1-dependent nucleosome remodeling and provides insights into its multifaceted role in chromatin remodeling.
The human ALC1 (Amplified in Liver Cancer 1) protein (also referred to as CHD1L or Chromodomain-Helicase-DNA-binding protein 1-Like) is a SNF2 family chromatin remodeling enzyme that functions together with the poly(ADP-ribose) polymerase PARP1 to catalyze ATP- and NAD+-dependent nucleosome remodeling. The ALC1 gene is amplified in a subset of hepatocellular carcinomas, and overexpression of the ALC1 protein leads to transformation of cultured cells and appearance of spontaneous tumors in mice (1, 2). Although the precise mechanism(s) by which ALC1 overexpression contributes to tumorigenesis remains unknown, ALC1 has been implicated in multiple DNA damage repair pathways (3–6). Several recent studies have shown that ALC1 overexpression confers resistance to PARP inhibitors used in treatment of DNA repair–deficient tumors, while loss or reduction of ALC1 expression renders cells exquisitely sensitive to these drugs (7–10). Hence, understanding the functional relationships between ALC1 and PARP1 is of considerable interest.
We and others initially demonstrated that ALC1 has cryptic DNA-dependent ATPase and ATP-dependent nucleosome sliding activities that are strongly activated in the presence of PARP1 and NAD+ (3, 11), which PARP1 and other PARPs use as substrate for synthesis of poly(ADP-ribose) (PAR) (12). ALC1 is unique among SNF2 family members in containing a macrodomain. The macrodomain, located at the enzyme’s C terminus, binds PAR chains containing three or more ADP-ribose residues (3, 11, 13–15). ALC1 macrodomain mutations that abolish PAR binding block ALC1 ATPase and nucleosome remodeling, indicating that PAR binding by the macrodomain is important for ALC1 activation. Recent studies have led to a working model for how binding of PAR to ALC1 macrodomain contributes to nucleosome remodeling. According to this model, ALC1 SNF2 ATPase domain interacts with and is held in an inactive state by the macrodomain. Upon binding of PAR to the macrodomain, this interaction is released, leading to structural changes in the ALC1 ATPase domain that relieve autoinhibition (13, 16). In subsequent steps, nucleosome binding by ALC1 stabilizes the catalytically active conformation of the ATPase, and a linker region between the ATPase and macrodomains contacts an acidic patch on nucleosomes to couple ATP hydrolysis to nucleosome sliding (17).
Although it is well established that one key role of PARP1 in ALC1-dependent nucleosome remodeling is to produce PAR, it is less clear whether it makes additional contributions. Recent findings indicating that free tri-ADP ribose is sufficient to activate ALC1 ATPase activity in the absence of PARP1 (13) suggest that the role of PARP1 might be limited merely to synthesizing PAR chains. In this case, PARP1 might act simply as a bystander in ALC1-dependent nucleosome remodeling. On the other hand, PARP1 possesses both nucleosome binding and histone chaperone activities (18). This, together with our previous evidence that ALC1 and PARP1 bind cooperatively to nucleosomes to form an ALC1–PARP1–nucleosome intermediate prior to remodeling (14), makes it tempting to speculate that PARP1 might play a more active role.
In the course of experiments investigating the mechanism(s) by which PARP1 contributes to ALC1-dependent nucleosome remodeling, we identified PARP1 mutants capable of activating ALC1 ATPase, but defective in supporting ALC1-catalyzed nucleosome remodeling. By investigating the properties of these and additional PARP1 mutants, we show that both the PARP1 C-terminal ADP-ribosyl transferase domain and its N-terminal region, which contains nucleosome binding activity, play important roles in ALC1-dependent nucleosome remodeling. We report these findings, which bring to light a role for PARP1 in chromatin remodeling.
Results
A PARP1 Mutant that Supports ALC1 ATPase but Not Nucleosome Remodeling.
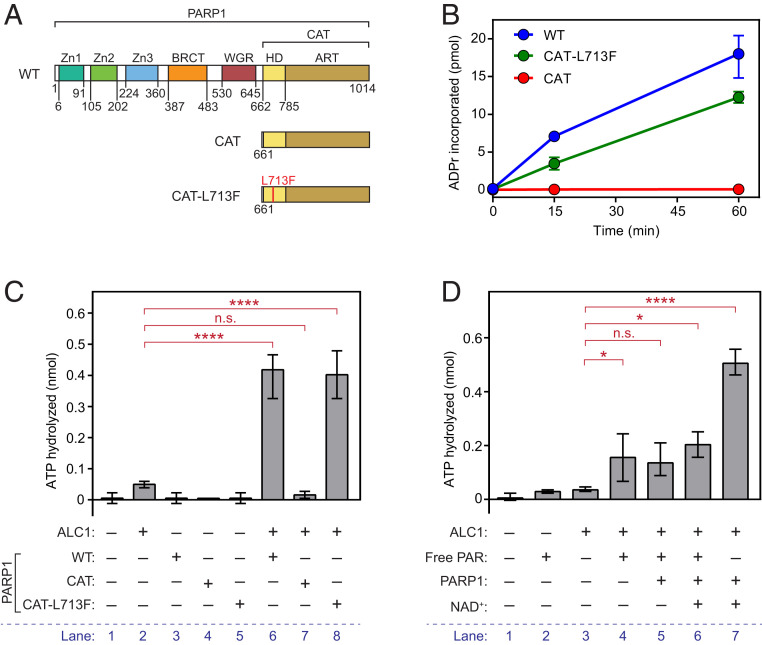
PARP1 consists of a C-terminal catalytic (CAT) domain and an N-terminal region needed for DNA-dependent allosteric activation of ADP-ribosyl transferase activity (19). As diagrammed in Fig. 1A, the N-terminal region includes three zinc finger domains (Zn1, Zn2, and Zn3), a BRCA1–C‐terminal domain (BRCT), and a tryptophan‐glycine‐arginine (WGR) domain. CAT is further divided into the catalytic ADP-ribosyl transferase subdomain (ART) and an autoinhibitory helical subdomain (HD), which sterically blocks access to the NAD+ binding site in ART (20). Binding of PARP1 N-terminal domains to DNA or nucleosomes drives unfolding of HD, leading to release of autoinhibition and productive NAD+ binding and PAR synthesis (21, 22).
Fig. 1.
The PARP1 noncatalytic domains are dispensable for activation of ALC1 ATPase. (A) Diagram showing domain architecture of wild-type (WT) PARP1 and CAT mutants. (B) PARylation activities of WT PARP1, CAT-L173F, and CAT. Incorporation of 32P-NAD+ into PAR was assayed in the presence of nucleosomes and quantitated by liquid scintillation counting. Data represent ADP-ribose incorporation (mean ± range) calculated from at least two independent replicates. (C) ALC1 ATPase activity in the presence of WT PARP1 or PARP1 CAT mutants was assayed as described. Each reaction contained nucleosomes and NAD+. (D) ALC1 ATPase activity in the presence of 1 µM free PAR (Trevigen 4336-100-01), PARP1, and/or NAD+ was assayed as described. Each reaction contained nucleosomes. For C and D, data presented are values of mean ± range from at least two independent experiments. Significance was determined using Tukey’s multiple comparison test. n.s., not significant; *P < 0.05; ****P < 10−4.
As part of our effort to explore the role of PARP1 in ALC1-dependent chromatin remodeling, we searched for PARP1 mutants capable of synthesizing PAR and activating ALC1 ATPase but not of promoting ALC1 catalyzed nucleosome sliding. We found that a PARP1 mutant that includes just CAT (residues 661 to 1,014; Fig. 1A and SI Appendix, Fig. S1A) has these properties. Consistent with previous results (21, 23), the isolated CAT domain is severely defective in PAR synthesis (Fig. 1B), as it lacks the N-terminal region needed for DNA-dependent release of HD-mediated autoinhibition (21, 23). We therefore exploited a well-characterized gain-of-function mutation, L713F. This mutation destabilizes HD and greatly increases the ability of isolated CAT to synthesize PAR (21, 23, 24). As shown in Fig. 1B, the rate of PAR synthesis in the presence of the gain-of-function mutant CAT-L713F approached that of wild-type PARP1. In addition, CAT-L713F, like wild-type PARP1, is subject to auto-PARylation (SI Appendix, Fig. S1C). Whereas the PARylation activity of wild-type PARP1 is strictly dependent on addition of nucleosomes to reaction mixtures, the rate of PAR synthesis by CAT-L713F is the same in either the presence or absence of nucleosomes (SI Appendix, Fig. S1D).
Consistent with previous findings (3, 11), we observed ALC1 ATPase activity is strongly stimulated by wild-type PARP1 in reactions containing NAD+ and nucleosomes (Fig. 1C, compare lanes 2 and 6). In these experiments, we used full-length human ALC1 (residues 1 to 897), expressed in and purified from Escherichia coli. PARP1 CAT had no detectable effect on ALC1 ATPase activity, consistent with its lack of detectable ADP-ribosyl transferase activity. In contrast, the catalytically active PARP1 mutant CAT-L713F activated ALC1 ATPase as well as wild-type PARP1, as might be expected given evidence that activation of ALC1 ATPase can be accomplished even by addition of free oligo(ADP-ribose), in the absence of any PARP1 (13). Indeed, we observe that free PAR can stimulate ALC1 ATPase in our assays, albeit to a level ∼30 to 40% lower than that observed in the presence of PARP1 and NAD+ (Fig. 1D). Addition of free PAR to reactions containing PARP1 and NAD+ reduced ALC1-dependent ATPase to about the same level as that seen with free PAR alone. At present, we do not know whether this is because free PAR competes with PAR on auto-PARylated PARP1 or because of the presence of inhibitory contaminants in free PAR.
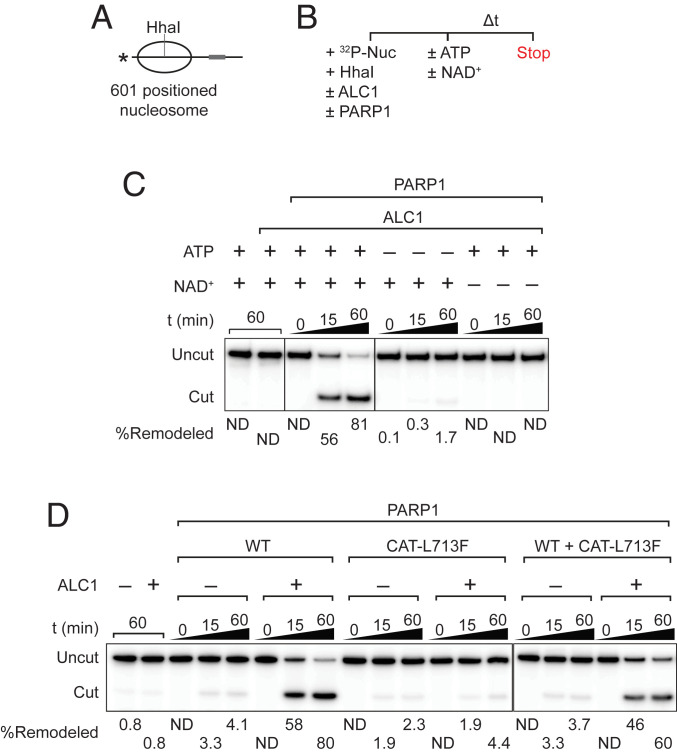
We next asked whether CAT-L713F could support ALC1-dependent nucleosome remodeling. In these assays, we used mononucleosomes reconstituted with recombinant human histone octamers assembled onto a radiolabeled DNA fragment bearing the 601 nucleosome positioning sequence (Fig. 2A and SI Appendix, Fig. S2A). As diagrammed in Fig. 2A, near the dyad of nucleosomes positioned on the 601 sequence is a HhaI restriction site (25), which is largely protected from digestion prior to nucleosome remodeling but which becomes accessible after ATP-dependent remodeling by ALC1 (3, 11). 32P-labeled nucleosomes were incubated with HhaI, with or without ALC1, PARP1, NAD+, and ATP as outlined in Fig. 2B. As expected, ATP-dependent nucleosome remodeling by ALC1, detected by conversion of the full-length, uncut nucleosomal DNA to the shorter, HhaI-cut fragment, is strongly dependent on PARP1 and NAD+ (Fig. 2C). In these assays, optimal nucleosome remodeling was observed when reactions contained 0.1 to 1 mM NAD+ and at least 1 mM ATP (SI Appendix, Fig. S2 B–D).
Fig. 2.
CAT-L713F fails to support ALC1-dependent nucleosome remodeling. (A) Scheme depicting a laterally positioned mononucleosome with a 32P-label at the 5′ end and a linker DNA. The gray box indicates the position of a GAL4 binding site sequence in the linker DNA, and the asterisk indicates the radiolabeled end of the DNA fragment. (B) HhaI deprotection assay for nucleosome remodeling. (C) ATP and NAD+ dependence of ALC1 nucleosome remodeling activity. (D) Nucleosome remodeling assays included ALC1, PARP1, and/or CAT-L713F as indicated in the figure. 32P-Nuc, 32P-labeled nucleosomes; ND, not detected; t, time.
Whereas CAT-L713F activated ALC1 ATPase similarly to wild-type PARP1, it was severely defective in supporting nucleosome remodeling (Fig. 2D). Arguing against the possibility that the failure of CAT-L713F to stimulate nucleosome remodeling was due to the presence of an inhibitory activity, addition of CAT-L713F to reactions containing wild-type PARP1 did not prevent ALC1-dependent nucleosome remodeling. Taken together, these findings argue that 1) PARP1 has an important function in ALC1 nucleosome remodeling apart from its role in activating ALC1 ATPase, and 2) this PARP1 function is housed in one or more of its N-terminal noncatalytic domains.
Evidence for PARylation-Independent Roles of Zn1, Zn3, and WGR Domains in ALC1 Nucleosome Remodeling.
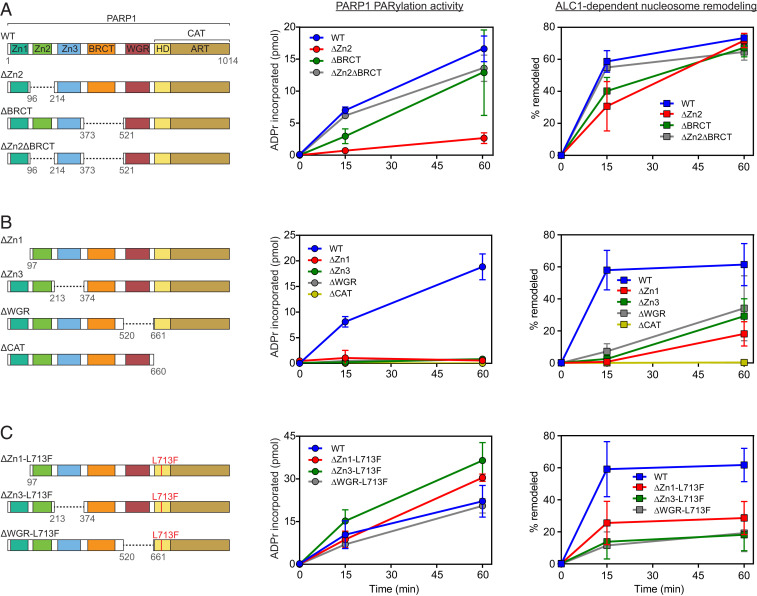
To explore in greater detail contributions of the N-terminal portion of PARP1 to ALC1-catalyzed nucleosome remodeling, we sought to identify the required domains. Guided by published structural information (23, 26–29), we expressed and purified a systematic series of PARP1 mutants possessing a wild-type catalytic domain but lacking the N-terminal Zn1, Zn2, Zn3, BRCT, or WGR domains (SI Appendix, Fig. S3 A and B) and compared their abilities to support PAR synthesis and nucleosome remodeling (Fig. 3).
Fig. 3.
Contributions of PARP1 N-terminal domains to ALC1-dependent nucleosome remodeling. The activities of WT PARP1 are compared to those of (A) PARP1 mutants lacking the Zn2, BRCT, or Zn2 and BRCT domains, (B) PARP1 mutants lacking the Zn1, Zn3, WGR, or CAT domains, and (C) various PARP1 mutants bearing the L713F mutation. The Left column of each panel shows a diagram of PARP1 mutants; the Middle column shows PARylation assays, and the Right column shows nucleosome remodeling. Data represent mean ± SD from at least three independent experiments.
PARP1 mutants containing a wild-type PARP1 catalytic domain but lacking Zn2 and/or BRCT support PAR synthesis (refs. 23, 27, 30 and Fig. 3 A, Middle). Similarly, we observe that mutants lacking the Zn2 and/or BRCT domains support activation of ALC1 nucleosome remodeling (Fig. 3 A, Right). For reasons that are not understood, the individual Zn2 and BRCT deletion mutants exhibit reduced PARylation and nucleosome remodeling activities, even though a double deletion mutant of PARP1 lacking both Zn2 and BRCT supports both PAR synthesis and ALC1 nucleosome remodeling as well as wild-type PARP1. Notably, there appears to be an imperfect correlation between the effect of deleting Zn2 alone on PARylation and nucleosome remodeling; deletion of the Zn2 domain alone has a substantially larger effect on PARP1 PARylation activity than on its ability to support nucleosome remodeling.
Unlike deletion of Zn2 and BRCT, deletion of Zn1, Zn3, or WGR leads to severe defects in the ability of PARP1 with a wild-type catalytic domain to support ALC1-dependent nucleosome remodeling and, as has been shown previously (23), to synthesize PAR. In our standard PARylation assays, we observed little or no PAR synthesis above background in the presence of the ΔZn1, ΔZn3, or ΔWGR mutants (Fig. 3B); however, using a more sensitive assay in which we analyzed 32P-labeled PAR isolated from these reactions by denaturing polyacrylamide gel electrophoresis (SI Appendix, Fig. S3D), we could detect very low levels of PAR synthesis by these mutants. Nucleosome remodeling in the presence of these mutants was also dramatically reduced and was preceded by a pronounced lag (Fig. 3 B, Right), resembling a lag seen in remodeling reactions performed with wild-type PARP1, at very low NAD+ concentrations where PAR is synthesized slowly (SI Appendix, Fig. S2B). Importantly, we observed no remodeling in the presence of a PARP1 mutant (ΔCAT) lacking the catalytic domain. These results suggest that despite their very low PARylation activity, ΔZn1, ΔZn3, and ΔWGR are able to drive weak ALC1 nucleosome remodeling once they have synthesized an amount of PAR that exceeds a threshold needed for activation of ALC1 ATPase.
We next sought to determine whether the Zn1, Zn3, and WGR domains are needed for optimal nucleosome remodeling solely because of their roles in DNA/nucleosome-dependent activation of PARP1 catalytic activity or whether they also contribute an additional function(s). To do so, we generated Zn1, Zn3, and WGR deletion mutants, all containing the L713F point mutation (SI Appendix, Fig. S3C), and compared their activities in nucleosome remodeling and PAR synthesis. We observed that the relative activities of wild-type PARP1 and ΔZn1-L713F, ΔZn3-L713F, and ΔWGR-L713F in PAR synthesis and in support of ALC1-dependent nucleosome remodeling were uncorrelated. In particular, all three mutants had activities in PAR synthesis that were equal to or better than wild-type PARP1 (Fig. 3C); as observed for CAT-L713F, PARylation activity of ΔZn1-L713F, ΔZn3-L713F, and ΔWGR-L713F did not depend on nucleosomes (SI Appendix, Fig. S4). Although introduction of the L713F mutation increased the initial rates of nucleosome remodeling by PARP1 lacking either the Zn1 or Zn3 domains, all three of these mutants supported less nucleosome remodeling by ALC1 than wild-type PARP1 in the first 15 min, and reactions with ΔZn1-L713F, ΔZn3-L713F, and ΔWGR-L713F plateaued after a substantially smaller fraction of nucleosomes had been remodeled. Taken together, these results indicate that no one domain within the PARP1 N-terminal region is essential for PARP1 activity in ALC1-dependent nucleosome remodeling. Importantly, however, the observation that none of these mutants support nucleosome remodeling as well as wild-type PARP1 suggests that the individual Zn1, Zn3, and WGR domains each make major contributions to a PARP1 function in ALC1-dependent nucleosome remodeling that cannot be explained simply by their requirement for PAR synthesis.
PARP1–Nucleosome Interactions in ALC1-Dependent Nucleosome Remodeling.
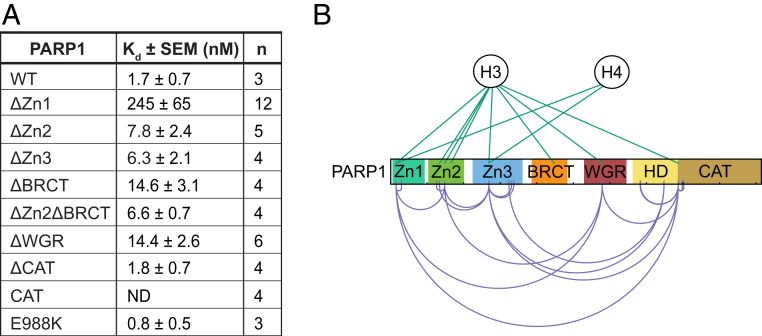
Previous studies have shown that the PARP1 N-terminal region binds nucleosomes (18, 31, 32). To begin to address the possibility that PARP1 nucleosome binding activity contributes to ALC1-dependent nucleosome remodeling, we asked whether the abilities of PARP1 mutants to support nucleosome remodeling correlates with their abilities to bind nucleosomes. To do so, we performed microscale thermophoresis (MST) to measure quantitatively the affinities for nucleosomes of PARP1 and PARP1 mutants lacking various domains at salt and nucleosome concentrations very similar to those used in our nucleosome remodeling assays. Wild-type PARP1 displayed a nucleosome-binding dissociation constant (Kd) of ∼2 nM under our assay conditions (Fig. 4A and SI Appendix, Fig. S5C). PARP1 deletion mutants lacking one or more of the N-terminal domains bound nucleosomes, but less well than wild type, with Kds ranging from about 6 to 15 nM (ΔZn2, ΔZn3, ΔZn2ΔBRCT, and ΔWGR) to ∼250 nM (ΔZn1) (Fig. 4A and SI Appendix, Fig. S5D). The substantial reduction in nucleosome binding observed upon deletion of the Zn1 domain (relative to PARP1 mutants lacking other N-terminal domains) stands in contrast to previous evidence ΔZn1 can bind with high affinity to free DNA (27) and suggests a particularly important role for the Zn1 domain in mediating nucleosome binding.
Fig. 4.
Contribution of PARP1 N-terminal domains to nucleosome binding. (A) Nucleosome binding affinities of PARP1 and mutants measured by MST. Listed are mean equilibrium dissociation constants derived from individual fitted nucleosome binding curves shown in SI Appendix, Fig. S5 C and D. (B) DSSO-mediated PARP1–histone (green lines) and PARP1–PARP1 (purple lines) cross-links identified by mass spectrometry in two independent experiments. Data were visualized using xiView (41). Kd, dissociation constant; n, number of replicates; ND, not detected.
Consistent with previous evidence that the PARP1 CAT domain does not bind DNA or nucleosomes without assistance from additional domains (32, 33), we were unable to detect nucleosome binding by CAT. We were also unable to detect binding of either CAT or CAT-L713F to nucleosomes using an electrophoretic mobility shift assay (SI Appendix, Fig. S6). Our observations that 1) CAT and CAT-L713F are the only PARP1 mutants tested with no detectable nucleosome binding activity and 2) CAT-L713F is fully active in PAR synthesis but has very little activity in nucleosome remodeling are consistent with the possibility that nucleosome binding by PARP1 contributes to ALC1-dependent nucleosome remodeling.
We also investigated the interaction of PARP1 with nucleosomes by performing mass spectrometry after cross-linking with disuccinimidyl sulfoxide (DSSO) (XL-MS) (34). Nearly all of the reproducible histone–PARP1 cross-links detected were between histones H3 and H4 and the PARP1 Zn1, Zn2, Zn3, BRCT, and WGR domains (Fig. 4B). Approximately 50% of these PARP1–histone cross-links fall on the H3 N-terminal tail (SI Appendix, Fig. S7 and Dataset S1), which, based on published structures, is located near the entry/exit sites of the nucleosome core particle (35, 36). This observation, together with the lack of reproducible PARP1 cross-links to either histones H2A or H2B, is consistent with previous studies that suggest PARP1 prefers to bind nucleosomal DNA at the nucleosome dyad and the entry/exit side of the nucleosome (37, 38). Interestingly, we observed several differences between histone–histone cross-links in free nucleosomes and nucleosomes bound by PARP1. Among these were reproducible loss of multiple cross-links between histones H3, H4, and H2B within the structured portion of the histone octamer and gain of cross-links between H3 and H4, H3 and H2A, and H4 and H2B. In addition, we observed replacement of a cross-link between H3K79 and H4K44 with one between H3K79 and PARP1 Zn3, a cross-link between H3K122 and H2BK108 with one between H3K122 and PARP1 Zn1, and two intramolecular cross-links (H4K31-H4K59 and H4K31-H4K91) with cross-links between H4K31 and the PARP1 Zn1 and Zn3 domains (SI Appendix, Figs. S7 and S8 and Dataset S1). While we cannot exclude the possibility these differences reflect run-to-run variation, they raise the possibility that interactions between PARP1 and nucleosomes induce subtle differences in the structure of the nucleosome core particle.
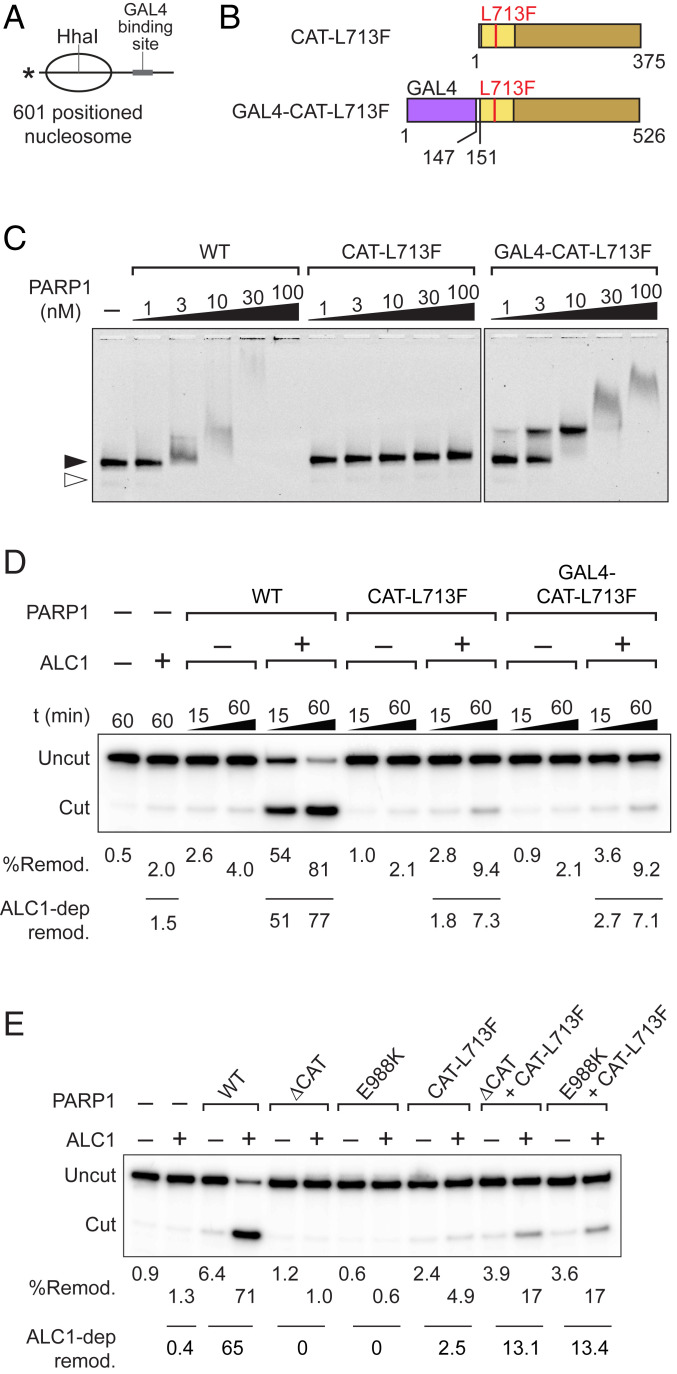
As noted earlier, ALC1 can be recruited to nucleosomes both in vitro and in cells via interaction of its macrodomain with nucleosome-bound, PARylated PARP1 (3, 11, 14). We considered the possibility that PARP1’s nucleosome binding domains might contribute to ALC1 nucleosome remodeling simply because they are needed to tether auto-PARylated PARP1 to nucleosomes. If this is the case, one might expect that tethering CAT-L713F to nucleosomes through an alternative DNA binding domain would increase the ability of CAT-L713F to support ALC1-dependent nucleosome remodeling. The nucleosomes used in our remodeling assays include a GAL4 binding site in the linker DNA (Fig. 5A). We therefore fused a GAL4 DNA binding domain to the N terminus of CAT L713F to generate GAL4-CAT-L713F (Fig. 5B and SI Appendix, Fig. S9A) and tested its ability to support ALC1-dependent nucleosome remodeling. In control experiments, we demonstrated that GAL4-CAT-L713F and free CAT-L713F have PARylation activities in either the presence or absence of nucleosomes (SI Appendix, Fig. S9B). In addition, GAL4-CAT-L713F, like CAT-L713F, strongly activates ALC1 ATPase activity (SI Appendix, Fig. S9C) and binds well to nucleosomes (Fig. 5C). Nevertheless, GAL4-CAT-L713F exhibits no more ability to support ALC1-dependent nucleosome remodeling than CAT-L713F alone (Fig. 5D). Thus, addition of an exogenous DNA binding domain that can recruit CAT-L713F to nucleosomal DNA was not sufficient to increase the ability of a catalytically active PARP1 fragment lacking PARP1’s nucleosome binding region to support ALC1-dependent remodeling.
Fig. 5.
Tethering CAT-L713F to nucleosomes fails to restore ALC1-dependent nucleosome remodeling. (A) Diagram of laterally positioned nucleosome showing the position of the GAL4 binding site sequence centered in the 69-bp linker DNA. (B) Diagram of GAL4-CAT-L713F mutant. (C) Nucleosome binding of GAL4-CAT-L713F assayed by agarose gel electrophoresis and fluorescence imaging. Filled and open arrowheads mark the position of nucleosomes and free DNA, respectively. (D) Nucleosome remodeling assay to test activation of ALC1-dependent nucleosome remodeling by GAL4-CAT-L713F. (E) Nucleosome remodeling assay to test activation of ALC1-dependent nucleosome remodeling by a combination of PARP1ΔCAT or PARP1-E988K and CAT-L713F mutants. D and E show results of the same type of HhaI deprotection assay and phosphorimaging, but reactions shown in E were incubated for only 30 min. In both panels, total level of nucleosome remodeling of each reaction calculated from a fraction of the HhaI cut is shown underneath the gel image, whereas levels of ALC1-dependent nucleosome remodeling are listed below the lines. Remod., remodeling.
These observations are consistent with the notion that binding of PARP1’s N-terminal domains to nucleosomes contributes more directly to PARP1 function in nucleosome remodeling, perhaps by helping to appropriately position ALC1 on nucleosomes or by modulating the architecture of nucleosomes in a way that makes them better substrates for ALC-dependent remodeling. If this is the case, it might be possible to reconstitute, or partially reconstitute, PARP1 function in remodeling by activating the ALC1 ATPase with the CAT-L713F mutant and supplying the PARP1 nucleosome binding domain in trans. To address this possibility, we tested two PARP1 mutants, PARP1-E988K (SI Appendix, Fig. S5B) and ΔCAT, which do not synthesize PAR but have intact nucleosome binding regions and bind nucleosomes with affinities similar to that of wild-type PARP1 (Fig. 4A and SI Appendix, Figs. S5D and S6). As shown earlier, there was little ALC1-dependent remodeling in the presence of CAT-L713F. As expected, there was no detectable ALC1-dependent remodeling with either ΔCAT or PARP1-E988K alone (Fig. 5E). However, providing either ΔCAT or PARP1-E988K in combination with CAT-L713F led to a synergistic increase in remodeling, partially restoring PARP1 function in ALC1-dependent nucleosome remodeling. Thus, the presence of a PARP1 nucleosome binding region, even when it is not covalently linked to an active CAT domain, increases the efficiency of ALC1-dependent nucleosome remodeling.
Discussion
In this report, we have investigated the role that PARP1 plays in ALC1-dependent nucleosome remodeling. We and others previously demonstrated that ALC1 possesses cryptic ATPase activity that is activated for nucleosome remodeling by binding through its macrodomain to PAR chains on auto-PARylated PARP1. More recently, it was shown that binding of tri-ADP-ribose to ALC1’s macrodomain can activate ALC1 in the absence of PARP1, by interfering with autoinhibitory interactions between the ALC1 SNF2 ATPase and macrodomains (13). This observation raised the question whether the sole function of PARP1 in ALC1-dependent nucleosome remodeling is to synthesize the PAR chains that activate ALC1 ATPase. In this report, we present evidence that PARP1 has additional functions in ALC1-dependent nucleosome remodeling.
Key evidence in support of this argument comes from our observation that a constitutively active PARP1 mutant, CAT-L713F, which includes only the PARP1 catalytic domain and is nearly as active in PAR synthesis as wild-type PARP1, strongly activates ALC1 ATPase but has little effect on its nucleosome remodeling activity. Thus, in addition to the PARP1 C-terminal ADP-ribosyl transferase domain required for PAR synthesis, one or more of the PARP1 noncatalytic domains must also contribute to ALC1-dependent nucleosome remodeling. In further experiments, we observed that no one of the PARP1 noncatalytic domains is absolutely required for nucleosome remodeling; however, PARP1 mutants ΔZn1-L713F, ΔZn3-L713F, and ΔWGR-L713F are all less active in remodeling than the wild-type enzyme, even though each of these has PARylation activity equal to or greater than wild-type PARP1. Hence, we suggest that optimal PARP1 function in remodeling likely involves the concerted action of the Zn1, Zn3, and WGR domains, in addition to the catalytic domain.
How do the PARP1 noncatalytic domains contribute to ALC1-dependent nucleosome remodeling? Previous studies have shown that they have DNA and nucleosome binding activities (18, 23, 27, 28, 31–33, 39, 40). Similar to the reduction in remodeling seen in reactions containing ΔZn1-L713F, ΔZn3-L713F, and ΔWGR-L713F, we observe that removal of the Zn1, Zn3, or WGR domain reduces nucleosome binding activity. In addition, based on results of XL-MS, each of these domains is in close proximity to the nucleosome core particle. Although no one of the N-terminal domains is essential for nucleosome binding, all appear to contribute to some degree. Indeed, our data indicate that the only mutant that fails to bind nucleosomes in both quantitative microscale thermophoresis experiments and in gel shift assays also supports little or no nucleosome remodeling.
How might PARP1 nucleosome binding activity contribute to remodeling? In light of previous evidence that PAR chains on nucleosome-bound auto-PARylated PARP1 can bind ALC1’s macrodomain and recruit ALC1 to regions of DNA damage in cells and to immobilized nucleosomes in vitro (3, 11, 14), one simple hypothesis is that the PARP1 noncatalytic domains just serve to recruit auto-PARylated PARP1 and ALC1 to nucleosomes. Arguing against this model, PARP1 nucleosome binding activity is clearly not needed to bring ALC1 to nucleosomes during activation of its ATPase, since ATPase can be activated either by CAT-L713F or by free PAR. In addition, we show here that simply tethering CAT-L713F to nucleosomes through an alternative DNA binding domain (GAL4) fails to restore nucleosome remodeling, even though GAL4-CAT-L713F binds nucleosomes and efficiently activates ALC1 ATPase.
An alternative hypothesis is that the PARP1 nucleosome binding activity plays a more direct role in ALC1-dependent remodeling. Consistent with the idea that specific binding of the PARP1 noncatalytic domains to nucleosomes is important for remodeling, we observe that ALC1-dependent nucleosome remodeling activity can be partially restored if CAT-L713F is supplemented with either of two catalytically inactive PARP1 mutants with intact nucleosome binding regions: PARP1-E988K, which has an inactivating point mutation in the catalytic domain, or PARP1ΔCAT, which lacks the CAT domain altogether. How PARP1 nucleosome binding activity facilitates remodeling remains to be determined. Of note, we observe that free PAR is unable to stimulate ALC1 nucleosome remodeling when added to reactions that include PARP1 but not NAD+ (SI Appendix, Fig. S10), raising the possibility that PARP1 auto-PARylation may be required. One possibility is that binding of ALC1 to nucleosome-bound, PARylated PARP1 helps to position ALC1 on the nucleosome in the proper orientation for remodeling. Another is that binding of PARylated PARP1 to nucleosomes might alter the structure of the nucleosome core particle in a way that facilitates ALC1-dependent remodeling. Relevant to the latter possibility, Muthurajan et al. (18) reported that auto-PARylation confers upon PARP1 the ability to function as a histone chaperone. Our finding that some histone–histone cross-links are altered in the presence of PARP1 would be consistent with the idea that PARP1 might induce subtle changes in core particle structure. Future studies will be needed to resolve these questions.
Materials and Methods
Detailed descriptions of plasmids and protocols for protein expression and purification, nucleosome preparation, cross-linking mass spectrometry, and for ATPase, nucleosome remodeling, nucleosome binding, and PARylation assays are provided in SI Appendix, Extended Materials and Methods.
Supplementary Material
Acknowledgments
We thank John Pascal and Marie-France Langelier for advice about PARP1 expression and purification and for a gift of an expression plasmid encoding wild-type PARP1. We are also grateful to Karolin Luger, Uma Muthurajan, and Maggie Chasse for the gift of purified PARP1, ΔZn2, and ΔZn2ΔBRCT mutants used in the initial phase of this study. We also thank Merry McLaird and Michaella Levy for help with PARP1 expression and purification and Aaron J. Gottschalk, Rushi Trivedi, and Boris Rubenstein for helpful discussions. This work was supported by the Stowers Institute for Medical Research and by a grant to the Stowers Institute from the Helen Nelson Medical Research Fund at the Greater Kansas City Community Foundation.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2107277118/-/DCSupplemental.
Data Availability
Raw data and search result files for the XL-MS experiments have been deposited to the Proteome Xchange (Accession number PXD025309) (42). Original data underlying this manuscript may be accessed from the Stowers Original Data Repository at https://www.stowers.org/research/publications/LIBPB-1616.
References
- 1.Ma N. F., et al., Isolation and characterization of a novel oncogene, amplified in liver cancer 1, within a commonly amplified region at 1q21 in hepatocellular carcinoma. Hepatology 47, 503–510 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Chen M., et al., Transgenic CHD1L expression in mouse induces spontaneous tumors. PLoS One 4, e6727 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahel D., et al., Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science 325, 1240–1243 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mejías-Navarro F., Rodríguez-Real G., Ramón J., Camarillo R., Huertas P., ALC1/eIF4A1-mediated regulation of CtIP mRNA stability controls DNA end resection. PLoS Genet. 16, e1008787 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pines A., et al., PARP1 promotes nucleotide excision repair through DDB2 stabilization and recruitment of ALC1. J. Cell Biol. 199, 235–249 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuda M., et al., ALC1/CHD1L, a chromatin-remodeling enzyme, is required for efficient base excision repair. PLoS One 12, e0188320 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blessing C., et al., The oncogenic helicase ALC1 regulates PARP inhibitor potency by trapping PARP2 at DNA breaks. Mol. Cell 80, 862–875.e6 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Hewitt G., et al., Defective ALC1 nucleosome remodeling confers PARPi sensitization and synthetic lethality with HRD. Mol. Cell 81, 767–783.e11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juhász S., et al., The chromatin remodeler ALC1 underlies resistance to PARP inhibitor treatment. Sci. Adv. 6, eabb8626 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma P., et al., ALC1 links chromatin accessibility to PARP inhibitor response in homologous recombination-deficient cells. Nat. Cell Biol. 23, 160–171 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottschalk A. J., et al., Poly(ADP-ribosyl)ation directs recruitment and activation of an ATP-dependent chromatin remodeler. Proc. Natl. Acad. Sci. U.S.A. 106, 13770–13774 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisemann T., Pascal J. M., Poly(ADP-ribose) polymerase enzymes and the maintenance of genome integrity. Cell. Mol. Life Sci. 77, 19–33 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh H. R., et al., A poly-ADP-ribose trigger releases the auto-inhibition of a chromatin remodeling oncogene. Mol. Cell 68, 860–871.e7 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Gottschalk A. J., Trivedi R. D., Conaway J. W., Conaway R. C., Activation of the SNF2 family ATPase ALC1 by poly(ADP-ribose) in a stable ALC1·PARP1·nucleosome intermediate. J. Biol. Chem. 287, 43527–43532 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dasovich M., et al., Identifying poly(ADP-ribose)-binding proteins with photoaffinity-based proteomics. J. Am. Chem. Soc. 143, 3037–3042 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehmann L. C., et al., Mechanistic insights into autoinhibition of the oncogenic chromatin remodeler ALC1. Mol. Cell 68, 847–859.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehmann L. C., et al., Mechanistic insights into regulation of the ALC1 remodeler by the nucleosome acidic patch. Cell Rep. 33, 108529 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muthurajan U. M., et al., Automodification switches PARP-1 function from chromatin architectural protein to histone chaperone. Proc. Natl. Acad. Sci. U.S.A. 111, 12752–12757 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langelier M. F., Eisemann T., Riccio A. A., Pascal J. M., PARP family enzymes: Regulation and catalysis of the poly(ADP-ribose) posttranslational modification. Curr. Opin. Struct. Biol. 53, 187–198 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langelier M. F., Zandarashvili L., Aguiar P. M., Black B. E., Pascal J. M., NAD+ analog reveals PARP-1 substrate-blocking mechanism and allosteric communication from catalytic center to DNA-binding domains. Nat. Commun. 9, 844 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawicki-McKenna J. M., et al., PARP-1 activation requires local unfolding of an autoinhibitory domain. Mol. Cell 60, 755–768 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogden T. E. H., et al., Dynamics of the HD regulatory subdomain of PARP-1; substrate access and allostery in PARP activation and inhibition. Nucleic Acids Res. 49, 2266–2288 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langelier M. F., Planck J. L., Roy S., Pascal J. M., Structural basis for DNA damage-dependent poly(ADP-ribosyl)ation by human PARP-1. Science 336, 728–732 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miranda E. A., Dantzer F., O’Farrell M., de Murcia G., de Murcia J. M., Characterisation of a gain-of-function mutant of poly(ADP-ribose) polymerase. Biochem. Biophys. Res. Commun. 212, 317–325 (1995). [DOI] [PubMed] [Google Scholar]
- 25.Lowary P. T., Widom J., New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J. Mol. Biol. 276, 19–42 (1998). [DOI] [PubMed] [Google Scholar]
- 26.Ali A. A. E., et al., The zinc-finger domains of PARP1 cooperate to recognize DNA strand breaks. Nat. Struct. Mol. Biol. 19, 685–692 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langelier M. F., Planck J. L., Roy S., Pascal J. M., Crystal structures of poly(ADP-ribose) polymerase-1 (PARP-1) zinc fingers bound to DNA: Structural and functional insights into DNA-dependent PARP-1 activity. J. Biol. Chem. 286, 10690–10701 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langelier M. F., Ruhl D. D., Planck J. L., Kraus W. L., Pascal J. M., The Zn3 domain of human poly(ADP-ribose) polymerase-1 (PARP-1) functions in both DNA-dependent poly(ADP-ribose) synthesis activity and chromatin compaction. J. Biol. Chem. 285, 18877–18887 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loeffler P. A., et al., Structural studies of the PARP-1 BRCT domain. BMC Struct. Biol. 11, 37 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altmeyer M., Messner S., Hassa P. O., Fey M., Hottiger M. O., Molecular mechanism of poly(ADP-ribosyl)ation by PARP1 and identification of lysine residues as ADP-ribose acceptor sites. Nucleic Acids Res. 37, 3723–3738 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark N. J., Kramer M., Muthurajan U. M., Luger K., Alternative modes of binding of poly(ADP-ribose) polymerase 1 to free DNA and nucleosomes. J. Biol. Chem. 287, 32430–32439 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wacker D. A., et al., The DNA binding and catalytic domains of poly(ADP-ribose) polymerase 1 cooperate in the regulation of chromatin structure and transcription. Mol. Cell. Biol. 27, 7475–7485 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steffen J. D., et al., Targeting PARP-1 allosteric regulation offers therapeutic potential against cancer. Cancer Res. 74, 31–37 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kao A., et al., Development of a novel cross-linking strategy for fast and accurate identification of cross-linked peptides of protein complexes. Mol. Cell. Proteomics 10, M110 002212 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davey C. A., Sargent D. F., Luger K., Maeder A. W., Richmond T. J., Solvent mediated interactions in the structure of the nucleosome core particle at 1.9 a resolution. J. Mol. Biol. 319, 1097–1113 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Luger K., Mäder A. W., Richmond R. K., Sargent D. F., Richmond T. J., Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 389, 251–260 (1997). [DOI] [PubMed] [Google Scholar]
- 37.Kim M. Y., Mauro S., Gévry N., Lis J. T., Kraus W. L., NAD+-dependent modulation of chromatin structure and transcription by nucleosome binding properties of PARP-1. Cell 119, 803–814 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Liu Z., Kraus W. L., Catalytic-independent functions of PARP-1 determine Sox2 pioneer activity at intractable genomic loci. Mol. Cell 65, 589–603.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eustermann S., et al., Structural basis of detection and signaling of DNA single-strand breaks by human PARP-1. Mol. Cell 60, 742–754 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurgina T. A., Anarbaev R. O., Sukhanova M. V., Lavrik O. I., A rapid fluorescent method for the real-time measurement of poly(ADP-ribose) polymerase 1 activity. Anal. Biochem. 545, 91–97 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Graham M., Combe C., Kolbowski L., Rappsilber J., xiView: A common platform for the downstream analysis of crosslinking mass spectrometry data. bioRxiv [Preprint] (2019) 10.1101/561829 (Accessed 15 March 2019). [DOI]
- 42.Ooi S.-K., et al., Multiple Roles for PARP1 in ALC1-dependent nucleosome remodeling. Proteome Xchange. http://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=pxd025309. Deposited 10 April 2021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data and search result files for the XL-MS experiments have been deposited to the Proteome Xchange (Accession number PXD025309) (42). Original data underlying this manuscript may be accessed from the Stowers Original Data Repository at https://www.stowers.org/research/publications/LIBPB-1616.